Abstract
Although it is most well-known for its roles in central nervous system (CNS) function, the vast majority of serotonin, or 5-hydroxytryptamine (5-HT), is produced in the gastrointestinal (GI) tract. 5-HT is synthesized mostly by enterochromaffin (EC) cells of the GI epithelium and, in small part, by neurons of the enteric nervous system (ENS). The GI tract contains an array of broadly distributed 5-HT receptors, which participate in functions such as motility, sensation, inflammation, and neurogenesis. The roles of 5-HT in these functions are reviewed, as well as its role in the pathophysiology of disorders of gut-brain interaction (DGBIs) and inflammatory bowel diseases (IBD).
Introduction
Although it is most well-known for its roles in central nervous system (CNS) function, serotonin, or 5-hydroxytryptamine (5-HT), was first discovered in the gastrointestinal (GI) tract. Vittorio Erspamer, the pharmacologist who discovered 5-HT, first named it “enteramine” after extracting it from rabbit gastric mucosa in 1937 (67), and later noted that it was present in the GI tract of every vertebrate animal he studied. Ten years later, Maurice Rapport, Arda Green, and Irvine Page identified this molecule as serotonin, named for its origins in serum (“sero”) and its ability to increase tone or vasoconstriction (“tonin”) (189, 190), and published its structure as 5-HT in 1949 (188). This research merged in 1952, when Erspamer confirmed that the enteramine molecule he had discovered in the mucosa of the GI tract was 5-HT (68). 5-HT was then found in the CNS and discovered to have roles in essential functions such as sleep, mood, and appetite (33). Although 5-HT has many important roles in the brain, the vast majority of the body’s 5-HT is produced in the gut, where it participates in functions such as motility, sensation, inflammation, and enteric neurogenesis (28, 74, 92, 158). The roles of 5-HT in these functions are reviewed, as well as its role in the pathophysiology of disorders of gut-brain interaction (DGBIs) and inflammatory bowel diseases (IBD).
5-HT Synthesis and Signaling
The gut contains the vast majority of the body’s 5-HT, synthesized mostly by EC cells of the mucosa and, in small part, by neurons of the enteric nervous system (ENS) (92, 119, 197). EC cells are a subtype of enteroendocrine cell that constitute less than 1% of the GI epithelium yet produce the vast majority of gut 5-HT (92). Serotonergic neurons make up only 2% to 3% of total ENS neurons but project a vast number of fibers throughout the myenteric plexus to elicit broad effects (52) (Figure 1).
Figure 1. Expression and distribution of serotonergic cells and axonal fibers in the colonic mucosa and the enteric nervous system (ENS).
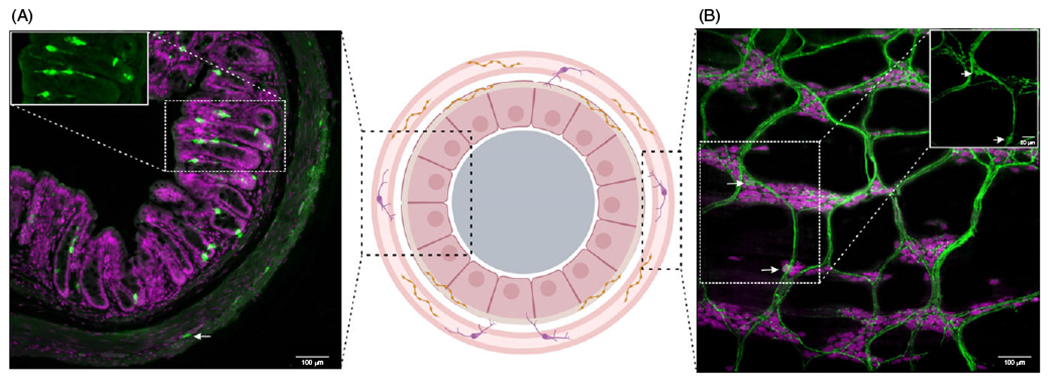
Serotonin (5-hydroxytryptamine, 5-HT)-positive cells (green) are present in both the enterochromaffin (EC) cells of the mucosa (A) and within myenteric plexus of the ENS (B). In the mucosa, 5-HT is expressed in EC cells (starred in inset) which make up less than 1% of gut epithelial cells (stained with nuclei marker, bisbenzimide, magenta). In the myenteric plexus, 5-HT+ neurons (arrow, inset) comprise 2% to 3% of total myenteric neurons (stained with pan-neuronal marker, ANNA-1, magenta) as observed in wholemount laminar preparation of the myenteric plexus (B) and a cross-section of the colon (arrow). In the ENS, 5-HT is also present in continuous presynaptic fibers that run across the enteric ganglia and along interganglionic fiber tracts, thus providing extensive innervation to other neurons within the ganglion. Made in © BioRender - biorender.com.
EC cells that synthesize 5-HT contain tryptophan hydroxylase 1 (TPH1), the rate-limiting enzyme that converts the precursor amino acid l-tryptophan into 5-hydroxytryptophan (5-HTP), which is then converted by a nonspecific aromatic l-amino acid decarboxylase to 5-HT (95, 228). 5-HT is stored in vesicular monoamine transporter 1 (VMAT1) within EC cells (163). Enteric neurons synthesize 5-HT in a similar fashion, but with tryptophan hydroxylase 2 (TPH2) as the rate-limiting enzyme and, within enteric neurons, 5-HT is stored in VMAT2 (193) (Figure 2). TPH2 is also present in serotonergic neurons of the CNS, primarily in the dorsal raphe nucleus (229, 241). A global loss of TPH1, in TPH1 knockout (KO) mice, results in complete depletion of intestinal epithelial 5-HT synthesis without any loss in the brain or the ENS, while knockout of TPH2 results in a loss of both brain and enteric neuronal 5-HT biosynthesis (228). Interestingly, loss of TPH1 does not affect total gastrointestinal transit time but does result in increased stool size and changes in colonic migrator motor complex patterns (107, 153). In contrast, although TPH2 synthesizes a much smaller amount of 5-HT in the gut, its deletion has profound effects on in vivo and ex vivo gut motility and enteric neurogenesis (140), which is explained in greater detail below. These data indicate differing roles of TPH1 and TPH2 in gut function and ENS development as well as differences in how epithelial versus neuronal 5-HT impact motility.
Figure 2. Serotonin (5-HT) biosynthesis and metabolism in the gastrointestinal tract.
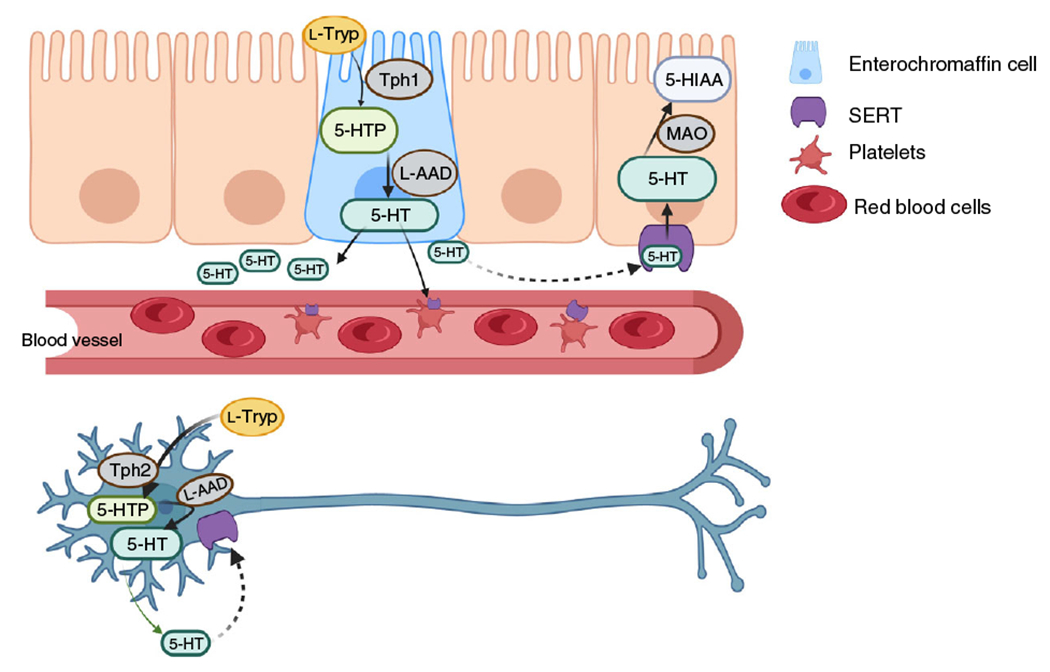
Biosynthesis of 5-HT in the gut is accomplished by two different isoforms of the rate-limiting enzyme, tryptophan hydroxylase (TPH). TPH1 is responsible for 5-HT synthesis in the mucosal enterochromaffin cells and TPH2 synthesizes 5-HT in enteric neurons. Both enzymes convert the amino acid l-tryptophan (l-Tryp) to 5-hydroxytryptophan (5-HTP). The enzyme l-amino acid decarboxylase (l-AAD) then converts 5-HTP to 5-HT where it is then released into the extracellular space (solid arrows) to act on 5-HT receptors and initiate downstream serotonergic signaling. Extracellular 5-HT is then taken back up into EC cells or enteric neurons via the 5-HT transporter, SERT (dashed arrows). Once intracellular, 5-HT is inactivated by monoamine oxidase (MAO), in the mitochondria, to 5-hydroxyindoleacetic acid (5-HIAA). Made in © BioRender - biorender.com.
After 5-HT is released and exerts effects on its receptors, it must be rapidly inactivated, as excess extracellular 5-HT can cause toxicity and/or receptor desensitization (46, 94). The enzyme, monoamine oxidase (MAO) is required for inactivation of 5-HT (181) but is located intracellularly. Outside of the cell, a high-affinity transporter is thus required to take up 5-HT because it is highly charged at a physiological pH (142). The plasmalemmal serotonin reuptake transporter (SERT) is the primary mechanism by which 5-HT is transported intracellularly. Once inside the cell, 5-HT is deaminated to 5-hydroxyindole acetaldehyde (5-HIAL), then oxidated by MAO to 5-hydroxyindoloacetic acid (5-HIAA) which is excreted by the kidneys (137). SERT is ubiquitously expressed in enterocytes that line the mucosa (98, 226), in some ENS neurons (92, 95), and in immune cells such as macrophages, mast cells, lymphocytes, and dendritic cells (108). Platelets circulating throughout the intestine also express SERT and thus contribute to 5-HT uptake in the gut (15, 138). Through blood circulation, platelets thus act as a vessel for endocrine signaling of 5-HT to targets such as liver (139) and bone (132). Platelets do not synthesize 5-HT because they do not contain TPH. The concentration of 5-HT in the blood is therefore likely an indicator of peripheral gut 5-HT production and SERT efficacy in platelets (77, 165).
5-HT Receptors in the GI Tract
At least 15 different 5-HT receptors have been characterized. Five of these receptor subclasses are present in the intestine: 5-HT1, 5-HT2, 5-HT3, 5-HT4, and 5-HT7 (118). These subtypes are all G-protein-coupled receptors except for 5-HT3, which is ionotropic (118, 157). These receptors are distributed in different cell types throughout the digestive tract, including in intrinsic neurons of the ENS and extrinsic primary afferent neurons (ExPANs) that project to the CNS (89). The roles of these receptors are detailed in the below sections and a summary of 5-HT receptor expression and function can be found in Table 1. Little is currently known about the whether multiple types of 5-HT receptors are present in the same cell; thus, future studies should examine co-expression of 5-HT receptors in ENS neurons and other cell types of the gut.
Table 1.
5-HT Receptor Expression and Function
| Receptor | Expression | Function | References |
|---|---|---|---|
| 5-HT1A | Enteric neurons | Inhibits neurons involved in peristalsis | (83, 85, 87) |
| 5-HT1P | Enteric neurons | Activates neurons involved in peristalsis | (4, 31, 51, 103, 231) |
| 5-HT2B | Enteric neurons | Activates neurons involved in peristalsis | (30) |
| Promotes neuronal development | (76) | ||
| Interstitial cells of Cajal | Promotes ICC proliferation | (216, 236) | |
| Extrinsic primary afferent neurons | Initiates pain signaling | (112, 178) | |
| 5-HT3 | Enteric neurons | Activates neurons involved in peristalsis | (110, 111) |
| Smooth muscle cells | Evokes muscle contraction | (158, 204) | |
| Extrinsic primary afferent neurons | Initiates pain signaling | (158, 204) | |
| Vagal afferent neurons | Evokes nausea and emesis | (168, 222) | |
| Enterochromaffin cells | Evokes 5-HT release | (158, 204) | |
| Interstitial cells of Cajal | Unknown | (235) | |
| Enterocytes | Facilitates secretion | (22) | |
| 5-HT4 | Enteric neurons | Present on presynaptic nerve terminals, activation evokes release of acetylcholine to initiate peristaltic contractions | (86, 218) |
| Smooth muscle cells | Evokes muscle contraction | (215) | |
| Extrinsic primary afferent neurons | Modulates pain signaling | (198) | |
| Enterocytes | Facilitates chloride secretion | (114) | |
| Enterochromaffin cells | Evokes 5-HT release | (114) | |
| Goblet cells | Evokes mucus discharge | (114) | |
| 5-HT7 | Enteric neurons | Mediates slow depolarizing responses in intrinsic primary afferent neurons | (59, 162, 219) |
| Smooth muscle cells | Inhibits smooth muscle cells, resulting in relaxation of smooth muscle | (44, 121, 184, 219) |
5-HT1 receptors
5-HT1 receptors (specifically 5-HT1A and 5-HT1P) are G-protein-coupled receptors localized to enteric neurons that exert inhibitory or excitatory actions, depending on the subtype. General 5-HT1 receptor activation with nonspecific agonists results in membrane hyperpolarization of postsynaptic neurons, thus inhibiting neurotransmitter release (83, 85, 87). Specific activation of the 5-HT1A receptor inhibits neurotransmitter release onto enteric motor neurons and disrupts motility, by diminishing colonic migrating motor complexes (CMMCs) (59). In contrast, the 5-HT1P receptor exerts excitatory effects; its activation induces neurotransmitter release from intrinsic primary afferent neurons (IPANs), which initiate peristaltic reflexes in the gut (31, 103, 231). 5-HT1P receptors on IPANs also mediate chloride secretion in the gut (51). A recent study demonstrated the presence of functional 5-HT1P receptors in the human small and large intestine; application of a 5-HT1P agonist resulted in long-lasting activation of human submucosal ENS neurons (4).
5-HT2 receptors
The 5-HT2B receptor is a G-protein-coupled receptor widely expressed in the GI tract where it is present in enteric neurons, smooth muscle cells, and interstitial cells of Cajal (ICCs) (30, 76). Ex vivo studies have shown that in rodent and human intestine, the excitatory effects of 5-HT are mediated in part by the 5-HT2B receptor (30). Activation of this receptor also contributes to the development of enteric neurons (76) as well as proliferation of ICCs (216, 236), which are the pacemaker cells of the gut that coordinate smooth muscle function (196). ICCs are required for normal gut motility; decreased ICC volume and/or impaired ICC functioning have been shown to contribute to dysmotility symptoms like constipation and gastroparesis (105, 239). 5-HT2B receptors are also expressed on ExPANs that innervate the gut and convey pain and other sensory information (112).
5-HT3 receptors
In contrast to the other 5-HT receptors present in the GI tract, which are G-protein-coupled, 5-HT3 receptors are ionotropic, enabling ligand-gated entry of cations including Ca2+ (180). These excitatory receptors are present in several subtypes of enteric neurons as well as ExPANs and vagal afferent fibers, ICCs, smooth muscle cells, enterocytes, and enterochromaffin cells (158, 215). The stimulation of 5-HT3 receptors evokes peristaltic contractions through stimulation of enteric neurons (110, 111). 5-HT3 receptor activation on enterocytes facilitates secretion (22) and the role of 5-HT3 receptors on ICCs is unknown, although activation results in rapid depolarization and therefore may initiate slow waves in the ICC network (235).
