Abstract
BACKGROUND:
Brolucizumab is a new anti-vascular endothelial growth factor (anti-VEGF) approved for treating neovascular age-related macular degeneration (nAMD). Multiple treatment regimens are available for treating nAMD. These regimens include manufacturer-recommended regimens, pro re nata (PRN) regimens, and treat-and-extend (T&E) regimens, which are based on clinical practice guidelines and data observed in the real-world clinical setting, classified as real-world evidence (RWE). Most budget impact models predict the financial consequences of adding a new drug to the formulary based on the manufacturer-recommended regimen. With different anti-VEGF treatment regimens being used in nAMD by ophthalmologists, it is
OBJECTIVE:
To estimate the budget impact of different treatment regimens of brolucizumab in nAMD from a US payer perspective.
METHODS:
A Microsoft Excel-based budget impact model was developed for different treatment regimens of brolucizumab over a 1-year time frame from a US payer perspective. A separate analysis was performed to estimate the budget impact from a US patient population perspective. Model inputs included drug costs, administration costs, physician visit costs, and disease monitoring costs. Outcomes in the budget impact model included the cost per member per month, annual health plan cost, and the US patient population-based annual cost. Based on the prevalence of nAMD in public and commercial health plans, a scenario analysis was conducted on the US population to account for the differences in the drug cost to the public and commercial payers. Further, 1-way sensitivity analyses were conducted to test model assumptions and uncertainty in model inputs.
RESULTS:
The addition of brolucizumab to the formulary increased the net budgetary impact under PRN and T&E regimens. The maximum increase in expenditure for a hypothetical health plan with 1 million enrollees was associated with the PRN regimen ($824,696), followed by the T&E regimen ($163,101). In contrast, using the manufacturer-recommended and RWE regimens led to an annual saving of $93,068 and $94,170 for the health plan, respectively. In the US patient population model, the introduction of brolucizumab resulted in savings in the manufacturer-recommended ($30.99 million) and RWE regimens ($31.35 million) but led to an increase in annual expenditures for the PRN ($274.58 million) and T&E ($54.30 million) regimens.
CONCLUSIONS:
Payers need to evaluate the cost impact of different treatment regimens of existing and new anti-VEGFs when making formulary decisions in nAMD management.
Plain language summary
Neovascular age-related macular degeneration (nAMD) is the leading cause of vision loss among older adults. Injectable drugs are used to treat nAMD, and their cost depends on the type of drug, the number of injections, and the number of physician office visits. The goal of nAMD-related treatment guidelines is to reduce the number of injections and physician office visits while maintaining the treatment outcomes in patients. This study demonstrates how different treatment guidelines can affect the overall cost of these injectable drugs.
Implications for managed care pharmacy
There is no clear preference for anti-vascular endothelial growth factor (anti-VEGF) treatment regimens in nAMD among physicians. This study provides valuable information to payers on cost related to various anti-VEGF treatment regimens. The study results show that the cost of adding brolucizumab to the formulary varies with the choice of its treatment regimen. In building a budget model for nAMD, payers should consider not only the anti-VEGF injection costs but also the different treatment regimens used by the providers. imperative that payers quantify the potential budget impact of different treatment regimens of brolucizumab.
Neovascular age-related macular degeneration (nAMD) is the leading cause of vision loss among older adults in developed countries, including the United States.1 The disease is characterized by the growth of new vessels under the macula, known as choroidal neovascularization, which in the end stage of disease results in fibrovascular scars, leading to central vision loss.2,3 Patients with nAMD experience poor quality of life and have reported higher stress and depression and lower physical activity than similarly aged individuals without nAMD.3 The societal economic burden due to nAMD-related blindness in the United States was estimated at $16 billion in 2020, with the cost projected to more than double by 2050. The societal burden included direct medical costs (eg, treatments and procedures), indirect costs (eg, productivity loss to patients and caregivers), and intangible costs (eg, years of life lost and quality-adjusted life years).4
Limited treatment options were available for nAMD in the 1980s and 1990s. These treatments were only able to stabilize and limit the patient’s vision loss and did not help in vision gain.5 The introduction of anti-vascular endothelial growth factor (anti-VEGF) in the early 2000s brought a paradigm shift in the management of nAMD, as these treatments were able to prevent vision loss and demonstrated effectiveness in vision gain. Currently, 4 anti-VEGF agents are available: ranibizumab, approved in 20 066; aflibercept in 20117; brolucizumab in 20198; and bevacizumab, which is used off label. Pivotal randomized clinical trials (RCTs) ANCHOR and MARINA showed significant visual gain and stability for ranibizumab compared with the early photodynamic therapy and placebo, respectively.9,10 Aflibercept demonstrated efficacy similar to ranibizumab in the RCTs VIEW 1 and 2.11,12 Similarly, brolucizumab has shown comparable outcomes to aflibercept in HAWK and HARRIER trials.13 Even though bevacizumab has not been approved to treat nAMD, it has demonstrated efficacy for visual gain similar to that of ranibizumab in the IVAN and CATT trials.14,15 Clinical guidelines16,17 in the United States recommend the use of anti-VEGFs as a first-line treatment for nAMD, which have now replaced older treatments, such as laser photocoagulation and photodynamic therapy.18
Anti-VEGFs are administered using a fixed- or variableinterval dosing. These drugs can be administered monthly, as needed, or at increasing intervals.19 Some RCTs used fixed-interval treatment regimens, and anti-VEGFs were administered at a regular interval, usually monthly.9,10,20-22 However, ophthalmologists reportedly use different injection frequencies when administering anti-VEGFs. In addition to fixed monthly dosing, pro re nata (PRN) and treat-and-extend (T&E) regimens are used based on their clinical experience and on the individual patient’s disease progression. The rationale for individualized treatment is rooted in reducing the overall treatment burden due to regular patient visits, pain and discomfort of intravitreal injections, and the cost of monthly injections.23,24 Two clinical practice guidelines adapting the injection frequency, PRN and T&E, have been robustly tested in multiple RCTs25-34 and have become popular among ophthalmologists.35,36
The PRN regimen includes monthly office visits, and after initial loading doses, injections are administered only when the disease is active25-28 Although studies have shown comparable visual outcomes between fixed monthly injection and PRN dosing, the monthly visit in PRN for evaluating disease status has led to a more proactive T&E regimen being adopted by ophthalmologists.35-37 The T&E regimen uses a more objective and formulated approach to calculate the patients’ subsequent return for injection rather than a fixed monthly follow-up.33,34 In the T&E regimen, an initial loading dose is given every 4 weeks for 3 consecutive visits. At subsequent visits, the patient follow-up interval is increased by 2 weeks, up to a maximum of 12 weeks if the nAMD is inactive (no blood or fluid noted on the exam and optical coherence tomography [OCT]).19 Conversely, if recurrent disease activity is noted, the follow-up interval is reduced by 2 weeks, with a minimum interval of 4 weeks.
