Abstract
The orbitofrontal cortex (OFC) plays a fundamental role in motivated behavior and decision-making. In humans, OFC structure and function is significantly disrupted in drug using and dependent individuals, including those exhibiting chronic alcohol use and alcoholism. In animal models, the OFC has been shown to significantly influence the seeking of non-alcohol drugs of abuse. However direct investigations of the OFC during alcohol seeking and use have been more limited. In the studies reported here, we inactivated lateral (lOFC) or medial OFC (mOFC) subregions in rats during multiple stages of alcohol seeking. After one month of intermittent access to homecage 20% ethanol (EtOH), rats were trained to self-administer EtOH under an FR3 schedule and implanted with cannulae directed to lOFC or mOFC. We inactivated OFC subregions with baclofen/muscimol during EtOH self-administration, extinction, cue-induced reinstatement, and progressive ratio testing to broadly characterize the influence of these subregions on alcohol seeking. There were no significant effects of mOFC or lOFC inactivation during FR3 self-administration, extinction, or progressive ratio self-administration. However, lOFC, and not mOFC, inactivation significantly decreased cue-induced reinstatement of EtOH seeking. These findings contribute new information to the specific impact of OFC manipulation on operant alcohol seeking, support previous studies investigating the role of OFC in seeking and consumption of alcohol and other drugs of abuse, and indicate a specific role for lOFC vs. mOFC in reinstatement.
Keywords: Addiction, Motivation, Alcohol use disorder, Prefrontal, Orbital
1. Introduction
Alcohol use is prevalent across the US and throughout the world, and alcohol abuse and alcohol use disorder (AUD) present major health problems (Lim et al., 2012; World Health Organization, 2014). In order to better understand alcohol use and to develop treatments for AUD, it is essential to identify and characterize brain functions underlying alcohol use such as motivation to acquire alcohol, both in the dependent and non-dependent states. As research has evolved throughout the years, there has been an increasing number of brain structures and systems associated with motivation to seek and consume alcohol (Koob, 2014). Among these nuclei, the prefrontal cortex is frequently implicated (Abernathy et al., 2010; Barker et al., 2015; George and Hope, 2017; Klenowski, 2018; Lu and Richardson, 2014; Zahr et al., 2017). The prefrontal cortex has multiple subregions, including the orbito-frontal cortex (OFC). Relative to other prefrontal cortical areas, the OFC has been somewhat less frequently studied in the context of alcohol use and AUD, particularly in animal models (Moorman, 2018).
The OFC plays a role in a number of functions associated with flexible decision making and motivation to acquire reward (Balleine et al., 2011; Izquierdo, 2017; Kringelbach, 2005; Mainen and Kepecs, 2009; McDannald et al., 2014; Noonan et al., 2012; O’Doherty, 2007; Padoa-Schioppa, 2011; Rolls and Grabenhorst, 2008; Rudebeck and Murray, 2014; Stalnaker et al., 2015; Wallis, 2011; Walton et al., 2011). One prominently described function of the OFC is to represent the quality and value of stimuli or outcomes, particularly ingested rewards (O’Doherty et al., 2001; Padoa-Schioppa, 2011; Padoa-Schioppa and Schoenbaum, 2015; Rolls, 2015; Tremblay and Schultz, 1999). Disruption of OFC function in humans and other animals results in impoverished decision making, in part through a role for the OFC in regulating appropriate associations between stimuli/actions and outcomes (Bechara, 2004; Dalton et al., 2016; Glascher et al., 2012; Rudebeck and Murray, 2014; Schoenbaum et al., 2009; Stolyarova and Izquierdo, 2017; Stopper et al., 2014; Zeeb et al., 2010), potentially in addition to other cognitive factors such as diminished behavioral flexibility or working memory. In addition, there is a growing appreciation that the OFC plays a key role in motivation to acquire non-alcohol drugs of abuse such as psychostimulants, and this has been shown in both humans (Capriles et al., 2003; Dom et al., 2005; Everitt et al., 2007; Fuchs et al., 2004; Hutcheson and Everitt, 2003; Kantak et al., 2009, 2013; Lasseter et al., 2009; London et al., 2000; Lucantonio et al., 2014; Porrino and Lyons, 2000; Schoenbaum and Shaham, 2008; Volkow and Fowler, 2000; Winstanley, 2007) and rodents (Capriles et al., 2003; Everitt et al., 2007; Fuchs et al., 2004; Hutcheson and Everitt, 2003; Kantak et al., 2009, 2013; Lasseter et al., 2009; Lucantonio et al., 2014; Porrino and Lyons, 2000; Schoenbaum and Shaham, 2008; Winstanley, 2007).
Studies of the OFC in AUDs have been less numerous than studies of other relevant brain areas, and have been less common than studies of OFC with respect to motivation for other drugs of abuse (Moorman, 2018). There are a number of reports of OFC disruption in human chronic alcohol users such as reduced cortical volume (Asensio et al., 2016; Beck et al., 2012; Cardenas et al., 2011; Crews and Boettiger, 2009; Demirakca et al., 2011; Durazzo et al., 2011; Harris et al., 2008; Jernigan et al., 1991; Kubota et al., 2001; Laakso et al., 2002; Le Berre et al., 2014; Matsuo et al., 2009; O’Neill et al., 2001; Pfefferbaum et al., 1988; Pfefferbaum et al., 1997; Pfefferbaum et al., 1998; Rando et al., 2011; Rosenbloom and Pfefferbaum, 2008; Sullivan and Pfefferbaum, 2005; Tanabe et al., 2009; Thayer et al., 2016; Wang et al., 2016; Zahr et al., 2017), and some disruption in baseline functional activation (Boettiger et al., 2007, 2009; Catafau et al., 1999; Forbes et al., 2014; Kuruoglu et al., 1996; Nicolas et al., 1993; Volkow and Fowler, 1994; Volkow et al., 1997). Furthermore, OFC is activated during alcohol craving in human heavy alcohol users (Blaine et al., 2017; Blaine and Sinha, 2017; Boettiger et al., 2007, 2009; Catafau et al., 1999; Claus et al., 2011; Forbes et al., 2014; Hermann et al., 2006; Kuruoglu et al., 1996; Myrick et al., 2004, 2008; Nicolas et al., 1993; Reinhard et al., 2015; Seo et al., 2011, 2013; Tapert et al., 2003; Volkow and Fowler, 1994; Volkow et al., 1997; Wrase et al., 2002). However, there have been only a small number of studies of the OFC in animal models of alcohol seeking or use. Acute ethanol inhibits OFC neurons (Badanich et al., 2013), and chronic ethanol exposure either enhances or suppresses OFC activation of neurons tested in vitro (Nimitvilai et al., 2016, 2017; Radke et al., 2017; Renteria et al., 2018), and produces altered OFC neuronal structure (McGuier et al., 2015) and function in vivo (McMurray et al., 2016).
There have been multiple reports of disruptions in OFC-associated behaviors after chronic ethanol. Chronic alcohol disrupts OFC-associated cognitive functions such as behavioral flexibility as measured by reversal learning and set-shifting tasks (Badanich et al., 2011; Coleman et al., 2014; Fernandez et al., 2017; Gass et al., 2014; Hu et al., 2015; Kroener et al., 2012; Obernier et al., 2002; Rodberg et al., 2017; Trantham-Davidson et al., 2014) and elevates risky decision making (Boutros et al., 2014; Clark et al., 2012; McMurray et al., 2014, 2016; Nasrallah et al., 2009, 2011; Schindler et al., 2014). OFC inactivation in mice had no effect on alcohol drinking, but increased drinking after chronic alcohol exposure (den Hartog et al., 2016). Somewhat in contrast, a recent study showed decreased context-driven reinstatement of ethanol seeking following OFC inactivation (Bianchi et al., 2018). Thus, although the number of studies indicating a role for OFC in ethanol seeking has increased in recent years, a number of specific details still need to be worked out.
The goal of the research reported here was to address a number of these outstanding details. Specifically, we compared the effect of OFC inactivation on a suite of operant behaviors: self-administration, extinction, reinstatement, and progressive-ratio seeking. We also investigated separate contributions of OFC subregions, as a growing number of studies have demonstrated that lateral (lOFC) and medial (mOFC) play different functions in natural behavior and drug seeking (Burton et al., 2014; Dalton et al., 2016; Fuchs et al., 2004; Gourley et al., 2010, 2016; Izquierdo, 2017; Lopatina et al., 2016, 2017; Mar et al., 2011; Stopper et al., 2014). We report that lateral, but not medial, OFC inactivation disrupted reinstatement and, to a lesser extent, progressive ratio, but not fixed-ratio self-administration or extinction. These results indicate that alcohol-reinforced seeking under relatively low effort requirements is not dependent on the OFC. However, when motivation levels are high and alcohol is not delivered, as seen during reinstatement, lOFC, becomes engaged. The data show that the OFC is an important brain area for the regulation of highly-motivated alcohol seeking, as seen in alcohol use disorders.
2. Materials and methods
2.1. Animals
Male Long Evans rats (n = 32; Charles River Laboratories, Wilmington, MA), weighing approximately 200–250 g upon arrival, were used in these experiments. Rats were single-housed in a temperature- and humidity-controlled room under reversed 12-h light/dark cycle (7:00 a.m. off to 7:00 p.m. on). Rats were given 6 days to acclimate before experiments began. Food and water were available ad libitum until rats weighed 300 g. Rats were then kept on 25 g of rat chow per day to prevent rapid weight gain. All protocols and procedures followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee at the University of Massachusetts Amherst.
2.2. Intermittent-access 20% ethanol drinking paradigm
Prior to operant training, rats received homecage intermittent-access to 20% ethanol (EtOH) (Carnicella et al., 2014; Moorman and Aston-Jones, 2009; Simms et al., 2008; Wise, 1973). Rats were given access to 20% EtOH during three 24-h sessions per week (Mondays, Wednesday and Fridays) with 24 or 48 h-ethanol deprivation between EtOH access days. During 24-h session, each rat received 40 ml of 20% EtOH in a 50 ml graduated plastic cylinder with ball point stainless-steel drinking spout. After 24 h, excluding the Friday session, the EtOH bottle was replaced with a similar bottle with 40 ml of tap water for the next 24 h. Over the weekend, rats did not receive either bottle. Rats were given intermittent-access to 20% EtOH for 12 sessions (4 weeks). 24-hour drinking session began during their dark cycle (from ~10:00 A.M.). Throughout this period, rats had access to their home-cage water bottle ad libtum. The weight of each rat was measured before beginning the 24-h EtOH drinking session in order to calculate the grams of EtOH consumed per kilogram of body weight. The amount of EtOH and water consumed was recorded after every 24-h session. EtOH solutions were prepared from 95% (v/v) EtOH (Fisher Scientific, Pittsburgh, PA) in tap water.
