Abstract
Background
The subcutaneous implantable cardioverter-defibrillator (S-ICD) has demonstrated safety and efficacy for the treatment of malignant ventricular arrhythmias. However, a limitation of the S-ICD lies in the inability to either pace-terminate ventricular tachycardia or provide prolonged bradycardia pacing support.
Objective
The rationale and design of a prospective, single-arm, multinational trial of an intercommunicative leadless pacing system integrated with the S-ICD will be presented.
Methods
A technical description of the modular cardiac rhythm management (mCRM) system (EMPOWER leadless pacemaker and EMBLEM S-ICD) and the implantation procedure is provided. MODULAR ATP (Effectiveness of the EMPOWER™ Modular Pacing System and EMBLEM™ Subcutaneous ICD to Communicate Antitachycardia Pacing) is a multicenter, international trial enrolling up to 300 patients at risk of sudden cardiac death at up to 60 centers trial design. The safety endpoint of freedom from major complications related to the mCRM system or implantation procedure at 6 months and 2 years are significantly higher than 86% and 81%, respectively, and all-cause survival is significantly >85% at 2 years.
Results
Efficacy endpoints are that at 6 months mCRM communication success is significantly higher than 88% and the percentage of subjects with low and stable thresholds is significantly higher than 80%. Substudies to evaluate rate-responsive features and performance of the pacing module are also described.
Conclusion
The MODULAR ATP global clinical trial will prospectively test the safety and efficacy of the first intercommunicating leadless pacing system with the S-ICD. This trial will allow for robust validation of device-device communication, pacing performance, rate responsiveness, and system safety.
Keywords: Leadless pacemaker, Subcutaneous ICD, Defibrillator, Transcatheter pacemaker, Antitachycardia pacing
Key Findings.
-
▪
The modular leadless pacemaker (EMPOWER) is the first intercommunicative, self-contained pacemaker that is designed for antitachycardia pacing in conjunction of the subcutaneous implantable cardioverter-defibrillator.
-
▪
The technology, design, and endpoints for a global clinical trial designed to assess the safety and efficacy of the mCRM Therapy System is outlined in this design paper.
-
▪
The safety and efficacy of this technology will be prospectively assessed with 2 primary and 1 secondary safety endpoints, as well as 2 primary and 1 secondary efficacy endpoints.
Introduction
A large body of prospective data has established the safety and efficacy of the totally subcutaneous implantable cardioverter-defibrillator (S-ICD).1, 2, 3, 4, 5, 6 The advantages of the S-ICD include improved lead longevity and the absence of transvenous hardware, which eliminates the risk for bacteremic seeding of leads and deterioration of venous and valvular structures. This technology has been successfully applied to many patient populations, including those who otherwise would not be ideal candidates for transvenous systems.7 While the initially higher rate of inappropriate therapies has been addressed with improved sensing algorithms,5,8,9 the remaining limitations of this device are the inability to either pace-terminate arrhythmias or provide long periods of pacing for intermittent bradycardia support.
The modular leadless pacemaker (LP) (EMPOWER; Boston Scientific, Marlborough, MA) is an intercommunicative, self-contained pacemaker that is intended to be able to deliver rapid burst pacing when commanded by the S-ICD sensing algorithm at rates of 170 beats/min and higher. Additionally, it is a functional transcatheter pacing system that is intended to provide rate-responsive bradycardia pacing support.10, 11, 12, 13 Preclinical evaluation of the LP pulse generator and its performance in concert with the S-ICD collectively known as the modular Cardiac Rhythm Management (mCRM) Therapy System (Boston Scientific) have been previously reported.14,15
Herein, we describe the technology, design, and endpoints for a global clinical trial designed to assess the safety and efficacy of the mCRM therapy system.
Methods
mCRM therapy system
The mCRM therapy system components consist of the LP and the S-ICD and its subcutaneous lead (Figure 1). The LP is an investigational device: a rate-responsive, single-chamber pacemaker with active-fixation nitinol tines. As previously described,14,15 the S-ICD uses unidirectional conductive communication to command the LP to deliver antitachycardia pacing (ATP). The LP is implanted in the right ventricle via a dedicated delivery catheter and introducer sheath and can be retrieved by a dedicated retrieval catheter. Both the S-ICD and the LP are programmed via an external programmer with investigational software installed (Supplemental Figure 1).
Figure 1.
Modular cardiac rhythm management (mCRM) therapy system. A: The mCRM therapy system: leadless pacemaker and subcutaneous implantable cardioverter-defibrillator pulse generators. B: Illustration of the implanted mCRM therapy system.
Study population
Up to 300 patients will be enrolled and implanted with the mCRM modular therapy system at up to 60 centers in the United States, Canada, the United Kingdom, and Europe. The inclusion and exclusion criteria are mentioned in Table 1. Patients with an ICD indication,7, 16 who do not require chronic pacing support are the primary patient population. Additionally, those with a previously implanted S-ICD with a risk of experiencing monomorphic ventricular tachycardia (VT) are eligible. While patients who would require chronic rate-responsive pacing therapy are excluded, the study design includes 2 substudies for data collection to support bradycardia and rate-responsive pacing.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | |
| Exclusion criteria |
|
MVT was defined as ventricular tachycardia >30 seconds.
