Abstract
Introduction
Although there have been many studies on stem cells, few have investigated how neurotransmitters and stem cell proliferation interact to regenerate dental pulp. Dental pulp regeneration is an innovative procedure for reviving dental pulp, if feasible for the entire tooth. Upon tooth injury, activated platelets release serotonin and dopamine in bulk to mobilize dental pulp stem cells to mediate natural dental repair. This has induced research on the role of neurotransmitters in increasing the proliferation rate of stem cells. This review also covers prospective future treatments for dental pulp regeneration.
Methods
A literature search was performed via PubMed and ScienceDirect from 2001 to 2022, using the keywords “neurotransmitter,” “stem cell,” “tooth regeneration,” “tooth repair,” “regenerative dentistry,” and “dental pulp.” Different inclusion/exclusion criteria were used, and the search was restricted to English articles.
Results
Nine publications reporting neurotransmitter interactions with stem cells for tooth and pulp regeneration were selected.
Conclusion
Neurotransmitters were found to interact with dental stem cells. Evidence pointing to neurotransmitters as a factor in the increased proliferation of stem cells was found. This review thus gives hope for tooth pulp regeneration and repair.
Keywords: Neurotransmitter, Stem cell, Tooth regeneration, Tooth repair, Regenerative dentistry, Dental pulp
1. Introduction
Tooth loss is typically caused by oral disorders, such as dental caries, periodontal disease, and trauma. The mental and physical anguish that may result from being in this predicament can significantly diminish a person’s quality of life (QOL) (Amar and Han, 2003). Maintaining healthy and functional teeth is important not only to enjoy meals and maintain one’s QOL but also to prevent dementia because mastication activates the brain (Nakahara and Ide, 2007). Thus, there is much interest in how teeth can grow back (Smith, 2004).
The 1960 discovery by Canadian scientists of the properties of stem cells that are capable of self-renewing and differentiating sparked interest in stem cell research (Sharkis, 2005). Stem cells are undifferentiated cells that can develop into any adult cell type. They are present in all multicellular organisms and may be identified through their capacity to do the foregoing (Narang and Sehgal, 2012). These cells have an unparalleled regenerative capacity and are thus widely used, either alone or in conjunction with scaffolds, to either replace or repair damaged cells (Dahake et al., 2020). There are two requirements that cells must meet to be considered stem cells. First, to generate clones of themselves, stem cells need to be capable of unlimited self-renewal. The proliferation of stem cells is strictly regulated compared to that of rapidly replicating cancer cells. Importantly, stem cells must be capable of giving rise to a specialized cell type that forms part of a healthy organism (Biehl and Russell, 2009).
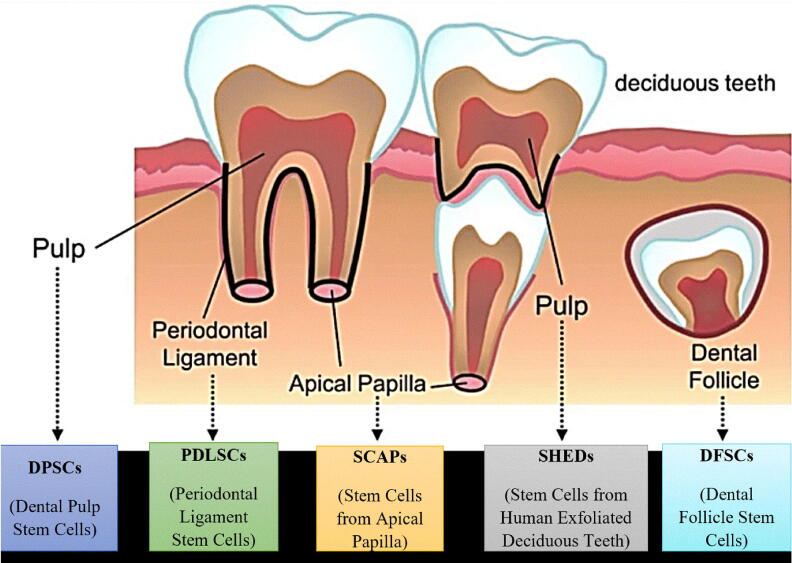
Two main sources of stem cells are used: embryonic stem cells (ES) and somatic stem cells, also known as adult stem cells. ES cells are obtained by harvesting the inner cell mass of a pre-implantation blastocyst (Evans and Kaufman, 1981, Martin, 1981). These cells are produced from rodents, primates, and humans. In dentistry, there are views that somatic (adult) stem cells are preferable to ES cells because they are readily available and their usage does not raise ethical issues such as the destruction of human embryos (Morsczeck et al., 2008). The different types of somatic dental stem cells in different locations in the mouth are shown in Fig. 1.
Fig. 1.
Dental stem cells in different locations in the mouth.
The names of different types of stem cells are based on their locations. Dental pulp stem cells (DPSCs) are located in the pulp of permanent teeth, while stem cells from human exfoliated deciduous teeth are located in the pulp of deciduous teeth. Periodontal ligament stem cells (PDLSCs) can be found in the periodontal ligament area, while stem cells from the apical papilla (SCAPs) are located at the apex of the tooth. Dental follicles are known as dental follicle stem cells.
