Abstract
BACKGROUND:
The discovery of specific oncogenic drivers in non–small cell lung cancer (NSCLC) has led to the development of highly targeted anaplastic lymphoma kinase tyrosine kinase inhibitors (ALKis). Brigatinib is a next-generation ALKi associated with prolonged progression-free survival in patients with ALKi-naive ALK+ NSCLC.
OBJECTIVE:
To estimate the cost-effectiveness of brigatinib compared with crizotinib and alectinib in patients with ALKi-naive ALK+ NSCLC, from a US payer perspective.
METHODS:
A lifetime area under the curve–partitioned survival model with 4 health states was used to evaluate the relative cost-effectiveness of brigatinib in the ALKi-naive ALK+ NSCLC setting. Brigatinib was compared with crizotinib within a cost-effectiveness framework and compared with alectinib in a cost-comparison framework, where all efficacy outcomes were assumed equal. The efficacy of brigatinib and crizotinib was informed by the ALTA-1L trial, and an indirect treatment comparison was performed to inform the efficacy of brigatinib vs alectinib owing to a lack of head-to-head data. Costs were derived from public sources. The main outcomes of the model were total costs, quality-adjusted life-years (QALYs), life-years, and incremental cost-effectiveness ratios. Univariate and probabilistic sensitivity analyses, in addition to multiple scenario analyses, were conducted to assess the robustness of the model outcomes.
RESULTS:
The improved outcomes observed in ALTA-1L translated into QALY gains (+0.97) in the comparison of brigatinib vs crizotinib. The superior efficacy profile was associated with increased time on treatment with brigatinib, which drove the increase in costs vs crizotinib (+$210,519). The resulting base-case incremental cost-effectiveness ratio was $217,607/QALY gained. Compared with alectinib, brigatinib was associated with a cost difference of −$8,546. Sensitivity analysis suggested that extrapolation of overall survival, the assumptions relating to time on treatment, and subsequent therapy costs were the most influential determinants of results. Probabilistic sensitivity analysis suggested brigatinib had the highest probability of being cost-effective beyond willingness-to-pay thresholds of $236,000 per QALY vs crizotinib and alectinib.
CONCLUSIONS:
At list prices and under base-case assumptions in the current analysis, brigatinib was associated with cost-savings vs alectinib, and QALY gains but at higher costs vs crizotinib. Additional research into the real-world efficacy of ALKis is warranted to further understand the comparative cost-effectiveness of these therapies.
Plain language summary
This study compares 3 lung cancer treatments in the United States. The results show that treatment A (brigatinib) is associated with a higher quality of life but higher costs than treatment B (crizotinib). Treatment A is associated with the same quality of life but lower costs than treatment C (alectinib). Further research is needed to understand how these lung cancer treatments affect patient well-being and costs.
Implications for managed care pharmacy
This is the first published economic evaluation comparing anaplastic lymphoma kinase inhibitors (ALKis; brigatinib, crizotinib, and alectinib) for the treatment of ALK + non–small cell lung cancer. Base-case results demonstrated variable quality-adjusted life-years and costs in a US setting. A greater understanding of the cost-effectiveness of US Food and Drug Administration–approved ALKis could better inform clinical trial investment, formulary, and US policy.
Lung cancer is one of the most commonly diagnosed cancers in the United States, accounting for 12.4% of all new cancer cases.1,2 Non–small cell lung cancer (NSCLC) is the most prevalent histologic class of lung cancer, accounting for approximately 85% of cases.3,4 Anaplastic lymphoma kinase (ALK) is a tyrosine kinase that is aberrantly expressed in 3%-7% of patients with NSCLC (referred to as ALK+ NSCLC).5,6 The presence of chromosomal rearrangements involving the ALK oncogene defines a molecular subset of NSCLC with distinct demographic features, including earlier age of onset and a higher prevalence in women.7,8 For patients diagnosed with stage IV ALK+ NSCLC, median overall survival (OS) is 81 months (range: 3-125+ months; median follow-up: 47 months).9
The discovery of specific oncogenic drivers in NSCLC has led to the development of highly targeted ALK tyrosine kinase inhibitors (herein referred to as ALK inhibitors [ALKis]).10 Crizotinib was the first ALKi to be approved for the treatment of metastatic ALK+ NSCLC by the US Food and Drug Administration (FDA) in 2011.11 Since then, a series of next-generation ALKis has been approved by the FDA for the frontline treatment of metastatic ALK+ NSCLC: ceritinib (2017),12 alectinib (2017),13 brigatinib (2020)14 (all second-generation), and lorlatinib (2021)15 (third-generation). Brigatinib is a central nervous system (CNS)-active, next-generation ALKi that targets a broad range of ALK-acquired resistance mutations. The ALTA-1L head-to-head trial of brigatinib vs crizotinib demonstrated that brigatinib is associated with prolonged progression-free survival (PFS) in patients with ALKi-naive ALK+ NSCLC.16,17
Many patients with ALK+ NSCLC are at risk of developing CNS metastases. CNS metastases are present in 22%-33% of patients at initial diagnosis and are the most common relapse site following ALKi treatment (45%-70%).18-22 CNS involvement can cause physical and psychological impairments and is typically managed with surgery, radiotherapy, and/or systemic therapy.23 CNS metastases remain a major cause of mortality among patients with ALK+ NSCLC, with only 4% of patients surviving for 5 or more years.24 CNS metastases are also associated with burdensome symptoms and a low health-related quality of life (HRQoL).19,24 Owing to CNS metastases constituting a considerable clinical burden and influencing treatment decisions, the consequences of CNS and non-CNS progression were captured separately in the economic model.
