Abstract
Introduction
Lithium disilicate glass–ceramic (LDC) restorations exhibit microorganism infiltration, recurrent caries, pulpal lesions, periodontal inflammation, and cement exposure to the oral environment over time. All these factors lead to restoration failure. This systematic review aimed to investigate the clinical outcomes of LDC full-coverage crowns (FCC) in permanent teeth compared with those of other full-coverage restoration materials.
Materials & Methods
Search strategies were developed for four databases: Web of Science, OVID, PubMed, and Scopus. Data extraction and quality appraisals were performed by two independent reviewers. Data on the presence of caries, post-operative sensitivity, and periodontal changes were extracted from the included clinical studies. In addition to the outcome measures, data on the sample size, study groups, method of restoration fabrication, type of impression, and type of abutment were recorded.
Results
We retrieved 3989 records for the title and abstract screening. Of these, 19 clinical studies met the inclusion criteria. The overall quality of the included studies indicates a low risk of bias. Most studies reported no pulpal involvement, recurrent caries, or post-operative sensitivity and presented a favorable periodontal response after the cementation of LDC-FCC during different follow-up periods.
Conclusion
Based on the endodontic and periodontic clinical responses of natural tooth abutments and their supporting periodontium, LDC-FCC can be considered a clinically successful restorative option.
Keywords: All-ceramic, Full-coverage crowns, Periodontal change, Post-operative sensitivity, Pulpal involvement, Recurrent caries
1. Introduction
Dental ceramics that are fabricated using modern manufacturing processes are commonly used in the construction of fixed dental prostheses (Li et al., 2014, Tinschert et al., 2004). Ceramic restorations offer satisfactory properties that are valued by patients in “modern-day” dentistry. These include excellent esthetics, functional quality, and biocompatibility (Santos et al., 2015, Suputtamongkol et al., 2008). The variability in dental ceramic restorations provides dental practitioners with a broad range of choices based on the patient’s clinical conditions and expectations (Seidel et al., 2020). Further advances in the mechanical and esthetic properties of dental ceramics have led to the introduction of lithium disilicate glass ceramics (LDC) (Ritter, 2010, Sanches et al., 2021). These consist of lithium oxide crystals embedded in a glassy matrix (Rizkalla & Jones, 2004). The interlocking orientation of the crystals prevents crack propagation, resulting in a superior flexural strength of up to 440 MPa (Rizkalla & Jones, 2004). Owing to their excellent biomechanical performance, favorable esthetics, and biocompatibility, LDC can be used safely in the production of monolithic restorations and as a core material for ceramic veneers (Maunula et al., 2017, Wendler et al., 2017).
All ceramic restorations, except LDC, can be classified according to their fabrication method, composition, fusing temperature, and microstructure (McLaren and Cao, 2009, Warreth and Elkareimi, 2020). Ceramic restorations can be fabricated through conventional stacking and sintering, heat- or dry-pressing methods, split casting and infusion techniques, or by computer-aided design and computer-aided manufacturing (CAD/CAM) technology (Anadioti et al., 2014, Höland et al., 2000, Sulaiman et al., 2015). All ceramic restorations can be categorized into three groups based on their composition:1) glass-based ceramics (such as feldspathic porcelain, leucite, and LDS), 2) glass-infiltration ceramics (e.g., in Ceram groups), and 3) non-glass-based ceramics (polycrystalline ceramics such as Alumina and Zirconia) (Warreth & Elkareimi, 2020). LDC is among the most commonly used glass-based ceramics, which can be used for two generations (Teichmann et al., 2017). The IPS Empress 2 (Ivoclar Vivadent, Schaan, Liechtenstein) belongs to the first generation and IPS e.max Press (Ivoclar, Vivadent, Schaan, Liechtenstein) represents the second generation. The second-generation LDC material signifies an improvement over the first-generation IPS Empress 2 in terms of strength, the assortment of translucency levels, and its enhanced ability to be used as a monolithic restoration (Teichmann et al., 2017). In terms of biocompatibility, all-ceramic materials such as LDC provide low solubility (Manicone et al., 2007) and high polishing ability. These help reduce plaque accumulation (Chan & Weber, 1986), and close marginal adaptation (Brawek et al., 2013) for long term function. Conversely, the presence of marginal gaps results in a vicious cycle of plaque biofilm accumulation, micro-infiltration of microbes, recurrent caries, pulpal lesions, periodontal inflammation, and cement exposure to the oral environment, ultimately leading to restoration failure (Demir et al., 2014, Dolev et al., 2019, Tan et al., 2008).
In light of these circumstances and to provide insights into the differing clinical applications of LDC restorations, data analysis based on outcomes after a sufficient duration of clinical service is necessary. Few systematic reviews have reported on the performance of LDC restorations based on the assessment of marginal adaptation and fatigue resistance (Nawafleh et al., 2016, Sanches et al., 2021). Moreover, clinical trials assessing recurrent caries occurrence and periodontal and endodontic responses in teeth restored with LDC are available (Schmitz et al., 2017, Fasbinder et al., 2010, Samer et al., 2017). However, to our knowledge, no systematic reviews have assessed these outcomes to provide an inclusive conclusion. Therefore, this systematic review aimed to investigate the clinical outcomes of single, full-coverage, lithium disilicate restorations in an adult population in comparison with other materials commonly used for single, full-coverage restorations such as zirconia, full metal, and porcelain-fused metal crowns.
2. Materials and methods
2.1. Research question
The PICO factors of this systematic review were:
-
•
Population: Adults requiring single, full-coverage, all-ceramic (lithium disilicate) restorations.
-
•
Intervention: Single, full-coverage all ceramic (lithium disilicate) restoration.
-
•
Comparator: Any other materials used for single, full-coverage restorations.
-
•
Clinical outcomes: pulpal involvement, recurrent caries, post-operative sensitivity, and periodontal changes.
The work focused on clinical outcomes such as pulpal involvement, recurrent caries, post-operative sensitivity, and periodontal changes. The authors followed The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for systematic reviews and meta-analyses (Liberati et al., 2009). The review protocol was predetermined but not published.
2.2. Search strategies
The search strategies were developed and applied by four authors (M. S. I., Y. A.D., N. M.A., and H. M.A.) to the four databases listed in Table 1. PubMed, SCOPUS, Web of Science, and OVID Medline were searched for published articles on March 1, 2021, with no restriction filters at this stage. All resulting citations and abstracts were downloaded and imported to the Covidence online platform (https://www.covidence.org/) for screening.
Table 1.
Electronic search strategies for Web of Science PubMed, Scopus and OVID Databases.
| Database: PubMed | |
| #1 | (“Milled ceramic” OR “Lithium disilicate” OR “Pressed ceramic” OR “E-max” OR “All-ceramic”) |
| #2 | (“Full coverage*” OR “Crown*” |
| #3 | #1 and #2 |
| Database: SCOPUS | |
| #1 | ( TITLE-ABS-KEY ( all-ceramic ) ) OR ( TITLE-ABS-KEY ( e-max ) ) OR ( TITLE-ABS-KEY ( pressed AND ceramic ) ) OR ( TITLE-ABS-KEY ( milled AND ceramic ) ) OR ( TITLE-ABS-KEY ( lithium AND disilicate ) |
| #2 | ( TITLE-ABS-KEY ( crown* ) ) OR ( TITLE-ABS-KEY ( full AND coverage* ) |
| #3 | #1 and #2 |
| Database: Web of Science | |
| #1 | TS= (All-ceramic OR Milled ceramic OR E-max OR Pressed ceramic OR Lithium disilicate) |
| #2 | TS=(Crown* OR Full coverage*) |
| #3 | #1 and #2 |
| Database: OVID (Ovid MEDLINE® and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Daily and Versions®1946 to 1 June 2020) | |
| #1 | All-ceramic.ti,ab. OR E-max.ti,ab. OR Pressed ceramic.ti,ab. OR Milled ceramic.ti,ab. OR lithium disilicate.ti,ab. |
| #2 | Crown*.ti,ab. OR full coverage*.ti,ab |
| #3 | #1 and #2 |
2.3. Inclusion and exclusion criteria
The primary inclusion criterion for this review was a clinical study that assessed clinical outcomes, including pulpal involvement, recurrent caries, post-operative sensitivity, and periodontal changes in tooth-supported, single, full-coverage, LDC prostheses. Case reports, case series, and review articles in addition to studies that exclusively assessed fixed-partial dentures, partial-coverage restorations, and non-tooth-supported restorations were excluded.
2.4. Screening and study selection
Two reviewers independently selected and screened the studies (N.M.A and H.M.A). The screening protocol included title and abstract screening, followed by full-text screening as shown in the PRISMA flow diagram (Fig. 1). Articles that did not meet the inclusion criteria were excluded. Conflicts between the reviewers were resolved by two senior reviewers (M. S. I. and Y. A.D.).
Fig. 1.
PRISMA flow diagram of study screening and selection.
2.5. Data extraction
Data were extracted by two reviewers (N. M.A. and H. M.A.) using a customized data extraction spreadsheet in Microsoft Excel (2020) software. Moreover, the results of the presence of pulpal involvement, recurrent caries, post-operative sensitivity, periodontal changes, and the degree of change were extracted from the included studies. Additionally, data related to sample size, including age and study groups, method of fabrication of the restoration, type of impression, and type of abutment, were recorded.
2.6. Quality Assessment
Two independent reviewers assessed the risk of bias in the included studies. Assessment tools for the included randomized clinical and observational studies were acquired from the Cochrane Assessment Tools. Studies with two or more “High risk of bias” categories in any domain were considered to have an overall high risk of bias.
