Abstract
Salmonella Typhi (S. Typhi), the invasive typhoidal serovar of Salmonella enterica that causes typhoid fever in humans, is a severe threat to global health. It is one of the major causes of high morbidity and mortality in developing countries. According to recent WHO estimates, approximately 11–21 million typhoid fever illnesses occur annually worldwide, accounting for 0.12–0.16 million deaths. Salmonella infection can spread to healthy individuals by the consumption of contaminated food and water. Typhoid fever in humans sometimes is accompanied by several other critical extraintestinal complications related to the central nervous system, cardiovascular system, pulmonary system, and hepatobiliary system. Salmonella Pathogenicity Island-1 and Salmonella Pathogenicity Island-2 are the two genomic segments containing genes encoding virulent factors that regulate its invasion and systemic pathogenesis. This Review aims to shed light on a comparative analysis of the virulence and pathogenesis of the typhoidal and nontyphoidal serovars of S. enterica.
Introduction
Salmonella enterica, a Gram-negative food-borne pathogen causing enteric fever and gastroenteritis, is one of the significant reasons for thousands of mortalities worldwide.1−3Salmonella is divided into two species: Salmonella bongori and Salmonella enterica.4 The species enterica is further categorized into six subspecies: enterica, salamae, diarizonae, indica, arizonae, and houtenae, with about 2700 serovars based on surface antigens. Almost 1500 serovars of S. enterica, which includes Typhimurium and Enteritidis, can cause infection in humans and animals after being consumed with contaminated food and water.5 On the contrary, S. bongori can only infect cold-blooded animals. Among all the serovars, the S. enterica serovar Typhi (S. Typhi) infection is strictly restricted to humans (known as typhoid fever). The host-restriction of S. Typhi is attributable to a 5% reduction of its genome due to the pseudogenization of 200–300 genes.6 Other typhoidal serovars, such as S. Paratyphi A, B, and C, are also associated with systemic infection in humans, known as paratyphoid fever.7
The nontyphoidal serovar (NTS) Salmonella Typhimurium (S. Typhimurium) can infect a wide range of hosts, including humans, plants, and animals.8,9 Other serovars of S. enterica, such as Enteritidis, Gallinarum, and Pullorum, are responsible for the outbreaks in poultry industries.10,11 Additionally, certain strains of Salmonella, such as sequence types ST313, ST34 (for Typhimurium), and ST11 (for Enteritidis), are associated with invasive bloodstream infection in humans.12 It has been reported that the multidrug-resistant S. Typhimurium ST313 causes bloodstream infection in adults and children with 20–25% fatality in sub-Saharan Africa.13,14Salmonella infection in humans can also cause extra-intestinal complications related to the central nervous system (3–35%), pulmonary system (1–6%), cardiovascular system (1–5%), and hepatobiliary system (1–26%).15 Owing to the excessive use of antibiotics, the rapid emergence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) strains of S. Typhi made Salmonella infection extremely difficult to treat.16 To study the in vivo pathogenesis of S. Typhimurium, many susceptible mouse strains, such as BALB/c and C57BL/6, mimicking the symptoms of typhoid fever in humans are extensively used.17,18 However, very few suitable models are available to study the in vivo pathogenesis and disease progression of S. Typhi. Additionally, a reduction in the size of its functional genome by either pseudogene formation or the absence of specific genes made our understanding of typhoid fever extremely difficult. Hence, a comprehensive discussion is required to explain the complexity of the interaction between S. Typhi and the human host. This Review will revisit the history, global epidemiology, pathogenicity, virulence, and extra-intestinal complications related to typhoid fever and recent developments in these research domains.
History of Typhoid Fever and Global Epidemiology
History of Typhoid Fever
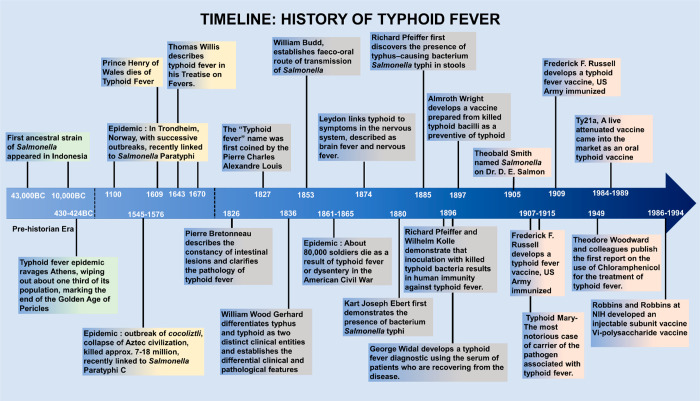
It is well established that Salmonella has existed and evolved on Earth with a long, multihued, and entrancing history of evolution. The name typhoid fever was derived from the Greek word “τνφοζ”, which stands for smoke, stupor, and obscurity associated with the diseased person, as they experienced stupor and neuropsychiatric symptoms in severe infection. Even though the description of typhoid fever was first made as early as 1643 by Sir Thomas Willis, it was often confused with typhus. However, typhus is a flea-borne illness caused by Rickettsia typhi and Rickettsia felis, with symptoms ranging from fever, headache, rash, and hepatitis to diverse organ prognosis.19 A brief timeline of the history of typhoid fever, therapeutic interventions, and vaccine development is depicted in Figure 1.
Figure 1.
A timeline history of typhoid fever.
In the context of typhoid vaccine designs, Vi capsular polysaccharides (Vi CPS) require only a single administration dose and elicits low reactogenicity. However, Vi CPS is a T-independent type 2 (Ti2) antigen that cannot be immunogenic in infants. New and future generations of vaccines include Vi glycoconjugate, O-antigen glycoconjugate, recombinant proteins, and proteins isolated and purified from whole Salmonella. These vaccine development advancements have improved the protection against Salmonella, allowing low reactogenicity and better memory response.20 As important as history is to understand this ancient pathogen, it is equally imperative to delve into the current global burden on healthcare and economies. Therefore, we will discuss global epidemiology in the next section.
Global Epidemiology
The overall burden of foodborne disease is enormous, accounting for 33 million deaths annually. Almost 1 in 10 people fall ill with foodborne illness, and Salmonella is one of the four predominant causes of diarrheal diseases globally. South-central Asia and Southeast Asia have the highest incidence of illness, followed by Latin America and Africa. In 2017, typhoid and paratyphoid fever alone accounted for 14.3 million illnesses globally. The same report suggested that S. Typhi caused around 76.3% of enteric fever, with a global fatality rate of 0.95% in 2017. Around 14.1% of total typhoidal cases are accounted for Southeast Asia, East Asia, and Oceania super-regions, followed by 12.1% of total typhoidal cases reported from sub-Saharan Africa.21 The available general case reports on the prevalence of Typhoidal fever in sub-Saharan Africa and Latin America are limited. However, a recent systemic review of sub-Saharan African cases suggests that the incidence of occurrence in African countries is highly heterogeneous and increases with better surveillance of healthcare systems with time.22 In Latin America, typhoid is more prevalent than paratyphoid.21
Typhoid and paratyphoid fevers are pervasive in young children (especially under the age of 5) and older adults in lower-income countries and are often life-threatening. Enteric fevers are endemic in developing countries, where adequate sanitation and acute clean water shortages hold paramount public health concerns.23,24 Even though typhoid and paratyphoid clinical symptoms are indistinguishable, the epidemiology, susceptibility to antibiotics, and geographical distributions are distinct.25,26 Paratyphoid to typhoid fever cases are higher in developed areas such as Central Europe (90.7%) and high-income subcontinents such as Australia, Asia Pacific, North America, and Western Europe. On the other hand, Central Asia, Eastern Europe, Latin America, North Africa, Middle East, South Asia, Southeast Asia, East Asia, Oceania, and Sub-Saharan Africa account for approximately 53–98% of typhoid fever of the reported enteric fever cases.21
A study from Cambodia showed that approximately 42.9% of isolates of S. Typhi demonstrated MDR, whereas only 11.5% of S. Paratyphi isolates displayed increased resistance to ciprofloxacin, and none of the isolates of S. Paratyphi exhibited MDR.26 Typhoidal and paratyphoid outbreaks in developed countries are usually linked to travel history to endemic areas.27 In the past few years, the rate of S. Paratyphi A infection has surged, accounting for nearly 50% of all cases, especially in the Southeast Asian population.24,28 A few studies also point out that genetic factors influence susceptibility to enteric fever. VAC14 is a phosphoinositide-regulating protein, and single nucleotide polymorphism (SNP) in VAC14 renders the host with an increased S. Typhi susceptibility. VAC14 SNP increases plasma membrane cholesterols, resulting in increased docking of S. Typhi to host cells.29 Studies from Jakarta, Indonesia, demonstrate that the gastric mucus lining expresses cystic fibrosis transmembrane conductance regulator (CFTR), which has been shown to act as adherent and facilitate the entry of S. Typhi but not S. Typhimurium into intestinal epithelial cells.30 Therefore, mutations in CFTR (such as F508del and IVS8CA) provide some protection against S. Typhi infection.31 Extraintestinal salmonellosis is strongly associated with the immune states of individuals. Thus, it affects immune-compromised persons.32,33
Symptoms and Diagnosis
The typhoidal and paratyphoidal fevers cannot be clinically differentiated. It takes about 8–14 days of incubation after infection. The incubation period also varies, with a range of 3–60 days. The disease can last from a few weeks to several months, depending upon the initial exposure to the infectious dose and the diagnosis and treatment time. These parameters also govern the subsequent severity of the disease and related complications. However, in NTS the infection is more localized, causing gastrointestinal inflammation. The incubation period for NTS is about 2–3 days, and the disease lasts for 3–7 days and is self-limiting in a healthy host without any underlying conditions.34−36 NTS serovars develop severe complications in immune-compromised individuals, such as systemic secondary-site infection, chronic conditions (reactive arthritis and irritable bowel syndrome), and other febrile illnesses.37 We have listed the common and differential typhoidal serovar and NTS infection symptoms for ease of interpretation in Table 1. Clinical diagnosis of typhoid involves a history of the patient’s residence and travel (from endemic areas) and the presentation of febrile illness for more than 3 days with gastrointestinal manifestations (pain, constipation, or diarrhea). The diagnosis is difficult in the first week of disease onset, but recent advancements have made it easier. In the early 21st century, despite the medical advances in developing countries, laboratory diagnosis can be divided into conventional typhoidal diagnosis (bacteriological culture and Widal test) and advanced typhoidal diagnosis (polymerase chain reaction (PCR) based, enzyme-linked immunosorbent assay (ELISA), radioimmunoassay (RIA), hemagglutination, and coagglutination). In the subsequent subsections, we will discuss the currently available diagnoses for typhoidal fevers.
Table 1. Symptoms of Typhoid Fever and Non-Typhoidal Salmonella Infection (or Salmonellosis).
| Salmonella enterica serovar | disease and symptoms |
|---|---|
| typhoidal (human-restricted) Typhi, Paratyphi, and Sendai | enteric fever, abdominal distention and pain, transient diarrhea or constipation, a maculopapular salmon-colored rash on the trunk |
| non-typhoidal (broad range) Typhimurium, Enteritidis, Dublin, Newport, et cetera. | gastro-intestinal, abdominal distention and pain, vomiting, inflammatory diarrhea |
Bacterial Culture-Based Diagnosis
The gold standard diagnostic tool for typhoidal serovar and NTS diseases is still culturing stool, blood, and bone marrow samples. The choice of samples, such as body fluids or feces, depends on the type of infection diagnosed early; for example, stool samples are usually taken for NTS infection associated with localized inflammation. However, this technique may become less sensitive once the infection becomes systemic.38 On the case of systemic enteric fevers such as typhoid and iNTS infection, blood or bone marrow is sampled during the first week of fever; later, urine or stool samples are evaluated. Getting culturable Salmonella in the first week of symptoms onset is particularly tricky. Thus, diagnostic laboratories perform the test multiple times, and with a large volume of blood the sensitivity of this diagnosis remains only about 40–80%.39−41 For genus-level identification, manual methods or automated systems such as MALDI-ToF are used.42S. Typhi usually gives weak H2S production and a negative ornithine decarboxylase upon biochemical aspect identification, whereas S. Paratyphi results in negative H2S, citrate, and lysine reactions.42
Serotyping and Serological Diagnosis
S. enterica, being a multiform pathogen, has over 2600 serovars identified to date. Therefore, the serotyping of the clinical isolates plays a significant role in central public health repositories for endemic serovar identification, epidemiological analysis, surveillance, and disease outbreak studies. The Salmonella subtyping is conducted according to the White–Kauffman–Le Minor scheme. The conventional scheme of serotyping of Salmonella evaluates the expression of surface antigens such as somatic (O), flagellar (H), and capsular (Vi) antigens. However, the Vi antigen is exclusively present in S. Typhi, S. Paratyphi C, and S. Dublin.43 Based on somatic antigen, S. enterica can be divided into 46 serogroups,44 and there are 114 individual flagellar antigens, resulting in 2600 serovars.45 The old serotyping methods were tedious and time-consuming for typing an isolate. Several new techniques aid the process of identification of Salmonella sp. serovars, such as pulsed-field gel electrophoresis (PFGE),46,47 ribotyping,48 clustered regularly interspaced short palindromic repeat (CRISPR),49,50 and multilocus sequence typing (MLST).51,52 The most performed serological diagnosis employs the Widal tube agglutination method. It is one of the primitive methods used for more than a century to diagnose typhoid or enteric fever. In this test, the killed S. Typhi and Paratyphi A bacteria are scored based on agglutinating antibodies to flagellar (H), LPS (O), and Vi antigens. However, the efficacy of this method is widely questionable due to its cross-reactivity with different serovars and low sensitivity.53,54 Reports of cross-reactivities with unrelated diseases like malaria, dengue fever, and brucellosis in enteric fever-endemic areas are prevalent. Despite all of the drawbacks, it is still extensively used in areas lacking public health infrastructure, mainly because of its low cost and ease of use.
Based on a similar principle, some commercially available diagnostic tests adopt the detection and even quantification of antibodies in the patient’s serum against several Salmonella antigens, such as outer membrane proteins or LPS. However, significant drawbacks remain, including low sensitivity (69%) and specificity (79%).55,56 Hemagglutination tests are also routinely used in various countries. A report from India has shown that the anti-LPS HA test has a sensitivity of 60% and a specificity of 98.2%.57 Similarly, 70% sensitivity and 92% specificity have been reported for the reverse-phase hemagglutination test (RPHA). These tests propose a convenient alternative for the Widal test to detect S. Typhi in endemic areas.58 Since there is an increasing need for rapid, reliable, and cost-efficient testing for Salmonella to meet the need of the current situation, less sensitive detection of bacteria by PCR from blood samples is widely conducted.
Advanced Diagnostic Techniques
The routine bacteriological culture has a 100% specificity and about 54% sensitivity, and the PCR-based ones have about 98.1% specificity and 85.4% sensitivity59 in samples from blood and bone marrow for the flagellin gene (fliC), Vi, O, and H antigens, hilA (an SPI-1 effector), and 23S rRNA, to name a few.40,60−70 Another widely used technique is multiplex PCR, which directly identifies gastrointestinal pathogens in clinical stool samples. Although it can target and simultaneously amplify an array of genes,71,72 the results still require further testing and validation through culturing for serotype classification. MALDI-TOF-based diagnosis is a newly emerging technique for reliable and rapid detection and typing of bacteria. Two of the FDA-approved MALDI-ToF systems for Salmonella detection are the Bruker Biotyper and bioMérieux Vitek MS systems. However, none of them can detect the serovar.73 Next-generation sequencing (NGS) for diagnostics and bacterial typing using whole-genome sequencing (WGS) has been on the rise late on as well due to a drastic reduction of cost and a significant increase in downstream tools and bioinformatics analysis for sequence assembly and comparative genomics. Apart from the contig arrangements and analysis, WGS data also facilitate identifying SNP and provides us with single-nucleotide changes among Salmonella serovars. This approach enables the study of phylogenetic relationships and disease outbreak epidemiology.74−76 WGS-based technologies are at the beginning of the exponential phase, and multiple technologies that have low cost, rapid, and reliability for clinical isolate typing are still in the pipeline, further leading to a shift in the current gold standard for clinical and public health sectors in phylogenetic relationship, origin of evolution, epidemiological, and geographical analyses of newly emerging and endemic serovars.
Models to Study Salmonella Infection
S. Typhihas is adapted to be highly specific to human hosts. Therefore, there are very few in vivo models for studying the infection prognosis. S. Typhimurium infection and colonization in natural resistance-associated macrophage protein-negative (Nramp1–/–) C57BL/6 or BALB/c mice induces typhoid fever-like symptoms and helps to unravel the infection biology of Salmonella Typhimurium.77−79 Since typhoidal serovar and NTS share only 89% of the genes, the findings in S. Typhimurium are extrapolated to S. Typhi.80 However, contradictory reports in the last two decades also indicate that, due to the loss of genes and pseudogenization in S. Typhi, all of the findings in S. Typhimurium cannot be directly applied to S. Typhi. In detail, we have discussed the loss of genes and pseudogenization in S. Typhi in the section on virulence determinants of Salmonella pathogenesis. The humanized mouse model in a severely immunodeficient (e.g., SCID or Rag2–/– γC –/–) mouse with the human immune system is the nearest animal model to study S. Typhi infection. These mice are engrafted with CD34+ human umbilical cord blood stem cells, human fetal hematopoietic stem and progenitor cells, or human leukocytes.81−84
More recent research in Salmonella biology sheds light on the organoid or enteroid models, which help fill the gap between in vivo and in vitro research systems. C. A. Nickerson et al. was among the first groups to show a significant difference in Salmonella infection in 2D and 3D organoid culture models of Int407.85 Two of the broad classifications of the 3D organoid model are mouse-derived and human-derived organoids. Zhang and colleagues performed one of the most primitive works with mouse-derived organoids. The observations revealed the bacteria-mediated tight junction disruption, NF-κB-induced inflammatory response, and downregulation of Lgr5 (stem marker). However, a faulty experimental setup led to infection from the basolateral side.86 The intestinal enteroid model also provided an excellent model for studying different cell types regulations such as Paneth cell (PC) degranulation upon LPS interaction and the antimicrobial role of Paneth cell α-defensins.87,88
Human-derived models help to further the studies, as they carry the specific human genetic makeup instead of mouse models. Few Salmonella pathogenesis studies utilize adult stem cells (ASCs) and pluripotent stem cells (PSCs) derived from human-enteroid models. In 2018, K. P. Nickerson et al. introduced another human-derived enteroid model system using human tissue biopsies and human ileum. The transcriptional profiling of the bacteria and host tissue revealed that S. Typhi downregulated critical host genes that are responsible for bacterial clearance, host immunity, and cytoskeleton rearrangements. The silent phase of infection associated with S. Typhi is attributed to the downregulation of SPI-1, which minimizes intestinal inflammation.89 Recent studies of S. Typhi in the C. elegans model system shed light on the usage of this system to answer research questions about S. Typhi research.90 Enteroid, humanized mouse, and cell culture models will be instrumental in answering the multiple open-ended questions about Salmonella.