It is estimated that US expenditure for lung cancer care was more than $14.1 billion in 2018.25 Analysis of 2 US claims databases demonstrated that substantially higher costs are incurred for patients with ALK+ NSCLC with CNS metastases vs patients without CNS metastases; mean total unadjusted costs per patient per month were $29,497 vs $22,791, respectively (inpatient, outpatient, and pharmacy use, up to 24 months’ follow-up, 2008-2015/6).26
Although advances in targeted therapies have improved survival in patients with ALK+ NSCLC,1,23,27 the disease remains incurable and annual lung cancer deaths (85% of which are NSCLC) in the United States are still greater than the combined deaths from colorectal, pancreatic, and breast cancers.1,3,4 The approval of multiple ALKis in the frontline setting has improved the treatment options available to patients.10,23,28 However, each ALKi has its own risk/benefit profile, and patients ultimately progress.26,28-30 Therefore, there is a continued need for novel efficacious frontline therapies to improve the patient journey and treatment pathway.
The objective of this study was to estimate the cost-effectiveness of brigatinib vs crizotinib and a cost comparison of brigatinib vs alectinib in patients with ALKi-naive ALK+ NSCLC, from a US payer perspective. These comparators were considered most appropriate as alectinib was considered the market-leading ALKi in the first-line setting at the time of analysis, and head-to-head data for brigatinib vs crizotinib were available from the ALTA-1L trial.29 Although a cost-effectiveness analysis of brigatinib vs crizotinib was possible because of the availability of head-to-head data,29 a cost comparison was undertaken vs alectinib owing to results from an indirect treatment comparison (ITC) being near unity, and following clinical opinion that brigatinib provides similar health benefits to alectinib. This study is the first economic evaluation comparing brigatinib, crizotinib, and alectinib for the treatment of ALK+ NSCLC in the United States.
Methods
MODEL STRUCTURE
The model structure reflects that of published cost-effectiveness evaluations for alectinib in the frontline ALK+ advanced NSCLC setting.31 The model was developed in Microsoft Excel 2010 as an area under the curve–partitioned survival model with 4 health states: preprogression, CNS progression, non-CNS progression, and death (Figure 1). CNS progression constitutes a considerable economic and humanistic burden and influences treatment decisions; hence, the consequences of CNS and non-CNS progression were captured separately.19,23,26
FIGURE 1.
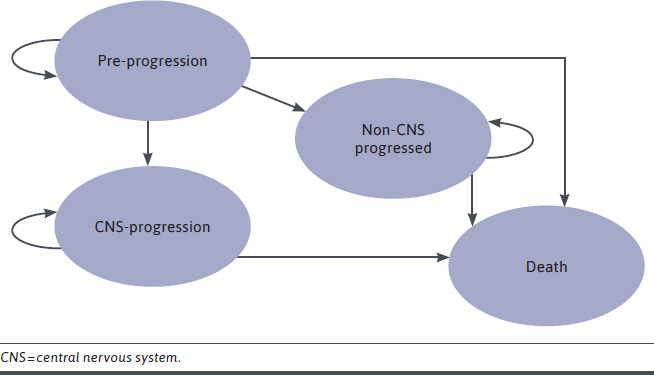
Model Structure
The model estimated the cost-effectiveness of brigatinib vs crizotinib and a cost comparison of brigatinib vs alectinib in patients with ALKi-naive ALK+ NSCLC. The area under the curve model extrapolated 3 endpoints for the comparison of brigatinib with crizotinib using the final data-cut from the ALTA-1L trial (PFS, CNS-PFS, and OS; median follow-up: 40.4 months, final data-cut).29 Owing to the lack of head-to-head data, an ITC was performed to inform the efficacy of brigatinib vs alectinib. Based on the results of the ITC, in which all results were near unity and nonsignificant, and following clinical feedback that suggested clinicians considered brigatinib to provide similar health benefits to alectinib, brigatinib was compared with alectinib in a cost-comparison framework. Within this framework, PFS, CNS-PFS, and OS for alectinib were assumed equal to those observed in the ALTA-1L trial for brigatinib (a cost-effectiveness analysis was considered in a scenario analysis).
The model considered a lifetime horizon (30 years) and a 28-day cycle length. Discounting was applied at 3% to the costs, quality-adjusted life-years (QALYs), and life-years gained in the base case.32,33
CLINICAL INPUTS
In the ALTA-1L clinical trial, patients who experienced progression as assessed by the blinded independent review committee (BIRC) or received radiotherapy to the brain while on crizotinib therapy could cross over to treatment with brigatinib. A total of 65 patients (47.1%) in the crizotinib arm formally crossed over to brigatinib. However, further informal crossover (crossover occurring at later lines or outside of the predefined window specifying an official crossover) was also observed; in total, 80 patients (58.0%) in the crizotinib arm received subsequent brigatinib.17 This is anticipated to reflect clinical practice in the United States, where brigatinib is available after crizotinib.23 Therefore, no adjustments were made in the base case. Scenario analyses considered the impact of removing efficacy and costs relating to subsequent brigatinib from the crizotinib arm using treatment switching methods.
The efficacy for brigatinib and crizotinib was informed by the final data-cut from the ALTA-1L trial (median follow-up 40.4 months).29 Systematic literature reviews, supplemented with targeted literature searches, identified the relevant data for the ITC with alectinib. The ITC was informed by 3 publications for alectinib vs crizotinib from the ALEX trial.30,34,35
Owing to the differences in a key treatment effect modifier (baseline CNS metastases) between the clinical trials, population-adjusted indirect methods (ie, anchored matched adjusted indirect comparisons [MAICs]) were considered with baseline CNS metastases included as the only treatment effect modifier, in addition to the standard network meta-analysis methodology.
