Abstract
Background
Hyaluronic acid is synthesised in plasma membranes and can be found in extracellular tissues. It has been suggested that the application of hyaluronic acid to chronic wounds may promote healing, and the mechanism may be due to its ability to maintain a moist wound environment which helps cell migration in the wound bed.
Objectives
To evaluate the effects of hyaluronic acid (and its derivatives) on the healing of chronic wounds.
Search methods
We used standard, extensive Cochrane search methods. The latest search date was February 2022.
Selection criteria
We included randomised controlled trials that compared the effects of hyaluronic acid (as a dressing or topical agent) with other dressings on the healing of pressure, venous, arterial, or mixed‐aetiology ulcers and foot ulcers in people with diabetes.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. We assessed the certainty of the evidence using the GRADE approach.
Main results
We included 12 trials (13 articles) in a qualitative synthesis, and were able to combine data from four trials in a quantitative analysis. Overall, the included trials involved 1108 participants (mean age 69.60 years) presenting 178 pressure ulcers, 54 diabetic foot ulcers, and 896 leg ulcers. Sex was reported for 1022 participants (57.24% female).
Pressure ulcers
It is uncertain whether there is a difference in complete healing (risk ratio (RR) 1.17, 95% confidence interval (CI) 0.58 to 2.35); change in ulcer size (mean difference (MD) 25.60, 95% CI 6.18 to 45.02); or adverse events (none reported) between platelet‐rich growth factor (PRGF) + hyaluronic acid and PRGF because the certainty of evidence is very low (1 trial, 65 participants). It is also uncertain whether there is a difference in complete healing between lysine hyaluronate and sodium hyaluronate because the certainty of evidence is very low (RR 2.50, 95% CI 0.71 to 8.83; 1 trial, 14 ulcers from 10 participants).
Foot ulcers in people with diabetes
It is uncertain whether there is a difference in time to complete healing between hyaluronic acid and lyophilised collagen because the certainty of evidence is very low (MD 16.60, 95% CI 7.95 to 25.25; 1 study, 20 participants). It is uncertain whether there is a difference in complete ulcer healing (RR 2.20, 95% CI 0.97 to 4.97; 1 study, 34 participants) or change in ulcer size (MD −0.80, 95% CI −3.58 to 1.98; 1 study, 25 participants) between hyaluronic acid and conventional dressings because the certainty of evidence is very low.
Leg ulcers
We are uncertain whether there is a difference in complete wound healing (RR 0.98, 95% CI 0.26 to 3.76), percentage of adverse events (RR 0.79, 95% CI 0.22 to 2.80), pain (MD 2.10, 95% CI −5.81 to 10.01), or change in ulcer size (RR 2.11, 95% CI 0.92 to 4.82) between hyaluronic acid + hydrocolloid and hydrocolloid because the certainty of evidence is very low (1 study, 125 participants). It is uncertain whether there is a difference in change in ulcer size between hyaluronic acid and hydrocolloid because the certainty of evidence is very low (RR 1.02, 95% CI 0.84 to 1.25; 1 study, 143 participants). We are uncertain whether there is a difference in complete wound healing between hyaluronic acid and paraffin gauze because the certainty of evidence is very low (RR 2.00, 95% CI 0.21 to 19.23; 1 study, 24 ulcers from 17 participants).
When compared with neutral vehicle, hyaluronic acid probably improves complete ulcer healing (RR 2.11, 95% CI 1.46 to 3.07; 4 studies, 526 participants; moderate‐certainty evidence); may slightly increase the reduction in pain from baseline (MD −8.55, 95% CI −14.77 to −2.34; 3 studies, 337 participants); and may slightly increase change in ulcer size, measured as mean reduction from baseline to 45 days (MD 30.44%, 95% CI 15.57 to 45.31; 2 studies, 190 participants). It is uncertain if hyaluronic acid alters incidence of infection when compared with neutral vehicle (RR 0.89, 95% CI 0.53 to 1.49; 3 studies, 425 participants). We are uncertain whether there is a difference in change in ulcer size (cm2) between hyaluronic acid and dextranomer because the certainty of evidence is very low (MD 5.80, 95% CI −10.0 to 21.60; 1 study, 50 participants).
We downgraded the certainty of evidence due to risk of bias or imprecision, or both, for all of the above comparisons. No trial reported health‐related quality of life or wound recurrence. Measurement of change in ulcer size was not homogeneous among studies, and missing data precluded further analysis for some comparisons.
Authors' conclusions
There is currently insufficient evidence to determine the effectiveness of hyaluronic acid dressings in the healing of pressure ulcers or foot ulcers in people with diabetes. We found evidence that hyaluronic acid probably improves complete ulcer healing and may slightly decrease pain and increase change in ulcer size when compared with neutral vehicle. Future research into the effects of hyaluronic acid in the healing of chronic wounds should consider higher sample size and blinding to minimise bias and improve the quality of evidence.
Keywords: Female, Humans, Male, Middle Aged, Bandages, Diabetic Foot, Diabetic Foot/therapy, Hyaluronic Acid, Hyaluronic Acid/therapeutic use, Pressure Ulcer, Pressure Ulcer/therapy, Randomized Controlled Trials as Topic, Wound Healing
Plain language summary
Hyaluronic acid for chronic wound healing
What is the aim of this review?
The aim of this review was to evaluate the effects of hyaluronic acid on the healing of chronic wounds. Hyaluronic acid is a naturally occurring molecule present in human cells. Chronic wounds are wounds that take a long time to heal. They include pressure ulcers, foot ulcers, and leg ulcers.
Key messages
We cannot be certain whether dressings and topical agents containing hyaluronic acid are more effective for healing pressure ulcers or foot ulcers in people with diabetes than other dressings and topical agents. When used in people with leg ulcers and compared with the inactive substance included in the dressing to serve as a means of delivering hyaluronic acid (neutral vehicle), hyaluronic acid probably improves complete ulcer healing and may slightly decrease pain and increase change in ulcer size. There was not enough information to be sure how dressings and topical agents containing hyaluronic acid compare with other dressings and topical agents in terms of potential side effects.
What was studied in the review?
Chronic wounds are hard‐to‐heal wounds that arise for a variety of reasons, including in response to an underlying disease. Treatment includes different types of wound dressing or topical agents with a variety of purposes, including: maintenance of a moist healing environment; reduction of bacteria present in the wound; and prevention of infection.
What did we do?
We searched the medical literature for studies that evaluated the effects of hyaluronic acid compared with other dressings. We compared the data obtained, summarised the results, and rated our confidence in the evidence, based on factors such as study methods and sizes. We only included randomised controlled trials, a type of study where people are assigned at random to receive different treatments, because they provide the most reliable health evidence.
What are the main results of the review?
We found 12 studies involving a total of 1108 participants. Sex was reported for 1022 participants (57.24% female). Mean age corresponded to 69.60 years. Dressings containing varying concentrations of hyaluronic acid, or containing hyaluronic acid in combination with another treatment, were compared with other dressing types.
It is uncertain whether hyaluronic acid is better or worse at healing pressure ulcers or foot ulcers in people with diabetes. It is also uncertain if there is any difference in effect between hyaluronic acid and other dressings on adverse events and pain in these types of wounds. This is due to scarcity of data to analyse or because of study limitations such as small sample sizes and methodological problems.
In leg ulcers, hyaluronic acid probably improves complete ulcer healing when compared with neutral vehicle (4 studies, 526 participants), and may slightly reduce pain (3 studies, 337 participants) and slightly increase change in ulcer size (2 studies, 190 participants). It is uncertain whether hyaluronic acid is better or worse at healing leg ulcers when compared with hydrocolloid (an agent that forms a gel when exposed to wound fluids), paraffin gauze, or dextranomer (a type of dressing that promotes wound healing).
No trial reported health‐related quality of life or wound recurrence.
What limited our confidence in the evidence?
Most studies were small (fewer than 100 participants), and most (9 out of 12) used methods that were likely to have introduced errors in their results. Follow‐up duration was short (9 out of 12 studies followed participants for 60 days or less), and studies were not designed to assess time to complete healing (only 1 study followed participants until complete healing).
How up‐to‐date is the review?
We searched for studies published up to February 2022.
Summary of findings
Summary of findings 1. Platelet‐rich growth factor + hyaluronic acid compared with platelet‐rich growth factor for pressure ulcers.
| Platelet‐rich growth factor + hyaluronic acid compared with platelet‐rich growth factor for pressure ulcers | ||||||
| Patient or population: pressure ulcer Setting: long‐stay hospital and geriatric centres Intervention: platelet‐rich growth factor + hyaluronic acid Comparison: platelet‐rich growth factor | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with platelet‐rich growth factor | Risk with platelet‐rich growth factor + hyaluronic acid | |||||
| Complete ulcer healing Follow‐up: 36 days |
Study population | RR 1.17 (0.58 to 2.35) | 65 (1 RCT) | ⊕⊝⊝⊝ Very low1 2 | It is uncertain if platelet‐rich growth factor + hyaluronic acid affects complete healing when compared with platelet‐rich growth factor. | |
| 320 per 1000 | 374 per 1000 (186 to 752) | |||||
| Time to complete wound healing ‐ not reported | No studies provided evidence for this outcome. | |||||
| Adverse events | No signs of infection in the pressure ulcers of either group | ‐ | 65 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 | It is uncertain if platelet‐rich growth factor + hyaluronic acid affects adverse events when compared with platelet‐rich growth factor. | |
| Health‐related quality of life | No studies provided evidence for this outcome. | |||||
| Pain | No studies provided evidence for this outcome. | |||||
| Change in ulcer size Follow‐up: 36 days | The per cent reduction in ulcer size was 54.80 | The per cent reduction in ulcer size was 80.4 | MD 25.60 cm higher (6.18 higher to 45.02 higher) | 65 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 | It is uncertain if platelet‐rich growth factor + hyaluronic acid affects change in ulcer size when compared with platelet‐rich growth factor. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded twice for risk of bias due to unclear blinding of participants and personnel and high risk of attrition bias. 2Downgraded twice for imprecision due to small numbers of participants and events and wide confidence intervals.
Summary of findings 2. Lysine hyaluronate compared with sodium hyaluronate for pressure ulcers.
| Lysine hyaluronate compared with sodium hyaluronate for pressure ulcers | ||||||
| Patient or population: people with pressure ulcers Setting: hospital Intervention: lysine hyaluronate Comparison: sodium hyaluronate | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with sodium hyaluronate | Risk with lysine hyaluronate | |||||
| Complete ulcer healing Follow‐up: 15 days | Study population | RR 2.50 (0.71 to 8.83) | 10 participants; 14 ulcers (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 | It is uncertain if lysine hyaluronate affects complete healing when compared with sodium hyaluronate. | |
| 286 per 1000 | 714 per 1000 (203 to 1000) | |||||
| Time to complete wound healing ‐ not reported | No studies provided evidence for this outcome. | |||||
| Adverse events ‐ not reported | No studies provided evidence for this outcome. | |||||
| Health‐related quality of life ‐ not reported | No studies provided evidence for this outcome. | |||||
| Pain | No studies provided evidence for this outcome. | |||||
| Change in ulcer size | Study authors reported the period required to reach 50% of ulcer healing; however, they did not provide means or standard deviation, thereby precluding further analysis. | ‐ | 10 participants; 14 ulcers (1 RCT) |
⊕⊝⊝⊝ Very low 1 2 | It is uncertain if lysine hyaluronate affects complete healing when compared with sodium hyaluronate. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded twice for risk of bias due to unclear risk for randomisation and allocation, and high risk for selective reporting. 2Downgraded twice for imprecision due to small numbers of participants and events and wide confidence intervals.
Summary of findings 3. Hyaluronic acid compared with lyophilised collagen for foot ulcers in people with diabetes.
| Hyaluronic acid compared with lyophilised collagen for foot ulcers in people with diabetes | ||||||
| Patient or population: foot ulcers in people with diabetes Setting: not reported Intervention: hyaluronic acid Comparison: lyophilised collagen | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with lyophilised collagen | Risk with hyaluronic acid | |||||
| Complete ulcer healing | ‐ | 20 (1 RCT) | ‐ | Study authors followed all participants until complete healing. | ||
| Time to complete healing | The mean time to complete healing was 32.4 days. | The mean time to complete healing was 49.0 days. | MD 16.60 days higher (7.95 higher to 25.25 higher) | 20 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 | It is uncertain if lyophilised collagen decreases time to complete healing when compared with hyaluronic acid. |
| Adverse events ‐ not reported | No studies provided evidence for this outcome. | |||||
| Health‐related quality of life ‐ not reported | No studies provided evidence for this outcome. | |||||
| Pain | Study authors did not provide quantitative analysis of pain, only a subjective assessment stating improvement of pain, itch, and paraesthesias in the collagen group. | ‐ | ‐ | ⊕⊝⊝⊝ Very low 1 2 | It is uncertain if lyophilised collagen decreases pain when compared with hyaluronic acid. | |
| Change in ulcer size | No studies provided evidence for this outcome. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded twice for risk of bias due to unclear risk of bias for randomisation, allocation, and blinding, and high risk of bias for attrition and selective reporting. 2Downgraded twice for imprecision due to small numbers of participants and events.
Summary of findings 4. Hyaluronic acid compared with conventional dressing (sterile petrolatum gauze) for foot ulcers in people with diabetes.
| Hyaluronic acid compared with conventional dressing (sterile petrolatum gauze) for foot ulcers in people with diabetes | ||||||
| Patient or population: foot ulcers in people with diabetes Setting: not reported Intervention: hyaluronic acid Comparison: conventional dressing/sterile petrolatum gauze | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with conventional dressing/sterile petrolatum gauze | Risk with hyaluronic acid | |||||
| Complete ulcer healing (12 weeks ‐ 84 days) | Study population | RR 2.20 (0.97 to 4.97) | 34 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 | It is uncertain if hyaluronic acid improves complete ulcer healing when compared with conventional dressing/sterile petrolatum. | |
| 294 per 1000 | 647 per 1000 (285 to 1000) | |||||
| Time to complete wound healing ‐ not reported | No studies provided evidence for this outcome. | |||||
| Adverse events ‐ not measured | Study authors reported severe adverse events in 1 case (5.9%) in the study group (infection followed by ray amputation) and 4 cases (23.5%) in the control group (2 amputations due to contralateral side infection, 1 cerebral vascular accident, 1 sepsis due to pneumonia). None of the events were considered to be related to the dressing material (e.g. infections were in contralateral side). | ‐ | ‐ | ⊕⊝⊝⊝ Very low 1 2 | It is uncertain if hyaluronic acid improves adverse events when compared with conventional dressing/sterile petrolatum. | |
| Health‐related quality of life ‐ not reported | No studies provided evidence for this outcome. | |||||
| Pain | No studies provided evidence for this outcome. | |||||
| Change in ulcer size | The mean change in ulcer size was 3.80 cm2. | The mean change in ulcer size was 3.00 cm2. | MD 0.80 cm2 lower (3.58 lower to 1.98 higher) | 25 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 | It is uncertain whether there is a difference in mean change in ulcer size between hyaluronic acid and conventional dressings. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded twice for risk of bias due to unclear risk of bias for allocation and blinding, and high risk of attrition bias. 2Downgraded twice for imprecision due to small numbers of participants and events and wide confidence intervals.
Summary of findings 5. Hyaluronic acid + hydrocolloid compared with hydrocolloid for leg ulcers.
| Hyaluronic acid + hydrocolloid compared with hydrocolloid for leg ulcers | ||||||
| Patient or population: people with leg ulcers Setting: inpatients or outpatients Intervention: hyaluronic acid + hydrocolloid Comparison: hydrocolloid | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with hydrocolloid | Risk with hyaluronic acid + hydrocolloid | |||||
| Complete ulcer healing (42 days) | Study population | RR 0.98 (0.26 to 3.76) | 125 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 | It is uncertain if hyaluronic acid + hydrocolloid affects complete ulcer healing when compared with hydrocolloid. | |
| 65 per 1000 | 63 per 1000 (17 to 243) | |||||
| Time to complete wound healing ‐ not reported | No studies provided evidence for this outcome. | |||||
| Adverse events | Study population | RR 0.79 (0.22 to 2.80) | 125 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 | It is uncertain if there is a difference in adverse events between hyaluronic acid + hydrocolloid and hydrocolloid. | |
| 81 per 1000 | 64 per 1000 (18 to 226) | |||||
| Health‐related quality of life ‐ not reported | No studies provided evidence for this outcome. | |||||
| Pain (VAS, mm) at follow‐up | The mean score was 10.0. | The mean score was 12.1. | MD 2.10 (5.81 lower to 10.01 higher) | 125 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 | It is uncertain if there is a difference in pain between treatments. |
| Change in ulcer size to at least 90% | Study population | RR 2.11 (0.92 to 4.82) | 125 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 | It is uncertain if there is a difference in change in ulcer size between treatments. | |
| 113 per 1000 | 238 per 1000 (104 to 544) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; VAS: visual analogue scale | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded twice for risk of bias due to high risk of bias for blinding of participants and personnel and outcome assessment. 2Downgraded twice for imprecision due to small numbers of participants and wide confidence intervals.
Summary of findings 6. Hyaluronic acid compared with hydrocolloid for leg ulcers.
| Hyaluronic acid compared with hydrocolloid for leg ulcers | ||||||
| Patient or population: people with leg ulcers Setting: general clinic (20 centres) Intervention: hyaluronic acid Comparison: hydrocolloid | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with hydrocolloid | Risk with hyaluronic acid | |||||
| Complete ulcer healing Follow‐up: 56 days | Data on complete wound healing were not properly presented at the endpoint (56 days). There was only a citation relating to 27 dropouts, including 12 due to ulcer healing, without specifying to which groups they belonged. | ‐ | 143 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 | It is uncertain if there is a difference in complete wound healing between treatments. | |
| Time to complete wound healing ‐ not reported | No studies provided evidence for this outcome. | |||||
| Adverse events Follow‐up: 56 days | The study report states that 77 adverse events were reported in 42 participants during the study; however, most of them were not localised to the ulcer. | ‐ | 143 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 | It is uncertain if there is a difference in adverse events between treatments. | |
| Health‐related quality of life ‐ not reported | No studies provided evidence for this outcome. | |||||
| Pain | No studies provided evidence for this outcome. | |||||
| Change in ulcer size > 40% | Study population | RR 1.02 (0.84 to 1.25) | 143 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 | It is uncertain if there is a difference in change in ulcer size between treatments. | |
| 718 per 1000 | 736 per 1000 (602 to 900) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded twice for risk of bias due to unclear risk of bias for allocation and high risk of bias for blinding of participants and personnel. 2Downgraded twice for imprecision due to small numbers of participants and events and wide confidence intervals.
Summary of findings 7. Hyaluronic acid compared with paraffin gauze for leg ulcers.
| Hyaluronic acid compared with paraffin gauze for leg ulcers | ||||||
| Patient or population: leg ulcers Setting: not reported Intervention: hyaluronic acid Comparison: paraffin gauze | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with paraffin gauze | Risk with hyaluronic acid | |||||
| Complete ulcer healing (56 days) | Study population | RR 2.00 (0.21 to 19.23) | 17 participants; 24 ulcers (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 | It is uncertain if hyaluronic acid increases ulcer healing when compared with paraffin gauze. | |
| 83 per 1000 | 167 per 1000 (17 to 1000) | |||||
| Time to complete wound healing ‐ not reported | No studies provided evidence for this outcome. | |||||
| Adverse events ‐ not reported | No studies provided evidence for this outcome. | |||||
| Health‐related quality of life ‐ not reported | No studies provided evidence for this outcome. | |||||
| Pain | No studies provided evidence for this outcome. | |||||
| Change in ulcer size | Study authors reported mean improvement in ulcer healing at 8 weeks; however, they did not provide standard deviations, thereby precluding further analysis. | ‐ | ‐ | ⊕⊝⊝⊝ Very low 1 2 | It is uncertain if hyaluronic acid improves ulcer healing when compared with paraffin gauze. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded twice for risk of bias due to unclear risk of bias for randomisation and allocation and high risk of bias for blinding of participants and personnel and outcome assessment and other bias. 2Downgraded twice for imprecision due to small numbers of participants and events and wide confidence intervals.
Summary of findings 8. Hyaluronic acid compared with neutral vehicle for leg ulcers.
| Hyaluronic acid compared with neutral vehicle for leg ulcers | ||||||
| Patient or population: people with leg ulcers Setting: patients' homes and care facilities; general centres Intervention: hyaluronic acid Comparison: neutral vehicle | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with neutral vehicle | Risk with hyaluronic acid | |||||
| Complete wound healing (60 days) | Study population | RR 2.11 (1.46 to 3.07) | 526 (4 RCTs) | ⊕⊕⊕⊝ Moderate 1 | Hyaluronic acid probably improves complete ulcer healing when compared with neutral vehicle. | |
| 130 per 1000 | 267 per 1000 (184 to 388) | |||||
| Time to complete wound healing |
Dereure 2012a, Mikosinski 2021a, and Mikosinski 2021b did not report this outcome. The authors of Humbert 2013 stated: "Other performance secondary endpoints (time to complete ulcer healing and global performance) were comparable between treatment groups, at any visit". However, no numbers were provided. |
‐ | 89 (1 RCT) | ⊕⊝⊝⊝ Very low 2 3 | It is uncertain if hyaluronic acid improves time to complete ulcer healing when compared with neutral vehicle. | |
| Adverse events ‐ incidence of infection | 122 per 1000 | 109 per 1000 (65 to 182) | RR 0.89 (0.53 to 1.49) | 425 (3 RCTs) | ⊕⊝⊝⊝ Very low 1 3 | It is uncertain if hyaluronic alters the incidence of infection when compared with neutral vehicle. |
| Health‐related quality of life ‐ not reported | No studies provided evidence for this outcome. | |||||
| Pain (VAS, mm), reduction from baseline | ‐ | MD 8.55 lower (14.77 lower to 2.34 lower) | ‐ | 337 (3 RCTs) | ⊕⊕⊝⊝ Low 1 4 | Hyaluronic acid may slightly increase reduction in pain from baseline when compared with neutral vehicle. |
| Change in ulcer size (45 days) | ‐ | MD 30.44 higher (15.57 higher to 45.31 higher) | ‐ | 190 (2 studies) | ⊕⊝⊝⊝ Low 1 4 | Hyaluronic acid may slightly increase change in ulcer size when compared with neutral vehicle. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; VAS: visual analogue scale | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded once for risk of bias due to unclear blinding of participants and personnel and high risk of attrition bias in one study. 2Downgraded twice for risk of bias. 3Downgraded twice for imprecision due to small sample size and wide or unknown confidence intervals. 4Downgraded once for imprecision due to small numbers of participants.
Summary of findings 9. Hyaluronic acid compared with dextranomer for leg ulcers.
| Hyaluronic acid compared with dextranomer for leg ulcers | ||||||
| Patient or population: people with leg ulcers Setting: hospitalised patients Intervention: hyaluronic acid Comparison: dextranomer | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with dextranomer | Risk with hyaluronic acid | |||||
| Complete ulcer healing ‐ not reported | No studies provided evidence for this outcome. | |||||
| Time to complete wound healing ‐ not reported | No studies provided evidence for this outcome. | |||||
| Adverse events | "There were five reports of side‐effects (local pain, a local burning sensation, panniculitis and a prickling sensation) in the HA group and two reports in the dextranomer group (surrounding eczema and local pain)." 1 participant (hyaluronic acid group) dropped out due to the onset of pain and a burning sensation. We were not able to estimate the rate of specific adverse events between groups. | ‐ | 50 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 | It is uncertain if hyaluronic acid increases adverse events compared with dextranomer. | |
| Health‐related quality of life ‐ not reported | No studies provided evidence for this outcome. | |||||
| Pain | No studies provided evidence for this outcome. | |||||
| Change in ulcer size (21 days) | The mean change was 4.2. | The mean change was 10.0. | MD 5.80 higher (10 lower to 21.60 higher) | 50 (1 RCT) | ⊕⊝⊝⊝ Very low 1 2 | It is uncertain if hyaluronic acid promotes a greater change in ulcer size when compared with dextranomer. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded once for risk of bias due to unclear risk of bias for randomisation, allocation concealment, and blinding of participants and personnel and outcome assessment. 2Downgraded twice for imprecision due to small numbers of participants and wide confidence intervals.
Background
Description of the condition
For definitions of technical terms, please see the glossary in Appendix 1.
Chronic wounds are wounds that "fail to proceed through an orderly and timely process to produce anatomic and functional integrity" (Lazarus 1994). Wound healing comprises a chronological sequence of four independent and overlapping steps: haemostasis (cessation of bleeding); inflammation; cell proliferation; and remodelling (Lazarus 1994; Schultz 2003). These steps involve many types of cells including fibroblasts and macrophages, as well as biochemical factors such as endogenous hyaluronic acid (HA) and metalloproteases of extracellular matrix (Chen 1999). Many factors influence the healing of chronic wounds including aetiology, comorbidities, nutrition, immobility, and medication.
Chronic wounds can be painful and infected (Schultz 2003; Velasco 2011). High levels of pain affect quality of life, Dias 2013; Siersma 2014, and people's ability to work and perform activities of daily living (Dumville 2013; O'Meara 2013). Treatment of chronic wounds includes different types of wound dressing and topical agents, with a variety of aims including maintenance of a moist healing environment (e.g. films, foam, hydrocolloids, alginates, hydrogel); reduction in bacterial load and infection (e.g. dressings and topical agents containing silver or iodine) (Bradley 1999; Powers 2013; Velasco 2011); or dressings and topical agents aiming to support healing that contain collagen, cellulose, and other factors (Velasco 2011).
Chronic wounds arise for a variety of reasons and usually in response to underlying disease (e.g. diabetes, venous disease, arterial disease, neurological conditions) or severe injury (e.g. burns, trauma, surgery) (Schultz 2003). Diabetic foot wounds and venous and pressure ulcers account for approximately 90% of chronic wounds (Kirketerp‐Møller 2011; Mustoe 2006). This review focuses on the treatment of pressure ulcers, leg ulcers, and diabetic foot ulcers.
Venous and arterial ulcers
Venous ulcers occur as a result of an impairment of venous return due to problems of the venous circulation in the legs (e.g. from deep venous thrombosis). In the UK, the prevalence of venous ulcers has been estimated at between 1 and 3 per 1000, and the figure is similar in Ireland (Agale 2013). Worldwide, the cost of venous ulcer treatments is higher than USD 1000 million (Margolis 2013). The standard, effective treatment for venous leg ulcers is compression therapy (O'Meara 2012); however, wound dressings are also used with the aim of promoting a healing environment, protecting the wound, absorbing exudate, and reducing infection.
Arterial ulcers affect approximately 1% of North Americans and develop due to impaired blood flow to the tissues, typically as a result of peripheral vascular disease (Collins 2010; Lazarus 2014; Porter 1995; Velasco 2011). The main treatment aim is to restore blood flow by revascularisation; however, wound dressings are used with the aim of protecting and healing the wound.
Foot ulcers in people with diabetes
Approximately 15% of people with diabetes will present a foot ulcer at some time in their lives (Barshes 2013; Boike 2017; Jeffcoate 2003). The US Centers for Disease Control and Prevention (CDC) have estimated that approximately 13% of people with diabetes have foot ulcers (CDC 2003), and the global costs of treating people with these wounds are high. In Sweden, the cost of treating foot infections in people with diabetes ranged from USD 30,000 without amputation to USD 58,000 with amputation (Peters 2013; Tennvall 2000). In England (2010/2011), the NHS spent GBP 639 to 662 million on the management of diabetic foot ulcers, representing approximately 0.7% of its budget (Kerr 2012). Wound dressings are used for the same reasons as described above, alongside removal of pressure and revascularisation where appropriate.
Pressure ulcers
Pressure ulcers are wounds that occur due to prolonged pressure, alone or in combination with shear. Risk factors for pressure ulcer development include immobility, poor nutrition, poor tissue perfusion, sensory impairment, and older age. Pressure ulcers are classified according to the depth of tissue affected (NPUAP/EPUAP/PPPIA 2014).
The prevalence of pressure ulcers varies according to the place where caring is provided, whether in hospitals, community, or long‐term facilities and depending on associated comorbidities (Amir 2013; Chen 2011; Gunningberg 2013). In a study in the USA, pressure ulcer prevalence was between 10% and 18% in acute care (including critical care and surgical rooms) and up to 29% in home care support services (Cuddigan 2001). In the Netherlands, the cost of pressure ulcer treatment varies from EUR 89 million to EUR 1900 million, or between 0.1% and 1% of the total amount spent by the Dutch health system (Makai 2010).
Description of the intervention
Karl Meyer discovered hyaluronic acid in 1930 (Meyer 1934). During the 1950s, Meyer and colleagues determined that hyaluronic acid was a linear polysaccharide (GlcNAc). It is a carbon hydrate (from disaccharide) that is easily dissolved, producing an aqueous gel (Nusgens 2010).
Hyaluronic acid is synthesised in the plasma membrane (Fraser 1997), and it can be found in extracellular tissues in many different concentrations (Collins 2013), mainly in articular fluids, tendon sheaths and bursae (Fraser 1997). It is involved with the lubrication, moisturising, and maintenance of tissue structure (Collins 2013; Pan 2013). Commercially produced hyaluronic acid comes from animal tissues (Oh 2010), and has growing importance in the development of biomaterial (Collins 2013). The British National Formulary (BNF) recognises sodium hyaluronate‐containing dressings, classifying them as a type of hydrogel for use directly in the wound or for application via a primary dressing (in both cases covered with a secondary dressing) (BNF 2017a). Hyaluronic acid can be combined with other dressing materials, such as hydrogel films (Boateng 2008), hydrocolloids (Zinoviev 2014), fibrin sheets (Anilkumar 2011), and alginates (Oh 2013). Sodium hyaluronate can be combined with antiseptics such as iodine to reduce bacterial load (BNF 2017a). It has been estimated that the annual global market of hyaluronic acid‐based products is approximately USD 1000 million (Pan 2013).
How the intervention might work
It has been suggested that the application of hyaluronic acid to chronic wounds may promote healing (Anilkumar 2011; Chen 1999), possibly via a role in the inflammation and granulation phases of healing (Chen 1999). One mechanism may be the ability of hyaluronic acid to maintain a moist wound environment that helps cell migration in the wound bed (e.g. migration of fibroblasts and endothelial cells). It has also been suggested that hyaluronic acid may reduce scarring and fibrosis and improve angiogenesis (Dicker 2014; Knudson 1993; Zhu 2006), and that it may have anti‐inflammatory effects (Chen 1999; Dicker 2014).
Why it is important to do this review