CIED = cardiac implantable electronic device; LA = left atrial; LVEF = left ventricular ejection fraction; MI = myocardial infarction; MVT = monomorphic ventricular tachycardia; S-ICD = subcutaneous implantable cardioverter-defibrillator; TV-ICD = transvenous implantable cardioverter-defibrillator; VA = ventricular arrhythmia.
TV-ICD system is expected to be fully explanted during or prior to full system implantation.
Patients with Model 1010 S-ICD or subject to electrical overstress field action are eligible if upgraded to BSC Model A209 or A219, or future BSC S-ICD Pulse Generator.
A scar involving at least 1 ventricular myocardial segment.
Study design
MODULAR ATP (NCT04798768) is a prospective, nonrandomized, single-arm, global study. It is designed to demonstrate safety, performance, and effectiveness of the mCRM modular therapy system.
Institutional review board approval at each participating institution will be obtained prior to patient enrollment. Subjects will be consented for follow-up visits every 6 months through at least 24 months. Subjects will continue to be followed every 6 months until the sponsor notifies sites that follow-up is complete. The total study duration is estimated to be 5 years.
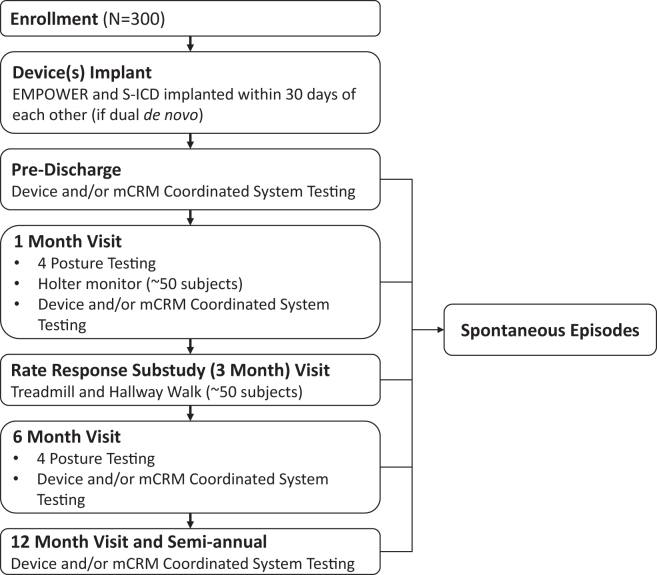
The MODULAR ATP design is summarized in Figure 2. In the case of de novo S-ICD implants, the LP may be implanted simultaneously or within 30 days from the S-ICD procedure. System testing will take place prior to discharge. All patients will undergo follow-up visits at 1, 6, and 12 months and subsequent semiannual visits. A Holter monitor substudy will take place at the 1-month visit and the rate response substudy will take place at the 3-month visit for a subset of patients. Data collection (Table 2) includes informed consent, patient history, implantation details, available imaging, device testing, and adverse events assessment.
Figure 2.
Study overview. mCRM = modular cardiac rhythm management; S-ICD = subcutaneous implantable cardioverter-defibrillator.
Table 2.
Data collection activities
| Procedure/assessment | Enrollment∗ | LP implant | S-ICD implant | mCRM coordinated system |
Follow-up in-office visits |
Additional visits§ | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| System testing† | Predischarge‡ | 1 mo | 3 mo | 6 mo | Semiannual | |||||
| All subjects | ||||||||||
| Informed consent | X | |||||||||
| Physical assessment, medical history | X | |||||||||
| S-ICD screening ECG | X|| | |||||||||
| Implantation details | X | X|| | ||||||||
| Fluoro/cine image | X | X | ||||||||
| Chest x-ray | X | |||||||||
| Communication threshold test | X | X¶ | X∗∗ | X†† | X†† | X†† | ||||
| Communication test | X∗∗ | X‡‡ | X‡‡,§§ | |||||||
| S-ICD conversion and sensing interaction testing | X | O | O | O | O | |||||
| Evaluation | X | X | X | X | X | X | X§§ | |||
| S-ICD evaluation | X | X | X | X | X | X§§ | ||||
| Adverse events assessment | X | X | X | X | X | X | X | X | X§§ | |
| Up to 59 subjects: rate response substudy | ||||||||||
| Rate response testing | X | |||||||||
| Up to 50 subjects: Holter substudy | ||||||||||
| Holter Monitor data recording | X | |||||||||
This table is intended to highlight key elements of study visits and does not include all study and substudy requirements.
ECG = electrocardiogram; LP = leadless pacemaker; mCRM = modular cardiac rhythm management; O = optional; S-ICD = subcutaneous implantable cardioverter-defibrillator; X = required.