The OS data in the crizotinib arm from the ALTA-1L clinical trial reflect a sequence of treatments (crizotinib followed by brigatinib), due to the high rate of crossover. Therefore, several methods were explored to adjust for the impact of crossover in the ALTA-1L clinical trial within the ITC framework, including inverse probability of censoring weighting, marginal structural models, and rank-preserving structural failure time models (RPSFTMs; with and without recensoring). These adjusted data were used in the ITCs to obtain relative efficacy estimates free of confounding from crossover in the crizotinib arm of the ALTA-1L trial. Full details of the methodology are reported elsewhere.36
The ITCs indicated similar outcomes under alternative assumptions for brigatinib vs alectinib for PFS BIRC (range for hazard ratios: 0.946-1.036) and OS (range for hazard ratios: 0.902-1.088). No ITC was possible on CNS-PFS outcomes because of differences in endpoint definitions in the ALTA-1L and ALEX trials.29,30,34,35 Clinical experts also communicated that brigatinib provides similar health benefits to alectinib. Thus, a cost-comparison analysis was conducted for brigatinib vs alectinib in the base case, in which PFS, OS, and CNS-PFS outcomes were assumed equivalent. A scenario analysis considered a cost-effectiveness framework for this comparison using the point estimates from the ITC. Additional scenarios explored the ITC methodologies, treatment switching adjustments, and the assessment body for PFS (ie, independent review vs investigator).
To inform the inputs for brigatinib and crizotinib in the model, data from ALTA-1L were extrapolated for the following outcomes: OS, PFS BIRC, and CNS-PFS (Figure 2).17 Assessment of proportional hazards was used to determine whether to use stratified or independent parametric models; clinical feedback indicated that because of the different mechanisms of action, they would not expect proportional hazards to hold between brigatinib and crizotinib. Therefore, independent parametric models were fitted to brigatinib and crizotinib data. However, for indirect comparisons, clinicians would expect proportional hazards to hold between brigatinib and alectinib based on similar mechanisms of action across the second-generation/third-generation ALKis.
FIGURE 2.
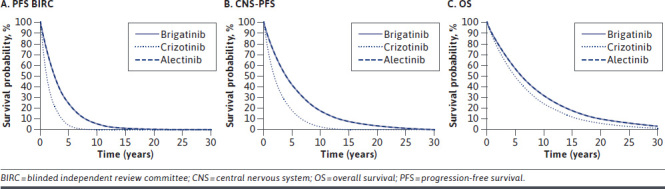
Extrapolated Outcomes for All Comparators
Seven parametric distributions (exponential, Weibull, Gompertz, γ, log-normal, log-logistic, and generalized γ) were fitted to the patient-level data for each outcome. The fit of each parametric model to the survival data was assessed through Akaike information criterion/Bayesian information criterion statistics, comparison with the Kaplan-Meier curves, and clinical expert opinion.
For all outcomes, there were limited differences in terms of how well each parametric curve fitted the observed data. The exponential curve was selected in the base case for both brigatinib and crizotinib for all outcomes. This represented the most conservative curve (ie, the lowest predicted survival), and the selection was validated by clinical experts.
HEALTH STATE UTILITIES
HRQoL was collected in ALTA-1L until 30 days after the last study dose with the European Organization for Research and Treatment of Cancer quality of life questionnaire (version 3.0)37 and the lung cancer–specific module (QLQ-LC13, version 3.0).38 European Organization for Research and Treatment of Cancer quality of life questionnaire data were mapped to European Quality of Life-five dimensions-3 levels utility scores (US tariff)39 using the best fitting response mapping algorithm published in Longworth et al.40 A mixed-effects regression model, to account for repeated measures, was then fitted to the data to estimate utility values for the different health states. Prognostic factors in the regression analysis included the presence of grade 3/4 adverse events (AEs), baseline European Quality of Life-five dimensions-3 levels index score, complete response, partial response, or stable disease (vs progressive disease). To reflect HRQoL throughout the model time horizon, and beyond the follow-up from ALTA-1L, multipliers sourced from the literature were applied accounting for the impact of CNS progression, chemotherapy, and best supportive care (Supplementary Table 1 (693.3KB, pdf) , available in online article). In addition, the model included a utility decrement associated with increasing age (−0.00026 each year).41
RESOURCE USE AND COSTS
All pharmacy costs were obtained from the Micromedex REDBOOK, and dosing schedules were aligned with marketing authorizations (Supplementary Table 5 (693.3KB, pdf) ).42-44 In the base case, dose interruptions/reductions associated with AEs or noncompliance were accounted for. The median relative dose intensities were 94.68% for brigatinib, 99.08% for crizotinib, and 100% for alectinib.17,45 Subsequent therapies were based on US clinical practice. Scenarios are presented that assume subsequent therapy use as per ALTA-1L (Supplementary Tables 2 and 8 (693.3KB, pdf) ) and exclude the use of dose intensity calculations.
Any-cause grade 3/4 AEs occurring in greater than or equal to 3% of patients in ALTA-1L and ALEX were included in the analysis. AEs were only modeled for patients on treatment and it was assumed that AEs for all therapies ceased once treatment was discontinued. It was further assumed that AEs lasted 1 model cycle (28 days). Where AEs were not reported in specific publications, the model assumed an event rate of 0. The costs associated with laboratory abnormalities were assumed to include the cost of 2 medical oncology outpatient visits and 2 blood tests (Supplementary Table 7 (693.3KB, pdf) ).
