Abstract
BACKGROUND:
The economic burden of major depressive disorder (MDD) is substantial and increasing; however, the impact of key clinical events (eg, hospitalization, suicide attempt/ideation, and treatment changes) on health care resource use and costs are less established.
OBJECTIVE:
To evaluate the health care utilization and costs among patients with MDD, particularly for those with key clinical events.
METHODS:
In this retrospective analysis, administrative health care claims from the IBM MarketScan Commercial Claims and Encounters Database were used to identify adults with a new diagnosis of MDD (January 1, 2009, to December 31, 2017). Patients with 12 months or more of continuous health care coverage before and after the initial medical claim with an MDD diagnosis (index date) and 1 or more pharmacy claims for an antidepressant within 60 days of any qualifying medical claim were included. The effect of post-index date key clinical events (eg, treatment changes, moderate to severe MDD, MDD-related emergency department [ED] visits, MDD-related hospitalizations, suicide attempt/ideation, severe mental health disorder, use of brain stimulation therapies) on all-cause total costs was assessed. Actual allcause costs were summarized descriptively and reported per patient per year (PPPY). Multivariable analyses compared differences in all-cause costs during follow-up, depending on whether patients experienced a key clinical event.
RESULTS:
A total of 455,082 patients met eligibility criteria. The average age was 41 years and 64% of patients were female. Mean (SD) all-cause PPPY costs during the follow-up period were $10,074 ($25,694). The most common key clinical events were treatment changes, moderate to severe MDD diagnosis, and MDD-related ED visits. The majority of patients (90.1%) experienced at least 1 treatment change, which was most commonly treatment discontinuation. Generally, mean costs for up to 90 days following an event were higher than those preceding the event. In multivariable analyses, patients with any key clinical events had 51% higher PPPY allcause health care costs compared with those who did not have any key clinical events. Compared with patients without key clinical events, follow-up costs were more than 2 times higher among patients with severe mental health disorder, MDD-related hospitalization, and suicide attempt/ideation. The most impactful key clinical event was treatment with electroconvulsive therapy, vagal nerve stimulation, or transcranial magnetic stimulation, in which patients incurred 4.3 times higher follow-up costs than those who did not receive one of these treatments.
CONCLUSIONS:
Key clinical events exacerbate health care resource use and costs among patients with MDD. Effective therapeutic regimens initiated optimally in the course of treatment may mitigate costly clinical events associated with MDD.
Plain language summary
Major depressive disorder (MDD), or depression, can be a costly condition. Certain events, like going to the hospital, are known to increase costs of depression. In this study, patients with certain events, such as emergency department visits for depression, had much higher health care costs than those who did not experience the events. The most common event was a change in depression treatment. High costs may be reduced with better depression care.
Implications for managed care pharmacy
This study assessed health care use and costs of patients with MDD, particularly those with certain key clinical events (eg, hospitalizations). Besides the event of treatment change, patients with the events had considerably higher costs than those without the events, suggesting that certain clinical events exacerbate costs. This research may inform managed care policy by promoting appropriate treatment early in the disease to mitigate the high costs associated with MDD.
Major depressive disorder (MDD) is a chronic, relapsing, and burdensome disease characterized by a variety of symptoms, including a persistent or chronic state of sadness, diminished interest or pleasure in activities, sleep and appetite disturbance, fatigue, indecision, psychomotor agitation or retardation, reduced ability to concentrate, and/or recurrent suicidal ideation.1 The prevalence of MDD in the United States has increased over the past 3 decades, with the current estimated lifetime prevalence standing at 20.6%.2
Current guidelines recommend antidepressant therapy for the initial treatment of MDD.3 The vast majority of currently available antidepressants act by modulating monoamine neurotransmitter systems.4,5 Selective serotonin reuptake inhibitors and serotonin norepinephrine reuptake inhibitors are the most common depression medication classes prescribed for initial treatment. Bupropion, mirtazapine, and multimodal agents (eg, vilazodone and vortioxetine) are also used. Antidepressants such as monoamine oxidase inhibitors and tricyclic antidepressants are typically reserved for later-line treatment because of tolerability issues.3 Commonly prescribed antidepressants often require several weeks of continued treatment before a clinical response is achieved,6 which can negatively impact compliance. Further, more than one-half of patients do not have an adequate response to initial treatment with antidepressants,7 and about one-third of patients achieve remission following initial treatment with antidepressants.7 Adjunctive treatment, including the addition of another antidepressant from a different class or an atypical antipsychotic to antidepressant therapy, may be initiated, particularly for patients who do not respond to initial treatment or for those with prior history of MDD (ie, recurrent MDD).3 Other later-line treatments may include brain stimulation therapies, such as electroconvulsive therapy (ECT), vagal nerve stimulation (VNS), and transcranial magnetic stimulation (TMS), which can be invasive and costly.
