Abstract
Background
Electrical lead abnormalities (ELAs) can result in device malfunction, leading to significant morbidity in patients with cardiac implantable electronic devices (CIEDs).
Objective
We sought to determine the prevalence and management of ELAs in patients with CIEDs.
Methods
This was a retrospective cohort study of patients implanted with a CIED between 2012 and 2019 at a tertiary care center. The primary outcome was ELA defined as increased capture threshold (≥2× implantation value), decreased sensing (≤0.5 implantation value), change in impedance (>50% over 3 months), or nonphysiologic potentials. A secondary outcome of device clinic utilization was also collected.
Results
There were 2996 unique patients (35% female) included with 4600 leads (57% Abbott, 43% Medtronic). ELAs were observed in 135 (3%) leads, including 124 (92%) Abbott and 10 (7%) Medtronic leads (hazard ratio 9.25, P < .001). Mean follow-up was 4.5 ± 2.2 years. ELAs were associated smaller lead French size, atrial location, and Abbott leads. Lead revision was required in 28% of cases. Patients with lead abnormalities had 38% more in-clinic visits per patient year of follow-up compared with those without (P < .001).
Conclusion
ELAs were more frequent in certain models, which increased rates of revision and follow-up. Identification of factors that mitigate these abnormalities to improve lead performance are required to improve care for these devices and provide efficient healthcare.
Keywords: Cardiac implantable electronic devices, Lead failure, Pacing leads, Tendril leads, Electrical noise
Key Findings.
-
▪
The incidence of electrical lead abnormality (ELA) was found to be significantly higher in Abbott compared with primarily Medtronic leads.
-
▪
The most common presentation of ELA was electrical noise, with the need for lead revision in 28%.
-
▪
Variables associated with ELA included lead French size and Abbott manufactured leads. Algorithms for sensing in Abbott devices did not account for all of the ELAs observed.
-
▪
The overall rate of lead revision was significantly higher in Abbott compared with Medtronic leads.
-
▪
Patients with ELAs had a higher rate of device clinic utilization, resulting in an increased burden due to this abnormality.
Introduction
Cardiac implantable electrical devices (CIEDs), including both pacemakers and implantable cardioverter-defibrillators, provide lifesaving therapy for patients with various cardiac conditions. It is critical that these devices and their components are rigorously monitored to ensure their quality and safety. Increases in device advisories have highlighted issues in bench testing, postmarketing surveillance, and processes for reporting of malfunctions. Product performance reports rely on voluntary reporting of device abnormalities by providers to device manufacturers and tends to result in an underestimation of the true incidence of lead failures, systematic surveillance, and lack of adjudication of events.1 Large prospective registries have been performed to mitigate this but are costly to do and may have limited follow-up.2
It is well known that leads are considered to be a weak link in the context of CIEDs.3 Previous studies have shown that CIED leads fail due to electrical abnormalities at a rate of about 2.3% to 5.5% over follow-up periods of at least 1 year.4, 5, 6 This study was designed to determine the prevalence and management of electrical lead abnormalities (ELAs) within a CIED population.
Methods
Design
This study was conducted as a retrospective cohort study including patients who underwent CIED implantation between January 1, 2012, to December 31, 2019, at the Queen Elizabeth II Health Sciences Centre. The study was approved by the institutional review board at the Nova Scotia Health Authority; a waiver of consent was obtained from the institutional review board, and individual consent was not needed. The research adheres to the STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) guidelines.
Data collection
Demographic data, clinical data, and device and lead characteristics were obtained through the electronic medical record. CIED follow-up was performed according to current guidelines, which uses blended in-clinic and remote follow-up for patients with high-voltage devices, and at least annual in-clinic follow-up for low-voltage devices.7, 8, 9, 10 The occurrence of ELAs and the proposed response was determined through review of device interrogations obtained at each follow-up visit contained in the Paceart database. This included remote and in-clinic visits. Both high- and low-voltage leads were included in this analysis.
Definitions
An ELA was defined as one of a sudden change in impedance (rise or drop >50% over a 3-month period), sensing of electrical noise from nonphysiologic potentials, decreased sensing (≤0.5 implantation value), or increased capture threshold (≥2× implantation value).11 A loose set screw was excluded at the time of replacement if a lead revision was performed. Lead dislodgements occurring within 90 days were excluded from this analysis as a potential contribution to an ELA occurrence. ELAs were verified independently by two electrophysiologists (JS, MG, CG, AA, DL, CM, or RP) using available information within the device. In-clinic, patients were evaluated with physical maneuvers to reproduce electrical noise (stretching and isometric exercise, device pocket manipulation, and straining) to differentiate between lead-related oversensing and myopotentials. Electromagnetic interference was diagnosed on the basis of the appearance of the electrical noise as repetitive, regular, high-frequency signals and by verifying sources of electromagnetic interference by history. Recordings due to electromagnetic interference or myopotential oversensing were excluded.