5-HT3 receptors play a critical role in evoking peristaltic contractions through stimulation of enteric neurons (110, 111); IPANs, which project to the mucosa and are involved in initiating peristaltic contractions, demonstrate responses to 5-HT that are exclusively mediated via 5-HT3 receptors (21). In isolated colons, addition of the 5-HT3 receptor antagonist, ondansetron abolishes spontaneous colonic contractions and diminishes peristaltic contractions triggered by mucosal stimulation, indicating that EC cell-released 5-HT activates IPANs via 5-HT3 receptors (106). These receptors also appear to be present in inhibitory motor neurons, which receive input from serotonergic interneurons and contribute to tonic inhibition of the circular muscle (59).
RNA sequencing studies have begun to show differences in 5-HT receptor expression throughout the length of the gut; it was recently demonstrated that 5-HT3 and 5-HT4 receptors are more highly expressed in the distal colon compared to proximal colon (61).
5-HT4 receptors
5-HT4 receptors are G-protein-coupled receptors widely expressed throughout the GI tract in enteric neurons, ExPANs, EC cells, and smooth muscle cells (215). In the ENS, 5-HT4 receptor agonists evoke peristaltic reflexes by acting on presynaptic nerve terminals to stimulate the release of excitatory neurotransmitters such as acetylcholine (71, 86, 141, 179, 218). 5-HT4 receptors are also expressed on colonic epithelial cells and their activation results in 5-HT release from EC cells, mucus discharge from goblet cells, and chloride secretion by enterocytes (114). 5-HT4 receptors have important neurotrophic roles as well; expression on enteric neurons and enteric neural crest-derived precursor cells (ENCDCs) mediates neuronal proliferation and differentiation (115, 141).
5-HT7 receptors
5-HT7 receptors are G-protein-coupled receptors that are present in cell bodies of a subset of enteric neurons and mediate slow depolarizing responses to 5-HT (162, 219). Recent RNAseq data confirm expression of 5-HT7 receptors in enteric neurons (240). 5-HT7 receptors have also been identified on smooth muscle cells in guinea pig ileum (44, 219), human ileum (184), and dog stomach (121). Pharmacological studies suggest they serve an inhibitory function, causing relaxation of the smooth muscle; however, functional 5-HT7 receptors have not been identified in smooth muscle of the mouse intestine (59).
Role of the Gut Microbiome in 5-HT Signaling
The gut microbiome comprises a dynamic population of trillions of microorganisms that reside at the epithelial interface of the gut. The microbiome has recently emerged as an important contributor to metabolism, immunity, and gut-brain signaling (149). Recent studies have demonstrated the presence of a bidirectional communication between 5-HT and gut microbiota. For the most part, EC cells secrete 5-HT basolaterally for purposes of signaling to surrounding neurons; however, some 5-HT is released on the apical side of EC cells directly into the lumen of the intestine (80). A recent study in mice demonstrated that increasing the amount of 5-HT available in the lumen increased the relative abundance of spore-forming microbes of the Clostridiaceae and Turicibacteraceae families (82). Further, a protein was detected on a particular bacterium, Turicibacter sanguinis, that is similar in structure and function to SERT, showing that bacteria can take up 5-HT and thus have a potential role in regulating 5-HT levels in the gut (82). Gut bacteria also secrete metabolites such as short-chain fatty acids (SCFAs) that have been shown to promote colonic motility in a 5-HT3 receptor-dependent manner (81). A more recent study demonstrated that colonization of bacteria in the mouse colon promoted TPH1 mRNA levels and increased 5-HT concentration and that this occurred via SCFA interactions with EC cells (191). Using calcium imaging techniques in primary murine EC cells, researchers found that SCFAs do not acutely (i.e., within 2 h) activate EC cells to induce 5-HT secretion (155). Microbiota regulation of gut 5-HT is thus more likely the result of ongoing interactions between bacterial metabolites and EC cells. Studies have also demonstrated that other microbial-specific metabolites such as tyramine, p-aminobenzoate, and α-tocopherol also promote TPH1 expression and 5-HT release from EC cells (238). Colonic EC cells express many receptors for these metabolites such as free fatty acid receptor 2 (FFAR2), which senses SCFAs, G-protein-coupled receptor 35 (GPR35), which senses small aromatic acids, and G-protein-coupled receptor 132 (GPR132), which senses lactate and acyl amides (145). Together these data suggest that there are abundant mechanisms by which microbes influence 5-HT production.
5-HT in ENS Development
In addition to its importance in gut function, 5-HT is also recognized for its role as a growth factor in enteric neuron development. Early-born enteric neurons contain 5-HT, which has been shown to promote both neurogenesis as well as the growth of specific subsets of later-born neurons, thus influencing ENS development. For example, in studies of cultured ENCDCs, the addition of 5-HT promotes the development of dopaminergic neurons. Conversely, mice lacking neuronal 5-HT (TPH2 KO mice) display ENS hypoplasia and deficits in later-born neurons, including those expressing gamma-aminobutyric acid (GABA), nitric oxide (NO), calcitonin gene-related peptide (CGRP), and tyrosine hydroxylase (TH, a marker of dopaminergic neurons) (140). ENS hypoplasia was also found in transgenic mice deficient in neuronal 5-HT due to a single nucleotide polymorphism (R439H) in the TPH2 enzyme (TPH2-R439H mice), a defect that was rescued by administration of slow-release 5-hydroxytryptophan (5-HTP) (120). In contrast, TPH1 KO mice do not display these deficits in ENS development, suggesting that mucosal 5-HT is not critical for proper ENS development. SERT, presumably through its modulation of 5-HT inactivation, also modulates ENS development. In transgenic mice with a substitution of glycine for alanine at the SERT locus (SERT Ala56 mice), this mutation results in lower levels of circulating 5-HT, due to excess SERT activity. Consequently, these mice display a hypoplastic ENS (151). Conversely, when SERT is knocked out globally (SERT KO mice) or when mice are exposed to selective serotonin reuptake inhibitors (SSRIs) during fetal development, mice display a hyperplastic ENS (151), supporting the idea that SERT blockade in development leads to enteric neuronal hyperplasia.
The receptors 5-HT2B and 5-HT4 have been shown to have important roles in mediating ENS development. The 5-HT2B receptor is expressed in subsets of enteric neurons and, in cultures of ENCDCs, stimulation of 5-HT2B enhances neuronal differentiation (76). 5-HT may therefore influence the fate of developing enteric neurons through activation of this receptor. ICCs also express the 5-HT2B receptor and require it for normal development of the ICC network (236), which acts as an intermediary between ENS neurons and smooth muscle to mediate slow wave propagation involved in gut motility (63). Activation of the 5-HT4 receptor has also been shown to be neurogenic; 5-HT4 agonism promotes neuronal survival and differentiation in vitro and neurogenesis in adult mice in vivo (141). The presence of stem cells in the mature gut makes this neurogenesis possible (27, 29, 134). Mice in which 5-HT4 receptors are knocked out (5-HT4 KO) show deficiencies in postnatal enteric neurogenesis that are exaggerated with age (141). 5-HT4 signaling also promotes postnatal enteric neural stem cell (ENSC) proliferation and neuronal differentiation. In colon explants cultured with ENSCs, addition of liposomal nanoparticles containing a 5-HT4 agonist, RS67506, increased neuronal proliferation and differentiation (115). Transplanting ENSCs along with these nanoparticles to the mouse colon in vivo also led to increased neuronal proliferation (115). The gut microbiota may also play a role in enteric neurogenesis that is dependent on 5-HT, as bacteria residing in the gut can induce 5-HT release from enteric neurons, via the 5-HT4 receptor, (58), which then influences ENS development.
Studies have demonstrated the therapeutic relevance of targeting the 5-HT4 receptor for neurogenesis (93). In studies where gut transection and anastomosis were carried out in murine models, oral delivery of the 5-HT4 agonist, mosapride citrate promoted reconstruction of enteric neural circuits, which restored defecation reflexes that had been disrupted (100, 212). These data show the potential mechanisms underlying the partial success of 5-HT4 receptor agonists as therapies for gut motility disorders. The 5-HT4 agonist prucalopride has been shown to increase neurogenesis in vivo and prevent deficiencies in ENS development caused by the SERT-Ala56 mutation (151). Prucalopride also exerts protective effects on enteric neurons exposed to oxidative stress challenge (25), indicating that it may have utility in treating degenerative disorders of the ENS, though this implication has not yet been extensively explored.
In addition to neurogenesis, enteric neuronal 5-HT also impacts the proliferation and maintenance of the intestinal mucosa. Mice lacking neuronal 5-HT (TPH2 KO) show diminished mucosal proliferation, as evidenced by reduced villus height, crypt depth, and proliferation index (104). TPH2-R439H mice display similar mucosal defects, which could be reversed by treatment with sustained-release 5-HTP (120). SERT Ala56 mice, which have lower 5-HT availability, also have an underdeveloped mucosa (151). In contrast, mice lacking mucosal 5-HT (TPH1 KO) did not display an under-developed mucosal layer; proliferation index in the gut epithelium of these mice was unchanged, whereas crypt depth and villus height were greater compared to wildtype littermate controls (104). Altogether, the available data are suggestive of the notion that 5-HT derived from ENS neurons, but not from EC cells, contributes to epithelial proliferation. Mice that lack SERT (SERT KO) or adult mice that have been administered SSRIs, show significantly more mucosal growth and proliferation of epithelial precursor cells (104). Administration of the 5-HT2A receptor antagonist, ketanserin reversed 5-HT-mediated mucosal proliferation; this effect is thus likely mediated by 5-HT2A receptors expressed on submucosal cholinergic neurons that innervate the mucosal layer (104). Consistent with these findings, mice lacking the 5-HT2A receptor have a smaller mucosal layer with shorter enterocytes (75). In contrast, mice exposed to SSRIs during embryonic development, through maternal administration, exhibit greater mucosal area (151). Finally, in vivo administration of the 5-HT4 agonist tegaserod increases proliferation of crypt epithelial cells in mice and promotes epithelial cell migration in vitro, as modeled in Caco-2 cell cultures (207). These data provide support of 5-HT4 agonists as a therapeutic that may enable growth and healing of the epithelial layer in conditions that damage the gut epithelium such as inflammatory diseases or chemotherapeutic regimens.
Role of 5-HT in Gut Motility
Peristaltic reflexes
Peristalsis is a complex set of interactions within the ENS responsible for propulsive contractions of the GI musculature that propel contents through the gut. Smooth muscle contraction on the oral end of the gut and relaxation on the aboral end is required to efficiently move digestive contents in a sequential manner. In 1899, Bayliss and Starling were the first to describe how a peristaltic reflex is initiated by an increase in intraluminal pressure; they found that they could reliably initiate peristaltic contractions via balloon distension of a dog intestine (13, 14). They observed that these reflexes remained intact after the intestine was severed from all extrinsic nerves and thus attributed the reflexes to a “local nervous mechanism” (13, 14). Trendelenburg coined the term “peristaltic reflex” in 1917, after confirming Bayliss and Starling’s results in isolated guinea pig intestine, also noting the intestine’s ability to produce reflexes independently of the brain (221). The “local nervous mechanism” is now known as the ENS.
Edith Bülbring and colleagues were the first to define the role of 5-HT in peristaltic reflexes and intestinal propulsion. In isolated guinea pig small intestine, they showed that application of 5-HT was sufficient to initiate peristaltic reflexes (35, 36) and that pressure-induced stimulation of the peristaltic reflex resulted in 5-HT release (37). They determined that 5-HT is released from EC cells in an intraluminal pressure-dependent manner (37). Later studies showed that other mechanical stimuli (e.g., brush stroking) applied to the mucosa also result in 5-HT release (19, 128), as do chemical stimuli that selectively activate EC cells (18, 172). 5-HT release from the mucosa has been shown to activate the ascending and descending circuits that comprise the peristaltic reflex and cause contraction and relaxation of smooth muscle, respectively. One way this may occur is via 5-HT released by EC cells, which act on IPANs, initiating neuronal reflexes necessary for contraction and relaxation (Figure 3) (23, 106, 123). The importance of mucosal 5-HT in peristalsis has been debated (201, 205), as neuronal 5-HT is also critical for normal gut motility patterns (140); however, mucosal 5-HT appears to have distinctive roles that are delineated below.
Figure 3. Mucosa and neuronal 5-HT in peristaltic reflexes and intestinal motility.
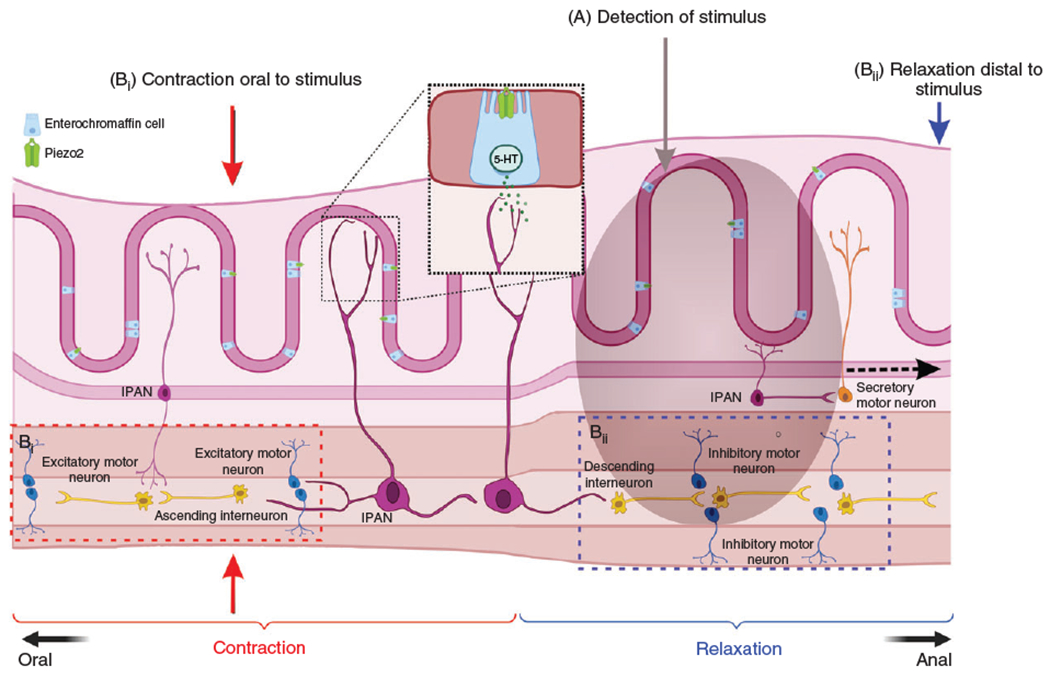
Mucosal 5-HT is released from enterochromaffin (EC) cells upon chemical or mechanical stimulation of the mucosa. A subpopulation of EC cells expresses the mechanoreceptor, Piezo2, that responds to distention/mechanical force causing 5-HT release (Inset). Mucosal 5-HT release causes downstream neuronal activation where intrinsic primary afferent neurons (IPAN) signal from the mucosa to ascending and descending neural pathways in the ENS to initiate contraction and relaxation respectively. Neuronal 5-HT present in a small population of enteric neurons (2% to 3%) project extensively and innervate different functional classes of neurons that drive intestinal motility. Neuronal 5-HT can mediate slow and fast excitatory synaptic transmission as well as play critical roles in the descending inhibitory pathways which culminates to intestinal motility. (A) A bolus of food in the gut lumen stimulates both ascending (Bi and descending (Bii) reflex pathways that work cohesively to propel the food through the gut. Ascending interneurons activate excitatory motor neurons upstream of the stimulus to initiate smooth muscle contraction (Bi, red dotted box) while descending interneurons activate inhibitory motor neurons downstream of the stimulus to initiate smooth muscle relaxation (Bii, blue dotted box). Made in © BioRender - biorender.com.