2.3. Ethanol self-administration training
Following the 12-session intermittent-access to 20% EtOH, rats were trained to self-administer 20% EtOH in sound-attenuated operant chambers (Med Associates, St. Albans, VT) for 1-h sessions during their active cycle. In each chamber, there were two nosepoke holes (active and inactive) on opposite sides of the chamber. Throughout each 1-h session, a fan provided white noise and ventilation, and at the beginning of each session, the active nosepoke hole was illuminated. Depending on the fixed ratio schedule, entries into the active nosepoke resulted in the 0.5 s presentation of a tone (1 kHz), termination of the nosepoke light, a 20 s presentation of the houselight, and delivery of 0.1 ml of 20% EtOH into a well located directly below the active nosepoke. Inactive nosepokes had no consequences but were recorded.
Rats were trained on a fixed ratio 1 (FR1) schedule for at least 5 days. After stable acquisition of FR1 responding, rats were trained on an FR2 schedule for at least 3 days until 85% of their nosepokes occurred when the houselight was off. The schedule requirement was then increased to FR3 for at least 3 days before surgery and testing. At the end of each session, reward wells were inspected to verify that animals consumed delivered ethanol. Because we almost always found empty reward wells, we assumed that delivered ethanol was always consumed and that rats regulated intake via nosepoke self-administration. However, because we did not directly measure amount of ethanol in-take, we refer to ethanol deliveries received as infusions.
2.4. Surgery
Rats were anesthetized with 5% isoflurane before being transferred to a stereotaxic instrument, where anesthesia was maintained with ~2.5% isoflurane in air through a nose-cone. Animals were given antibiotic (0.1 ml cefazolin, i.m.) and analgesic (meloxicam, 1 mg/kg, s.c.) prior to incisions. The skull was exposed and incision points were covered with 2% lidocaine. Rats were implanted with bilateral stainless-steel guide cannulas (26 gauge; Plastics One, Roanoke, VA) that were lowered in pre-drilled bilateral holes in either the lateral or medial OFC (lOFC: +3.2 mm anteroposterior (AP), ± 2.5 mm mediolateral (ML), −4.6 mm dorsoventral (DV); mOFC: +4.2 mm anteroposterior (AP), ± 0.6 mm mediolateral (ML), −4.3 mm dorsoventral (DV), relative to bregma). Three additional holes were drilled, screws were implanted, and dental cement was used to secure the cannulae. Rats were given at least 6 days to recover post-surgery while weight, activity and eating behavior were monitored and recorded. After recovery, rats were re-trained on FR3 to self-administer 20% EtOH for at least 4 days prior to testing.
2.5. Intracranial drug infusions
For drug infusions, stainless-steel injectors (33 gauge; Plastics One) were inserted into the bilaterally implanted guide cannulas and extended 1 mm beyond the tip of the cannulas. Injectors were connected to 10 μl Hamilton syringes (Hamilton Company, Reno, NV) which were mounted on microinfusion pumps (UMP3/Micro 4, World Precision Instruments, Sarasota, FL). Inactivation of the OFC was achieved by infusing a combination of GABAB and GABAA agonists baclofen and muscimol (B+M; 1.0 and 0.1 mM, respectively; Tocris Bioscience, Bristol, UK) in artificial cerebrospinal fluid (aCSF). Vehicle infusion was performed using aCSF. Infusions were delivered at a volume of 0.6 μl (lOFC) or 0.3 μl (mOFC) per hemisphere over 2 min. After infusion, injectors were left in place for 1 min to allow diffusion. This rate, volume and concentration of drug infusion have been shown to influence behavior when infused into the medial or lateral OFC (Fuchs et al., 2004). Animals received both B+M and aCSF on separate days, separated by intervening non-treatment days.
2.6. Experiment 1: Effect of bilateral inactivation of the OFC on operant EtOH self-administration
Following post-surgical self-administration retraining, rats received sham infusions (injector was placed in the cannula for 3 min but no drugs were infused) into the lOFC or mOFC. After 10 min, rats were placed in the operant chambers for an hour under FR3. Rats then received two FR3 test days (B+M and aCSF), which were separated by a day of no infusion on FR3. On each test day, rats received infusions of B +M or the vehicle into the lOFC or mOFC before being placed in the chamber for an hour on FR3. The order in which the drugs were infused was randomized across subject.
After the last day of operant EtOH self-administration, rats were trained during daily hour-long extinction sessions. Throughout each session, the fan remained on and active nosepokes did not result in any programmed results, but all nosepokes were recorded. Rats were trained for at least 3 days until they had ≤15 nosepokes per session for 2 consecutive days (extinction criteria).
2.7. Experiment 2: Effect of bilateral inactivation of the OFC on the reinstatement of alcohol-seeking behavior
To examine the effect of bilateral inactivation on cue-induced reinstatement of alcohol-seeking, rats received two reinstatement test days after reaching extinction criterion. On either test day, rats were infused with B+M or vehicle. After 10 min, rats were placed in the chamber for an hour. Throughout each 1-h session, the fan was on, and at the beginning of each session, the active nosepoke hole was illuminated. Three active nosepokes resulted in the 0.5 s presentation of a tone (dB, 1 kHz), termination of the nosepoke light, a 20 s presentation of the houselight, but EtOH was not delivered. Entries into the inactive nosepoke hole had no consequences but were recorded. In between test days, rats underwent extinction training until they re-reached extinction criteria (< 15 nosepokes per session for 2 consecutive days).
2.8. Experiment 3: Effect of bilateral inactivation of the OFC on the extinction of alcohol-seeking behavior
After completion of reinstatement test days, rats underwent a second round of extinction training. To examine the effect of bilateral inactivation on the extinction of alcohol-seeking behavior, rats received two test days after reaching extinction criterion. Extinction inactivation was conducted after reinstatement inactivation to prevent any possible influence of extinction inactivation on reinstatement behavior.
2.9. Experiment 4: Effect of bilateral inactivation of the OFC on progressive ratio breakpoint
After completion of extinction tests, rats underwent three days of 1-h FR3 sessions. Rats then received two test days of progressive ratio (PR) testing. The PR test environment was the same as for FR3, but the number of nose pokes required to receive alcohol increased on each trial based on the equation: Response ratio (rounded to the nearest integer) = [5e (injection number x 0.2)] – 5 (Richardson and Roberts, 1996). Rats were bilaterally infused with B+M and aCSF prior to testing on separate PR testing days. PR testing lasted either 6 h, or until 60 min of no nose pokes occurred. In between test days, rats underwent daily 1-h FR3 sessions, for at least 2 days.
2.10. Histology
Rats were perfused transcardially with 0.9% saline and 4% formaldehyde solutions at the end of each study. Brains were removed and stored in 4% formaldehyde for 24 h then transferred into sucrose azide until sectioning. Brains were sectioned coronally at a thickness of 40 μm using a cryostat (Leica CM3050 S; Leica Biosystems, Buffalo Grove, IL). Sections were mounted on slides, stained with neutral red and cover slipped. Cannula placements were verified by comparing cannula damage to a rat brain atlas (Paxinos and Watson, 2007). Animals with misplaced cannulae were excluded from analysis.
2.11. Data analysis
Statistical analyses were conducted using Prism (GraphPad, San Diego, CA) and Matlab (Mathworks, Natick, MA). Analysis of homecage drinking was performed using repeated measures ANOVA. The effects of OFC inactivation on alcohol self-administration, extinction, reinstatement, and progressive ratio (active/inactive lever responses, reinforced/non-reinforced well entries) were assessed using separate paired t-tests (baclofen/muscimol vs. vehicle). An alpha value of 0.05 was considered significant for all statistical tests.
3. Results
3.1. Intermittent access increased homecage ethanol consumption
Male rats (n = 32) were trained to drink 20% EtOH using intermittent access (see Methods). Rats reliably increased alcohol intake over the course of 1 month of homecage drinking (Fig. 1A; F(11, 341) = 5.97, p < 0.0001, mixed-effects model with Geisser-Greenhouse correction, main effect of drinking session). Rats in the mOFC-implant group drank slightly but significantly more than lOFC rats (F (1, 30) = 4.377, p = 0.045, main effect of treatment group). However, there was no significant interaction effect (F (11, 329) = 1.318, p = 0.21) indicating that both groups escalated over time. To confirm this, we performed independent repeated-measures ANOVAs on each group and found that significant increases in drinking were observed in rats to be implanted with mOFC cannulae (F(11, 165) = 4.00, p = 0.007) and lOFC cannulae (F(11, 165) = 2.84, p = 0.027). Due to variability within and across subjects, only a small number of sessions exhibited significant differences from one another (Tukey). However, the main effect of session combined with the overall greater levels of drinking in later vs. earlier sessions indicated overall escalation of drinking sufficient to demonstrate motivation to consume ethanol in both cohorts.
Fig. 1.
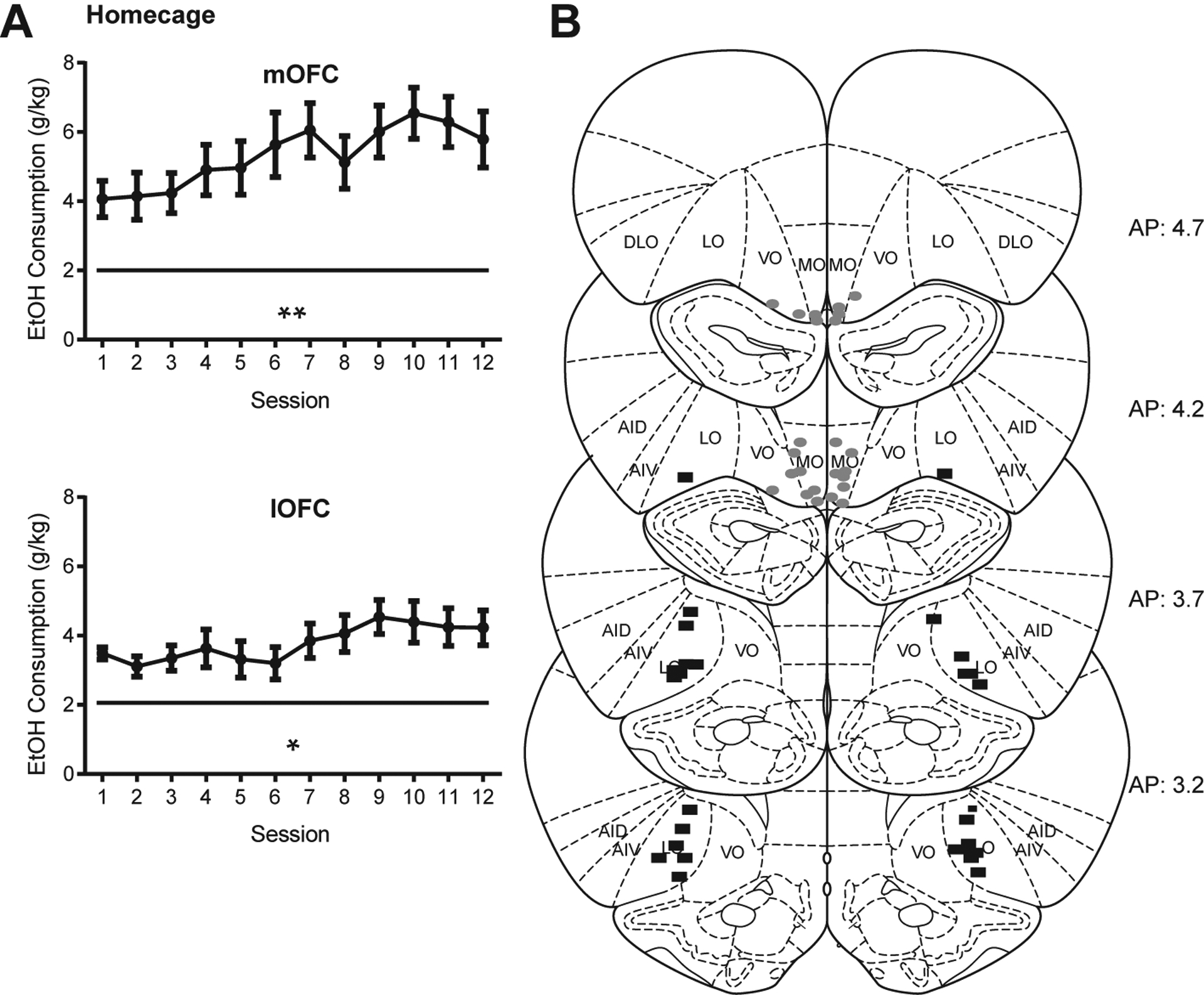
(A) Rats increased drinking over the course of homecage intermittent access. Mean ± SE EtOH in-take in g/kg on each drinking day over the course of 4 weeks of homecage drinking (pre-cannula implant) for mOFC implanted rats (n = 16, top) and lOFC implanted rats (n = 16, bottom). Significant effects observed across all animals (F(11, 341) = 5.97, p < 0.0001, mixed-effects model with Geisser-Greenhouse correction, main effect of drinking session) and within each group (mOFC (F(11, 165) = 4.00, p = 0.007) and lOFC (F(11, 165) = 2.84, p = 0.027, repeated-measures ANOVAs). See main text for additional analyses. (B) Cannula placements based on damage caused by injector cannulae. Gray ovals = mOFC placements. Black rectangles = lOFC placements. One rat was removed due to missed cannula placements. *p < 0.05, **p < 0.01.