Data from real-world evidence (RWE) studies rarely match the evidence from the RCTs, irrespective of the monthly or clinical guideline-based treatment regimen. Unlike RCTs, patients in RWE studies are not limited to rigid inclusion/exclusion criteria, often resulting in diverse patients with advanced disease.38 Several RWE studies in nAMD have reported poor visual improvement among patients treated with anti-VEGF.39-44 Poor visual outcomes could be attributed to undertreatment of patients (fewer visits and/or fewer injections) or patients with advanced diseases. More importantly, RWE studies have consistently shown fewer mean injections and office visits than pivotal RCTs that corelate with poor outcomes.41,45,46 A metaanalysis of ranibizumab involving 42 global RWE studies showed a mean of 6.3 injections and 8.4 visits in the first year, with a subsequent decrease in both injections and visits for the second and third years.47 A positive association between injection frequency and the visual outcomes was also reported, which suggested injection frequency as an important independent indicator of visual improvement.47
Several studies of anti-VEGFs involving aflibercept and ranibizumab have shown noninferiority of both PRN and T&E regimens compared with a monthly regimen.25-27,48-50 A 2020 Cochrane review of 15 RCTs evaluating visual outcome based on treatment regimen showed no difference in visual acuity gain between monthly and T&E regimens at 1-year follow-up and minor but clinically insignificant gain between monthly and PRN regimens.19 Considering that the outcomes of various anti-VEGF injection patterns are similar, assessing the cost associated with these drugs becomes imperative. Cost-effectiveness studies from the United States51-54 and international perspectives55-62 have been published for older anti-VEGFs, which have shown bevacizumab as the most cost-effective option, followed by aflibercept and ranibizumab. A 2018 review of economic studies reported that the cost-effectiveness comparison of aflibercept and ranibizumab was inconclusive and that the economic dominance between the 2 drugs was dependent on the injection frequency and the medication cost assumptions.63 Another major premise often seen in nAMD economic studies is analyzing the outcome using a 1-eye model. As nAMD is a bilateral disease and can affect both eyes, a 1-eye model underestimates per patient cost. Despite this, only a few studies have used a 2-eye model,52,61,64,65 presumably, because of the unavailability of the needed data and the requirement of sophisticated modeling expertise.63,66
Besides the limitation of existing economic evidence due to injection frequency and the limited use of 2-eye economic models, only 1 economic study in the United States has evaluated the cost effectiveness of brolucizumab.67 This study showed brolucizumab to be less costly and more effective than aflibercept and ranibizumab. Furthermore, the budgetary impact of adding brolucizumab to a formulary has not been reported in the published literature. Therefore, this study will estimate the budget impact of different treatment regimens of brolucizumab in nAMD from a US payer perspective.
Methods
Two Microsoft Excel-based budget impact models (BIMs) were developed: one for a health plan with hypothetical enrollees and the other based on actual US populations using 2022 US census data. Both models estimated the budget impact of brolucizumab using 4 treatment regimens over a 1-year time frame. The 4 treatment regimens included (1) the manufacturer-recommended regimen, as prescribed in the US Food and Drug Administration (FDA) label; (2) PRN; (3) T&E; and (4) the RWE regimen using data from RWE studies. The direct medical cost associated with anti-VEGFs in the 4 regimens differed primarily on the annual ophthalmologist visits and the number of anti-VEGF injections. Figure 1 shows the schematic representation of these models.
FIGURE 1.

Schematic Representation of the Budget Impact Model for the Brolucizumab in nAMD Treatment Based on Different Treatment Regimens
TARGET POPULATION
A 1-year BIM utilized a health plan with 1 million hypothetical enrollees. The treatment eligibility was based on nAMD prevalence (1.2%)66 and the proportion of the health plan population aged at least 50 years (36.04%), which was derived from the US census data.68 The treatment-eligible population was further divided into 1- (83.5%) and fellow-eye (16.5%) involvement based on the literature.69 The uptake of anti-VEGF among the eligible population in the base model was assumed to be 80%. The budget impact for the health plan was reported as total annual cost and per member per month (PMPM) cost. The BIM model with the US population used a population aged at least 50 years based on 2022 census data.68 Besides the baseline population for the base case population model, all other eligibility criteria and model inputs were the same for both the BIM models.
TREATMENT REGIMENS
Treatment regimens reported in the literature were modeled to reflect anti-VEGF use, and the net budget impact was based on the differences in the annual dose and direct medical cost related to the administration and monitoring of the different regimens of the same drug. The first regimen was based on the manufacturer recommendation and used FDA prescribing labels6-8 for all the drugs except bevacizumab, which is prescribed off label. For bevacizumab, the number of injections reported in the CATT trial was used.14,28 For PRN and T&E, the brolucizumab dose was taken from the first-year data of the HAWK and HARRIER RCTs, and the number of injections was determined by accounting for the proportion of patients treated every 12 weeks if the disease was stable and every 8 weeks if not stable.31,32,70 For the RWE regimen, the brolucizumab dose for 12 months was estimated from Bilgic and colleagues’ 2021 study that reported a mean follow-up of 10.4 months.71 The doses for other anti-VEGFs under RWE regimen were taken as a weighted means from Ciulla et al41 and Rao et al39 that used claims data. The doses for the remainder of the drug regimens, not described here, were taken directly from RCTs and did not require any adjustment or calculation (Table 1). Multicenter and international studies were used when US data were not available.
TABLE 1.
Population, Treatment Regimens, and the Market Share Input
| Value | Sources, details, and assumptions | |
|---|---|---|
| Population parameters | ||
| US population 2022, n | 332,941,000 | US Census Bureau68 |
| Enrollee in a health care plan, n | 1,000,000 | Assumption |
| Population aged at least 50 years, % | 36.04 | US Census Bureau68 |
| Prevalence of nAMD in people aged at least 50 years, % | 1.20 | Rudnicka et al (2015)81 |
| Patients with 1-eye involvement, % | 83.5 | Zarbin et al (2020)69 |
| Proportion of patients on anti-VEGF therapy before launch of brolucizumab, % | 80 | Assumption |
| nAMD population on public insurance, % | 73 | Assumption based on Rao et al (2018)39 |
| Treatment regimens, dosage (packs/year for 1 eye) | ||
| Manufacturer regimen | ||
| Ranibizumab | 12 | 0.5 mg (0.05 mL) monthly6 |
| Aflibercept | 8.02 | 2 mg (0.05 mL)/4 weeks for the first 3 doses, followed by the same doses once every 8 weeks7 |
| Bevacizumab | 12 | 1.25 mg (0.05 mL) monthly14,28 |
| Brolucizumab | 7.01 | 6 mg/0.05 mL/4 weeks for the first 3 doses, followed by every 8-12 weeks (10 weeks taken for calculation)8 |
| Pro re nata | ||
| Ranibizumab | 6.9 | CATT RCT28,29 |
| Aflibercept | 5.1 | Veritti et al (AFFIRMED study)89 |
| Bevacizumab | 7.7 | CATT RCT28,29 |
| Brolucizumab | 7.13 | HAWK & HARRIER RCTs30-32 |
| Treat and extend | ||
| Ranibizumab | 8.00 | LUCAS RCT33 |
| Aflibercept | 8.00 | ATLAS RCT34 |
| Bevacizumab | 8.90 | LUCAS RCT33 |
| Brolucizumab | 7.13 | HAWK & HARRIER RCTs31,32,70 |
| Real-world evidence | ||
| Ranibizumab | 7.23 | Ciulla et al (2020)41; Rao et al (2018)39 |
| Aflibercept | 7.04 | Ciulla et al (2020)41; Rao et al (2018)39 |
| Bevacizumab | 6.91 | Ciulla et al (2020)41; Rao et al (2018)39 |
| Brolucizumab | 5.55 | Bilgic et al (2021) (REBA study)71 |
| Market shares, % | ||
| Anti-VEGF market shares before brolucizumab launch | ||
| Ranibizumab | 24.56 | Parikh et al (2018)72; Ciulla et al (2020)41; Rao et al (2018)39 |
| Aflibercept | 17.48 | Parikh et al (2018)72; Ciulla et al (2020)41; Rao et al (2018)39 |
| Bevacizumab | 57.96 | Parikh et al (2018)72; Ciulla et al (2020)41; Rao et al (2018)39 |
| Brolucizumab uptake in 1 year after launch | ||
| Brolucizumab uptake in 1 year after launch | 3.84 | Zarbin et al (2020)69 |
| Percentage of Brolucizumab-switch | 92.51 | Zarbin et al (2020)69 |
| Percentage of Brolucizumab-naive | 7.49 | Zarbin et al (2020)69 |
| Proportion of switch from anti-VEGF to brolucizumab | ||
| Ranibizumab to brolucizumab | 15.41 | Zarbin et al (2020)69 |
| Aflibercept to brolucizumab | 71.15 | Zarbin et al (2020)69 |
| Bevacizumab to brolucizumab | 13.44 | Zarbin et al (2020)69 |
| Anti-VEGF market shares after brolucizumab launched (switch patients) | ||
| Ranibizumab | 24.01 | Calculated from input data |
| Aflibercept | 14.95 | Calculated from input data |
| Bevacizumab | 57.48 | Calculated from input data |
| Brolucizumab | 3.56 | Calculated from input data |
nAMD = neovascular age-related macular degeneration; RCT = randomized clinical trial; VEGF = vascular endothelial growth factor.
