Abstract
This work synthesized three new CrAz2, MnAz2, and FeAz2 complexes and investigated them using IR, mass, UV spectroscopy, elemental analysis, conductivity and magnetic tests, and thermogravimetric analysis. The azo-ligand, 4-(2-hydroxyphenylAzo)-1-naphthol (Az), couples with metal ions via its nitrogen (in −N=N– bonds) and oxygen (in hydroxyl group) atoms, according to the IR spectra of these complexes. Through thermal examination (TG/TGA), the number and location of water in the complexes were also determined. Density functional theory (DFT) theory is applied to ameliorate the structures of the ligand (Az) and metal complexes and analyze the quantum chemical characteristics of these complexes. The antifungal and antibacterial activity of the ligand and its complexes opposed to several hazardous bacteria and fungi was investigated in vitro. Metal complexes were discovered to have a higher inhibitory impact on some organisms than the free ligand. The MnAz2 complex exhibited the best activity among the studied materials, whereas the CrAz2 complex had the lowest. The compounds’ binding affinity to the E. coli (PDB ID: 1hnj) structure was predicted using molecular docking. Binding energies were calculated by analyzing protein-substrate interactions. These encouraging findings imply that these chemicals may have physiological effects and may be valuable for a variety of medical uses in the future.
1. Introduction
Over the course of the past four decades, azo dyes that include heterocyclic rings have made substantial contributions to the field of coordination chemistry.1 Research in the fields of biology, electrochemistry, and analysis may all make use of these substances in a variety of different ways.2 Because of their potential cytotoxicity as well as their antibacterial, antifungal, and antioxidant capabilities, they have garnered a lot of studies.3,4 There has been a significant amount of research done on the production of metal(II) complexes of azo compounds, as well as their biological activity.5,6 Because their azo group possesses powerful medicinal properties, azo dyes are advantageous chelating agents for a variety of metal ions,7,8 due to the azo group of these dyes. In addition to this, it has been demonstrated that azo metal complexes can be beneficial as metal anticorrosion agents and as thermally stable optical storage devices,9,10
There is an urgent need for new metal-based compounds that can prevent the development of hazardous germs that have become worldwide spread. These microorganisms have the potential to pollute food, water, and soil, as well as cause sickness in humans, animals, and plants.11,12 In recent years, bioinorganic chemistry, which is the study of coordination chemistry with biological ligands, has experienced tremendous expansion. Bio-metals such as manganese, iron, cobalt, nickel, and copper have been major contributors to this rise. The study of metal-bioligand complexes is essential to gaining an understanding of the processes that occur in living organisms.13,14 Because coordination chemistry has a history of being successful in the field of pharmacotherapy, academics and researchers have been keenly interested in the topic for a significant amount of time.
Metal ions are selected based on their broad biological history. Previously, complexes of Cr(III), Mn(II), and Fe(III) with azo ligands were reported to show anticancer, antioxidant, and antibacterial activity.7,15−18 Therefore, we speculate that complexes of Cr(III), Mn(II), and Fe(III) with azo ligands will show more antibacterial and antifungal activity. These findings prompted us to start a project on Cr(III), Mn(II), and Fe(III)-azo-ligand complexes, involving an investigation of their structural characterization, antibacterial, and antifungal properties.
As a result, the purpose of this work is to develop novel coordination compounds based on azo dyes that contain CrAz2, MnAz2, and FeAz2 and then analyze the bioactivity of these compounds in vitro and computationally. In addition, density functional theory (DFT) simulations were utilized so that the three-dimensional (3D) chemical structure of the complexes could be determined. Authors anticipate that the current effort may result in the discovery of a new family of chemicals that due to the ease with which they can be produced and the remarkable efficacy they possess, may represent promising candidates for the treatment of bacterial and fungal diseases.
2. Experimental Section
2.1. Synthesis
2.1.1. Materials and Reagents
The purity of the chemicals used was suitable for use as analytical reagents (AR). These included 4-(2-hydroxyphenylAzo)-1-naphthol, chromium(III) chloride, manganese(II) chloride, and iron(III) chloride, in addition to organic solvents, such as EtOH and CH3CN.
2.1.2. Synthesis of the 1-(2-Hydroxyphenylazo)-2-naphthol Azo-Ligand (Az)
1-(2-Hydroxyphenylazo)-2-naphthol as the azo-ligand (Az) was prepared as previously reported19 with slight modifications.
2.1.2.1. Step I: Preparation of Diazonium Salt of 2-Aminophenolas Azo Molecules
A cooled solution (0 °C) of NaNO2 (0.69 g, 0.01 mol) in 25 mL of water was gradually added to a cooled solution of 1.09 g (0.01 mol) of 2-aminophenol and 36 mL of HCl under stirring in an ice bath for 20 min. The diazonium salt solution was stored in the refrigerator at all times (Scheme 1).
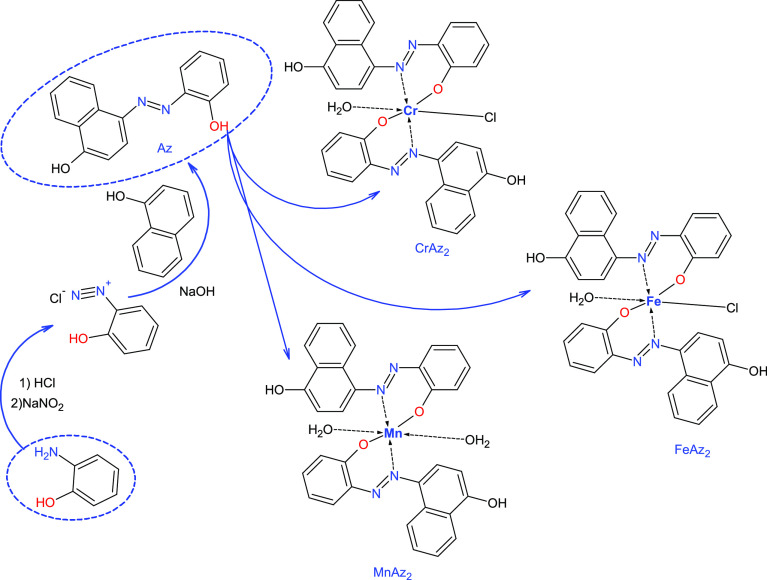
Scheme 1. Preparation of Az Ligands and Its Complexes.
2.1.2.2. Step II: Coupling Procedure
The cooled solution of diazonium salt was gradually added to the cooled solution (0 °C) of 1-naphthol (1.44 g, 0.01 mol) in NaOH (10% w/v) in an ice bath. Then, the azo dye will be precipitated. The final azo product was produced by filtration of the crude precipitate, multiple cold water washes, and recrystallization from the suitable solvent; Scheme 1.
2.1.2.3. 4-(2-Hydroxyphenyl azo)-1-naphthol
Brown–red solid; yield (91%); mp. 190–191 °C; IR (cm–1): 3114–3218 (OH), 3001 (Ar-H); 1H NMR (400 MHz, DMSO-d6) δ (ppm): 11.25 (s, 1H, OH), 8.56 (s, 1H, OH), 8.26–6.98 (m, 10H, Ar-H); 13C NMR (100 MHz, DMSO-d6) δ (ppm): 157.99, 154.55, 139.65, 132.84, 128.50, 125.93,123.20, 123.07, 122.43, 120.31, 118.34, 115.53, 109.05; elemental analysis for C16H12N2O2 (calcd/found); C, 72.72/72.62; H, 4.55/4.41; N, 10.60/10.71.
2.1.3. Preparation of the Metal Complexes
During the process of heating the combination, a solution of the metal salt (2.0 mmol) in water (10 mL) was progressively added to ethanol that already contained the ligand (4.0 mmol). The solution was stirred consistently during the ten-hour process of refluxing in a water bath at 90 °C. The product was recrystallized after it had been filtered, dried, and washed with a water–ethanol mixture with a ratio of 1:2. It was established how much of each product could be obtained as well as the melting and decomposition temperatures; Scheme 1.
2.2. Characterization
The various physicochemical techniques used for the characterization of the free Az ligand and its metal complexes are listed in the Supplementary data file.
2.3. DFT Calculations
To optimize the geometry of the AZ ligand and its complexes, the cc-pVQZ,20,21 the basis set as a full-electron basis set for all elements composing the investigated compounds in conjunction with the hybrid correlation functional (B3LYP),22−24 was used in ethanol as the solvent. The solvent (ethanol) effect was solved using the polarizable constant model (IEFPCM).25,26 The DFT calculations27 were done using Gaussian 09 W,28 and the optimized structures, highest occupied molecular orbitals (HOMO)–lowest unoccupied molecular orbitals (LUMO) orbitals, and molecular electrostatic potential (MEP) map were visualized using GaussView 5.29 Also, natural bond orbital (NBO) analysis of the current compounds was performed using the NBO 3.1 programme30 as applied in the Gaussian 09 program. To determine quantum chemical parameters such as ionization potential (IP = −EHOMO), electronegativity (EN = (I.P. + E.A.)/2), the energy gap (ΔE = ELUMO – EHOMO), chemical potential (CP = −EN), electron affinity (EA = −ELUMO), softness (S = 1/CH), chemical hardness (CH = (I.P. – E.A.)/2)), and electrophilicity index (EP = EN2/2CH), the LUMO and HOMO energies of the relevant compounds were utilized.31,32
2.4. In Vitro Investigation of Bioactivity
The antimicrobials activity of the compounds was examined using the disc diffusion method,33,34 against a panel of bacteria (Escherichia coli (−ve), Pseudomonas aeruginosa (−ve), Bacillus cereus (+ve), and Staphylococcus aureus (+ve)) and fungi. The results showed that the compounds were effective against all of the bacteria and fungi tested (Trichophyton rubrum, Candida albicans, and Aspergillus flavus). The inhibitory zone that surrounded the disc was measured in millimeters and the compounds’ activity was compared to that of the standard chloramphenicol antibiotic using the percentage activity index (AI% = IZ of the test compound/IZ of the standard) multiplied by 100.35,36
2.5. Molecular Docking
Molecular docking simulations of the specified compounds were performed against the 1HNJ protein to validate the therapeutic efficacy of these compounds. 1HNJ is the structural representation of the FabH-CoA complex in E. coli. It is believed that the FabH receptor is targeted with a view to learning about the potential antibacterial properties of chemicals that are present in nature.37,38 The enzyme FabH plays a role in the synthesis of fatty acids.
The three-dimensional structure of the objective protein receptor was attained by consulting the protein database (http://www.rcsb.org). The chemicals that are researched are utilized as a substrate. The molecular docking investigation is carried out with the use of a molecular overeating environment (MOE).39
After creating a new database, optimizing each chemical by reducing the amount of energy required for substrate preparation, and finally storing the results in the MDB format, in order to prepare the receptor, the target receptor underwent processes including the addition of hydrogen atoms, the connection of receptor types, the fixing of potential energy, and, finally, the manufacture of active pockets and dummies.40,41 Docking patterns and interaction parameters were exported so that interaction features could be analyzed, and inhibitory activity could be ranked according to a scoring function (S, kcal/mol).42,43
3. Results and Discussion
3.1. Structural Configuration of the Azo-Ligand Ligand (Az)
The chemical structure of the azo-ligand (Az) was assured by its spectral (IR, 1H NMR, and 13C NMR) and elemental analyses. During the Az analysis, a wide OH absorption band was found between 3114 and 3218 cm–1, and an aromatic C–H band was identified at 3001 cm–1. At 1542 cm–1, the characteristic band that can be produced by the N=N group during stretching vibration absorption was also seen.
