Abstract
Introduction
Cephalometry is the study of skull measurements for clinical evaluation, diagnosis, and surgical planning. Machine learning (ML) algorithms have been used to accurately identify cephalometric landmarks and detect irregularities related to orthodontics and dentistry. ML-based cephalometric imaging reduces errors, improves accuracy, and saves time.
Method
In this study, we conducted a meta-analysis and systematic review to evaluate the accuracy of ML software for detecting and predicting anatomical landmarks on two-dimensional (2D) lateral cephalometric images. The meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for selecting and screening research articles. The eligibility criteria were established based on the diagnostic accuracy and prediction of ML combined with 2D lateral cephalometric imagery. The search was conducted among English articles in five databases, and data were managed using Review Manager software (v. 5.0). Quality assessment was performed using the diagnostic accuracy studies (QUADAS-2) tool.
Result
Summary measurements included the mean departure from the 1–4-mm threshold or the percentage of landmarks identified within this threshold with a 95% confidence interval (CI). This meta-analysis included 21 of 577 articles initially collected on the accuracy of ML algorithms for detecting and predicting anatomical landmarks. The studies were conducted in various regions of the world, and 20 of the studies employed convolutional neural networks (CNNs) for detecting cephalometric landmarks. The pooled successful detection rates for the 1-mm, 2-mm, 2.5-mm, 3-mm, and 4-mm ranges were 65%, 81%, 86%, 91%, and 96%, respectively. Heterogeneity was determined using the random effect model.
Conclusion
In conclusion, ML has shown promise for landmark detection in 2D cephalometric imagery, although the accuracy has varied among studies and clinicians. Consequently, more research is required to determine its effectiveness and reliability in clinical settings.
Keywords: Machine learning, Convolutional neural network, Artificial intelligence, Lateral cephalometry, Orthodontics, Accuracy
1. Introduction
Utilizing oral radiology can be lucrative in various fields of dentistry, such as endodontics, periodontology, and orthodontics (Abdinian and Baninajarian, 2017, Mehdizadeh et al., 2022). Cephalometry is the study of skull dimensions using linear and angular measurements of anatomical and constructed landmarks on standardized two-dimensional (2D) lateral head films. The linear and angular measurements from cephalometry can be used in facial recognition and forensic identification (Hlongwa 2019). However, cephalometry is used most frequently in orthodontics and oral surgery for the diagnosis of malocclusion and treatment planning. It is used in combination with facial form evaluation and model analysis to identify the location of skeletal and dental anomalies that can be improved with braces and/or surgery (Durão et al., 2013).
Currently, detecting irregularities related to orthodontics and dentistry has become possible owing to advancements in artificial intelligence (AI) (Pattanaik 2019). AI technology has been incorporated into cephalometry to resolve accurate diagnosis and surgical planning issues (Shin and Kim 2022). Cephalometry combined with AI may be able to assist practitioners with determination of bone age, extraction decisions, orthognathic surgical prediction, and temporomandibular bone segmentation (Mohammad-Rahimi et al., 2021, Mehdizadeh et al., 2022, Ebadian et al., 2023). Cephalometry and AI are often combined with other diagnostic tools, such as facial form analysis and model analysis; thus, the time-consuming task of orthodontic diagnosis can be made more efficient, accurate, and objective (Ruizhongtai Qi 2020).
Decision-making models can hopefully be used in computerized analysis to acquire accurate and consistent data in a timely fashion and then utilize this data to formulate treatment strategies. This type of computerized diagnosis and treatment planning is still in its infancy despite several technical advancements in AI (Juneja et al., 2021). This technology would be a major advancement for diagnosis since the introduction of cephalometry by Broadbent and Hofrath in the 1930s (Helal et al., 2019, Park and Pruzansky, 2019, Palomo et al., 2021, Tanna et al., 2021).
In the last few decades, ML approaches have been implicated in anatomical landmarks detection, computerized diagnosis, and data mining related to medical assessments. ML algorithms have commonly been used extensively for decision-making and in various fields to solve real-world data-related issues (Bollen, 2019, Jodeh et al., 2019). Research has indicated that cephalometric analysis provides detailed images of anatomical structural points. This improves reliability by maximizing the identifying points' accuracy (Kök et al., 2019). However, there is still uncertainty regarding the accuracy of cephalometric imaging results in detecting anatomical landmarks; thus, the algorithm’s accuracy is unclear and should be addressed by analyzing previous studies. In this study, we conducted a meta-analysis and systematic review to assess the accuracy of machine learning (ML) software for detecting and predicting anatomical landmarks on 2D lateral cephalometric images.
2. Materials and methods
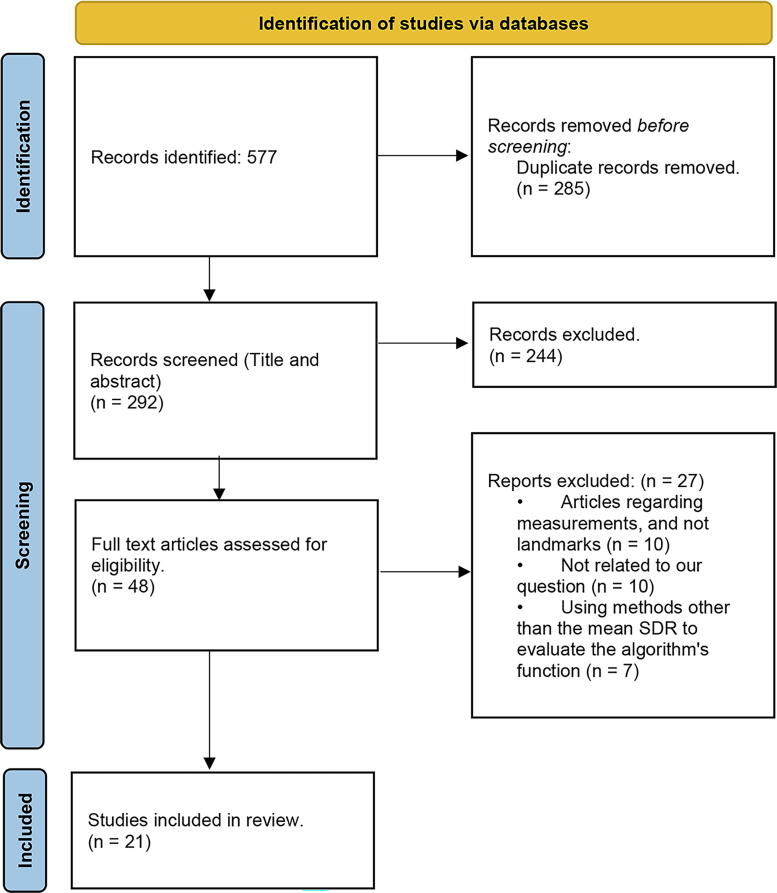
The meta-analysis conducted in this study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al., 2009) for extracting, selecting, and screening the included research articles (Fig. 1). After the initial screening phase, the study protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) with code CRD42023399216 (Alshamrani et al., 2022). The population, intervention, control, and outcomes (PICO) question was as follows:
Fig. 1.