Virulence Determinants of Salmonella Pathogenesis
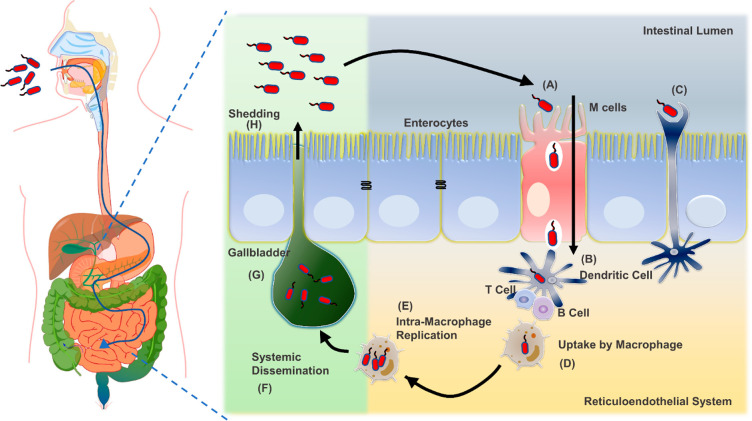
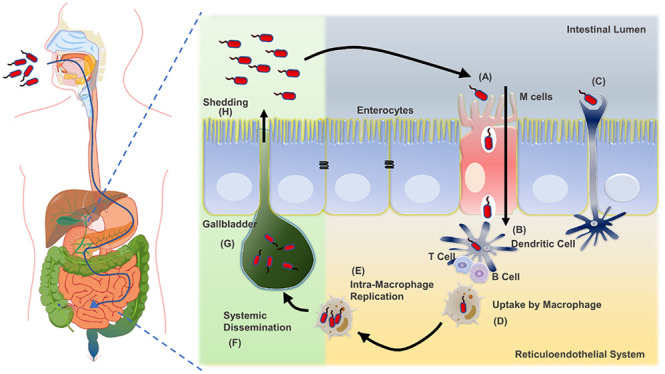
Unless otherwise stated, we aim to shed light upon Salmonella pathogenesis here, pertaining to typhoidal serovar and NTS. Upon ingestion of contaminated food and water, bacteria enter the digestive tract and overcome the acidic pH of the stomach with an excellent acid tolerance system called phoPQ. In the subsequent events, they reach the intestinal milieu, where they further escape several host-innate immune arsenals such as digestive enzymes, bile salts,91 secretory IgA,92,93 antimicrobial peptides (AMPs).94−97Salmonella then traverses the intestinal mucus layer to reach the epithelium beneath. In the small intestine, Salmonella is taken up preferentially by microfold (M) cells (Figure 2). The M cells are specialized cells that help sample food antigens from the intestinal lumen through pinocytosis. This sampling further channelizes the transfer of Salmonella to various immune cells in the Peyer’s patches.98,99 Alongside Salmonella inducing its uptake through bacterial-mediated endocytosis in nonphagocytic cells, Salmonella-mediated endocytosis into the enterocytes is initiated by the adhesion of bacteria to enterocytes by various molecules like fimbrial adhesins. Postadhesion, Salmonella can mediate its uptake by inducing membrane ruffling due to extensive modulation of the actin cytoskeleton of enterocytes.
Figure 2.
The course of Salmonella infection and long-term carriage. Initially, (A) Salmonella preferentially enters M cells, (B) which transport them to the lymphoid cells (T and B) in the underlying Peyer’s patches. (C) It can also be taken up by dendritic cells, elongated dendrites through the intestinal epithelial barrier. (D) Once across the epithelium, Salmonella serotypes associated with systemic illness enter intestinal macrophages and (E) undergo intramacrophage replication and (F) disseminate throughout the reticuloendothelial system, leading to systemic infections. (G) Further colonization of the gallbladder leads to (H) chronic carriage and the bacterium’s shedding through this mechanism. The green background shade depicts the GI tract near the gallbladder opening, and the yellow background depicts the GI tract’s intestinal areas.
On the other hand, CD18+ phagocytes can also phagocytose Salmonella from the intestinal lumen, thereby translocating it to Peyer’s patches.100 Once the bacteria reach the reticuloendothelial system, the macrophages can phagocytose the typhoidal serovar and NTS through phagocytosis and macropinocytosis. In the upcoming sections, we discuss the subsequent events about macrophages. The NTS elicits a strong Th1 immune response in immune-competent individuals due to pathogen-associated molecular patterns (PAMPs). It is characterized by IL-18-mediated recruitment of bone marrow-derived phagocytes, neutrophils, and B and T cells in the lamina propria, leading to phagocytosis of invading Salmonella. This response limits the dissemination of NTS from becoming a systemic infection, leading to self-limiting gastroenteritis.101
Upon entering the macrophages, Salmonella senses the phagosomal environment, activating various virulence arsenals to thrive and replicate in the macrophages in otherwise microbicidal niches and leading to dissemination throughout the reticuloendothelial system.102−109S. Typhi can surpass the host immune checkpoints, including lymph nodes that restrict the infection.110 Induction of Salmonella pathogenicity island 1 (SPI-1) transcription and Vi-polysaccharide downregulates flagella to subvert the early inflammatory response in S. Typhi, one of its well-known PAMPs.111,112 In S. Typhi, the Vi capsular antigen synthesis begins once the bacterium marks its entry into the ileum region of the gut.113S. Paratyphi A does not express Vi, yet it is capable of causing typhoid-like illness.25 According to reports, S. Typhi lacking the Vi antigen is capable of causing infection,114 indicating that Vi capsular polysaccharide is not entirely indispensable for the pathogenesis of typhoidal serovars.
The molecular mechanisms of Salmonella pathogenesis are highly complex and well-coordinated. Pathogenic strains of Salmonella spp. differ from their nonpathogenic relatives by harboring specific pathogenicity genes organized into clusters called pathogenicity islands.115−117 Pathogenic islands are gene clusters incorporated into the genome, in either the chromosome or a plasmid, with variable GC content and codon usage compared to the surrounding regions. They are also flanked by direct repeats, insertion sequences, or tRNA genes, acting as sites for DNA recombination. Pathogenic strains of Salmonella have acquired these islands from other pathogenic bacteria during evolution by horizontal gene transfer. Some are conserved throughout the Salmonella genus, whereas others are exclusively found in specific serovars. To date, 24 SPIs have been identified, of which SPI-1 and SPI-2 have been vastly characterized. SPI-1 is required for the bacterium to invade the nonphagocytic epithelial cells, whereas SPI-2 is necessary for bacterial survival and replication inside the macrophages.118 The remaining SPIs contribute to other pathogenic attributes like fimbrial expression, magnesium and iron uptake, multiple antibiotic resistance, intracellular survival, and systemic dissemination.119
S. Typhi is an example of reductive evolution where the serovar has undergone functional inactivation of genes (pseudogenization of 200–300 genes) to adapt specifically to the human host.120S. Typhi and S. Typhimurium have 11 SPIs in common (1–6, 9, 11, 12, 13, and 16). SPI-8 and SPI-10 were initially found in S. Typhi and are considered absent in S. Typhimurium. There is only one SPI specific to S. Typhimurium, viz, SPI-14, and four SPIs specific to S. Typhi, viz., SPI-7, SPI-15, SPI-17, and SPI-18.121 SPI-19, SPI-20, and SPI-21 are absent in both serovars (S. Typhi and S. Typhimurium).122 SPI-7 and SPI-11 code for the Vi antigen and the typhoid toxin, two specific virulence determinants of S. Typhi.123 A type IVB pilus encoded by SPI-7 is usually used by S. Typhi while adhering to human monocytes and epithelial cells by interacting with the CFTR receptor.120 Other virulence determinants include the flagella, which activate innate immune response via the recognition of monomeric flagellin by the NLR family, apoptosis inhibitory proteins (NAIP), and TLR5 receptors.123
This section discusses how the coordinated expression and functioning of virulance determinants in Salmonella aid in its entry, survival, and dissemination in the host body. Research in nonphagocytic cells and Salmonella interaction has focused chiefly on S. Typhimurium, even in cell culture models. Therefore, in the upcoming section with nonphagocytic cells, most of the work is on S. Typhimurium until otherwise stated.
Interaction of Salmonella with Nonphagocytic Cells
Much literature suggests that entry into the nonphagocytic cells holds immense importance in disease manifestations, since the primary site of infection resides in the intestinal epithelial cells. Therefore, we have elaborately discussed the current knowledge of the pathogenesis of S. Typhimurium in nonphagocytic cells.
SPI-1 Facilitates Entry into Nonphagocytic Cells
In 1989, Galan and Curtiss discovered that the genes responsible for mediating entry into the nonphagocytic cells are encoded by SPI-1.124,125 SPI-1 encodes a molecular syringe capable of injecting an array of bacterial effector proteins directly into the host cells. These effectors modulate host cell signaling (especially inflammatory ones) and induce the uptake of the bacteria into nonphagocytic cells. The general theory is that SPI-1 type 3 secretion system (T3SS) mediated uptake of Salmonella into the nonphagocytic host cells occurs through the injection of bacterial effectors, which are responsible for the rearrangement of the cytoskeleton resulting in the formation of membrane ruffles, a process similar to macro-pinocytosis. SopB, SopE, and SopE2 are critical determinants for the uptake of Salmonella in epithelial cell uptake, and combined mutation in all three genes shows defects in entry.126 SopB is an inositol phosphate phosphatase that acts on phosphatidylinositol species to remove 4′- and 5′-phosphates, which is essential for membrane ruffling.127 There is a SopB-dependent increase in phosphatidylinositol-3-phosphate (PI3P), phosphatidylinositol-3,4-bisphosphate (PI(3,4)P2) and phosphatidylinositol-3,4,5-triphosphate (PI(3,4,5)P3) in infected cultured cells and on the SCV membrane.128,129 Accumulation of these PIP lipids species leads to the recruitment of several host factors, such as guanine nucleotide exchange factors (GEFs), that further activate an essential Rho GTPase.130 SopB also activates Akt or protein kinase B (PKB)-YAP, which facilitates S. Typhimurium’s growth inside host cells and inhibits apoptosis.131,132 The activity of SopB assisted by Vps34 and Rab5 on a Salmonella-containing vacuole (SCV) membrane leads to increased charge on the membrane, leading to lysosomal fusion inhibition conferring protection to SCV from acid hydrolases of the lysosome.128 SopB also reduces the overall lysosomal numbers, giving the pathogen an upper hand compared to the host.129
On the other hand, Salmonella effectors such as SopE2 and SopE mimic host GEFs that activate Rho GTPase Cdc42,133 and SopE can also activate Rac1.134,135 Host Rho GTPase is significant in bacterial uptake by nonphagocytic cells such as enterocytes and epithelial cells in the gallbladder. In conjunction with these effectors, SPI-1-encoded effectors such as SipA and SipC also facilitate uptake by directly interacting with actin-binding proteins. SipC is a part of SPI-1 translocon and can nucleate actin filament polymerization and bundle pre-existing actin.136−138 SipA initiates actin nucleation and the bundling activity of SipC by the high-affinity binding and stabilization of actin filaments. Further, SipA also prevents depolymerization by host ADF and cofilin proteins.139,140 However, SipA deletion mutants only have reduced invasion capacity, suggesting that SipA is not entirely indispensable but does facilitate the entry of the bacteria.141 In contrast, a recent report challenges the existing model of ruffle-mediated entry into the cells. The report suggests that entry into the mouse gut epithelium occurs through the “discrete-invasion” model. This discrete invasion is facilitated by SipA, which is capable of manifesting significant elongation of local microvilli, and does not favor cooperative invasion.142 SipB, SipC, and SipD are essential in forming the needle complex. Besides this, they have other functions, e.g., SipB is necessary for Salmonella-induced caspase-1-dependent apoptosis and the release of IL-18143−145 and SipC interacts with F-actin, which helps in pathogen internalization.138 A potential functionality difference might appear between the typhoidal and nontyphoidal serovars due to amino acid substitutions in the SipD translocon of S. Typhi.146 Although these effectors together orchestrate the uptake of the bacteria, several redundant pathways of the host are stealthily exploited by Salmonella for its benefit and to gain access to nonphagocytic cells (Table 2).
Table 2. Host Factors That Are Exploited by Salmonella Effectors to Facilitate Its Uptake into Non-Phagocytic Cells.
| host complex targeted | bacterial effectors | mechanism | refs |
|---|---|---|---|
| the WAVE regulatory complex (WRC) | SopB, SopE, SptP | the WRC complex is essential for the formation of lamellipodia, which is governed by Rac1 and Arf1 activity. GEF mimic SopE can activate Rac1, and Arf1 is activated by activity of SopB, which helps in the accumulation of PI (3,4,5) P3 and ARNO at the site of entry, leading to the recruitment of WRC and the promotion of the Arp2/3 complex for actin remodelling. SptP is responsible for the inactivation of Rac1 and returning the cell to the resting stage. | (267−275) |
| the WASH complex | not identified | the WASH (Wiskott–Aldrich syndrome protein and scar homologue) complex, also responsible for activating Arp2/3-dependent actin polymerization, acts downstream of RhoA and accumulates at the Salmonella entry site. | (270, 276, 277) |
| myosin-mediated contractility | SopB | actin stress fibers are decorated with myosin IIA and IIB upon infection. SopB helps in hijacking and activating myosin II through the activation of RhoA and downstream Rho kinases (Rocks). the exact molecular mechanism remains elusive, but altered phosphoinositide dynamics might be involved. | (128, 278) |
| formins | SopB, SopE/E2 | formins are a family of proteins that are nucleators of actin polymerization independently of Arp2/3. FHOD1 also lies downstream of RhoA and can be activated by Rac1. SPIRE1 (pathogen entry) and SPIRE2 (replicative niche) are also involved in cytoskeleton remodelling, as these help in nucleating the assembly/bundling of straight actin fiber. | (130, 135, 279, 280) |
| the exocyst complex | SipC, SopE/E2 | Exo70 is part of a hetero-octameric exocyst complex that helps in vesicle transport, and it interacts with SipC. assembly of an exocyst complex requires activation of RelA by SopE/E2. the exact molecular mechanism for this complex-mediated entry also remains elusive, however, it is proposed that it may fulfill the extra phospholipids requirement during the formation of membrane ruffles. | (281) |
| IQGAP1 | SopE/E2, SopB | IQGAP1 has a homologous domain of GTPase activating protein (GAP, inactivators of Rho GTPases), thus it binds GTPases (Rac1 and Cdc42) and prevents its inactivation. IQGAP1 also acts as a molecular scaffold for downstream MAPK/ERK signaling and various phosphoinositide kinases that might be in relation to SopB. | (282−285) |
| annexins | SopE/E2, SopB | annexins (A1, A2 and A5) are calcium-dependent membrane=binding proteins that indirectly regulate cytoskeleton membrane dynamics, and they accumulate at the site of Salmonella-mediated entry. A2 along with p11 protein binds AHNAK (a phosphoprotein regulator of membrane). exact molecular dynamics have yet to be elucidated. | (286) |
| myosin VI | SopE/E2, SopB | myosin VI plays a key role in endocytosis, and SopE specifically recruits it at the site of entry via Rac1-WRC and Rac1-p21activated kinase (PAK) pathway. myosin VI also recruits a PI3P-binding, actin-binding and GEF for Cdc42 called frabin at the site of membrane ruffles. | (128, 272, 287−289) |
| villin | SipA, SptP | villin, an actin-binding and severing protein, is required for Salmonella to enter into the polarized enterocytes. it is suggested that severing leads to the generation of barbed ends, which are essential for new filament growth. the activity of villin is regulated by SipA and SptP. | (290) |
The invasion may also be influenced by bile, Mg2+, and short-chain fatty acids.147 The liver produces bile continuously and helps in the digestion and absorption of fats. Before its release into the intestines, bile is stored in the gall bladder at high concentrations. It is an essential environmental cue to upregulate virulence gene expression during infection within the host’s gastrointestinal tract. Salmonella regulates the production of virulence factors following bile exposure. BarA is the sensor kinase that responds to environmental stimuli and acts through its cognate response regulator SirA to induce the expression of invasion genes. This pathway includes the induction of hilA expression and a hilA-independent effect on invF expression.148 Bile represses the invasion capability of S. Typhimurium via BarA/SirA,149,150 and in vitro studies have shown that bile exposure increased the invasive capability of S. Typhi in HeLa cells. A protein stability assay reveals that this observation is attributed to the more excellent stability of one of the chief regulators of SPI-1, HilD, upon bile exposure in S. Typhi.151
The temperature differentially regulates the expression of T3SS in typhoidal and nontyphoidal serovars. During fever, an increase in body temperature (39–42 °C) in an infected host reduces the invasion and motility in typhoidal serovars. SPI-2 also has a temperature-dependent regulation. Higher temperatures (42 °C) increased SPI-2 expression in both S. Paratyphi A and S. Typhimurium, although a significant increase in SPI-2-mediated intracellular replication was found only in S. Paratyphi A.152 This is another example of adaptation, since fever marks the beginning of systemic dissemination of typhoidal serovars, where intracellular survival is more important than invasion.125
The Dual Lifestyle of Salmonella in Nonphagocytic Cells
After entering the epithelial cells, the pathogen can lead a dual lifestyle, either cytosolic or vacuolar. Salmonella remains encapsulated within a host membrane compartment, SCV, or it might quit the vacuole to thrive in the host cytosol. Salmonella modulates the intricate pathways of host endosomal–lysosomal maturation with SPI-2 effectors to establish a successful replicative niche. Once inside the cell, there is the downregulation of the expression of SPI-1 and the upregulation of another molecular syringe SPI-2-encoded T3SS, which spans the vacuolar membrane and bacterial membrane and can inject several effectors from the bacterial cytosol to the host cytosol. The central cues for the induction of SPI-2 are limited nutrients, low pH, antimicrobial peptide accumulation in the vacuolar lumen, and decreased Mg2+ concentration.153 The pH sensing mechanism by intracellular Salmonella is mediated by a protein complex comprising SsaM, SpiC, and SsaL encoded by SPI-2. This complex can sense low and neutral pH and regulate effector translocation.154
A recent review on the genetic tuning of S. enterica describes all the aspects involving genetic switches of Salmonella to thrive in hostile host niches.155 SPI-2 injects as many as 43 different effector proteins into the host cytoplasm, assisting in SCV maturation and establishing a replicative niche.156−158 The SCV maturation is comprised of three different stages: early (<30 min postinfection), intermediate (30 min to 5 h postinfection), and late (>5 h postinfection). Each stage is distinct regarding bacterial and host proteins recruited on SCVs. Apart from mediating entry, SopB also serves a highly vital role in the initial biogenesis of SCV: it alters the phosphoinositide dynamics of the SCV membrane through hydrolysis of PI(3,5)P2 and PI(3,4,5)P3 to PI(3)P.159−161 It is also responsible for recruiting GTPases, Rab5, and PI(3) kinase Vps34, which induces rapid remodelling of the membrane through early endosome antigen 1 (EEA1) mediated fusions with early endosomes within as little as 5 min of entry.128,160,162 Recently, it was reported that SopB triggers rapid recruitment of cytosolic sorting nexin 18 (SNX18; an SH3-PX-BAR domain sorting nexin protein) that further assists SopB in its phosphoinositide phosphatase activity.163 Next, the SCV membrane acquires several endocytic host protein markers, such as transferrin receptors (TfnR), Rab4, and Rab11,164,165 which act as protective shelters in an otherwise hostile host environment.
