Abstract
Treatment with gamma-interferon (IFN-γ) is associated with reduced frequency and severity of infections in chronic granulomatous disease (CGD), but the mechanism is unknown. Since the inducible nitric oxide (NO) synthase can be amplified by IFN-γ in murine macrophages, for example, we hypothesized that IFN-γ might modulate NO release from polymorphonuclear neutrophils (PMNs) in patients with CGD. Eight patients with CGD and eight healthy controls were studied. Each patient was given either 50 or 100 μg of IFN-γ per m2 on two consecutive days. The production of NO from N-formyl-methionyl-leucyl-phenylalanine (fMLP)-stimulated PMNs was assessed as the NG-monomethyl-l-arginine-inhibitable oxidation of oxyhemoglobin to methemoglobin in the presence of catalase and superoxide dismutase. Prior to IFN-γ treatment, the PMNs from CGD patients produced 372 ± 27 (mean ± standard error of the mean) pmol of NO/106 PMNs at 45 min, while the control PMNs produced 343 ± 44 pmol. On day 1 after IFN-γ treatment, NO production increased to 132% ± 25% of that for controls, and on day 3 it reached 360% ± 37% (P < 0.001) of that for controls. On day 8, the values still remained higher, 280% ± 78% more than the control values. Likewise, the bactericidal capacity of PMNs increased on day 3. The present data show that IFN-γ treatment of CGD patients is associated with an increased production of NO from PMNs when activated by fMLP. Since these PMNs lack the capacity to produce superoxide anions, it is conceivable that this increase in NO release could be instrumental in augmenting host defense.
Chronic granulomatous disease (CGD) is a rare X-linked or autosomal inherited disease characterized by recurrent life-threatening infections (27). The basic defect is an inability of phagocytic cells to produce superoxide anions and hydrogen peroxide, as a result of a defect in one of the subcomponents of the NADPH oxidase in these cells. In patients with the X-linked form of CGD, cells lack a membrane-associated part of the oxidase gp91phox protein (27). Patients with the autosomal recessive form of CGD fail to demonstrate the presence of either one of two cytosolic factors, p47phox and p67phox, or the membrane-bound p22phox (27).
A multicenter study showed that recombinant human gamma interferon (IFN-γ) administered subcutaneously (s.c.) three times a week significantly reduced serious infections (17). This regimen has therefore been recommended since 1991 as prophylaxis against infections in patients with CGD. However, the mechanisms of action of IFN-γ in CGD are poorly understood. Although some early reports on variant forms of CGD showed a partially restored oxidative metabolism in cells of CGD patients after IFN-γ treatment (10, 11), studies of more common CGD phenotypes could not corroborate that observation (17, 25, 28, 30).
Human polymorphonuclear neutrophils (PMNs) produce and release nitric oxide (NO) spontaneously (31) or following activation (21); both inducible and constitutive isoforms of NO synthase (NOS) have been purified from human PMNs (6, 29). Several lines of evidence imply that this function is of importance for host defense (22), possibly in CGD and furthermore in the inflammatory response as reviewed previously (14, 24). NO has been shown to possess cytotoxic (5) and bactericidal actions, particularly against intracellular pathogens (4, 13). In healthy individuals, NO released from PMNs reacts rapidly with superoxide anion-forming peroxynitrite (3). However, in CGD this reaction is not possible, and thus, NO by itself may be of a relatively larger importance. In murine systems, IFN-γ is a potent inducer of NOS (24). Inducible NOS activity in vivo has been demonstrated in PMNs from the urine of patients with urinary tract infections (29), a situation where PMNs are exposed to not only bacterial products, such as chemotactic oligopeptides, but also bacteria and a considerable presence of cytokines. Also, PMNs from CGD patients (herein referred to as CGD PMNs) have been shown to produce NO in vitro (7). Thus, we asked if the mechanism for improved host defense in CGD induced by IFN-γ could be associated with effects on PMN NO release, activated by a synthetic analogue to naturally occurring bacterial oligopeptides, and compared these data with those of a simultaneously run PMN microbicidal assay.