PRISMA flowchart for screening and selection of standardized research articles.
Is 2D lateral cephalometric imagery suitable for detecting and predicting anatomical landmarks using ML software? What is the accuracy?
2.1. Eligibility criteria
The meta-analysis included the following inclusion criteria: (1) studies employing the diagnostic accuracy and prediction of ML, (2) evaluation and assessment of 2D cephalometric imagery analysis, such as 2D lateral radiographs with relevant landmarks that provide detection and prediction accuracy, (3) reporting the outcome as the mean successful detection rate (SDR), (4) those published after 2000 until February 2023, as we expected ML-related data to be included, and (5) articles published only in the English language. Only studies that met the above criteria were included.
Studies were excluded if they (1) already conducted a systematic review and meta-analysis or scoping review, (2) reported other methods for the function of the algorithms rather than SDR, (3) were studies of cephalometry-irrelevant landmarks or used other methods for non-radiographic data, or (4) were articles published in other languages.
2.2. Research strategy and screening
The search and screening of research articles were systematically performed using five databases, including PubMed, Scopus, Scopus Secondary, Embase, and Web of Science (WOS), for studies published from January 2000 to February 2023 in English. The meta-analysis utilized PRISMA systematic review and meta-analysis guidelines for screening and selecting the included studies. The overall search was designed to analyze the different publications across different disciplines; the keywords for each database are outlined in Table 1.
Table 1.
Keywords for each database.
| Database | Keyword | Result |
|---|---|---|
| Pubmed | (“Artificial Intelligence”[Mesh] OR “Machine Learning”[Mesh] OR “Neural Networks, Computer”[Mesh] OR “Deep Learning”[Mesh]) AND (“lateral cephalometry” OR “lateral cephalometric”) | 129 |
| Scopus | (TITLE-ABS-KEY (“Artificial Intelligence”) OR TITLE-ABS-KEY (“Machine Learning”) OR TITLE-ABS-KEY (“Neural Networks”) OR TITLE-ABS-KEY (“Deep Learning”)) AND (TITLE-ABS-KEY (“Cephalometry”) OR TITLE-ABS-KEY (“lateral cephalometry”) OR TITLE-ABS-KEY (“lateral cephalometric”)) | 193 |
| Scopus secondary | (TITLE-ABS-KEY (“Artificial Intelligence”) OR TITLE-ABS-KEY (“Machine Learning”) OR TITLE-ABS-KEY (“Neural Networks”) OR TITLE-ABS-KEY (“Deep Learning”)) AND (TITLE-ABS-KEY (“Cephalometry”) OR TITLE-ABS-KEY (“lateral cephalometry”) OR TITLE-ABS-KEY (“lateral cephalometric”)) | 5 |
| Embase | ('artificial intelligence'/exp OR 'artificial intelligence' OR 'machine learning'/exp OR 'machine learning' OR 'artificial neural network'/exp OR 'artificial neural network' OR 'neural networks'/exp OR 'neural networks' OR 'deep learning'/exp OR 'deep learning') AND ('cephalometry'/exp OR cephalometry OR 'lateral cephalometry' OR 'lateral cephalometric') | 191 |
| WOS | (ALL=(“Artificial Intelligence” OR “Machine Learning” OR “Neural Networks” OR “Deep Learning”)) AND ALL=(“Cephalometry” OR “lateral cephalometry” OR “lateral cephalometric”) | 59 |
The titles and abstracts were screened independently by reviewers, and the third reviewer resolved disagreements. All included studies met the eligibility criteria in full and were those for which the full text was available.
2.3. Data collection and synthesis
The information extracted from research papers is displayed in Table 1. The extracted information was based on study characteristics, including author, year of publication, country of study, imagery (2D lateral radiographs), objective, number of landmarks detected, and findings, as shown in Table 2. Studies were fully extracted if the article mentioned several test datasets or models.
Table 2.
Data extraction.
