Abstract
Introduction
Radiotherapy is one of the treatment modalities for the treatment of head and neck cancer (HNC). However, it leads to the development of chronic and acute side effects. These side effects impact negatively on the patient’s quality of life and oral functioning. This clinical review aims to provide basic information about HNC, understand the impact of radiotherapy on oral health, and explain the role of dental care providers for HNC patients during the pre-and post-radiotherapy time.
Materials and Methods
Electronic databases (i.e., PubMed, Scopus, and Google Scholar) were searched using defined keywords. The main inclusion criteria were any studies describing “dental management of patients with head and neck cancer” and “dental management of patients treated with radiotherapy.”
Results
Thematic analysis was used to summaries the findings of the included studies (n = 102) into main headings and subheadings. All studies were published between 1970 and 2023.
Conclusion
The number of HNC patients is increasing. This necessitates the need for raising the awareness of dental care providers to the side-effects of HNC therapy which includes treatment with radiotherapy, chemotherapy, and/or surgery. Dental care providers should understand the common side-effects and their treatments besides their role in the pre- (i.e., dental extraction of teeth with poor prognosis and maintaining good oral hygiene) and post- (i.e., oral rehabilitation and post-HNC dental care) radiotherapy dental care.
Keywords: Head and neck neoplasm, Dry mouth, Oral mucositis, Radiation caries, Osteoradionecrosis, Radiotherapy
1. Introduction
The National Cancer Institute (NCI) describes head and neck cancer (HNC) as the types of cancers that arise in the tissues and organs of the head and neck region, which includes the larynx, pharynx, throat, lips, tongue, tonsils, nasal cavity, paranasal sinuses, and salivary glands (NCI, 2016).
Squamous cell carcinomas of the head and neck region are the sixth most common malignancy in the world, accounting for over 500,000 new cases annually worldwide (Alsahafi et al., 2019). It represents 3% of all cancers in the United States (US) (Siegel RL, 2017) and 3% in the United Kingdom (UK) (Cancer Research UK, 2017). Forty percent of HNCs affect the oral cavity as primary tumours, while 60% appear in the other organs of the head and neck regions (Ray-Chaudhuri et al., 2013).
In 2016, an epidemiological study found that HNC is increasing with a significant variation based on the cancer site. For example, this epidemiological study found that the larynx was the most common type of HNC (23%), followed by the oral cavity (21%). The research team also found that the age-standardised incidence has increased among men by 40% and by 87% among women. Incidence was also higher in those living in more deprived areas of London. Females from Asian countries (mainly Bangladeshi) presented with a higher incidence of oral, laryngeal and thyroid cancers compared with their non-Asian counterparts, while both genders of Chinese origin had a higher incidence of nasopharyngeal cancer (Tataru et al., 2016).
2. Materials and Methods
Electronic databases (i.e., PubMed, Scopus, and Google Scholar) were searched using the following keywords: head and neck neoplasm/cancer, dentistry, dental management, osteoradionecrosis, oral mucositis, dry mouth, truisms, loss of taste and radiation caries. The search included in vivo and in vitro studies published in English. The main inclusion criteria were any studies describing “dental management of patients with head and neck cancer” and “dental management of patients treated with radiotherapy.”
3. Results
Thematic analysis was used to summarise the findings of the included studies into main headings and subheadings. This clinical review considered 102 articles/studies that discussed the dental management of head and neck cancer patients. All studies were published between 1970 and 2023. A summary of the basic details about head and neck cancer besides the negative impact of radiotherapy on oral and dental health and their dental management are discussed below.
3.1. Aetiology and risk factors of developing HNC
There are three main risk factors that increase the risk of developing HNC: lifestyle, genetic factors, and environmental factors (Ghantous and Elnaaj, 2017).
Lifestyle (i.e. exposure to carcinogenic agents such as tobacco use, betel quid chewing, alcohol and consumption of low nutritional diet) is the most common reason for developing HNC (Gupta et al., 2017, Ghantous and Elnaaj, 2017, Kumar et al., 2016). It is common in both developed and undeveloped countries. Carcinogenicity is dose-dependent and people who have more exposure to carcinogenic agents are more susceptible to develop HNC than people who do not smoke or drink alcohol. Furthermore, some factors act synergistically so that the use of both smoking and consuming alcohol is far greater than the sum of their individual risks (Mello et al., 2019).
Genetically, some families are more susceptible to develop oral cancer in relation to their genetic inherited traits and weak immunity to fight cancer cells (Fernández-Mateos et al., 2019). For example, inability to prevent the recovery of damaged deoxyribonucleic acid (DNA) as a result of a genetic inherited impairment (Alsahafi et al., 2019, Fernández-Mateos et al., 2019). Another example is people with a genetic disorder (i.e. Fanconi anaemia) have a high chance to develop squamous cell carcinoma (Machiels et al., 2020, Hopkins et al., 2008). Environmentally, infection with high risk of viruses such as human papillomavirus (HPV) might lead to development of oropharyngeal cancer (Alsahafi et al., 2019). Oropharyngeal cancer mainly affects white men with a higher socioeconomic status and minimal history of smoking (median age at the time of diagnosis = 54 years) (Elrefaey et al., 2014).
3.2. Prognosis of HNC
Generally, the prognosis of HNC is highly dependent on the stage of cancer with early-stage HNC having a better prognosis (eight-year survival rate for early-stage HNC is 70.9%) than advanced-stage cancer (eight-year survival rate for advanced-stage HNC is 30.2) (Chow, 2020). Level of tumour extension, level of invasion, spread of tumour to other organs (metastasis), and number of involved lymph nodes determine the stage of HNC (Platzek et al., 2014). Clinical examination, histopathology tests, and imaging techniques are used to determine the stage of tumour and the prognosis (Leemans et al., 2011). For example, the Tumour, lymph node, and metastasis (TNM) staging system, the use of HPV status and the history of tobacco use are important factors that determine the prognosis of oropharyngeal cancers (Anantharaman et al., 2018, Guily et al., 2017). Unfortunately, the rate of developing a second primary tumour in the head and neck region is higher than other types of malignancies, meaning poor outcomes with a high mortality rate (Leoncini et al., 2018).
Besides the clinical multidisciplinary work in HNC management, the investigation of the impact of the microbiome in the development of HNC has increased dramatically. Studying oncogenic characteristics of cancer, HNC inflammatory mediators and immunology, and the role of anti-HNC therapy showed promising findings. This is crucial as recent studies on the microbiome and HNC showed controversial findings when compared to previous works. For example, a retrospective case-matched study on adult patients (<40 years old) who received treatment for squamous cell carcinoma reported no difference between younger and older patients when compared to overall, cancer-specific, or progression-free survival. Accordingly, this study concluded that further studies are needed to identify oncogenic factors that play a crucial factor in raising the prognosis of HNC and reducing the failure rate of the treatment (Blanchard et al., 2017). This indeed will also have a positive impact on the prevention of HNC as well as increase the success rate of the anti-cancer therapy. However, the direct relationship between the microbiome and HNC has not yet been fully understood and further studies are indeed required (Orlandi et al., 2019).
3.3. Treatment modalities
Different treatment modalities have been recognised in the field of HNC, such as surgical removal of the tumour, radiotherapy, chemotherapy, or a combination of these. The recommended modality for HNC depends on the tumour’s grade, TNM stage, site and the number of affected lymph nodes (Platzek et al., 2014). Radiotherapy has the advantage of organ preservation. It may also be used for inaccessible or inoperable tumours. It works by using high-energy radiation from x-rays, gamma x-rays, protons, neutrons and other sources, which destroy cancer cells and reduce the size of the tumours (Delaney et al., 2005). Moreover, radiotherapy could be used as adjunctive treatment following the surgical removal of the primary tumour or after the surgical removal of the affected lymph nodes (Chow, 2020). Recent advances in radiotherapy techniques (i.e. intensity-modulated radiation therapy (IMRT) accompanied by a better understanding of the tissue’s reaction to radiation therapy make radiotherapy the best treatment modality for most HNCs (De Felice et al., 2016). The ionising radiations work through direct damage to the nucleic part of any cell (DNA) or indirectly because of the interaction between the water and radiation (free radicals). Thereby, its effect is not limited to the cancer cells, and it also damages the other healthy cells. High doses of radiotherapy produce more damage to the normality and functionality of non-affected cells as the damage is dose-related and depends on the turnover survival rate of the tissue’s stems cells, and in turn high volume will produce more significant side-effects (Chang et al., 2014, Abdollahi, 2016). Tissues in the oral cavity have a rapid cell turnover, meaning that the oral mucosa is affected by the acute and chronic toxicity form of the radiotherapy (Bhandari et al., 2020). The success rate of treatment is significantly higher if modern radiotherapy (i.e. IMRT) is applied for early-stage cancer (Sowder et al., 2017).