Health state resource use was defined by whether a patient received frontline treatment (ie, on-treatment), had discontinued frontline treatment (ie, off-treatment), or had CNS progression. Resource use inputs were informed by Zhou et al,46 and associated costs were sourced from the Centers for Medicare and Medicaid Services Physician Lookup47 (Supplementary Tables 4 and 6 (693.3KB, pdf) ).
The cost of end-of-life care was applied to all patients who entered the death health state as a one-off cost. The one-off cost of terminal care was $1,863, calculated as the average management cost associated with the 8 weeks prior to death, including hospitalization, hospice care, and outpatient patient costs.48
ASSESSING UNCERTAINTY
One-way sensitivity analyses were performed to evaluate the sensitivity of model results to individual inputs. Each parameter was independently varied within a plausible range defined by the 95% CI or a credible interval. The results for the most influential parameters were plotted on a tornado diagram. Scenarios exploring the following assumptions were also performed: population, parametric curve fits for OS, PFS BIRC, PFS investigator-assessed, CNS-PFS, and treatment switching adjustments. To characterize joint uncertainty in model inputs, a probabilistic sensitivity analysis (PSA) was performed. A total of 10,000 PSA iterations were performed and results plotted on a cost-effectiveness acceptability curve, which reports the probability that each therapy is the most cost-effective treatment option at alternative willingness-to-pay (WTP) thresholds.
Results
BRIGATINIB VS CRIZOTINIB
Table 1 presents the base-case results. The improved outcomes observed in ALTA-1L translated into QALY gains (+0.97) in the comparison of brigatinib vs crizotinib; this gain was observed despite the use of brigatinib as a subsequent therapy for the majority of patients progressing in the crizotinib arm. The superior efficacy profile was associated with increased time on treatment with brigatinib, which increased the costs vs crizotinib (+$210,519). The resulting base-case incremental cost-effectiveness ratio (ICER) was $217,607/QALY gained—aligning with the probabilistic ICER of $218,991/QALY gained. Scenarios exploring adjusted OS data (ie, removing the effect of subsequent brigatinib) led to incremental QALYs up to +1.50.
TABLE 1.
Base-Case Results
| Technology | Total costs ($)a | Total LYG | Total QALYs | Incremental costs ($) | Incremental LYG | Incremental QALYs | ICER brigatinib vs comparator ($/QALY) |
|---|---|---|---|---|---|---|---|
| Brigatinib | 968,833 | 6.87 | 4.54 | — | — | — | — |
| Crizotinib | 758,314 | 5.86 | 3.58 | 210,519 | 1.01 | 0.97 | 217,607 |
| Alectinib | 977,379 | 6.87 | 4.55 | −8,546 | 0.00 | 0.00 | Cost comparison |
aDisaggregated costs are provided in Supplementary Table 9 (693.3KB, pdf) .
ICER = incremental cost-effectiveness ratio; LYG = life-year gained; QALY = quality-adjusted life-year.
As reflected in the scenario analyses, the ICER was most sensitive to the choice of OS parametric curve and the assumptions relating to time on treatment. The exponential curve predicted OS outcomes in the base case; this is a conservative assumption because it was associated with the highest ICER, as other parametric forms reduced the ICER as low as $169,483/QALY gained (Gompertz). The base case assumed patients receive treatment until progression—again, this is considered a conservative assumption because using the time on treatment data from the ALTA-1L clinical trial reduced the ICER to $98,436/QALY gained. Furthermore, alternative assumptions relating to duration of treatment led to ICERs that ranged from $211,011/QALY gained (treat for 3 cycles after progression) to $217,607/QALY gained (treatment until progression). ICERs for scenarios exploring treatment switching adjustments ranged from $215,143/QALY gained (adjusted for official switchers only using the RPSFTM and recensoring) to $246,051/QALY gained (adjusted for all switchers using RPSFTM without recensoring). One-way sensitivity analysis (Supplementary Figure 1 (693.3KB, pdf) ) demonstrated that the cost of subsequent therapies was also a key driver of results. A scenario exploring the use of subsequent therapies based on those observed in ALTA-1L reduced the ICER to $165,990/QALY gained. Further scenarios exploring subsequent therapy with ceritinib at a dose of 750 mg and assuming 100% dose intensity for crizotinib led to ICERs of $215,138/QALY gained and $240,176/QALY gained, respectively. Key base-case assumptions relating to the comparison of brigatinib vs crizotinib are conservative, as alternative assumptions generally improved the ICER in favor of brigatinib (Supplementary Table 2 (693.3KB, pdf) ).
BRIGATINIB VS ALECTINIB
Compared with alectinib, brigatinib was associated with a cost difference of −$8,546 attributable to the subsequent therapy distributions (the cost-comparison analysis assumed OS, PFS, and CNS-PFS outcomes were identical between brigatinib and alectinib). In the scenario that sourced subsequent therapy distributions from ALTA-1L and relevant clinical trials, brigatinib was associated with a cost increase of +$72,740. Treatment duration scenarios led to incremental cost savings that ranged from −$8,338 (treat for 3 cycles after progression) to −$8,477 (treat for 1 cycle after progression). Assuming 100% dose intensity for alectinib led to a marginal cost increase with brigatinib of +$21,743 (Supplementary Table 2 (693.3KB, pdf) ).