The decision to replace a lead was left to the treating physician’s discretion. The risk-benefit of lead revision was considered in this decision-making process: the risks of lead revision being infection, intraprocedural complications, and subsequent lead dislodgment and the benefit being having a lead without ELA.12 For example, patients who had ventricular leads with ELA and were dependent with resultant pacing inhibition would have undergone revision, as compared with lead in the atrium, where the effect of lack of atrial pacing due to inhibition is of little risk to the patient.
Outcomes
The primary outcome measures included time to detection of ELA, response to the abnormality, rate of revision, and complications associated with lead revision (if revision was required). Secondary outcome measures included number of clinic visits comparing patients with and without ELAs.
Statistical analysis
Descriptive statistics were reported as count and percentage for categorical variables, mean ± SD for normally distributed continuous variables, and median (interquartile range [IQR]) for non-normally distributed continuous variables. Number of clinic visits, remote visits, overall visits, and rates of visits per year of follow-up were calculated and described for the overall cohort and by study group. Patient characteristics were compared between primary device manufacturer, mortality outcome, and ELA. Chi-square or Fisher’s exact tests were used to compare categorical variables between groups and the Wilcoxon rank sum test was used for nonparametric continuous variables. Survival from time of device implantation to death or date of last follow-up was analyzed using Kaplan-Meier plots and log-rank tests to compare the survival distribution between groups. Kaplan-Meier survival estimates were also generated at 1-year and 5-year time points with 95% confidence intervals (CIs), and pointwise P values were generated to compare survival estimates between the groups. A competing risks analysis was performed for the primary outcome of ELA with mortality as competing risk. The cumulative incidence function was compared between groups using Gray’s method. Differences in ELA rates and mortality were compared using a Fine-Gray subdistribution hazard model for competing risks regression. Robust sandwich covariance estimate is used in presence of multiple outcomes per unique patient ID. Univariate analysis using competing risks regression was performed on a priori selected clinical factors, followed by multivariate regression modeling. The proportional hazards assumption was tested using the Kolmogorov-type supremum test. Incidence rate ratios for clinic visits, remote visits, and overall visits per study group were estimated using a negative binomial regression model. Statistical analyses were performed using SAS STAT 14.3 version 9.4 (SAS Institute, Cary, NC). A 2-sided P value of <.05 was the threshold for statistical significance unless otherwise specified.
Results
This study included 4600 leads in 2996 patients, 35% were women, and 71.7% of patients had low-voltage devices and 28.2% had high-voltage devices. Lead characteristics are summarized in Table 1. The lead location was distributed as follows: 39% right atrium and 61% right ventricle. Lead access was 39% subclavian, 36% cephalic, and 6% other (Table 2). Of the leads analyzed, 57% were manufactured by Abbott Laboratories (Chicago, IL), 42% by Medtronic (Minneapolis, MN), and 1% by other manufacturers; 37% of leads were in pacemaker-dependent patients at the time of this analysis. The distribution of lead models was Tendril (26%; Abbott), Isoflex (24%; Abbott), CapSure Fix (30%; Medtronic), and other (20%). High-voltage leads comprised 18.6% of leads analyzed. Mismatch between device manufacturer and lead manufacturer was present in 2.9% of Abbott devices (1.7% Medtronic leads, 1.2% Pacesetter, 0.04% unknown) and 12.7% of Medtronic devices (12.5% Abbott leads, 0.14% Guidant). There were 16 different implanters over the course of the study period. The mean length of follow-up was 4.5 ± 2.2 years.
Table 1.