MDD is associated with significant increases in functional disability, lost productivity, and higher health care utilization.8 As such, the economic burden of MDD is substantial and increasing; it is currently estimated to be approximately $210 billion annually in both direct and indirect costs in the United States alone.9 Studies have shown that costs associated with MDD are further amplified by the occurrence of clinical events, such as treatment changes, brain stimulation therapies, hospitalizations, suicidal behavior, and severe mental health diagnoses.9-11 Such events are typically preceded by poor response to oral antidepressant treatment.3,10
The objective of this retrospective study was to better understand the health care utilization and costs among patients with MDD, particularly for those with key clinical events. These events included treatment changes, moderate to severe MDD diagnosis, MDD-related emergency department (ED) visits, MDD-related hospitalizations, suicide attempt or ideation, severe mental health disorder, and the use of ECT, VNS, or TMS.
Methods
STUDY DESIGN
This retrospective analysis was conducted using health insurance claims from the IBM MarketScan Commercial Claims and Encounters Database. The database contained deidentified, patient-specific health data of reimbursed health care claims for employees, retirees, and their dependents of more than 250 medium and large employers and health plans in the United States.
The index date was defined as the date of the first MDD diagnosis claim after a 12-month “washout” period without MDD diagnoses or prescription claims for MDD treatment. The baseline period was defined as the 12 continuous months prior to the index date, and the follow-up period was defined as 12 continuous months (or longer) after the index date (Figure 1). The follow-up period ended in the event of inpatient death (given limited reporting of outpatient deaths in claims data), end of continuous enrollment, or end of the study period.
FIGURE 1.
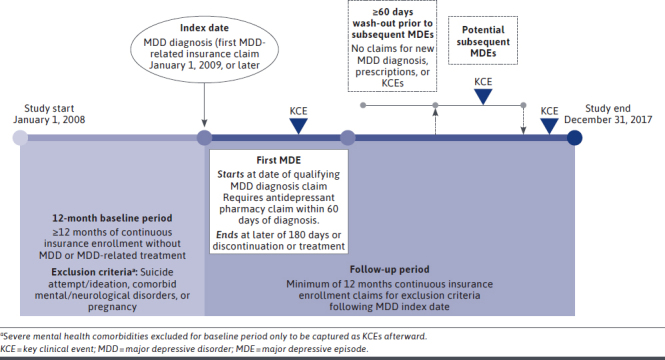
Study Design
STUDY POPULATION
Patients aged 18 years and older with a new diagnosis of MDD (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM]: 296.20–296.24, 296.30–296.34, 300.4, 311; ICD-10-CM: F32.*, F33.*, F34.1) between January 1, 2009, and December 31, 2017, were potentially eligible for the analysis. Analyzed patients were required to have at least 1 outpatient claim with an MDD diagnosis in any position or at least 1 inpatient claim with an MDD diagnosis in the primary position (index date), at least 1 major depressive episode (MDE), and at least 12 months of continuous enrollment in medical and pharmacy benefits before and after the index date. An MDE was defined as 1 or more inpatient or outpatient medical claims with an MDD diagnosis claim, with 1 or more antidepressant pharmacy claims occurring within 60 days of the qualifying inpatient or outpatient medical claim. An MDE ended at the later of 180 days or discontinuation of treatment. A washout period (no new claims for MDD diagnoses, prescriptions, or key clinical events) between first and subsequent MDEs was applied for at least 60 days prior to each MDE. Patients with a diagnosis of bipolar or other mood disorders or a claim for major neurocognitive/neurodevelopmental disorders any time during the entire study period were excluded from analysis. Additionally, patients with claims for the following conditions, medications, or procedures during the 12 months prior to the index date were excluded: pregnancy; suicide attempt or evidence of suicidal ideation; severe mental health disorders (schizophrenia/schizophrenic disorders, psychosis-related disorders and paranoid states, drug-induced depression, depressive-type psychosis); ECT, VNS, or TMS use; or ketamine use.