2.7. Data synthesis
A qualitative summary of the characteristics and findings of the included studies was reported. A quantitative meta-analysis was not conducted due to the small number of included studies and variations in the brand and type of LDS, fabrication method, impression technique, and cementation protocol used.
3. Results
3.1. Study selection
We obtained 7742 records through an initial database search. After removing duplicate studies, short communications, letters to the editor, and technical notes, 3989 records were identified. The title and abstract screening of 3989 records yielded 238 studies for full-text screening. Eventually, 19 articles met the inclusion criteria and were considered in this systematic review (Fig. 1).
3.2. Characteristics of the included studies
The characteristics of the included studies are summarized in Table 2. The reviewed studies comprised both retrospective and prospective clinical datasets, and only nine of the 19 studies had control groups (Aziz et al., 2019, Batson et al., 2014, Esquivel-Upshaw et al., 2013, Hammoudi et al., 2022; Seidel et al., 2020a; Seydler and Schmitter, 2015, Taskonak and Sertgöz, 2006, Teichmann et al., 2017). The sample sizes were heterogeneous between the included studies and within the groups in individual studies. In some of the included studies, demographic data, such as age, health status, and sex of the participants were missing (Aziz et al., 2019, Batson et al., 2014, Esquivel-Upshaw et al., 2013, Fasbinder et al., 2010, Forrer et al., 2020, Gehrt et al., 2013, Schmitz et al., 2017, Scutella et al., 2020; Seidel et al., 2020a; Seydler and Schmitter, 2015, Taskonak and Sertgöz, 2006, Teichmann et al., 2017, Toman and Toksavul, 2015). While there was heterogeneity among the studies in terms of the impression technique used (conventional vs. digital), some studies did not mention the impression technique used (Forrer et al., 2020, Samer et al., 2017, Schmitz et al., 2017). Similarly, variation was observed concerning the ceramic prosthesis fabrication technique, wherein 10 studies used CAD/CAM milling, 7 used the press technique, and 3 did not report it (Esquivel-Upshaw et al., 2013, Hammoudi et al., 2022, Schmitz et al., 2017). The mean follow-up period in the included studies ranged from one month to nine years.
Table 2.
Characteristics of included studies.
| No. | Study | Type of Study Clinical trial/retrospective | Group Sample Size (# of crowns) | Participants | Intervention | Control | Method of impression | Method of fabrication Pressed/milled | Follow-up Time |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ahn et al., 2020 (Ahn et al., 2022) | Clinical trial |
CEREC, TRIOS N = 12 EZIS: N:16 |
Health status: without any general disease Age: 24 to 83 y Gender: 10 male and 30 female |
MLD based on CAD/CAM system: CEREC EZIS TRIOS |
– | Digital impression CEREC group: CEREC Bluecam EZIS group: EZIS PO TRIOS group: TRIOS 3 |
CAD/CAM Milled |
Base line, 1 month, 2 mmonts, 3 months & 6 months |
| 2 | Hammoudi et al., 2020 (Hammoudi et al., 2022) | Clinical trial |
LDC N = 362 TZ N = 351 |
Health status: Good overall dental health Age: 25 to 63 years Gender: 45 males and 17 females |
LDC | TZ | Conventional (Polyether material) | Not mentioned | Base line, 14 months, 31 months, 39 months, 54 months & 65 months |
| 3 | Forrer et al., 2020 (Forrer et al., 2020) | Retrospective study |
CC: N = 20 CP: N = 39 CV: N = 16 |
Health status: Not mentioned Age: Not mentioned Gender: 30 males and 52 females |
CC CP CV |
– | Not mentioned | CAD/CAM Milled | Base line & 4.08 ± 0.36 years |
| 4 | Scutella et al., 2020 (Scutella et al., 2020) | Retrospective study | N = 122 | Health status: Not mentioned Age: Mean age 56.4 years Gender: 29 male and 58 female, |
LDC | – | Digital impressions (CEREC 3D Blue-cam) | Chairside CAD/CAM Milled | 5 years |
| 5 | Seidel et al., 2020 (Seidel et al., 2020) | Clinical trial | N = 15 | Health status: Good Age: 18 years and above Gender: Not mentioned |
MLD | ZVGC | Conventional (polyvinylsiloxane material) | CAD/CAM Milled | Base line, 1 year, 2 years & 3 years |
| 6 | Aziz et al., 2019 (Aziz et al., 2019) | Retrospective clinical study | N = 40 | Health Status: Not mentioned Age: 29–79 years Gender: 12 males and 20 females |
LDGC CAD-CAM | CL | Digital impression (CEREC Omnicam) | CAD/CAM Milled | 4 years |
| 7 | Samer et al., 2017 (Samer et al., 2017) | Cross sectional study/retrospective | N = 88 | Health status: Medically fit Age: 18–64 years Gender: 16 males and 31 females |
LDC | – | Not mentioned | Pressed | Not clearly mentioned |
| 8 | Schimtz et al., 2017 (Schmitz et al., 2017) | Retrospective clinical study | N: 627 | Health status: Not mentioned Age: Not mentioned Gender: Not mentioned |
MLD | – | Not mentioned | Not mentioned | 48.17 ± 27.72 months |
| 9 | Rauch et al., 2017 (Rauch et al., 2017) | Clinical trial | N = 41 | Health status: Healthy Age: 26.2–73.8 years Gender: 13 males and 21 females |
LDC | – | Digital impressions (Cerec 3 units) | CAD/CAM milled | Base line, 6 months, 1 year, 2 years, 3 years, 4 years, 5 years, & 6 years |
| 10 | Teichmann et al., 2017 (Teichmann et al., 2017) | Prospective clinical study |
LDFDP: N = 33 LDC: N = 106 LDIMP: N = 32 Other-LD: N = 13 |
Health status: Not mentioned Age: LDC: mean age 23.9 LDFDP: mean age 40.7 LDIM: mean age 40.7 Gender: 30 males and 38 females |
LDC | -LDFDP -LDIMP - Other-LD |
Conventional (Polyether or A-Silicon material) | Pressed | Base line, 6 months, 1 year, 2 years, 3 years, 5 years & 7 years |
| 11 | Seydler et al., 2015 (Seydler & Schmitter, 2015) | Clinical trial | N = 30 | Health status: Not mentioned Age: Less than 18 years Gender: 22 males and 38 Females |
LDC | VZ | Digital Impression (Blue cam; Sirona Dental Systems) | CAD/CAM Milled | 1 year & 2 years |
| 12 | Toman et al., 2015 (Toman & Toksavul, 2015) | Clinical trial | N = 125 | Health status: Not mentioned Age: Not mentioned Gender: 13 males and 21 females |
LDC | – | Conventional (Venylpolyvinyl siloxane material) | Pressed | Base line, 6 months, then annually |
| 13 | Batson et al., 2014 (Batson et al., 2014) | Clinical trial |
MC: N = 12 LDC, ZC N = 10 |
Health status: Not mentioned Age: Not mentioned Gender: Not mentioned |
LDC | MC ZC CL tooth |
Digital Impression: iTero |
CAD/ CAM Milled |
Base line, 1 month & 6 months |
| 14 | Esquivel-Upshaw et al., 2013 (Esquivel-Upshaw et al., 2013) | Clinical trial | N = 12 | Health status: Good overall dental health Age: Above 18 Gender: Not mentioned |
LDC LDCV |
MC | Conventional (Polyvinyl siloxane) | Not mentioned | Base line, 1 year, 2 years & 3 years |
| 15 | Cortellini et al., 2012 (Cortellini & Canale, 2012) | Clinical trial | N = 235 | Health status: Healthy Age: 20–61 years Gender: 32 males and 44 females |
LDC | – | Conventional (Polyether material) | -CAD/CAM Milled -Pressed |
Base line, 1 year, 2 years & 3 years |
| 16 | Gehrt et al., 2012 (Gehrt et al., 2013) | Clinical trial | Adhesively luted:N = 72 Glass-ionomer cement: N = 32 |
Health status: Not mentioned Age: Mean age, 34 ± 9.6 years Gender: 15 males and 26 females |
LDC | – | Conventional (Polyether material) | Pressed | Base line, 5 years, 6 years, 7 years, 8 years & 9 years |
| 17 | Fasbinder et al., 2010 (Fasbinder et al., 2010) | Clinical trial |
LDC-4: N = 23 LDC-5: N = 39 |
Health status: Not mentioned Age: Not mentioned Gender: Not mentioned |
LDC: -LDC-4 -LDC-5 |
– | Digital impression | CAD/CAM Milled | Base line, 6 months, 1 year & 2 years |
| 18 |
Suputtamongkol et al., 2008 (Suputtamongkol et al., 2008) |
Clinical trial | N = 10 | Health status: Good to excellent overall dental health. Age: 20–56 years Gender: 5 males & 25 females |
LLD: -LDC-1 -LDC-2 -LDC-3 |
– | Conventional (Polyvinyl siloxane Material) | Pressed | Base line, 1 year & 2 years |
| 19 | Taskonak et al., 2006 (Taskonak & Sertgöz, 2006) | Clinical trial | N = 20 | Health status: Not mentioned Age: 21–59 years Gender: 3 males and 12 females |
LDC | LDFPD |
Conventional (Polyvinyl siloxane material) | Pressed | Base line, 1 year & 2 years |
MLD: Monolithic Lithium Disilicate crown, VZ: zirconia frameworks veneered with CAD/CAM-produced lithium disilicate ceramic, D: Day, M: Month, Y: Year, B: Baseline, NA: Not applicable, LLD: Layered lithium disilicate crowns, LDC-1: Lithium Disilicate crown cemented with Variolink II resin cement (Ivoclar Vivadent), LDC-2: Lithium Disilicate crown cemented with temporary cement. retrieved after 1 year of insertion., LDC-3: Lithium Disilicate crown cemented with temporary cement. retrieved after 2 years of insertion, LDC-4: Lithium Disilicate crown cemented with a self-etching, dual-curing cement with a self-etching primer and adhesive, LDC-5: Lithium Disilicate crown cemented with an experimental self-adhesive, dual-curing cement, ZVGC: zirconia coping hand- veneered with glass–ceramic, LD: Lithium disilicate, LDGC CAD-CAM: Lithium disilicate glass–ceramic (LDGC) computer-aided design (CAD)-computer- aided manufacturing (CAM), CL: Contralateral, CP: Pressed Lithium Disilicate Crowns, CV: veneered pressed lithium disilicate crowns, CC: computer-aided design and computer-aided manufacturing lithium disilicate crowns, FPDC: Veneered zirconia based FPD, FPDM: Metal Ceramic FPD, MC: Metal Crowns, LDC: Lithium Disilicate Crowns, ZC: Zirconia Crowns, LDFDP: Lithium disilicate fixed dental prosthesis, LDIMP: Lithium disilicate implant-supported crowns, Other-LD: resin-bonded FDPs, inlay-retained FDP, implant FDP, ILD: In direct lithium disilicate restorations (inlay, partial crown and crown), LDCV: Lithium disilicate core ceramic/veneer ceramic crowns, ZFPD: Zirconia Fixed Partial Denture, ILDC: implant supported lithium disilicate crowns, TZ: Translucent zirconia crown.