Lead implant characteristics
| Characteristic | All leads (N = 4600) | No ELA (n = 4465) | ELA (n = 135) | P value |
|---|---|---|---|---|
| Lead location∗ | ||||
| Right atrium | 1770 (39) | 1699 (38) | 71 (53) | .0007 |
| Right ventricle | 2819 (61) | 2755 (62) | 64 (47) | |
| Lead access† | ||||
| Cephalic | 1659 (44) | 1599 (44) | 60 (48) | .6661 |
| Subclavian | 1816 (49) | 1758 (49) | 58 (46) | |
| Other | 257 (7) | 250 (7) | 7 (6) | |
| Lead type‡ | .0222 | |||
| Passive | 1773 (39) | 1734 (39) | 39 (29) | |
| Active | 2823 (61) | 2728 (61) | 95 (71) | |
| Lead manufacturer | <.0001 | |||
| Abbott | 2602 (57) | 2478 (56) | 124 (92) | |
| Medtronic | 1956 (43) | 1946 (44) | 10 (7) | |
| Other | 42 (1) | 41 (1) | 1 (1) | |
| Lead French size§ | .0053 | |||
| <7F | 2736 (60) | 2648 (59) | 88 (66) | |
| 7–8F | 1259 (27) | 1218 (27) | 41 (31) | |
| >8F | 597 (13) | 592 (13) | 5 (3.7) | |
| Lead model | <.0001 | |||
| Tendril | 1192 (26) | 1120 (25) | 72 (53) | |
| 1688TC | 1 | 1 | 0 | |
| 1882TC | 611 | 568 | 43 | |
| 1888TC | 2 | 2 | 0 | |
| 2088TC | 498 | 473 | 25 | |
| LPA1200M | 80 | 76 | 4 | |
| Isoflex | 1090 (24) | 1052 (24) | 38 (28) | |
| 1944 | 179 | 167 | 12 | |
| 1948 | 911 | 885 | 26 | |
| CapSure Fix | 1381 (30) | 1376 (31) | 5 (3.7) | |
| 4073 | 1 | 0 | 1 | |
| 4074 | 277 | 277 | 0 | |
| 4076 | 122 | 120 | 2 | |
| 4574 | 168 | 168 | 0 | |
| 5038 | 24 | 24 | 0 | |
| 5054 | 121 | 121 | 0 | |
| 5076 | 552 | 550 | 2 | |
| 5086 | 71 | 71 | 0 | |
| 5092 | 2 | 2 | 0 | |
| 5554 | 43 | 43 | 0 | |
| Other | 937 (20) | 917 (21) | 20 (15) | |
| Pacing dependent | 1707 (37) | 1633 (37) | 74 (55) | <.0001 |
| High-voltage leads|| | 855 (19) | 836 (19) | 19 (14) | .1820 |
Values are n (%) or n.
ELA = electrical lead abnormality.
11 missing.
868 missing.
4 missing.
8 missing.
3 missing.
Table 2.
Presentation and management of electrical lead abnormality by lead manufacturer
| Variable | All (n = 135) | Abbott (n = 124) | Medtronic (n = 10) | Other (n = 1) |
|---|---|---|---|---|
| Presentation | ||||
| Electrical noise only | 114 (84.4) | 108 | 6 | 0 |
| Increased threshold only | 2 (1.5) | 1 | 1 | 0 |
| Electrical noise and impedance change | 4 (3.0) | 1 | 2 | 1 |
| Electrical noise and increased threshold | 6 (4.5) | 6 | 0 | — |
| Electrical noise and decreased sensing | 7 (5.3) | 7 | 0 | — |
| Impedance change and increased threshold | 1 (0.7) | 1 | 0 | — |
| Electrical noise, impedance change and increased threshold | 1 (0.7) | 0 | 1 | — |
| Management | ||||
| Observation | 77 (57) | 75 (60) | 2 (20) | 0 |
| Reprogram | 20 (15) | 18 (14) | 2 (20) | 0 |
| Revision | 38 (28) | 31 (25) | 6 (60) | 1 (100) |
Electrical lead abnormalities
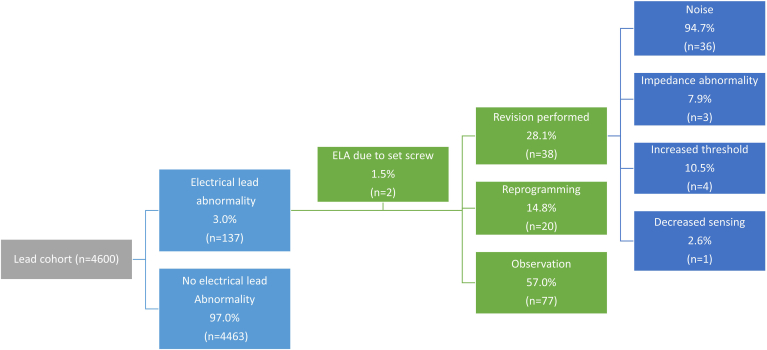
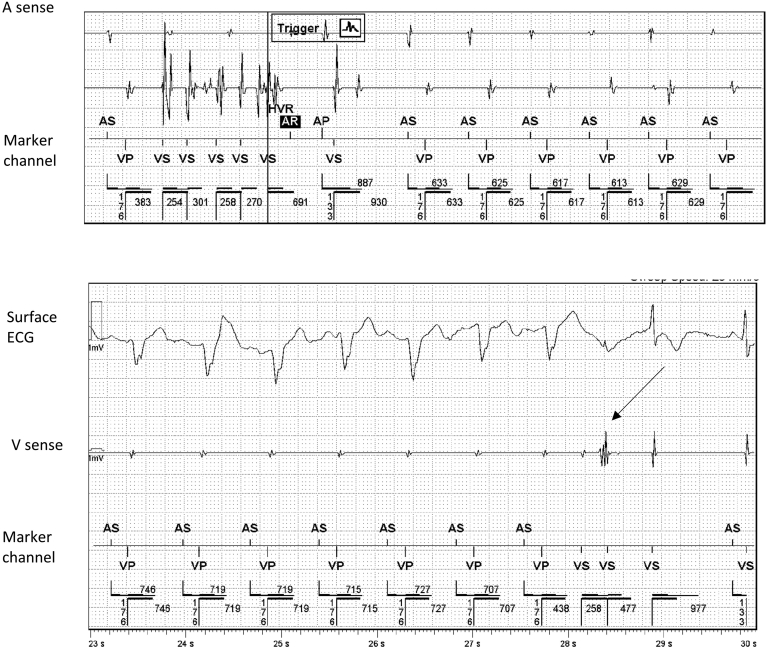