≤30 days prior to LP and/or S-ICD implantation.
The date that the mCRM Coordinated System is implanted (successful implantation of both the S-ICD system and LP).
Prior to hospital discharge with mCRM Coordinated System.
If associated with a reportable adverse event, spontaneous episode, or device deficiency.
Only needed if de novo S-ICD.
Upright posture only.
4 postures, see Supplemental Appendix.
In case of communication test failure.
1 posture only; upright is recommended.
Required, if applicable.
Implantation procedure
Eligible investigators must complete a prespecified training program for device implantation and retrieval and an assessment by qualified co-investigators. Procedure-related medications (anesthesia/sedatives, anticoagulants, antiplatelets, and antibiotics) will be at the discretion of the investigator.
The LP is delivered under fluoroscopy using the dedicated delivery catheter (Supplemental Figure 1) through a ≥21F inner diameter introducer sheath. After deployment, the LP’s stability is then assessed by applying gentle traction via the tether. Electrical tests are performed to assure electrical parameters are within recommended values (Supplemental Table 1). The LP can be recaptured and repositioned if stability is not adequate. After stability is assessed, the LP is released from the tether. Data collection at implantation and system testing includes implant images, paced QRS morphology, sensing, and communication threshold testing. Finally, ventricular fibrillation is induced while the pulse generator is programmed to pace asynchronously to ensure appropriate detection of VF by the S-ICD (Table 2).
Acceptable pacing and sensing parameters will be guided by study personnel (Supplemental Tables 2–4), but acceptable final parameters before tether removal will ultimately be made for each patient individually by the implanting physician. Recapture and redeployment will be allowed as necessary if initial parameters are deemed unacceptable. Venous closure and hemostasis methods will be at the physician’s discretion.
Study organization
Study oversight includes a Steering Committee with members from various geographies, who advise on study design and execution. The data collection and monitoring are managed by the sponsor. An independent Clinical Events Committee, consisting of physician experts in cardiology, will determine the mCRM therapy system– and procedure-related complications.
A separate Data Monitoring Committee (DMC) is responsible for the oversight review of all safety data. The DMC will include leading experts in cardiology and biostatistics who are independent of the study and the sponsor. The DMC will review accumulating safety data to monitor the incidence of Clinical Events Committee events and other trends that would warrant study modification or termination.
Statistical methods and sample size calculation
Sample size calculations were performed using Cytel East 6 software (Cytel, Cambridge, MA). A sample size of 300 provides >90% power to test the study’s safety endpoints 1 and 2 and is greater than the sample size required to test all objectives at the designed power levels, as summarized in Table 3.
Table 3.
Study endpoints
| Endpoint | Time frame (mo) | Description | Expected value | Performance goal | Statistical test | α level (%) | Power level (%) | Subjects | Assumed attrition (%) | Subjects with assumed attrition | Lower confidence limit |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Safety endpoint 1 | 6 | Major mCRM system- and procedure-related complication–free rate | 93% | 86% | K-M | Overall: 2.5 | 90 | — | 25 | Interim: 179 | — |
| Interim: 1.2 | Interim: 134 | 98.8% 1-sided, pointwise | |||||||||
| Final: 1.9 | Final: 223 | Final:298 | 98.1% 1-sided, pointwise | ||||||||
| Safety endpoint 2 | 12 | Major mCRM system- and procedure-related complication–free rate | 92% | 81% | K-M | 2.5 | 90 | 112 | 30 | 160 | 97.5% 1-sided, pointwise |
| 99.8 | 210 | 300 | |||||||||
| Primary effectiveness 1 | 6 | Communication Success between S-ICD and LP | 95% | 88% | Repeated-measures logistic regression | 2.5 | 80 | 152 tests/38 subjects | 30 | 220 tests/55 subjects | 97.5% 1-sided, pointwise |
| Primary effectiveness 2 | 6 | % subjects with adequate pacing capture threshold ≤2 V @ 0.4 ms | 95% | 80% | Binomial | 2.5 | 90 | 57 | 30 | 82 | Binomial, 2.5% 1-sided, exact |
| Secondary safety | 24 | All-cause survival | 92.7% | 85% | K-M | 2.5 | 80 | 123 | 40 | 275 | 5% 1-sided, pointwise |
| Secondary effectiveness | 3 | LP rate response function | 0.65 ≤ mean MCR slope ≤ 1.35 | 0.85 ≤ mean MCR slope ≤ 1.15 | Kay-Wilkoff model22,23 | 2.5 | 90 | 35 | 40% | 59 | 95%, lower limit |
K-M = Kaplan-Meier; LP = leadless pacemaker; MCR = metabolic-chronotropic relation.