Following one month of intermittent access to homecage EtOH, rats were trained to self-administer 20% EtOH on an FR1 (5–10 days), then FR2 (3–6 days), and then FR3 (3–5 days) schedule. Following stable acquisition of FR3 self-administration, rats were stereotaxically implanted with cannulae directed to either lOFC or mOFC (n = 16 each). At the end of all studies, animals were perfused and OFC sections were stained to identify cannula location (Fig. 1B). One animal was removed from the mOFC group based on missed placements, and one animal died during surgery, leaving 16 lOFC and 14 mOFC subjects included in the analyses below.
3.2. Neither lOFC or mOFC inactivation altered ethanol self-administration
Following recovery from surgery, rats were retrained to self-administer 20% EtOH for 4–10 days to reach pre-surgical levels of operant responding. After the final day of retraining, rats received a sham infusion (injector cannula connected to guide cannula but no solution infused) followed by a test session. On the following day, rats received 0.6 (lOFC) or 0.3 (mOFC) μl of either B+M or aCSF into each hemisphere. After 10 min, rats were tested on FR3 EtOH seeking. The next day rats performed FR3 EtOH seeking with no treatment. The day after that, rats received the opposite treatment (aCSF or B+M) and performed a final day of FR3 EtOH self-administration. There were no extinction sessions preceding FR3 tests. As shown in Fig. 2, there were no effects of B+M treatment in either mOFC or lOFC on active or inactive nosepokes or number of infusions received (mOFC nosepokes: (t(13) = 0.37, p = 0.72; mOFC infusions: (t(13) = 0.61, p = 0.55; lOFC nosepokes: (t(15) = 0.60, p = 0.56; lOFC infusions: (t(15) = 0.43, p = 0.68). Thus, neither mOFC nor lOFC inactivation influenced reinforced EtOH seeking on a FR3 schedule.
Fig. 2.
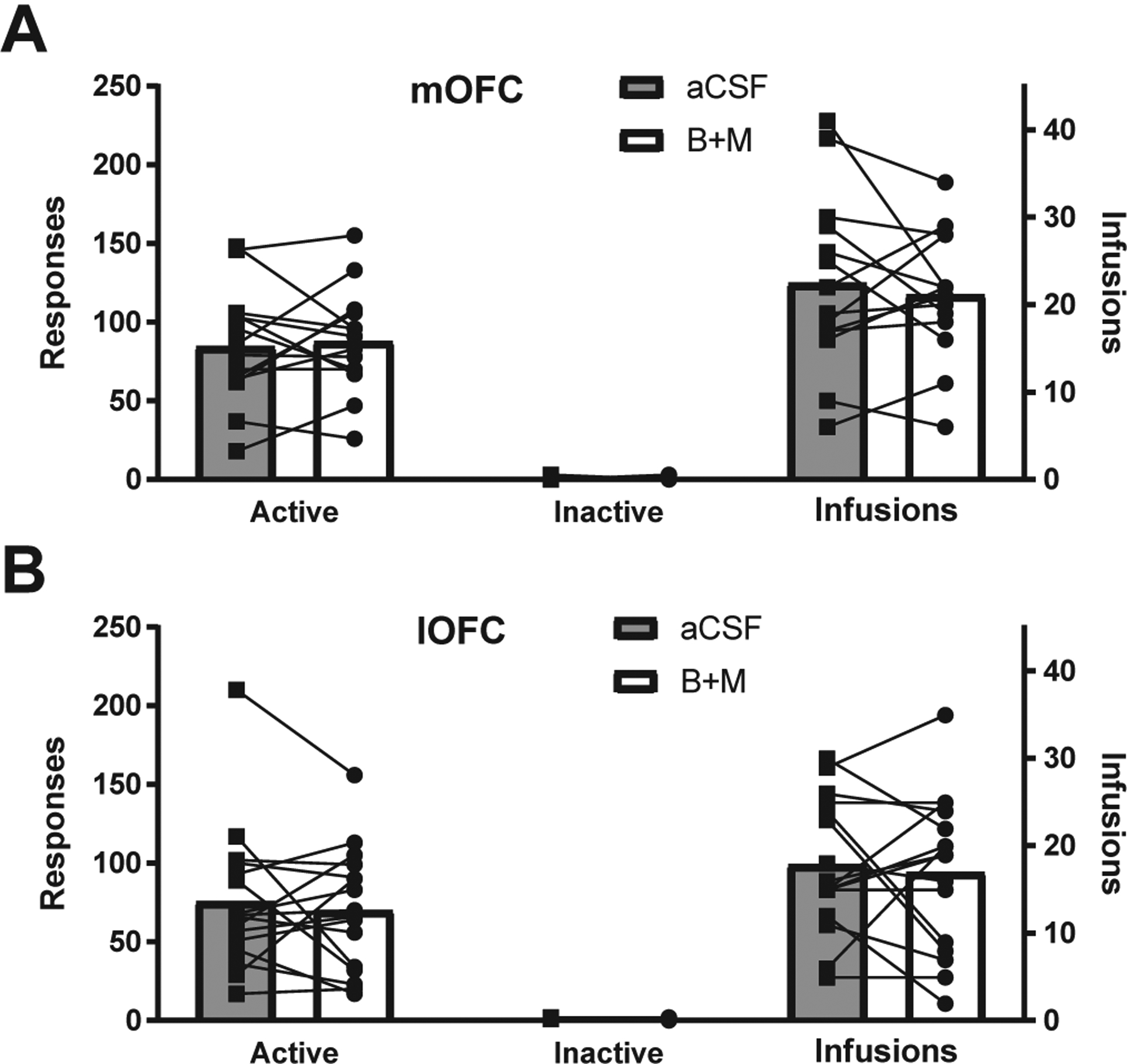
OFC inactivation did not significantly influence EtOH self-administration. There were no significant decreases (as measured by paired t-tests) in active nosepokes, infusions, or inactive nosepokes after inactivation of either (A) mOFC (n = 14) or (B) lOFC (n = 16).
3.3. lOFC, but not mOFC inactivation decreased cue-induced reinstatement of ethanol seeking
Rats were then extinguished to the point of performing fewer than 15 nosepokes per session for 2 consecutive days. During extinction sessions, neither active nor inactive nosepokes produced any outcomes, but both responses were recorded. Rats extinguished in 10.3 ± 1.3 days on average, but extinction rates were variable (min = 4, max = 32 days). Number of extinction sessions to reach criteria were variable across individuals due to the use of a set response criteria, as opposed to a set number of days. Although using a set number of days would produce equivalent exposure across rats, it would potentially result in variable degrees of extinction learning, so we chose to use response-based criteria. The last four days of extinction responding are shown in Fig. 3A and B. Upon reaching extinction criteria, rats received cue-induced reinstatement during which active nosepokes produced the tone previously associated with EtOH, but no EtOH was delivered. Prior to testing rats received an infusion of B+M or aCSF into mOFC or lOFC. Rats were then re-extinguished back to criteria and tested with a second cue-induced reinstatement after receiving the opposite treatment (aCSF or B+M). As shown in Fig. 3D, lOFC inactivation significantly decreased active nosepokes during reinstatement to extinction levels (B +M vs. saline: t(15) = 3.31, p = 0.0048, B+M vs. extinction: t(15) = 1.44, p = 0.17) and number of “infusions” (i.e., cues elicited) (t(15) = 3.25, p = 0.0054). Although mOFC inactivation did slightly decrease reinstatement nosepokes and alcohol cues elicited, this effect was not significant (nosepokes: (t(13) = 1.33, p = 0.21); infusions: (t(13) = 0.93, p = 0.37), and inactivation-associated poking was significantly higher than during extinction (t(13) = 6.08, p < 0.0001), Fig. 3C. There was a small, but significant decrease in inactive nose-poking following medial inactivation (t(13) = 2.69, p = 0.02; mean of 0.66 vs. 0 inactive nosepokes), but not lateral inactivation (t(15) = 0.22, p = 0.83; mean of 0.25 vs. 0.19 inactive nosepokes). Reinstatement responding was greater in mOFC vs. lOFC treated animal on average, both under aCSF and B+M treatment (B+M: t(28) = 4.24, p = 0.0002; aCSF: t(28) = 3.19, p = 0.0035).
Fig. 3.