Singlet broad signals for the −OH group were seen in the 1H NMR spectra of Az at 11.25 (s, 1H, OH) and 8.56 (s, 1H, OH), as well as aromatic signals at 8.26–6.98 ppm (m, 10H, Ar–H). Signals were seen in the13C NMR spectra at the following frequencies: 157.99, 154.55, 139.65, 132.84, 128.50, 125.93, 123.20, 123.07, 122.43, 120.31, 118.34, 115.53, 109.05 which is characteristic of CH of phenyl and naphthyl moiety, Supporting data; Figure S1. Also, the elemental analysis of the Az ligand referred to good agreement between the found and calculated values for C, H, and N, Table 1, which confirms the proposed structure; Scheme 1.
Table 1. Physical Properties, UV–Vis, Conductivity, Magnetic, and FT-IR Results.
| Az | CrAz2 | MnAz2 | FeAz2 | ||
|---|---|---|---|---|---|
| physical properties | color | brown–red | white–green | white yellow | dark-violet |
| melting point (°C) | 190–191 | >300 | >300 | >300 | |
| yield (%) | 97 | 90 | 87 | 89 | |
| conductivity μv, Ω–1 cm2 mol–1 | Acetonitrile | 11.78 | 12.04 | 11.44 | |
| DMF | 10.15 | 9.48 | 10.07 | ||
| assignment | Non-electrolyte | ||||
| UV–vis | λmax, nm | 380 | 615 | 410 | 505 |
| magnetic | μeff (B.M) | 3.84 | 1.96 | 1.84 | |
| assignment | d3 (t2g3) | d5 low spin (t2g5) | d5 (t2g5) | ||
| stoichiometry | M:L | 1:2 | 1:2 | 1:2 | |
| IR spectra | υ (−OH) | 3318 | 3478 | 3488 | 3460 |
| υ (−N=N) | 1542 | 1464 | 1460 | 1470 | |
| υ (M–O) | 551 | 547 | 544 | ||
| υ (M–N) | 485 | 478 | 484 | ||
| EA found (calc.) % | C | 72.62 (72.72) | 60.47 (60.81) | 62.53 (62.24) | 60.28 (60.45) |
| H | 4.41 (4.55) | 3.55 (3.83) | 4.66 (4.24) | 3.66 (3.80) | |
| N | 10.71 (10.60) | 8.69 (8.86) | 8.87 (9.07) | 8.62 (8.81) | |
| M | 8.54 (8.23) | 8.72 (8.90) | 8.99 (8.78) | ||
3.2. Structural Clarification of the Metal Complexes
3.2.1. Conductivity Measurements and Elemental Analysis
The metal complexes that are formed are stable even when the temperature is kept at room temperature. Although they are insoluble in water, they are dissolvable in DMF and acetonitrile. The molar conductivity (10–3 M in both DMF and acetonitrile solution) as well as the elemental analysis of the generated compounds is displayed in Table 1. The theoretical and experimental elemental analyses of metal complexes are very well in agreement with one another. The proposed formula of the title complexes, from the correlation of the elemental analyses data, are C32H24ClCrN4O5, C32H26MnN4O6, and C32H24ClFeN4O5, for the Cr(III), Mn(II), and Fe(III) complexes, respectively. The metal complexes do not have an electrolytic character, which may be deduced from the low molar conductivity values.
3.2.2. IR Spectra
The purpose of the infrared spectral collection was to achieve a deeper comprehension of the relationship that exists between the Az ligand and the metal ion. The most significant infrared bands that may be used to determine the ligand–metal combination that works best are outlined in Table 1 which can be found here. These bands are almost certainly the result of interactions between the coordinating sites of the ligand and the metal ions in the molecule.
At a frequency of 1542 cm–1, the azo (−N=N−) band was identified. This band showed up in the IR spectra of the Az ligand after coordination with the metal ion, but it did so at a lower wave number, 1460–1470, as shown in Table 1. This indicates that coordination occurred with the azo-nitrogen.
In the infrared spectra of the unbound ligand, the phenolic–OH band could be seen anywhere between 3114 and 3218 cm–1 in wavelength. The vibration phenolic–OH was similarly eliminated in the metal complexes, which lends credence to the theory that the phenolic oxygen of the ligand plays a role in the formation of the C–O–M bond during deprotonation;44,45Table 1.
The broadband in the complexes was positioned at >3400 cm–1; it was determined that this band was caused by −OH groups on water molecules.46,47 In addition, the complexes have two additional spectral bands, which may be found at 478–485 and 544–551, respectively, and are referred to as (M–N)48,49 and (M–O);50,51Table 1.
Our research shows that the Azo-ligand, Az, functions as a mononegatively bi-dentate ligand, meaning that it generates complexes by way of both its azo-nitrogen (−N=N−) and phenolic oxygen (−OH) atoms. This is the conclusion that we have drawn from our data.
3.2.3. Magnetic Moment and Electronic Spectra Measurements
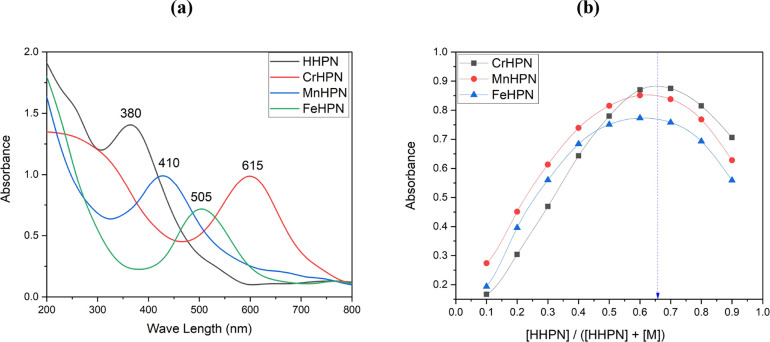
The UV–V of the Az ligand and its metal complexes were measured in acetonitrile between 200 and 800 nanometers. Electronic transitions that may be attributed to n → π* states were observed to take place in the unbound Az ligand when it was observed at 380 nm. When a metal ion was present, the band shifted to longer wavelengths than it normally would have been. With a view to determining the internal structure of the transition metal complexes, the effective magnetic moment, denoted by the formula μeff = 2.83 (((Xg*Mwt) – (dia magnetic correction*T))0.5), was utilized as another method.
In the case of the CrAz2 complex, the electronic spectrum band at 615 nm was connected to the transition of 4A2g(F) → 4T2g(F) in the octahedral geometry complex.52 The 3.84 B.M magnetic moment found for the CrAz2 complex may be described by the occurrence of a d3 (t2g3) electron configuration in an octahedral geometry around the Cr(III) center. The results from the electronic transitions are supplemented by an effective magnetic moment value (3.84 μB), which is within the experimentally acceptable range (3.70–3.90 μB) for Cr(III) complexes.53
In the case of the MnAz2 complex, the band in its electronic spectra that is located at 24390.24 cm–1 may be ascribed to 6A1g(F) → 4A1g(G) transition, which hints at an octahedral geometry around the Mn(II) center;54,55Table 1. The value of 1.96 B.M for the μeff of the MnAz2 complex is indicative of the occurrence of a d5 low-spin (t2g5) electron configuration in an octahedral geometry around the center of Mn(II);56Table 1.
The electronic spectrum of FeAz2 shows a transition band at 19,801.98 cm–1. This visible band is assigned to the ligand-to-metal charge transfer (LMCT) band characteristic of octahedral iron(III) complexes. In general, high-spin Fe(III) complexes have magnetic moment values of around 5.90 μB with no possibility of orbital contribution, while low-spin complexes (S = 1/2) have values close to 1.70 μB.57 The μeff value for FeAz2 is found to be 1.84 μB, which is compatible with a return to the d5 (t2g5) configuration;58Table 1. This suggests that the complex has an octahedral geometry with Fe(III) as its core.
3.2.4. Metal Complexes Stoichiometry
The continuous variation method developed by Jobs was applied in the process of determining the chemical formula of metal complexes.59 The detailed procedures for the Jobs method of continuous variation are listed in the Supporting Information. The absorbance curve that was generated by using this method demonstrated the highest level of absorption at a mole fraction of the ligand equal to 0.65. This finding hints that the complex was formed with a metal-to-ligand ratio of 1:2 (as seen in Figure 1), as was previously mentioned.
Figure 1.
(a) UV–Vis Spectral and (b) stoichiometry of the CrAz2, MnAz2, and FeAz2 complexes.
3.2.5. Mass Spectra
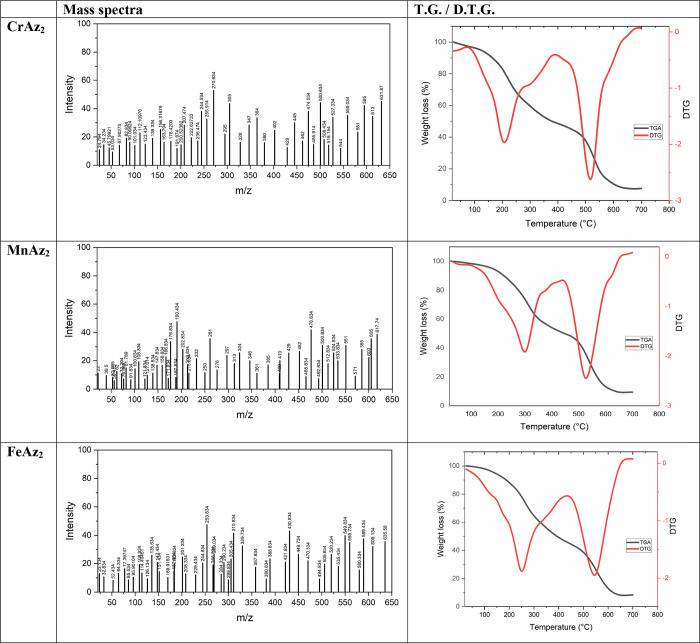
The patterns of mass spectra are analyzed to determine the proportional amounts of the constituent parts of a substance. During the course of our investigation, we looked at the mass spectra of several metal complexes, as can be shown in Figure 2. The molecular ion peak, denoted by the symbol M+, can be found in the mass spectra of the CrAz2, MnAz2, and FeAz2 complexes, and its values (631.87, 617.74, and 635.58) are excellent matches for the values that were predicted (631.5, 617, and 635.5). It is possible that the particular characteristics of the metal complexes are responsible for the presence of other peaks in the mass spectrum. The results of our analysis of the mass spectrum are in line with the previously established values for carbon, hydrogen, and nitrogen, as well as the hypothesized structure.
Figure 2.
Mass spectra and TG/ DTG curves of the CrAz2, MnAz2, and FeAz2 complexes.
3.2.6. Thermal Analysis of the Prepared Complexes
It is necessary to ascertain the proportion of coordinated to uncoordinated water molecules in order to get an understanding of the structural transformations that take place in metal complexes as a result of heat treatment (Figure 2 and Table 2).60,37,61
Table 2. Thermal Decomposition of the Prepared Complexes.
| TG (°C) | DTG (°C) | mass loss (%) |
assignment | residue | ||
|---|---|---|---|---|---|---|
| found | calculated | |||||
| CrAz2 | 30–430 | 215 | 53.84 | 53.76 | 1H2O + C20H14O2Cl | Cr |
| 430–650 | 520 | 38.22 | 38.01 | C12H8O2N4 | ||
| MnAz2 | 30–420 | 295 | 52.29 | 52.19 | 2H2O + C20H14O2 | Mn |
| 420–640 | 530 | 39.13 | 38.90 | C12H8O2N4 | ||
| FeAz2 | 30–440 | 255 | 53.24 | 53.42 | 1H2O + C20H14O2Cl | Fe |
| 440–680 | 540 | 37.91 | 37.77 | C12H8O2N4 | ||
The first stage of deterioration was observed at temperatures between 30 and 430 °C, 30 and 420 °C, and 30 and 440 °C, with calculated weight loss percentages of (53.84 (53.76), 52.29 (52.19)), and (37.91 (37.77)) corresponding to the removal of 1H2O + C20H14O2Cl, 2H2O + C20H14O2, and 1H2O + C20H14O2Cl for CrAz2, MnAz2, and FeAz2 complexes, respectively (Figure 2 and Table 2).