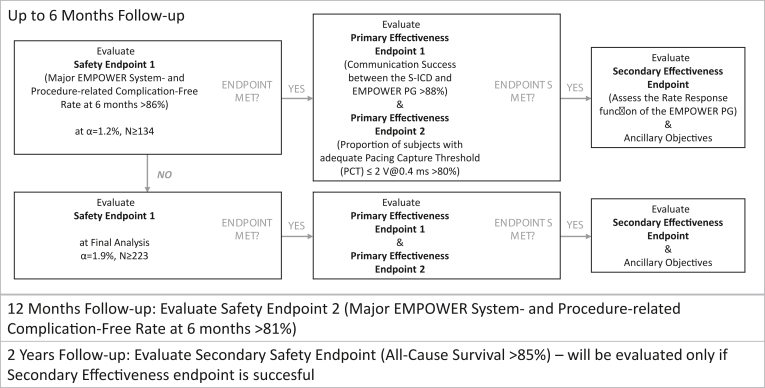
An interim endpoint analysis will occur after ≥134 patients have undergone system implantation and have been followed for 6 months. If the safety endpoint 1 is met during interim analysis, then the remaining endpoints using up to a 6-month follow-up period can be evaluated with this patient dataset (Figure 3).
Figure 3.
Endpoint analysis. Temporal and conditional map of endpoints, designed to minimize type I errors. S-ICD = subcutaneous implantable cardioverter-defibrillator; PG = pulse generator.
To address differing pre- and postmarket regulatory needs across different geographies, safety endpoint 2 will be evaluated, with ≥112 subjects that have been followed for 12 months with >90% power and all 300 subjects enrolled, resulting in ≥210 subjects followed for 12 months with >99.8% power.
In order to control the overall study type I error rate, the secondary effectiveness endpoint, the accelerometer-based rate response function of the LP, will only be evaluated if safety endpoint 1 and the primary effectiveness endpoint are successful. The secondary safety endpoint, all-cause survival through 2 years, will only be evaluated if all of these endpoints are successful (Figure 3).
Study endpoints
Study endpoints (Table 3) are designed to evaluate the overall mCRM therapy system safety and efficacy.17, 18, 19 Outcomes will be assessed among the following 3 groups: (1) the unidirectional transmission of conductive communication signals from the S-ICD system and LPs to deliver pacing commands; (2) the LP’s fixation mechanism and fixation stability; and (3) the LP’s accelerometer performance as the basis for accelerometer-based rate-responsive pacing (see secondary effectiveness endpoint). All endpoints are met if the results are significantly greater than the performance goal, using the lower confidence limits outlined for each endpoint, as outlined in Table 3.
Safety endpoint 1
Safety endpoint 1 will be met if freedom from major complications through 6 months postimplantation is >86%. Major complications are defined as any complication related to the LP or its implantation procedure that results in system revision, permanent loss of LP function, hospitalization, or death. Similar safety endpoints have been used for other leadless pacing systems. Specifically, the major complication-free performance goal was 86% for the LEADLESS II study (Safety and Effectiveness Trial for the Nanostim Leadless Pacemaker) of Nanostim20 and 83% for Micra,21 which were in turn based on transvenous pacing systems.
Interim analysis of this endpoint will take place after at least 134 subjects undergo system implantation and are followed for at least 6 months. This interim analysis allows for early evaluation of endpoints at acceptable power level and low α level of 1.2% (Statistical Methods and Sample Size Calculation and Table 3), which in turn provides an opportunity for earlier regulatory device approval request in some geographies. During this analysis, if the safety endpoint is met, then primary and secondary effectiveness endpoints will also be evaluated at this time. If the endpoint is not met, then the safety endpoint will be evaluated again on the entire study cohort along with the other endpoints (Figure 3).
Safety endpoint 2
The safety endpoint 2 is freedom from major complications for all patients through 12 months post–system implantation and will be met if the complication-free rate is >81%.24 The results of this analysis will be the basis of pre- or postmarket regulatory approval in U.S. and European Union geographies, as appropriate.
Primary effectiveness endpoint 1
The primary efficacy objective is to assess the robustness of mCRM system communication. During communication tests, the S-ICD will command the LP to deliver pacing at a rate ∼10 beats/min faster than the patient’s intrinsic rhythm. To meet the efficacy endpoint, the rate of successful communication attempts from the S-ICD to the LP must be >88%.
Because device communication can be affected by the relative orientation of the devices, the primary effectiveness endpoint will demonstrate communication success varied orientations by altering the patient’s body posture. More information regarding communication tests is provided in the Supplemental Appendix.
