Abstract
BACKGROUND:
The rising prevalence and associated public health burden of obesity has led to advancements in pharmaceuticals for weight management. Semaglutide 2.4 mg, an anti-obesity medication (AOM) recently approved by the US Food and Drug Administration, has demonstrated clinically relevant weight loss in its phase 3 clinical trials. Economic evaluation comparing semaglutide 2.4 mg with other available weight management therapies is essential to inform payers for decision-making.
OBJECTIVES:
To assess the cost-effectiveness of semaglutide 2.4 mg in the treatment of adult patients with obesity (ie, body mass index [BMI] ≥ 30) and adult patients who are overweight (ie, BMI 27-29.9) with 1 or more weight-related comorbidities from a US third-party payer perspective.
METHODS:
A cohort Markov model was constructed to compare semaglutide 2.4 mg with the following comparators: no treatment, diet and exercise (D&E), and 3 branded AOMs (liraglutide 3 mg, phentermine-topiramate, and naltrexone-bupropion). All AOMs, including semaglutide 2.4 mg, were assumed to be taken in conjunction with D&E. Changes in BMI, blood pressure, cholesterol level, experience of acute and chronic obesity-related complications, costs, and quality-adjusted life years (QALYs) were simulated over 30 years based on pivotal trials of the AOMs and other relevant literature. Drug and health care prices reflect 2021 standardized values. Cost-effectiveness was examined with a willingness-to-pay (WTP) threshold of $150,000 per QALY gained. Sensitivity analyses were conducted to test the robustness of the cost-effectiveness results to plausible variation in model inputs.
RESULTS:
In the base-case analysis, treatment with semaglutide 2.4 mg was estimated to improve QALYs by 0.138 to 0.925 and incur higher costs by $3,254 to $25,086 over the 30-year time horizon vs comparators. Semaglutide 2.4 mg is cost-effective against all comparators at the prespecified WTP threshold, with the incremental cost per QALY gained ranging from $23,556 to $144,296 per QALY gained. In the sensitivity analysis, extended maximum treatment duration, types of subsequent treatment following therapy discontinuation, and weight-rebound rates were identified as key drivers for model results. The estimated probability of semaglutide 2.4 mg being cost-effective compared with comparators ranged from 67% to 100% when varying model parameters and assumptions.
CONCLUSIONS:
As a long-term weight management therapy, semaglutide 2.4 mg was estimated to be cost-effective compared with no treatment, D&E alone, and all other branded AOM comparators under a WTP threshold of $150,000 per QALY gained over a 30-year time horizon.
Plain language summary
Having obesity can be expensive given the high risk of many related health conditions. Semaglutide 2.4 mg is a new medication for obesity that resulted in more weight loss in clinical trials than other approved obesity medications. This study found that semaglutide 2.4 mg was a cost-effective treatment.
Implications for managed care pharmacy
Given the rising prevalence and profound economic burden associated with obesity and the evolving landscape of treatment options for weight management, comprehensive economic evaluations are crucial to inform decision-making for payers. Under a willingness-to-pay threshold of $150,000, semaglutide 2.4 mg was estimated to be cost-effective compared with no treatment, diet and exercise, and anti-obesity medications over a 30-year horizon. This finding may support coverage and reimbursement decisions for patients with obesity or overweight and weight-related comorbidities.
Obesity (body mass index [BMI] ≥ 30 [calculated as weight in kilograms divided by the square of height in meters]) and overweight (BMI 25-29.9) raise considerable public health concerns of global significance given their high prevalence and disease burden.1,2 Obesity and overweight are significantly associated with incidence of a wide spectrum of health conditions (eg, type 2 diabetes [T2D], gallbladder disease, cardiovascular disease, colon cancer, breast cancer, and endometrial cancer), which lead to impairment in quality of life for patients and substantial health care costs.3,4 The United States is among the countries with the highest prevalence of obesity and overweight, with prevalence rates projected to increase to nearly 50% and 30% by 2030, respectively.5 It is estimated that individuals with obesity and overweight in the United States incurred $1.72 trillion in total direct health care and indirect costs in 2016.6
Prevention and management of obesity and overweight are often challenging, as the etiology is complex and multifactorial, involving genetic, physiological, behavioral, and environmental factors.3,7 Emerging treatment options now allow physicians and patients to personalize treatments. Guideline-recommended therapies include lifestyle and behavioral interventions, pharmacotherapy, and bariatric surgeries.8,9 Although lifestyle interventions (ie, diet and exercise [D&E]) can result in significant health benefits, patients typically only achieve weight loss of up to 8 kg over 6-12 months of frequent and comprehensive treatment, and weight regain is common because of challenges with metabolic adaptation and maintaining D&E and ongoing counseling.10
For patients who struggle with weight management, treatment with an anti-obesity medication (AOM) (eg, naltrexone-bupropion, phentermine-topiramate, and liraglutide 3 mg) may be considered as add-on treatment to D&E.9,11 Semaglutide 2.4 mg, approved in 2021 by the US Food and Drug administration (FDA), is a long-acting, glucagon-like peptide-1 analogue that promotes weight loss via increased satiety and satiation and reduced hunger.12,13 In the pivotal, phase 3, placebo-controlled Semaglutide Treatment Effect in People with Obesity (STEP) 1 trial, semaglutide 2.4 mg had clinically significant weight loss (at least a 5% reduction in weight from baseline level) compared with placebo (weight reduction: 14.9% vs 2.4% at week 68; P < 0.001).11,14-16 The STEP 2 trial demonstrated similar weight loss benefits among adults with overweight/obesity and T2D,17 whereas the STEP 4 trial highlighted the continued weight loss achieved among patients who completed 68 weeks of treatment of semaglutide 2.4 mg vs patients who withdrew from semaglutide 2.4 mg at week 20.18
As weight management strategies evolve, it is important to estimate the cost-effectiveness of new medications to support coverage and reimbursement decisions. In this study, we aimed to estimate the cost-effectiveness of semaglutide 2.4 mg in the treatment of adult patients with obesity (ie, BMI ≥ 30) and adult patients with overweight (ie, BMI 27-29.9) and 1 or more weight-related comorbidities (ie, hypertension, dyslipidemia, obstructive sleep apnea, or cardiovascular disease).