3.3. Risk of bias appraisal
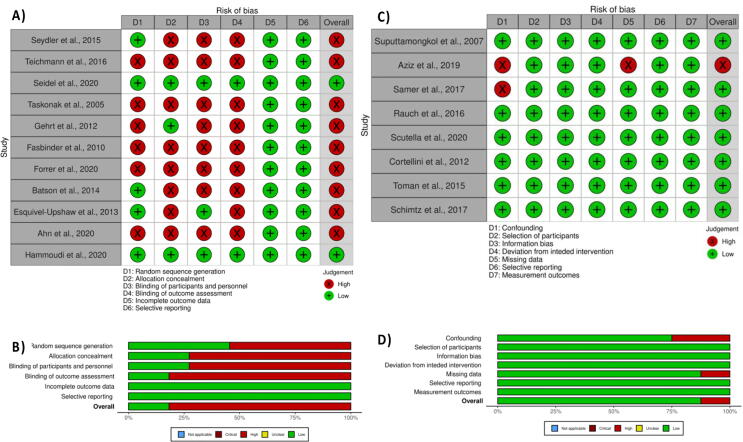
Most of the reviewed randomized clinical studies did not provide information about sample randomization, allocation, blinding of participants, or outcome assessments (Fig. 2). However, most of the included observational studies showed an overall low risk of bias (Fig. 2). Two of the eight included observational studies showed a high risk of bias in the confounding bias domain owing to variations in clinical skills among the operators (undergraduate or postgraduate students) who prepared and placed the crowns. One study also showed a high risk of bias as a higher percentage of participants refused to report for recall visits.
Fig. 2.
(A) Risk of bias appraisal for randomized clinical studies, (B) overall risk of bias for each domain of for randomized clinical studies, (C) Risk of bias appraisal for observational studies, (D) overall risk of bias for each domain of for randomized clinical studies.
3.4. Outcomes of the included studies
3.4.1. Pulpal involvement
Of the 19 included studies, only six assessed pulpal involvement during the follow-up period. These findings are summarized in Table 3A. Two studies reported no pulpal involvement during the follow-up periods. Four studies showed a very low percentage of caries recurrence under LDC after one year or longer. Overall, most studies reported no pulpal involvement with the placement of LDC, and in the few crowns associated with pulpal involvement, the involvement occurred after a long follow-up period.
Table 3.
Included Studies Reporting Outcomes.
| No. | Study | Assessment Method | Intervention: N (%) | Control: N (%) | Summary of Results (p-value) |
|---|---|---|---|---|---|
| |||||
| 1 |
Forrer et al., 2020 (Forrer et al., 2020) |
Presence of pulpal involvement | CC: Base line: 04 yers: 1 (11.1) CP Base line: 0 4 years: 1 (2.9) CV Base line: 0 4 years: 0 |
– | There was no pulpal involvement noticed on teeth with CC, CP, CV after 4 years of follow up. |
| 2 | Schimtz et al., 2017 (Schmitz et al., 2017) | Presence of pulpal involvement | MLD Base line: NA 48.17 months: 1 (0.16) |
– | There was pulpal involvement noticed on a tooth with MLD after 48.17 m of follow up. |
| 3 |
Teichmann et al., 2017 (Teichmann et al., 2017) |
Presence of pulpal involvement | LDC: 4.2 years: 1 (0.94) 7.3 years: 1 (0.94) 10 years: 1 (0.94) 12.3 years: 1 (0.94) 12.6 years: 2 (1.88) |
-LDFDP: 6.7 years: 1 (3) -LDIMP: NA -Other-LD: Not mentioned |
Among the LDC group, pulpal involvement was found in 1 tooth at all follow up intervals. In the LDFDP group, only one sample had pulpal involvement at 6.7 y follow up interval. |
| 4 | Seydler et al., 2015 (Seydler & Schmitter, 2015) |
Presence of pulpal involvement | MLD Base line: NA 1 year: 2 (6.6) 2 years: 0 (0) |
VZ B:NA 1 year: 2 (6.6) 2 years: 0 (0) |
Pulpal involvement was found after 1 year in both MLD and VZ groups. No pulpal involvement was found after two years of follow among all groups. |
| 5 | Gehrt et al., 2012 (Gehrt et al., 2013) |
Presence of pulpal involvement | LDC: 40.2 months: 1 (1.1) 94.7 months: 2 (2.1) |
– | There was one tooth with LDC with pulpal involvement at 40.2 m follow up, and two at 94.7 m follow up. |
| 6 |
Aziz et al., 2019 (Aziz et al., 2019) |
Presence of pulpal involvement | LDGC CAD-CAM: 4 years: 0 (0) |
– | There was no pulpal involvement noticed on teeth with LDGC CAD-CAM after 4 years of follow up. |
| |||||
| 1 | Hammoudi et al., 2020 (Hammoudi et al., 2022) |
Presence of recurrent caries | LDCBase line: 0 (0) 14 months: 0 (0) 31 months: 0 (0) 39 months: 0 (0) 54 months: 0 (0) 65 months: 0 (0) |
TZBase line: 0 (0) 14 months: 0 (0) 31 months: 0 (0) 39 months: 0 (0) 54 months: 0 (0) 65 months: 0 (0) |
There was no recurrent caries noticed on teeth with LDC and TZ at all follow up intervals (No p-value was reported). |
| 2 |
Forrer et al., 2020 (Forrer et al., 2020) |
Presence of recurrent caries | CC:Base line: 0 (0) 4 years: 0 (0) CPBase line: 0 (0) 4 years: 0 (0) CVBase line: 0 (0) 4 years: 0 (0) |
– | There was no recurrent caries noticed on teeth with CC, CP, CV at 4 years of follow up (No p-value was reported). |
| 3 |
Scutella et al., 2020 (Scutella et al., 2020) |
Presence of recurrent caries | LDC: 5 years: 0 (0) |
– | There was no recurrent caries noticed on teeth with LDC after 5 years of follow up (No p-value was reported). |
| 4 |
Seidel et al., 2020 (Seidel et al., 2020b) |
Presence of recurrent caries | MLD:Base line: 0 (0) 1 year: 0 (0) 2 years: 0 (0) 3 years: 0 (0) |
ZVGC:Base line: 0 (0) 1 year: 0 (0) 2 years: 0 (0) 3 years: 0 (0) |
There was no recurrent caries noticed on teeth with MLD and ZVGC after 1, 2, and 3 years of follow up (No p-value was reported). |
| 5 |
Samer et al., 2017 (Samer et al., 2017) |
Presence of recurrent caries | LDC:0 (0) |
– | There was no recurrent caries noticed on teeth with LDC (No p-value was reported). |
| 6 | Schimtz et al., 2017 (Schmitz et al., 2017) |
Presence of recurrent caries | MLD Base line: NA 48.17 months: 0 (0) |
– | There was no recurrent caries noticed on teeth with MLD at 48.17 months of follow up (No p-value was reported). |
| 7 | Rauch et al., 2016 (Rauch et al., 2017) |
Presence of recurrent caries | LDC:Base line: 0 (0) 6 months: 0 (0) 1 year: 0 (0) 2 years: 1 (2.4) 3 years: 1 (2.4) 4 years: 2 (4.9) 5 years: 1 (2.4) 6 years: 1 (2.4) |
– | There was no recurrent caries noticed on teeth with LDC at 6 months and 1 year follow up (No p-value was reported).One tooth with LDC had recurrent caries at 2, 3, 5 and 6 y of follow-up. Two teeth with LDC had recurrent caries at 4 y of follow-up (No p-value was reported). |
| 8 | Teichmann et al., 2017 (Teichmann et al., 2017) | Presence of recurrent caries | LDC: 5.5 years: 1 10.3 years: 2 |
-LDFDP N: 5.5 years: 1 -LDIMP: NA -Other-LD: Not mentioned |
LDIMP and LDC had a significantly higher survival than LDFDP (LDIMP vs. LDFDP: both tests p-value = 0.001; LDC vs. LDFDP: p-value = 0.001 and p-value = 0.005) at different follow-up periods. |
| 9 | Seydler et al., 2015 (Seydler & Schmitter, 2015) |
Presence of recurrent caries | MLD Base line: NA 1 year: 0 (0) 2 years: 0 (0) |
VZ Base line:NA 1 year: 0 (0) 2 years: 0 (0) |
There was no recurrent caries noticed on teeth with MLD and VZ at all follow-up periods (No p-value was reported). |
| 10 | Toman et al., 2015 (Toman & Toksavul, 2015) |
Presence of recurrent caries | LDC:0 (0) |
– | There was no recurrent caries noticed on teeth with LDC at all follow-up periods (No p-value was reported). |
| 11 |
Esquivel-Upshaw et al., 2013 (Esquivel-Upshaw et al., 2013) |
Presence of recurrent caries | LDC Base line: NA 1 year: 0 (0) 2 years: 0 (0) 3 years: 0 (0) LDCV: Base line: NA 1 year: 0 (0) 2 years: 0 (0) 3 years: 0 (0) |
MC: Base line: NA 1 year: 1 (8) 2 years: 1 (8) 3 years: 2 (18) |
Statistically insignificant differences were found at 1 y follow-up with p-value of 0.99, at 2 y follow-up with p-value of 0.99 and at 3 y follow-up with p-value of 0.32. |
| 12 | Cortellini et al., 2012 (Cortellini & Canale, 2012) |
Presence of recurrent caries | LDC: 42.59 months: 0 (0) 31.2 months: 0 (0) 17.49 months: 0 (0) 8.12 months: 0 (0) |
– | There was no recurrent caries noticed on teeth with LDC at all follow-up periods. |