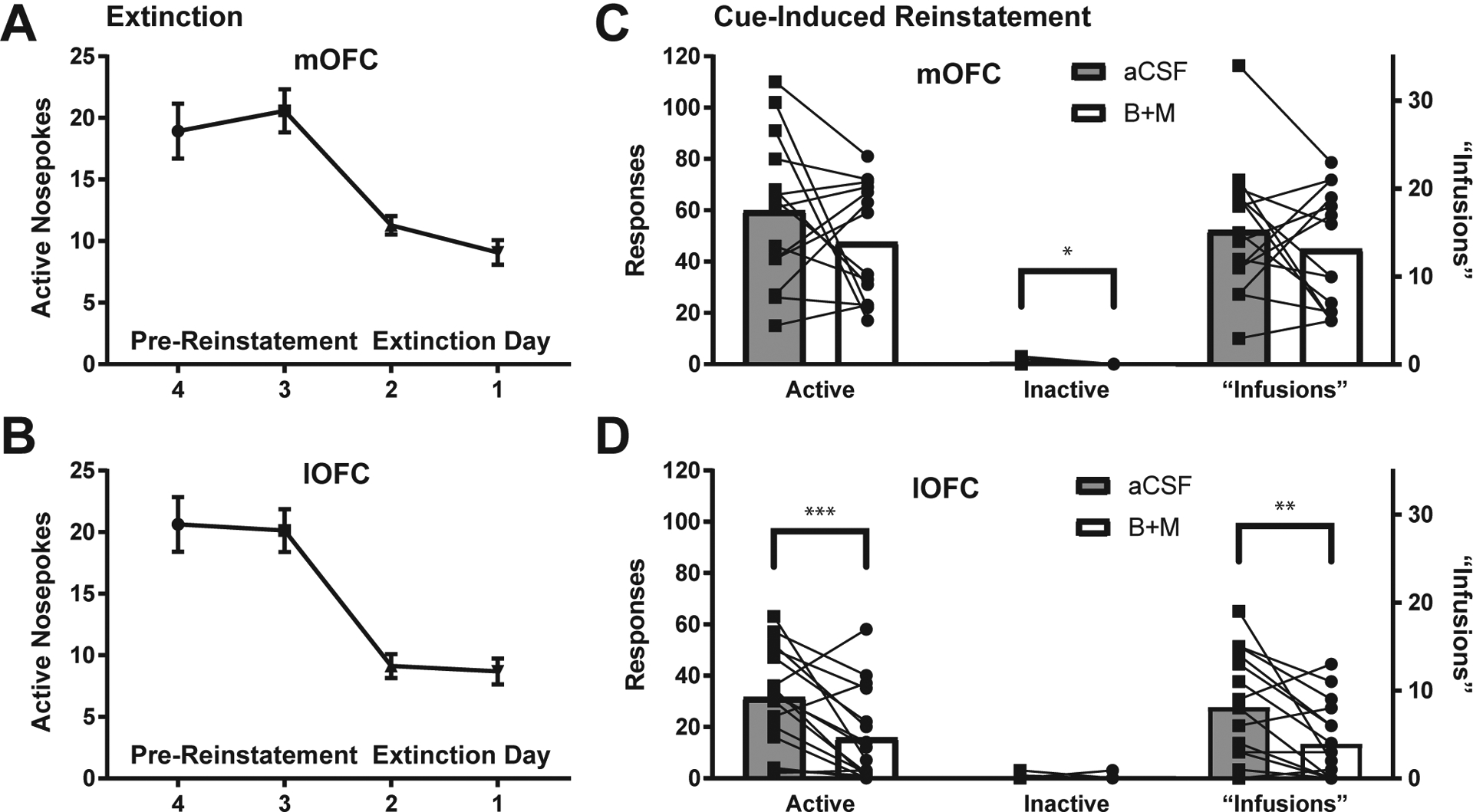
lOFC (n = 16), but not mOFC (n = 14) inactivation significantly decreased cue-induced reinstatement of EtOH seeking. (A) Extinction curve for mOFC-implanted rats. (B) Extinction curve for lOFC-implanted rats. (C) mOFC inactivation did not significantly alter active nosepokes or ethanol-cues received. Inactive nosepokes were slightly, but significantly decreased after mOFC inactivation (t(13) = 2.69, p = 0.02, paired t-test). (D) lOFC inactivation significantly decreased active nosepokes (t(15) = 3.31, p = 0.0048, paired t-test), and “infusions” received, i.e., cues elicited (t(15) = 3.25, p = 0.0054)., but not inactive nosepokes. See main text for additional analyses. *p < 0.05, **p < 0.01, ***p < 0.005.
3.4. Neither lOFC or mOFC inactivation altered extinction of ethanol seeking
Following reinstatement testing, 14 mOFC-implanted and 8 lOFC-implanted rats were re-extinguished to criteria and the effect of B+M or aCSF was tested on extinction responding. Extinction inactivation was performed after reinstatement to prevent any possible influence of extinction inactivation on reinstatement behavior. As shown in Fig. 4, there was no effect of either mOFC or lOFC inactivation on active or inactive nosepokes during extinction (mOFC: (t(13) = 0.84, p = 0.41; lOFC: t(7) = 0.46, p = 0.66). Infusions are not shown because no alcohol or cues were delivered.
Fig. 4.
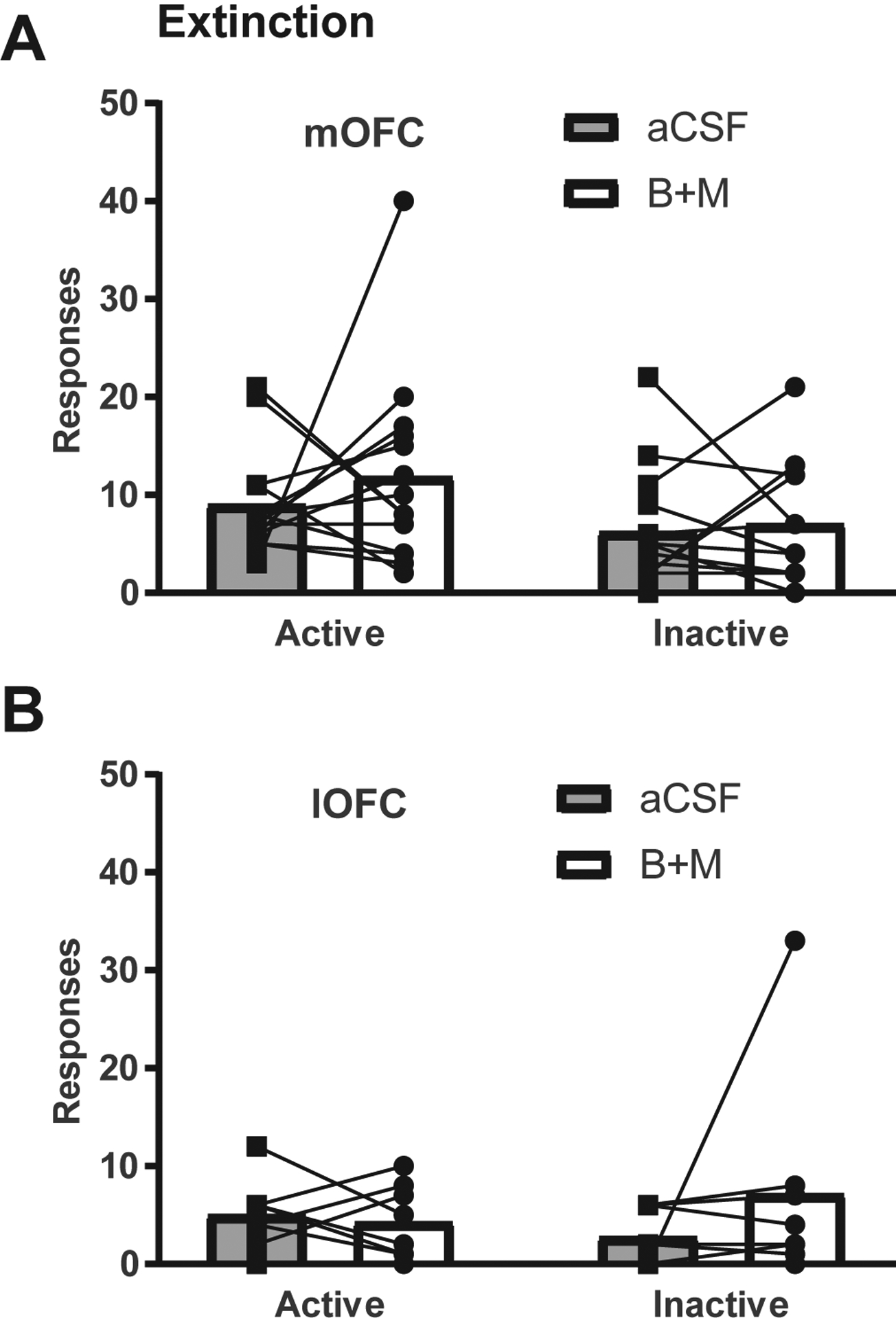
OFC inactivation did not significantly affect extinction of EtOH seeking. There were no significant effects of inactivation of (A) mOFC (n = 14) or (B) lOFC (n = 8) on active or inactive nosepokes.
3.5. Neither lOFC or mOFC inactivation significantly altered ethanol progressive ratio responding
After extinction inactivations, 14 mOFC-implanted and 8 lOFC-implanted rats were re-trained on FR3 self-administration for EtOH and then tested on progressive ration EtOH seeking (PR). Rats received at least 3 days of FR3 followed by PR testing under either B+M or aCSF treatment. Rats then received 2 days of FR3 followed by a second PR test with alternate treatment (aCSF or B+M). The impact of OFC inactivation on PR responding is shown in Fig. 5. mOFC inactivation produced no consistent effects (nosepokes: (t(13) = 0.002, p = 0.998; infusions: (t(13) = 0.08, p = 0.93). lOFC inactivation decreased active nosepokes in 7/8 rats tested, but this effect was not significant (nose-pokes: (t(7) = 2.16, p = 0.068; infusions: (t(7) = 2.00, p = 0.09).
Fig. 5.
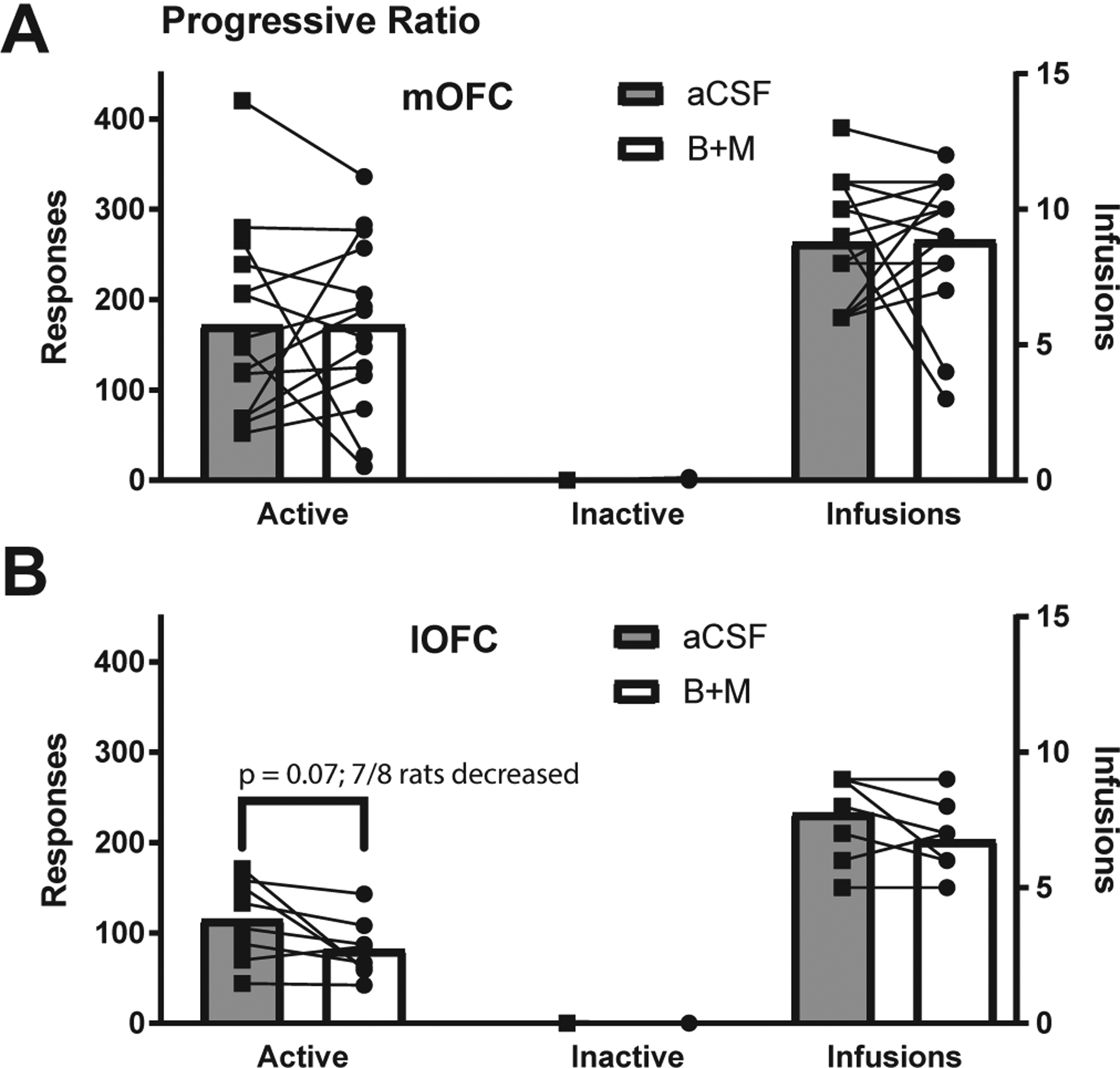
OFC inactivation did not significantly affect EtOH seeking under a progressive ratio schedule. (A) mOFC inactivation (n = 14) did not influence nosepokes or infusions. (B) lOFC inactivation (n = 8) decreased active nose-pokes and infusions, but the effect was not significant (nosepokes: (t(7) = 2.16, p = 0.068; infusions: (t(7) = 2.00, p = 0.09, paired t-test).
4. Discussion
A growing amount of research to date indicates that the OFC is involved in alcohol use, abuse, and use disorder in humans – OFC structure and function is disrupted after chronic alcohol use and craving for alcohol activates OFC in heavy drinkers (Moorman, 2018). Causal work relating OFC function to alcohol use in animal models to date has been more limited in scope. The goal of this study was to characterize the effect of OFC inactivation on a range of alcohol seeking behaviors. A second goal was to determine potential differences between OFC sub-regions and alcohol seeking. Our results show that lOFC plays a role in alcohol seeking, but primarily during reinstatement seeking, and potentially during progressive ratio testing, when motivation levels are high and either no or limited primary reinforcer is available to guide behavior. In contrast, we found no significant effects of mOFC inactivation on alcohol seeking, either when alcohol was available or during reinstatement.
Our results are novel in that they are the first to demonstrate, in animal models, a role for OFC in instrumental alcohol seeking, to characterize contributions to specific aspects of alcohol-seeking behavior, and to show that there are regional differences in OFC regulation of alcohol seeking. Previous studies have shown that chronic alcohol disrupts OFC-associated cognitive functions such as behavioral flexibility (Badanich et al., 2011; Coleman et al., 2014; Fernandez et al., 2017; Gass et al., 2014; Hu et al., 2015; Kroener et al., 2012; Obernier et al., 2002; Rodberg et al., 2017; Trantham-Davidson et al., 2014) and elevates risky decision making (Boutros et al., 2014; Clark et al., 2012; McMurray et al., 2014, 2016; Nasrallah et al., 2009, 2011; Schindler et al., 2014). Acute alcohol increases OFC activation as measured by c-Fos expression (Knapp et al., 2001; Li et al., 2010; Ryabinin et al., 1997; Vilpoux et al., 2009) and inhbits OFC neuronal activity as measured by in vitro electrophysiology (Badanich et al., 2013). Chronic ethanol exposure either increases (Nimitvilai et al., 2016; Radke et al., 2017) or decreases (Renteria et al., 2018) OFC excitation in vitro and results in spine morphological changes (McGuier et al., 2015). Although there is some variability in the results of mechanistic measures of the effect of alcohol on OFC function in animal models, there are clearly profound influences.
Because the OFC is well known to drive motivated behavior for both natural and drug reinforcers, we were interested in what function the OFC plays in vivo during alcohol seeking. To date there have been few studies addressing this question. Expression of c-Fos and mTORC1 is increased in cue-induced alcohol seeking (Barak et al., 2013; Jupp et al., 2011; Laguesse et al., 2017). den Hartog and colleagues showed that either lesions or DREADD inactivation of lOFC increased alcohol drinking in male adult C57Bl/6J mice (den Hartog et al., 2016). However, these effects were only seen in mice treated with chronic intermittent ethanol vapor exposure – there was no impact of OFC manipulation in ethanol drinking mice unexposed to this model of dependence. Importantly, the authors observed less suppression of drinking by the presence of normally-aversive quinine in lOFC lesioned mice, suggesting that a key role for lOFC is to regulate or control drinking, and that when it is disrupted, animals switch to compulsive or habitual drinking. Other studies have identified a role for OFC in goal directed behavior (Barker et al., 2015; Gremel and Costa, 2013). For example, DREADD activation of OFC has recently been shown to restore goal directed behavior for food that is disrupted following chronic intermittent ethanol exposure (Renteria et al., 2018).
Our studies did not directly test goal-directed vs. habitual seeking per se – techniques such as random ratios or devaluation were not employed. However, our results are in alignment with the role of OFC in motivation for alcohol. Previous studies have demonstrated a role for OFC in encoding outcome value and signaling motivation (Balleine et al., 2011; Dalley et al., 2004; Kringelbach, 2005; Mainen and Kepecs, 2009; Moorman and Aston-Jones, 2014; Noonan et al., 2012; O’Doherty, 2007; Padoa-Schioppa, 2011; Rolls and Grabenhorst, 2008; Rudebeck and Murray, 2014; Schoenbaum et al., 2009; Schoenbaum et al., 2011; van Duuren et al., 2009; van Wingerden et al., 2010; Wallis, 2011; Walton et al., 2011). In particular, lOFC inactivation has been shown to reduce cue-and context-induced reinstatement of cocaine seeking (Arguello et al., 2017; Fuchs et al., 2004; Lasseter et al., 2009, 2011). The OFC is also well positioned to regulate highly motivated seeking via connectivity with BLA, NAc, LH, and VTA, among other brain areas historically associated with reward/reinforcement, including ethanol, seeking (Floyd et al., 2001; Gabbott et al., 2005; Heilbronner et al., 2016; Hoover and Vertes, 2011; Reep et al., 1996; Reynolds and Zahm, 2005; Schoenbaum et al., 2006). Exactly why lOFC inactivation had no effect on FR3 alcohol seeking is not clear. However, this finding is in line with the work of den Hartog and colleagues, who showed no effect of lOFC manipulation in regulated drinking (den Hartog et al., 2016). One intriguing possibility is that OFC plays multiple roles in guiding behavior that are differentially revealed depending on context or testing regimen. So contexts in which goal-vs. habit-related behaviors are investigated may emphasize a role of OFC in goal-directed behavior, tests of intensity of motivation may support a role in particularly high levels of reinforcer seeking, and tests of behavioral flexibility and decision-making will support a role of OFC in these functions. This idea is in line with conceptualization of OFC as representing a map of cognitive states (Lopatina et al., 2017; Schuck et al., 2016; Wilson et al., 2014), only some of which are tested in each experimental paradigm. An important line of future research will be to integrate these multiple roles of the OFC in the context of chronic alcohol to understand how OFC disruption contributes to AUD.
Bianchi and colleagues recently showed that OFC inactivation (broadly, focused more on the medial border between vOFC and lOFC) decreased context-induced reinstatement for ethanol seeking (Bianchi et al., 2018). This generally supports a role for OFC in ethanol reinstatement, as shown here for cue-induced reinstatement. Whether these effects were driven by mOFC vs. lOFC are unclear as this was not directly tested. The results presented here extend those findings in three ways. First, we showed that lOFC inactivation disrupted cue-induced reinstatement of ethanol seeking. Second, we showed that lateral, but not medial OFC inactivation disrupted reinstatement of ethanol seeking. Third we showed that OFC-inactivation selectively disrupted reinstatement, but not ethanol-reinforced ethanol seeking or extinction. Of interest, recent work by Morisot and colleagues showed that mTORC1 inhibition in lOFC decreased ethanol seeking during extinction, but not during reinforced ethanol seeking, generally supporting both our findings and those of Bianchi and colleagues (Morisot et al., 2019). The fact that we observed no impact of OFC inactivation during extinction does slightly differentiate our findings from theirs, but the overall observation that OFC seems important for non-reinforced seeking is a common theme that suggests an important set of questions to follow-up on in future work.
We noted some limitations in the current study. Differences in levels of alcohol seeking between lOFC and mOFC groups may have confounded interpretation of null effects in mOFC-inactivated animals. However, there were no significant correlations between effects of OFC manipulation and basal motivation, as measured by homecage alcohol consumption or nosepokes during any stage of operant testing (all tests p > 0.05, data not shown). Furthermore, we observed no reliable inhibition of responding in either high- or low-drinking/seeking mOFC-inactivated rats, and lOFC inactivation decreased reinstatement in both high- and low-drinking/seeking rats. Together, these results support the proposal that our findings are based more on region-specific differences, as opposed to individual differences. Ultimately, further studies should be done to disentangle aspects of motivation and OFC function, through the use of inactivation and seeking studies such as investigated here. Those studies are currently underway in our laboratory (Hernandez et al., 2017). An additional limitation is the lower number of lOFC inactivation rats tested during progressive ratio (8 vs. 16 in other circumstances). We observed a decrease in seeking under progressive ratio conditions in 7/8 rats tested, but this effect was not significant. Whether this effect would be more reliable if larger numbers of animals are tested remains to be explored, but future tests along these lines will help strengthen our understanding of the relationship between OFC function and motivation to acquire alcohol. Despite these limitations, this series of studies is the first to demonstrate a causal effect of OFC manipulation on alcohol seeking in an operant context, specifically during cue-induced reinstatement.