Roles of neuronal versus mucosal 5-HT in motility
Recent studies using a combination of genetic and pharmacological techniques have sought to define how both neuronal and mucosal 5-HT contribute to gut motility. Experiments have revealed that neuronal 5-HT is key for normal motility; in TPH2 KO mice, in vivo GI transit is significantly slower in terms of total transit time, colonic transit, and small intestinal transit (120, 140). This slower transit is also observed in mice lacking both TPH1 and TPH2 (TPH1/2 double KO), and in TPH2-R439H mice, which are deficient in neuronal 5-HT (85, 94). As previously mentioned, mice lacking TPH2 have multiple defects in ENS and mucosal development; therefore, it is difficult to decipher whether the slowed motility demonstrated in these animals is due to a lack of neuronal 5-HT or to an underdeveloped ENS. TPH2 is also present in neurons of the CNS; therefore, it is possible that lack of 5-HT in the CNS alters extrinsic pathways that could contribute to these defects in motility. Studies have shown that 5-HT contributes to slow and fast excitatory synaptic transmission and the resulting propagating contractions, suggesting that 5-HT in the ENS is a critical mediator of motility (66, 169, 213, 227). Although only a small proportion of neurons release 5-HT (2%–3%), these neurons have vast projections throughout the ENS, making connections with other 5-HT neurons, as well as other key mediators of motility including inhibitory nitric oxide synthase (NOS)-containing neurons, and ICCs (176). It is thus possible that the slowed motility observed in TPH2 deficient mice results from both developmental defects and/or the ongoing lack of neuronal 5-HT signaling. 5-HT neurons, which reside only in the myenteric plexus of the ENS, are thought of as interneurons that receive sensory input and signal to motor neurons to facilitate motility, but the close associations of 5-HT neuronal fibers with ICCs and blood vessels suggest that they have a motor function in signaling to these cell types (176). 5-HT neurons in the ENS also project to the submucosal layer, where they have a role in coupling motility with secretion (202). Recent RNA-seq data have classified 5-HT neurons with multiple morphological types, suggesting additional roles that require more study (164). A recently published study demonstrated that a pharmacological increase of enteric neuronal 5-HT availability does not affect CMMCs, suggesting that more research is also needed in this area to fully elucidate the roles of neuronal 5-HT in normal motility (154).
In contrast to TPH2-deficient mice, mice lacking mucosal 5-HT do not display defects in in vivo GI transit time, as measured in both TPH1 KO mice (140) and mice that were administered nonabsorbable TPH inhibitor drugs that restrict peripheral 5-HT production (24, 153). Further, with the mucosa removed from ex vivo colon preparations, peristaltic contractions could still be initiated with adequate stretching of the tissue, demonstrating that depletion of mucosal 5-HT does not eradicate CMMCs (126, 204). When peristaltic contractions were measured ex vivo, however, it was demonstrated that TPH1 KO mice displayed irregular CMMCs that often propagated in the retrograde direction (9, 107, 203), suggesting that mucosal 5-HT has a more subtle, yet important, role in regulating peristalsis. Taken together with previous data showing that EC cell-derived 5-HT can initiate contractions (38), this research suggests that mucosal 5-HT is not necessary for initiating propulsive contractions but is important for ensuring that these contractions are fully effective. A recent study in which EC cells were selectively ablated in adult mice showed that loss of EC cells resulted in delayed gastric emptying, total GI transit, and colonic transit, as well as reduced CMMC frequency (232), suggesting that EC cells are important for normal motility postdevelopment. Finally, studies have also shown that applying 5-HT receptor antagonists can block peristaltic contractions even in colons devoid of a mucosal layer, raising questions about the importance of endogenous signaling between EC cells and surrounding ENS neurons in motility (200).
Studies are now beginning to focus on how EC cells sense contents passing through the gut and, in turn, release 5-HT. It was recently discovered that subpopulations of EC cells express Piezo2 (2, 230), a mechanoreceptor that is also expressed in peripheral sensory neurons as well as specialized cells of the skin epidermis (53, 187, 234). Piezo2 senses mechanical stimulation, endowing gut epithelial cells with the ability to respond to distension and small mechanical forces imposed by luminal contents. EC cells release 5-HT downstream of Piezo2 activation (2), likely communicating with surrounding ENS neurons responsible for initiating peristaltic contractions. A more recent study used single-cell RNAseq to show that the vast majority of Piezo2-expressing cells in the gut epithelium also express TPH1, highlighting the significance of 5-HT in gut mechanosensation (145). Mice lacking mucosal Piezo2 displayed irregular small intestinal and colonic transit depending on the size of the intraluminal contents (220), suggesting that Piezo2 is required for modulating motility patterns in response to smaller intraluminal stimuli. Piezo2-expressing EC cells therefore contribute to the “fine-tuning” necessary for effective motility.
EC cells express synaptic proteins (18, 220), indicating that they may communicate rapidly with intrinsic and extrinsic neurons rather than via slower paracrine mechanisms; however, a recent anatomical study in which spinal ExPAN fibers were anterogradely labeled suggested that these fibers terminate at a distance from EC cells that would not enable synaptic communication (60). Optogenetic studies have shown that specific activation of all colonic epithelial cells, including EC cells, initiates ExPAN firing and local colonic contractions, which could be blocked by a combination of 5-HT3 and 5-HT4 receptor antagonists (146, 167). The latency between epithelial stimulation and neuronal activation varied greatly in these studies, indicating that communication between colonic epithelial cells and surrounding neurons occurred via fast and slow paracrine mechanisms. Future studies should investigate whether the pattern and numbers of EC cells innervated by extrinsic and intrinsic neurons differ depending on region of the gut.
Role of SERT in motility
Several transgenic mouse models and pharmacological interventions have shown the importance of SERT in gut motility. Mice in which SERT is genetically ablated (SERT KO mice) display abnormalities in GI transit, varying from slowed transit to faster transit, indicative of alternating constipation and diarrhea-like phenotypes, respectively (45). This variation may be due to desensitization of 5-HT receptors involved in producing motility. When averaged, SERT KO mice do not display changes in total GI transit time compared to wild-type littermates but do show slowed in vivo colonic motility as well as ex vivo peristaltic abnormalities (reduced CMMC length) (151). Mice exposed to the SSRI fluoxetine during development had slower intestinal transit compared to control-treated mice, including longer total GI transit and colonic transit times (151). The SERT KO and SSRI treatments have similar effects in inhibiting 5-HT inactivation and thus increasing 5-HT availability; however, SERT KO mice have a congenital deletion of SERT lasting into adulthood and the SSRI-treated mice were only exposed to fluoxetine during development (in utero and up to postnatal day 21) and not in adulthood when motility was assessed, which could explain the somewhat diverse results observed in these studies. The motility abnormalities in both SERT KO mice and fluoxetine-exposed mice were reversed by chemical sympathectomy with systemic 6-hydroxydopamine administration (151), suggesting that deletion or inhibition of SERT can increase central sympathetic input to the intestine, likely contributing to the slowing of motility (1). In a recent study of adult mouse colonic motility, ex vivo CMMC frequency was decreased after bath application of the SSRI fluoxetine, an effect that was abolished when the mucosa was removed, suggesting the importance of mucosal 5-HT in regulating peristaltic contractions (154).
Mice harboring the SERT Ala56 mutation, in which SERT is hyperfunctional and less 5-HT is available, display slower in vivo GI transit as well as slower ex vivo colonic motility, compared to wild-type littermates (151). As mentioned above, these mice develop a hypo-ganglionated ENS, which could partially explain these deficiencies in motility (151). The impact of SERT on gastrointestinal motility has relevance in several clinical areas. For example, multiple SERT mutations have been identified in autism spectrum disorder (ASD) (182, 183, 208), with SERT Ala56 being the most common (224). GI motility problems, especially constipation, are approximately four-fold more common in children with ASD (160). In addition to slowed motility, SERT Ala56 mice also display communication deficits and repetitive behaviors reminiscent of ASD (130, 208, 223, 224), suggesting that the SERT Ala56 mutation may result in developmental perturbations of 5-HT signaling relevant to multiple features of ASD. SERT Ala56 mice also have hyperserotonemia (224), which results from high levels of 5-HT in the blood and is common in individuals with ASD (50, 88, 166, 192). Another area of clinical relevance is exposure to SSRIs in utero. Several studies have supported the hypothesis that gestational exposure to anti-depressants, including SSRIs, impacts ENS development and GI function in humans; retrospective and prospective studies have shown that this exposure increases the likelihood of GI disturbances in childhood (170, 171, 194).
Therapeutic Targets for 5-HT in GI Function
TPH1 and SERT
DGBIs such as irritable bowel syndrome (IBS) are characterized by recurring abdominal pain or discomfort and altered stool patterns (144). No consistent biomarkers have been identified for IBS and thus it is diagnosed based on symptoms and according to stool patterns, as designated by the Rome IV criteria, as constipation-predominant (IBS-C), diarrhea-predominant (IBS-D), or mixed phenotype (IBS-M) (55).
Altered serotonergic signaling may contribute to the disordered motility and/or pain associated with IBS. Several studies that have examined levels of 5-HT or its modulatory factors (SERT, TPH1, EC cells) in IBS patients have yielded inconsistent results (Table 2). One study demonstrated that SERT expression is decreased in colon biopsies from both IBS-D and IBS-C patients (48); however, EC cell density and tissue agitation-evoked 5-HT release did not differ from healthy controls. Subsequent studies have confirmed the finding that SERT mRNA levels in both the colon and small intestine are lower in IBS patients (72, 77, 129), although one study found that there were no differences (41). In patients with IBS-D, mucosal 5-HT content and TPH1 mRNA levels were also found to be decreased (48). Another study confirmed that colon samples from IBS-D and IBS-C patients did not have differences in EC cell density compared to healthy controls; however, postinfectious IBS patient samples showed significantly higher EC cell density, indicating that gut 5-HT may play a more prominent role in this subtype of IBS (136). In another analysis of IBS patient samples of all phenotypes, there were no differences in EC cell count, SERT mRNA levels, or TPH1 mRNA levels when comparing patients with and without hypersensitivity, demonstrating that mucosal 5-HT content may not directly correlate with the painful symptoms of IBS (129).
Table 2.
Alterations of 5-HT in Gastrointestinal Disorders
| GI disorder | EC cell density | TPH1 expression | SERT expression | Postprandial 5-HT levels | References |
|---|---|---|---|---|---|
| IBS-D | No difference | Decrease | Decrease | (48) | |
| Decrease | (72, 77, 129) | ||||
| No difference | (41) | ||||
| Increase | (7, 64, 116) | ||||
| IBS-C | No difference | Decrease | (48) | ||
| Decrease | (72, 77, 129) | ||||
| No difference | (72) | ||||
| Decrease | (64) | ||||
| No difference | (7) | ||||
| Postinfectious IBS | Increase | (136) | |||
| Increase | (64) | ||||
| Ulcerative colitis | Increase | Increase | Decrease | (16, 48, 65) | |
| Crohn’s disease | Increase | Increase | Decrease | (16, 48, 65) |
Mucosal SERT impacts circulating 5-HT levels; therefore, blood 5-HT concentration can also indicate gut mucosal 5-HT availability. Postprandial 5-HT levels in platelet-poor plasma samples are reported to be increased in patients with IBS-D (7, 64, 116) or postinfectious IBS (64), but decreased (64) or unchanged (7) in IBS-C patients. These studies may be challenging to interpret, however, because the vast majority of 5-HT in the blood is localized to the platelets (161). Studies have also shown that fasting blood 5-HT levels are reduced in IBS-D patients (79) but drastically increased in IBS-C patients compared to healthy controls (7). A more recent study of patients of all IBS phenotypes demonstrated that fasting plasma 5-HT concentrations did not differ between healthy controls and IBS patients; however, levels of the metabolite 5-hydroxy indoleacetic acid (5-HIAA) were significantly decreased in IBS patients (217). Further studies are necessary to understand how 5-HT release in the GI tract is altered in IBS and whether disordered motility is a cause or the consequence of altered 5-HT signaling.
As described above, 5-HT released from EC cells initiates sensory and motor signaling in the gut; therefore, inhibiting 5-HT release may attenuate symptoms of IBS-D. Drugs have been developed to inhibit TPH synthesis in a peripherally restricted manner, so that 5-HT release is prevented at the level of the intestinal epithelium (i.e., from EC cells). Compounds such as LX-1031 have been shown to lower 5-HT levels in the mouse GI tract without affecting brain and enteric neuronal 5-HT levels (143, 153). In human trials, LX-1031 improved stool consistency as well as pain and discomfort in IBS-D and IBS-M patients who displayed a dose-dependent decrease in urine 5-HIAA levels (32, 40).
SSRI drugs, such as fluoxetine, have commonly been used in the treatment of IBS, but the mechanism by which they improve symptoms is unclear, as they inhibit SERT in both the brain and gut (214). SSRIs act centrally and alleviate anxiety and depression, which are often coincident with IBS and may exacerbate IBS symptoms (62, 109). SSRIs may also increase 5-HT availability in the gut and therefore affect sensorimotor function, potentially slowing gut transit time in IBS-D patients (99). Human trials show conflicting results about their efficacy in alleviating abdominal pain (39, 156), with one study showing that a limited course of SSRIs did not affect rectal sensitivity (135). Further studies are required to establish the efficacy of SSRIs and determine the differential effects of inhibiting SERT in the gut versus the CNS.
5-HT1 receptors
The 5-HT1 receptor plays a role in gastric motility; in humans, the 5-HT1 agonist, sumatriptan delays gastric emptying and causes relaxation of the gastric fundus, enabling accommodation of larger volumes of food (54, 117, 210). In rodent studies, peripheral administration of the 5-HT1A agonist buspirone had an inhibitory effect on fundic tone (237). Although 5-HT1 receptor agonist drugs showed promise for treating the impaired fundic tone and the early satiety symptoms associated with functional dyspepsia, their use in dyspeptic patients has been accompanied by undesired off-target effects such as enhanced visceral sensitivity and constriction of coronary arteries (57). In contrast, the 5-HT1P receptor exerts excitatory effects; its activation induces neurotransmitter release from IPANs, which initiate peristaltic reflexes in the gut (31, 103, 231). 5-HT1P expression on IPANs also mediates chloride secretion in the gut (51). A recent study demonstrated functional 5-HT1P receptors in human small and large intestines; application of a 5-HT1P agonist resulted in long-lasting activation of human submucosal ENS neurons (4).
5-HT2 receptors
Recent studies have shown that 5-HT2B receptors on ICCs play an important role in colonic motility (122). Diabetic mice with a constipation-like phenotype displayed significant decreases in ICC 5-HT2B receptor expression compared to nondiabetic mice, providing a possible explanation for slowed colonic motility in these mice (122). Conversely, ICC activity was stimulated by the 5-HT2B receptor agonist BW723C86, and treatment with this drug increased colonic transit speed in vivo, correcting the slowed motility in diabetic mice (122). 5-HT2B receptor antagonists can also modulate colonic motility; in studies performed in mice, high doses of the 5-HT2B receptor antagonist RS-127445 reduced the frequency and amplitude of colonic contractions and reduced fecal output (12). 5-HT2B receptors are also expressed on ExPANs and are involved in mediating pain (112). Visceral pain is a common symptom of IBS and studies performed in rats have shown that 5-HT2B receptor antagonists can diminish stress-induced visceral hypersensitivity, as measured by visceromotor responses to colorectal distension (178).
5-HT3 receptors
5-HT3 receptor antagonists, such as alosetron, have shown efficacy in alleviating both diarrhea and abdominal pain associated with IBS-D (3, 186). Such drugs slow motility by blocking 5-HT3 receptors on IPANs that are stimulated by EC cell-released 5-HT and also by inhibiting the 5-HT3 receptors on interneurons and motor neurons that contribute to fast EPSPs (84). These actions result in decreased propulsive motility and local secretion, which alleviates diarrhea. The 5-HT3 receptor antagonists likely inhibit pain by blocking 5-HT3 receptors on extrinsic primary afferent nerves that convey pain and discomfort (10, 124, 147). Rodent studies have shown that alosetron inhibits nociceptive responses to colorectal distension and decreases activation of dorsal horn neurons in the spinal cord that are involved in pain signaling (133). Alosetron has been found to be more effective in female IBS-D patients than in male patients; clinical studies show that alosetron alleviated pain and discomfort and improved stool consistency in females yet did not improve symptoms in male patients (11, 43). Alosetron was removed from the market in 2000 due to GI side effects such as ischemic colitis but later reintroduced for treating only severe cases of IBS-D in female patients (73). The 5-HT3 receptor also has a role in mediating nausea and vomiting. Stimulation of 5-HT3 receptors present on the vagus nerve can lead to nausea and thus specific 5-HT3 receptor antagonists, such as granisetron, have been used to treat nausea and emesis induced by chemotherapy and radiation therapy (168, 222).