OUTCOMES AND ANALYSES
Key Clinical Events. Qualifying key clinical events measured during the follow-up period comprised changes in treatment, moderate to severe MDD diagnosis, MDD-related hospitalizations or ED visits, MDD-related suicide attempt or ideation, severe mental health disorder, and ECT, VNS, or TMS use. Changes in treatment were defined as discontinuation (>30-day gap in treatment), switch (initiation of new antidepressant treatment within 30 days of the discontinuation date of the previous treatment), addition of adjunctive therapy (eg, ≥1 antidepressants, atypical antipsychotics, mood stabilizers, thyroid hormone with at least 30-day overlap with current antidepressant monotherapy regimen), and change in adjunctive therapy. Patients were classified as having a moderate to severe MDD diagnosis if they experienced a qualifying diagnostic code (ICD-9-CM/ICD-10-CM) at the initial MDD diagnosis or at any point during follow-up. MDD-related suicide attempt or ideation and ECT, VNS, or TMS use were also identified based on coding at any point throughout follow-up. Severe mental health disorders were defined as 1 or more inpatient or outpatient claims with an ICD-9-CM/ICD-10-CM diagnostic code for 1 of the following conditions: schizophrenia/schizophrenic disorder, psychosis-related disorders and paranoid states, drug-induced depression, and depressive-type psychosis. MDD-related hospitalizations or ED visits were identified if the patient had evidence of a hospitalization or ED visit, respectively, with an MDD diagnosis code in the primary position. Key clinical events were not mutually exclusive, and multiple events could have occurred for the same patient.
Costs. Costs were measured during the 12-month baseline period, during follow-up periods, and before and after key clinical events. All-cause costs were reported per patient per year (PPPY) based on paid amounts of adjudicated claims, which included insurer and health plan payments, as well as patient cost-sharing in the form of copayment, deductible, and coinsurance.
Statistical Analyses. All-cause costs were summarized descriptively. Multivariable analyses were conducted to compare differences in all-cause costs during follow-up between patients with and without each of the key clinical events while controlling for confounding variables, such as baseline demographics (clinical characteristics), and baseline costs. A multivariable analysis assessing cost differences between patients with and without any key clinical event was also created. In this model, change of therapy was not included as a key clinical event, given that nearly all patients had a change in therapy (mostly comprising therapy discontinuation), which may not reflect MDD manifestation or progression. A complete list of confounding variables is shown in Supplementary Table 1 (114.1KB, pdf) (available in online article). Associations between key clinical events and costs were estimated using separate gamma-family generalized linear models with a log link; length of follow-up and pre-index cost covariates were log transformed prior to modeling to resolve overly influential outliers, and differences in covariate-adjusted costs were calculated between subgroups within each patient characteristic or clinical condition using the recycled predictions method.
Results
PATIENT ATTRITION
Between January 1, 2009, and December 31, 2017, a total of 10,485,754 patients with an MDD diagnosis were identified, and 455,082 (4.3%) met all eligibility criteria and were included in the analysis (Supplementary Figure 1 (114.1KB, pdf) ).
DEMOGRAPHICS AND CLINICAL CHARACTERISTICS
Details on patient demographics and clinical characteristics at baseline are shown in Supplementary Table 2 (114.1KB, pdf) .
Among eligible patients, 290,959 (64%) were female, and the mean (SD) age was 41.2 (12.9) years. The mean (SD) length of follow-up was 1,095 (632.1) days, or approximately 3 years, with almost 2% of patients having at least 8 years of follow-up. In the 12-month baseline period, nearly one-third of patients (141,714) had some type of chronic pain diagnosis; 46,791 (10%) anxiety disorders; 41,202 (9%) sleep disorders; and 29,423 (6%) substance abuse disorders.