| Author/year | Country | Architecture | Objective | Sample size | SDR (successful detection rate) |
|---|---|---|---|---|---|
| Alshamrani et al. (2022) (Alshamrani et al., 2022) | Saudi Arabia | CNN (autoencoder-based Inception layers) | Generate a Bjork–Jarabak and Ricketts cephalometrics automatically. | 100 | Basic autoencoder model trained on Set 1 2.0 mm: 64% 2.5 mm: 69% 3.0 mm: 72% 4.0 mm: 77% |
| 150 | Model autoencoder wider Paddup box set 2 2.0 mm: 71% 2.5 mm: 75% 3.0 mm: 78% 4.0 mm: 84% |
||||
| El-Fegh et al. (2008) (El-Fegh et al., 2008) | Libya/ Canada | CNN | A new approach to cephalometric X-ray landmark localization | > 80 | 2.0 mm: 91% |
| El-Feghi et al. (2003) (El-Feghi et al., 2003) | Canada | MLP | A novel algorithm based on the use of the Multi-layer Perceptron (MLP) to locate landmarks on a digitized X-ray of the skull | 134 | 2.0 mm: 91.6% |
| Hwang et al. (2021) (Hwang et al., 2021) | South Korea | CNN (YOLO version 3) |
To compare an automated cephalometric analysis based on the latest deep learning method | 200 | 2.0 mm: 75.45% 2.5 mm: 83.66% 3.0 mm: 88.92% 4.0 mm: 94.24% |
| Jiang et al. (2023) (Jiang et al., 2023) | China | CNN (A cascade framework “CephNet”) | Utilizing artificial intelligence (AI) to achieve automated landmark localization in patients with various malocclusions | 259 | 1.0 mm: 66.15% 2.0 mm: 91.73% 3.0 mm: 97.99% |
| Kafieh et al. (2009) (Kafieh et al., 2009) | Iran | ASM | As a new method for automatic landmark detection in cephalometry, they propose two different methods for bony structure discrimination in cephalograms. | 63 | 1.0 mm: 24.00% 2.0 mm: 61.00% 5.0 mm: 93.00% |
| Kim et al. (2020) (Kim et al., 2020) | South Korea | CNN | Develop a fully automated cephalometric analysis method using deep learning and a corresponding web-based application that can be used without high-specification hardware. | 100 | 2.0 mm: 84.53% 2.5 mm: 90.11% 3.0 mm: 93.21% 4.0 mm: 96.79% |
| Kim et al. (2021) (Kim et al., 2021) | South Korea | CNN | Propose a fully automatic landmark identification model based on a deep learning algorithm using real clinical data | 50 | 2.0 mm: 64.30% 2.5 mm: 77.30% 3.0 mm: 85.50% 4.0 mm: 95.10% |
| Lee et al. (2020) (Lee et al., 2020) | South Korea | BCNN | Develop a novel framework for locating cephalometric landmarks with confidence regions | 250 | 2.0 mm: 82.11% 2.5 mm: 88.63% 3.0 mm: 92.28% 4.0 mm: 95.96% |
| Oh et al. (2021) (Oh et al., 2020) | South Korea | CNN | They proposed a novel framework DACFL that enforces the FCN to understand a much deeper semantic representation of cephalograms | 150 | 2.0 mm: 86.20% 2.5 mm: 91.20% 3.0 mm: 94.40% 4.0 mm: 97.70% |
| 100 | 2.0 mm: 75.90% 2.5 mm: 83.40% 3.0 mm: 89.30% 4.0 mm: 94.70% |
||||
| Ramadan et al. (2022) (Ramadan et al., 2022) | Saudi Arabia | CNN | Detection of the cephalometric landmarks automatically | 150 | 2.0 mm: 90.39% 3.0 mm: 92.37% |
| 100 | 2.0 mm: 82.66% 3.0 mm: 84.53% |
||||
| Song et al. (2020) (Song et al., 2020) | Japan | CNN (with a backbone of ResNet50) | A two-step method for the automatic detection of cephalometric landmarks | 150 | 2.0 mm: 86.40% 2.5 mm: 91.70% 3.0 mm: 94.80% 4.0 mm: 97.80% |
| 100 | 2.0 mm: 74.00% 2.5 mm: 81.30% 3.0 mm: 87.50% 4.0 mm: 94.30% |
||||
| Song et al. (2021) (Song et al., 2021) | Japan/ China | CNN (Deep convolutional neural networks) | A coarse-to-fine method to detect cephalometric landmarks | 150 | 2.0 mm: 85.20% 2.5 mm: 91.20% 3.0 mm: 94.40% 4.0 mm: 97.20% |
| 100 | 2.0 mm: 72.20% 2.5 mm: 79.50% 3.0 mm: 85.00% 4.0 mm: 93.50% |
||||
| Song et al. (2020) (Song et al., 2019) | Japan/ China | CNN (Resnet50) | A semi-automatic method for detection of cephalometric landmarks using deep learning. | 150 | 2.0 mm: 85.00% 2.5 mm: 90.70% 3.0 mm: 94.50% 4.0 mm: 98.40% |
| 100 | 2.0 mm: 81.80% 2.5 mm: 88.06% 3.0 mm: 93.80% 4.0 mm: 97.95% |
||||
| Tanikawa et al. (2009) (Tanikawa et al., 2009) | Japan | N/A | Evaluate the reliability of a system that performs automatic recognition of anatomic landmarks and adjacent structures on lateral cephalograms using landmark-dependent criteria unique to each landmark | 65 | 88.00% |
| Ugurlu, (2022) (Uğurlu 2022) | Turkey | CNN | Develop an artificial intelligence model to detect cephalometric landmark, automatically enabling the automatic analysis of cephalometric radiographs | 180 | 2.0 mm: 76.20% 2.5 mm: 83.50% 3.0 mm: 88.20% 4.0 mm: 93.40% |
| Wang et al. (2018) (Wang et al., 2018) | China | Multiscale decision tree regression voting using SIFTbased patch | Develop a fully automatic system of cephalometric analysis, including cephalometric landmark detection and cephalometric measurement in lateral cephalograms. | 150 | 2.0 mm: 73.37% 2.5 mm: 79.65% 3.0 mm: 84.46% 4.0 mm: 90.67% |
| 165 | 2.0 mm: 72.08% 2.5 mm: 80.63% 3.0 mm: 86.46% 4.0 mm: 93.07% |
||||
| Yao et al. (2022) (Yao et al., 2022) | China | CNN | Develop an automatic landmark location system to make cephalometry more convenient | 100 | 1.0 mm: 54.05% 1.5 mm: 91.89% 2.0 mm: 97.30% 2.5 mm: 100.00% 3.0 mm: 100.00% 4.0 mm: 100.00% |
| Yoon et al. (2022) (Yoon et al., 2022) | South Korea | CNN (EfficientNetB0 (Eff-UNet B0) model) | Evaluate the accuracy of a cascaded two-stage (CNN) model in detecting upper airway soft tissue landmarks in comparison with the skeletal landmarks on lateral cephalometric images | 100 | 1.0 mm: 74.71% 2.0 mm: 93.43% 3.0 mm: 97.29% 4.0 mm: 98.71% |
| Yue et al. (2006) (Yue et al., 2006) | China | ASM | Craniofacial landmark localization and structure tracing are addressed in a uniform framework. | 86 | 2.0 mm: 71.00% 4.0 mm: 88.00% |
| Zeng et al. (2021) (Zeng et al., 2021) | China | CNN | A novel approach with a cascaded three-stage convolutional neural networks to predict cephalometric landmarks automatically. | 150 | 2.0 mm: 81.37% 2.5 mm: 89.09% 3.0 mm: 93.79% 4.0 mm: 97.86% |
| 100 | 2.0 mm: 70.58% 2.5 mm: 79.53% 3.0 mm: 86.05% 4.0 mm: 93.32% |
CNN: convolutional neural network, ASM: Active shape model, BCNN: Bayesian Convolutional Neural Networks, MLP: Multi-layer Perceptron.
2.4. Quality assessment
The quality assessment of diagnostic accuracy studies (QUADAS-2) tool (Whiting et al., 2011) was utilized to evaluate risk bias, which accounted for risk bias (data selection, index test, and reference test) and applicability concerns (no flow or timing, data selection, index test, and reference test). Two reviewers assessed the bias risk in the included studies and interpreted the results.