MARKET SHARE (TREATMENT MIX)
The market share of anti-VEGFs was determined before and after the introduction of brolucizumab. The market share before brolucizumab was determined using 664,766 eye-level data taken from 3 RWE studies that used patient registries and health plan data.39,41,72 A 1-year uptake of brolucizumab was assumed to be 3.84% based on a 6-month uptake of 1.92% reported in a patient registry study involving more than 1.15 million patients with nAMD.69 Further, in 1 year, 92.51% of the brolucizumab population (3.84%), switched from existing anti-VEGFs to brolucizumab, and the remaining were considered treatment naive.69 Treatmentnaive patients were those eligible for the anti-VEGF but who were not on anti-VEGF therapy prior to the launch of brolucizumab. The proportion of patients switching from existing anti-VEGFs to brolucizumab is provided in Table 1.
COST
The BIM included direct medical costs related to drug acquisition, office visits, administration, and disease monitoring (Table 2) . The per-unit drug costs in the base case analysis was based on the wholesale acquisitions cost (WAC) listed in the IBM Micromedex RedBook.73 The cost associated with drug administration, office visits, and monitoring were based on per-visit service costs using Current Procedural Terminology codes reported in the Centers for Medicare and Medicaid Services physician fee schedule national payment.74 The intravitreal injection cost for both eyes was estimated at 1.5 times the cost of 1 eye injection using Medicare’s multiple surgery payment guidelines.75,76 The cost of fluorescein angiography for both eyes was estimated at twice the cost of 1 eye based on a recently published study.74 The number of annual office visits and OCT examinations were assumed to be equivalent to the number of injections for all regimens except PRN. For the PRN regimen, both the monthly clinical visits and OCT examination at each visit were considered based on PRN treatment guidelines. The models assumed fluorescein angiography only at the initial visit.
TABLE 2.
Cost Input for the Model
| Services | Rate | Cost per unit | CPT and HCPCS codes |
|---|---|---|---|
| Drug costs | |||
| Wholesale acquisitions cost | |||
| Ranibizumab 0.5 mg/0.05 mL | Manufacturer: 12, PRN: 6.9, T&E: 8, RWE: 7.23 | $1,950.00 | IBM Micromedex Red Book73 |
| Aflibercept 2 mg/0.05 mL | Manufacturer: 8.02, PRN: 5.1, T&E: 8, RWE: 7.04 | $1,850.00 | IBM Micromedex Red Book73 |
| Bevacizumab 1.25 mg/0.05 mL | Manufacturer: 12, PRN: 7.7, T&E: 8.9, RWE: 6.91 | $73.20 | IBM Micromedex Red Book73 |
| Brolucizumab 6 mg/0.05 mL | Manufacturer: 7.01, PRN: 7.13, T&E: 7.13, RWE: 5.55 | $1,850.00 | IBM Micromedex Red Book73 |
| Average sales price | Same rate as used for WAC | ||
| Ranibizumab 0.5 mg/0.05 mL | $1,376.00 | HCPCS J277880 | |
| Aflibercept 2 mg/0.05 mL | $1,826.00 | HCPCS J017880 | |
| Bevacizumab 1.25 mg/0.05 mL | $73.00 | IBM Micromedex Red Book73 | |
| Brolucizumab 6 mg/0.05 mL | $1,872.00 | HCPCS J017980 | |
| Administration costs | |||
| Cost of intravitreal injection in 1 eye | Based on the treatment regimen | $114.20 | CPT 6702874 |
| Cost of intravitreal injection in both eyes (1.5x the cost of 1 eye) | — | $171.30 | Medicare multiple surgery payment guidelines75,76 |
| Physician visits | |||
| Cost of an initial office visit | 1 | $169.57 | CPT 9920474 |
| Cost of subsequent office visit | PRN is monthly; other regimens rate is determined by dosages | $128.39 | CPT 9201474 |
| Lab and disease monitoring costs | |||
| Optical coherence tomography at each visit | PRN is monthly; other regimens rates is same as their dosages | $41.18 | CPT 9213474 |
| Fluorescein angiography, at initial evaluation | 1 | $127.70 | CPT 92235,74 rate assumed to be one for the first visit |
| Cost of fluorescein angiography involving fellow eye (2x the cost of 1 eye) | $255.40 | Assumed based on a study Yu et al (2021)67 | |
CPT = Current Procedural Terminology; HCPCS = Healthcare Common Procedure Coding System; PRN = pro re nata; RWE = real-world evidence; T&E = treat and extend; WAC = wholesale acquisitions cost.
SCENARIO ANALYSIS (BASED ON MEDICARE ALLOWABLE PAYMENTS FOR PART B DRUGS)
A scenario analysis was conducted for the US population model by incorporating the percentage of patients with nAMD enrolled in public and commercial health plans. The reimbursement for public insurance was based on Medicare allowable payment for Part B using the average sales price (ASP). The remaining population on anti-VEGFs was considered enrolled in a commercial health plan, and the WAC was used for cost as described in the base case model. Based on Rao et al, 73% of patients were estimated to be enrolled in public insurance.39 If patients had dual coverage (private and Medicare/Medicaid), as mentioned in Rao et al, their reimbursement was considered to be under the public-Medicare plan. This assumption was made as the proportion of patients enrolled in public insurance reported in Rao et al closely matched the proportion of nAMD enrollees in a Medicare fee-for-service reported in the literature.77
The unit cost under public insurance for all drugs except bevacizumab was determined using the ASP. For bevacizumab, the WAC was used, as Medicare reimbursement varies among Medicare administrative contractors because of its off-label use, and no invariant national reimbursement cost data were available.77,78 The actual cost to Medicare was determined by using the Medicare allowable payment for Part B drugs, as described by Glasser et al77 and Werble et al.79 For Part B drug payment, Medicare pays 80% to an ASP and an additional 6% of ASP (ASP + 6% × ASP). To this amount, a 2% sequester Medicare payment reduction is applied under the Budget Control Act of 2011. As an example, in our study, the ASP of $1,872 for brolucizumab will be reimbursed at $1,556 ($1,872 × 106% × 80% × 98%) by Medicare. Table 1 includes the ASP cost for the drugs based on the Healthcare Common Procedure Coding System using 2022 ASP drug pricing files from the Centers for Medicare and Medicaid Services.80
SENSITIVITY ANALYSIS
A deterministic sensitivity analysis was conducted using the upper and lower bounds of the selected model parameters to assess their net budgetary impact. The parameters included the prevalence of nAMD in patients aged at least 50 years (base case: 1.2%; upper/lower bound: 0.8%-1.7%),81 the proportion of patients on anti-VEGFs (base case: 80%; upper/lower bound: ±10%), patients with 1- or 2-eye involvement (for 1 eye, base case: 83.5%; upper/lower bound: ±10%), brolucizumab drug costs (WAC and ASP ±10%), and the brolucizumab market share (base case: 3.84%; upper/lower bound: ±10%-20%). The changes in the net budgetary impact are presented as tornado diagrams (Figure 2).
FIGURE 2.