To increase propulsive motility and secretion in the gut to alleviate constipation, 5-HT3 receptor agonists, such as pumosetrag, have been developed (69). Efforts have been focused on partial agonists because 5-HT3 receptor activation on extrinsic primary afferent fibers and vagal fibers can lead to pain and nausea, respectively. Results from clinical trials showed that pumosetrag was effective in relieving constipation and also reducing reflux events in individuals with gastroesophageal reflux disease (GERD) (47, 69).
5-HT4 receptors
5-HT4 receptor agonist drugs promote motility in the ENS by evoking peristaltic reflexes by acting on presynaptic nerve terminals to stimulate the release of neurotransmitters that mediate peristaltic contractions (71, 86, 141, 179, 218). This indirect action is thought to enhance naturally occurring reflexes rather than directly generate neurotransmission, which can ultimately inhibit propulsive motility (113). Transgenic mice that lack 5-HT4 receptors display slowed colonic motility (141), suggesting that these receptors are critical in propulsive motility; however, these defects may also result from altered ENS development, as 5-HT4 receptors have important neurotrophic roles as well.
The 5-HT4 receptor has been exploited for treating IBS; 5-HT4 receptor agonists have proven to be effective in relieving pain and constipation associated with IBS-C and can also accelerate the rate of gastric emptying (70, 159). These agonists activate 5-HT4 receptors on presynaptic nerve terminals, evoking acetylcholine release and enhancing propulsive contractions (71, 86, 141, 179, 218). They likely also promote gut motility and alleviate constipation by acting on 5-HT4 receptors on the intestinal epithelium to cause increased mucus discharge and chloride secretion (114). 5-HT4 receptors are also present on ExPANs, but it is not clear how 5-HT4 agonists alleviate visceral pain. Studies show that these agonists have an inhibitory effect on mechanosensory signaling, as they reduce firing of ExPANs in response to distension (198) and reduce visceral hypersensitivity (114).
The earlier developed 5-HT4 receptor agonists, such as cisapride and tegaserod, were shown to be effective in treating constipation but were accompanied by cardiovascular side effects due to effects on other targets such as hERG potassium channels (56, 209). More targeted agonists, such as prucalopride, have since been developed for treatment of IBS-C (42, 185, 211), which have a favorable safety profile and show efficacy in both male and female patients with constipation (177). Studies have shown that 5-HT4 receptor agonists have maximal effects when applied to the mucosal, rather than serosal, surface of the colon in ex vivo motility assays (78, 102, 114, 123); thus, studies are now aimed at developing nonabsorbable agonists that would only act on cells of the mucosal layer, which may be a better-targeted treatment.
5-HT and inflammation
The intestine provides an important interface between the body and the external environment and, as such, contains major components of the body’s immune system. The gut is equipped to simultaneously defend against numerous potentially pathogenic organisms and to also maintain a commensal microbiome (101, 127, 195). Although the gut microbiome is an important contributor to functions such as GI motility (191) and nervous system development (175), the immune system must constantly defend against microbial invasion (150). Interactions between neurons and immune cells in the gut are important in regulating these immune responses. 5-HT is an important paracrine messenger that participates in crosstalk between enteric neurons and immune cells and has a significant role in intestinal inflammation (34, 225). Immune cells express the 5-HT receptors 2, 3, 4, and 7 (199) and, via these receptors, 5-HT influences immune cell trafficking, chemotaxis, activation, and proliferation (5, 6, 8, 96). Some immune cells secrete 5-HT themselves, namely macrophages and T cells (8) and, in mice and rats, mast cells synthesize and release 5-HT and take up 5-HT via SERT (148).
Animal models of IBD have revealed how mucosal and enteric neuronal 5-HT may differentially modulate inflammatory processes in the gut. The most studied rodent IBD models include colitis induced by trinitrobenzene sulfonic acid (TNBS), which mimics pathological features of Crohn’s disease, and dextran sulfate sodium (DSS), which more closely resembles ulcerative colitis. Infections caused by Citrobacter rodentium, Trichinella spiralis, and Escherichia coli have also been studied (20, 34, 173, 174, 233). All these inflammation-based models result in increased EC cell density and decreased levels of epithelial SERT in the intestine. Studies have demonstrated that mucosal 5-HT is proinflammatory; in TPH1 KO mice and mice administered a peripherally restricted TPH inhibitor, colitis induced by TNBS or DSS is significantly diminished (97, 153, 199). Downregulation of SERT could be either a cause or a consequence of gut inflammation. In cultures of Caco-2 human epithelial cells, addition of the proinflammatory cytokines interferon-gamma and tumor necrosis factor reduced levels of SERT (14). In SERT KO mice, TNBS-induced colitis is exacerbated, suggesting that increased 5-HT availability in the gut contributes to inflammation (26).
In contrast to mucosal 5-HT, enteric neuronal 5-HT appears to have anti-inflammatory actions in the gut. This is best demonstrated in mice lacking neuronal 5-HT (TPH2 KO), which, after DSS-induced colitis, had increased mortality, greater inflammation severity, and higher pro-inflammatory cytokine levels in the colon, compared to wild-type littermates (91). The function of neuronal 5-HT may therefore be to counteract inflammation-induced damage to ENS neurons; however, this area of study should be investigated using more targeted techniques.
Studies show that 5-HT may modulate gut inflammation via the 5-HT4 receptor. In animal models of colitis, activation of 5-HT4 receptors on the colonic epithelium reduces the development of colitis and accelerates recovery (207) Colitis also promotes 5-HT4 receptor-mediated neurogenesis that drives glial cells to transdifferentiate into neurons (17). Consistent with these findings, ENS hyperplasia is found in inflamed bowel segments of IBD patients and, in animal models, ENS hyperplasia increases predisposition to both TNBS and DSS-induced colitis (152). Although hyperplasia appears to increase susceptibility to inflammation, neurogenesis may also be protective, as 5-HT4 receptors promote neuroprotection against oxidative stress associated with inflammation (17, 100, 141). To develop future therapies for IBD, it is critical to further investigate how 5-HT4 receptor-mediated neurogenesis impacts inflammatory states.
Several aspects of 5-HT signaling have been noted to be altered in patients with IBD (Table 2). The density of EC cells is increased in intestinal biopsies taken from individuals with either Crohn’s disease (CD) or ulcerative colitis (UC) (16, 48, 65). EC cells isolated from such biopsies secrete significantly more of the proinflammatory cytokine interleukin 1p, and release more 5-HT in response to treatment with lipopolysaccharide, compared to healthy controls (131). Ileum and colon biopsies from patients with either UC or CD also displayed higher TPH1 mRNA levels and significantly lower SERT mRNA levels compared to healthy controls (125). Patients with celiac disease and postinfectious IBS also display increases in EC cell number, as well as increases in postprandial 5-HT levels (49, 90, 206). All these conditions are also associated with a decrease in epithelial SERT expression levels (48), further supporting the idea that increased mucosal 5-HT availability may contribute to intestinal inflammation.
Conclusion
Research over the last 60 years has revealed the important roles of 5-HT in GI function. Early studies demonstrated that addition of 5-HT to the intestine evokes motility patterns and the mechanisms underlying this response have proven to be increasingly complex. EC cells of the GI epithelium and some ENS neurons release 5-HT, and multiple cell types that reside in the gut modulate 5-HT signaling via SERT and the expression of 5-HT receptors. This diversity of cell types and receptor functions has made it difficult to determine precisely how 5-HT mediates GI motility while simultaneously contributing to an array of other functions such as sensory signaling, epithelial homeostasis, inflammation, and neurogenesis. Studies should further examine the distribution of 5-HT receptors throughout the gut and their physiological relevance to these functions. This knowledge could reveal cell type-specific treatments for DGBIs; for example, 5-HT3 receptor activation stimulates peristalsis and also mediates pain signaling, and it is not yet known whether treatments can target one function but not the other. Further, although studies have revealed potential functions of each 5-HT receptor, much of this knowledge has not been translated into effective therapies. For example, activation of the 5-HT4 receptor ameliorates inflammation in mouse models of colitis, but there is not yet an effective 5-HT4 receptor agonist drug for IBD. Similarly, 5-HT is known to be a potent growth factor in the ENS, but it is unclear how its signaling may be targeted to ENS regeneration in aging and neurodegenerative disorders. The challenge of future research will be to better understand the different components of the serotonergic system of the GI tract, how they work together, and how they contribute to pathophysiology underlying GI diseases.
Didactic Synopsis.
Major teaching points
The gut contains the vast majority of the body’s 5-HT, synthesized mostly by enterochromaffin (EC) cells of the mucosa and, in small part, by neurons of the enteric nervous system (ENS).
5-HT availability in the gut is modulated by the serotonin reuptake transporter (SERT), which is expressed throughout the gut epithelium, in ENS neurons, and in circulating platelets.
Enteric neuronal 5-HT acts as a growth factor in development of the ENS and proliferation of the intestinal mucosa.
Both mucosal and neuronal 5-HT are involved in gastrointestinal (GI) motility; mucosal 5-HT is not necessary for initiating peristaltic contractions but is important for ensuring these contractions are fully effective.
The 5-HT receptors present throughout the GI tract that have roles in motility are 5-HT1, 5-HT2, 5-HT3, 5-HT4, and 5-HT7, which are G-protein-coupled receptors except for the 5-HT3 receptor, which is ionotropic.
5-HT signaling appears to be altered in disorders of gut-brain interaction (DGBIs), such as irritable bowel syndrome (IBS), and has been targeted for treatment of abdominal pain and disordered gut motility, the two main symptoms of DGBIs.
Mucosal 5-HT promotes inflammation in the gut and is upregulated in inflammatory bowel diseases.
Acknowledgements
The authors are supported by National Institutes of Health grants R01 DK126644 (KGM), R01 DK130517 (KGM), R01 DK130518 (KGM), F32 DK132810 (SAN), and Department of Defense grant PR160365 (KGM).
References
- 1.Adamec R, Burton P, Blundell J, Murphy DL, Holmes A. Vulnerability to mild predator stress in serotonin transporter knockout mice. Behav Brain Res 170: 126–140, 2006. DOI: 10.1016/j.bbr.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 2.Alcaino C, Knutson KR, Treichel AJ, Yildiz G, Strege PR, Linden DR, Li JH, Leiter AB, Szurszewski JH, Farrugia G, Beyder A. A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proc Natl Acad Sci USA 115: E7632–E7641, 2018. DOI: 10.1073/pnas.1804938115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andresen V, Montori VM, Keller J, West CP, Layer P, Camilleri M. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: A systematic review and meta-analysis of randomized controlled trials. Clin Gastroenterol Hepatol 6: 545–555, 2008. DOI: 10.1016/j.cgh.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Annaházi A, Berger TE, Demir IE, Zeller F, Müller M, Anneser M, Skerra A, Michel K, Schemann M. Metabotropic 5-HT receptor-mediated effects in the human submucous plexus. Neurogastroenterol Motil 34: e14380, 2022. DOI: 10.1111/nmo.14380. [DOI] [PubMed] [Google Scholar]
- 5.Arreola R, Becerril-Villanueva E, Cruz-Fuentes C, Velasco-Velázquez MA, Garcés-Alvarez ME, Hurtado-Alvarado G, Quintero-Fabian S, Pavón L. Immunomodulatory effects mediated by serotonin. J Immunol Res 2015: 354957, 2015. DOI: 10.1155/2015/354957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Askenase PW, Herzog WR, Millet I, Paliwal V, Ramabhadran R, Rochester C, Geba GP, Ptak W. Serotonin initiation of delayed-type hypersensitivity: Mediation by a primitive Thy-1+ antigen-specific clone or by specific monoclonal IgE antibody. Skin Pharmacol 4 (Suppl 1): 25–42, 1991. DOI: 10.1159/000210981. [DOI] [PubMed] [Google Scholar]
- 7.Atkinson W, Lockhart S, Whorwell PJ, Keevil B, Houghton LA. Altered 5-hydroxytryptamine signaling in patients with constipationand diarrhea-predominant irritable bowel syndrome. Gastroenterology 130: 34–43, 2006. DOI: 10.1053/j.gastro.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 8.Baganz NL, Blakely RD. A dialogue between the immune system and brain, spoken in the language of serotonin. ACS Chem Neurosci 4:48–63, 2013. DOI: 10.1021/cn300186b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balasuriya GK, Hill-Yardin EL, Gershon MD, Bornstein JC. Asexually dimorphic effect of cholera toxin: Rapid changes in colonic motility mediated via a 5-HT3 receptor-dependent pathway in female C57Bl/6 mice. J Physiol 594:4325–4338, 2016. DOI: 10.1113/JP272071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, di Nardo G, Trevisani M, Campi B, Geppetti P, Tonini M, Bunnett NW, Grundy D, Corinaldesi R. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology 132: 26–37, 2007. DOI: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 11.Bardhan KD, Bodemar G, Geldof H, Schütz E, Heath A, Mills JG, Jacques LA. A double-blind, randomized, placebo-controlled dose-ranging study to evaluate the efficacy of alosetron in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther 14 (1): 23–34, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Bassil A, Taylor C, Bolton V, Gray K, Brown J, Cutler L, Summerfield S, Bruton G, Winchester W, Lee K, Sanger G. Inhibition of colonic motility and defecation by RS-127445 suggests an involvement of the 5-HT2B receptor in rodent large bowel physiology. Br J Pharmacol 158: 252, 2009. DOI: 10.1111/J.1476-5381.2009.00155.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayliss WM, Starling EH. The movements and innervation of the small intestine. J Physiol 24: 99, 1899. DOI: 10.1113/JPHYSIOL.1899.SP000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayliss WM, Starling EH. The movements and the innervation of the large intestine. J Physiol 26: 107, 1900. DOI: 10.1113/JPHYSIOL.1900.SP000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beikmann BS, Tomlinson ID, Rosenthal SJ, Andrews AM. Serotonin uptake is largely mediated by platelets versus lymphocytes in peripheral blood cells. ACS Chem Neurosci 4: 161–170, 2013. DOI: 10.1021/CN300146W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belai A, Boulos PB, Robson T, Burnstock G. Neurochemical coding in the small intestine of patients with Crohn’s disease. Gut 40: 767–774, 1997. DOI: 10.1136/gut.40.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belkind-Gerson J, Hotta R, Nagy N, Thomas AR, Graham H, Cheng L, Solorzano J, Nguyen D, Kamionek M, Dietrich J, Cherayil BJ, Goldstein AM. Colitis induces enteric neurogenesis through a 5-HT4-dependent mechanism. Inflamm Bowel Dis 21: 870–878, 2015. DOI: 10.1097/MIB.0000000000000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellono NW, Bayrer JR, Leitch DB, Castro J, Zhang C, O’Donnell TA, Brierley SM, Ingraham HA, Julius D. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 170: 185–198.e16, 2017. DOI: 10.1016/J.CELL.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertrand PP. Real-time detection of serotonin release from enterochromaffin cells of the guinea-pig ileum. Neurogastroenterol Motil 16: 511–514, 2004. DOI: 10.1111/J.1365-2982.2004.00572.X. [DOI] [PubMed] [Google Scholar]