In the cost-effectiveness scenarios, ICERs for brigatinib vs alectinib ranged from $21,524/QALY gained to $304,461/QALY gained using different ITC and treatment switching methodologies. This included ICERs of $92,898/QALY gained under an anchored MAIC (intention-to-treat [ITT], OS, and PFS BIRC) adjusting for treatment switching in all switchers, without recensoring scenario; $122,218/QALY gained under an anchored MAIC (ITT, OS, and PFS BIRC) adjusting for treatment switching in all switchers and including recensoring scenario; and $149,298/QALY gained under an unanchored MAIC (ITT, OS, and PFS BIRC) scenario (Supplementary Table 3 (693.3KB, pdf) ). However, these should be interpreted carefully as the very small difference in incremental QALYs—driven by the limited differences in efficacy between the 2 drugs—results in an inflated ICER.
Within PSA based on list prices, brigatinib was associated with the highest probability of being cost-effective at all WTP thresholds above $236,000 (Supplementary Figure 2 (693.3KB, pdf) ).
Discussion
The availability of next-generation ALKis means that many patients can potentially maintain disease control for several years.10,23 Although the overarching mechanisms of action of available ALKis are comparable, there are differences with respect to pharmacological structure, binding specificities to the ALK kinase, and kinase inhibition potency.28,49 These characteristics are reflected in variable efficacy and safety profiles,28,49 as well as their cost-effectiveness, as reported in this analysis.
The ALTA-1L head-to-head trial demonstrated prolonged PFS with brigatinib vs crizotinib, translating to additional discounted life-years of 1.01 and additional QALYs of 0.97 within the model.29 The base case included the 58.0% of patients in the crizotinib arm of the ALTA-1L trial who received second-line brigatinib.17 This was not adjusted for in the base case as brigatinib is available and used in this setting in the United States.23 However, when removing the impact of second-line brigatinib from the crizotinib arm, the additional life-years and QALYs gained with brigatinib increased to 2.26 and 1.40, respectively. Therefore, it should be considered that the base case reflects frontline brigatinib vs a sequence of treatments—ie, crizotinib followed by brigatinib—and that the efficacy differences would be greater if such sequences of treatments were unavailable. This emphasizes the importance of using an efficacious ALKi in the frontline setting. Within the model, improved efficacy with brigatinib caused patients to remain in the progression-free health state with improved HRQoL for longer and incur less routine care costs vs crizotinib. However, this also means that patients remain on treatment with brigatinib for longer, leading to higher costs than with crizotinib (+$210,519), which are partly offset by subsequent therapy and terminal care cost savings with brigatinib.
The cost comparison of brigatinib vs alectinib demonstrated that brigatinib, in the ALKi-naive ALK+ NSCLC setting, is associated with cost savings of −$8,546 per patient attributable to the subsequent therapy distributions. Although time on frontline treatment and the distribution of patients progressing on brigatinib or alectinib are expected to be similar, patients would not be expected to receive the same treatment again. Therefore, the subsequent therapy distribution in the model was assumed to be equal, with the exception of 30% of patients receiving alectinib after brigatinib and 30% of patients receiving brigatinib after alectinib. Although highly sensitive to subsequent therapy assumptions, alectinib is associated with higher subsequent therapy costs, as the pack price of brigatinib is greater than that of alectinib ($16,364 vs $15,396 for brigatinib vs alectinib, respectively). Additionally, time on treatment with second-line brigatinib is longer than that with second-line alectinib, as brigatinib has been shown to be highly efficacious in this setting (again leading to higher subsequent therapy costs than alectinib).50,51 Assuming a dose intensity of 100% for brigatinib led to brigatinib being associated with a marginal cost increase of +$21,743 vs alectinib, further illustrating that cost savings with brigatinib are dependent on drug acquisition costs.
There are some important considerations regarding treatment with brigatinib that are not reflected within the model. Brigatinib is administered as a single tablet once daily with or without food. Therefore, brigatinib provides dosing advantages compared with alectinib (administered as 4 capsules twice daily with food) and crizotinib (administered twice daily).46,47 The ease of brigatinib dosing offers greater convenience compared with other ALKis owing to its flexibility and reduced pill burden, which could positively impact adherence and convenience for patients, many of whom are of working age.
Many patients treated with crizotinib progress within 2 years, with the CNS being the most common relapse site.18-22 Indeed, crizotinib has limited efficacy in the treatment of CNS metastases.30,52 However, next-generation ALKis (brigatinib and alectinib) have greater diffusion across the blood-brain barrier, thereby reducing CNS involvement.53,54 Thus, there are considerable clinical and economic benefits of treating patients upfront with the best possible ALKi.17,49
A key strength of this analysis is the robust direct comparison between brigatinib and crizotinib in the phase 3 ALTA-1L trial.17,29 In addition to efficacy and safety results, ALTA-1L also provided HRQoL data for analysis. It is noteworthy that clinician feedback suggests that this trial is highly representative of real-world clinical practice, including prior systemic chemotherapy, baseline disease characteristics (eg, patients with CNS metastases), and allowing crossover from crizotinib to brigatinib. The data from ALTA-1L and ALEX were based on final and latest datacuts, respectively, for each trial. Differences between study populations used in the ITC were explored using a MAIC methodology. Additional strengths of the analysis include the model structure reflecting outcomes of importance to patients and clinicians (eg, CNS-PFS) and extensive sensitivity analyses being conducted to explore the assumptions and uncertainty associated with different data sources and methods.