| 13 | Gehrt et al., 2012 (Gehrt et al., 2013) |
Presence of recurrent caries | LDC: 58.6 months: 1 |
– | Not mentioned clearly |
| 14 |
Fasbinder et al., 2010 (Fasbinder et al., 2010) |
Presence of recurrent caries | LDC-4 (%): 6 months: 0 (0) 1 year: 0 (0) 2 years: 0 (0)LDC-5 (%) : 6 months: 0 (0) 1 year: 0 (0) 2 years: 0 (0) |
– | There was no recurrent caries noticed on teeth with LDC-4 and LDC-5 at all follow-up periods. |
| 15 | Aziz et al., 2007 (Aziz et al., 2019) |
Presence of recurrent caries | LDGC CAD-CAM: 24 months: 1 (2.5) |
– | There was one sample with recurrent caries noticed on teeth with LDGC CAD-CAM at 24-month of follow up. |
| 16 |
Suputtamongkol et al., 2008 (Suputtamongkol et al., 2008) |
Presence of recurrent caries | LLD:Base line: 0 (0) 1 year: 0 (0) |
– | There was no recurrent caries noticed on teeth with LLD after 1 year of follow-up. |
| 17 | Taskonak et al., 2006 (Taskonak & Sertgöz, 2006) |
Presence of recurrent caries | LDC (%):Base line: 0 (0) 1 year: 0 (0) 2 years: 0 (0) |
LDFDP (%):Base line: 0 (0) 1 year: 0 (0) 2 years: 0 (0) |
There was no recurrent caries noticed on teeth with LDC and LDFPD after 1 and 2 years of follow-up. |
| |||||
| 1 |
Samer et al., 2017 (Samer et al., 2017) |
Presence of post-operative sensitivity | LDC:0 (0) |
– | There was no post-operative sensitivity noticed on teeth with LDC at different follow-up periods. |
| 2 | Schimtz et al., 2017 (Schmitz et al., 2017) |
Presence of post-operative sensitivity | MLD Base line: NA 48.17 months: 4 (0.64) |
– | (No p-value was reported). |
| 3 | Seydler et al., 2015 (Seydler & Schmitter, 2015) |
Absence of post-operative sensitivity | MLD Base line: NA 1 year: 22 (73) 2 years: 28 (93) |
VZ Base line:NA 1 year: 24 (80) 2 years: 29 (96) |
(No p-value was reported). |
| 4 | Toman et al., 2015 (Toman & Toksavul, 2015) |
Presence of post-operative sensitivity | LDC:0 (0) |
– | There was no post-operative sensitivity noticed on teeth with LDC at different follow-up periods. |
| 5 |
Esquivel-Upshaw et al., 2013 (Esquivel-Upshaw et al., 2013) |
Presence of post-operative sensitivity | LDC Base line: NA 1 year: 1 (8) 2 years: 1 (8) 3 years: 0 (0) LDCV: Base line: NA 1 year: 0 (0) 2 years: 1 (8) 3 years: 0 (0) |
MC: Base line: NA 1 year: 2 (15) 2 years: 1 (8) 3 years: 2 (18) |
Statistically insignificant differences were found at 1 y follow-up with p-value of 0.99, at 2 y follow-up with p-value of 0.99 and at 3 y follow-up with p-value of 0.31. |
| 6 | Cortellini et al., 2012 (Cortellini & Canale, 2012) |
Presence of post-operative sensitivity | LDC: 42.59 months: 0 (0) 31.2 months: 0 (0) 17.49 months: 0 (0) 8.12 months: 0 (0) |
– | There was no post-operative sensitivity noticed on teeth with LDC at all follow-up periods. |
| 7 |
Fasbinder et al., 2010 (Fasbinder et al., 2010) |
Presence of post-operative sensitivity | LDC-4: 6 months: 2 (8.7) 1 year: 0 (0) 2 years: 0 (0) LDC-5: 6 months: 3 (7.7) 1 year: 0 (0) 2 years: 0 (0) |
– | (No p-value was reported). |
| 8 | Aziz et al., 2007 (Aziz et al., 2019) |
Presence of post-operative sensitivity | LDGC CAD-CAM: Base line: NA 2 months: 1 |
– | There was no post-operative sensitivity noticed on teeth with LDGC CAD-CAM after 2 months of follow-up. |
| 9 |
Suputtamongkol et al., 2008 (Suputtamongkol et al., 2008) |
Presence of post-operative sensitivity | LLD: Base line: NA 1 year: 0 (0) |
– | There was no post-operative sensitivity noticed on teeth with LLD after 1 year of follow-up. |
| 10 | Taskonak et al., 2006 (Taskonak & Sertgöz, 2006) |
Absence of post-operative sensitivity | LDC (%):Base line: (95) 1 year: (95) 2 years: (1 0 0) |
LDFDP (%):Base line: (90) 1 years: (90) 2 years: (1 0 0) |
(No p-value was reported). |
| |||||
| 1 | Ahn et al., 2020 (Ahn et al., 2022) |
Mean scores of:PD (mm)BI (score)PI (score) |
CEREC: PD: Base line: 1.4 ± 0.4 1 month: 1.5 ± 0.5 3 months: 1.6 ± 0.7 6 months: 1.9 ± 0.6 BI: Base line: 0.3 ± 0.5 1 month: 0.7 ± 0.8 3 months: 0.8 ± 0.7 6 months: 0.7 ± 0.6 PI: Base line: 0.5 ± 0.5 1 m: 0.8 ± 0.7 3 months: 1.1 ± 0.8 6 months: 1.1 ± 0.8 EZIS: PD: Base line: 1.2 ± 0.6 1 months: 1.4 ± 0.4 3 months: 1.5 ± 0.5 6 months: 1.7 ± 0.6 BI: Base line: 0.2 ± 0.4 1 m: 0.3 ± 0.5 3 months: 0.4 ± 0.5 6 months: 0.3 ± 0.6 PI: Base line: 0.6 ± 0.5 1 m: 0.6 ± 0.6 3 months: 0.5 ± 0.6 6 months: 0.5 ± 0.6 TRIOS: PD: Base line: 1.5 ± 0.4 1 m: 1.6 ± 0.4 3 months: 1.8 ± 0.5 6 months: 2.1 ± 0.5 BI: Base line: 0.2 ± 0.4 1 m: 0.2 ± 0.4 3 months: 0.4 ± 0.5 6 months: 0.6 ± 0.9 PI: Base line: 0.7 ± 0.5 1 m: 0.6 ± 0.5 3 months: 0.7 ± 0.5 6 months: 0.7 ± 0.7 |
– | Statistical insignificant differences were found between the groups in the PD, BI, and PI indices with p-value of > 0.05. |
| 2 |
Forrer et al., 2020 (Forrer et al., 2020) |
Mean change in: PI BoP Recession PD CAL |
CP: PI: Base line: 0.17 ± 0.14 4 years: 0.03 ± 0.07 BoP: Base line: 0.24 ± 0.30 4 years: 0.25 ± 0.28 Recession: Base line: 0.09 ± 0.14 4 years: 0.16 ± 0.27 PD: Base line: 2.62 ± 0.40 4 years: 2.61 ± 0.65 CAL: Base line: 3.76 ± 0.74 4 years: 2.77 ± 0.70 CV: PI: Base line: 0.12 ± 0.14 4 years: 0.08 ± 0.11 BoP: Base line:0.44 ± 0.33 4 years: 0.18 ± 0.19 Recession: Base line: 0.06 ± 0.08 4 years: 0.12 ± 0.13 PD: Base line: 2.38 ± 0.57 4 years: 2.40 ± 0.80 CAL: Base line: 3.44 ± 0.94 4 years: 2.52 ± 0.82 CC: PI: Base line: 0.23 ± 0.24 4 years: 0.01 ± 0.04 BoP: Base line: 0.25 ± 0.28 4 years: 0.12 ± 0.13 Recession: Base line: 0.36 ± 0.36 4 years: 0.59 ± 0.47 PD: Base line: 3.01 ± 0.46 4 years: 2.60 ± 0.90 CAL: Base line: 4.40 ± 1.08 4 years: 3.9 ± 1.10 |
– | Periodontal parameters (mean sites with plaque, mean sites with BoP) significantly improved at the follow up appointment (mean sites with plaque p-value < 0.001, mean sites with BOP with p-value = 0.017). Statistical significant reduction in PPD with p-value = 0.031 and increase in recession with p-value = 0.018 was noticed in the CC group. |
| 3 |
Teichmann et al., 2017 (Teichmann et al., 2017) |
Presence of periodontal disease | LDC (N): 1 year: 1 3.9 years: 1 4 years: 1 4.5 years: 1 7.6 years: 1 10.3–10.6 years: 4 |
-LDFDP: 3.1 years: 2 -LDIMP: 13.4 years: 1 14.8 years: 2 -Other-LD: Not mentioned |
LDIMP and LDC had significantly higher survival than LDFDP at different follow-up intervals. LDIMP vs. LDFDP: p-value = 0.001 at different follow-up intervals. LDC vs. LDFDP: p-value = 0.001 and p-value = 0.005 at different follow-up intervals. |
| 4 | Seydler et al., 2015 (Seydler & Schmitter, 2015) |
Presence of periodontal disease | MLD Base line: NA 1 year: 0 (0) 2 years: 0 (0) |
VZ Base line:NA 1 year: 3 (10) 2 years: 1 (0.3) |
(No p-value was reported). |
| 5 |
Batson et al., 2014 (Batson et al., 2014) |
Mean Change in: Buccal GCF Lingual GCF Presence of BoP |
LDC Buccal GCF: Base line: 32.00 ± 9.56 1 month: 44.13 ± 24.51 6 months: 43.33 ± 19.10 Lingual GCF: Base line: 39.40 ± 16.77 1 month: 56.88 ± 23.74 6 months: 39.83 ± 15.72 BoP:Base line: 5 (50) 1 month: 2 (25) 6 months: 2 (33) |
MC: Buccal GCF: Base line: 46.25 ± 23.95 1 month: 43.75 ± 14.06 6 months: 32.67 ± 14.51 Lingual GCF: Base line: 33.25 ± 12.17 1 month: 43.17 ± 17.48 6 months: 37.50 ± 20.05 BoP:Base line: 8 (67) 1 month: 5 (42) 6 months: 3 (50) ZC: Buccal GCF: Base line: 47.90 ± 22.79 1 month: 39.00 ± 12.12 6 months: 39.29 ± 14.40 Lingual GCF: Base line: 44.10 ± 22.49 1 month: 37.00 ± 17.61 6 months: 42.57 ± 23.73 BoP:Base line: 6 (60) 1 month: 5 (56) 6 months: 2 (29) |