- 20.Bertrand PP, Barajas-Espinosa A, Neshat S, Bertrand RL, Lomax AE. Analysis of real-time serotonin (5-HT) availability during experimental colitis in mouse. Am J Physiol Gastrointest Liver Physiol 298:G446–G455, 2010. DOI: 10.1152/ajpgi.00318.2009. [DOI] [PubMed] [Google Scholar]
- 21.Bertrand PP, Kunze WA, Furness JB, Bornstein JC. The terminals of myenteric intrinsic primary afferent neurons of the guinea-pig ileum are excited by 5-hydroxytryptamine acting at 5-hydroxytryptamine-3 receptors. Neuroscience 101: 459–469, 2000. DOI: 10.1016/s0306-4522(00)00363-8. [DOI] [PubMed] [Google Scholar]
- 22.Bhattarai Y, Schmidt BA, Linden DR, Larson ED, Grover M, Beyder A, Farrugia G, Kashyap PC. Human-derived gut microbiota modulates colonic secretion in mice by regulating 5-HT3 receptor expression via acetate production. Am J Physiol Gastrointest Liver Physiol 313: G80–G87, 2017. DOI: 10.1152/ajpgi.00448.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bian X-C, Bornstein JC, Bertrand PP. Nicotinic transmission at functionally distinct synapses in descending reflex pathways of the rat colon. Neurogastroenterol Motil 15 (2): 161–171, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Bian Z-X, Qin H-Y, Tian S-L, Qi S-D, Bian Z, Qin H, Tian S. Combined effect of early life stress and acute stress on colonic sensory and motor responses through serotonin pathways: Differences between proximal and distal colon in rats. Stress 14 (4): 448–458, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Bianco F, Bonora E, Natarajan D, Vargiolu M, Thapar N, Torresan F, Giancola F, Boschetti E, Volta U, Bazzoli F, Mazzoni M, Seri M, Clavenzani P, Stanghellini V, Sternini C, de Giorgio R. Prucalopride exerts neuroprotection in human enteric neurons. Am J Physiol Gastrointest Liver Physiol 310: G768–G775, 2016. DOI: 10.1152/AJPGI.00036.2016/ASSET/IMAGES/LARGE/ZH30081670760004.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bischoff SC, Mailer R, Pabst O, Weier G, Sedlik W, Li Z, Chen JJ, Murphy DL, Gershon MD. Role of serotonin in intestinal inflammation: Knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am J Physiol Gastrointest Liver Physiol 296: G685–G695, 2009. DOI: 10.1152/ajpgi.90685.2008. [DOI] [PubMed] [Google Scholar]
- 27.Bixby S, Kruger GM, Mosher JT, Joseph NM, Morrison SJ. Cell-intrinsic differences between stem cells from different regions of the peripheral nervous system regulate the generation of neural diversity. Neuron 35: 643–656, 2002. DOI: 10.1016/s0896-6273(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 28.Blaschko H, Burkard WP, Carlsson A, Cohen LH, Erspamer V, Gey KF, Gyermek L, Hagen PB, Hanson A, Levine WG, Mantegazzini P, Pletscher A, Stacey RS, Vialli M. 5-Hydroxytryptamine and related Indolealkylamines. In: Erspamer V, editor. Handbook of Experimental Pharmacology. New York: Springer-Verlag, 1966. [Google Scholar]
- 29.Bondurand N, Natarajan D, Thapar N, Atkins C, Pachnis V. Neuron and glia generating progenitors of the mammalian enteric nervous system isolated from foetal and postnatal gut cultures. Development 130: 6387–6400, 2003. DOI: 10.1242/dev.00857. [DOI] [PubMed] [Google Scholar]
- 30.Borman RA, Tilford NS, Harmer DW, Day N, Ellis ES, Sheldrick RLG, Carey J, Coleman RA, Baxter GS. 5-HT(2B) receptors play a key role in mediating the excitatory e€ects of 5-HT in human colon in vitro. Br J Pharmacol 135 (5): 1144–1151, 2002. www.nature.com/bjp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Branchek TA, Mawe GM, Gershon MD. Characterization and localization of a peripheral neural 5-hydroxytryptamine receptor subtype (5-HT1P) with a selective agonist, 3H-5-hydroxyindalpine. J Neurosci 8: 2582–2595, 1988. DOI: 10.1523/JNEUROSCI.08-07-02582.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown PM, Drossman DA, Wood AJJ, Cline GA, Frazier KS, Jackson JI, Bronner J, Freiman J, Zambrowicz B, Sands A, Gershon MD. The tryptophan hydroxylase inhibitor LX1031 shows clinical benefit in patients with nonconstipating irritable bowel syndrome. Gastroenterology 141: 507–516, 2011. DOI: 10.1053/j.gastro.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brummelte S, Mc Glanaghy E, Bonnin A, Oberlander TF. Developmental changes in serotonin signaling: Implications for early brain function, behavior and adaptation. Neuroscience 342: 212–231, 2017. DOI: 10.1016/J.NEUROSCIENCE.2016.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buhner S, Schemann M. Mast cell-nerve axis with a focus on the human gut. Biochim Biophys Acta 1822: 85–92, 2012. DOI: 10.1016/j.bbadis.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Bülbring E, Crema A. Observations concerning the action of 5-hydroxytryptamine on the peristaltic reflex. Br J Pharmacol Chemother 13 (4): 444–457, 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bülbring E, Crema A. The action of 5-hydroxytryptamine, 5-hydroxytryptophan and reserpine on intestinal peristalsis in anaesthetized guinea-pigs. J Physiol 146 (1): 29–53, 1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bülbring E, Crema A. The release of 5-hydroxytryptamine in relation to pressure exerted on the intestinal mucosa. J Physiol 146: 18–28, 1959. DOI: 10.1113/JPHYSIOL.1959.SP006175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bülbring E, Lin RCY. The effect of intraluminal application of 5-hydroxytryptamine and 5-hydroxytryptophan on peristalsis; the local production of 5-ht and its release in relation to intraluminal pressure and propulsive activity. J Physiol 140 (3): 381, 1958. [PMC free article] [PubMed] [Google Scholar]
- 39.Bundeff AW, Woodis CB. Selective serotonin reuptake inhibitors for the treatment of irritable bowel syndrome. Ann Pharmacother 48: 777–784, 2014. DOI: 10.1177/1060028014528151. [DOI] [PubMed] [Google Scholar]
- 40.Camilleri M LX-1031, a tryptophan 5-hydroxylase inhibitor, and its potential in chronic diarrhea associated with increased serotonin. Neurogastroenterol Motil 23 (3): 193–200, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Camilleri M, Andrews CN, Bharucha AE, Carlson PJ, Ferber I, Stephens D, Smyrk TC, Urrutia R, Aerssens J, Thielemans L, Göhlmann H, van den Wyngaert I, Coulie B. Alterations in expression of p11 and SERT in mucosal biopsy specimens of patients with irritable bowel syndrome. Gastroenterology 132: 17–25, 2007. DOI: 10.1053/j.gastro.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Camilleri M, Kerstens R, Rykx A, Vandeplassche L. A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med 358 (22): 2344–2354, 2008. www.nejm.org. [DOI] [PubMed] [Google Scholar]
- 43.Camilleri M, Mayer EA, Drossmanà DA, Heath A, Dukes GE, Mcsorley D, Kong S, Mangel AW, Northcutt AR. Improvement in pain and bowel function in female irritable bowel patients with alosetron, a 5-HT 3 receptor antagonist. Aliment Pharmacol Ther 13 (9): 1149–1159, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Carter D, Champney M, Hwang B, Eglen RM. Characterization of a postjunctional 5-HT receptor mediating relaxation of guinea-pig isolated ileum. Eur J Pharmacol 280: 243–250, 1995. DOI: 10.1016/0014-2999(95)00195-q. [DOI] [PubMed] [Google Scholar]
- 45.Chen JJ, Li Z, Pan H, Murphy DL, Tamir H, Koepsell H, Gershon MD. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. J Neurosci 21: 6348–6361, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen JX, Pan H, Rothman TP, Wade PR, Gershon MD. Guinea pig 5-HT transporter: Cloning, expression, distribution, and function in intestinal sensory reception. Am J Phys 275, 1998. DOI: 10.1152/AJPGI.1998.275.3.G433. [DOI] [PubMed] [Google Scholar]
- 47.Choung RS, Ferguson DD, Murray JA, Kammer PP, Dierkhising RA, Zinsmeister AR, Nurbhaià S, Landauà SB, Talley NJ. A novel partial 5HT3 agonist DDP733 after a standard refluxogenic meal reduces reflux events: A randomized, double-blind, placebo-controlled pharmacodynamic study. Aliment Pharmacol Ther 27 (5): 404–411, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM, Moses PL. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 126: 1657–1664, 2004. DOI: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 49.Coleman NS, Foley S, Dunlop SP, Wheatcroft J, Blackshaw E, Perkins AC, Singh G, Marsden CA, Holmes GK, Spiller RC. Abnormalities of serotonin metabolism and their relation to symptoms in untreated celiac disease. Clin Gastroenterol Hepatol 4: 874–881, 2006. DOI: 10.1016/j.cgh.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 50.Cook EH, Arora RC, Anderson GM, Berry-Kravis EM, Yan S, ya Yeoh HC, Sklena PJ, Charak DA, Leventhal BL. Platelet serotonin studies in hyperserotonemic relatives of children with autistic disorder. Life Sci 52: 2005–2015, 1993. DOI: 10.1016/0024-3205(93)90685-V. [DOI] [PubMed] [Google Scholar]
- 51.Cooke HJ, Sidhu M, Wang YZ. Activation of 5-HT1P receptors on submucosal afferents subsequently triggers VIP neurons and chloride secretion in the guinea-pig colon. J Auton Nerv Syst 66: 105–110, 1997. DOI: 10.1016/s0165-1838(97)00075-1. [DOI] [PubMed] [Google Scholar]
- 52.Costa M, Brookes SJ, Steele PA, Gibbins I, Burcher E, Kandiah CJ. Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience 75: 949–967, 1996. DOI: 10.1016/0306-4522(96)00275-8. [DOI] [PubMed] [Google Scholar]
- 53.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330: 55–60, 2010. DOI: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coulie B, Tack J, Mae B, Geypens B, de Roo M, Janssens J. Sumatriptan, a selective 5-HT1 receptor agonist, induces a lag phase for gastric emptying of liquids in humans. Am J Phys 272 (4 Pt 1): G902–G908, 1997. [DOI] [PubMed] [Google Scholar]
- 55.Crowell MD, Shetzline MA, Moses PL, Mawe GM, Talley NJ. Enterochromaffin cells and 5-HT signaling in the pathophysiology of disorders of gastrointestinal function. Curr Opin Investig Drugs 5: 55–60, 2004. [PubMed] [Google Scholar]
- 56.de Maeyer JH, Lefebvre RA, Schuurkes JAJ. 5-HT 4 receptor agonists: Similar but not the same. Neurogastroenterol Motil 20 (2): 99–112, 2008. [DOI] [PubMed] [Google Scholar]
- 57.de Ponti F, Ponti D. Pharmacology of serotonin: What a clinician should know. Gut 53: 1520–1535, 2004. DOI: 10.1136/gut.2003.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Vadder F, Grasset E, Holm LM, Karsenty G, Macpherson AJ, Olofsson LE, Bäckhed F. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc Natl Acad Sci USA 115: 6458–6463, 2018. DOI: 10.1073/PNAS.1720017115/-/DCSUPPLEMENTAL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dickson EJ, Heredia DJ, Smith TK. Critical role of 5-HT 1A , 5-HT 3 , and 5-HT 7 receptor subtypes in the initiation, generation, and propagation of the murine colonic migrating motor complex. Am J Physiol Gastrointest Liver Physiol 299: 144–157, 2010. DOI: 10.1152/ajpgi.00496.2009.-The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dodds KN, Travis L, Kyloh MA, Jones LA, Keating DJ, Spencer NJ. The gut-brain axis: Spatial relationship between spinal afferent nerves and 5-HT-containing enterochromaffin cells in mucosa of mouse colon. Am J Physiol Gastrointest Liver Physiol 322: G523–G533, 2022. DOI: 10.1152/AJPGI.00019.2022. [DOI] [PubMed] [Google Scholar]
- 61.Drokhlyansky E, Smillie CS, van Wittenberghe N, Ericsson M, Griffin GK, Eraslan G, Dionne D, Cuoco MS, Goder-Reiser MN, Sharova T, Kuksenko O, Aguirre AJ, Boland GM, Graham D, Rozenblatt-Rosen O, Xavier RJ, Regev A. The human and mouse enteric nervous system at single-cell resolution. Cell 182: 1606–1622.e23, 2020. DOI: 10.1016/j.cell.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology 123: 2108–2131, 2002. DOI: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 63.Du P, O’Grady G, Gibbons SJ, Yassi R, Lees-Green R, Farrugia G, Cheng LK, Pullan AJ. Tissue-specific mathematical models of slow wave entrainment in wild-type and 5-HT2B knockout mice with altered interstitial cells of Cajal networks. Biophys J 98: 1772, 2010. DOI: 10.1016/J.BPJ.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dunlop SP, Coleman NS, Blackshaw E, Perkins AC, Singh G, Marsden CA, Spiller RC. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol 3: 349–357, 2005. DOI: 10.1016/s1542-3565(04)00726-8. [DOI] [PubMed] [Google Scholar]
- 65.El-Salhy M, Danielsson A, Stenling R, Grimelius L. Colonic endocrine cells in inflammatory bowel disease. J Intern Med 242: 413–419, 1997. DOI: 10.1046/j.1365-2796.1997.00237.x. [DOI] [PubMed] [Google Scholar]
- 66.Erde SM, Sherman D, Gershon MD. Morphology and serotonergic innervation of physiologically identified cells of the guinea pig’s myenteric plexus. J Neurosci 5: 617–633, 1985. DOI: 10.1523/JNEUROSCI.05-03-00617.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Erspamer V Pharmacology of enteramine. I. Action of acetone extract of rabbit stomach mucosa on blood pressure and on surviving isolated organs. Arch Exp Pathol Pharmakol 196: 343–346, 1940. [Google Scholar]
- 68.Erspamer V, Asero B. Identification of enteramine, the specific hormone of the enterochromaffin cell system, as 5-hydroxytryptamine. Nature 169: 800–801, 1952. DOI: 10.1038/169800b0. [DOI] [PubMed] [Google Scholar]
- 69.Evangelista S Drug evaluation: Pumosetrag for the treatment of irritable bowel syndrome and gastroesophageal reflux disease. Curr Opin Investig Drugs 8: 416–422, 2007. [PubMed] [Google Scholar]
- 70.Evans BW, Clark WK, Moore DJ, Whorwell PJ. Tegaserod for the treatment of irritable bowel syndrome and chronic constipation. Cochrane Database Syst Rev 17: CD003960, 2007. DOI: 10.1002/14651858.CD003960.pub3. [DOI] [PubMed] [Google Scholar]
- 71.Fang X, Liu S, Wang X-Y, Gao N, Hu H-Z, Wang G-D, Cook CH, Needleman BJ, Mikami DJ, Xia Y, Fei G-J, Hicks GA, Wood JD. Neurogastroenterology of tegaserod (HTF 919) in the submucosal division of the guinea-pig and human enteric nervous system. Neurogastroenterol Motil 20: 80–93, 2008. DOI: 10.1111/j.1365-2982.2007.00983.x. [DOI] [PubMed] [Google Scholar]
