Abstract
Background
Balloon pulmonary angioplasty (BPA) is rapidly evolving therapeutic option for patients with nonsurgical chronic thromboembolic pulmonary hypertension (CTEPH). There are few U.S. studies that have reported on the outcomes of this novel therapeutic option.
Objectives
The authors sought to evaluate the efficacy and safety of refined BPA in the treatment of patients with CTEPH.
Methods
This is a retrospective study of CTEPH patients that underwent BPA. The primary efficacy endpoint was the change in pulmonary vascular resistance after BPA as compared to baseline and the primary safety endpoint was the rate of hemoptysis within 24 hours. Secondary endpoints included death, World Health Organization functional class, and 6-minute walk distance. Logistic regression was used to evaluate factors associated with a hemodynamic and functional response.
Results
A total of 211 BPA sessions were performed on 77 patients (average 2.7 ± 1.7 sessions/patient). After BPA the mean pulmonary vascular resistance improved by 26% (6.5 ± 3.4 WU to 4.8 ± 2.9 WU, P < 0.001). The mean 6-minute walk distance improved by 71.7 m (P < 0.001), and WHO functional class improved by 1 functional class (P < 0.001). There was one death related to reperfusion lung injury. Ten sessions (4.7%) were complicated by hemoptysis. Independent factors associated with improved functional and hemodynamic response included preprocedural use of riociguat, reduce baseline PA compliance, and >3 BPA sessions/patient.
Conclusions
This single center study from the United States showed that BPA with refined techniques in patients with CTEPH was relatively safe and was associated with significant improvements in pulmonary hemodynamics and functional capacity.
Key words: balloon pulmonary angioplasty, chronic thromboembolic pulmonary disease, chronic thromboembolic pulmonary hypertension
Central Illustration
Chronic thromboembolic pulmonary hypertension (CTEPH) and chronic thromboembolic pulmonary disease (CTEPD) are obstructive pulmonary vascular disorders that affect patients who have experienced a pulmonary embolism (PE).1 Although most PE events resolve with minimal residual pulmonary vascular abnormalities, there is a subset of patients who go on to develop symptomatic pulmonary vascular obstruction due to organized thrombus. If left untreated, this may result in pulmonary hypertension and right ventricular dysfunction associated with considerable morbidity as well as a mortality risk.2,3
Though the treatment of choice for CTEPH is surgical pulmonary thromboendarterectomy (PTE),4 for patients deemed inoperable or at very high risk for PTE, balloon pulmonary angioplasty (BPA) is rapidly evolving as an effective therapeutic option. Recent 2022 European Society of Cardiology and European Respiratory Society guidelines have recommended BPA as Class I treatment for patients with inoperable disease or those with residual symptomatic CTEPH after PTE.5 BPA has been studied as a modality of treatment in such patients since early 2000.6,7 The early results from the United States showed a high rate of major complications including mortality, hemoptysis, and the need for positive pressure ventilation, which led to poor adoption of BPA in this country.6 During the past decade in Japan and Europe, the technique of BPA has been evolving including use of pressure wire guidance and under sizing balloons in initial sessions, which has been referred to as “refined BPA”.7 These refinements have resulted in notable improvements in safety, cardiopulmonary hemodynamics, as well as functional capacity.8 These results have encouraged the adoption of this procedure in the United States. However there is very limited data from the United States, describing the outcomes of BPA in these patients.9,10
In this retrospective study, we evaluated the effects of BPA on pulmonary vascular resistance (PVR) and World Health Organization (WHO) functional class among all patients who underwent BPA in our tertiary care referral center. We further evaluated the parameters that were associated with a positive functional and hemodynamic response to BPA.
Methods
Study design and patient selection
A retrospective chart review was conducted of all patients who underwent BPA after a comprehensive evaluation by the Temple University Hospital Pulmonary Hypertension, Right Heart Failure, and CTEPH program within the Temple Heart and Vascular Institute. Patients were evaluated by an expert multidisciplinary CTEPH team, which includes PH/CTEPH cardiology experts, interventional cardiology, cardiothoracic surgery, and diagnostic radiology. Patients provided informed consent for the procedure, and the study was approved by our Institutional Review Board. Consecutive patients who underwent BPA for CTEPH and CTEPD between August 2015 and May 2022 were included. The primary efficacy endpoint was reduction in PVR and the primary safety endpoint was the rate of hemoptysis within 24 hours of the procedure. Secondary effectiveness endpoints included change in WHO functional class, 6-minute walk distance (6MWD) (in meters), change in cardiac output and index, mean pulmonary artery (PA) pressure, PA compliance, and plasma B-type natriuretic peptide (BNP) levels. The secondary safety endpoints included any procedure-related adverse events including reperfusion lung injury, PA dissection without hemoptysis, hemoptysis after 24 hours of BPA to 30 days, and death. We also performed a subgroup analysis to assess the factors associated with a positive hemodynamic and/or functional response.
Preoperative evaluation
All patients with CTEPH and CTEPD were presented at a weekly multidisciplinary CTEPH conference with extensive case reviews. History and physical examinations were reviewed, including history of previous venous thromboembolism and pulmonary hypertension (PH) medical therapy. Imaging studies reviewed included echocardiography, contrast enhanced lung computed tomography, ventilation/perfusion (V/Q) lung scintigraphy, invasive hemodynamic parameters, catheter-directed pulmonary angiography, and when available, invasive or noninvasive cardiopulmonary exercise testing. The multidisciplinary team then selected patients for BPA, consisting of patients with inoperable CTEPH, those with residual PH post-PTE or patients who were deemed too high risk for PTE surgery. All patients had 6MWD, BNP, baseline right heart catheterization, and selective pulmonary angiography before BPA. Patients with normal resting hemodynamics underwent cardiopulmonary exercise testing prior to BPA.