PREVALENCE OF KEY CLINICAL EVENTS
The most common key clinical events during the study period were treatment changes, moderate to severe MDD diagnosis, and MDD-related ED visits (Figure 2). Most patients (410,159; 90.1%) experienced at least 1 treatment change. Additionally, 23.5% of patients had a moderate to severe MDD diagnosis at least once during follow-up. Six percent of patients had at least 1 MDD-related ED visit and 2.5% had at least 1 hospitalization. Two percent of patients (10,564) had at least 1 occurrence of suicide attempt or ideation, and 1.1% (5,119) were diagnosed at least once with a severe mental health disorder. Treatment with ECT, VNS, or TMS was rare, as only 0.2% of patients (718) were treated with these procedures.
FIGURE 2.
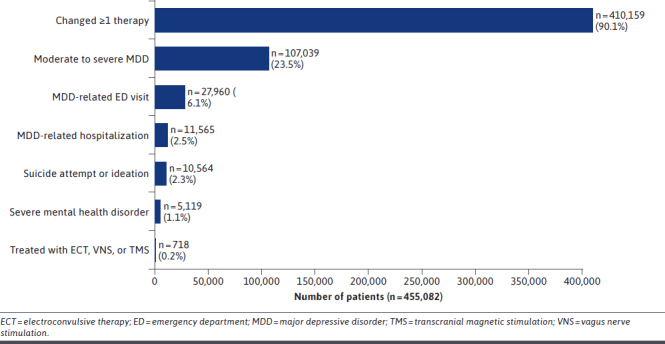
Prevalence of Key Clinical Events
Among all patients, there was a mean (SD) of 1.6 (1.0) treatment changes, and among patients with at least 1 adjunctive therapy, there was a mean (SD) of 3.2 (1.8) changes. Approximately one-quarter of patients had 2 or more treatment changes during the study period. Discontinuation was the most common type of treatment change during the study period and was experienced by 390,783 patients (85.9%), the majority of whom discontinued an antidepressant monotherapy regimen. Only 12% of all patients had a treatment switch during follow-up, and approximately one-third of patients reinitiated treatment. During the study period, few patients received adjunctive therapy (40,315 patients [8.9%]). Of the patients who received adjunctive therapy throughout follow-up, the majority received antidepressant combination therapy (>75% of all adjunctive regimens); only 8,024 patients (<2% of all patients) had their MDD treatment augmented with an atypical antipsychotic.
HEALTH CARE COSTS
Annualized All-Cause Costs. Mean (SD) all-cause PPPY costs for all patients during the follow-up period, regardless of the presence of key clinical events, were $10,074 ($25,694) (Table 1). These costs were approximately $2,200 higher than mean all-cause PPPY costs during the baseline period.
TABLE 1.
Mean Annualized All-Cause Health Care Costs Per Patient
| Annualized all-cause health care costs per patient | Mean (SD), $ |
|---|---|
| All patientsa | |
| Inpatient | 2,160 (13,985) |
| Outpatient | 5,934 (15,196) |
| Pharmacy | 1,980 (6,976) |
| By key clinical event | |
| Changed ≥ 1 therapy | 9,960 (25,650) |
| Moderate to severe MDD | 11,509 (25,568) |
| MDD-related ED visit | 15,700 (31,179) |
| MDD-related hospitalization | 15,941 (29,562) |
| Suicide attempt or ideation | 14,681 (24,312) |
| Severe mental health disorder | 23,096 (49,958) |
| Treated with ECT, VNS, or TMS | 49,121 (94,962) |
a Annualized all-cause costs were measured during the follow-up period and include all patients, regardless of key clinical event presence.
ECT = electroconvulsive therapy; ED = emergency department; MDD = major depressive disorder; TMS = transcranial magnetic stimulation; VNS = vagus nerve stimulation.
Compared with the overall patient population, patients with the following key clinical events incurred higher mean all-cause costs: moderate to severe MDD diagnosis, MDD-related ED visits, MDD-related hospitalizations, suicide attempt or ideation, severe mental health disorder, and ECT, VNS, or TMS use (Table 1). Patients with an event of 1 or more treatment changes had mean all-cause costs comparable to that of the overall patient population ($9,960 vs $10,074). Mean costs during the first 30, 60, and 90 days following a key clinical event were considerably higher than those preceding the event (Figure 3). The 1 exception was that costs were slightly higher during the 30, 60, and 90 days preceding the event of change in treatment.