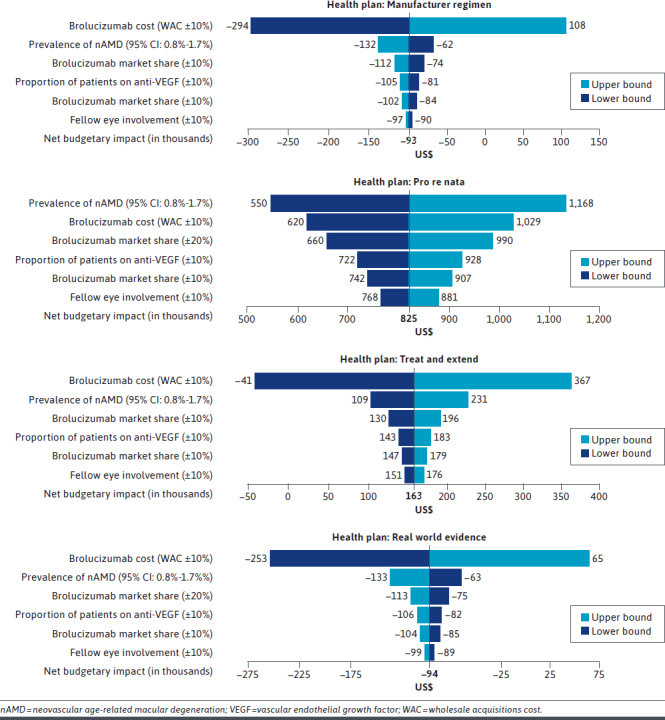
One-Way Sensitivity Analysis of the Budget Impact Model for the Brolucizumab in nAMD Treatment Based on Different Treatment Regimens
Results
POPULATION
For a health plan with a hypothetical 1 million enrollees, 3,460 patients (1-eye = 2,888 and fellow-eye = 572) with nAMD were estimated to be on anti-VEGFs. For the 2022 US population, 1,151,923 patients (1-eye = 961,418 and fellow-eye = 190,504) with nAMD were estimated to be on anti-VEGFs.
COSTS
Ranibizumab had the highest drug and administration cost per patient in all the regimens under the WAC. The ASP (scenario analysis) was highest for aflibercept under the T&E and RWE regimens, whereas the cost for ranibizumab and brolucizumab was highest for the manufacturer and the PRN regimens, respectively. The cost of bevacizumab was lowest for all 4 regimens, primarily because of a low WAC per unit. Although the drug administration could differ based on the clinical progress, the monitoring cost in the PRN regimen remains the same for all the drugs, as this regimen involves monitoring patients regularly at a monthly interval. The cost per patient for an annual treatment with anti-VEGFs in nAMD based on the WAC and ASP using different treatment regimens is described in Supplementary Table 1 (203.2KB, pdf) (available in online article).
BASE CASE BUDGET IMPACT
In the health plan model, introducing brolucizumab as a treatment for nAMD increased the net budgetary impact for PRN and T&E and decreased the net budgetary impact for the manufacturer-recommended and RWE regimens. The maximum increase in expenditure was associated with the PRN regimen (health plan = $824,696 and PMPM = $0.069), followed by the T&E regimen (health plan = $163,101 and PMPM = $0.014). The manufacturer-recommended regimen led to an annual savings of $93,068 (PMPM =$0.008), and the RWE regimen led to an annual savings of $94,170 (PMPM = $0.008). In the US population model, the introduction of brolucizumab resulted in a savings of $30.99 million and $31.35 million in the manufacturer and RWE regimens, respectively. In contrast, it increased annual expenditures for PRN and T&E (Table 3).
TABLE 3.
Base Case Model
| Manufacturer regimen | PRN regimen | T&E regimen | RWE regimen | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| US population | Health plan | PMPM | US population | Health plan | PMPM | US population | Health plan | PMPM | US population | Health plan | PMPM | |
| Without brolucizumab | ||||||||||||
| Drug cost and administration cost | ||||||||||||
| One eye | $9,750 M | $29.3 M | $2.44 | $5,851 M | $17.6 M | $1.46 | $7,470 M | $22.4 M | $1.87 | $6,571 M | $19.7 M | $1.64 |
| Fellow eye | $3,741 M | $11.2 M | $0.94 | $2,242 M | $6.7 M | $0.56 | $2,868 M | $8.6 M | $0.72 | $2,528 M | $7.6 M | $0.63 |
| Monitoring cost | ||||||||||||
| One eye | $2,005 M | $6.0 M | $0.50 | $2,119 M | $6.4 M | $0.53 | $1,552 M | $4.7 M | $0.39 | $1,306 M | $3.9 M | $0.33 |
| Fellow eye | $422 M | $1.3 M | $0.11 | $444 M | $1.3 M | $0.11 | $332 M | $1.0 M | $0.08 | $283 M | $0.9 M | $0.07 |
| Total cost | $15,917 M | $47.8 M | $3.98 | $10,656 M | $32.0 M | $2.67 | $12,221 M | $36.7 M | $3.06 | $10,687 M | $32.1 M | $2.67 |
| With brolucizumab | ||||||||||||
| Drug cost and administration cost | ||||||||||||
| One eye | $9,734 M | $29.2 M | $2.44 | $6,043 M | $18.1 M | $1.51 | $7,510 M | $22.6 M | $1.88 | $6,553 M | $19.7 M | $1.64 |
| Fellow eye | $3,735 M | $11.2 M | $0.93 | $2,317 M | $7.0 M | $0.58 | $2,884 M | $8.7 M | $0.72 | $2,521 M | $7.6 M | $0.63 |
| Monitoring cost | ||||||||||||
| One eye | $1,997 M | $6.0 M | $0.50 | $2,125 M | $6.4 M | $0.53 | $1,550 M | $4.7 M | $0.39 | $1,300 M | $3.9 M | $0.33 |
| Fellow eye | $420 M | $1.3 M | $0.11 | $445 M | $1.3 M | $0.11 | $331 M | $1.0 M | $0.08 | $282 M | $0.8 M | $0.07 |
| Total cost | $15,886 M | $47.7 M | $3.98 | $10,930 M | $32.8 M | $2.74 | $12,275 M | $36.9 M | $3.07 | $10,656 M | $32.0 M | $2.67 |
| Budget impact | −$30.99 M | −$93,068 | −$0.008 | $274.58 M | $824,696 | $0.069 | $54.30 M | $163,101 | $0.014 | −$31.35 M | −$94,170 | −$0.008 |
PMPM budget impact cost in the last row is presented to 3 decimals for accuracy of interpretation, and rest are presented up to 2 decimals. A negative value indicates a net budgetary decrease (cost saving), whereas a positive value indicates a net budgetary increase with the introduction of brolucizumab in the market.
M = million; PMPM = per member per month; PRN = pro re nata; RWE = real-world evidence; T&E= treat and extend.
SCENARIO ANALYSIS BUDGET IMPACT (MEDICARE PAYMENT FOR PART B)
In this analysis, 840,903 individuals received anti-VEGFs under the Medicare allowable payment for Part B drugs using ASP and 311,019 individual received anti-VEGFs under commercial health plans based on the WAC. Introducing brolucizumab as a treatment for nAMD increased the net budgetary impact for all the treatment regimens except RWE, which resulted in a savings of $3.14 million. The maximum increase in expenditure was associated with the PRN regimen ($267.93 million), followed by T&E ($76.41 million), and manufacturer regimen ($10.08 million). A major difference was noted in the manufacturer regimen, which showed an increased budgetary impact ($10.08 million) in the Medicare-adjusted model compared with a decreased budgetary impact (−$30.99) in the base population model.
SENSITIVITY ANALYSIS
In the health plan model, varying the brolucizumab WAC by 10% had the most significant impact for all treatment regimens except PRN, which showed the prevalence of nAMD as having the highest impact on the budget. The major impact of input variability was observed in the PRN and T&E regimens for the prevalence of nAMD and brolucizumab WAC, respectively. In the PRN regimen, an increased prevalence of nAMD resulted in a budgetary increase of $1,168,319 compared with a budgetary increase of $824,696 in the base case model. In the T&E regimen, a decrease in brolucizumab WAC by 10% resulted in a net budgetary decrease and annual savings of $41,177 compared with the base model budgetary increase of $163,101. Finally, the variability in the percentage of fellow-eye involvement had a negligible impact on all treatment regimens. One-way sensitivity analyses of the budgetary impact of the health plan are presented as tornado diagrams (Figure 2), and the sensitivity analysis of the population model, including the Medicare payment for the Part B adjusted model (scenario analysis), is presented in Supplementary Table 2 (203.2KB, pdf) .