Methods
MODEL OVERVIEW
A cohort Markov model (Figure 1) was developed in Microsoft Excel to estimate the cost-effectiveness of semaglutide 2.4 mg vs comparators in patients with obesity and overweight from a US third-party payer perspective. The model design for the current study was informed by a review of prior health technology assessment submissions and published economic evaluations for the management of obesity and overweight.19-22 Comparators considered in the model included no treatment, D&E alone, and branded AOMs that are standard-of-care for long-term weight management (ie, liraglutide 3 mg, phentermine-topiramate, and naltrexonebupropion).3 AOMs that have been withdrawn from the US market were not included in the analysis. All AOMs, including semaglutide 2.4 mg, are used in conjunction with D&E. Bariatric surgery was evaluated as an acute event, rather than as a comparator, because it is recommended only for patients with more severe obesity (ie, BMI ≥ 40 or ≥ 35 with obesity-related comorbidities).9
FIGURE 1.
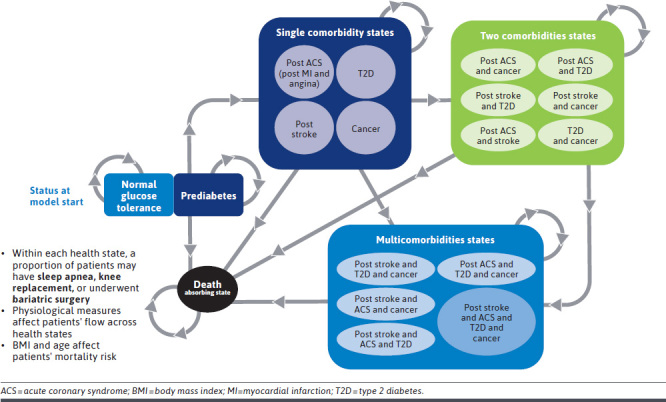
Model Structure and Schematic
Patients’ physiological parameters, obesity complications, health care costs, and utility were simulated over 30 years, which allows the model to capture outcomes along patients’ disease course from treatment initiation (at age of 46 years based on STEP 1 trial data) to reaching stable weight (approximately age 65-70 years).14,23 A cycle length of 3 months was applied in the first year, allowing for more accurate representation of treatment effects immediately following treatment initiation. Annual cycles were applied after the first year. Half-cycle correction was applied to estimate occurrence of state transitions in the middle of each cycle. An annual discount rate of 3% was applied to costs and quality-adjusted life expectancy, as recommended in the Institute for Clinical and Economic Review reference case.24 Key model assumptions are described in Supplementary Table 1 (1.4MB, pdf) , available in online article.
Efficacy (ie, changes in weight, blood pressure, and cholesterol) was applied for all patients receiving semaglutide 2.4 mg from treatment initiation and for responders to comparator AOMs on response evaluation. The response evaluation periods for comparator AOMs were based on corresponding prescribing information, including a nonresponder stopping rule, which was not required for semaglutide 2.4 mg based on the FDA label.25-28 Drug costs were incurred throughout the treatment duration, including during the response evaluation period. Patients could discontinue AOM treatment because of any reason before the 2-year maximum treatment duration, at which point all patients were assumed to stop AOM treatment. This assumption was based on the real-world observation that the majority of patients discontinued AOM treatments within 2 years.29 Patients discontinuing AOMs were assumed to continue lifestyle intervention (ie, D&E) until death or the end of the 30-year model time horizon. After AOM treatment discontinuation, weight loss benefit (represented by BMI reduction) is expected to diminish (ie, weight rebounds) at a more rapid rate than natural weight gain.21 The rebound rate was applied until patients’ BMI returned to the baseline level.21 After returning to baseline BMI, patients experienced natural weight gain until age 68 years.23 Other physiological parameters also returned to baseline level after treatment discontinuation but were assumed to maintain at baseline levels until death or the end of the modeled time horizon. For D&E, we assumed patients would benefit from lifestyle intervention with weight loss for approximately 2 years. Afterward, patients’ BMI were assumed to rebound back to baseline and experience natural weight gain. For the no treatment arm, patients experienced natural weight gain from model start until they reached stable weight at age 68 years.
Chronic conditions associated with obesity were captured by health states. Acute conditions and procedures were captured as events that could occur within health states. Patients could transition between the following 5 mutually exclusive categories of health states: no comorbidity (ie, normal glucose tolerance or prediabetes), single comorbidity (ie, postacute coronary syndrome, T2D, poststroke, and cancer), dual comorbidity, multicomorbidity, and death. Health events and acute complications considered in the model included bariatric surgery, acute coronary syndrome (myocardial infarction and angina), stroke (including transient ischemic attack), obstructive sleep apnea, and knee replacement. Transition probabilities between health states and the incidence of health events were derived from published risk equations accounting for factors such as physiological parameters (eg, BMI), medical history, and demographics. Additional details and equations are further described in Supplementary Table 2 (1.4MB, pdf) . A BMI adjustment factor estimated by meta-analysis was applied to age-sex–stratified natural mortality to estimate mortality risk.30
Health care costs for obesity treatment, consultation, management of comorbidities, and obesity treatment-related adverse events (AEs) were included in the base-case analysis. A scenario analysis from the societal perspective considering both medical costs and costs of BMI-related productivity loss was also explored. BMI-specific utility and disutility weights (ie, quality-of-life decrement) of obesity-related comorbidities were included in the model. Model outputs included life-years (LYs), quality-adjusted life-years (QALYs), and total and disaggregated costs (in 2021 US dollars). Incremental cost-effectiveness ratios (ICERs) were calculated in terms of incremental cost per QALY gained.
MODEL INPUTS
Key clinical, utility, and cost inputs are summarized in Table 1 and Supplementary Table 3 (1.4MB, pdf) .
TABLE 1.