No significant difference was found among the three crown systems for GCF volumes or BOP. |
| 6 |
Esquivel-Upshaw et al., 2013 (Esquivel-Upshaw et al., 2013) |
Presence of periodontal disease | LDC Base line: NA 1 year: 0 (0) 2 years: 0 (0) 3 years: 0 (0) LDCV: Base line: NA 1 year: 0 (0) 2 years: 0 (0) 3 years: 0 (0) |
MC: Base line: NA 1 year: 1 (1.2) 2 years: 1 (1.2) 3 years: 1 (1.2) |
Statistically significant differences were found at 1 y follow-up with p-value of 0.99, at 2 y follow-up with p-value of 0.99 and at 3 y follow-up with p-value of 0.99. |
| 7 | Cortellini et al., 2012 (Cortellini & Canale, 2012) |
GI PI |
LDC: GI Score = 1: 18 (7.6) PI: Score = 1: 12 (5) |
– | GI and PI score of 1 was found in 18 cases and 12 cases, respectively at different follow-up periods. |
| 8 | Gehrt et al., 2012 (Gehrt et al., 2013) |
Presence of periodontal disease | LDC:Base line: 0 (0) 5 years: 0 (0) 6 years: 0 (0) 7 years: 0 (0) 8 years: 0 (0) 9 years: 0 (0) |
– | (No p-value was reported). |
| 9 |
Aziz et al., 2019 (Aziz et al., 2019) |
Presence of periodontal disease | LDGC CAD-CAM: 4 years: 0 (0) |
CL: 4 years: 0 (0) |
Statistically insignificant differences were found among the groups at before and after insertion follow-up with p-value of 0.452 and 0.486, respectively. Compared to control with p-value of 0.727 and 0.430, respectively. |
| 10 | Suputtamongkol et al., 2007 (Suputtamongkol et al., 2008) | Presence of periodontal disease | LLD:Base line: 0 (0) 1 year: 0 (0) |
– | (No p-value was reported). |
| 11 | Taskonak et al., 2005 (Taskonak & Sertgöz, 2006) |
GI = 0 PI = 0 |
LDC: GI %: Base line: 95 1 year: 80 2 years: 75 PI %: Base line: 100 1 year: 70 2 years: 60 |
LDFDP: GI %: Base line: 90 1 year: 85 2 years: 85 PI %: Base line: 100 1 year: 67 2 years: 80 |
There was an increase in GI and PI in both intervention and control groups at all follow-up periods. |
NA: Not applicable, CC: computer-aided design and computer-aided manufacturing lithium disilicate crowns, CP: Pressed Lithium Disilicate Crowns, CV: veneered pressed lithium disilicate crowns, MLD: Monolithic Lithium Disilicate crown, LDC: Lithium Disilicate Crowns, LDGC CAD-CAM: Lithium disilicate glass–ceramic (LDGC) computer-aided design (CAD)-computer- aided manufacturing (CAM), LDFDP: Lithium disilicate fixed dental prosthesis, LDIMP: Lithium disilicate implant-supported crowns, Other-LD: resin-bonded FDPs, inlay-retained FDP, implant FDP, VZ: zirconia frameworks veneered with CAD/CAM-produced lithium disilicate ceramic, TZ: Translucent zirconia crown, ZVGC: zirconia coping hand- veneered with glass–ceramic, MC: Metal Crowns, LDCV: Lithium Disilicate Core ceramic/veneer ceramic crowns, LDC-4: Lithium Disilicate crown cemented with a self-etching, dual-curing cement with a self-etching primer and adhesive, LDC-5: Lithium Disilicate crown cemented with an experimental self-adhesive, dual-curing cement, LLD: Layered lithium disilicate crowns, PD: Pocket Depth, BoP: Bleeding on Probing, PI: Plaque Index, GI: Gingival index, CAL: Clinical Attachment Loss, GCF: Gingival Crevicular Fluid, BI: Bleeding Index, CEREC: CEREC Bluecam, CEREC AC, CEREC MC, computer-aided design and computer-aided manufacturing system, EZIS: EZIS PO, EZIS VR, EZIS HM computer-aided design and computer-aided manufacturing system, TRIOS: TRIOS 3, EXO-CAD, ARUM-4X computer-aided design and computer-aided manufacturing system, CL: Contralateral.
3.4.2. Recurrent caries
Of the 19 studies assessed, 17 studies reported recurrent dental caries after LDC placement. Only 6 of the 17 studies had control groups that were used to compare the performance. These findings are summarized in Table 3B. Fourteen out of the 19 studies reported no recurrent caries during the follow-up period. Overall, most studies reported minimal to no recurrent caries upon the use of LDC. Only four patients showed a very low percentage of recurrent caries under LDC after two years or longer.
3.4.3. Post-operative sensitivity
Of the 19 included studies, only 10 assessed postoperative sensitivity. A summary of these findings is provided in Table 3C. Five of the studies reported no postoperative sensitivity to LDC, and those that reported sensitivity to LDC showed the disappearance of sensitivity with time.
3.4.4. Periodontal changes
Out of the 19 studies, periodontal changes were assessed as an outcome in 11 studies, as shown in Table 3D. Among these 11 studies, no periodontal changes were reported during the follow-up period after LDC placement in six studies. While Ahn et al. (2022) reported no significant difference in the pocket depth (PD), bleeding index (BI), and plaque index (PI) among all groups of LDC crowns after a six-month follow-up period, Forrer et al. (2020) reported a statistically significant improvement in PI and bleeding on propping (BoP) at all follow-up periods after the placement of LDC crowns. They also reported a statistically significant decrease in PD and a significant increase in recession in the CC group. Similarly, Batson et al., (2014) indicated no significant differences in buccal and lingual gingival crevicular fluid (GCF) volumes or BoP among all study groups at the different follow-up periods. While Cortellini & Canale (2012) reported a gingival index (GI) and a PI score of “1″ in 18 cases and 12 cases, respectively, Taskonak & Sertgöz (2006) reported an increase in GI and PI in both the intervention and control groups at all follow-up periods.
4. Discussion
Lithium disilicate-based ceramics have been widely used as indirect restorative materials because of their esthetically pleasing appearance, ability to mimic natural teeth, and ease of fabrication using computer-aided design and manufacturing technology (Rauch et al., 2018). Although clinicians frequently face various interventions, the paucity of evidence for each intervention hinders the decision of the optimal option for a patient’s needs (Schwartz et al., 1997). The two-year and five-year survival rates of LDC crowns are reportedly 100% and 97.8%, respectively (Pieger et al., 2014). Nevertheless, these crowns are associated with common complications such as chipping, cracking, and fracture of the veneering porcelain (Raigrodski et al., 2012, Sax et al., 2011). Several studies have compared the mechanical and optical properties of LDC crowns used for full-coverage restorations with those of other similar restorations such as zirconia and porcelain-fused metal crowns (Al-Thobity et al., 2021, Amaral et al., 2020, Nam et al., n.d., Zarone et al., 2019, Ziyad et al., 2021). However, the current systematic review was novel as it discussed the presence of recurrent caries, as well as periodontal and pulpal outcomes of single, full-coverage lithium disilicate restorations. Early and late biological complications are critical and can affect the clinical outcomes of these restorations. Postoperative hypersensitivity and pulpal injuries are common early complications induced by cavity preparation and cementation techniques (Fotiadou et al., 2021, Wisithphrom et al., 2006). Recurrent caries is the most common cause of late failure (Hickel et al., 2007).