ELAs were observed in 137 (3.0%) leads. Two of these were found to be due set screws at the time of revision and were excluded from the remainder of the analysis for ELAs, leaving 135 (2.9%) leads in the cohort (Figure 1). Abnormalities were seen in 124 (4.8%) of 2602 of Abbott leads and 10 (0.5%) of 1956 of Medtronic leads. There were 19 high-voltage leads with ELA: 16 presented with electrical noise (n = 16 of 19, 84.2%), of which 14 (73.7%) were Abbott manufactured and 5 (26.3%) were Medtronic manufactured. There was no statistically significant difference in the rates of electrical noise in high-voltage leads between manufacturers (P = 1.000). One patient presented with inappropriate shocks. The most common presentation of ELA was electrical noise in 97.0% (example in Figure 2), followed by increased capture threshold (7.4%), change in sensing (5.2%), and impedance change (4.4%) (see example in Figure 3; Table 2). The majority of leads with ELA presented with only 1 abnormality (n = 116 of 135, 86%), with 114 of those leads presenting with electrical noise only. The remaining leads with ELA therefore presented with more than 1 ELA (13%) (Table 2). The mean annual failure rate for Medtronic leads was 0.079%, while for Abbott leads it was 0.72% annually. The cumulative incidence of ELA was significantly higher in Abbott leads when compared with Medtronic leads (HR 9.25, 95% CI 4.93–17.36) (Figure 4A). The number of ELAs seen within the first 3 months was 5 (4.0%) of 124 for Abbott leads and 1 (10.0%) of 10 for Medtronic leads (P = .3779).
Figure 1.
Flow diagram of electrical lead abnormalities (ELAs), response to lead abnormality and presentation of lead abnormality for the entire lead cohort (n = 4600).
Figure 2.
Example electrogram demonstrating oversensing. The top panel is an electrogram stored by the device as a high ventricular rate episode. The V sense channel demonstrates high frequency potentials classified as electrical noise, sensed by the device, thereby inhibiting pacing in this patient who was pacemaker dependent. The bottom panel demonstrates that in-clinic isometric exercises reproduced the noise (see arrow) as seen on the V sense channel, with no evidence of physiologic signals on the electrocardiogram (ECG).
Figure 3.
Example electrogram demonstrating abrupt rise in impedance, as indicated by the red circle. RV = right ventricular.
Figure 4.
Cumulative incidence of electrical lead abnormalities by lead manufacturer over time (A) and lead revision by lead manufacturer over time (B). Medtronic is represented in blue and Abbott in black. A: Hazard ratio of 9.25 (95% confidence interval 4.93–17.36, P value of <.001 by Gray K-sample test). B: Hazard ratio 0.68 (95% confidence interval 0.28–1.69, P value of .4391 by Gray K-sample test).
There were 2326 Abbott leads connected to an Abbott device, of which 121 (5.20%) had ELA. In contrast, of the 275 Abbott leads connected to a Medtronic device, there were 3 (1.09%) that had ELA. There was a significant association between device manufacturer and Abbott leads with ELA (P = .0025). Of the Medtronic leads with an Abbott device, 1 (2.5%) had an ELA compared with 9 (0.47%) of the Medtronic leads with a Medtronic device. There was no evidence to suggest an association between device manufacturer and ELA withing the subset of Medtronic leads (P = .1871).