MATERIALS AND METHODS
Percoll and Sephadex G-25 were from Pharmacia Fine Chemicals (Uppsala, Sweden). Endotoxin-free water and Hanks’ balanced salt solution (HBSS) were from Gibco (Paisley, Scotland). l-Arginine, catalase, bovine hemoglobin, and N-formyl-methionyl-leucyl-phenylalanine (fMLP) were from Sigma (St. Louis, Mo.). Superoxide dismutase (SOD) was from Boehringer Mannheim (Mannheim, Germany). NG-Monomethyl-l-arginine (l-NMMA) was a kind gift from Wellcome Research Laboratories (Beckenham, United Kingdom). Tetrakis(3-methoxy-4-hydroxyphenyl)nickel(II)-porphyrin was from Interchim (Montluçon, France). Nafion and pure NO gas were from Aldrich (Milwaukee, Wis.).
PMNs were prepared from venous blood by Percoll density centrifugation essentially as described previously (15). The cells (purity was >99%; viability by trypan blue exclusion was >98%) were resuspended in HBSS at 2 × 106/ml. After suspension in HBSS, the cells were kept in aliquots at 4°C until 60 min prior to analysis. At that time, SOD, catalase, and either l-arginine or the NOS inhibitor l-NMMA were added and the cells were placed in a shaking water bath at 37°C until use.
Nitroblue tetrazolium reduction and chemiluminescence augmented by luminol were assessed as previously described (15, 23).
Patients.
The eight CGD patients tested (Table 1) had a history of recurrent infections since childhood. They had a negative nitroblue tetrazolium test and chemiluminescence reaction. All patients were free from infection at the time of the study. Details about the patients, e.g., mutations, are published in reference 1. Prior to this study, they had not been treated with IFN-γ. Some were on treatment with a prophylactic antibiotic; however, the same antibiotic dosage regimen was used throughout the study. Healthy members of the laboratory staff served as controls; however, they did not receive IFN-γ.
TABLE 1.
CGD patient characteristics and IFN-γ dosea
| Patient no. | Age (yr)/genderb | CGD typec | Dose of IFN-γ (μg/m2) |
|---|---|---|---|
| 1 | 6/F | Autosomal, not defined | 100 |
| 2 | 7/M | X910 | 50 |
| 3 | 17/M | X910 | 100 |
| 4 | 9/M | X910 | 50 |
| 5 | 17/M | X910 | 50 |
| 6 | 16/F | A670 | 50 |
| 7 | 33/M | A470 | 100 |
| 8 | 22/M | A470 | 50 |
No patient had an oxidative capacity.
F, female; M, male.
The numbers 91, 47, and 67 indicate the defective phox proteins. X, X-linked inheritance; A, autosomal recessive; superscript zero, undetectable protein on immunoblot.
Study design.
After informed consent was obtained, patients were randomized to receive either 50 or 100 μg of IFN-γ (Imukin; Boehringer Ingelheim, Ingelheim, Germany) per m2 on two consecutive days, assuming that this regimen would induce a rapid and transient increase of the cell functions studied and yet have tolerable side effects. IFN-γ was administered s.c. by a nurse, and the doses were given in a double-blinded manner. Venous blood samples were obtained prior to IFN-γ administration and on days 1, 3, and 8 after the last dose, between 8 and 9 a.m. Cells from the controls were consistently assessed simultaneously. The randomization code was broken after all results were registered. CGD patients were divided into different subgroups to determine whether there were any differences in the responses of the PMNs.
The study was approved by the local ethical committee and the Swedish Board for Pharmaceutical Trials.
Preparation of oxyhemoglobin (HbO2) was performed essentially as described previously (21). A solution of bovine hemoglobin in distilled water was made at a concentration of 1 mM. The hemoglobin solution was oxygenated by bubbling with O2 for 5 min. Subsequently, the hemoglobin was reduced with 1.5 mM sodium dithionite, prepared in deoxygenated doubly distilled water, and reoxygenated by bubbling with O2 for 20 min. The sodium dithionite was removed on a Sephadex G-25 column. Fresh oxyhemoglobin was prepared for each day of the experiment, and the concentration was determined spectrophotometrically at 415 nm (12).
Measurement of methemoglobin was performed essentially as described previously (21) but with some important differences. Briefly, this was performed spectrophotometrically at 401 versus 411 nm (12, 21) at 37°C in a Perkin-Elmer Lambda 7 spectrophotometer. Cells were treated with l-arginine or l-NMMA for 45 min at 37°C in a shaking water bath. During the incubation, SOD (30 μg/ml) and/or catalase (15 μg/ml) was present. Oxyhemoglobin was added immediately prior to analysis. After the background absorbance was read, the stimulus was added and the reaction was monitored continuously for up to 4 h. All samples were assessed as the difference between cells with l-arginine and cells with l-NMMA, thus directly giving the amount of NO formed. The following equation was used to calculate the amount of methemoglobin formed: ɛ = 19.7 mM−1 cm−1 (12). The viability of the cells was repeatedly determined with trypan blue exclusion and was not affected by methemoglobin, SOD, catalase, or the arginines. Pretrial experiments had shown that the major part of NO release was over after 25 min, and at 45 min no further net NO generation was detected.