2. Materials and methods
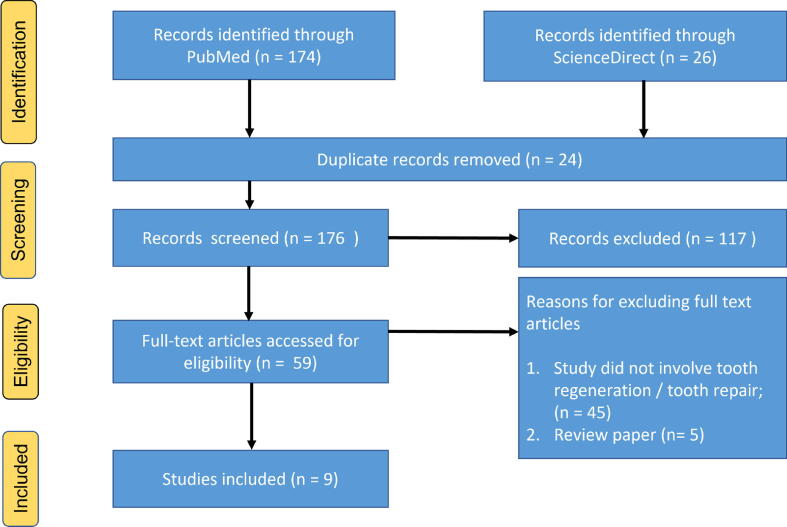
Using the keywords “neurotransmitter,” “stem cell,” “tooth regeneration,” “tooth repair,” “regenerative dentistry,” and “dental pulp,” a literature search was performed via PubMed and ScienceDirect from 2001 to 2022. After the application of additional exclusion criteria, nine publications reporting neurotransmitter interactions with stem cells for tooth and pulp regeneration were included in this review. The selection criteria are shown in Fig. 2.
Fig. 2.
PRISMA chart 2020 for the selection criteria at every step.
3. Results
Dental pulp is susceptible to injuries of varying severity. All of these traumas are responsible for dental pulp changes (Chmilewsky et al., 2014). The pulp is mesenchymal soft tissue located between the central chamber and the root tip. Typical healthy dental pulp tissue is composed of layered cells that can divide and differentiate (Shah et al., 2020). It also consists of odontoblasts, fibroblasts, and immunological cells (Goldberg et al., 2008). Tissue repair, regeneration, and regulated inflammatory response are all under the control of pulp cells (Eming et al., 2007). Pulp cells may start an inflammatory response to begin the healing process, although this depends on the kind and severity of bacterial infection. This is true regardless of whether the bacteria have colonized the whole pulp or have not done so.
3.1. Neurotransmitters and stem cells
3.1.1. Serotonin
Serotonin, also known as 5-hydroxytryptamine or 5-HT, is a monoamine neurotransmitter that plays a role in neuroendocrine function control, depressive-symptom modulation, anxiety and temperature regulation. The production of 5-HT involves two primary processes. The first process involves the formation of 5-hydroxytryptophan from tryptophan through the action of tryptophan hydroxylase. The second process involves the conversion of 5-hydroxytryptophan into 5-HT through the action of L-aromatic amino acid decarboxylase enzyme (Tyce, 1990). Moreover, 5-hydroxytryptophan is a necessary precursor of 5-HT found in the cell’s cytoplasm (Deutch, 2013).
5-HT plays a role in dentin repair by modulating endogenous pulpal stem cells. A robust quantitative measure of dentin restoration was used by Baudry (Baudry et al., 2015) to examine the impact of the in vivo pharmacological inhibition of 5-HT and dopamine (3,4-dihydroxyphenethylamine or DA) receptors on tooth healing at day 30 after pulp damage. Soon after the damage, gelatin hydrogel microspheres containing selective 5-HT1D (SB714786), 5-HT2B (RS127445), or 5-HT7 (SB269970) receptor antagonists were implanted inside the pulp of the first rat molar (final concentrations: 100 nM). The increase in Ca2+ concentration that normally happens during dentin regeneration was inhibited by blocking 5-HT2B and, to a lesser extent, 5-HT7 receptors. The Ca2+ accumulation in the mesial pulp was unaffected by antagonistic 5-HT1D receptors. These results validate the presence and activity of 5-HT2B and 5-HT7 receptors on the surfaces of odontogenic cells, as demonstrated in vitro (Baudry et al., 2015).
Odontogenic stem cells have a dual serotonergic identity, and the A4 and H8 pulpal cell lines exhibit significant quantities of brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and insulin-like growth factor 1 (IGF-1), all of which are stored in enormous dense core vesicles (Baudry et al., 2019). The growth hormones BDNF, NGF, and IGF-1 have all been shown to promote axon guidance and elongation and neuronal survival (Decourtye et al., 2017, Lykissas et al., 2007). Pulp stem cells secrete neurotrophins that may facilitate dentin remineralization, root formation, and tooth restoration. In addition to producing 5-HT and DA, the A4 and H8 pulpal cell lines display the fully functional enzymes necessary for complete 5-HT/DA processing. Included in this group are the rate-limiting enzymes in the biosynthesis of 5-HT and DA, tyrosine hydroxylase and tryptophane hydroxylase, and the monoamine oxidases essential for bioamine catabolism.