Discussion
Anti-VEGFs have become the standard of care in the management of nAMD, as they not only prevent vision loss but also improve visual acuity.82,83 From a payer perspective, there is also a need to estimate the financial consequences of adoption and diffusion of a new treatment in a health care setting, as it allows payers to allocate resources where needed. Most BIMs use the manufacturer-recommended regimen when seeking drug formulary approval. The manufacturer-recommended regimen uses a regular dosing interval for anti-VEGFs as commonly reported in the RCTs. However, regular treatments by ophthalmologists have used variable frequency treatment regimens, such as T&E and PRN. The outcomes related to anti-VEGFs in RCTs are also different than those reported in real-world studies, and one of the reasons for this discrepancy is attributed to the variable frequency regimens.84
For payers, managing health care costs is critical, and they need to assess the impact of different treatment regimens for anti-VEGFs on their budgets. This is the first study in the United States or otherwise that has assessed the affordability of brolucizumab and other anti-VEGFs based on variable frequency regimens. Our results show that the annual cost per patient for treating nAMD is lowest when using off-label bevacizumab. Besides bevacizumab, in the health plan model using the WAC, brolucizumab has the lowest cost in all the regimens except PRN, which showed the lowest cost for aflibercept. However, when accounting for the market share and switching from existing anti-VEGFs to brolucizumab, the per-patient cost savings translates into a net budgetary savings for the manufacturer-recommended and RWE regimens. Although administering bevacizumab has the least cost and has comparable efficacy to other anti-VEGFs,28,29 it is only approved for treating various cancers and is available in very high dosages. Therefore, administering bevacizumab for nAMD requires an adjustment of dosage by compounding pharmacists, which carries the potential risk of contamination.19
The differences in the outcome of the existing nAMD economic studies are primarily due to the differences in the cost and/or frequency of injections, as anti-VEGFs have shown similar outcomes in RCTs.61,63 Besides the cost and frequency of injections, a difference in the economic outcomes could arise because of the 1-eye vs 2-eye model, with 2-eye models generating a more accurate estimation of economic outcomes.63,65 Although the majority of the economic studies have used a 1-eye model, the studies performed using 2-eye models have shown mixed results for the economic outcomes.
When looking at the cost component from the 2-eye model studies, our results for bevacizumab and monthly ranibizumab are similar to other studies as the least and most costly anti-VEGFs, respectively, while considering the WAC.52,61 When considering the WAC for drugs, our findings differ from the study by Ghosh et al that showed ranibizumab T&E as less costly than the manufacturer-recommended guideline for aflibercept. Our study found a slightly higher cost associated with the ranibizumab T&E.64 However, when considering the ASP, our findings were similar to the study by Ghosh et al.64 No economic studies have been conducted using the 2-eye model for brolucizumab. The results of the sensitivity analysis suggest that even after varying the data input ranges for different regimens, results were stable for most variables except for the WAC and disease prevalence.
The study findings will assist payers in adjusting their budgets by taking into consideration the different variable treatment regimens and their impact on nAMD-related spending. Although the study highlights cost savings and additional expenditures associated with the treatment regimens, the decision to include new drugs in the formulary should not be solely based on their budgetary impact. Instead, the health plan should consider treatment outcomes, providers’ opinions, and patients’ preferences when adding a drug to the formulary. In the management of nAMD, an ophthalmologist may not choose the same regimen for all their patients. Rather, they may tailor the treatment based on the individual patient’s age, fear and anxiety for intravitreal injections, the convenience of monthly followup, and, most importantly, disease progression. Adherence to treatments also needs to be emphasized, as it has been shown to correlate with maintaining or gaining visual acuity.85
There are some recent developments in nAMD treatment, including a new drug delivery system (Susvimo), an ocular implant port delivery system with ranibizumab,86 and a new drug (faricimab-svoa). Susvimo was approved in October 2021 and is surgically inserted in the eye and continuously delivers ranibizumab, requiring refilling every 6 months.86,87 Faricimab-svoa received FDA approval in January 2022 and is a bispecific antibody and inhibits angiopoietin 2 in addition to VEGF.88 We excluded Susvimo and faricimab-svoa from the analysis because of a lack of data on market penetration and the number of injections for faricimab-svoa under different regimens.
LIMITATIONS
Although our study is the first to conduct a budget impact analysis of variable frequency treatment regimens for anti-VEGFs in nAMD, there are some limitations to consider. First, we used the data from multicenter (including the United States) and international studies when data from the United States were not available. The HAWK and HARRIER RCTs were performed in a multicenter setting, and the LUCAS and AFFIRM RCTs were conducted in Europe. In addition, the RWE study for the brolucizumab dose was conducted in Germany and India, with the majority of patients (87%) being from Germany. Second, the study did not include adverse event-related cost differences among anti-VEGFs. Costs associated with side effects were excluded, as the study compared different anti-VEGF treatment regimens and comparing the side effects from the clinical setting (RWE) and research setting (RCTs) was not feasible because of different objectives of the 2 settings. Third, an assumption was made for the market share of brolucizumab, and its impact on the existing anti-VEGFs in the market was extrapolated based on 1-year data. Finally, 100% adherence to treatments and the stable prevalence of nAMD were assumed.
Conclusions
The cost impact of adding brolucizumab to the formulary in treating nAMD is dependent on the treatment regimens. Although manufacturer-recommended and RWE regimens resulted in cost savings, payers should be wary of the additional cost they could incur when accounting for clinical guidelines and real-world settings. The study provides additional economic inputs for payers to consider when evaluating the impact of a new treatment that may have a variable frequency treatment regimen, such as anti-VEGFs in the management of nAMD. The budget impact of new nAMD treatments will assist payers in developing treatment and monitoring guidelines to improve nAMD-related care.