Following extensive validation of outcomes by clinical experts, the exponential curves for OS, PFS, and CNS-PFS were the only plausible extrapolations that provided internal consistency for these outcomes within the model. The exponential distribution was the most conservative curve choice for OS outcomes for brigatinib vs crizotinib. The predicted life-years from the exponential curves also align with published economic evaluations and literature55—other parametric curve selections were found to overpredict long-term survival.
LIMITATIONS
The analysis is subject to several limitations. CNS-PFS data are not currently available for alectinib; given that CNS metastases are a major cause of mortality and are associated with a low HRQoL, the analysis would benefit from the inclusion of these data.19,24
The ALTA-1L trial defined a progression event as a Response Evaluation Criteria in Solid Tumors progression, radiotherapy for CNS metastases, or death, whichever occurred first,17 whereas the ALEX trial defined a PFS event as a Response Evaluation Criteria in Solid Tumors progression or death, whichever occurred first.30,34,35 The number of events defined by radiotherapy to the brain was small in the ALTA-1L trial: 3 (2.2%) and 9 (6.5%) in the brigatinib and crizotinib arms, respectively, for PFS BIRC.17,29 Thus, although the impact of this on PFS BIRC, PFS, and CNS-PFS outcomes should be considered, it was not thought to be a driver of results. In addition, the base case assumed that patients are treated until progression; in a real-world clinical setting, patients may be treated beyond progression, which reduces the ICER in favor of brigatinib.
There are incomplete data from both trials regarding the subsequent therapies received after frontline therapy. The unadjusted OS data reflect a sequence of treatments, with the effects of the subsequent therapies confounding relative efficacy estimates of frontline therapies. The crizotinib arm in the ALTA-1L study was particularly impacted by this owing to the subsequent use of brigatinib in addition to other subsequent ALKi use. Although methods were explored to disentangle the effects of crossover using the ALTA-1L data, it was not possible to do this for other subsequent therapies given the small patient numbers supporting the complex methodology and the lack of information from the ALEX trial. The authors are unable to comment on which direction this would drive results.
Conclusions
The ALTA-1L head-to-head trial demonstrated prolonged PFS with brigatinib vs crizotinib, translating to additional life-years and QALYs within the economic model.17 Brigatinib incurred higher costs than crizotinib, mostly explained by improved efficacy with brigatinib, which resulted in patients remaining progression-free and receiving treatment for longer. The cost comparison of brigatinib vs alectinib assumed OS, PFS, and CNS-PFS outcomes were equal and demonstrated that brigatinib was cost-saving under base-case assumptions. Additional research into the real-world efficacy of ALKi therapy is warranted to further understand comparative cost-effectiveness.
ACKNOWLEDGMENTS
The authors are grateful to Teodor G. Paunescu, who is an employee of Takeda Pharmaceuticals U.S.A., Inc., and provided insights that greatly assisted the preparation of the manuscript. The authors also recognize the valuable contribution of Phillipa White, who is an employee of Source Health Economics and contributed to the writing and editing of the manuscript. This research is a cost-effectiveness evaluation and does not involve human subjects; as such, institutional review board review was not required.
REFERENCES
- 1.American Cancer Society. Cancer Statistics Centre 2021 estimates. Accessed March 2021. https://cancerstatisticscenter.cancer.org/#!/
- 2.National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Lung and Bronchus Cancer. Accessed July 2021. https://seer.cancer.gov/statfacts/html/lungb.html
- 3.Navada S, Lai P, Schwartz A, Kalemkerian G. Temporal trends in small cell lung cancer: Analysis of the national Surveillance, Epidemiology, and End-Results (SEER) database. J Clin Oncol. 2006;24(18_suppl):7082. doi: 10.1200/jco.2006.24.18_suppl.7082 [DOI] [Google Scholar]
- 4.Sher T, Dy GK, Adjei AA. Small cell lung cancer. Mayo Clin Proc. 2008;83(3):355-67. doi: 10.4065/83.3.355 [DOI] [PubMed] [Google Scholar]
- 5.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4–ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561-6. doi: 10.1038/nature05945 [DOI] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma: Nature. 2014;511(7511):543-50. doi: 10.1038/nature13385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chia PL, Mitchell P, Dobrovic A, John T. Prevalence and natural history of ALK positive non-small-cell lung cancer and the clinical impact of targeted therapy with ALK inhibitors. Clin Epidemiol. 2014;6:423. doi: 10.2147/CLEP.S69718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Passaro A, Lazzari C, Karachaliou N, et al. Personalized treatment in advanced ALK-positive non-small cell lung cancer: From bench to clinical practice. Onco Targets Ther. 2016;9:6361-76. doi: 10.2147/OTT.S98347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pacheco JM, Gao D, Smith D, et al. Natural history and factors associated with overall survival in stage IV ALK-rearranged non–small cell lung cancer. J Thorac Oncol. 2019;14(4):691-700. doi: 10.1016/j.jtho.2018.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casaluce F, Sgambato A, Maione P, et al. ALK inhibitors: A new targeted therapy in the treatment of advanced NSCLC. Target Oncol. 2013;8(1):55-67. doi: 10.1007/s11523-012-0250-9 [DOI] [PubMed] [Google Scholar]
- 11.Malik SM, Maher VE, Bijwaard KE, et al. US Food and Drug Administration approval: Crizotinib for treatment of advanced or metastatic non-small cell lung cancer that is anaplastic lymphoma kinase positive. Clin Cancer Res. 2014;20(8):2029-34. doi: 10.1158/1078-0432.CCR-13-3077 [DOI] [PubMed] [Google Scholar]
- 12.U.S. Food and Drug Administration. FDA broadens ceritinib indication to previously untreated ALK-positive metastatic NSCLC. 2017. Accessed March 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-broadens-ceritinib-indication-previously-untreated-alk-positive-metastatic-nsclc
- 13.US Food and Drug Administration. Alectinib approved for (ALK) positive metastatic non-small cell lung cancer (NSCLC). 2017. Accessed March 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/alectinib-approved-alk-positive-metastatic-non-small-cell-lung-cancer-nsclc
- 14.US Food and Drug Administration. FDA approves brigatinib for ALK-positive metastatic NSCLC. 2020. Accessed March 2021. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-brigatinib-alk-positive-metastatic-nsclc
- 15.US Food and Drug Administration. FDA approves lorlatinib for metastatic ALK-positive NSCLC. 2021. Accessed March 2021. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-lorlatinib-metastatic-alk-positive-nsclc
- 16.Camidge DR, Kim HR, Ahn M-J, et al. Brigatinib versus crizotinib in ALKpositive non–small-cell lung cancer. N Engl J Med. 2018;379(21):2027-39. doi: 10.1200/JCO.20.00505 [DOI] [PubMed] [Google Scholar]
- 17.Takeda Pharmaceuticals Ltd. Data on file. Clinical Study Report AP26113-13-301: A phase 3 multicenter open-label study of brigatinib (AP26113) versus crizotinib in patients with ALK-positive advanced lung cancer. 2020.