In clinical studies, a control group serves as the baseline for determining the effectiveness of the study treatment (Schwartz et al., 1997). In this review, of the 19 included studies, 11 did not have control groups. This condition made it unfeasible to perform a meta-analysis comparing LDC with other restorative materials (Ahn et al., 2022, Cortellini and Canale, 2012, Fasbinder et al., 2010, Forrer et al., 2020, Gehrt et al., 2013, Rauch et al., 2017, Samer et al., 2017, Schmitz et al., 2017, Scutella et al., 2020, Suputtamongkol et al., 2008, Toman and Toksavul, 2015).
Different techniques have been used to manufacture dental restorations. CAD/CAM has recently gained popularity in modern dentistry over conventional fabrication methods (Kollmuss et al., 2016, Susic et al., 2017). Different philosophies exist regarding these manufacturing methods with variable expected outcomes (Kollmuss et al., 2016). However, three of the included studies did not mention the method used to fabricate the prosthesis (Esquivel-Upshaw et al., 2013, Samer et al., 2017, Schmitz et al., 2017). A previous review reported that the quality of CAD/CAM restorations is inconsistent and does not demonstrate superior performance compared with existing conventional techniques (Ahmed, 2018).
We identified 11 studies that did not mention the overall health status of their participants (Aziz et al., 2019, Batson et al., 2014, Fasbinder et al., 2010, Forrer et al., 2020, Gehrt et al., 2013, Schmitz et al., 2017, Scutella et al., 2020, Seydler and Schmitter, 2015, Taskonak and Sertgöz, 2006, Teichmann et al., 2017, Toman and Toksavul, 2015). In this context, knowing the participants’ medical histories and medications was essential. Certain systemic conditions, such as poorly controlled diabetes mellitus, can reportedly alter the periodontal tissue (Lalla & Papapanou, 2011). Moreover, the intake of certain medications can adversely affect the periodontal tissue and its healing ability (Hughes and Bartold, 2018, Khalid et al., 2018). Iatrogenic gingival and periodontal tissue injuries can occur during tooth preparation, especially while creating a subgingival boundary finish line (Harish et al., 2015). Thus, the healing of the gingival attachment apparatus after tooth preparation in a patient with systemic compromise is expected to differ from that of a healthy patient.
None of the included studies reported adverse pulpal reactions following the cementation of LD crowns. This may be due to the minimally invasive tooth preparation design required for LD restorations (Cortellini & Canale, 2012; N. A. Nawafleh et al., 2017). In contrast, extensive tooth preparation is required for certain other restorative materials, leading to open dentinal tubules that contain the terminals of the pulpal nerve fibers, which are ultimately connected to the pulp (Liu et al., 2020, Mantzourani and Sharma, 2013). Trauma during tooth preparation is usually associated with extensive tooth structure removal, which exposes more dentinal tubules and adverse pulpal effects such as restoration-induced pulp hyperemia (Liu et al., 2020, Mantzourani and Sharma, 2013).
The marginal fit of full-coverage crowns is among the main predictors of long-term biological success and longevity (Riccitiello et al., 2018). The adverse consequences of crowns with marginal discrepancies include high rates of microleakage and bacterial biofilm formation. This increases the risk of dentinal demineralization and periodontal inflammation (Riccitiello et al., 2018). The emergence of CAD/CAM technology has improved the marginal adaptation of full-coverage crowns, thereby overcoming the dilemma of recurrent caries (Contrepois et al., 2013, Zeltner et al., 2017). In the current review, 10 of the 19 studies reported the use of CAD/CAM milling techniques in the fabrication of LDC. However, seven studies reported the use of pressed LDC. Additionally, four studies reported a low percentage of recurrent caries. Of them, two were pressed LDC, and two were fabricated using CAD/CAM milling. Furthermore, no consensus was obtained on the comparison between the marginal fit of CAD/CAM restorations and those fabricated using conventional techniques due to insufficient clinical data and the heterogeneity of measurement protocols (Contrepois et al., 2013, Zeltner et al., 2017).
The studies included in this review concluded that no postoperative sensitivity was found with LDC and that those who had initial postoperative sensitivity reported a resolution with time. All studies used resin-based cement for their subjects. The relationship between cement type, post-operative sensitivity, and patient satisfaction has been a popular topic in the literature. The major disadvantages of other cement types include thermal damage to the pulp associated with Zinc Phosphate cement and low initial pH of Glass Ionomer Cement (GIC), which are all risk factors for post-operative sensitivity (Blatz et al., 2013). On the other hand, resin cement has been proven to have a higher initial pH and low solubility, thereby contributing to lower pulpal irritation and post-operative sensitivity (Blatz et al., 2013).
A main advantage of LDCs is their biocompatibility with oral soft tissues (Forster et al., 2014). The findings of the current review revealed a favorable periodontal response after LDC cementation. The high polishability of the lithium disilicate surface promotes the adhesion and proliferation of gingival fibroblasts and epithelial cells (Tetè et al., 2014). Similarly, little to no inflammatory reactions in the gingival crevicular fluid have been reported after the insertion of lithium disilicate restorations in the oral cavity (Ariaans et al., 2016). In addition, culture data have shown the natural reaction of the soft tissue surrounding subgingival restorations fabricated with lithium disilicate glass ceramics (Forster et al., 2014). Thus, LDC is an excellent option for restoring teeth that require a full-coverage restoration.
Due to the limited number of included studies, several factors were related to the fabrication of LDC. These included the brand and type of LDS, fabrication method, impression technique, and cementation protocol. Another limitation was the lack of information about the exact time when complications occurred during the follow-up period, as knowing the time of failure could aid in their categorization as early or delayed complications. To our knowledge, this is the first systematic review to evaluate the available evidence in the literature regarding the clinical outcomes of lithium disilicate full-coverage crowns. This review also identified the gaps and opportunities for future research as it provided implications for conducting well-designed clinical trials in the area of restorative dentistry.
5. Conclusion
In dentistry, LDC is considered clinically successful after assessing several biological responses of the natural tooth abutment and the surrounding periodontium. We identified successful clinical outcomes in terms of pulpal involvement, recurrent caries, post-operative sensitivity, and periodontal changes. The current review suggests that LDC is a good biocompatible restorative material for oral soft tissues. Owing to the limited number of studies, further clinical trials with longer follow-up periods are required for future meta-analyses.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Contributor Information
Yousif A. Al-Dulaijan, Email: yaaldulaijan@iau.edu.sa.
Hussain M. Aljubran, Email: 2170004534@iau.edu.sa.
Nabras M. Alrayes, Email: 2170003511@iau.edu.sa.
Hajer A. Aldulaijan, Email: haldulaijan@ksu.edu.sa.
Mishali AlSharief, Email: msalsharief@iau.edu.sa.
Faisal E. Aljofi, Email: fealjofi@iau.edu.sa.
Maria S. Ibrahim, Email: msibrahim@iau.edu.sa.
