Abstract
BACKGROUND:
Antineutrophil cytoplasmic antibody (ANCA) vasculitis (AV) is a complex group of autoimmune disorders affecting blood vessels in multiple organ systems. Delays in diagnosis are common because AV symptoms can be nonspecific and present heterogeneously. This may result in increased health care utilization in the months preceding diagnosis.
OBJECTIVE:
To examine whether Medicare beneficiaries with AV experienced increased health care utilization and costs in the year before the first diagnosis recorded in claims, relative to beneficiaries without AV.
METHODS:
This retrospective cohort study used 2015-2016 Medicare Part A/B claims and Part D prescription drug data. Beneficiaries with newly diagnosed AV were identified by having 1 or more inpatient claims or 2 or more noninpatient claims 7 or more days apart in 2016 with an International Classification of Diseases, Tenth Revision, Clinical Modification code for AV, with no AV claims in the year prior. Beneficiaries with AV were matched 1:1 on age and sex to beneficiaries without any diagnoses for any type of systemic vasculitis in 2016. Beneficiaries with Part A/B coverage (AB, n = 1,460) and Part A/B/D coverage (ABD, n = 3,252) were analyzed separately. We estimated generalized linear mixed models with a negative binomial distribution to compare health care costs and utilization by AV status.
RESULTS:
Beneficiaries with AV had approximately 3 times higher Medicare Part A/B payments (incidence rate ratio [95% CI]: AB: 2.94 [2.44-3.53]; ABD: 2.95 [2.64-3.29]) and 2.5 times higher beneficiary Part A/B payments (AB: 2.47 [2.14-2.84]; ABD: 2.62 [2.40-2.87]) vs beneficiaries without AV. Beneficiaries with AV experienced significantly higher utilization across all categories, with the largest differences observed in hospital outpatient visits (AB: 2.69 [2.22-3.27]; ABD: 3.08 [2.73-3.47]).
CONCLUSIONS:
In the year prior to AV diagnosis, Medicare beneficiaries have significantly higher health care costs and utilization than beneficiaries without AV.
Plain language summary
Medicare patients had significant increases in health care costs and use in the year prior to a new antineutrophil cytoplasmic antibody vasculitis (AV) diagnosis, compared with patients without AV. These data suggest that health care use may escalate because of worsening AV symptoms that lead to more frequent appointments and testing. Our study illustrates the high burden of AV to the health care system even before formal diagnosis.
Implications for managed care pharmacy
The economic burden of patients with AV may be underestimated owing to the difficulty of diagnosing systemic autoimmune diseases. Our study found that in Medicare patients with AV, the increased health care utilization and costs begin at least 1 year prior to formal diagnosis. Future research is needed to accelerate detection of AV for earlier intervention and ultimately improve health outcomes for patients with AV.
Antineutrophil cytoplasmic antibody (ANCA) vasculitis (AV) is a group of rare, systemic autoimmune diseases with 3 subtypes: granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (EGPA). Although clinical presentation of each subtype can vary, all are characterized by inflammation and necrosis of small- and medium-sized blood vessels, often causing severe damage to multiple organs.1-4 Pulmonary involvement and glomerulonephritis are common clinical manifestations of GPA and MPA, whereas EGPA presents with eosinophilia and asthma.5 AV was once fatal in up to 80% of patients within a year; however, the use of effective immunosuppressive medications now results in a chronic, relapsing-remitting disease course and 5-year survival rates of 73%-83% for GPA, 45%-85% for MPA, and 89%-97% for EGPA.6,7
Early diagnosis of AV is imperative, as delays in diagnosis are associated with irreversible organ damage.4 Although there is variation in the treatment strategy for remission induction depending on disease subtype and initial disease severity, there is consensus that prompt immunosuppressive treatment is necessary to improve morbidity and mortality.3,4 The latest US guidelines from the American College of Rheumatology and the Vasculitis Foundation recommend initial treatment with cyclophosphamide (an alkylating antineoplastic agent) or rituximab (a monoclonal antibody) for severe AV, with pulse intravenous glucocorticoids or high-dose daily glucocorticoids as an additional treatment option.5 For initial treatment of nonsevere disease, glucocorticoids plus methotrexate are preferred for GPA and MPA, whereas glucocorticoids plus mepolizumab are preferred for EGPA; cyclophosphamide and rituximab can also be used for nonsevere disease in all subtypes. Other treatment options include azathioprine and mycophenolate mofetil, typically combined with glucocorticoids.
Unfortunately, health care providers frequently have difficulty establishing the diagnosis, and patients with AV often experience delays in receiving treatment.2 This is likely due to the prodromal phase of AV, characterized by nonspecific symptoms, (eg, fatigue, fevers, and arthralgias), which poses a challenge for early identification. Furthermore, patients are likely to first seek treatment with general practitioners, who may be less familiar with AV. Studies have shown that prolonged intervals from first symptoms to diagnosis are associated with end-stage kidney disease (ESKD) and mortality.8,9 After emergence of symptoms, patients’ use of health care services and associated costs may increase, as diagnosis may require multiple visits with various subspecialty providers.
A previous study reported that Medicare beneficiaries diagnosed with any type of systemic vasculitis (SV), including AV, have double the annual health care costs compared with beneficiaries without SV but did not examine utilization or costs before diagnosis.10 The increased utilization likely begins prior to formal diagnosis, and there is limited literature regarding the burden of AV before diagnosis. One study conducted in commercially insured patients found that 33% and 61% of patients with new diagnoses of GPA and MPA, respectively, experienced a major symptom of AV in the 6 months before first diagnosis.11 Investigators noted that health care costs for patients newly diagnosed with MPA increased before diagnosis, peaking at 3 months after diagnosis. The extent to which these findings generalize to patients with other AV subtypes and with primary Medicare insurance coverage, the most common US insurer of hospitalized patients with SV, is unknown owing to differences between populations (eg, age, health status, and socioeconomic status).12
To our knowledge, burden of AV manifestation during the prediagnosis period has not been characterized in the Medicare population. This study aimed to evaluate whether Medicare fee-for-service (FFS) beneficiaries newly diagnosed with AV had higher health care costs and utilization in the 12 months before diagnosis than Medicare beneficiaries without AV.