Electrochemical detection of NO release was performed with a three-electrode potentiostatic Biopulse system (Tacussel, Lyon, France). The working electrode was a carbon fiber (8 μm in diameter and approximately 1 mm in length) coated with tetrakis(3-methoxy-4-hydroxyphenyl)nickel(II)-porphyrin and Nafion films, and NO was produced as described previously (19, 20). The suspension of PMNs (1.75 × 106 to 3.5 × 106/ml in HBSS with Ca2+) was incubated for 20 min at 37°C with l-arginine or l-NMMA (both at 1 mM) and SOD (15 μg/ml). fMLP at 100 nM was added when a stable current baseline was reached, and NO production was monitored for 5 min. There were no changes in baseline current when l-NMMA, l-arginine, or SOD was added to unstimulated PMNs, either for different concentrations of H2O2 or upon preincubation with catalase (data not shown). The electrode was calibrated with standard NO solutions as described previously (19, 20), and a standard curve with PMNs present was made for each experiment and at the end of each measurement. All samples were assessed as the difference between samples with l-arginine and identical samples with l-NMMA. Thus, only the L-NMMA-inhibitable response was assessed and taken to represent the net amount of NO released from PMNs.
Mini-bactericidal assay.
A miniaturized bactericidal assay was used as described previously (9). Staphylococcus aureus was cultured in soy broth at 37°C and then washed. PMNs (1.4 × 106) in 70 μl of NaCl with 0.1% bovine serum albumin, 106 bacteria in 10 μl of HBSS with 0.1% bovine serum albumin, and 20 μl of human AB− plasma were mixed in sterile 96-well plates. The plates were incubated in a shaking water bath for 90 min at 37°C, and samples were taken at 0, 45, and 90 min. Two ml of sterile water was added to 10 μl of test solution to lyse PMNs; 100 μl of this solution was added to 1 ml of sterile water. This solution (100 μl) was then put on blood agar plates and incubated overnight in 37°C; subsequently, CFU were counted. All samples were run in duplicate. For comparison, the control PMNs from the laboratory staff were tested for bactericidal capacity, and bacterial samples in HBSS without PMN, as well as PMN samples without bacteria, were run on agar plates to study normal growth and to exclude contamination.
Statistical assessment was performed with Student’s t test for paired samples where appropriate.
RESULTS
Three patients received 100 μg of IFN-γ per m2, and five patients received 50 μg/m2 (Table 1). Few adverse effects were reported, and IFN-γ was well tolerated, even in the group receiving 100 μg/m2.
PMN NO production.
First, we assessed if the conditions that the PMNs were subjected to in this study affected our ability to detect the release of NO. To this end, we applied both an electrochemical method, based on an NO-sensitive porphyrinic electrode, and the spectrophotometric detection of NO-dependent, i.e., l-NMMA-inhibitable, oxidation of HbO2 to methemoglobin. When activated with fMLP (1 μM), the production of NO from control PMNs over 5 min (the reliable life span of the electrode under these conditions) was 55.7 ± 18.3 pmol of NO/106 PMNs/min (n = 6). This corresponded well with similar measurements by the HbO2 method, as applied in this study, on other aliquots of cells from the same cell preparations producing 60.9 ± 15.2 pmol of NO/106 PMNs/min (n = 6). The release of NO from buffer-exposed cells was not detectable with the electrode or with the HbO2 method. Due to the paucity of cells and the limited measurement times possible with the electrochemical method, the HbO2 method was preferred for the measurements of the NO production of the IFN-γ-treated patients.