FIGURE 3.
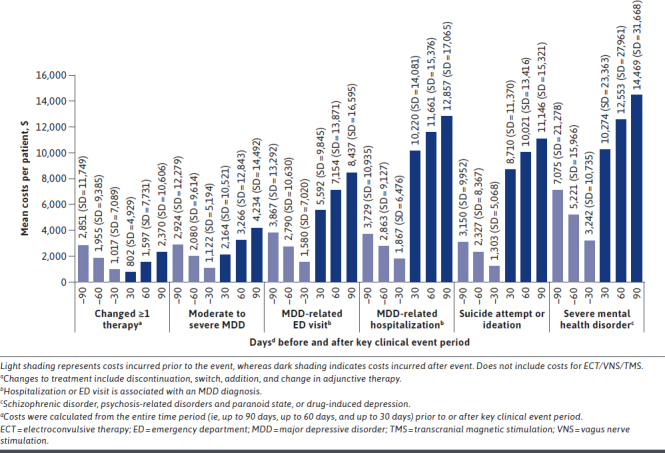
Mean Costs Preceding and Following Key Clinical Events
Comparing Patients With and Without Events. After multivariable analysis, patients with any key clinical events had 51% higher PPPY all-cause health care costs compared with those who did not have any key clinical events (Figure 4). The most impactful key clinical event was treatment with ECT, VNS, or TMS, in which patients incurred 4.3 times higher follow-up costs than those who did not receive 1 of these treatments. Compared with patients without the event, follow-up costs were 2.4 times higher among patients with a severe mental health disorder, 2.2 times higher among those with MDD-related hospitalization, 2.1 times higher among those with suicide attempt or ideation, 1.8 times higher among those with MDD-related ED visit, and 1.4 times higher among those with moderate to severe MDD. Compared with those who did not experience at least 1 treatment change, patients who did experience this event incurred higher all-cause costs PPPY ($10,254 vs $9,846; cost difference = $408).
FIGURE 4.
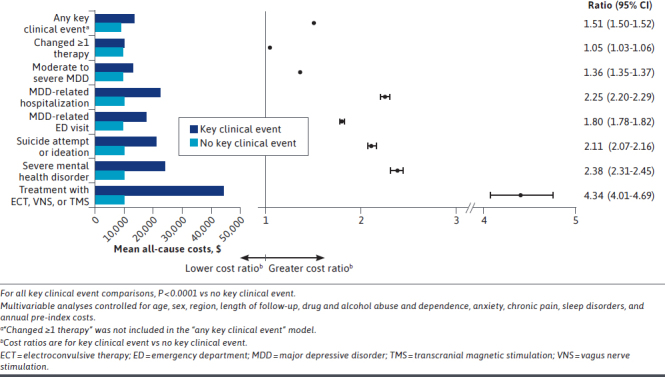
Multivariable Analysis of All-Cause Costs: With Key Clinical Event vs Without Key Clinical Event
Discussion
This retrospective study assessed health care utilization and costs among patients newly diagnosed with MDD, particularly for those with key clinical events. More than 90% of patients had at least 1 key clinical event (ie, treatment change, moderate to severe MDD, MDD-related ED visit or hospitalization, severe mental health disorder, suicide attempt/ideation, or treatment with ECT, VNS, or TMS). Overall, patients who had key clinical events incurred greater costs compared with those who did not. Regardless of the presence of key clinical events, mean annualized allcause costs were approximately 20% higher at follow-up than at baseline. All-cause hospitalization and outpatient pharmacy costs each comprised around 20% of total costs at follow-up, with other outpatient services accounting for the remaining 60% of costs. Patients who had at least 1 occurrence of a key clinical event, with the exception of treatment change, had higher average annual follow-up health care costs than the overall patient population, in which the presence of key clinical events was not considered; further, these patients had 51% higher costs than patients who did not have any key clinical events.
Treatment change was the most common key clinical event, and the vast majority of treatment changes were discontinuations. Discontinuation may have been a function of remission in some patients; however, remission codes are not used consistently in claims data and were not used to identify patients in this study. After adjusting for potential confounders, costs were slightly higher for patients who had a treatment change event vs those who did not, indicating that patients who experienced a change in treatment did not incur considerably higher costs. Further, the cost of treatment changes was numerically similar to those of moderate to severe MDD events, less than those of MDD-related hospitalizations/ED visits and suicide attempt or ideation events, and far less than those of severe mental health disorder and treatment with ECT, VNS, or TMS events. In this study, the key clinical event of treatment change was defined broadly, and future research is needed to better understand the nuances of the types of treatment changes that occur in clinical practice and the impact that treatment changes could have on costs associated with MDD.
During the 90 days prior to and following a key clinical event, average costs after the event were generally higher than costs preceding the event. The only exception was the key clinical event of treatment change, in which observed costs were slightly lower after the event. This finding was further supported in the multivariable analysis, as patients with at least 1 treatment change incurred only modestly higher costs than those without at least 1 treatment change. It is possible that there were other events not captured in our study that may have contributed to costs before and after the event. Additional research is needed to evaluate other potentially relevant events and how they contribute to pre-event and post-event costs to better understand costs associated with MDD care.
The costliest key clinical event was treatment with ECT, VNS, or TMS. Although only a small percentage of patients were treated with these therapies, the associated costs were striking, representing a 3- to 4-fold increase in all-cause costs over those of patients without any key clinical event. These procedures are typically more invasive and costly than other events,12 which likely accounted for the majority of costs. However, patients treated with these therapies may have more severe, treatment-resistant MDD and/or costly comorbidities that result in high costs. Because patients could have multiple key clinical events, it is possible that these patients may have also experienced costly events, such as ED visits or hospitalizations.
Our results highlight the high cost of key clinical events for patients with MDD. It is feasible that costly clinical events could be avoided if the patient is provided with effective care earlier in the course of treatment. For example, patients who are unresponsive or partially responsive to standard antidepressant therapy may benefit from the addition of atypical antipsychotics, which have demonstrated robust efficacy when added to antidepressants for MDD,13,14 instead of cycling through antidepressants with similar mechanisms of action. There is evidence that treatment patterns are often inconsistent with clinical guidelines for MDD.15 As such, 1 potential strategy to lower costs associated with MDD may be better adherence to treatment guidelines3 (eg, initiate adjunctive therapy after the first line of unresponsive therapy lasting 1-2 months). Another strategy may be to identify which subgroup of patients would benefit from adjunctive treatments earlier in the disease course, as many patients do not achieve remission with antidepressant monotherapy and combination therapy7. Focusing on patient treatment history may deter clinicians from prescribing potentially ineffective medications and reduce the overall costs associated with MDD.
LIMITATIONS
Limitations of this study include the use of a database restricted to claims in the United States, which may increase the difficulty of extrapolating the results to other populations. Also, large databases can be subject to data input errors, which could potentially compromise the results. The use of insurance claims data limited the ability to distinguish whether treatment changes were according to standard of care or a result of inadequate response or treatment resistance. For this reason, treatment changes may not be considered as significant as receipt of ECT as a key clinical event, which is supported by our cost results (ie, the average cost for patients with treatment change was similar to the overall population). Additional research in leveraging electronic medical records along with physician notes may help further refine the analysis and definition of key clinical events. During the study period, ICD codes transitioned from ICD-9-CM to ICD-10-CM, which may have influenced clinical event rates captured in this study. The event of suicidal attempt and ideation may be underreported, and, therefore, the estimate in this analysis is likely conservative. Further, treatment data (eg, changed 1 therapy) were ascertained from filled prescriptions, which do not provide information about whether patients had taken their medications; this is important to note because treatment nonadherence is common in patients with MDD.16 The multivariable analyses were not all-inclusive, as only claims data were included (eg, demographics, geographic location); therefore, not all confounding factors may have been accounted for in the analysis. By design, multivariable analyses are expressly descriptive, and our findings should be not interpreted as causal. Finally, cancer diagnosis, which was not accounted for in our analysis, may affect the antidepressant treatment choice.