Under the assumption that confounds did not obscure a legitimate lOFC/mOFC difference, our results speak to the growing recognition of OFC regional heterogeneity. As noted above, lOFC but not mOFC inactivation decreased reinstatement of cocaine seeking. Other differences in rodent OFC region have identified separable roles in high-vs. low-value outcome representation or behavioral task structure (Burton et al., 2014; Lopatina et al., 2015, 2016, 2017), and response-inhibition vs. outcome representation (Dalton et al., 2016; Gourley et al., 2010, 2016; Mar et al., 2011; Stopper et al., 2014), and in cocaine seeking (Fuchs et al., 2004). An important question that remains is whether effects of lateral OFC inactivation specifically diminishes reinstatement of drug seeking, as opposed to natural reinforcers. Previous OFC lesion studies have found limited influence on reinstatement of food seeking (Grakalic et al., 2010) but work from our lab indicates that OFC inactivation does suppress reinstatement of seeking of a sucrose solution (Hernandez et al., 2017). Thus this question remains an important line of future investigation.
Results from human studies of chronic alcohol users repeatedly demonstrate an involvement of the OFC in aspects of particularly highly-motivated alcohol use. The details of which functions specific OFC subregions are playing in alcohol use or dependence still remain to be fully characterized. The results of the current study, combined with other recent findings related to the OFC and alcohol use (Moorman, 2018), confirm that OFC function, potentially driving elevated alcohol seeking, or dysfunction, potentially disrupting other flexible goal-directed decisions and behaviors, during alcohol use is an important topic to pursue and may lead to further insights related to the neural basis of AUD.
HIGHLIGHTS.
Orbitofrontal cortex (OFC) is involved in motivation for natural and drug reinforcers.
There is growing interest in OFC contributions to alcohol seeking.
Rat OFC inactivation decreased cue-induced alcohol reinstatement.
This effect was selective for lateral vs. medial OFC.
There were no significant effects on alcohol seeking during FR3 self-administration, progressive ratio, or extinction.
Acknowledgments
Thanks to Dr. Stan Floresco for helpful comments on the manuscript.
Funding
This work was supported by National Institutes of Health research grants AA024571, AA025481, and DA041674 and a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation.
Footnotes
CRediT authorship contribution statement
Ifeyinwa Arinze: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing - original draft, Writing - review & editing. David E. Moorman: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing.
References
- Abernathy K, Chandler LJ, Woodward JJ, 2010. Alcohol and the prefrontal cortex. Int. Rev. Neurobiol 91, 289–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arguello AA, Richardson BD, Hall JL, Wang R, Hodges MA, Mitchell MP, Stuber GD, Rossi DJ, Fuchs RA, 2017. Role of a lateral orbital frontal cortex-basolateral amygdala circuit in cue-induced cocaine-seeking behavior. Neuropsychopharmacology 42, 727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asensio S, Morales JL, Senabre I, Romero MJ, Beltran MA, Flores-Bellver M, Barcia JM, Romero FJ, 2016. Magnetic resonance imaging structural alterations in brain of alcohol abusers and its association with impulsivity. Addiction Biol. 21, 962–971. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Becker HC, Woodward JJ, 2011. Effects of chronic intermittent ethanol exposure on orbitofrontal and medial prefrontal cortex-dependent behaviors in mice. Behav. Neurosci 125, 879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badanich KA, Mulholland PJ, Beckley JT, Trantham-Davidson H, Woodward JJ, 2013. Ethanol reduces neuronal excitability of lateral orbitofrontal cortex neurons via a glycine receptor dependent mechanism. Neuropsychopharmacology 38, 1176–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Leung BK, Ostlund SB, 2011. The orbitofrontal cortex, predicted value, and choice. Ann. N. Y. Acad. Sci 1239, 43–50. [DOI] [PubMed] [Google Scholar]
- Barak S, Liu F, Ben Hamida S, Yowell QV, Neasta J, Kharazia V, Janak PH, Ron D, 2013. Disruption of alcohol-related memories by mTORC1 inhibition prevents relapse. Nat. Neurosci 16, 1111–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Corbit LH, Robinson DL, Gremel CM, Gonzales RA, Chandler LJ, 2015. Corticostriatal circuitry and habitual ethanol seeking. Alcohol 49, 817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, 2004. The role of emotion in decision-making: evidence from neurological patients with orbitofrontal damage. Brain Cognit. 55, 30–40. [DOI] [PubMed] [Google Scholar]
- Beck A, Wustenberg T, Genauck A, Wrase J, Schlagenhauf F, Smolka MN, Mann K, Heinz A, 2012. Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Arch. Gen. Psychiatr 69, 842–852. [DOI] [PubMed] [Google Scholar]
- Bianchi PC, Carneiro de Oliveira PE, Palombo P, Leao RM, Cogo-Moreira H, Planeta CDS, Cruz FC, 2018. Functional inactivation of the orbitofrontal cortex disrupts context-induced reinstatement of alcohol seeking in rats. Drug Alcohol Depend. 186, 102–112. [DOI] [PubMed] [Google Scholar]
- Blaine SK, Sinha R, 2017. Alcohol, stress, and glucocorticoids: from risk to dependence and relapse in alcohol use disorders. Neuropharmacology 122, 136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine SK, Seo D, Sinha R, 2017. Peripheral and prefrontal stress system markers and risk of relapse in alcoholism. Addiction Biol. 22, 468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger CA, Mitchell JM, Tavares VC, Robertson M, Joslyn G, D’Esposito M, Fields HL, 2007. Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-O-methyltransferase 158(Val/Val) genotype. J. Neurosci 27, 14383–14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger CA, Kelley EA, Mitchell JM, D’Esposito M, Fields HL, 2009. Now or Later? An fMRI study of the effects of endogenous opioid blockade on a decision-making network. Pharmacol. Biochem. Behav 93, 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros N, Semenova S, Liu W, Crews FT, Markou A, 2014. Adolescent intermittent ethanol exposure is associated with increased risky choice and decreased dopaminergic and cholinergic neuron markers in adult rats. Int. J. Neuropsychopharmacol 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton AC, Kashtelyan V, Bryden DW, Roesch MR, 2014. Increased firing to cues that predict low-value reward in the medial orbitofrontal cortex. Cerebr. Cortex 24, 3310–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J, 2003. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology 168, 66–74. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Durazzo TC, Gazdzinski S, Mon A, Studholme C, Meyerhoff DJ, 2011. Brain morphology at entry into treatment for alcohol dependence is related to relapse propensity. Biol. Psychiatr 70, 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ron D, Barak S, 2014. Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol 48, 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catafau AM, Etcheberrigaray A, Perez de los Cobos J, Estorch M, Guardia J, Flotats A, Berna L, Mari C, Casas M, Carrio, 1999. Regional cerebral blood flow changes in chronic alcoholic patients induced by naltrexone challenge during detoxification. J. Nucl. Med 40, 19–24. [PubMed] [Google Scholar]
- Clark JJ, Nasrallah NA, Hart AS, Collins AL, Bernstein IL, Phillips PE, 2012. Altered risk-based decision making following adolescent alcohol use results from an imbalance in reinforcement learning in rats. PloS One 7 e37357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus ED, Kiehl KA, Hutchison KE, 2011. Neural and behavioral mechanisms of impulsive choice in alcohol use disorder. Alcohol Clin. Exp. Res 35, 1209–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG Jr., Liu W, Oguz I, Styner M, Crews FT, 2014. Adolescent binge ethanol treatment alters adult brain regional volumes, cortical extracellular matrix protein and behavioral flexibility. Pharmacol. Biochem. Behav 116, 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Boettiger CA, 2009. Impulsivity, frontal lobes and risk for addiction. Pharmacol. Biochem. Behav 93, 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW, 2004. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci. Biobehav. Rev 28, 771–784. [DOI] [PubMed] [Google Scholar]
- Dalton GL, Wang NY, Phillips AG, Floresco SB, 2016. Multifaceted contributions by different regions of the orbitofrontal and medial prefrontal cortex to probabilistic reversal learning. J. Neurosci 36, 1996–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirakca T, Ende G, Kammerer N, Welzel-Marquez H, Hermann D, Heinz A, Mann K, 2011. Effects of alcoholism and continued abstinence on brain volumes in both genders. Alcohol Clin. Exp. Res 35, 1678–1685. [DOI] [PubMed] [Google Scholar]
- den Hartog C, Zamudio-Bulcock P, Nimitvilai S, Gilstrap M, Eaton B, Fedarovich H, Motts A, Woodward JJ, 2016. Inactivation of the lateral orbitofrontal cortex increases drinking in ethanol-dependent but not non-dependent mice. Neuropharmacology 107, 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dom G, Sabbe B, Hulstijn W, van den Brink W, 2005. Substance use disorders and the orbitofrontal cortex: systematic review of behavioural decision-making and neuroimaging studies. Br. J. Psychiatry 187, 209–220. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Tosun D, Buckley S, Gazdzinski S, Mon A, Fryer SL, Meyerhoff DJ, 2011. Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: relationships to relapse and extended abstinence. Alcohol Clin. Exp. Res 35, 1187–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Hutcheson DM, Ersche KD, Pelloux Y, Dalley JW, Robbins TW, 2007. The orbital prefrontal cortex and drug addiction in laboratory animals and humans. Ann. N. Y. Acad. Sci 1121, 576–597. [DOI] [PubMed] [Google Scholar]
- Fernandez GM, Lew BJ, Vedder LC, Savage LM, 2017. Chronic intermittent ethanol exposure leads to alterations in brain-derived neurotrophic factor within the frontal cortex and impaired behavioral flexibility in both adolescent and adult rats. Neuroscience 348, 324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R, 2001. Orbitomedial prefrontal cortical projections to hypothalamus in the rat. J. Comp. Neurol 432, 307–328. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Rodriguez EE, Musselman S, Narendran R, 2014. Prefrontal response and frontostriatal functional connectivity to monetary reward in abstinent alcohol-dependent young adults. PloS One 9 e94640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, See RE, 2004. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J. Neurosci 24, 6600–6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ, 2005. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J. Comp. Neurol 492, 145–177. [DOI] [PubMed] [Google Scholar]
- Gass JT, Glen WB Jr., McGonigal JT, Trantham-Davidson H, Lopez MF, Randall PK, Yaxley R, Floresco SB, Chandler LJ, 2014. Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacology 39, 2570–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Hope BT, 2017. Cortical and amygdalar neuronal ensembles in alcohol seeking, drinking and withdrawal. Neuropharmacology 122, 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glascher J, Adolphs R, Damasio H, Bechara A, Rudrauf D, Calamia M, Paul LK, Tranel D, 2012. Lesion mapping of cognitive control and value-based decision making in the prefrontal cortex. Proc. Natl. Acad. Sci. U. S. A 109, 14681–14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Lee AS, Howell JL, Pittenger C, Taylor JR, 2010. Dissociable regulation of instrumental action within mouse prefrontal cortex. Eur. J. Neurosci 32, 1726–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Zimmermann KS, Allen AG, Taylor JR, 2016. The medial orbitofrontal cortex regulates sensitivity to outcome value. J. Neurosci 36, 4600–4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grakalic I, Panlilio LV, Quiroz C, Schindler CW, 2010. Effects of orbitofrontal cortex lesions on cocaine self-administration. Neuroscience 165, 313–324. [DOI] [PubMed] [Google Scholar]