The 2nd degradation step was seen at temperatures ranging from 430 to 650 °C, 420 to 640 °C, and 440 to 680 °C, with calculated weight loss percentages of (38.22 (38.01)), (39.13 (38.90)), and (37.91 (37.77)), which corresponded to the elimination of C12H8O2N4 for both CrAz2 and MnAz2 complexes, respectively; Figure 2 and Table 2.
At the conclusion of the thermal degrading process, the metal remained as a metallic residue, as seen in Figure 2 and Table 2. Following analysis of the TG and DTG curves, it was concluded that none of the CrAz2, MnAz2, or FeAz2 complexes contain any water molecules that contribute to their hydration despite the fact that the coordination spheres of the CrAz2, MnAz2, and FeAz2 complexes each contain one, two, and one coordinated water molecules, respectively.
3.3. DFT Calculations
3.3.1. Geometry Optimization
The Az free ligand and its metal complexes are shown in Figure 3, each with their optimal structural representations. It was found that all of the CrAz2, MnAz2, and FeAz2 complexes have hexa-coordinate geometry as [Cr(Az)2(Cl)(H2O)], [Mn(Az)2(H2O)2], and [Fe(HPN)2(Cl)(H2O)]. The bond parameters (bond length, bond angles, and dihedral angles) of the free ligand and its metal complexes are found in the supplementary data file (Supporting Information data file).
Figure 3.
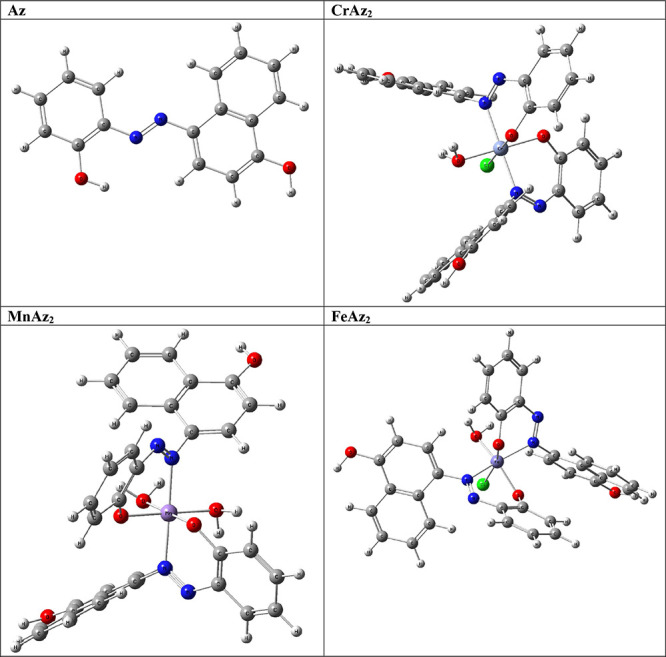
3D optimized structure of the subject compounds.
The properties in terms of total energy (ETotal), energy gap (ΔE), hardness (CH), and softness (S) were calculated for free ligand and compared with that in its metal complexes.
To perform a comparative study of the relative stability of the studied complexes, the binding energy (BE) or the complexation energy of the studied complexes was calculated, Table S1, as follows:62
The more negative BE corresponds to the most stable complex. From the calculated values, Table S1, one can notice that all complexes are more stable than their corresponding ligands, and the order of the complex’s stability is [Fe(HPN)2(Cl)(H2O)] −692.261 eV) > [Mn(Az)2(H2O)2] (−589.062 eV) > [Cr(Az)2(Cl)(H2O)] (−475.781 eV).
3.3.2. Frontier Molecular Orbitals (FMOs) and Reactivity Parameters
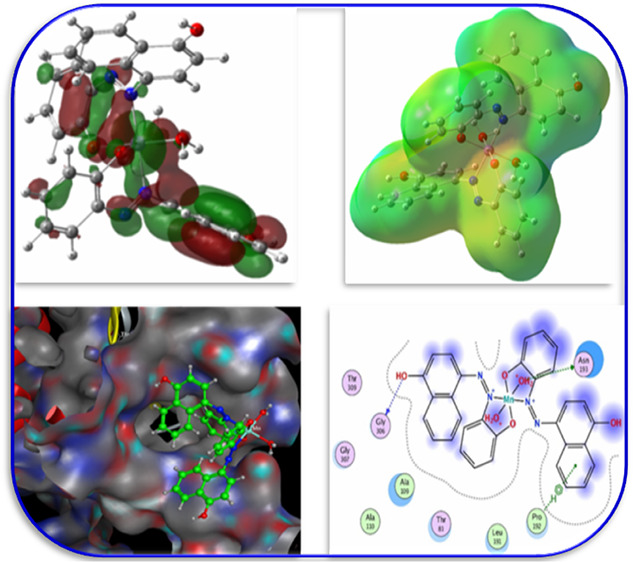
The FMOs, especially the HOMOs and the LUMOs, have been widely used to understand the chemical stability, optical, reactivity, and electrical features of various chemical systems.63,64 The plots of the HOMO and LUMO are provided in Figure 4.
Figure 4.
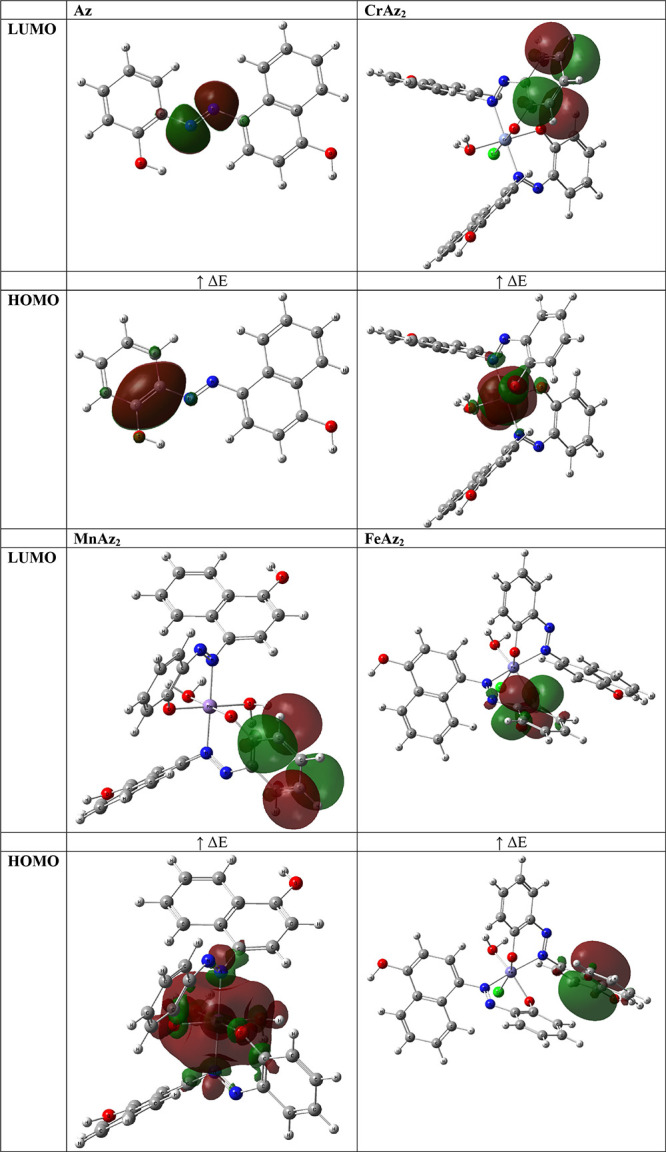
HOMO–LUMO of the optimized structures.
In this particular piece of research, HOMO–LUMO energies were used to calculate a number of different properties, including the energy gap, ionization potential, electronegativity, electron affinity, chemical hardness, chemical potentials, softness, and electrophilicity index. The stability, biological activity, polarizability, reactivity, and hardness–softness of a molecule are all affected by the HOMO and LUMO orbitals.65,66
It is also possible to forecast the reactivity of a molecule by making use of the energy gap that exists between these orbitals. As a consequence of this, molecules that have a lower ΔE are more conducive to the docking process (MnAz2 > FeAz2 > CrAz2 > Az).
Hard acids react more frequently with strong bases, whereas soft acids react more frequently with weak bases, according to the hard-soft acid–base rule, which may be used to forecast the chance of one molecule interacting with another. In interactions with biological molecules, soft molecules are preferred over hard molecules, which is to be expected given the prevalence of soft molecules in living things. As a consequence of this, it is possible that soft molecules interact with biological molecules more effectively than hard molecules.67,68 As a direct consequence of this, the ranking of MnAz2, FeAz2, CrAz2, and Az in Table 3 is as follows: MnAz2 > FeAz2 > CrAz2 > Az.
Table 3. Calculated EHOMO (eV), ELUMO (eV), ionization Potential (IP, eV), Electronegativity (EN, eV), the Energy Gap (ΔE, eV), Chemical Potential (CP, eV), electron Affinity (EA, eV), Softness (S, eV), Chemical Hardness (CH, eV), and Electrophilicity Index (EP, eV) of the Subject Compounds.
| EHOMO (eV) | ELUMO (eV) | ΔE (eV) | IP (eV) | EA (eV) | EN (eV) | CP (eV) | CH (eV) | S (eV–1) | EP (eV) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Az | –10.22 | –1.17 | 9.05 | 10.22 | 1.17 | 5.69 | –5.69 | 4.52 | 0.11 | 3.58 |
| CrAz2 | –14.10 | –8.14 | 5.96 | 14.10 | 8.14 | 11.12 | –11.12 | 2.98 | 0.34 | 20.76 |
| MnAz2 | –17.83 | –12.35 | 5.48 | 17.83 | 12.35 | 15.09 | –15.09 | 2.74 | 0.37 | 41.58 |
| FeAz2 | –10.26 | –4.61 | 5.65 | 10.26 | 4.61 | 7.44 | –7.44 | 2.83 | 0.35 | 9.78 |
The chemical potential and electrophilicity index of the compounds in question represent both the substances’ inherent stability as well as their predisposition for engaging in the electrophilic activity.52,69
The electrophilicity and nucleophilicity indices are also crucial. The electrophilicity index indicates electron-accepting capacity, whereas the nucleophilicity index demonstrates electron-donating ability.53,70 As an electrophile, the chemical reactivity ranks in the same direction as a growing electrophilicity index. Thus, in terms of chemical reactivity, MnAz2 is more reactive as an electrophile, followed by CrAz2, than FeAz2 and finally the Az ligand.
3.3.3. Molecular Electrostatic Potential (MEP)
Electrostatic potential (MEP) is a measure of the force acting on a positive test charge (a proton) near a molecule due to the electrical charge distribution of the molecule’s electrons and nuclei. MEP can be used to visualize the charge distribution of molecules and how they interact with each other. MEP can also be used as a general and versatile indicator for electronic substituent effects in various chemical reactions,71,72 A color-coded map is used in MEP representations to highlight the various charge values of the electronic potential that was present at the surface of the molecule. These charge values were observed. The region that is lacking electrons is depicted as a positively charged zone in blue, whilst the region that is abundant in electrons is depicted as a negatively charged region in red. The mapping MEP of the compounds under study were explored theoretically and is provided in Figure 5. In spite of the fact that the majority of the positive sites were found on the hydrogen atoms, while the negative charges were found on hetero oxygen and nitrogen atoms. This indicated that the ligand is an electron donor through the hetero oxygen and nitrogen atoms.
Figure 5.
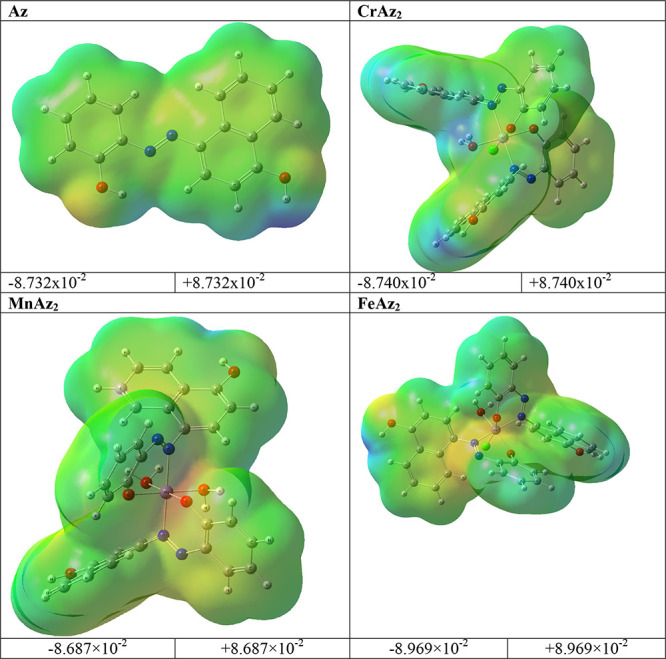
MEP of the title compounds.
3.3.4. Natural Charge Analysis
Because they represent the physicochemical characteristics of a molecule (the electronic structure, vibrational spectra, dipole moment, polarizability, and other molecular properties), atomic charges play a significant role in molecules,73,74 The atomic charges of the free ligand and its Cr(III), Mn(II), and Fe(III) complexes in the current investigation were calculated using NBO analysis at the B3LYP/cc-pVQZ level of theory in ethanol as the solvent. The findings are presented in Figure 6 and in the Supporting Information file.
Figure 6.
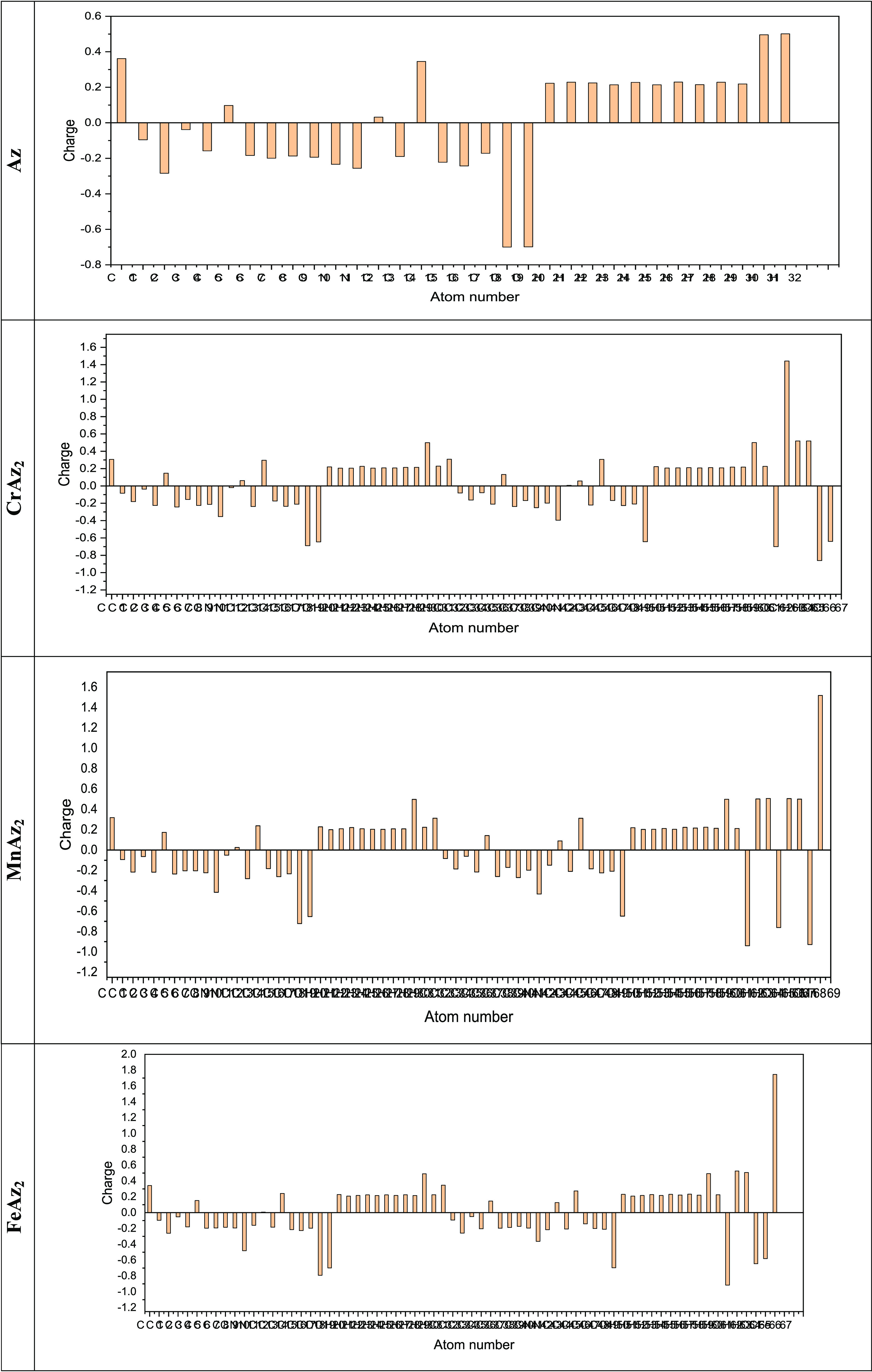
Plot of natural charge distribution of the free ligand and its Cr(III), Mn(II), and Fe(III) complexes computed using the B3LYP/cc-pVQZ level of theory in ethanol as the solvent.
Understanding electronegativity equalization and charge transfer in the chemical reactivity of the title molecules is made easier with the use of NBO analysis. The NBO analysis of the molecules reveals that carbon atoms in the free ligand and its Cr(III), Mn(II), and Fe(III) complexes contain both positive and negative charges. Positive carbons are seen for carbon atoms coupled to the electron-withdrawing oxygen and nitrogen atoms, as illustrated in Figure 6, and the values are tabulated in the Supporting Information file, including (C1, C15, C6, C13), (C32, C46, C1, C15, C6, C37, C13, C44), (C1, C32, C46, C15, C6, C37, C44, C13), and (C32, C1, C46, C15, C6, C37, C44, C13) atoms in the tiled free ligand and its Cr(III), Mn(II), and Fe(III) complexes, respectively. On the other hand, the other carbon atoms including carbon atoms (C3, C17, C16, C8, C10, C14, C9, C7, C18, C5, C2, C4), (C40, C7, C38, C14, C17, C48, C5, C9, C45, C10, C18, C36, C49, C41, C3, C16, C39), (C14, C40, C17, C38, C7, C18, C48, C10, C5, C3, C36, C45, C49, C8, C9, C41, C34), and (C3, C34, C17, C16, C49, C45, C36, C48, C38, C7, C18, C41, C10, C8, C39) have a negative charge in the tiled free ligand and its Cr(III), Mn(II), and Fe(III) complexes, respectively. More specifically, the C1 (0.36179 e), C32 (0.30866 e), C1 (0.31891 e), and C32 (0.34781 e) atoms are the largest positive carbons, while the carbon atoms C3 (−0.28405 e), C40 (−0.25088 e), C14 (−0.28139 e), C3 (−0.25998 e) atoms are the largest negative carbons, for the free ligand and its Cr(III), Mn(II), and Fe(III) complexes, respectively. All the hydrogen atoms in the free ligand and its Cr(III), Mn(II), and Fe(III) complexes have positive charges. Specifically, the H32 (0.50109 e), H65 (0.51891 e), H64 (0.50612 e), H63 (0.52542 e) atoms are the positive hydrogen atoms in the free ligand and its Cr(III), Mn(II), and Fe(III) complexes, respectively.
The net charges of the Cr(III), Mn(II), and Fe(III) ions in the Cr(III), Mn(II), and Fe(III) complexes are (1.4412 e), (1.51852 e), and (1.74594 e), respectively, whereas the valence of the free Cr(III), Mn(II), and Fe(III) ions is +3, +2, and +3, respectively. The decrease in the positive charge of the Cr(III), Mn(II), and Fe(III) ions in the Cr(III), Mn(II), and Fe(III) complexes was due to accumulation of the electronic charges from the associated coordinated ions.
3.3.5. NBO Analysis
The NBO study gives evidence for interactions between donors and acceptors and predicts the energy required for their stabilization with second-order perturbations.75 In order to demonstrate the donor–acceptor interactions that take place between the HOMO and LUMO levels of the molecule, the NBO analysis was carried out using the B3LYP/cc-pVQZ method. Supporting Information data provide a summary of the natural population analysis that was calculated as well as the natural electronic configuration of the investigated compounds.
The NBO method is well recognized as a fast approach to learning about the features of the electronic structure. Donor–acceptor analysis is facilitated by this method, as is analysis of charge transfer, delocalization, and conjugative interactions in molecules.76
The NBO analysis tool is a helpful method for investigating charge transfer or hyper-conjugative interactions, as well as for analyzing intra- and intermolecular bonding. The NBO quantitatively examines bonding and anti-bonding interactions caused by second-order disturbance by expressing the energy of these interactions as E(2).77−79 To calculate the off-diagonal NBO matrix element E(2) = ΔEij = qi(F(i,j)2/Ej – Ei), where Ei and Ej are the diagonal elements, qi is donor orbital occupancy and Fi,j is the NBO off-diagonal matrix element.
According to observations of perturbation energy E(2) for various transitions between these donors and acceptors, the Supporting Information file, the following transitions for free ligands are extremely likely to occur; for free ligands; C1–C3 → C2–C4 (132.62 kJ/mol, π → π*), C13–C15 → C17–C18 (116.7 kJ/mol, π → π*), C13–C15 → C14–C16 (76.25 kJ/mol, π → π*), O19 → C13–C15 (27.81 kJ/mol, LP → π*), for Cr(II) complex; C7–C8 → C9–C10 (88.93 kJ/mol, π → π*), C35–C37 → C40–C41 (53.76 kJ/mol, π → π*), C1–C2 → C7–C8 (41.96 kJ/mol, π → π*), C4–C6 → C9–C10 (38.89 kJ/mol, π → π*), for Mn(II) complex; C1–C2 → C4–C6 (720.14 kJ/mol, π → π*), C32–C33 → C35–C37 (400.09 kJ/mol, π → π*), C34–C36 → C35–C37 (338.46 kJ/mol, π → π*), C44–C46 → C45–C47 (62.76 kJ/mol, π → π*), for Fe(III) complex; C13–C15 → C17–C18 (82.91 kJ/mol, π → π*), C13–C15 → C14–C16 (62.76 kJ/mol, π → π*), C44–C46 → C45–C47 (44.13 kJ/mol, π → π*), C44–C46 → C48–C49 (38.44 kJ/mol, π → π*), are the most probable transitions.