Primary effectiveness endpoint 2
The second primary effectiveness endpoint is pacing capture threshold stability, intended to reflect the LP’s fixation stability. The percentage of patients with adequate pacing capture threshold (defined as ≤2 V @ 0.4 ms) collected at the 6-month visit must be >80%, which is similar to performance criteria for other transcatheter pacing systems.20,21
Secondary safety endpoint
The long-term safety of the LP and overall mCRM therapy system will be assessed by testing the hypothesis that all-cause survival through 2 years postimplantation, for the entire patient cohort, is >85%. Estimated survival is 92.7%, a weighted average of results from S-ICD4,5 and recent transvenous ICD (TV-ICD) studies, including the APPRAISE ATP (Assessment of Primary Prevention Patients Receiving An ICD - Systematic Evaluation of ATP), ENABLE MRI (Magnetic Resonance Imaging) Access for Patients With New and Existing ICDs (Implantable Cardioverter Defibrillator) and CRT-Ds (Cardiac Resynchronization Therapy Defibrillator), MANAGE-HF (Multiple Cardiac Sensors for the Management of Heart Failure), and PREEMT-HF (PRospective Evaluation of Electrocardiographic Voltage Changes and Six Minute Walk Test for Predicting Readmissions in Heart Failure) clinical trials. This endpoint will only be evaluated if safety endpoint 1 as well as the primary and secondary effectiveness are successful (Figure 3).
Secondary effectiveness endpoint
Up to 59 subjects who are able to perform a treadmill walk protocol will be offered enrollment in the treadmill substudy using a modified Minnesota Pacemaker Response Exercise Protocol. The LP will be programmed in VVIR mode and data will be collected to evaluate the correlation between the patient’s paced heart rate and the patient’s workload as evaluated throughout every stage of the treadmill test.
Holter substudy
To assess LP pacing performance, 50 patients will undergo overdrive pacing while wearing a Holter monitor for 25 to 30 minutes during an in-office visit. Patients will then wear the Holter monitor in the ambulatory setting for 16 to 24 hours.
Discussion
The MODULAR ATP global clinical trial will prospectively test the first-of-its-kind leadless pacing system that communicates with the S-ICD. The technology assessed by this study is critical to the evolution of pacing and defibrillation in general due to the clinical barriers that transvenous leads pose to the care of patients. ICDs have proven to be lifesaving devices with remarkable mortality benefit in appropriate patient populations. However, the most important source of morbidity and mortality from these systems involves the transvenous lead. It was recognized decades ago that the transvenous ICD lead was particularly vulnerable to failure compared with pacemaker leads.25 Indeed, 2 subsequent major transvenous ICD lead failures resulted in serious patient morbidity and mortality.26,27
Leadless technology has emerged as the next frontier for all forms of cardiac pacing and defibrillation and would be a potentially valuable adjunct to the S-ICD. The seminal trials of 2 transcatheter pacing systems were originally described simultaneously in 2015.20,28 Since then, widespread adoption of this technology has occurred with acceptable safety and efficacy. The mCRM is the first transcatheter pacing system that wirelessly communicates with a subcutaneous defibrillator platform and, if validated, will add to the utility of leadless pacing devices by offering (1) a leadless modality for pace termination of ventricular arrhythmias and (2) pacing at energy levels that do not lead to pain or discomfort.29
It is clear that patients with ICDs who receive shocks have significantly worsened quality of life, functional status, and mortality compared with those who do not receive shocks.30,31 In the PRAETORIAN (A PRospective, rAndomizEd Comparison of subcuTaneOous and tRansvenous ImplANtable Cardioverter Defibrillator Therapy) trial, while patients randomized to the S-ICD arm were 1.5 times more likely to receive shocks than those receiving transvenous systems, the PRAETORIAN trial also showed no reduction in total appropriate shocks in the transvenous, ATP-capable arm vs the S-ICD arm with ATP unavailable.32 However, it is not known whether pace termination of ventricular arrhythmias mitigates these variables. The ongoing APPRAISE ATP trial33 is a randomized controlled trial to assess the overall benefit of ATP in primary prevention patients and identify patient characteristics who most benefit from ATP. The PRAETORIAN trial showed 46% of VT episodes were successfully terminated by ATP in the transvenous arm, which was comparable to rates in other recent studies.32,34,35 The PainFREE Rx II (Pacing Fast VT Reduces Shock Therapies) trial demonstrated an 85% reduction in appropriate ICD shocks in those randomized to the ATP arm, but in the MADIT-RIT (Multicenter Automatic Defibrillator Implantation Trial - Reduce Inappropriate Therapy) trial, subjects randomized to delayed therapy ICD programming had very small amounts of ATP and had substantially improved mortality.36,37 Despite this controversy, a proportion of patients who require ICDs are spared from ICD shock with ATP-capable devices. In the PARTITA (Does Timing of VT Ablation Affect Prognosis in Patients With an Implantable Cardioverter-defibrillator?) trial, among 517 patients undergoing ICD implantation with ischemic and nonischemic substrates, 154 (29.8%) patients had sustained VT and 112 patients received ATP therapy with contemporary device programming.38
While only a proportion of patients with defibrillators receive pace termination of ventricular arrhythmias, it is noteworthy that patients who get successful ATP receive therapy multiple times. In one published series, 248 ATP episodes occurred in only 47 patients,12 which suggests that certain patients with ICDs are especially spared from ICD shocks by ATP-capable platforms. In this trial, selection criteria were designed to target this particularly vulnerable population by including those with prior VT or with substrates at risk for pace-terminable VT.