- Gremel CM, Costa RM, 2013. Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions. Nat. Commun 4, 2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GJ, Jaffin SK, Hodge SM, Kennedy D, Caviness VS, Marinkovic K, Papadimitriou GM, Makris N, Oscar-Berman M, 2008. Frontal white matter and cingulum diffusion tensor imaging deficits in alcoholism. Alcohol Clin. Exp. Res 32, 1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronner SR, Rodriguez-Romaguera J, Quirk GJ, Groenewegen HJ, Haber SN, 2016. Circuit-based corticostriatal homologies between rat and primate. Biol. Psychiatr 80, 509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann D, Smolka MN, Wrase J, Klein S, Nikitopoulos J, Georgi A, Braus DF, Flor H, Mann K, Heinz A, 2006. Blockade of cue-induced brain activation of abstinent alcoholics by a single administration of amisulpride as measured with fMRI. Alcohol Clin. Exp. Res 30, 1349–1354. [DOI] [PubMed] [Google Scholar]
- Hernandez JS, Binette A, Moorman DE, 2017. Selective Effects of Chemogenetic Inhibition of Orbitofrontal Cortex on Operant Ethanol Seeking. Annual Meeting of the Society for Neuroscience, Washington, D.C: p. Program No. 76.05. [Google Scholar]
- Hoover WB, Vertes RP, 2011. Projections of the medial orbital and ventral orbital cortex in the rat. J. Comp. Neurol 519, 3766–3801. [DOI] [PubMed] [Google Scholar]
- Hu W, Morris B, Carrasco A, Kroener S, 2015. Effects of acamprosate on attentional set-shifting and cellular function in the prefrontal cortex of chronic alcohol-exposed mice. Alcohol Clin. Exp. Res 39, 953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson DM, Everitt BJ, 2003. The effects of selective orbitofrontal cortex lesions on the acquisition and performance of cue-controlled cocaine seeking in rats. Ann. N. Y. Acad. Sci 1003, 410–411. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, 2017. Functional heterogeneity within rat orbitofrontal cortex in reward learning and decision making. J. Neurosci 37, 10529–10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Schafer K, Butters N, Cermak LS, 1991. Magnetic resonance imaging of alcoholic Korsakoff patients. Neuropsychopharmacology 4, 175–186. [PubMed] [Google Scholar]
- Jupp B, Krstew E, Dezsi G, Lawrence AJ, 2011. Discrete cue-conditioned alcohol-seeking after protracted abstinence: pattern of neural activation and involvement of orexin(1) receptors. Br. J. Pharmacol 162, 880–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Mashhoon Y, Silverman DN, Janes AC, Goodrich CM, 2009. Role of the orbitofrontal cortex and dorsal striatum in regulating the dose-related effects of self-administered cocaine. Behav. Brain Res 201, 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Yager LM, Brisotti MF, 2013. Impact of medial orbital cortex and medial subthalamic nucleus inactivation, individually and together, on the maintenance of cocaine self-administration behavior in rats. Behav. Brain Res 238, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenowski PM, 2018. Emerging role for the medial prefrontal cortex in alcohol-seeking behaviors. Addict. Behav 77, 102–106. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Braun CJ, Duncan GE, Qian Y, Fernandes A, Crews FT, Breese GR, 2001. Regional specificity of ethanol and NMDA action in brain revealed with FOS-like immunohistochemistry and differential routes of drug administration. Alcohol Clin. Exp. Res 25, 1662–1672. [PubMed] [Google Scholar]
- Koob GF, 2014. Neurocircuitry of alcohol addiction: synthesis from animal models. Handb. Clin. Neurol 125, 33–54. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, 2005. The human orbitofrontal cortex: linking reward to hedonic experience. Nat. Rev. Neurosci 6, 691–702. [DOI] [PubMed] [Google Scholar]
- Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, Chandler LJ, 2012. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PloS One 7 e37541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota M, Nakazaki S, Hirai S, Saeki N, Yamaura A, Kusaka T, 2001. Alcohol consumption and frontal lobe shrinkage: study of 1432 non-alcoholic subjects. J. Neurol. Neurosurg. Psychiatry 71, 104–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuruoglu AC, Arikan Z, Vural G, Karatas M, Arac M, Isik E, 1996. Single photon emission computerised tomography in chronic alcoholism. Antisocial personality disorder may be associated with decreased frontal perfusion. Br. J. Psychiatry 169, 348–354. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Gunning-Dixon F, Vaurio O, Repo-Tiihonen E, Soininen H, Tiihonen J, 2002. Prefrontal volumes in habitually violent subjects with antisocial personality disorder and type 2 alcoholism. Psychiatr. Res 114, 95–102. [DOI] [PubMed] [Google Scholar]
- Laguesse S, Morisot N, Phamluong K, Ron D, 2017. Region specific activation of the AKT and mTORC1 pathway in response to excessive alcohol intake in rodents. Addiction Biol. 22, 1856–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Ramirez DR, Xie X, Fuchs RA, 2009. Involvement of the lateral orbitofrontal cortex in drug context-induced reinstatement of cocaine-seeking behavior in rats. Eur. J. Neurosci 30, 1370–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Wells AM, Xie X, Fuchs RA, 2011. Interaction of the basolateral amygdala and orbitofrontal cortex is critical for drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology 36, 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre AP, Rauchs G, La Joie R, Mezenge F, Boudehent C, Vabret F, Segobin S, Viader F, Allain P, Eustache F, Pitel AL, Beaunieux H, 2014. Impaired decision-making and brain shrinkage in alcoholism. Eur. Psychiatr 29, 125–133. [DOI] [PubMed] [Google Scholar]
- Li J, Cheng Y, Bian W, Liu X, Zhang C, Ye JH, 2010. Region-specific induction of FosB/DeltaFosB by voluntary alcohol intake: effects of naltrexone. Alcohol Clin. Exp. Res 34, 1742–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, Hoy D 3rd, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, Powles J 3rd, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stockl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA, 2012. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, Ernst M, Grant S, Bonson K, Weinstein A, 2000. Orbitofrontal cortex and human drug abuse: functional imaging. Cerebr. Cortex 10, 334–342. [DOI] [PubMed] [Google Scholar]
- Lopatina N, McDannald MA, Styer CV, Sadacca BF, Cheer JF, Schoenbaum G, 2015. Lateral orbitofrontal neurons acquire responses to upshifted, downshifted, or blocked cues during unblocking. Elife 4 e11299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatina N, McDannald MA, Styer CV, Peterson JF, Sadacca BF, Cheer JF, Schoenbaum G, 2016. Medial orbitofrontal neurons preferentially signal cues predicting changes in reward during unblocking. J. Neurosci 36, 8416–8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatina N, Sadacca BF, McDannald MA, Styer CV, Peterson JF, Cheer JF, Schoenbaum G, 2017. Ensembles in medial and lateral orbitofrontal cortex construct cognitive maps emphasizing different features of the behavioral landscape. Behav. Neurosci 131, 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YL, Richardson HN, 2014. Alcohol, stress hormones, and the prefrontal cortex: a proposed pathway to the dark side of addiction. Neuroscience 277, 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucantonio F, Caprioli D, Schoenbaum G, 2014. Transition from ‘model-based’ to ‘model-free’ behavioral control in addiction: involvement of the orbitofrontal cortex and dorsolateral striatum. Neuropharmacology 76 Pt B, 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainen ZF, Kepecs A, 2009. Neural representation of behavioral outcomes in the orbitofrontal cortex. Curr. Opin. Neurobiol 19, 84–91. [DOI] [PubMed] [Google Scholar]
- Mar AC, Walker AL, Theobald DE, Eagle DM, Robbins TW, 2011. Dissociable effects of lesions to orbitofrontal cortex subregions on impulsive choice in the rat. J. Neurosci 31, 6398–6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K, Nicoletti M, Nemoto K, Hatch JP, Peluso MA, Nery FG, Soares JC, 2009. A voxel-based morphometry study of frontal gray matter correlates of impulsivity. Hum. Brain Mapp 30, 1188–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannald MA, Jones JL, Takahashi YK, Schoenbaum G, 2014. Learning theory: a driving force in understanding orbitofrontal function. Neurobiol. Learn. Mem 108, 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuier NS, Padula AE, Lopez MF, Woodward JJ, Mulholland PJ, 2015. Withdrawal from chronic intermittent alcohol exposure increases dendritic spine density in the lateral orbitofrontal cortex of mice. Alcohol 49, 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray MS, Amodeo LR, Roitman JD, 2014. Effects of voluntary alcohol intake on risk preference and behavioral flexibility during rat adolescence. PloS One 9 e100697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray MS, Amodeo LR, Roitman JD, 2016. Consequences of adolescent ethanol consumption on risk preference and orbitofrontal cortex encoding of reward. Neuropsychopharmacology 41, 1366–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, 2018. The role of the orbitofrontal cortex in alcohol use, abuse, and dependence. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 87, 85–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G, 2009. Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol–preferring Sprague–Dawley rats. Alcohol 43, 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G, 2014. Orbitofrontal cortical neurons encode expectation-driven initiation of reward-seeking. J. Neurosci 34, 10234–10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisot N, Phamluong K, Ehinger Y, Berger AL, Moffat JJ, Ron D, 2019. mTORC1 in the orbitofrontal cortex promotes habitual alcohol seeking. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, George MS, 2004. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology 29, 393–402. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K, 2008. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch. Gen. Psychiatr 65, 466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah NA, Yang TW, Bernstein IL, 2009. Long-term risk preference and suboptimal decision making following adolescent alcohol use. Proc. Natl. Acad. Sci. U. S. A 106, 17600–17604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah NA, Clark JJ, Collins AL, Akers CA, Phillips PE, Bernstein IL, 2011. Risk preference following adolescent alcohol use is associated with corrupted encoding of costs but not rewards by mesolimbic dopamine. Proc. Natl. Acad. Sci. U. S. A 108, 5466–5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas JM, Catafau AM, Estruch R, Lomena FJ, Salamero M, Herranz R, Monforte R, Cardenal C, Urbano-Marquez A, 1993. Regional cerebral blood flow-SPECT in chronic alcoholism: relation to neuropsychological testing. J. Nucl. Med 34, 1452–1459. [PubMed] [Google Scholar]