3.4. Implications of radiotherapy for the oral cavity
Treatment of HNC with radiotherapy can have long-term side-effects, which puts oral health at risk. Two types of toxicity related to radiotherapy might develop; acute/early form toxicity and chronic/late form toxicity (Bhandari et al., 2020). Acute adverse effects include oral mucositis, taste disturbance, xerostomia (dry mouth) and trismus. The chronic adverse effects include radiation caries and osteoradionecrosis (ORN).
3.4.1. Acute form toxicity
3.4.1.1. Oral mucositis
Oral mucositis is an early complication following radiotherapy, chemotherapy or a combination of both. Clinically this appears as mucosal inflammation with sloughing erythematous, and pain related to the mucosal tissues that line the gastrointestinal tract (GIT) (Bhandari et al., 2020). In the head and neck region, areas that are lined with mucosal tissues include larynx, pharynx, nasal cavity, paranasal sinuses, oropharyngeal region and the oral cavity. Oral mucositis usually starts within 12–15 days post-radiotherapy (The Royal College of Surgeons of England/The Britsh Society for Disability and Oral Health (RCS/BSDH), 2018). On some occasions, it is combined with atrophy and ulceration that affect the patient’s social life (Jung et al., 2019). A significant number of patients suffer whilst eating, which in turn affects their nutritional status and reduces their food intake with difficulties in swallowing (Nakajima et al., 2015, Ray-Chaudhuri et al., 2013). There are different levels of oral mucositis, which depends on multiple factors such as radiation technique used, field of radiation and dose of the radiation (Sheibani et al., 2015). Treatment of oral mucositis involves either pharmacological or non-pharmacological treatments. Difflam (15% Benzydamine oral rinse) is one of the most common medications used in the management of oral mucositis (15 ml every eight hours and up to three weeks post-radiotherapy (Riley, 2017)). The pharmacodynamics of the drug is described by its local action with high therapeutic range. It acts as an analgesic and an anti-inflammatory agent as an inhibitor to the pro-inflammatory cytokines such as tumour necrosis factor and interleukine (Sonis et al., 2000). Non-pharmacological approaches have been advocated; for instance, ice cubes may help to prevent oral mucositis associated with the treatment. However, this shows significantly better results with patients post-chemotherapy rather than the patients who received radiotherapy (Nawi et al., 2018). Patients with HNC following radiation who develop oral mucositis often have difficulty maintaining good oral hygiene. Brushing teeth can become a painful behaviour, thereby, a soft toothbrush or oral swabs are suggested to maintain good oral hygiene (Kumar et al., 2013).
3.4.1.2. Taste disturbance
Direct ionising radiation damages the majority of taste buds in all parts of the oral cavity. Three types of taste disturbance might develop in HNC patients post-radiotherapy, which are altered taste (dysgeusia), diminished ability to taste (hypogeusia), or complete loss of taste (ageusia) (Bhandari et al., 2020). Taste disturbance usually starts early after beginning radiotherapy and before oral mucositis (Vissink et al., 2003). Taste bud irritation usually appears around the fifth week post-radiotherapy (Deshpande et al., 2018). A systematic review which included seven cross-sectional and 11 cohort studies concluded that 70–100% of HNC patients have taste disturbance (Deshpande et al., 2018). Taste disturbance reduces food intake and leads to further complications in chewing (Bhandari et al., 2020). Recovery of normal taste level in HNC patients post-radiotherapy varies from patient to patient, and may be achieved after cessation of radiation, but permanent damage has been observed in some cases (Deshpande et al., 2018).
3.4.1.3. Xerostomia (dry mouth)
Xerostomia (dry mouth) is a common and significant chronic oral complication in HNC patients (Samim et al., 2016). Dry mouth starts to develop at 26 Gy and above (Tribius et al., 2013). The exact incidence and prevalence of dry mouth is still unknown and reported differently worldwide, however, a reduction in cases secondary to advanced radiotherapy techniques, for example IMRT, can help reduce damage to surrounding soft tissues such as salivary glands (Burke et al., 2012, Nutting et al., 2011).
In the UK, research conducted in Cardiff (The Holme Tower Marie Curie Centre) reported the prevalence of dry mouth as 77% of 197 patients (112 men, 85 women) who were terminally ill with different types of cancers admitted to hospital (Jobbins et al., 1992). In the Netherlands, an explorative study (Jager-Wittenaar et al., 2011) stated that all of 116 patients with oral/oropharyngeal cancer receiving different cumulative radiation volumes to the salivary glands suffered a moderate to severe dry mouth. Another survey in the Netherland reported 65% of 39 long-term survival patients with a malignancy in head and neck region experienced a moderate to severe degree of dry mouth post-radiotherapy (Wijers et al., 2002). In the US, a retrospective study in the University of Texas reported 29% of 748 patients diagnosed with several types of HNCs to have different levels of dry mouth, each with differing perceptions of quality of life (QoL) (Hanna et al., 2015).
These differences in both prevalence and incidence result from variable measuring tools, differences in radiation doses, varying tumour locations within the head and neck region, tumour stages, timing of the measurement and its effect on major or minor salivary glands (Hanna et al., 2015). Additionally, advanced radiotherapy techniques such as IMRT have significantly improved the severity of oral dryness and may account for some differences between studies (Owosho et al., 2017).
Xerostomia increases the risk of dental caries and specifically radiation caries. This could be explained by the indirect effect of reduced saliva, buffering changes (more acidic media), and reduced innate protection mechanism of the saliva (Arrifin et al., 2018). A systematic review has found that the level of fungal infection is also significantly increased in patients receiving cancer therapy (Lalla et al., 2010).
Oral health providers have a clear role to help reduce dry mouth in HNC patients (Burke et al., 2012). Two approaches are usually considered for managing patients with xerostomia. The dental team frequently advise patients to take regular sips of water and use sugar-free chewing gum to stimulate the remaining saliva acinar cells (Kaae et al., 2016). In addition, a systematic review and meta-analysis which included three studies concluded that using pharmacological treatment (i.e. Pilocarpine®) can help to stimulate parasympathetic system and stimulate salivary acinar cells significantly (Cheng et al., 2016). Artificial saliva can help to reduce the patient’s perception of mouth dryness and acts as a lubricant (Burke et al., 2012, Łysik et al., 2019). Further, as noted above, using IMRT has helped to reduce the radiation side-effects including xerostomia (Wang and Eisbruch, 2016).
3.4.1.4. Trismus
Trismus is defined as difficulty to open the mouth to the normal range of opening due to fibrosis of the masticatory muscles (Bhandari et al., 2020). Dijkstra et al. (2006) described trismus as the reduction of the mouth opening less than 35 mm, due to the inflammation of the elevator muscles of the mandible resulting from ionising radiation. A systematic review concluded that the prevalence of trismus was found to be 17% at baseline, 44% at six months post-radiotherapy, 32% at 12 months post-radiotherapy and 32% at 3–10 years post-radiotherapy (Watters et al., 2019). It appears clinically two months post-radiotherapy with a reduction of 2–4% in the mouth opening per month (Bhandari et al., 2020). As a result of trismus, patients can have difficulties in maintaining self and professional dental care and this affects their QoL negatively (Burke et al., 2012).
Jawad et al. (2015a) recommend patients with HNC to exercise the masticatory muscles before the treatment with radiotherapy, as this may help to prevent trismus post-radiotherapy treatment. A systematic review found that early exercise therapy is crucial for successful results (Kamstra et al., 2017). For example, using wooden tongue depressors between the upper and lower teeth is suggested to help increase the opening of mouth post-radiotherapy, increasing the number of wooden tongue depressors over the time (Jawad et al., 2015b). Another example is the Therabite appliance©, which works in the same way (Kamstra et al., 2013). Using Therabite appliance© and wooden tongue depressors helps to increase mouth opening significantly if used together (Ezzat et al., 2020). On the other hand, some oral surgeons suggest early extraction of posterior teeth if the radiation dose is high and trismus is expected to develop. On some occasions, patients with severe trismus may be seen by physiotherapists, speech, and language therapists, or all of them for advice (Burke et al., 2012).