Methods
STUDY DESIGN AND DATA SOURCES
This study was approved by the University of North Carolina Institutional Review Board. We conducted a retrospective, observational study using 2015-2016 Medicare Part A and Part B medical claims, Part D prescription data, and the Master Beneficiary Summary File obtained under a data use agreement from the Centers for Medicare & Medicaid Services (CMS). We obtained these data for a cohort of all FFS beneficiaries with at least 1 medical claim of any type (inpatient, outpatient, skilled nursing, home health, hospice, durable medical equipment, or carrier/professional services) in 2016 with an International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis code for any type of SV in any position (N = 148,742), including AV subtypes as well as other forms of SV affecting other types of blood vessels. Supplementary Table 1 (144.2KB, pdf) (available in online article) contains a complete list of SV diagnosis codes. We also obtained data for a random sample of beneficiaries without any claims with an ICD-9-CM or ICD-10-CM diagnosis code for SV in 2015-2016 (N = 851,257). This combined cohort of 999,999 Medicare beneficiaries made up our source population for the study sample. Per the data use agreement with CMS, these data are confidential and cannot be shared outside of the research team.
STUDY SAMPLE
Construction of the study sample from the source population is summarized in Figure 1. To identify incident cases of AV in 2016 for our primary analysis, we included beneficiaries aged 18 years and older who had 1 or more inpatient claims or 2 or more noninpatient claims at least 7 days apart in 2016 with an ICD-10-CM code for GPA (M31.30 or M31.31), MPA (M31.7), or EGPA (M30.1), with no claims with an ICD-9-CM or ICD-10-CM diagnosis code for these conditions or arteritis unspecified (447.6, I77.6) in the 12 months prior to index. In addition, the 2 noninpatient claims had to be for the same qualifying ICD-10-CM code. We restricted to beneficiaries who were continuously enrolled in Medicare Parts A and B, with no Medicare Advantage enrollment in the 12 months prior to index, and then stratified these beneficiaries into 2 subgroups: (1) beneficiaries with Parts A/B medical coverage only (AB sample) and (2) beneficiaries with continuous Part D prescription drug coverage in addition to Part A/B medical coverage (ABD sample). Medicare Advantage beneficiaries were excluded because of incomplete availability of medical claims.
FIGURE 1.
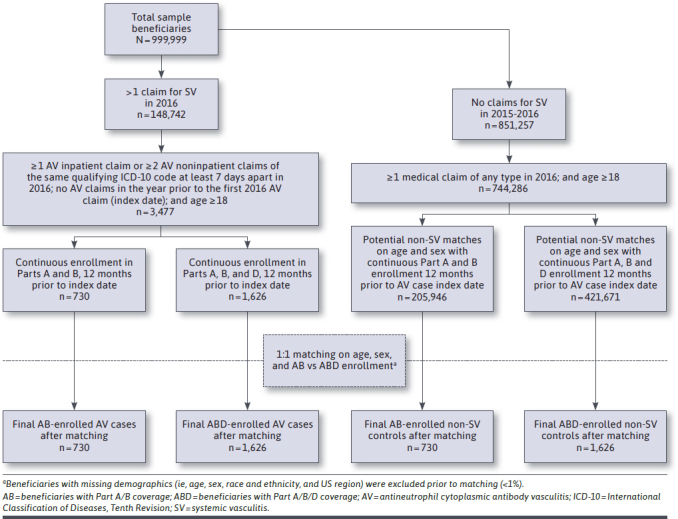
Sample Construction Diagram
We then identified all beneficiaries in the non-SV source population with at least 1 medical claim of any type in 2016 to obtain comparison groups for both the AB and ABD samples. Beneficiaries with AV were matched 1:1 on age (calculated in months) and sex to beneficiaries without diagnosis codes for any type of SV in 2015 or 2016, without replacement. The study index date for beneficiaries with AV was the date of the first AV claim in 2016, which also served as the index date for the matched control (non-AV). Beneficiaries with missing demographics (ie, age, sex, race and ethnicity, or US census region) were excluded prior to matching (less than 1%).
DEPENDENT VARIABLES
We summarized beneficiaries’ annual health care utilization and costs (rounded to the nearest whole dollar) in the 12 months prior to the index date in 2016 as dependent variables. These included total annual Medicare and beneficiary Parts A and B payments, total annual Medicare and beneficiary Part B drug payments, and annual counts of specific utilization events, including acute inpatient stays, hospital outpatient visits, emergency department visits, Part B (infused) drug events, Part B physician office services, imaging, tests, other procedures, durable medical equipment events, and other Part B events. We defined these variables using publicly available specifications established by CMS to summarize utilization and costs on a calendar year basis.13 However, we applied these definitions to the year prior to the index date rather than the calendar year. In addition, total annual medical and drug costs for the AB and ABD samples were measured in 3-month intervals (quarterly) to examine trends over time in the year leading up to the first AV diagnosis.
For the ABD sample, we measured total annual Medicare and beneficiary Part D drug payments and the number of Part D prescription fills, standardized to a duration of 30 days. We also determined the use of specific medication classes of interest (corticosteroids, opioids, nonsteroidal anti-inflammatory drugs, and immunosuppressants), defined as having at least one Part B or D event for a medication in these classes. These medications were identified by linking National Drug Codes and Healthcare Common Procedure Coding System codes in Part D event files and medical claims to therapeutic class information in the Lexi-data Basic Database (Wolters Kluwer Health, Inc.).14 Immunosuppressants included agents recommended in AV treatment guidelines for remission induction or maintenance, including cyclophosphamide, azathioprine, methotrexate, mycophenolate, leflunomide, and rituximab.15-19
COVARIATES
Demographics, insurance coverage, and comorbidities were measured at time of study index. Demographics were obtained from the Master Beneficiary Summary File and included age (calculated at index date in months), sex, race and ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, or Other/Unknown), and US region (Northeast, Midwest, South, and West).20 For insurance coverage, we determined whether the beneficiary was dually enrolled in Medicaid (which provides assistance with Part A, B, and D copays and premiums), not dually enrolled in Medicaid but a recipient of the Part D low-income subsidy (which provides full or partial assistance with Part D copays and premiums), or neither. We also determined whether the beneficiary originally qualified for Medicare based on disability (rather than age). To characterize overall comorbidity burden, we calculated a weighted Quan-Charlson Comorbidity Index (Quan-CCI).21 Presence of ESKD in beneficiaries with and without AV were reported among the combined AB and ABD sample.
STATISTICAL ANALYSES
Data were analyzed using SAS 9.4 (SAS Institute Inc.) and Stata (Version 16.1, StataCorp LLC). All analyses were conducted separately for the AB and ABD sample. Demographic, insurance, and clinical characteristics were described and compared between beneficiaries with and without AV. To test for differences in these characteristics by AV vs non-AV status, we used the Wilcoxon signed rank test for continuous variables and conditional logistic regression for categorical variables.