Salmonella-Containing Vacuole (SCV) Maturation and Replication
The SCV maturation mimics host endocytic pathways by shedding off early, sorting, and recycling membrane markers within 30 min postinfection and acquiring a set of late endocytic marker proteins such as vacuolar-ATPase, Rab7, Rab9, lysosomal glycoproteins (LAMP1, LAMP2, and LAMP3/CD63), and RILP (Rab-interacting lysosomal protein).166−170 Rab7 is the primary small GTPase acting in late endosomal and lysosomal vesicles, and Rab7 facilitates the recruitment of lysosomal glycoproteins, RILP, FYVE (another Rab-interacting lysosomal protein), and coiled-coil domain-containing protein 1 (FYCO-1).171−173 Rab7 and its effector RILP recruit the motor protein dynein to SCV to initiate its centripetal movement, resulting in peri-Golgi localization of SCV. However, it is very compelling that Salmonella selectively excludes a few late endocytic markers such as mannose-6-phosphate receptors (M6PR), lysobisphosphatidic acid (LBPA), and lysosomal hydrolases (cathepsins) from the SCV membrane in the process of SCV maturation.166,167,174,175
S. enterica forms a highly complex stabilized network of tubules called Salmonella-induced tubules (SITs). Some of the well-studied SITs are Salmonella-induced filaments (SIFs). SIF formation occurs mainly in epithelial cells, and SIFs are the only SITs harboring LAMPs. Salmonella-induced filament A (SifA) is responsible for forming SIFs, and the SifA mutant cannot induce SIFs. The host protein that interacts with the SPI2 effector SifA is the SifA- and kinesin-interacting protein (SKIP, aka PLEKHM2). The association of SifA-SKIP inhibits the recruitment of M6PR by sequestering Rab9, which is essential for the maturation of endocytic vesicles. The reduced M6PR onto the SCV membrane leads to reduced delivery of lysosomal enzymes inside the SCV, thereby conferring protection from various host degradative enzymes.176 SopD2, another SPI-2 effector, disrupts the Rab7-mediated recruitment of RILP and FYCO1 at the late stage of SCV maturation, leading to the shedding of RILP and FYCO1.172,173,177 SseF and SseG form a tethering complex along with the Golgi-associated protein ACBD3.178,179 SIFs also have access to the host cell’s endocytosed content,180−182 suggesting that SIFs enable nutrient acquisition. Long filamentous structures are potent in providing larger surface areas for more nutrient uptake and promoting bacterial survival and, more importantly, replication along with SCV. A recent study by Hensel and colleagues demonstrated a continuum between the SIFs and SCVs.183 Moreover, auxotrophic mutants, incapable of inducing SIFs, cannot access the host nutrients.184 There is a dearth of research on the exact molecular mechanism of Salmonella replication inside the host cellular niche. However, Salmonella perturbs the SCV and the lysosome ratio to successfully thrive inside the host niche by replicating with the SCV to maintain one bacterium per vacuole. The requirement of a dedicated vacuolar compartment per bacterium in the host cell increases the vacuolar load. Simultaneously, the SCV remains nonbulky, which helps avoid lysosomal fusion.185 About 10–20% of intracellular bacteria quit their vacuolar niche to enter the cytosol.186−188 About half of the SCVs are essential targets for host xenophagy and are subsequently eliminated, and the other half escape this by hyper-replication.179,189 The cytosolic bacterial population triggers host cell pyroptosis and extrusion of enterocytes from the intestinal lumen by inducing a local burst. The cytosolic lifestyle gives Salmonella an advantage over the host, as hyper-replication in the enterocytes causes localized inflammation, and the bacteria can silently replicate in the vacuolar niche and remain hidden from host immune arsenals.
Salmonella Modulates Host Endocytic Protein
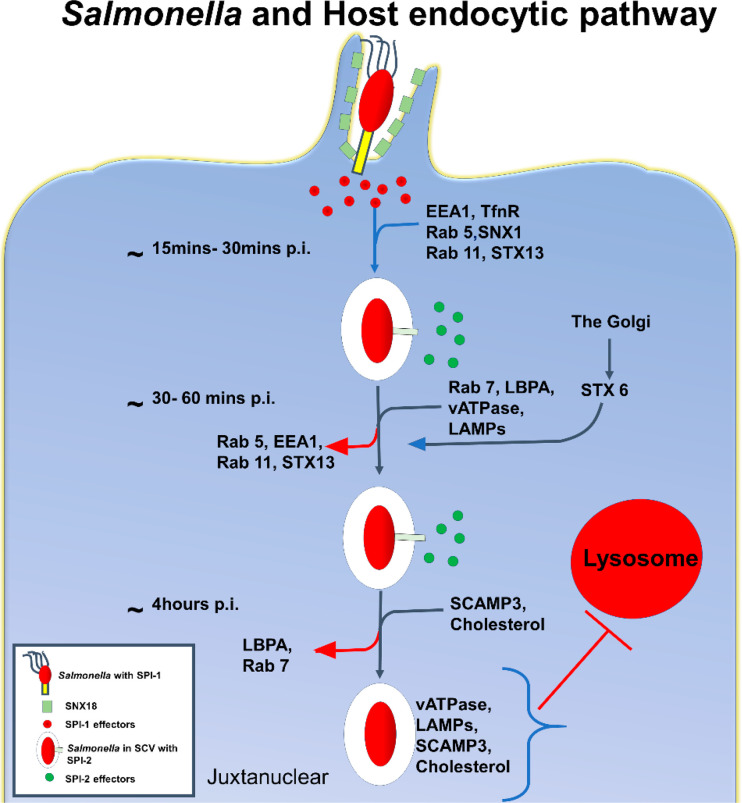
Apart from these host factors, another set of host proteins plays a role in SCV maturation and biogenesis, called soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) proteins. SNAREs are the proteins that help endocytic vesicular fusion and fission and serve an intricate function in maintaining host endolysosomal pathway homeostasis. Newer insights into the role of SNAREs in SCV biogenesis suggest that Salmonella also modulates the host endolysosomal pathways to facilitate its survival and replicative niche. A recent report suggests that a host SNARE called syntaxin 8 interacts with SipA (SipA mimics cognate SNARE of syntaxin 8) to promote its fusion to early endosomes, thereby recruiting other syntaxins such as syntaxin 7 and syntaxin 13 to the SCV membrane, which potentially arrest its maturation and further assist in survival.190 The SCV and infection-associated macro-pinosomes (IAMs) fuse with the help of SNARE proteins (SNAP25 and STX4) to maintain dynamic SCV growth and shrinkage.191 SPI-2 mediates the SCV division in a syntaxin 3-dependent manner to facilitate Salmonella survival.192 SipC recruits syntaxin 6, which helps acquire LAMP1 on SCV from the Golgi apparatus.193Salmonella can partially trigger autophagy in epithelial cells via SPI-1-encoded T3SS-1-mediated damage to the SCV membrane, exposing the SCV lumen to various host cytoplasmic ubiquitin ligases. Apart from this, the damaged SCV can also be recognized by galectin 8, which is capable of recruiting autophagic machinery.194 SCV damage also leads to the release of Salmonella in the cytosol, which the ubiquitin systems of host cells can identify. Subsequently, cytosolic Salmonella leads to the bacteria’s polyubiquitination, which is recognized by NDP52, OPTN, and p62 adaptor molecules linked to LC3B autophagic capture.171 In Figure 3, we have described the necessary steps involved in SCV biogenesis, maturation, and interaction with host–endosomal–lysosomal pathways. However, it is interesting that if the SCV membrane remains undamaged, Salmonella can subvert the host autophagic machinery and thrive inside the host macrophages by employing SopB-mediated mechanisms.195
Figure 3.
Salmonella-containing vacuole (SCV) biogenesis, maturation, and interaction with host endolysosomal pathways.
Interaction of Salmonella with Phagocytes
The phagocytes engulf Salmonella by a process called phagocytosis and initiate killing by mounting an antimicrobial response in the form of reactive oxygen species (ROS), reactive nitrogen species (RNS), cationic antimicrobial peptides (CAMPs), metal ion starvation, and acid stress.196 Professional antigen-presenting cells such as dendritic cells and macrophages can prime CD4+/ CD8+ T lymphocytes by presenting bacterial antigens via major histocompatibility complexes (MHC) I and II. IL-18 influences the activation of CD4+ T lymphocytes, resulting in the secretion of IFN-γ and the clearance of S. Typhimurium infection from the spleen of mice in a MHC-II-independent manner.197 CD8+ T lymphocytes are essential in clearing the infection caused by cytosolic bacteria such as Listeria monocytogenes.198,199 However, the same is unknown during the infection of vacuolar pathogens such as S. Typhimurium.200,201 The successful onset of typhoid fever in humans by S. Typhi depends on its ability to evade and eventually establish an intracellular proliferating niche in phagocytes. In the following part of this Review, we discuss the intelligent approaches employed by S. Typhi and S. Typhimurium to deceive the immune system and initiate systemic infections in humans and mice.
Intracellular Life of Salmonella in the Macrophage
The virulence of S. Typhi is unique in comparison with that of S. Typhimurium, which is capable of thriving inside a wide range of host cells, including murine macrophages (RAW264.7 and J774A.1 cells), human monocyte-derived macrophages (THP-1 and U937 cells),202−204 dendritic cells,205,206 and B lymphocytes.207−209 To escape the immune response, invasive strains of Salmonella, such as S. Typhi Ty2 and S. Typhimurium D23580, downregulate their metabolic activity and start consuming lipids over carbohydrates.210 After entering macrophages, S. Typhimurium stays inside modified membrane-bound SCVs.211,212 The mature SCV recruits several proteins like LAMP-1 and Rab7 and inhibits their fusion with the lysosome, similar to nonphagocytic cells.185,211,212 The presence of the SCV membrane protects the bacteria from intracellular threats such as ROS, RNS, CAMPs, etc.213,214 Unlike the nontyphoidal serovars, S. Typhi prefers to invade and survive only inside human monocyte-derived macrophages.215,216
Host Restriction of S. Typhi during Macrophage Infection
The inability of S. Typhi to thrive inside murine macrophages can be attributed to the absence of virulent gene gtgE that codes for a cysteine protease GtgE which is secreted into the host cell cytosol by SPI-2 T3SS.217,218 Additionally, S. Typhi cannot produce the GTPase-activating protein SopD2 due to pseudogenization.219 While staying in murine macrophages, S. Typhimurium employs functionally active GtgE and SopD2 to inhibit the recruitment of Rab29, Rab32, and Rab38 GTPases on the SCV membrane.219,220 Rab29, Rab32, and Rab38 and their cognate guanine nucleotide exchange factors BLOC1, BLOC2, and BLOC3 are associated with the transportation of melanin-synthesizing enzymes and their phenyl oxidase intermediates with potential antimicrobial activities into lysosome-like organelle called melanosomes.219,221 In murine macrophage and dendritic cells, the Rab32–BLOC3 pathway helps deliver itaconate, a metabolite that has higher antimicrobial activity and is produced in mitochondria from aconitate decarboxylase, into SCV to restrict the intracellular growth of S. Typhi.222 The pseudogenes in the S. Typhi genome facilitate bacterial evolution and enhance their survival fitness inside a human host.
Role of Salmonella Pathogenicity Islands of S. Typhi in Intramacrophage Survival
Contrary to SPI-1 of S. Typhi, SPI-2-encoded T3SS and antimicrobial resistance genes showed markedly increased expression. Unlike nontyphoidal serovars, S. Typhi can activate the inflammasome in monocyte-derived macrophages two hours postinfection in a SPI-1-dependent manner.223 As an early response to Salmonella sp. infection in macrophages, the antibacterial oxidative burst is induced by NADPH phagocytic oxidase, followed by the activation of inducible nitric oxide synthase (iNOS), which creates a prolonged bacteriostatic nitrosative burst.196 Interestingly, macrophage-resident S. Typhimurium impairs the NADPH phagocytic oxidase-dependent respiratory burst in an SPI-2-dependent manner.104,224
On the contrary, S. Typhi infection in human monocyte-derived macrophage cells is independent of SPI-2-encoded translocon protein SseB and basal secretion apparatus SsaR.225 Using the transposon-directed insertion site sequencing (TraDIS) technique, Karlinsey et al. proved that SPI-2-encoded T3SS of S. Typhi is dispensable for the induction of systemic typhoid fever in the humanized mouse model.226 Expression of the functionally active protein of the S. Typhimurium-encoded marT-fidL operon in S. Typhi (where the same gene is present in the form of a nonfunctional pseudogene) enhanced its susceptibility toward in vitro peroxide stress by suppressing the expression of surV and aggravated its killing in human monocyte-derived macrophages.227 The type IVB pili encoded by the pil operon located in SPI-7 of S. Typhi is responsible for augmenting the expression of NF-ΚB and proinflammatory cytokine IL-6 in THP-1 cells in a protein kinase C-dependent manner.228 The inhibition of type IVB pili of S. Typhi using a high-affinity single-stranded RNA aptamer (S-PS8.4) impaired bacterial entry in THP-1 cells, implicating an essential role of pili in mediating bacterial interaction with the host macrophage.229
Role of Typhoid Toxin in Intramacrophage Survival of S. Typhi
The SPI-11 of S. Typhi expresses an A2B5-type typhoid toxin. It is a multimeric protein complex having catalytically active CdtB (with DNaseI activity) and PltA (with ADP ribosyl transferase activity) domains and five PltB monomers that constitute the base of the toxin.123,230 The low Mg2+ ion concentration of SCV triggers the PhoPQ two-component system, which activates the cdtB, pltA, and pltB operons encoding the structural and functional subunits of the typhoid toxin and suppresses the expression of histone-like protein H-NS.231 Deleting the phoP gene from S. Typhi attenuated its intracellular survival in epithelial and macrophage cells.215 Upon secretion of the typhoid toxin from the lumen of SCV, it is transported to the cell membrane with the help of Rab29.232 After being secreted from the infected cells, it intoxicates the surrounding target cells in an autocrine or paracrine fashion and increases the severity of typhoid fever.230 The typhoid toxin induces DNA damage in THP-1 cells, eventually resulting in the exhaustion of RPA, a sensor of ssDNA/DNA replication, and the formation of γH2AX foci, which brings senescence. The mechanism behind the replication and senescence has yet to be understood; however, it could be a reason for the higher spread of S. Typhi infection.233 Williams et al. proved that the expression of the CdtB toxin in the nontyphoidal serovar of Salmonella sp. Salmonella Javiana induces autophagy and cell death in infected macrophages.234 However, despite the high amino acid sequence similarity between Javiana and Typhoid toxins, there is a difference in the evolutionary host tissue adaptation and cell tropism of both the organisms.235
Role of Other Virulent Genes of S. Typhi in Intramacrophage Survival
A plethora of virulent genes assist in the survival of S. Typhi in macrophages. The global gene expression profile of S. Typhi showed that it could also express both SPI-1- and SPI-2-encoded type III secretion systems and virulence factors (SPI-1, prg, sip, spa, and inv; SPI-2, pipB and sifB) during infection and survival within macrophages. In addition, Salmonella Typhi can upregulate the expression of several virulence determinants (two-component systems and regulators), such as phoPQ, ssrAB, and slyA, while infecting macrophages.236 Transposon mutagenesis revealed that mutation in ssaQ, ssaP, and ssaN impaired the intracellular survival of Salmonella Typhi in macrophages.237 In contrast, the uninterrupted intracellular growth of SPI-2-deficient S. Typhi showed that neither PhoPQ nor SPI-2 was required for infection in THP-1 cells.226.225
The alternative σ-factor rpoS that regulates the expression of spv (Salmonella plasmid virulence) during the systemic infection of S. Typhimurium plays a vital role in the virulence of S. Typhi. Deleting rpoS from the S. Typhi genome makes the bacteria susceptible to NO-dependent host defense even inside both murine (RAW264.7) macrophages.238 Wang et al. reported that deleting sufC enhances Salmonella Typhi’s susceptibility toward oxidative stress and reduces its survival inside the macrophages.239 The virulence-associated capsular polysaccharide (Vi antigen) of S. Typhi plays a diverse role in its survival inside phagocytes. It can abolish the nitric oxide- and reactive oxygen species-dependent innate immune response in monocyte-derived macrophage cells and neutrophils.240 In S. Typhi, the expression of the Vi capsular antigen is positively regulated by OxyR, which eventually induces hydrogen peroxide inducible genes.241,242 Further, the Vi capsular antigen results in reduced binding of complement component 3 (C3) on the surface of S. Typhi, thus rendering protection against complement-mediated phagocytosis and preventing its clearance from the liver, spleen, and blood of mice.243 The Vi antigen-dependent abrogation of IL-8 expression in infected THP-1 cells is the reason behind the deficiency of neutrophils in intestinal infiltrates of patients suffering from typhoid fever.244 Due to the presence of the Vi capsular antigen around its surface, S. Typhi can restrict the recruitment of complement component 5a (C5a) and thus inhibit the C5aR-dependent chemotactic movement of neutrophils.245S. Typhi fimbriae also regulate phagocytosis and intracellular survival in THP-1 cells.246S. Paratyphi A uses the O2 antigen to prevent the binding of immunoglobulin IgM and thus inhibit the respiratory burst in primary murine and human neutrophils.247
Karlinsey and colleagues showed the importance of iron acquisition and utilization during in vivo infection caused by S. Typhi, where the iroCDEN mutant could not induce typhoid fever in hu-SRC-SCID mice.226 While infecting THP-1 macrophages, wild-type S. Typhi secretes eukaryote-like serine-threonine kinase from SCV into the host cytoplasm, which phosphorylates myelin basic proteins (MBPs) and eventually enhances the expression of proinflammatory cytokines like TNF-α and IL-6.248 The bacterial noncoding RNA (ncRNAs) malS are crucial in regulating S. Typhi virulence inside the macrophage. The intramacrophage life of S. Typhi was notably hampered by the overexpression of 5′ UTR of malS (malS-5′UTR), which downregulated the expression of mgtC that maintains ATP homeostasis in the bacteria.249 A study conducted by He et al. implicated that pRST98, a hybrid resistance–virulence plasmid of S. Typhi, is responsible for suppressing autophagy and promoting the survival of the bacteria in infected macrophages.250 Recent studies demonstrated that nontyphoidal Salmonella serovars widely use numerous novel virulence factors, such as outer membrane proteins (OmpA and PgtE), YdcP, and a novel putative collagenase, to interact with host macrophages.251−254 High sequential homology for these novel virulence factors between Salmonella Typhi and Typhimurium indicated their involvement during typhoid fever. However, thorough and detailed research is required to annotate their exact functions in S. Typhi.
S. Typhi Infection in Dendritic Cells
During chronic Salmonella infection, the greater abundance of macrophages, neutrophils, and dendritic cells in the spleen indicated that S. Typhi could reside in dendritic cells (DCs), neutrophils, and B lymphocytes.255 Dendritic cells can capture bacteria from the lumen of the intestine. With the help of cell surface MHC II molecules and other costimulatory molecules like CD40 receptors, CD80, and CD86 ligands, DCs present bacterial antigens to the CD4+ helper T lymphocyte population and induce an adaptive immune response. While infecting murine DCs, S. Typhimurium delays the recruitment of NOX2 on SCV and impairs antigen presentation by K63-linked ubiquitination of MHC-II and subsequent degradation by endosomal proteases.256S. Typhimurium infection in murine DCs enhances the expression and activity of iNOS (NOS2), which produces RNS to limit the growth of the bacteria in the SIRT2-dependent manner.257 During S. Typhi infection, intestinal inflammation is dictated by the pathogen and gut microbiota’s interactions with intestinal DCs. The coinfection of intestinal DCs with a probiotic strain of Bifidobacterium breve CNCM I-4034 and human pathogen S. Typhi enhanced the expression of proinflammatory cytokines and chemokines like TNF-α, IL-8, and RANTES in a TLR-2-dependent manner.258 Infection of bone marrow-derived dendritic cells (BMDCs) with S. Typhi bacterial ghost (STG, which is an inactivated vaccine candidate expressing the lysisE gene from DNA phage ϕX174), triggered the maturation of CD11c+ BMDCs by increasing the expression of CD40, CD80, and MHC-II molecules.259 Furthermore, in DCs, the viable S. Typhi deploys several virulence factors to manipulate the host signaling process to favor its growth. The pRST98 of S. Typhi suppresses the surface expression of several costimulatory molecules, such as CD40, CD80, and CD86, inhibiting autophagy in DCs. Wild-type S. Typhi can escape the Th1 response by downregulating IL-12 and IFN-γ and upregulating IL-10.260 In line with this observation, BALB/c mice infected with S. Typhimurium SR11 x3337 bearing the pRST98 plasmid of S. Typhi showed suppression of dendritic and T cell activation.261 On the contrary, human dendritic cells cocultured with the PBMCs infected with S. Typhi produced a higher level of proinflammatory cytokines like 12p70 and IFN-γ and augmented the antigen presentation to primed CD3+ and CD8+ effector/memory T cells.262S. Typhi utilizes TviA, a regulatory protein associated with flagellin expression (fliC), to invade the intestinal mucosa and escape antigen presentation by dendritic cells. The expression of S. Typhi TviA in S. Typhimurium diminished antigen presentation by dendritic cells to CD4+ T lymphocytes by lowering the availability of flagellar antigen in the Nramp+/+ C57BL/6 mouse.263 However, the complex behavior of S. Typhi in dendritic cells has been poorly understood, and a considerable amount of research is required to unveil the mechanisms employed by the pathogen to modulate DCs and to thrive inside an otherwise hostile niche. Table 3 summarizes the strategies that Salmonella uses to emerge as a successful pathogen.
Table 3. Salmonella Pathogenicity Islands (SPIs), Genomic Structure, and Functions in S. Typhi.
| SPI | effectors | known function | (modified) function in S. Typhi | refs |
|---|---|---|---|---|
| SPI-3 (17-kb pathogenicity island located at 82 min, just behind the selC tRNA locus of Salmonella) | in the mgtCB operon, the gene mgtC is specific for Salmonella. mgtB which is a Mg2+ transporting ATPase. Another Mg2+ transporter coded by SPI-3 is mgtA. SPI-3. It also harbors the marT-fidL operon, encoding MarT transcriptional regulator and a hypothetical protein FidL | in the mgtCB operon, the gene mgtC is specific for Salmonella, facilitates intramacrophage survival, growth in low Mg2+ media, and survival in acidic environments of endosomes resulting from SPI-1-mediated invasion. MisL, coded by SPI-3, is an extracellular matrix adhesin involved in intestinal colonization | the region adjacent to the selC tRNA region hosts the maximum amount of variations between S. Typhi and S. Typhimurium along with some pseudogenes like STY4024 (cigR), STY4027 (marT), STY4030 (misL), STY4034, STY4035, and STY4037. a few more pseudogenes are also noted in other regions of SPI-3 like STY4012, STY4007, and STY4003. in brief, MisL, its regulator MarT, and an unknown putative transcriptional regulator (STY4012) are inactivated in S. Typhi. expression of S. Typhimurium marT-fidL in S. Typhi negatively affected the survival of the bacterium inside macrophage cells but not epithelial cells | (291−295) |
| SPI-4 (24 kb fragment located next to a potential tRNA-like gene at centisome 92) | SPI-4 encodes a type 1 secretion system (siiCDF), which encodes the almost 600 kDa protein (encoded by SiiE) | SiiE is a nonfimbrial adhesin protein, 600 kDa in size, containing 53 repeats of Ig domains. SiiE is secreted into the culture medium and mediates contact-dependent adhesion to the epithelial cell surface | SPI-4 has role in the intracellular uptake and survival in macrophages for S. Typhi. SiiE is coded by only one ORF in S. Typhimurium (STM4261), unlike in S. Typhi, where it is segmented into two ORFs in S. Typhi (STY4458 and STY4459) due to the presence of a stop codon. this implies that siiE is a pseudogene in S. Typhi that correlates with the loss of function of adhesin responsible for intestinal colonization by S. Typhimurium | (237, 296, 297) |
| SPI-5 (approximately 7.6 kb long and has been found next to serT tRNA) | encodes the effector proteins for both the T3SS system of SPI-1 and SPI-2 | SopB translocated by SPI-1 T3SS helps in membrane ruffling and invasion. PipA contributes in the development of systemic infection. PipB translocated by SPI-2-encoded T3SS facilitates accumulation of lipid rafts and helps in intramacrophage survival | no difference has been observed between the two serovars apart from an additional ORF (STY114) that putatively encodes a transposase in S. Typhi | (117, 298, 299) |
| SPI-6 (located next to aspV tRNA gene at centisome 47 and it extends for 47 kb and 59 kb in both S. Typhi and S. Typhimurium) | SPI-6 has the saf gene and the pagN gene | the saf gene codes for fimbriae and the pagN gene codes for the invasion protein in both the serovars | a fragment 10 kb in size is present downstream of the saf operon only in S. Typhi, which includes putative transposase remnants (STY0343 and STY0344, both of which are pseudogenes), the fimbrial operon tcfABCD, and genes tinR (STY0349) and tioA (STY0350). although the T6SS gene cluster is intact in S. Typhi, the protein complex is thought to be nonfunctional due to the presence of a pseudogene form of SciI (VipB homologue). Wang et al. have disproved this assumption and have identified the genes sciA, sciG, sciS, and vrgS as important for T6SS function | (299, 300) |
| SPI-7 (largest SPI, 133 kb, located between two partially duplicated copies of the tRNA-pheU gene and contains about 150 genes) | encodes the capsular polysaccharides or the Vi antigen. it contains the pil gene cluster along with some of the genes of the conjugative plasmid like tra and sam | the pil gene cluster that is responsible for coding many virulence factors along with the SopE prophage (ST44) that encodes the SPI-1 effector SopE | the Vi capsule also acts to inhibit flagellin expression, causes limited recognition of Salmonella by NAIP, and helps to reduce levels of pyroptosis and IL-1β secretion of macrophages. Along with this, Vi has been reported to bind to cell surface, thus dampening the inflammation caused by MAPK signaling or IL-8 production | (301−303) |
| SPI-8 (6.8 kb and has been next to pheV tRNA gene that is found next to SPI-13) | the exact function of SPI-8 is yet to be elucidated but it is known to code for putative virulence factors | (120, 304) | ||
| SPI-9 | encodes for virulence factors of the type I secretion system and encodes RTX-like protein. It shares a 40% nucleotide homology to siiCDEF genes from SPI-4 | arranged in an operon whose promoter was upregulated in low pH and high osmolarity conditions in a RpoS-dependent fashion. All the proteins encoded by this pathogenicity island localize in the membrane faction, supporting their putative role as T1SS. SPI-9 contributed to the adherence of S. Typhi to epithelial cells under high osmolarity or low pH or in situation mimicking conditions of the small intestine | (80, 298, 305) | |
| SPI-10 (32.8 kb, next to leuX tRNA gene at centisome 93) | In S. Typhimurium, it is substituted by a 20 kb uncharacterized island without any SPI annotation that comprises functionally unrelated genes sharing little homology to sequences from the genomic database | there is possible relationship of this sequence with DNA repair, deletion of this island in S. Typhimurium 14028 attenuated the virulence of the strain | a full P4-related phage named ST46 that harbors prpZ as cargo genes responsible for encoding the Ser-Thr protein involved in S. Typhi survival in macrophages. many other pseudogenes are also found in S. Typhi, namely, STY4835 (IS1230), STY4836 (sefA), STY4839 (sefD), STY4841 (sefR), STY4845 (a thiol–disulfide interchange protein), and STY4848 (putative transposase). the homologous ORFs STY4842–4846 of S. Typhi includes the srgA gene. The srgA gene encodes a functional disulfide oxidoreductase in S. Typhimurium unlike that in S. Typhi, where it is a pseudogene (STY4845). it contributes to virulence factors that lead to the formation of Sef fimbriae, a phenomenon restricted to S. Typhi and S. Enteritidis. The role of cryptic bacteriophage is yet to be elucidated | (304, 306, 307) |
| SPI-11 (6.7 kb in S. Typhimurium and 10 kb in S. Typhi) | S. Typhi lacks the putative envelope lipoprotein envF, but it retains six additional ORFs (STY1884–1891), including the typhoid toxin CdtB. the CdtB toxin is elaborated in the section Interaction of Salmonella Typhi with Phagocytes | (308) | ||
| SPI-12 (is located next to proL tRNA centisome 48, 6.3 kb long in S. Typhi) | SPI-12 is responsible for causing systemic infection in mice for S. Typhimurium 14028 | in S. Typhi, three ORFs are pseudogenes, viz., STY2466a, STY2468, and STY2469. Only the sspH2 gene is functional in this island | (146, 309) | |
| SPI-13 (next to the pheV tRNA gene at centisome 67 in S. Typhimurium and S. Typhi.; there is an 8 kb fragment different in the serovars, which corresponds to SPI-8 in S. Typhi) | In S. Typhimurium, the ORFs STM3117–3123 code for virulence-associated genes | the genes are involved in intracellular replication in murine macrophages and contribute to systemic infection of mice | S. Typhi SPI-8 harbors two bacteriocin immunity proteins (STY3281 and STY3283) and four pseudogenes. The virulence properties of SPI-8 are unknown. The conserved 17 kb in SPI-13 has not been found to contribute to virulence | (6, 310, 311) |
| SPI-14 | the function of this SPI has yet to be understood | although these are found in S. Enteritidis and S. Typhimurium, they are absent from S. Typhi and S. Paratyphi A | (304) | |
| SPI-15 (6.5 kb island that is present near the glyU tRNA gene in S. Typhi) | absent in S. Typhimurium | constitutes five islands encoding hypothetical proteins, which have been identified by bioinformatic analysis | (312) | |
| SPI-16 (4.5 kb fragment located next to argU tRNA) | five ORFs in S. Typhimurium | seven ORFs in S. Typhi. Four of these genes are pseudogenes in S. Typhi. the three remaining ORFs in S. Typhi have a high level of similarity with P22 phage involved in seroconversion. they are suggested to cause O-antigen glycosylation and cell surface variation. | (312, 313) | |
| SPI-17 (inserted next to an argW tRNA site) | this pathogenicity island contains seroconversion genes homologous to P22 phage | it showed a high similarity to genes of SPI-16, including a putative lipopolysaccharide modification acyltransferase | most of these genes are pseudogenes in S. Typhi | (312) |
| SPI-18 | This genomic island is missing in S. Typhimurium | codes for two putative genes. One of these is hlyE, encoding a hemolysin related to the Escherichia coli K12 HlyE hemolysin. Fuentes et al. have shown that S. Typhi hlyE mutants are impaired in their capability to invade human epithelial cells in vitro, and its heterologous expression in S. Typhimurium has been seen to improve the colonization of deep organs in mice. additionally, a secreted 27 kDa invasin, coded by taiA (STY1499), increases bacterial uptake by human macrophages | (304, 314, 315) |
Extra-Intestinal Infections Caused by S. Typhi
Salmonella multiplies in reticuloendothelial cells and macrophages of the liver, spleen, lymph nodes, and bone marrow during the asymptomatic phase of infection. If left untreated, bacteria reach a threshold level and are released in the blood, initiating secondary bacteremia with cytokine secretion during the symptomatic phase of typhoid fever.264 This secondary persistent bacteremia phase leads to the seeding of other organs, which may further complicate the situation by developing intestinal perforations, hepatitis, pneumonia, and tissue abscesses, usually during the second and fourth weeks of illness.40,41,265,266
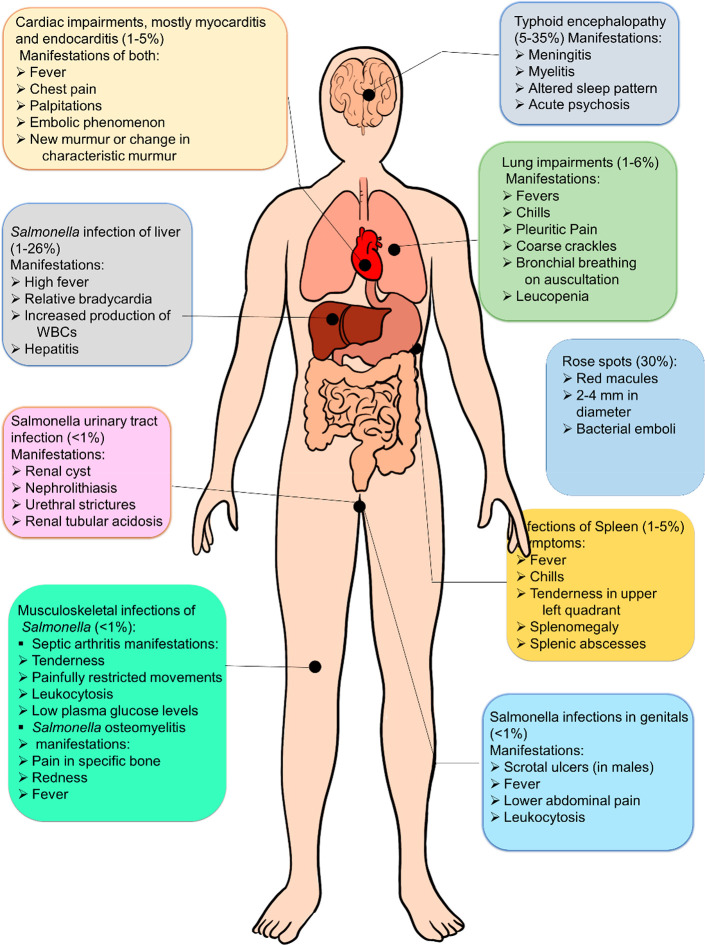
Hence, it will be appropriate to comment that once bacteremia sets in following an S. Typhi infection, almost all organs have the potential to host bacterial foci. Quite frequently diagnosis of this infection is ignored, which leads to fatal outcomes. Imaging techniques such as magnetic resonance imaging (MRI), ultrasonography (USG), and radiographs have facilitated the diagnostic process. MRI is a medical imaging technique used in radiology, and it can visualize the anatomy and physiological processes of the body. USG can help diagnose systemic manifestations like enlarged mesenteric lymph nodes (MLNs), mural thickening of terminal patients, splenomegaly, and acute acalculous cholecystitis associated with Salmonella infection. However, culturing the pathogen from the site of infection is the ultimate gold-standard diagnostic tool. Figure 4 and Table 4 demonstrate summarys of the extraintestinal infections of Salmonella and their manifestations.
Figure 4.
Extra-intestinal infections caused by Salmonella Typhi. The spleen is located behind the stomach, and kidneys are located at the back of the trunk’s middle and have been marked accordingly. The percentage of occurrence is expressed concerning total gastrointestinal infections caused by the pathogen.
Table 4. Extra-Intestinal Infections by S. Typhi.
| organ infected | complications | symptoms | pre-disposing factors | diagnosis | treatment | refs |
|---|---|---|---|---|---|---|
| brain | “typhoid encephalopathy” under severe conditions, Parkinson’s syndrome, motor-neuron disease, transient amnesia, symmetrical sensory-motor neurotrophy, schizophreniform psychosis, and Guillain-Barre syndrome | altered consciousness, delirium, and confusion along with other rare neurological symptoms like myelitis, altered sleep pattern, meningitis, and acute psychosis | neuroimaging with magnetic resonance imaging (MRI), angiography, computed tomography (CT), and/or radionuclide scans might be handy for diagnosis of brain abscesses, epidural empyemas, and subdural empyemas | (316−317,318,319) | ||
| cardiac impairments | major complications like (i) myocarditis and (ii) endocarditis to pericarditis and arteritis as rare complications | (i) ,icrocirculatory disturbances, edema, inflammation of the heart intramural vessels, lymphocytic, and macrophage infiltration of the stroma, sometimes with the formation of granulomas, and dystrophic and necrotic changes | underlying cardiac complexities like heart valvular abnormalities, congenital heart disease, or rheumatic heart disease | (i) abnormal doppler signal, ECG, CMR, indicators of cardiac injury (CK, AST, and LDH), electromyocardial biopsy (EMB). (ii) culturing the pathogen from the blood. transthoracic or transesophageal echocardiography might reveal the presence of vegetation | antibiotics and supportive management. | (320, 321) |
| lung disease | bronchopneumonia, lobar pneumonia, pleurisy with effusion, and empyema | fever, chills, coughs (with or without sputum), pleuritic pain, coarse crackles and bronchial breathing on auscultation, diarrhea, and leukopenia | prior abnormalities of lung or pleura with the presence of lung malignancies in some cases. mortality is usually high in geriatric patients, with underlying malignancies and immunosuppression due to antineoplastic treatments | chest radiographs or by isolation of the bacterium from sputum, protected specimen brush (PSB) material of bronchial secretions | Salmonella pneumonia calls for at least 2 weeks of antimicrobial therapy. under unresponsive antibiotic therapy, lobectomy might be an option | (322−324) |
| liver | hepatitis | high fever, relative bradycardia, and left shift of WBCs | liver damage markers like elevated serum alanine and aspartate transaminase levels, alkaline phosphatase levels, and γ-glutamyl transferase levels. abdominal ultrasonography (USG) or MR cholangiography. blood culture and serological tests for Salmonella | (325) | ||
| spleen | fever, chills, tenderness in the left upper quadrant, and splenomegaly | hemoglobinopathies, impaired host resistance, subacute bacterial endocarditis, IV drug abuse, trauma, diabetes mellitus, skin sepsis, respiratory tract infections, immunodeficiency, and urinary tract infections | ultrasonography (USG), USG-guided aspiration followed by culturing the pathogen, Widal test, stool culture, and blood culture | drainage of the largest abscesses along with antibiotic regimen is the favorable treatment. percutaneous drainage preferred. Patients with multiple abscesses unresponsive to percutaneous drainage might call for a splenectomy | (326, 327) | |
| urinary tract | abscesses in kidney and nephrons. rare complications are IgA nephropathy, hemolytic-uremic syndrome, acute tubular necrosis, and Henoch-Schpnlein purpura | structural or functional abnormalities, pyelonephritis, dermoid cyst, calculi, dermoid cyst, and renal transplant | diagnosis is possible by culturing the pus from the abscesses | needs the proper antimicrobial regime. in the case of a perinephric abscess, a drainage procedure might also be required in addition to the administration of antibiotics | (328) | |
| genital tract | pelvic inflammatory diseases (PID). along with the pelvis, Salmonella can form abscesses in pelvic bones and intraperitoneal spaces | fever, lower abdominal pain, and leukocytosis | most of the cases had underlying structural deformities like ovarian or dermoid cysts or were immunocompromised | increase in WBCs, ultrasonography. laparoscopic evaluation of the pelvic organs is the ideal method for diagnosing tubo-ovarian abscesses. isolation of Salmonella Typhi from purulent abscesses may make definitive diagnosis possible | 7–14 days of antibiotic treatment in the case of genitourinary infections or longer if underlying complications as stone or abscess collection are seen | (329, 330) |
| musculoskeletal system | (i) osteomyelitis and (ii) septic arthirits | (i) Ttypically, an infection of the long bones like femur and humerus, it might also affect the lumbar vertebrae, tibia, radius, and ulna. (ii) the chondrosternal junctions, the hip, the sacroiliac joints, the spine, and the knee | (i) predilection for patients with systemic lupus erythematosus (SLE), diabetes mellitus, lymphoma, liver and cardiovascular diseases, previous surgery or trauma, and patients on steroids. (ii) immunosuppressed individuals or children with congenital diseases where bacteremia has the propensity to spread via hematogenous route to larger joints | Salmonella osteomyelitis can be confirmed by simultaneous isolation of Salmonella spp. from blood and pus of affected musculoskeletal site, MRI, CT, and radiographs | antimicrobial therapy and surgical debridement | (331) |
| skin | Rose spots | red, blanchable macular lesions 2–4 mm in diameter, which show up in 50% of the cases of enteric fever. within the seventh to twelfth day from the onset of symptoms, rose-spots are seen to occur in crops of 5–10 lesions on the lower chest and upper abdomen and may last for 3–4 days | Salmonella can be cultured from the puncture biopsies | cured with antibiotics alone without surgical intervention | (15) |
Conclusion
Salmonella enterica infection remains a significant public health concern worldwide, especially in third-world countries where Salmonella infection is often endemic due to various socio-economic factors. The history of epidemics, genetic makeup, and stealthy strategies against the host makes Salmonella a superbug. Its adaptation to infect a range of hosts often makes eradicating this notorious pathogen several times difficult. Despite significant progress in medical science over the last centuries, Salmonella causes significant mortality and morbidity pertaining mainly to its adaptation to host defense and the evolution of drug-resistant strains. Salmonella can cleverly manipulate host defense arsenals, thereby mounting an intense infection in the host system. This Review discussed the essential aspects that help the bacteria achieve this, especially horizontally acquired islands (SPIs). Therefore, understanding the biology of Salmonella infection is essential and will help us to reduce the burden of Salmonella infection globally.
Although typhoidal and nontyphoidal Salmonella serovars belong to the same biological species, these two serovars mount and trigger distinct acute infections in the host. The situation worsens due to extraintestinal manifestations, carriage, and recurrent infections in the population. The field of Salmonella biology has an immense amount of literature on understanding the molecular mechanisms of virulence. Nevertheless, there is a dearth of accurate information regarding the in vivo scenario due to host restrictions and the unavailability of permissive animal models. Therefore, there is a pressing need for a different animal model, a collaborative system of biology insights, and advanced techniques to decipher the systemic effects of Salmonella infection and single-cell approaches. This will further help us to develop plausible novel therapeutics and vaccination strategies against Salmonella infections.
Acknowledgments
We are thankful for the financial support from the Department of Biotechnology (DBT) and Department of Science and Technology (DST), Ministry of Science and Technology. D.C. acknowledges DAE for the SRC outstanding investigator award and funds, a TATA fellowship, and ASTRA Chair Professorship funds. The authors jointly acknowledge the DBT-IISc Partnership Programme. Infrastructure support from ICMR (Center for Advanced Study in Molecular Medicine), DST (FIST), and UGC-CAS (special assistance) is acknowledged. R.C. duly acknowledges the CSIR-SRF fellowship. A.R.C. acknowledges Shamrao M. Kaikini and Krishna S. Kaikini’s fellowship. D.M. acknowledges the IISc fellowship.
Author Present Address
§ Adjunct Faculty - School of Biological Sciences, Indian Institute of Science Education and Research, Thiruvananthapuram, Kerala 695551, India
Author Contributions
# These authors contributed equally.
The authors declare no competing financial interest.
References
- Knodler L. A.; Elfenbein J. R. Salmonella enterica. Trends Microbiol 2019, 27 (11), 964–965. 10.1016/j.tim.2019.05.002. [DOI] [PubMed] [Google Scholar]
- Mutz Y. D. S.; et al. Salmonella enterica: A hidden risk for dry-cured meat consumption?. Crit Rev. Food Sci. Nutr 2020, 60 (6), 976–990. 10.1080/10408398.2018.1555132. [DOI] [PubMed] [Google Scholar]
- Rau R. B.; et al. Salmonella enterica mcr-1 Positive from Food in Brazil: Detection and Characterization. Foodborne Pathog Dis 2020, 17 (3), 202–208. 10.1089/fpd.2019.2700. [DOI] [PubMed] [Google Scholar]
- Lamas A.; et al. A comprehensive review of non-enterica subspecies of Salmonella enterica. Microbiol Res. 2018, 206, 60–73. 10.1016/j.micres.2017.09.010. [DOI] [PubMed] [Google Scholar]
- Ferrari R. G. Worldwide Epidemiology of Salmonella Serovars in Animal-Based Foods: a Meta-analysis. Appl. Environ. Microbiol. 2019, 85 (14), e00591-19. 10.1128/AEM.00591-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhill J.; et al. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 2001, 413 (6858), 848–52. 10.1038/35101607. [DOI] [PubMed] [Google Scholar]
- Fookes M.; et al. Salmonella bongori provides insights into the evolution of the Salmonellae. PLoS Pathog 2011, 7 (8), e1002191 10.1371/journal.ppat.1002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks F.; et al. Incidence of invasive salmonella disease in sub-Saharan Africa: a multicentre population-based surveillance study. Lancet Glob Health 2017, 5 (3), e310–e323. 10.1016/S2214-109X(17)30022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikora A.; Garcia A. V.; Hirt H. Plants as alternative hosts for Salmonella. Trends Plant Sci. 2012, 17 (5), 245–9. 10.1016/j.tplants.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Jajere S. M. A review of Salmonella enterica with particular focus on the pathogenicity and virulence factors, host specificity and antimicrobial resistance including multidrug resistance. Vet World 2019, 12 (4), 504–521. 10.14202/vetworld.2019.504-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afshari A.; et al. Salmonella Enteritidis and Salmonella Typhimorium identification in poultry carcasses. Iran J. Microbiol 2018, 10 (1), 45–50. [PMC free article] [PubMed] [Google Scholar]
- Stanaway J. D.; et al. The global burden of non-typhoidal salmonella invasive disease: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis 2019, 19 (12), 1312–1324. 10.1016/S1473-3099(19)30418-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feasey N. A.; et al. Invasive non-typhoidal salmonella disease: an emerging and neglected tropical disease in Africa. Lancet 2012, 379 (9835), 2489–2499. 10.1016/S0140-6736(11)61752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack B.; et al. Invasive non-typhoidal Salmonella infections in sub-Saharan Africa: a systematic review on antimicrobial resistance and treatment. BMC Med. 2020, 18, 212. 10.1186/s12916-020-01652-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D. B.; DuPont H. L. Problem pathogens: extra-intestinal complications of Salmonella enterica serotype Typhi infection. Lancet Infect Dis 2005, 5 (6), 341–8. 10.1016/S1473-3099(05)70138-9. [DOI] [PubMed] [Google Scholar]
- Chowdhury A. R.; et al. Loss of outer membrane protein A (OmpA) impairs the survival of Salmonella Typhimurium by inducing membrane damage in the presence of ceftazidime and Meropenem. J. Antimicrob. Chemother. 2022, 77 (12), 3376–3389. 10.1093/jac/dkac327. [DOI] [PubMed] [Google Scholar]
- Majee S.; et al. Spatiotemporal evaporating droplet dynamics on fomites enhances long term bacterial pathogenesis. Commun. Biol. 2021, 4, 1173. 10.1038/s42003-021-02711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan V.; et al. phoP maintains the environmental persistence and virulence of pathogenic bacteria in mechanically stressed desiccated droplets. iScience 2023, 26 (5), 106580. 10.1016/j.isci.2023.106580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstead G. M. History, Rats, Fleas, and Opossums: The Ascendency of Flea-Borne Typhus in the United States, 1910–1944. Trop Med. Infect. Dis. 2020, 5 (1), 37. 10.3390/tropicalmed5010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan C. A.; Martin L. B.; Micoli F. Vaccines against invasive Salmonella disease: current status and future directions. Hum Vaccin Immunother 2014, 10 (6), 1478–93. 10.4161/hv.29054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanaway J. D.; et al. The global burden of typhoid and paratyphoid fevers: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis 2019, 19 (4), 369–381. 10.1016/S1473-3099(18)30685-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H.; et al. A Systematic Review of Typhoid Fever Occurrence in Africa. Clin Infect Dis 2019, 69 (Supplement_6), S492–S498. 10.1093/cid/ciz525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer E.; Schwartz E. Enteric fever: a travel medicine oriented view. Curr. Opin Infect Dis 2010, 23 (5), 432–7. 10.1097/QCO.0b013e32833c7ca1. [DOI] [PubMed] [Google Scholar]
- Ochiai R. L.; et al. Salmonella paratyphi A rates, Asia. Emerg Infect Dis 2005, 11 (11), 1764–6. 10.3201/eid1111.050168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näsström E.; et al. Salmonella Typhi and Salmonella Paratyphi A elaborate distinct systemic metabolite signatures during enteric fever. Elife 2014, 3, e03100. 10.7554/eLife.03100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers L. M. F.; et al. The clinical and microbiological characteristics of enteric fever in Cambodia, 2008–2015. PLoS Negl Trop Dis 2017, 11 (9), e0005964 10.1371/journal.pntd.0005964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M. F.; et al. Typhoid fever in the United States, 1999–2006. JAMA 2009, 302 (8), 859–65. 10.1001/jama.2009.1229. [DOI] [PubMed] [Google Scholar]
- Woods C. W.; et al. Emergence of Salmonella enterica serotype Paratyphi A as a major cause of enteric fever in Kathmandu, Nepal. Trans R Soc. Trop Med. Hyg 2006, 100 (11), 1063–7. 10.1016/j.trstmh.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Alvarez M. I.; et al. Human genetic variation in VAC14 regulates Salmonella invasion and typhoid fever through modulation of cholesterol. Proc. Natl. Acad. Sci. U. S. A. 2017, 114 (37), E7746–E7755. 10.1073/pnas.1706070114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B.; et al. Salmonella typhi uses CFTR to enter intestinal epithelial cells. Nature 1998, 393 (6680), 79–82. 10.1038/30006. [DOI] [PubMed] [Google Scholar]
- van de Vosse E.; et al. Susceptibility to typhoid fever is associated with a polymorphism in the cystic fibrosis transmembrane conductance regulator (CFTR). Hum. Genet. 2005, 118 (1), 138–40. 10.1007/s00439-005-0005-0. [DOI] [PubMed] [Google Scholar]
- Marzel A.; et al. Integrative analysis of Salmonellosis in Israel reveals association of Salmonella enterica Serovar 9,12:l,v:- with extraintestinal infections, dissemination of endemic S. enterica Serovar Typhimurium DT104 biotypes, and severe underreporting of outbreaks. J. Clin Microbiol 2014, 52 (6), 2078–88. 10.1128/JCM.00399-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfall E. J.; et al. Trends in antimicrobial drug resistance in Salmonella enterica serotypes Typhi and Paratyphi A isolated in Europe, 1999–2001. Int. J. Antimicrob. Agents 2003, 22 (5), 487–91. 10.1016/S0924-8579(03)00262-0. [DOI] [PubMed] [Google Scholar]
- Gal-Mor O.; Boyle E. C.; Grassl G. A. Same species, different diseases: how and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front. Microbiol. 2014, 5, 391. 10.3389/fmicb.2014.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M. P.; Humphrey T. J.; Maskell D. J. Molecular insights into farm animal and zoonotic Salmonella infections. Philos. Trans R Soc. Lond B Biol. Sci. 2009, 364 (1530), 2709–23. 10.1098/rstb.2009.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsolis R.; et al. From bench to bedside: Stealth of enteroinvasive pathogens. Nature reviews. Microbiology 2008, 6, 883–92. 10.1038/nrmicro2012. [DOI] [PubMed] [Google Scholar]
- Haselbeck A. H.; et al. Current perspectives on invasive nontyphoidal Salmonella disease. Curr. Opin Infect Dis 2017, 30 (5), 498–503. 10.1097/QCO.0000000000000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman R.; Levine M. M. Summary of an international workshop on typhoid fever. Rev. Infect Dis 1986, 8 (3), 329–49. 10.1093/clinids/8.3.329. [DOI] [PubMed] [Google Scholar]
- Miguet E.; Cormack J. R. Miguet and Cormack on Creosote. Edinb. Med. Surg J. 1836, 46 (129), 428–442. [PMC free article] [PubMed] [Google Scholar]
- Crump J. A.; et al. Epidemiology, Clinical Presentation, Laboratory Diagnosis, Antimicrobial Resistance, and Antimicrobial Management of Invasive Salmonella Infections. Clin Microbiol Rev. 2015, 28 (4), 901–37. 10.1128/CMR.00002-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry C. M.; et al. Typhoid fever. N Engl J. Med. 2002, 347 (22), 1770–82. 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- Humphries R. M.; Linscott A. J. Practical Guidance for Clinical Microbiology Laboratories: Diagnosis of Bacterial Gastroenteritis. Clin Microbiol Rev. 2015, 28 (1), 3–31. 10.1128/CMR.00073-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner F. W.; et al. Salmonella nomenclature. J. Clin Microbiol 2000, 38 (7), 2465–7. 10.1128/JCM.38.7.2465-2467.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B.; et al. Structural diversity in Salmonella O antigens and its genetic basis. FEMS Microbiol Rev. 2014, 38 (1), 56–89. 10.1111/1574-6976.12034. [DOI] [PubMed] [Google Scholar]
- Issenhuth-Jeanjean S.; et al. Supplement 2008–2010 (no. 48) to the White-Kauffmann-Le Minor scheme. Res. Microbiol 2014, 165 (7), 526–30. 10.1016/j.resmic.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Schwartz D. C.; Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell 1984, 37 (1), 67–75. 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Gerner-Smidt P.; et al. PulseNet USA: a five-year update. Foodborne Pathog Dis 2006, 3 (1), 9–19. 10.1089/fpd.2006.3.9. [DOI] [PubMed] [Google Scholar]
- Esteban E.; et al. Use of ribotyping for characterization of Salmonella serotypes. J. Clin Microbiol 1993, 31 (2), 233–7. 10.1128/jcm.31.2.233-237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre L.; et al. CRISPR typing and subtyping for improved laboratory surveillance of Salmonella infections. PLoS One 2012, 7 (5), e36995 10.1371/journal.pone.0036995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F.; et al. Novel virulence gene and clustered regularly interspaced short palindromic repeat (CRISPR) multilocus sequence typing scheme for subtyping of the major serovars of Salmonella enterica subsp. enterica. Appl. Environ. Microbiol. 2011, 77 (6), 1946–56. 10.1128/AEM.02625-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M.; et al. Multilocus sequence typing as a replacement for serotyping in Salmonella enterica. PLoS Pathog 2012, 8 (6), e1002776 10.1371/journal.ppat.1002776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan C. B.; Diggle M. A.; Clarke S. C. Multilocus sequence typing. Molecular Biotechnology 2005, 29 (3), 245. 10.1385/MB:29:3:245. [DOI] [PubMed] [Google Scholar]
- Ley B.; et al. Evaluation of the Widal tube agglutination test for the diagnosis of typhoid fever among children admitted to a rural hdospital in Tanzania and a comparison with previous studies. BMC Infect Dis 2010, 10, 180. 10.1186/1471-2334-10-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olopoenia L. A.; King A. L. Widal agglutination test - 100 years later: still plagued by controversy. Postgrad Med. J. 2000, 76 (892), 80–4. 10.1136/pmj.76.892.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thriemer K.; et al. A systematic review and meta-analysis of the performance of two point of care typhoid fever tests, Tubex TF and Typhidot, in endemic countries. PLoS One 2013, 8 (12), e81263 10.1371/journal.pone.0081263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijedoru L.; Mallett S.; Parry C. M.; et al. Rapid diagnostic tests for typhoid and paratyphoid (enteric) fever. Cochrane Database Syst. Rev. 2017, 5, CD008892. 10.1002/14651858.CD008892.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S.; Chitnis D. S. Haemagglutination system for the simultaneous detection of LPS and anti LPS antibodies of S.typhi. Indian J. Med. Sci. 1997, 51 (8), 265–269. [PubMed] [Google Scholar]
- Kalhan R.; et al. Rapid diagnosis of typhoid fever. Indian Journal of Pediatrics 1998, 65 (4), 561–564. 10.1007/BF02730895. [DOI] [PubMed] [Google Scholar]
- Lin L. H.; et al. Rectal swab sampling followed by an enrichment culture-based real-time PCR assay to detect Salmonella enterocolitis in children. Clin Microbiol Infect 2011, 17 (9), 1421–5. 10.1111/j.1469-0691.2010.03450.x. [DOI] [PubMed] [Google Scholar]
- Tennant S. M.; et al. Identification by PCR of non-typhoidal Salmonella enterica serovars associated with invasive infections among febrile patients in Mali. PLoS Negl Trop Dis 2010, 4 (3), e621 10.1371/journal.pntd.0000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J. H.; et al. Detection of Salmonella typhi in the blood of patients with typhoid fever by polymerase chain reaction. J. Clin Microbiol 1993, 31 (6), 1439–43. 10.1128/jcm.31.6.1439-1443.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A.; et al. Multiplex PCR for differential diagnosis of emerging typhoidal pathogens directly from blood samples. Epidemiol Infect 2009, 137 (1), 102–7. 10.1017/S0950268808000654. [DOI] [PubMed] [Google Scholar]
- Kumar A.; et al. Detection of Salmonella typhi by polymerase chain reaction: implications in diagnosis of typhoid fever. Infect Genet Evol 2002, 2 (2), 107–10. 10.1016/S1567-1348(02)00090-4. [DOI] [PubMed] [Google Scholar]
- Prakash P.; et al. Evaluation of nested PCR in diagnosis of typhoid fever. J. Clin Microbiol 2005, 43 (1), 431–2. 10.1128/JCM.43.1.431-432.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nga T. V.; et al. The sensitivity of real-time PCR amplification targeting invasive Salmonella serovars in biological specimens. BMC Infect Dis 2010, 10, 125. 10.1186/1471-2334-10-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar G.; et al. Use of urine with nested PCR targeting the flagellin gene (fliC) for diagnosis of typhoid fever. J. Clin Microbiol 2012, 50 (6), 1964–7. 10.1128/JCM.00031-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darton T. C.; et al. Blood culture-PCR to optimize typhoid fever diagnosis after controlled human infection identifies frequent asymptomatic cases and evidence of primary bacteraemia. J. Infect 2017, 74 (4), 358–366. 10.1016/j.jinf.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Jimenez M. M.; Cardona-Castro N. Validation of a PCR for diagnosis of typhoid fever and salmonellosis by amplification of the hilA gene in clinical samples from Colombian patients. J. Med. Microbiol 2004, 53, 875–878. 10.1099/jmm.0.45630-0. [DOI] [PubMed] [Google Scholar]
- Van Lint P.; et al. Evaluation of a real-time multiplex PCR for the simultaneous detection of Campylobacter jejuni, Salmonella spp., Shigella spp./EIEC, and Yersinia enterocolitica in fecal samples. Eur. J. Clin Microbiol Infect Dis 2015, 34 (3), 535–42. 10.1007/s10096-014-2257-x. [DOI] [PubMed] [Google Scholar]
- Kumar A.; et al. Quick PCR based diagnosis of typhoid using specific genetic markers. Biotechnol. Lett. 2010, 32 (5), 707–12. 10.1007/s10529-010-0211-2. [DOI] [PubMed] [Google Scholar]
- Khare R.; et al. Comparative evaluation of two commercial multiplex panels for detection of gastrointestinal pathogens by use of clinical stool specimens. J. Clin Microbiol 2014, 52 (10), 3667–73. 10.1128/JCM.01637-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson N. W.; Buchan B. W.; Ledeboer N. A. Comparison of the BD MAX enteric bacterial panel to routine culture methods for detection of Campylobacter, enterohemorrhagic Escherichia coli (O157), Salmonella, and Shigella isolates in preserved stool specimens. J. Clin Microbiol 2014, 52 (4), 1222–4. 10.1128/JCM.03099-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y.; et al. Mass spectrometry biotyper system identifies enteric bacterial pathogens directly from colonies grown on selective stool culture media. J. Clin Microbiol 2010, 48 (11), 3888–92. 10.1128/JCM.01290-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang S.; et al. Genetic relationships of phage types and single nucleotide polymorphism typing of Salmonella enterica Serovar Typhimurium. J. Clin Microbiol 2012, 50 (3), 727–34. 10.1128/JCM.01284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schork N. J.; Fallin D.; Lanchbury J. S. Single nucleotide polymorphisms and the future of genetic epidemiology. Clin Genet 2000, 58 (4), 250–64. 10.1034/j.1399-0004.2000.580402.x. [DOI] [PubMed] [Google Scholar]
- Collins A.; Lonjou C.; Morton N. E. Genetic epidemiology of single-nucleotide polymorphisms. Proc. Natl. Acad. Sci. U. S. A. 1999, 96 (26), 15173–7. 10.1073/pnas.96.26.15173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche G.; et al. Slc11a1 (Nramp1) impairs growth of Salmonella enterica serovar typhimurium in macrophages via stimulation of lipocalin-2 expression. J. Leukoc Biol. 2012, 92 (2), 353–9. 10.1189/jlb.1111554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecher B.; et al. Chronic Salmonella enterica serovar Typhimurium-induced colitis and cholangitis in streptomycin-pretreated Nramp1+/+ mice. Infect. Immun. 2006, 74 (9), 5047–57. 10.1128/IAI.00072-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunrath O.; Bumann D. Host resistance factor SLC11A1 restricts Salmonella growth through magnesium deprivation. Science 2019, 366 (6468), 995–999. 10.1126/science.aax7898. [DOI] [PubMed] [Google Scholar]
- McClelland M.; et al. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 2001, 413 (6858), 852–6. 10.1038/35101614. [DOI] [PubMed] [Google Scholar]
- Mian M. F.; et al. Humanized mice for Salmonella typhi infection: new tools for an old problem. Virulence 2011, 2 (3), 248–52. 10.4161/viru.2.3.16133. [DOI] [PubMed] [Google Scholar]
- Song J.; et al. A mouse model for the human pathogen Salmonella typhi. Cell Host Microbe 2010, 8 (4), 369–76. 10.1016/j.chom.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firoz Mian M.; et al. Humanized mice are susceptible to Salmonella typhi infection. Cell Mol. Immunol 2011, 8 (1), 83–7. 10.1038/cmi.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby S. J.; et al. Humanized nonobese diabetic-scid IL2rgammanull mice are susceptible to lethal Salmonella Typhi infection. Proc. Natl. Acad. Sci. U. S. A. 2010, 107 (35), 15589–94. 10.1073/pnas.1005566107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson C. A.; et al. Three-dimensional tissue assemblies: novel models for the study of Salmonella enterica serovar Typhimurium pathogenesis. Infect. Immun. 2001, 69 (11), 7106–20. 10.1128/IAI.69.11.7106-7120.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. G.; et al. Salmonella-infected crypt-derived intestinal organoid culture system for host-bacterial interactions. Physiol Rep 2014, 2 (9), e12147. 10.14814/phy2.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. S.; et al. A small intestinal organoid model of non-invasive enteric pathogen-epithelial cell interactions. Mucosal Immunol 2015, 8 (2), 352–61. 10.1038/mi.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farin H. F.; et al. Paneth cell extrusion and release of antimicrobial products is directly controlled by immune cell-derived IFN-gamma. J. Exp Med. 2014, 211 (7), 1393–405. 10.1084/jem.20130753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson K. P.; et al. Salmonella Typhi Colonization Provokes Extensive Transcriptional Changes Aimed at Evading Host Mucosal Immune Defense During Early Infection of Human Intestinal Tissue. EBioMedicine 2018, 31, 92–109. 10.1016/j.ebiom.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra K.; et al. GH18 family glycoside hydrolase Chitinase A of Salmonella enhances virulence by facilitating invasion and modulating host immune responses. PLoS Pathog 2022, 18 (4), e1010407 10.1371/journal.ppat.1010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prouty A. M.; et al. Bile-salt-mediated induction of antimicrobial and bile resistance in Salmonella typhimurium. Microbiology 2004, 150 (4), 775–783. 10.1099/mic.0.26769-0. [DOI] [PubMed] [Google Scholar]
- Richards A. F.; et al. Inhibition of invasive salmonella by orally administered IgA and IgG monoclonal antibodies. PLoS Negl Trop Dis 2020, 14 (3), e0007803 10.1371/journal.pntd.0007803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michetti P.; et al. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect. Immun. 1992, 60 (5), 1786–92. 10.1128/iai.60.5.1786-1792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted M. E.; et al. Enteric defensins: antibiotic peptide components of intestinal host defense. J. Cell Biol. 1992, 118 (4), 929–36. 10.1083/jcb.118.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matamouros S.; Miller S. Typhimurium strategies to resist killing by cationic antimicrobial peptides. Biochim. Biophys. Acta 2015, 1848 (11), 3021–3025. 10.1016/j.bbamem.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-del Portillo F.; Foster J. W.; Finlay B. B. Role of acid tolerance response genes in Salmonella typhimurium virulence. Infect. Immun. 1993, 61 (10), 4489–92. 10.1128/iai.61.10.4489-4492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suar M.; Ryan D. Small RNA in the acid tolerance response of Salmonella and their role in virulence. Virulence 2015, 6 (2), 105–6. 10.4161/21505594.2014.988543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohbata S.; Yokoyama H.; Yabuuchi E. Cytopathogenic effect of Salmonella typhi GIFU 10007 on M cells of murine ileal Peyer’s patches in ligated ileal loops: an ultrastructural study. Microbiol. Immunol. 1986, 30 (12), 1225–37. 10.1111/j.1348-0421.1986.tb03055.x. [DOI] [PubMed] [Google Scholar]
- Jones B. D.; Ghori N.; Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer’s patches. J. Exp Med. 1994, 180 (1), 15–23. 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Torres A.; et al. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature 1999, 401 (6755), 804–8. 10.1038/44593. [DOI] [PubMed] [Google Scholar]
- Haraga A.; Ohlson M. B.; Miller S. I. Salmonellae interplay with host cells. Nat. Rev. Microbiol 2008, 6 (1), 53–66. 10.1038/nrmicro1788. [DOI] [PubMed] [Google Scholar]
- Bader M. W.; et al. Regulation of Salmonella typhimurium virulence gene expression by cationic antimicrobial peptides. Mol. Microbiol. 2003, 50 (1), 219–30. 10.1046/j.1365-2958.2003.03675.x. [DOI] [PubMed] [Google Scholar]
- Miao E. A.; Freeman J. A.; Miller S. I. Transcription of the SsrAB regulon is repressed by alkaline pH and is independent of PhoPQ and magnesium concentration. J. Bacteriol. 2002, 184 (5), 1493–7. 10.1128/JB.184.5.1493-1497.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Torres A.; et al. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science 2000, 287 (5458), 1655–8. 10.1126/science.287.5458.1655. [DOI] [PubMed] [Google Scholar]
- Shea J. E.; et al. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc. Natl. Acad. Sci. U. S. A. 1996, 93 (6), 2593–7. 10.1073/pnas.93.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H.; et al. Identification of a pathogenicity island required for Salmonella survival in host cells. Proc. Natl. Acad. Sci. U. S. A. 1996, 93 (15), 7800–4. 10.1073/pnas.93.15.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpuche-Aranda C. M.; et al. Salmonella stimulate macrophage macropinocytosis and persist within spacious phagosomes. J. Exp Med. 1994, 179 (2), 601–8. 10.1084/jem.179.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpuche Aranda C. M.; et al. Salmonella typhimurium activates virulence gene transcription within acidified macrophage phagosomes. Proc. Natl. Acad. Sci. U. S. A. 1992, 89 (21), 10079–83. 10.1073/pnas.89.21.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. I.; Kukral A. M.; Mekalanos J. J. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc. Natl. Acad. Sci. U. S. A. 1989, 86 (13), 5054–8. 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C. H.; et al. A cross-sectional seroepidemiological survey of typhoid fever in Fiji. PLoS Negl Trop Dis 2017, 11 (7), e0005786 10.1371/journal.pntd.0005786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong M.; Yan H.; Li Y. Flagellin: a unique microbe-associated molecular pattern and a multi-faceted immunomodulator. Cell Mol. Immunol 2017, 14 (10), 862–864. 10.1038/cmi.2017.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhadad D.; et al. Flagellin Is Required for Host Cell Invasion and Normal Salmonella Pathogenicity Island 1 Expression by Salmonella enterica Serovar Paratyphi A. Infect. Immun. 2015, 83 (9), 3355–68. 10.1128/IAI.00468-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Q. T.; et al. The Salmonella enterica serotype Typhi Vi capsular antigen is expressed after the bacterium enters the ileal mucosa. Infect. Immun. 2010, 78 (1), 527–35. 10.1128/IAI.00972-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. L.; Jeza V. T.; Pan Q. Salmonella typhi: from a human pathogen to a vaccine vector. Cell Mol. Immunol 2008, 5 (2), 91–7. 10.1038/cmi.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos R. L.; et al. Pathogenesis of Salmonella-induced enteritis. Braz. J. Med. Biol. Res. 2003, 36 (1), 3–12. 10.1590/S0100-879X2003000100002. [DOI] [PubMed] [Google Scholar]
- Schmidt H.; Hensel M. Pathogenicity islands in bacterial pathogenesis. Clin Microbiol Rev. 2004, 17 (1), 14–56. 10.1128/CMR.17.1.14-56.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyas B.; Tsai C. N.; Coombes B. K. Evolution of Salmonella-Host Cell Interactions through a Dynamic Bacterial Genome. Front Cell Infect Microbiol 2017, 7, 428. 10.3389/fcimb.2017.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng R. A.; Eade C. R.; Wiedmann M. Embracing Diversity: Differences in Virulence Mechanisms, Disease Severity, and Host Adaptations Contribute to the Success of Nontyphoidal Salmonella as a Foodborne Pathogen. Front. Microbiol. 2019, 10, 1368. 10.3389/fmicb.2019.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siriken B. [Salmonella pathogenicity islands]. Mikrobiyol Bul 2013, 47 (1), 181–8. 10.5578/mb.4138. [DOI] [PubMed] [Google Scholar]
- Sabbagh S. C.; et al. So similar, yet so different: uncovering distinctive features in the genomes of Salmonella enterica serovars Typhimurium and Typhi. FEMS Microbiol Lett. 2010, 305 (1), 1–13. 10.1111/j.1574-6968.2010.01904.x. [DOI] [PubMed] [Google Scholar]
- Shah D. H.; et al. Identification of Salmonella gallinarum virulence genes in a chicken infection model using PCR-based signature-tagged mutagenesis. Microbiology 2005, 151 (12), 3957–3968. 10.1099/mic.0.28126-0. [DOI] [PubMed] [Google Scholar]
- Galyov E. E.; et al. A secreted effector protein of Salmonella dublin is translocated into eukaryotic cells and mediates inflammation and fluid secretion in infected ileal mucosa. Mol. Microbiol. 1997, 25 (5), 903–12. 10.1111/j.1365-2958.1997.mmi525.x. [DOI] [PubMed] [Google Scholar]
- Johnson R.; Mylona E.; Frankel G. Typhoidal Salmonella: Distinctive virulence factors and pathogenesis. Cell Microbiol 2018, 20 (9), e12939 10.1111/cmi.12939. [DOI] [PubMed] [Google Scholar]
- Collazo C. M.; Galan J. E. The invasion-associated type-III protein secretion system in Salmonella--a review. Gene 1997, 192 (1), 51–9. 10.1016/S0378-1119(96)00825-6. [DOI] [PubMed] [Google Scholar]
- Galan J. E.; Curtiss R. 3rd Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc. Natl. Acad. Sci. U. S. A. 1989, 86 (16), 6383–7. 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D.; et al. A Salmonella inositol polyphosphatase acts in conjunction with other bacterial effectors to promote host cell actin cytoskeleton rearrangements and bacterial internalization. Mol. Microbiol. 2001, 39 (2), 248–59. 10.1046/j.1365-2958.2001.02230.x. [DOI] [PubMed] [Google Scholar]
- Piscatelli H. L.; Li M.; Zhou D. Dual 4- and 5-phosphatase activities regulate SopB-dependent phosphoinositide dynamics to promote bacterial entry. Cellular Microbiology 2016, 18 (5), 705–719. 10.1111/cmi.12542. [DOI] [PubMed] [Google Scholar]
- Mallo G. V.; et al. SopB promotes phosphatidylinositol 3-phosphate formation on Salmonella vacuoles by recruiting Rab5 and Vps34. J. Cell Biol. 2008, 182 (4), 741–52. 10.1083/jcb.200804131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee R.; et al. Deceiving The Big Eaters: Salmonella Typhimurium SopB subverts host cell Xenophagy in macrophages via dual mechanisms. Microbes and Infection 2023, 25, 105128. 10.1016/j.micinf.2023.105128. [DOI] [PubMed] [Google Scholar]
- Patel J. C.; Galan J. E. Differential activation and function of Rho GTPases during Salmonella-host cell interactions. J. Cell Biol. 2006, 175 (3), 453–63. 10.1083/jcb.200605144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knodler L. A.; Finlay B. B.; Steele-Mortimer O. The Salmonella effector protein SopB protects epithelial cells from apoptosis by sustained activation of Akt. J. Biol. Chem. 2005, 280 (10), 9058–64. 10.1074/jbc.M412588200. [DOI] [PubMed] [Google Scholar]
- Garcia-Gil A.; et al. SopB activates the Akt-YAP pathway to promote Salmonella survival within B cells. Virulence 2018, 9 (1), 1390–1402. 10.1080/21505594.2018.1509664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stender S.; et al. Identification of SopE2 from Salmonella typhimurium, a conserved guanine nucleotide exchange factor for Cdc42 of the host cell. Mol. Microbiol. 2000, 36 (6), 1206–21. 10.1046/j.1365-2958.2000.01933.x. [DOI] [PubMed] [Google Scholar]
- Friebel A.; et al. SopE and SopE2 from Salmonella typhimurium activate different sets of RhoGTPases of the host cell. J. Biol. Chem. 2001, 276 (36), 34035–40. 10.1074/jbc.M100609200. [DOI] [PubMed] [Google Scholar]
- Hardt W. D.; et al. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell 1998, 93 (5), 815–26. 10.1016/S0092-8674(00)81442-7. [DOI] [PubMed] [Google Scholar]
- Hayward R. D.; Koronakis V. Direct nucleation and bundling of actin by the SipC protein of invasive Salmonella. EMBO J. 1999, 18 (18), 4926–34. 10.1093/emboj/18.18.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collazo C. M.; Galan J. E. The invasion-associated type III system of Salmonella typhimurium directs the translocation of Sip proteins into the host cell. Mol. Microbiol. 1997, 24 (4), 747–56. 10.1046/j.1365-2958.1997.3781740.x. [DOI] [PubMed] [Google Scholar]
- Kaniga K.; et al. Homologs of the Shigella IpaB and IpaC invasins are required for Salmonella typhimurium entry into cultured epithelial cells. J. Bacteriol. 1995, 177 (14), 3965–71. 10.1128/jb.177.14.3965-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhie E. J.; Hayward R. D.; Koronakis V. Control of Actin Turnover by a Salmonella Invasion Protein. Mol. Cell 2004, 13 (4), 497–510. 10.1016/S1097-2765(04)00053-X. [DOI] [PubMed] [Google Scholar]
- McGhie E. J.; Hayward R. D.; Koronakis V. Cooperation between actin-binding proteins of invasive Salmonella: SipA potentiates SipC nucleation and bundling of actin. EMBO Journal 2001, 20 (9), 2131–2139. 10.1093/emboj/20.9.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepson M. A.; Kenny B.; Leard A. D. Role of sipA in the early stages of Salmonella typhimurium entry into epithelial cells. Cell Microbiol 2001, 3 (6), 417–26. 10.1046/j.1462-5822.2001.00124.x. [DOI] [PubMed] [Google Scholar]
- Fattinger S. A.; et al. Salmonella Typhimurium discreet-invasion of the murine gut absorptive epithelium. PLoS Pathog 2020, 16 (5), e1008503 10.1371/journal.ppat.1008503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh D.; et al. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. U. S. A. 1999, 96 (5), 2396–401. 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher D.; et al. Salmonella virulence factor SipB induces activation and release of IL-18 in human dendritic cells. J. Leukoc Biol. 2002, 72 (4), 743–51. 10.1189/jlb.72.4.743. [DOI] [PubMed] [Google Scholar]
- Obregon C.; et al. Human alveolar macrophages infected by virulent bacteria expressing SipB are a major source of active interleukin-18. Infect. Immun. 2003, 71 (8), 4382–8. 10.1128/IAI.71.8.4382-4388.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eswarappa S. M.; et al. Differentially evolved genes of Salmonella pathogenicity islands: insights into the mechanism of host specificity in Salmonella. PLoS One 2008, 3 (12), e3829 10.1371/journal.pone.0003829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altier C. Genetic and environmental control of salmonella invasion. J. Microbiol 2005, 43, 85–92. [PubMed] [Google Scholar]
- Altier C.; et al. Characterization of two novel regulatory genes affecting Salmonella invasion gene expression. Mol. Microbiol. 2000, 35 (3), 635–46. 10.1046/j.1365-2958.2000.01734.x. [DOI] [PubMed] [Google Scholar]
- Prouty A. M.; Gunn J. S. Salmonella enterica serovar typhimurium invasion is repressed in the presence of bile. Infect. Immun. 2000, 68 (12), 6763–9. 10.1128/IAI.68.12.6763-6769.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellermeier J. R.; Slauch J. M. Adaptation to the host environment: regulation of the SPI1 type III secretion system in Salmonella enterica serovar Typhimurium. Curr. Opin Microbiol 2007, 10 (1), 24–9. 10.1016/j.mib.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Johnson R.; et al. Comparison of Salmonella enterica Serovars Typhi and Typhimurium Reveals Typhoidal Serovar-Specific Responses to Bile. Infect. Immun. 2018, 86, e00490-17 10.1128/IAI.00490-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhadad D.; et al. Feverlike Temperature is a Virulence Regulatory Cue Controlling the Motility and Host Cell Entry of Typhoidal Salmonella. J. Infect Dis 2015, 212 (1), 147–56. 10.1093/infdis/jiu663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaus T.; et al. SseBCD proteins are secreted by the type III secretion system of Salmonella pathogenicity island 2 and function as a translocon. J. Bacteriol. 2001, 183 (20), 6036–45. 10.1128/JB.183.20.6036-6045.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X. J.; et al. pH sensing by intracellular Salmonella induces effector translocation. Science 2010, 328 (5981), 1040–3. 10.1126/science.1189000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee R.; et al. Enteropathogens: Tuning Their Gene Expression for Hassle-Free Survival. Front. Microbiol. 2019, 9, 3303. 10.3389/fmicb.2018.03303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRock D. L.; Chaudhary A.; Miller S. I. Salmonellae interactions with host processes. Nat. Rev. Microbiol 2015, 13 (4), 191–205. 10.1038/nrmicro3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueira R.; Holden D. W. Functions of the Salmonella pathogenicity island 2 (SPI-2) type III secretion system effectors. Microbiology 2012, 158 (5), 1147–1161. 10.1099/mic.0.058115-0. [DOI] [PubMed] [Google Scholar]
- Galan J. E. Salmonella interactions with host cells: type III secretion at work. Annu. Rev. Cell Dev Biol. 2001, 17, 53–86. 10.1146/annurev.cellbio.17.1.53. [DOI] [PubMed] [Google Scholar]
- Steele-Mortimer O.; et al. Activation of Akt/protein kinase B in epithelial cells by the Salmonella typhimurium effector sigD. J. Biol. Chem. 2000, 275 (48), 37718–24. 10.1074/jbc.M008187200. [DOI] [PubMed] [Google Scholar]
- Malik-Kale P.; et al. Salmonella - at home in the host cell. Front. Microbiol. 2011, 2, 125. 10.3389/fmicb.2011.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terebiznik M. R.; et al. Elimination of host cell PtdIns(4,5)P(2) by bacterial SigD promotes membrane fission during invasion by Salmonella. Nat. Cell Biol. 2002, 4 (10), 766–73. 10.1038/ncb854. [DOI] [PubMed] [Google Scholar]
- McGhie E. J.; et al. Salmonella takes control: effector-driven manipulation of the host. Curr. Opin Microbiol 2009, 12 (1), 117–24. 10.1016/j.mib.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl D.; et al. SopB-Mediated Recruitment of SNX18 Facilitates Salmonella Typhimurium Internalization by the Host Cell. Front. Cell. Infect. Microbiol. 2017, 7, 257. 10.3389/fcimb.2017.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. C.; et al. Interaction of the Salmonella-containing vacuole with the endocytic recycling system. J. Biol. Chem. 2005, 280 (26), 24634–41. 10.1074/jbc.M500358200. [DOI] [PubMed] [Google Scholar]
- Steele-Mortimer O.; et al. Biogenesis of Salmonella typhimurium-containing vacuoles in epithelial cells involves interactions with the early endocytic pathway. Cell Microbiol 1999, 1 (1), 33–49. 10.1046/j.1462-5822.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- Meresse S.; et al. Controlling the maturation of pathogen-containing vacuoles: a matter of life and death. Nat. Cell Biol. 1999, 1 (7), E183–8. 10.1038/15620. [DOI] [PubMed] [Google Scholar]