For urgent or elective dental treatment of patients with trismus, the dental team has to plan to accommodate dental treatment. For instance, short dental appointments, resting-time during the dental procedure, and use of a mouth prop with paediatric dental burs (short bur lengths) with paediatric headpieces may be required (Burke et al., 2012).
3.4.2. Chronic form toxicity
3.4.2.1. Radiation caries
Radiation caries in patients with HNC develops rapidly and aggressively post-radiotherapy (Burke et al., 2012). It affects around 30% of HNC patients post-radiotherapy (Palmier et al., 2020). It has unique features and appears differently from the usual characteristics and clinical presentation of dental caries in healthy patients of the general population (Hegde and John, 2018). It has an unusual appearance on smooth surfaces of the tooth and at the cusp tips. Radiation caries is characterised by its distribution along the lines of the cemento-enamel junction of the tooth, which leads to severe crown destruction around the gingival margin (Palmier et al., 2020). Clinically, this destruction can lead to crown amputation if it is not managed properly in the early stage (Burke et al., 2012).
Some patients with HNC complain of less severe dental pain post-radiotherapy, which does not reflect the severe dental status. This could be explained by a reduction of the vascularity of the dental pulp with fibrotic tissues developed as a result of exposure to ionising radiation (Abed, 2021).
Providing dental restorative treatment to caries in irradiated teeth requires careful management and treatment planning to help prevent early loss of the teeth. Advanced adhesive techniques in dentistry (i.e. chemical bonding of the tooth with the glass ionomer materials) and atraumatic restorative technique (minimal invasive dentistry) is best used in patients with radiation caries (Jawad et al., 2015a). For example, using spoon excavator or dye gel to remove dental caries through advanced adhesive technique and minimal invasive dentistry will help to provide dental treatment for HNC patients who cannot tolerate hand-pieces and/or mouth opening for a long time because of mouth trismus post-radiotherapy (Moore et al., 2012).
3.4.2.2. Osteoradionecrosis
The definition of ORN has not been clearly agreed. Most published definitions rely on the clinical features of the lesion, but inclusion of radiographs has been suggested to help diagnosis of ORN (Støre and Boysen, 2000). The major difference is the duration of bone exposure before the lesion can be recognised as ORN. Marx (1983b) described ORN as “an area of exposed, devitalized bone present for greater than three months in an area that has been irradiated, with no signs of local neoplastic disease”. It may be asymptomatic or symptomatic causing pain, swelling, discharge, erythema, and skin fistula (Nabil and Samman, 2012). This may be superimposed with infection by a microbial organism if it is left undiagnosed and untreated (Madrid et al., 2010). High dose of radiation can lead to early development of ORN (Jawad et al., 2015a), while late development of ORN is related to traumatic procedures such as tooth extraction (Nabil and Samman, 2012). A systematic review by Nabil and Samman (2011) concluded that seven of 100 patients having dental extraction post-radiotherapy will develop ORN and that two of 100 teeth extracted post-radiotherapy will develop ORN (Nabil and Samman, 2011). Table 1 presents the summary of ORN classification developed by Lyons and Brennan (2014).
Table 1.
The summary of ORN classification developed by Lyons and Bernnan (2014).
| Stage | Description | Clinical picture |
|---|---|---|
| 1 | <2.5 cm length, asymptomatic exposed bone; required medical treatment only. | 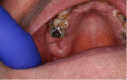 |
| 2 | >2.5 cm length asymptomatic exposed bone including pathological fracture and/or inferior alveolar nerve canal involvement; required medical treatment only, unless dental sepsis or obviously loose necrotic bone. | 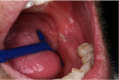 |
| 3 | >2.5 cm length, symptomatic exposed bone, but with no other features despite medical treatment; consider debridement and local pedicle flap. | 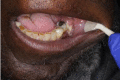 |
| 4 | >2.5 cm length, symptomatic exposed bone with pathological fracture and/or inferior alveolar nerve canal or orocutaneous fistula. Reconstruct with free flap. | 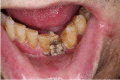 |
3.4.2.2.1. Factors affecting the development of ORN
The reported incidence of ORN is different between studies, and is influenced by radiation dose, presence of local trauma, field of radiation, and location of the tumour (mandible or maxilla) (Nabil and Samman, 2012). Each of these are now discussed in turn.
-
(a)
Radiation dose
The radiation dose is the most important risk factor for the development of ORN (Beumer et al., 1984, Morrish et al., 1981). The higher the dose received, the higher the risk of developing ORN (Thorn et al., 2000, Clayman, 1997, Widmark et al., 1989, Curi and Lauria, 1997). Nabil and Samman (2011) found that no patients receiving 60 Gy or less had developed ORN. However, this conclusion should be interpreted with caution, because data in their systematic review was based on a limited sample size and the risk is not eliminated when doses are below 60 Gy (Thorn et al., 2000, Widmark et al., 1989). A recent case series study found that the occurrence of ORN in patients who received 57.3 Gy and above is high (Iqbal and Kyzas, 2020).
-
(b)
Presence of local trauma
Dental extraction has been considered as one of the major risk factors for the development of ORN (Nabil and Samman, 2012). There is still debate regarding the best time to perform the dental extractions in relation to radiotherapy. For example, Lajolo et al. (2020) reported that dental extraction prior to treatment with radiotherapy would increase the risk of developing ORN, with an incidence of 2.2%, while Nabil and Samman (2011) found that dental extraction post-radiotherapy is a more risky procedure and will increase risk of development ORN, with an incidence of 7%. Pre-radiotherapy dental assessment, patient’s education, excellent oral hygiene, use of high fluoride-containing toothpaste (5000 ppm), and careful attention to diet and nutrition will reduce dental treatment post-radiotherapy (i.e. dental extraction) (Burke et al., 2012), but these efforts will not totally prevent the need for dental extraction in irradiated populations (Banjar et al., 2023).
-
(c)
Field of radiation
It is known that an area included within the field of radiation will have a higher chance to develop ORN. Thorn et al. (2000) reported that only one patient out of 80 patients with HNC had developed ORN outside the field of radiation. Treatment with IMRT may also help to eliminate the possibility of ORN of the extracted tooth/teeth in the area outside the field of radiation as it works by focusing the radiation on the region affected by cancer cells and protects the non-affected region (Nutting et al., 2011, Burke et al., 2012). However, a recent study found that the rate of ORN in the era of IMRT still is unknown and requires more studies (Caparrotti et al., 2017).
-
(d)
Location of the tumour
Proximity of the mandible or maxilla to the tumour region has been reported to increase the risk of development of ORN. This is explained by the pattern of blood supply to the mandible being less than in the maxilla. Additionally, the mandible is included usually in the radiation field more than the maxilla (Nabil and Samman, 2012). Thereby, dental extraction in the mandible has to be considered as higher risk for developing ORN post-radiotherapy. The exact incidence and prevalence of ORN is still unknown and reported differently worldwide; however, a reduction in cases secondary to advanced radiotherapy techniques, for example, IMRT, can help reduce the development of ORN (Banjar et al., 2023).
3.4.2.2.2. Theories of the development of ORN
Three are three theories about the development of ORN. Corresponding treatment modalities were developed and based on the theories. These are explained below.
-
(a)
Radiation trauma-infection theory
Meyer (1970) defined ORN as an oral lesion that developed due to the combination of ionising radiation, present of local trauma and infection. He suggested that the trauma provided the portal for the invasion by bacteria into the underlying irradiated bone. His theory lasted for a decade and became the foundation for the popular use of antibiotics with surgery to treat ORN.
-
(b)
Hypoxic-hypocellular-hypovascular theory
The availability of oxygen atoms, the ability of the body’s cells to proliferate and the presence of blood supply are major key factors for a successful healing process. Marx (1983a) developed the hypoxic-hypocellular-hypovascular theory of the development of the ORN. The pathophysiology sequence suggested by Marx is the presence of the ionising radiation, in addition to the reduction of the oxygen level at the cellular level, reduced cells proliferation and reduced blood supply.