Key Clinical, Utility, and Cost Inputs
| Cohort inputs | Value | Source |
|---|---|---|
| Baseline cohort characteristics | ||
| Age, y | 46.0 | Wilding et al,14 2021 |
| Female sex, % | 74.1 | |
| Male sex, % | 25.9 | |
| BMI | 37.9 | |
| SBP, mmHg | 126.0 | |
| Total cholesterol, mg/dL | 190.4 | |
| HDL cholesterol, mg/dL | 49.4 | |
| Proportion with prediabetes, % | 43.7 | |
| History of cardiovascular disease, % | 9.8 | |
| Clinical inputs | ||
| Minimum BMI for bariatric surgery | 35.0 | American Society for Metabolic and Bariatric Surgery49 |
| Annual incidence of bariatric surgery, % | 1.1 | English et al,50 2020 |
| Natural weight increase per year, kg | 0.5 | Ara et al,21 2012 |
| Cost inputs (2021 USD) | ||
| Cost of bariatric surgery (including costs associated with preoperative management, procedure, postsurgery follow-up, and complications) | $31,965.23 | Weiner et al,51 2013; Kizy et al,52 2017; Alsumali et al,53 2018; Lee et al,39 2020 |
| Cost of obesity monitoring per year | $622.50 | CMS Physician Fee Schedule 2021 |
| Utility inputs | ||
| BMI-specific utility | Utility range as 0.97-0.93 for the BMI domain of 30-42 (higher BMI is associated with lower utility) | Pi-Sunyer et al,35 2015 |
| Comorbidity disutilities | ||
| T2D | −0.07 | Shah et al,54 2020 |
| Post-ACS | −0.02 | Bhatt et al,55 2020 |
| OSA | −0.24 | Pietzsch et al,56 2015 |
| Cancer | −0.07 | Gough et al,57 2009 |
| Poststroke | −0.07 | Bhatt et al,55 2020 |
| Acute event disutilities | ||
| Bariatric surgery | −0.22 | Campbell et al,58 2010 |
| ACS | −0.03 | Bhatt et al,55 2020 |
| Knee replacement | −0.18 | Shah et al,54 2020 |
| Stroke | −0.09 | Shah et al,54 2020 |
| TIA | −0.07 | Bhatt et al,55 2020 |
| Treatment-specific inputs | Semaglutide 2.4 mg | Liraglutide 3 mg | Phentermine-topiramate | Naltrexone-bupropion | D&E | |
| Clinical inputs | ||||||
| Source | Wilding et al,14 2021 | Pi-Sunyer et al,35 2015, NNI data on file | Gadde et al,32 2011; Garvey et al,33 2012 | Greenway et al,34 2010; Apovian et al,31 2013 | Wilding et al,14 2021 | |
| Percentage change in BMI, % from baseline, mean (SE) | ||||||
| Month 6 | −11.7 (0.2) | −7.4 (0.1) | −8.7 (2.2) | −7.8 (0.2) | −2.8 (0.2) | |
| Year 1 | −14.9 (3.7) | −8.0 (0.1) | −9.6 (2.4) | −8.2 (2.1) | −2.4 (0.6) | |
| Year 2 | −17.6 (4.4) | −7.1 (0.2) | −9.3 (2.3) | −9.7 (2.4) | −2.8 (0.7) | |
| Response evaluation period, wk | 21 | 16 | 14 | 15 | 12 | |
| Titration period, wk | 16 | 5 | 2 | 3 | 0 | |
| Proportion of patients achieving response, % | — | 68.4 | 55.3 | 45.4 | 24.9 | |
| Years required to rebound to baseline weight after treatment discontinuation, y | 3 | 2 | 2 | 2 | 1 | |
| Cost inputs (2021 USD) | ||||||
| Source | IBM Redbook 2021,59 FDA Package Label 2021,25,28 FDA Package Label 2020,26,27 | |||||
| Titration period treatment cost, $ | 1,939.22 | 632.26 | 43.40 | 106.26 | — | |
| Annual maintenance treatment cost, $ | 17,597.48 | 16,459.72 | 2,264.55 | 3,696.33 | — | |
BMI was calculated as weight in kilograms divided by the square of height in meters.
— = not applicable; ACS = acute coronary syndrome; BMI = body mass index; D&E = diet and exercise; FDA = US Food and Drug Administration; HDL = high-density lipoprotein; IBM = International Business Machines Corporation; OSA = obstructive sleep apnea; SBP = systolic blood press; TIA = transient ischemic attack; T2D = type 2 diabetes; USD = United States dollar; wk = weeks; y = years.
Clinical Inputs. Treatment efficacy of AOMs and D&E were captured by changes in BMI, systolic blood pressure, high-density lipoprotein cholesterol, and total cholesterol. Response evaluation periods for AOM comparators were informed by US prescribing information, and efficacy inputs for the AOMs and D&E arms were informed by their respective pivotal phase 3 trials (Supplementary Table 4 (1.4MB, pdf) ).14,31-35 Given the limited availability of long-term efficacy data from AOM trials, assumptions based on the latest time point for which data were available were applied when data for specific time points were unavailable (detailed assumptions and extrapolation methods are described in Supplementary Table 2 (1.4MB, pdf) ).
Treatment discontinuation rates were obtained from the STEP 1 and Satiety and Clinical Adiposity–Liraglutide Evidence in Nondiabetic and Diabetic Individuals (SCALE) trials for semaglutide 2.4 mg and liraglutide 3 mg, respectively (Supplementary Table 5 (1.4MB, pdf) ).14,35 Given the limited longitudinal data regarding treatment discontinuation in the phase 3 trials of naltrexone-bupropion and phentermine-topiramate, treatment discontinuation for these AOMs were derived using hazard ratios (HRs) of treatment discontinuation among patients prescribed with liraglutide 3 mg, phentermine-topiramate (HR vs liraglutide 3 mg = 1.56), and naltrexone-bupropion (HR vs liraglutide 3 mg = 2.08) observed in a real-world study and trial-observed rates for liraglutide 3 mg.29,35 Discontinuation rates were extrapolated to the maximum treatment duration modeled in the base case (ie, 2 years). Patients were assumed to continue D&E throughout the modeled time horizon in the AOM and D&E groups in the base case.
The annual weight-rebound rate was defined as the proportion of the trial-observed efficacy benefit that diminished per year after treatment discontinuation. Weight-rebound rates were estimated based on total weight loss reported from the corresponding trial of each treatment14,31-35 and the weight increase per year observed in the STEP 4 trial in the base case18 or the STEP 1 trial for a scenario analysis evaluating discontinuation of both AOMs and D&E.14 Natural weight gain (0.47 kg/year) used in the model was estimated by Ara et al, using the General Practice Research Database.21
All-grade AE rates sourced from prescribing information were included. Since severity of AEs was not reported for the other AOMs and severe AE rates were required for the model, a percent severity multiplier was applied to capture the proportion of patients with severe AEs, which was based on the STEP 1 and STEP 2 trials of semaglutide 2.4 mg.14,17 It was assumed that the proportion of patients with severe AEs would be consistent across all treatments.