REFERENCES
- 1.Smith W, Assink J, Klein R, et al. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology. 2001;108(4):697-704. doi: 10.1016/s0161-6420(00)00580-7 [DOI] [PubMed] [Google Scholar]
- 2.Gregori G, Wang F, Rosenfeld PJ, et al. Spectral domain optical coherence tomography imaging of drusen in nonexudative age-related macular degeneration. Ophthalmology. 2011;118(7):1373-9. doi: 10.1016/j.ophtha.2010.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell P, Liew G, Gopinath B, Wong TY. Age-related macular degeneration. Lancet. 2018;392(10153):1147-59. doi: 10.1016/S0140-6736(18)31550-2 [DOI] [PubMed] [Google Scholar]
- 4.Moshfeghi AA, Lanitis T, Kropat G, et al. Social cost of blindness due to AMD and diabetic retinopathy in the United States in 2020. Ophthalmic Surg Lasers Imaging Retina. 2020;51(4):S6-14. doi: 10.3928/23258160-20200401-01 [DOI] [PubMed] [Google Scholar]
- 5.Argon laser photocoagulation for neovascular maculopathy: Three-year results from randomized clinical trials. Macular Photocoagulation Study Group. Arch Ophthalmol. 1986;104(5):694-701. [PubMed] [Google Scholar]
- 6.Genentech, Inc. Highlights of prescribing information: LUCENTIS (ranibizumab injection) intravitreal injection; initial U.S. approval 2006. U.S. Food and Drug Administration. Published 2014. Accessed April 9, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125156s105lbl.pdf [Google Scholar]
- 7.Regeneron Pharmaceuticals, Inc. Highlights of prescribing information: EYLEA (aflibercept) injection, for intravitreal use; initial U.S. approval 2011. U.S. Food and Drug Administration. Published 2019. Accessed April 9, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125387s061lbl.pdf [Google Scholar]
- 8.Novartis Pharmaceuticals Corporation. Highlights of prescribing information: BEOVU (brolucizumab-dbll) injection, for intravitreal injection; initial U.S. approval 2019. U.S. Food and Drug Administration. Published 2019. Accessed April 15, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/761125Orig1s000Lbl.pdf [Google Scholar]
- 9.Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology. 2006;116(1):57-65.e5. doi: 10.1016/j.ophtha.2008.10.018 [DOI] [PubMed] [Google Scholar]
- 10.Rosenfeld PJ, Brown DM, Heier JS, et al. ; MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355(14):1419-31. doi: 10.1056/NEJMoa054481 [DOI] [PubMed] [Google Scholar]
- 11.Dixon JA, Oliver SCN, Olson JL, Mandava N. VEGF Trap-Eye for the treatment of neovascular age-related macular degeneration. Expert Opin Investig Drugs. 2009;18(10):1573-80. doi: 10.1517/13543780903201684 [DOI] [PubMed] [Google Scholar]
- 12.Do DV. Antiangiogenic approaches to age-related macular degeneration in the future. Ophthalmology. 2009;116(10):S24-6. doi: 10.1016/j.ophtha.2009.06.049 [DOI] [PubMed] [Google Scholar]
- 13.Dugel PU, Koh A, Ogura Y, et al. ; HAWK and HARRIER Study Investigators. HAWK and HARRIER: Phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72-84. doi: 10.1016/j.ophtha.2019.04.017 [DOI] [PubMed] [Google Scholar]
- 14.Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ; CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897-908. doi: 10.1056/NEJMoa1102673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakravarthy U, Harding SP, Rogers CA, et al. ; IVAN study investigators. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382(9900):1258-67. [DOI] [PubMed] [Google Scholar]
- 16.Bakri SJ, Thorne JE, Ho AC, et al. Safety and efficacy of anti-vascular endothelial growth factor therapies for neovascular age-related macular degeneration: A report by the American Academy of Ophthalmology. Ophthalmology. 2019;126(1):55-63. doi: 10.1016/j.ophtha.2018.07.028 [DOI] [PubMed] [Google Scholar]
- 17.Flaxel CJ, Adelman RA, Bailey ST, et al. Age-related macular degeneration preferred practice pattern. Ophthalmology. 2020;127(1):P1-65. doi: 10.1016/j.ophtha.2019.09.024 [DOI] [PubMed] [Google Scholar]
- 18.McLaughlin MD, Hwang JC. Trends in vitreoretinal procedures for medicare beneficiaries, 2000 to 2014. Ophthalmology. 2017;124(5):667-73. doi: 10.1016/j.ophtha.2017.01.001 [DOI] [PubMed] [Google Scholar]
- 19.Li E, Donati S, Lindsley KB, Krzystolik MG, Virgili G. Treatment regimens for administration of anti-vascular endothelial growth factor agents for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2020;(5):CD012208. doi: 10.1002/14651858.CD012208.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakravarthy U, Harding SP, Rogers CA, et al. ; IVAN Study Investigators. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: One-year findings from the IVAN randomized trial. Ophthalmology. 2012;119(7):1399-411. doi: 10.1016/j.ophtha.2012.04.015 [DOI] [PubMed] [Google Scholar]
- 21.Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T; ANCHOR Study Group. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology. 2009;116(1): 57-65.e5. doi: 10.1016/j.ophtha.2008.10.018 [DOI] [PubMed] [Google Scholar]
- 22.Busbee BG, Ho AC, Brown DM, et al. ; HARBOR Study Group. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013;120(5):1046-56. doi: 10.1016/j.ophtha.2012.10.014 [DOI] [PubMed] [Google Scholar]
- 23.Spaide R. Ranibizumab according to need: A treatment for age-related macular degeneration. Am J Ophthalmol. 2007;143(4):679-80. doi: 10.1016/j.ajo.2007.02.024 [DOI] [PubMed] [Google Scholar]
- 24.Hussain RM, Ciulla TA, Hariprasad SM. Treatment burden in neovascular AMD: Visual acuity outcomes are associated with anti-VEGF injection frequency. Ophthalmic Surg Lasers Imaging Retina. 2017;48(10):780-4. doi: 10.3928/23258160-20170928-01 [DOI] [PubMed] [Google Scholar]
- 25.Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: Ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121(1):193-201. doi: 10.1016/j.ophtha.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 26.U.S. National Library of Medicine. Vascular endothelial growth factor VEGF Trap-Eye: investigation of efficacy and safety in wet age-related macular degeneration (AMD) (VIEW1). Identifier NCT00509795. ClinicalTrials.gov. Updated December 28, 2012. Accessed January 1, 2021. https://clinicaltrials.gov/ct2/show/NCT00509795
- 27.U.S. National Library of Medicine. Vascular endothelial growth factor (VEGF) Trap-Eye: investigation of efficacy and safety in wet age-related macular degeneration (AMD) (VIEW 2). Identifier NCT00637377. ClinicalTrials.gov. Updated December 12, 2014. Accessed January 1, 2021. https://clinicaltrials.gov/ct2/show/NCT00637377
- 28.U.S. National Library of Medicine. Comparison of age-related macular degeneration treatments trials: Lucentis-Avastin trial. Identifier NCT00593450. ClinicalTrials.gov. Updated August 21, 2017. Accessed July 1, 2021. https://clinicaltrials.gov/ct2/show/results/NCT00593450
- 29.Martin DF, Maguire MG, Fine SL, et al. Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group . Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: Two-year results. Ophthalmology. 2012;119(7):1388-98. doi: 10.1016/j.ophtha.2012.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dugel PU, Singh RP, Koh A, et al. HAWK and HARRIER: Ninety-six-week outcomes from the phase 3 trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2021;128(1):89-99. doi: 10.1016/j.ophtha.2020.06.028 [DOI] [PubMed] [Google Scholar]
- 31.U.S. National Library of Medicine. Efficacy and safety of RTH258 versus aflibercept - study 1 (HAWK). Identifier NCT02307682. ClinicalTrials.gov. Updated January 13, 2020. Accessed April 4, 2021. https://clinicaltrials.gov/ct2/show/NCT02307682
- 32.U.S. National Library of Medicine. Efficacy and safety of RTH258 versus aflibercept - study 2 (HARRIER). Identifier NCT02434328. ClinicalTrials.gov. Updated September 11, 2020. Accessed April 4, 2021. https://clinicaltrials.gov/ct2/show/NCT02434328