These sequences lead to persistent hypoxia and tissue breakdown that cause a chronic non-healing wound. This pathophysiology gives a clear explanation why hyperbaric oxygen (HBO) is used in the treatment of the ORN. HBO has a long history in the management of ORN started by Mainous and his colleague (Mainous et al., 1973). In 1983, Marx supported the use of HBO in the management of ORN based on the hypoxic-hypocellular-hypovascular theory by its efficacy as bactericidal and bacteriostatic. In 1987, Marx and Johnson found that HBO plays a very important role in increasing the fibroblast cells, collagen and angiogenesis (Marx and Johnson, 1987). It requires one session at least prior to dental extraction to help reduce the development of ORN.
Recently, there has been a debate about the availability and efficacy of HBO. For example, one session of HBO lasts for 90 min, and it cannot be used in patients with acute pain who required urgent dental extraction (Vudiniabola et al., 1999). Additionally, the Marx protocol suggests 30 dives +/-10 for one patient and one dive costs £233 (Patel et al., 2017). HBO has some reported common complications such as middle ear barotrauma and myopia. Other less common complications are pneumothorax, oxygen toxicity seizure and acute pulmonary disease (Camporesi, 2014). Therefore, a significant number of clinicians do not recommend HBO as an effective treatment for ORN.
-
(c)
Radiation-induced fibro-atrophic theory (RIF)
Radiation-induced fibro-atrophic theory was proposed by Delanian and Lefaix (2004). They found that the total cellular depletion of fibroblast cells might contribute to the development of ORN (Delanian and Lefaix, 2004). Three phases of the RIF had been recognised. The first one is defined by the pre-fibrotic phase (change of the endothelia cells and establishment of acute inflammation). The second phase is the constitutive organised phase, characterised by the disorganisation of the extracellular matrix and functional of the abnormal fibroblast called “myofibroblast”. The last phase is the formation of the fibro-atrophic phase (Delanian and Lefaix, 2004).
The main pharmacodynamics goal of the treatment of ORN by considering the RIF theory is the regulation of fibroblast cells function. A combination of pentoxifylline (400 mg/ twice per day), which is an antioxidant drug, and vitamin E (1000 IU/ once daily) works as a potent anti-fibrotic agent, and it showed a significant result recently (Kolokythas et al., 2019).
4. Discussion
4.1. The role of the oral healthcare providers with HNC patients
The Calman–Hine report of 1995 examined cancer services in the UK and suggested a restructuring of cancer services to help achieve equitable level of access to high levels of expertise throughout the country. The report highlighted the need to focus cancer treatment and medical management regimens on both longevity and QoL (Calman and Hine, 1995). Maintenance of good oral healthcare is an important factor to post-cancer treatment adaptation and QoL (Santos et al., 2017). Thus, oral healthcare providers have an important role in improving the QoL of patients with HNC. They are involved in a multidisciplinary team (MDT) who promote consistent high standards of oral care through an integrated care pathway. The patient should be referred to the dental department for pre-treatment review once the cancer is diagnosed and radiotherapy is planned (The National Institute for Health and Care Excellence (NICE), 2004). The role of the dentist who is involved in the MDT is to diagnose oral diseases, manage patients before, during and after the cancer treatment, and also help to rehabilitate the oral function during any stages of HNC treatment. Their role is to work with other team members to provide prompt advice and treatment at all stages of the patient pathway from diagnosis to discharge. The Royal College of Surgeons of England/The Britsh Society for Disability and Oral Health (RCS/BSDH) (2018) published clear clinical guidelines to help provide effective oral and dental management of cancer patients receiving radiotherapy, chemotherapy and/or haematopoietic stem cell transplant; see Table 2.
Table 2.
The RCS/BSDH recommendations for the oral management of oncology patients requiring radiotherapy, chemotherapy, and haematopoietic stem cell transplant.
| Cancer therapy stage | Dental recommendations |
|---|---|
| Pre-cancer therapy | 1. Elimination of oral infection and potential risk of oral disease or discomfort such as sharp teeth or ill-fitting dentures. |
| 2. Achieving optimal healing after any tooth extraction. | |
| 3. Oral hygiene instructions. | |
| 4. Impressions of the mouth are taken for study casts to construct applicator trays and where appropriate for obturators planning. | |
| During cancer therapy | 1. Recommendations to use an alcohol-free chlorhexidine mouthwash. |
| 2. Reducing the side-effects such as xerostomia and mucositis. | |
| 3. If the mouth is too painful for cleaning with a soft toothbrush, the tissues can be cleaned with oral sponges or gauze moistened with alcohol-free mouthwash. | |
| Post-cancer therapy | 1. Regular radiographs, oral health advice and preventive regime reinforcement. |
| 2. Strategies for dealing with xerostomia continue. | |
| 3. High fluoride toothpaste should be used. | |
| 4. Jaw exercises are implemented in the event of trismus. |
4.2. The pre-radiotherapy dental assessment of HNC patients
The dental needs of HNC patients have been noted to be higher than the general population yet may have had irregular dental attendance. For example, in a Brazilian study, 207 (135 of whom were non-edentulous) HNC patients were assessed before they started radiotherapy (Jham et al., 2008). The study reported that 89% of the non-edentulous patients had periodontal diseases and among them 63% needed urgent periodontal treatment, a further 33% of the 207 patients had remaining roots that required dental extraction and 18% had dental caries that required dental restorations. Another example of a cohort study at the Royal London Hospital on 100 HNC patients (66 male and 34 female patients) during the pre-radiotherapy dental assessment found that of all dentate patients (n = 98), 71% were diagnosed with periodontal diseases and 61% presented with one or more carious teeth (Critchlow et al., 2014). Recently, a large retrospective observational study of 886 HNC patients who received treatment with radiotherapy for the three most common HNC subsites (i.e. larynx, oral cavity and oropharynx) at Guy’s and St Thomas’ NHS Foundation Trust between 2011 and 2017, found that the number of decayed, missed and filled teeth (DMFT) across three subsites were statistically significant with those with laryngeal cancer having an average of 18.2 on DMFT index (SD = 6.3) compared to 16.5 in those with oral cavity (SD = 7.8) and 15.4 in those with oropharynx (SD = 7.8) cancers (DMFT index ranges from 0 to 28) (Patel et al., 2020). Patel and colleagues concluded that oral profiles of HNC patients vary substantially, and for this reason dental protocols should be tailored accordingly.
The dental team and patients may experience problematic dental appointments at this stage. A significant number of patients are in a state of shock after being diagnosed with cancer. Patients have also reported that they have little information given to them (Ray-Chaudhuri et al., 2013). Therefore, clear, concise information in writing is indeed recommended. It is essential for the dental team to discuss with the patient why they have to be assessed by a dentist before starting the treatment with radiotherapy. Additionally, patients need to know about future dental appointments (post-radiotherapy dental assessment) until the end of the cancer therapy regimen (Burke et al., 2012). Dental assessment should start with a thorough oral and dental examination, and investigation (i.e. pulp testing and dental radiographs) to help approach definitive oral and dental diagnosis for each tooth (Ray-Chaudhuri et al., 2013). This should be followed by the usual standard assessment such as chief complaints, standard histories (i.e. medical history, dental history, social history, and family history), diet analysis, and short- and long-term treatment plans. Then an honest discussion with the patient about the likely side-effects of ionising radiation on the head and neck regions, and specifically on oral and dental health, is crucial (Burke et al., 2012).