Conclusions
The overall costs of MDD are significant, and the results of this retrospective study indicate that these costs are exacerbated by key clinical events in patients with MDD. Effective medication regimens initiated optimally in the course of treatment may mitigate costly clinical events and reduce the substantial economic burden of MDD.
ACKNOWLEDGMENTS
The authors would like to thank Amy Tung, PharmD, MS, of AbbVie for her contributions to this analysis. Writing and editorial assistance was provided to the authors by Prescott Medical Communications Group (Chicago, IL), which was funded by AbbVie.
REFERENCES
- 1.Otte C, Gold SM, Penninx BW, et al. Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065. doi: 10.1038/nrdp.2016.65 [DOI] [PubMed] [Google Scholar]
- 2.Hasin DS, Sarvet AL, Meyers JL, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018;75(4):336-46. doi: 10.1001/jamapsychiatry.2017.4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Practice guidelines for the treatment of patients with major depressive disorder. American Psychiatric Association. 2010. [Google Scholar]
- 4.Murrough JW, Charney DS. Is there anything really novel on the antidepressant horizon? Curr Psychiatry Rep. 2012;14(6):643-9. doi: 10.1007/s11920-012-0321-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newport DJ, Carpenter LL, McDonald WM, et al. Ketamine and other NMDA antagonists: Early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950-66. doi: 10.1176/appi.ajp.2015.15040465 [DOI] [PubMed] [Google Scholar]
- 6.Warden D, Rush AJ, Trivedi MH, Fava M, Wisniewski SR. The STAR*D Project results: A comprehensive review of findings. Curr Psychiatry Rep. 2007;9(6):449-59. doi: 10.1007/s11920-007-0061-3 [DOI] [PubMed] [Google Scholar]
- 7.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163(11):1905-17. doi: 10.1176/ajp.2006.163.11.1905 [DOI] [PubMed] [Google Scholar]
- 8.Egede LE. Major depression in individuals with chronic medical disorders: Prevalence, correlates and association with health resource utilization, lost productivity and functional disability. Gen Hosp Psychiatry. 2007;29(5):409-16. doi: 10.1016/j.genhosppsych.2007.06.002 [DOI] [PubMed] [Google Scholar]
- 9.Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76(2):155-62. doi: 10.4088/JCP.14m09298 [DOI] [PubMed] [Google Scholar]
- 10.Citrome L, Jain R, Tung A, Landsman-Blumberg PB, Kramer K, Ali S. Prevalence, treatment patterns, and stay characteristics associated with hospitalizations for major depressive disorder. J Affect Disord. 2019;249:378-84. doi: 10.1016/j.jad.2019.01.044 [DOI] [PubMed] [Google Scholar]
- 11.Gauthier G, Guerin A, Zhdanava M, et al. Treatment patterns, healthcare resource utilization, and costs following first-line antidepressant treatment in major depressive disorder: a retrospective US claims database analysis. BMC Psychiatry. 2017;17(1):222. doi: 10.1186/s12888-017-1385-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cusin C, Dougherty DD. Somatic therapies for treatment-resistant depression: ECT, TMS, VNS, DBS. Biol Mood Anxiety Disord. 2012;2:14. doi: 10.1186/2045-5380-2-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: A meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009;166(9):980-91. doi: 10.1176/appi.ajp.2009.09030312 [DOI] [PubMed] [Google Scholar]
- 14.Papakostas GI, Shelton RC, Smith J, Fava M. Augmentation of antidepressants with atypical antipsychotic medications for treatment-resistant major depressive disorder: A meta-analysis. J Clin Psychiatry. 2007;68(6):826-31. doi: 10.4088/jcp.v68n0602 [DOI] [PubMed] [Google Scholar]
- 15.Kern DM, Cepeda MS, Defalco F, Etropolski M. Treatment patterns and sequences of pharmacotherapy for patients diagnosed with depression in the United States: 2014 through 2019. BMC Psychiatry. 2020;20(1):4. doi: 10.1186/s12888-019-2418-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein-Shvachman I, Karpas DS, Werner P. Depression treatment non-adherence and its psychosocial predictors: Differences between young and older adults? Aging Dis. 2013;4(6):329-36. doi: 10.14336/AD.2013.0400329 [DOI] [PMC free article] [PubMed] [Google Scholar]