Conclusion
The MODULAR ATP global clinical trial will be the first to test leadless pacing technology that intercommunicates with the entirely subcutaneous implantable defibrillator. This technology is critical to the evolution of cardiac pacing and defibrillation.
Acknowledgments
Funding Sources
This trial is fully funded by Boston Scientific Corporation.
Disclosures
Michael S. Lloyd has served as a consultant for and received research support from Medtronic, Boston Scientific, Biosense Webster, and Abbott. Amy J. Brisben, Ursula Appl, Julie West, Nathan Carter, Kenneth M. Stein are full-time employees of Boston Scientific. Vivek Y. Reddy has served as a consultant for Abbott, AtriAN, Biosense Webster, BioTel Heart, Biotronik, Cairdac, Cardiofocus, Cardionomic, CoreMap, Fire1, Gore & Associates, Impulse Dynamics, Novartis, Novo Nordisk, Philips, and Pulse Biosciences; owns equity in Manual Surgical Sciences, Newpace, Nyra Medical, Surecor, and Vizaramed; and has served as a consultant for and owns equity in Ablacon, Acutus Medical, Anumana, APN Health (apnhealth.com), Aquaheart, Atacor, Autonomix, Axon Therapies, Backbeat, BioSig, Boston Scientific, CardiaCare, CardioNXT/AFTx, Circa Scientific, CoRISMA, Corvia Medical, DiNovA EP Technology, East End Medical, EP Frontiers, EpiEP, Field Medical, Focused Therapeutics, Hudson River Trading, Intershunt, Javelin, Kardium, Keystone Heart, LuxMed, Medlumics, Medtronic, Middlepeak, Biosense Webster, Oracle Health, Philips, Restore Medical, Sirona Medical, SoundCath, and Valcare; and has received grant support from Boston Scientific. Carina Blomström-Lundqvist has served as a consultant and/or received honoraria from Bayer, Medtronic, CathPrint, Philips, Sanofi Aventis, Boston Scientific, Abbott, Milestone, Organon, and Merck Sharp & Dohme. Lucas V.A. Boersma's affiliations have received compensation for services rendered to Boston Scientific, Medtronic, Adagio, Philips, and Acutus. Maria Grazia Bongiorni has served as a consultant for Boston Scientific, Abbott, and Biotronik. Martin C. Burke has received honoraria and research grants from Boston Scientific and AtaCor Medical; and owns equity in AtaCor Medical. Daniel J. Cantillon has served as a consultant for Abbott and Boston Scientific. Rahul Doshi has served as a consultant for Boston Scientific. Paul A. Friedman owns a possible financial benefit from the commercialization of AI ECG algorithms by Anumana, Eko health, and AliveCor. Daniel Gras has served as a consultant for Boston Scientific, Biotronik, and Zoll. Steven P. Kutalek has served as a consultant for Boston Scientific. Petr Neuzil has received grant support from Boston Scientific. Paul R. Roberts has received research funding from Boston Scientific; and honoraria from Boston Scientific and Medtronic. D.J. Wright has received speaker fees from and served as a consultant for Boston Scientific and Medtronic. Lluis Mont has received unrestricted grants, received support for fellowship programs, and served as a consultant for Abbott, Biosense Webster, Boston Scientific, and Medtronic. Reinoud E. Knops has received compensation for services and research grants from Abbott, Boston Scientific, and Medtronic.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
All subjects will provide informed consent.
Ethics Statement
This trial will be conducted according to the local regulatory bodies of each institution and follow the Helsinki Declaration guidelines on human research.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2023.05.004.