4.2.1. Pre-radiotherapy dental extraction
It has been recommended that any teeth with a poor prognosis, if they are included in the field of radiation, should be extracted prior to treatment with radiotherapy (Burke et al., 2012). Additionally, removing any teeth with pocket depth of more than 7 mm was also recommended (Scully et al., 2007). Additionally, removing the teeth that are unopposed or will become unopposed after dental extraction of other teeth has been recommended (Ray-Chaudhuri et al., 2013). This is to help prevent the need for non-recommended and invasive dental treatment that might lead to development of ORN post-radiotherapy (i.e. dental extraction, oral and periodontal surgery, and any procedures have an impact on the bone). Every patient should have their assessment based on their risk factors and after careful analysis of the advantages and disadvantages of dental extraction. Unfortunately, there are no clear guidelines regarding the type of teeth that should be extracted and no standard definition for the meaning of “hopeless tooth/teeth”. This may explain why there is a difference between oral and dental care providers in the UK regarding the pre-radiotherapy dental extraction, with some of them favouring extraction of posterior teeth to reduce the possible needs of that post-radiotherapy and hence increases risk of ORN (Ray-Chaudhuri et al., 2013). For example, patients who are planning to receive ionising radiation to the posterior part of their mouth (i.e. molar teeth are included in the field of radiation) have their posterior teeth extracted and patients are left with short dental arch (i.e. from the lower right second premolar to the lower left second premolar). Clinicians who supported this point found that a short dental arch could provide enough function in a significant number of patients (Käyser, 1981, Witter et al., 1990). This part of dental treatment (i.e. dental extraction of posterior teeth prior to treatment with radiotherapy) is less understood by the patients and their carers and can add additional psychological trauma to the diagnosis with HNC (Clough et al., 2018).
4.3. Post-radiotherapy regular review and oral rehabilitation
Patients diagnosed with HNC and treated with radiotherapy require review appointments to check their understanding regarding the cancer therapy, and their compliance with the suggested oral health regime. Post-radiotherapy dental care mainly focuses on the management of post-radiotherapy complications. This is usually achieved by the patient’s local general dental practitioner who should receive full details from the secondary care centre about the patient’s medical condition and their needs. A thorough oral examination is required to check for any suspicious new pathology or local recurrence for early referral to a secondary care centre. At this stage, patients usually attend with many questions regarding their oral rehabilitation, as they have been left without posterior teeth or even sometimes anterior teeth (Ray-Chaudhuri et al., 2013). Therefore, some patients are left with difficulty swallowing and eating related to their missing teeth (Abed et al., 2019). Each patient should be assessed for oral rehabilitation and a decision made based on the best interest of each patient. For some patients, no attempt at prosthetic treatment may be the option of choice, especially where patients may not be able to tolerate a denture and may wish to continue as they are (Abed et al., 2023). If teeth are to be replaced, different options might be considered such as removable partial dentures, fixed prosthesis, or dental implants with/without retained fixed or removable prostheses (Siddall et al., 2012).
5. Strengths and limitations
This narrative review showed what exactly has been properly accomplished regarding the dental aspects of HNC patients, hence showing the gaps that need to be addressed. Also, it gave a summary of the dental amendment of HNC and great insight into the dental management of HNC patients for new dentists. On the other hand, this narrative review lacks comprehensive and systematic searching; therefore, valuable studies on the field could be missed. Additionally, this narrative review did not have a clear method; hence the review’s reliability is low (Grant and Booth, 2009).
6. Conclusion
The number of HNC patients is increasing. This necessitates the need for raising the awareness of dental care providers to the side-effects of HNC therapy which includes treatment with radiotherapy, chemotherapy, and/or surgery. Treatment with radiotherapy includes acute (i.e., dry mouth, oral mucositis, loss of taste, and trismus) and chronic (i.e., radiation caries and ORN) side-effects. These side-effects affect the HNC patients’ quality of life negatively. Dental care providers should understand the common side-effects and their treatments besides their role in the pre- (i.e., dental extraction of teeth with poor prognosis and maintaining good oral hygiene) and post- (i.e., oral rehabilitation and post-HNC dental care) radiotherapy dental care.
CRediT authorship contribution statement
Hassan Abed: Conceptualization, Methodology, Data curation, Writing – original draft, Visualization, Investigation, Validation, Writing – review & editing.
Declaration of Competing Interest
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
References
- Abdollahi H. Basic Radiotherapy Physics and Biology. J. Med. Phys./Assoc. Medical Physicists of India. 2016;41:77–79. [Google Scholar]
- Abed H., Reilly D., Burke M., Daly B. Patients with head and neck cancers' oral health knowledge, oral health-related quality of life, oral health status, and adherence to advice on discharge to primary dental care: a prospective observational study. Spec. Care Dentist. 2019;39:593–602. doi: 10.1111/scd.12418. [DOI] [PubMed] [Google Scholar]
- Abed H., Mannocci F., Bakhsh A. The impact of radiotherapy on pulp vitality in patients with head and neck cancer: a systematic review. Saudi Endodontic J. 2021;11:123–128. [Google Scholar]
- Abed H., Reilly D., Burke M., Sharka R., Daly B. The association between dental arch length and oral health-related quality of life in head and neck cancer patients post-radiotherapy. Spec. Care Dentist. 2023;43:111–118. doi: 10.1111/scd.12755. [DOI] [PubMed] [Google Scholar]
- Alsahafi E., Begg K., Amelio I., Raulf N., Lucarelli P., Sauter T., Tavassoli M. Clinical update on head and neck cancer: molecular biology and ongoing challenges. Cell Death Dis. 2019;10:1–17. doi: 10.1038/s41419-019-1769-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharaman D., Billot A., Waterboer T., Gheit T., Abedi-Ardekani B., Lagiou P., Lagiou A., Ahrens W., Holcátová I., Merletti F. Predictors of oropharyngeal cancer survival in Europe. Oral Oncol. 2018;81:89–94. doi: 10.1016/j.oraloncology.2018.04.016. [DOI] [PubMed] [Google Scholar]
- Arrifin A., Heidari E., Burke M., Fenlon M.R., Banerjee A. The effect of radiotherapy for treatment of head and neck cancer on oral flora and saliva. J. Oral Health Preventive Dentistry. 2018;16:425–429. doi: 10.3290/j.ohpd.a41364. [DOI] [PubMed] [Google Scholar]
- Banjar A., Patel V., Abed H. Pentoxifylline and tocopherol (vitamin E) with/without clodronate for the management of osteoradionecrosis: a scoping review. Oral Dis. 2023;29(1):29–39. doi: 10.1111/odi.14058. [DOI] [PubMed] [Google Scholar]
- Beumer J., Harrison R., Sanders B., Kurrasch M. Osteoradionecrosis: predisposing factors and outcomes of therapy. Head Neck Surg. 1984;6:819–827. doi: 10.1002/hed.2890060404. [DOI] [PubMed] [Google Scholar]
- Bhandari S., Soni B.W., Bahl A., Ghoshal S. Radiotherapy-induced oral morbidities in head and neck cancer patients. Spec. Care Dentist. 2020;40:238–250. doi: 10.1111/scd.12469. [DOI] [PubMed] [Google Scholar]
- Blanchard P., Belkhir F., Temam S., El Khoury C., De Felice F., Casiraghi O., Patrikidou A., Mirghani H., Levy A., Even C., Gorphe P. Outcomes and prognostic factors for squamous cell carcinoma of the oral tongue in young adults: a single-institution case-matched analysis. Eur. Arch. Otorhinolaryngol. 2017;274:1683–1690. doi: 10.1007/s00405-016-4419-1. [DOI] [PubMed] [Google Scholar]
- Burke M.C., Fenlon M.R., Banerjee A. The role of the general dental practitioner in managing the oral care of head and neck oncology patients. Dent. Update. 2012;39:694–702. doi: 10.12968/denu.2012.39.10.694. [DOI] [PubMed] [Google Scholar]
- Calman K.C., Hine D. Department of Health; 1995. A policy framework for commissioning cancer services. A report by the expert advisory group on cancer to the chief medical officers of England and Wales: guidance for purchasers and providers of cancer services. [Google Scholar]
- Camporesi E.M. Side effects of hyperbaric oxygen therapy. J. Undersea Hyperbaric Medical Soc. 2014;41:253–257. [PubMed] [Google Scholar]
- CANCER RESEARCH UK, 2017. Head and neck cancer statistics [Online]. Available: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/head-and-neck-cancers#heading-One [Accessed 30/09/2020].