Utility Inputs. Utility varied by BMI level and comorbid conditions occurring throughout the modeled time horizon. In the base case, BMI-associated utility was estimated based on a linear model with a fixed utility decrement per unit BMI increase, accounting for age, prior history of heart disease, hypertension, and smoking status using patient-reported outcome scores from the SCALE trial for liraglutide 3 mg, which were mapped to utility scores.35 Disutility weights associated with comorbidities, acute events (including bariatric surgery), and treatment-related AEs were also accounted for and were informed by the literature (Table 1 and Supplementary Table 6 (1.4MB, pdf) ).
Cost Inputs. The model considered costs of drug acquisition, bariatric surgery, health states, acute events, and AEs (Table 1 and Supplementary Table 7 (1.4MB, pdf) ). Cost inputs were converted to 2021 US dollars, as necessary, using the consumer price index for medical services.36
SENSITIVITY ANALYSES
Deterministic sensitivity analysis (DSA) and scenario analyses were conducted to examine the influence of specific inputs and assumptions on model results (Supplementary Tables 8 and 9 (1.4MB, pdf) ). Key model parameters were varied by the 95% CI if such information was reported; if 95% CI was not available, model parameters were varied by ± 25% of the base-case value. Scenario analyses were performed to explore alternative treatment discontinuation assumptions, maximum treatment durations, bariatric surgery consideration, time horizons, discount rates, treatment discontinuation rates, baseline utilities by BMI, and natural weight-gain rates. A scenario analysis was also conducted from the societal perspective to account for the impact of treatment on indirect costs due to estimated BMI-related productivity loss, including absenteeism, presenteeism, disability benefits, and workers’ compensation. In addition, the cost-effectiveness of semaglutide 2.4 mg was examined among subgroup populations, including patients with BMI class I (30 ≤ BMI < 35), BMI class II (35 ≤ BMI < 40), BMI class III (BMI ≥ 40), or T2D.
A probabilistic sensitivity analysis was conducted using a Monte-Carlo simulation, with 1,000 iterations to estimate the probability of semaglutide 2.4 mg being cost-effective compared with comparator treatments under a willingness-to-pay (WTP) threshold of $150,000 per QALY, based on the value assessment framework of the Institute for Clinical and Economic Review.24 In each iteration, inputs were randomly drawn from specified distributions, using standard errors from original data sources, where available. In the absence of data on the variability around point estimates, variability was assumed as 25% of the mean value. The probabilistic sensitivity analysis results were presented in a cost-effectiveness acceptability curve comparing semaglutide 2.4 mg with each comparator (distributional assumptions are described in Supplementary Table 10 (1.4MB, pdf) ).
This article followed the Consolidated Health Economic Evaluation Reporting Standards Checklist for reports of economic evaluations of health interventions.
Results
BASE-CASE RESULTS
Estimated mean BMI trajectories over the modeled time horizon for each treatment arm are presented in Supplementary Figure 1 (1.4MB, pdf) . Total and breakdown costs, LY, QALY, and ICER estimates for each treatment arm are depicted in Table 2. Treatment with semaglutide 2.4 mg was estimated to be cost-effective against the current standard of care, D&E, with an ICER of $122,549 given improved QALYs of 0.181 and additional costs of $22,138 over a 30-year horizon. Semaglutide 2.4 mg was also estimated to be cost-effective compared with no treatment and other AOMs, with ICERs ranging from $27,113 (vs no treatment) to $144,296 (vs phentermine-topiramate).
TABLE 2.
Base-Case Cost-Effectiveness Results
| Semaglutide 2.4 mg | Liraglutide 3 mg | Phentermine-topiramate | Naltrexone-bupropion | D&E | No treatment | |
|---|---|---|---|---|---|---|
| Total costs, $ (2021 USD) | 130,040 | 126,786 | 109,078 | 109,977 | 107,902 | 104,954 |
| Obesity treatment costs | 26,399 | 20,455 | 2,249 | 3,021 | 0 | 0 |
| Obesity monitoring costs | 11,928 | 11,818 | 11,691 | 11,724 | 11,660 | 0 |
| Costs of chronic complications | 75,628 | 77,711 | 78,408 | 78,441 | 79,329 | 87,509 |
| Costs of acute events | 16,085 | 16,802 | 16,730 | 16,790 | 16,913 | 17,445 |
| Total QALYs | 13.492 | 13.354 | 13.347 | 13.335 | 13.311 | 12.567 |
| Total LYs | 17.071 | 17.018 | 17.022 | 17.015 | 17.010 | 16.601 |
| Incremental costs, $ (2021 USD) | — | 3,254 | 20,962 | 20,063 | 22,138 | 25,086 |
| Incremental QALYs | — | 0.138 | 0.145 | 0.157 | 0.181 | 0.925 |
| ICER, $ (incremental cost per QALY) | — | 23,556 | 144,296 | 127,518 | 122,549 | 27,113 |
— = not applicable; D&E = diet and exercise; ICER = incremental cost-effectiveness ratio; LY = life-year; QALY = quality-adjusted life-year; USD = United States dollar.
SENSITIVITY AND SCENARIO ANALYSES
Across all scenarios evaluated, the ICER for semaglutide 2.4 mg ranged from $30,540 to $253,206 compared with D&E. The model was most sensitive to maximum treatment duration and time horizon, followed by regimen after treatment discontinuation, weight-rebound rate, and semaglutide 2.4 mg efficacy on BMI. The tornado diagram in Figure 2 presents the 20 most influential sensitivity analyses in the comparison between semaglutide 2.4 mg and D&E. DSA results for semaglutide 2.4 mg compared with other comparators are described in Supplementary Figure 2 (1.4MB, pdf) . Of note, if all patients discontinued D&E, in addition to AOMs, after the 2-year maximum treatment duration, ICERs ranged from $7,287 (vs liraglutide 3 mg) to $51,025 (vs phentermine-topiramate). The ICERs for the comparisons with liraglutide 3 mg and no treatment were consistently below the $150,000/QALY WTP threshold over a 30-year horizon under all scenarios examined in the DSA. The results for the cost-effectiveness of semaglutide 2.4 mg among subgroup populations are described in Supplementary Tables 11-14 (1.4MB, pdf) . Notably, semaglutide 2.4 mg was estimated to be particularly cost-effective compared with D&E, no treatment, and other AOMs in the subgroup of patients with obesity class III (ICERs ranged from $8,094 for liraglutide 3 mg to $85,024 for phentermine-topiramate). In contrast, ICERs for semaglutide 2.4 mg was higher in the subgroup of patients with T2D (ranging from $87,211 for liraglutide 3 mg to $225,171 for phentermine-topiramate).