2.5. Summary measures and data synthesis
To be considered for meta-analysis, a study had to report either the deviation from a 1-, 2-, 3-, and 4-mm estimated error criterion (in mm) or the percentage of landmarks accurately predicted within this 1-, 2-, 3-, and 4-mm prediction error thresholds (Higgins and Thompson 2002). Our final measurements were the mean deviation from the 1-, 2-, 3-, and 4-mm thresholds (in mm) or the percentage of landmarks identified within the 1-, 2-, 3-, and 4-mm thresholds, both with their 95% confidence intervals (CI). The meta-analysis was conducted using Review Manager version 5.0, and heterogeneity was evaluated using Cochrane's Q and I2 statistics using the random effect model (Viechtbauer 2010).
3. Results
3.1. Identified studies
The meta-analysis yielded approximately 577 research articles on the accuracy of ML algorithms for detecting and predicting anatomical landmarks from the abovementioned databases. According to the inclusion criteria, 48 papers were determined to be relevant, reliable, and in line with the study's objectives. Through the exclusion criteria, 27 papers were eliminated of the 48 studies. Approximately 21 of the remaining articles met the aforementioned criteria and were included.
The reasons for exclusion were as follows:
-
•
Studies on measurements and not landmarks = 10
-
•
Those not related to our question = 10
-
•
Those using methods other than the mean SDR to evaluate the algorithm's function = 7
3.2. Descriptive analysis of identified studies
Among the 577 studies selected, 21 articles were included in the data extraction phase. These studies were model-based studies conducted in Korea, Saudi Arabia, Iran, Israel, Canada, Bosnia, China, Turkey, the USA, and Italy, representing different world regions. Furthermore, they included studies on ML cephalometric landmark detection through CNN, and the outcomes were successful detection rates.
3.3. Risk of bias
The risk of bias in the included studies was assessed using the QUADAS-2 tool in two main domains: risk of bias and applicability issues. The risk of bias assessment demonstrated that some of the included articles exhibit a high risk of bias in data selection (n = 11, 52.38%), reference tests (n = 6, 28.57%), index tests (n = 1.4, 76%), and timing (n = 2.9, 52%). The majority of the presented studies had applicability issues for data selection (n = 5.23, 8%), reference tests (n = 0), and index tests (n = 2.95, 2%). A detailed assessment of the risk of bias and applicability concerns is provided in Table 3.
Table 3.
Bias risk assessment.
| Risk of bias |
Applicability concerns |
|||||||
|---|---|---|---|---|---|---|---|---|
| Authors | Year | Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard |
| Kim et al. (Kim et al., 2021) | 2021 | Low | Low | Low | Low | Low | Low | Low |
| Kafieh et al. (Kafieh et al., 2009) | 2009 | High | Low | High | Unclear | Low | Low | High |
| Oh et al. (Oh et al., 2020) | 2021 | Low | Low | Low | Low | Low | Low | Low |
| Ramadan et al. (Ramadan et al., 2022) | 2022 | High | Low | Low | Low | High | Low | Low |
| El-Fegh (El-Fegh et al., 2008) | 2008 | High | Low | Low | High | High | Low | Low |
| El-Feghi et al. (El-Feghi et al., 2003) | 2003 | High | Low | Low | High | High | Low | Low |
| Lee et al. (Lee et al., 2020) | 2020 | Low | Low | Low | Low | Low | Low | Low |
| Kim et al. (Kim et al., 2020) | 2020 | Low | Low | Low | Low | Low | Low | Low |
| Alshamrani et al. (Alshamrani et al., 2022) | 2022 | High | Low | High | Low | High | Low | Low |
| Hwang et al. (Hwang et al., 2021) | 2021 | Low | Unclear | Low | Low | Low | Unclear | Low |
| Jiang et al. (Jiang et al., 2023) | 2022 | Low | Low | Low | Low | Low | Low | Low |
| Song et al. (Song et al., 2020) | 2020 | High | Low | Low | Low | Low | Low | Low |
| Song et al. (Song et al., 2019) | 2019 | High | Low | High | Unclear | High | Low | High |
| Tanikawa et al. (Tanikawa et al., 2009) | 2009 | Low | Low | High | Low | Low | Low | Low |
| Yao et al. (Yao et al., 2022) | 2022 | Low | Low | Low | Low | Low | Low | Low |
| Wang et al. (Wang et al., 2018) | 2018 | High | Low | Low | Unclear | Low | Low | Low |
| Yue et al. (Yue et al., 2006) | 2006 | High | Low | Low | Low | Low | Low | Low |
| Yoon et al. (Yoon et al., 2022) | 2022 | Low | Low | High | Low | Low | Low | Low |
| Song et al. (Song et al., 2021) | 2021 | High | High | Low | Low | Low | Unclear | Low |
| Zeng et al. (Zeng et al., 2021) | 2021 | High | Low | Low | Low | Low | Low | Low |
| Ugurlu (Uğurlu 2022) | 2022 | Low | Low | High | Low | Low | Low | Low |
3.4. Architecture of AI
The majority of the included studies use various modalities of CNNs as the architecture for detecting landmarks on radiographs (n = 15, 71.4%) followed by the active shape model (ASM) at 9% (n = 2). Further information is provided in Table 2.