- Caparrotti F., Huang S.H., Lu L., Bratman S.V., Ringash J., Bayley A., Cho J., Giuliani M., Kim J., Waldron J. Osteoradionecrosis of the mandible in patients with oropharyngeal carcinoma treated with intensity-modulated radiotherapy. Cancer. 2017;123:3691–3700. doi: 10.1002/cncr.30803. [DOI] [PubMed] [Google Scholar]
- Chang D.S., Lasley F.D., Das I.J., Mendonca M.S., Dynlacht J.R. Springer; 2014. Basic Radiotherapy Physics and Biology. [Google Scholar]
- Cheng C.-Q., Xu H., Liu L., Wang R.-N., Liu Y.-T., Li J., Zhou X.-K. Efficacy and safety of pilocarpine for radiation-induced xerostomia in patients with head and neck cancer: a systematic review and meta-analysis. J. Am. Dent. Assoc. 2016;147:236–243. doi: 10.1016/j.adaj.2015.09.014. [DOI] [PubMed] [Google Scholar]
- Chow L.Q. Head and neck cancer. N. Engl. J. Med. 2020;382:60–72. doi: 10.1056/NEJMra1715715. [DOI] [PubMed] [Google Scholar]
- Clayman L. Management of dental extractions in irradiated jaws: A protocol without hyperbaric oxygen therapy. J. Oral Maxillofac. Surg. 1997;55:275–281. doi: 10.1016/s0278-2391(97)90542-5. [DOI] [PubMed] [Google Scholar]
- Clough S., Burke M., Daly B., Scambler S. The impact of pre-radiotherapy dental extractions on head and neck cancer patients: a qualitative study. Br. Dent. J. 2018;225:28–32. doi: 10.1038/sj.bdj.2018.442. [DOI] [PubMed] [Google Scholar]
- Critchlow S., Morgan C., Leung T. The oral health status of pre-treatment head and neck cancer patients. Br. Dent. J. 2014;216:1–5. doi: 10.1038/sj.bdj.2013.1246. [DOI] [PubMed] [Google Scholar]
- Curi M.M., Lauria L. Osteoradionecrosis of the jaws: a retrospective study of the background factors and treatment in 104 cases. J. Oral Maxillofac. Surg. 1997;55:540–544. doi: 10.1016/s0278-2391(97)90478-x. [DOI] [PubMed] [Google Scholar]
- de Felice F., Thomas C., Patel V., Connor S., Michaelidou A., Sproat C., Kwok J., Burke M., Reilly D., McGurk M. Osteoradionecrosis following treatment for head and neck cancer and the effect of radiotherapy dosimetry: the Guy's and St Thomas' Head and Neck Cancer Unit experience. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:28–34. doi: 10.1016/j.oooo.2016.01.007. [DOI] [PubMed] [Google Scholar]
- Delaney G., Jacob S., Featherstone C., Barton M. The role of radiotherapy in cancer treatment. Cancer. 2005;104:1129–1137. doi: 10.1002/cncr.21324. [DOI] [PubMed] [Google Scholar]
- Delanian S., Lefaix J.-L. The radiation-induced fibroatrophic process: therapeutic perspective via the antioxidant pathway. Radiother. Oncol. 2004;73:119–131. doi: 10.1016/j.radonc.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Deshpande T.S., Blanchard P., Wang L., Foote R.L., Zhang X., Frank S.J. Radiation-related alterations of taste function in patients with head and neck cancer: a systematic review. Curr. Treat. Options Oncol. 2018;19:72–87. doi: 10.1007/s11864-018-0580-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra P., Huisman P., Roodenburg J. Criteria for trismus in head and neck oncology. Int. J. Oral Maxillofac. Surg. 2006;35:337–342. doi: 10.1016/j.ijom.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Elrefaey S., Massaro M., Chiocca S., Chiesa F., Ansarin M. HPV in oropharyngeal cancer: the basics to know in clinical practice. Acta Otorhinolaryngol. Ital. 2014;34:299–309. [PMC free article] [PubMed] [Google Scholar]
- Ezzat Y.E., Sharka R.M., Huzaimi A.A., Al-Zahrani K.M., Abed H.H. The role of exercise therapy in managing post-radiotherapy trismus in head and neck cancer. J. Taibah Univ. Med. Sci. 2020 doi: 10.1016/j.jtumed.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Mateos J., Seijas-Tamayo R., Adansa Klain J.C., Pastor Borgoñón M., Pérez-Ruiz E., Mesía R., Del Barco E., Salvador Coloma C., Rueda Dominguez A., Caballero Daroqui J., Fernández Ruiz E. Genetic susceptibility in head and neck squamous cell carcinoma in a Spanish population. Cancers. 2019;11:493–504. doi: 10.3390/cancers11040493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghantous Y., Elnaaj A. Global incidence and risk factors of oral cancer. Harefuah. 2017;156:645–649. [PubMed] [Google Scholar]
- Grant M.J., Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info. Libr. J. 2009;26:91–108. doi: 10.1111/j.1471-1842.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Gupta B., Bray F., Kumar N., Johnson N.W. Associations between oral hygiene habits, diet, tobacco and alcohol and risk of oral cancer: a case–control study from India. Cancer Epidemiol. 2017;51:7–14. doi: 10.1016/j.canep.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Hanna E.Y., Mendoza T.R., Rosenthal D.I., Gunn G.B., Sehra P., Yucel E., Cleeland C.S. The symptom burden of treatment-naive patients with head and neck cancer. Cancer. 2015;121:766–773. doi: 10.1002/cncr.29097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde M.N., John A. Radiation induced caries: an overview. J. Health Allied Sci. 2018;8:28–33. [Google Scholar]
- Hopkins J., Cescon D.W., Tse D., Bradbury P., Xu W., Ma C., Wheatley-Price P., Waldron J., Goldstein D., Meyer F., Bairati I., Liu G. Genetic polymorphisms and head and neck cancer outcomes: a review. Cancer Epidemiol. Prevention Biomarkers. 2008;17:490–499. doi: 10.1158/1055-9965.EPI-07-2714. [DOI] [PubMed] [Google Scholar]
- Iqbal Z., Kyzas P. Analysis of the critical dose of radiation therapy in the incidence of Osteoradionecrosis in head and neck cancer patients: a case series. Br. Dent. J. 2020;6:1–6. doi: 10.1038/s41405-020-00044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager-Wittenaar H., Dijkstra P.U., Vissink A., van Oort R.P., van der Laan B.F., Roodenburg J.L. Malnutrition in patients treated for oral or oropharyngeal cancer—prevalence and relationship with oral symptoms: an explorative study. Support. Care Cancer. 2011;19:1675–1683. doi: 10.1007/s00520-010-1001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawad H., Hodson N., Nixon P. A review of dental treatment of head and neck cancer patients, before, during and after radiotherapy: part 1. Br. Dent. J. 2015;218:65–68. doi: 10.1038/sj.bdj.2015.28. [DOI] [PubMed] [Google Scholar]
- Jawad H., Hodson N.A., Nixon P.J. A review of dental treatment of head and neck cancer patients, before, during and after radiotherapy: part 2. Br. Dent. J. 2015;218:69–74. doi: 10.1038/sj.bdj.2015.29. [DOI] [PubMed] [Google Scholar]
- Jham B.C., Reis P.M., Miranda E.L., Lopes R.C., Carvalho A.L., Scheper M.A., Freire A.R. Oral health status of 207 head and neck cancer patients before, during and after radiotherapy. Clin. Oral Invest. 2008;12:19–24. doi: 10.1007/s00784-007-0149-5. [DOI] [PubMed] [Google Scholar]