FIGURE 2.
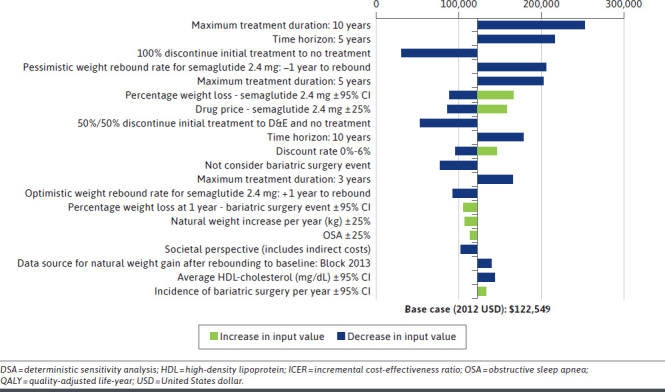
Tornado Diagram Based on DSA/Scenario Analyses for Semaglutide 2.4 mg vs Diet & Exercise
At a WTP threshold of $150,000 per QALY, the estimated probability of semaglutide 2.4 mg being cost-effective was 82% compared with D&E, 98% compared with liraglutide 3 mg, 64% compared with phentermine-topiramate, 74% compared with naltrexone-bupropion, and 100% compared with no treatment over a 30-year horizon (Figure 3, Supplementary Figure 3 (1.4MB, pdf) ).
FIGURE 3.
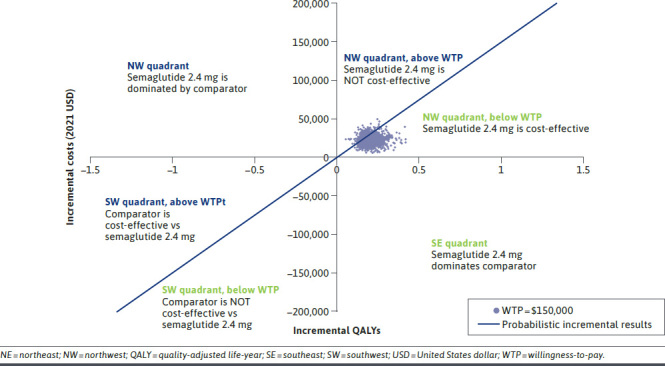
Cost-Effectiveness Plane for Semaglutide 2.4 mg vs Diet and Exercise
Discussion
Semaglutide 2.4 mg has demonstrated a well-tolerated safety profile and rapidly achieved and sustained weight loss in pivotal clinical trials.14 In the phase 3 STEP 1 trial, average weight loss was 14.9% after 68 weeks of semaglutide 2.4 mg use, with 69% of patients achieving more than 10% weight loss.14 This represents clinically significant and greater weight loss than those of other approved AOMs, which reported lower proportions of patients achieving sustained weight loss from their respective pivotal trials (eg, proportion of patients who achieved ≥ 10% weight loss at 1 year was 28% for naltrexone-bupropion and 48% for phentermine-topiramate).25,32 In addition, nonresponder stopping rules by regulatory agencies were only applied to other approved AOMs given the anticipated low success rates for nonresponders to achieve clinically meaningful weight loss with continued treatment.37 Adding to the demonstrated clinical benefit, the current study found that semaglutide 2.4 mg was estimated to be cost-effective at the WTP of $150,000 per QALY gained compared with all study comparators, including no treatment, D&E alone, and other AOMs over the 30-year horizon.
To our knowledge, this is the first cost-effectiveness analysis (CEA) of semaglutide 2.4 mg. Other AOMs have been evaluated in a limited number of CEAs, though aside from phentermine-topiramate in one study and phentermine in another,38,39 most have not demonstrated cost-effectiveness compared with lifestyle and behavioral interventions.40,41
Deterministic and probabilistic sensitivity analyses indicate the cost-effectiveness estimates were robust, although results are subject to more variation because of changes in a few parameters and assumptions. ICERs of semaglutide 2.4 mg vs comparators increased over longer treatment durations in part because of the higher drug cost incurred in the semaglutide 2.4 mg arm and higher treatment persistence compared with other AOMs. ICERs also increased with a 5-year time horizon analysis, suggesting that a short-term model may not capture the full and long-term benefits of semaglutide 2.4 mg treatment. Indeed, given that obesity is a complex and chronic disease associated with various long-term comorbidities, a shorter-term time horizon is insufficient to capture the effects of obesity treatments on the risk of chronic comorbidities in the long term and the associated impact on health care costs and quality of life.3,4 Thus, a 30-year time horizon was used to cover all relevant health outcomes and costs of obesity intervention. Additionally, since natural weight gain typically levels off at approximately 65-70 years,23 a 30-year horizon is sufficient to cover the time between treatment initiation (at age 46 years based on the STEP 1 trial)14 and this natural weight-gain plateau. Lastly, based on the scenario analyses, semaglutide 2.4 mg can be cost-effective below the $150,000 per QALY WTP threshold over a 30-year horizon when patients are assumed to also stop D&E after discontinuing AOMs, which may better reflect real-world practice. These findings have important implications in supporting the treatment decision-making process for payers.
This study contributes important insight to obesity management and economic evaluation by estimating the cost-effectiveness of semaglutide 2.4 mg with a model aiming to capture the real-world disease course of patients with obesity and overweight. In particular, a comprehensive list of key obesity-related comorbidities that may manifest over the natural course of disease were captured by health states or health events in the model. These comorbidities were identified via a systematic literature review of medical conditions with moderate to strong correlation with obesity. Incidence of chronic and acute comorbidities were estimated using well-established risk equations based on large-scale cohort studies and longitudinal metabolic parameters from phase 3 trials of AOMs. Costs and disutility values of obesity-related comorbidities based on published data were applied.