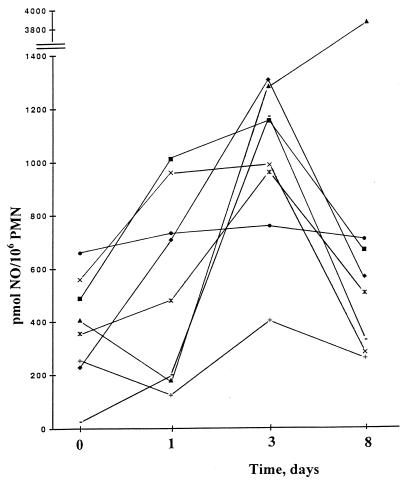
Prior to IFN-γ treatment, the CGD PMNs produced slightly more NO following stimulation with fMLP than the controls did, 372 ± 27 (mean ± standard error of the mean) pmol of NO/106 PMNs at 45 min, compared with 343 ± 44 pmol of NO/106 PMNs for control PMNs (i.e., 108% ± 16% of the control value) (Fig. 1). On day 1 after IFN-γ treatment, there was a slight increase of this function in the CGD PMNs, to 132% ± 25% of the control value. On day 3 there was a maximal enhancement of NO production, to 360% ± 37% (P < 0.001) of the control value. On day 8, the values were still elevated, i.e., they were 280% ± 78% of the control value.
FIG. 1.
NO production in CGD PMNs following in vivo IFN-γ administration and stimulation with fMLP. Results are individual values for CGD patients on each day of the experiment. ⧫, patient 1; ■, patient 2; ▴, patient 3; ×, patient 4; ✕⃒, patient 5; ●, patient 6; +, patient 7; −, patient 8.
There was no significant difference in the increase of NO production between the two different doses, since the patients receiving the higher dose (n = 3), 100 μg/m2, increased their PMN NO production on day 3 by a mean of 337% and the low-dose group (n = 5) increased their NO production by a mean of 350% after fMLP stimulation. There was no significant difference between the improvement of the patients with X-linked disease and the improvement of the patients with autosomal recessive disease.
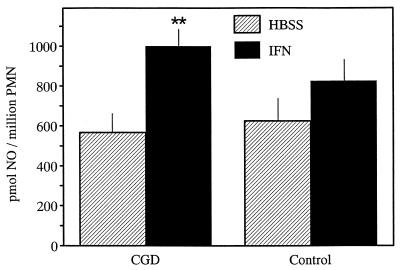
We also tested whether IFN-γ might facilitate NO release from PMNs in vitro. NO production following in vitro incubation of CGD PMNs with 1,000 U of IFN-γ (Fig. 2) revealed a 173% increase following fMLP stimulation (compared to samples without IFN-γ), while control PMNs increased their NO release only 130% (Fig. 2) following incubation with IFN-γ and fMLP stimulation.
FIG. 2.
Effect of IFN-γ treatment of PMNs for 45 min in vitro on activated NO release from PMNs. Cells were obtained from CGD patients (n = 3) or healthy volunteers (controls) (n = 2) and activated with fMLP at 1 μM. The release of NO was monitored continuously for 45 min by the HbO2 method, as described in Materials and Methods. For each experimental condition, samples were run in duplicate. All values given are the l-NMMA-inhibitable responses and are expressed as means ± standard errors of the means. ∗∗, P < 0.001.
PMN bactericidal capacity.
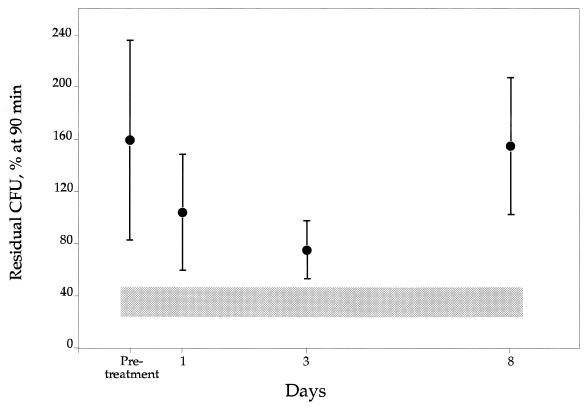
Bacterial growth without PMNs present resulted in a 249% ± 122% (mean ± standard deviation [SD]) increase in CFU after 90 min (compared to the starting sample, with 100%). There was no contamination of bacteria in PMN preparations or in HBSS in any of the samples. Control PMNs killed bacteria efficiently, leaving 35.5% ± 11% residual bacteria alive after 90 min of incubation (Fig. 3). In contrast, CGD PMNs showed no bactericidal capacity prior to IFN-γ treatment, as evidenced by a growth level of 159% ± 80% CFU after 90 min, a figure close to the bacterial growth in the absence of PMNs. In the IFN-γ-treated CGD patients, there was an improvement in the bactericidal capacity of the PMNs on day 1 after IFN-γ treatment, with 93% ± 45% residual bacteria after 90 min (Fig. 3). However, the highest bactericidal capacity of the PMNs was on day 3, with 75% ± 14% residual bacteria (Fig. 3). On day 8 the bactericidal capacity of the CGD PMNs was back to pretreatment values. There was no significant difference between the improvement of the patients with X-linked disease and the improvement of the patients with autosomal recessive disease. Neither was there any significant difference between low- and high-dose treatments.