- Meresse S.; et al. The rab7 GTPase controls the maturation of Salmonella typhimurium-containing vacuoles in HeLa cells. EMBO J. 1999, 18 (16), 4394–403. 10.1093/emboj/18.16.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-del Portillo F.; Finlay B. B. Targeting of Salmonella typhimurium to vesicles containing lysosomal membrane glycoproteins bypasses compartments with mannose 6-phosphate receptors. J. Cell Biol. 1995, 129 (1), 81–97. 10.1083/jcb.129.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drecktrah D.; et al. Salmonella trafficking is defined by continuous dynamic interactions with the endolysosomal system. Traffic 2007, 8 (3), 212–25. 10.1111/j.1600-0854.2006.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuzon C. R.; et al. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J. 2000, 19 (13), 3235–49. 10.1093/emboj/19.13.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostowy S.; et al. p62 and NDP52 proteins target intracytosolic Shigella and Listeria to different autophagy pathways. J. Biol. Chem. 2011, 286 (30), 26987–95. 10.1074/jbc.M111.223610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankiv S.; et al. FYCO1 is a Rab7 effector that binds to LC3 and PI3P to mediate microtubule plus end-directed vesicle transport. J. Cell Biol. 2010, 188 (2), 253–69. 10.1083/jcb.200907015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R. E.; et al. Salmonella impairs RILP recruitment to Rab7 during maturation of invasion vacuoles. Mol. Biol. Cell 2004, 15 (7), 3146–54. 10.1091/mbc.e04-02-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumell J. H.; et al. Characterization of Salmonella-Induced Filaments (Sifs) Reveals a Delayed Interaction Between Salmonella-Containing Vacuoles and Late Endocytic Compartments. Traffic 2001, 2 (9), 643–653. 10.1034/j.1600-0854.2001.20907.x. [DOI] [PubMed] [Google Scholar]
- Knodler L. A.; Steele-Mortimer O. The Salmonella effector PipB2 affects late endosome/lysosome distribution to mediate Sif extension. Mol. Biol. Cell 2005, 16 (9), 4108–23. 10.1091/mbc.e05-04-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGourty K.; et al. Salmonella inhibits retrograde trafficking of mannose-6-phosphate receptors and lysosome function. Science 2012, 338 (6109), 963–7. 10.1126/science.1227037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordens I.; et al. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr. Biol. 2001, 11 (21), 1680–1685. 10.1016/S0960-9822(01)00531-0. [DOI] [PubMed] [Google Scholar]
- Yu X. J.; Liu M.; Holden D.W. Salmonella Effectors SseF and SseG Interact with Mammalian Protein ACBD3 (GCP60) To Anchor Salmonella-Containing Vacuoles at the Golgi Network. mBio 2016, 7 (4), e00474-16 10.1128/mBio.00474-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham C. L.; et al. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J. Biol. Chem. 2006, 281 (16), 11374–83. 10.1074/jbc.M509157200. [DOI] [PubMed] [Google Scholar]
- Zhao W.; et al. The Salmonella effector protein SifA plays a dual role in virulence. Sci. Rep 2015, 5, 12979. 10.1038/srep12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajashekar R.; et al. Dynamic remodeling of the endosomal system during formation of Salmonella-induced filaments by intracellular Salmonella enterica. Traffic 2008, 9 (12), 2100–16. 10.1111/j.1600-0854.2008.00821.x. [DOI] [PubMed] [Google Scholar]
- Drecktrah D.; et al. Dynamic behavior of Salmonella-induced membrane tubules in epithelial cells. Traffic 2008, 9 (12), 2117–29. 10.1111/j.1600-0854.2008.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liss V.; et al. Salmonella enterica Remodels the Host Cell Endosomal System for Efficient Intravacuolar Nutrition. Cell Host Microbe 2017, 21 (3), 390–402. 10.1016/j.chom.2017.02.005. [DOI] [PubMed] [Google Scholar]
- Popp J.; et al. Role of host cell-derived amino acids in nutrition of intracellular Salmonella enterica. Infect. Immun. 2015, 83 (12), 4466–75. 10.1128/IAI.00624-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eswarappa S. M.; et al. Division of the Salmonella-containing vacuole and depletion of acidic lysosomes in Salmonella-infected host cells are novel strategies of Salmonella enterica to avoid lysosomes. Infect. Immun. 2010, 78 (1), 68–79. 10.1128/IAI.00668-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knodler L. A.; Nair V.; Steele-Mortimer O. Quantitative assessment of cytosolic Salmonella in epithelial cells. PLoS One 2014, 9 (1), e84681 10.1371/journal.pone.0084681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik-Kale P.; Winfree S.; Steele-Mortimer O. The bimodal lifestyle of intracellular Salmonella in epithelial cells: replication in the cytosol obscures defects in vacuolar replication. PLoS One 2012, 7 (6), e38732 10.1371/journal.pone.0038732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin A. J.; et al. Recognition of bacteria in the cytosol of Mammalian cells by the ubiquitin system. Curr. Biol. 2004, 14 (9), 806–11. 10.1016/j.cub.2004.04.033. [DOI] [PubMed] [Google Scholar]
- Knodler L. A.; et al. Dissemination of invasive Salmonella via bacterial-induced extrusion of mucosal epithelia. Proc. Natl. Acad. Sci. U. S. A. 2010, 107 (41), 17733–8. 10.1073/pnas.1006098107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P. K.; et al. Salmonella SipA mimics a cognate SNARE for host Syntaxin8 to promote fusion with early endosomes. J. Cell Biol. 2018, 217 (12), 4199–4214. 10.1083/jcb.201802155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenin V.; et al. Dynamic Growth and Shrinkage of the Salmonella-Containing Vacuole Determines the Intracellular Pathogen Niche. Cell Rep 2019, 29 (12), 3958–3973.e7. 10.1016/j.celrep.2019.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee R.; et al. Syntaxin 3 SPI-2 dependent crosstalk facilitates the division of Salmonella containing vacuole. Traffic 2023, 24 (7), 270–283. 10.1111/tra.12887. [DOI] [PubMed] [Google Scholar]
- Madan R.; et al. Salmonella acquires lysosome-associated membrane protein 1 (LAMP1) on phagosomes from Golgi via SipC protein-mediated recruitment of host Syntaxin6. J. Biol. Chem. 2012, 287 (8), 5574–87. 10.1074/jbc.M111.286120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston T. L.; et al. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 2012, 482 (7385), 414–8. 10.1038/nature10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee R.; et al. Deceiving The Big Eaters: Salmonella Typhimurium SopB subverts host cell Xenophagy in macrophages via dual mechanisms. Microbes Infect 2023, 25, 105128. 10.1016/j.micinf.2023.105128. [DOI] [PubMed] [Google Scholar]
- Gogoi M.; Shreenivas M. M.; Chakravortty D. Hoodwinking the Big-Eater to Prosper: The Salmonella-Macrophage Paradigm. J. Innate Immun 2019, 11 (3), 289–299. 10.1159/000490953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan A.; et al. Innate immune activation of CD4 T cells in salmonella-infected mice is dependent on IL-18. J. Immunol 2007, 178 (10), 6342–9. 10.4049/jimmunol.178.10.6342. [DOI] [PubMed] [Google Scholar]
- Chavez-Arroyo A.; Portnoy D. A. Why is Listeria monocytogenes such a potent inducer of CD8+ T-cells?. Cell. Microbiol. 2020, 22 (4), e13175 10.1111/cmi.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana A.; et al. RIPK3 and Caspase-1/11 Are Necessary for Optimal Antigen-Specific CD8 T Cell Response Elicited by Genetically Modified Listeria monocytogenes. Front. Immunol. 2020, 11, 536. 10.3389/fimmu.2020.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. J.; Dunmire S.; McSorley S. J. MHC class-I-restricted CD8 T cells play a protective role during primary Salmonella infection. Immunol. Lett. 2012, 148 (2), 138–43. 10.1016/j.imlet.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Medina M.; et al. Salmonella impairs CD8 T cell response through PD-1: PD-L axis. Immunobiology 2015, 220 (12), 1369–80. 10.1016/j.imbio.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Achouri S.; et al. The frequency and duration of Salmonella-macrophage adhesion events determines infection efficiency. Philos. Trans R Soc. Lond B Biol. Sci. 2015, 370 (1661), 20140033. 10.1098/rstb.2014.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathrop S. K.; et al. Replication of Salmonella enterica Serovar Typhimurium in Human Monocyte-Derived Macrophages. Infect. Immun. 2015, 83 (7), 2661–71. 10.1128/IAI.00033-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis M. J.; et al. A macrophage-based screen identifies antibacterial compounds selective for intracellular Salmonella Typhimurium. Nat. Commun. 2019, 10, 197. 10.1038/s41467-018-08190-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swart A. L.; Hensel M. Interactions of Salmonella enterica with dendritic cells. Virulence 2012, 3 (7), 660–7. 10.4161/viru.22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno S. M.; et al. The capacity of Salmonella to survive inside dendritic cells and prevent antigen presentation to T cells is host specific. Immunology 2008, 124 (4), 522–33. 10.1111/j.1365-2567.2008.02805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales-Reyes R.; et al. Survival of Salmonella enterica serovar Typhimurium within late endosomal-lysosomal compartments of B lymphocytes is associated with the inability to use the vacuolar alternative major histocompatibility complex class I antigen-processing pathway. Infect. Immun. 2005, 73 (7), 3937–44. 10.1128/IAI.73.7.3937-3944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souwer Y.; et al. Selective infection of antigen-specific B lymphocytes by Salmonella mediates bacterial survival and systemic spreading of infection. PLoS One 2012, 7 (11), e50667 10.1371/journal.pone.0050667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Medina M.; et al. Salmonella modulates B cell biology to evade CD8(+) T cell-mediated immune responses. Front. Immunol. 2014, 5, 586. 10.3389/fimmu.2014.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.; et al. Single-Cell and Time-Resolved Profiling of Intracellular Salmonella Metabolism in Primary Human Cells. Anal. Chem. 2019, 91 (12), 7729–7737. 10.1021/acs.analchem.9b01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr T.; et al. The phorbol 12-myristate-13-acetate differentiation protocol is critical to the interaction of THP-1 macrophages with Salmonella Typhimurium. PLoS One 2018, 13 (3), e0193601 10.1371/journal.pone.0193601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V.; Schwerk P.; Tedin K. Rapid Isolation of intact Salmonella-containing vacuoles using paramagnetic nanoparticles. Gut. Pathog. 2018, 10, 33. 10.1186/s13099-018-0256-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden J.; et al. Direct measurement of oxidative and nitrosative stress dynamics in Salmonella inside macrophages. Proc. Natl. Acad. Sci. U. S. A. 2015, 112 (2), 560–5. 10.1073/pnas.1414569112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan A.; Schnare M.; Chakravortty D. Of Men Not Mice: Bactericidal/Permeability-Increasing Protein Expressed in Human Macrophages Acts as a Phagocytic Receptor and Modulates Entry and Replication of Gram-Negative Bacteria. Front. Immunol. 2016, 7, 455. 10.3389/fimmu.2016.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murret-Labarthe C.; et al. New Roles for Two-Component System Response Regulators of Salmonella enterica Serovar Typhi during Host Cell Interactions. Microorganisms 2020, 8 (5), 722. 10.3390/microorganisms8050722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spano S. Host restriction in Salmonella: insights from Rab GTPases. Cell Microbiol 2014, 16 (9), 1321–8. 10.1111/cmi.12327. [DOI] [PubMed] [Google Scholar]
- Spano S.; Galan J. E. A Rab32-dependent pathway contributes to Salmonella typhi host restriction. Science 2012, 338 (6109), 960–3. 10.1126/science.1229224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtel R.; et al. The protease GtgE from Salmonella exclusively targets inactive Rab GTPases. Nat. Commun. 2018, 9, 44. 10.1038/s41467-017-02110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan J. E. Typhoid toxin provides a window into typhoid fever and the biology of Salmonella Typhi. Proc. Natl. Acad. Sci. U. S. A. 2016, 113 (23), 6338–44. 10.1073/pnas.1606335113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spano S.; et al. A Bacterial Pathogen Targets a Host Rab-Family GTPase Defense Pathway with a GAP. Cell Host Microbe 2016, 19 (2), 216–26. 10.1016/j.chom.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Angelica E. C. The building BLOC(k)s of lysosomes and related organelles. Curr. Opin Cell Biol. 2004, 16 (4), 458–64. 10.1016/j.ceb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Chen M.; et al. Itaconate is an effector of a Rab GTPase cell-autonomous host defense pathway against Salmonella. Science 2020, 369 (6502), 450–455. 10.1126/science.aaz1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. H.; et al. Hyperexpression of type III secretion system of Salmonella Typhi linked to a higher cytotoxic effect to monocyte-derived macrophages by activating inflammasome. Microb Pathog 2020, 146, 104222. 10.1016/j.micpath.2020.104222. [DOI] [PubMed] [Google Scholar]
- Gallois A.; et al. Salmonella pathogenicity island 2-encoded type III secretion system mediates exclusion of NADPH oxidase assembly from the phagosomal membrane. J. Immunol 2001, 166 (9), 5741–8. 10.4049/jimmunol.166.9.5741. [DOI] [PubMed] [Google Scholar]
- Forest C. G.; et al. Intracellular survival of Salmonella enterica serovar Typhi in human macrophages is independent of Salmonella pathogenicity island (SPI)-2. Microbiology 2010, 156 (12), 3689–3698. 10.1099/mic.0.041624-0. [DOI] [PubMed] [Google Scholar]
- Karlinsey J. E.; et al. Genome-wide Analysis of Salmonella enterica serovar Typhi in Humanized Mice Reveals Key Virulence Features. Cell Host Microbe 2019, 26 (3), 426–434.e6. 10.1016/j.chom.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega A. P.; et al. Lose to win: marT pseudogenization in Salmonella enterica serovar Typhi contributed to the surV-dependent survival to H2O2, and inside human macrophage-like cells. Infect Genet Evol 2016, 45, 111–121. 10.1016/j.meegid.2016.08.029. [DOI] [PubMed] [Google Scholar]
- Wang F.; et al. Type IVB piliated Salmonella typhi enhance IL-6 and NF-kappaB production in human monocytic THP-1 cells through activation of protein kinase C. Immunobiology 2005, 210 (5), 283–93. 10.1016/j.imbio.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Pan Q.; et al. Aptamers that preferentially bind type IVB pili and inhibit human monocytic-cell invasion by Salmonella enterica serovar typhi. Antimicrob. Agents Chemother. 2005, 49 (10), 4052–60. 10.1128/AAC.49.10.4052-4060.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spano S.; Ugalde J. E.; Galan J. E. Delivery of a Salmonella Typhi exotoxin from a host intracellular compartment. Cell Host Microbe 2008, 3 (1), 30–8. 10.1016/j.chom.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Fowler C. C.; Galan J. E. Decoding a Salmonella Typhi Regulatory Network that Controls Typhoid Toxin Expression within Human Cells. Cell Host Microbe 2018, 23 (1), 65–76.e6. 10.1016/j.chom.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. J.; Song J.; Galan J. E. Receptor-Mediated Sorting of Typhoid Toxin during Its Export from Salmonella Typhi-Infected Cells. Cell Host Microbe 2016, 20 (5), 682–689. 10.1016/j.chom.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibler A. E. M.; et al. Typhoid toxin exhausts the RPA response to DNA replication stress driving senescence and Salmonella infection. Nat. Commun. 2019, 10, 4040. 10.1038/s41467-019-12064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K.; et al. Cytotoxic mechanism of cytolethal distending toxin in nontyphoidal Salmonella serovar (Salmonella Javiana) during macrophage infection. DNA Cell Biol. 2015, 34 (2), 113–24. 10.1089/dna.2014.2602. [DOI] [PubMed] [Google Scholar]
- Lee S.; et al. Salmonella Typhoid Toxin PltB Subunit and Its Non-typhoidal Salmonella Ortholog Confer Differential Host Adaptation and Virulence. Cell Host Microbe 2020, 27 (6), 937–949.e6. 10.1016/j.chom.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucher S. P.; et al. Transcriptome of Salmonella enterica serovar Typhi within macrophages revealed through the selective capture of transcribed sequences. Proc. Natl. Acad. Sci. U. S. A. 2006, 103 (6), 1906–11. 10.1073/pnas.0509183103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh S. C.; et al. Selection of Salmonella enterica serovar Typhi genes involved during interaction with human macrophages by screening of a transposon mutant library. PLoS One 2012, 7 (5), e36643 10.1371/journal.pone.0036643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M. S.; et al. Involvement of Salmonella enterica serovar Typhi RpoS in resistance to NO-mediated host defense against serovar Typhi infection. Microb Pathog 2006, 40 (3), 116–25. 10.1016/j.micpath.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Wang M.; et al. SufC may promote the survival of Salmonella enterica serovar Typhi in macrophages. Microb Pathog 2015, 85, 40–3. 10.1016/j.micpath.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Hahn M. M.; Gunn J.S. Salmonella Extracellular Polymeric Substances Modulate Innate Phagocyte Activity and Enhance Tolerance of Biofilm-Associated Bacteria to Oxidative Stress. Microorganisms 2020, 8 (2), 253. 10.3390/microorganisms8020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; et al. OxyR positively and directly regulates Vi polysaccharide capsular antigen in Salmonella enterica serovar Typhi. Microb Pathog 2018, 124, 191–197. 10.1016/j.micpath.2018.08.050. [DOI] [PubMed] [Google Scholar]
- Christman M. F.; Storz G.; Ames B. N. OxyR, a positive regulator of hydrogen peroxide-inducible genes in Escherichia coli and Salmonella typhimurium, is homologous to a family of bacterial regulatory proteins. Proc. Natl. Acad. Sci. U. S. A. 1989, 86 (10), 3484–8. 10.1073/pnas.86.10.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. P.; et al. The Vi capsular polysaccharide prevents complement receptor 3-mediated clearance of Salmonella enterica serotype Typhi. Infect. Immun. 2011, 79 (2), 830–7. 10.1128/IAI.00961-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffatellu M.; et al. The Vi capsular antigen of Salmonella enterica serotype Typhi reduces Toll-like receptor-dependent interleukin-8 expression in the intestinal mucosa. Infect. Immun. 2005, 73 (6), 3367–74. 10.1128/IAI.73.6.3367-3374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangdi T.; et al. The Vi capsular polysaccharide enables Salmonella enterica serovar typhi to evade microbe-guided neutrophil chemotaxis. PLoS Pathog 2014, 10 (8), e1004306 10.1371/journal.ppat.1004306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufresne K.; Saulnier-Bellemare J.; Daigle F. Functional Analysis of the Chaperone-Usher Fimbrial Gene Clusters of Salmonella enterica serovar Typhi. Front. Cell. Infect. Microbiol. 2018, 8, 26. 10.3389/fcimb.2018.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyoshi H.; et al. Mechanisms to Evade the Phagocyte Respiratory Burst Arose by Convergent Evolution in Typhoidal Salmonella Serovars. Cell Rep 2018, 22 (7), 1787–1797. 10.1016/j.celrep.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theeya N.; et al. An inducible and secreted eukaryote-like serine/threonine kinase of Salmonella enterica serovar Typhi promotes intracellular survival and pathogenesis. Infect. Immun. 2015, 83 (2), 522–33. 10.1128/IAI.02521-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong F.; et al. The malS-5′UTR weakens the ability of Salmonella enterica serovar Typhi to survive in macrophages by increasing intracellular ATP levels. Microb Pathog 2018, 115, 321–331. 10.1016/j.micpath.2017.12.072. [DOI] [PubMed] [Google Scholar]