To examine the unadjusted and adjusted differences in total annual payments and annual counts of utilization events in beneficiaries with AV compared with beneficiaries without AV, we estimated generalized linear mixed models. We used a negative binomial distribution with a log link to account for the skewed distribution of the utilization and cost variables. To account for the nonindependence of beneficiaries within matched pairs, we included a random effect for the pair and calculated robust SEs. The multivariable model controlled for demographic and insurance variables, including the matching variables of age and sex, as well as race and ethnicity, dual Medicaid enrollment or Part D low-income subsidy, entitlement due to disability, and US region. We adjusted for these demographic and insurance variables to control for external factors unrelated to AV disease manifestation. We reported the incident rate ratio (IRR) of the mean utilization events and health care costs, with 95% CIs. These ratios were used to calculate predictive margins, ie, the annual predicted events and payments. We used mixed effects logistic regression with a random effect for the beneficiary pair to compare the use of specific medication groups by AV status and reported the unadjusted and fully adjusted odds ratio (ORs) with 95% CI. We calculated predictive margins from the fully adjusted models to estimate the predicted probability of use of each medication class.
Total costs (comprising both Medicare and beneficiary payments) were also described by quarter. We examined whether the differences in cost varied across quarters, by adding quarter-by-AV status interaction terms to the negative binomial regression model and testing their significance using a Wald test.
SENSITIVITY ANALYSIS
To evaluate the robustness of our results to different case-finding algorithms for incident AV, we applied a more restrictive definition for newly diagnosed AV, requiring at least 2 inpatient or noninpatient claims at least 7 days apart in 2016. The AB and ABD samples were redefined using the restricted study sample and analyzed separately using the same analyses as described for the primary analysis. We also conducted sensitivity analyses excluding matched pairs in which one beneficiary had ESKD, to test the possibility that pre-existing ESKD could drive utilization and costs differences.
Results
STUDY POPULATION
After applying patient selection criteria and matching on age and sex, there were a total of 1,460 beneficiaries in the AB sample (730 AV, 730 non-AV; Table 1). The AB sample had a mean age of 71.8 years, and 52.2% were female. Beneficiaries with AV were more likely to be White and non-Hispanic and have a higher Quan-CCI compared with the matched controls.
TABLE 1.
Demographic and Clinical Characteristics of Beneficiaries by Insurance Coverage and AV Diagnosis
| Demographics | Medicare Part AB sample | Medicare Part ABD sample | ||||
|---|---|---|---|---|---|---|
| Overall (n = 1,460) | Beneficiaries without AV (n = 730) | Beneficiaries with AV (n = 730) | Overall (n = 3,252) | Beneficiaries without AV (n = 1,626) | Beneficiaries with AV (n = 1,626) | |
| Age, years, mean (SD) | 71.8 (11.4) | 71.8 (11.4) | 71.8 (11.4) | 70.4 (11.8) | 70.4 (11.8) | 70.4 (11.8) |
| Female | 762 (52.2) | 381 (52.2) | 381 (52.2) | 2,014 (61.9) | 1,007 (61.9) | 1,007 (61.9) |
| Male | 698 (47.8) | 349 (47.8) | 349 (47.8) | 1238 (38.1) | 619 (38.1) | 619 (38.1) |
| Race | ||||||
| Non-Hispanic White (reference) | 1,210 (82.9) | 594 (81.4) | 616 (84.4) | 2,577 (79.2) | 1,277 (78.5) | 1,300 (80.0) |
| Non-Hispanic Black | 90 (6.2) | 62 (8.5) | 28 (3.8)a | 285 (8.8) | 173 (10.6) | 112 (6.9)a |
| Hispanic | 98 (6.7) | 44 (6.0) | 54 (7.4) | 236 (7.3) | 103 (6.3) | 133 (8.2) |
| Other/unknown | 62 (4.3) | 30 (4.1) | 32 (4.4) | 154 (4.7) | 73 (4.5) | 81 (5.0) |
| Region | ||||||
| South (reference) | 621 (42.5) | 339 (46.4) | 282 (38.6) | 1,300 (40.0) | 710 (43.7) | 590 (36.3) |
| Northeast | 238 (16.3) | 109 (14.9) | 129 (17.7)b | 615 (18.9) | 279 (17.2) | 336 (20.7)a |
| Midwest | 309 (21.2) | 138 (18.9) | 171 (23.4)b | 733 (22.5) | 341 (21.0) | 392 (24.1)b |
| West | 292 (20.0) | 144 (19.7) | 148 (20.3) | 604 (18.6) | 296 (18.2) | 308 (18.9)b |
| Comorbidities | ||||||
| Quan-CCI, median (IQR) | 2 (1-5) | 1 (0-4) | 3 (2-6)a | 3 (1-6) | 1 (0-5) | 4 (2-7)a |
| Originally enrolled in Medicare because of disability | 325 (22.3) | 165 (22.6) | 160 (21.9) | 839 (25.8) | 454 (27.9) | 385 (23.7)a |
| Insurance coveragec | ||||||
| Dual-enrolled in Medicaid | 32 (2.2) | 19 (2.6) | 13 (1.8) | — | — | — |
| Part D enrollment | ||||||
| Medicaid (and LIS) | — | — | — | 855 (26.3) | 455 (28.0) | 400 (24.6)b |
| LIS | — | — | — | 198 (6.1) | 85 (5.2) | 113 (7.0) |
| Neither Medicaid nor LIS (reference) | — | — | — | 2,199 (67.6) | 1,086 (66.8) | 1,113 (68.5) |
Data are presented as n (%) unless otherwise noted.
a P < 0.001 for comparison of beneficiaries with AV vs without AV.
b P < 0.05 for comparison of beneficiaries with AV vs without AV.
c Dual enrollment into Medicaid was used for the AB analysis; Medicaid and LIS were used for the ABD analysis.
AB = beneficiaries with Part A/B coverage; ABD = beneficiaries with Part A/B/D coverage; AV = antineutrophil cytoplasmic antibody vasculitis; IQR = interquartile range; LIS = low-income subsidy; Quan-CCI = Quan-Charlson Comorbidity Index.
For the ABD sample, a total of 3,252 beneficiaries (1,626 AV, 1,626 non-AV) were included after applying the same patient selection criteria. The ABD sample had a mean age of 70.4 years, and 61.9% were female (Table 1). Beneficiaries with AV were more likely to be White and non-Hispanic, have a higher Quan-CCI, and be originally enrolled in Medicare because of disability compared with the matched controls. Higher percentages of beneficiaries without AV were dually enrolled in Medicaid.
GPA was the most frequent AV type in both samples (AB: 72.7%; ABD: 72.6%), followed by MPA (AB: 16.3%; ABD: 17.5%; Supplementary Table 2 (144.2KB, pdf) ). In the combined AB and ABD sample, there were a total of 285 (12.1%) beneficiaries with AV (N = 2,356) that were also diagnosed with ESKD, compared with 24 (1.0%) beneficiaries without AV (N = 2,356), with a P value of < 0.001 (data not shown in table).
TOTAL UTILIZATION AND COSTS
AB Sample. In the 12 months prior to index, beneficiaries with AV had mean (SD) unadjusted total Part A/B payments of $18,772 ($28,049) and $3,414 ($4,063) respectively, compared with $8,568 ($23,802) and $1,513 ($2,585) for the matched controls (Table 2). In adjusted regression models, Medicare Part A/B payments were almost 3 times higher (IRR = 2.94 [95% CI = 2.44-3.53]) in beneficiaries with AV (predicted payments: $21,582 [95% CI = $18,908-$24,255]) compared with beneficiaries without AV (predicted payments: $7,346 [95% CI = $6,165-$8,528]). Similarly, beneficiary Part A/B payments were approximately 2.5 times higher (IRR = 2.47 [95% CI = 2.14-2.84]), with predicted payments of $3,563 (95% CI = $3,248-$3,878) vs $1,444 (95% CI = $1,277-$1,611) for beneficiaries with and without AV, respectively. Total Medicare Part B drug payments were significantly higher at an IRR of 2.31 (95% CI = 1.65-3.23) and 3.79 (95% CI = 2.20-6.52), respectively.
TABLE 2.
Total 1-Year Utilization and Costs Prior to Diagnosis for Beneficiaries With AV and Matched Controls (AB Sample)
| Descriptive statistics | Adjusted regression modela | ||||||
|---|---|---|---|---|---|---|---|
| Beneficiaries without AV (N = 730) | Beneficiaries with AV (N = 730) | IRRs | Beneficiaries without AV (N = 730) | Beneficiaries with AV (N = 730) | |||
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | IRR (95% CI) | Predicted means (95% CI) | Predicted means (95% CI) | |
| Medical service payments, USDb | |||||||
| Total Medicare Parts A and B payments | 8,568 (23,802) | 1-977 (601-7,392) | 18,772 (28,049) | 8,254 (2,497-24,799) | 2.94c (2.44-3.53) | 7,346 (6,165-8,528) | 21,582 (18,908-24,255) |
| Total beneficiary Parts A and B payments | 1,513 (2,585) | 619 (263-1,818) | 3,414 (4,063) | 2,128 (783-4,233) | 2.47c (2.14-2.84) | 1,444 (1,277-1,611) | 3,563 (3,248-3,878) |
| Drug payments, USDb | |||||||
| Total Medicare Part B drug payments | 260 (1,652) | 16 (0-68) | 627 (3,147) | 38 (0-207) | 2.31c (1.65-3.23) | 231 (156-306) | 534 (356-711) |
| Total beneficiary Part B drug payments | 58 (421) | 0 (0-3) | 152 (809) | 0 (0-16) | 3.79c (2.20-6.52) | 61 (21-101) | 232 (46-418) |
| Health care utilization | |||||||
| Acute inpatient stays | 0.22 (0.63) | 0 (0-0) | 0.61 (1.08) | 0 (0-1) | 2.77c (2.19-3.51) | 0.22 (0.18-0.27) | 0.61 (0.53-0.69) |
| Hospital outpatient visits | 6.87 (18.86) | 2 (0-6) | 22.78 (53.00) | 5 (1-15) | 2.69c (2.22-3.27) | 7.46 (6.35-8.57) | 20.08 (16.71-23.45) |
| Emergency department visits | 0.73 (1.96) | 0 (0-1) | 1.06 (1.97) | 0 (0-1) | 1.57c (1.28-1.94) | 0.70 (0.58-0.82) | 1.10 (0.95-1.26) |
| Part B drug events | 2.88 (6.28) | 2 (0-3) | 4.73 (12.89) | 2 (0-4) | 1.47c (1.23-1.76) | 2.97 (2.55-3.38) | 4.36 (3.67-5.06) |
| Part B physician office services | 7.67 (7.75) | 6 (2-10) | 12.09 (10.71) | 10 (5-16) | 1.62c (1.47-1.79) | 7.57 (7.02-8.13) | 12.27 (11.08-13.47) |
| Imaging | 4.36 (6.28) | 2 (0-6) | 9.70 (9.90) | 7 (3-14) | 2.40c (2.11-2.71) | 4.22 (3.80-4.64) | 10.11 (9.31-10.90) |
| Tests | 14.13 (25.09) | 7.5 (1-18) | 27.49 (32.24) | 16.5 (5-38) | 2.08c (1.80-2.39) | 13.72 (12.19-15.24) | 28.47 (25.14-31.80) |
| Other procedures | 7.77 (21.51) | 2 (0-7) | 10.58 (19.68) | 5 (1-12) | 1.72c (1.41-2.11) | 6.87 (5.74-8.00) | 11.83 (10.19-13.47) |
| Durable medical equipment | 2.36 (6.75) | 0 (0-1) | 3.33 (7.52) | 0 (0-2) | 1.54d (1.16-2.03) | 2.28 (1.80-2.76) | 3.50 (2.85-4.15) |
| Other Part B carrier events | 5.00 (21.13) | 1 (0-4) | 7.88 (27.05) | 3 (0-9) | 1.82c (1.50-2.21) | 4.48 (3.71-5.25) | 8.15 (6.91-9.39) |
a Adjusted for age, sex, race, dual Medicaid enrollment or Part D low-income subsidy, entitlement due to disability, and US region.
b Health care costs were measured in the 1-year period prior to the index date in 2016, resulting in a combination of 2015 and 2016 USD.
c P < 0.001.
d P = 0.003.
AB = beneficiaries with Part A/B coverage; AV = antineutrophil cytoplasmic antibody vasculitis; IQR = interquartile range; IRR = incidence rate ratio; USD = US dollars.
