Abstract
Background
Cardiovascular disease (CVD) is the leading cause of death worldwide. The aim of this study was to examine if CVD affects the mortality of women after a breast cancer diagnosis and population controls differently.
Methods
The analysis included a total of 3,555 women, diagnosed with primary stage 1–3 breast cancer or in situ carcinoma between 2002 and 2005 and 7,334 controls breast cancer-free at recruitment, all aged 50–74 years, who were followed-up in a German breast cancer case–control study until June, 30 2020. Kaplan–Meier and cumulative incidence function were calculated for all-cause mortality and mortality from any cancer, stratified for case–control status and CVD, separately for women aged < 65 and ≥ 65 years. Cox regression and Fine-Gray subdistribution hazard models were used to estimate hazard ratios (HR) and 95% confidence intervals (95% CI) for the association between case–control-status, CVD and mortality from all causes/any cancer.
Results
The median follow-up was 16.1 years. In total, 1,172 cases (33.0%) and 1,401 initial controls (19.1%) died. CVD prevalence at recruitment was 15.2% in cases and controls. Cases with CVD had the highest and controls without CVD the lowest mortality during the entire observation period in both age groups (< 65 and ≥ 65 years). CVD was identified as a risk factor for all-cause mortality in both cases and controls aged < 65 years (HR 1.22, 95%CI 0.96–1.55 and HR 1.79, 95%CI 1.43–2.24) as well as at ages of ≥ 65 years (HR 1.44, 95%CI 1.20–1.73 and HR 1.59, 95%CI 1.37–1.83). A significant association of CVD and cancer mortality was found only for cases aged ≥ 65 years.
Conclusion
CVD was significantly associated with all-cause mortality of both cases and controls and CVD was identified as a risk factor for cancer mortality of cases aged ≥ 65 years at recruitment. Therefore, attention should be paid on monitoring and preventing CVD in breast cancer patients, especially in those diagnosed at older ages.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13058-023-01680-x.
Keywords: Breast cancer, Mortality, Cardiovascular disease, Case–control study
Introduction
In 2020, 2.26 million new diagnoses of breast cancer and 685,000 deaths were observed worldwide. Breast cancer is among the top five cancer-related causes of death [1] but breast cancer mortality in European Countries declined during the last decades, especially in the Northern and Western countries [2, 3]. Improvement in treatments, diagnosis and disease management as well as systematic screening programs have contributed towards the reduction in mortality [2, 4–7]. In the growing group of aging breast cancer survivors, other comorbidities, especially cardiovascular diseases (CVD), are of great importance [8, 9].
Breast cancer patients with prevalent comorbidities, including CVD, have poorer survival outcomes than patients without comorbidities [10]. In a large U.S. breast cancer cohort (n = 63,566), breast cancer patients with CVD had a 1.24-fold higher risk of dying from breast cancer compared to patients without CVD [11]. As a consequence of cardiotoxicity, breast cancer therapies such as radiotherapy can promote incident cardiac events in the short and long-term [12]. Shared risk factors of breast cancer and cardiovascular disease, such as lifestyle factors including tobacco or alcohol use, obesity and a lack of physical activity may worsen cardiotoxicity of cancer treatments [13].
In 2018, the American Heart Association published a scientific statement on CVD and breast cancer to give an overview of the intersection of both diseases including shared risk factors and cardiotoxicity of treatments. The authors pointed out that in older women CVD represents a greater risk for mortality than the cancer diagnosis itself [14].
Ten years after diagnosis, the probability of dying from other causes than breast cancer, of which heart diseases were most common (1,727 of 7,271 deaths), was 0.20 whereas the probability of dying from breast cancer was only 0.04 [15]. Furthermore, two case–control studies of Bradshaw et al. and Ramin et al. showed that survivors of breast cancer had a higher risk of dying from CVD more than 7 and 8 years after diagnosis, respectively, compared to women without breast cancer [16, 17]. A systematic review from 2017, including 14 studies of different designs, also showed a higher CVD mortality among women with breast cancer compared to the general population [18].
Although many studies compared CVD mortality in breast cancer survivors and controls, there is a lack of studies investigating the impact of CVD on mortality, especially with focussing on the difference between women after breast cancer diagnosis and population controls.
The aim of this study was to investigate whether cardiovascular diseases (CVD) affect all-cause mortality and cancer-specific mortality differently in women after a breast cancer diagnosis (cases) and controls without a breast cancer diagnosis from the German MARIE-study (Mamma Carcinoma Risk Factor Investigation).
Materials and methods
Study population and design
We used data from 11,154 women enrolled in the prospective population-based case–control study MARIE (Mamma Carcinoma Risk Factor Investigation) which aimed to investigate risk factors for breast cancer. Details of the study design have been described elsewhere [19]. In short, cases aged 50 to 74 years (n = 3,813) who were diagnosed with histologically confirmed stage 1 to 4 primary invasive breast cancer or carcinoma in situ between 2001 and 2005 were extracted from hospitals and pathology records in Hamburg and the region Rhine-Neckar-Karlsruhe and from the cancer registry Hamburg. Population-based age- and region-matched controls were selected from registration offices, of which 7,341 completed the baseline interview. Patients diagnosed with stage 4 breast cancer, pre-OP chemo (n = 255) or missing stage (n = 2), controls diagnosed with breast cancer before 2006 (n = 6) as well as women with missing information on CVD at baseline (n = 2) were excluded from the analyses, resulting in 3,555 cases and 7,334 controls for this analysis.
Assessment of cardiovascular disease and covariates
Information on covariates was obtained at a standardized face-to-face interview at recruitment.The participants were asked if they have ever been diagnosed with any of the comorbidities listed in the questionnaire. Other diseases could be specified via free text fields. In our analysis, the binary exposure variable CVD was defined as having ever been diagnosed with at least one of the following diseases until recruitment: myocardial infarction, congestive heart failure, peripheral vascular disease, angina pectoris, arrhythmia or stroke.
Assessment of vital status and causes of death
Vital status (alive, dead, lost to follow-up) and exact dates of death and lost to follow-up were obtained from the German registration offices for every woman on 30th June 2020. Death certificates were requested from the local health offices for the entire observation period. Causes of death were coded according to the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10 WHO).
Statistical analyses
The observation period was from the date of the baseline interview until death, last date known to be alive or the 30th of June 2020, which ever came first. Following descriptive analyses for relevant variables, Kaplan–Meier survival curves for all-cause mortality and cumulative incidence functions for mortality of any cancer (referred to as "cancer mortality") were estimated, stratified for case–control status and CVD and separately for women aged < 65 years and ≥ 65 years at recruitment. Median follow-up time was calculated using reverse Kaplan–Meier method.
Cox regression models were used to estimate hazard ratios (HR) with 95% confidence intervals (95% CIs) for the association of CVD at baseline, including the interaction with case–control status, and all-cause mortality. Due to the known interaction between cancer (treatments) and CVD [20], cancer mortality was investigated separately using Cox regression (cause-specific HRs) and Fine-Gray models (subdistribution HRs). Age was used as the time-scale (start: age at baseline, end: age at date of death or censoring).
As mentioned above, two separate models were calculated for the age groups < 65 years and ≥ 65 years, since the population of people aged ≥ 65 will rapidly grow in the next decades and the CVD burden will increase [21]. Age is one of the most significant factors influencing survival of breast cancer patients in general as well as the risk of CVD and dying from CVD [9, 18].
Covariates to be entered in the models were chosen based on hypotheses concerning their relationship with CVD and mortality, using directed acyclic graphs (DAG) [22]. Based on the DAG (Additional file 1: Figure S1) we adjusted for the following baseline variables: body mass index (BMI, continuous), education (low, middle, high), smoking status (never, former, current), alcohol consumption (abstinent, < 19 g/day, ≥ 19 g/day), physical activity since age of 50 years based on walking, cycling and recreational physical activity (MET hours/week, continuous; if information was missing and women were aged < 56, information about physical activity since age of 30 years was used), diabetes, cancer other than breast cancer prior to baseline and living with a partner (yes/no).
For supplemental analysis, CVD mortality was compared between cases and controls using Cox regression (cause-specific) and Fine-Gray models, stratified by age groups (< 65 years and ≥ 65 years) and adjusted for the above mentioned covariates.
All analyses were performed using SAS Software, version 9.4 of the SAS system for Windows (Copyright © 2002–2012 SAS Institute Inc., Cary, NC, USA).
Results
Characteristics of the study population
A total of 10,889 women (3,555 cases, 7,334 controls) were included in the analyses. Overall, case and control characteristics were comparable, but there were differences between women aged < 65 years (n = 6,480) and those ≥ 65 years (n = 4,409) at baseline.
The BMI at baseline was slightly higher in the elderly. The proportions of women having a low educational level, being never smokers and being abstinent were higher in those aged ≥ 65 years than in the younger women but similar in cases and controls. While only 11.8% (cases) and 11.4% (controls) of the older women were current smokers, 25.2% (cases) and 25.3% (controls) of the younger women were current smokers. Physical activity was about 50 METs per week in cases and controls in both age groups (Table 1).
Table 1.
Characteristics of the study population at baseline, stratified for age groups and case–control status, n = 10,889
| < 65 years | ≥ 65 years | |||
|---|---|---|---|---|
| Cases (%) n = 2123 |
Controls (%) n = 4357 |
Cases (%) n = 1432 |
Controls (%) n = 2977 |
|
| Study region (missing = 0) | ||||
| Hamburg | 1175 (55.3%) | 2358 (54.1%) | 825 (57.6%) | 1644 (55.2%) |
| Rhine-Neckar-Karlsruhe | 948 (44.7%) | 1999 (45.9%) | 607 (42.4%) | 1333 (44.8%) |
|
Age (mean ± SD) (missing = 0) |
58.6 (± 3.9) | 58.5 (± 4.0) | 68.6 (± 2.9) | 68.6 (± 2.9) |
| BMI (mean ± SD) (missing = 29) | 25.7 (± 4.5) | 25.9 (± 4.8) | 26.2 (± 4.3) | 26.6 (± 4.7) |
| Educationa (missing = 1) | ||||
| Low | 1108 (52.2%) | 2271 (52.1%) | 908 (63.4%) | 1905 (64.0%) |
| Middle | 626 (29.5%) | 1303 (29.9%) | 370 (25.8%) | 768 (25.8%) |
| High | 388 (18.3%) | 783 (18.0%) | 154 (10.8%) | 304 (10.2%) |
| Living with a partner (missing = 13) | ||||
| No | 529 (25.0%) | 1111 (25.5%) | 515 (36.0%) | 1073 (36.1%) |
| Yes | 1589 (75.0%) | 3242 (74.5%) | 915 (64.0%) | 1902 (63.9%) |
| Smoking status (missing = 3) | ||||
| Never | 976 (46.0%) | 1868 (42.9%) | 891 (62.2%) | 1887 (63.4%) |
| Former | 613 (28.9%) | 1384 (31.8%) | 372 (26.0%) | 748 (25.1%) |
| Current | 534 (25.2%) | 1104 (25.3%) | 169 (11.8%) | 340 (11.4%) |
| Alcohol consumption (missing = 7) | ||||
| Abstinent | 438 (20.6%) | 832 (19.1%) | 356 (24.9%) | 720 (24.2%) |
| < 19 g/day | 1318 (62.1%) | 2815 (64.6%) | 892 (62.4%) | 1852 (62.3%) |
| ≥ 19 g/day | 367 (17.3%) | 708 (16.3%) | 181 (12.7%) | 403 (13.5%) |
| Physical activityb (mean ± SD) (missing = 135) | 50.6 (± 37.1) | 51.8 (± 38.1) | 49.5 (± 35.4) | 51.2 (± 36.6) |
| Chronic diseasesc | ||||
| Cardivascular diseased | 245 (11.5%) | 503 (11.5%) | 295 (20.6%) | 614 (20.6%) |
| Hypertension | 720 (34.0%) | 1465 (33.8%) | 738 (51.6%) | 1474 (49.8%) |
| Diabetes | 127 (6.0%) | 220 (5.1%) | 176 (12.3%) | 293 (9.9%) |
| Any cancer (excluding breast) | 110 (5.2%) | 198 (4.5%) | 96 (6.7%) | 192 (6.4%) |
aMeasured by combining school education and professional education
bMET hours/week from sports, cycling and walking from the age of 50 +
cDiagnosis between birth and baseline
dIncluding myocardial infarction, heart failure, arrhythmia, angina pectoris, stroke, peripheral vascular disease
CVD prevalence at baseline was twice as high in women aged ≥ 65 years compared to the younger ones (20.6% vs. 11.5%). Diabetes prevalence was higher in cases than in controls and also higher in women aged ≥ 65 than in the younger group (Table 1).
Breast cancer specific characteristics of the cases are shown in Additional file 4: Table S1.
Mortality by case–control status, CVD and age
The median follow-up time was 16.1 years. In total, 1172 cases (33.0%) and 1401 controls (19.1%) died. A total of 48 women (0.4%) were lost to follow-up. The proportion of deceased women was higher among the elderly than among younger women (Table 2). The most common cause of death among cases was breast cancer, with the proportion of those who died from breast cancer being higher in cases aged < 65 years than in cases aged ≥ 65 years (53.6% vs. 39.9%). The proportion of those who died from CVD was greater in older than in younger cases (19.9% vs. 10.4%). In younger controls, other cancer was the primary cause of death (46.0%) whereas in the older ones, it was CVD (32.3%) (Table 2).
Table 2.
Vitalstatus and causes of death by age group at recruitment
| < 65 years | ≥ 65 years | |||
|---|---|---|---|---|
| Cases (%) n = 2123 |
Controls (%) n = 4357 |
Cases (%) n = 1432 |
Controls (%) n = 2977 |
|
| Alive at 30/06/2020 | 1563 (73.6%) | 3844 (88.2%) | 807 (56.4%) | 2054 (69.0%) |
| Deceased (total) | 548 (25.8%) | 489 (11.2%) | 624 (43.6%) | 912 (30.6%) |
| Of these: cause of deatha | ||||
| Breast cancer | 294 (53.6%) | 22 (4.5%) | 249 (39.9%) | 28 (3.1%) |
| Other cancer | 119 (21.7%) | 225 (46.0%) | 114 (18.3%) | 238 (26.1%) |
| CVD | 57 (10.4%) | 90 (18.4%) | 124 (19.9%) | 295 (32.3%) |
| Chronic lung disease | 13 (2.4%) | 28 (5.7%) | 27 (4.3%) | 55 (6.0%) |
| Other/unknown | 58 (10.6%) | 109 (22.3%) | 107 (17.1%) | 279 (30.6%) |
| Missing death certificate | 7 (1.3%) | 15 (3.1%) | 3 (0.5%) | 17 (1.9%) |
| Lost to follow-up | 12 (0.6%) | 24 (0.6%) | 1 (0.1%) | 11 (0.4%) |
aPercentages for causes of death refer to numbers of deceased (total) (100%)
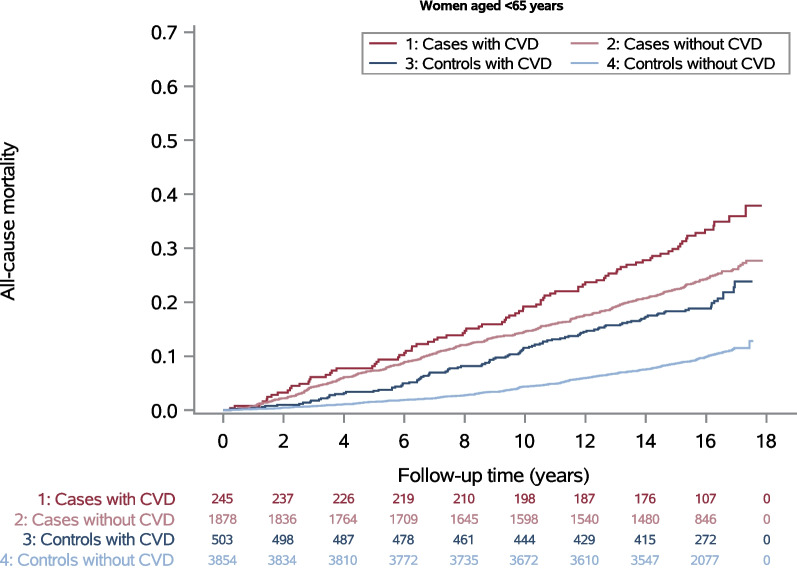
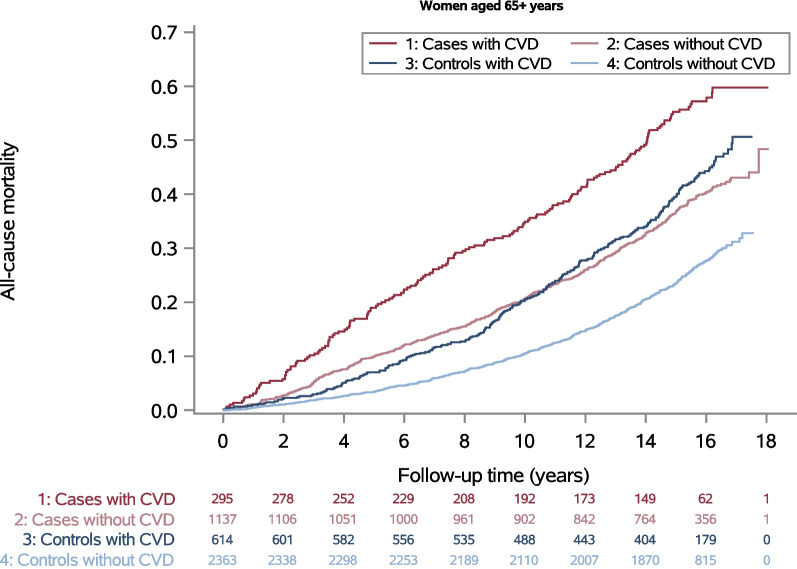
All-cause mortality was higher in cases than in controls and also higher in women with CVD than in women without CVD among both age groups (Figs. 1 and 2). However, in the older age group controls with CVD tended to have a higher mortality than cases without CVD after ten years of follow-up (Fig. 2). For both age groups, the highest all-cause mortality during the entire observation period was found for cases with CVD and the lowest for controls without CVD (Figs. 1 and 2). Mortality from any cancer including breast cancer, was higher in cases than in controls. A relevantly higher cancer mortality in those with CVD compared to those without CVD was found only among cases aged ≥ 65 years at diagnosis.
Fig. 1.
All-cause mortality and numbers at risk for women aged < 65 years
Fig. 2.
All-cause mortality and numbers at risk for women aged ≥ 65 years
Association between case-control status, cardiovascular diseases and mortality
CVD at baseline was associated with all-cause mortality in controls < 65 years and in cases and controls ≥ 65 years (Table 3). In the younger age group, there was an interaction between case–control status and CVD (P = 0.020), indicating differential effects of CVD on all-cause mortality in cases and controls. Cases with CVD at baseline had a 1.22-fold higher risk of dying from any cause (95% CI 0.96–1.55) compared to cases without CVD whereas the HR for controls with CVD vs. controls without CVD was 1.79 (95% CI 1.43–2.24). In women ≥ 65 years, the HR for CVD vs. no CVD was 1.44 (95% CI 1.20–1.73) in cases and 1.59 (95% CI 1.37–1.83) in controls (P for interaction = 0.42) (Table 3). We did not find an interaction between cases-control status and CVD for cancer-specific mortality. Point estimates were higher for controls than for cases in women < 65 and vice versa in women ≥ 65 (Table 3). Cases aged ≥ 65 years with CVD had a 1.31 times higher risk of dying from any cancer compared to those without CVD (95%CI 1.02–1.68 for subdistribution HR; cause-specific HR 1.43, 95% CI 1.12–1.82). Subdistribution and cause-specific hazard ratios pointed in the same direction with estimates for cause-specific HRs being slightly higher (Table 3).
Table 3.
Hazard Ratios and 95% confidence intervals for all-cause mortality and cancer mortality
| < 65 years | ≥ 65 years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Endpoint | Group | HRa | 95% CIb | Pc | HRa | 95% CIb | Pc | |||
| All-cause mortality | Cases | No CVD | Ref | 0.020 | Ref | 0.42 | ||||
| CVD | 1.22 | 0.96 | 1.55 | 1.44 | 1.20 | 1.73 | ||||
| Controls | No CVD | Ref | Ref | |||||||
| CVD | 1.79 | 1.43 | 2.24 | 1.59 | 1.37 | 1.83 | ||||
| Cancer mortality | Cases | No CVD | Ref | 0.46 | Ref | 0.26 | ||||
| CVD | 1.05 | 0.79 | 1.42 | 1.43 | 1.12 | 1.82 | ||||
| Controls | No CVD | Ref | Ref | |||||||
| CVD | 1.26 | 0.88 | 1.80 | 1.15 | 0.86 | 1.54 | ||||
| Endpoint | Group | SHRd | 95% CIb | Pc | SHRd | 95% CIb | Pc | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Cancer mortality | Cases | No CVD | Ref | 0.52 | Ref | 0.27 | ||||
| CVD | 1.02 | 0.75 | 1.39 | 1.31 | 1.02 | 1.68 | ||||
| Controls | No CVD | Ref | Ref | |||||||
| CVD | 1.19 | 0.83 | 1.71 | 1.06 | 0.80 | 1.42 | ||||
Models adjusted for the baseline variables age, BMI, education, living with a partner, smoking status, alcohol consumption, physical activity, diabetes and tumors other than breast cancer. The model includes the interaction term of CVD and case–control status
aHR Hazard ratio
bCI Confidence interval
cP value for interaction between case–control status and CVD
dSHR Subdistribution hazard ratio
Unadjusted hazard ratios were somewhat larger (Additional file 4; Table S2), indicating the confounding effect of the variables considered in the adjusted model were not large.
Mortality from CVD was higher in cases compared to controls in women < 65 years (cause-specific HR 1.51, 95%CI 1.08–2.13; SHR 1.33, 95%CI 0.95–1.87) but not in those aged ≥ 65 years (Additional file 4: Table S3).
Discussion
To our knowledge, there are many studies focusing on the risk of death from CVD, but no studies comparing the impact of CVD on all-cause or cancer specific mortality in breast cancer survivors and population controls. Our study showed an effect of CVD at baseline on all-cause mortality in cases and controls after 16 years of follow-up. The relative effect given as hazard ratio appeared to be larger in controls, in particular in women aged < 65 years. The absolute risk difference was increasing over the observation period and in women aged < 65 years it was also larger in controls than in cases. The results therefore do not support the hypothesis that a CVD diagnosis until baseline is a stronger risk factor for total mortality in women diagnosed with breast cancer than in women without this diagnosis. Within the first ten years, cases aged ≥ 65 years without CVD still had a higher mortality than controls with CVD at the same age (Fig. 2). Afterwards, mortality of controls with CVD was little higher than mortality of cases without CVD. An effect of CVD at baseline on cancer mortality was only found for cases aged ≥ 65 years.
When interpreting the results, one has to bear in mind that cases had a higher level of all-cause mortality, and therefore the HR has to be interpreted with caution. Furthermore, when looking at the Kaplan–Meier curves in Figs. 1 and 2, it is noticeable that the risk difference (calculated as difference between the blue, respectively red, curves) was greater in controls aged ≥ 65 than in controls aged < 65 years, whereas the HR for controls aged ≥ 65 was smaller (HR 1.59, 95%CI 1.37–1.83) than for controls aged < 65 years (HR 1.79, 95%CI 1.43–2.24). Even if the difference is small in relative terms, a large absolute difference means that the exposure, in this case CVD, leads to a high number of additional deaths, which could be reduced if the exposure were absent. For our example, this means that the number of additional deaths from CVD is higher in the older controls compared to the younger ones, although this is not reflected in the HR. Therefore, from a public health perspective, it is important to consider both relative and absolute differences.
Furthermore, in controls, the proportion of deaths from CVD among all causes of deaths was higher than in cases (Table 2, 18.4% vs. 10.4% for ages < 65 and 32.4% vs. 19.9% for ages ≥ 65). It is expected that women with known CVD are also at higher risk of dying from CVD and that the presence of CVD has a greater effect on death from CVD than on death from other causes, what is supported by our finding of a higher impact of CVD at baseline on all-cause mortality in controls than in cases.
There are already a number of studies comparing the mortality from CVD in breast cancer cases and controls. The authors of a systematic review of 14 studies on the risk of death from cardiovascular disease and breast cancer summarized that women with breast cancer had a higher CVD mortality than the general population [18]. A nested case–control study published in 2021 reported similar results and found that breast cancer survivors had an increased CVD mortality risk than controls 8 years after diagnosis [17].
In our study, we also found a higher CVD mortality in cases than in controls, but only in women aged < 65 years. Even if breast cancer was the most common cause of death in younger cases, competing risk analysis showed that they also had a higher CVD mortality than controls. We did not observe this for cases ≥ 65 years. This might be explained by adverse events due to cancer treatment. At older ages, CVD is one of the most common causes of death in the general population, so that breast cancer has less impact on CVD mortality than the patients’ age.
Cases and controls had comparable CVD prevalences at baseline, 11.5% in both cases and controls aged < 65 years and 20.6% in both groups aged ≥ 65 years. Self-reported CVD prevalence was relatively high in our study compared to others (12.8% in ≥ 66 US breast cancer patients [11]; 8.5%/18.6% in 60–69/70–79 year old breast cancer patients from the German PASSOS study [23]). Despite the adjustment for baseline CVD prevalence, there was an increased risk of CVD death in younger cases, suggesting that there was an increased incidence of CVD after breast cancer diagnosis in this group, leading to increased CVD mortality.
Two main explanations for the higher CVD mortality in breast cancer patients are given in the literature: firstly, breast cancer and CVD have shared risk factors such as diabetes, resulting in a higher prevalence of these risk factors in women with breast cancer compared to the general population [24]. Secondly, cancer treatments such as radiotherapy can have cardiotoxic effects and cause CVD and cardiovascular death [12, 14].
A register based study by Patnaik et al. including 63,566 women with breast cancer from the U.S. found that CVD at diagnosis had an impact on other cause mortality (HR 1.87, 95%CI 1.80–1.93) as well as on breast cancer mortality (HR 1.24, 95%CI 1.13–1.26). The authors concluded that the reduction of CVD is important in the long-term care of breast cancer patients as it contributes to overall as well as to breast cancer specific mortality [11].
The impact of CVD at diagnosis on mortality, or worse prognosis for women with breast cancer and CVD could be explained by therapy guideline violations in patients with underlying comorbidities [25].
Strengths and limitations
The data we used for our analyses came from a large population-based case–control study with a median follow-up time of 16.1 years that allows conclusions about long-term effects of CVD. Information on CVD and covariates at baseline were self-reported via standardized face-to-face interviews. A validation study of Kropp et al. showed a good agreement between self-reported hormone therapy data in the MARIE study and information on hormone therapies from attending physicians [26]. It can be assumed that the reliability of data on chronic diseases in MARIE is similarly high.
Furthermore, we accounted for shared risk factors of breast cancer and CVD, including age, obesity/BMI and lifestyle factors such as tobacco use and alcohol consumption.
We only examined the impact of CVD diagnosis between birth and recruitment. Since breast cancer therapies can cause cardiac events and promote the development of CVD [12], we may have missed to demonstrate a differential impact of incident CVD on mortality in the time-course after treatment.
Conclusion
The highest mortality rates were observed in ≥ 65 years old cases with CVD diagnosis until recruitment. CVD was identified as a risk factor for all-cause mortality of both cases and controls. However CVD at baseline did not appear to be a stronger risk factor in women diagnosed with breast cancer than in women without the diagnosis. CVD was also associated with increased cancer mortality of cases aged ≥ 65 years at recruitment. CVD will become increasingly relevant among the growing and aging group of breast cancer survivors. Since breast cancer therapies may contribute to CVD, attention should be paid to monitoring and preventing CVD and its risk factors in breast cancer patients, especially in older women.
Supplementary Information
Additional file 1. Directed acyclic graph for the association of CVD and all-cause mortality. Green arrow= causal path, red arrows= biasing paths.
Additional file 2. Mortality from any cancer for women aged <65 years.
Additional file 3. Mortality from any cancer for women aged ≥65 years.
Additional file 4. Supplemental Tables 1–3
Acknowledgements
We acknowledge D. Flesch-Janys for his many years of function as a PI in the MARIE study, U. Eilber for her work as data manager and AK. Ozga for her statistical support. Furthermore, we would like to thank the MARIE study team as well as all participants for their long-term cooperation.
Abbreviations
- BMI
Body mass index
- CVD
Cardiovascular disease
- HR
Hazard ratio
- MARIE
Mamma carcinoma risk factor investigation
- MET
Metabolic equivalent of task
- SHR
Subdistributional hazard ratio
- 95%CI
95% Confidence interval
Author contributions
HB, JCC and NO were responsible for the project administration and supervision, the methodology, and did the critical revision. SB, FF and AM conducted the mortality follow-up and prepared the data. AK and BH coded the causes of death. AM analyzed the data and wrote the manuscript. HB, JCC, NO, KG, SB, FF, AK and BH read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. We acknowledge financial support from the Open Access Publication Fund of UKE - Universitätsklinikum Hamburg-Eppendorf and DFG – German Research Foundation. The study was funded by the Deutsche Krebshilfe e.V. (#70–2892-BR I, #108253 and #108419, #110826 and #110828), the Hamburger Krebsgesellschaft, the Hamburger Stiftung zur Förderung der Krebsbekämpfung and the Bundesministerium für Bildung und Forschung (Grant number 01ER1901 PERGOLA2).
Availability of data and materials
The datasets analysed during the current study are not publicly available due to data protection fundamentals but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Written informed consent from all participants has been obtained. The MARIE study was approved by the ethics committees of the University of Heidelberg, the Hamburg Medical Council, and the Medical Board of the State of Rhineland-Palatinate and was conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World health organization: Cancer. Fact sheet no. 297 https://www.who.int/en/news-room/fact-sheets/detail/cancer (2022). Assessed 15.12.2022.
- 2.Autier P, Boniol M, LaVecchia C, Vatten L, Gavin A, Héry C, et al. Disparities in breast cancer mortality trends between 30 European countries: retrospective trend analysis of WHO mortality database. BMJ (Clin Res Ed) 2010;341:c3620. doi: 10.1136/bmj.c3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carioli G, Malvezzi M, Rodriguez T, Bertuccio P, Negri E, La Vecchia C. Trends and predictions to 2020 in breast cancer mortality in Europe. The Breast. 2017;36:89–95. doi: 10.1016/j.breast.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson SG, Wilcox M. The benefits and harms of breast cancer screening: an independent review. Br J Cancer. 2013;108:2205–2240. doi: 10.1038/bjc.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalager M, Zelen M, Langmark F, Adami H-O. Effect of screening mammography on breast-cancer mortality in Norway. N Engl J Med. 2010;363:1203–1210. doi: 10.1056/NEJMoa1000727. [DOI] [PubMed] [Google Scholar]
- 6.Blanks RG, Moss SM, McGahan CE, Quinn MJ, Babb PJ. Effect of NHS breast screening programme on mortality from breast cancer in England and Wales, 1990–8: comparison of observed with predicted mortality. BMJ (Clin Res Ed) 2000;321:665–669. doi: 10.1136/bmj.321.7262.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jatoi I, Miller AB. Why is breast-cancer mortality declining? Lancet Oncol. 2003;4:251–254. doi: 10.1016/S1470-2045(03)01037-4. [DOI] [PubMed] [Google Scholar]