Using NBO calculations, significant knowledge on the structures and characteristics of the Cr(III), Mn(II), and Fe(III) complexes was collected. The NBO analysis of the compounds indicated substantial inter- and intramolecular interactions between the acceptor NBOs and the donor NBOs. These interactions were between the donor NBOs and the acceptor NBOs.
3.4. In Vitro Antimicrobial Activity
The newly synthesized compounds’ antibacterial and antifungal activity was tested against a diversity of types of bacteria and fungi strains, such as Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli, Aspergillus flavus, Bacillus cereus, Trichophyton rubrum, and Candida albicans. The purpose of these tests was to gain a better understanding of the newly synthesized compounds. The biological activity of each compound was evaluated by determining how much of an impact it had on the size of the inhibition zone (IZ). This zone serves as a measurement of how effective the chemical is in inhibiting the development of microorganisms. Table 4 demonstrates that the metal complexes had an inferior minimum inhibitory concentration (MIC) and a broader inhibition zone compared to the pristine ligand.
Table 4. Anti-Bacterial and Anti-Fungal Activity of the Titled Compounds in Terms of Inhibition Zone (IZ, mm) and Activity Index (%).
| anti-bacterial
activity | |||||
|---|---|---|---|---|---|
| bacterial strains | Az | CrAz2 | MnAz2 | FeAz2 | |
| Pseudomonas aeruginosa | IZ | 9 | 15 | 16 | 16 |
| % | 50.00 | 83.33 | 88.89 | 88.89 | |
| MIC | 50 | 12.5 | 6.25 | 6.25 | |
| Escherichia coli (−ve) | IZ | 9 | 17 | 18 | 17 |
| % | 45.00 | 85.00 | 90.00 | 85.00 | |
| MIC | 25 | 6.25 | 6.25 | 6.25 | |
| Staphylococcus aureus (+ve) | IZ | 8 | 15 | 15 | 15 |
| % | 44.44 | 83.33 | 83.33 | 83.33 | |
| MIC | 25 | 12.5 | 6.25 | 6.25 | |
| Bacillus cereus (+ve) | IZ | 9 | 14 | 15 | 14 |
| % | 50.00 | 77.78 | 83.33 | 77.78 | |
| MIC | 50 | 6.25 | 6.25 | 6.25 | |
| anti-fungal activity | |||||
|---|---|---|---|---|---|
| fungal strains | Az | CrAz2 | MnAz2 | FeAz2 | |
| Aspergillus flavus | IZ | 9 | 16 | 16 | 16 |
| % | 47.37 | 84.21 | 84.21 | 84.21 | |
| MIC | 25 | 12.5 | 6.25 | 6.25 | |
| Trichophyton rubrum | IZ | 9 | 17 | 18 | 17 |
| % | 40.91 | 77.27 | 81.82 | 77.27 | |
| MIC | 50 | 12.5 | 6.25 | 12.5 | |
| Candida albicans | IZ | 9 | 17 | 17 | 17 |
| % | 42.86 | 80.95 | 80.95 | 80.95 | |
| MIC | 50 | 12.5 | 6.25 | 6.25 | |
The chelation hypothesis80−82 proposes that metal ions have a positive charge that can be neutralized through the process of chelation or the creation of a complex with a ligand. This theory is one of several that have been proposed as potential explanations for this phenomenon. This electron delocalization across the ring might make the molecule more lipophilic, which would make it easier for it to enter the lipid bilayer that makes up the cell membrane. Once inside, the complex may cause the binding sites to become unstable and may change the metabolic pathways, which may ultimately result in the bacterium’s death.
The inhibition zone values of the newly found compounds are compared in Table 4 with those of the chloramphenicol antibiotic, which has a high activity index and is therefore expected to have a high inhibition zone value. Strong antibacterial activity was shown by the MnAz2 complex against Escherichia coli (−ve), Pseudomonas aeruginosa (−ve), Bacillus cereus (+ve), and Staphylococcus aureus (+ve), respectively, with the activity index (%) of 88.89, 83.33, and 90.00, respectively. Additionally, it demonstrated significant antifungal effectiveness against Trichophyton rubrum, Aspergillus flavus, and Candida albicans, with activity indexes (%) of 81.82, 84.21, and 80.95, respectively.
The MIC also known as the lowermost dose of a drug that may prevent the growth of bacteria or fungus is frequently used as a starting point in larger preclinical trials of potential antibacterial drugs. These trials are often conducted in preparation for clinical testing. The MIC value was found by repeatedly diluting a sample until the desired concentration was reached. The MIC values that were calculated for the drugs being studied are presented in Table 4. This table demonstrates that the metal complexes had a lower MIC (about 6.25 ppm) than the free ligand did (approximately 50 ppm).
To make a comparison between the biological activity of the compounds that are the subject of this research and that of the metal complexes that have been described in the previous scientific literature,83−89 Supporting Information; Table S2. The findings indicated that the current compounds had significant values of biological activity which suggests that they could be effective antimicrobials.
3.5. Molecular Docking
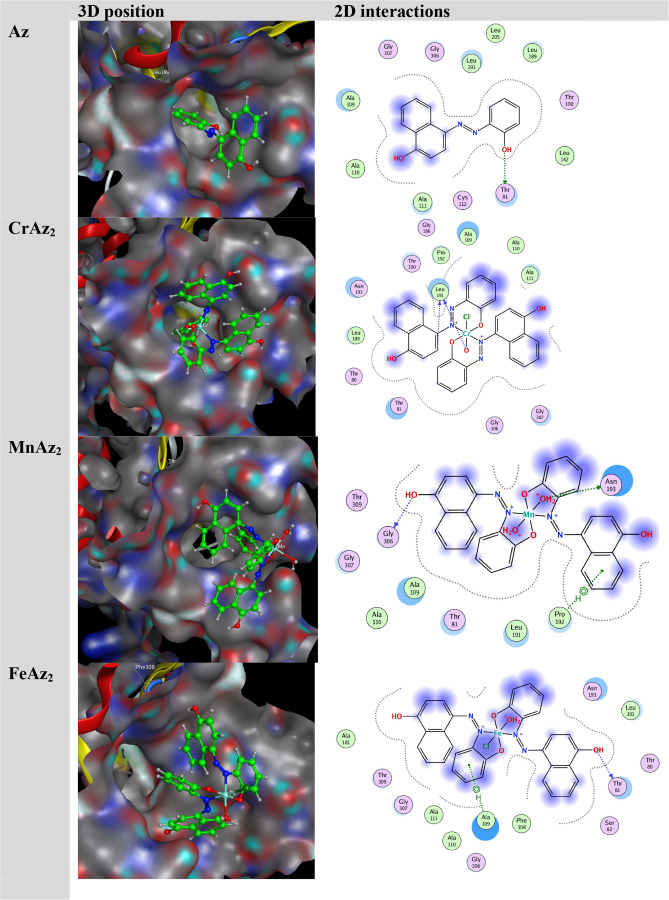
To provide a deep understanding of the interactions between the synthesized complexes and target protein E. coli (PDB ID: 1hnj), we performed the molecular docking study by using the MOE program. The docking score (S) and hydrogen bonding (H) interactions were visualized, and interaction energies were calculated for the title complexes. The obtained energy results are presented in Table 5. Figure 7 demonstrates the structures of synthesized complexes interacting with amino-acid residues through some hydrogen bonding interactions. The significance of the metal ion and ligand is figured out by the energy values of hydrogen bonding interactions forming between the same amino-acid residues and noncoordinated parts of ligands around metal ions.90,49
Table 5. Molecular Docking Data.
| ligand | receptor | interaction | distance | E (kcal/mol) | S (kcal/mol) | Ki (μM) | |
|---|---|---|---|---|---|---|---|
| Az | O 19 | THR 81 | H-donor | 2.85 | –1.40 | –6.55 | 16.17 |
| CrAz2 | C 5 | LEU 191 | H-donor | 3.21 | –0.80 | –7.26 | 4.85 |
| O 65 | LEU 191 | H-donor | 2.65 | –5.10 | |||
| MnAz2 | O 20 | GLY 06 | H-donor | 2.90 | –0.90 | –7.98 | 1.43 |
| O 65 | ASN 193 | H-donor | 2.66 | –12.60 | |||
| 6-ring | PRO 192 | pi–H | 3.62 | –0.70 | |||
| FeAz2 | O 52 | THR 81 | H-donor | 2.90 | –2.50 | –7.88 | 1.69 |
| 6-ring | ALA 09 | pi-H | 3.81 | –0.60 |
Figure 7.
3D orientation of the substrate–protein complex.
The docking data showed that the active site of the protein had strong interactions with the substrates, including hydrophobic and hydrogen bonding interactions.91 After all the compounds were investigated, it was found that MnAz2 had the greatest capacity to inhibit, followed by FeAz2, CrAz2, and Az. As can be seen in Table 5 and Figure 7, the most efficient molecule, MnAz2, interacted with the protein through the formation of hydrogen bonds as well as hydrophobic interactions.
The inhibition constant, often known as the Ki value, of a molecule, can be utilized to evaluate the drug’s potential as a hit, lead, or therapeutic candidate.92,93 A higher Ki rating denotes a more powerful action, which is advantageous because of its increased potency. Throughout the course of this study, the Ki values for the compounds ranged anywhere from 1.43 for MnAz2 to 4.85 for CrAz2. According to these data, MnAz2 appears to be a potential therapy method due to the fact that it has a low Ki value.
The findings indicate that molecular docking may be used to predict chemical binding affinity to an antimicrobial target protein and provide potential treatment choices. In general, these findings illustrate that molecular docking can be used. These findings will need to be validated via more research and the efficacy of these chemicals as potential medicines will also need to be evaluated.
4. Conclusions
In this work, we synthesized CrAz2, MnAz2, and FeAz2 complexes and thoroughly analyzed them using a variety of spectroscopic and physicochemical methods. The Az ligand acted as a monobasic bi-dentate NO ligand with 1:2 molar ratios when attached to Cr(III), Mn(II), and Fe(III). Several analytical and spectroscopic techniques were employed to ascertain the geometric structures of the CrAz2, MnAz2, and FeAz2 complexes. Our results showed that the CrAz2, MnAz2, and FeAz2 complexes all have octahedral geometries. To further our understanding of the properties of the CrAz2, MnAz2, and FeAz2 complexes, we also used quantum chemistry methods to estimate their theoretically perfect molecular structures. As a result, we were able to foresee and verify the electronic structures of these complexes, which shed light on their prospective medicinal chemistry uses. In addition to analyzing the properties of the free ligand and its metal complexes, we looked into their potential antibacterial and antifungal effects. To test the effects of the free ligand and its metal complexes, we used pathogenic bacterial and fungal strains that are frequently found in Egypt’s polluted environments. Based on the results, metal complexes have the potential to be used as efficient antimicrobial agents as they are more active against bacteria than the free ligand. At last, we used molecular docking to examine whether or not the free ligand and its metal complexes were effective in restraining E. coli growth (PDB ID: 1hnj). By testing several compounds, we were able to see if any of them were able to bind to the receptor of interest and perhaps alter its function. One of the most promising strategies for inhibiting E. coli growth is the MnAz2 complex, which has the highest affinity to the receptor. Our study has elucidated the structure and antimicrobial activity of CrAz2, MnAz2, and FeAz2 complexes in great detail. Having this information can help researchers create and perfect these complexes for use in medicinal chemistry.
Acknowledgments
The authors acknowledge the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research at King Faisal University, Saudi Arabia, for financial support under the annual funding track (GRANT 3191).