Importantly, the unique design of the STEP trials allows us to estimate weight rebound posttreatment cessation, a key driver of the cost-effectiveness estimates. The STEP 4 trial followed patients who received semaglutide 2.4 mg for 20 weeks and were subsequently randomly assigned to placebo with D&E for 48 weeks,18 thereby informing estimates of weight rebound for patients who discontinued AOM but remained on D&E. In the scenario analysis of patients who discontinued both AOM and D&E, data from the STEP 1 trial were used since the trial followed patients who received semaglutide 2.4 mg for 68 weeks and then discontinued both semaglutide 2.4 mg and D&E for 7 weeks.14 The STEP trial data are currently the only available data to inform posttreatment cessation weight-rebound rate.
Our model was developed following best practice guidelines with a comparable structure and design with the Core Obesity Model, which was validated by Lopes et al, per the International Society for Pharmacoeconomics and Outcomes Research and the Society for Medical Decision Making.42 The Lopes validation concluded that the disease course of obesity and obesity-related comorbid conditions were accurately predicted by the Core Obesity Model. Furthermore, implementation accuracy, including model structure, assumptions, and parameters, was validated by an academic expert in economic evaluation. External validation with a clinician specializing in obesity management was also conducted to ensure the model reflected the clinical practice and outcomes in the United States, including inputs of observed and extrapolated efficacy, posttreatment discontinuation weight-rebound rates, utility, and costs. Moreover, the model took a conservative approach to estimate the cost of semaglutide 2.4 mg by incorporating a longer titration period of semaglutide 2.4 mg (16 weeks) relative to the comparators and thus the associated additional titration costs. In addition, the cost-effectiveness of comparator AOMs estimated in the current model are broadly aligned with prior economic evaluation. The extensive validation steps and the comparable results for other AOMs with literature indicate that the current model was well-designed and fit for the purpose of supporting the economic assessment of semaglutide 2.4 mg vs relevant alternative strategies in the treatment of obesity.
LIMITATIONS
Certain limitations apply to this model and the input data. Baseline cohort characteristics were based on the STEP 1 trial population, which may not be representative of the general population with obesity. Treatment efficacies were derived from randomized controlled trials, with simulated results over 30 years, which may have limited generalizability to the real-world disease course of obesity. Naive treatment comparison based on respective pivotal trial data for semaglutide 2.4 mg and other AOMs was used to inform treatment efficacy as head-to-head and indirect treatment comparisons between semaglutide 2.4 mg and comparators were not available (the STEP 8 trial comparing semaglutide 2.4 mg vs liraglutide 3 mg was published after study completion).43 Treatment inputs for D&E were derived from STEP 1, in which patients received individual counseling to support calorie reduction and increased physical activity, which may not be representative of typical D&E in the real world. Although trial design and target population of the pivotal phase 3 trials were broadly comparable across semaglutide 2.4 mg and other AOMs, differences in trial populations existed. Notably, the CONQUER and SEQUEL trials of phentermine-topiramate included patients with T2D at baseline,32,33 whereas patients with T2D were excluded from the trials for naltrexone-bupropion and semaglutide 2.4 mg.14,31,34 Since weight loss efficacy is generally lower in patients with T2D,44 this may have impacted the comparison between phentermine-topiramate and semaglutide 2.4 mg. In addition, the phentermine-topiramate trials excluded patients with uncomplicated obesity,32,33 whereas such patients were included in the trials for naltrexone-bupropion and semaglutide 2.4 mg.14,31,34 Nevertheless, given the lack of head-to-head comparative studies of these AOMs at the time of analysis, the clinical efficacy inputs were deemed appropriate by clinicians. Long-term efficacy extrapolation was performed for all AOMs beyond the trial observation period, which was validated by clinicians and an academic methodologist. Studies of AOMs with extended follow-up periods and direct comparisons between AOMs are warranted to contribute longer-term efficacy data to the growing body of obesity literature, including the STEP 5 trial; the findings of which were presented after completion of the current study and may be incorporated in future iterations of cost-effectiveness modeling.45
Moreover, real-world data on weight-rebound rates after treatment discontinuation were not available. Therefore, trial-based data were used, with an assumption that rebound rates remained constant until weight returned to baseline value. Additionally, although the 2-year maximum treatment duration was informed by real-world use of comparator AOMs,29 this may not reflect real-world use of semaglutide 2.4 mg, in which treatment duration may be extended given its favorable weight loss benefit and safety profile. Of note, our study used trial-based treatment discontinuation rates in the base-case analysis, as these data correspond with the trial-observed efficacy data. However, treatment discontinuation was extrapolated using real-world HR-based derivation for phentermine-topiramate and naltrexone-bupropion because of lack of longitudinal data from their respective phase 3 trial publications. Uncertainties may be introduced by the derivation, although the HR-based derivation is a well-established approach to extrapolate time-to-event variables when observed data are not available.46
Assumptions that may not be reflective of the real-world situation were used in the model when lacking clinical data. For instance, patients were assumed to continue D&E throughout the modeled time horizon, irrespective of AOM treatments in the base case, although lifestyle intervention may be challenging to maintain over the long-term. The AEs considered in the model were only those that were specific to glucagon-like peptide-1 AOMs, which represents a conservative estimate since additional costs for AE management of phentermine-topiramate and naltrexone-bupropion (eg, tachycardia and hypertension) were not included. Additionally, real-world usage of AOMs is not represented in this study; AOMs are known to be underused in clinical practice because of physician hesitancy to prescribe, limited health insurance coverage, and consequently high out-of-pocket costs for the patients.47,48 Moreover, approved, branded AOMs indicated for long-term use were evaluated in this study, so the study findings may not be generalizable to the short-term use of generic AOMs (eg, phentermine) or off-label generic components of the branded AOM combination formulations.
Lastly, the model simulated outcomes over 30 years, and most patients would transition from commercial insurance to Medicare starting from the age of 65 years; however, the model did not differentiate health care resource use or cost based on commercial insurance coverage and Medicare coverage after the patients reached the age of 65. From a commercial insurance payer perspective, this model may overestimate the longterm costs after patients reaching the age of 65. In addition, drug costs for the AOMs represent acquisition costs and do not account for rebates, copay, or coinsurance, as these vary widely in the United States.
Conclusions
In this economic evaluation, semaglutide 2.4 mg use was associated with higher life expectancy and QALYs compared with no treatment, D&E, and all other branded AOMs over a 30-year time horizon. Under a WTP threshold of $150,000, semaglutide 2.4 mg was estimated to be cost-effective relative to all comparators evaluated among adult patients with obesity and adult patients with overweight and 1 or more weight-related comorbidities. Semaglutide 2.4 mg has the potential to fulfill a significant unmet need and represents a cost-effective treatment option for adults with obesity in the United States.
ACKNOWLEDGMENTS
Medical writing support was provided by a professional medical writer, Christine Tam, an employee of Analysis Group, Inc. Support for this assistance was provided by Novo Nordisk Inc. The authors thank Dr Josh Carlson for validating the design and implementation of the cost-effectiveness model.
REFERENCES
- 1.Centers for Disease Control and Prevention. Overweight and obesity: Adult obesity facts. 2021. Accessed May 21, 2021. https://www.cdc.gov/obesity/data/adult.html
- 2.Fryar C, Carroll M, Afful J. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960–1962 Through 2017–2018. 2021. https://www.cdc.gov/nchs/data/hestat/obesity-adult-17-18/obesity-adult.htm#Citation
- 3.Bray GA, Fruhbeck G, Ryan DH, Wilding JP. Management of obesity. Lancet. 2016;387(10031):1947-56. doi: 10.1016/S0140-6736(16)00271-3 [DOI] [PubMed] [Google Scholar]
- 4.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of comorbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward ZJ, Bleich SN, Cradock AL, et al. Projected US state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381(25):2440-50. doi: 10.1056/NEJMsa1909301 [DOI] [PubMed] [Google Scholar]
- 6.Waters H, Graf M. America’s obesity crisis: The health and economic costs of excess weight. Milken Institute; 2018. Accessed May 21, 2021. https://milkeninstitute.org/report/americas-obesity-crisis-health-and-economic-costs-excess-weight [Google Scholar]
- 7.Centers for Disease Control and Prevention. Defining adult overweight & obesity. 2021. Accessed July 19, 2021. https://www.cdc.gov/obesity/adult/defining.html
- 8.Wyatt HR. Update on treatment strategies for obesity. J Clin Endocrinol Metab. 2013;98(4):1299-306. doi: 10.1210/jc.2012-3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan DH, Kahan S. Guideline recommendations for obesity management. Med Clin North Am. 2018;102(1):49-63. doi: 10.1016/j.mcna.2017.08.006 [DOI] [PubMed] [Google Scholar]
- 10.American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Obesity Expert Panel. Expert panel report: Guidelines (2013) for the management of overweight and obesity in adults. Obesity (Silver Spring). 2014;22 Suppl 2:S41-410. doi: 10.1002/oby.20660 [DOI] [PubMed] [Google Scholar]
- 11.Daneschvar HL, Aronson MD, Smetana GW. FDA-approved anti-obesity drugs in the United States. Am J Med. 2016;129(8):879.e871-76. doi: 10.1016/j.amjmed.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 12.Blundell J, Finlayson G, Axelsen M, et al. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes Metab. 2017;19(9):1242-51. doi: 10.1111/dom.12932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibbons C, Blundell J, Tetens Hoff S, Dahl K, Bauer R, Baekdal T. Effects of oral semaglutide on energy intake, food preference, appetite, control of eating and body weight in subjects with type 2 diabetes. Diabetes Obes Metab. 2021;23(2):581-88. doi: 10.1111/dom.14255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilding JP, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989. doi: 10.1056/NEJMoa2032183 [DOI] [PubMed] [Google Scholar]
- 15.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403. doi: 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Blair SN, Church TS. Effects of clinically significant weight loss with exercise training on insulin resistance and cardiometabolic adaptations. Obesity (Silver Spring). 2016;24(4):812-19. doi: 10.1002/oby.21404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies M, Faerch L, Jeppesen OK, et al. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): A randomised, doubleblind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397(10278): 971-84. doi: 10.1016/S0140-6736(21)00213-0 [DOI] [PubMed] [Google Scholar]
- 18.Rubino D, Abrahamsson N, Davies M, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: The STEP 4 Randomized Clinical Trial. JAMA. 2021;325(14):1414-25. doi: 10.1001/jama.2021.3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institute for Health and Care Excellence (NICE). Technology appraisal guidance [TA664]. Liraglutide for managing overweight and obesity. 2020. Accessed May 31, 2021. https://www.nice.org.uk/guidance/ta664
- 20.Ara R, Blake L. PSY36 modelling the cost-effectiveness of orlistat as a treatment for obesity in primary care. Value Health. 2011;14(7):A416-17. doi: 10.1016/j.jval.2011.08.1008 [DOI] [Google Scholar]
- 21.Ara R, Blake L, Gray L, et al. What is the clinical effectiveness and cost-effectiveness of using drugs in treating obese patients in primary care? A systematic review. Health Technology Assess. 2012;16(5):iii-xiv. doi: 10.3310/hta16050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis L, Taylor M, Broom J, Johnston K. The cost-effectiveness of the LighterLife weight management programme as an intervention for obesity in England. Clin Obe. 2014;4(3):180-88. doi: 10.1111/cob.12060 [DOI] [PubMed] [Google Scholar]
- 23.National Institute for Health and Care Excellence (NICE). Single Technology Appraisal: Liraglutide for Managing Overweight and Obesity [ID740]. NICE; 2020. [Google Scholar]
- 24.Institute for Clinical and Economic Review (ICER). 2020-2023 Value Assessment Framework. ICER; 2020. [Google Scholar]
- 25.Contrave. Package insert. Currax Pharmaceuticals LLC. 2021. [Google Scholar]
- 26.Qsymia. Package insert. VIVUS, Inc. 2020. [Google Scholar]
- 27.Saxenda. Package insert. Novo Nordisk Inc. 2020. [Google Scholar]
- 28.Wegovy. Package insert. Novo Nordisk Inc. 2021. [Google Scholar]
- 29.Ganguly R, Tian Y, Kong SX, et al. Persistence of newer anti-obesity medications in a real-world setting. Diabetes Res Clin Pract. 2018;143:348-56. doi: 10.1016/j.diabres.2018.07.017 [DOI] [PubMed] [Google Scholar]
- 30.Di Angelantonio E, Bhupathiraju SN, Wormser D, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776-86. doi: 10.1016/S0140-6736(16)30175-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring). 2013;21(5):935-43. doi: 10.1002/oby.20309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): A randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341-52. doi: 10.1016/S0140-6736(11)60205-5 [DOI] [PubMed] [Google Scholar]