3.1.2. Dopamine
DA, a catecholamine, operates as a neurotransmitter throughout a broad evolutionary span. It is known to control motor circuits, evaluate sensory stimuli, and mediate reward or reinforcement signals in the mammalian brain (Miller, 2020).
DA takes part in dental repair via D1 and D3 receptors. Early after the lesion, the first rat molar pulp was implanted with gelatin hydrogel microspheres containing selective antagonists of D1 (SCH23390) and D3 (S33084) receptors (final concentrations: 100 nM). On day 30 after pulp damage, the mesial pulpal Ca2 + concentration was used as a reliable quantitative measure of dentin restoration. The increase in Ca2 + concentration that generally takes place during dentin healing was inhibited by blocking the D1 and D3 receptors. These findings imply that D1 and D3 receptors are present and active on the surfaces of odontogenic cells, thus validating the in vitro findings. These wide data imply that DA and 5-HT co-released by platelets and the subsequent activation of all D1, D3, 5-HT2B, and 5-HT7 receptors are necessary for tooth regeneration.
The osteogenic induction of PDLSCs was established by cultivating cells on a Mussel-inspired polydopamine (PDA) film or an uncoated polystyrene surface as a control, following Lee et al. (2014). To determine whether PDLSC underwent osteogenic differentiation, the intracellular calcium levels, alkaline phosphatase activity, and protein expression of osteocalcin, osterix, and runt-related transcription factor 2 were analyzed. The PDLSCs grown on PDA film had more osteogenic activity than those grown on the control surface. In addition, compared to the control cells, the PDLSCs grown on PDA film showed greater levels of integrin adhesion receptors integrin 5 and 1 in their respective proportions. One isoform of the intracellular signaling protein phosphatidylinositol-3-kinase (PI3K), p110, was expressed by PDLSCs on PDA film in a PDA dose–dependent manner. This signaling protein was shown to interact with integrin 1, demonstrating that integrin is required for PI3K activation in response to PDA. Finally, the PDA-induced osteogenic activity of PDLSCs was reduced by PI3K inhibition (Lee et al., 2014).
3.1.3. Acetylcholine
The data about the role of acetylcholine in pulp regeneration and dental stem cell mobilization are insufficient, but its role as a modulator of fiber outgrowth was studied by Biagioni et al. (2000), and its role as a modulator of neuronal development was investigated under two experimental conditions. First, a choline acetyltransferase construct was transfected into a neuroblastoma cell line that cannot synthesize any neurotransmitters. Second, acetylcholine production was stimulated, followed by enhanced expression of neuronal traits. In rat dissociated retinal cell cultures, small amounts of acetylcholine were discovered spontaneously (Lipton, 1988). This confirms amacrine cells’ regulation of ganglion cell fiber elongation through the release of acetylcholine.
Acetylcholine production begins early in motor neuron development, and its potential function in regulating subsequent processes of neuron differentiation and interaction with target cells has been investigated (Vaca and Pilar, 1979). The initial activation of choline acetyltransferase (ChAT) in development, the availability of quantifiable levels of acetylcholine, and the presence and localization of muscarinic acetylcholine receptors imply that the activation of acetylcholine production in dorsal root ganglia may be associated with acethylcholine ability to regulate neurite elongation, presumably in combination with neurotrophic factors. The presence of ChAT-transfected neuroblastoma clones indicates that acetylcholine acts as a modulator of fiber development (Landgraf et al., 2010, Resende and Adhikari, 2009, Ulrich and Majumder, 2006).
3.2. Potential of dental stem cells for use in future pulp therapy
Angiogenesis and tissue mineralization are keys to the formation of new dentin or pulp-like tissue (Gong et al., 2016). Quick recovery of blood flow to the transplant locations is essential for the longevity of tissue-engineered implants. This blood supply acts as a conduit for the transfer of nutrients, the delivery of oxygen, and the removal of metabolic waste. This explanation also applies to dentin and pulp regeneration because the opening at the root apex is too tiny for intracanal blood infusion (Murray et al., 2007). Long-term engraftment of stem cells may be less effective as a therapy if nutrients and oxygen are not given on time. There is also a chance that the transplanted cells will die from a lack of oxygen, a condition called hypoxia-induced apoptosis.
In the past, it was common practice to wait for access to the transplanted replacement within the canal region and for the ingrowth of new vasculature. However, if a dense, multicellular structure that restricts blood flow develops, the technique may not be effective. Furthermore, because the host-derived vascular networks consisting of the initial migration and proliferation of endothelial cells, the angiogenic sprouting of new blood vessels, and the ultimate phase of vascular stabilization take a long time to set up, bioengineered replacement grafts are likely to fail from ischemia and necrosis before they integrate with the host tissue (Yuan et al., 2015). Pre-vascularization has become an option for treating this problem. With the help of vessel plexuses made ahead of time, bioengineered grafts may be able to connect to the vascular system of the host faster after transplantation.