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic Supplementary Material.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c01413.
1H NMR of the AZ free ligand; 13C NMR of the AZ free ligand; binding energy of the studied complexes based on DFT/B3LYP with the cc-PVQZ basis set in ethanol as the solvent; comparison between the minimum inhibition concentrations (MIC) of the current compounds with previously reported compounds in the literature survey against E. coli; bond parameters (bond length, bond angles, and dihedral angles) of the free ligand and its metal complexes; summary of natural population analysis; NBO data of the ligand; summary of natural population analysis of the Cr(III) complex; NBO data of the Cr(III) complex; summary of Natural Population Analysis of the Mn(II) complex; NBO data of the Mn(II) complex; summary of natural population analysis of the Fe(III) complex; and NBO data of the Fe(III) complex (PDF)
Author Contributions
H.M.A.E.-L.: Investigation, Funding acquisition, Writing-original draft, Writing-review and editing. M.M.K.: Investigation, Methodology, Resources, Formal analysis, Data curation, Funding acquisition, Writing-original draft, Writing-review and editing. M.K.: Writing-original draft, Writing-review and editing. A.A.: Investigation, Supervision, Methodology, Resources, Formal analysis, Data curation, Funding acquisition, Writing-original draft, Writing-review and editing. A.A.A.: Investigation, Supervision, Methodology, Resources, Formal analysis, Data curation, Funding acquisition, Writing-original draft, Writing-review and editing. A.A.A.: Investigation, Supervision, Methodology, Resources, Formal analysis, Data curation, Funding acquisition, Writing-original draft, Writing-review and editing. All authors have read and agreed to the published version of the manuscript.
This work was funded by the Deanship of Scientific Research, at King Faisal University, Saudi Arabia (GRANT 3191).
The authors declare no competing financial interest.
Supplementary Material
References
- Abouzayed F. I.; Abouel-Enein S. A.; Hammad A. M. Synthesis of Some Novel Nanosized Chelates of Anchoring Bisazo Dye 5-[5-(4, 6-Dioxo-2-thioxo-hexahydro-pyrimidin-5-ylazo)-naphthalen-1-ylazo]-2-mercapto-1 H-pyrimidine-4, 6-dione and Their Applications as Antioxidant and Antitumor Agents. ACS Omega 2021, 6, 27737–27754. 10.1021/acsomega.1c02989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T. L.; Gigant N.; Joseph D. Advances in direct metal-catalyzed functionalization of azobenzenes. ACS Catal. 2018, 8, 1546–1579. 10.1021/acscatal.7b03583. [DOI] [Google Scholar]
- Eltaboni F.; Bader N.; El-Kailany R.; Elsharif N.; Ahmida A. Chemistry and Applications of Azo Dyes: A Comprehensive Review. J. Chem. Rev. 2022, 4, 313–330. 10.22034/jcr.2022.349827.1177. [DOI] [Google Scholar]
- Bakr E. A.; Atteya E. H.; Al-Hefnawy G. B.; El-Attar H. G.; El-Gamil M. M. A novel azo-azomethine benzoxazole-based ligand and its transition metal (II),(III),(IV) complexes: Synthesis, characterization, theoretical studies, biological evaluation, and catalytic application. Appl. Organomet. Chem. 2023, 37, e7042 10.1002/aoc.7042. [DOI] [Google Scholar]
- Kumar S. S.; Sadasivan V.; Meena S. S.; Sreepriya R.; Biju S. Synthesis, structural characterization and biological studies of Ni (II), Cu (II) and Fe (III) complexes of hydrazone derived from 2-(2-(2, 2-dimethyl-4, 6-dioxo-1, 3-dioxan-5-ylidene) hydrazinyl) benzoic acid. Inorg. Chim. Acta. 2022, 536, 120919 10.1016/j.ica.2022.120919. [DOI] [Google Scholar]
- Mehrparvar S.; Scheller Z. N.; Wölper C.; Haberhauer G. Design of azobenzene beyond simple on–off behavior. J. Am. Chem. Soc. 2021, 143, 19856–19864. 10.1021/jacs.1c09090. [DOI] [PubMed] [Google Scholar]
- Deghadi R. G.; Mahmoud W. H.; Mohamed G. G. Metal complexes of tetradentate azo-dye ligand derived from 4, 4′-oxydianiline: Preparation, structural investigation, biological evaluation and MOE studies. Appl. Organomet. Chem. 2020, 34, e5883 10.1002/aoc.5883. [DOI] [Google Scholar]
- Kongot M.; Reddy D. S.; Singh V.; Patel R.; Singhal N. K.; Kumar A. A manganese (II) complex tethered with S-benzyldithiocarbazate Schiff base: Synthesis, characterization, in-vitro therapeutic activity and protein interaction studies. Spectrochim. Acta, Part A 2020, 231, 118123 10.1016/j.saa.2020.118123. [DOI] [PubMed] [Google Scholar]
- Yousef T.; Khairy M. Synthesis, Characterization, Optical, DFT, TD DFT Studies and In silico ADME Predictions of Thiosemicarbazone ligand and its Au (III) Complex. Orient. J. Chem. 2022, 38, 380303 10.13005/ojc/380303. [DOI] [Google Scholar]
- Wang M.; Zhao Y.; Chang M.; Ding B.; Deng X.; Cui S.; Hou Z.; Lin J. Azo initiator loaded black mesoporous titania with multiple optical energy conversion for synergetic photo-thermal-dynamic therapy. Appl. Mater. Interfaces 2019, 11, 47730–47738. 10.1021/acsami.9b17375. [DOI] [PubMed] [Google Scholar]
- Elkanzi N. A.; Hrichi H.; Salah H.; Albqmi M.; Ali A. M.; Abdou A. Synthesis, physicochemical properties, biological, molecular docking and DFT investigation of Fe (III), Co (II), Ni (II), Cu (II) and Zn (II) complexes of the 4-[(5-oxo-4, 5-dihydro-1, 3-thiazol-2-yl) hydrazono] methyl} phenyl 4-methylbenzenesulfonate Schiff-base ligand. Polyhedron 2023, 230, 116219 10.1016/j.poly.2022.116219. [DOI] [Google Scholar]
- Alghuwainem Y. A.; Abd El-Lateef H. M.; Khalaf M. M.; Abdelhamid A. A.; Alfarsi A.; Gouda M.; Abdelbaset M.; Abdou A. Synthesis, structural, DFT, antibacterial, antifungal, anti-inflammatory, and molecular docking analysis of new VO (II), Fe (III), Mn (II), Zn (II), and Ag (I) complexes based on 4-((2-hydroxy-1-naphthyl) azo) benzenesulfonamide. J. Mol. Liq. 2023, 369, 120936 10.1016/j.molliq.2022.120936. [DOI] [Google Scholar]
- Rana M.; Cho H.-J.; Roy T. K.; Mirica L. M.; Sharma A. K. Azo-dyes based small bifunctional molecules for metal chelation and controlling amyloid formation. Inorg. Chim. Acta 2018, 471, 419–429. 10.1016/j.ica.2017.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea M.; Emandi A.; Marinescu D.; Cristurean E.; Olar R.; Braileanu A.; Budrugeac P.; Segal E. Thermal stability of some azo-derivatives and their complexes. J. Therm. Anal. Calorim. 2003, 72, 525–531. 10.1023/A:1024517430630. [DOI] [Google Scholar]
- Khedr A. M.; El-Ghamry H. A.; El-Sayed Y. S. Nano-synthesis, solid-state structural characterization, and antimicrobial and anticancer assessment of new sulfafurazole azo dye-based metal complexes for further pharmacological applications. Appl. Organomet. Chem. 2022, 36, e6548 10.1002/aoc.6548. [DOI] [Google Scholar]
- Mahmoud W. H.; Sayed F. N.; Mohamed G. G. Azo dye with nitrogen donor sets of atoms and its metal complexes: synthesis, characterization, DFT, biological, anticancer and Molecular docking studies. Appl. Organomet. Chem. 2018, 32, e4347 10.1002/aoc.4347. [DOI] [Google Scholar]
- Mahmoud W. H.; Sayed F. N.; Mohamed G. G. Synthesis, characterization and in vitro antimicrobial and anti-breast cancer activity studies of metal complexes of novel pentadentate azo dye ligand. Appl. Organomet. Chem. 2016, 30, 959–973. 10.1002/aoc.3529. [DOI] [Google Scholar]
- Saad F. A.; Elghalban M. G.; El-Metwaly N. M.; El-Ghamry H.; Khedr A. M. Density functional theory/B3LYP study of nanometric 4-(2, 4-dihydroxy-5-formylphen-1-ylazo)-N-(4-methylpyrimidin-2-yl) benzenesulfonamide complexes: Quantitative structure–activity relationship, docking, spectral and biological investigations. Appl. Organomet. Chem. 2017, 31, e3721 10.1002/aoc.3721. [DOI] [Google Scholar]
- Mohammed H. Synthesis, identification, and biological study for some complexes of azo dye having theophylline. Sci. World J. 2021, 2021, 9943763 10.1155/2021/9943763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg K. B. Basis set effects on calculated geometries: 6-311++ G** vs. aug-cc-pVDZ. J. Comput. Chem. 2004, 25, 1342–1346. 10.1002/jcc.20058. [DOI] [PubMed] [Google Scholar]
- Martin J. M.; Taylor P. R. Basis set convergence for geometry and harmonic frequencies. Are h functions enough?. Chem. Phys. Lett. 1994, 225, 473–479. 10.1016/0009-2614(94)87114-0. [DOI] [Google Scholar]
- Becke A. D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098. 10.1103/PhysRevA.38.3098. [DOI] [PubMed] [Google Scholar]
- Lee C.; Yang W.; Parr R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. 10.1103/PhysRevB.37.785. [DOI] [PubMed] [Google Scholar]
- Raghavachari K. Perspective on “Density functional thermochemistry. III. The role of exact exchange” Becke AD (1993) J Chem Phys 98: 5648–52. Theor. Chem. Acc. 2000, 103, 361–363. 10.1007/s002149900065. [DOI] [Google Scholar]
- Khodiev M.; Holikulov U.; Jumabaev A.; Issaoui N.; Lvovich L. N.; Al-Dossary O. M.; Bousiakoug L. G. Solvent effect on the self-association of the 1, 2, 4-triazole: A DFT study. J. Mol. Liq. 2023, 382, 121960 10.1016/j.molliq.2023.121960. [DOI] [Google Scholar]
- Pecul M.; Lamparska E.; Cappelli C.; Frediani L.; Ruud K. Solvent effects on Raman optical activity spectra calculated using the polarizable continuum model. J. Phys. Chem. A. 2006, 110, 2807–2815. 10.1021/jp056443c. [DOI] [PubMed] [Google Scholar]
- Kohn W.; Sham L. J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133. 10.1103/PhysRev.140.A1133. [DOI] [Google Scholar]
- Tomberg A.Gaussian 09w tutorial. In An introduction to computational chemistry using G09W and Avogadro software, 2013; pp 1–36.