3.5. Successful detection rates
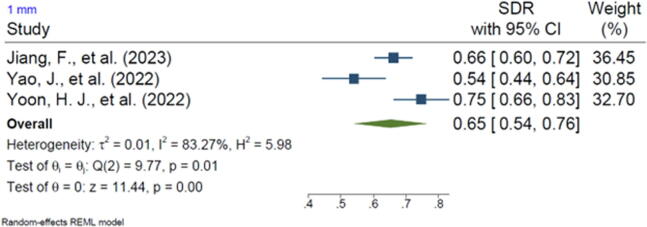
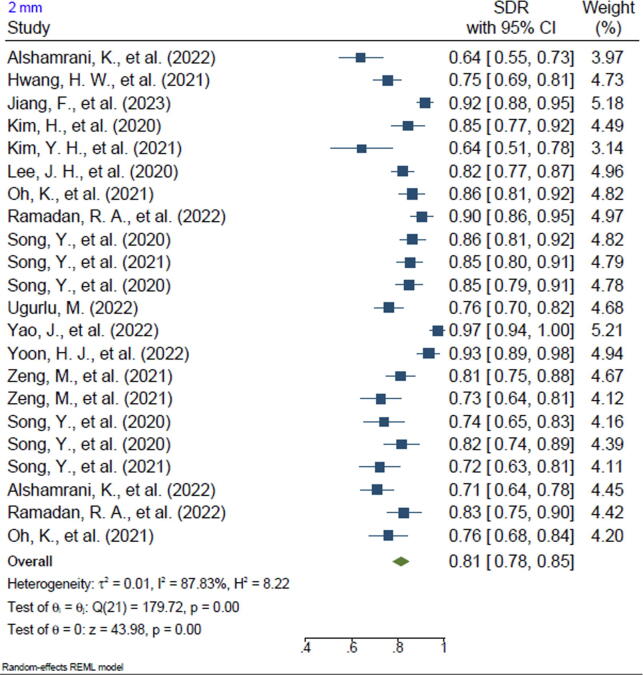
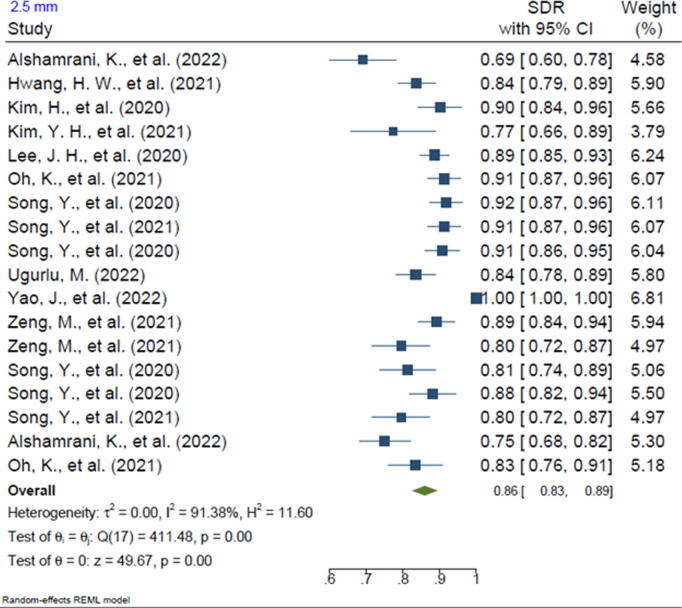
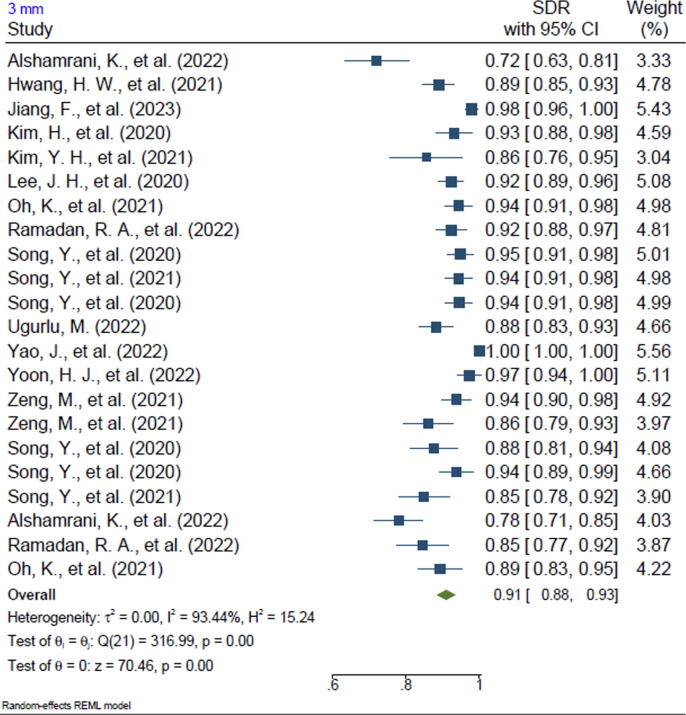
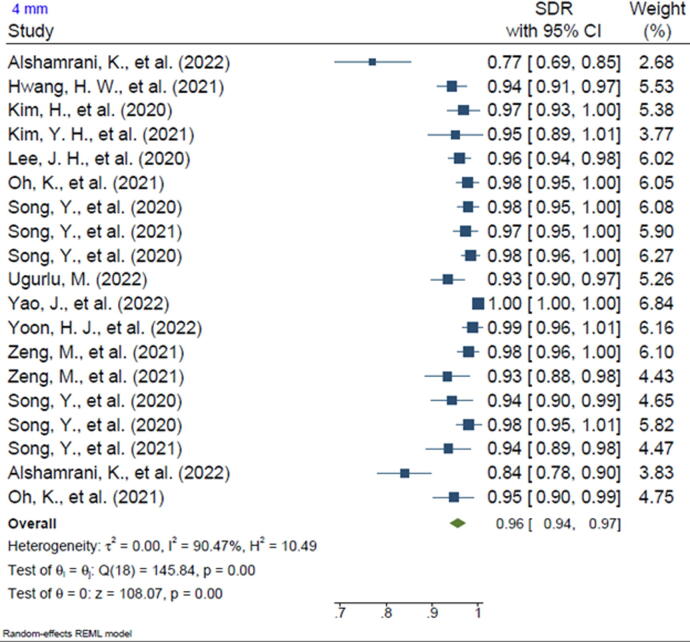
Twenty-one of the included studies reported the SDR of anatomical landmarks in different ranges. Most studies reported the SDR for the range of 2 mm (n = 20, 95.2%). In addition, 13 of the included studies reported the SDR for the 2.5-mm range (61.9%), 16 studies reported the SDR for the 3-mm range (76.2%), 15 studies reported the SDR for the 4-mm range (71.4%), and 3 studies reported the SDR for the 1-mm range (14.2%). The pooled SDR for the 1-mm, 2-mm, 2.5-mm, 3-mm, and 4-mm ranges were 65%, 81%, 86%, 91%, and 96%, respectively, the supplementary files for Figures 2-6. Table 4 presents further findings of each meta-analysis.
Table 4.