Appendix. Supplementary Data
References
- 1.Knops R.E., Olde Nordkamp L.R.A., Delnoy P.H.M., et al. Subcutaneous or transvenous defibrillator therapy. N Engl J Med. 2020;383:526–536. doi: 10.1056/NEJMoa1915932. [DOI] [PubMed] [Google Scholar]
- 2.Healey J.S., Krahn A.D., Bashir J., et al. Perioperative safety and early patient and device outcomes among subcutaneous versus transvenous implantable cardioverter defibrillator implantations: a randomized, multicenter trial. Ann Intern Med. 2022;175:1658–1665. doi: 10.7326/M22-1566. [DOI] [PubMed] [Google Scholar]
- 3.Weiss R., Knight B.P., Gold M.R., et al. Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation. 2013;128:944–953. doi: 10.1161/CIRCULATIONAHA.113.003042. [DOI] [PubMed] [Google Scholar]
- 4.Lambiase P.D., Theuns D.A., Murgatroyd F., et al. Subcutaneous implantable cardioverter-defibrillators: long-term results of the EFFORTLESS study. Eur Heart J. 2022;43:2037–2050. doi: 10.1093/eurheartj/ehab921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gold M.R., Lambiase P.D., El-Chami M.F., et al. Primary results from the Understanding Outcomes With the S-ICD in Primary Prevention Patients With Low Ejection Fraction (UNTOUCHED) trial. Circulation. 2021;143:7–17. doi: 10.1161/CIRCULATIONAHA.120.048728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burke M.C., Aasbo J.D., El-Chami M.F., et al. 1-Year prospective evaluation of clinical outcomes and shocks: the Subcutaneous ICD Post Approval Study. J Am Coll Cardiol EP. 2020;6:1537–1550. doi: 10.1016/j.jacep.2020.05.036. [DOI] [PubMed] [Google Scholar]
- 7.Al-Khatib S.M., Stevenson W.G., Ackerman M.J., et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018;72:e91–e220. doi: 10.1016/j.jacc.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 8.Brisben A.J., Burke M.C., Knight B.P., et al. A new algorithm to reduce inappropriate therapy in the S-ICD system. J Cardiovasc Electrophysiol. 2015;26:417–423. doi: 10.1111/jce.12612. [DOI] [PubMed] [Google Scholar]
- 9.Theuns D., Brouwer T.F., Jones P.W., et al. Prospective blinded evaluation of a novel sensing methodology designed to reduce inappropriate shocks by the subcutaneous implantable cardioverter-defibrillator. Heart Rhythm. 2018;15:1515–1522. doi: 10.1016/j.hrthm.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Huang J., Patton K.K., Prutkin J.M. Concomitant use of the subcutaneous implantable cardioverter defibrillator and a permanent pacemaker. Pacing Clin Electrophysiol. 2016;39:1240–1245. doi: 10.1111/pace.12955. [DOI] [PubMed] [Google Scholar]
- 11.Ljungstrom E., Brandt J., Mortsell D., Borgquist R., Wang L. Combination of a leadless pacemaker and subcutaneous defibrillator with nine effective shock treatments during follow-up of 18 months. J Electrocardiol. 2019;56:1–3. doi: 10.1016/j.jelectrocard.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed F.Z., Cunnington C., Motwani M., Zaidi A.M. Totally leadless dual-device implantation for combined spontaneous ventricular tachycardia defibrillation and pacemaker function: a first report. Can J Cardiol. 2017;33(1066):e1065–e1066.e7. doi: 10.1016/j.cjca.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Ito R., Kondo Y., Winter J., et al. Combination of a leadless pacemaker and subcutaneous implantable cardioverter defibrillator therapy for a Japanese patient with prosthetic valve endocarditis. J Arrhythm. 2019;35:311–313. doi: 10.1002/joa3.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breeman K.T.N., Swackhamer B., Brisben A.J., et al. Long-term performance of a novel communicating antitachycardia pacing-enabled leadless pacemaker and subcutaneous implantable cardioverter-defibrillator system: A comprehensive preclinical study. Heart Rhythm. 2022;19:837–846. doi: 10.1016/j.hrthm.2022.01.024. [DOI] [PubMed] [Google Scholar]
- 15.Tjong F.V.Y., Brouwer T.F., Koop B., et al. Acute and 3-month performance of a communicating leadless antitachycardia pacemaker and subcutaneous implantable defibrillator. J Am Coll Cardiol EP. 2017;3:1487–1498. doi: 10.1016/j.jacep.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Habib G., Lancellotti P., Antunes M.J., et al. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM) Eur Heart J. 2015;36:3075–3128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 17.Wathen M. Implantable cardioverter defibrillator shock reduction using new antitachycardia pacing therapies. Am Heart J. 2007;153:44–52. doi: 10.1016/j.ahj.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 18.Wilkoff B.L., Fauchier L., Stiles M.K., et al. 2015 HRS/EHRA/APHRS/SOLAECE expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Heart Rhythm. 2016;13:e50–e86. doi: 10.1016/j.hrthm.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 19.Stiles M.K., Fauchier L., Morillo C.A., Wilkoff B.L. 2019 HRS/EHRA/APHRS/LAHRS focused update to 2015 expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. J Interv Card Electrophysiol. 2020;59:135–144. doi: 10.1007/s10840-019-00662-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reddy V.Y., Exner D.V., Cantillon D.J., et al. Percutaneous implantation of an entirely intracardiac leadless pacemaker. N Engl J Med. 2015;373:1125–1135. doi: 10.1056/NEJMoa1507192. [DOI] [PubMed] [Google Scholar]