3.2.1. Hypoxia for angiogenesis
Hypoxia is a normal occurrence in both pathological and nonpathological circumstances involving tooth pulp tissue. Dental pulp cells (DPCs) are often vulnerable to ischemia when the surrounding vascular bundles are extensively damaged in traumatic injuries. In addition, because of their unique anatomical structure (surrounded by tough dentin and having a small opening at the apices), their blood supply may be cut off during the restoration process. This occurs when vasoconstrictors found in local anesthetics reduce the microcirculation of blood flow. Ischemia disrupts the tooth pulp’s oxygen equilibrium by reducing the amount of oxygen that can reach the pulp from the circulation. Furthermore, dental caries’ rising inflammatory reactions typically elevate intracanal pressure, forcing the oxygen out. The root canal area has a high oxygen tension even in the absence of pathology, and ex vivo pulp cell growth and expansion are regularly observed. Consequently, a number of studies have sought to simulate pulp hypoxia to examine how DPCs react to low oxygen levels.
Amemiya et al. (2003) observed that when canine DPCs were subjected to low oxygenation circumstances, they formed more formazan and expanded more quickly than pulp cells cultured under normoxic conditions. Following that, many studies on human dental pulp cells (hDPCs) revealed that those grown in hypoxic settings exhibited higher rates of proliferation, higher proportions of side populations, stronger angiogenic potential, and higher in vitro erythropoietin expression than those grown in normal conditions (Aranha et al., 2010, Wang et al., 2010). Iida et al. (2010) proved the odontogenic capacity of hDPCs in vitro and in vivo by efficiently isolating them from the inflamed teeth of elderly adults exposed to hypoxia. Recent research investigating extensive vessel-like networks emerging in a SCAP–human umbilical vein endothelial cell coculture group under hypoxic settings and found that the created hypoxic environment drastically boosted the production of hypoxia-inducible factor-1, ephrinB2, and vascular endothelial growth factor (Yuan et al., 2015). Hypoxia may be a practical and efficient technique for priming the reparative and regenerative pulp tissue angiogenic potential of DPSCs and for improving the possibility of separating healthy DPCs from pulp tissue that has been destroyed or replaced.
3.2.2. Scaffoldless delivery approach with cell sheet technology
Stem cells are often pre-mixed with foreign scaffold materials to create a three-dimensional (3D) built structure before being implanted into target areas. Due to poor cell migration and retention inside the supporting scaffolds, which exacerbate the issues associated with biomaterials, the effectiveness of this well-known method has been questioned for some time. The presented biomaterials generally cannot mimic the native extracellular matrix (ECM) or implanted device surfaces. Using a 3D in vitro culture method, Iohara et al. (2004) created DPCs over a decade ago. This method requires only one round of centrifugation to remove cell pellets or aggregates, which is a significant improvement over the prior methods. Odontogenic differentiation and ECM accumulation were both significantly higher in the 3D pellet than in the monolayer culture. DPC pellet transplantation with recombinant human bone morphogenetic protein-2 therapy effectively promoted dentin synthesis in canine tooth pulp following amputation (Iohara et al., 2004). As the centrifugation stage may add extra force that changes how cells behave, Syed-Picard et al. (2014) came up with a self-assembly method in which cells could build and keep their preferred 3D microenvironment on their own. This 3D, self-assembled device was implanted into the canal space of human tooth root segments, where it proceeded to create vascularized dental pulp–like tissue in scaffoldless DPC engineered tissue (Syed-Picard et al., 2014).
Cell sheet technology (CST), a comparable but distinct novel “scaffold-free” technique created for regenerative medicine, has recently been the main focus of periodontal tissue development research (Iwata et al., 2015, Iwata et al., 2009, Wang et al., 2014). Thermoresponsive polymers, such as poly(ethylene glycol), are the bases of CST, which is a non-invasive technique (N-isopropylacrylamide; PIPAAm). Unlike conventional methods of cell separation, which often include the use of dissociative enzymes or mechanical forces, this method does not compromise cell viability in the process. A small temperature shift is required to remove cells that have been grown in a continuous monolayer while maintaining their structure and extracellular components on a PIPAAm-treated surface (Elloumi-Hannachi et al., 2010, Matsuura et al., 2014). However, necrosis is likely to occur in the graft if the ECM is not deposited and nutrients are not acquired, as the cell sheets in such a restricted tubular space are difficult to manipulate. Na et al. (2016) successfully regenerated pulp tissue throughout a complete empty tooth canal by transforming a cell sheet structure into a more malleable 3D pellet system using a cell sheet–derived pellet and via ectopic implantation into SCIDM (Na et al., 2016). While these findings are intriguing, a more in-depth in vivo study of the scaffoldless administration strategy in large animal models is still needed.
3.2.3. Local regeneration of dentin and pulp tissue after pulpotomy
According to Shimizu et al., 2003, Julovi et al., 2008, hyaluronic acid (HA), a glycosaminoglycan found in large amounts in the human body, is known to play an important role in maintaining shape and reducing inflammation. It is also a good material for tissue engineering (Ramamurthi and Vesely, 2002). The aforementioned researchers conducted in vitro and in vivo investigations to determine if the HA sponge is beneficial as a scaffold for dentin–pulp complex regeneration treatment and discovered that it has all the required properties (Inuyama et al., 2010). Local regeneration of the dentin–pulp complex following pulpotomy in older teeth may be challenging in contrast to young teeth, which have a plentiful blood supply and cells. Nonetheless, establishing the optimum growth factor combination and developing a delivery mechanism for growth factors and cell scaffolds will boost dentin–pulp complex regeneration treatment after pulpotomy (Morotomi et al., 2015).
3.2.4. Cell homing strategy for pulp regeneration
Activation of stem/progenitor cells from the periapical tissue around the apical region of the root facilitates cell homing. Growth factor–impregnated scaffolds are placed into root canals through an enlarged apical foramen to encourage endogenous stem/progenitor cells situated close to the root apex to migrate, multiply, and differentiate (Kim et al., 2013). As it is not necessary to identify or manipulate stem cells in vitro, cell homing may be simpler to implement in clinical settings than cell transplantation (Kim et al., 2013). Adult teeth, unlike embryonic teeth, lack pluripotent dental papilla cells. This strategy may rely on the development of novel techniques for producing stem cells surrounding the root apex, including periodontal ligament stem cells (Morotomi et al., 2019).
3.2.5. Potential role of neutrophil extracellular traps
Neutrophil extracellular traps have recently been revealed as a new bacterial killing mechanism that involves reactive oxygen species signaling and results in cellular DNA extrusions, leading to microbial entrapment and death (Cooper et al., 2017). The assessment of their levels inside diseased pulp may be used to target the implementation of innovative disease management techniques. They may be useful in managing pulpal infections. More research is needed to determine how these structures affect pulp vitality and healing responses (Srivastava, 2019).
3.2.6. Low-intensity pulsed ultrasound treatment
Low-intensity pulsed ultrasound (LIPUS) treatment may activate mesenchymal stem cells (MSCs) in dental tissues, thus providing a therapeutic method for promoting dental tissue regeneration (Man et al., 2012). The process is not entirely understood, but it is thought to be due to non-thermal biomechanical effects. In particular, LIPUS may exert an effect on the cytoskeleton and cell membrane, initiating downstream signaling processes via acoustic microstreaming and physical radiation. As a result, this simple, low-cost method may provide an appropriate dental tissue regeneration method in the dental clinic (Scheven et al., 2009).
4. Discussion
In this review, we chose articles that contained important information about the relationship between neurotransmitters and stem cells for future dental pulp regeneration. As few studies have investigated the role of neurotransmitters in the regeneration of dental pulp, therefore we included studies related to the interaction of neurotransmitters with stem cell proliferation.
Baudry et al. (2015) demonstrated the presence of 5-HT and DA autoreceptors in pulpal stem cells, indicating a dual bioaminergic identity. It was discovered that to mobilize endogenous stem cells for tooth healing, active blood platelets in injured pulp must produce both 5-HT and DA. Further research revealed that systemic 5-HT and DA signals are required for the development of stem cells containing 5-HT and DA receptors, which are essential for tooth repair in vivo. Lee et al. (2014) reported that PDLSCs cultivated on PDA films could have increased cell proliferation and osteogenic potential due to integrin–PI3K interaction. DA polymerization in alkaline pH circumstances led to the formation of the PDA coating, which was then linked to the simultaneous adsorption of several biomolecules and the attachment of cells.
Furthermore, in Rim et al. (2012) study, human MSCs cultivated on PDA-coated poly(L-lactide) (PLLA) fibers showed greater osteogenic differentiation than MSCs cultured on non-coated PLLA fibers. Landgraf et al. (2010) reported that in their research on the function of acetylcholine in embryonic stem cell survival, proliferation, and death, ACh increased the survivability of embryonic stem cells but inhibited cell proliferation. They demonstrated that ACh might regulate cell death and proliferation by attaching to particular receptors on stem cells.
Among the three neurotransmitters discussed in this review, both 5-HT and DA are capable of enhancing cell proliferation, while ACh decreases proliferation. Thus, further studies focusing on dental pulp regeneration with 5-HT and DA should be conducted in the future. Overall, additional studies in the field of regenerative dentistry should continue to achieve significant accomplishments in dentin and pulp regeneration.
5. Conclusion
Research on dental stem cells as promising tools for tooth regeneration in regenerative dental medicine is emerging and progressing significantly. The discovery of the interaction of neurotransmitters with dental stem cells in this review raises expectations for tooth pulp repair and regeneration. The data suggest that neurotransmitters contribute to an increase in stem cell proliferation. Neurotransmitters and dental stem cells may hold the key to improving dental treatment in the future, although further studies are needed to confirm this.
Funding
This review was fully funded by Universiti Sains Malaysia Short Term Research Grant 304.PPSG.6315578.