FIG. 3.
Bactericidal capacity of CGD PMNs after IFN-γ treatment in vivo. CFU were counted after 90 min of incubation with S. aureus. The shaded area illustrates the bactericidal capacity of control PMNs (± SD); controls were not treated with IFN-γ. Pretreatment there was no killing capacity of CGD PMNs at all, leaving 159% residual bacteria, i.e., a bacterial growth. However, on day 3 a maximal killing capacity, leaving 75% residual bacteria, was noted. Error bars indicate SDs.
DISCUSSION
In this study we have evaluated the effects of IFN-γ, given s.c. on two consecutive days, on the NO production and on the bactericidal capacity of CGD PMNs. Our finding of enhanced NO production after this treatment is contradictory to the conclusions of another study (8), where no such effect could be found. However, profound differences in study design and methods used for NO detection may explain the differing results. In our study, we measured the release of NO per se, whereas the other study used the measurement of the metabolites NO2− and NO3−. Furthermore, our study evaluated the effects of IFN-γ on NO production and microbicidal capacity after two consecutive doses, and three patients received a higher dose than normally used (100 μg/m2). The idea with that dose regimen was to elicit a more powerful response and yet have tolerable side effects. It has previously been shown that PMNs do not produce NO2− or NO3− in the absence of azide (18). However, a question which cannot be resolved by the present data is the effect of IFN-γ treatment over a prolonged period of time on PMN NOS activity.
Since CGD is mainly a defect in oxidative functions, initial reports on oxidative functions of CGD patients after IFN-γ treatment suggested enhanced superoxide production and increased gene expression for the oxidase components, i.e., cytochrome b558 (10, 11). Some of these early studies were performed on variant forms of CGD (defined as CGD PMNs with some NADPH rest activity), revealing for some patients a normalization of superoxide anion production (11). No such patients were included in our study. In the placebo-controlled multicenter study of IFN-γ treatment of CGD patients (17) and also in a follow-up study (28), neither superoxide anion production nor bactericidal capacity was improved in the IFN-γ group after a prolonged time of treatment. In this study there was an effect on the PMN bactericidal capacity that coincided timewise with the peak in NO production on day 3; however, we made evaluations only of two consecutive doses to previously untreated patients. Publications on the Aspergillus-damaging capacity of CGD PMNs after IFN-γ treatment report that this function is increased (26). Furthermore, a previous study performed by some of our group (2) revealed that the maximal Aspergillus-damaging capacity seems to peak timewise with the production of NO, as shown in this study on day 3 after IFN-γ treatment. Other investigators have looked at other possible explanations for the beneficial effect of IFN-γ on these patients, for instance, antimicrobial proteins in the PMNs, but have found none (25). Also, Fcγ receptor I expression is increased after this treatment, as previously shown (2, 16).
Thus, we conclude that the present data strongly support the concept that IFN-γ treatment of CGD patients is associated with an increased production of NO from PMNs. Since these PMNs lack the capacity to produce superoxide anions, it is conceivable that this increase in NO release is one function of PMNs which could be considered to be, at least in part, instrumental in augmenting host defense.
ACKNOWLEDGMENTS
This study was supported by grants from the Swedish Children’s Cancer Association, Stiftelsen Samariten, the Swedish Medical Research Council (grants 19X-05991 and 19P-8884), the Funds of the Karolinska Institute, Tore Nilson’s Fund, the Funds of the Swedish Medical Association, the Swedish Heart and Lung Fund, the Funds for Medical Development in Southern Stockholm, the Swedish Association Against Rheumatism, and Boehringer Ingelheim AB, Stockholm, Sweden.
REFERENCES
- 1.Åhlin A, de Boer M, Roos D, Leusen J, Smith C I E, Sundin U, Rabbani H, Palmblad J, Elinder G. Prevalence, genetics and clinical presentation of chronic granulomatous disease in Sweden. Acta Paediatr. 1995;84:1386–1394. doi: 10.1111/j.1651-2227.1995.tb13575.x. [DOI] [PubMed] [Google Scholar]
- 2.Åhlin A, Elinder G, Palmblad J. Dose dependent enhancements by interferon-gamma on functional responses of neutrophils from chronic granulomatous disease patients. Blood. 1997;89:3396–3401. [PubMed] [Google Scholar]
- 3.Beckman J S, Beckman T W, Chen J, Marshall P A, Freeman B A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–1626. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boockvar K S, Granger D L, Poston R M, Maybodi M, Washington M K, Hibbs J B, Jr, Kurlander R L. Nitric oxide produced during murine listeriosis is protective. Infect Immun. 1994;62:1089–1100. doi: 10.1128/iai.62.3.1089-1100.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bratt J, Gyllenhammar H. The role for nitric oxide in lipoxin A4-induced polymorphonuclear neutrophil-dependent cytotoxicity towards human vascular endothelium, in vitro. Arthritis Rheum. 1995;38:768–776. doi: 10.1002/art.1780380609. [DOI] [PubMed] [Google Scholar]
- 6.Bryant J L, Mehta P, Vonderporten A, Mehta J L. Co-purification of 130 kd nitric oxide synthase and a 22 kd link protein from human neutrophils. Biochem Biophys Res Commun. 1992;189:558–563. doi: 10.1016/0006-291x(92)91594-g. [DOI] [PubMed] [Google Scholar]
- 7.Condino-Neto A, Muscara M N, Grumach A S, Carneiro-Sampaio M M S, De Nucci G. Neutrophils and mononuclear cells from patients with chronic granulomatous disease release nitric oxide. Br J Clin Pharmacol. 1993;35:485–490. doi: 10.1111/j.1365-2125.1993.tb04174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Condino-Neto A, Muscara M, Bellinati-Pires R, Carneiro-Sampaio M M, Brandao A C, Grumach A S, De Nucci G. Effect of therapy with recombinant human interferon-gamma on the release of nitric oxide by neutrophils and mononuclear cells from patients with chronic granulomatous disease. J Interferon Cytokine Res. 1996;16:357–364. doi: 10.1089/jir.1996.16.357. [DOI] [PubMed] [Google Scholar]
- 9.Cross A S, Zollinger W, Manrell R, Gemski P, Sadoff J. Evaluation of immunotherapeutic approaches for the potential treatment of infections caused by K1-positive Escherichia coli. J Infect Dis. 1983;147:68–76. doi: 10.1093/infdis/147.1.68. [DOI] [PubMed] [Google Scholar]
- 10.Ezekowitz R A B, Orkin S H, Newburger P E. Recombinant interferon gamma augments phagocyte superoxide production and chronic granulomatous disease gene expression in X-linked variant chronic granulomatous disease. J Clin Investig. 1987;80:1009–1016. doi: 10.1172/JCI113153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ezekowitz R A B, Dinauer M C, Jaffe H S, Stuart H O, Newburger P E. Partial correction of the phagocyte defect in patients with X-linked chronic granulomatous disease by subcutaneous interferon gamma. N Engl J Med. 1988;319:146–151. doi: 10.1056/NEJM198807213190305. [DOI] [PubMed] [Google Scholar]
- 12.Feelisch M, Noack E A. Correlation between nitric oxide formation during degradation of organic nitrates and activation of guanylate cyclase. Eur J Pharmacol. 1987;139:19–30. doi: 10.1016/0014-2999(87)90493-6. [DOI] [PubMed] [Google Scholar]
- 13.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon-gamma in resistance to mycobacterium-tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorbunov N, Esposito E. Nitric oxide as a mediator of inflammation. Int J Immunopathol Pharmacol. 1993;6:67–76. [Google Scholar]
- 15.Gyllenhammar H. Lucigenin chemiluminescence in the assessment of neutrophil superoxide production. J Immunol Methods. 1987;97:209–215. doi: 10.1016/0022-1759(87)90461-3. [DOI] [PubMed] [Google Scholar]
- 16.Huizinga T W, van der Schoot C E, Roos D, Weening R. Induction of neutrophil Fc-gamma receptor expression can be used as a marker for biologic activity of recombinant interferon-gamma in vivo. Eur J Clin Investig. 1991;21:48–50. [PubMed] [Google Scholar]