- 18.Guérin A, Sasane M, Zhang J, et al. Economic burden and treatments of progression to metastatic disease in ALK+ NSCLC patients: Metastatic non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;90(5):S44-5. doi: 10.1016/j.ijrobp.2014.08.231 [DOI] [Google Scholar]
- 19.Aldea M, Besse B, Hendriks LE. ALK inhibitors in ALK-positive NSCLC with central nervous system metastases. Eur Oncol Haematol. 2020;16(1):18-21. [Google Scholar]
- 20.Mezquita L, Planchard D. The role of brigatinib in crizotinib-resistant nonsmall cell lung cancer. Cancer Manag Res. 2018;10:123-30. doi: 10.2147/CMAR.S129963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preusser M, Winkler F, Valiente M, et al. Recent advances in the biology and treatment of brain metastases of non-small cell lung cancer: Summary of a multidisciplinary roundtable discussion. ESMO Open. 2018;3(1):e000262. doi: 10.1136/esmoopen-2017-000262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toyokawa G, Seto T, Takenoyama M, Ichinose Y. Insights into brain metastasis in patients with ALK+ lung cancer: Is the brain truly a sanctuary? Cancer Metastasis Rev. 2015;34(4):797-805. doi: 10.1007/s10555-015-9592-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Comprehensive Cancer Network. Clinical practice guidelines in non-small cell lung cancer. March 2021. Accessed March 2021. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- 24.Guérin A, Sasane M, Zhang J, et al. Brain metastases in patients with ALK+ non-small cell lung cancer: clinical symptoms, treatment patterns and economic burden. J Med Econ. 2015;18(4):312-22. doi: 10.3111/13696998.2014.1003644 [DOI] [PubMed] [Google Scholar]
- 25.National Cancer Institute. Cancer trends progress report. Financial burden of cancer case, US 2018. Accessed March 2021. https://progressreport.cancer.gov/after/economic_burden
- 26.Burudpakdee C, Wong W, Seetasith A, et al. Economic impact of preventing brain metastases with alectinib in ALK-positive non-small cell lung cancer. Lung Cancer. 2018;119:103-11. doi: 10.1016/j.lungcan.2018.03.008 [DOI] [PubMed] [Google Scholar]
- 27.Camidge DR, Kim HR, Ahn M-J, et al. Brigatinib versus crizotinib in advanced ALK inhibitor-naive ALK-positive nonsmall cell lung cancer: Second interim analysis of the phase III ALTA-1L trial. 38. 2020:3592-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itchins M, Chia PL, Hayes S, et al. Treatment of ALK-rearranged non–small cell lung cancer: A review of the landscape and approach to emerging patterns of treatment resistance in the Australian context. Asia-Pacific Journal of Clinical Oncology. 2017;13:3-13. [DOI] [PubMed] [Google Scholar]
- 29.Camidge DR, Kim HR, Ahn M-J, et al. Brigatinib versus crizotinib in ALK inhibitor–naive advanced ALK-positive NSCLC: Final results of phase 3 ALTA-1L trial. J Thorac Oncol. 2021;16(12):2091-108. doi: 10.1016/j.jtho.2021.07.035 [DOI] [PubMed] [Google Scholar]
- 30.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non–small-cell lung cancer. N Engl J Med. 2017;377(9):829-38. doi: 10.1056/NEJMoa1704795 [DOI] [PubMed] [Google Scholar]
- 31.National Institute for Health and Care Excellence. Alectinib for untreated ALKpositive advanced non-small-cell lung cancer TA536. August 2018. Accessed July 2021. https://www.nice.org.uk/guidance/TA536
- 32.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093-103. doi: 10.1001/jama.2016.12195 [DOI] [PubMed] [Google Scholar]
- 33.Institute for Clinical Economic Review. ICER’s reference case for economic evaluations: Principles and rationale. January 31, 2020. Accessed December 2021. https://icer.org/wp-content/uploads/2020/10/ICER_Reference_Case_013120.pdf
- 34.Camidge DR, Dziadziuszko R, Peters S, et al. Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced Non–Small cell lung cancer in the global phase III ALEX study. J Thorac Oncol. 2019;14(7):1233-43. doi: 10.1016/j.jtho.2019.03.007 [DOI] [PubMed] [Google Scholar]
- 35.Mok T, Shaw A, Camidge R, et al. Final PFS, updated OS and safety data from the randomised, phase III ALEX study of alectinib (ALC) versus crizotinib (CRZ) in untreated advanced ALK + NSCLC. Ann Oncol. 2019;30:v607. doi: 10.1093/annonc/mdz260.006 [DOI] [Google Scholar]
- 36.Cranmer H, Liu Y, Kay S, Shen J. Overall survival estimates adjusting for treatment crossover in patients previously untreated with an ALK inhibitor with ALK+ NSCLC using data from the Alta-1L clinical trial. Abstract presented at: ISPOR Europe, 2021. Abstract number PCN23. https://www.ispor.org/heor-resources/presentations-database/presentation/euro2020-3282/107597 [Google Scholar]