Ethical statement
This narrative review was conducted ethically in all aspects. It is clear from any kind of plagiarism; all reviewed relevant articles and resources were properly cited. As this is a narrative review which highlights the use of the future pulp therapy of dental stem cells, it did not require approval from the Institutional Ethical Committee.
CRediT authorship contribution statement
Hidayah Ramli: Writing – review & editing. Norhayati Yusop: Writing – original draft, Writing – review & editing. Rosmaliza Ramli: Writing – original draft, Writing – review & editing. Zurairah Berahim: Writing – original draft, Writing – review & editing. Roshan Peiris: Writing – original draft, Writing – review & editing. Nurhafizah Ghani: Conceptualization, Writing – original draft, Writing – review & editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sdentj.2023.05.004.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Amar S., Han X. The impact of periodontal infection on systemic diseases. Med. Sci. Monit. 2003;9:Ra291-9. [PubMed] [Google Scholar]
- Amemiya K., Kaneko Y., Muramatsu T., Shimono M., Inoue T. Pulp cell responses during hypoxia and reoxygenation in vitro. Eur. J. Oral Sci. 2003;111:332–338. doi: 10.1034/j.1600-0722.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- Aranha A.M.F., Zhang Z., Neiva K.G., Costa C.A.S., Hebling J., Nör J.E. Hypoxia enhances the angiogenic potential of human dental pulp cells. J. Endod. 2010;36:1633–1637. doi: 10.1016/j.joen.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Baudry A., Alleaume-Butaux A., Dimitrova-Nakov S., Goldberg M., Schneider B., Launay J.-M., Kellermann O. Essential roles of dopamine and serotonin in tooth repair: functional interplay between odontogenic stem cells and platelets. Stem Cells. 2015;33:2586–2595. doi: 10.1002/stem.2037. [DOI] [PubMed] [Google Scholar]
- Baudry A., Schneider B., Launay J.-M., Kellermann O. Serotonin in stem cell based-dental repair and bone formation: a review. Biochimie. 2019;161:65–72. doi: 10.1016/j.biochi.2018.07.030. [DOI] [PubMed] [Google Scholar]
- Biagioni S., Tata A.M., De Jaco A., Augusti-Tocco G. Acetylcholine synthesis and neuron differentiation. Int. J. Dev. Biol. 2000;44:689–697. [PubMed] [Google Scholar]
- Biehl J.K., Russell B. Introduction to Stem Cell Therapy. J. Cardiovasc. Nurs. 2009;24 doi: 10.1097/JCN.0b013e318197a6a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmilewsky F., Jeanneau C., Dejou J., About I. Sources of dentin-pulp regeneration signals and their modulation by the local microenvironment. J. Endod. 2014;40:S19–S25. doi: 10.1016/j.joen.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Cooper P.R., Chicca I.J., Holder M.J., Milward M.R. Inflammation and regeneration in the dentin-pulp complex: net gain or net loss? J. Endod. 2017;43:S87–S94. doi: 10.1016/j.joen.2017.06.011. [DOI] [PubMed] [Google Scholar]
- Dahake P.T., Panpaliya N.P., Kale Y.J., Dadpe M.V., Kendre S.B., Bogar C. Response of stem cells from human exfoliated deciduous teeth (SHED) to three bioinductive materials – An in vitro experimental study. Saudi Dental J. 2020;32:43–51. doi: 10.1016/j.sdentj.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decourtye L., Mire E., Clemessy M., Heurtier V., Ledent T., Robinson I.C., Mollard P., Epelbaum J., Meaney M.J., Garel S., Le Bouc Y., Kappeler L. IGF-1 Induces GHRH neuronal axon elongation during early postnatal life in mice. PLoS One. 2017;12:e0170083. doi: 10.1371/journal.pone.0170083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutch A.Y. In: Fundamental Neuroscience. 4th ed. Squire L.R., Berg D., Bloom F.E., Du Lac S., Ghosh A., Spitzer N.C., editors. Academic Press; San Diego: 2013. Chapter 6 - Neurotransmitters. [Google Scholar]
- Elloumi-Hannachi I., Yamato M., Okano T. Cell sheet engineering: a unique nanotechnology for scaffold-free tissue reconstruction with clinical applications in regenerative medicine. J. Intern. Med. 2010;267:54–70. doi: 10.1111/j.1365-2796.2009.02185.x. [DOI] [PubMed] [Google Scholar]
