Abstract
BACKGROUND:
Among patients with human epidermal growth factor receptor 2-positive (HER2+) metastatic breast cancer (MBC), incidence of brain metastases (BMs) is relatively high and increasing. Despite the high unmet need for patients with HER2+ MBC and BMs, real-world data on treatment patterns and outcomes for these patients are limited.
OBJECTIVE:
To compare treatment patterns and overall survival (OS) among patients with HER2+ MBC with and without BMs in the United States.
METHODS:
This was a real-world retrospective cohort study in which adults diagnosed with HER2+ MBC between January 1, 2016, and May 31, 2019, were identified in the Flatiron Health electronic health records database. The cohort was stratified by presence of BMs at MBC diagnosis (baseline) and before the initiation of each line of therapy (LOT). Key outcomes were OS and systemic therapy/regimen used within each LOT. An adjusted Cox proportional hazards model was used to evaluate the impact of BMs on OS.
RESULTS:
Of 1,755 included patients, 173 (9.9%) had BMs at baseline. Trastuzumab+ pertuzumab–based regimens were the most common first- (n = 689, 44.3%) and second-line (n = 316, 35.3%) treatments for all patients. Among patients with BMs, trastuzumab emtansine was the most common third-line regimen (n = 18, 23.4%). Lapatinib-based regimens were used more frequently among patients with BMs but were used by less than 20% of patients with BMs within any LOT. Median OS was 22.3 and 37.3 months for patients with and without BMs at baseline, respectively. Patients with BMs had a higher risk of death compared with patients without BMs (HR, 3.2; 95% CI = 2.6-3.8).
CONCLUSIONS:
BMs are associated with an increased risk of mortality among patients with HER2+ MBC. Further studies are needed to evaluate the extent to which novel systemic therapies for HER2+ MBC address the unmet need among patients with BMs.
Plain language summary
Of patients with human epidermal growth factor receptor 2–positive (HER2+) breast cancer that had spread, in 10% it had spread to their brain when diagnosed. National Comprehensive Cancer Network (NCCN) Central Nervous System guidelines recommend systemic tucatinib- or lapatinib-based treatment for patients with cancer that has spread to their brain. Overall, 40% received recommended treatments, but only 20% of those with cancer that had spread to their brain. Patients with cancer that had spread to their brain were more likely to die sooner.
Implications for managed care pharmacy
In this real-world study of patients with HER2+ metastatic breast cancer (MBC), 10% had brain metastases (BMs) at diagnosis. Overall, less than 20% of patients with BMs received the NCCN-recommended regimens. Patients with BMs had shorter overall survival and a 3-fold greater risk of death, highlighting the greater disease burden and unmet need in patients with HER2+ MBC and BMs.
Breast cancer (BC) is the most frequently diagnosed cancer in women worldwide and is the leading cause of cancer death in women.1 In the United States, there were an estimated 276,480 new cases of BC and 42,170 BC deaths in 2020.2 The human epidermal growth factor receptor 2-positive (HER2+) BC subtype is an aggressive form of the disease associated with an increased likelihood of recurrence and metastasis.3 Approximately 15% of women diagnosed with early (stages 1-3) BC and 26% of women diagnosed with metastatic disease have the HER2+ molecular subtype.4
Among BC subtypes, incidence of brain metastases (BMs) is highest for HER2+ and triple-negative BC.5 Evidence suggests that up to 50% of patients with HER2+ BC will develop brain or central nervous system (CNS) metastases over the course of their disease6-9; however, because screening of asymptomatic patients for BMs is not recommended in clinical practice, the incidence of BMs is likely underestimated.10-12 Since the introduction of systemic HER2-directed agents for BC, which are now standard of care, the incidence of BMs has been increasing in patients with HER2+ metastatic breast cancer (MBC).7,13 This is hypothesized to be due to multiple factors, including improved systemic disease and a lack of HER2-directed therapies that effectively penetrate the blood-brain barrier to delay the development of BMs. Additionally, there is a biological predisposition of HER2+ tumors to metastasize to the brain.14,15 As patients with HER2+ MBC live longer as a result of systemic therapies, there is more time for development of BMs.14-16
Current treatment approaches for patients with HER2+ MBC and BMs include the use of systemic therapies recommended for HER2+ MBC and local therapies such as surgical resection, stereotactic radiosurgery/radiotherapy (SRS), and whole-brain radiation therapy (WBRT).10-12,17-19 Surgical resection may be followed by SRS or WBRT for improved local control.19-21 However, patients who undergo WBRT often experience progressive and irreversible cognitive deterioration, including decline in learning capacity, processing speed, memory, and attention.11,22 Seizures and balance disorders are also neurological sequelae from radiation therapy.10,23-25 For some patients (eg, those with a limited number of BM lesions), SRS is preferred to WBRT as a less toxic local therapy option.11,19 Local therapies are often added to systemic HER2-directed therapies.26 In addition to the appropriate local therapy, clinical