- 33.Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): A randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2):297-308. doi: 10.3945/ajcn.111.024927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595-605. doi: 10.1016/S0140-6736(10)60888-4 [DOI] [PubMed] [Google Scholar]
- 35.Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11-22. doi: 10.1056/NEJMoa1411892 [DOI] [PubMed] [Google Scholar]
- 36.United States Department of Labor, Bureau of Labor Statistics. Consumer price index - all urban consumers. Accessed February 21, 2021. https://data.bls.gov/timeseries/CUUR0000SAM
- 37.Rebello CJ, O’Neil PM, Horn DB, Greenway FL. Timing the discussion of antiobesity medications during obesity treatment. Obesity (Silver Spring). 2016;24(10):2027-28. doi: 10.1002/oby.21614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finkelstein EA, Kruger E, Karnawat S. Cost-effectiveness analysis of qsymia for weight loss. Pharmacoeconomics. 2015;33(7):699-706. doi: 10.1007/s40273-014-0182-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee M, Lauren BN, Zhan T, et al. The cost-effectiveness of pharmacotherapy and lifestyle intervention in the treatment of obesity. Obes Sci Pract. 2020;6(2): 162-70. doi: 10.1002/osp4.390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finkelstein EA, Verghese NR. Incremental cost-effectiveness of evidence-based non-surgical weight loss strategies. Clin Obes. 2019;9(2):e12294. doi: 10.1111/cob.12294 [DOI] [PubMed] [Google Scholar]
- 41.Nuijten M, Marczewska A, Araujo Torres K, Rasouli B, Perugini M. A health economic model to assess the cost-effectiveness of OPTIFAST for the treatment of obesity in the United States. J Med Econ. 2018;21(9):835-44. doi: 10.1080/13696998.2018.1468334 [DOI] [PubMed] [Google Scholar]
- 42.Lopes S, Johansen P, Lamotte M, McEwan P, Olivieri AV, Foos V. External validation of the core obesity model to assess the cost-effectiveness of weight management interventions. Pharmacoeconomics. 2020;38(10):1123-33. doi: 10.1007/s40273-020-00941-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubino DM, Greenway FL, Khalid U, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: The STEP 8 Randomized Clinical Trial. JAMA. 2022;327(2):138-50. doi: 10.1001/jama.2021.23619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pi-Sunyer FX. Weight loss in type 2 diabetic patients. Diabetes Care. 2005;28(6):1526-27. doi: 10.2337/diacare.28.6.1526 [DOI] [PubMed] [Google Scholar]
- 45.Garvey WT, Batterham RL, Bhatta M, et al. Two-year effect of semaglutide 2.4 mg vs placebo in adults with overweight or obesity: STEP 5. Paper presented at 39th Annual Meeting of The Obesity Society 2021. [Google Scholar]
- 46.Vickers A. An evaluation of survival curve extrapolation techniques using long-term observational cancer data. Med Decis Making. 2019;39(8):926-38. doi: 10.1177/0272989X19875950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gomez G, Stanford FC. US health policy and prescription drug coverage of FDA-approved medications for the treatment of obesity. Int J Obes (Lond). 2018;42(3):495-500. doi: 10.1038/ijo.2017.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.United States Government Accountability Office. Obesity drugs: Few adults used prescription drugs for weight loss and insurance coverage varied. 2019. Accessed May 21, 2021. https://www.gao.gov/products/gao-19-577
- 49.American Society for Metabolic and Bariatric Surgery. Who is a candidate for bariatric surgery? Accessed May 26, 2020. https://asmbs.org/patients/who-is-a-candidate-for-bariatric-surgery
- 50.English WJ, DeMaria EJ, Hutter MM, et al. American Society for Metabolic and Bariatric Surgery 2018 estimate of metabolic and bariatric procedures performed in the United States. Surg Obes Relat Dis. 2020;16(4):457-63. doi: 10.1016/j.soard.2019.12.022 [DOI] [PubMed] [Google Scholar]
- 51.Weiner JP, Goodwin SM, Chang HY, et al. Impact of bariatric surgery on health care costs of obese persons: A 6-year follow-up of surgical and comparison cohorts using health plan data. JAMA Surg. 2013;148(6):555-62. doi: 10.1001/jamasurg.2013.1504 [DOI] [PubMed] [Google Scholar]
- 52.Kizy S, Jahansouz C, Downey MC, Hevelone N, Ikramuddin S, Leslie D. National trends in bariatric surgery 2012-2015: Demographics, procedure selection, readmissions, and cost. Obes Surg. 2017;27(11):2933-39. doi: 10.1007/s11695-017-2719-1 [DOI] [PubMed] [Google Scholar]
- 53.Alsumali A, Eguale T, Bairdain S, Samnaliev M. Cost-effectiveness analysis of bariatric surgery for morbid obesity. Obes Surg. 2018;28(8):2203-14. doi: 10.1007/s11695-017-3100-0 [DOI] [PubMed] [Google Scholar]
- 54.Shah CH, Brown JD. Reliability and validity of the short-form 12 item version 2 (SF-12v2) health-related quality of life survey and disutilities associated with relevant conditions in the US older adult population. J Clin Med. 2020;9(3):661. doi: 10.3390/jcm9030661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhatt DL, Briggs AH, Reed SD, et al. Cost-effectiveness of alirocumab in patients with acute coronary syndromes: The ODYSSEY OUTCOMES Trial. J Am Coll Cardiol. 2020;75(18):2297-308. doi: 10.1016/j.jacc.2020.03.029 [DOI] [PubMed] [Google Scholar]
- 56.Pietzsch JB, Liu S, Garner AM, Kezirian EJ, Strollo PJ. Long-term cost-effectiveness of upper airway stimulation for the treatment of obstructive sleep apnea: A model-based projection based on the STAR Trial. Sleep. 2015;38(5):735-44. doi: 10.5665/sleep.4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gough SC, Kragh N, Ploug UJ, Hammer M. Impact of obesity and type 2 diabetes on health-related quality of life in the general population in England. Diabetes Metab Syndr Obes. 2009;2:179-84. doi: 10.2147/dmsott.s7088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Campbell J, McGarry LA, Shikora SA, Hale BC, Lee JT, Weinstein MC. Cost-effectiveness of laparoscopic gastric banding and bypass for morbid obesity. Am J Manag Care. 2010;16(7):e174-87. [PubMed] [Google Scholar]
- 59.RED BOOK Online. IBM Micromedex [database online]. 2020. Accessed May 31, 2021. https://www.micromedexsolutions.com