Factors associated with ELAs
On univariate analysis, factors associated with a greater risk of ELAs included atrial location (4.0% vs 2.3%, P = .0007), active fixation (3.4% vs 2.2%, P = .0222), Abbott manufacturer (4.8% vs 0.51%, P < .0001), smaller lead French size, Tendril model (6.0%) (when compared with Isoflex [3.5%], CapSure Fix [0.4%], and other [2.1%]; P < .0001) and pacing dependence (Table 1). A significant association between ELA and lead manufacturer remained even after accounting for clustering of implant provider. On multivariable analysis, variables associated with ELA included chamber location (HR 1.68, 95% CI 1.06–2.67, P = .0270) and Abbott leads (HR 10.44, 95% CI 5.15–21.17, P < .001) (Table 3). Lead French size, passive vs active fixation, and chamber location were highly correlated; only lead French size and chamber location were kept in the multivariate model. When examining pacing leads only, risk of ELA was associated with atrial lead location on univariate analysis, with a higher rate in the atrial location (HR 1.85, P = .015).
Table 3.
Multivariate competing risk regression for time to ELA
| Variable | ELA (95% CI) | P value |
|---|---|---|
| Primary device type (pacemaker vs ICD) | 0.964 (0.554–1.679) | .8972 |
| Sex (female vs male) | 1.052 (0.731–1.516) | .7841 |
| Age (by decile) | 0.915 (0.764–1.097) | .3367 |
| Lead chamber (RA vs RV) | 1.683 (1.061–2.670) | .0270 |
| Lead manufacturer (Abbott vs Medtronic) | 10.443 (5.151, 21.170) | <.001 |
| Lead French size (by 1 unit) | 0.955 (0.641–1.423) | .8199 |
| Lead access | .7131∗ | |
| Cephalic vs other | 0.808 (0.325–2.010) | .6467 |
| Subclavian vs other | 0.874 (0.357–2.801) | .7682 |
| Cephalic vs subclavian | 0.924 (0.639–1.338) | .6775 |
CI = confidence interval; ELA = electrical lead abnormality; ICD = implantable cardioverter-defibrillator; RA = right atrium; RV = right ventricle.
Global P value for overall difference between all categories in variable.
Clinical response to ELAs
Clinical response to ELA was observation in 57.0%, reprogramming in 14.8%, and revision in 28.1% (Table 3). The overall rate of lead revision was found to be significantly higher in Abbott leads (1.2%), compared with Medtronic leads (0.3%) (P < .001). The median time to lead revision in those requiring revision was 3.0 (IQR 2.0–4.2) years for Abbott leads and 1.8 (IQR 1.1–6.0) years for Medtronic leads, which was found to be a nonsignificant difference (P = .4391) (Figure 4B).
There were 10 patients who had symptoms related to lead noise, 8 of whom were pacemaker dependent. The symptoms included 6 patients with presyncope and 2 with syncope. There was 1 patient with congenital complete heart block with pacing inhibition but no symptoms and 1 patient with long QT syndrome in which inhibition of pacing was felt to be detrimental. There were 27 patients with no symptoms, 21 of whom were pacing dependent, 5 of whom had reproducible noise in clinic, and 1 of whom revised due to right ventricular lead noise alone. Complications with revision occurred in 5 (13.2%) patients, and no patient deaths due to lead abnormalities were observed.
Device clinic utilization
Patients with no ELA had a mean of 2.2 ± 4.13 in-clinic visits per patient year, whereas patients with ELA had a mean of 2.4 ± 0.91 (Figure 4). Patients with ELA had 38.3% more in-clinic visits per year of follow-up when compared with those without ELA (P < .001). When considering those who experienced ELA, this group was found to have significantly more in-clinic, remote, and total visits per year follow-up after the identification of ELA when compared with before ELA (P < .001, P = .0138, and P < .001 for in-clinic, remote, and total visits, respectively). In response to ELA, mean clinic visits were 2.25 ± 0.73 for observation, 2.13 ± 0.72 for reprogramming, and 2.86 ± 1.22 for revision (Figure 5A). The incident rate ratio of clinic visits for observation compared with revision was lowest (0.77, 95% CI 0.96–0.88, P = .0002) when compared with those that were revised, with no significant difference in those that underwent observation or reprogramming (P = .35) (Figure 5B). Similarly, the incident rate ratio for reprogramming compared with revision was 0.70 (95% CI 0.58–0.86, P = .0006).
Figure 5.
A: In-clinic visits per patient year in patients with and without electrical lead abnormalities (ELAs). Bars represent the minimum and maximum values, excluding outliers. The no ELA group is shown in blue and the ELA group is shown in red. Mean clinic visits were 2.2 ± 4.13 and 2.4 ± 0.91, respectively. The mean incident rate ratio for ELA compared with no ELA was 1.38 (95% confidence interval [CI] 1.25–1.53, P < .001). B: In-clinic visits according to response to ELA. Bars represent the minimum and maximum values, excluding outliers. An observation is shown in blue, revision is shown in red, and reprogramming is shown in green. Mean clinic visits were 2.25 ± 0.73, 2.15 ± 0.73, and 2.83 ± 1.21, respectively. The incident rate ratio of clinic visits for observation compared with revision was lowest (0.77, 95% CI 0.58–0.88; P = .0003), with no significant difference in those that underwent observation compared with reprogramming (P = .40). Similarly, the incident rate ratio for reprogramming compared with revision was 0.72 (95% CI 0.58–0.88; P = .0014).