- 21.Ritter P., Duray G.Z., Zhang S., et al. Micra Transcatheter Pacing Study Group. The rationale and design of the Micra Transcatheter Pacing Study: safety and efficacy of a novel miniaturized pacemaker. Europace. 2015;17:807–813. doi: 10.1093/europace/euv026. [DOI] [PubMed] [Google Scholar]
- 22.Kay G.N. Quantitation of chronotropic response: comparison of methods for rate-modulating permanent pacemakers. J Am Coll Cardiol. 1992;20:1533–1541. doi: 10.1016/0735-1097(92)90447-u. [DOI] [PubMed] [Google Scholar]
- 23.Wilkoff B.L., Corey J., Blackburn G. A mathematical model of the cardiac chronotropic response to exercise. J Electrophysiol. 1989;3:176–180. [Google Scholar]
- 24.Medicines and Healthcare products Regulatory Agency Leadless cardiac pacemaker therapy: design of pre- and post-market clinical studies. 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/956252/Leadless-EAG-guidance.pdf Available at:
- 25.Lawton J.S., Wood M.A., Gilligan D.M., Stambler B.S., Damiano R.J., Jr., Ellenbogen K.A. Implantable transvenous cardioverter defibrillator leads: the dark side. Pacing Clin Electrophysiol. 1996;19:1273–1278. doi: 10.1111/j.1540-8159.1996.tb04204.x. [DOI] [PubMed] [Google Scholar]
- 26.Hauser R.G., McGriff D., Retel L.K. Riata implantable cardioverter-defibrillator lead failure: analysis of explanted leads with a unique insulation defect. Heart Rhythm. 2012;9:742–749. doi: 10.1016/j.hrthm.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 27.Krahn A.D., Champagne J., Healey J.S., et al. Outcome of the Fidelis implantable cardioverter-defibrillator lead advisory: a report from the Canadian Heart Rhythm Society Device Advisory Committee. Heart Rhythm. 2008;5:639–642. doi: 10.1016/j.hrthm.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds D., Duray G.Z., Omar R., et al. A leadless intracardiac transcatheter pacing system. N Engl J Med. 2016;374:533–541. doi: 10.1056/NEJMoa1511643. [DOI] [PubMed] [Google Scholar]
- 29.Friedman P., Murgatroyd F., Boersma L.V.A., et al. Efficacy and safety of an extravascular implantable cardioverter-defibrillator. N Engl J Med. 2022;387:1292–1302. doi: 10.1056/NEJMoa2206485. [DOI] [PubMed] [Google Scholar]
- 30.Schron E.B., Exner D.V., Yao Q., et al. Quality of life in the antiarrhythmics versus implantable defibrillators trial: impact of therapy and influence of adverse symptoms and defibrillator shocks. Circulation. 2002;105:589–594. doi: 10.1161/hc0502.103330. [DOI] [PubMed] [Google Scholar]
- 31.Larsen G.K., Evans J., Lambert W.E., Chen Y., Raitt M.H. Shocks burden and increased mortality in implantable cardioverter-defibrillator patients. Heart Rhythm. 2011;8:1881–1886. doi: 10.1016/j.hrthm.2011.07.036. [DOI] [PubMed] [Google Scholar]
- 32.Knops R.E., van der Stuijt W., Delnoy P., et al. Efficacy and safety of appropriate shocks and antitachycardia pacing in transvenous and subcutaneous implantable defibrillators: analysis of all appropriate therapy in the PRAETORIAN trial. Circulation. 2022;145:321–329. doi: 10.1161/CIRCULATIONAHA.121.057816. [DOI] [PubMed] [Google Scholar]
- 33.Schuger C.D., Ando K., Cantillon D.J., et al. Assessment of primary prevention patients receiving an ICD - Systematic evaluation of ATP: APPRAISE ATP. Heart Rhythm O2. 2021;2:405–411. doi: 10.1016/j.hroo.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arenal A., Proclemer A., Kloppe A., et al. Different impact of long-detection interval and anti-tachycardia pacing in reducing unnecessary shocks: data from the ADVANCE III trial. Europace. 2016;18:1719–1725. doi: 10.1093/europace/euw032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuger C., Daubert J.P., Zareba W., et al. Reassessing the role of antitachycardia pacing in fast ventricular arrhythmias in primary prevention implantable cardioverter-defibrillator recipients: results from MADIT-RIT. Heart Rhythm. 2021;18:399–403. doi: 10.1016/j.hrthm.2020.11.019. [DOI] [PubMed] [Google Scholar]
- 36.Wathen M.S., DeGroot P.J., Sweeney M.O., et al. PainFREE Rx III Trial Investigators. Prospective randomized multicenter trial of empirical antitachycardia pacing versus shocks for spontaneous rapid ventricular tachycardia in patients with implantable cardioverter-defibrillators: Pacing Fast Ventricular Tachycardia Reduces Shock Therapies (PainFREE Rx II) trial results. Circulation. 2004;110:2591–2596. doi: 10.1161/01.CIR.0000145610.64014.E4. [DOI] [PubMed] [Google Scholar]
- 37.Moss A.J., Schuger C., Beck C.A., et al. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012;367:2275–2283. doi: 10.1056/NEJMoa1211107. [DOI] [PubMed] [Google Scholar]
- 38.Della Bella P., Baratto F., Vergara P., et al. Does timing of ventricular tachycardia ablation affect prognosis in patients with an implantable cardioverter defibrillator? results from the multicenter randomized PARTITA trial. Circulation. 2022;145:1829–1838. doi: 10.1161/CIRCULATIONAHA.122.059598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.