- He P.; et al. Salmonella enterica serovar Typhi plasmid pR ST98 enhances intracellular bacterial growth and S. typhi-induced macrophage cell death by suppressing autophagy. Braz J. Infect Dis 2012, 16 (3), 262–6. 10.1590/S1413-86702012000300008. [DOI] [PubMed] [Google Scholar]
- Hajra D.; et al. Salmonella Typhimurium U32 peptidase, YdcP, promotes bacterial survival by conferring protection against in vitro and in vivo oxidative stress. Microb. Pathog. 2022, 173, 105862. 10.1016/j.micpath.2022.105862. [DOI] [PubMed] [Google Scholar]
- Roy Chowdhury A.; et al. Salmonella Typhimurium outer membrane protein A (OmpA) renders protection from nitrosative stress of macrophages by maintaining the stability of bacterial outer membrane. PLoS Pathog 2022, 18 (8), e1010708 10.1371/journal.ppat.1010708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee R.; et al. Salmonella Typhimurium PgtE is an essential arsenal to defend against the host resident antimicrobial peptides. Microbiol Res. 2023, 271, 127351. 10.1016/j.micres.2023.127351. [DOI] [PubMed] [Google Scholar]
- van der Heijden J.; et al. Salmonella Rapidly Regulates Membrane Permeability To Survive Oxidative Stress. mBio 2016, 7 (4), e01238-16 10.1128/mBio.01238-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson C.; Ingman M.; Jo Wick M. Elevated neutrophil, macrophage and dendritic cell numbers characterize immune cell populations in mice chronically infected with Salmonella. Microb Pathog 2006, 41 (2–3), 49–58. 10.1016/j.micpath.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Gogoi M.; et al. Salmonella escapes antigen presentation through K63 ubiquitination mediated endosomal proteolysis of MHC II via modulation of endosomal acidification in dendritic cells. Pathog. Dis. 2018, 76 (2), ftx125. 10.1093/femspd/ftx125. [DOI] [PubMed] [Google Scholar]
- Gogoi M.; et al. Salmonella escapes adaptive immune response via SIRT2 mediated modulation of innate immune response in dendritic cells. PLoS Pathog 2018, 14 (11), e1007437 10.1371/journal.ppat.1007437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez-Brito M.; et al. Cell-free culture supernatant of Bifidobacterium breve CNCM I-4035 decreases pro-inflammatory cytokines in human dendritic cells challenged with Salmonella typhi through TLR activation. PLoS One 2013, 8 (3), e59370 10.1371/journal.pone.0059370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won G.; et al. A Salmonella Typhi ghost induced by the E gene of phage phiX174 stimulates dendritic cells and efficiently activates the adaptive immune response. J. Vet Sci. 2018, 19 (4), 536–542. 10.4142/jvs.2018.19.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L.; et al. Salmonella enterica serovar Typhi plasmid impairs dendritic cell responses to infection. Curr. Microbiol. 2012, 65 (2), 133–40. 10.1007/s00284-012-0136-1. [DOI] [PubMed] [Google Scholar]
- Wei L.; et al. Suppression of dendritic cell and T-cell activation by the pRST(9)(8) Salmonella plasmid. Mol. Med. Rep 2015, 11 (3), 2306–14. 10.3892/mmr.2014.2919. [DOI] [PubMed] [Google Scholar]
- Salerno-Goncalves R.; Sztein M. B. Priming of Salmonella enterica serovar typhi-specific CD8(+) T cells by suicide dendritic cell cross-presentation in humans. PLoS One 2009, 4 (6), e5879 10.1371/journal.pone.0005879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atif S. M.; et al. Salmonella enterica serovar Typhi impairs CD4 T cell responses by reducing antigen availability. Infect. Immun. 2014, 82 (6), 2247–54. 10.1128/IAI.00020-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan C.; et al. Interleukin (IL)-12 and IL-23 are key cytokines for immunity against Salmonella in humans. J. Infect Dis 2004, 190 (10), 1755–7. 10.1086/425021. [DOI] [PubMed] [Google Scholar]
- Crum N. F. Current trends in typhoid Fever. Curr. Gastroenterol Rep 2003, 5 (4), 279–86. 10.1007/s11894-003-0064-0. [DOI] [PubMed] [Google Scholar]
- Sejvar J.; et al. Neurologic manifestations associated with an outbreak of typhoid fever, Malawi--Mozambique, 2009: an epidemiologic investigation. PLoS One 2012, 7 (12), e46099 10.1371/journal.pone.0046099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisi S.; et al. Membrane and actin dynamics interplay at lamellipodia leading edge. Curr. Opin Cell Biol. 2013, 25 (5), 565–73. 10.1016/j.ceb.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Chen Z.; et al. Structure and control of the actin regulatory WAVE complex. Nature 2010, 468 (7323), 533–8. 10.1038/nature09623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. A.; et al. Active Arf6 recruits ARNO/cytohesin GEFs to the PM by binding their PH domains. Mol. Biol. Cell 2007, 18 (6), 2244–53. 10.1091/mbc.e06-11-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänisch J.; et al. Molecular dissection of Salmonella-induced membrane ruffling versus invasion. Cell Microbiol 2010, 12 (1), 84–98. 10.1111/j.1462-5822.2009.01380.x. [DOI] [PubMed] [Google Scholar]
- Humphreys D.; et al. Arf6 coordinates actin assembly through the WAVE complex, a mechanism usurped by Salmonella to invade host cells. Proc. Natl. Acad. Sci. U. S. A. 2013, 110 (42), 16880–5. 10.1073/pnas.1311680110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys D.; et al. Salmonella virulence effector SopE and Host GEF ARNO cooperate to recruit and activate WAVE to trigger bacterial invasion. Cell Host Microbe 2012, 11 (2), 129–39. 10.1016/j.chom.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J. S.; et al. Caveolin-1 mediates Salmonella invasion via the regulation of SopE-dependent Rac1 activation and actin reorganization. J. Infect Dis 2014, 210 (5), 793–802. 10.1093/infdis/jiu152. [DOI] [PubMed] [Google Scholar]
- Koronakis V.; et al. WAVE regulatory complex activation by cooperating GTPases Arf and Rac1. Proc. Natl. Acad. Sci. U. S. A. 2011, 108 (35), 14449–54. 10.1073/pnas.1107666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V.; et al. Arf GTPase interplay with Rho GTPases in regulation of the actin cytoskeleton. Small GTPases 2019, 10 (6), 411–418. 10.1080/21541248.2017.1329691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R.; et al. Wash functions downstream of Rho and links linear and branched actin nucleation factors. Development 2009, 136 (16), 2849–60. 10.1242/dev.035246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linardopoulou E. V.; et al. Human subtelomeric WASH genes encode a new subclass of the WASP family. PLoS Genet 2007, 3 (12), e237 10.1371/journal.pgen.0030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch J.; et al. Activation of a RhoA/myosin II-dependent but Arp2/3 complex-independent pathway facilitates Salmonella invasion. Cell Host Microbe 2011, 9 (4), 273–85. 10.1016/j.chom.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Truong D.; et al. Formin-mediated actin polymerization promotes Salmonella invasion. Cell Microbiol 2013, 15 (12), 2051–63. 10.1111/cmi.12173. [DOI] [PubMed] [Google Scholar]
- Westendorf J. J. The formin/diaphanous-related protein, FHOS, interacts with Rac1 and activates transcription from the serum response element. J. Biol. Chem. 2001, 276 (49), 46453–9. 10.1074/jbc.M105162200. [DOI] [PubMed] [Google Scholar]
- Nichols C. D.; Casanova J. E. Salmonella-directed recruitment of new membrane to invasion foci via the host exocyst complex. Curr. Biol. 2010, 20 (14), 1316–20. 10.1016/j.cub.2010.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S.; et al. Agonist-stimulated phosphatidylinositol-3,4,5-trisphosphate generation by scaffolded phosphoinositide kinases. Nat. Cell Biol. 2016, 18 (12), 1324–1335. 10.1038/ncb3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T.; Wang S.; Kaibuchi K. IQGAPs as Key Regulators of Actin-cytoskeleton Dynamics. Cell Struct Funct 2015, 40 (2), 69–77. 10.1247/csf.15003. [DOI] [PubMed] [Google Scholar]
- Kim H.; et al. Salmonella enterica serotype Typhimurium usurps the scaffold protein IQGAP1 to manipulate Rac1 and MAPK signalling. Biochem. J. 2011, 440 (3), 309–18. 10.1042/BJ20110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. D.; et al. IQGAP1 regulates Salmonella invasion through interactions with actin, Rac1, and Cdc42. J. Biol. Chem. 2007, 282 (41), 30265–72. 10.1074/jbc.M702537200. [DOI] [PubMed] [Google Scholar]
- Jolly C.; et al. The Annexin A2/p11 complex is required for efficient invasion of Salmonella Typhimurium in epithelial cells. Cell Microbiol 2014, 16 (1), 64–77. 10.1111/cmi.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A. B.; et al. MYO6 is targeted by Salmonella virulence effectors to trigger PI3-kinase signaling and pathogen invasion into host cells. Proc. Natl. Acad. Sci. U. S. A. 2017, 114 (15), 3915–3920. 10.1073/pnas.1616418114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss F.; Spudich G.; Kendrick-Jones J. Myosin VI: cellular functions and motor properties. Annu. Rev. Cell Dev Biol. 2004, 20, 649–76. 10.1146/annurev.cellbio.20.012103.094243. [DOI] [PubMed] [Google Scholar]
- Ono Y.; et al. Two actions of frabin: direct activation of Cdc42 and indirect activation of Rac. Oncogene 2000, 19 (27), 3050–8. 10.1038/sj.onc.1203631. [DOI] [PubMed] [Google Scholar]
- Lhocine N.; et al. Apical invasion of intestinal epithelial cells by Salmonella typhimurium requires villin to remodel the brush border actin cytoskeleton. Cell Host Microbe 2015, 17 (2), 164–77. 10.1016/j.chom.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsey C. W.; et al. Salmonella enterica serotype Typhimurium MisL is an intestinal colonization factor that binds fibronectin. Mol. Microbiol. 2005, 57 (1), 196–211. 10.1111/j.1365-2958.2005.04666.x. [DOI] [PubMed] [Google Scholar]
- Fierer J.; Guiney D. G. Diverse virulence traits underlying different clinical outcomes of Salmonella infection. J. Clin Invest 2001, 107 (7), 775–80. 10.1172/JCI12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L.; et al. Magnesium transport in Salmonella typhimurium: regulation of mgtA and mgtCB during invasion of epithelial and macrophage cells. Microbiology 1998, 144 (7), 1835–1843. 10.1099/00221287-144-7-1835. [DOI] [PubMed] [Google Scholar]
- Tukel C.; et al. MarT activates expression of the MisL autotransporter protein of Salmonella enterica serotype Typhimurium. J. Bacteriol. 2007, 189 (10), 3922–6. 10.1128/JB.01746-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snavely M. D.; Miller C. G.; Maguire M. E. The mgtB Mg2+ transport locus of Salmonella typhimurium encodes a P-type ATPase. J. Biol. Chem. 1991, 266 (2), 815–823. 10.1016/S0021-9258(17)35246-8. [DOI] [PubMed] [Google Scholar]
- Kiss T.; Morgan E.; Nagy G. Contribution of SPI-4 genes to the virulence of Salmonella enterica. FEMS Microbiol Lett. 2007, 275 (1), 153–9. 10.1111/j.1574-6968.2007.00871.x. [DOI] [PubMed] [Google Scholar]
- Gerlach R. G.; et al. Salmonella pathogenicity island 4-mediated adhesion is coregulated with invasion genes in Salmonella enterica. Infect. Immun. 2007, 75 (10), 4697–709. 10.1128/IAI.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan E.; et al. Identification of host-specific colonization factors of Salmonella enterica serovar Typhimurium. Mol. Microbiol. 2004, 54 (4), 994–1010. 10.1111/j.1365-2958.2004.04323.x. [DOI] [PubMed] [Google Scholar]
- Knodler L. A.; et al. Salmonella effectors within a single pathogenicity island are differentially expressed and translocated by separate type III secretion systems. Mol. Microbiol. 2002, 43 (5), 1089–103. 10.1046/j.1365-2958.2002.02820.x. [DOI] [PubMed] [Google Scholar]
- Wang M.; et al. Molecular Characterization of a Functional Type VI Secretion System in Salmonella enterica serovar Typhi. Curr. Microbiol. 2011, 63 (1), 22. 10.1007/s00284-011-9935-z. [DOI] [PubMed] [Google Scholar]
- Maurya P.; Gulati A. K.; Nath G. Status of Vi gene, its expression and Salmonella Pathogenicity Island (SPI-7) in Salmonella Typhi in India. Southeast Asian J. Trop Med. Public Health 2010, 41 (4), 913–919. [PubMed] [Google Scholar]
- Wain J.; et al. Vi antigen expression in Salmonella enterica serovar Typhi clinical isolates from Pakistan. J. Clin Microbiol 2005, 43 (3), 1158–65. 10.1128/JCM.43.3.1158-1165.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard D.; et al. Composition, acquisition, and distribution of the Vi exopolysaccharide-encoding Salmonella enterica pathogenicity island SPI-7. J. Bacteriol. 2003, 185 (17), 5055–65. 10.1128/JB.185.17.5055-5065.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card R.; et al. Virulence Characterisation of Salmonella enterica Isolates of Differing Antimicrobial Resistance Recovered from UK Livestock and Imported Meat Samples. Front. Microbiol. 2016, 7, 640. 10.3389/fmicb.2016.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez J. C.; et al. SPI-9 of Salmonella enterica serovar Typhi is constituted by an operon positively regulated by RpoS and contributes to adherence to epithelial cells in culture. Microbiology 2016, 162 (8), 1367–1378. 10.1099/mic.0.000319. [DOI] [PubMed] [Google Scholar]
- Faucher S. P.; et al. The prpZ gene cluster encoding eukaryotic-type Ser/Thr protein kinases and phosphatases is repressed by oxidative stress and involved in Salmonella enterica serovar Typhi survival in human macrophages. FEMS Microbiol Lett. 2008, 281 (2), 160–6. 10.1111/j.1574-6968.2008.01094.x. [DOI] [PubMed] [Google Scholar]
- Bouwman C. W.; et al. Characterization of SrgA, a Salmonella enterica serovar Typhimurium virulence plasmid-encoded paralogue of the disulfide oxidoreductase DsbA, essential for biogenesis of plasmid-encoded fimbriae. J. Bacteriol. 2003, 185 (3), 991–1000. 10.1128/JB.185.3.991-1000.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; et al. Recognition and Ubiquitination of Salmonella Type III Effector SopA by a Ubiquitin E3 Ligase, HsRMA1. J. Biol. Chem. 2005, 280 (46), 38682–38688. 10.1074/jbc.M506309200. [DOI] [PubMed] [Google Scholar]
- Miao E. A.; et al. Salmonella typhimurium leucine-rich repeat proteins are targeted to the SPI1 and SPI2 type III secretion systems. Mol. Microbiol. 1999, 34 (4), 850–64. 10.1046/j.1365-2958.1999.01651.x. [DOI] [PubMed] [Google Scholar]
- Haneda T.; et al. Genome-wide identification of novel genomic islands that contribute to Salmonella virulence in mouse systemic infection. FEMS Microbiol Lett. 2009, 297 (2), 241–9. 10.1111/j.1574-6968.2009.01686.x. [DOI] [PubMed] [Google Scholar]
- Shi L.; et al. Proteomic analysis of Salmonella enterica serovar typhimurium isolated from RAW 264.7 macrophages: identification of a novel protein that contributes to the replication of serovar typhimurium inside macrophages. J. Biol. Chem. 2006, 281 (39), 29131–40. 10.1074/jbc.M604640200. [DOI] [PubMed] [Google Scholar]
- Vernikos G. S.; Parkhill J. Interpolated variable order motifs for identification of horizontally acquired DNA: revisiting the Salmonella pathogenicity islands. Bioinformatics 2006, 22 (18), 2196–203. 10.1093/bioinformatics/btl369. [DOI] [PubMed] [Google Scholar]
- Bogomolnaya L. M.; et al. Form variation’ of the O12 antigen is critical for persistence of Salmonella Typhimurium in the murine intestine. Mol. Microbiol. 2008, 70 (5), 1105–19. 10.1111/j.1365-2958.2008.06461.x. [DOI] [PubMed] [Google Scholar]
- Fuentes J. A.; et al. The Salmonella Typhi hlyE gene plays a role in invasion of cultured epithelial cells and its functional transfer to S. Typhimurium promotes deep organ infection in mice. Res. Microbiol 2008, 159 (4), 279–87. 10.1016/j.resmic.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Darwin K. H.; Miller V. L. The putative invasion protein chaperone SicA acts together with InvF to activate the expression of Salmonella typhimurium virulence genes. Mol. Microbiol. 2000, 35 (4), 949–60. 10.1046/j.1365-2958.2000.01772.x. [DOI] [PubMed] [Google Scholar]
- Jain K. C.; Mahapatra A. K. Subdural empyema due to salmonella infection. Pediatr Neurosurg 1998, 28 (2), 89–90. 10.1159/000028627. [DOI] [PubMed] [Google Scholar]
- Kapoor K.; et al. A rare neurological complication of typhoid fever: Guillain-Barre’ syndrome. J. Pediatr Neurosci 2014, 9 (2), 148–9. 10.4103/1817-1745.139323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehndiratta S.; Rajeshwari K.; Dubey A. P. Guillain-Barre syndrome as a complication of typhoid fever in a child. Neurol India 2012, 60 (4), 433–5. 10.4103/0028-3886.100722. [DOI] [PubMed] [Google Scholar]
- May W.; Senitiri I. Guillain-Barré syndrome associated with typhoid fever. A case study in the Fiji Islands. Pac. Health Dialog 2010, 16 (2), 85–88. [PubMed] [Google Scholar]
- Villablanca P.; et al. Salmonella Berta myocarditis: Case report and systematic review of non-typhoid Salmonella myocarditis. World J. Cardiol 2015, 7 (12), 931–7. 10.4330/wjc.v7.i12.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer B.; et al. Cardiac troponin T in patients with clinically suspected myocarditis. J. Am. Coll Cardiol 1997, 30 (5), 1354–9. 10.1016/S0735-1097(97)00317-3. [DOI] [PubMed] [Google Scholar]
- Tong M. J.; Youel D. B.; Cotten C. L. Acute pneumonia in tropical infections. Am. J. Trop Med. Hyg 1972, 21 (2), 50–7. 10.4269/ajtmh.1972.21.50. [DOI] [PubMed] [Google Scholar]
- Cohen J. I.; Bartlett J. A.; Corey G. R. Extra-intestinal manifestations of salmonella infections. Medicine (Baltimore) 1987, 66 (5), 349–88. 10.1097/00005792-198709000-00003. [DOI] [PubMed] [Google Scholar]
- Aguado J. M.; et al. Pleuropulmonary infections due to nontyphoid strains of Salmonella. Arch Intern Med. 1990, 150 (1), 54–6. 10.1001/archinte.1990.00390130070008. [DOI] [PubMed] [Google Scholar]
- Karoli R.; et al. Salmonella hepatitis: an uncommon complication of a common disease. J. Family Med. Prim Care 2012, 1 (2), 160–2. 10.4103/2249-4863.104992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhongle N. N.; Nagdeo N. V.; Thombare V. R. A Splenic Abscess which was Caused by Salmonella Typhi in a Non Sickler Patient: A Rare Case Finding. J. Clin Diagn. Res. 2013, 7 (3), 537–538. 10.7860/JCDR/2013/4563.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharavanan P.; et al. Salmonella typhi Splenic Abscess Following Blunt Abdominal Injury: A Case Report. J. Clin Diagn. Res. 2016, 10 (7), DD01–DD02. 10.7860/JCDR/2016/19789.8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjunath R.; et al. Typhoid-associated acute kidney injury masquerading as a relapse of Takayasu arteritis. Saudi J. Kidney Dis Transpl 2017, 28 (1), 178–180. 10.4103/1319-2442.198270. [DOI] [PubMed] [Google Scholar]
- Valayatham V. Salmonella: the pelvic masquerader. Int. J. Infect Dis 2009, 13 (2), e53–5. 10.1016/j.ijid.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Lareau S. M.; Beigi R. H. Pelvic inflammatory disease and tubo-ovarian abscess. Infect Dis Clin North Am. 2008, 22 (4), 693–708. 10.1016/j.idc.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Rohilla R.; et al. Salmonella osteomyelitis: A rare extraintestinal manifestation of an endemic pathogen. J. Lab Physicians 2019, 11 (2), 164–170. 10.4103/JLP.JLP_165_18. [DOI] [PMC free article] [PubMed] [Google Scholar]