Meta-analysis results.
| Diameter range | Detection percentage | 95% confidence interval | I2 | P-value heterogeneity |
|---|---|---|---|---|
| 1 mm | 65% | 54–76 | 83.27 | 0.01 |
| 2 mm | 81% | 78–85 | 87.83 | 0.00 |
| 2.5 mm | 86% | 83–89 | 91.38 | 0.00 |
| 3 mm | 91% | 88–93 | 93.44 | 0.00 |
| 4 mm | 96% | 94–97 | 90.47% | 0.00 |
4. Discussion
This study's systematic review revealed that ML algorithms for anatomical landmarking of 2D cephalometric images have been implicated as an active radiography resource, as 20 of 21 are studies that reported accuracy, which were typically published between 2006 and 2023. Fifteen studies used varied modalities of CNN, and six studies utilized other AI architectures, such as ASM and Bayesian convolutional neural networks (BCNN). Most of the studies reported SDR for the 2-mm (95.2%), 2.5-mm (61.9%), 3-mm (76.2%), and 4-mm (71.4%) ranges. The overall reported SDR for the 1-mm range was 65% followed by 81% for 2 mm, 86% for 2.5 mm, 91% for 3 mm, and 96% for 4 mm.
Even though these assessments are based on landmarks, it is impossible to systematically determine a total systematic error from landmark machine translation errors. The overall standard deviation might be decreased or increased based on landmark coordinate values, which alters the therapeutic relevance of the findings. Consequently, there is a shortage of data on the diagnosis accuracy of computerized three-dimensional (3D) cephalometry.
Another study found that, compared to other radiographic techniques, cephalograms provide quantitative and qualitative results for anatomical landmark detection (Bichu et al., 2021, Joda and Pandis, 2021, Liu et al., 2021, Auconi et al., 2022). Skeletal landmark detection improves the accuracy of quantitative analyses as it identifies reference points. Thus, the landmarks' precise source must be determined to produce relevant results. The current study assessed research that utilized 2D cephalometric images and ML for landmark detection.
The efficacy of ML, as demonstrated by experimental trials, has transformed the implications of ML for cephalometric analysis. However, it requires considerable attention due to the association of certain challenges in orthodontics and other medical assessments. One such difficulty is the presence of “black-box” characteristics in ML, which necessitates improving the visuals and gaining the confidence of physicians and patients before the clinical implementation of ML (Su et al., 2020, Du et al., 2022). Moreover, trial techniques are needed to manage bias risk. For instance, performing consistency evaluations is crucial to assess consistency. Allocation plans also need to be free of personal prejudices. Furthermore, a few other issues, such as a reliability crisis, underfitting, and inadequacy of data, have limited the use of ML in cephalometry (Asiri et al., n.d., Tandon et al., 2020, Palanivel et al., 2021, Tanikawa et al., 2021).
Montufar et al. (Montúfar et al., 2018) conducted automatic cephalometric analysis for landmark detection using cone beam computed tomography (CBCT) images and an active surface AI model. They determined the accuracy of this process to be 3.64 mm on average at 18 anatomical points.
Several studies have reported the risk of more errors while detecting irregular structures through cephalometric analysis. Patcas et al. (Patcas et al., 2019) conducted a 2D hybrid cephalometric analysis to acquire CBCT images. Approximately 18 anatomical landmark points were identified with a mean error of 2.51 mm via holistic three-dimensional cephalometric detection. Yu et al. (Yu et al., 2014) evaluated the accuracy of cephalometric analysis using the ML method and reported the interaction between landmark detection and facial attractiveness identification algorithms.
Similarly, Patcas et al. (Patcas et al., 2019) performed a study using AI to assess the accuracy of landmark detection through cephalometric analysis before treatment or decision-making before surgery. For approximately 146 patients that underwent orthognathic surgery, their starting and final images were evaluated using algorithms for facial beauty and appearance. Their study suggested that patients undergoing orthognathic surgery might be assessed for facial symmetry and chronological age using ML.
This meta-analysis had several limitations. First, we focused on ML to detect anatomical landmarks, and a comparison with automated landmarking procedures was not conducted. Second, we excluded several studies, following the inclusion criteria, e.g., those utilizing DL to detect cephalometric analysis, whose full texts were unavailable and did not comprehensively address the study objectives. Third, a variety of risk biases existed in the included studies. Data selection produced limited and potentially unrepresentative groups; most studies utilized the same dataset. Conclusive evidence for predictive data value was relatively poor, particularly for 3D images; images in the test dataset typically were from only a few individuals. Fourth, as previously stated, the limited generalizability was because only a few researchers tested the established DL models on fully independent datasets, such as those from different centers, people, or image processors. Finally, most studies relied on precision estimations rather than other, obviously comparable outcome measures, such as variations in millimeters, pixels, or percentages (primarily as a result of our inclusion criteria) (Gupta et al., 2016).
The use of an ML tool in primary care and its impact on diagnostic and treatment practices, the efficacy, and safety were not documented as additional outcomes that would have been relevant to physicians, patients, or other users. Future research should consider expanding the outcome set and thoroughly testing the applicability of DL in various contexts and situations (e.g., observational studies in clinical care and randomized controlled trials). Of note, the criteria for AI-based cephalometric evaluations could change based on the resulting treatment decisions.
One of the limitations of this study was not including books, other types of literature, and articles that were not in English. To obtain a more accurate outcome, further studies should include more databases, such as Google Scholar, and gray literature.
5. Conclusion
This study demonstrated that ML is significant for detecting landmarks through cephalometric 2D imagery. Most included studies focused on 2D imagery generated by automated cephalometric analysis of ML, which shows promise for the future. The accuracy of landmark detection using ML was heterogeneous across the included studies; however, the accuracy rates of clinicians varied significantly. While generally consistent, the overall evidence shows low generalizability and consistent accuracy, and the clinical utility of ML has yet to be demonstrated. The use of AI for accurately detecting cephalometric landmarks with the extremely low certainty of the findings is intriguing. However, future research should focus on establishing its efficacy and reliability in various samples.
CRediT authorship contribution statement
Jimmy Londono: Conceptualization, Writing – review & editing. Shohreh Ghasemi: Writing – original draft, Writing – review & editing. Altaf Hussain Shah: Investigation, Writing – original draft. Amir Fahimipour: Methodology, Formal analysis. Niloofar Ghadimi: Methodology, Writing – original draft. Sara Hashemi: Methodology, Formal analysis. Zohaib Khurshid Sultan: Investigation, Writing – original draft. Mahmood Dashti: Conceptualization, Methodology, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
Footnotes
Peer review under responsibility of King Saud University. Production and hosting by Elsevier
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sdentj.2023.05.014.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary figure 1.
Supplementary figure 2.
Supplementary figure 3.
Supplementary figure 4.
Supplementary figure 5.