- Nielsen A.; Holder A.. Gauss view 5.0, user’s reference; GAUSSIAN Inc.: Pittsburgh, 2009. [Google Scholar]
- Tian L.; Feiwu C. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580. 10.1002/jcc.22885. [DOI] [PubMed] [Google Scholar]
- Seleem H.; El-Shetary B.; Shebl M. Synthesis and characterization of a novel series of metallothiocarbohydrazone polymers and their adducts. Heteroat. Chem. 2007, 18, 100–107. 10.1002/hc.20239. [DOI] [Google Scholar]
- Shokr E. K.; Kamel M. S.; Abdel-Ghany H.; Ali El-Remaily M. A. E. A. A.; Abdou A. Synthesis, characterization, and DFT study of linear and non-linear optical properties of some novel thieno [2, 3-b] thiophene azo dye derivatives. Mater. Chem. Phys. 2022, 290, 126646 10.1016/j.matchemphys.2022.126646. [DOI] [Google Scholar]
- Shebl M.; Khalil S. M. Synthesis, spectral, X-ray diffraction, antimicrobial studies, and DNA binding properties of binary and ternary complexes of pentadentate N 2 O 3 carbohydrazone ligands. Monatsh. Chem. 2015, 146, 15–33. 10.1007/s00706-014-1302-x. [DOI] [Google Scholar]
- Latif M.; Ahmed T.; Hossain M. S.; Chaki B.; Abdou A.; Kudrat-E-Zahan M. Synthesis, Spectroscopic Characterization, DFT Calculations, Antibacterial Activity, and Molecular Docking Analysis of Ni (II), Zn (II), Sb (III), and U (VI) Metal Complexes Derived from a Nitrogen-Sulfur Schiff Base. Russ. J. Gen. Chem. 2023, 93, 389–397. 10.1134/S1070363223020214. [DOI] [Google Scholar]
- Morgan S. M.; El-Sonbati A.; Eissa H. Geometrical structures, thermal properties and spectroscopic studies of Schiff base complexes: Correlation between ionic radius of metal complexes and DNA binding. J. Mol. Liq. 2017, 240, 752–776. 10.1016/j.molliq.2017.05.114. [DOI] [Google Scholar]
- Elkanzi N. A.; Ali A. M.; Albqmi M.; Abdou A. New Benzimidazole-Based Fe (III) and Cr (III) Complexes: Characterization, Bioactivity Screening, and Theoretical Implementations Using DFT and Molecular Docking Analysis. Appl. Organomet. Chem. 2022, 36, e6868 10.1002/aoc.6868. [DOI] [Google Scholar]
- Prakash A.; Singh B. K.; Bhojak N.; Adhikari D. Synthesis and characterization of bioactive zinc (II) and cadmium (II) complexes with new Schiff bases derived from 4-nitrobenzaldehyde and acetophenone with ethylenediamine. Spectrochim. Acta, Part A 2010, 76, 356–362. 10.1016/j.saa.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Abdou A. Synthesis, Structural, Molecular Docking, DFT, Vibrational Spectroscopy, HOMO-LUMO, MEP Exploration, antibacterial and antifungal activity of new Fe (III), Co (II) and Ni (II) hetero-ligand complexes. J. Mol. Struct. 2022, 1262, 132911 10.1016/j.molstruc.2022.132911. [DOI] [Google Scholar]
- Scholz C.; Knorr S.; Hamacher K.; Schmidt B. DOCKTITE— A Highly Versatile Step-by-Step Workflow for Covalent Docking and Virtual Screening in the Molecular Operating Environment. J. Chem. Inf. Model. 2015, 55, 398–406. 10.1021/ci500681r. [DOI] [PubMed] [Google Scholar]
- Abd El-Lateef H. M.; Khalaf M. M.; Kandeel M.; Amer A. A.; Abdelhamid A. A.; Abdou A. Designing, Characterization, biological, DFT, and molecular docking analysis for new FeAZD, NiAZD, and CuAZD complexes incorporating 1-(2-hydroxyphenylazo)-2-naphthol (H2AZD). Comput. Biol. Chem. 2023, 105, 107908 10.1016/j.compbiolchem.2023.107908. [DOI] [PubMed] [Google Scholar]
- Abd El-Lateef H. M.; Khalaf M. M.; Kandeel M.; Amer A. A.; Abdelhamid A. A.; Abdou A. New Mixed-ligand Thioether-quinoline complexes of Nickel (II), Cobalt (II), and Copper (II): Synthesis, Structural Elucidation, DFT, antimicrobial activity and Molecular docking exploration. Appl. Organomet. Chem. 2023, 37, e7134 10.1002/aoc.7134. [DOI] [Google Scholar]
- Al-Ayash S.; Al-Noor T.; Abdou A. Synthesis and Characterization of Metals Complexes with Uracil and Uracil Derivatives (A Review). Russ. J. Gen. Chem. 2023, 93, 987–995. 10.1134/S107036322304028X. [DOI] [Google Scholar]
- Alghuwainem Y. A.; El-Lateef H. M. A.; Khalaf M. M.; Amer A. A.; Abdelhamid A. A.; Alzharani A. A.; Alfarsi A.; Shaaban S.; Gouda M.; Abdou A. Synthesis, DFT, Biological and Molecular Docking Analysis of Novel Manganese (II), Iron (III), Cobalt (II), Nickel (II), and Copper (II) Chelate Complexes Ligated by 1-(4-Nitrophenylazo)-2-naphthol. Int. J. Mol. Sci. 2022, 23, 15614. 10.3390/ijms232415614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Gaber M. A. I.; Abd El-Lateef H. M.; Khalaf M. M.; Shaaban S.; Shawky M.; Mohamed G. G.; Abdou A.; Gouda M.; Abu-Dief A. M. Design, Synthesis, Spectroscopic Inspection, DFT and Molecular Docking Study of Metal Chelates Incorporating Azo Dye Ligand for Biological Evaluation. Materials 2023, 16, 897. 10.3390/ma16030897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdou A.; Mostafa H. M.; Abdel-Mawgoud A.-M. M. Seven metal-based bi-dentate NO azocoumarine complexes: Synthesis, physicochemical properties, DFT calculations, drug-likeness, in vitro antimicrobial screening and molecular docking analysis. Inorg. Chim. Acta 2022, 539, 121043 10.1016/j.ica.2022.121043. [DOI] [Google Scholar]
- Marković M.; Judaš N.; Sabolović J. Combined Experimental and Computational Study of cis-trans Isomerism in Bis (l-valinato) copper (II). Inorg. Chem. 2011, 50, 3632–3644. 10.1021/ic102585f. [DOI] [PubMed] [Google Scholar]
- Bacher F.; Enyedy E.; Nagy N. R. V.; Rockenbauer A.; Bognár G. M.; Trondl R.; Novak M. S.; Klapproth E.; Kiss T. S.; Arion V. B. Copper (II) complexes with highly water-soluble L-and D-Proline–thiosemicarbazone conjugates as potential inhibitors of topoisomerase IIα. Inorg. Chem. 2013, 52, 8895–8908. 10.1021/ic401079w. [DOI] [PubMed] [Google Scholar]
- Shiju C.; Arish D.; Kumaresan S. Synthesis, characterization, cytotoxicity, DNA cleavage, and antimicrobial activity of lanthanide (III) complexes of a Schiff base ligand derived from glycylglycine and 4-nitrobenzaldehyde. Arab. J. Chem. 2017, 10, S2584–S2591. 10.1016/j.arabjc.2013.09.036. [DOI] [Google Scholar]
- Najar A. M.; Eswayah A.; Moftah M. B.; Mk R. O.; Bobtaina E.; Najwa M.; Elhisadi T. A.; Tahani A.; Tawati S. M.; Khalifa A. M. Rigidity and flexibility of pyrazole, s-triazole, and v-triazole derivative of chloroquine as potential therapeutic against COVID-19. J. Med. Chem. Sci. 2023, 6, 2056–2084. 10.26655/JMCHEMSCI.2023.9.14. [DOI] [Google Scholar]
- Ikram M.; Rehman S.; Faiz A. Synthesis, characterization and antimicrobial studies of transition metal complexes of imidazole derivative. Bull. Chem. Soc. Ethiop. 2010, 24, 201. 10.4314/bcse.v24i2.54743. [DOI] [Google Scholar]
- Aiyelabola T.; Ojo I.; Adebajo A.; Ogunlusi G.; Oyetunji O.; Akinkunmi E.; Adeoye A. Synthesis, characterization and antimicrobial activities of some metal (II) amino acids’ complexes. Adv. Biol. Chem. 2012, 2, 268. 10.4236/abc.2012.23034. [DOI] [Google Scholar]
- Fekri A.; Zaky R. Solvent-free synthesis and computational studies of transition metal complexes of the aceto-and thioaceto-acetanilide derivatives. J. Organomet. Chem. 2016, 818, 15–27. 10.1016/j.jorganchem.2016.05.015. [DOI] [Google Scholar]
- Kumar A.; Kumar D.; Kumari K.; Mkhize Z.; Seru L. K.; Bahadur I.; Singh P. Metal-ligand complex formation between ferrous or ferric ion with syringic acid and their anti-oxidant and anti-microbial activities: DFT and molecular docking approach. J. Mol. Liq. 2021, 322, 114872 10.1016/j.molliq.2020.114872. [DOI] [Google Scholar]
- Hashem H. E.; Nath A.; Kumer A. Synthesis, molecular docking, molecular dynamic, quantum calculation, and antibacterial activity of new Schiff base-metal complexes. J. Mol. Struct. 2022, 1250, 131915 10.1016/j.molstruc.2021.131915. [DOI] [Google Scholar]
- Aljohani E. T.; Shehata M. R.; Alkhatib F.; Alzahrani S. O.; Abu-Dief A. M. Development and structure elucidation of new VO2+, Mn2+, Zn2+, and Pd2+ complexes based on azomethine ferrocenyl ligand: DNA interaction, antimicrobial, antioxidant, anticancer activities, and molecular docking. Appl. Organomet. Chem. 2021, 35, e6154 10.1002/aoc.6154. [DOI] [Google Scholar]
- Liu J.-Q.; Chen J.; Cheng F.; Luo D.; Huang J.; Ouyang J.; Nezamzadeh-Ejhieh A.; Peng Y.; Khan M. S. Recent advances in Ti-based MOFs in the biomedical applications. Dalton Trans. 2022, 51, 14817. 10.1039/d2dt03196e. [DOI] [PubMed] [Google Scholar]
- Al-Khateeb Z. T.; Karam F. F.; Al-Adilee K. Synthesis and characterization of some metals complexes with new heterocyclic azo dye ligand 2-[2-(5-Nitro thiazolyl) azo]-4-methyl-5-nitro phenol and their biological activities. J. Phys.: Conf. Ser. 2019, 1294, 052043 10.1088/1742-6596/1294/5/052043. [DOI] [Google Scholar]
- Modhavadiya V. Synthesis, characterization, spectral studies, biocidal activities of Fe (II) and Cu (II) complexes of azo dye ligand derived from sulfamethoxazole and substituted p-cresol. Orient. J. Chem. 2012, 28, 921. 10.13005/ojc/280236. [DOI] [Google Scholar]
- Rezaeivala M.; Keypour H. Schiff base and non-Schiff base macrocyclic ligands and complexes incorporating the pyridine moiety–The first 50 years. Coord. Chem. Rev. 2014, 280, 203–253. 10.1016/j.ccr.2014.06.007. [DOI] [Google Scholar]
- Job P. Recherches sur la formation de complexes minéraux en solution, et sur leur stabilité. Ann. Chim. 1928, 9, 113–203. [Google Scholar]