Beneficiaries with AV also had significantly higher utilization counts across all categories. The largest difference was observed in acute inpatient stays (IRR = 2.77 [95% CI = 2.19-3.51]), followed by hospital outpatient visits (IRR = 2.69 [95% CI = 2.22-3.27]) and imaging events (IRR = 2.40 [95% CI = 2.11-2.71]). For beneficiaries with AV, this translates to a predicted 20.08 hospital outpatient visits [95% CI = 16.71-23.45] and 10.11 [95% CI = 9.31-10.90] imaging events, compared with a predicted 7.46 hospital outpatient visits [95% CI = 6.35-8.57] and 4.22 imaging events [95% CI = 3.80-4.64], respectively, for beneficiaries without AV.
ABD Sample. Beneficiaries with AV had mean (SD) unadjusted total Part A/B payments of $23,445 ($33,647) and $4,381 ($5,064), respectively, compared with $10,136 ($19,945) and $1,907 ($3,292) for the matched controls in the year prior to index (Table 3). The adjusted regression shows that beneficiaries with AV had significantly higher Medicare Part A/B (IRR = 2.95 [95% CI = 2.64-3.29]) and beneficiary Part A/B payments (IRR = 2.62 [95% CI = 2.40-2.87]) than beneficiaries without AV. Beneficiaries with AV had predicted Medicare Part A/B payments of $26,643 (95% CI = $24,680-$28,606) and beneficiary Part A/B payments of $4,669 (95% CI = $4,392-$4,946), whereas beneficiaries without AV had predicted Medicare Part A/B payments of $9,035 (95% CI = $8,212-$9,858) and beneficiary A/B payments of $1,779 (95% CI = $1,648-$1,910). Total Medicare Part B drug spending was significantly higher for beneficiaries with AV compared with beneficiaries without AV (IRR = 2.47 [95% CI = 1.99-3.06] and 4.80 [95% CI = 3.38-6.82]), amounting to predicted payments of $1,009 (95% CI = $802-$1,217) and $477 (95% CI = $257-$697). In addition, beneficiaries with AV also had higher Medicare Part D drug payments (IRR = 1.65 [95% CI = 1.44-1.89]) and beneficiary Part D drug payments (IRR = 1.49 [95% CI = 1.35-1.65]).
TABLE 3.
Total 1-Year Utilization and Costs Prior to Diagnosis for Beneficiaries With AV and Matched Controls (ABD Sample)
| Descriptive statistics | Adjusted regression modela | ||||||
|---|---|---|---|---|---|---|---|
| Beneficiaries without AV (N = 1,626) | Beneficiaries with AV (N = 1,626) | IRRs | Beneficiaries without AV (N = 1,626) | Beneficiaries with AV (N = 1,626) | |||
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | IRR (95% CI) | Predicted means (95% CI) | Predicted means (95% CI) | |
| Medical service payments, USDb | |||||||
| Total Medicare Parts A and B payments | 10,136 (19,945) | 2,991 (1,131-8,896) | 23,445 (33,647) | 11,095 (4,052-31,413) | 2.95c (2.64-3.29) | 9,035 (8,212-9,858) | 26,643 (24,680-28,606) |
| Total beneficiary Parts A and B payments | 1,907 (3,292) | 800 (366-2,080) | 4,381 (5,064) | 2,582 (1,106-5,360) | 2.62c (2.40-2.87) | 1,779 (1,648-1,910) | 4,669 (4,392-4,946) |
| Drug payments, USDb | |||||||
| Total Medicare Part B drug payments | 493 (3,081) | 47 (0-206) | 1,216 (5,670) | 64 (8-283) | 2.47c (1.99-3.06) | 409 (325-493) | 1,009 (802-1,217) |
| Total beneficiary Part B drug payments | 114 (790) | 0 (0-8) | 302 (1,451) | 3 (0-49) | 4.80c (3.38-6.82) | 99 (62-136) | 477 (257-697) |
| Total Medicare Part D drug payments | 3,430 (12,568) | 811 (108-2,372) | 5,090 (16,042) | 1,476 (333-3,123) | 1.65c (1.44-1.89) | 3,146 (2,720-3,572) | 5,194 (4,506-5,883) |
| Total beneficiary Part D drug payments | 581 (1,098) | 204 (56-643) | 1,047 (3,430) | 354 (89-1,026) | 1.49c (1.35-1.65) | 620 (570-671) | 925 (838-1,011) |
| Health care utilization | |||||||
| Acute inpatient stays | 0.28 (0.75) | 0 (0-0) | 0.66 (1.20) | 0 (0-1) | 2.60c (2.23-3.02) | 0.27 (0.23-0.30) | 0.70 (0.63-0.76) |
| Hospital outpatient visits | 8.39 (17.05) | 3 (1-9) | 29.35 (56.29) | 8 (3-21) | 3.08c (2.73-3.47) | 8.97 (8.18-9.75) | 27.59 (24.88-30.31) |
| Emergency department visits | 0.80 (2.12) | 0 (0-1) | 1.35 (2.98) | 0 (0-2) | 2.10c (1.81-2.44) | 0.72 (0.63-0.81) | 1.51 (1.35-1.68) |
| Part B drug events | 4.09 (8.67) | 2 (0-4) | 6.96 (13.31) | 3 (1-6) | 1.67c (1.50-1.85) | 4.07 (3.72-4.41) | 6.77 (6.20-7.34) |
| Part B physician office services | 9.12 (8.21) | 7 (3-13) | 14.05 (11.90) | 11 (6-19) | 1.56c (1.47-1.65) | 9.08 (8.68-9.47) | 14.14 (13.55-14.72) |
| Imaging | 4.89 (6.26) | 3 (1-7) | 11.01 (10.82) | 8 (4-15) | 2.40c (2.22-2.60) | 4.74 (4.45-5.03) | 11.39 (10.83-11.95) |
| Tests | 18.02 (24.99) | 11 (3-23) | 32.23 (34.15) | 21 (9-43) | 1.90c (1.76-2.06) | 17.50 (16.41-18.59) | 33.27 (31.49-35.04) |
| Other procedures | 7.76 (16.32) | 2 (0-7) | 11.85 (20.49) | 6 (2-13) | 1.74c (1.55-1.95) | 7.22 (6.53-7.91) | 12.53 (11.52-13.53) |
| Durable medical equipment | 3.55 (8.48) | 0 (0-2) | 4.93 (9.17) | 0 (0-6) | 1.48c (1.27-1.71) | 3.46 (3.06-3.86) | 5.11 (4.57-5.64) |
| Other Part B carrier events | 5.71 (11.98) | 2 (0-6) | 11.09 (29.09) | 4 (1-11) | 2.04c (1.81-2.31) | 5.49 (4.97-6.02) | 11.23 (10.16-12.30) |
| Number of 30-day day of supply | 53.29 (38.39) | 47.07 (25.47-74.54) | 63.57 (40.46) | 58.70 (34.6-86.9) | 1.23c (1.17-1.29) | 52.54 (50.74-54.34) | 64.50 (60.80-68.21) |
| Drug class utilization | N (%) with 1 claim | N (%) with 1 claim | Odds ratio (95% CI) | Predicted probabilities (95% CI) | Predicted probabilities (95% CI) | ||
| Any corticosteroid use | 562 (34.56) | 994 (61.13) | 3.18c (2.71-3.74) | 0.34 (0.32-0.37) | 0.61 (0.59-0.64) | ||
| Any NSAID use | 207 (12.73) | 203 (12.48) | 1.04 (0.83-1.29) | 0.12 (0.11-0.14) | 0.13 (0.11-0.14) | ||
| Any immunosuppressant use | 41 (2.52) | 248 (15.25) | 6.97c (4.79-10.14) | 0.03 (0.02-0.03) | 0.15 (0.13-0.17) | ||
| Any opioid use | 661 (40.65) | 797 (49.02) | 1.53c (1.32-1.77) | 0.40 (0.38-0.42) | 0.50 (0.47-0.52) | ||
a Adjusted for age, sex, race, dual Medicaid enrollment or Part D low-income subsidy, entitlement due to disability, and US region.