- Jobbins J., Bagg J., Finlay I.G., Addy M., Newcombe R. Oral and dental disease in terminally ill cancer patients. BMJ. Br. Med. J. 1992;304:1612. doi: 10.1136/bmj.304.6842.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y.-S., Park E.-Y., Sohn H.-O. Oral health status and oral health-related quality of life according to presence or absence of mucositis in head and neck cancer patients. J. Cancer Prevention. 2019;24:43–47. doi: 10.15430/JCP.2019.24.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaae J.K., Stenfeldt L., Eriksen J.G. Xerostomia after radiotherapy for oral and oropharyngeal cancer: increasing salivary flow with tasteless sugar-free chewing gum. Front. Oncol. 2016;6:111–116. doi: 10.3389/fonc.2016.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamstra J.I., Roodenburg J.L., Beurskens C.H., Reintsema H., Dijkstra P.U. TheraBite exercises to treat trismus secondary to head and neck cancer. Support. Care Cancer. 2013;21:951–957. doi: 10.1007/s00520-012-1610-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamstra J.I., van Leeuwen M., Roodenburg J.L., Dijkstra P.U. Exercise therapy for trismus secondary to head and neck cancer: a systematic review. Head Neck. 2017;39:2352–2362. doi: 10.1002/hed.24859. [DOI] [PubMed] [Google Scholar]
- Käyser A. Shortened dental arches and oral function. J. Oral Rehabil. 1981;8:457–462. doi: 10.1111/j.1365-2842.1981.tb00519.x. [DOI] [PubMed] [Google Scholar]
- Kolokythas A., Rasmussen J., Reardon J., Feng C. Management of osteoradionecrosis of the jaws with pentoxifylline–tocopherol: a systematic review of the literature and meta-analysis. Int. J. Oral Maxillofac. Surg. 2019;48:173–180. doi: 10.1016/j.ijom.2018.08.007. [DOI] [PubMed] [Google Scholar]
- Kumar N., Brooke A., Burke M., John R., O'Donnell A., Soldani F. The oral management of oncology patients requiring radiotherapy, chemotherapy and/or bone marrow transplantation. Faculty Dental J. 2013;4:200–203. [Google Scholar]
- Kumar M., Nanavati R., Modi T.G., Dobariya C. Oral cancer: etiology and risk factors: a review. J. Cancer Res. Ther. 2016;12:458–463. doi: 10.4103/0973-1482.186696. [DOI] [PubMed] [Google Scholar]
- Lajolo C., Gioco G., Rupe C., Troiano G., Cordaro M., Lucchese A., Paludetti G., Giuliani M. Tooth extraction before radiotherapy is a risk factor for developing osteoradionecrosis of the jaws: a systematic review. Oral Dis. 2020 doi: 10.1111/odi.13485. [DOI] [PubMed] [Google Scholar]
- Lalla R.V., Latortue M.C., Hong C.H., Ariyawardana A., D’Amato-palumbo S., Fischer D.J., Martof A., Nicolatou-Galitis O., Patton L.L., Elting L.S. A systematic review of oral fungal infections in patients receiving cancer therapy. Support. Care Cancer. 2010;18:985–992. doi: 10.1007/s00520-010-0892-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans C.R., Braakhuis B.J., Brakenhoff R.H. The molecular biology of head and neck cancer. Nat. Rev. Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- Leoncini E., Vukovic V., Cadoni G., Giraldi L., Pastorino R., Arzani D., Petrelli L., Wünsch-Filho V., Toporcov T.N., Moyses R.A. Tumour stage and gender predict recurrence and second primary malignancies in head and neck cancer: a multicentre study within the INHANCE consortium. Eur. J. Epidemiol. 2018;33:1205–1218. doi: 10.1007/s10654-018-0409-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons A., Brennan P. Osteoradionecrosis a new classification. Br. J. Oral Maxillofac. Surg. 2014;52:64–65. doi: 10.1016/j.bjoms.2014.02.017. [DOI] [PubMed] [Google Scholar]
- Łysik D., Niemirowicz-Laskowska K., Bucki R., Tokajuk G., Mystkowska J. Artificial saliva: challenges and future perspectives for the treatment of xerostomia. Int. J. Mol. Sci. 2019;20:3199–3215. doi: 10.3390/ijms20133199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid C., Abarca M., Bouferrache K. Osteoradionecrosis: an update. Oral Oncol. 2010;46:471–474. doi: 10.1016/j.oraloncology.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Mainous E., Boyne P., Hart G. Elimination of sequestrum and healing of osteoradionecrosis of the mandible after hyperbaric oxygen therapy: report of a case. J. Oral Surg. 1973;31:336–339. [PubMed] [Google Scholar]
- Marx R.E. A new concept in the treatment of osteoradionecrosis. J. Oral Maxillofac. Surg. 1983;41:351–357. doi: 10.1016/s0278-2391(83)80005-6. [DOI] [PubMed] [Google Scholar]
- Marx R.E. Osteoradionecrosis: a new concept of its pathophysiology. J. Oral Maxillofac. Surg. 1983;41:283–288. doi: 10.1016/0278-2391(83)90294-x. [DOI] [PubMed] [Google Scholar]
- Marx R.E., Johnson R.P. Studies in the radiobiology of osteoradionecrosis and their clinical significance. Oral Surgery, Oral Med. Oral Pathol. 1987;64:379–390. doi: 10.1016/0030-4220(87)90136-8. [DOI] [PubMed] [Google Scholar]
- Mello F.W., Melo G., Pasetto J.J., Silva C.A.B., Warnakulasuriya S., Rivero E.R.C. The synergistic effect of tobacco and alcohol consumption on oral squamous cell carcinoma: a systematic review and meta-analysis. Clin. Oral Invest. 2019;23:2849–2859. doi: 10.1007/s00784-019-02958-1. [DOI] [PubMed] [Google Scholar]
- Meyer I. Infections diseases of the jaws. J. Oral Surg. 1970;28:17–26. [PubMed] [Google Scholar]
- Moore S., Burke M.C., Fenlon M.R., Banerjee A. The role of the general dental practitioner in managing the oral care of head and neck oncology patients. Dent. Update. 2012;39:694–702. doi: 10.12968/denu.2012.39.10.694. [DOI] [PubMed] [Google Scholar]
- Morrish R.B., Chan E., Silverman S., Meyer J., Fu K.K., Greenspan D. Osteonecrosis in patients irradiated for head and neck carcinoma. Cancer. 1981;47:1980–1983. doi: 10.1002/1097-0142(19810415)47:8<1980::aid-cncr2820470813>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Nabil S., Samman N. Incidence and prevention of osteoradionecrosis after dental extraction in irradiated patients: a systematic review. Int. J. Oral Maxillofac. Surg. 2011;40:229–243. doi: 10.1016/j.ijom.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Nabil S., Samman N. Risk factors for osteoradionecrosis after head and neck radiation: a systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:54–69. doi: 10.1016/j.tripleo.2011.07.042. [DOI] [PubMed] [Google Scholar]
- Nakajima N., Watanabe S., Kiyoi T., Tanaka A., Suemaru K., Araki H. Evaluation of edaravone against radiation-induced oral mucositis in mice. J. Pharmacol. Sci. 2015;127:339–343. doi: 10.1016/j.jphs.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Nawi R.I.M., Chui P.L., Ishak W.Z.W., Chan C.M.H. Oral Cryotherapy: prevention of oral mucositis and pain among patients with colorectal cancer undergoing chemotherapy. Clin. J. Oncol. Nurs. 2018;22:555–560. doi: 10.1188/18.CJON.555-560. [DOI] [PubMed] [Google Scholar]
- NATIONAL CANCER INSTITUTE (NCI), 2016. Oral complications of chemotherapy and head/neck radiation [Online]. Available: https://www.ncbi.nlm.nih.gov/books/NBK65881/#_NBK65881_pubdet_ [Accessed 09/02/2018].