- 8.Parry C, Kent EE, Mariotto AB, Alfano CM, Rowland JH. Cancer survivors: a booming population. Cancer Epidemiol Biomarkers Prev. 2011;20:1996–2005. doi: 10.1158/1055-9965.EPI-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blaes AH, Konety SH. Cardiovascular disease in breast cancer survivors: an important topic in breast cancer survivorship. J Nat Cancer Inst. 2021;113:105–106. doi: 10.1093/jnci/djaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards BK, Noone A-M, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, et al. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290–1314. doi: 10.1002/cncr.28509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patnaik JL, Byers T, Diguiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13:R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. New Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 13.Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133:1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laxmi S. Mehta, MD, FAHA [Chair], Karol E. Watson, MD, PhD, FAHA [Vice Chair], Ana Barac, MD, PhD, Theresa M. Beckie, PhD, FAHA, Vera Bittner, MD, MSPH, FAHA, Salvador Cruz-Flores, MD, MPH, FAHA, Susan Dent, MD, Lavanya Kondapalli, MD, Bonnie Ky, MD, MSCE, Tochukwu Okwuosa, DO, Ileana L. Piña, MD, MPH, FAHA, Annabelle Santos Volgman, MD, FAHA American heart association cardiovascular disease in women and special populations committee of the council on clinical cardiology, council on cardiovascular and stroke nursing, and council on quality of care and outcomes research: cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American heart association. Circulation 2018; 137: e30–e66. [DOI] [PMC free article] [PubMed]
- 15.Hanrahan EO, Gonzalez-Angulo AM, Giordano SH, Rouzier R, Broglio KR, Hortobagyi GN, et al. Overall survival and cause-specific mortality of patients with stage T1a, bN0M0 breast carcinoma. J Clin Oncol. 2007;25:4952–4960. doi: 10.1200/JCO.2006.08.0499. [DOI] [PubMed] [Google Scholar]
- 16.Bradshaw PT, Stevens J, Khankari N, Teitelbaum SL, Neugut AI, Gammon MD. Cardiovascular disease mortality among breast cancer survivors. Epidemiology. 2016;27:6–13. doi: 10.1097/EDE.0000000000000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramin C, Schaeffer ML, Zheng Z, Connor AE, Hoffman-Bolton J, Lau B, et al. All-cause and cardiovascular disease mortality among breast cancer survivors in clue ii, a long-standing community-based cohort. J Natl Cancer Inst. 2021;113:137–145. doi: 10.1093/jnci/djaa096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gernaat SAM, Ho PJ, Rijnberg N, Emaus MJ, Baak LM, Hartman M, et al. Risk of death from cardiovascular disease following breast cancer: a systematic review. Breast Cancer Res Treat. 2017;164:537–555. doi: 10.1007/s10549-017-4282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flesch-Janys D, Slanger T, Mutschelknauss E, Kropp S, Obi N, Vettorazzi E, et al. Risk of different histological types of postmenopausal breast cancer by type and regimen of menopausal hormone therapy. Int J Cancer. 2008;123:933–941. doi: 10.1002/ijc.23655. [DOI] [PubMed] [Google Scholar]
- 20.Aleman BMP, Moser EC, Nuver J, Suter TM, Maraldo MV, Specht L, et al. Cardiovascular disease after cancer therapy. EJC Suppl. 2014;12:18–28. doi: 10.1016/j.ejcsup.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. 2012;110:1097–1108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. Int J Epidemiol. 2016;45:1887–1894. doi: 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- 23.Lorenz E, Blettner M, Lange B, Schmidt M, Schneider A, Schwentner L, et al. Prevalence of cardiac disease in breast cancer patients at time of diagnosis compared to the general female population in Germany. Breast Care (Basel) 2018;13:264–271. doi: 10.1159/000487261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weaver KE, Foraker RE, Alfano CM, Rowland JH, Arora NK, Bellizzi KM, et al. Cardiovascular risk factors among long-term survivors of breast, prostate, colorectal, and gynecologic cancers: a gap in survivorship care? J Cancer Surviv. 2013;7:253–261. doi: 10.1007/s11764-013-0267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wollschläger D, Meng X, Wöckel A, Janni W, Kreienberg R, Blettner M, et al. Comorbidity-dependent adherence to guidelines and survival in breast cancer-Is there a role for guideline adherence in comorbid breast cancer patients? A retrospective cohort study with 2137 patients. Breast J. 2018;24:120–127. doi: 10.1111/tbj.12855. [DOI] [PubMed] [Google Scholar]
- 26.Kropp S, Terboven T, Hedicke J, Mutschelknauss E, Slanger T, Braendle W, et al. Good agreement between physician and self-reported hormone therapy data in a case-control study. J Clin Epidemiol. 2007;60:1280–1287. doi: 10.1016/j.jclinepi.2007.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Directed acyclic graph for the association of CVD and all-cause mortality. Green arrow= causal path, red arrows= biasing paths.
Additional file 2. Mortality from any cancer for women aged <65 years.
Additional file 3. Mortality from any cancer for women aged ≥65 years.
Additional file 4. Supplemental Tables 1–3
Data Availability Statement
The datasets analysed during the current study are not publicly available due to data protection fundamentals but are available from the corresponding author on reasonable request.