Discussion
This study demonstrates that ELAs are more commonly observed with Abbott leads among the leads studied in this analysis. These abnormalities occurred with both the Isoflex and Tendril lead models but more frequently with the Tendril leads. Observation was the most frequent response to an ELA, but revision was required in some cases, with greater frequency in the case of Abbott lead models. Smaller French size leads, atrial location, and Abbott-manufactured leads were found to be associated with a higher rate of ELA. Patients with ELAs had a higher burden of device clinic visits, compared with those without, as did those who required revision, compared with observation or reprogramming.
Prior studies have examined the risk of ELAs and have observed varying results. Khatiwala and colleagues13 performed a meta-analysis examining non–implantation-related lead malfunction rates in 14,579 leads, finding lead abnormalities in 5% of leads, with a higher incidence in Abbott leads (relative risk 7.81, 95% CI 3.21–19.0). The most common observation was lead noise with normal impedance.13 Abbott leads connected to a non-Abbott generator did have a lower incidence of abnormalities (4.7% vs 8.0%), but this remained higher than that of other manufacturers (1.1%–5.5%).13 We found a higher incidence of ELA in Abbott leads connected to Abbott devices than those with Medtronic devices, as well as higher rates of ELA in atrial leads compared with right ventricular leads. This corroborates previous studies, which suggested that a proportion of lead noise observed is due to Abbott device algorithms that are more sensitive to detection of electrical lead noise, as may be seen with atrial-specific algorithms. This does not explain all of the observed incidences of ELAs, as the rate of ELA in Abbott leads with Medtronic devices was still 2-fold greater than what was observed in Medtronic devices with Medtronic leads (1.09% vs 0.47%).14 The current study offers a balanced representation of Abbott and Medtronic leads, at 57% and 43% of leads analyzed, respectively. This is in contrast to the prior study in which 75% of the leads analyzed were Abbott manufactured,13 leading to a potential for selection bias due to overrepresentation of Abbott leads.15 Several other studies have reported similar findings with the Tendril pacing lead and posited whether the Optim insulation may explain some of these observations.4,14,16 As for the higher incidence of ELA in the atrial position, it is possible that there may be a true higher failure rate of leads in this position. El-Chami and colleagues4 made a similar observation, in which leads implanted in an atrial location had an odds ratio of 2.28 for malfunction compared with leads implanted in the right ventricle. Kusumoto and colleagues17 suggested that the targeted annual failure rate for pacing leads is 0.2%.17 In this study the mean annual failure rate for Medtronic leads was 0.079%, compared with Abbott leads which was 0.72%. Understanding the mechanisms of these differences is crucial to preventing further lead issues in all manufactured leads, but particularly in the current Abbott models used.
Lead design may contribute to some of the ELAs that occur with higher rates, although changes in the electrode-myocardial interface, extrinsic to lead design, can also result in ELAs, as defined in this study. The presence of electrical noise indicates some lack of integrity in the lead structure that may be due to fracture or abrasion, while threshold and impedance changes could be due to changes in the lead-tissue interface. The finding that smaller lead size was associated with ELAs is likely related to the specific design of Abbott pacing leads. The reduced outer insulation in Abbott leads, which led to the smaller lead design, could explain the findings in this study, as well as prior issues that led to a recall with the Riata lead (Abbott).18 The Sprint Fidelis lead (Medtronic) was a 6.6F lead that was found to be prone to lead fracture due to lead crush between the clavicle and first rub, as well as ring-cable fractures in the intracardiac region, which may have resulted from not only the smaller lead size, but also the intrinsic design.19,20 The newer high-voltage Durata lead (Abbott) has an extra layer of Optim on the proximal portion to prevent insulation-can abrasion, making the proximal lead body larger due to the extra layer. Other issues that may impact the occurrence of ELAs include lead handling, lead slack, and venous access site.19,21 In our study, there was no specific clustering of ELAs by operator or venous access. The Tendril leads most prone to ELAs have been those surrounded by Optim.4 Leads covered with polyurethane alone are known to be susceptible to chemical degradation (environmental stress cracking and metal ion–induced oxidation), leading to major recalls.22 Silicone alone has also been prone to abrasion vulnerability, as seen with the most recent Riata and Riata ST recall.23 Haeberlin and colleagues24 have reported higher rates of ELAs of the Beflex/Vega leads as well, hypothesizing that a stiffer lead design due to minor design differences resulted in this observation. Conversely, one of the smallest leads available, the Medtronic 3830 (4F), has to date reported excellent performance.25
Lead abnormalities are a complex CIED issue, as clinicians must manage the risks of replacement with the resultant consequences if complete device failure occurs. In some cases, the risk of ELA, such as pacing dependence, can be catastrophic, such that revision with extraction or abandonment must be performed. The main ELA observed was lead noise without changes in electrical impedance. This led to lead revisions in 28% of patients, mostly in patients who were pacing dependent, and inhibition of pacing occurred due to the presence of the noise. The complication rate in our study was similar to prior reports that have indicated that complication rates with lead revision at the time of a pulse generator change to vary from 11.1% to 18.7%, depending on the type of lead requiring revision.12 Aside from complications from revision, our study demonstrated that the detection of ELAs led to a significant increase in device clinic visits, compared with those patients without abnormalities. Improving device clinic utilization has been a focus of many efforts due to the increased demand on clinics with burgeoning numbers of patients with active CIEDs. Use of remote monitoring and leadless devices may improve some of the challenges that device clinics face in managing the lead-related issues, which will continue to arise, until leadless device technology overtakes conventional technology.