BPA technique
After obtaining informed consent (which includes a discussion of the unconfirmed benefit and risks of hemoptysis and death), a right heart catheterization usually via a common femoral vein approach was performed using a 7-F Swan Ganz catheter with a 0.035-inch guidewire lumen. The catheter is exchanged over a 0.035-inch storq wire for a 7-F 70-cm long sheath whose distal tip is positioned in the lobar artery. Through this sheath a 6-F guiding catheter (usually multipurpose or JR-4 for the right PA branches and AL1 for the left PA branches) is used to selectively cannulate segmental vessels and a baseline angiography is performed (Figure 1). The location of these selective angiograms is decided based on the review of the baseline studies including computed tomography angiography of chest, V/Q scan, and nonselective pulmonary angiograms. Once the lesion is identified, a 300 cm long radi pressure wire (Abbott Laboratories) is used to cross the lesion and assess the distal pressure and waveform. If the exact location of the lesion is not clear angiographically, gradual pullback of the pressure wire is performed. When the pressure transducer reaches the site of the lesion, the pressure tracing suddenly changes into a ventricularized pressure waveform; further pullback proximal to the lesion, will demonstrate a normal PA pressure waveform similar to the one transduced by the guiding catheter (Central Illustration). Following this the wire is re-advanced again into the distal vessel. The BPA is then performed over the pressure wire usually with a small over-the-wire balloon (eg, 2.0 × 20 mm noncompliant balloon) and its effect on distal pressure is monitored continuously. We continue to dilate until the mean distal pressure reaches a value around 35 mm Hg,8 there is restoration of pulsatile flow and adequate pulmonary venous return is seen. For lesions that cannot be crossed with a pressure wire, we switch over to 0.014-inch workhorse wires like a Hi-Torque balance middle weight or Runthrough wire. Our rationale for routinely using the pressure wire in these cases included the following: 1) we can accurately identify the location of the lesions that are not angiographically well visualized; 2) in patients with high PA pressures (>35 mm Hg) we keep the pressure distal to the lesion <35 mm Hg mean, which has been shown to reduce reperfusion pulmonary edema;8 and 3) we have found the exchange length pressure wire over a balloon is able to cross two-third of these lesions, which in turn avoids an added step of exchanging out the pressure or support catheter.
Figure 1.
Selective Angiogram of Right Pulmonary Artery Segmental Branches
Selective angiogram of the right pulmonary artery segmental arteries shows distal disease (arrows) in these branches which is not well visualized in the nonselective angiograms. CTEPH = chronic thromboembolic pulmonary hypertension.
Central Illustration.
Refined Balloon Pulmonary Angioplasty Technique and Outcomes
(A) This figure shows a typical pressure waveform (red arrows) as measured by a pressure wire distal to the lesion, within the lesion where the waveform is ventricularized and distal to the lesion after balloon inflation with an undersized balloon and after dilatation with optimal balloon diameter. (B) Shows the effect of BPA on pulmonary vascular resistance, 6 minute walk distance, B-type natriuretic peptide, and on pulmonary artery compliance. BPA = balloon pulmonary angioplasty.
The number of segments that we revascularized in one session depended on the risks of reperfusion lung injury. If patient had very high PVR (>12 WU) we would limit revascularization to one lobe and not treat chronic total occlusions in the first session. On the other hand, if a patient had low PVR (<6 WU) we will revascularize more than one lobe in a single session. We use Iodixanol 320 (Visipaque, GE Healthcare), which is diluted with 30% normal saline. In patients with abnormal renal function, we would limit the contrast volume to 3 times the glomerular filtration rate. We prefer to revascularize lower lobes first, as the volume of alveolar perfusion is greatest in the lower lobes. All procedures were done under conscious sedation and supplemental oxygen to keep oxygen saturations >98%. Outpatient anticoagulation regimen was held prior to BPA and intravenous heparin is used during the procedure with a goal ACT of >200. Therapeutic anticoagulation was resumed 1 hour after sheath removal unless the patient experienced a bleeding complication.
Postprocedural management
Following completion of BPA, patients are given a dose of intravenous diuretic before they leave the catheterization laboratory. Patients were monitored in the cardiac intensive care unit during our early experience (from August 2015 to December 2019) and after that most of the patients were monitored on the PH and heart failure telemetry floor service barring any intraprocedural complications. PH-specific medical therapy and diuretics were continued as deemed necessary by the PH/CTEPH cardiologists. Chest radiography was obtained the following morning and patients were monitored overnight for hemoptysis, reperfusion lung injury, dyspnea, and hypoxia. After discharge, patients had follow-up outpatient evaluation with our PH/CTEPH cardiology experts. Follow-up outpatient studies included transthoracic echocardiography, V/Q scan, right heart catheterization, and a 6MWD. However, during the peak of the COVID-19 pandemic between 2020 and 2021, many patients were not able to get these tests or right heart catheterization as they were evaluated virtually via video telehealth encounter.
Hemodynamic and functional responders
In absence of an established definition, we defined hemodynamic responders as those patients whose PVR decreased by >10%. Functional responders were defined as those patients who improved their WHO functional class by ≥1. We performed multivariate logistic regression to identify factors associated with a hemodynamic and/or functional response.
Statistical and sensitivity analysis
Continuous variables were reported as mean ± SD and categorical variables were reported as percentages. Continuous variables were compared using a 2-sample student’s t-test and categorical variables were compared using Pearson chi-square test. Univariate analysis was used to identify factors with a hemodynamic and or functional response and if significant, these variables were then tested in a multivariate logistic regression analysis model. All statistical analyses were considered statistically significant if the P values were <0.05. Sensitivity analysis was performed using multiple imputation (MI) to address missing data for follow-up PVR.11 The MI was performed in the following 3 steps: 1) the missing follow-up PVR data (n = 13 [16.9%]) were filled in 100 times to generate 100 complete data sets; 2) the 100 data sets were analyzed by paired t-tests to compare baseline and post-BPA PVR values; and 3) the results from the 100 complete data sets were combined for the MI inference.11 SAS PROC MI and PROC MIANALYZE were used for the multiple-imputation analysis. The software used for all the analysis was SAS version 9.4 (SAS Institute Inc).
Results
Seventy-seven patients underwent a total of 211 BPA sessions at Temple University Hospital between August 2015 and May 2022. Baseline characteristics of the study population are shown in Table 1. The mean age of the patients was 62 ± 14.1 years, and 40 (51.9%) were female. Twenty-one (27.3%) patients had undergone prior PTE, 33 (42.9%) had inoperable CTEPH, 21 (27.3%) were determined to be at prohibitively high surgical risk due to comorbidities, and 2 (2.6%) declined PTE. Fifteen (19.2%) patients are still undergoing their BPA treatment course.
Table 1.
Baseline Characteristics of Total Cohort (N = 77)
| Age (y) | 61.9 ± 14.1 |
| Female | 40 (51.9) |
| Race | |
| Asian | 1 (1.3) |
| Black | 19 (24.7) |
| Hispanic | 7 (9.1) |
| White | 50 (64.9) |
| Body mass index (kg/m2) | 30.3 ± 8.2 |
| WHO functional class | |
| I | 6 (7.9) |
| II | 17 (22.4) |
| III | 35 (46.1) |
| IV | 18 (23.7) |
| 6 min walk distance (m) | 391 ± 248 |
| Baseline PVR <4 WU | 23 (29.8) |
| Baseline PVR <3 WU | 11 (14.2) |
| Comorbidities | |
| History of deep venous thrombosis | 32 (41.6) |
| History of pulmonary embolism | 55 (71.4) |
| May Thurner syndrome | 3 (3.9) |
| Uterine fibroid | 6 (7.8) |
| Pacemaker/venous port | 7 (9.1) |
| Splenectomy | 6 (7.8) |
| Hemoglobinopathy | 6 (7.8) |
| Coagulopathy | 8 (10.5) |
| Venous malformation | 4 (5.2) |
| History of cancer | 16 (20.8) |
| Atrial fibrillation | 6 (7.9) |
| Diabetes mellitus | 10 (13) |
| Thyroid disease | 10 (13) |
| Asthma | 10 (13) |
| Home oxygen use | 15 (19.5) |
| Hypertension | 34 (44.2) |
| Chronic kidney disease | 9 (11.7) |
| Chronic obstructive pulmonary disease | 15 (19.5) |
| Liver disease | 1 (1.3) |
| Coronary artery disease | 5 (6.5) |
| Stroke | 3 (3.9) |
| Sleep disorders | 20 (26) |
| Tobacco use | 29 (37.7) |
| Drug abuse | 2 (2.6) |
Values are mean ± SD or n (%).
PVR = pulmonary vascular resistance; WHO = World Health Organization.
The remaining 62 patients completed all their BPA sessions, which was determined by having achieved clinical goals, like WHO functional class 2 or less, PVR of <3, right ventricle (RV) function by echocardiography normal or near normal, or comorbidities that would limit any further functional improvement or patient refused any further BPA sessions.
Forty-eight of the 77 patients (62.3%) were receiving PH medical therapies, the most common being riociguat (39.0%), with phosphodiesterase 5 inhibitor (PDE5i) therapy (sildenafil or tadalafil) in 22.1% of patients (Table 2). Twenty-two (28.5%) patients were on warfarin, 47 (61%) on direct oral anticoagulants, and 6 (7.8%) were on enoxaparin. Eleven (14.3%) patients were also taking aspirin; 2 (2.6%) were taking clopidogrel. Eleven patients had an invasive CPET pre-BPA (mild to moderate CTEPH = 7 and CTEPD = 4), the findings of which are in the Supplemental Table 1.
Table 2.
Pulmonary Hypertension Medications (N = 77)
| None | 30 (39.0) |
| Riociguat | 30 (39.0) |
| Sildenafil | 12 (15.6) |
| Macitentan | 10 (13.0) |
| Ambrisentan | 3 (3.9) |
| Tadalafil | 5 (6.5) |
| Parenteral treprostinil | 1 (1.3) |
| Oral treprostinil | 1 (1.3) |
| Selexipag | 1 (1.3) |
| Treprostinil | 1 (1.3) |
| 1 PH medication | 33 (42.9) |
| 2 PH medications | 11 (14.3) |
| 3 PH medications | 3 (3.3) |
Values are n (%).
PH = pulmonary hypertension.
The average number of BPA sessions per patient was 2.7 ± 1.7 sessions (Figure 2). The mean number of lesions treated per session was 5.03 ± 3.35 lesions. Thirty five patients (45.4%) underwent >3 sessions and 42 patients had only 1 or 2 BPA sessions. More than 60% of the lesions were crossed with pressure wire, with pressure wire measurements being mostly qualitative, given the majority of patients had a pressure waveform distal to the stenosis similar to pulmonary capillary wedge pressure tracing before BPA intervention. Following BPA intervention, the distal pressure waveform would change into a pulsatile tracing, and it was this pressure we monitored with progressively increasing balloon sizes, with the goal to keep distal mean PA pressure <35 mm Hg in those patients whose mean pulmonary arterial pressure (mPAP) was >35 mm Hg.8 The balloon sizes ranged from 1.5 to 9 mm (mean: 2.75 ± 1.2 mm). In the initial sessions we undersized the balloon just to restore the flow into an occluded segment and then in subsequent sessions we used appropriately sized balloons. We use intravascular ultrasound for balloon sizing for proximal lesions in the interlobar or truncus lesions but avoid this for the distal lesions, where we use visual estimation. Post-PTE patients accounted for 24.4% (52/211) of all the sessions. Other procedural characteristics are shown in Table 3.
Figure 2.
Number of Sessions per Patient
This figure shows how many sessions each patient had during the study period. The median number of sessions was 2.0 and the average number of sessions was 2.7. Seven patients had more than 4 sessions.
Table 3.
Procedural Characteristics (N = 211)
| Mean number of BPA sessions per patient | 2.7 ± 1.7 |
| Mean number of lesions treated per BPA session | 5.03 ± 3.35 |
| No. of patients treated with ≥3 BPA sessions | 35 (45.0) |
| Mean number of lesions treated per patient | 12.8 (9.3) |
| Mean number of CTOs treated per patient | 0.9 (1.3) |
| Median contrast used per BPA session (cc) | 270 (200-330) |
| Median fluoroscopy time per session (min) | 59 (45-70) |
| Median dose of radiation (mGy) | 429 (258-628) |
| Guide catheters used (in the order of frequency) | MP, JR4, and AL1 |
| Wires used most commonly (in the order of frequency) | Radi, BMW, and Runthrough |
| Mean balloon size (minimum to maximum), mm | 2.75 ± 1.2 (1.5-9.0) |
Values are mean ± SD, n (%), or median (IQR [25th-75th]).
BMW = balance middle weight; BPA = balloon pulmonary angioplasty; CTO = chronic total occlusions.
The primary endpoint of the change in mean PVR showed a decrease from 6.5 ± 3.4 WU to 4.8 ± 2.9 WU (mean change: 1.7 ± 2.8; P < 0.001). The mean 6MWD improved from 354.5 ± 131.4 m to 426.2 ± 124.3 m (P < 0.0001). WHO functional class improved from a median of 3 at baseline to 2 after BPA (P < 0.0001), Figure 3. The mean BNP level decreased from 206.7 pg/mL to 83.5 pg/mL (P < 0.0001). PA compliance increased by 23.8% from 1.89 ± 0.9 mL/mm Hg to 2.34 ± 1.2 mL/mm Hg (increase of 0.45 ± 1.2; P = 0.003) (Central Illustration). Cardiac index increased from 2.43 ± 0.5 L/min/m2 to 2.53 ± 0.4 L/min/m2 (increase of 0.11 ± 0.48) which was not statistically significant (P = 0.07). The mean PA pressure decreased from 41.0 ± 13.6 mm Hg to 34.6 ± 11.3 mm Hg (P < 0.0001) (Table 4).
Figure 3.
WHO Functional Class Before and After Balloon Pulmonary Angioplasty
This figure shows the improvement in WHO functional class before and after the balloon pulmonary angioplasty. BPA = balloon pulmonary angioplasty; WHO = World Health Organization.
Table 4.
Clinical and Hemodynamic Data Before and After Balloon Pulmonary Angioplasty
| Baseline | Post BPA | Change | P Value | |
|---|---|---|---|---|
| WHO functional class | (n = 65) | (n = 65) | ||
| Median | III | II | I | |
| Mean ± SD | 2.85 ± 0.85 | 1.89 ± 0.92 | 0.95 ± 0.94 | <0.001 |
| I | 4 (6.2) | 26 (40.0) | ||
| II | 17 (26.2) | 25 (38.5) | ||
| III | 29 (44.6) | 9 (13.8) | ||
| IV | 15 (23.1) | 5 (7.7) | ||
| 6 min walk distance (m) | (n = 40) | (n = 40) | ||
| 354.5 ± 131.4 | 426.2 ± 124.3 | 71.7 ± 100.9 | <0.001 | |
| B-type natriuretic peptide (pg/mL) | (n = 48) | (n = 48) | ||
| Median (IQR, 25-75) | 114 (53-235) | 40 (21-98.6) | ||
| Mean ± SD | 206.7 ± 197.8 | 83.5 ± 93.7 | 123.2 ± 183.2 | <0.001 |
| Hemodynamics | (n = 65) | (n = 65) | ||
| Right atrial pressure (mm Hg) | 9.0 ± 4.4 | 9.0 ± 11.9 | 0.0 ± 12.0 | 1.00 |
| Systolic PA pressure (mm Hg) | 65.8 ± 19.2 | 57.6 ± 20.8 | 8.2 ± 16.2 | <0.001 |
| Diastolic PA pressure (mm Hg) | 24.0 ± 8.0 | 21.0 ± 7.2 | 3.0 ± 7.7 | 0.0028 |
| Mean PA pressure (mm Hg) | 41.0 ± 13.6 | 34.6 ± 11.3 | 6.4 ± 13.3 | <0.001 |
| Pulmonary capillary wedge pressure (mm Hg) | 11.9 ± 3.5 | 11.8 ± 3.9 | 0.12 ± 4.2 | 0.81 |
| Pulmonary vascular resistance (WU) | 6.5 ± 3.4 | 4.8 ± 2.9 | 1.7 ± 2.8 | <0.001 |
| Systemic vascular resistance (dynes/s/cm−5) | 1,299 ± 631 | 1,364 ± 318 | 65 ± 631 | 0.51 |
| Cardiac output (L/min) | 4.72 ± 0.98 | 4.96 ± 1.1 | 0.24 ± 0.98 | 0.05 |
| Cardiac index (L/min/m2) | 2.43 ± 0.5 | 2.53 ± 0.4 | 0.11 ± 0.48 | 0.07 |
| PA compliance (mL/mm Hg) | 1.89 ± 0.9 | 2.34 ± 1.2 | 0.45 ± 1.2 | 0.003 |
Values are mean ± SD or n (%) unless otherwise indicated. Since these are paired tests and the number of pairs that data was available and tested for comparison are shown.
BPA = balloon pulmonary angioplasty; PA = pulmonary artery; WHO = World Health Organization.
While follow-up echocardiography data were limited to a smaller cohort within our study, the available data in 41 patients demonstrated significant improvements in the degree of RV function as quantified by tricuspid annular systolic excursion, RV size, degree of tricuspid regurgitation, PA systolic pressure estimation, the degree of systolic interventricular septal flattening, and the RV base:apex ratio (Table 5).
Table 5.
Echocardiographic Outcomes
| Baseline (n = 41) | Post BPA (n = 41) | P Value (Paired t-Test) | |
|---|---|---|---|
| Pulmonary artery systolic pressure (mm Hg) | 53.4 ± 20.9 | 48.4 ± 17.8 | <0.001 |
| Left ventricular ejection fraction (%) | 61.8 ± 3.6 | 63.8 ± 4.5 | <0.001 |
| TAPSE (CM) | 1.83 ± 0.34 | 2.1 ± 0.38 | <0.001 |
| Septal flattening | 0.003 | ||
| None | 4 (9.8) | 3 (18.8) | |
| Mild | 17 (41.5) | 11 (68.8) | |
| Moderate | 13 (31.7) | 2 (12.5) | |
| Severe | 6 (14.6) | 0 (0) | |
| RV size | 0.351 | ||
| Normal | 2 (4.9) | 8 (50.0) | |
| Mild enlargement | 7 (17.1) | 1 (6.5) | |
| Moderate enlargement | 26 (63.4) | 6 (37.5) | |
| Severe enlargement | 5 (12.2) | 4 (6.3) | |
| RV apex to base ratio | 0.012 | ||
| Normal | 10 (24.4) | 10 (62.5) | |
| Mild | 12 (29.3) | 5 (31.3) | |
| Moderate | 17 (41.5) | 1 (6.3) | |
| Severe | 1 (2.4) | 0 (0) | |
| RV function | 0.974 | ||
| Normal | 5 (7.0) | 7 (43.8) | |
| Mildly dysfunctional | 32 (45.1) | 8 (50) | |
| Moderately dysfunctional | 24 (33.8) | 1 (6.3) | |
| Severely dysfunctional | 10 (14.1) | 0 (0) | |
| RVOT notch | N/A | ||
| None | 6 (14.6) | 12 (80.0) | |
| Late systolic notching | 25 (60.9) | 1 (6.7) | |
| Mid systolic notching | 9 (21.9) | 2 (13.3) | |
| Tricuspid valve regurgitation | 0.035 | ||
| Mild | 25 (60.9) | 7 (43.8) | |
| Moderate | 9 (21.9) | 8 (50.0) | |
| Severe | 1 (19.4) | 1 (6.3) |
Values are mean ± SD or n (%).
BPA = balloon pulmonary angioplasty; RV = right ventricle; RVOT = right ventricular outflow tract; TAPSE = tricuspid annular systolic excursion.
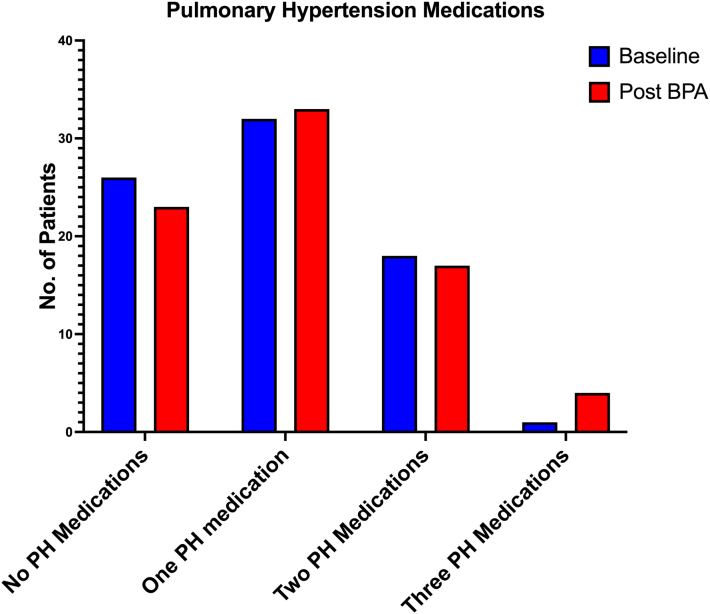
The average follow-up duration after the first BPA session was 15.0 ± 17.9 months. Patient overall survival rate was 92%. There were 6 deaths in total of which 1 was related to BPA and CTEPH, while as 5 others were from motor vehicle accident, recreational drug overdose, severe left heart failure, and 2 were from COVID-19. Seven (47%) of the 15 patients who were on home oxygen before the BPA, were able to come off the oxygen after the BPA. There was no change in the overall number of PH medications before and after BPA. Three patients were able to discontinue all their PH medications whereas 3 patients with incomplete BPA’s were treated with one additional PH medication (Figure 4).
Figure 4.
Pulmonary Hypertension Medications Before and After Balloon Pulmonary Angioplasty
This figure shows that the number pulmonary hypertension medications were similar before and after the Balloon pulmonary angioplasty. BPA = balloon pulmonary angioplasty.
Procedure-related complications
Three patients (1.4%) had major procedure-related adverse events, which included 1 death related to reperfusion lung injury, 1 pulmonary hemorrhage, and 1 mild hemoptysis that was treated with mechanical ventilation. Ten BPA sessions (4.7%) were complicated by hemoptysis, and all occurred at the time of the procedure (Table 6). The hemoptysis rate was 5.7% (3/52) in post-PTE patients and it was 4.3% (7/161) in patients who did not have prior PTE. Nine patients had mild hemoptysis of <50 cc by visual estimation and one had major hemoptysis (pulmonary hemorrhage) of >50 cc which required mechanical ventilation and embolization of the PA branch which was performed immediately by the interventional cardiologists performing the BPA. The only other patient that required mechanical ventilation experienced mild hemoptysis and was intubated as a precautionary measure, as this was very early in our BPA experience. This patient later returned for repeat BPA sessions without any subsequent adverse events. Both patients were extubated within 24 hours and discharged home. None of the patients had hemoptysis after 24 hours of BPA. The third serious complication occurred in one patient that led to death and was related to reperfusion lung injury requiring extracorporeal membrane oxygenation. This patient had been turned down multiple times by the surgical team due to comorbidities including a body mass index of 80 kg/m2, obesity hypoventilation, reactive airway disease, and severe physical deconditioning placing her at an unacceptably high operative risk. Although her anticipated BPA risk was also high due to comorbidities, her hemodynamic status, RV function, and exercise capabilities were so severely impaired despite PH medical therapy that the patient and the multidisciplinary CTEPH team opted to pursue BPA. None of the patients developed acute kidney injury.
Table 6.
Procedural Complications (N = 211)
| Death related to BPA | 1/77 (1.3) |
| Hemoptysis, mild | 9/211 (4.2) |
| Hemoptysis, moderate to severe | 1/211 (0.47) |
| Mechanical ventilation | 2/211 (0.95) |
| Use of ECMO | 1/211 (0.47) |
| Delayed onset lung injury | 1/77 (1.3) |
| Acute kidney injury | 0/211 (0) |
| Major adverse event related to BPA | 3/77 (3.8) |
Values are n/N (%).
BPA = balloon pulmonary angioplasty; ECMO = extracorporeal membrane oxygenation.
Factors associated with hemodynamic and/or functional response
The factors associated with a favorable hemodynamic and/or functional response to the BPA included the following: use of riociguat prior to BPA, higher number of BPA sessions (for every additional BPA session the odds ratio of functional and/or hemodynamic response was 3.3-fold higher), lower baseline PA compliance (an increase of 1 in baseline PA compliance reduces the chances of being a responder by 55%) (Table 7). The baseline PVR >4.0 was also associated with a positive response; however, it was not statistically significant (Figure 5). On multivariate logistic regression, the independent variables associated with a hemodynamic and/or functional response included the number of BPA sessions the patient had and baseline riociguat treatment prior to BPA. The median number of sessions amongst responders was 3.0 and in nonresponders was 1.0 BPA sessions, which most likely reflects the burden of PA obstruction and its role in causing patient’s symptoms. The hemodynamic and/or functional responders had an increase in PA compliance of 0.5 mL/mm Hg as compared to nonresponders whose PA compliance increased by only 0.2 mL/mm Hg after BPA. The variable associated with a poor response was the normal baseline PA compliance. The mean reduction in PVR in hemodynamic responders was 34.3% ± 18.8% (median 32.8%, IQR: 17.3%-48.8%)
Table 7.
The Univariate Factors of Hemodynamic and or Functional Response to Balloon Pulmonary Angioplasty
| Overall (N = 72) | Responder (n = 61) | Nonresponders (n = 11) | P Value | Odds Ratio (95% CI) | Increment | |
|---|---|---|---|---|---|---|
| Riociguat use prior to BPA | 27 (37.5) | 27 (100.0) | 0 (0.0) | 0.0034 | 11.6 (2.28–342) | |
| Baseline PVR wood units | 6.2 ± 3.3 | 6.5 ± 3.4 | 4.3 ± 2.4 | 0.048 | 1.85 (1.01–3.41) | 2 |
| Baseline PVR >4.0 WU | 50 (69.4) | 45 (90) | 5 (10.0) | 0.07 | 3.38 (0.90–12.59) | 2 |
| Number of sessions per patient | 2.0 (2.0-4.0) | 3.0 (2.0-4.0) | 1.0 (1.0-2.0) | 0.008 | 3.33 (1.36–8.13) | 1 |
| Baseline PA compliance | 1.9 ± 1.0 | 1.8 ± 0.7 | 2.8 ± 1.8 | 0.015 | 0.45 (0.23–0.86) | 1 |
| Baseline PA compliance >2.3 mL/mm Hg | 21 (29.3) | 15 (71.4) | 6 (28.6) | 0.053 | 0.27 (0.07–1.02) | 1 |
| No. of segments per patient | 8.2 ± 4.4 | 8.7 ± 4.3 | 5.5 ± 4.3 | 0.033 | 1.78 (1.05–3.04) | 3 |
| No. of vessels per patient | 12.8 ± 9.3 | 13.8 ± 9.4 | 7.7 ± 7.6 | 0.061 | 1.93 (0.97–3.84) | 7 |
| No. of CTO per patient | 0.9 ± 1.3 | 1.0 ± 1.4 | 0.4 ± 0.5 | 0.18 | 1.72 (0.77–3.86) | 1 |
Values are n (%), mean ± SD, or median (IQR).
BPA = balloon pulmonary angioplasty; CTO = chronic total occlusion; PA = pulmonary artery; PVR = pulmonary vascular resistance.
Figure 5.
Factors Associated With a Functional and Hemodynamic Response Following Balloon Pulmonary Angioplasty
This is a Forrest plot showing the log of the odds ratios associated with the functional and hemodynamic response after a balloon pulmonary angioplasty in patients with CTEPH. In the multivariable logistic regression analysis, the independent variables of this response were use of riociguat and number of BPA sessions. Another variable associated with a lack of response was the normal pulmonary artery compliance. BPA = balloon pulmonary angioplasty; CTO = chronic total occlusion; PA = pulmonary artery; PVR = pulmonary vascular resistance.
Sensitivity analysis and missingness
There are 65 patients with follow-up PVR and 12 patients without follow-up PVR. Demographics and clinical baseline characteristics were generally similar between the 2 groups. We addressed the missingness of the data using MIs method (see the results in Supplemental Appendix). The results of our study were similar by MI.
Discussion
This single center observational study represents one of the largest, reported experiences of American patients treated with BPA. We found that BPA using refined techniques was associated with a significant improvement in pulmonary hemodynamic parameters as defined by a reduction in both PVR and mPAP. Patients treated with BPA experienced improvement in functional class and exercise capabilities, with relatively low rates of procedure-related complications. The use of riociguat prior to BPA was associated with a beneficial response. A novel finding in our study was that normal baseline PA compliance of >2.3 mL/mm Hg was associated with lack of hemodynamic and functional response to BPA.
BPA was associated with significant improvements in invasive hemodynamic parameters with a 26% (1.7 WU) improvement in PVR and a 15% (6.4 mm Hg) reduction in mPAP. Though these data are similar to the results from recently published randomized controlled studies, the degree of improvement in those studies were larger with regards to reduction in PVR (26% vs 60%) and mPAP (6.4 mm Hg vs 15 mm Hg improvement).12, 13, 14 This difference may be due to several reasons, the most important one being the lower baseline PVR and lesser number of BPA sessions in our cohort. In our cohort, 30% of the subjects had a PVR <4 WU, while comparative studies have only included patients with a PVR >4 WU. In the current study the average number of BPA sessions was 2.7 as compared 4.7 sessions in multicenter randomized controlled trial based on BPA and 7.7 sessions per patient in the RACE (Rate Control vs Electrical Cardioversion for Persistent Atrial Fibrillation Study) trial.13,14 Other possible factors include operator experience, differences in patient population, and differences in inoperability criteria. Additionally 27% of patients in our cohort were post PTE patients, a group that has traditionally lower magnitude of reduction in PVR.15
Recently, pulmonary arterial compliance (PAC) has been shown to be an important measure of RV afterload along with PVR, and closely correlates with prognosis and functional improvement.16, 17, 18, 19 This study demonstrated an improvement in PAC after BPA, which further supports the hemodynamic as well as functional status efficacy of BPA intervention. This is especially relevant considering that thromboembolic disease is often a multicompartmental pulmonary vascular pathology, affecting both small and large arterial segments, with reduced PAC being present in some CTEPH patients despite a lower or normal resting PVR.20 One novel finding of this study was that normal PA compliance at baseline was associated with lack of hemodynamic and functional status benefit. Future studies should consider evaluating this parameter prospectively to assess if a normal compliance may suggest futility of BPA in these patients.
All qualitative echo-Doppler features that reflect a reduction in PVR including RV base:apex ratio, right ventricular outflow tract pulse wave Doppler notching, and systolic interventricular septal flattening, showed improvements in this study. In addition, there were significant improvements noted in RV size and function. Published echo-Doppler outcomes after BPA are limited. Prior studies have shown these variables correlate favorably with clinical and hemodynamic outcomes including survival and functional capacity in patients with CTEPH.21 In this study echo-Doppler data seem to parallel our PTE experience,22 and support the notion that BPA intervention led to physiologically significant improvements in right heart afterload, and in turn improved RV systolic function and reverse remodeling of the right heart.
BPA treatment was associated with significant improvements in WHO functional class and 6MWD without any change in the number of PH specific medications. There was an improvement in mean 6MWD by 71.7 m after BPA, and an improvement in mean WHO class by 1 level. These results are consistent with those of previous studies.7,8,12,23, 24, 25, 26, 27, 28 The improvements we observed in exercise and functional capacity after BPA are promising, and at times life-changing, particularly in the young and physically active patients. In addition almost one-half of the patients that were on home oxygen were able to come off oxygen, which can dramatically improve the quality of life in these patients.29
One of the most notable findings of this study is the low procedure-related complications with a hemoptysis rate of 4.7% and procedure related mortality of (1.3%). Other studies have reported a hemoptysis rate ranging from 6% to 22%.13,14,30,31 Refinements of technique have made BPA a safer procedure.8 The low rates of adverse events in this study may be related to the routine use of pressure wire guidance in our interventions. Prior studies have shown that treating target lesions to a goal distal mean PA pressure of 35 mm Hg or less resulted in fewer complications.8,32,33 Contemporary published experiences in BPA do not utilize this approach.7,8,12,23,24,26, 27, 28 Our center attempts to always start with a pressure wire and use other wires when a 300 cm pressure wire with a balloon support cannot cross a lesion. We adhere to this approach, performing multiple staged pressure wire-guided BPA sessions in close proximity, with progressive dilatation using larger balloon diameters during the subsequent sessions. Pretreating patients medically in an attempt to achieve lower PVR prior to BPA could be another reason for reduced procedural complications.14
Prior use of riociguat was a predictor of a favorable response to BPA in our cohort. This finding is consistent with the finding of a recently published randomized controlled RACE trial,14 and may speak to both a more robust PH phenotype seen in patients selected to receive riociguat prior to BPA as well as a potential salient effect of medication pretreatment prior to BPA. In addition, the greater the number of sessions, number of segments, number of vessels revascularized, the better the hemodynamic and functional response.
Study Limitations
First, it is a retrospective, single center cohort study with well-known limitations of this study design. Second, this is an initial report of our ongoing experience with BPA. The modest PVR reduction seen in this study may be related to few BPA sessions per patient and the fact that some of the patients had there last invasive hemodynamics assessed prior to the last BPA session. This is corroborated by the significant improvements noted in the functional, echocardiographic, and biomarkers observed weeks or months after the last recorded BPA session. Third, a portion of our patients have yet to complete their BPA treatment course or follow-up studies, with many currently due for further BPA sessions. In an attempt to remedy this, future studies may contain a subgroup analysis dedicated to the cohort of patients who have completed revascularization in order to best assess the clinical and hemodynamic effects of BPA. Another limitation of this nonblinded study design with follow-up at varied time points is the interventionalist’s bias regarding start and end times of revascularizations. Lastly, our study was conducted during COVID 19 pandemic which significantly affected our in-person clinical follow-up of these patients, and therefore, missing information on tests like 6MWD, echocardiogram, or invasive hemodynamics.
Conclusions
In this single center early BPA experience within the United States, eligible patients undergoing BPA experienced significant improvements in PVR, 6MWD, WHO functional class, and BNP levels following the treatment. This refined BPA technique was safe and well tolerated with a relatively low rate of complications. In conjunction with medical therapy, BPA could be considered a viable treatment option for the inoperable CTEPH/CTEPD and recurrent or persistent PH after PTE.
PERSPECTIVES.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: BPA is a rapidly evolving therapeutic option for patients with nonsurgical CTEPH. There are few U.S. studies that have reported on the outcomes of this novel therapeutic option using refined techniques. The BPA with refined techniques in the US patients with CTEPH was safe and was associated with significant improvements in pulmonary vascular resistance, right ventricular function, and functional capacity.
TRANSLATIONAL OUTLOOK: To assess comparative effectiveness of refined BPA with PH-specific medical therapy as compared to PH-specific medical therapy alone in patients with nonsurgical chronic thromboembolic pulmonary disease and CTEPH using a randomized controlled trial design.
Funding support and author disclosures
The study was supported by Temple Heart and Vascular Institute. Dr Bashir is the co-inventor of the Bashir endovascular catheter and has equity interest in Thrombolex, Inc; and is supported by a NHLBI grant under the SBIR grant mechanism. Dr Brisco-Bacik is currently employed by GSK plc. Dr Auger is a consultant for Janssen pharmaceuticals and Neptune Medical; and is an invited speaker for MERCK and Bayer. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgment
The authors would like to thank Dr Hiromi Matsubara for his guidance and mentoring, while we were getting our BPA program started at Temple University Hospital.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental materials and table, please see the online version of this paper.
Supplementary data
References
- 1.Humbert M., Kovacs G., Hoeper M.M., et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2023;61:2200879. doi: 10.1183/13993003.00879-2022. [DOI] [PubMed] [Google Scholar]
- 2.Galie N., Humbert M., Vachiery J.L., et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015;46:903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 3.Lang I.M., Pesavento R., Bonderman D., Yuan J.X. Risk factors and basic mechanisms of chronic thromboembolic pulmonary hypertension: a current understanding. Eur Respir J. 2013;41:462–468. doi: 10.1183/09031936.00049312. [DOI] [PubMed] [Google Scholar]
- 4.Mayer E., Jenkins D., Lindner J., et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg. 2011;141:702–710. doi: 10.1016/j.jtcvs.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 5.Humbert M., Kovacs G., Hoeper M.M., et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618–3731. doi: 10.1093/eurheartj/ehac237. [DOI] [PubMed] [Google Scholar]
- 6.Feinstein J.A., Goldhaber S.Z., Lock J.E., Ferndandes S.M., Landzberg M.J. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation. 2001;103:10–13. doi: 10.1161/01.cir.103.1.10. [DOI] [PubMed] [Google Scholar]
- 7.Mizoguchi H., Ogawa A., Munemasa M., Mikouchi H., Ito H., Matsubara H. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv. 2012;5:748–755. doi: 10.1161/CIRCINTERVENTIONS.112.971077. [DOI] [PubMed] [Google Scholar]
- 8.Inami T., Kataoka M., Shimura N., et al. Pressure-wire-guided percutaneous transluminal pulmonary angioplasty: a breakthrough in catheter-interventional therapy for chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol Intv. 2014;7:1297–1306. doi: 10.1016/j.jcin.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Anand V., Frantz R.P., DuBrock H., et al. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: initial single-center experience. Mayo Clin Proc Innov Qual Outcomes. 2019;3:311–318. doi: 10.1016/j.mayocpiqo.2019.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahmud E., Patel M., Ang L., Poch D. Advances in balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Pulm Circ. 2021;11 doi: 10.1177/20458940211007385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubin D.B. John Wiley & Sons, Inc; 1987. Multiple Imputation for Nonresponse in Surveys. [Google Scholar]
- 12.Ogawa A., Satoh T., Fukuda T., et al. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: results of a multicenter registry. Circ Cardiovasc Qual Outcomes. 2017;10 doi: 10.1161/CIRCOUTCOMES.117.004029. [DOI] [PubMed] [Google Scholar]
- 13.Kawakami T., Matsubara H., Shinke T., et al. Balloon pulmonary angioplasty versus riociguat in inoperable chronic thromboembolic pulmonary hypertension (MR BPA): an open-label, randomised controlled trial. Lancet Respir Med. 2022;10:949–960. doi: 10.1016/S2213-2600(22)00171-0. [DOI] [PubMed] [Google Scholar]
- 14.Jais X., Brenot P., Bouvaist H., et al. Balloon pulmonary angioplasty versus riociguat for the treatment of inoperable chronic thromboembolic pulmonary hypertension (RACE): a multicentre, phase 3, open-label, randomised controlled trial and ancillary follow-up study. Lancet Respir Med. 2022;10:961–971. doi: 10.1016/S2213-2600(22)00214-4. [DOI] [PubMed] [Google Scholar]
- 15.Hug K.P., Gerry Coghlan J., Cannon J., et al. Serial right heart catheter assessment between balloon pulmonary angioplasty sessions identify procedural factors that influence response to treatment. J Heart Lung Transplant. 2021;40:1223–1234. doi: 10.1016/j.healun.2021.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Mahapatra S., Nishimura R.A., Sorajja P., Cha S., McGoon M.D. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol. 2006;47:799–803. doi: 10.1016/j.jacc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 17.Dupont M., Mullens W., Skouri H.N., et al. Prognostic role of pulmonary arterial capacitance in advanced heart failure. Circ Heart Fail. 2012;5:778–785. doi: 10.1161/CIRCHEARTFAILURE.112.968511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Godinas L., Bonne L., Budts W., et al. Balloon pulmonary angioplasty for the treatment of nonoperable chronic thromboembolic pulmonary hypertension: single-center experience with low initial complication rate. J Vasc Interv Radiol. 2019;30:1265–1272. doi: 10.1016/j.jvir.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 19.Akaslan D., Atas H., Aslanger E., et al. Change in pulmonary arterial compliance and pulmonary pulsatile stress after balloon pulmonary angioplasty. Anatol J Cardiol. 2022;26:43–48. doi: 10.5152/AnatolJCardiol.2021.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umemoto S., Abe K., Hosokawa K., et al. Increased pulmonary arterial compliance after balloon pulmonary angioplasty predicts exercise tolerance improvement in inoperable CTEPH patients with lower pulmonary arterial pressure. Heart Lung. 2022;52:8–15. doi: 10.1016/j.hrtlng.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Vaidya A., Golbus J.R., Vedage N.A., Mazurek J., Raza F., Forfia P.R. Virtual echocardiography screening tool to differentiate hemodynamic profiles in pulmonary hypertension. Pulm Circ. 2020;10 doi: 10.1177/2045894020950225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raza F., Vaidya A., Lacharite-Roberge A.S., et al. Initial clinical and hemodynamic results of a regional pulmonary thromboendarterectomy program. J Cardiovasc Surg (Torino) 2018;59:428–437. doi: 10.23736/S0021-9509.17.10188-6. [DOI] [PubMed] [Google Scholar]
- 23.Wiedenroth C.B., Ghofrani H.A., Adameit M.S.D., et al. Sequential treatment with riociguat and balloon pulmonary angioplasty for patients with inoperable chronic thromboembolic pulmonary hypertension. Pulm Circ. 2018;8 doi: 10.1177/2045894018783996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minatsuki S., Kiyosue A., Kodera S., et al. Effectiveness of balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension despite having lesion types suitable for surgical treatment. J Cardiol. 2020;75:182–188. doi: 10.1016/j.jjcc.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Brenot P., Jais X., Taniguchi Y., et al. French experience of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Eur Respir J. 2019;53:1802095. doi: 10.1183/13993003.02095-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olsson K.M., Wiedenroth C.B., Kamp J.C., et al. Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension: the initial German experience. Eur Respir J. 2017;49:1602409. doi: 10.1183/13993003.02409-2016. [DOI] [PubMed] [Google Scholar]
- 27.Andreassen A.K., Ragnarsson A., Gude E., Geiran O., Andersen R. Balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Heart. 2013;99:1415–1420. doi: 10.1136/heartjnl-2012-303549. [DOI] [PubMed] [Google Scholar]
- 28.Kataoka M., Inami T., Kawakami T., Fukuda K., Satoh T. Balloon pulmonary angioplasty (percutaneous transluminal pulmonary angioplasty) for chronic thromboembolic pulmonary hypertension: a Japanese perspective. J Am Coll Cardiol Intv. 2019;12:1382–1388. doi: 10.1016/j.jcin.2019.01.237. [DOI] [PubMed] [Google Scholar]
- 29.Sakamoto H., Goda A., Tobita K., et al. EmPHasis-10 health-related quality of life and exercise capacity in chronic thromboembolic pulmonary hypertension after balloon angioplasty. J Am Heart Assoc. 2022;11 doi: 10.1161/JAHA.122.026400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang W., Wen L., Song Z., Shi W., Wang K., Huang W. Balloon pulmonary angioplasty vs riociguat in patients with inoperable chronic thromboembolic pulmonary hypertension: a systematic review and meta-analysis. Clin Cardiol. 2019;42:741–752. doi: 10.1002/clc.23212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerges C., Friewald R., Gerges M., et al. Efficacy and safety of percutaneous pulmonary artery subtotal occlusion and chronic total occlusion intervention in chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv. 2021;14 doi: 10.1161/CIRCINTERVENTIONS.120.010243. [DOI] [PubMed] [Google Scholar]
- 32.Kinutani H., Shinke T., Nakayama K., et al. High perfusion pressure as a predictor of reperfusion pulmonary injury after balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Int J Cardiol Heart Vasc. 2016;11:1–6. doi: 10.1016/j.ijcha.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roik M., Wretowski D., Labyk A., et al. Refined balloon pulmonary angioplasty driven by combined assessment of intra-arterial anatomy and physiology--Multimodal approach to treated lesions in patients with non-operable distal chronic thromboembolic pulmonary hypertension--Technique, safety and efficacy of 50 consecutive angioplasties. Int J Cardiol. 2016;203:228–235. doi: 10.1016/j.ijcard.2015.10.116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.