References
- Ahn J.-J., Bae E.-B., Lee J.-J., Choi J.-W., Jeon Y.-C., Jeong C.-M., Yun M.-J., Lee S.-H., Lee K.-B., Huh J.-B. Clinical evaluation of the fit of lithium disilicate crowns fabricated with three different CAD-CAM systems. J. Prosthet. Dent. 2022;127(2):239–247. doi: 10.1016/j.prosdent.2020.06.031. [DOI] [PubMed] [Google Scholar]
- Ahmed K.E. We’re Going Digital: The Current State of CAD/CAM Dentistry in Prosthodontics. Primary Dental Journal. 2018;7(2):30–35. doi: 10.1177/205016841800700205. [DOI] [PubMed] [Google Scholar]
- Al-Thobity A.M., Gad M.M., Farooq I., Alshahrani A.S., Al-Dulaijan Y.A. Acid effects on the physical properties of different CAD/CAM ceramic materials: an in vitro analysis. J. Prosthodont.: Off. J. Am. College Prosthodont. 2021;30(2):135–141. doi: 10.1111/jopr.13232. [DOI] [PubMed] [Google Scholar]
- Amaral B., Tomaz De Almeida R., Fagundes De Oliveira K., Caldas R.A. Mechanical and optical properties of feldspathic ceramics and lithium disilicate: literature review. Rev. Bras. Odontol. 2020;77:1427. doi: 10.18363/rbo.v77.2020.e1427. [DOI] [Google Scholar]
- Anadioti E., Aquilino S.A., Gratton D.G., Holloway J.A., Denry I., Thomas G.W., Qian F. 3D and 2D marginal fit of pressed and CAD/CAM lithium disilicate crowns made from digital and conventional impressions. J. Prosthodont. 2014;23(8):610–617. doi: 10.1111/jopr.12180. [DOI] [PubMed] [Google Scholar]
- Ariaans K., Heussen N., Schiffer H., Wienert A.-L., Plümäkers B., Rink L., Wolfart S. Use of molecular indicators of inflammation to assess the biocompatibility of all-ceramic restorations. J. Clin. Periodontol. 2016;43(2):173–179. doi: 10.1111/jcpe.12500. [DOI] [PubMed] [Google Scholar]
- Aziz A., El-Mowafy O., Tenenbaum H.C., Lawrence H.P., Shokati B. Clinical performance of chairside monolithic lithium disilicate glass-ceramic CAD-CAM crowns. J. Esthet. Restor. Dent. 2019;31(6):613–619. doi: 10.1111/jerd.12531. [DOI] [PubMed] [Google Scholar]
- Batson E.R., Cooper L.F., Duqum I., Mendonça G. Clinical outcomes of three different crown systems with CAD/CAM technology. J. Prosthet. Dent. 2014;112(4):770–777. doi: 10.1016/j.prosdent.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Blatz M.B., Mante F.K., Saleh N., Atlas A.M., Mannan S., Ozer F. Postoperative tooth sensitivity with a new self-adhesive resin cement–a randomized clinical trial. Clin. Oral Invest. 2013;17(3):793–798. doi: 10.1007/s00784-012-0775-4. [DOI] [PubMed] [Google Scholar]
- Brawek P.K., Wolfart S., Endres L., Kirsten A., Reich S. The clinical accuracy of single crowns exclusively fabricated by digital workflow–the comparison of two systems. Clin. Oral Invest. 2013;17(9):2119–2125. doi: 10.1007/s00784-013-0923-5. [DOI] [PubMed] [Google Scholar]
- Chan C., Weber H. Plaque retention on teeth restored with full-ceramic crowns: a comparative study. J. Prosthet. Dent. 1986;56(6):666–671. doi: 10.1016/0022-3913(86)90140-x. [DOI] [PubMed] [Google Scholar]
- Schwartz C.E., Chesney M.A., Irvine M.J., Keefe F.J. The control group dilemma in clinical research: applications for psychosocial and behavioral medicine trials. Psychosomatic Medicine. 1997;59(4):362–371. doi: 10.1097/00006842-199707000-00005. [DOI] [PubMed] [Google Scholar]
- Contrepois, M., Soenen, A., Bartala, M., Laviole, O., 2013. Marginal adaptation of ceramic crowns: a systematic review. The Journal of Prosthetic Dentistry 110 (6), 447-454.e10. https://doi.org/10.1016/j.prosdent.2013.08.003. [DOI] [PubMed]
- Cortellini D., Canale A. Bonding lithium disilicate ceramic to feather-edge tooth preparations: a minimally invasive treatment concept. J. Adhes. Dent. 2012;14(1):7–10. doi: 10.3290/j.jad.a22708. [DOI] [PubMed] [Google Scholar]
- Demir N., Ozturk A.N., Malkoc M.A. Evaluation of the marginal fit of full ceramic crowns by the microcomputed tomography (micro-CT) technique. Eur. J. Dent. 2014;8(4):437–444. doi: 10.4103/1305-7456.143612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolev E., Bitterman Y., Meirowitz A. Comparison of marginal fit between CAD-CAM and hot-press lithium disilicate crowns. J. Prosthet. Dent. 2019;121(1):124–128. doi: 10.1016/j.prosdent.2018.03.035. [DOI] [PubMed] [Google Scholar]
- Esquivel-Upshaw J., Rose W., Oliveira E., Yang M., Clark A.E., Anusavice K. Randomized, controlled clinical trial of bilayer ceramic and metal-ceramic crown performance. J. Prosthodont. 2013;22(3):166–173. doi: 10.1111/j.1532-849X.2012.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasbinder, D.J., Dennison, J.B., Heys, D., Neiva, G., 2010. A clinical evaluation of chairside lithium disilicate CAD/CAM crowns: a two-year report. Journal of the American Dental Association (1939) 141 (Suppl 2), 10S-4S. https://doi.org/10.14219/jada.archive.2010.0355. [DOI] [PubMed]
- Forrer F.A., Schnider N., Brägger U., Yilmaz B., Hicklin S.P. Clinical performance and patient satisfaction obtained with tooth-supported ceramic crowns and fixed partial dentures. J. Prosthet. Dent. 2020;124(4):446–453. doi: 10.1016/j.prosdent.2019.08.012. [DOI] [PubMed] [Google Scholar]
- Forster A., Ungvári K., Györgyey Á., Kukovecz Á., Turzó K., Nagy K. Human epithelial tissue culture study on restorative materials. J. Dent. 2014;42(1):7–14. doi: 10.1016/j.jdent.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Fotiadou C., Manhart J., Diegritz C., Folwaczny M., Hickel R., Frasheri I. Longevity of lithium disilicate indirect restorations in posterior teeth prepared by undergraduate students: A retrospective study up to 8.5 years. J. Dent. 2021;105 doi: 10.1016/j.jdent.2020.103569. [DOI] [PubMed] [Google Scholar]
- Gehrt M., Wolfart S., Rafai N., Reich S., Edelhoff D. Clinical results of lithium-disilicate crowns after up to 9 years of service. Clin. Oral Invest. 2013;17(1):275–284. doi: 10.1007/s00784-012-0700-x. [DOI] [PubMed] [Google Scholar]
- Hammoudi W., Trulsson M., Svensson P., Smedberg J.-I. Long-term results of a randomized clinical trial of 2 types of ceramic crowns in participants with extensive tooth wear. J. Prosthet. Dent. 2022;127(2):248–257. doi: 10.1016/j.prosdent.2020.08.041. [DOI] [PubMed] [Google Scholar]
- Harish, P., Joseph, S.A., Sirajuddin, S., Gundapaneni, V., Chungkham, S., A., 2015. Iatrogenic Damage to the Periodontium Caused by Fixed Prosthodontic Treatment Procedures. The Open Dentistry Journal 9 (1), 190–196. https://doi.org/10.2174/1874210601509010190.
- Hickel R., Roulet J.-F., Bayne S., Heintze S.D., Mjör I.A., Peters M., Rousson V., Randall R., Schmalz G., Tyas M., Vanherle G. Recommendations for conducting controlled clinical studies of dental restorative materials. Science Committee Project 2/98–FDI World Dental Federation study design (Part I) and criteria for evaluation (Part II) of direct and indirect restorations including onlays and partial crowns. J. Adhes. Dent. 2007;9(Suppl 1):121–147. [PubMed] [Google Scholar]
- Höland W., Schweiger M., Frank M., Rheinberger V. A comparison of the microstructure and properties of the IPS Empress 2 and the IPS Empress glass-ceramics. J. Biomed. Mater. Res. 2000;53(4):297–303. doi: 10.1002/1097-4636(2000)53:4<297::aid-jbm3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Hughes, F.J., Bartold, P.M., 2018. Periodontal complications of prescription and recreational drugs. Periodontology 2000 78 (1), 47–58. https://doi.org/10.1111/prd.12230. [DOI] [PubMed]
- Khalid S., Chatzistavrianou D., Blair F. The impact of medication on the periodontium: a review of the literature. Dent. Update. 2018;45(3):256–265. doi: 10.12968/denu.2018.45.3.256. [DOI] [Google Scholar]
- Kollmuss M., Kist S., Goeke J.E., Hickel R., Huth K.C. Comparison of chairside and laboratory CAD/CAM to conventional produced all-ceramic crowns regarding morphology, occlusion, and aesthetics. Clin. Oral Invest. 2016;20(4):791–797. doi: 10.1007/s00784-015-1554-9. [DOI] [PubMed] [Google Scholar]
- Lalla E., Papapanou P.N. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat. Rev. Endocrinol. 2011;7(12):738–748. doi: 10.1038/nrendo.2011.106. [DOI] [PubMed] [Google Scholar]
- Li R.W.K., Chow T.W., Matinlinna J.P. Ceramic dental biomaterials and CAD/CAM technology: state of the art. J. Prosthodont. Res. 2014;58(4):208–216. doi: 10.1016/j.jpor.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clin. Res. Ed.) 2009;339 doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.-X., Tenenbaum H.C., Wilder R.S., Quock R., Hewlett E.R., Ren Y.-F. Pathogenesis, diagnosis and management of dentin hypersensitivity: an evidence-based overview for dental practitioners. BMC Oral Health. 2020;20(1):220. doi: 10.1186/s12903-020-01199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manicone P.F., Rossi Iommetti P., Raffaelli L. An overview of zirconia ceramics: basic properties and clinical applications. J. Dent. 2007;35(11):819–826. doi: 10.1016/j.jdent.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Mantzourani M., Sharma D. Dentine sensitivity: past, present and future. J. Dent. 2013;41(Suppl 4):S3–S. doi: 10.1016/S0300-5712(13)70002-2. [DOI] [PubMed] [Google Scholar]
- Maunula H., Hjerppe J., Lassila L.L.V., Närhi T.O. Optical properties and failure load of thin CAD/CAM ceramic veneers. Eur. J. Prosthodont. Restor. Dent. 2017;25(2):86–92. doi: 10.1922/EJPRD_01677Maunula07. [DOI] [PubMed] [Google Scholar]
- McLaren E.A., Cao P.T. Ceramics in dentistry—part I: classes of materials. Inside Dent. 2009:94–105. [Google Scholar]
- Nam, S.-J., Yoon, M.-J., Kim, W.-H., Ryu, G.-J., Bang, M.-K., Huh, J.-B., n.d.. Marginal and Internal Fit of Conventional Metal-Ceramic and Lithium Disilicate CAD/CAM Crowns. The International Journal of Prosthodontics 28 (5), 519–521. https://doi.org/10.11607/ijp.4089. [DOI] [PubMed]
- Nawafleh N., Hatamleh M., Elshiyab S., Mack F. Lithium disilicate restorations fatigue testing parameters: a systematic review. J. Prosthodont.: Off. J. Am. College Prosthodont. 2016;25(2):116–126. doi: 10.1111/jopr.12376. [DOI] [PubMed] [Google Scholar]
- Nawafleh N.A., Hatamleh M.M., Öchsner A., Mack F. Fracture load and survival of anatomically representative monolithic lithium disilicate crowns with reduced tooth preparation and ceramic thickness. J. Adv. Prosthodont. 2017;9(6):416–422. doi: 10.4047/jap.2017.9.6.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieger S., Salman A., Bidra A.S. Clinical outcomes of lithium disilicate single crowns and partial fixed dental prostheses: a systematic review. J. Prosthet. Dent. 2014;112(1):22–30. doi: 10.1016/j.prosdent.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Raigrodski A.J., Hillstead M.B., Meng G.K., Chung K.-H. Survival and complications of zirconia-based fixed dental prostheses: a systematic review. J. Prosthet. Dent. 2012;107(3):170–177. doi: 10.1016/S0022-3913(12)60051-1. [DOI] [PubMed] [Google Scholar]
- Rauch A., Reich S., Schierz O. Chair-side generated posterior monolithic lithium disilicate crowns: clinical survival after 6 years. Clin. Oral Invest. 2017;21(6):2083–2089. doi: 10.1007/s00784-016-1998-6. [DOI] [PubMed] [Google Scholar]
- Rauch A., Reich S., Dalchau L., Schierz O. Clinical survival of chair-side generated monolithic lithium disilicate crowns:10-year results. Clin. Oral Invest. 2018;22(4):1763–1769. doi: 10.1007/s00784-017-2271-3. [DOI] [PubMed] [Google Scholar]
- Riccitiello F., Amato M., Leone R., Spagnuolo G., Sorrentino R. In vitro evaluation of the marginal fit and internal adaptation of zirconia and lithium disilicate single crowns: micro-CT comparison between different manufacturing procedures. Open Dent. J. 2018;12:160–172. doi: 10.2174/1874210601812010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter R.G. Multifunctional uses of a novel ceramic-lithium disilicate. J. Esthet. Restor. Dent. 2010;22(5):332–341. doi: 10.1111/j.1708-8240.2010.00362.x. [DOI] [PubMed] [Google Scholar]
- Rizkalla A., Jones D. Mechanical properties of commercial high strength ceramic core materials. Dent. Mater. 2004;20(2):207–212. doi: 10.1016/S0109-5641(03)00093-9. [DOI] [PubMed] [Google Scholar]
- Samer M.S., Faraz Q., Al-Dubai S.A.R., Vohra F., Abdullah H., Taiyeb-Ali T.B., Saub R. Clinical outcomes and predictors of satisfaction in patients with improved lithium disilicate all-ceramic crowns. Med. Princ. Pract. 2017;26(5):470–479. doi: 10.1159/000481864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanches I.B., Metzker T.C., Kappler R., Oliveira M.V., Carvalho A.O., Castor Xisto Lima E.M. Marginal adaptation of CAD-CAM and heat-pressed lithium disilicate crowns: a systematic review and meta-analysis. J. Prosthet. Dent. 2021 doi: 10.1016/j.prosdent.2021.03.021. [DOI] [PubMed] [Google Scholar]
- Santos, M.J.M.C., Costa, M.D., Rubo, J.H., Pegoraro, L.F., Santos, G.C., 2015. Current all-ceramic systems in dentistry: a review. Compendium of Continuing Education in Dentistry (Jamesburg, N.J. : 1995) 36 (1), 31–37; quiz 38, 40. [PubMed]
- Sax C., Hämmerle C.H.F., Sailer I. 10-year clinical outcomes of fixed dental prostheses with zirconia frameworks. Int. J. Comput. Dent. 2011;14(3):183–202. [PubMed] [Google Scholar]
- Schmitz, J.H., Cortellini, D., Granata, S., Valenti, M., 2017. Monolithic lithium disilicate complete single crowns with feather-edge preparation design in the posterior region: A multicentric retrospective study up to 12 years. Quintessence International (Berlin, Germany : 1985), 601–608. https://doi.org/10.3290/j.qi.a38678. [DOI] [PubMed]
- Scutella F., Weinstein T., Redaelli S., Cerutti A., Testori T., Özcan M. Reliability of chair-side monolithic CAD-CAM generated lithium disilicate single crowns with knife- edge finish line: up to 5-year retrospective analysis of clinical performance. Eur. J. Prosthodont. Restor. Dent. 2020;28(2) doi: 10.1922/EJPRD_1930Scutella04. [DOI] [PubMed] [Google Scholar]
- Seidel A., Belli R., Breidebach N., Wichmann M., Matta R.E. The occlusal wear of ceramic fixed dental prostheses: 3-Year results in a randomized controlled clinical trial with split-mouth design. J. Dent. 2020;103 doi: 10.1016/j.jdent.2020.103500. [DOI] [PubMed] [Google Scholar]
- Seydler B., Schmitter M. Clinical performance of two different CAD/CAM-fabricated ceramic crowns: 2-Year results. J. Prosthet. Dent. 2015;114(2):212–216. doi: 10.1016/j.prosdent.2015.02.016. [DOI] [PubMed] [Google Scholar]
- Sulaiman T.A., Delgado A.J., Donovan T.E. Survival rate of lithium disilicate restorations at 4 years: a retrospective study. J. Prosthet. Dent. 2015;114(3):364–366. doi: 10.1016/j.prosdent.2015.04.011. [DOI] [PubMed] [Google Scholar]
- Suputtamongkol K., Anusavice K.J., Suchatlampong C., Sithiamnuai P., Tulapornchai C. Clinical performance and wear characteristics of veneered lithia-disilicate-based ceramic crowns. Dent. Mater. 2008;24(5):667–673. doi: 10.1016/j.dental.2007.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susic I., Travar M., Susic M. The application of CAD / CAM technology in dentistry. IOP Conf. Ser.: Mater. Sci. Eng. 2017;200 doi: 10.1088/1757-899X/200/1/012020. [DOI] [Google Scholar]
- Tan P.L., Gratton D.G., Diaz-Arnold A.M., Holmes D.C. An in vitro comparison of vertical marginal gaps of CAD/CAM titanium and conventional cast restorations. J. Prosthodont. : Off. J. Am. College Prosthodont. 2008;17(5):378–383. doi: 10.1111/j.1532-849X.2008.00302.x. [DOI] [PubMed] [Google Scholar]
- Taskonak B., Sertgöz A. Two-year clinical evaluation of lithia-disilicate-based all-ceramic crowns and fixed partial dentures. Dent. Mater. 2006;22(11):1008–1013. doi: 10.1016/j.dental.2005.11.028. [DOI] [PubMed] [Google Scholar]
- Teichmann M., Göckler F., Weber V., Yildirim M., Wolfart S., Edelhoff D. Ten-year survival and complication rates of lithium-disilicate (Empress 2) tooth-supported crowns, implant-supported crowns, and fixed dental prostheses. J. Dent. 2017;56:65–77. doi: 10.1016/j.jdent.2016.10.017. [DOI] [PubMed] [Google Scholar]
- Tetè S., Zizzari V.L., Borelli B., De Colli M., Zara S., Sorrentino R., Scarano A., Gherlone E., Cataldi A., Zarone F. Proliferation and adhesion capability of human gingival fibroblasts onto zirconia, lithium disilicate and feldspathic veneering ceramic in vitro. Dent. Mater. J. 2014;33(1):7–15. doi: 10.4012/dmj.2013-185. [DOI] [PubMed] [Google Scholar]
- Tinschert J., Natt G., Hassenpflug S., Spiekermann H. Status of current CAD/CAM technology in dental medicine. Int. J. Comput. Dent. 2004;7(1):25–45. [PubMed] [Google Scholar]
- Toman, M., Toksavul, S., 2015. Clinical evaluation of 121 lithium disilicate all-ceramic crowns up to 9 years. Quintessence International (Berlin, Germany : 1985) 46 (3), 189–197. https://doi.org/10.3290/j.qi.a33267. [DOI] [PubMed]
- Warreth A., Elkareimi Y. All-ceramic restorations: a review of the literature. Saudi Dent. J. 2020;32(8):365–372. doi: 10.1016/j.sdentj.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendler M., Belli R., Petschelt A., Mevec D., Harrer W., Lube T., Danzer R., Lohbauer U. Chairside CAD/CAM materials. part 2: flexural strength testing. Dent. Mater. 2017;33(1):99–109. doi: 10.1016/j.dental.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Wisithphrom K., Murray P.E., About I., Windsor L.J. Interactions between cavity preparation and restoration events and their effects on pulp vitality. Int. J. Periodont. Restor. Dent. 2006;26(6):596–605. [PubMed] [Google Scholar]
- Zarone F., Di Mauro M.I., Ausiello P., Ruggiero G., Sorrentino R. Current status on lithium disilicate and zirconia: a narrative review. BMC Oral Health. 2019;19(1):134. doi: 10.1186/s12903-019-0838-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeltner M., Sailer I., Mühlemann S., Özcan M., Hämmerle C.H.F., Benic G.I. Randomized controlled within-subject evaluation of digital and conventional workflows for the fabrication of lithium disilicate single crowns. Part III: marginal and internal fit. J. Prosthet. Dent. 2017;117(3):354–362. doi: 10.1016/j.prosdent.2016.04.028. [DOI] [PubMed] [Google Scholar]
- Ziyad, T.A., Abu-Naba’a, L.A., Almohammed, S.N., 2021. Optical properties of CAD-CAM monolithic systems compared: three multi-layered zirconia and one lithium disilicate system. Heliyon 7 (10), e08151. https://doi.org/10.1016/j.heliyon.2021.e08151 [DOI] [PMC free article] [PubMed]