- Nutting C.M., Morden J.P., Harrington K.J., Urbano T.G., Bhide S.A., Clark C., Miles E.A., Miah A.B., Newbold K., Tanay M. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–136. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi E., Iacovelli N.A., Tombolini V., Rancati T., Polimeni A., De Cecco L., Valdagni R., De Felice F. Potential role of microbiome in oncogenesis, outcome prediction and therapeutic targeting for head and neck cancer. Oral Oncol. 2019;99 doi: 10.1016/j.oraloncology.2019.104453. [DOI] [PubMed] [Google Scholar]
- Owosho A.A., Thor M., Oh J.H., Riaz N., Tsai C.J., Rosenberg H., Varthis S., Yom S.H.K., Huryn J.M., Lee N.Y. The role of parotid gland irradiation in the development of severe hyposalivation (xerostomia) after intensity-modulated radiation therapy for head and neck cancer: Temporal patterns, risk factors, and testing the QUANTEC guidelines. J. Cranio-Maxillofac. Surg. 2017;45:595–600. doi: 10.1016/j.jcms.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmier N.R., Migliorati C.A., Prado-Ribeiro A.C., de Oliveira M.C.Q., Vechiato Filho A.J., de Goes M.F., Brandao T.B., Lopes M.A., Santos-Silva A.R. Radiation-related caries: current diagnostic, prognostic, and management paradigms. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;130:52–62. doi: 10.1016/j.oooo.2020.04.003. [DOI] [PubMed] [Google Scholar]
- Patel V., Ormondroyd L., Lyons A., McGurk M. The financial burden for the surgical management of osteoradionecrosis. Br. Dent. J. 2017;222:177–180. doi: 10.1038/sj.bdj.2017.121. [DOI] [PubMed] [Google Scholar]
- Patel V., Patel D., Browning T., Patel S., McGurk M., Sassoon I., Guerrero Urbano T., Fenlon M. Pre-radiotherapy presenting dental status of the three most common head and neck cancer subsites in a novel radiation era. Faculty Dental J. 2020;11:52–57. [Google Scholar]
- Platzek I., Beuthien-Baumann B., Schneider M., Gudziol V., Kitzler H.H., Maus J., Schramm G., Popp M., Laniado M., Kotzerke J. FDG PET/MR for lymph node staging in head and neck cancer. Eur. J. Radiol. 2014;83:1163–1168. doi: 10.1016/j.ejrad.2014.03.023. [DOI] [PubMed] [Google Scholar]
- Ray-Chaudhuri A., Shah K., Porter R. The oral management of patients who have received radiotherapy to the head and neck region. Br. Dent. J. 2013;214:387–393. doi: 10.1038/sj.bdj.2013.380. [DOI] [PubMed] [Google Scholar]
- Riley E. Understanding oral mucositis: Causes and treatments. J. Community Nursing. 2017;31:69–72. [Google Scholar]
- THE ROYAL COLLEGE OF SURGEONS OF ENGLAND/THE BRITSH SOCIETY FOR DISABILITY AND ORAL HEALTH (RCS/BSDH). 2018. The oral management of oncology patients requiring radiotherapy, chemotherapy and / or bone marrow transplantation [Online]. Available: https://www.bsdh.org/index.php/component/edocman/the-oral-management-of-oncology-patients-requiring-radiotherapy-chemotherapy-and-or-bone-marrow-transplantation [Accessed 01/03/2019].
- Samim F., Epstein J.B., Zumsteg Z.S., Ho A.S., Barasch A. Oral and dental health in head and neck cancer survivors. Cancers of the Head & Neck. 2016;1:1–7. doi: 10.1186/s41199-016-0015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos P.S., Cremonesi A.L., Quispe R.A., Rubira C.M. The impact of oral health on quality of life in individuals with head and neck cancer after radiotherapy: the importance of dentistry in psychosocial issues. Acta Odontol Latinoam. 2017;30:62–67. [PubMed] [Google Scholar]
- Scully C., Dios P.D., Kumar N. Elsevier; London: 2007. Special Care In Dentistry. [Google Scholar]
- Sheibani K.M., Mafi A.R., Moghaddam S., Taslimi F., Amiran A., Ameri A. Efficacy of benzydamine oral rinse in prevention and management of radiation-induced oral mucositis: a double-blind placebo-controlled randomized clinical trial. Asia Pac. J. Clin. Oncol. 2015;11:22–27. doi: 10.1111/ajco.12288. [DOI] [PubMed] [Google Scholar]
- Siddall K.Z., Rogers S.N., Butterworth C.J. The prosthodontic pathway of the oral cancer patient. Dent. Update. 2012;39:98–106. doi: 10.12968/denu.2012.39.2.98. [DOI] [PubMed] [Google Scholar]
- Siegel, R.L., Miller, K.D., Fuchs, H.E., 2017. Cancer Statistic [Online]. American Cancer Society. Available: https://www.ncbi.nlm.nih.gov/pubmed?term=28055103 [Accessed 02/09/2019].
- Sonis S., Peterson R., Edwards L., Lucey C., Wang L., Mason L., Login G., Ymamkawa M., Moses G., Bouchard P. Defining mechanisms of action of interleukin-11 on the progression of radiation-induced oral mucositis in hamsters. Oral Oncol. 2000;36:373–381. doi: 10.1016/s1368-8375(00)00012-9. [DOI] [PubMed] [Google Scholar]
- Sowder J.C., Cannon R.B., Buchmann L.O., Hunt J.P., Hitchcock Y., Lloyd S., Grossmann K.F., Monroe M.M. Treatment-related determinants of survival in early-stage (T1–2N0M0) oral cavity cancer: a population-based study. Head Neck. 2017;39:876–880. doi: 10.1002/hed.24679. [DOI] [PubMed] [Google Scholar]
- St Guily J.L., Rousseau A., Baujat B., Périé S., Schultz P., Barry B., Dufour X., Malard O., Pretet J.L., Clavel C., Birembaut P. Oropharyngeal cancer prognosis by tumour HPV status in France: the multicentric Papillophar study. Oral Oncol. 2017;67:29–36. doi: 10.1016/j.oraloncology.2017.01.012. [DOI] [PubMed] [Google Scholar]
- Støre G., Boysen M. Mandibular osteoradionecrosis: clinical behaviour and diagnostic aspects. Clin. Otolaryngol. 2000;25:378–384. doi: 10.1046/j.1365-2273.2000.00367.x. [DOI] [PubMed] [Google Scholar]
- Tataru D., Mak V., Simo R., Davies E., Gallagher J. Trends in the epidemiology of head and neck cancer in London. Clin. Otolaryngol. 2016;42:104–114. doi: 10.1111/coa.12673. [DOI] [PubMed] [Google Scholar]
- THE NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE (NICE). 2004. Improving outcomes in head and neck cancers [Online]. Available: https://www.nice.org.uk/guidance/csg6 [Accessed 10/10/2017].
- Thorn J.J., Hansen H.S., Specht L., Bastholt L. Osteoradionecrosis of the jaws: clinical characteristics and relation to the field of irradiation. J. Oral Maxillofac. Surg. 2000;58:1088–1093. doi: 10.1053/joms.2000.9562. [DOI] [PubMed] [Google Scholar]
- Tribius S., Sommer J., Prosch C., Bajrovic A., Muenscher A., Blessmann M., Kruell A., Petersen C., Todorovic M., Tennstedt P. Xerostomia after radiotherapy. Strahlenther. Onkol. 2013;189:216–222. doi: 10.1007/s00066-012-0257-2. [DOI] [PubMed] [Google Scholar]
- Vissink A., Jansma J., Spijkervet F., Burlage F., Coppes R. Oral sequelae of head and neck radiotherapy. Crit. Rev. Oral Biol. Med. 2003;14:199–212. doi: 10.1177/154411130301400305. [DOI] [PubMed] [Google Scholar]
- Vudiniabola S., Pirone C., Williamson J., Goss A.N. Hyperbaric oxygen in the prevention of osteoradionecrosis of the jaws. Aust. Dent. J. 1999;44:243–247. doi: 10.1111/j.1834-7819.1999.tb00227.x. [DOI] [PubMed] [Google Scholar]
- Wang X., Eisbruch A. IMRT for head and neck cancer: reducing xerostomia and dysphagia. J. Radiat. Res. 2016;57:69–75. doi: 10.1093/jrr/rrw047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters A.L., Cope S., Keller M.N., Padilla M., Enciso R. Prevalence of trismus in patients with head and neck cancer: A systematic review with meta-analysis. Head Neck. 2019;41:3408–3421. doi: 10.1002/hed.25836. [DOI] [PubMed] [Google Scholar]
- Widmark G., Sagne S., Heikel P. Osteoradionecrosis of the jaws. Int. J. Oral Maxillofac. Surg. 1989;18:302–306. doi: 10.1016/s0901-5027(89)80100-6. [DOI] [PubMed] [Google Scholar]
- Wijers O.B., Levendag P.C., Braaksma M.M., Boonzaaijer M., Visch L.L., Schmitz P.I. Patients with head and neck cancer cured by radiation therapy: a survey of the dry mouth syndrome in long-term survivors. Head Neck. 2002;24:737–747. doi: 10.1002/hed.10129. [DOI] [PubMed] [Google Scholar]
- Witter D., Cramwinckel A., van Rossum G., Käyser A. Shortened dental arches and masticatory ability. J. Dent. 1990;18:185–189. doi: 10.1016/0300-5712(90)90107-p. [DOI] [PubMed] [Google Scholar]