- 72.Faure C, Patey N, Gauthier C, Brooks EM, Mawe GM. Serotonin signaling is altered in irritable bowel syndrome with diarrhea but not in functional dyspepsia in pediatric age patients. Gastroenterology 139: 249–258, 2010. DOI: 10.1053/j.gastro.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fayyaz M, Lackner JM. Serotonin receptor modulators in the treatment of irritable bowel syndrome. Ther Clin Risk Manag 4 (1): 41–48, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Feldberg W, Toh CC. Distribution of 5-hydroxytryptamine (serotonin, enteramine) in the wall of the digestive tract. J Physiol 119: 352–362, 1953. DOI: 10.1113/JPHYSIOL.1953.SP004850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fiorica-Howells E, Hen R, Gingrich J, Li Z, Gershon MD. 5-HT2A receptors: Location and functional analysis in intestines of wild-type and 5-HT2A knockout mice. Am J Physiol Gastrointest Liver Physiol 282: 877–893, 2002. DOI: 10.1152/AJPGI.00435.2001/ASSET/IMAGES/LARGE/H30520811013.JPEG. [DOI] [PubMed] [Google Scholar]
- 76.Fiorica-Howells E, Maroteaux L, Gershon MD. Serotonin and the 5-HT(2B) receptor in the development of enteric neurons. J Neurosci 20 (1): 294–305, 2000. DOI: 10.1523/JNEUROSCI.20-01-00294.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Foley S, Garsed K, Singh G, Duroudier NP, Swan C, Hall IP, Zaitoun A, Bennett A, Marsden C, Holmes G, Walls A, Spiller RC. Impaired uptake of serotonin by platelets from patients with irritable bowel syndrome correlates with duodenal immune activation. Gastroenterology 140, 2011. DOI: 10.1053/J.GASTRO.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 78.Foxx-Orenstein AE, Jin JG, Grider JR. 5-HT4 receptor agonists and delta-opioid receptor antagonists act synergistically to stimulate colonic propulsion. Am J Phys 275: G979–G983, 1998. DOI: 10.1152/ajpgi.1998.275.5.G979. [DOI] [PubMed] [Google Scholar]
- 79.Franke L, Schmidtmann M, Riedl A, Riedl A, van der Voort I, Uebelhack R, Mönnikes H. Serotonin transporter activity and serotonin concentration in platelets of patients with irritable bowel syndrome: Effect of gender. J Gastroenterol 45: 389–398, 2010. DOI: 10.1007/s00535-009-0167-y. [DOI] [PubMed] [Google Scholar]
- 80.Fujimiya M, Okumiya K, Kuwahara A. Immunoelectron microscopic study of the luminal release of serotonin from rat enterochromaffin cells induced by high intraluminal pressure. Histochem Cell Biol 108: 105–113, 1997. DOI: 10.1007/s004180050151. [DOI] [PubMed] [Google Scholar]
- 81.Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, Eubanks S, Harris M, Pappas TN, Takahashi T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Phys Regul Integr Comp Phys 284: R1269–R1276, 2003. DOI: 10.1152/AJPREGU.00442.2002. [DOI] [PubMed] [Google Scholar]
- 82.Fung TC, Vuong HE, Luna CDG, Pronovost GN, Aleksandrova AA, Riley NG, Vavilina A, Mcginn J, Rendon T, Forrest LR, Hsiao EY. Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat Microbiol 4: 2064–2073, 2019. DOI: 10.1038/s41564-019-0540-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Galligan JJ. Differential inhibition of cholinergic and noncholinergic neurogenic contractions by mu opioid and alpha-2 adrenergic agonists in guinea pig ileum. J Pharmacol Exp Ther 264: 375–383, 1993. [PubMed] [Google Scholar]
- 84.Galligan JJ, LePard KJ, Schneider DA, Zhou X. Multiple mechanisms of fast excitatory synaptic transmission in the enteric nervous system. J Auton Nerv Syst 81: 97–103, 2000. DOI: 10.1016/s0165-1838(00)00130-2. [DOI] [PubMed] [Google Scholar]
- 85.Galligan JJ, North RA. Opioid, 5-HT1A and alpha 2 receptors localized to subsets of guinea-pig myenteric neurons. J Auton Nerv Syst 32: 1–11, 1991. DOI: 10.1016/0165-1838(91)90229-v. [DOI] [PubMed] [Google Scholar]
- 86.Galligan JJ, Pan H, Messori E. Signalling mechanism coupled to 5-hydroxytryptamine4 receptor-mediated facilitation of fast synaptic transmission in the guinea-pig ileum myenteric plexus Neurogastroenterol Motil 15: 523–529, 2003. DOI: 10.1046/j.1365-2982.2003.00428.x. [DOI] [PubMed] [Google Scholar]
- 87.Galligan JJ, Surprenant A, Tonini M, North RA. Differential localization of 5-HT1 receptors on myenteric and submucosal neurons. Am J Phys 255: G603–G611, 1988. DOI: 10.1152/ajpgi.1988.255.5.G603. [DOI] [PubMed] [Google Scholar]
- 88.Geller E, Yuwiler A, Freeman BJ, Ritvo E. Platelet size, number, and serotonin content in blood of autistic, childhood schizophrenic, and normal children. J Autism Dev Disord 18 (1): 119–126, 1988. [DOI] [PubMed] [Google Scholar]
- 89.Gershon MD. Review article: Serotonin receptors and transporters - roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther 20 (Suppl 7): 3–14, 2004. DOI: 10.1111/J.1365-2036.2004.02180.X. [DOI] [PubMed] [Google Scholar]
- 90.Gershon MD. Nerves, reflexes, and the enteric nervous system: Pathogenesis of the irritable bowel syndrome. J Clin Gastroenterol 39: S184–S193, 2005. DOI: 10.1097/01.mcg.0000156403.37240.30. [DOI] [PubMed] [Google Scholar]
- 91.Gershon MD. Serotonin is a sword and a shield of the bowel: Serotonin plays offense and defense. Trans Am Clin Climatol Assoc 123: 268, 2012. [PMC free article] [PubMed] [Google Scholar]
- 92.Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes 20: 14–21, 2013. DOI: 10.1097/MED.0B013E32835BC703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gershon MD. 5-HT4-mediated neuroprotection: A new therapeutic modality on the way? Am J Physiol Gastrointest Liver Physiol 310: G766–G767, 2016. DOI: 10.1152/AJPGI.00120.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gershon MD, Ross LL. Studies on the relationship of 5-hydroxytryptamine and the enterochromaffin cell to anaphylactic shock in mice. J Exp Med 115: 367–382, 1962. DOI: 10.1084/JEM.115.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gershon MD, Tack J. The serotonin signaling system: From basic understanding to drug development for functional GI disorders. Gastroenterology 132: 397–414, 2007. DOI: 10.1053/J.GASTRO.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 96.Gershon RK, Askenase PW, Gershon MD. Requirement for vasoactive amines for production of delayed-type hypersensitvity skin reactions. J Exp Med 142: 732–747, 1975. DOI: 10.1084/jem.142.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ghia J-E, Li N, Wang H, Collins M, Deng Y, El-Sharkawy RT, Côté F, Mallet J, Khan WI. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology 137: 1649–1660, 2009. DOI: 10.1053/j.gastro.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 98.Gill RK, Pant N, Saksena S, Singla A, Nazir TM, Vohwinkel L, Turner JR, Goldstein J, Alrefai WA, Dudeja PK. Function, expression, and characterization of the serotonin transporter in the native human intestine. Am J Physiol Gastrointest Liver Physiol 294, 2008. DOI: 10.1152/AJPGI.00354.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gorard DA, Libby GW, Farthing MJG. Influence of antidepressants on whole gut and orocaecal transit times in health and irritable bowel syndrome. Aliment Pharmacol Ther 8 (2): 159–166, 1994. [DOI] [PubMed] [Google Scholar]
- 100.Goto K, Kawahara I, Inada H, Misawa H, Kuniyasu H, Nabekura J, Takaki M. Activation of 5-HT4 receptors facilitates neurogenesis from transplanted neural stem cells in the anastomotic ileum. J Physiol Sci 66: 67–76, 2016. DOI: 10.1007/S12576-015-0396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goyal MS, Venkatesh S, Milbrandt J, Gordon JI, Raichle ME. Feeding the brain and nurturing the mind: Linking nutrition and the gut microbiota to brain development. Proc Natl Acad Sci USA 112: 14105–14112, 2015. DOI: 10.1073/pnas.1511465112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grider JR, Foxx-Orenstein AE, Jin JG. 5-Hydroxytryptamine4 receptor agonists initiate the peristaltic reflex in human, rat, and guinea pig intestine. Gastroenterology 115: 370–380, 1998. DOI: 10.1016/s0016-5085(98)70203-3. [DOI] [PubMed] [Google Scholar]
- 103.Grider JR, Kuemmerle JF, Jin JG. 5-HT released by mucosal stimuli initiates peristalsis by activating 5-HT4/5-HT1p receptors on sensory CGRP neurons. Am J Phys 270: G778–G782, 1996. DOI: 10.1152/AJPGI.1996.270.5.G778. [DOI] [PubMed] [Google Scholar]
- 104.Gross ER, Gershon MD, Margolis KG, Gertsberg ZV, Cowles RA. Neuronal serotonin regulates growth of the intestinal mucosa in mice. Gastroenterology 143: 408–417.e2, 2012. DOI: 10.1053/J.GASTRO.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.He C-L, Burgart L, Wang L, Pemberton J, Young-Fadok T, Szurszewski J, Farrugia G. Decreased interstitial cell of Cajal volume in patients with slow-transit constipation. Gastroenterology 118 (1): 14–21, 2000. [DOI] [PubMed] [Google Scholar]
- 106.Heredia DJ, Dickson EJ, Bayguinov PO, Hennig GW, Smith TK. Localized release of serotonin (5-hydroxytryptamine) by a fecal pellet regulates migrating motor complexes in murine colon. Gastroenterology 136: 1328–1338, 2009. DOI: 10.1053/J.GASTRO.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Heredia DJ, Gershon MD, Koh SD, Corrigan RD, Okamoto T, Smith TK. Important role of mucosal serotonin in colonic propulsion and peristaltic reflexes: In vitro analyses in mice lacking tryptophan hydroxylase 1. J Physiol 591: 5939–5957, 2013. DOI: 10.1113/JPHYSIOL.2013.256230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Herr N, Bode C, Duerschmied D. The effects of serotonin in immune cells. Front Cardiovasc Med 4, 2017. DOI: 10.3389/FCVM.2017.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Herschbach P, Henrich G, von Rad M. Psychological factors in functional gastrointestinal disorders: Characteristics of the disorder or of the illness behavior? Psychosom Med 61: 148–153, 1999. DOI: 10.1097/00006842-199903000-00005. [DOI] [PubMed] [Google Scholar]
- 110.Hillsley K, Grundy D. Sensitivity to 5-hydroxytryptamine in different afferent subpopulations within mesenteric nerves supplying the rat jejunum. J Physiol 509 (Pt 3): 717–727, 1998. DOI: 10.1111/j.1469-7793.1998.717bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hillsley K, Kirkup AJ, Grundy D. Direct and indirect actions of 5-hydroxytryptamine on the discharge of mesenteric afferent fibres innervating the rat jejunum. J Physiol 506 (Pt 2): 551–561, 1998. DOI: 10.1111/j.1469-7793.1998.551bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hockley JF, Taylor TS, Callejo G, Wilbrey AL, Gutteridge A, Bach K, Winchester WJ, Bulmer DC, McMurray G, Smith ESJ. Single-cell RNAseq reveals seven classes of colonic sensory neuron. Gut 68: 633–644, 2019. DOI: 10.1136/gutjnl-2017-315631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hoffman JM, McKnight ND, Sharkey KA, Mawe GM. The relationship between inflammation-induced neuronal excitability and disrupted motor activity in the guinea pig distal colon. Neurogastroenterol Motil 23: 673–e279, 2011. DOI: 10.1111/j.1365-2982.2011.01702.x. [DOI] [PubMed] [Google Scholar]
- 114.Hoffman JM, Tyler K, MacEachern SJ, Balemba OB, Johnson AC, Brooks EM, Zhao H, Swain GM, Moses PL, Galligan JJ, Sharkey KA, Greenwood-Van Meerveld B, Mawe GM. Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology 142: 844–854.e4, 2012. DOI: 10.1053/j.gastro.2011.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hotta R, Cheng LS, Graham HK, Nagy N, Belkind-Gerson J, Mattheolabakis G, Amiji MM, Goldstein AM. Delivery of enteric neural progenitors with 5-HT4 agonist-loaded nanoparticles and thermosensitive hydrogel enhances cell proliferation and differentiation following transplantation in vivo. Biomaterials 88: 1, 2016. DOI: 10.1016/J.BIOMATERIALS.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Houghton LA, Atkinson W, Whitaker RP, Whorwell PJ, Rimmer MJ. Increased platelet depleted plasma 5-hydroxytryptamine concentration following meal ingestion in symptomatic female subjects with diarrhoea predominant irritable bowel syndrome. Gut 52: 663–670, 2003. DOI: 10.1136/gut.52.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Houghton LA, Fowler P, Keene ON, Read NW. Effect of sumatriptan, a new selective 5HT1-like agonist, on liquid gastric emptying in man. Aliment Pharmacol Ther 6: 685–691, 1992. DOI: 10.1111/j.1365-2036.1992.tb00732.x. [DOI] [PubMed] [Google Scholar]
- 118.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav 71: 533–554, 2002. DOI: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 119.Humenick A, Chen BN, Wattchow DA, Zagorodnyuk VP, Dinning PG, Spencer NJ, Costa M, Brookes SJH. Characterization of putative interneurons in the myenteric plexus of human colon. Neurogastroenterol Motil 33, 2021. DOI: 10.1111/nmo.13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Israelyan N, del Colle A, Li Z, Park Y, Xing A, JPR J, Luna RA, Jensen DD, Madra M, Saurman V, Rahim R, Latorre R, Law K, Carson W, Bunnett NW, Caron MG, Margolis KG. Effects of serotonin and slow-release 5-hydroxytryptophan on gastrointestinal motility in a mouse model of depression. Gastroenterology 157: 507–521.e4, 2019. DOI: 10.1053/J.GASTRO.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Janssen P, Prins NH, Meulemans AL, Lefebvre RA. Pharmacological characterization of the 5-HT receptors mediating contraction and relaxation of canine isolated proximal stomach smooth muscle. Br J Pharmacol 136 (2): 321–329, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jin B, Ha SE, Wei L, Singh R, Zogg H, Clemmensen B, Heredia DJ, Gould TW, Sanders KM, Ro S. Colonic motility is improved by the activation of 5-HT2B receptors on interstitial cells of Cajal in diabetic mice. Gastroenterology 161: 608–622.e7, 2021. DOI: 10.1053/J.GASTRO.2021.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jin J-G, Foxx-Orenstein AE, Grider JR. Propulsion in guinea pig colon induced by 5-hydroxytryptamine (HT) via 5-HT4 and 5-HT3 receptors. J Pharmacol Exp Ther 288: 93–97, 1999. [PubMed] [Google Scholar]
- 124.Johanson JF. Options for patients with irritable bowel syndrome: Contrasting traditional and novel serotonergic therapies. Neurogastroenterol Motil 16: 701–711, 2004. DOI: 10.1111/j.1365-2982.2004.00550.x. [DOI] [PubMed] [Google Scholar]
- 125.Jørandli JW, Thorsvik S, Skovdahl HK, Kornfeld B, Sæterstad S, Gustafsson BI, Sandvik AK, van Beelen GA. The serotonin reuptake transporter is reduced in the epithelium of active Crohn’s disease and ulcerative colitis. Am J Physiol Gastrointest Liver Physiol 319: G761–G768, 2020. DOI: 10.1152/ajpgi.00244.2020. [DOI] [PubMed] [Google Scholar]
- 126.Keating DJ, Spencer NJ. Release of 5-hydroxytryptamine from the mucosa is not required for the generation or propagation of colonic migrating motor complexes. Gastroenterology 138 (659–670): 670.e1–670.e2, 2010. DOI: 10.1053/j.gastro.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 127.Kedees MH, Guz Y, Grigoryan M, Teitelman G. Functional activity of murine intestinal mucosal cells is regulated by the glucagon-like peptide-1 receptor. Peptides (NY) 48: 36–44, 2013. DOI: 10.1016/j.peptides.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 128.Kellum JM, Albuquerque FC, Stoner MC, Harris RP. Stroking human jejunal mucosa induces 5-HT release and Cl− secretion via afferent neurons and 5-HT4 receptors. Am J Physiol Gastrointest Liver Physiol 277: G515–G520, 1999. DOI: 10.1152/AJPGI.1999.277.3.G515/ASSET/IMAGES/LARGE/AGIJ30815004X.JPEG. [DOI] [PubMed] [Google Scholar]