Limitations of this study include small numbers for lead manufacturers other than Abbott and Medtronic, as well as a lack of correlation of electrical findings with the returned lead product. As a retrospective study, the treatment pathway to deal with ELAs was determined on an individual basis by the treating physician which could have led to heterogeneity in the decision to observe, reprogram, or revise the lead. The ability to discern whether lead implantation characteristics (lead slack, lead placement in pocket) or more sensitive detection algorithms may have contributed to these findings is unknown.
Conclusion
ELAs were more common with Abbott leads in this study, primarily in comparison with Medtronic leads, resulting in higher rates of revision and healthcare resource utilization. Further identification of factors that may improve management of lead abnormalities, to mitigate burden on device clinics and improve algorithms that may help differentiate the appropriate threshold for noise detection from true electrical lead dysfunction, is required to prevent morbidity for patients with CIEDs.
Acknowledgments
Funding Sources
This work was supported by a Dalhousie Medical Research Foundation Leo Alexander Studentship, Halifax, Canada.
Disclosures
Ratika Parkash has research grants from Medtronic and Abbott. There are no additional conflicts of interest to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
A waiver of consent was obtained from the institutional review board; individual consent was not needed.
Ethics Statement
The study was approved by the institutional review board at the Nova Scotia Health Authority. The research reported in this study adheres to the STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) guidelines.
References
- 1.Parkash R., Bennett M. In: In: Clinical Cardiac Pacing, Defibrillation and Resynchronization Therapy. 5th ed. Ellenbogen K.A., Wilkoff B.L., Kay G.N., Lau C.P., Auricchio A., editors. Elsevier; New York, NY: 2017. 42 - Managing advisories of cardiac implantable electronic devices; pp. 1175–1190. [Google Scholar]
- 2.Cairns J.A., Epstein A.E., Rickard J., et al. Prospective long-term evaluation of Optim-insulated (Riata ST Optim and Durata) implantable cardioverter-defibrillator leads. Heart Rhythm. 2014;11:2156–2162. doi: 10.1016/j.hrthm.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 3.Maisel W.H. Transvenous implantable cardioverter-defibrillator leads: the weakest link. Circulation. 2007;115:2461–2463. doi: 10.1161/CIRCULATIONAHA.107.698597. [DOI] [PubMed] [Google Scholar]
- 4.El-Chami M.F., Rao B., Shah A.D., et al. Long-term performance of a pacing lead family: a single-center experience. Heart Rhythm. 2019;16:572–578. doi: 10.1016/j.hrthm.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 5.Khattak F., Gupta A., Alluri K., Shariff N., Saba S. Rate and predictors of electrical failure in non-recalled defibrillator leads. Indian Pacing Electrophysiol J. 2019;19:100–103. doi: 10.1016/j.ipej.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghani A., Delnoy P.P.H.M., Ramdat Misier A.R., et al. Incidence of lead dislodgement, malfunction and perforation during the first year following device implantation. Neth Heart J. 2014;22:286–291. doi: 10.1007/s12471-014-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sridhar A.R.M., Patton K.K. In: Cardiac Pacing and ICDs. Ellenbogen K.A., Kaszala K., editors. Wiley; London, United Kingdom: 2020. Follow-up of the patient with a CIED; pp. 417–439. [Google Scholar]
- 8.Yee R., Verma A., Beardsall M., Fraser J., Philippon F., Exner D.V. Canadian Cardiovascular Society/Canadian Heart Rhythm Society joint position statement on the use of remote monitoring for cardiovascular implantable electronic device follow-up. Can J Cardiol. 2013;29:644–651. doi: 10.1016/j.cjca.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 9.Fraser J.D., Gillis A.M., Irwin M.E., Nishimura S., Tyers G.F., Philippon F. Guidelines for pacemaker follow-up in Canada: a consensus statement of the Canadian Working Group on Cardiac Pacing. Can J Cardiol. 2000;16(355–363):367–376. [PubMed] [Google Scholar]