Chronic wounds are extremely common globally and costly to manage. Hyaluronic acid‐containing wound treatments may promote chronic wound healing, and a rigorous, comprehensive systematic review of relevant research is needed to determine their contribution to healing. A chronic wound is a complex clinical situation that causes considerable economic impact and adversely affects the quality of life of those affected. There is no current, rigorously derived summary of the evidence to inform clinicians of the effects of hyaluronic acid dressings in treating chronic wounds. This review systematically analyses data on the effects of hyaluronic acid on chronic wound healing.
Objectives
To evaluate the effects of hyaluronic acid (and its derivatives) on the healing of chronic wounds.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) that compared the effects of hyaluronic acid (as a dressing or topical agent) with no hyaluronic acid on the healing of pressure ulcers, venous, arterial or mixed‐aetiology leg ulcers and foot ulcers in people with diabetes. Studies were eligible irrespective of the language of publication. We excluded studies that used quasi‐random methods of allocation (e.g. alternation). For future updates, we plan to include cross‐over trials, but will only consider the effects of the first randomised intervention.
Types of participants
We included adults in any care setting (e.g. hospital patients, outpatients, long‐term care facilities, home care) who had pressure ulcers, leg ulcers (of venous, arterial, or mixed aetiology) or foot ulcers (including people with diabetes and foot ulcers). We accepted study authors' diagnostic criteria for wound aetiology. We analysed and presented data by each wound type separately. We planned to include trials that recruited people with different types of chronic wound (e.g. people with leg ulcers of different aetiologies or combined data from people with both leg and pressure ulcers). If the data were not presented separately by type of wound (or if trialists could not provide this information), we would analyse these studies grouped as 'mixed chronic wounds'; however, no studies fell into this category.
Types of interventions
The intervention of interest was any type of wound dressing or topical agent containing hyaluronic acid or any of its derivatives (hyaluronan‐based scaffold, hylan polymers, and sodium hyaluronate). We included studies comparing dressings or topical agents that contain hyaluronic acid with any other type of dressing, topical agent, placebo, or standard treatment. Only RCTs in which the presence or absence of a hyaluronic acid dressing was the only systematic difference between treatment groups were eligible. We also included studies comparing topical agents and dressings containing different concentrations or types of hyaluronic acid delivery. To simplify the comparisons, we categorised dressings according to the Nurse Prescribers’ Formulary (see Appendix 2) (BNF 2017b).
Types of outcome measures
Primary outcomes
Complete wound healing. We considered the proportion of ulcers healed during follow‐up, as presented by the trial authors.
Time to complete wound healing, correctly analysed using survival, time‐to‐event approaches, ideally with adjustment for relevant covariate such as the baseline size.
Adverse events (e.g. the presence of wound infection and signs and symptoms of clinical infection).
Secondary outcomes
Health‐related quality of life (measured using a standardised generic questionnaire such as EQ, 36‐item Short Form Health Survey (SF‐36), SF‐12, SF‐6 or a disease‐specific questionnaire).
Pain (e.g. at dressing change, between dressing changes, or over the course of treatment) was included only if measured by reliable and validated instruments such as surveys, questionnaires, data capture process, or visual analogue scales.
Wound recurrence rate (number of weeks or months without wounds, when available).
Change (and the rate of change) of the wound size and area, expressed as absolute changes (e.g. changes of surface area in cm2 since baseline) or relative changes (e.g. a percentage change in the area relative to baseline).
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to identify reports of relevant clinical trials:
Cochrane Wounds Specialised Register (searched 10 February 2022);
Cochrane Central Register of Controlled Trials (CENTRAL; 2022, Issue 1) in the Cochrane Library (searched 10 February 2022);
MEDLINE Ovid including In‐Process & Other Non‐Indexed Citations (1946 to 10 February 2022);
Embase Ovid (1974 to 10 February 2022);
CINAHL Plus EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to 10 February 2022).
The search strategies for the Cochrane Wounds Specialised Register, CENTRAL, MEDLINE Ovid, Embase Ovid, and CINAHL Plus EBSCO can be found in Appendix 3. In MEDLINE Ovid we combined the subject‐specific strategy with the sensitivity‐ and precision‐maximising version of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (2008 revision) (Lefebvre 2021). We combined the Embase Ovid search with the Embase Ovid filter developed by Cochrane UK (Lefebvre 2021). We combined the CINAHL Plus EBSCO search with the trial filter developed by Glanville 2019. There were no restrictions with respect to language, date of publication, or study setting.
We also searched the following clinical trials registries:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 15 February 2022);
World Health Organization International Clinical Trials Registry Platform (www.who.int/clinical-trials-registry-platform; searched 15 February 2022);
EU Clinical Trials Register (www.clinicaltrialsregister.eu/ctr-search/search; searched 13 May 2022).
Search strategies for clinical trial registries can be found in Appendix 3.
Searching other resources
We attempted to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, as well as relevant systematic reviews, meta‐analyses, and health technology assessment reports.
We contacted wound care specialists and manufacturers of dressings and topical agents containing hyaluronic acid to obtain data on unpublished studies or studies in progress. When necessary, we contacted authors of key papers and abstracts to request further information.
We did not perform a separate search for adverse effects of dressings or topical agents containing hyaluronic acid. We considered adverse effects described in the included studies only.
Data collection and analysis
We performed data collection and analysis according to methods stated in the published protocol (Roehrs 2016), which were based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022).
Selection of studies
Two review authors (HR and GB) independently assessed the titles and abstracts of studies identified by the searches for potential relevance. Any disagreements were discussed during consensus meetings with a third review author (JGDS). Two review authors (HR and GB) examined the full‐text reports of those studies deemed potentially relevant. Studies that fulfilled the eligibility criteria were included in the review. Any disagreements were discussed at consensus meetings with a third review author (JGDS).
When more than one publication was linked to the same study, all the papers were included, with one marked as the primary source of information. We extracted data from all the papers (maximal data extraction), taking care not to double‐count participants.
Data extraction and management
Two review authors (HR and MJM) independently collected data using predefined forms. In the case of missing information, we contacted the study authors.
We extracted the following data.
Research design
Care setting (e.g. hospital, long‐term care home)
Country of origin
Publication source
Year of publication
Duration of follow‐up
Sources of funding
Unit of randomisation
Unit of analysis
Inclusion criteria and exclusion criteria
Participants
Characteristics of the examined group (number of participants; sex; age)
Details of the intervention
Co‐interventions
Duration of treatment
Primary outcomes and secondary outcomes
Losses to follow‐up
Assessment of risk of bias in included studies
Two review authors (JGDS and FP) independently assessed the methodological quality of the included studies using Cochrane's risk of bias assessment tool (Higgins 2017), and we compiled a risk of bias table for each eligible study. The tool addresses six specific domains, namely, sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias) and blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other issues (e.g. extreme baseline imbalance or inappropriate administration of the intervention). We also included conflicts of interest as part of this last domain; however, as recommended in the Cochrane Handbook, we considered its impact as a whole in the study design and risk of bias, and did not restrict its impact to this specific domain (Boutron 2022). See Appendix 4 for details of the criteria on which the risk of bias assessment was based. We classified each domain as being at a low, high, or unclear risk of bias. We considered a trial to be at high overall risk of bias if any of the following three key criteria were not met: adequate sequence generation, adequate allocation concealment, and blinding of outcome assessors. We considered that all outcomes were equally impacted by unblinded assessment and incompleteness of outcome data.
Any disagreements between review authors were discussed and consensus was achieved during the final assessment.
We presented the risk of bias assessment using a risk of bias summary figure, presenting all evaluations in a cross‐tabulation of the study by entry. Where possible, when the absence of reported information prevented a clear decision, we contacted the trial authors for clarification.
Measures of treatment effect
We extracted data to calculate summary measures, and where these were not available we extracted summary measures as reported. We pooled data according to wound type. We presented dichotomised data (e.g. complete healing) as a risk ratio (RR) with a 95% confidence interval (CI). We used RR rather than odds ratio (OR) because OR (when interpreted as RR) can give an inflated impression of the effect size when event rates are high, as is the case for many trials of treatments of chronic wounds (Deeks 2002). We expressed continuous data (e.g. a reduction in the wound size or area) as mean differences (MD) with 95% CI. For future updates, we will attempt to calculate standardised mean differences (SMD) from measures of the same outcome when different methods were used to collect data (e.g. health‐related quality of life) (Deeks 2022). In trials that did not present data for change in pain from baseline, but presented data for pain at baseline and at the end of treatment, we estimated the change in pain calculating the MD between these time points and therefore comparing treatment groups; however, caution is advised in interpreting the results because samples were treated as independent.
Unit of analysis issues
We recorded whether trials measured outcomes in relation to an ulcer, a foot, a participant, or whether multiple ulcers on the same participant were studied (Dumville 2013). Where studies were randomised at the participant level and outcomes measured at the wound level, we treated the participant as the unit of analysis when the number of wounds assessed appeared to be equal to the number of participants (e.g. one wound per person). In cases where the included studies contained some clustered data (randomisation carried out at the participant level, with the allocated treatment used on multiple wounds per participant, but data presented and analysed per wound), we reported this, noting whether data had been (incorrectly) treated as independent. We did not include these data in meta‐analyses but reported them separately. We recorded this as part of the risk of bias assessment. For the outcomes adverse effects and pain, we treated the participant as the unit of analysis when the number of wounds assessed appeared to be equal to the number of participants (e.g. one wound per person).
Dealing with missing data
Missing data in trials of low quality are common. Randomisation may be compromised when participants are excluded from the postrandomisation analysis, or when participants are lost during follow‐up. In the case of missing data, we contacted the original investigators to request the information where possible. No additional data were provided by study authors, therefore no data inclusions are reported. In individual studies that presented data on the proportion of healed wounds, we assumed that if the randomised participant was not included in the analysis, there was no ulcer healing (the person was considered in the denominator but not in the numerator). If a study did not specify the number of participants in groups before dropout, then only complete data were presented. Secondary outcomes were presented as complete‐case analysis. For studies that presented SEM (standard errors of mean), we calculated the value of the SD (standard deviation) using SD = SEM x sqrt(n).
Assessment of heterogeneity
We considered clinical heterogeneity (participant characteristics, outcome definitions, interventions, and evaluation of results) and methodological details (variability in study quality and risk of bias) of the included studies. We supplemented this assessment of clinical and methodological heterogeneity with information regarding statistical heterogeneity, which we evaluated using the Chi2 test. We considered a P value of less than 0.10 as indicative of statistically significant heterogeneity given that the Chi2 test has low power, particularly in the case where studies included in a meta‐analysis have small sample size. We carried out this statistical assessment in conjunction with the I2 statistic, considering that I2 values of 25% or less may indicate a low level of heterogeneity, and values of 75% or more may indicate very high heterogeneity (Higgins 2003).
Assessment of reporting biases
Had more than 10 studies been included in any meta‐analysis, we would have attempted to check for the existence of publication bias by constructing a funnel plot. For future updates, if we detect evidence of asymmetry, we will explore possible explanations, such as publication bias, selective outcome reporting, poor methodological design, inadequate analysis, and true heterogeneity (Page 2022).
Data synthesis
We presented a narrative overview of the studies reviewed, and synthesised included data by using meta‐analysis where applicable employing Review Manager 5 (Review Manager 2020). We grouped the included trials by type of chronic wound and by intervention versus comparison (e.g. hyaluronic acid compared with dressings, or topical treatments containing hyaluronic acid with any other type of dressing or topical agent, or with placebo or standard treatment). We considered clinical and methodological heterogeneity and undertook pooling when studies appeared appropriately similar in terms of participants, type of wound, intervention, and outcome type. We pooled results using a random‐effects model and reported the pooled estimate together with its 95% CI. Conducting meta‐analysis with a fixed‐effect model in the presence of even minor heterogeneity may provide overly narrow CIs. We planned only to use a fixed‐effect approach when clinical and methodological heterogeneity was found to be minimal. We used Chi² and I² to quantify heterogeneity, but did not use these statistics to guide the choice of a model for meta‐analysis. For dichotomous outcomes, we presented the summary estimate as an RR with 95% CI. Where continuous outcomes were measured, we presented an MD with 95% CI. We planned to pool SMD estimates where studies measured the same outcome using different methods, such as health‐related quality of life data; however, this outcome was not reported in the included studies.
Subgroup analysis and investigation of heterogeneity
Leg ulcers are mainly due to venous leg insufficiency. Compression therapy during venous leg ulcer (VLU) treatment is strongly recommended. We therefore planned to carry out subgroup analyses according to the presence or absence of compression therapy, independent of type (elastic or inelastic) or level (moderate or high) in trials including ulcers from venous aetiology. However, all four trials combined for the meta‐analysis that investigated hyaluronic acid compared with neutral vehicle used compression as a standard treatment, therefore we did not perform subgroup analyses. For future updates, we will compare the magnitude of effect on the primary outcomes between a subset of studies that applied compression to a subset of studies where no compression was used. We will assess the magnitude of effect analysing the CIs of the summary estimates in the two subgroups (Section 9.6.3.1; Higgins 2017). If the presence or absence of compression therapy is not clearly indicated in a trial report, we will not include these trials in this subgroup analysis.
Sensitivity analysis
We planned to perform sensitivity analyses for each comparison that had a meta‐analysis according to the risk of bias of each RCT to assess the effect on the overall estimate of excluding studies with high risk of bias (those classified as high risk of bias in any of the three key domains: generation of random sequence, adequate allocation concealment, and blinding of outcome assessor). However, none of the trials combined for meta‐analysis presented high risk of bias for the above‐mentioned domains, therefore we did not perform sensitivity analyses.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE system to assess the certainty of evidence, size of interventions, and the sum of available data for the main results. We carried out a GRADE assessment on all eligible outcomes where possible and included complete wound healing, time to complete wound healing, adverse events, health‐related quality of life, pain, and change in ulcer size in the summary of findings tables (see Differences between protocol and review). This allowed a more comprehensive assessment of important outcomes that may impact decision‐making in health care. The GRADE approach defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the quantity in question. The assessment of the certainty of a body of evidence involves consideration of the within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates, and risk of the publication bias (Schünemann 2022). For the risk of bias domain, we downgraded one level if studies presented unclear risk of bias for all outcomes, and one or two levels when studies were assessed as at high risk of bias for one or more domains (Schünemann 2022). We followed the methods described by Guyatt and colleagues when downgrading for imprecision: either considering both the optimal information size (OIS) and the 95% CI of each meta‐analysis if they were estimable, or considering the sample size, the number of events, and other eIectiveness indicators if the calculation of OIS and undertaking a meta‐analysis were not applicable (Guyatt 2011). We downgraded twice for imprecision when there were very few events and CIs around effects included both appreciable benefit and appreciable harm. The results of the review are presented in summary of findings tables.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies.
Results of the search
Our database searches resulted in 207 records. We also identified 19 additional possible inclusions from checking the reference lists of included trials. After removing 18 duplicates, we assessed the title and abstracts of 208 records.
Full‐text screening of 25 records led to the identification of 13 reports from 12 studies. We therefore included 12 trials (13 articles) in qualitative analysis. We were able to combine data from four trials for quantitative analysis (see Figure 1).
1.
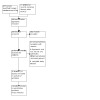
Study flow diagram.
Included studies
We included 13 publications originating from 12 RCTs, dating from 1991 to 2021, in the review (Dereure 2012a; Dereure 2012b; Di Mauro 1991; Felzani 2011; Humbert 2013; Lee 2016; Meaume 2008; Mikosinski 2021a; Mikosinski 2021b; Ortonne 1996; Ramos‐Torrecillas 2015; Taddeucci 2004).
See Characteristics of included studies and Table 10 for further details.
1. Summary of comparisons.
| Comparison | Number of studies | Number of randomised participants | Number of ulcers |
| Pressure ulcer | |||
| Lysine hyaluronate vs sodium hyaluronate | 1 | 50 | 54 |
| PRGF + HA vs PRGF | 1 | 115 | 124 |
| Foot ulcer | |||
| HA vs LC | 1 | 20 | 20 |
| HA vs conventional dressing (sterile petrolatum gauze) | 1 | 34 | 34 |
| Leg ulcer | |||
| HA + HC vs HC | 1 | 125 | 125 |
| HA vs HC | 1 | 170 | 170 |
| HA vs paraffin gauze | 1 | 17 | 24 |
| HA vs neutral vehicle | 4 | 526 | 526 |
| HA vs dextranomer | 1 | 51 | 51 |
HA: hyaluronic acid; HC: hydrocolloid; LC: lyophilised collagen; PRGF: platelet‐rich growth factor
Study design and settings
The majority of included studies used a parallel‐group design. One trial had four arms that included a control group (standard care including hydrogel), a group receiving one dose of platelet‐rich growth factor (PRGF), a group receiving two doses of PRGF, and a group receiving two doses of PRGF + hyaluronic acid (Ramos‐Torrecillas 2015); we were therefore able to extract data for comparison from the group treated with two doses of PRGF and the group treated with two doses of PRGF + hyaluronic acid (as hyaluronic acid was the only systematic difference between these two groups). Seven studies were multicentre (Dereure 2012a; Dereure 2012b; Humbert 2013; Meaume 2008; Mikosinski 2021a; Mikosinski 2021b; Ortonne 1996), and five were single‐centre RCTs (Di Mauro 1991; Felzani 2011; Lee 2016; Ramos‐Torrecillas 2015; Taddeucci 2004).
The studies including people with pressure ulcers were conducted in Spain, Ramos‐Torrecillas 2015, and Italy, Felzani 2011. The patient care setting was a long‐stay hospital and four geriatric centres in Ramos‐Torrecillas 2015 and a hospital in Felzani 2011. The studies including people with diabetic foot ulcers were conducted in South Korea as reported by Lee 2016, and assumed to be carried out in Italy due to the affiliations of Di Mauro 1991; care settings were not described. Studies involving people with leg ulcers were conducted in France and Poland in Dereure 2012a, Dereure 2012b, Mikosinski 2021a, and Mikosinski 2021b; France, Italy, and Switzerland in Meaume 2008; Italy in Taddeucci 2004; France, Morocco, and Poland in Humbert 2013; and France in Ortonne 1996. Trials included inpatients and outpatients (Dereure 2012a; Dereure 2012b; Meaume 2008); only outpatients (Mikosinski 2021a; Mikosinski 2021b; Taddeucci 2004); only hospitalised patients (Ortonne 1996); and people in home and care facilities (Humbert 2013).
Types of participants
A total of 1108 participants were randomised from sample sizes ranging from 17 participants, Taddeucci 2004, to 170 participants, Dereure 2012b. Of 1022 participants in RCTs that reported sex, 585 were female (57.24%) and 437 were male (42.76%). Mean age corresponded to 69.60 years and was calculated from 1009 participants from studies that provided participant age. Participants presented 178 pressure ulcers, 54 diabetic foot ulcers, and 896 leg ulcers.
Severity of pressure ulcers were stages (European Ulcer Advisory Panel) I to III in Felzani 2011 and stages II and III in Ramos‐Torrecillas 2015. In trials involving people with diabetic foot ulcers, Lee 2016 described minimal size ≥ 1 cm2 and at least six weeks of duration, while Di Mauro 1991 did not specify severity or chronicity. Trials involving people with leg ulcers recruited participants with ulcers present for at least two months and with an initial area ranging from 3 to 12 cm2, in Taddeucci 2004, to 5 to 40 cm2 (Dereure 2012a; Dereure 2012b; Humbert 2013; Meaume 2008; Mikosinski 2021a; Mikosinski 2021b). Trials included leg ulcers of venous aetiology (Ortonne 1996; Taddeucci 2004), or of venous and mixed aetiologies (venous and arterial, with a predominant venous component, i.e. volunteers with ankle‐brachial index > 0.8) (Dereure 2012a; Dereure 2012b; Humbert 2013; Meaume 2008; Mikosinski 2021a; Mikosinski 2021b).
Types of interventions
Pressure ulcers
One four‐arm study investigated the effects of a PRGF and hyaluronic acid (Ramos‐Torrecillas 2015).
Another study used lysine hyaluronate (Lys‐HA) (Lysial) as an alternative to the more commonly used salt sodium hyaluronate (Felzani 2011). Study duration was 36 days in Ramos‐Torrecillas 2015 and 15 days in Felzani 2011.
Foot ulcers
Di Mauro 1991 compared hyaluronic acid medicated gauze with lyophilised collagen, and Lee 2016 compared the effects of hyaluronic acid dressing with conventional moisture‐retentive dressing (sterile petrolatum gauze). Participants were followed up for 12 weeks in Lee 2016 and to wound healing in Di Mauro 1991.
Leg ulcers
The dressings comparisons evaluated by the included RCTs were as follows.
Hyaluronic acid + hydrocolloid compared with hydrocolloid alone (Meaume 2008).
Hyaluronic acid‐impregnated compared with hydrocolloid (Dereure 2012b).
Hyaluronic acid (Hyalofill‐F) compared with paraffin gauze (Taddeucci 2004).
Hyaluronic acid compared with neutral vehicle (Dereure 2012a; Humbert 2013; Mikosinski 2021a; Mikosinski 2021b).
Hyaluronic acid gauze pad impregnated (0.05% sodium hyaluronate) compared with dextranomer paste (Ortonne 1996).
Study duration was 56 days or until complete healing in Dereure 2012b; 42 days in Meaume 2008; 8 weeks or until the ulcer healed (whichever occurred first) in Taddeucci 2004; 60 days or until complete healing in Dereure 2012a and Humbert 2013; 21 days in Ortonne 1996; and 23 weeks in Mikosinski 2021a and Mikosinski 2021b.
Funding sources
Eight studies received full or partial funding from pharmaceutical companies that produced the dressing (Dereure 2012a; Dereure 2012b; Humbert 2013; Lee 2016; Meaume 2008; Mikosinski 2021a; Mikosinski 2021b; Ortonne 1996). The other four trials did not report funding sources (Di Mauro 1991; Felzani 2011; Ramos‐Torrecillas 2015; Taddeucci 2004).
Excluded studies
We excluded 12 studies for the following reasons (see Characteristics of excluded studies): four studies were not RCTs (Edmonds 2000; Galasso 1978; Mekkes 2001; Prosdocimi 2012), and eight studies had an ineligible study design (i.e. hyaluronic acid was not the only systematic difference between treatment groups) (Abbruzzese 2009; Caravaggi 2003; Caridi 2016; Cuevas 2007; Maggio 2012; Romanelli 2007; Uccioli 2011; You 2014).
Ongoing studies
We did not identify any ongoing studies.
Studies awaiting classification
We did not identify any studies awaiting classification.
Risk of bias in included studies
A summary of the risk of bias assessment is presented in Figure 2 and Figure 3 and Characteristics of included studies.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Generation of the randomisation sequence
All 12 studies were described as randomised; however, only eight of these studies reported using an appropriate method to generate the randomisation sequence and were therefore assessed as at low risk of bias (Dereure 2012a; Dereure 2012b; Humbert 2013; Lee 2016; Meaume 2008; Mikosinski 2021a; Mikosinski 2021b; Ramos‐Torrecillas 2015). Six studies used a randomisation list that was prepared using validated SAS software (Institute Inc) (Dereure 2012a; Dereure 2012b; Humbert 2013; Lee 2016; Mikosinski 2021a; Mikosinski 2021b), while Meaume 2008 and Ramos‐Torrecillas 2015 reported using a computer‐generated randomisation sequence. The remaining four studies did not specify the method of randomisation and were assessed as at unclear risk of bias (Di Mauro 1991; Felzani 2011; Ortonne 1996; Taddeucci 2004).
Concealment of the allocation process
Eight trials did not provide a clear description of allocation concealment and were therefore assessed as at unclear risk of bias (Dereure 2012b; Di Mauro 1991; Felzani 2011; Humbert 2013; Lee 2016; Ortonne 1996; Ramos‐Torrecillas 2015; Taddeucci 2004). In one RCT (Meaume 2008), sealed envelopes containing the treatment code for each individual patient were given to the investigator at each centre. The investigator could only open the envelope after having included a patient in the study, and would only then know to which treatment group that patient had been allocated. However, the authors did not describe if the envelopes were sequentially numbered and opaque, therefore we also judged this trial as at unclear risk of bias. Three studies stated that the groups were allocated according to a central randomisation list and were thus assessed as at low risk of bias (Dereure 2012a; Mikosinski 2021a; Mikosinski 2021b).
Blinding
Performance bias
We assessed four RCTs as being at unclear risk of bias because they did not provide details regarding blinding of participants or personnel (Di Mauro 1991; Humbert 2013; Lee 2016; Ortonne 1996). We assessed four studies as being at low risk of bias because the products used in the intervention were provided in identical containers, shape, and texture in order to maintain double‐blinding (Dereure 2012a; Felzani 2011; Mikosinski 2021a; Mikosinski 2021b). We assessed four RCTs as being at high risk of bias for this domain (Dereure 2012b; Meaume 2008; Ramos‐Torrecillas 2015 and Taddeucci 2004), either because they were open‐label studies (Meaume 2008; Ramos‐Torrecillas 2015; Taddeucci 2004), or because blinding was not possible due to the different appearance of the treatments (Dereure 2012b).
Detection bias
We assessed two studies as being at unclear risk of bias either because information about the blinding of outcome assessors was lacking, or because the information provided was insufficient to permit a judgement (Di Mauro 1991; Ortonne 1996). Seven trials reported blinding of the outcome assessor and were judged as being at low risk of bias (Dereure 2012a; Dereure 2012b; Felzani 2011; Humbert 2013; Lee 2016; Mikosinski 2021a; Mikosinski 2021b). Three studies were open‐label studies with no blinding and were therefore assessed as at high risk of bias (Meaume 2008; Ramos‐Torrecillas 2015; Taddeucci 2004).
Incomplete outcome data
We assessed six RCTs as being at low risk of bias (Dereure 2012a; Dereure 2012b; Felzani 2011; Meaume 2008; Mikosinski 2021a; Ortonne 1996). We assessed five RCTs as at high risk of bias because they did not report withdrawals; had high numbers of losses to follow‐up; and because some participants did not complete the full treatment (Di Mauro 1991; Humbert 2013; Lee 2016;Ramos‐Torrecillas 2015; Taddeucci 2004). In one trial, there was inconsistency in the numbers and reasons for dropouts, therefore we judged this trial to be at unclear risk of bias for this domain (Mikosinski 2021b).
Selective reporting
We assessed 10 RCTs as being at low risk for this domain (Dereure 2012a; Dereure 2012b; Humbert 2013; Lee 2016; Meaume 2008; Mikosinski 2021a; Mikosinski 2021b; Ortonne 1996; Ramos‐Torrecillas 2015; Taddeucci 2004). We were able to obtain the protocol from two studies (Humbert 2013; Lee 2016). Protocols for the other studies were not available; however, by assessing data from published articles we were able to confirm that all planned outcomes described in the methods section were reported in the results section.
We assessed two studies as being at high risk of bias (Di Mauro 1991; Felzani 2011). In Felzani 2011, the authors did not present mean (or corrected mean by covariate) and a measure of variability such as SD for ulcer area and percentage change in wound area, nor did they present measurement of statistical variability for time to reach 50% wound healing. Di Mauro 1991 did not mention any methods for quantification of symptoms such as pain or paraesthesia, nor were pain and paraesthesia described as measured outcomes; however, in the results section the authors state: “In the group treated with collagen, a significant improvement was shown in symptoms such as reduction of pain, itch and paraesthesia.”
Other potential sources of bias
We assessed six studies as being at low risk of bias (Dereure 2012a; Humbert 2013; Lee 2016; Meaume 2008; Mikosinski 2021a; Mikosinski 2021b). We assessed three studies as being at unclear risk of bias because we were not able to assess whether there was an imbalance between experimental groups or any other potential sources of bias (Dereure 2012b; Di Mauro 1991; Ortonne 1996). We assessed three RCTs as being at high risk of bias because they included multiple ulcers in the same participant and the unit of randomisation was the participant, and analysis was not adjusted for clustered data (Felzani 2011; Ramos‐Torrecillas 2015; Taddeucci 2004).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9
For the main comparisons, see Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9.
In this section, we have reported the effects of hyaluronic acid compared with different interventions separated by wound type.
We attempted to contact study authors for further information on the outcomes of this review; however, we obtained no further information during the course of conducting the review.
Comparison 1: pressure ulcers: platelet‐rich growth factor (PRGF) + hyaluronic acid versus PRGF (1 trial, 115 participants, 124 wounds)
Only one study with a 36‐day follow‐up period presented results for this comparison (Ramos‐Torrecillas 2015). We were able to pool data from two arms of the study where hyaluronic acid was the only systematic difference between treatments, therefore 65 participants (40 participants in the PRGF + hyaluronic acid group) were included in our analysis. The study described randomisation at the level of participants; however, the number of ulcers was greater than the number of participants. There was no accounting for non‐independence of data in the analysis, resulting in a unit of analysis issue.
Primary outcomes
Complete ulcer healing
Complete wound healing was observed in 37.50% (15 out of 40) of pressure ulcers treated with PRGF + hyaluronic acid and in 32.00% (8 out of 25) of those treated with PRGF alone. It is uncertain whether there is a difference in complete healing between PRGF + hyaluronic acid versus PRGF because the certainty of evidence is very low (risk ratio (RR) 1.17, 95% confidence interval (CI) 0.58 to 2.35; 1 trial, 65 participants; Analysis 1.1) (Ramos‐Torrecillas 2015). We downgraded the certainty of evidence twice due to risk of bias and twice due to imprecision.
1.1. Analysis.