References
- Abdinian M., Baninajarian H. The accuracy of linear and angular measurements in the different regions of the jaw in cone-beam computed tomography views. Dental Hypotheses. 2017;8:100. doi: 10.4103/denthyp.denthyp_29_17. [DOI] [Google Scholar]
- Alshamrani K., Alshamrani H., Alqahtani F.F., et al. Automation of Cephalometrics Using Machine Learning Methods. Comput. Intell. Neurosci. 2022;2022:3061154. doi: 10.1155/2022/3061154. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Asiri, S.N., Tadlock, L.P., Schneiderman, E., et al., Applications of artificial intelligence and machine learning in orthodontics. APOS Trends in Orthodontics. 10, 10.25259/APOS_117_2019. [DOI]
- Auconi P., Gili T., Capuani S., et al. The validity of machine learning procedures in orthodontics: what is still missing? J Pers Med. 2022;12 doi: 10.3390/jpm12060957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichu Y.M., Hansa I., Bichu A.Y., et al. Applications of artificial intelligence and machine learning in orthodontics: a scoping review. Prog. Orthod. 2021;22:18. doi: 10.1186/s40510-021-00361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen A.-M. Cephalometry in orthodontics: 2D and 3D. Am. J. Orthod. Dentofac. Orthop. 2019;156:161. [Google Scholar]
- Du W., Bi W., Liu Y., et al. Res Sq; 2022. Decision Support System for Orthgnathic diagnosis and treatment planning based on machine learning. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durão A.R., Pittayapat P., Rockenbach M.I., et al. Validity of 2D lateral cephalometry in orthodontics: a systematic review. Prog. Orthod. 2013;14:31. doi: 10.1186/2196-1042-14-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebadian B., Fathi A., Tabatabaei S. Stress distribution in 5-Unit fixed partial dentures with a pier abutment and rigid and nonrigid connectors with two different occlusal schemes: a three-dimensional finite element analysis. Int. J. Dent. 2023;2023:3347197. doi: 10.1155/2023/3347197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Fegh I., Galhood M., Sid-Ahmed M., et al. Automated 2-D cephalometric analysis of X-ray by image registration approach based on least square approximator. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2008;2008:3949–3952. doi: 10.1109/iembs.2008.4650074. [DOI] [PubMed] [Google Scholar]
- El-Feghi I., Sid-Ahmed M.A., Ahmadi M. Automatic identification and localization of craniofacial landmarks using multi layer neural network. Int. Conf. Medical Image Comput. Computer-Assisted Intervention. 2003 [Google Scholar]
- Gupta A., Kharbanda O.P., Sardana V., et al. Accuracy of 3D cephalometric measurements based on an automatic knowledge-based landmark detection algorithm. Int. J. Comput. Assist. Radiol. Surg. 2016;11:1297–1309. doi: 10.1007/s11548-015-1334-7. [DOI] [PubMed] [Google Scholar]
- Helal N.M., Basri O.A., Baeshen H.A. Significance of cephalometric radiograph in orthodontic treatment plan decision. J. Contemp. Dent. Pract. 2019;20:789–793. [PubMed] [Google Scholar]
- Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Hlongwa P. Cephalometric analysis: manual tracing of a lateral cephalogram. S Afr. Dent. J. 2019;74 doi: 10.17159/2519-0105/2019/v74no6a6. [DOI] [Google Scholar]
- Hwang H.W., Moon J.H., Kim M.G., et al. Evaluation of automated cephalometric analysis based on the latest deep learning method. Angle Orthod. 2021;91:329–335. doi: 10.2319/021220-100.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F., Guo Y., Yang C., et al. Artificial intelligence system for automated landmark localization and analysis of cephalometry. Dentomaxillofac. Radiol. 2023;52:20220081. doi: 10.1259/dmfr.20220081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joda T., Pandis N. The challenge of eHealth data in orthodontics. Am. J. Orthod. Dentofac. Orthop. 2021;159:393–395. doi: 10.1016/j.ajodo.2020.12.002. [DOI] [PubMed] [Google Scholar]
- Jodeh D.S., Kuykendall L.V., Ford J.M., et al. Adding depth to cephalometric analysis: comparing two- and three-dimensional angular cephalometric measurements. J. Craniofac. Surg. 2019;30:1568–1571. doi: 10.1097/scs.0000000000005555. [DOI] [PubMed] [Google Scholar]
- Juneja M., Garg P., Kaur R., et al. A review on cephalometric landmark detection techniques. Biomed. Signal Process. Control. 2021;66 [Google Scholar]
- Kafieh, R., Sadri, S., Mehri, A., et al., 2009. Discrimination of bony structures in cephalograms for automatic landmark detection. In: Advances in Computer Science and Engineering: 13th International CSI Computer Conference, CSICC 2008 Kish Island, Iran, March 9-11, 2008 Revised Selected Papers, Springer.
- Kim M.-J., Liu Y., Oh S.H., et al. Automatic cephalometric landmark identification system based on the multi-stage convolutional neural networks with CBCT combination images. Sensors. 2021;21:505. doi: 10.3390/s21020505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Shim E., Park J., et al. Web-based fully automated cephalometric analysis by deep learning. Comput. Methods Programs Biomed. 2020;194 doi: 10.1016/j.cmpb.2020.105513. [DOI] [PubMed] [Google Scholar]
- Kök H., Acilar A.M., İzgi M.S. Usage and comparison of artificial intelligence algorithms for determination of growth and development by cervical vertebrae stages in orthodontics. Prog. Orthod. 2019;20:41. doi: 10.1186/s40510-019-0295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Yu H.J., Kim M.J., et al. Automated cephalometric landmark detection with confidence regions using Bayesian convolutional neural networks. BMC Oral Health. 2020;20:270. doi: 10.1186/s12903-020-01256-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Chen Y., Li S., et al. Machine learning in orthodontics: challenges and perspectives. Adv. Clin. Exp. Med. 2021;30:1065–1074. doi: 10.17219/acem/138702. [DOI] [PubMed] [Google Scholar]
- Mehdizadeh M., Tavakoli Tafti K., Soltani P. Evaluation of histogram equalization and contrast limited adaptive histogram equalization effect on image quality and fractal dimensions of digital periapical radiographs. Oral. Radiol. 2022 doi: 10.1007/s11282-022-00654-7. [DOI] [PubMed] [Google Scholar]
- Mohammad-Rahimi H., Nadimi M., Rohban M.H., et al. Machine learning and orthodontics, current trends and the future opportunities: a scoping review. Am. J. Orthod. Dentofac. Orthop. 2021;160(170–192):e174. doi: 10.1016/j.ajodo.2021.02.013. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 2009;151(264–269) doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- Montúfar J., Romero M., Scougall-Vilchis R.J. Automatic 3-dimensional cephalometric landmarking based on active shape models in related projections. Am. J. Orthod. Dentofac. Orthop. 2018;153:449–458. doi: 10.1016/j.ajodo.2017.06.028. [DOI] [PubMed] [Google Scholar]
- Oh K., Oh I.-S., Le V.N.T., et al. Deep anatomical context feature learning for cephalometric landmark detection. IEEE J. Biomed. Health Inform. 2020;25:806–817. doi: 10.1109/JBHI.2020.3002582. [DOI] [PubMed] [Google Scholar]
- Palanivel J., Davis D., Srinivasan D., et al. Artificial intelligence-creating the future in orthodontics-a review. J. Evol. Med. Dent. Sci. 2021;10:2108–2114. [Google Scholar]
- Palomo, J.M., El, H., Stefanovic, N., et al., 2021. 3D Cephalometry. 3D Diagnosis and Treatment Planning in Orthodontics: An Atlas for the Clinician, pp. 93-127.