guidelines for management of advanced HER2+ BC with BMs recommend that patients whose systemic disease is not progressive at the time of BM diagnosis should remain on their current systemic therapy, whereas patients whose systemic disease is progressive at BM diagnosis should receive HER2-directed therapy according to treatment algorithms.10,11,18,19 The National Comprehensive Cancer Network (NCCN)-recommended first-line (1L) standard of care for patients with HER2+ MBC is trastuzumab + pertuzumab in combination with a taxane, followed by trastuzumab emtansine (T-DM1) in patients who progress after 1L treatment.10,11,27 There is no established standard of care in third-line (3L) treatment; however, other treatment options include lapatinib + trastuzumab or capecitabine, as well as trastuzumab with chemotherapy.10,11,27
Despite improvements in outcomes with the widespread use of targeted therapies for HER2+ MBC in recent decades, prognosis after a diagnosis of BMs remains poor.16 In the Systemic Therapies for HER2-positive Metastatic Breast Cancer (SystHERs) study, a prospective cohort of HER2+ MBC patients (N = 977) in the United States enrolled from 2012 to 2016, the risk of death was higher in patients with CNS metastases at diagnosis vs patients who never developed CNS metastases (hazard ratio [HR] = 2.86; 95% CI = 2.05-4.00).8 Additionally, progression of BMs/CNS metastases is the cause of death among 61%-70% of HER2+ MBC patients with BMs/CNS metastases.28,29
Symptoms (eg, seizures, visual impairment, headaches, nausea/vomiting) and neurological progression associated with BMs can be debilitating, affecting patients’ health-related quality of life (HRQoL) and ability to carry out daily activities.30 In the SystHERs study, patients with CNS metastases at MBC diagnosis reported lower HRQoL at enrollment, greater impairment in daily activities, and greater severity of cognitive dysfunction compared with patients without CNS metastases at diagnosis.8
Despite the high unmet need among patients with HER2+ MBC and BMs, real-world data on treatment patterns and outcomes for these patients are limited. The objective of this study was to compare real-world treatment patterns and overall survival (OS) among HER2+ MBC patients in the United States with and without BMs.
Methods
STUDY DESIGN AND DATA SOURCE
This was a retrospective cohort study, as well as an exploratory treatment effectiveness study, using the Flatiron Health database (a nationwide, longitudinal, demographically and geographically diverse, deidentified database derived from electronic health record (EHR) data from more than 280 cancer clinics in the United States).31 Flatiron’s Enhanced Metastatic Breast Cancer Datamart includes structured data (eg, laboratory values, prescribed drugs), in addition to unstructured data (eg, biomarker levels), collected via technology-enabled chart abstraction from physician’s notes and other unstructured documents. Institutional review board approval was not required, as the study was noninterventional and only deidentified patient records were used.
PATIENT POPULATION
The study included patients diagnosed with HER2+ MBC between January 1, 2016, and May 31, 2019, who were aged 18 years or older at diagnosis and had follow-up on or after their MBC diagnosis date. Patients with BMs were identified based on the evidence of BMs in Flatiron’s abstracted sites of metastasis table extracted from patients’ EHR records. Outcomes were assessed based on presence of BMs at MBC diagnosis (baseline) as well as before the initiation of each line of therapy (LOT). Patients were followed until their last activity date, at which point they were censored. No exclusion criteria were applied to the cohort and clinical trial patients were not excluded from the study.
LOT AND TREATMENT REGIMEN DEFINITIONS
Flatiron Health’s LOT rules were used to characterize treatment patterns.31 Flatiron defines each LOT as the first eligible drug episode plus other eligible drugs administered within 28 days. An “episode” of systemic therapy is defined as an administration or a noncanceled order for nonabstracted therapies. If a patient was prescribed 2 or more of the hormone therapies letrozole, anastrozole, exemestane, or tamoxifen in a LOT, only 1 is included in the LOT according to which was received last in the 28-day period. This same rule applies to patients who receive 2 or more lines of leuprolide, goserelin, or triptorelin.
Treatment regimens in each LOT were categorized into mutually exclusive groups on a hierarchical basis: “trastuzumab + pertuzumab–based” regimens were defined as receipt of trastuzumab + pertuzumab in the same line but no lapatinib or T-DM1; “trastuzumab-based” regimens were defined as receipt of trastuzumab in a line but no pertuzumab, lapatinib, or T-DM1; “T-DM1–based” regimens were defined as receipt of T-DM1 in a line but no pertuzumab, trastuzumab, or lapatinib; “lapatinib-based” regimens were defined as receipt lapatinib in a line but no trastuzumab, pertuzumab, or T-DM1; and “hormone + chemotherapy” regimens were defined as receipt of hormone therapy + chemotherapy in the same line but no trastuzumab, pertuzumab, T-DM1, or lapatinib. If patients received only hormone therapy or only chemotherapy in a line, the regimen was categorized as “hormone” or “chemotherapy,” respectively. All other combinations were categorized as “other.”
COVARIATES
Covariates of interest included sex, age, race and ethnicity, stage of initial BC diagnosis, de novo or recurrent MBC at baseline, Eastern Cooperative Oncology Group performance status (ECOG PS) score, hormone receptor status, and number and sites of metastases at baseline.
OUTCOMES
The primary outcome of interest was OS, defined as time from baseline to death or last visit date. Patients without a death recorded during follow-up were censored at their last activity date. Patient mortality data are verified within the Flatiron dataset by cross-checking of both EHR structured data, such as date of death, and unstructured EHR data sources, including medical care notes.32 Secondary outcomes included the proportion of patients with BMs as the first site of metastatic progression and type of systemic therapy/regimen within each LOT.
STATISTICAL ANALYSIS
Descriptive statistics for continuous variables included mean, median, SD, and IQR. Categorical variables were presented as frequency counts and percentages. Survival analyses were conducted using the Kaplan-Meier method. An adjusted Cox proportional hazards model was used to assess the impact of BMs on OS. The model was adjusted for age, hormone receptor status, year of metastatic diagnosis, number of non-BM sites at baseline (< 2 and ≥ 2), body mass index, baseline ECOG PS (0, 1, and ≥ 2), and recurrent vs de novo MBC. The presence of BMs was treated as a time-varying covariate to capture BMs that developed over time. A P value of < 0.05 was considered statistically significant.
Results
Of 8,333 patients with an MBC diagnosis between January 1, 2016, and May 31, 2019, a total 1,755 (21.1%) met the inclusion criteria. Of these, 173 (9.9%) had evidence of BM diagnosis at baseline (Supplementary Figure 1 (157.7KB, pdf) , available in online article). Median follow-up time was 9.6 months for patients with BMs and 15.6 months for patients without BMs at baseline. Patients with BMs at baseline were younger, on average, than those without BMs at baseline (56 vs 63 years) (Table 1). Compared with patients without BMs at baseline, a greater proportion of patients with BMs at baseline had recurrent MBC.
TABLE 1.
Patient Demographic and Clinical Characteristics
| Characteristic | Total (N = 1,755) | BMs at baseline (n = 173) | No BMs at baseline (n = 1,582) |
|---|---|---|---|
| Sex | |||
| Female | 99.3 (1,742) | 99.4 (172) | 99.2 (1,570) |
| Male | 0.7 (13) | 0.6 (1) | 0.8 (12) |
| Median age (in years) | 62.0 | 56.0 | 63.0 |
| Age 18-64 years | 52.9 (928) | 64.7 (112) | 51.6 (816) |
| Age ≥ 65 years | 47.1 (827) | 35.3 (61) | 48.4 (766) |
| Race and ethnicity a | |||
| Non-Hispanic White | 64.9 (1,026) | 62.8 (98) | 65.1 (928) |
| Non-Hispanic Black | 12.1 (191) | 8.3 (13) | 12.5 (178) |
| Other | 13.7 (217) | 16.7 (26) | 13.4 (191) |
| Hispanic or Latino | 9.3 (148) | 12.2 (19) | 9.0 (129) |
| Unknown | (173) | (17) | (156) |
| Stage at initial diagnosis a | |||
| 1 | 10.3 (168) | 9.1 (15) | 10.4 (153) |
| 2 | 25.1 (411) | 28.7 (47) | 24.7 (364) |
| 3 | 23.9 (391) | 35.4 (58) | 22.6 (333) |
| 4 | 40.7 (667) | 26.8 (44) | 42.3 (623) |
| Unknown/missing | 118 | 9 | 109 |
| De novo or recurrent MBC | |||
| De novo | 38.0 (667) | 25.4 (44) | 39.4 (623) |
| Recurrent | 62.0 (1,088) | 74.6 (129) | 60.6 (959) |
| ECOG score a | |||
| 0 | 51.1 (291) | 54.1 (33) | 50.8 (258) |
| 1 | 34.3 (195) | 37.7 (23) | 33.9 (172) |
| ≥ 2 | 14.6 (83) | 8.2 (5) | 15.4 (78) |
| Missing | 1,186 | 112 | 1,074 |
| Hormone receptor status a | |||
| HR+ (ER+ or PR+) | 67.5 (945) | 55.8 (67) | 68.6 (878) |
| HR- (ER- and PR-) | 32.5 (455) | 44.2 (53) | 31.4 (402) |
| Unknown/missing | 355 | 53 | 302 |
| Number of metastatic sites | |||
| < 3 | 79.5 (1,396) | 63.6 (110) | 81.3 (1,286) |
| ≥ 3 | 20.5 (359) | 36.4 (63) | 18.7 (296) |
| Nonbrain metastatic sites b | |||
| Bone | 53.3 (935) | 30.1 (52) | 55.8 (883) |
| Lung | 33.9 (595) | 31.8 (55) | 34.1 (540) |
| Distant lymph node | 29.9 (524) | 18.5 (32) | 31.1 (492) |
| Liver | 28.8 (506) | 22.5 (39) | 29.5 (467) |
| Follow-up time (in months) | 15.0 (0.0-44.4) | 9.6 (0.2-43.1) | 15.6 (0.0-44.4) |
| Received NCCN guideline–recommended treatment c | |||
| First line | 41.8 (651) | 11.8 (17) | 44.9 (634) |
| Second line | 14.5 (130) | 13.0 (16) | 14.8 (114) |
| Third line | 17.2 (76) | 16.9 (13) | 17.2 (63) |
| Fourth line | 14.8 (31) | 9 (4) | 16.3 (27) |
| Fifth line | 13.5 (15) | 0 (0) | 16.9 (15) |
Data presented as % (n), n, or median (minimum-maximum) unless otherwise indicated.