Comparison 1: Pressure ulcer: platelet‐rich growth factor + hyaluronic acid versus platelet‐rich growth factor, Outcome 1: Complete ulcer healing (36 days)
Time to complete healing
No studies provided evidence for this outcome.
Adverse events
The authors reported no signs of infection in the pressure ulcers of both groups during the 36‐day follow‐up period (Ramos‐Torrecillas 2015). However, it is uncertain if PGRF + hyaluronic acid impacts adverse events compared with PGRF because the certainty of evidence is very low. We downgraded the certainty of evidence twice due to risk of bias and twice due to imprecision.
Secondary outcomes
Change in ulcer size
We cannot be certain if there is a difference in changes in ulcer size (% from baseline) between treatments because the certainty of evidence is very low (mean difference (MD) 25.60, 95% CI 6.18 to 45.02; 1 study, 65 participants; Analysis 1.2). We downgraded the certainty of the evidence twice due to risk of bias and twice for imprecision.
1.2. Analysis.

Comparison 1: Pressure ulcer: platelet‐rich growth factor + hyaluronic acid versus platelet‐rich growth factor, Outcome 2: Change in ulcer size
The other secondary outcomes were not reported.
Comparison 2: pressure ulcers: lysine hyaluronate versus sodium hyaluronate (1 trial, 50 participants, 54 wounds)
Only one study presented results for this comparison (Felzani 2011). The trial recruited 59 participants and included 50 participants (randomisation reported at the level of participant) and reported data analysis from 54 ulcers. There was no accounting for non‐independence of data in the analysis, resulting in a unit of analysis issue.
Primary outcomes
Complete ulcer healing
Felzani 2011 assessed wound healing in stage I to III pressure ulcers, but only provided quantitative data for complete wound healing during the follow‐up period for stage III wounds. It is uncertain whether there is a difference in complete healing between lysine hyaluronate and sodium hyaluronate because the certainty of evidence is very low (RR 2.50, 95% CI 0.71 to 8.83; 1 trial, 14 ulcers from 10 participants; Analysis 2.1) (Felzani 2011). We downgraded the certainty of the evidence twice due to risk of bias and twice due to imprecision.
2.1. Analysis.

Comparison 2: Pressure ulcer: lysine hyaluronate versus sodium hyaluronate, Outcome 1: Complete ulcer healing
Time to complete healing
No studies provided evidence for this outcome.
Adverse events
No studies provided evidence for this outcome.
Secondary outcomes
Change in ulcer size
Felzani 2011 reported the treatment period necessary to reach 50% regression of the lesion between groups; however, the trial authors did not provide means or SD, thereby precluding further analysis. The authors reported that this period was shorter in the lysine hyaluronate group compared with the sodium hyaluronate group for stage I ulcers ("9 days versus 15 days, P < 0.05"); stage II ulcers ("9.50 versus 15 days, P < 0.05"); and stage III ulcers ("12.90 days versus 19.20 days, P < 0.05"). It is uncertain whether there is a difference in change in ulcer size between lysine hyaluronate and sodium hyaluronate because the certainty of evidence is very low. We downgraded the certainty of the evidence twice due to risk of bias and twice due to imprecision.
The other secondary outcomes were not reported.
Comparison 3: foot ulcers in people with diabetes: hyaluronic acid versus lyophilised collagen (1 trial, 20 participants)
Only one study presented results for this comparison (Di Mauro 1991).
Primary outcomes
Complete ulcer healing
Participants were followed until complete healing.
Time to complete healing
It is uncertain whether there is a difference in time to complete healing between hyaluronic acid and lyophilised collagen because the certainty of evidence is very low (MD 16.60, 95% CI 7.95 to 25.25; 1 study, 20 participants; Analysis 3.1). We downgraded the certainty of the evidence twice due to risk of bias and twice for imprecision.
3.1. Analysis.

Comparison 3: Foot ulcer in people with diabetes: hyaluronic acid versus lyophilised collagen, Outcome 1: Time to complete healing
Adverse events
No studies provided evidence for this outcome.
Secondary outcomes
Pain
Di Mauro 1991 did not provide quantitative analysis of pain, only a subjective assessment stating improvement of pain, itch, and paraesthesias in the collagen group. It is uncertain whether there is a difference in pain between hyaluronic acid and lyophilised collagen because the certainty of evidence is very low. We downgraded the certainty of the evidence twice due to risk of bias and twice for imprecision.
The other secondary outcomes were not reported.
Comparison 4: foot ulcers in people with diabetes: hyaluronic acid versus conventional dressing (sterile petrolatum gauze) (1 trial, 34 participants)
Only one study with a 12‐week follow‐up period presented results for this comparison (Lee 2016).
Primary outcomes
Complete ulcer healing
Complete wound healing was observed in 64.71% (11 out of 17) of foot ulcers treated with hyaluronic acid and 29.41% (5 out of 17) of those treated with conventional dressing. It is uncertain whether there is a difference in complete ulcer healing between hyaluronic acid and conventional dressing because the certainty of evidence is very low (RR 2.20, 95% CI 0.97 to 4.97; 1 study, 34 participants; Analysis 4.1) (Lee 2016). We downgraded the certainty of the evidence twice due to risk of bias and twice for imprecision.
4.1. Analysis.

Comparison 4: Foot ulcer in people with diabetes: hyaluronic acid versus conventional dressing/sterile petrolatum gauze, Outcome 1: Complete ulcer healing (12 weeks)
Time to complete healing
No studies provided evidence for this outcome.
Adverse events
Study authors reported severe adverse events in one case (5.90%) in the study group (infection followed by ray amputation) and four cases (23.50%) in the control group (two amputations due to contralateral side infection, one cerebral vascular accident, one sepsis due to pneumonia) (Lee 2016). None of the events were considered to be related to the dressing material. It is uncertain whether there is a difference in adverse events between hyaluronic acid and conventional dressing because the certainty of evidence is very low. We downgraded the certainty of the evidence twice due to risk of bias and twice for imprecision.
Secondary outcomes
Change in ulcer size
A mean reduction from baseline in ulcer area observed was 3.00 cm2 (SD 2.55) in the hyaluronic acid group and 3.80 cm2 (SD 4.25) in the conventional dressing group. The authors stated that the change in ulcer size was also analysed using the analysis of covariance (ANCOVA) model considering baseline ulcer size as covariate, and that these analyses showed no significant differences (reported P = 0.116). It is uncertain whether there is difference in mean change in ulcer size between hyaluronic acid and conventional dressing because the certainty of evidence is very low (MD −0.80, 95% CI −3.58 to 1.98; 1 study, 25 participants; Analysis 4.2). We downgraded the certainty of the evidence twice due to risk of bias and twice for imprecision.
4.2. Analysis.

Comparison 4: Foot ulcer in people with diabetes: hyaluronic acid versus conventional dressing/sterile petrolatum gauze, Outcome 2: Change in ulcer size
The authors also reported that the duration needed to achieve 50% reduction in area was 28.60 ± 19.20 days in the hyaluronic acid group and 49.50 ± 21.40 days in the conventional dressing group (reported P = 0.04). Healing velocity (%/week) was 12.99 ± 6.52 in the hyaluronic acid group and 7.53 ± 3.66 in the conventional dressing group (reported P = 0.022).
The other secondary outcomes were not reported.
Comparison 5: leg ulcers: hyaluronic acid + hydrocolloid versus hydrocolloid (1 trial, 125 participants)
Only one study with a 42‐day follow‐up period presented results for this comparison (Meaume 2008).
Primary outcomes
Complete ulcer healing
Complete wound healing was observed in 6.35% (4 out of 63) of leg ulcers treated with hyaluronic acid + hydrocolloid and in 6.45% (4 out of 62) of those treated with hydrocolloid alone. We are uncertain whether there is a difference in complete wound healing between treatments because the certainty of evidence is very low (RR 0.98, 95% CI 0.26 to 3.76; 1 study, 125 participants; Analysis 5.1). We downgraded the certainty of the evidence twice due to risk of bias and twice for imprecision.
5.1. Analysis.

Comparison 5: Leg ulcer: hyaluronic acid + hydrocolloid versus hydrocolloid, Outcome 1: Complete ulcer healing (42 days)
Time to complete healing
No studies provided evidence for this outcome.
Adverse events
Adverse events considered to be related to treatment were reported in four participants (6.40%) treated with hyaluronic acid and hydrocolloid (itching and oedema, erosion of peri‐ulcer skin, exfoliation and rash, pain) and five participants (8.10%) treated with hydrocolloid (heavy exudates and erosion, pruritus and eczema, eczema and purpura, two presented systemic infection) (Meaume 2008). We are uncertain whether there is a difference in adverse events between treatments because the certainty of evidence is very low (RR 0.79, 95% CI 0.22 to 2.80; 1 study, 125 participants; Analysis 5.2). We downgraded the certainty of the evidence twice due to risk of bias and twice for imprecision.
5.2. Analysis.

Comparison 5: Leg ulcer: hyaluronic acid + hydrocolloid versus hydrocolloid, Outcome 2: Adverse events
Secondary outcomes
Pain
Pain and itching was assessed using a 100‐millimetre visual analogue scale.
Itching and pain after 42 days of treatment were reported to be of little clinical significance in both treatment groups. Mean (± standard error of mean) for itching was 6.50 ± 2.50 and 8.40 ± 2.50 in the hyaluronic acid + hydrocolloid group and hydrocolloid group, respectively (reported P = 0.20). We are uncertain whether there is a difference in pain between hyaluronic acid + hydrocolloid and hydrocolloid because the certainty of evidence is very low (MD 2.10, 95% CI −5.81 to 10.01; 1 study, 125 participants; Analysis 5.3). We downgraded the certainty of the evidence twice due to risk of bias and twice for imprecision.
5.3. Analysis.

Comparison 5: Leg ulcer: hyaluronic acid + hydrocolloid versus hydrocolloid, Outcome 3: Pain (VAS, mm) at follow‐up
Change in ulcer size
The median percentage reduction of ulcer area provided by Meaume 2008 was 42.60 (95% CI 66.60 to 5.70) and 31.0 (95% CI 51.60 to 8.80) in the hyaluronic acid + hydrocolloid group versus the hydrocolloid group. The comparison of those reductions using the Wilcoxon test for medians provided by the study authors shows no significant differences between treatment groups. We are uncertain whether there is a difference in change in ulcer size (to at least 90%) between hyaluronic acid + hydrocolloid and hydrocolloid because the certainty of evidence is very low (RR 2.11, 95% CI 0.92 to 4.82; 1 study, 125 participants; Analysis 5.4). We downgraded the certainty of the evidence twice due to risk of bias and twice for imprecision.
5.4. Analysis.

Comparison 5: Leg ulcer: hyaluronic acid + hydrocolloid versus hydrocolloid, Outcome 4: Change in ulcer size to at least 90%
The other secondary outcomes were not reported.
Comparison 6: leg ulcers: hyaluronic acid versus hydrocolloid (1 trial, 170 participants, 143 included in per‐protocol analysis)
Only one non‐inferiority study with a 56‐day follow‐up period presented results for this comparison (Dereure 2012b).
Primary outcomes
Complete ulcer healing
Data on complete wound healing were not properly presented at the endpoint (56 days). There was only a statement reporting 27 dropouts, including 12 dropouts due to ulcer healing, without specifying to which groups they belonged. It is uncertain whether there is a difference in complete ulcer healing between hyaluronic acid and hydrocolloid because the certainty of evidence is very low. We downgraded the certainty of the evidence twice due to risk of bias and twice for imprecision.
Time to complete ulcer healing
No studies provided evidence for this outcome.
Adverse events
The study report stated that 77 adverse events were reported in 42 participants during the study, without specifying to which groups they belonged. However, most of the adverse events were not localised to the ulcer (see Table 2 of the article), and no serious adverse events were reported (Dereure 2012b). It is uncertain whether there is a difference in adverse events between hyaluronic acid and hydrocolloid because the certainty of evidence is very low. We downgraded the certainty of the evidence twice due to risk of bias and twice for imprecision.
Secondary outcomes
Pain
No studies provided evidence for this outcome. Dereure 2012b only reported pain at baseline.
Change in ulcer size
Study authors calculated the percentage of participants with ulcer size reduction ≥ 40% in each group as the primary endpoint. The observed percentage was 73.61% (53 out of 72) in the hyaluronic acid group and 71.83% (51 out of 71) in the hydrocolloid group. It is uncertain whether there is a difference in change in ulcer size between hyaluronic acid and hydrocolloid because the certainty of evidence is very low (RR 1.02, 95% CI 0.84 to 1.25; 1 study, 170 participants, 143 included in per‐protocol analysis; Analysis 6.1). We downgraded the certainty of the evidence twice due to risk of bias and twice for imprecision.
6.1. Analysis.