- 17.International Chronic Granulomatous Disease Cooperative Study Group. A controlled trial of interferon gamma to prevent infection in chronic granulomatous disease. N Engl J Med. 1991;324:509–516. doi: 10.1056/NEJM199102213240801. [DOI] [PubMed] [Google Scholar]
- 18.Klebanoff S J, Nathan C F. Nitrite production by stimulated human polymorphonuclear leukocytes supplemented with azide and catalase. Biochem Biophys Res Commun. 1993;197:192–198. doi: 10.1006/bbrc.1993.2459. [DOI] [PubMed] [Google Scholar]
- 19.Lantoine F, Trevin S, Bedioui F, Devynck J. Selective and sensitive electrochemical measurement of nitric oxide in aqueous solution: discussion and new results. J Electroanal Chem. 1995;392:85–93. [Google Scholar]
- 20.Lantoine F, Brunet A, Bedioui F, Devynck J. Direct measurement of nitric oxide production in platelets: relationship with cytosolic Ca2+ concentration. Biochem Biophys Res Commun. 1995;215:842–849. doi: 10.1006/bbrc.1995.2540. [DOI] [PubMed] [Google Scholar]
- 21.Lärfars G, Gyllenhammar H. Measurement of methemoglobin formation from oxyhemoglobin. A real-time, continuous assay of nitric oxide release by human polymorphonuclear leukocytes. J Immunol Methods. 1995;184:53–62. doi: 10.1016/0022-1759(95)00074-k. [DOI] [PubMed] [Google Scholar]
- 22.Malawista S E, Montgomery R R, van Blaricom B G. Evidence for reactive nitrogen intermediates in killing of staphylococci by human neutrophil cytoplasts. A new microbicidal pathway for polymorphonuclear leukocytes. J Clin Investig. 1992;90:631–636. doi: 10.1172/JCI115903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meerhof L J, Roos D. Heterogeneity in chronic granulomatous disease detected with an improved nitroblue tetrazolium slide test. J Leukoc Biol. 1986;39:699–711. doi: 10.1002/jlb.39.6.699. [DOI] [PubMed] [Google Scholar]
- 24.Moncada S, Higgs E A. Endogenous nitric oxide: physiology, pathology and clinical relevance. Eur J Clin Investig. 1991;21:361–371. doi: 10.1111/j.1365-2362.1991.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 25.Mühlebach T J, Gabay J, Nathan C F, Erny C, Dopfer G, Schroten H, Wahn V, Seger R A. Treatment of patients with chronic granulomatous disease with recombinant human interferon-gamma does not improve neutrophil oxidative metabolism, cytochrome b558 content or levels of four anti-microbial proteins. Clin Exp Immunol. 1992;88:203–206. doi: 10.1111/j.1365-2249.1992.tb03062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rex J H, Bennett J E, Gallin J I, Malech H L, DeCarlo E S. In vivo interferon-γ therapy augments the in vitro ability of chronic granulomatous disease neutrophils to damage Aspergillus hyphae. J Infect Dis. 1991;163:849–852. doi: 10.1093/infdis/163.4.849. [DOI] [PubMed] [Google Scholar]
- 27.Thrasher A J, Keep N H, Wientjes F, Segal A. Chronic granulomatous disease. Biochim Biophys Acta. 1994;1227:1–24. doi: 10.1016/0925-4439(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 28.Weening R S, Leitz G J, Seger R A. Recombinant human interferon-gamma in patients with chronic granulomatous disease—European follow up study. Eur J Pediatr. 1995;154:295–298. doi: 10.1007/BF01957365. [DOI] [PubMed] [Google Scholar]
- 29.Wheeler M A, Smith S D, Garcia-Cardena G, Nathan C F, Weiss R M, Sessa W M. Bacterial infection induces nitric oxide synthase in human neutrophils. J Clin Investig. 1997;99:110–116. doi: 10.1172/JCI119121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodman R C, Erickson R W, Rae J, Jaffe H S, Curnutte J T. Prolonged recombinant interferon-γ therapy in chronic granulomatous disease: evidence against enhanced neutrophil oxidase activity. Blood. 1992;79:1558–1562. [PubMed] [Google Scholar]
- 31.Wright C D, Muelsh A, Busse R, Osswald H. Generation of nitric oxide by human neutrophils. Biochem Biophys Res Commun. 1989;160:813–819. doi: 10.1016/0006-291x(89)92506-0. [DOI] [PubMed] [Google Scholar]