b Health care costs were measured in the 1-year period prior to the index date in 2016, resulting in a combination of 2015 and 2016 USD.
c P < 0.001.
ABD = beneficiaries with Part A/B/D coverage; AV = antineutrophil cytoplasmic antibody vasculitis; IQR = interquartile range; IRR = incidence rate ratio; NSAID = nonsteroidal anti-inflammatory drug; USD = US dollars.
Similar to the AB sample, ABD-enrolled beneficiaries with AV experienced significantly higher utilization counts across all categories. The largest difference was observed in hospital outpatient visits with an IRR of 3.08 (95% CI = 2.73-3.47), followed by acute inpatient stays (IRR = 2.60 [95% CI = 2.23-3.02]) and imaging events (IRR = 2.40 [95% CI = 2.22-2.60]). For beneficiaries with AV, this translates to a predicted 27.59 (95% CI = 24.88-30.31) hospital outpatient visits and 11.39 (95% CI = 10.83-11.95) imaging events, compared with beneficiaries without AV with a predicted 8.97 (95% CI = 8.18-9.75) hospital outpatient visits and 4.74 (95% CI = 4.45-5.03) imaging events.
We observed a significantly greater mean (SD) number of 30-day supply prescriptions for beneficiaries with AV compared with beneficiaries without AV, 63.57 (40.46) and 53.29 (38.39), respectively (Table 3). The adjusted regression shows that beneficiaries with AV had a predicted 64.50 (95% CI = 60.80-68.21) 30-day supply prescriptions vs 52.54 (95% CI = 50.74-54.34) 30-day supply prescriptions in beneficiaries without AV. There was also a higher percentage of beneficiaries with AV with at least 1 claim for corticosteroids, opioids, and immunosuppressants. Compared with beneficiaries without AV, beneficiaries with AV had more than 3 times the odds of corticosteroid use (OR = 3.18 [95% CI = 2.71-3.74]), 7 times the odds of immunosuppressant use (OR = 6.97 [95% CI = 4.79-10.14]), and 53% higher odds of opioid use (OR = 1.53 [95% CI = 1.32-1.77]). No significant difference between groups was observed for the use of nonsteroidal anti-inflammatory drugs.
QUARTERLY COSTS
AB Sample. After adjusting for demographic and insurance factors, total expenditures for beneficiaries with AV were significantly higher in each quarter when compared with the matched controls (Table 4). Furthermore, the quarter-by-AV status interaction terms were significant (χ2 = 53.31, P < 0.001). In the 0-3 months immediately before AV diagnosis, beneficiaries with AV experienced almost 4.5 times higher total expenditures compared with beneficiaries without AV (IRR = 4.43 [95% CI = 3.52-5.57]). In the more distal three quarters, IRRs for total expenditures ranged from 2.01 to 2.47 (Table 4).
TABLE 4.
Total Costs Prior to Diagnosis for Beneficiaries With AV and Matched Controls, Cost by Quarter
| Payments, USDa | Beneficiaries without AVb | Beneficiaries with AVb | Adjusted regression modelc | ||
|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | IRR (95% CI) | |
| AB sample | |||||
| Q1: 10-12 months prior to diagnosis | 1,876 (4,913) | 399 (75-1,279) | 4,476 (11,290) | 661 (165-2,934) | 2.47d (1.92-3.18) |
| Q2: 7-9 months prior to diagnosis | 2,238 (5,485) | 379 (101-1,326) | 4,351 (13,139) | 747 (155-3,109) | 2.01d (1.55-2.60) |
| Q3: 4-6 months prior to diagnosis | 2,824 (12,208) | 409 (95-1,530) | 4,906 (10,384) | 859 (174-3,976) | 2.45d (1.92-3.13) |
| Q4: 0-3 months prior to diagnosis | 3,232 (12,711) | 460 (108-1,699) | 9,043 (14,207) | 3,524 (963-12,942) | 4.43d (3.52-5.57) |
| ABD sample | |||||
| Q1: 10-12 months prior to diagnosis | 3,618 (7,754) | 1,219 (418-3,129) | 6,993 (12,947) | 2,296 (846-7,265) | 2.16d (1.92-2.42) |
| Q2: 7-9 months prior to diagnosis | 3,850 (9,453) | 1,191 (458-3,082) | 7,084 (12,993) | 2,334 (875-7,982) | 2.14d (1.91-2.40) |
| Q3: 4-6 months prior to diagnosis | 4,289 (10,116) | 1,223 (426-3,122) | 8,116 (13,797) | 2,665 (1,012-10,014) | 2.40d (2.14-2.71) |
| Q4: 0-3 months prior to diagnosis | 4,414 (9,885) | 1,251 (408-3,328) | 12,578 (17,774) | 6,093 (2,222-15,957) | 3.85d (3.43-4.32) |
a Health care costs were measured in the 1-year period prior to the index date in 2016, resulting in a combination of 2015 and 2016 USD.
b N = 730 for the AB sample; N = 1,626 for the ABD sample.
c Adjusted for age, sex, race, dual Medicaid enrollment or Part D low-income subsidy, entitlement due to disability, and US region.
d P < 0.001.