- Nimitvilai S, Lopez MF, Mulholland PJ, Woodward JJ, 2016. Chronic intermittent ethanol exposure enhances the excitability and synaptic plasticity of lateral orbito-frontal cortex neurons and induces a tolerance to the acute inhibitory actions of ethanol. Neuropsychopharmacology 41, 1112–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimitvilai S, Lopez MF, Mulholland PJ, Woodward JJ, 2017. Ethanol dependence abolishes monoamine and GIRK (Kir3) channel inhibition of orbitofrontal cortex excitability. Neuropsychopharmacology 42, 1800–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MP, Kolling N, Walton ME, Rushworth MF, 2012. Re-evaluating the role of the orbitofrontal cortex in reward and reinforcement. Eur. J. Neurosci 35, 997–1010. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, 2007. Lights, camembert, action! the role of human orbitofrontal cortex in encoding stimuli, rewards, and choices. Ann. N. Y. Acad. Sci 1121, 254–272. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F, 2001. Representation of pleasant and aversive taste in the human brain. J. Neurophysiol 85, 1315–1321. [DOI] [PubMed] [Google Scholar]
- O’Neill J, Cardenas VA, Meyerhoff DJ, 2001. Effects of abstinence on the brain: quantitative magnetic resonance imaging and magnetic resonance spectroscopic imaging in chronic alcohol abuse. Alcohol Clin. Exp. Res 25, 1673–1682. [PubMed] [Google Scholar]
- Obernier JA, White AM, Swartzwelder HS, Crews FT, 2002. Cognitive deficits and CNS damage after a 4-day binge ethanol exposure in rats. Pharmacol. Biochem. Behav 72, 521–532. [DOI] [PubMed] [Google Scholar]
- Padoa-Schioppa C, 2011. Neurobiology of economic choice: a good-based model. Annu. Rev. Neurosci 34, 333–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Schoenbaum G, 2015. Dialogue on economic choice, learning theory, and neuronal representations. Curr. Opin. Behav. Sci 5, 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 2007. The Rat Brain in Stereotaxic Coordinates. Academic Press/Elsevier, Amsterdam ; Boston. [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Crusan K, Jernigan TL, 1988. Brain CT changes in alcoholics: effects of age and alcohol consumption. Alcohol Clin. Exp. Res 12, 81–87. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO, 1997. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin. Exp. Res 21, 521–529. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO, 1998. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Arch. Gen. Psychiatr 55, 905–912. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, 2000. Orbital and medial prefrontal cortex and psychostimulant abuse: studies in animal models. Cerebr. Cortex 10, 326–333. [DOI] [PubMed] [Google Scholar]
- Radke AK, Jury NJ, Kocharian A, Marcinkiewcz CA, Lowery-Gionta EG, Pleil KE, McElligott ZA, McKlveen JM, Kash TL, Holmes A, 2017. Chronic EtOH effects on putative measures of compulsive behavior in mice. Addiction Biol. 22, 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando K, Hong KI, Bhagwagar Z, Li CS, Bergquist K, Guarnaccia J, Sinha R, 2011. Association of frontal and posterior cortical gray matter volume with time to alcohol relapse: a prospective study. Am. J. Psychiatr 168, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reep RL, Corwin JV, King V, 1996. Neuronal connections of orbital cortex in rats: topography of cortical and thalamic afferents. Exp. Brain Res 111, 215–232. [DOI] [PubMed] [Google Scholar]
- Reinhard I, Lemenager T, Fauth-Buhler M, Hermann D, Hoffmann S, Heinz A, Kiefer F, Smolka MN, Wellek S, Mann K, Vollstadt-Klein S, 2015. A comparison of region-of-interest measures for extracting whole brain data using survival analysis in alcoholism as an example. J. Neurosci. Methods 242, 58–64. [DOI] [PubMed] [Google Scholar]
- Renteria R, Baltz ET, Gremel CM, 2018. Chronic alcohol exposure disrupts top-down control over basal ganglia action selection to produce habits. Nat. Commun 9, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Zahm DS, 2005. Specificity in the projections of prefrontal and insular cortex to ventral striatopallidum and the extended amygdala. J. Neurosci 25, 11757–11767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC, 1996. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J. Neurosci. Methods 66, 1–11. [DOI] [PubMed] [Google Scholar]
- Rodberg EM, den Hartog CR, Anderson RI, Becker HC, Moorman DE, Vazey EM, 2017. Stress facilitates the development of cognitive dysfunction after chronic ethanol exposure. Alcohol Clin. Exp. Res 41, 1574–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, 2015. Taste, olfactory, and food reward value processing in the brain. Prog. Neurobiol 127–128, 64–90. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F, 2008. The orbitofrontal cortex and beyond: from affect to decision-making. Prog. Neurobiol 86, 216–244. [DOI] [PubMed] [Google Scholar]
- Rosenbloom MJ, Pfefferbaum A, 2008. Magnetic resonance imaging of the living brain: evidence for brain degeneration among alcoholics and recovery with abstinence. Alcohol Res. Health 31, 362–376. [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Murray EA, 2014. The orbitofrontal oracle: cortical mechanisms for the prediction and evaluation of specific behavioral outcomes. Neuron 84, 1143–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabinin AE, Criado JR, Henriksen SJ, Bloom FE, Wilson MC, 1997. Differential sensitivity of c-Fos expression in hippocampus and other brain regions to moderate and low doses of alcohol. Mol. Psychiatr 2, 32–43. [DOI] [PubMed] [Google Scholar]
- Schindler AG, Tsutsui KT, Clark JJ, 2014. Chronic alcohol intake during adolescence, but not adulthood, promotes persistent deficits in risk-based decision making. Alcohol Clin. Exp. Res 38, 1622–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Shaham Y, 2008. The role of orbitofrontal cortex in drug addiction: a review of preclinical studies. Biol. Psychiatr 63, 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA, 2006. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci. 29, 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK, 2009. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nat. Rev. Neurosci 10, 885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Takahashi Y, Liu TL, McDannald MA, 2011. Does the orbitofrontal cortex signal value? Ann. N. Y. Acad. Sci 1239, 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck NW, Cai MB, Wilson RC, Niv Y, 2016. Human orbitofrontal cortex represents a cognitive map of state space. Neuron 91, 1402–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Jia Z, Lacadie CM, Tsou KA, Bergquist K, Sinha R, 2011. Sex differences in neural responses to stress and alcohol context cues. Hum. Brain Mapp 32, 1998–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Lacadie CM, Tuit K, Hong KI, Constable RT, Sinha R, 2013. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatr. 70, 727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE, 2008. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin. Exp. Res 32, 1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker TA, Cooch NK, Schoenbaum G, 2015. What the orbitofrontal cortex does not do. Nat. Neurosci 18, 620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolyarova A, Izquierdo A, 2017. Complementary contributions of basolateral amygdala and orbitofrontal cortex to value learning under uncertainty. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopper CM, Green EB, Floresco SB, 2014. Selective involvement by the medial orbitofrontal cortex in biasing risky, but not impulsive, choice. Cerebr. Cortex 24, 154–162. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A, 2005. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology 180, 583–594. [DOI] [PubMed] [Google Scholar]
- Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T, Banich M, 2009. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biol. Psychiatr 65, 160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Meloy MJ, Brown SA, 2003. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Arch. Gen. Psychiatr 60, 727–735. [DOI] [PubMed] [Google Scholar]
- Thayer RE, Hagerty SL, Sabbineni A, Claus ED, Hutchison KE, Weiland BJ, 2016. Negative and interactive effects of sex, aging, and alcohol abuse on gray matter morphometry. Hum. Brain Mapp 37, 2276–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trantham-Davidson H, Burnett EJ, Gass JT, Lopez MF, Mulholland PJ, Centanni SW, Floresco SB, Chandler LJ, 2014. Chronic alcohol disrupts dopamine receptor activity and the cognitive function of the medial prefrontal cortex. J. Neurosci 34, 3706–3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay L, Schultz W, 1999. Relative reward preference in primate orbitofrontal cortex. Nature 398, 704–708. [DOI] [PubMed] [Google Scholar]
- van Duuren E, van der Plasse G, Lankelma J, Joosten RN, Feenstra MG, Pennartz CM, 2009. Single-cell and population coding of expected reward probability in the orbitofrontal cortex of the rat. J. Neurosci 29, 8965–8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wingerden M, Vinck M, Lankelma J, Pennartz CM, 2010. Theta-band phase locking of orbitofrontal neurons during reward expectancy. J. Neurosci 30, 7078–7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilpoux C, Warnault V, Pierrefiche O, Daoust M, Naassila M, 2009. Ethanol-sensitive brain regions in rat and mouse: a cartographic review, using immediate early gene expression. Alcohol Clin. Exp. Res 33, 945–969. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, 1994. Brain-imaging studies of the combined use of cocaine and alcohol and of the pharmacokinetics of cocaethylene. NIDA Res. Monogr 138, 41–56. [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, 2000. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cerebr. Cortex 10, 318–325. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Overall JE, Hitzemann R, Fowler JS, Pappas N, Frecska E, Piscani K, 1997. Regional brain metabolic response to lorazepam in alcoholics during early and late alcohol detoxification. Alcohol Clin. Exp. Res 21, 1278–1284. [PubMed] [Google Scholar]
- Wallis JD, 2011. Cross-species studies of orbitofrontal cortex and value-based decision-making. Nat. Neurosci 15, 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Behrens TE, Noonan MP, Rushworth MF, 2011. Giving credit where credit is due: orbitofrontal cortex and valuation in an uncertain world. Ann. N. Y. Acad. Sci 1239, 14–24. [DOI] [PubMed] [Google Scholar]
- Wang J, Fan Y, Dong Y, Ma M, Ma Y, Dong Y, Niu Y, Jiang Y, Wang H, Wang Z, Wu L, Sun H, Cui C, 2016. Alterations in brain structure and functional connectivity in alcohol dependent patients and possible association with impulsivity. PloS One 11 e0161956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RC, Takahashi YK, Schoenbaum G, Niv Y, 2014. Orbitofrontal cortex as a cognitive map of task space. Neuron 81, 267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, 2007. The orbitofrontal cortex, impulsivity, and addiction: probing orbitofrontal dysfunction at the neural, neurochemical, and molecular level. Ann. N. Y. Acad. Sci 1121, 639–655. [DOI] [PubMed] [Google Scholar]
- Wise RA, 1973. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia 29, 203–210. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2014. Global Status Report on Alcohol and Health.
- Wrase J, Grusser SM, Klein S, Diener C, Hermann D, Flor H, Mann K, Braus DF, Heinz A, 2002. Development of alcohol-associated cues and cue-induced brain activation in alcoholics. Eur. Psychiatr 17, 287–291. [DOI] [PubMed] [Google Scholar]
- Zahr NM, Pfefferbaum A, Sullivan EV, 2017. Perspectives on fronto-fugal circuitry from human imaging of alcohol use disorders. Neuropharmacology 122, 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, Floresco SB, Winstanley CA, 2010. Contributions of the orbitofrontal cortex to impulsive choice: interactions with basal levels of impulsivity, dopamine signalling, and reward-related cues. Psychopharmacology 211, 87–98. [DOI] [PubMed] [Google Scholar]