Comparison 6: Leg ulcer: hyaluronic acid versus hydrocolloid, Outcome 1: Change in ulcer size > 40%
The other secondary outcomes were not reported.
Comparison 7: leg ulcers: hyaluronic acid versus paraffin gauze (1 trial, 17 participants, 24 ulcers)
Only one study with an eight‐week follow‐up period presented results for this comparison (Taddeucci 2004). The study described randomisation at the level of participants; however, the number of ulcers was greater than the number of participants. There was no accounting for non‐independence of data in the analysis, resulting in a unit of analysis issue.
Primary outcomes
Complete ulcer healing
Complete wound healing was observed in 16.67% (2 out of 12) of leg ulcers treated with hyaluronic acid and 8.33% (1 out of 12) of those treated with paraffin gauze. We are uncertain whether there is a difference in complete wound healing between hyaluronic acid and paraffin gauze because the certainty of evidence is very low (RR 2.00, 95% CI 0.21 to 19.23; 1 study, 17 participants, 24 ulcers; Analysis 7.1). We downgraded the certainty of the evidence twice due to risk of bias and twice for imprecision.
7.1. Analysis.

Comparison 7: Leg ulcer: hyaluronic acid versus paraffin gauze, Outcome 1: Complete ulcer healing (56 days)
Time to complete healing
No studies provided evidence for this outcome.
Adverse events
No studies provided evidence for this outcome.
Secondary outcomes
Change in ulcer size
The only information provided by the authors of Taddeucci 2004 was that the ulcers in the hyaluronic acid group exhibited a mean improvement of 8.10 cm2 (33% area decrease) at week 8, compared with 0.40 cm2 (1.80% decrease) in the paraffin gauze group (reporting P = 0.002); however, the study authors did not present SDs, thereby precluding further analysis. We are uncertain whether there is a difference in change in ulcer size between hyaluronic acid and paraffin gauze because the certainty of evidence is very low. We downgraded the certainty of the evidence twice due to risk of bias and twice for imprecision.
The other secondary outcomes were not reported.
Comparison 8: leg ulcers: hyaluronic acid versus neutral vehicle (4 trials, 526 participants)
Four studies presented results for this comparison: Dereure 2012a and Humbert 2013 with a 60‐day follow‐up period, and Mikosinski 2021a and Mikosinski 2021b with a 23‐week follow‐up period.
Primary outcomes
Complete ulcer healing
We were able to combine results from four studies for complete ulcer healing analysis (Dereure 2012a; Humbert 2013; Mikosinski 2021a; Mikosinski 2021b). Combined results demonstrated low statistical heterogeneity among studies (I2 = 0%, P = 0.49); however, studies assessed healing at different time points, therefore we used a random‐effects model. Hyaluronic acid probably improves complete ulcer healing when compared with neutral vehicle (RR 2.11, 95% CI 1.46 to 3.07; 4 studies, 526 participants; Analysis 8.1). The certainty of evidence is moderate, downgraded once for risk of bias.
8.1. Analysis.

Comparison 8: Leg ulcer: hyaluronic acid versus neutral vehicle, Outcome 1: Complete wound healing (from 60 days up to 23 weeks)
Time to complete healing
Dereure 2012a, Mikosinski 2021a, and Mikosinski 2021b did not report this outcome.
In Humbert 2013, the study authors stated: "Other performance secondary endpoints (time‐to‐complete ulcer healing and global performance) were comparable between treatment groups, at any visit"; however, no numbers were provided. We are uncertain whether there is a difference in time to complete ulcer healing between hyaluronic acid and conventional dressing because the certainty of evidence is very low. We downgraded the certainty of the evidence twice for risk of bias and twice for imprecision.
Adverse events
We were able to pool data for incidence of infection in a meta‐analysis. Infection was observed in 2.22% (1 out of 45) of leg ulcers treated with hyaluronic acid and in 0% (0 out of 44) of those treated with neutral vehicle in Humbert 2013. Mikosinski 2021a reported infection in 14.60% (12 out of 82) of leg ulcers treated with hyaluronic acid and in 15.11% (13 out of 86) of those treated with vehicle. Infection was observed in 11.08% (10 out of 85) of leg ulcers treated with hyaluronic acid and in 15.70% (13 out of 83) of those treated with neutral vehicle in Mikosinski 2021b.
It is uncertain if hyaluronic acid alters the incidence of infection when compared with neutral vehicle because the certainty of evidence is very low (RR 0.89, 95% CI 0.53 to 1.49; I2 = 0%; 3 studies, 425 participants; Analysis 8.2). We downgraded the certainty of the evidence once due to risk of bias and twice for imprecision.
8.2. Analysis.

Comparison 8: Leg ulcer: hyaluronic acid versus neutral vehicle, Outcome 2: Adverse events ‐ infection
The studies also reported the number of adverse events; however, it was not specified in all cases if adverse events were related to the treatment, systemic or restricted to the wound. In some cases multiple adverse events were counted in the same participant. We were therefore not able to pool data for analysis or properly interpret the information.
In Humbert 2013, the study authors stated that adverse events were mainly mild or moderate (75%), with only 12 (25%) rated as severe (eight in the hyaluronic acid group versus four in the neutral vehicle group); however, the severe adverse events in the hyaluronic acid group were mostly reported by one participant (6/8 adverse events), and only one was reported as treatment‐related (pain).
In Dereure 2012a, the study authors reported that adverse events were mainly mild or moderate (88%). Nine adverse events (11%) were rated as severe, five in the hyaluronic acid group versus four in the control group (application site burn, inflammation or pain, and aggravated condition), and two adverse events were rated as serious, one in each group (neither was considered to be treatment‐related).
In Mikosinski 2021b, a total of 64 treatment‐emergent adverse events were reported by 34 participants (40.00%) in the hyaluronic acid cream group, and 84 were reported by 38 participants (45.80%) in the neutral cream group. In both cases, these were mostly mild to moderate events.
In Mikosinski 2021a, a total of 43 treatment‐emergent adverse events were reported by 27 participants (32.90%) in the hyaluronic acid gauze pad group, and 44 were reported by 34 participants (39.50%) in the neutral gauze pad group.
Secondary outcomes
Pain
All studies measured pain using the 100‐millimetre visual analogue scale (VAS), where minor pain is 0 and greatest pain is 100 mm.
Dereure 2012a reported reduction in pain in the hyaluronic acid group compared with the neutral vehicle group, and we were able to calculate the reduction in pain in Humbert 2013 and Mikosinski 2021a using data reported at baseline and after follow‐up. We were therefore able to pool data for the pain reduction from baseline. Hyaluronic acid may slightly increase reduction in pain from baseline compared with neutral vehicle (MD −8.55, 95% CI −14.77 to −2.34; 3 studies, 337 participants; Analysis 8.3). The certainty of evidence was low, downgraded once due to risk of bias and once for imprecision.
8.3. Analysis.

Comparison 8: Leg ulcer: hyaluronic acid versus neutral vehicle, Outcome 3: Pain (VAS) reduction from baseline
Mikosinski 2021b did not present numerical data for pain, only reporting that "the mean VAS score for pain intensity diminished over time during the study period, in a similar manner in both groups".
Change in ulcer size
We were able to combine data from two studies for change in ulcer area from baseline to 45 days of follow‐up (Dereure 2012a; Humbert 2013). We were not able to combine data for the longest follow‐up (60 days) because the data collected in Humbert 2013 were incomplete.
Hyaluronic acid may slightly promote greater change in ulcer size when compared with neutral vehicle, measured as mean reduction from baseline to 45 days (MD 30.44%, 95% CI 15.57 to 45.31; 2 studies, 190 participants; Analysis 8.4). The certainty of evidence was low, downgraded once due to risk of bias and once for imprecision.
8.4. Analysis.

Comparison 8: Leg ulcer: hyaluronic acid versus neutral vehicle, Outcome 4: Change in ulcer size (45 days)
The other secondary outcomes were not reported.
Comparison 9: leg ulcers: hyaluronic acid versus dextranomer (1 trial, 51 participants)
Only one non‐inferiority study with a 21‐day follow‐up period presented results for this comparison (Ortonne 1996). Complete data were reported for 50 participants (1 dropout).
Primary outcomes
Complete ulcer healing
No studies provided evidence for this outcome.
Time to complete healing
No studies provided evidence for this outcome.
Adverse events
The authors of Ortonne 1996 described that there were five reports of side effects (local pain, two cases of a local burning sensation, panniculitis and a prickling sensation) in the hyaluronic acid group and two reports of side effects in the dextranomer group (surrounding eczema and local pain). Data were not sufficiently detailed or comparable to permit quantitative analysis. We are uncertain whether there is a difference in adverse events between hyaluronic acid and dextranomer because the certainty of evidence is very low. We downgraded the certainty of the evidence once for risk of bias and twice for imprecision.
Secondary outcomes
Pain
Ortonne 1996 reported a reduction of the number of participants showing symptoms of pain on days 0, 7, 14, and 21; however, the study authors did not provide quantitative data that would have allowed further analysis.
Change in ulcer size
The SD of mean difference was not available in the study, therefore it was calculated considering that the baseline data and the 21‐day data were independent samples (a conservative way to calculate this value). We are uncertain whether there is a difference in change in ulcer size (cm2) between hyaluronic acid and dextranomer because the certainty of evidence is very low (MD 5.80, 95% CI −10.0 to 21.60; 1 study, 50 participants; Analysis 9.1). We downgraded the certainty of the evidence once for risk of bias and twice for imprecision.
9.1. Analysis.

Comparison 9: Leg ulcer: hyaluronic acid versus dextranomer, Outcome 1: Change in wound size (21 days)
The other secondary outcomes were not reported.
Discussion
Summary of main results
We included 12 RCTs (13 reports) assessing the healing of pressure ulcers (2 trials), diabetic foot ulcers (2 trials), and leg ulcers of venous or mixed aetiology (8 trials). In trials investigating pressure ulcers or diabetic foot ulcers, hyaluronic acid was compared with different dressings among studies. The certainty of evidence was very low, precluding us from combining data and performing meta‐analysis. Consequently, there is currently insufficient evidence to determine the effectiveness of hyaluronic acid dressings in the healing of pressure ulcers or diabetic foot ulcers.
For leg ulcers, hyaluronic acid was compared with hydrocolloid, paraffin gauze, dextranomer, and neutral vehicle. We were able to combine data for the comparison hyaluronic acid versus neutral vehicle in leg ulcers. Hyaluronic acid probably improves complete ulcer healing (4 studies, 526 participants; moderate‐certainty evidence) and may slightly increase reduction in pain from baseline (3 studies, 337 participants; low‐certainty evidence). Hyaluronic acid may also slightly increase change in ulcer size (2 studies, 190 participants; low‐certainty evidence); however, it is uncertain if hyaluronic acid alters the incidence of infection for this comparison (3 studies, 425 participants; very low‐certainty evidence). For the comparisons of hyaluronic acid versus hydrocolloid, paraffin gauze, and dextranomer, or when hyaluronic acid + hydrocolloid was compared with hydrocolloid, we were not able to perform meta‐analysis, and the certainty of the evidence was very low; consequently, there is currently insufficient evidence to determine the effectiveness of hyaluronic acid compared with these dressings in the healing of leg ulcers.
None of the trials reported health‐related quality of life or wound recurrence, therefore we could not assess the effect of hyaluronic acid on these outcomes.
Overall completeness and applicability of evidence
The objective of this review was to assess the effectiveness of hyaluronic acid in the healing of chronic wounds. We identified multiple interventions and reported wound healing in pressure ulcers, foot ulcers in people with diabetes, and leg ulcers. We found studies investigating the effectiveness of hyaluronic acid for all types of chronic prespecified for inclusion in the review. Our primary outcome was assessed in all but two of the included trials. The evidence is currently applicable because most of the dressings compared with hyaluronic acid in this review are still on the market. Most of the use of hyaluronic acid was in leg ulcers, predominantly due to venous disease; however, we were able to combine data from only four studies. Given our assessment of the certainty of the evidence, we are uncertain whether there is a difference in the healing of pressure ulcers or foot ulcers in people with diabetes when hyaluronic acid is used in comparison with all other interventions assessed in this review. However, we found evidence that hyaluronic acid probably improves complete ulcer healing and may slightly increase reduction in pain from baseline and promote greater change in ulcer size when compared with neutral vehicle. We did not perform subgroup analyses because all studies in the meta‐analysis used compression therapy as standard care. Additionally, we did not perform sensitivity analyses because no studies included in meta‐analysis were considered to have an overall high risk of bias.
One limitation of the included studies was the variation in duration of follow‐up, with 23 weeks being the longest time point (only one study reported time to complete ulcer healing). This impacted our assessment of the effectiveness of hyaluronic acid in the treatment of chronic wounds. The results of this systematic review demonstrate the need for additional RCTs with high methodological quality addressing the effect of hyaluronic acid on chronic wound healing, especially in pressure ulcers and foot ulcers.
Quality of the evidence
This systematic review was limited by the quality of the existing data. The following points must be considered when analysing the results of this review: the small number of included studies, small sample size, and some methodological aspects that increased the risk of bias.
Our assessment of the certainty of evidence was very low for most comparisons and outcomes, except for the outcomes complete ulcer healing, pain, and change in ulcer size for the comparison hyaluronic acid versus neutral vehicle. We downgraded the certainty of evidence due to high risk of bias and imprecision. Additionally, we identified some methodological issues, in particular blinding of personnel and outcome assessors and imprecision due to few included studies and several studies with small sample sizes. Some study reports did not provide sufficient information for assessment of outcomes or for quantitative analysis.
Potential biases in the review process
We attempted to apply robust methods in the process of analysing the search, collecting data, performing meta‐analysis, and assessing risk of bias. We intensively searched other sources for references. Whenever possible, we adopted intention‐to‐treat analysis. However, incomplete outcome data limited the analysis, since these data could not be obtained from study authors and could not be entered into a meta‐analysis.
When authors did not report change in pain for the comparison hyaluronic acid versus neutral vehicle in leg ulcers, we estimated the magnitude of change using data from the longest follow‐up and baseline in two cases (Humbert 2013; Mikosinski 2021a); however, we had to consider the means from those time points as independent groups. We recognise that these calculated data might be inaccurate.
Agreements and disagreements with other studies or reviews
Two other reviews also assessed the effect of hyaluronic acid on chronic wounds (Shaharudin 2016; Voigt 2012), and Voigt 2012 also assessed the effect on acute wounds (burns). Voigt 2012 assessed the effects of hyaluronic acid in venous leg ulcer and diabetic foot ulcers; however, most of the trials included in our review were not included in their review. This is because most of them were published posteriorly. Shaharudin 2016 was designed to assess the effects of hyaluronic acid in leg ulcers, pressure ulcers, and diabetic foot ulcers. Even though Shaharudin 2016 reported that they planned to assess pressure ulcers, they did not include any trials of pressure ulcers in the review. We found two trials involving pressure ulcers, from which were able to extract data.
We only included studies where the only systematic difference between treatments was hyaluronic acid. In some RCTs included in Shaharudin 2016 and Voigt 2012, hyaluronic acid was not the only systematic difference between groups, as in the case of the studies assessing diabetic foot ulcers where a hyaluronic acid pad was used as a substrate for later autologous tissue graft (Caravaggi 2003; Uccioli 2011). In our opinion this could have impacted the conclusions of the review and potentially overestimated the effect of hyaluronic acid. We did not include these trials in our review.
Shaharudin 2016 performed an analysis of combined data from all RCTs that reported a specific outcome (e.g. number of wounds healed at follow‐up). The authors reported there was no evidence of the effect of hyaluronic acid on ulcer healing, but the pooled data included comparing hyaluronic acid with different dressings. In our review, in order to avoid clinical heterogeneity, we did not combine studies for meta‐analysis when hyaluronic acid was compared with different dressings. Like Shaharudin 2016, we did not include Romanelli 2007 in our review, and we included a trial, Lee 2016, that was not included in the Shaharudin 2016 review.
In Voigt 2012, the authors concluded that "there appears to be an overall positive effect of HA [hyaluronic acid] in the healing of chronic wounds from various etiologies ...", and Shaharudin 2016 concluded that "the evidence does not support claims for beneficial effects of HA or its derivatives towards improvement of chronic wound healing even though there is some evidence on their effectiveness especially on reducing pain intensity". Neither Shaharudin 2016 nor Voigt 2012 assessed the certainty of evidence using GRADE. We view this as a limitation because it impacts data interpretation. Consequently, the conclusions of Shaharudin 2016 are only partially similar to our findings, and we did not reach the same conclusion as Voigt 2012.
Our findings partially agree with the observations reported in a recently published network meta‐analysis (Norman 2018), which concluded that insufficient data prevented a determination that any one dressing type was more effective than another in healing venous leg ulcers. However, including recently published data not assessed in this meta‐analysis (Mikosinski 2021a; Mikosinski 2021b), we found moderate‐certainty evidence that hyaluronic acid probably improves complete ulcer healing and may slightly reduce pain and slightly promote greater change in ulcer size when compared with neutral vehicle.
Authors' conclusions
Implications for practice.
There is currently insufficient evidence to determine the effectiveness of hyaluronic acid dressings in the healing of pressure ulcers or foot ulcers in people with diabetes. Practitioners may, therefore, consider other issues such as cost and symptom management when choosing between dressings. However, we did find evidence that hyaluronic acid probably improves complete ulcer healing and may slightly decrease pain and increase change in ulcer size when compared with neutral vehicle.
Implications for research.
Future studies assessing the effects of hyaluronic acid on wound healing should consider using all the steps from the CONSORT statement in addition to improving the reporting of findings and avoiding small sample size. Follow‐up periods should be longer than the period presented in the included studies (on average 30 to 60 days), or studies should consider time to complete ulcer healing as an outcome. Adverse events should also be reported. In order to minimise bias, a clear method of randomisation and allocation should be adopted, as well blinding of participants and personnel and, in particular, outcome assessor.
In terms of treatment choice, any investment in future primary research must maximise its value to patients, healthcare professionals, service commissioners, and other decision‐makers. Given the large number of treatment options, the design of future trials should be driven by high‐priority questions from patients and other decision‐makers.
History
Protocol first published: Issue 5, 2016
Acknowledgements
The authors would like to acknowledge the contribution of the following peer referees who provided feedback on the protocol: Susan O'Meara, Richard Kirubakaran, Clifford Richardson, Laura Bolton, and Anne Lyddiatt; and the Brazilian Cochrane Centre (Maria Regina Torloni and Gustavo Porfirio) for advice on the protocol.
The authors would also like to acknowledge the contribution of the following peer referees who provided feedback on the review: Chunhu Shi, Beryl De Souza, and Amanda Roberts.
The authors would like to acknowledge the contribution of Patricia Ziegelmann for her assistance in performing statistical analyses and Karla Crozeta for her role as author on the protocol, substantial contributions to the conception and design of the review, and initial analysis of the data.
Appendices
Appendix 1. Glossary
Ankle‐Brachial Pressure Index (ABPI): the ratio of blood pressure at the ankle to that in the arm. This ratio provides a measure of the degree of arterial disease in the legs, where a value of 1.0 indicates that there is no reduction in blood supply to the legs, compared with the arm. A ratio lower than 0.9 indicates reduced blood supply to the lower limb.
Anti‐inflammatory: a drug or treatment designed to reduce inflammation (i.e. redness, heat, swelling, etc.).
Chronic: marked by long duration, by frequent recurrence over a long time, and often by slowly progressing deterioration; having a slow progressive course of indefinite duration. Examples of chronic wounds are pressure ulcers, leg ulcers, and diabetic foot ulcers.
Compression therapy: the application of external pressure to a limb, to help venous blood or lymph circulation. Compression can be applied using bandages, elastic stockings, or inflatable sleeves.
Necrotic tissue: dead or dying tissue, which may be caused by an interruption of the blood supply.
Shear: force acting along the line of the edge of the skin. One of three factors known to contribute to the development of pressure ulcers.
Definitions taken from the Medical Dictionary.
Appendix 2. Nurse Prescribers' Formulary 2011 categories of dressings
Basic wound contact dressings
Low‐adherence dressings and wound contact materials: usually cotton pads that are placed directly in contact with the wound, these can be non‐medicated (e.g. paraffin gauze dressing) or medicated (e.g. containing povidone iodine or chlorhexidine). Examples include paraffin gauze dressing, BP 1993, and Xeroform (Covidien) dressing, a non‐adherent petrolatum blend with 3% bismuth tribromophenate on fine mesh gauze.
Absorbent dressings: applied directly to the wound and may also be used as secondary absorbent layers in the management of heavily exuding wounds. Examples include Primapore (Smith & Nephew), Megapore (Mölnlycke), and absorbent cotton gauze (BP 1988).
Advanced wound dressings
Hydrocolloid dressings: usually composed of an absorbent hydrocolloid matrix on a vapor‐permeable film or foam backing. Examples include: Granuflex (Convatec) and NU DERM (Systagenix). Fibrous alternatives have been developed that resemble alginates and are not occlusive: Aquacel (Convatec).
Hydrogel sheet and amorphous dressings: consist of a starch polymer and up to 96% water. These dressings can absorb wound exudate or rehydrate a wound depending upon the moisture levels. They are supplied as either flat sheets or amorphous hydrogel. Examples of hydrogel sheet dressings include: Actiformcool (Activa) and Aquaflo (Covidien). Examples of amorphous hydrogel dressings include: Purilon Gel (Coloplast) and NuGel (Systagenix).
Sodium hyaluronate dressings: sodium hyaluronate products are thought to hydrate the wound. The dressings can be applied directly to the wound or to a primary dressing.
Films ‐ permeable film and membrane dressings: permeable to water vapour and oxygen, but not to liquid water or micro‐organisms. Examples include Tegaderm (3M) and Opsite (Smith & Nephew).
Soft polymer dressings: dressings composed of a soft silicone polymer held in a non‐adherent layer, these are moderately absorbent. Examples include: Mepitel (Mölnlycke) and Urgotul (Urgo).
Foam dressings: contain hydrophilic polyurethane foam and are designed to absorb wound exudate and maintain a moist wound surface. A variety of versions exist, some of which include additional absorbent materials such as viscose and acrylate fibres, or particles of super‐absorbent polyacrylate, or are silicone‐coated for non‐traumatic removal. Examples include: Allevyn (Smith & Nephew), Biatain (Coloplat), and Tegaderm (3M).
Alginate dressings: highly absorbent dressings composed of calcium alginate or calcium sodium alginate, which can be combined with collagen. The alginate forms a gel while in contact with the wound surface, which can be lifted off at dressing removal or rinsed away with sterile saline. Bonding to a secondary viscose pad increases absorbency. Examples include: Curasorb (Covidien), SeaSorb (Coloplast), and Sorbsan (Unomedical).
Capillary‐action dressings: consist of an absorbant core of hydrophilic fibres held between two low‐adherent contact layers. Examples include: Advadraw (Advancis) and Vacutx (Protex).
Odour‐absorbent dressings: dressings that contain charcoal and are used to absorb wound odour. Often this type of dressing is used in conjunction with a secondary dressing to improve absorbency. Examples include: CarboFLEX (Convatec).
Antimicrobial dressings
Honey‐impregnated dressings: contain medical‐grade honey which is proposed to have antimicrobial and anti‐inflammatory properties and can be used for acute or chronic wounds. Examples include: Medihoney (Medihoney) and Activon Tulle (Advancis).
Iodine‐impregnated dressings: when exposed to wound exudate these release free iodine, which is thought to act as a wound antiseptic. One example is Iodozyme (Insense).
Silver‐impregnated dressings: used to treat infected wounds, as silver ions are thought to have antimicrobial properties. Silver versions of most dressing types are available (e.g. silver foam, silver hydrocolloid, etc.). Examples include: Acticoat (Smith & Nephew) and Urgosorb Silver (Urgo).
Other antimicrobial dressings: these dressings are composed of a gauze or low‐adherent dressing impregnated with an ointment thought to have antimicrobial properties. Examples include: chlorhexidine gauze dressing (Smith & Nephew) and Cutimed Sorbact (BSN Medical).
Specialist dressings
Protease‐modulating matrix dressings: designed to alter the activity of proteolytic enzymes (i.e. breakdown of protein or dead skin) in chronic wounds. Examples include: Promogran (Systagenix) and Sorbion (H & R).
Silicone keloid dressing: designed to reduce or prevent hypertrophic or keloid scarring. Examples include: Cica‐Care (Smith & Nephew) and Clitech (Su‐med).
Appendix 3. Search strategies
Cochrane Wounds Specialised Register
1 MESH DESCRIPTOR Hyaluronic Acid EXPLODE ALL AND INREGISTER
2 (hyaluron* or hyaluran* or Hyalofil* or Hyalomatr*) AND INREGISTER
3 #1 OR #2 AND INREGISTER
4 MESH DESCRIPTOR Chronic Disease EXPLODE ALL AND INREGISTER
5 MESH DESCRIPTOR Wound Healing EXPLODE ALL AND INREGISTER
6 #4 AND #5 AND INREGISTER
7 MESH DESCRIPTOR Skin Ulcer EXPLODE ALL AND INREGISTER
8 MESH DESCRIPTOR Leg Ulcer EXPLODE ALL AND INREGISTER
9 MESH DESCRIPTOR Pressure Ulcer EXPLODE ALL AND INREGISTER
10 MESH DESCRIPTOR Foot Ulcer EXPLODE ALL AND INREGISTER
11 MESH DESCRIPTOR Diabetic Foot EXPLODE ALL AND INREGISTER
12 (skin ulcer* or foot ulcer* or diabetic foot or diabetic feet or leg ulcer* or varicose ulcer* or venous ulcer* or stasis ulcer* or ulcus cruris or crural ulcer* or arterial ulcer* or neuropathic ulcer*) AND INREGISTER
13 ((ischaemic or ischemic) next (wound* or ulcer*)) AND INREGISTER
14 (wound* or ulcer*) next (ischaemic or ischemic) AND INREGISTER
15 (bed sore* or bedsore* or pressure sore* or pressure ulcer* or decubitus ulcer*) AND INREGISTER
16 (chronic next (wound* or ulcer*)) AND INREGISTER
17 #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 AND INREGISTER
18 #17 AND #3 AND INREGISTER
Trial register specific search of The Cochrane Central Register of Controlled Clinical Trials (CENTRAL) via Cochrane Register of Studies
1 MESH DESCRIPTOR Hyaluronic Acid EXPLODE ALL AND CENTRAL:TARGET
2 (hyaluron* or hyaluran* or Hyalofil* or Hyalomatr*) AND CENTRAL:TARGET
3 #1 OR #2 AND CENTRAL:TARGET
4 MESH DESCRIPTOR Chronic Disease EXPLODE ALL AND CENTRAL:TARGET
5 MESH DESCRIPTOR Wound Healing EXPLODE ALL AND CENTRAL:TARGET
6 #4 AND #5 AND CENTRAL:TARGET
7 MESH DESCRIPTOR Skin Ulcer EXPLODE ALL AND CENTRAL:TARGET
8 MESH DESCRIPTOR Leg Ulcer EXPLODE ALL AND CENTRAL:TARGET
9 MESH DESCRIPTOR Pressure Ulcer EXPLODE ALL AND CENTRAL:TARGET
10 MESH DESCRIPTOR Foot Ulcer EXPLODE ALL AND CENTRAL:TARGET
11 MESH DESCRIPTOR Diabetic Foot EXPLODE ALL AND CENTRAL:TARGET
12 (skin ulcer* or foot ulcer* or diabetic foot or diabetic feet or leg ulcer* or varicose ulcer* or venous ulcer* or stasis ulcer* or ulcus cruris or crural ulcer* or arterial ulcer* or neuropathic ulcer*) AND CENTRAL:TARGET
13 ((ischaemic or ischemic) next (wound* or ulcer*)) AND CENTRAL:TARGET
14 (wound* or ulcer*) next (ischaemic or ischemic) AND CENTRAL:TARGET
15 (bed sore* or bedsore* or pressure sore* or pressure ulcer* or decubitus ulcer*) AND CENTRAL:TARGET
16 (chronic next (wound* or ulcer*)) AND CENTRAL:TARGET
17 #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 AND CENTRAL:TARGET
18 #17 AND #3 AND CENTRAL:TARGET
19 (NCT0* or ACTRN* or ChiCTR* or DRKS* or EUCTR* or eudract* or IRCT* or ISRCTN* or JapicCTI* or JPRN* or NTR0* or NTR1* or NTR2* or NTR3* or NTR4* or NTR5* or NTR6* or NTR7* or NTR8* or NTR9* or SRCTN* or UMIN0*):AU AND CENTRAL:TARGET
20 http*:SO AND CENTRAL:TARGET
21 #19 OR #20 AND CENTRAL:TARGET
22 #18 AND #21
The Cochrane Central Register of Controlled Clinical Trials (CENTRAL)
#1 MeSH descriptor: [Hyaluronic Acid] explode all trees
#2 (hyaluron* or hyaluran* or hyalofil* or hyalomatr*):ti,ab,kw
#3 {or #1‐#2}
#4 MeSH descriptor: [Chronic Disease] explode all trees
#5 MeSH descriptor: [Wound Healing] explode all trees
#6 {and #4‐#5}
#7 MeSH descriptor: [Skin Ulcer] explode all trees
#8 MeSH descriptor: [Diabetic Foot] explode all trees
#9 (skin next ulcer*) or (foot next ulcer*) or (diabetic next foot) or (diabetic next feet) or (leg next ulcer*) or (varicose next ulcer*) or (venous next ulcer*) or (stasis next ulcer*) or (arterial next ulcer*) or (ulcer next cruris) or (ulcus next cruris) or (crural next ulcer*):ti,ab,kw
#10 ((ischaemic or ischemic) next (wound* or ulcer*)):ti,ab,kw
#11 ((bed next sore*) or bedsore* or (pressure next sore*) or (pressure next ulcer*) or (decubitus next ulcer*)):ti,ab,kw
#12 chronic next wound*:ti,ab,kw
#13 (chronic next ulcer*):ti,ab,kw
#14 {or #6‐#13}
#15 {and #3, #14} in Trials
Ovid MEDLINE
1 exp Hyaluronic Acid/
2 (hyaluron* or hyaluran*).tw.
3 (Hyalofil* or Hyalomatr*).tw.
4 or/1‐3
5 exp Chronic Disease/
6 exp Wound Healing/
7 and/5‐6
8 exp Skin Ulcer/
9 exp Leg Ulcer/
10 exp Pressure Ulcer/
11 exp Foot Ulcer/
12 exp Diabetic Foot/
13 (skin ulcer* or foot ulcer* or diabetic foot or diabetic feet or leg ulcer* or varicose ulcer* or venous ulcer* or stasis ulcer* or ulcus cruris or crural ulcer* or arterial ulcer* or neuropathic ulcer*).tw.
14 ((ischaemic or ischemic) adj (wound* or ulcer*)).tw.
15 (bed sore* or pressure sore* or pressure ulcer* or decubitus ulcer*).tw.
16 (chronic adj (wound* or ulcer*)).tw.
17 or/7‐16
18 and/4,17
19 randomized controlled trial.pt.
20 controlled clinical trial.pt.
21 randomized.ab.
22 placebo.ab.
23 drug therapy.fs.
24 randomly.ab.
25 trial.ab.
26 groups.ab.
27 or/19‐26
28 exp animals/ not humans.sh.
29 27 not 28
30 18 and 29
Ovid Embase
1 hyaluronic acid/
2 (hyaluron* or hyaluran*).tw.
3 (Hyalofil* or Hyalomatr*).tw.
4 or/1‐3
5 exp Chronic Disease/
6 exp Wound Healing/
7 and/5‐6
8 exp Chronic Wound/
9 chronic wound*.tw.
10 (chronic adj3 ulcer*).tw.
11 exp Skin Ulcer/
12 exp Diabetic Foot/
13 (skin ulcer* or foot ulcer* or diabetic foot or diabetic feet or leg ulcer* or varicose ulcer* or venous ulcer* or stasis ulcer* or ulcus cruris or crural ulcer* or arterial ulcer* or neuropathic ulcer*).tw.
14 ((ischaemic or ischemic) adj (wound* or ulcer*)).tw.
15 (bed sore* or pressure sore* or pressure ulcer* or decubitus ulcer*).tw.
16 or/7‐15
17 and/4,16
18 Randomized controlled trial/
19 Controlled clinical study/
20 Random$.ti,ab.
21 randomization/
22 intermethod comparison/
23 placebo.ti,ab.
24 (compare or compared or comparison).ti.
25 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab.
26 (open adj label).ti,ab.
27 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab.
28 double blind procedure/
29 parallel group$1.ti,ab.
30 (crossover or cross over).ti,ab.
31 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 orintervention$1 or patient$1 or subject$1 or participant$1)).ti,ab.
32 (assigned or allocated).ti,ab.
33 (controlled adj7 (study or design or trial)).ti,ab.
34 (volunteer or volunteers).ti,ab.
35 human experiment/
36 trial.ti.
37 or/18‐36
38 (random$ adj sampl$ adj7 (cross section$ or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.)
39 Cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.)
40 (((case adj control$) and random$) not randomi?ed controlled).ti,ab.
41 (Systematic review not (trial or study)).ti.
42 (nonrandom$ not random$).ti,ab.
43 Random field$.ti,ab.
44 (random cluster adj3 sampl$).ti,ab.
45 (review.ab. and review.pt.) not trial.ti.
46 we searched.ab. and (review.ti. or review.pt.)
47 update review.ab.
48 (databases adj4 searched).ab.
49 (rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/
50 Animal experiment/ not (human experiment/ or human/)
51 or/38‐50
52 37 not 51
53 17 and 52
EBSCO CINAHL Plus
S37 S13 AND S36
S36 S35 NOT S34
S35 S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28
S34 S32 NOT S33
S33 MH (human)
S32 S29 OR S30 OR S31
S31 TI (animal model*)
S30 MH (animal studies)
S29 MH animals+
S28 AB (cluster W3 RCT)
S27 MH (crossover design) OR MH (comparative studies)
S26 AB (control W5 group)
S25 PT (randomized controlled trial)
S24 MH (placebos)
S23 MH (sample size) AND AB (assigned OR allocated OR control)
S22 TI (trial)
S21 AB (random*)
S20 TI (randomised OR randomized)
S19 MH cluster sample
S18 MH pretest‐posttest design
S17 MH random assignment
S16 MH single‐blind studies
S15 MH double‐blind studies
S14 MH randomized controlled trials
S13 S4 AND S12
S12 S5 or S6 or S7 or S8 or S9 or S10 or S11
S11 TI ( chronic wound* or chronic ulcer* ) or AB ( chronic wound* or chronic ulcer* )
S10 TI ( bed sore* or pressure sore* or pressure ulcer* or decubitus ) or AB ( bed sore* or pressure sore* or pressure ulcer* or decubitus )
S9 AB skin ulcer* or foot ulcer* or diabetic foot* or diabetic feet or leg ulcer* or varicose ulcer* or venous ulcer* or stasis ulcer* or arterial ulcer* or ischemic ulcer* or ischaemic ulcer* or ulcus cruris or ulcer cruris
S8 TI skin ulcer* or foot ulcer* or diabetic foot* or diabetic feet or leg ulcer* or varicose ulcer* or venous ulcer* or stasis ulcer* or arterial ulcer* or ischemic ulcer* or ischaemic ulcer* or ulcus cruris or ulcer cruris
S7 (MH "Diabetic Foot")
S6 (MH "Skin Ulcer+")
S5 (MH "Wounds, Chronic")
S4 S1 OR S2 OR S3
S3 TI ( (Hyalofil* or Hyalomatr*) ) OR AB ( (Hyalofil* or Hyalomatr*) )
S2 TI ( (hyaluron* or hyaluran*) ) OR AB ( (hyaluron* or hyaluran*) )
S1 (MH "Hyaluronic Acid")
US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov)
hyaluronic acid OR Hyaluronan OR Hyaluronate sodium OR hyalofil or Hyalomatr | chronic wound OR chronic ulcer OR wound Healing
World Health Organization International Clinical Trials Registry Platform
hyaluronic acid OR Hyaluronan OR Hyaluronate sodium OR hyalofil or Hyalomatr [intervention] AND wound [title]
hyaluronic acid OR Hyaluronan OR Hyaluronate sodium OR hyalofil or Hyalomatr [intervention] AND wound [condition]
EU Clinical Trials Register
hyaluronic acid AND wounds
Appendix 4. Risk of bias table judgement criteria
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process provided to permit a judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not have foreseen assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based, and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly have foreseen assignments and thus introduced selection bias, such as allocation based on: use of an open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. envelopes were unsealed, not opaque, or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information to permit a judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definitive judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque, and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following:
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of study personnel ensured, and it is unlikely that the blinding could have been broken.
Study personnel were not blinded, but outcome assessment was blinded, and the non‐blinding of others was unlikely to have introduced bias.
High risk of bias
Any one of the following:
No blinding or incomplete blinding, and the outcome or outcome measurement was likely to be influenced by lack of blinding.
Blinding of personnel attempted, but it is likely that the blinding could have been broken.
Study personnel were not blinded, and the non‐blinding of others was likely to have introduced bias.
Unclear
Either of the following:
Insufficient information provided to permit a judgement of low or high risk of bias.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following:
No missing outcome data.
Reasons for missing outcome data were unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes was not enough to have a clinically relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following:
Reason for missing outcome data is likely to be related to true outcome, with either an imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk was enough to induce clinically relevant bias in the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes was enough to induce clinically relevant bias in the observed effect size.
‘As‐treated’ analysis done with substantial departure to the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Either of the following:
Insufficient reporting of attrition/exclusions to permit a judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of the suggestion of selective outcome reporting?
Low risk of bias
Either of the following:
The study protocol is available, and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way.
The study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following:
Not all of the study’s prespecified primary outcomes have been reported.
One or more primary outcomes are reported using measurements, analysis methods, or subsets of the data (e.g. subscales) that were not prespecified.
One or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information to permit a judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Comparability of treatment groups in relation to baseline ulcer surface area.
Choice of analysis where multiple ulcers on the same individuals(s) are studied.
Choice of analysis in cluster‐randomised trials.
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Data and analyses
Comparison 1. Pressure ulcer: platelet‐rich growth factor + hyaluronic acid versus platelet‐rich growth factor.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Complete ulcer healing (36 days) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2 Change in ulcer size | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 2. Pressure ulcer: lysine hyaluronate versus sodium hyaluronate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Complete ulcer healing | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 3. Foot ulcer in people with diabetes: hyaluronic acid versus lyophilised collagen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Time to complete healing | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 4. Foot ulcer in people with diabetes: hyaluronic acid versus conventional dressing/sterile petrolatum gauze.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Complete ulcer healing (12 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.2 Change in ulcer size | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 5. Leg ulcer: hyaluronic acid + hydrocolloid versus hydrocolloid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Complete ulcer healing (42 days) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.2 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.3 Pain (VAS, mm) at follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.4 Change in ulcer size to at least 90% | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 6. Leg ulcer: hyaluronic acid versus hydrocolloid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Change in ulcer size > 40% | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 7. Leg ulcer: hyaluronic acid versus paraffin gauze.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Complete ulcer healing (56 days) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 8. Leg ulcer: hyaluronic acid versus neutral vehicle.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Complete wound healing (from 60 days up to 23 weeks) | 4 | 526 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [1.46, 3.07] |
| 8.2 Adverse events ‐ infection | 3 | 425 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.53, 1.49] |
| 8.3 Pain (VAS) reduction from baseline | 3 | 337 | Mean Difference (IV, Random, 95% CI) | ‐8.55 [‐14.77, ‐2.34] |
| 8.4 Change in ulcer size (45 days) | 2 | 190 | Mean Difference (IV, Random, 95% CI) | 30.44 [15.57, 45.31] |
Comparison 9. Leg ulcer: hyaluronic acid versus dextranomer.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Change in wound size (21 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dereure 2012a.
| Study characteristics | ||
| Methods |
Research design: RCT; parallel; prospective; multicentre; comparative; randomised; double‐blind Care setting: inpatients and outpatients of 24 centres in France (17 centres) and Poland (7 centres) Country of origin: France and Poland Publication source:Journal of Wound Care Year of publication: 2012 Duration of follow‐up: 60 days or until complete healing Sources of funding: Laboratoires Genévrier Unit of randomisation: participant Unit of analysis: participant Inclusion criteria: aged 18 or over; with at least 1 leg ulcer venous or mixed aetiology present for more than 2 months and less than 4 years; ulcer surface area 5 to 40 cm2, with no necrotic tissue; wound was deemed suitable for compression; documented past history of deep venous thrombosis of the lower limbs and/or clinical evidence of post‐thrombotic syndrome with chronic oedema and lipodermatosclerosis, and/or available data of an arterial‐venous Doppler examination performed within the previous 6 months and showing post‐phlebitic sequels (residual thrombosis), and/or a superficial or profound reflux on the venous system; no local use of hyaluronic acid within the 3 months prior to inclusion; albuminaemia ≥ 25 g/L; ABPI of ≥ 0.8; daily use of compression therapy for ambulatory patients, as recommended by the French Health Authorities (long‐stretch elastic bandage or multilayer bandage) Exclusion criteria: participants with an ulcer of non‐vascular origin, or due to a general cause; significant arterial insufficiency (ABPI < 0.8); clinical suspicion of local and/or systemic infection; hepatic or renal failure; recent history of venous thrombosis (< 3 months); diabetic patients; participants who were allergic to local anaesthetics or components of 2 treatments, or were receiving treatment that delayed the healing process |
|
| Participants |
Number of participants: 101 participants were included in the ITT population (group 1: 50 participants; group 2: 51 participants). 75 participants were considered in the per‐protocol population (group 1: 38 participants; group 2: 37 participants). Female gender: group 1: 54% (n = 27); group 2: 57% (n = 29) Age: group 1: 68.6 ± 12.4 (n = 50); group 2: 69.7 ± 14.7 (n = 51) |
|
| Interventions |
Details of the intervention: both treatments were supplied in the same form, external packaging shape, odour, and texture, in order to maintain the double blinding Group 1: 0.2% hyaluronic acid‐based topical (ialuset cream; Laboratories Genévrier) Group 2: neutral vehicle (same formulation as ialuset cream, but without hyaluronic acid, obtained by emulsion of a fat and aqueous phase; Laboratories Genévrier) Co‐interventions: in the majority of cases (90%), compression was primarily long‐stretch elastic or multilayer bandages. Compression was applied in the morning and removed before going to bed. Systemic antibiotics could be used in the event of clinically relevant infection. Systemic analgesics were authorised, provided they were interrupted at least 10 hours before each visit. Duration of treatment: 60 days, or until complete healing |
|
| Outcomes |
Primary outcomes of the review: complete wound healing; adverse events Secondary outcomes: percentage of wound size reduction; pain, assessed using a VAS |
|
| Notes | Losses to follow‐up: 101 participants constituted the ITT population, 27 withdrew from the study (n = 11 for group 1 and n = 16 for group 2). Major protocol deviations were reported during the study for 12 participants in group 1 and 14 participants in group 2. These participants were therefore excluded from the per‐protocol group, which thus comprised 75 participants (n = 38 in group 1, n = 37 in group 2). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation list was prepared using a validated SAS software (Institute Inc.) by an independent provider appointed for this study (Axonal). Randomisation was stratified by centre" |
| Allocation concealment (selection bias) | Low risk | Quote: "The HA acid treatment cream and neutral vehicle were allocated according to a randomisation list balanced per blocks of four". "Treatment allocation and evaluation were assessed by a blinded physician." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both treatment were supplied in the same form, external packaging, shape, odour, and texture, in order to maintain the double blinding" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Two independent readers, blind to treatment allocation, measured the wound size based on the drawings on sterile tracing papers, in a centralised fashion and using planimetrics system. The percentage reduction of the wound area between day 0 and day 14, day 28 and day 56, was calculated". "Treatment allocation and evaluation were assessed by a blinded physician" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "101 participants were included in the intention‐to‐treat (ITT) population (group 1: 50 participants; group 2: 51 participants). 75 patients were considered in the per protocol (PP) population (group 1: 38 participants; group 2: 37 participants)" |
| Selective reporting (reporting bias) | Low risk | Outcomes in methods section were described in results section. |
| Other bias | Low risk | Groups were balanced at baseline and only one ulcer was selected in volunteers with multiple ulcers. The study was sponsored by Laboratories Genévrier and authors received honoraria for their contributions to the study. |
Dereure 2012b.
| Study characteristics | ||
| Methods |
Research design: RCT, prospective, multicentre, comparative, parallel‐group, randomised, controlled, blind‐observer, non‐inferiority clinical trial Care setting: 20 centres, selected by the study sponsor, 4 centres in France and 16 in Poland Country of origin: France and Poland Publication source:Journal of Wound Care Year of publication: 2012 Duration of follow‐up: 56 days, or until complete healing Sources of funding: Laboratoires Genévrier Unit of randomisation: participant Unit of analysis: participant Inclusion criteria: surface of the selected target ulcer 5 to 40 cm2, with no necrotic tissue; wound consistent with the use of an appropriate compression device; documented past history of deep venous thrombosis of the lower limbs and/or clinical evidence of post‐thrombotic syndrome with chronic oedema and lipodermatosclerosis and/or available data of an arterial‐venous Doppler examination performed within the previous 6 months and showing post‐phlebitic sequels (residual thrombosis), and/or a superficial or profound reflux on the venous system; ABPI ≥ 0.8; daily use of compression devices for ambulatory patients; no local use of HA within the previous 3 months; albuminaemia ≥ 25 g/L; participants covered by a health insurance system; women of childbearing age had to use a reliable contraceptive method for at least 1 year Exclusion criteria: participants with an ulcer of non‐vascular origin, or due to a general cause; diabetic patients; with significant arterial insufficiency (ABPI < 0.8); with a clinical suspicion of local and/or general infection; with hepatic or renal failure, with a recent history of venous thrombosis (less than 3 months); pregnant or breastfeeding woman, or woman planning to be pregnant; with known allergies to local anaesthetics or to investigational treatments components, or under treatment delaying the healing process; participants who had participated in a clinical investigation within the 2 months preceding the inclusion visit |
|
| Participants |
Number of participants: 170 participants were included in the ITT population (n = 2 in France and n = 168 in Poland; group 1: 85 participants; group 2: 85 participants). 143 participants constituted the per‐protocol population (group 1: 72 participants; group 2: 71 participants). Female gender: group 1: 61% (n = 44); group 2: 59% (n = 42) Age: group 1: 64.2 ± 14.4 (n = 72); group 2: 68.5 ± 13.1 |
|
| Interventions |
Details of intervention Group 1: 0.05% HA‐impregnated cotton gauze pad (ialuset gauze pad; Laboratoires Genévrier) Group 2: HC dressing (DuoDERM E; Convatec) Dressing procedure: the ulcer was cleaned with physiological serum, and the assigned dressing was then applied by a nurse or by the investigator. The gauze pad (group 1) was applied to the wound every day, covered with sterile gauze. The HC dressing (group 2) was directly applied to the wound every 2 to 3 days. Co‐interventions: both treatments were then covered by an adapted and efficient compression bandage, prescribed by investigators according to the standard care recommended by French Health Authorities (HAS) on June 2006 (grade 2; 3); low‐elasticity bandages with short stretch (< 20%), elastic bandages with long stretch (> 20%), multilayered bandages and compression stockings. Wound excision procedures were authorised if necessary. Systemic antibiotics could be used in the event of clinically relevant infection. Systemic analgesics were authorised, provided they were interrupted at least 10 hours before each visit. Duration of treatment: 56 days, or until complete healing |
|
| Outcomes |
Primary outcomes of the review: percentage of participants with completely healed ulcer; adverse events Secondary outcomes: reduction of at least 40% of the initial wound surface after 56 days of treatment; percentage wound size reduction; pain (only at baseline) |
|
| Notes | Losses to follow‐up: 27 participants (15%) did not complete the study (group 1: n = 13; group 2: n = 14) primarily due to ulcer healing (n = 12, 46%). 7 participants (27%) dropped out due to treatment‐related adverse event. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation list was prepared using a validated SAS software (Institute Inc.) by an independent provider appointed for this study (Axonal). Randomisation was stratified by centre" |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation was described, however there was no mention of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible due to different appearances of treatments. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Two independent readers, blind to treatment allocation, measured the wound size based on the drawings on sterile tracing papers, in a centralised fashion and using planimetrics system. The percentage reduction of the wound area between day 0 and day 14, day 28 and day 56, was calculated" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A total of 170 patients were included (n=2 in France and n=168 in Poland) ...". "Overall, 26 patients (15%) did not complete the study (n=13 for HA, n=13 for HC dressing) primarily due to ulcer healing (n=12; 46%). Seven patients (27%) dropped out for treatment related AE" Comments: participants withdrawal were justified and balanced between groups. |
| Selective reporting (reporting bias) | Low risk | Outcomes in methods section were described in results section. |
| Other bias | Unclear risk | Groups were balanced at baseline and only one ulcer was selected in volunteers with multiple ulcers, however, only 2 volunteers were included from 4 eligible centres in France and authors did not provide reason for this imbalance. The study was sponsored by Laboratories Genévrier and authors received honoraria for their contributions to the study. |
Di Mauro 1991.
| Study characteristics | ||
| Methods |
Research design: randomised, comparative, clinical trial Care setting: not described Country of origin: not described Publication source:Drugs under Experimental and Clinical Research Year of publication: 1991 Duration of follow‐up: until wound healing Sources of funding: not described Unit of randomisation: participant Unit of analysis: participant Inclusion criteria: participants affected by non‐insulin‐dependent diabetes and ulcers Exclusion criteria: not described |
|
| Participants |
Number of participants: 20 participants (ITT and per‐protocol). The groups were assumed to be the same size (n = 10 in each arm) based on the description from the trialist: "Twenty patients (twelve males and eight females, age range 60‐78 years) affected by non‐insulin‐dependent diabetes and ulcer were, consecutively and at random, treated with LC or hyaluronic acid medicated gauze". Male gender: 60% (n = 12) Age: 60 to 78 years 19 participants had foot ulcers, and 1 participant had a post‐traumatic ulcer at the volar surface of the wrist. |
|
| Interventions |
Details of the intervention Group 1: hyaluronic acid medicated gauze. Participants in this group were treated according the same general procedures of local therapy. Group 2: lyophilised type I collagen was applied on the surface of the ulcers or inside the fistulas. The tablets were moistened with saline or antibiotic solution when applied on the surface of the ulcers; tablets were dry, cut, and suitably moulded when inserted in the fistulas. Dressing was renewed every 2 days. Co‐interventions: ulcers were treated by debridement, repeated saline solution washings, and local antibiotic therapy |
|
| Outcomes |
Primary outcomes of the review: time to complete wound healing Secondary outcomes: pain |
|
| Notes | Losses to follow‐up: not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Consecutively and at random" Comments: unclear risk of bias, authors did not specify the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Did not provide a clear description of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Personnel were probably not blinded (did not mention if involved personnel were different people treating different groups) because lyophilised collagen were tablets that needed to be moistened and moulded when applied on wounds. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Authors did not report withdrawals or statistical methods. |
| Selective reporting (reporting bias) | High risk | Authors did not mention any methods for quantification of symptoms such as pain or paraesthesia or described pain and paraesthesia as a measured outcome; however, in the results section authors state: "In the group treated with collagen, a significant improvement was shown in symptoms such as reduction of pain, itch and paraesthesia" |
| Other bias | Unclear risk | It is not possible to assess imbalance in treatment groups based on presented data. Authors did not report source of funding. |
Felzani 2011.
| Study characteristics | ||
| Methods |
Research design: RCT, double‐blind, single‐centre Care setting: hospitalised patients Country of origin: Italy Publication source:Advances in Therapy, Springer Healthcare Year of publication: 2011 Duration of follow‐up: 15 days Sources of funding: not described Unit of randomisation: participant Unit of analysis: ulcers Inclusion criteria: hospitalised patients of both sexes; aged above 18 years, with a foreseen hospitalisation period of longer than 15 days, with grade 1 to 3 decubitus ulcers Exclusion criteria: patients that could not co‐operate with the hygienic measures to be adopted for the treatment of sores and those with a history of intolerance to hyaluronic acid; need of concomitant local and/or general antibiotic therapy for skin lesions or for systemic diseases |
|
| Participants |
Number of participants: 59 participants were recruited, and 50 participants with 54 pressure ulcers were included in analysis. Participants to be treated were divided into 3 groups based on ulcer stage: first group (stage I), 20 participants; second group (stage II), 20 participants; third group (stage III), 10 participants. Among participants in the third group, 2 participants had 2 lesions, and 1 participant had 3 lesions. Therefore, 14 decubitus ulcers were treated. Male gender: 42% (n = 21) Age: 56 ± 7 years |
|
| Interventions |
Details of the intervention Group 1: hyaluronic acid (Lys‐HA; Lysial, Fatai‐Nyl Srl; Jasper LLC, Lugano, Switzerland) Group 2: sodium hyaluronate Dressing procedure: wounds were initially thoroughly cleaned with saline. Blood clots, foreign bodies, and excess necrotic tissue were removed with gauze. Macerated skin borders were surgically removed. After these cleaning operations, the cream was applied as a thin layer across the ulcer surface. For the secondary medication, fat gauzes were preferred for direct contact with the wound, whereas final dressing was performed with sterile gauzes. Dressing changes were made daily during the first week of the study, and every other day during the second week. Co‐interventions: nutrition supplements and patient mobilisation and turning were provided according to the standard of care Duration of treatment: 15 days |
|
| Outcomes |
Primary outcomes of the review: complete wound healing for stage III ulcers Secondary outcomes: time necessary to reach 50% lesion size regression |
|
| Notes | Losses to follow‐up: 100% of participants completed the treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This single‐centre randomised controlled trial (RCT) was conducted double‐blinded" Comments: did not specify the method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The products were provided in identical containers, the only difference being the batch number |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The nursing team was unaware of the study treatment allocation." "Lesion analysis was performed in a blinded manner by expert specialized investigators and the following quantitative criteria were examined: lesion size (area), and regression time of 50% of lesion size" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "specifically, the first group (stage 1 decubitus ulcers) was initially formed by 25 patients; 5 participants were considered as not assessable since treatment was suspended. In 3 cases, this was because patients left the institute, in the other 2 cases it was because worsening of their condition due to underlying disease requiring antibiotic therapy. The second group (stage 2 decubitus ulcers) was initially formed by 24 patients; of those, 2 were considered as not assessable due to spontaneous suspension of study treatment, and 2 were excluded due to worsening of their condition requiring antibiotic therapy." Comment: participants withdrawal were justified and balanced between groups |
| Selective reporting (reporting bias) | High risk | Authors did not present mean (or corrected mean by covariate) and a measure of variability such as standard deviation for ulcer area or degree of area changes nor measure of variability for time to reach 50% lesion size regression. Complete ulcer healing was reported only for stage III wounds. |
| Other bias | High risk | Data analysis was based on number of ulcers that exceed the number of randomised participants. |
Humbert 2013.
| Study characteristics | ||
| Methods |
Research design: RCT, parallel, multicentre, comparative, randomised, double‐blind clinical trial Care setting: participants' home and care facilities (29 centres participated in the study: 18 centres in France, 3 in Morocco, and 8 in Poland) Country of origin: France, Morocco, and Poland Publication source:International Wound Journal Year of publication: 2013 Duration of follow‐up: 60 days or until complete healing Sources of funding: Laboratoires Genévrier, with the support of local contract research organisations in France, Morocco, and Poland Unit of randomisation: participant Unit of analysis: participant Inclusion criteria: male or female inpatients or outpatients; aged 18 years or over; diagnosis of leg ulcers of venous or mixed arterial/venous origin present for > 2 months and < 4 years; wound with surface area of the selected target ulcer comprised between 5 and 40 cm2; without necrotic tissue; documented past history of deep venous thrombosis of the lower limbs and/or clinical evidence of post‐thrombotic syndrome with chronic oedema and lipodermatosclerosis and/or available data of an arterial‐venous Doppler examination performed within the previous 6 months and showing post‐phlebitic sequels (residual thrombosis), and/or a superficial or profound reflux on the venous system; with no local HA treatment within the 3 months before inclusion; with albuminaemia ≥ 25 g/L; with ankle/brachial Doppler systolic pressure index ≥ 0.8; with an adapted compression treatment which was worn all during the study; covered by a health insurance system; women of childbearing age had to use a reliable contraceptive method for at least 3 months before and during the study Exclusion criteria: pregnant or breastfeeding women or women planning to be pregnant in the course of the study; with an ulcer of non‐vascular origin (phagedenic pyodermatitis); with clinical evidence of significant arterial insufficiency (claudication, pain at decubitus); with an ulcer due to a general cause (haematological cause); with any type of diabetes; suffering from hepatic disorders (ALAT/ASAT ≥ 2.5 ULN); suffering from renal disorders (creatinine clearance < 30 mL/min); with known allergy to local anaesthetics such as to Xylocaıne, Lidocaıne or Prilocaıne; with a clinical suspicion of general infection (erysipelas, phlegmon); presence of at least 1 of the following symptoms, reminiscent of the local and/or general infection: peri‐ulcerous inflammation, odorous and purulent flow, adenopathy, lymphangitis, fever, unexpected healing interruption; presence of a recent venous thrombosis (< 3 months); known allergy to 1 of the components of the investigational medical devices; under treatments delaying the healing process: systemic corticosteroids, cytostatic drugs, immunosuppressive agents; participation in any type of clinical investigation concurrently or within the 2 months preceding the inclusion visit |
|
| Participants |
Number of participants: 89 participants were included in the analysis (ITT population), instead of the 140 participants previously calculated (group 1: 45 participants; group 2: 44 participants). In addition, 72 participants were defined as per‐protocol population (group 1: 38 participants; group 2: 34 participants). Characteristics of the examined groups: the study was conducted with inpatients or outpatients with 1 or several leg ulcers of venous or mixed arterial/venous origin Female gender: group 1: 44.4% (n = 20); group 2: 54.5% (n = 20) Age: group 1: 59.4 ± 2.5; group 2: 64.1 ± 2.7 |
|
| Interventions |
Details of the intervention Group 1: 0.05% HA impregnated cotton gauze pad (ialuset gauze pad manufactured by Laboratoires Genévrier, Sophia‐Antipolis, France) Group 2: neutral vehicle (same formulation as ialuset gauze pad but without HA) Dressing procedure: the ulcer was cleaned with physiological serum, and the assigned dressing was then applied by a nurse at the participant’s home (for outpatients), or in various care facilities (for inpatients) except during evaluation visits when the dressing was applied by the investigator. The gauze pad was applied to the wound, covered with sterile gauze, and then covered with an appropriate bandage. Co‐interventions: surgical wound excision procedures were authorised if necessary with or without previous local anaesthesia. Systemic antibiotics could be used in case of clinically relevant infection. Systemic analgesics were authorised, provided they were interrupted at least 10 hours before each visit to allow a proper evaluation of wound‐related pain. The use of high‐dosage systemic corticosteroids, cytostatic and immunosuppressive drugs, and local use of proteolytic enzymes for wound debridement were not permitted during the study. Duration of treatment: 60 days or until complete healing |
|
| Outcomes |
Primary outcomes of the review: complete wound healing (45 days); time to complete wound healing; adverse effect Secondary outcomes: percentage of wound size reduction (after 45 days of treatment); pain was assessed according to VAS |
|
| Notes | Losses to follow‐up: 28 participants did not compete the study (n = 18 in group 1; n = 10 in group 2) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation list was prepared by Data Management & Statistics Unit of IBSA Institut Biochimique SA, Switzerland using a validated software from SAS Institute Inc., Cary, NC in accordance with international standards" |
| Allocation concealment (selection bias) | Unclear risk | Allocation method was properly described but there was no mention of concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Authors state it is a double‐blinded study, however, there is no description of the method for blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Two independent readers, equally blind to treatment, measured the wound size based on the drawings on sterile tracing papers, in a centralised fashion and using a digital planimetrics system, Visitrak" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Sample size calculation resulted in 140 volunteers. An interim analysis was intended to be performed upon 80 subjects completing the study, however, authors reported that 28 individuals did not complete the study from 89 originally included in the ITT population (10 individuals did not meet inclusion criteria); therefore, less than 80. Authors reported that the study was then stopped based on the significance difference observed for the primary performance parameter (area reduction after 45 days of treatment) in this interim analysis. |
| Selective reporting (reporting bias) | Low risk | All proposed outcomes described in methods section are presented and properly analysed. |
| Other bias | Low risk | Participants were not significantly different between the 2 arms as regards gender, age and body mass index, frequency of medical and/or surgical background, localization and duration of target ulcer, proportion of fibrinous or granulation tissue. Only 1 ulcer was assessed per volunteer. Project management and monitoring of the study was carried out by the sponsor, Laboratoiries Genevrier, with the support of local contract research organisations in France, Morocco and Poland. |
Lee 2016.
| Study characteristics | ||
| Methods |
Research design: RCT, prospective, randomised, placebo‐controlled, single‐centre Care setting: not reported Country of origin: Korea Publication source:Wound Repair and Regeneration Year of publication: 2016 Duration of follow‐up: 12 weeks Sources of funding: Genewel (Seoul, South Korea) Unit of randomisation: participant Unit of analysis: participant Inclusion criteria: those with type 1 or 2 diabetes mellitus; aged over 20 years; those with an ulcer size ≥ 1 cm2 for more than 6 weeks, without signs of healing; those with an ulcer graded as Wagner stage 1 or 2; those with adequate circulation in the foot confirmed by transcutaneous partial pressure of oxygen (TcPO2) ≥ 30 mmHg or palpable pulses at the ankle (dorsalis pedis artery or posterior tibial artery); those with diabetic peripheral neuropathy diagnosed with the Michigan Neuropathy Screening Instrument (MNSI score of ≥ 2.5); those without local or systemic signs of DFU infection (local tenderness, erythema, fever, and leukocytosis); and those who signed the written consent form after full description of the clinical trial Exclusion criteria: diagnosis of presented osteomyelitis, systemic inflammatory disease, or autoimmune disease (e.g. rheumatoid arthritis, gout, systemic lupus erythematosus, and ankylosing spondylitis) and deep vein thrombosis; patients who were pregnant, were undergoing immunosuppressant treatment, or had any systemic wasting disease (e.g. chronic obstructive pulmonary disease, chronic heart failure, and malignancy) |
|
| Participants |
Number of participants: 34 (ITT) participants were enrolled and randomised into the 2 groups with a 1:1 ratio (17 participants in each group). 25 (per‐protocol) participants were included in the final analysis (group 1: 13 participants; group 2: 12 participants). Male gender: group 1: 84% (n = 11); group 2: 66% (n = 8) Age: group 1: 57.08 ± 13.92; group 2: 57.58 ± 13.01 |
|
| Interventions |
Details of the intervention Group 1: hyaluronic acid dressing material (Healoderm, Genewel, Seoul, South Korea) Group 2: conventional moisture‐retentive dressing (sterile petrolatum gauze, SungKwang, Cheonan‐si, South Korea) Dressing procedure: thorough cleansing of the wound bed and margin with normal saline, additional debridement to remove necrotic tissues and expose healthy bleeding margin if necessary, trimming of the corresponding dressing material according to the size and shape of the ulcer, and direct application of the dressing material to the wound bed and then covering with polyurethane foam (Medifoam, Genewel). The dressing change was performed during the scheduled weekly follow‐up. However, depending on the amount of exudate, additional dressing change was performed 2 to 3 times per week. Both dressing materials were prepared in an identical packaging with the same label to ensure blinding. However, due to different morphology of the dressing materials, the investigators were not blind to the dressing materials. Co‐interventions: according to participants' clinical presentation (general strength, balance, gait pattern, and daily accommodation), an orthopaedic shoe with rigid sole, crutches, and/or wheelchairs were additionally prescribed Duration of treatment: 12 weeks |
|
| Outcomes |
Primary outcomes of the review: complete wound healing (12 weeks); rate of adverse effects and events Secondary outcomes: change of wound size and area; velocity of healing was also reported |
|
| Notes | Losses to follow‐up: 9 participants (group 1: n = 4; group 2: n = 5) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A biostatistician who was blinded to the purpose of the study conducted a stratified permuted block randomizations using the SAS system" |
| Allocation concealment (selection bias) | Unclear risk | Authors do not describe how the allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Both the dressing materials were prepared in an identical packaging with the same label to ensure blinding. However, due to different morphology of the dressing materials, the investigators were not blind to the dressing materials" Comments: there was no personnel blinding due to the characteristics of the dressing materials. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "... the ulcer size was measured by a trained orthopaedic fellow who was blind and independent of this study. The ulcer size was measured on a weekly basis, for 12 weeks, ..." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High number of participants lost in follow‐up, 9 out of 34 (26%). Authors did not use intention to treat analysis. |
| Selective reporting (reporting bias) | Low risk | All proposed outcomes described in methods section are presented and properly analysed. |
| Other bias | Low risk | The demographics, medical status, and baseline DFU characteristics of the 2 groups were similar. The authors declare that there are no conflicts of interest. |
Meaume 2008.
| Study characteristics | ||
| Methods |
Research design: randomised, parallel‐group, prospective, open, multicentre Care setting: inpatients or outpatients, 18 centres in 3 countries (France, 15 centres; Italy, 2 centres; Switzerland, 1 centre) Country of origin: France, Italy, and Switzerland Publication source:Current Medical Research and Opinion Year of publication: 2008 Duration of follow‐up: 42 days Sources of funding: IBSA Institut Biochimique SA (Pambio‐Noranco, Switzerland) and Laboratoires Genévrier (Antibes, France) Unit of randomisation: participant Unit of analysis: participant Inclusion criteria: inpatients or outpatients of any gender, of 18 years of age or more; with life expectation longer than the duration of the study and having given their written informed consent to the participation in the study; participants should have 1 or more leg ulcers of varicose, post‐thrombotic or mixed venous‐arterial origin assessed by clinical criteria and venous Doppler examination, showing signs of post‐phlebitic, residual thrombosis or either a superficial or a profound return flow on the venous system; ulcers must have been present for more than 2 months but less than 1 year, and sized between 5 and 40 cm2 Exclusion criteria: the presence of fibrin was tolerated if corresponding to less than 50% of the lesion area, whereas the presence of necrotic tissue was reason for exclusion; serum albumin values < 25 g/L; a systolic pressure index < 0.8 (ankle pressure/humeral pressure); ulcers of non‐vascular origin or caused by a systemic disease, or patients suffering from uncontrolled diabetes; suspicion of infection (e.g. erysipelas, phlegmon); a venous thrombosis in the previous 3 months, presence of diffuse eczema around the ulcer; known hypersensitivity to 1 of the hydrocolloid dressings components; use of local treatments (e.g. topical proteolytic enzymes) other than the tested medical devices, concomitant use of drugs that can negatively influence the wound‐healing process such as systemic corticosteroids, cytostatics, and immunosuppressants; patients having participated in another trial during the 2 months preceding the inclusion in our study; pregnant or breastfeeding women; and participants not willing or not able to respect the protocol restrictions |
|
| Participants |
Number of participants: 125 participants (group 1: 62 participants; group 2: 63 participants). All 125 included participants were assessed for efficacy and safety in the ITT analysis, whereas the per‐protocol analysis was performed on the 108 participants (group 1: 56 participants; group 2: 52 participants who had either achieved a complete wound healing before day 42 or completed the 42‐day treatment period without major protocol violations). Male gender: group 1: 30.15% (n = 19); group 2: 56.45% (n = 35) Age: group 1: 73 ± 1.4; group 2: 75 ± 1.4 |
|
| Interventions |
Details of the intervention Group 1: hydrocolloid dressing containing 0.2% of HA Group 2: hydrocolloid dressing not containing HA Dressing procedure: "at the inclusion visit (day 1), a surgical debridement of the ulcer area was performed when necessary. For this purpose, use of local anaesthetics – either in liquid or cream forms – was allowed. The lesion was then cleaned with physiological solution at each control visit, before the application of the hydrocolloid dressing. For participants having several ulcers or a bilateral ulcer, the investigator selected 1 lesion only to be treated with the tested medical devices according the above‐mentioned criteria, whereas the other ulcers were treated according to standard treatment protocols of the centres." Co‐interventions: "a suitable and individually adapted, effective elastic stocking was prescribed by the investigator and worn by all participants. A secondary bandage was used only if strictly necessary, according to the judgement of the investigator." Duration of treatment: for a maximum treatment period of 6 consecutive weeks (42 days). Once included, participants were always assessed by the same personnel in charge of the study at each control visit, after 7, 14, 28, and 42 days or until a complete wound healing was recorded. |
|
| Outcomes |
Primary outcomes of the review: complete healing; adverse events Secondary outcomes: reduction of the wound area; evolution of the wound bed conditions; pain and itching were assessed by participants on a 100‐millimetre VAS |
|
| Notes | Losses to follow‐up: 22 participants dropped out before day 42: complete healing of their ulcer (group 1: n = 4; group 2: n = 4) and due to adverse events (group 1: n = 3; group 2: n = 4) or other reason (group 1: n = 4; group 2: n = 3) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Following inclusion, patients were randomly assigned to one of the two arms of treatment, both in form of a hydrocolloid dressing of 10X10 cm, according to a computer generated randomisation list" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Sealed envelopes containing the treatment code for each individual patient were given to the investigator at each centre. The investigator could open the envelope only after having included a patient in the study and only then know to which treatment group that patient had been allocated" Comment: authors did not specify if envelops were sequentially numbered and opaque. We could not obtain this information from authors; therefore, we judged this study as unclear for this domain. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study was open‐label. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Twenty‐two patients stopped the treatment before day 42: 8 patients (4 in each treatment arm) for the complete healing of their ulcer, and 14 patients (7 in each experimental group) due to AEs or other reason. No statistical difference was found between the 2 groups as far as the number of patients withdrawn and the reason for withdrawal were concerned. Three patients were identified as protocol violators, all in the HC group: 1 had an ulcer area of 80 cm2 at inclusion (inclusion criterion was a maximum ulcer area of 40 cm2), and 2 other patients used non‐authorized local treatments." |
| Selective reporting (reporting bias) | Low risk | All proposed outcomes described in methods section are presented and properly analysed. |
| Other bias | Low risk | The proportion of males was higher in the control group but other participants characteristics were balanced between groups. Only 1 ulcer was assessed per volunteer. No conflict of interest was reported by authors. |
Mikosinski 2021a.
| Study characteristics | ||
| Methods |
Research design: RCT, parallel, prospective, multicentre, multinational, randomised, double‐blind, clinical study conducted between 13 June 2017 and 31 December 2018 Care setting: 14 centres, 2 in France and 12 in Poland Country of origin: France and Poland Publication source:Wounds Year of publication: 2021 Duration of follow‐up: 23 weeks or until complete healing Sources of funding: IBSA Institut Biochimique S.A Unit of randomisation: participant Unit of analysis: participant Inclusion criteria: adult males and females older than 18 years were eligible for the study if they experienced 1 or more chronic leg ulcers of venous or mixed (venous and arterial, with a predominant venous component) origin of more than 2 months and less than 4 years’ duration. In participants presenting with more than 1 ulcer, the investigator selected a target ulcer. Exclusion criteria: patients with an ulcer of non‐vascular origin or related to a general cause, evidence of dominant significant arterial insufficiency and/or ABPI that was not between 0.8 and 1.2, diabetes mellitus, hepatic or renal failure, clinical suspicion of wound infection, an ulcer with exposed tendon or bone or due to malignancy, recent history of venous thrombosis, and/or ongoing treatment with drugs known to adversely affect the healing process were excluded |
|
| Participants |
Number of participants: 189 patients were screened, 169 were randomised, and 168 (82 in the HA gauze pad group and 86 in the neutral gauze pad group) were eventually enrolled in the study and received at least 1 application of the IMD (safety analysis set). Of these, 164 (83 in the HA gauze pad group and 81 in the neutral gauze pad group) were included in the full analysis set (all people in the safety analysis set who received 1 or more postbaseline efficacy assessment). Female gender: HA gauze pad group: 54.9% (n = 45); neutral gauze pad group: 57% (n = 49) Age: HA gauze pad group: 72.6 ± 13.80 (n = 82); neutral gauze pad group: 67.20 ± 12.48 (n = 86) |
|
| Interventions |
Details of the intervention Group 1: the HA‐containing gauze pad was a 10 cm x 10 cm, sterile, ready‐to‐use, fixed‐dose dressing for topical use, impregnated with 0.05% sodium hyaluronate Group 2: the neutral comparator contained the same ingredients except for HA and had identical visual and physical characteristics to the test product Dressing procedure: study treatments were used in conjunction with standard local therapy that specified cleansing the target ulcer with sterile saline before each application, with or without the use of surgical debridement or local anaesthesia as necessary. In the event of clinical evidence of infection (confirmed on swabbing), therapy with systemic antibiotics was considered, but the use of topical antimicrobials and antiseptics was prohibited. After wound cleansing, the HA gauze pad or neutral comparator was applied directly to the target ulcer once daily by the study nurse or authorised study personnel either at the participant’s home or at the clinic. During the study visits, the gauze pad was applied by the investigator (or designee). The gauze pads were placed onto the entire cavity of the target ulcer, and the wound area was then covered with a sterile dressing and finally completed by appropriate pressure bandaging. Wound debridement, dressing, and compression were only applied by personnel with knowledge and experience in the assessment and management of patients with leg ulcers. Co‐interventions: standard care (i.e. ulcer cleansing, debridement/anaesthesia as necessary, and optimised compression) Duration of treatment: 20 weeks, or until complete healing |
|
| Outcomes |
Primary outcomes of the review: ulcer healing as evaluated by the central assessor blinded to the product used and confirmed 3 weeks after the end of treatment Secondary outcomes: the percentage of completely healed target ulcers as assessed by the investigator and at all other scheduled study visits as assessed by the blinded central assessor. Endpoints also included target ulcer residual area relative to baseline, calculated as percentage relative to the ulcer area at the time of randomisation, the condition of the peri‐ulcerous skin, the total amount of analgesics used, the percentage of participants presenting with infection after application of the first IMD confirmed by swabbing of the target ulcer, patient adherence to treatment, time to achieve complete healing as centrally assessed, and pain intensity as measured by VAS. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization list was prepared by the Data Management and Statistics Department of the sponsor according to standard operating procedures, using validated software (SAS Institute Inc)." |
| Allocation concealment (selection bias) | Low risk | Quote: "The study was conducted in double‐blind fashion, with treatment allocation hidden to the participants, investigator, sponsor, contract research organization team, and the central assessor, located separately from any of the study sites. Strict procedures were adopted to maintain the blind throughout the study." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Strict procedures were adopted to maintain the blind throughout the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All personnel involved in the trial were blinded to the treatment, and the HA gauze pads and neutral gauze pads, packaging, and labelling were indistinguishable from one another. In addition, the clinical assessment of the primary efficacy variable (target ulcer healing) was centrally and independently performed by an experienced, blinded assessor judging clinical results on standardised photography of the target ulcer. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Overall, 144 patients (71 in the HA gauze pad group; 73 in the neutral gauze pad group) completed the study; 25 discontinued treatment (13 in the HA group; 12 in the neutral gauze pad group). Of those, 18 withdrew (9 in each group) for personal reasons—3 in the HA group (2 for AEs and 1 for other reasons) and 2 in the neutral gauze pad group due to lack of efficacy. One person in each group was lost to follow‐up. Among the 5 participants who had more than 1 major protocol deviation (3 in the HA group; 2 in the neutral gauze pad group), the most common reason was violation of exclusion criteria." |
| Selective reporting (reporting bias) | Low risk | Outcomes in methods section were described in results section. |
| Other bias | Low risk | The study was sponsored and funded by IBSA Institut Biochimique S.A. The project was managed and monitored by the contract research organisation CROMSOURCE through its local organisations in Poland and France. Participants and target ulcer were similar between groups. |
Mikosinski 2021b.
| Study characteristics | ||
| Methods |
Research design: RCT, parallel‐group, prospective, multicentre, randomised, double‐blind, clinical study conducted between 13 June 2017 and 17 April 2019 Care setting: 20 centres in Poland Country of origin: Poland Publication source:Wounds Year of publication: 2021 Duration of follow‐up: 23 weeks or until complete healing Sources of funding: IBSA Institut Biochimique S.A Unit of randomisation: participant Unit of analysis: participant Inclusion criteria: adult males and females 18 years or older were eligible for inclusion in the study if the following criteria were met: 1 or more chronic leg ulcers of venous (varicose or post‐thrombotic) or mixed (venous and arterial) origin with a predominance of venous origin, with a duration of at least 2 months but less than 4 years; the target ulcer area was at least 5 cm2 but no more than 40 cm2, with less than 50% of necrotic tissue; an arteriovenous Doppler examination showing superficial or profound venous reflux and/or a well‐documented history of deep venous thrombosis and/or clinical evidence of post‐thrombotic syndrome with chronic oedema and lipodermatosclerosis Exclusion criteria: the presence of a non‐vascular ulcer or a general cause (e.g. haematologic cause), ankle‐brachial index less than 0.8 or higher than 1.2 and/or dominant significant arterial insufficiency, diabetes mellitus of any type, hepatic or renal failure, presence of wound infection, an ulcer with exposed tendon or bone or due to malignancy, a recent history of venous thrombosis, and ongoing treatment with drugs known to adversely affect the healing process |
|
| Participants |
Number of participants: a total of 199 European participants were screened, and 168 (85 in the HA cream group and 83 in the neutral cream group) were eventually enrolled in the study and received at least 1 application of the IMD (safety analysis set). Of these participants, 164 were included in the full analysis set (all participants in the safety analysis set who had at least 1 postbaseline efficacy assessment), with 83 in the HA cream group and 81 in the neutral cream group. A total of 144 participants (HA cream group, 70; neutral cream group, 74) completed the study. Female gender: HA cream group: 61.2% (n = 52); neutral cream group: 60.2% (n = 50) Age: HA cream group: 68.9 ± 12.95 (n = 85); neutral cream group: 70.0 ± 12.17 (n = 83) |
|
| Interventions |
Details of the intervention Group 1: the active treatment (HA cream) contained HA 0.2% intended for topical use and was supplied for the study in 100‐gram tubes Group 2: the neutral comparator cream contained the same ingredients, with the exception of HA, and had visual and physical characteristics identical to those of the active cream Dressing procedure: after wound cleansing, the HA cream or neutral comparator cream was applied directly to the target ulcer once daily by the study nurse or authorised study personnel at either the participant's home or clinic. Following application of the cream, the wound area was covered with a sterile gauze dressing and an appropriate long‐stretch graduated elastic bandage with stirrup (BIFLEX 16+ PRACTIC bandage), which was provided to all sites; this regimen was applied to all participants according to the standard of care. Co‐interventions: standard care (i.e. ulcer cleansing, debridement/anaesthesia as necessary, and optimised compression) Duration of treatment: 20 weeks, or until complete healing |
|
| Outcomes |
Primary outcomes of the review: the primary efficacy endpoint was ulcer healing, defined as 100% re‐epithelialisation of the wound area at 20 weeks or at any earlier visit if healing occurred before week 20, as evaluated by the central blinded assessor and confirmed 3 weeks after initial healing achievement Secondary outcomes: secondary efficacy endpoints included the percentage of completely healed target ulcers as assessed by the investigator, and at all other scheduled study visits as assessed by the central blinded assessor; target ulcer area relative to baseline at each study visit; condition of the peri‐ulcerous skin; total amount of analgesics used; rate of infection of the target ulcer; adherence to treatment; time to achieve complete healing as centrally assessed; and pain intensity as self‐assessed by the participant on a VAS at each study visit |
|
| Notes | Dropouts: a total of 144 participants (HA cream group, 70; neutral cream group, 74) completed the study. 26 participants (HA cream group, n = 17; neutral cream group, n = 9) discontinued treatment. Of those, 14 participants (HA cream group, n = 9; neutral cream group, n = 5) withdrew from the study for personal reasons. 3 participants (HA cream group, n = 2; neutral cream group, n = 1) withdrew due to adverse events and 3 (HA cream group, n = 1; neutral cream group, n = 2) due to serious adverse events. 3 participants in the HA cream group withdrew because of a protocol violation (n = 1) or were lost to follow‐up (n = 2), and 3 participants (HA cream group, n = 2; neutral cream group, n = 1) withdrew for other reasons. 1 participant in each group was lost to follow‐up. A total of 5 participants (HA cream group, n = 4; neutral cream group, n = 1) had at least 1 major protocol deviation, most commonly for prohibited concomitant medication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization list was prepared using validated software (SAS Institute Inc) by the Data Management and Statistics Department of the sponsor and stored in electronic form in a secure directory to ensure confidentiality in full respect of standard operating procedures." |
| Allocation concealment (selection bias) | Low risk | Quote: "The study was double‐blind; treatment allocation was kept hidden to the subject, investigator, sponsor, contract research organization team, and central assessor. Strict procedures were followed throughout the study to maintain blinding." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The active treatment (HA cream) contained HA 0.2% intended for topical use and was supplied for the study in 100‐g tubes. The neutral comparator cream contained the same ingredients, with the exception of HA, and had visual and physical characteristics identical to those of the active cream. All personnel involved in the trial were blinded to the treatment, and the presentation, packaging, and labeling of the HA cream and neutral comparator cream were fully indistinguishable." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "To minimize bias, the test and comparator treatments were randomly allocated using a standard central randomization system, and access to the randomization code information was strictly regulated and monitored, and the clinical assessment of the primary efficacy variable was centrally and independently performed by an experienced, blinded assessor judging clinical results via standardized photography of the target ulcer. All personnel involved in the trial were blinded to the treatment, and the presentation, packaging, and labeling of the HA cream and neutral comparator cream were fully indistinguishable." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "A total of 144 subjects (HA cream group, 70; neutral cream group, 74) completed the study. Twenty‐six subjects (HA cream group, n = 17; neutral cream group, n =9) discontinued treatment. Of those, 14 subjects (HA cream group, n = 9; neutral cream group, n = 5) withdrew from the study for personal reasons. Three subjects (HA cream group, n = 2; neutral cream group, n = 1) withdrew for AEs and 3 (HA cream group, n = 1; neutral cream group, n = 2) for SAEs. Three subjects in the HA cream group withdrew because of a protocol violation (n =1) or were lost to follow‐up (n = 2), and 3 subjects (HA cream group, n = 2; neutral cream group, n = 1) withdrew for other reasons. One subject in each group was lost to follow‐up. A total of 5 subjects (HA cream group, n = 4; neutral cream group, n = 1) had at least 1 major protocol deviation, most commonly for prohibited concomitant medication." Comment: the sum for the number of participants in the description does not match the number of dropout reported. |
| Selective reporting (reporting bias) | Low risk | Comments: all outcomes described in the methods section were described in the results section. |
| Other bias | Low risk | Participants and target ulcer were similar between groups. The study was sponsored and funded by IBSA Institut Biochimique S.A. |
Ortonne 1996.
| Study characteristics | ||
| Methods |
Research design: multicentre controlled study Care setting: hospitalised patients Country of origin: France Publication source:Journal of Dermatological Treatment Year of publication: 2008 Duration of follow‐up: 21 days Sources of funding: IBSA Institut Biochimique SA (Lugano, Switzerland) Unit of randomisation: participant Unit of analysis: participant Inclusion criteria: hospitalised patients; male and female; presenting with 1 or more varicose ulcers of venous or post‐thrombotic origin; between 3 and 12 cm in diameter; with a systolic pressure index > 0.9 mmHg; and which had been present for more 3 months Exclusion criteria: patients with traumatic wounds; ulcers of arterial origin, ulcers due to necrotic angiodermatitis, distal necrosis, non‐stabilised cardiac insufficiency, non‐stable venous insufficiency; those treated with arterial vasodilator drugs within the previous 7 days; pregnant women and bedridden patients |
|
| Participants |
Number of participants: 51 (ITT) participants presenting with 1 or more varicose ulcers of venous or post‐thrombotic origin (group 1: 26 participants; group 2: 24 participants), 50 (per‐protocol) Male gender: group 1: 37% (n = 10); group 2: 29% (n = 7) Age: group 1: 66.2 ± 3.1; group 2: 69.7 ± 3.6 |
|
| Interventions |
Details of the intervention Group 1: HA gauze pad (each 10 cm x 10 cm gauze pad was impregnated with 4 g of cream containing 0.05% sodium hyaluronate) per day for 21 days Group 2: dextranomer paste daily (each individual dose sachet contained 6.4 g dextranomer) Dressing procedure: a preliminary ulcer debridement was performed before the start of the study. Ulcers were cleaned with a physiological solution before each daily application. Co‐interventions: venotonic treatment (15 participants; group 1: n = 9, group 2: n = 6) Duration of treatment: 21 days |
|
| Outcomes |
Primary outcomes of the review: adverse effects Secondary outcomes: evolution of ulcer (ulcer dimensions, sclerous edges, re‐epithelialised edges, budding zone), pain |
|
| Notes | Losses to follow‐up: 1 participant (group 1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible patients were randomised into two groups of equal size" Comments: the authors reported that the study was randomised, however, they do not describe how the randomising process occurred. |
| Allocation concealment (selection bias) | Unclear risk | Authors did not describe the allocation method and concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study presented 51 participants with loss during follow‐up of 1 participant (2%). Considered as low risk. It does not refer to intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | The protocol is not available but all proposed outcomes described in methods section are presented and properly analysed. |
| Other bias | Unclear risk | Baseline characteristics of volunteers were balanced, however insufficiently described. Did not report conflicts of interest. |
Ramos‐Torrecillas 2015.
| Study characteristics | ||
| Methods |
Research design: randomised, open‐label, clinical trial Care setting: 1 long‐stay hospital and 4 geriatric centres Country of origin: Granada (Spain) Publication source:Biological Research for Nursing Year of publication: 2014 Duration of follow‐up: 36 days Sources of funding: none Unit of randomisation: participant Unit of analysis: ulcer Inclusion criteria: the presence, for more than 8 weeks, of stage II or III pressure ulcers (European Pressure Ulcer Advisory Panel classification), with the largest diameter ≤ 10 cm and showing presence of granulation tissue and the absence of infection and/or necrotic tissue Exclusion criteria: the receipt of immunosuppressive treatment or the presence of cancer, HIV infection, hepatitis, systemic infection or clinical signs compatible with active local infection, active vasculitis, systemic erythematous lupus, or cryoglobulinaemia |
|
| Participants |
Number of participants: 115 participants (ITT), rendering a final study sample of 100 participants (per‐protocol) with 124 stage II to III pressure ulcers. We were able to pool data from 2 arms of the study where hyaluronic acid was the only systematic difference between treatments. Female gender: 60% (n = 60) Age: 82.5 ± 4.7 |
|
| Interventions |
Details of the intervention: Group 1: control group (standard pressure ulcer care) (25 pressure ulcers) Group 2: 1 dose of PRGF (34 pressure ulcers) Group 3: 2 doses of PRGF (25 pressure ulcers) Group 4: 2 doses of PRGF plus HA (40 pressure ulcers) Multiple pressure ulcers in the same participant were treated with the same procedure. Dressing procedure: throughout the study, all participants were treated every 3 days according to the standard hospital protocol: ulcer debridement, cleaning with physiological saline and sterile gauze, application of liquid hydrogel (Intrasite1 Gel, Smith & Nephew, Barcelona, Spain), and placement of a polyurethane dressing (Mepilex Border Lite1, Molnlycke Health Care, Madrid, Spain). PRGF was applied before placement of the dressing on day 0 of the study in treatment group 1 and on days 0 and 15 of the study in treatment groups 3 and 4, in combination with HA in the case of group 3. Co‐interventions: all participants were turned every 2 hours, and the progression of the ulcer was followed using the Pressure Ulcer Scale for Healing (PUSH) Duration of treatment: 36 days |
|
| Outcomes |
Primary outcomes of the review: percentage of completely healed pressure ulcers; adverse effects Secondary outcomes: change in ulcer size |
|
| Notes | Losses to follow‐up: 15 participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We used a computer‐generated randomisation table to randomly assign participants to a control group (standard PU care) or treatment group A (one dose of PRGF), B (two doses of PRGF), or C (two doses of PRGF plus HA)" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reported the numbers who withdrew per study but did not provide reasons or further details (15 lost to follow‐up, 13%). Did not conduct an intention‐to‐treat analysis and multiple ulcers were assessed in the same volunteer. |
| Selective reporting (reporting bias) | Low risk | The protocol is not available but all proposed outcomes described in methods section are presented and properly analysed. |
| Other bias | High risk | Data analysis was based on number of ulcers that exceed the number of randomised participants. There is not enough detail for participants characteristics in each group at baseline (such as demographics, anthropometrics, etc). |
Taddeucci 2004.
| Study characteristics | ||
| Methods |
Research design: open, single‐centre, randomised, parallel, comparative study Care setting: outpatients Country of origin: Italy Publication source:Journal of Wound Care Year of publication: 2004 Duration of follow‐up: 8 weeks or until the ulcer healed, whichever occurred first Sources of funding: none Unit of randomisation: participant Unit of analysis: ulcer Inclusion criteria: consenting patients aged over 18 years; venous ulceration present for at least 3 months Exclusion criteria: arterial, metabolic, or traumatic ulcers; infected ulcers with signs of cellulitis; immunosuppressive, corticosteroid, or cytostatic therapy within 4 weeks prior to study enrolment; insulin‐dependent diabetes; concomitant diseases such as tumours or metabolic diseases; pregnancy or suspected pregnancy |
|
| Participants |
Number of participants: 17 participants with 24 ulcers (group 1: 12 ulcers; group 2: 12 ulcers) Male gender: not described Age: not described |
|
| Interventions |
Details of the intervention Group 1: Hyaluronic acid (Hyalofill‐F, a hyaluronan derivative) covered with sterile gauze and the compression bandage Pehacrepp E Group 2: paraffin gauze (control treatment; standard therapy in Italy) covered with sterile gauze and the same compression bandage Dressing procedure: during the assessment visits the investigator cleansed the ulcer with sterile saline, applied the treatment dressing and then the compression Co‐interventions: followed by compression bandaging Duration of treatment: 8 weeks or until the ulcer healed, whichever occurred first |
|
| Outcomes |
Primary outcomes of the review: complete wound healing Secondary outcomes: change in wound size, pain |
|
| Notes | Losses to follow‐up: 6 participants (group 1: n = 1; group 2: n = 5) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors report the randomisation, however they do not make clear the method used. There is a reference to "... assigned sequentially to one of two treatments:", however we are not sure if this is a referring to a pre‐determined sequence or if they used alternation (which would not be a true randomisation method). We were not able to obtain the answer to this question and the trial was maintained in the review. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High percentage of losses (25%) in the study (withdrawn reported based on ulcers, not subjects). Not clear how many volunteers dropped‐out. |
| Selective reporting (reporting bias) | Low risk | All proposed outcomes described in methods section are presented and properly analysed. |
| Other bias | High risk | Data analysis was based on number of ulcers that exceed the number of randomised participants. Baseline characteristics of volunteers in each group was not reported. |
ABPI: Ankle‐Brachial Pressure Index; ASAT/ALAT: aspartate amino transferase/alanine amino transferase ratio; DFU: diabetic foot ulcer; HA: hyaluronic acid; HC: hydrocolloid; IMD: investigational medical devices; ITT: intention‐to‐treat; PRGF: platelet‐rich plasma growth factor; RCT: randomised controlled trial; ULN: upper limit of normal; VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbruzzese 2009 | HA was not the only systematic difference between treatment groups. Intervention: Vulnamin vs neutral vehicle |
| Caravaggi 2003 | HA was not the only systematic difference between treatment groups. Intervention: HYAFF 11–based autologous dermal and epidermal grafts |
| Caridi 2016 | HA was not the only systematic difference between treatment groups. Intervention: polynucleotides and hyaluronic acid gel (PNHA) (Nucliaskin S) |
| Cuevas 2007 | HA was not the only systematic difference between treatment groups. Intervention: combined hyaluronic acid and antibacterial effect of zinc sulfonamide |
| Edmonds 2000 | Ineligible study design |
| Galasso 1978 | Ineligible study design |
| Maggio 2012 | HA was not the only systematic difference between treatment groups. Intervention: Vulnamin vs calcium alginate |
| Mekkes 2001 | Ineligible study design |
| Prosdocimi 2012 | Ineligible study design |
| Romanelli 2007 | HA was not the only systematic difference between treatment groups. Intervention ‐ OASIS® wound matrix versus hyaloskin |
| Uccioli 2011 | HA was not the only systematic difference between treatment groups. Intervention ‐ autologous grafting using HYAFF scaffolds |
| You 2014 | HA was not the only systematic difference between treatment groups. Intervention ‐ autologous fibroblast‐hyaluronic acid complex |
HA: hyaluronic acid
Differences between protocol and review
We carried out a GRADE assessment on all eligible outcomes where possible and included complete wound healing, time to complete wound healing, adverse events, health‐related quality of life, pain, and change in ulcer size in the summary of findings tables. This allowed a more complete evaluation of the quality of the evidence base. We originally planned to apply Kappa measures to verify the level of agreement among review authors; however, we decided not to do this at the final review stage because it is not a standard procedure in Cochrane Reviews. For the studies that presented standard errors of mean (SEM), we calculated the value of the standard deviation (SD) using SD = SEM x sqrt(n).
Contributions of authors
Hellen Roehrs: conceived the review; designed the review; co‐ordinated the review; extracted data; checked quality of data extraction; analysed or interpreted data; checked quality assessment; performed statistical analysis; produced the first draft of the review; contributed to writing or editing the review; wrote to study authors/experts/companies; performed translations; approved the final review prior to submission. Janislei GD Stocco: designed the review; extracted data; checked quality of data extraction; undertook quality assessment; produced the first draft of the review; contributed to writing or editing the review; approved the final review prior to submission. Franciele Pott: designed the review; extracted data; checked quality of data extraction; undertook quality assessment; produced the first draft of the review; contributed to writing or editing the review; approved the final review prior to submission. Gisely Blanc: designed the review; analysed or interpreted data; produced the first draft of the review; approved the final review prior to submission. Marineli J Meier: conceived the review; designed the review; co‐ordinated the review; extracted data; checked quality of data extraction; analysed or interpreted data; checked quality assessment; checked quality of statistical analysis; produced the first draft of the review; contributed to writing or editing the review; advised on the review; approved the final review prior to submission; is guarantor of the review. Fernando AL Dias: designed the review; co‐ordinated the review; checked quality of data extraction; analysed or interpreted data; undertook quality assessment; checked quality assessment; performed statistical analysis; checked quality of statistical analysis; produced the first draft of the review; contributed to writing or editing the review; advised on the review; performed translations; approved the final review prior to submission; is guarantor of the review.
Contributions of the editorial base
Gill Norman (Editor): edited the review; advised on methodology, interpretation, and content.
Gill Rizzello (Managing Editor): co‐ordinated the editorial process; advised on interpretation and content; edited the protocol and review.
Naomi Shaw, Reetu Child, and Sophie Bishop (Information Specialists): designed and edited the search strategy and edited the search methods sections of the protocol and review.
Tom Patterson (Editorial Assistant): edited the Plain language summary and reference sections of the review.
Sources of support
Internal sources
-
National Council for Scientific and Technological Development CNPq, Brazil
Grant ‐ Bolsa de Produtividade em Desenvolvimento Tecnológico e Extensão Inovadora for Fernando Dias
External sources
-
The National Institute for Health Research (NIHR), UK
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Wounds. The views and opinions expressed are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health and Social Care.
Declarations of interest
Hellen Roehrs: none known
Janislei Stocco: none known
Franciele Pott: works as a health professional
Gisely Blanc: none known
Marineli Meier: none known
Fernando Dias: none known
New
References
References to studies included in this review
Dereure 2012a {published data only}
- Dereure O, Czubek M, Combemale P. Efficacy and safety of hyaluronic acid in treatment of leg ulcers: a double-blind RCT. Journal of Wound Care 2012;21(3):131-2, 134-6, 138-9. [22399081] [DOI] [PubMed] [Google Scholar]
Dereure 2012b {published data only}
- Dereure O, Mikosinki J, Zegota Z, Allaert FA. RCT to evaluate a hyaluronic acid containing gauze pad in leg ulcers of venous or mixed aetiology. Journal of Wound Care 2012;21(11):539-42, 544, 546-7. [DOI] [PubMed] [Google Scholar]
Di Mauro 1991 {published data only}
- Di Mauro C, Ossino AM, Trefiletti M, Polosa P, Beghè F. Lyophilized collagen in the treatment of diabetic ulcer. Drugs under Experimental and Clinical Research 1991;7(XVII):371-3. [PubMed]
Felzani 2011 {published data only}
- Felzani G, Spoletini I, Convento A, Di Lorenzo B, Rossi P, Miceli M, et al. Effect of lysine hyaluronate on the healing of decubitus ulcers in rehabilitation patients. Advances in Therapy 2011;28(5):439-45. [DOI] [PubMed] [Google Scholar]
Humbert 2013 {published data only}
- Humbert P, Mikosinki J, Benchikhi H, Allaert FA. Efficacy and safety of a gauze pad containing hyaluronic acid in treatment of leg ulcers of venous or mixed origin: a double-blind, randomised, controlled trial. International Wound Journal 2013;10(2):159-66. [DOI: 10.1111/j.1742-481X.2012.00957.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2016 {published data only}
- Lee M, Han SH, Choi JW, Chung KH, Lee JW. Hyaluronic acid dressing (Healoderm) in the treatment of diabetic foot ulcer: a prospective, randomized, placebo-controlled, single-center study. Wound Repair and Regeneration 2016;24(3):581-8. [DOI: 10.1111/wrr.12428] [DOI] [PubMed] [Google Scholar]
Meaume 2008 {published data only}
- Meaume S, Ourabah Z, Romanelli M, Manopulo R, De Vathaire F, Salomon D, et al. Efficacy and tolerance of a hydrocolloid dressing containing hyaluronic acid for the treatment of leg ulcers of venous or mixed origin. Current Medical Research and Opinion 2008;24(10):2729-39. [DOI] [PubMed] [Google Scholar]
Mikosinski 2021a {published data only}
- Mikosinski J, Di Landro A, Łuczak-Szymerska K, Soriano E, Caverzasio C, Binelli D, et al. Efficacy and safety of a hyaluronic acid-containing gauze pad in the treatment of chronic venous or mixed-origin leg ulcers: a prospective, multicenter, randomized controlled trial. Wounds 2021;33(6):147-57. [DOI: 10.25270/wnds/040721.02] [DOI] [PubMed] [Google Scholar]
Mikosinski 2021b {published data only}
- Mikosinski J, Di Landro A, Kasztalska-Kazmierczak K, Soriano E, Caverzasio C, Binelli D, et al. Efficacy and safety of a hyaluronic acid-containing cream in the treatment of chronic, venous, or mixed-origin leg ulcers: a prospective, multicenter randomized controlled trial. Wounds 2021;33(11):285-95. [DOI: 10.25270/wnds/100521.01] [DOI] [PubMed] [Google Scholar]
Ortonne 1996 {published data only}
- Ortonne JP. A controlled study of the activity of hyaluronic acid in the treatment of venous leg ulcers. Journal of Dermatological Treatment 1996;7(2):75-81. [Google Scholar]
- Ortonne JP. Comparative study of the activity of hyaluronic acid and dextranomer in the treatment of leg ulcers of venous origin. Annales de Dermatologie et de Vénéréologie 2001;Suppl:13-6. [PMID: ] [PubMed] [Google Scholar]
Ramos‐Torrecillas 2015 {published data only}
- Ramos-Torrecillas J, Garcia-Martínez O, De Luna-Bertos E, Ocaña-Peinado FM, Ruiz C. Effectiveness of platelet-rich plasma and hyaluronic acid for the treatment and care of pressure ulcers. Biological Research for Nursing 2014;17(2):152-8. [DOI] [PubMed] [Google Scholar]
Taddeucci 2004 {published data only}
- Taddeucci P, Pianigiani E, Colletta V, Torasso F, Andreassi L, Andreassi A, et al. An evaluation of Hyalofill-F plus compression bandaging in the treatment of chronic venous ulcers. Journal of Wound Care 2004;13(5):202-4. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abbruzzese 2009 {published data only}
- Abbruzzese L, Rizzo L, Fanelli G, Tedeschi A, Scatena A, Goretti C, et al. Effectiveness and safety of a novel gel dressing in the management of neuropathic leg ulcers in diabetic patients: a prospective double-blind randomized trial. International Journal of Lower Extremity Wounds 2009;8(3):134-40. [DOI: 10.1177/1534734609344140] [DOI] [PubMed] [Google Scholar]
Caravaggi 2003 {published data only}
- Caravaggi C, De Giglio R, Pritelli C, Sommaria M, Dalla Noce S, Faglia E, et al. HYAFF 11-based autologous dermal and epidermal grafts in the treatment of noninfected diabetic plantar and dorsal foot ulcers: a prospective, multicenter, controlled, randomized clinical trial. Diabetes Care 2003;23(10):2853-9. [DOI] [PubMed] [Google Scholar]
Caridi 2016 {published data only}
- Caridi G, Massara M, Acri I, Zavettieri S, Grande R, Butrico L, et al. Trophic effects of polynucleotides and hyaluronic acid in the healing of venous ulcers of the lower limbs: a clinical study. International Wound Journal 2014;13(1):754-8. [DOI: 10.1111/iwj.12368] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cuevas 2007 {published data only}
- Cuevas FR, Méndez AA, Andrade IC. Zinc hyaluronate effects on ulcers in diabetic patients. Gerokomos 2007;2(18):91-105. [Google Scholar]
Edmonds 2000 {published data only}
- Edmonds M, Foster A. Hyalofill: a new product for chronic wound management. Diabetic Foot Journal 2000;3(1):29-30. [Google Scholar]
Galasso 1978 {published data only}
- Galasso U, Fiumanò F, Cloro L, Strati V. Use of hyaluronic acid in the therapy of varicose ulcers of the lower limbs. Minerva Chirurgica 1978;33(21):1581-96. [PMID: ] [PubMed] [Google Scholar]
Maggio 2012 {published data only}
- Maggio G, Armenio A, Ruccia F, Giglietto D, Pascone M, Ribatti D. A new protocol for the treatment of the chronic venous ulcers of the lower limb. Clinical and Experimental Medicine 2012;12(1):55-60. [DOI] [PubMed] [Google Scholar]
Mekkes 2001 {published data only}
- Mekkes JR, Nahuys M. Induction of granulation tissue formation in chronic wound by hyaluronic acid. Wounds 2001;4(13):159-64. [Google Scholar]
Prosdocimi 2012 {published data only}
- Prosdocimi M, Bevilacqua C. Impaired wound healing in diabetes: the rationale for clinical use of hyaluronic acid plus silver sulfadiazine. Minerva Medica 2012;103(6):533-9. [PMID: ] [PubMed] [Google Scholar]
Romanelli 2007 {published data only}
- Romanelli M, Dini V, Bertone M, Barbanera S, Brilli C. OASIS wound matrix versus Hyaloskin in the treatment of difficult-to-heal wounds of mixed arterial/venous aetiology. International Wound Journal 2007;4(1):3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Uccioli 2011 {published data only}
- Uccioli L, Giurato L, Ruotolo V, Ciavarella A, Grimaldi MS, Piaggesi A, et al. Two-step autologous grafting using HYAFF scaffolds in treating difficult diabetic foot ulcers: results of a multicenter, randomized controlled clinical trial with long-term follow-up. International Journal of Lower Extremity Wounds 2011;10(2):80-5. [DOI] [PubMed] [Google Scholar]
You 2014 {published data only}
- You HJ, Han SK, Rhie JW. Randomised controlled clinical trial for autologous fibroblast-hyaluronic acid complex in treating diabetic foot ulcers. Journal of Wound Care 2014;23(11):521-30. [DOI] [PubMed] [Google Scholar]
Additional references
Agale 2013
- Agale SV. Chronic leg ulcers: epidemiology, aetiopathogenesis and management. Ulcers 2013;44(6):449-56. [DOI: 10.1155/2013/413604] [DOI] [Google Scholar]
Amir 2013
- Amir Y, Halfens RJ, Lohrmann C, Schols JM. Pressure ulcer prevalence and quality of care in stroke patients in an Indonesian hospital. Journal of Wound Care 2013;22(5):254-60. [DOI] [PubMed] [Google Scholar]
Anilkumar 2011
- Anilkumar TV, Muhamed J, Anumol J, Arun J, Mohananc PV, Lissy KK. Advantages of hyaluronic acid as a component of fibrin sheet for care of acute wound. Biologicals 2011;39(2):81-8. [DOI] [PubMed] [Google Scholar]
Barshes 2013
- Barshes NR, Sigireddi M, Wrobel JS, Mahankali A, Robbins JM, Kougias P, et al. The system of care for the diabetic foot: objectives, outcomes, and opportunities. Diabetic Foot and Ankle 2013;4:21847. [DOI: 10.3402/dfa.v4i0.21847] [DOI] [PMC free article] [PubMed] [Google Scholar]
BNF 2017a
- British Medical Association and Royal Pharmaceutical Society of Great Britain. British National Formulary: sodium hyaluronate dressings. www.medicinescomplete.com/mc/bnf/current/PHP101087-sodium-hyaluronate-dressings.htm (accessed 20 October 2017).
BNF 2017b
- British Medical Association and Royal Pharmaceutical Society of Great Britain. British National Formulary: wound management products and elasticated garments. www.medicinescomplete.com/mc/bnf/current/PHP101071-wound-management-products-and-elasticated-garments.htm (accessed 20 October 2017).
Boateng 2008
- Boateng JS, Matthews KH, Stevens HN, Eccleston GM. Wound healing dressings and drug delivery systems: a review. Journal of Pharmaceutical Sciences 2008;97(8):2892-923. [DOI] [PubMed] [Google Scholar]
Boike 2017
- Boike A, Maier M, Logan D. Prevention and treatment of leg and foot ulcers in diabetes mellitus. June 2017. www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/endocrinology/prevention-treatment-diabetic-leg-and-foot-ulcers/ (accessed 20 October 2017).
Boutron 2022
- Boutron I, Page MJ, Higgins JP, Altman DG, Lundh A, Hróbjartsson A. Chapter 7: Considering bias and conflicts of interest among the included studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Bradley 1999
- Bradley M, Cullum N, Nelson EA, Petticrew M, Sheldon T, Torgerson D. Systematic reviews of wound care management: dressing and topical agents used in the healing of chronic wounds. Health Technology Assessment 1999;3(17):1-131. [PubMed] [Google Scholar]
CDC 2003
- US Centers for Disease Control and Prevention (CDC). Morbidity and mortality weekly report: history of foot ulcer among persons with diabetes, United States 2000-2002. November 2003. www.cdc.gov/mmwr/preview/mmwrhtml/mm5245a3.htm (accessed 20 October 2017). [PubMed]
Chen 1999
- Chen WY, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair and Regeneration 1999;7(2):79-99. [DOI] [PubMed] [Google Scholar]
Chen 2011
- Chen CC, Yen CJ, Dai YT, Wang C, Huang GH. Prevalence of geriatric conditions: a hospital-wide survey of 455 geriatric in patients in a tertiary medical center. Archives of Gerontology and Geriatrics 2011;52(1):46-50. [DOI] [PubMed] [Google Scholar]
Collins 2010
- Collins L, Seraj S. Diagnosis and treatment of venous ulcers. American Family Physician 2010;81(8):989-96. [PubMed] [Google Scholar]
Collins 2013
- Collins MN, Birkinshaw C. Hyaluronic acid based scaffolds for tissue engineering: a review. Carbohydrate Polymers 2013;92(2):1262–79. [DOI] [PubMed] [Google Scholar]
Cuddigan 2001
- Cuddigan J, Berlowitz DR, Ayello EA. Pressure ulcer in America: prevalence, incidence and implications for the future. An executive summary of the National Pressure Ulcer Advisory Panel monograph. Advances in Skin and Wound Care 2001;14(4):208-15. [DOI] [PubMed] [Google Scholar]
Deeks 2002
- Deeks JJ. Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcome. Statistics in Medicine 2002;21:1575-600. [DOI] [PubMed] [Google Scholar]
Deeks 2022
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Dias 2013
- Dias TY, Costa IK, Liberato SM, Souza AJ, Mendes FR, Torres GV. Quality of life for venous ulcer patients: a comparative study in Brazil/Portugal. Online Brazilian Journal of Nursing 2013;12(3):491-500. [Google Scholar]
Dicker 2014
- Dicker KT, Gurski LA, Pradhan-Bhatt S, Witt RL, Farach-Carson MC, Jia X. Hyaluronan: a simple polysaccharide with diverse biological functions. Acta Biomaterialia 2014;10(4):1558–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dumville 2013
- Dumville JC, Deshpande S, O'Meara S, Speak K. Foam dressings for healing diabetic foot ulcers. Cochrane Database of Systematic Reviews 2013, Issue 6. Art. No: CD009111. [DOI: 10.1002/14651858.CD009111.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fraser 1997
- Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. Journal of Internal Medicine 1997;242(1):27-33. [DOI] [PubMed] [Google Scholar]
Glanville 2019
- Glanville J, Dooley G, Wisniewski S, Foxlee R, Noel-Storr A. Development of a search filter to identify reports of controlled clinical trials within CINAHL Plus. Health Information and Libraries Journal 2019;36(1):73-90. [DOI] [PubMed] [Google Scholar]
Gunningberg 2013
- Gunningberg L, Hommel A, Bååth C, Idvall E. The first national pressure ulcer prevalence survey in county council and municipality settings in Sweden. Journal of Evaluation in Clinical Practice 2013;19(5):862-7. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence - study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64:407-15. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Higgins 2022
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Jeffcoate 2003
- Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet 2003;361(9368):1545-51. [DOI] [PubMed] [Google Scholar]
Kerr 2012
- Kerr M, on behalf of NHS diabetes. Foot care for people with diabetes in the NHS in England: the economic case for change. March 2012. diabetes-resources-production.s3-eu-west-1.amazonaws.com/diabetes-storage/2017-08/Factsheet%20Footcare.pdf (accessed 20 October 2017).
Kirketerp‐Møller 2011
- Kirketerp-Møller K, Zulkowski K, James C. Chronic wound colonization, infection, and biofilms. In: Bjarnsholt T, Jensen PØ, Moser C, Høiby N, editors(s). Biofilm Infections. 1st edition. New York (NY): Springer-Verlag, 2011:11-24. [DOI: 10.1007/978-1-4419-6084-9] [DOI] [Google Scholar]
Knudson 1993
- Knudson CB, Knudson W. Hyaluronan-binding proteins in development, tissue homeostasis, and disease. FASEB Journal 1993;7(13):1233-41. [PubMed] [Google Scholar]
Lazarus 1994
- Lazarus GS, Cooper DM, Knighton DR, Percoraro RE, Rodeheaver G, Robson MC. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair and Regeneration 1994;2(3):165-70. [DOI] [PubMed] [Google Scholar]
Lazarus 2014
- Lazarus G, Valle MF, Malas M, Umair Q, Maruthur NM, Doggett D, et al. Chronic venous leg ulcer treatment: future research needs. Wound Repair and Regeneration 2014;22(1):34-42. [DOI] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Featherstone R, Littlewood A, Marshall C, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Makai 2010
- Makai P, Koopmanschap M, Bal R, Nieboer AP. Cost-effectiveness of a pressure ulcer quality collaborative. Cost Effectiveness and Resource Allocation 2010;8(11):1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Margolis 2013
- Margolis DJ. Epidemiology of wounds. In: Mani R, Romanelli M, Shukla V, editors(s). Measurements in Wound Healing. 1st edition. London (UK): Springer-Verlag, 2013:145–53. [DOI: 10.1007/978-1-4471-2987-5] [DOI] [Google Scholar]
Medical Dictionary
- The Free Dictionary. Medical dictionary. medical-dictionary.thefreedictionary.com/ (accessed 20 October 2017).
Meyer 1934
- Meyer K, Palmer JW. Polysaccharide of vitreous humor. Journal of Biological Chemistry 1934;107:629-34. [Google Scholar]
Mustoe 2006
- Mustoe TA, O'Shaughnessy K, Kloeters O. Chronic wound pathogenesis and current treatment strategies: a unifying hypothesis. Plastic and Reconstructive Surgery 2006;117(7 Suppl):35-41S. [DOI] [PubMed] [Google Scholar]
Norman 2018
- Norman G, Westby MJ, Rithalia AD, Stubbs N, Soares MO, Dumville JC. Dressings and topical agents for treating venous leg ulcers. Cochrane Database of Systematic Reviews 2018, Issue 6. Art. No: CD012583. [DOI: 10.1002/14651858.CD012583.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
NPUAP/EPUAP/PPPIA 2014
- National Pressure Ulcer Advisory Panel (NPUAP), European Pressure Ulcer Advisory Panel (EPUAP), Pan Pacific Pressure Injury Alliance (PPPIA). Prevention and treatment of pressure ulcers: quick reference guide. October 2014. www.npuap.org/wp-content/uploads/2014/08/Updated-10-16-14-Quick-Reference-Guide-DIGITAL-NPUAP-EPUAP-PPPIA-16Oct2014.pdf (accessed 20 October 2017).
Nusgens 2010
- Nusgens BV. Hyaluronic acid and extracellular matrix: a primitive molecule? Annales de Dermatologie et de Vénéréologie 2010;1 Suppl:3-8. [DOI] [PubMed] [Google Scholar]
O'Meara 2012
- O'Meara S, Cullum N, Nelson EA, Dumville JC. Compression for venous leg ulcers. Cochrane Database of Systematic Reviews 2012, Issue 11. Art. No: CD000265. [DOI: 10.1002/14651858.CD000265.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Meara 2013
- O'Meara S, Martyn-St James M, Adderley U. Foam dressings for venous leg ulcers. Cochrane Database of Systematic Reviews 2013, Issue 5. Art. No: CD009907. [DOI: 10.1002/14651858.CD009907.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oh 2010
- Oh EJ, Park K, Kim KS, Kim J, Yang JA, Kong JH, et al. Target specific and long-acting delivery of protein, peptide, and nucleotide therapeutics using hyaluronic acid derivatives. Journal of Controlled Release 2010;141(1):2-12. [DOI] [PubMed] [Google Scholar]
Oh 2013
- Oh SH, Na SY, Song KS, Lee JH. Sprayable powder of hyaluronate embedded in mildly cross-linked alginate as a post-surgical tissue adhesion barrier. Macromolecular Research 2013;21(11):1263–9. [Google Scholar]
Page 2022
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Pan 2013
- Pan NC, Vignoli JA, Baldo C, Celligoi MA. Hyaluronic acid: characteristics, microbial production and industrial applications. Biochemistry and Biotechnology Reports 2013;2(4):42-58. [DOI: 10.5433/2316-5200] [DOI] [Google Scholar]
Peters 2013
- Peters EJ, Lipsky BA. Diagnosis and management of infection in the diabetic foot. Medical Clinics of North America 2013;97(5):911-46. [DOI] [PubMed] [Google Scholar]
Porter 1995
- Porter JM, Moneta GL, International Consensus Committee on Chronic Venous Disease. Reporting standards in venous disease: an update. Journal of Vascular Surgery 1995;21(4):635-45. [DOI] [PubMed] [Google Scholar]
Powers 2013
- Powers JG, Morton LM, Phillips TJ. Dressings for chronic wounds. Dermatologic Therapy 2013;26:197-206. [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Schultz 2003
- Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K, et al. Wound bed preparation: a systematic approach to wound management. Wound Repair and Regeneration 2003;11(1 Suppl):1-28. [DOI] [PubMed] [Google Scholar]
Schünemann 2022
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Shaharudin 2016
- Shaharudin A, Aziz Z. Effectiveness of hyaluronic acid and its derivatives on chronic wounds: a systematic review. Journal of Wound Care 2016;25(10):585-92. [DOI] [PubMed] [Google Scholar]
Siersma 2014
- Siersma V, Thorsen H, Holstein PE, Kars M, Apelqvist J, Jude EB, et al. Health-related quality of life predicts major amputation and death, but not healing, in people with diabetes presenting with foot ulcers: the Eurodiale study. Diabetes Care 2014;37(3):694-700. [DOI] [PubMed] [Google Scholar]
Tennvall 2000
- Tennvall GR, Apelqvist J, Eneroth M. Cost of deep foot infections in patients with diabetes mellitus. Pharmacoeconomics 2000;18(3):225-38. [DOI] [PubMed] [Google Scholar]
Velasco 2011
- Velasco M. Diagnostic and treatment of leg ulcers [Aspectos diagnósticos y terapéuticos de las úlceras de las piernas]. Actas Dermo-Sifiliográficas 2011;102(10):780-90. [DOI] [PubMed] [Google Scholar]
Voigt 2012
- Voigt J, Driver VR. Hyaluronic acid derivatives and their healing effect on burns, epithelial surgical, wounds, and chronic wounds: a systematic review and meta-analysis of randomized controlled trials. Wound Repair and Regeneration 2012;20(3):317-31. [DOI] [PubMed] [Google Scholar]
Zhu 2006
- Zhu H, Mitsuhashi N, Klein A, Barsky LW, Weinberg K, Barr ML, et al. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells 2006;24(4):928–35. [DOI: 10.1634/stemcells.2005-0186] [DOI] [PubMed] [Google Scholar]
Zinoviev 2014
- Zinoviev EV, Rakhmatullin RR, Almazov IA. New bioplastic material based on hyaluronic acid hydrocolloid. Clinical and Experimental Dermatology Research 2014;5(2):215-6. [DOI: 10.4172/2155-9554.1000215] [DOI] [Google Scholar]
References to other published versions of this review
Roehrs 2016
- Roehrs H, Stocco JG, Pott F, Blanc G, Crozeta K, Meier MJ, et al. Dressings and topical agents containing hyaluronic acid for chronic wound healing. Cochrane Database of Systematic Reviews 2016, Issue 5. Art. No: CD012215. [DOI: 10.1002/14651858.CD012215] [DOI] [PMC free article] [PubMed] [Google Scholar]