AB = beneficiaries with Part A/B coverage; ABD = beneficiaries with Part A/B/D coverage; AV = antineutrophil cytoplasmic antibody vasculitis; IQR = interquartile range; USD = US dollars.
ABD Sample. Similar to the AB sample, the total Parts A, B, and D expenditures for beneficiaries with AV were also significantly higher in each quarter compared with the matched controls (Table 4), with a significant quarter-by-AV status interaction term (χ2 = 136.06, P < 0.0001). In the 0-3 months immediately before AV diagnosis, beneficiaries with AV experienced almost 4 times higher total Part A, B, and D expenditures compared with beneficiaries without AV (IRR=3.85 [95% CI = 3.43-4.32]). IRRs for total Part A, B, and D expenditures in the prior three quarters ranged from 2.14 to 2.40 (Table 4).
SENSITIVITY ANALYSIS
With the alternative case-finding algorithm for selecting beneficiaries with incident AV, there were a slightly lower number of beneficiaries with AV in the study sample (AB: total n = 1,230, 615 AV and 615 non-AV; ABD: total n = 2,760, 1,380 AV and 1,380 non-AV). We observed a similar magnitude of differences in health care utilization and costs between beneficiaries with AV and beneficiaries without AV compared with the primary study sample (Supplementary Tables 3-7 (144.2KB, pdf) ). When excluding matched pairs in which either beneficiary had ESKD (AB: n = 1,262; 631 AV and 631 non-AV; ABD: n = 2,844; 1,422 AV and 1,422 non-AV), utilization and cost differences persisted (Supplementary Tables 8-10 (144.2KB, pdf) ).
Discussion
This analysis used a national cohort of newly diagnosed Medicare beneficiaries with AV in FFS Part A/B and beneficiaries with additional Part D coverage to examine the 12-month period prior to formal AV diagnosis. This is the first study to describe the prediagnosis health care costs and utilization of beneficiaries with AV in the Medicare population. Our findings suggest that greater health care utilization and costs previously observed for patients with vasculitis after diagnosis become apparent in the year prior to diagnosis with AV.10
Overall and across all categories of care, we found substantial increases in health care costs and utilization in beneficiaries in the year prior to new AV diagnosis relative to beneficiaries without AV. After controlling for age, sex, race, US region, dual Medicaid enrollment or Part D low-income subsidy, and entitlement due to disability, our analysis showed that beneficiaries with AV have almost 3 times the Medicare Part A/B payments compared with beneficiaries without AV in both the AB and ABD sample. In addition, the beneficiaries with AV in both samples had approximately 2.5 times higher beneficiary Part A/B payments. Consistent with the postdiagnosis study conducted by Thorpe et al, hospital outpatient visits and tests were the highest utilization categories in the year prior to diagnosis.
To investigate whether beneficiaries with AV experienced a higher level of cost burden throughout the entire 12-month period, we evaluated costs by quarter and observed a significant increase in every quarter for both samples. These results are comparable with a previous study in patients with commercial insurance or Medicare supplemental coverage that reported that patients with MPA incurred higher costs in the 0-3 months before diagnosis compared with 4-6 months before diagnosis.11 We similarly observed a considerable increase in the 0-3 months immediately prior to diagnosis in the beneficiaries with AV in our study. These data suggest that health care utilization may escalate owing to worsening symptoms that drive more frequent testing and ultimately lead to a diagnosis. This trend is consistent between the populations, but costs are considerably higher in the commercial insurance or Medicare supplemental population compared with the sample of FFS Medicare beneficiaries in this study. This may be due to differences in reimbursement as commercial insurance has been reported to have higher payments compared with traditional Medicare.22
Ambiguity in diagnosing systemic autoimmune diseases is common owing to overlapping symptoms and nonspecific autoantibodies.23 We observed an increase in the 30-day supplies of medications and higher use of corticosteroids, opioids, and immunosuppressants in the 12 months prior to first AV diagnosis. These medications may have been prescribed for concern of other autoimmune diseases or to manage nonspecific AV symptoms. Because regimens may not necessarily be optimized for targeted therapy of AV, nonspecific prescribing can lead to inefficient use of medical services and subsequently contribute to the increased utilization and costs observed in our study.
Early treatment and prompt detection are necessary in this population, as unaddressed AV symptoms may lead to irreversible organ damage. In our total study sample, there was a clinically and statistically significant difference in beneficiaries with AV (12.1%) that were also diagnosed with ESKD before their first AV diagnosis code compared with beneficiaries without AV (1.0%). One study evaluating the biopsy-proven AV population reported that 18% of patients with AV required dialysis at initial presentation.24 In addition, another study described that 53.8% and 37.1% of patients with MPA and GPA had a diagnosis of either acute or chronic renal failure in the 6-month period prior to diagnosis.11 This likely demonstrates the substantial impact that delayed diagnosis of AV has on patient outcomes in addition to the increased health care costs reported in our study. Improvements need to be made in the detection of AV to allow for timely treatment, especially given the severe physical and socioeconomic repercussions that delays have to both the patients and health care system.
LIMITATIONS
This study has several limitations that should be considered. The analysis did not control for comorbidities, as it would be difficult to distinguish whether these were related to the disease manifestation of AV or independent factors. However, the demographic and insurance variables that were used in the adjusted model should minimize the external variables unrelated to AV. In addition, large utilization and cost differences remained even after excluding beneficiaries with ESKD, ruling out the possibility that pre-existing ESKD drove differences. These sensitivity results should be viewed as conservative, as prior research suggests that some patients develop kidney failure in the months before diagnosis. Medicare Advantage beneficiaries were not included in this analysis. Therefore, these results may not be generalizable to this population, as beneficiaries may have different usage for health care services. In addition, there are inherent constraints of observational database studies. Misclassification may have occurred because of miscoding; in particular, there is no established algorithm to identify patients with AV in claims databases. We have mitigated these limitations by conducting sensitivity analyses with a more restrictive definition of AV, requiring at least 2 claims at least 7 days apart, and observed similar results. In addition, we also used established CMS definitions of health care utilization and cost categories. Finally, given the observational nature of the study, we cannot definitively say that these costs are directly attributable to the symptoms of AV.
Conclusions
Although AV is a rare set of conditions, this study illustrates the high burden of AV to the health care system even before formal diagnosis. Earlier identification of AV is necessary to guide patients to appropriate care and potentially reduce costs and morbidity. Future studies should further explore how delays in AV diagnosis affect long-term patient outcomes and if specific health care utilization could be used to develop a predictive risk tool that allows providers to detect the nonspecific symptoms of AV.