- 129.Kerckhoffs APM, ter Linde JJM, Akkermans LMA, Samsom M. SERT and TPH-1 mRNA expression are reduced in irritable bowel syndrome patients regardless of visceral sensitivity state in large intestine. Am J Physiol Gastrointest Liver Physiol 302: G1053–G1060, 2012. DOI: 10.1152/ajpgi.00153.2011. [DOI] [PubMed] [Google Scholar]
- 130.Kerr TM, Muller CL, Miah M, Jetter CS, Pfeiffer R, Shah C, Baganz N, Anderson GM, Crawley JN, Sutcliffe JS, Blakely RD, Veenstra-Vanderweele J. Genetic background modulates phenotypes of serotonin transporter Ala56 knock-in mice. Mol Autism 4: 1–11, 2013. DOI: 10.1186/2040-2392-4-35/FIGURES/4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kidd M, Gustafsson BI, Drozdov I, Modlin IM. IL1beta- and LPS-induced serotonin secretion is increased in EC cells derived from Crohn’s disease. Neurogastroenterol Motil 21: 439–450, 2009. DOI: 10.1111/j.1365-2982.2008.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kode A, Obri A, Paone R, Kousteni S, Ducy P, Karsenty G. Lrp5 regulation of bone mass and serotonin synthesis in the gut. Nat Med 20: 1228–1229, 2014. DOI: 10.1038/NM.3698. [DOI] [PubMed] [Google Scholar]
- 133.Kozlowski CM, Green A, Grundy D, Boissonade FM, Bountra C. The 5-HT 3 receptor antagonist alosetron inhibits the colorectal distention induced depressor response and spinal c-fos expression in the anaesthetised rat. Gut 46: 474–480, 2000. DOI: 10.1136/gut.46.4.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kruger GM, Mosher JT, Bixby S, Joseph N, Iwashita T, Morrison SJ. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron 35: 657–669, 2002. DOI: 10.1016/s0896-6273(02)00827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kuiken SD, Tytgat GNJ, Boeckxstaens GEE. The selective serotonin reuptake inhibitor fluoxetine does not change rectal sensitivity and symptoms in patients with irritable bowel syndrome: A double blind, randomized, placebo-controlled study. Clin Gastroenterol Hepatol 1: 219–228, 2003. DOI: 10.1053/cgh.2003.50032. [DOI] [PubMed] [Google Scholar]
- 136.Lee KJ, Kim YB, Kim JH, Kwon HC, Kim DK, Cho SW. The alteration of enterochromaffin cell, mast cell, and lamina propria T lymphocyte numbers in irritable bowel syndrome and its relationship with psychological factors. J Gastroenterol Hepatol 23: 1689–1694, 2008. DOI: 10.1111/j.1440-1746.2008.05574.x. [DOI] [PubMed] [Google Scholar]
- 137.Leopoldo M, Guzel T, Mirowska-Guzel D. The role of serotonin neurotransmission in gastrointestinal tract and pharmacotherapy. Molecules 27 (5): 1680, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lesch K-P, Wolozin BL, Murphy DL, Riederer P. Primary structure of the human platelet serotonin uptake site: Identity with the brain serotonin transporter. J Neurochem 60: 2319–2322, 1993. DOI: 10.1111/J.1471-4159.1993.TB03522.X. [DOI] [PubMed] [Google Scholar]
- 139.Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, Gachet C, Bader M, Clavien PA. Platelet-derived serotonin mediates liver regeneration. Science 312: 104–107, 2006. DOI: 10.1126/SCIENCE.1123842. [DOI] [PubMed] [Google Scholar]
- 140.Li Z, Chalazonitis A, Huang YY, Mann JJ, Margolis KG, Yang QM, Kim DO, Côté F, Mallet J, Gershon MD. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci 31: 8998–9009, 2011. DOI: 10.1523/JNEUROSCI.6684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu MT, Kuan YH, Wang J, Hen R, Gershon MD. 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci 29: 9683–9699, 2009. DOI: 10.1523/JNEUROSCI.1145-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu M-T, Rayport S, Jiang Y, Murphy DL, Gershon MD. Expression and function of 5-HT3 receptors in the enteric neurons of mice lacking the serotonin transporter. Am J Physiol Gastrointest Liver Physiol 283: G1398–G1411, 2002. DOI: 10.1152/ajpgi.00203.2002. [DOI] [PubMed] [Google Scholar]
- 143.Liu Q, Yang Q, Sun W, Vogel P, Heydorn W, Yu X-Q, Hu Z, Yu W, Jonas B, Pineda R, Calderon-Gay V, Germann M, O’Neill E, Brommage R, Cullinan E, Platt K, Wilson A, Powell D, Sands A, Zambrowicz B, Shi Z-C. Discovery and characterization of novel tryptophan hydroxylase inhibitors that selectively inhibit serotonin synthesis in the gastrointestinal tract. J Pharmacol Exp Ther 325: 47–55, 2008. DOI: 10.1124/jpet.107.132670. [DOI] [PubMed] [Google Scholar]
- 144.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 130: 1480–1491, 2006. DOI: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 145.Lund ML, Egerod KL, Engelstoft MS, Dmytriyeva O, Theodorsson E, Patel BA, Schwartz TW. Enterochromaffin 5-HT cells - A major target for GLP-1 and gut microbial metabolites. Mol Metab 11: 70–83, 2018. DOI: 10.1016/j.molmet.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Makadia PA, Najjar SA, Saloman JL, Adelman P, Feng B, Margiotta JF, Albers KM, Davis BM. Optogenetic activation of colon epithelium of the mouse produces high-frequency bursting in extrinsic colon afferents and engages visceromotor responses. J Neurosci 38: 5788–5798, 2018. DOI: 10.1523/JNEUROSCI.0837-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mangel AW, Northcutt AR. Review article: the safety and efficacy of alosetron, a 5-HT3 receptor antagonist, in female irritable bowel syndrome patients. Aliment Pharmacol Ther 13: 77–82, 1999. [DOI] [PubMed] [Google Scholar]
- 148.Manning BM, Meyer AF, Gruba SM, Haynes CL. Single-cell analysis of mast cell degranulation induced by airway smooth muscle-secreted chemokines. Biochim Biophys Acta 1850: 1862–1868, 2015. DOI: 10.1016/j.bbagen.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Margolis KG, Cryan JF, Mayer EA. The microbiota-gut-brain axis: From motility to mood. Gastroenterology 160: 1486, 2021. DOI: 10.1053/J.GASTRO.2020.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Margolis KG, Gershon MD. Neuropeptides and inflammatory bowel disease. Curr Opin Gastroenterol 25: 503–511, 2009. DOI: 10.1097/MOG.0b013e328331b69e. [DOI] [PubMed] [Google Scholar]
- 151.Margolis KG, Li Z, Stevanovic K, Saurman V, Israelyan N, Anderson GM, Snyder I, Veenstra-VanderWeele J, Blakely RD, Gershon MD. Serotonin transporter variant drives preventable gastrointestinal abnormalities in development and function. J Clin Invest 126: 2221–2235, 2016. DOI: 10.1172/JCI84877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Margolis KG, Stevanovic K, Karamooz N, Li ZS, Ahuja A, D’Autréaux F, Saurman V, Chalazonitis A, Gershon MD. Enteric neuronal density contributes to the severity of intestinal inflammation. Gastroenterology 141 (588–598): 598.e1–598.e2, 2011. DOI: 10.1053/j.gastro.2011.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Margolis KG, Stevanovic K, Li Z, Yang QM, Oravecz T, Zambrowicz B, Jhaver KG, Diacou A, Gershon MD. Pharmacological reduction of mucosal but not neuronal serotonin opposes inflammation in mouse intestine. Gut 63: 928–937, 2014. DOI: 10.1136/GUTJNL-2013-304901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Martin AM, Jones LA, Wei L, Spencer NJ, Sanders KM, Ro S, Keating DJ. Distinguishing the contributions of neuronal and mucosal serotonin in the regulation of colonic motility. Neurogastroenterol Motil 34 (8): e14361, 2022. [DOI] [PubMed] [Google Scholar]
- 155.Martin AM, Lumsden AL, Young RL, Jessup CF, Spencer NJ, Keating DJ. Regional differences in nutrient-induced secretion of gut serotonin. Phys Rep 5: 13199, 2017. DOI: 10.14814/PHY2.13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Masand PS, Pae C-U, Krulewicz S, Peindl K, Mannelli P, Varia IM, Patkar AA. A double-blind, randomized, placebo-controlled trial of paroxetine controlled-release in irritable bowel syndrome. Psychosomatics 50: 78–86, 2009. DOI: 10.1176/appi.psy.50.1.78. [DOI] [PubMed] [Google Scholar]
- 157.Mawe GM, Branchek TA, Gershon MD. Peripheral neural serotonin receptors: Identification and characterization with specific antagonists and agonists. Proc Natl Acad Sci USA 83 (24): 9799–9803, 1986. (autonomic nervous system/enteric nervous system/gut) [Online]. https://www.pnas.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mawe GM, Hoffman JM. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol 10: 473–486, 2013. DOI: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.McCallum RW, Prakash C, Campoli-Richards DM, Goa KL. Cisapride. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use as a prokinetic agent in gastrointestinal motility disorders. Drugs 36: 652–681, 1988. DOI: 10.2165/00003495-198836060-00002. [DOI] [PubMed] [Google Scholar]
- 160.McElhanon BO, McCracken C, Karpen S, Sharp WG. Gastrointestinal symptoms in autism spectrum disorder:A meta-analysis. Pediatrics 133 (5): 872–883, 2014. [DOI] [PubMed] [Google Scholar]
- 161.Mercado CP, Kilic F. Molecular mechanisms of SERT in platelets: regulation of plasma serotonin levels. Mol Interv 10: 231–241, 2010. DOI: 10.1124/mi.10.4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Monro RL, Bornstein JC, Bertrand PP. Slow excitatory post-synaptic potentials in myenteric AH neurons of the guinea-pig ileum are reduced by the 5-hydroxytryptamine7 receptor antagonist SB 269970. Neuroscience 134: 975–986, 2005. DOI: 10.1016/j.neuroscience.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 163.Montesinos MS, Machado JD, Camacho M, Diaz J, Morales YG, de La Rosa DA, Carmona E, Castañeyra A, Viveros OH, O’Connor DT, Mahata SK, Borges R. The crucial role of chromogranins in storage and exocytosis revealed using chromaffin cells from chromogranin A null mouse. J Neurosci 28: 3350–3358, 2008. DOI: 10.1523/JNEUROSCI.5292-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Morarach K, Mikhailova A, Knoflach V, Memic F, Kumar R, Li W, Ernfors P, Marklund U. Diversification of molecularly defined myenteric neuron classes revealed by single-cell RNA sequencing. Nat Neurosci 24: 34–46, 2021. DOI: 10.1038/s41593-020-00736-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Morrissey JJ, Walker MN, Lovenberg W. The absence of tryptophan hydroxylase activity in blood platelets. Proc Soc Exp Biol Med 154: 496–499, 1977. DOI: 10.3181/00379727-154-39702. [DOI] [PubMed] [Google Scholar]
- 166.Mulder EJ, Anderson GM, Kema IP, de Bildt A, van Lang NDJ, den Boer JA, Minderaa RB. Platelet serotonin levels in pervasive developmental disorders and mental retardation: Diagnostic group differences, within-group distribution, and behavioral correlates. J Am Acad Child Adolesc Psychiatry 43: 491–499, 2004. DOI: 10.1097/00004583-200404000-00016. [DOI] [PubMed] [Google Scholar]
- 167.Najjar SA, Edwards BS, Albers KM, Davis BM, Smith-Edwards KM. Optogenetic activation of the distal colon epithelium engages enteric nervous system circuits to initiate motility patterns. Am J Physiol Gastrointest Liver Physiol 321: G426–G435, 2021. DOI: 10.1152/ajpgi.00026.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Navari RM. 5-HT3 receptors as important mediators of nausea and vomiting due to chemotherapy. Biochim Biophys Acta Biomembr 1848: 2738–2746, 2015. DOI: 10.1016/J.BBAMEM.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 169.Neal KB, Parry LJ, Bornstein JC. Strain-specific genetics, anatomy and function of enteric neural serotonergic pathways in inbred mice. J Physiol 587: 567–586, 2009. DOI: 10.1113/JPHYSIOL.2008.160416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nijenhuis CM, ter Horst PGJ, de Jong-Van Den Berg LTW, Wilffert B. Disturbed development of the enteric nervous system after in utero exposure of selective serotonin re-uptake inhibitors and tricyclic antidepressants. Part 1: Literature review Correspondence Keywords enteric nervous system, norepinephrine transporter, ontogeny, selective serotonin re-uptake inhibitor, serotonin re-uptake transporter, tricyclic antidepressants. Br J Clin Pharmacol 73 (1): 126–134, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nijenhuis CM, ter Horst PGJ, van Rein N, Wilffert B, Lolkje TW, de Jong-Van Den Berg TW, de Jong-Van Den Berg W. Disturbed development of the enteric nervous system after in utero exposure of selective serotonin re-uptake inhibitors and tricyclic antidepressants. Part 2: Testing the hypotheses. Br J Clin Pharmacol 73 (1): 126–134, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nozawa K, Kawabata-Shoda E, Doihara H, Kojima R, Okada H, Mochizuki S, Sano Y, Inamura K, Matsushime H, Koizumi T, Yokoyama T, Ito H. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc Natl Acad Sci USA 106: 3408–3413, 2009. DOI: 10.1073/PNAS.0805323106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.O’Hara JR, Lomax AE, Mawe GM, Sharkey KA. Ileitis alters neuronal and enteroendocrine signalling in guinea pig distal colon. Gut 56: 186–194, 2007. DOI: 10.1136/gut.2006.102780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.O’Hara JR, Skinn AC, MacNaughton WK, Sherman PM, Sharkey KA. Consequences of Citrobacter rodentium infection on enteroendocrine cells and the enteric nervous system in the mouse colon. Cell Microbiol 8: 646–660, 2006. DOI: 10.1111/j.1462-5822.2005.00657.x. [DOI] [PubMed] [Google Scholar]
- 175.Obata Y, Pachnis V. The effect of microbiota and the immune system on the development and organization of the enteric nervous system. Gastroenterology 151: 836–844, 2016. DOI: 10.1053/j.gastro.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Okamoto T, Barton MJ, Hennig GW, Birch GC, Grainger N, Corrigan RD, Koh SD, Sanders KM, Smith TK. Extensive projections of myenteric serotonergic neurons suggest they comprise the central processing unit in the colon. Neurogastroenterol Motil 26: 556–570, 2014. DOI: 10.1111/nmo.12302. [DOI] [PubMed] [Google Scholar]
- 177.Omer A, Quigley EMM. An update on prucalopride in the treatment of chronic constipation. Ther Adv Gastroenterol 10: 877–887, 2017. DOI: 10.1177/1756283X17734809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Oõmahony SM, Bulmer DC, Coelho A-M, Fitzgerald P, Bongiovanni C, Lee K, Winchester W, Dinan TG, Cryan JF. 5-HT 2B receptors modulate visceral hypersensitivity in a stress-sensitive animal model of brain-gut axis dysfunction. Neurogastroenterol Motil 22 (5): 573–578, 2010. [DOI] [PubMed] [Google Scholar]
- 179.Pan H, Galligan JJ. 5-HT1A and 5-HT4 receptors mediate inhibition and facilitation of fast synaptic transmission in enteric neurons. Am J Phys 266: G230–G238, 1994. DOI: 10.1152/ajpgi.1994.266.2.G230. [DOI] [PubMed] [Google Scholar]
- 180.Peters JA, Kelley SP, Dunlop JI, Kirkness EF, Hales TG, Lambert JJ. The 5-hydroxytryptamine type 3 (5-HT3) receptor reveals a novel determinant of single-channel conductance. Biochem Soc Trans 32: 547–552, 2004. DOI: 10.1042/BST0320547. [DOI] [PubMed] [Google Scholar]