a Patients with missing/unknown status omitted from percentage calculations.
b Categories are not mutually exclusive.
c NCCN guideline–recommended treatments defined as lapatinib-based for patients with BMs at any LOT, trastuzumab and pertuzumab–based for patients without BMs at start of 1L, and T-DM1–based for patients without BMs at start of second line/third line/fourth line/fifth line.
BM = brain metastasis; ECOG = Eastern Cooperative Oncology Group; ER = estrogen receptor; LOT = line of therapy; MBC = metastatic breast cancer; NCCN = National Comprehensive Cancer Network; PR = progesterone receptor.
Over the study period, we observed an increase in the percentage of patients who progressed from early BC presenting with BMs as the first site of metastasis (10.5% in 2016 to 18.4% in 2019) (Figure 1).
FIGURE 1.

Percentage of HER2+ Patients Who Progressed From Early BC to Metastatic BC With the Brain as the First Site of Metastasis Compared With Any Other Site of Metastasis, by Year
The data that support the findings of this study were obtained under license from Flatiron Health, New York, NY, and are not publicly available.
TREATMENT PATTERNS
The most common 1L and second-line (2L) treatments for all patients were trastuzumab + pertuzumab–based regimens (1L, n = 689 patients, [44.3%]; 2L, n = 316 patients, [35.3%]), though NCCN guidelines recommend this regimen for 1L, and trastuzumab deruxtecan and T-DM1 are recommended 2L treatments. In both 1L and 2L, the proportion of patients using trastuzumab + pertuzumab–based regimens was higher for patients without BMs compared with patients with BMs (1L, 44.9% vs 38.2% and 2L, 36.9% vs 25.2%, respectively) (Figure 2). Although the NCCN guidelines for HER2+ MBC do not specify recommended treatments for patients with BMs, the NCCN CNS guidelines recommend systemic treatment with tucatinib- or lapatinib-based regimens. The most common 3L therapies were T-DM1–based regimens (n = 18, 23.4%) among patients with BMs and trastuzumab + pertuzumab–based regimens (n = 90, 24.6%) among patients without BMs, though NCCN guidelines recommend T-DM1 in 2L. Trastuzumab-based regimens, which are included in NCCN guidelines for later lines of therapy, were the most commonly used therapies for patients with and without BMs in both the fourth-line (4L) (25.0% and 24.1%, respectively) and fifth-line (5L) (36.4% and 29.2%, respectively) therapies. In 1L through 4L, lapatinib-based regimens were used more frequently among patients with BMs, in line with the NCCN CNS guidelines. However, lapatinib-based regimens were used by less than 20% of patients with BMs within any LOT, which is consistent with the NCCN HER2+ MBC guidelines. There was no lapatinib use in 5L, despite NCCN CNS guidelines recommending it for BM patients regardless of LOT.
FIGURE 2.
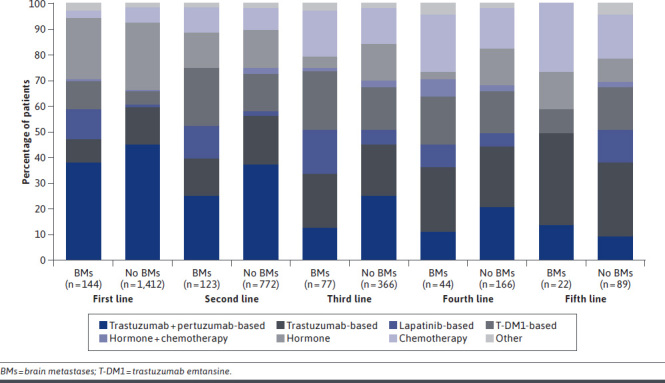
Treatment Patterns by Line of Therapy in Patients With and Without BMs
OS
Median OS was 22.3 months (95% CI = 15.3-25.9) for patients with BMs at baseline and 37.3 months (95% CI = 34.3-39.9) for patients without BMs (Figure 3). Median OS was shorter for patients with BMs compared with patients without BMs across all lines of therapy (Figure 4). Patients with BMs had a higher risk of death compared with patients without BMs (HR = 3.2; 95% CI = 2.6-3.8).
FIGURE 3.
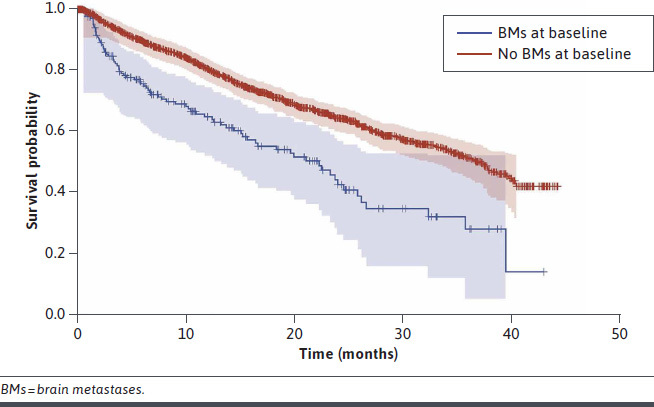
Overall Survival by the Presence of BMs at Baseline
FIGURE 4.

Median Overall Survival by LOT for Patients With and Without Brain Metastases
Discussion
This real-world retrospective study examined treatment patterns and OS in patients with HER2+ MBC with and without BMs from January 2016 to May 2019. Our results indicate that treatment patterns in this population are heterogeneous, especially in later lines of therapy. Overall, higher use of trastuzumab + pertuzumab–based therapy in 1L is consistent with current international clinical practice guideline recommendations.10,11,27 Trastuzumab + pertuzumab–based regimens were most commonly used in 1L and 2L, and use was greater among patients without BMs compared with patients with BMs (44.9% vs 38.2% in 1L, respectively). In 3L, T-DM1–based therapy was most common for patients with BMs, whereas trastuzumab–based therapy was the most common for patients without BMs. Trastuzumab–based therapy was used most commonly for all patients in 4L and 5L. Overall, there was a high use of trastuzumab in all lines of therapy in both cohorts. Although lapatinib + capecitabine is a recommended regimen for HER2+ patients with BMs,19 lapatinib-based regimens were used by less than 20% of patients with BMs within any LOT.
Our treatment pattern findings are generally consistent with those observed in other real-world studies. In the SystHERs registry, trastuzumab + pertuzumab–based therapy was also the most common 1L regimen. Relative to our findings of 1L use, a higher proportion of patients in the SystHERs registry received a trastuzumab + pertuzumab–based regimen: 52.9% and 74.8% with and without CNS metastases at diagnosis, respectively.8 Also similar to our findings, compared with patients without CNS metastases at diagnosis, a smaller proportion of patients with CNS metastases at diagnosis received trastuzumab (92.8% and 70.1%, respectively) and a larger proportion of patients received lapatinib (2.5% and 23.0%, respectively) as part of their 1L regimen. Shewade et al examined 1L treatment following diagnosis of CNS metastases among patients with HER2+ MBC and found that the most common treatments were trastuzumab + pertuzumab–containing (25.8%) or trastuzumab-containing (15.0%) regimens.33 Lapatinib-containing (20.5%) and T-DM1–containing (16.7%) regimens were also commonly used. Relative to our findings among patients with BMs in 1L, use of trastuzumab + pertuzumab–containing regimens was somewhat lower and use of lapatinib- and T-DM1–containing regimens was higher in this study.
We also observed that risk of death among patients with BMs was 3 times higher than risk of death among patients without BMs, which is consistent with findings from the SystHERs registry study, in which patients with CNS metastases at MBC diagnosis had significantly shorter median OS and almost 3 times higher risk (HR = 2.86) of death than those who never developed CNS metastases.8
To our knowledge, 3 studies have addressed the change in the pattern of metastatic occurrence in patients with HER2+ MBC and BMs. The brain is increasingly becoming the first site of metastatic disease,34 particularly among patients with HER2+ BC. In a retrospective analysis of HER2+ MBC patients from 2 US academic hospitals, BMs were present at MBC diagnosis in 8% of patients diagnosed between 2000 and 2007 compared with 16% of those diagnosed between 2008 and 2014.35 Our study findings showed that the proportion of BMs as the first site of metastasis increased during the study period from 10.5% to 18.4%.
Recent studies of novel therapies for the treatment of HER2+ MBC have explored outcomes among patients with CNS metastases/BMs.36-38 For example, in the phase 3 NALA clinical trial of neratinib + capecitabine vs lapatinib + capecitabine in patients with HER2+ MBC previously treated with 2 or more HER2-directed regimens, time to intervention (most commonly radiotherapy) for symptomatic CNS disease was delayed with neratinib + capecitabine compared with lapatinib + capecitabine (cumulative incidence of CNS intervention: 22.8% vs 29.2%, respectively; P = 0.043).36 However, this study excluded patients with unstable or symptomatic CNS disease. A post hoc exploratory analysis of T-DM1 clinical activity and safety in 2L+ HER2+ MBC patients with asymptomatic or stable BMs at baseline was conducted in the singlearm, phase 3b KAMILLA clinical trial.37 Of the 398 patients with baseline BMs, 126 (31.7%) had measurable BMs for the evaluation of treatment response. Median progression-free survival and OS were 5.5 months (95% CI = 5.3-5.6) and 18.9 months (95% CI = 17.1-21.3), respectively, in patients with baseline BMs compared with 7.7 months (95% CI = 6.8-8.1) and 30.0 months (95% CI = 27.6-31.2) in those without.37
Until recently, there have been limited clinical data on the outcomes of HER2+ MBC patients with BMs because trials of HER2-directed therapies typically exclude patients with BMs, particularly symptomatic or active BMs. The international, randomized, double-blind HER2CLIMB pivotal trial of tucatinib + trastuzumab and capecitabine vs placebo + trastuzumab and capecitabine included 291 (47.5% of the total study population) patients with untreated or previously treated progressing BMs.39 Based on positive results from the HER2CLIMB trial, tucatinib (TUKYSA, Seagen Inc.) received US Food and Drug Administration approval in April 2020,40 in combination with trastuzumab and capecitabine, for adult patients with advanced unresectable or metastatic HER2+ BC, including patients with BMs who have received 1 or more prior HER2-directed regimens in the metastatic setting, and it is now a recommended treatment option for these patients.19,41 In February 2021, tucatinib was approved by the European Medicines Agency, in combination with trastuzumab and capecitabine, for the treatment of adult patients with HER2+ locally advanced or MBC who have received at least 2 prior anti-HER2 treatment regimens. Exploratory analyses from the HER2CLIMB trial showed that the tucatinib regimen reduced the risk of brain/intracranial progression or death by 68% (HR = 0.32; 95% CI = 0.22-0.48) and reduced the risk of death by 42% (HR = 0.58; 95% CI = 0.40-0.85) in patients with BMs.42 In another exploratory analysis of HER2CLIMB patients, time to new brain lesions or death was reduced by 48% in the tucatinib arm (HR = 0.52; 95% CI = 0.33-0.82; P = 0.005).43 To date, this is the first regimen to demonstrate improved antitumor activity in patients with HER2+ MBC and BMs in a randomized controlled trial. Given the evolving treatment landscape for HER2+ MBC patients, including those with BMs, further real-world studies are needed to understand how new and emerging therapies will influence treatment patterns and outcomes for patients with HER2+ MBC and BMs.
LIMITATIONS
The primary limitation of this study is that the validity of our findings depend on the completeness of treatment records and documentation. Additionally, there is potential for misclassification of LOT because of Flatiron Health’s rule-based algorithm. However, we would expect this to be nondifferential in nature. Inherent to real-world data is the possibility of misclassification, both for death and BMs. However, these would both be nondifferential in nature and would, therefore, result in an attenuated effect estimate. Additionally, a previously published validation analysis of mortality endpoint reporting by Flatiron reported a sensitivity of 97.7% for patients with MBC, suggesting that any misclassification of death is unlikely to have significantly impacted the outcomes of this study.44 It may be that some patients with BMs were included in the patients without BMs cohort, which would have reduced the difference in survival outcomes between cohorts.
Conclusions
Findings from this study show that BMs are associated with an increased risk of mortality among patients with HER2+ MBC, representing a substantial clinical burden. Additional real-world studies are needed to evaluate the extent to which recently introduced novel systemic therapies for HER2+ MBC address the need for systemic treatments that are effective for patients with BMs.
ACKNOWLEDGMENTS
Medical writing support was provided by Philip Ruane of Curo Consulting, a division of Envision Pharma Group, and funded by Seagen Inc.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 3.Ignatov A, Eggemann H, Burger E, Ignatov T. Patterns of breast cancer relapse in accordance to biological subtype. J Cancer Res Clin Oncol. 2018;144(7):1347-55. doi: 10.1007/s00432-018-2644-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gong Y, Liu YR, Ji P, Hu X, Shao ZM. Impact of molecular subtypes on metastatic breast cancer patients: A SEER population-based study. Sci Rep. 2017;7:45411. doi: 10.1038/srep45411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim YJ, Kim JS, Kim IA. Molecular subtype predicts incidence and prognosis of brain metastasis from breast cancer in SEER database. J Cancer Res Clin Oncol. 2018;144(9):1803-16. doi: 10.1007/s00432-018-2697-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brufsky AM, Mayer M, Rugo HS, et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: Incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17(14):4834-43. doi: 10.1158/1078-0432.CCR-10-2962 [DOI] [PubMed] [Google Scholar]
- 7.Darlix A, Louvel G, Fraisse J, et al. Impact of breast cancer molecular subtypes on the incidence, kinetics and prognosis of central nervous system metastases in a large multicentre real-life cohort. Br J Cancer. 2019;121(12):991-1000. doi: 10.1038/s41416-019-0619-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurvitz SA, O’Shaughnessy J, Mason G, et al. Central nervous system metastasis in patients with HER2-positive metastatic breast cancer: Patient characteristics, treatment, and survival from SystHERs. Clin Cancer Res. 2019;25(8):2433-41. doi: 10.1158/1078-0432.CCR-18-2366 [DOI] [PubMed] [Google Scholar]
- 9.Pestalozzi BC, Holmes E, de Azambuja E, et al. CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: A retrospective substudy of the HERA trial (BIG 1-01). Lancet Oncol. 2013;14(3):244-48. doi: 10.1016/S1470-2045(13)70017-2 [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Breast Cancer Version 2.2021. 2021. Accessed September 1, 2021. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1419
- 11.Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 5). Ann Oncol. 2020;31(12):1623-49. doi: 10.1016/j.annonc.2020.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardoso F, Senkus E, Costa A, et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4). Ann Oncol. 2018;29(8):1634-57. doi: 10.1093/annonc/mdy192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witzel I, Oliveira-Ferrer L, Pantel K, Müller V, Wikman H. Breast cancer brain metastases: Biology and new clinical perspectives. Breast Cancer Res. 2016;18(1):8. doi: 10.1186/s13058-015-0665-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22(17):3608-17. doi: 10.1200/JCO.2004.01.175 [DOI] [PubMed] [Google Scholar]
- 15.Lin NU, Winer EP. Brain metastases: The HER2 paradigm. Clin Cancer Res. 2007;13(6):1648-55. doi: 10.1158/1078-0432.CCR-06-2478 [DOI] [PubMed] [Google Scholar]
- 16.Mounsey LA, Deal AM, Keith KC, et al. Changing natural history of HER2-positive breast cancer metastatic to the brain in the era of new, targeted therapies. Clin Breast Cancer. 2018;18(1):29-37. doi: 10.1016/j.clbc.2017.07.017 [DOI] [PubMed] [Google Scholar]
- 17.Brosnan EM, Anders CK. Understanding patterns of brain metastasis in breast cancer and designing rational therapeutic strategies. Ann Transl Med. 2018;6(9):163. doi: 10.21037/atm.2018.04.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramakrishna N, Temin S, Chandarlapaty S, et al. Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2018;36(27):2804-07. doi: 10.1200/JCO.2018.79.2713 [DOI] [PubMed] [Google Scholar]
- 19.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Central Nervous System Cancers Version 2.2020. 2020. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1425
- 20.Churilla TM, Chowdhury IH, Handorf E, et al. Comparison of local control of brain metastases with stereotactic radiosurgery vs surgical resection: A secondary analysis of a randomized clinical trial. JAMA Oncol. 2019;5(2):243-47. doi: 10.1001/jamaoncol.2018.4610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahajan A, Ahmed S, McAleer MF, et al. Post-operative stereotactic radio-surgery versus observation for completely resected brain metastases: A single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1040-48. doi: 10.1016/S1470-2045(17)30414-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutierrez-Valencia E, Sanchez-Rodriguez I, Baderrama-Ibarra R, et al. Diagnosis and management of brain metastases: An updated review from a radiation oncology perspective. J Cancer Metastasis Treat. 2019;5:54. doi: 10.20517/2394-4722.2019.20 [DOI] [Google Scholar]
- 23.Riva G, Ferrari A, Durante S, et al. Clinical outcome in brain metastases from breast cancer treated with stereotactic radiotherapy. Radiother Oncol. 2019;133:S671. doi: 10.1016/s0167-8140(19)31632-9 [DOI] [Google Scholar]
- 24.Eberst L, Morelle M, Sunyach MP, et al. Focalized treatment strategy for patients with 1 to 5 breast cancer brain metastasis: A retrospective study of 70 patients treated with surgery or stereotactic radiosurgery. Ann Oncol. 2016;127(Suppl 6):vi77. 10.1093/annonc/mdw365.28 [DOI] [Google Scholar]
- 25.Cao KI, Lebas N, Gerber S, et al. Phase II randomized study of whole-brain radiation therapy with or without concurrent temozolomide for brain metastases from breast cancer. Ann Oncol. 2015;26(1):89-94. doi: 10.1093/annonc/mdu488 [DOI] [PubMed] [Google Scholar]
- 26.Duchnowska R, Loibl S, Jassem J. Tyrosine kinase inhibitors for brain metastases in HER2-positive breast cancer. Cancer Treat Rev. 2018;67:71-77. doi: 10.1016/j.ctrv.2018.05.004 [DOI] [PubMed] [Google Scholar]
- 27.Giordano SH, Temin S, Davidson NE. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: ASCO Clinical Practice Guideline Update summary. J Oncol Pract. 2018;14(8):501-04. doi: 10.1200/JOP.18.00290 [DOI] [PubMed] [Google Scholar]
- 28.Le Scodan R, Massard C, Jouanneau L, et al. Brain metastases from breast cancer: Proposition of new prognostic score including molecular subtypes and treatment. J Neurooncol. 2012;106(1): 169-76. doi: 10.1007/s11060-011-0654-x [DOI] [PubMed] [Google Scholar]
- 29.Baffert S, Cottu P, Kirova YM, et al. Treatment patterns, clinical outcomes and health care costs associated with her2-positive breast cancer with central nervous system metastases: A French multicentre observational study. BMC Health Serv Res. 2013;13:456. doi: 10.1186/1472-6963-13-456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oehrlich NE, Spineli LM, Papendorf F, Park-Simon TW. Clinical outcome of brain metastases differs significantly among breast cancer subtypes. Oncol Lett. 2017;14(1):194-200. doi: 10.3892/ol.2017.6166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flatiron Health. For life sciences. Accessed September 1, 2021. https://flatiron.com/real-world-evidence/
- 32.Curtis MD, Griffith SD, Tucker M, et al. Development and validation of a high-quality composite real-world mortality endpoint. Health Serv Res. 2018;53(6):4460-76. doi: 10.1111/1475-6773.12872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shewade A, Hsieh AF-C, Mani A, et al. Real-world (RW) characteristics, treatment (tx) patterns, and overall survival (OS) in US patients (pts) with metastatic breast cancer (mBC) and CNS metastases (CNS mets). J Clin Oncol. 2018;36(15_suppl):1037. doi: 10.1200/JCO.2018.36.15suppl.1037 [DOI] [Google Scholar]
- 34.van den Hurk CJ, Eckel R, van de Poll-Franse LV, et al. Unfavourable pattern of metastases in M0 breast cancer patients during 1978-2008: A population-based analysis of the Munich Cancer Registry. Breast Cancer Res Treat. 2011;128(3):795-805. doi: 10.1007/s10549-011-1372-y [DOI] [PubMed] [Google Scholar]
- 35.Strulov Shachar S, Deal A, Vaz-Luis I, et al. Abstract P1-12-08: The incidence and outcomes of brain metastases in HER2-positive metastatic breast cancer with the advent of modern anti-HER2 therapies. Cancer Res. 2017;77(4 Suppl):P1-12-08. 10.1158/1538-7445.SABCS16-P1-12-08 [DOI] [Google Scholar]
- 36.Saura C, Oliveira M, Feng Y-H, et al. Neratinib + capecitabine versus lapatinib + capecitabine in patients with HER2+ metastatic breast cancer previously treated with ≥ 2 HER2-directed regimens: Findings from the multinational, randomized, phase III NALA trial. J Clin Oncol. 2019;37(15_suppl):1002. doi: 10.1200/JCO.2019.37.15suppl.1002 [DOI] [Google Scholar]
- 37.Montemurro F, Delaloge S, Barrios CH, et al. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: Exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial(*). Ann Oncol. 2020;31(10):1350-58. doi: 10.1016/j.annonc.2020.06.020 [DOI] [PubMed] [Google Scholar]
- 38.Jerusalem G, Park YH, Yamashita T, et al. CNS metastases in HER2-positive metastatic breast cancer treated with trastuzumab deruxtecan: DESTINY-Breast01 subgroup analyses. Ann Oncol. 2020;31(Suppl 2):S63-64. 10.1016/j.annonc.2020.03.239 [DOI] [Google Scholar]
- 39.Murthy R, Borges VF, Conlin A, et al. Tucatinib with capecitabine and trastuzumab in advanced HER2-positive metastatic breast cancer with and without brain metastases: A non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19(7):880-88. doi: 10.1016/S1470-2045(18)30256-0 [DOI] [PubMed] [Google Scholar]
- 40.US Food & Drug Administration. FDA approves tucatinib for patients with HER2-positive metastatic breast cancer. 2020. Accessed February 2, 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tucatinib-patients-her2-positive-metastatic-breast-cancer
- 41.Seagen Inc. TUKYSA (tucatinib) [package insert]. 2020. Accessed September 1, 2021. https://www.seagen.com/medicines/tukysa
- 42.Lin NU, Borges V, Anders C, et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB Trial. J Clin Oncol. 2020;38(23):2610-19. doi: 10.1200/JCO.20.00775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bachelot T, Lin NU, Murthy RK, et al. Impact of tucatinib on progression free survival in patients with HER2+ metastatic breast cancer and stable or active brain metastases. Ann Oncol. 2020;31(Suppl 4):S359-60. 10.1016/j.annonc.2020.08.395 [DOI] [Google Scholar]
- 44.Zhang Q, Gossai A, Monroe S, Nussbaum NC, Parrinello CM. Validation analysis of a composite real-world mortality endpoint for patients with cancer in the United States. Health Serv Res. 2021;56(6):1281-87. doi: 10.1111/1475-6773.13669 [DOI] [PMC free article] [PubMed] [Google Scholar]