- 33.Berg K, Pedersen TR, Sandvik L, Bragadóttir R. Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology. 2015;122(1):146-52. doi: 10.1016/j.ophtha.2014.07.041 [DOI] [PubMed] [Google Scholar]
- 34.DeCroos FC, Reed D, Adam MK, et al. Treat-and-extend therapy using aflibercept for neovascular age-related macular degeneration: A prospective clinical trial. Am J Ophthalmol. 2017;180:142-50. doi: 10.1016/j.ajo.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 35.Singh RP. ASRS global trends in retina - 2019. American Society of Retina Specialists. Accessed November 10, 2021. https://www.asrs.org/content/documents/2019-global-trends-survey-for-website.pdf
- 36.Singer M. Which anti-VEGF do I choose as first-line therapy? Retinal Physician. Published September 2020. Accessed September 5, 2021. https://www.retinalphysician.com/newsletter/retina-minute/september-2020
- 37.Zarranz-Ventura J, Liew G, Johnston RL, et al. United Kingdom Age-Related Macular Degeneration Electronic Medical Records Users Group . The neovascular age-related macular degeneration database: report 2: incidence, management, and visual outcomes of second treated eyes. Ophthalmology. 2014;121(10):1966-75. doi: 10.1016/j.ophtha.2014.04.026 [DOI] [PubMed] [Google Scholar]
- 38.Roach L. Treat-and-extend strategy: is there a consensus? EyeNet Magazine. Published January 2016. Accessed March 21, 2022. https://www.aao.org/eyenet/article/treat-extend-strategy-is-there-consensus
- 39.Rao P, Lum F, Wood K, et al. Real-world vision in age-related macular degeneration patients treated with single anti-VEGF drug type for 1 year in the IRIS registry. Ophthalmology. 2018;125(4):522-8. doi: 10.1016/j.ophtha.2017.10.010 [DOI] [PubMed] [Google Scholar]
- 40.Ciulla TA, Huang F, Westby K, Williams DF, Zaveri S, Patel SC. Real-world outcomes of anti-vascular endothelial growth factor therapy in neovascular age-related macular degeneration in the United States. Ophthalmol Retin. 2018;2(7):645-53. doi: 10.1016/j.oret.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 41.Ciulla TA, Hussain RM, Pollack JS, Williams DF. Visual acuity outcomes and anti-vascular endothelial growth factor therapy intensity in neovascular age-related macular degeneration patients: A real-world analysis of 49 485 eyes. Ophthalmol Retin. 2020;4(1):19-30. doi: 10.1016/j.oret.2019.05.017 [DOI] [PubMed] [Google Scholar]
- 42.Arevalo JF, Lasave AF, Wu L, et al. Pan-American Collaborative Retina Study Group (PACORES) . Intravitreal bevacizumab for choroidal neovascularization in age-related macular degeneration: 5-year results of the Pan-American Collaborative Retina Study Group. Retina. 2016;36(5):859-67. doi: 10.1097/IAE.0000000000000827 [DOI] [PubMed] [Google Scholar]
- 43.Holz FG, Tadayoni R, Beatty S, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. 2015;99(2):220-6. doi: 10.1136/bjophthalmol-2014-305327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rasmussen A, Bloch SB, Fuchs J, et al. A 4-year longitudinal study of 555 patients treated with ranibizumab for neovascular age-related macular degeneration. Ophthalmology. 2013;120(12):2630-6. doi: 10.1016/j.ophtha.2013.05.018 [DOI] [PubMed] [Google Scholar]
- 45.Fong DS, Custis P, Howes J, Hsu JW. Intravitreal bevacizumab and ranibizumab for age-related macular degeneration: A multicenter, retrospective study. Ophthalmology. 2010;117(2):298-302. doi: 10.1016/j.ophtha.2009.07.023 [DOI] [PubMed] [Google Scholar]
- 46.Holekamp NM, Liu Y, Yeh WS, et al. Clinical utilization of anti-VEGF agents and disease monitoring in neovascular age-related macular degeneration. Am J Ophthalmol. 2014;157(4):825-33.e1. doi: 10.1016/j.ajo.2013.12.018 [DOI] [PubMed] [Google Scholar]
- 47.Kim LN, Mehta H, Barthelmes D, Nguyen V, Gillies MC. Metaanalysis of real-world outcomes of intravitreal ranibizumab for the treatment of neovascular age-related macular degeneration. Retina. 2016;36(8):1418-31. doi: 10.1097/IAE.0000000000001142 [DOI] [PubMed] [Google Scholar]
- 48.Almuhtaseb H, Kanavati S, Rufai SR, Lotery AJ. One-year real-world outcomes in patients receiving fixed-dosing aflibercept for neovascular age-related macular degeneration. Eye (Lond). 2017;31(6):878-83. doi: 10.1038/eye.2017.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barthelmes D, Nguyen V, Daien V, et al. ; Fight Retinal Blindness Study Group. Two year outcomes of “treat and extend” intravitreal therapy using aflibercept preferentially for neovascular age-related macular degeneration. Retina. 2018;38(1):20-8. doi: 10.1097/IAE.0000000000001496 [DOI] [PubMed] [Google Scholar]
- 50.Silva R, Berta A, Larsen M, Macfadden W, Feller C, Monés J, TREND Study Group . Treat-and-extend versus monthly regimen in neovascular age-related macular degeneration: Results with ranibizumab from the TREND study. Ophthalmology. 2018;125(1):57-65. doi: 10.1016/j.ophtha.2017.07.014 [DOI] [PubMed] [Google Scholar]
- 51.Brown GC, Brown MM, Rapuano S, Boyer D. Cost-utility analysis of vegf inhibitors for treating neovascular age-related macular degeneration. Am J Ophthalmol. 2020;218:225-41. doi: 10.1016/j.ajo.2020.05.029 [DOI] [PubMed] [Google Scholar]
- 52.Hernandez L, Lanitis T, Cele C, Toro-Diaz H, Gibson A, Kuznik A. Intravitreal aflibercept versus ranibizumab for wet age-related macular degeneration: A cost-effectiveness analysis. J Manag Care Spec Pharm. 2018;24(7):608-16. doi: 10.18553/jmcp.2018.24.7.608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown GC, Brown MM, Lieske HB, Turpcu A, Rajput Y. The comparative effectiveness and cost-effectiveness of ranibizumab for neovascular macular degeneration revisited. Int J Retin Vitr. 2017;3:5. doi: 10.1186/s40942-016-0058-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stein JD, Newman-Casey PA, Mrinalini T, Lee PP, Hutton DW. Cost-effectiveness of bevacizumab and ranibizumab for newly diagnosed neovascular macular degeneration. Ophthalmology. 2014;121(4):936-45. doi: 10.1016/j.ophtha.2013.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gheorghe A, Mahdi L, Musat O. Age-related macular degeneration. Rom J Ophthalmol. 2015;59(2):74-7. [PMC free article] [PubMed] [Google Scholar]
- 56.Dakin HA, Wordsworth S, Rogers CA, et al. ; IVAN Study Investigators. Cost-effectiveness of ranibizumab and bevacizumab for age-related macular degeneration: 2-year findings from the IVAN randomised trial. BMJ Open. 2014;4(7):e005094. doi: 10.1136/bmjopen-2014-005094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elshout M, van der Reis MI, Webers CAB, Schouten JSAG. The cost-utility of aflibercept for the treatment of age-related macular degeneration compared to bevacizumab and ranibizumab and the influence of model parameters. Graefes Arch Clin Exp Ophthalmol. 2014;252(12):1911-20. doi: 10.1007/s00417-014-2641-3 [DOI] [PubMed] [Google Scholar]
- 58.Nunes RP, Hirai FE, Rodrigues EB, Farah ME. Cost-effectiveness of Anti-VEGF treatments for age-related macular degeneration: A Brazilian perspective. Arq Bras Oftalmol. 2020;83(1):48-54. doi: 10.5935/0004-2749.20200020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Panchmatia HR, Clements KM, Hulbert E, et al. Aflibercept vs. ranibizumab: Cost-effectiveness of treatment for wet age-related macular degeneration in Sweden. Acta Ophthalmol. 2016;94(5):441-8. doi: 10.1111/aos.12964 [DOI] [PubMed] [Google Scholar]
- 60.Van Asten F, Michels CTJ, Hoyng CB, et al. The cost-effectiveness of bevacizumab, ranibizumab and aflibercept for the treatment of age-related macular degeneration—A cost-effectiveness analysis from a societal perspective. PLoS One. 2018;13(5):e0197670. doi: 10.1371/journal.pone.0197670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vottonen P, Kankaanpää E. Cost-effectiveness of treating wet age-related macular degeneration at the Kuopio University Hospital in Finland based on a two-eye Markov transition model. Acta Ophthalmol. 2016;94(7):652-6. doi: 10.1111/aos.13185 [DOI] [PubMed] [Google Scholar]