- 181.Prah A, Purg M, Stare J, Vianello R, Mavri J. How monoamine oxidase a decomposes serotonin: An empirical valence bond simulation of the reactive step. J Phys Chem B 124: 8259, 2020. DOI: 10.1021/ACS.JPCB.0C06502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Prasad HC, Steiner JA, Sutcliffe JS, Blakely RD. Enhanced activity of human serotonin transporter variants associated with autism. Philos Trans R Soc Lond Ser B Biol Sci 364 (1514): 163–173, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Prasad HC, Zhu C-B, Mccauley JL, Samuvel DJ, Ramamoorthy S, Shelton RC, Hewlett WA, Sutcliffe JS, Blakely RD. Human serotonin transporter variants display altered sensitivity to protein kinase G and p38 mitogen-activated protein kinase. Proc Natl Acad Sci USA 102 (32): 11545–11550, 2005. www.pnas.orgcgidoi10.1073pnas.0501432102. Accessed June 26, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Prins NH, Briejer MR, van Bergen PJE, Akkermans LMA, Schuurkes JAJ. Evidence for 5-HT 7 receptors mediating relaxation of human colonic circular smooth muscle. Br J Pharmacol 128 (4): 849–852, 1999. http://www.stockton-press.co.uk/bjp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Quigley EMM, Vandeplassche L, Kerstens R, Ausma J. Clinical trial: the efficacy, impact on quality of life, and safety and tolerability of prucalopride in severe chronic constipation-a 12-week, randomized, double-blind, placebo-controlled study. Aliment Pharmacol Ther 29 (3): 315–328, 2009. [DOI] [PubMed] [Google Scholar]
- 186.Rahimi R, Nikfar S, Abdollahi M. Efficacy and tolerability of alosetron for the treatment of irritable bowel syndrome in women and men: A meta-analysis of eight randomized, placebo-controlled, 12-week trials. Clin Ther 30: 884–901, 2008. DOI: 10.1016/j.clinthera.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 187.Ranade SS, Woo S-H, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Bégay V, Coste B, Mainquist J, Wilson AJ, Francisco AG, Reddy K, Qiu Z, Wood JN, Lewin GR, Patapoutian A. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516: 121–125, 2014. DOI: 10.1038/nature13980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rapport MM. Serum vasoconstrictor (serotonin) the presence of creatinine in the complex; a proposed structure of the vasoconstrictor principle. J Biol Chem 180: 961–969, 1949. [PubMed] [Google Scholar]
- 189.Rapport MM, Green AA, Page IH. Purification of the substance which is responsible for the vasoconstrictor activity of serum. Fed Proc 6: 184, 1947. [PubMed] [Google Scholar]
- 190.Rapport MM, Green AA, Page IH. Partial purification of the vasoconstrictor in beef serum. J Biol Chem 174: 735–741, 1948. [PubMed] [Google Scholar]
- 191.Reigstad CS, Salmonson CE, Rainey JF, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G, Kashyap PC. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J 29: 1395–1403, 2015. DOI: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ritvo ER, Yuwiler A, Geller E, Ornitz EM, Saeger K, Plotkin S. Increased Blood Serotonin and Platelets in Early Infantile Autism. Arch Gen Psychiatry 23 (6): 566–572, 1970. https://jamanetwork.com/. [DOI] [PubMed] [Google Scholar]
- 193.Sakowski SA, Geddes TJ, Thomas DM, Levi E, Hatfield JS, Kuhn DM. Differential tissue distribution of tryptophan hydroxylase isoforms 1 and 2 as revealed with monospecific antibodies. Brain Res 1085: 11–18, 2006. DOI: 10.1016/J.BRAINRES.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 194.Salisbury AL, Papandonatos GD, Stroud LR, Smith AK, Brennan PA. Prenatal antidepressant exposures and gastrointestinal complaints in childhood: A gut-brain axis connection? Dev Psychobiol 62: 816–828, 2020. DOI: 10.1002/dev.21966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sánchez de Medina F, Romero-Calvo I, Mascaraque C, Martínez-Augustin O. Intestinal inflammation and mucosal barrier function. Inflamm Bowel Dis 20: 2394–2404, 2014. DOI: 10.1097/MIB.0000000000000204. [DOI] [PubMed] [Google Scholar]
- 196.Sanders KM, Ward SM, Koh SD. Interstitial cells: Regulators of smooth muscle function. Physiol Rev 94: 859–907, 2014. DOI: 10.1152/PHYSREV.00037.2013/ASSET/IMAGES/LARGE/Z9J0031426970010.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Schemann M, Schaaf C, Mäder M. Neurochemical coding of enteric neurons in the guinea pig stomach. J Comp Neurol 353: 161–178, 1995. DOI: 10.1002/CNE.903530202. [DOI] [PubMed] [Google Scholar]
- 198.Schikowski A, Thewissen M, Mathis C, Ross H-G, Enck P. Serotonin type-4 receptors modulate the sensitivity of intramural mechanoreceptive afferents of the cat rectum. Neurogastroenterol Motil 14: 221–227, 2002. DOI: 10.1046/j.1365-2982.2002.00328.x. [DOI] [PubMed] [Google Scholar]
- 199.Shajib MS, Khan WI. The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol (Oxford) 213: 561–574, 2015. DOI: 10.1111/apha.12430. [DOI] [PubMed] [Google Scholar]
- 200.Sia TC, Whiting M, Kyloh M, Nicholas SJ, Oliver J, Brookes SJ, Dinning PG, Wattchow DA, Spencer NJ. 5-HT3 and 5-HT4 antagonists inhibit peristaltic contractions in guinea-pig distal colon by mechanisms independent of endogenous 5-HT. Front Neurosci 7: 136, 2013. DOI: 10.3389/fnins.2013.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Smith TK, Gershon MD. CrossTalk proposal: 5-HT is necessary for peristalsis. J Physiol 593: 3225–3227, 2015. DOI: 10.1113/JP270182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Smith TK, Koh SD. A model of the enteric neural circuitry underlying the generation of rhythmic motor patterns in the colon: The role of serotonin. Am J Physiol Gastrointest Liver Physiol 312: 1–14, 2017. DOI: 10.1152/ajpgi.00337.2016.-We. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Smith TK, Park KJ, Hennig GW. Colonic migrating motor complexes, high amplitude propagating contractions, neural reflexes and the importance of neuronal and mucosal serotonin. J Neurogastroenterol Motil 20: 423–446, 2014. DOI: 10.5056/jnm14092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Spencer NJ, Nicholas SJ, Robinson L, Kyloh M, Flack N, Brookes SJ, Zagorodnyuk VP, Keating DJ. Mechanisms underlying distensionevoked peristalsis in guinea pig distal colon: Is there a role for enterochromaffin cells? Am J Physiol Gastrointest Liver Physiol 301: G519–G527, 2011. DOI: 10.1152/ajpgi.00101.2011. [DOI] [PubMed] [Google Scholar]
- 205.Spencer NJ, Sia TC, Brookes SJ, Costa M, Keating DJ. CrossTalk opposing view: 5-HT is not necessary for peristalsis. J Physiol 593: 3229, 2015. DOI: 10.1113/JP270183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Spiller R. Serotonin, inflammation, and IBS: Fitting the jigsaw together? J Pediatr Gastroenterol Nutr 45 (Suppl 2): S115–S119, 2007. DOI: 10.1097/MPG.0b013e31812e66da. [DOI] [PubMed] [Google Scholar]
- 207.Spohn SN, Bianco F, Scott RB, Keenan CM, Linton AA, O’Neill CH, Bonora E, Dicay M, Lavoie B, Wilcox RL, MacNaughton WK, de Giorgio R, Sharkey KA, Mawe GM. Protective actions of epithelial 5-hydroxytryptamine 4 receptors in normal and inflamed colon. Gastroenterology 151: 933, 2016. DOI: 10.1053/J.GASTRO.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sutcliffe JS, Delahanty RJ, Prasad HC, McCauley JL, Han Q, Jiang L, Li C, Folstein SE, Blakely RD. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. Am J Hum Genet 77: 265–279, 2005. DOI: 10.1086/432648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tack J, Camilleri M, Chang L, Chey WD, Galligan JJ, Lacy BE, Müller-Lissner S, EM MQ, Schuurkes J, de Maeyer JH, Stanghellini V, Arbor A. Systematic review: Cardiovascular safety profile of 5-HT 4 agonists developed for gastrointestinal disorders. Aliment Pharmacol Ther 35 (7): 745–767, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tack J, Coulie B, Wilmer A, Andrioli A, Janssens J. Influence of sumatriptan on gastric fundus tone and on the perception of gastric distension in man. Gut 46: 468–473, 2000. DOI: 10.1136/gut.46.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tack J, van Outryve M, Beyens G, Kerstens R, Vandeplassche L. Prucalopride (Resolor) in the treatment of severe chronic constipation in patients dissatisfied with laxatives. Gut 58: 357–365, 2009. DOI: 10.1136/gut.2008.162404. [DOI] [PubMed] [Google Scholar]
- 212.Takaki M, Goto K, Kawahara I. The 5-hydroxytryptamine 4 receptor agonist-induced actions and enteric neurogenesis in the gut. J Neurogastroenterol Motil 20: 17–30, 2014. DOI: 10.5056/JNM.2014.20.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Takaki M, Mawe GM, Barasch JM, Gershon MD, Gershon MD. Physiological responses of guinea-pig myenteric neurons secondary to the release of endogenous serotonin by tryptamine. Neuroscience 16: 223–240, 1985. DOI: 10.1016/0306-4522(85)90059-4. [DOI] [PubMed] [Google Scholar]
- 214.Talley NJ. SSRIs in IBS: Sensing a dash of disappointment. Clin Gastroenterol Hepatol 1: 155–159, 2003. DOI: 10.1053/cgh.2003.50023. [DOI] [PubMed] [Google Scholar]
- 215.Terry N, Margolis KG. Serotonergic mechanisms regulating the GI tract: Experimental evidence and therapeutic relevance. Handb Exp Pharmacol 239: 319–342, 2017. DOI: 10.1007/164_2016_103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Tharayil VS, Wouters MM, Stanich JE, Roeder JL, Lei S, Beyder A, Gomez-Pinilla PJ, Gershon MD, Maroteaux L, Gibbons SJ, Farrugia G. Lack of serotonin 5-HT 2B receptor alters proliferation and network volume of interstitial cells of Cajal in vivo. Neurogastroenterol Motil 22 (4): 462–469, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Thijssen AY, Mujagic Z, Jonkers DMAE, Ludidi S, Keszthelyi D, Hesselink MA, Clemens CHM, Conchillo JM, Kruimel JW, Masclee AAM. Alterations in serotonin metabolism in the irritable bowel syndrome. Aliment Pharmacol Ther 43: 272–282, 2016. DOI: 10.1111/apt.13459. [DOI] [PubMed] [Google Scholar]
- 218.Tonini M, Galligan JJ, North RA. Effects of cisapride on cholinergic neurotransmission and propulsive motility in the guinea pig ileum. Gastroenterology 96: 1257–1264, 1989. DOI: 10.1016/s0016-5085(89)80012-5. [DOI] [PubMed] [Google Scholar]
- 219.Tonini M, Vicini R, Cervio E, de Ponti F, de Giorgio R, Barbara G, Stanghellini V, Dellabianca A, Sternini C. 5-HT7 receptors modulate peristalsis and accommodation in the guinea pig ileum. Gastroenterology 129 (5): 1557–1566, 2005. [DOI] [PubMed] [Google Scholar]
- 220.Treichel AJ, Finholm I, Knutson KR, Alcaino C, Whiteman ST, Brown MR, Matveyenko A, Wegner A, Kacmaz H, Mercado-Perez A, Gajdos GB, Ordog T, Grover M, Szurszewski J, Linden DR, Farrugia G, Beyder A. Specialized mechanosensory epithelial cells in mouse gut intrinsic tactile sensitivity. Gastroenterology 162: 535–547.e13, 2022. DOI: 10.1053/j.gastro.2021.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Trendelenburg P. Physiological and pharmacological investigations of small intestinal peristalsis. Naunyn Schmiedeberg’s Arch Pharmacol 373: 101–133, 2006. DOI: 10.1007/s00210-006-0052-7. [DOI] [PubMed] [Google Scholar]
- 222.Tyers MB, Freeman AJ. Mechanism of the anti-emetic activity of 5-HT3 receptor antagonists. Oncology 49: 263–268, 1992. DOI: 10.1159/000227054. [DOI] [PubMed] [Google Scholar]
- 223.Veenstra-Vanderweele J, Jessen TN, Thompson BJ, Carter M, Prasad HC, Steiner JA, Sutcliffe JS, Blakely RD. Modeling rare gene variation to gain insight into the oldest biomarker in autism: Construction of the serotonin transporter Gly56Ala knock-in mouse. J Neurodev Disord 1 (2): 158–171, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, Cohen J, Mannangatti P, Jessen T, Thompson BJ, Ye R. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc Natl Acad Sci USA 109: 5469–5474, 2012. DOI: 10.1073/pnas.1112345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Verheijden S, de Schepper S, Boeckxstaens GE. Neuron-macrophage crosstalk in the intestine: a “microglia” perspective. Front Cell Neurosci 9: 403, 2015. DOI: 10.3389/fncel.2015.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wade PR, Chen J, Jaffe B, Kassem IS, Blakely RD, Gershon MD. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci 16: 2352–2364, 1996. DOI: 10.1523/JNEUROSCI.16-07-02352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wade PR, Tamir H, Kirchgessner AL, Gershon MD. Analysis of the role of 5-HT in the enteric nervous system using anti-idiotopic antibodies to 5-HT receptors. Am J Phys 266, 1994. DOI: 10.1152/AJPGI.1994.266.3.G403. [DOI] [PubMed] [Google Scholar]
- 228.Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol 66: 1673–1680, 2003. DOI: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- 229.Walther DJ, Peter J-U, Bashammakh S, Hörtnagl H, Voits M, Fink H, Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 299: 76, 2003. DOI: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 230.Wang F, Knutson K, Alcaino C, Linden DR, Gibbons SJ, Kashyap P, Grover M, Oeckler R, Gottlieb PA, Li HJ, Leiter AB, Farrugia G, Beyder A. Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. J Physiol 595: 79–91, 2017. DOI: 10.1113/JP272718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wang LC, Cai SR, Huang ZX, Shao QL, Ma RC. 5-HT1P receptor-mediated slow depolarization in neurons of guinea pig inferior mesenteric ganglion. Zhongguo Yao Li Xue Bao 20: 505–508, 1999. [PubMed] [Google Scholar]
- 232.Wei L, Singh R, Ha SE, Martin AM, Jones LA, Jin B, Jorgensen BG, Zogg H, Chervo T, Gottfried-Blackmore A, Nguyen L, Habtezion A, Spencer NJ, Keating DJ, Sanders KM, Ro S. Serotonin deficiency is associated with delayed gastric emptying. Gastroenterology 160: 2451, 2021. DOI: 10.1053/J.GASTRO.2021.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wheatcroft J, Wakelin D, Smith A, Mahoney CR, Mawe G, Spiller R. Enterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunction. Neurogastroenterol Motil 17: 863–870, 2005. DOI: 10.1111/j.1365-2982.2005.00719.x. [DOI] [PubMed] [Google Scholar]
- 234.Woo S-H, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, Stucky CL, Patapoutian A. Piezo2 is required for Merkel-cell mechanotransduction. Nature 509: 622–626, 2014. DOI: 10.1038/nature13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wouters MM, Farrugia G, Schemann M. 5-HT receptors on interstitial cells of Cajal, smooth muscle and enteric nerves. Neurogastroenterol Motil 19 (Suppl 2): 5–12, 2007. DOI: 10.1111/j.1365-2982.2007.00963.x. [DOI] [PubMed] [Google Scholar]
- 236.Wouters MM, Gibbons SJ, Roeder JL, Distad M, Ou Y, Strege PR, Szurszewski JH, Farrugia G. Exogenous serotonin regulates proliferation of interstitial cells of Cajal in mouse jejunum through 5-HT2B receptors. Gastroenterology 133: 897–906, 2007. DOI: 10.1053/J.GASTRO.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 237.Xue L, Camilleri M, Locke GR III, Schuurkes JA, Meulemans A, Coulie BJ, Szurszewski JH, Farrugia G. Serotonergic modula-tion of murine fundic tone. Am J Physiol Gastrointest Liver Physiol 291: 1180–1186, 2006. DOI: 10.1152/ajpgi.00224.2006.-Fundic. [DOI] [PubMed] [Google Scholar]
- 238.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161: 264–276, 2015. DOI: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zárate N, Mearin F, Wang X-Y, Huizinga JD. Severe idiopathic gastroparesis due to neuronal and interstitial cells of Cajal degeneration: Pathological findings and management. Gut 52 (7): 966–970, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zeisel A, Hochgerner H, Lönnerberg P, Johnsson A, Memic F, van der Zwan J, Häring M, Braun E, Borm LE, la Manno G, Codeluppi S, Furlan A, Lee K, Skene N, Harris KD, Hjerling-Leffler J, Arenas E, Ernfors P, Marklund U, Linnarsson S. Molecular architecture of the mouse nervous system. Cell 174: 999–1014.e22, 2018. DOI: 10.1016/J.CELL.2018.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zhang X, Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science 305: 217, 2004. DOI: 10.1126/SCIENCE.1097540.4868. [DOI] [PubMed] [Google Scholar]