REFERENCES
- 1.Robson J, Doll H, Suppiah R, et al. Damage in the anca-associated vasculitides: Long-term data from the European vasculitis study group (EUVAS) therapeutic trials. Ann Rheum Dis. 2015;74(1):177-84. doi: 10.1136/annrheumdis-2013-203927 [DOI] [PubMed] [Google Scholar]
- 2.Poulton CJ, Nachman PH, McGregor JG, Jennette JC, Falk RJ, Hogan SL. Pathways to renal biopsy and diagnosis among patients with ANCA small-vessel vasculitis. Clin Exp Rheumatol. 2013;31(1 Suppl 75):S32-7. [PMC free article] [PubMed] [Google Scholar]
- 3.Hogan SL, Falk RJ, Chin H, et al. Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med. 2005;143(9):621-31. doi: 10.7326/0003-4819-143-9-200511010-00005 [DOI] [PubMed] [Google Scholar]
- 4.Flossmann O. Risks of treatments and long-term outcomes of systemic ANCA-associated vasculitis. Presse Med. 2015;44(6 Pt 2):e251-7. doi: 10.1016/j.lpm.2015.02.019 [DOI] [PubMed] [Google Scholar]
- 5.Chung SA, Langford CA, Maz M, et al. 2021. American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Arthritis Care Res (Hoboken). 2021;73(8):1088-105. doi: 10.1002/acr.24634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan JA, Dehghan N, Chen W, Xie H, Esdaile JM, Avina-Zubieta JA. Mortality in ANCA-associated vasculitis: A meta-analysis of observational studies. Ann Rheum Dis. 2017;76(9):1566-74. doi: 10.1136/annrheumdis-2016-210942 [DOI] [PubMed] [Google Scholar]
- 7.Walton EW. Giant-cell granuloma of the respiratory tract (Wegener’s granulomatosis). Br Med J. 1958;2(5091):265-70. doi: 10.1136/bmj.2.5091.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houben E, Groenland SL, van der Heijden JW, Voskuyl AE, Doodeman HJ, Penne EL. Relation between duration of the prodromal phase and renal damage in ANCA-associated vasculitis. BMC Nephrol. 2017;18(1):378. doi: 10.1186/s12882-017-0797-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koldingsnes W, Nossent H. Predictors of survival and organ damage in Wegener’s granulomatosis. Rheumatology (Oxford). 2002;41(5):572-81. doi: 10.1093/rheumatology/41.5.572 [DOI] [PubMed] [Google Scholar]
- 10.Thorpe CT, Thorpe JM, Jiang T, et al. Healthcare utilization and expenditures for United States Medicare beneficiaries with systemic vasculitis. Semin Arthritis Rheum. 2018;47(4):507-19. doi: 10.1016/j.semarthrit.2017.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raimundo K, Farr AM, Kim G, Duna G. Clinical and economic burden of antineutrophil cytoplasmic antibody-associated vasculitis in the United States. J Rheumatol. 2015;42(12):2383-91. doi: 10.3899/jrheum.150479 [DOI] [PubMed] [Google Scholar]
- 12.Thorpe CT, Thorpe JM, Kang Y, Carpenter DM, McGregor JG, Hogan SL. Characteristics of hospitalizations for systemic vasculitis in the United States: National estimates using data from the Healthcare Cost and Utilization Project (HCUP). Moderated poster presented at: 17th International Vasculitis and ANCA Workshop; 2015; London, England, April 19-22, 2015. [Google Scholar]
- 13.Centers for Medicare & Medicaid Services. Chronic Condition Data Warehouse Master Beneficiary Summary File Cost & Use Segment Codebook. 2017. Accessed March 13, 2020. https://www2.ccwdata.org/web/guest/data-dictionaries
- 14.Wolters Kluwer Health. Lexi-Data Basic, version I.25.0. 2016.
- 15.Ntatsaki E, Carruthers D, Chakravarty K, et al. BSR and BHPR guideline for the management of adults with ANCA-associated vasculitis. Rheumatology (Oxford). 2014;53(12):2306-9. doi: 10.1093/rheumatology/ket445 [DOI] [PubMed] [Google Scholar]
- 16.McGeoch L, Twilt M, Famorca L, et al. CanVasc recommendations for the management of antineutrophil cytoplasm antibody-associated vasculitides. J Rheumatol. 2016;43(1):97-120. doi: 10.3899/jrheum.150376 [DOI] [PubMed] [Google Scholar]
- 17.Yates M, Watts RA, Bajema IM, et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis. 2016;75(9):1583-94. doi: 10.1136/annrheumdis-2016-209133 [DOI] [PubMed] [Google Scholar]
- 18.de Souza AWS, Calich AL, de Ataíde Mariz H, et al. Recommendations of the Brazilian Society of Rheumatology for the induction therapy of ANCA-associated vasculitis. Rev Bras Reumatol Engl Ed. 2017;57 Suppl 2:484-96. [DOI] [PubMed] [Google Scholar]
- 19.Charles P, Bienvenu B, Bonnotte B, et al. Rituximab: Recommendations of the French Vasculitis Study Group (FVSG) for induction and maintenance treatments of adult, antineutrophil cytoplasm antibody-associated necrotizing vasculitides. Presse Med. 2013;42(10):1317-30. doi: 10.1016/j.lpm.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 20.Eicheldinger C, Bonito A. More accurate racial and ethnic codes for Medicare administrative data. Health Care Financ Rev. 2008;29(3):27-42. [PMC free article] [PubMed] [Google Scholar]
- 21.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-9. doi: 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 22.Maeda JLK, Nelson L. How do the hospital prices paid by Medicare Advantage plans and commercial plans compare with Medicare fee-for-service prices? Inquiry. 2018;55:46958018779654. doi: 10.1177/0046958018779654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narain S, Richards HB, Satoh M, et al. , Diagnostic accuracy for lupus and other systemic autoimmune diseases in the community setting. Arch Intern Med. 2004;164(22):2435-41. doi: 10.1001/archinte.164.22.2435 [DOI] [PubMed] [Google Scholar]
- 24.Lionaki S, Hogan SL, Jennette CE, et al. The clinical course of ANCA small-vessel vasculitis on chronic dialysis. Kidney Int. 2009;76(6):644-51. doi: 10.1038/ki.2009.218 [DOI] [PMC free article] [PubMed] [Google Scholar]