- Park, J.H., Pruzansky, D.P., 2019. Imaging and Analysis for the Orthodontic Patient. Craniofacial 3D Imaging: Current Concepts in Orthodontics and Oral and Maxillofacial Surgery. 71-83.
- Patcas R., Bernini D.A.J., Volokitin A., et al. Applying artificial intelligence to assess the impact of orthognathic treatment on facial attractiveness and estimated age. Int. J. Oral Maxillofac. Surg. 2019;48:77–83. doi: 10.1016/j.ijom.2018.07.010. [DOI] [PubMed] [Google Scholar]
- Pattanaik S. Evolution of cephalometric analysis of orthodontic diagnosis. Indian J. Forensic. Med. Toxicol. 2019;13 [Google Scholar]
- Ramadan R., Khedr A., Yadav K., et al. Convolution neural network based automatic localization of landmarks on lateral x-ray images. Multimed. Tools Appl. 2022;81 doi: 10.1007/s11042-021-11596-3. [DOI] [Google Scholar]
- Ruizhongtai Qi, C., 2020. Deep learning on 3D data. 3D Imaging, Analysis and Applications. 513-566.
- Shin S., Kim D. Comparative validation of the mixed and permanent dentition at web-based artificial intelligence cephalometric analysis. J. Korean Acad. Pedtatric. Dent. 2022;49:85–94. [Google Scholar]
- Song, Y., Qiao, X., Iwmoto, Y., et al., 2019. Semi-automatic Cephalometric Landmark Detection on X-ray Images Using Deep Learning Method. In: International Conference on Natural Computation, Fuzzy Systems and Knowledge Discovery.
- Song Y., Qiao X., Iwamoto Y., et al. Automatic cephalometric landmark detection on X-ray images using a deep-learning method. Appl. Sci. 2020;10:2547. [Google Scholar]
- Song Y., Qiao X., Iwamoto Y., et al. An efficient deep learning based coarse-to-fine cephalometric landmark detection method. IEICE Trans. Inf. Syst. 2021;E104.D:1359–1366. doi: 10.1587/transinf.2021EDP7001. [DOI] [Google Scholar]
- Su M., Feng G., Liu Z., et al. Tapping on the black box: how is the scoring power of a machine-learning scoring function dependent on the training set? J. Chem. Inf. Model. 2020;60:1122–1136. doi: 10.1021/acs.jcim.9b00714. [DOI] [PubMed] [Google Scholar]
- Tandon D., Rajawat J., Banerjee M. Present and future of artificial intelligence in dentistry. J. Oral Biol. Craniofac. Res. 2020;10:391–396. doi: 10.1016/j.jobcr.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanikawa C., Yagi M., Takada K. Automated cephalometry: system performance reliability using landmark-dependent criteria. Angle Orthod. 2009;79:1037–1046. doi: 10.2319/092908-508r.1. [DOI] [PubMed] [Google Scholar]
- Tanikawa C., Kajiwara T., Shimizu Y., et al. Machine/deep learning for performing orthodontic diagnoses and treatment planning. Mach. Learn. Dentistry. 2021:69–78. [Google Scholar]
- Tanna N.K., AlMuzaini A., Mupparapu M. Imaging in Orthodontics. Dent. Clin. N. Am. 2021;65:623–641. doi: 10.1016/j.cden.2021.02.008. [DOI] [PubMed] [Google Scholar]
- Uğurlu M. Performance of a convolutional neural network- based artificial intelligence algorithm for automatic cephalometric landmark detection. Turk. J. Orthod. 2022;35:94–100. doi: 10.5152/TurkJOrthod.2022.22026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010;36:1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- Wang S., Li H., Li J., et al. Automatic analysis of lateral cephalograms based on multiresolution decision tree regression voting. J. Healthc. Eng. 2018;2018:1797502. doi: 10.1155/2018/1797502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting P.F., Rutjes A.W., Westwood M.E., et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- Yao J., Zeng W., He T., et al. Automatic localization of cephalometric landmarks based on convolutional neural network. Am. J. Orthod. Dentofac. Orthop. 2022;161:e250–e259. doi: 10.1016/j.ajodo.2021.09.012. [DOI] [PubMed] [Google Scholar]
- Yoon H.J., Kim D.R., Gwon E., et al. Fully automated identification of cephalometric landmarks for upper airway assessment using cascaded convolutional neural networks. Eur. J. Orthod. 2022;44:66–77. doi: 10.1093/ejo/cjab054. [DOI] [PubMed] [Google Scholar]
- Yu X., Liu B., Pei Y., et al. Evaluation of facial attractiveness for patients with malocclusion: a machine-learning technique employing Procrustes. Angle Orthod. 2014;84:410–416. doi: 10.2319/071513-516.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue W., Yin D., Li C., et al. Automated 2-D cephalometric analysis on X-ray images by a model-based approach. I.E.E.E. Trans. Biomed. Eng. 2006;53:1615–1623. doi: 10.1109/TBME.2006.876638. [DOI] [PubMed] [Google Scholar]
- Zeng M., Yan Z., Liu S., et al. Cascaded convolutional networks for automatic cephalometric landmark detection. Med. Image Anal. 2021;68 doi: 10.1016/j.media.2020.101904. [DOI] [PubMed] [Google Scholar]