- Fan R.; Sumitani R.; Mochida T. Synthesis and Reactivity of Cyclopentadienyl Ruthenium (II) Complexes with Tris (alkylthio) benzenes: Transformation between Dinuclear and Sandwich-Type Complexes. ACS Omega 2020, 5, 2034–2040. 10.1021/acsomega.9b04272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arafath M. A.; Adam F.; Ahamed M. B. K.; Karim M. R.; Uddin M. N.; Yamin B. M.; Abdou A. Ni (II), Pd (II) and Pt (II) complexes with SNO-group thiosemicarbazone and DMSO: Synthesis, characterization, DFT, molecular docking and cytotoxicity. J. Mol. Struct. 2023, 1278, 134887 10.1016/j.molstruc.2022.134887. [DOI] [Google Scholar]
- Rahmouni N. T.; el Houda Bensiradj N.; Megatli S. A.; Djebbar S.; Baitich O. B. New mixed amino acids complexes of iron (III) and zinc (II) with isonitrosoacetophenone: Synthesis, spectral characterization, DFT study and anticancer activity. Spectrochim. Acta, Part A 2019, 213, 235–248. 10.1016/j.saa.2019.01.042. [DOI] [PubMed] [Google Scholar]
- Parr R. G.; Yang W. Density functional approach to the frontier-electron theory of chemical reactivity. J. Am. Chem. Soc. 1984, 106, 4049–4050. 10.1021/ja00326a036. [DOI] [Google Scholar]
- Yu J.; Su N. Q.; Yang W. Describing Chemical Reactivity with Frontier Molecular Orbitalets. JACS Au 2022, 2, 1383–1394. 10.1021/jacsau.2c00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrichi H.; Elkanzi N. A.; Ali A. M.; Abdou A. A novel colorimetric chemosensor based on 2-[(carbamothioylhydrazono) methyl] phenyl 4-methylbenzenesulfonate (CHMPMBS) for the detection of Cu (II) in aqueous medium. Res. Chem. Intermed. 2023, 49, 2257–2276. 10.1007/s11164-022-04905-4. [DOI] [Google Scholar]
- Hossain M.; Khushy K.; Latif M.; Hossen M. F.; Asraf M. A.; Kudrat-E-Zahan M.; Abdou A. Co (II), Ni (II), and Cu (II) Complexes Containing Isatin-Based Schiff Base Ligand: Synthesis, Physicochemical Characterization, DFT Calculations, Antibacterial Activity, and Molecular Docking Analysis. Russ. J. Gen. Chem. 2022, 92, 2723–2733. 10.1134/S1070363222120222. [DOI] [Google Scholar]
- Abu-Dief A. M.; Alotaibi N. H.; Al-Farraj E. S.; Qasem H. A.; Alzahrani S.; Mahfouz M. K.; Abdou A. Fabrication, structural elucidation, theoretical, TD-DFT, vibrational calculation and molecular docking studies of some novel adenine imine chelates for biomedical applications. J. Mol. Liq. 2022, 365, 119961 10.1016/j.molliq.2022.119961. [DOI] [Google Scholar]
- Abdou A.; Abdel-Mawgoud A. M. M. Synthesis, structural elucidation, and density functional theory investigation of new mononuclear Fe (III), Ni (II), and Cu (II) mixed-ligand complexes: Biological and catalase mimicking activity exploration. Appl. Organomet. Chem. 2022, 36, e6600 10.1002/aoc.6600. [DOI] [Google Scholar]
- Abdou A.; Omran O. A.; Nafady A.; Antipin I. S. Structural, spectroscopic, FMOs, and non-linear optical properties exploration of three thiacaix (4) arenes derivatives. Arab. J. Chem. 2022, 15, 103656 10.1016/j.arabjc.2021.103656. [DOI] [Google Scholar]
- Chattaraj P. K.; Roy D. R. Update 1 of: electrophilicity index. Chem. Rev. 2007, 107, PR46–PR74. 10.1021/cr078014b. [DOI] [Google Scholar]
- Khan I. M.; Shakya S. Exploring colorimetric real-time sensing behavior of a newly designed CT complex toward nitrobenzene and Co2+: spectrophotometric, DFT/TD-DFT, and mechanistic insights. ACS Omega 2019, 4, 9983–9995. 10.1021/acsomega.9b01314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varukolu M.; Palnati M.; Nampally V.; Gangadhari S.; Vadluri M.; Tigulla P. New Charge Transfer Complex between 4-Dimethylaminopyridine and DDQ: Synthesis, Spectroscopic Characterization, DNA Binding Analysis, and Density Functional Theory (DFT)/Time-Dependent DFT/Natural Transition Orbital Studies. ACS Omega 2022, 7, 810–822. 10.1021/acsomega.1c05464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouaket A.; Chraka A.; Raissouni I.; El Amrani M. A.; Berrada M.; Knouzi N. Synthesis, spectroscopic (13C/1H-NMR, FT-IR) investigations, quantum chemical modelling (FMO, MEP, NBO analysis), and antioxidant activity of the bis-benzimidazole molecule. J. Mol. Struct. 2022, 1259, 132729 10.1016/j.molstruc.2022.132729. [DOI] [Google Scholar]
- Gadhe C. G.; Kothandan G.; Cho S. J. Large variation in electrostatic contours upon addition of steric parameters and the effect of charge calculation schemes in CoMFA on mutagenicity of MX analogues. Mol. Simul. 2012, 38, 861–871. 10.1080/08927022.2012.659182. [DOI] [Google Scholar]
- Kargar H.; Fallah-Mehrjardi M.; Behjatmanesh-Ardakani R.; Munawar K. S. Synthesis, spectra (FT-IR, NMR) investigations, DFT, FMO, MEP, NBO analysis and catalytic activity of MoO2 (VI) complex with ONO tridentate hydrazone Schiff base ligand. J. Mol. Struct. 2021, 1245, 131259 10.1016/j.molstruc.2021.131259. [DOI] [Google Scholar]
- Weinhold F.; Landis C. R. Natural bond orbitals and extensions of localized bonding concepts. Chem. Educ. Res. Pract. 2001, 2, 91–104. 10.1039/B1RP90011K. [DOI] [Google Scholar]
- Tanak H.; Ağar A.; Yavuz M. Combined experimental and computational modeling studies on 4-[(2-hydroxy-3-methylbenzylidene) amino]-1, 5-dimethyl-2-phenyl-1, 2-dihydro-3H-pyrazol-3-one. Int. J. Quantum Chem. 2011, 111, 2123–2136. 10.1002/qua.22504. [DOI] [Google Scholar]
- Premkumar S.; Jawahar A.; Mathavan T.; Dhas M. K.; Sathe V.; Benial A. M. F. DFT calculation and vibrational spectroscopic studies of 2-(tert-butoxycarbonyl (Boc)-amino)-5-bromopyridine. Spectrochim. Acta, Part A 2014, 129, 74–83. 10.1016/j.saa.2014.02.147. [DOI] [PubMed] [Google Scholar]
- Premkumar S.; Rekha T.; Rajkumar B. J.; Asath R. M.; Jawahar A.; Mathavan T.; Benial A. M. F. Vibrational spectroscopic and structural investigations of 2-amino-6-methoxy-3-nitropyridine: a DFT approach. Braz. J. Phys. 2015, 45, 621–632. 10.1007/s13538-015-0365-4. [DOI] [Google Scholar]
- Schatz A.; Martin J. J.; Fosdick L.; Leicester H. M. The proteolysis-chelation theory of dental caries. J. Am. Dent. Assoc. 1962, 65, 368–375. 10.14219/jada.archive.1962.0265. [DOI] [PubMed] [Google Scholar]
- Wang X. A chelate theory for the mechanism of action of aspirin-like drugs. Med. Hypotheses 1998, 50, 239–251. 10.1016/S0306-9877(98)90024-X. [DOI] [PubMed] [Google Scholar]
- Kubota Y.; Tanaka S.; Funabiki K.; Matsui M. Synthesis and fluorescence properties of thiazole–boron complexes bearing a β-ketoiminate ligand. Org. Lett. 2012, 14, 4682–4685. 10.1021/ol302179r. [DOI] [PubMed] [Google Scholar]
- Skotnicka A.; Kolehmainen E.; Czeleń P.; Gawinecki R.; Valkonen A. Tautomeric equilibria in solutions of 2-phenacylbenzoxazoles. Int. J. Mol. Sci. 2013, 14, 4444–4460. 10.3390/ijms14034444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimizadeh M.; Eshghi H.; Shiri A.; Ghadamyari Z.; Matin M. M.; Oroojalian F.; Pordeli P. Fe (HSO 4) 3 as an efficient catalyst for diazotization and diazo coupling reactions. J. Korean Chem. Soc. 2012, 56, 716–719. 10.5012/jkcs.2012.56.6.716. [DOI] [Google Scholar]
- Shomali A.; Valizadeh H.; Noorshargh S. New Generation of Nitrite Functionalized Star-like Polyvinyl Imidazolium Compound: Application as a Nitrosonium Source and Three Dimensional Nanocatalyst for the Synthesis of Azo Dyes. Lett. Org. Chem. 2017, 14, 409–418. 10.2174/1570178614666170321125216. [DOI] [Google Scholar]
- Akki M.; Reddy D. S.; Katagi K. S.; Kumar A.; Devarajegowda H. C.; Babagond V.; Mane S.; Joshi S. D. Synthesis of coumarin-thioether conjugates as potential anti-tubercular agents: Their molecular docking and X-ray crystal studies. J. Mol. Struct. 2022, 1266, 133452 10.1016/j.molstruc.2022.133452. [DOI] [Google Scholar]
- Perveen F.; Qureshi R.; Ansari F. L.; Kalsoom S.; Ahmed S. Investigations of drug–DNA interactions using molecular docking, cyclic voltammetry and UV–Vis spectroscopy. J. Mol. Struct. 2011, 1004, 67–73. 10.1016/j.molstruc.2011.07.027. [DOI] [Google Scholar]
- Deghadi R. G.; Abbas A. A.; Mohamed G. G. Theoretical and experimental investigations of new bis (amino triazole) schiff base ligand: Preparation of its UO2 (II), Er (III), and La (III) complexes, studying of their antibacterial, anticancer, and molecular docking. Appl. Organomet. Chem. 2021, 35, e6292 10.1002/aoc.6292. [DOI] [Google Scholar]
- Jarad A. J.; Dahi M. A.; Al-Noor T. H.; El-ajaily M. M.; AL-Ayash S. R.; Abdou A. Synthesis, spectral studies, DFT, biological evaluation, molecular docking and dyeing performance of 1-(4-((2-amino-5-methoxy) diazenyl) phenyl) ethanone complexes with some metallic ions. J. Mol. Struct. 2023, 1287, 135703 10.1016/j.molstruc.2023.135703. [DOI] [Google Scholar]
- Murugan T.; Venkatesh R.; Geetha K.; Abdou A. Synthesis, Spectral Investigation, DFT, Antibacterial, Antifungal and Molecular Docking Studies of Ni(II), Zn(II), Cd(II) Complexes of Tetradentate Schiff-Base Ligand. Asian J. Chem. 2023, 35, 1509–1517. 10.14233/ajchem.2023.27808. [DOI] [Google Scholar]
- Elkanzi N. A.; Ali A. M.; Hrichi H.; Abdou A. New mononuclear Fe (III), Co (II), Ni (II), Cu (II), and Zn (II) complexes incorporating 4-{[(2 hydroxyphenyl) imino] methyl} phenyl-4-methylbenzenesulfonate (HL): Synthesis, characterization, theoretical, anti-inflammatory, and molecular docking investigation. Appl. Organomet. Chem. 2022, 36, e6665 10.1002/aoc.6665. [DOI] [Google Scholar]
- Shaaban S.; Abdou A.; Alhamzani A. G.; Abou-Krisha M. M.; Al-Qudah M. A.; Alaasar M.; Youssef I.; Yousef T. A. Synthesis and in silico investigation of organoselenium-clubbed schiff bases as potential Mpro inhibitors for the SARS-CoV-2 replication. Life 2023, 13, 912. 10.3390/life13040912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic Supplementary Material.