- Eming S.A., Krieg T., Davidson J.M. Inflammation in wound repair: molecular and cellular mechanisms. J. Invest. Dermatol. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- Evans M.J., Kaufman M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Goldberg M., Farges J.C., Lacerda-Pinheiro S., Six N., Jegat N., Decup F., Septier D., Carrouel F., Durand S., Chaussain-Miller C., Denbesten P., Veis A., Poliard A. Inflammatory and immunological aspects of dental pulp repair. Pharmacol. Res. 2008;58:137–147. doi: 10.1016/j.phrs.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong T., Heng B.C., Lo E.C., Zhang C. Current advance and future prospects of tissue engineering approach to dentin/pulp regenerative therapy. Stem Cells Int. 2016;2016:9204574. doi: 10.1155/2016/9204574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida K., Takeda-Kawaguchi T., Tezuka Y., Kunisada T., Shibata T., Tezuka K. Hypoxia enhances colony formation and proliferation but inhibits differentiation of human dental pulp cells. Arch. Oral Biol. 2010;55:648–654. doi: 10.1016/j.archoralbio.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Inuyama Y., Kitamura C., Nishihara T., Morotomi T., Nagayoshi M., Tabata Y., Matsuo K., Chen K.K., Terashita M. Effects of hyaluronic acid sponge as a scaffold on odontoblastic cell line and amputated dental pulp. J. Biomed. Mater. Res. B Appl. Biomater. 2010;92:120–128. doi: 10.1002/jbm.b.31497. [DOI] [PubMed] [Google Scholar]
- Iohara K., Nakashima M., Ito M., Ishikawa M., Nakasima A., Akamine A. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J. Dent. Res. 2004;83:590–595. doi: 10.1177/154405910408300802. [DOI] [PubMed] [Google Scholar]
- Iwata T., Yamato M., Tsuchioka H., Takagi R., Mukobata S., Washio K., Okano T., Ishikawa I. Periodontal regeneration with multi-layered periodontal ligament-derived cell sheets in a canine model. Biomaterials. 2009;30:2716–2723. doi: 10.1016/j.biomaterials.2009.01.032. [DOI] [PubMed] [Google Scholar]
- Iwata T., Washio K., Yoshida T., Ishikawa I., Ando T., Yamato M., Okano T. Cell sheet engineering and its application for periodontal regeneration. J. Tissue Eng. Regen. Med. 2015;9:343–356. doi: 10.1002/term.1785. [DOI] [PubMed] [Google Scholar]
- Julovi S.M., Ito H., Hiramitsu T., Yasuda T., Nakamura T. Hyaluronan inhibits IL-1β-stimulated collagenase production via down-regulation of phosphorylated p38 in SW-1353 human chondrosarcoma cells. Mod. Rheumatol. 2008;18:263–270. doi: 10.1007/s10165-008-0067-7. [DOI] [PubMed] [Google Scholar]
- Kim S.G., Zheng Y., Zhou J., Chen M., Embree M.C., Song K., Jiang N., Mao J.J. Dentin and dental pulp regeneration by the patient's endogenous cells. Endod Topics. 2013;28:106–117. doi: 10.1111/etp.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf D., Barth M., Layer P.G., Sperling L.E. Acetylcholine as a possible signaling molecule in embryonic stem cells: studies on survival, proliferation and death. Chem. Biol. Interact. 2010;187:115–119. doi: 10.1016/j.cbi.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Lee J.S., Yi J.K., An S.Y., Heo J.S. Increased osteogenic differentiation of periodontal ligament stem cells on polydopamine film occurs via activation of integrin and PI3K signaling pathways. Cell. Physiol. Biochem. 2014;34:1824–1834. doi: 10.1159/000366381. [DOI] [PubMed] [Google Scholar]
- Lipton S.A. Spontaneous release of acetylcholine affects the physiological nicotinic responses of rat retinal ganglion cells in culture. J. Neurosci. 1988;8:3857–3868. doi: 10.1523/JNEUROSCI.08-10-03857.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykissas M.G., Batistatou A.K., Charalabopoulos K.A., Beris A.E. The role of neurotrophins in axonal growth, guidance, and regeneration. Curr. Neurovasc. Res. 2007;4:143–151. doi: 10.2174/156720207780637216. [DOI] [PubMed] [Google Scholar]
- Man J., Shelton R.M., Cooper P.R., Scheven B.A. Low-intensity low-frequency ultrasound promotes proliferation and differentiation of odontoblast-like cells. J. Endod. 2012;38:608–613. doi: 10.1016/j.joen.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Martin G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. U.S.A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura K., Utoh R., Nagase K., Okano T. Cell sheet approach for tissue engineering and regenerative medicine. J. Control. Release. 2014;190:228–239. doi: 10.1016/j.jconrel.2014.05.024. [DOI] [PubMed] [Google Scholar]
- Miller M.W. Dopamine as a multifunctional neurotransmitter in gastropod molluscs: an evolutionary hypothesis. Biol. Bull. 2020;239:189–208. doi: 10.1086/711293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morotomi T., Tabata Y., Kitamura C. Dentin-Pulp complex regeneration therapy following pulp amputation. Adv. Techniques Biol. Med. 2015;3:1–5. [Google Scholar]
- Morotomi T., Washio A., Kitamura C. Current and future options for dental pulp therapy. Jpn Dent. Sci. Rev. 2019;55:5–11. doi: 10.1016/j.jdsr.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsczeck C., Schmalz G., Reichert T.E., Völlner F., Galler K., Driemel O. Somatic stem cells for regenerative dentistry. Clin. Oral Invest. 2008;12:113–118. doi: 10.1007/s00784-007-0170-8. [DOI] [PubMed] [Google Scholar]