- 37.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365-76. doi: 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 38.Bergman B, Aaronson N, Ahmedzai S, Kaasa S, Sullivan M. The EORTC QLQ-LC13: A modular supplement to the EORTC core quality of life questionnaire (QLQ-C30) for use in lung cancer clinical trials. Eur J Cancer. 1994;30(5):635-42. doi: 10.1016/0959-8049(94)90535-5 [DOI] [PubMed] [Google Scholar]
- 39.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: Development and testing of the D1 valuation model. Med Care. 2005;43(3):203-20. doi: 10.1097/00005650-200503000-00003 [DOI] [PubMed] [Google Scholar]
- 40.Longworth L, Yang Y, Young T, et al. Use of generic and condition-specific measures of health-related quality of life in NICE decision-making: A systematic review, statistical modelling and survey. Health Technol Assess. 2014;18(9):1-224. doi: 10.3310/hta18090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ara R, Brazier JE. Using health state utility values from the general population to approximate baselines in decision analytic models when condition-specific data are not available. Value Health. 2011;14(4):539-45. doi: 10.1016/j.jval.2010.10.029 [DOI] [PubMed] [Google Scholar]
- 42.European Medicines Agency. Crizotinib summary of product characteristics. Accessed July 2021. https://www.ema.europa.eu/en/documents/product-information/xalkori-epar-product-information_en.pdf
- 43.European Medicines Agency. Alecensa summary of product characteristics. Accessed July 2021. https://www.ema.europa.eu/en/documents/product-information/alecensa-epar-product-information_en.pdf
- 44.European Medicines Agency. Brigatinib summary of product characteristics. Accessed August 2021. https://www.ema.europa.eu/en/documents/product-information/alunbrig-epar-product-information_en.pdf
- 45.National Institute for Health and Care Excellence. TA524 committee papers. Brentuximab vedotin for treating CD30positive Hodgkin lymphoma. Accessed July 15, 2019. https://www.nice.org.uk/guidance/ta524
- 46.Zhou Z-Y, Mutebi A, Han S, et al. Cost-effectiveness of ceritinib in previously untreated anaplastic lymphoma kinase-positive metastatic non-small cell lung cancer in the United States. J Med Econ. 2018;21(6):577-86. doi: 10.1080/13696998.2018.1443111 [DOI] [PubMed] [Google Scholar]
- 47.Centers for Medicare and Medicaid Services. Physician fee schedule look-up tool. Accessed August 2021. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PFSlookup
- 48.Duncan I, Ahmed T, Dove H, Maxwell TL. Medicare cost at end of life. Am J Hosp Palliat Care. 2019;36(8):705-10. doi: 10.1177/1049909119836204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.National Institute for Health and Care Excellence. Single technology appraisal committee papers: Brigatinib for ALK-positive advanced nonsmall-cell lung cancer that has not been previously treated with an ALK inhibitor [ID1468]. May 2020.
- 50.Kim D-W, Tiseo M, Ahn M-J, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: A randomized, multicenter phase II trial. J Clin Oncol. 2017;35(22):2490-8. doi: 10.1200/JCO.2016.71.5904 [DOI] [PubMed] [Google Scholar]
- 51.Ou S-HI, Ahn JS, De Petris L, et al. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: A phase II global study. J Clin Oncol. 2016;34(7):661-8. doi: 10.1200/jco.2015.63.9443 [DOI] [PubMed] [Google Scholar]
- 52.Zhou C, Kim S-W, Reungwetwattana T, et al. Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): A randomised phase 3 study. Lancet Respir Med. 2019;7(5): 437-46. doi: 10.1016/S2213-2600(19)30053-0 [DOI] [PubMed] [Google Scholar]
- 53.Wrona A. Management of CNS disease in ALK-positive non-small cell lung cancer: Is whole brain radiotherapy still needed? Cancer Radiother. 2019;23(5): 432-8. doi: 10.1016/j.canrad.2019.03.009 [DOI] [PubMed] [Google Scholar]
- 54.Metro G, Lunardi G, Floridi P, et al. CSF concentration of crizotinib in two ALK-positive non-small-cell lung cancer patients with CNS metastases deriving clinical benefit from treatment. J Thorac Oncol. 2015;10(5):e26-7. doi: 10.1097/JTO.0000000000000468 [DOI] [PubMed] [Google Scholar]
- 55.Hao X, Shen A, Wu B. Cost-effectiveness of nivolumab plus ipilimumab as first-line therapy in advanced non-small-cell lung cancer. Front Pharmacol. 2021;12:573852. doi: 10.3389/fphar.2021.573852 [DOI] [PMC free article] [PubMed] [Google Scholar]