- 62.Yanagi Y, Fukuda A, Barzey V, Adachi K. Cost-effectiveness of intravitreal aflibercept versus other treatments for wet age-related macular degeneration in Japan. J Med Econ. 2017;20(2):204-12. doi: 10.1080/13696998.2016.1245196 [DOI] [PubMed] [Google Scholar]
- 63.Elshout M, Webers CAB, van der Reis MI, Schouten JSAG. A systematic review on the quality, validity and usefulness of current cost-effectiveness studies for treatments of neovascular age-related macular degeneration. Acta Ophthalmol. 2018;96(8):770-8. doi: 10.1111/aos.13824 [DOI] [PubMed] [Google Scholar]
- 64.Ghosh W, Wickstead R, Claxton L, et al. The cost-effectiveness of ranibizumab treat and extend regimen versus aflibercept in the UK. Adv Ther. 2016;33(9):1660-76. doi: 10.1007/s12325-016-0367-9 [DOI] [PubMed] [Google Scholar]
- 65.Claxton L, Hodgson R, Taylor M, Malcolm B, Pulikottil Jacob R. Simulation modelling in ophthalmology: application to cost effectiveness of ranibizumab and aflibercept for the treatment of wet age-related macular degeneration in the United Kingdom. Pharmacoeconomics. 2017;35(2):237-48. doi: 10.1007/s40273-016-0459-z [DOI] [PubMed] [Google Scholar]
- 66.Hodgson R, Reason T, Trueman D, et al. Challenges associated with estimating utility in wet age-related macular degeneration: A novel regression analysis to capture the bilateral nature of the disease. Adv Ther. 2017;34(10):2360-70. doi: 10.1007/s12325-017-0620-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu JS, Carlton R, Agashivala N, Hassan T, Wykoff CC. Brolucizumab vs aflibercept and ranibizumab for neovascular age-related macular degeneration: A cost-effectiveness analysis. J Manag Care Spec Pharm. 2021;27(6):743-52. doi: 10.18553/jmcp.2021.27.6.743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.United States Census Bureau. U.S. and world population clock. Accessed July 31, 2022. https://www.census.gov/popclock/
- 69.Zarbin M, Basavarajaiah G, Yu J, et al. Profiles and early outcomes of patients who initiated brolucizumab for neovascular (wet) age-related macular degeneration (AMD) in the IRIS Registry and Komodo database. Presented at: American Academy of Ophthalmology 2020 Virtual Congress. Session: PO395. Accessed January 25, 2022. https://aao.scientificposters.com/epsSearchAAO.cfm [Google Scholar]
- 70.Dugel PU, Koh A, Ogura Y, et al. ; HAWK and HARRIER Study Investigators. HAWK and HARRIER: Phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72-84. doi: 10.1016/j.ophtha.2019.04.017 [DOI] [PubMed] [Google Scholar]
- 71.Bilgic A, Kodjikian L, March de Ribot F, et al. Real-world experience with brolucizumab in wet age-related macular degeneration: The REBA study. J Clin Med. 2021;10(13):2758. doi: 10.3390/jcm10132758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parikh R, Feng PW, Del Priore LV, Adelman RA, Chaudhry NA. Relationship between claims data and the preferences and trends survey: An analysis of anti-vascular endothelial growth factor medication choice for age-related macular degeneration. J Vitreoretin Dis. 2018;2(2):96-9. doi: 10.1177/2474126417753433 [DOI] [Google Scholar]
- 73.IBM Watson Health. IBM Micromedex RED BOOK. IBM. Accessed January 4, 2021. https://www.micromedexsolutions.com/home/dispatch/ssl/true
- 74.Centers for Medicare & Medicaid Services. Physician fee schedule. CMS.gov. Accessed August 1, 2022. https://www.cms.gov/medicare/physician-fee-schedule/search
- 75.American Academy of Opthalmology. Coding for injectable drugs. Accessed April 11, 2022. https://www.aao.org/practice-management/coding/injectable-drugs
- 76.Centers for Medicare & Medicaid Services. Billing and coding: Intraocular bevacizumab. Accessed November 2, 2022. https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleid=53008&ver=40&Date=&DocID=A53008&SearchType=Advanced&bc=EgAAAAgAAAAA&=#
- 77.Glasser DB, Parikh R, Lum F, Williams GA. Intravitreal anti-vascular endothelial growth factor cost savings achievable with increased bevacizumab reimbursement and use. Ophthalmology. 2020;127(12):1688-92. doi: 10.1016/j.ophtha.2020.06.012 [DOI] [PubMed] [Google Scholar]
- 78.Levinson DR. Medicare payments for drugs used to treat wet age-related macular degeneration. OEI-03-10-00360. Department of Health and Human Services. Published April 2012. Accessed January 24, 2014. https://oig.hhs.gov/oei/reports/oei-03-10-00360.pdf [Google Scholar]
- 79.Werble C, Wilensky G, Goldstein D. Health Policy Brief: Medicare Part B. Health Aff. 2017;5:1-4. doi: 10.1377/hpb2017.6 [DOI] [Google Scholar]
- 80.Centers for Medicare & Medicaid Services. 2022 ASP drug pricing files. Updated June 15, 2022. Accessed August 1, 2022. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/2022-asp-drug-pricing-files
- 81.Rudnicka AR, Kapetanakis VV, Jarrar Z, et al. Incidence of late-stage age-related macular degeneration in American Whites: Systematic review and meta-analysis. Am J Ophthalmol. 2015;160(1):85-93.e3. doi: 10.1016/j.ajo.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 82.Peden MC, Suñer IJ, Hammer ME, Grizzard WS. Long-term outcomes in eyes receiving fixed-interval dosing of antivascular endothelial growth factor agents for wet age-related macular degeneration. Ophthalmology. 2015;122(4):803-8. doi: 10.1016/j.ophtha.2014.11.018 [DOI] [PubMed] [Google Scholar]
- 83.Qin VL, Young J, Silva FQ, Conti FF, Singh RP. Outcomes of patients with exudative age-related macular degeneration treated with antivascular endothelial growth factor therapy for three or more years: A review of current outcomes. Retina. 2018;38(8):1500-8. doi: 10.1097/IAE.0000000000001753 [DOI] [PubMed] [Google Scholar]
- 84.Ciulla TA. “Real-world" outcomes of anti-VEGF therapy in neovascular AMD in the United States. Differences between real-world and clinical trial outcomes continue to confound. Retinal Physician. Published April 1, 2019. Accessed September 10, 2021. https://www.retinalphysician.com/issues/2019/april-2019/8220;real-world-8221;-outcomes-of-anti-vegf-the
- 85.Ehlken C, Helms M, Böhringer D, Agostini HT, Stahl A. Association of treatment adherence with real-life VA outcomes in AMD, DME, and BRVO patients. Clin Ophthalmol. 2018;12:13-20. doi: 10.2147/OPTH.S151611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Genentech, Inc. Highlights of prescribing information: Susvimo (ranibizumab injection) for intravitreal use via SUSVIMO ocular implant. Initial US approval: 2006. US Food and Drug Administration. Published 2011. Accessed April 15, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761197s000lbl.pdf [Google Scholar]
- 87.Holekamp NM, Campochiaro PA, Chang MA, et al. all Archway Investigators . Archway randomized phase 3 trial of the port delivery system with ranibizumab for neovascular age-related macular degeneration. Ophthalmology. 2022;129(3):295-307. doi: 10.1016/j.ophtha.2021.09.016 [DOI] [PubMed] [Google Scholar]
- 88.Genentech, Inc. Highlights of prescribing information: Vabysmo (faricimab-svoa) injection, for intravitreal use. Initial US approval: 2022. US Food and Drug Administration. Published 2022. Accessed April 22, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761235s000lbl.pdf [Google Scholar]
- 89.Veritti D, Sarao V, Missiroli F, Ricci F, Lanzetta P. Twelve-month outcomes of intravitreal aflibercept for neovascular age-related macular degeneration: Fixed versus as-needed dosing. Retina. 2019;39(11):2077-83. doi: 10.1097/IAE.0000000000002299 [DOI] [PubMed] [Google Scholar]