- Murray P.E., Garcia-Godoy F., Hargreaves K.M. Regenerative endodontics: a review of current status and a call for action. J. Endod. 2007;33:377–390. doi: 10.1016/j.joen.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Na S., Zhang H., Huang F., Wang W., Ding Y., Li D., Jin Y. Regeneration of dental pulp/dentine complex with a three-dimensional and scaffold-free stem-cell sheet-derived pellet. J. Tissue Eng. Regen. Med. 2016;10:261–270. doi: 10.1002/term.1686. [DOI] [PubMed] [Google Scholar]
- Nakahara T., Ide Y. Tooth regeneration: implications for the use of bioengineered organs in first-wave organ replacement. Hum. Cell. 2007;20:63–70. doi: 10.1111/j.1749-0774.2007.00031.x. [DOI] [PubMed] [Google Scholar]
- Narang S., Sehgal N. Stem cells: a potential regenerative future in dentistry. Indian J. Hum. Genet. 2012;18:150–154. doi: 10.4103/0971-6866.100749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthi A., Vesely I. Smooth muscle cell adhesion on crosslinked hyaluronan gels. J. Biomed. Mater. Res. 2002;60:195–205. doi: 10.1002/jbm.10061. [DOI] [PubMed] [Google Scholar]
- Resende R.R., Adhikari A. Cholinergic receptor pathways involved in apoptosis, cell proliferation and neuronal differentiation. Cell Commun. Signal. 2009;7:20. doi: 10.1186/1478-811X-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rim N.G., Kim S.J., Shin Y.M., Jun I., Lim D.W., Park J.H., Shin H. Mussel-inspired surface modification of poly(L-lactide) electrospun fibers for modulation of osteogenic differentiation of human mesenchymal stem cells. Colloids Surf. B Biointerfaces. 2012;91:189–197. doi: 10.1016/j.colsurfb.2011.10.057. [DOI] [PubMed] [Google Scholar]
- Scheven B., Shelton R., Cooper P., Walmsley A., Smith A. Therapeutic ultrasound for dental tissue repair. Med. Hypotheses. 2009;73:591–593. doi: 10.1016/j.mehy.2009.05.032. [DOI] [PubMed] [Google Scholar]
- Shah D., Lynd T., Ho D., Chen J., Vines J., Jung H.D., Kim J.H., Zhang P., Wu H., Jun H.W., Cheon K. Pulp-Dentin tissue healing response: a discussion of current biomedical approaches. J. Clin. Med. 2020;9 doi: 10.3390/jcm9020434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkis S.J. Canadian stem cell scientists take the prize. Cell. 2005;122:817–819. doi: 10.1016/j.cell.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Yasuda T., Nakagawa T., Yamashita E., Julovi S.M., Hiramitsu T., Nakamura T. Hyaluronan inhibits matrix metalloproteinase-1 production by rheumatoid synovial fibroblasts stimulated by proinflammatory cytokines. J. Rheumatol. 2003;30:1164–1172. [PubMed] [Google Scholar]
- Smith A.J. Tooth tissue engineering and regeneration–a translational vision! J. Dent. Res. 2004;83:517. doi: 10.1177/154405910408300701. [DOI] [PubMed] [Google Scholar]
- Srivastava S. Current and future perspectives for dentin-pulp tissue engineering - an update. S. Afr. Dent. J. 2019;74:110–114. [Google Scholar]
- Syed-Picard F.N., Ray H.L., Jr., Kumta P.N., Sfeir C. Scaffoldless tissue-engineered dental pulp cell constructs for endodontic therapy. J. Dent. Res. 2014;93:250–255. doi: 10.1177/0022034513517901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyce G.M. Origin and metabolism of serotonin. J. Cardiovasc. Pharmacol. 1990;16(Suppl 3):S1–S7. [PubMed] [Google Scholar]
- Ulrich H., Majumder P. Neurotransmitter receptor expression and activity during neuronal differentiation of embryonal carcinoma and stem cells: from basic research towards clinical applications. Cell Prolif. 2006;39:281–300. doi: 10.1111/j.1365-2184.2006.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaca K., Pilar G. Mechanisms controlling choline transport and acetylcholine synthesis in motor nerve terminals during electrical stimulation. J. Gen. Physiol. 1979;73:605–628. doi: 10.1085/jgp.73.5.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wei X., Ling J., Huang Y., Gong Q. Side population increase after simulated transient ischemia in human dental pulp cell. J. Endod. 2010;36:453–458. doi: 10.1016/j.joen.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Wang J., Zhang R., Shen Y., Xu C., Qi S., Lu L., Wang R., Xu Y. Recent advances in cell sheet technology for periodontal regeneration. Curr. Stem Cell Res. Ther. 2014;9:162–173. doi: 10.2174/1574888x09666140213150218. [DOI] [PubMed] [Google Scholar]
- Yuan C., Wang P., Zhu L., Dissanayaka W.L., Green D.W., Tong E.H., Jin L., Zhang C. Coculture of stem cells from apical papilla and human umbilical vein endothelial cell under hypoxia increases the formation of three-dimensional vessel-like structures in vitro. Tissue Eng. A. 2015;21:1163–1172. doi: 10.1089/ten.tea.2014.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.