- 10.Gillis A.M., Philippon F., Cassidy M.R., et al. Guidelines for implantable cardioverter defibrillator follow-up in Canada: a consensus statement of the Canadian Working Group on Cardiac Pacing. Can J Cardiol. 2003;19:21–37. [PubMed] [Google Scholar]
- 11.Nair S.G., Swerdlow C.D. Monitoring for and diagnosis of lead dysfunction. Card Electrophysiol Clin. 2018;10:573–599. doi: 10.1016/j.ccep.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Poole J.E., Gleva M.J., Mela T., et al. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation. 2010;122:1553–1561. doi: 10.1161/CIRCULATIONAHA.110.976076. [DOI] [PubMed] [Google Scholar]
- 13.Khatiwala R.V., Mullins E., Fan D., Srivatsa U.N., Dhruva S.S., Oesterle A. Electrical abnormalities with St. Jude/Abbott pacing leads: a systematic review and meta-analysis. Heart Rhythm. 2021;18:2061–2069. doi: 10.1016/j.hrthm.2021.08.026. [DOI] [PubMed] [Google Scholar]
- 14.Narui R., Nakamura T., Nakajima I., et al. Detection of high-frequency artifact as a function of pulse generator algorithms and outer-insulation material. Heart Rhythm. 2019;16:1855–1861. doi: 10.1016/j.hrthm.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 15.Darden D., Birgersdotter-Green U. To the Editor–Denominator neglect in meta-analysis: electrical abnormalities in St. Jude/Abbott pacing leads. Heart Rhythm. 2021;18:2226. doi: 10.1016/j.hrthm.2021.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Adelstein E., Zhang L., Nazeer H., Loka A., Steckman D. Increased incidence of electrical abnormalities in a pacemaker lead family. J Cardiovasc Electrophysiol. 2021;32:1111–1121. doi: 10.1111/jce.14941. [DOI] [PubMed] [Google Scholar]
- 17.Kusumoto F.M., Schoenfeld M.H., Wilkoff B.L., et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017;14:e503–e551. doi: 10.1016/j.hrthm.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Parkash R., Tung S., Champagne J., et al. Insight into the mechanism of failure of the Riata lead under advisory. Heart Rhythm. 2015;12:574–579. doi: 10.1016/j.hrthm.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Birnie D.H., Parkash R., Exner D.V., et al. Clinical predictors of Fidelis lead failure: report from the Canadian Heart Rhythm Society Device Committee. Circulation. 2012;125:1217–1225. doi: 10.1161/CIRCULATIONAHA.111.053744. [DOI] [PubMed] [Google Scholar]
- 20.Krahn A.D., Champagne J., Healey J.S., et al. Outcome of the Fidelis implantable cardioverter-defibrillator lead advisory: a report from the Canadian Heart Rhythm Society Device Advisory Committee. Heart Rhythm. 2008;5:639–642. doi: 10.1016/j.hrthm.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 21.Krahn A.D., Morissette J., Lahm R., et al. Radiographic predictors of lead conductor fracture. Circ Arrhythm Electrophysiol. 2014;7:1070–1077. doi: 10.1161/CIRCEP.114.001612. [DOI] [PubMed] [Google Scholar]
- 22.Lee J.Z., Vaidya V., Mulpuru S.K. In: Cardiac Pacing and ICDs. Ellenbogen K.A., Kaszala K., editors. Wiley; London, United Kingdom: 2020. Basics of cardiac pacing: components of pacing, defibrillation, and resynchronization therapy systems; pp. 33–68. [Google Scholar]
- 23.Hayes D., Freedman R., Curtis A.B., et al. Prevalence of externalized conductors in Riata and Riata ST silicone leads: results from the prospective, multicenter Riata Lead Evaluation Study. Heart Rhythm. 2013;10:1778–1782. doi: 10.1016/j.hrthm.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 24.Haeberlin A., Anwander M.T., Kueffer T., et al. Unexpected high failure rate of a specific MicroPort/LivaNova/Sorin pacing lead. Heart Rhythm. 2021;18:41–49. doi: 10.1016/j.hrthm.2020.08.010. [DOI] [PubMed] [Google Scholar]
- 25.3830 SelectSecure Medtronic CRHF Product Performance eSource. https://wwwp.medtronic.com/productperformance/model/3830-selectsecure.html Available at: