Abstract
Purpose
Improved life expectancy has increased the likelihood for long-term complications from chemotherapy among cancer survivors. One burdensome complication is chemotherapy-induced peripheral neuropathy (CIPN). We evaluated rates of CIPN outcomes in the Detroit Research on Cancer Survivorship (ROCS) cohort.
Methods
The population included 1,034 African American (AA) survivors who received chemotherapy for breast, colorectal, lung or prostate cancer. CIPN prevalence was based on initial occurrence of worsening of self-reported pain, numbness or tingling after chemotherapy. Current CIPN included symptoms still present at the time of the survey, and persistent CIPN symptoms were present 12 or more months post-chemotherapy. CIPN severity was ranked as mild, moderate or severe. Logistic regression was utilized to evaluate sociodemographic and clinical factors associated with the various categories of CIPN.
Results
CIPN prevalence was 68%, with 53% current and 52% persistent. The symptom severity distribution based on prevalent CIPN included 32.2% mild, 30.8% moderate, and 36.9% severe. Factors associated with prevalent CIPN (odds ratio, 95% confidence interval) included primary cancer site (breast: 3.88, 2.02–7.46); and (colorectal: 5.37, 2.69–10.73), lower risk for older age at diagnosis (0.66, 0.53–0.83) and divorced/separated marital status (2.13, 1.42–3.21). Current CIPN was in addition, associated with more advanced stage disease trend (1.34, 1.08–1.66) and greater number of co-morbid medical conditions trend (1.23, 1.09–1.40), as was persistent CIPN. Severity of prevalent CIPN was associated with history of arthritis (1.55, 1.06–2.26) and severity of persistent CIPN with higher BMI (1.58, 1.07–2.35).
Conclusions
CIPN is a common and persistent complication in AA cancer survivors. Further research is needed to improve our understanding of CIPN predictors in all groups of cancer survivors.
Keywords: Peripheral Neuropathy, Chemotherapy-induced peripheral neuropathy, Severity, Cancer, Survivorship, Chemotherapy, Risk factors
Introduction
According to 2019 data from the National Cancer Institute (NCI), breast, lung, prostate, and colorectal cancer (CRC) in the order listed, are the four leading types of malignancy in the US; with each also contributing to the top five causes of cancer-associated mortality [1]. While new treatments have improved disease-free survival for many patients with cancer [2], a significant proportion of cancer survivors experience burdensome comorbidities and short and long-term side effects from chemotherapy which reduce quality of life [3–5], functionality [6] and at times survival [7]. One side effect of particular concern is chemotherapy-induced peripheral neuropathy (CIPN) which can confer significant debilitating effects on patient functionality.
CIPN is often seen following administration of specific classes of chemotherapy including taxanes, vinca alkaloids and platinum agents which elicit either new-onset or exacerbate pre-existing peripheral neuropathy (PN) [8]. Although the pathophysiology of CIPN is not completely understood [9], interference with axonal transport induced by microtubule-interfering agents plays a major role [10]. Taxanes (e.g., paclitaxel, docetaxel, nab-paclitaxel) and platinum agents are used in the adjuvant, neoadjuvant, and metastatic setting for multiple solid malignancies including breast, lung, prostate and CRC [11]. Based on prior studies, it is estimated that 30% of women with breast cancer develop CIPN within 6 months of completing chemotherapy [12]. In a large adjuvant breast cancer trial, 41.9% of participants reported CIPN 2 years after completing chemotherapy [13]. A meta-analysis evaluating predictors of CIPN among patients with a variety of solid tumors reported a relationship between CIPN and advancing age, type of taxane therapy, and history of diabetes, however this analysis did not report cancer-specific rates of CIPN [14]. In another analysis from the Women’s Health Initiative (WHI), Life and Longevity after Cancer (LILAC) cohort, 17% of women with local or regional stage breast cancer developed CIPN and, of these, 74% reported persistent CIPN symptoms at a median of 6.5 years after diagnosis [15].
We previously reported on factors associated with CIPN in the Detroit Research on Cancer Survivors (ROCS) cohort, showing that 52% of participants reported current CIPN, meaning continued symptoms of CIPN at the time of the ROCS survey [16]. The present analysis extends our prior findings by evaluating CIPN in four different ways including our primary outcome variables (prevalent and current CIPN), and secondary outcome variables CIPN severity and persistent CIPN. Predictor variables included detailed sociodemographic and clinical data available in ROCS. Given the paucity of data on CIPN in the general population [17], and specifically in African American (AA) cancer survivors, our report advances the literature on CIPN in a vulnerable, underserved cancer survivorship population.
Methods
Study design
Detroit ROCS is a population-based AA survivorship cohort in Southeast Michigan developed to evaluate sociodemographic, clinical and other factors that impact cancer survivorship. All participants completed baseline surveys providing detailed socio-demographic and clinical information. Data collected by participant questionnaires and cancer registry review include information on risk factors, medical history, cancer diagnoses (histopathological information, staging and phenotypic markers), symptoms related to cancer and cancer treatment modalities, and quality of life. The assessment included-specific information on symptoms associated with peripheral neuropathy (PN) or CIPN [16, 18]. The current analysis will use a retrospective cohort design including AA men and women diagnosed with lung, breast, prostate, or colorectal (CRC) enrolled in the Detroit ROCS study.
Figure 1 presents a CONSORT diagram of ROCS participants included in the analysis. Of 4,017 ROCS participants for whom information was available, we excluded 1,964 individuals who did not receive chemotherapy, 662 who received version 1 of the ROCS survey which did not include questions about CIPN, 319 who had younger-onset cancers other than breast, lung, prostate or colorectal or who had uterine cancer, 27 who had incomplete information about worsening symptoms, and 11 with incomplete information about CIPN, leading to a final analytic cohort of 1,034. Informed consent was obtained from all participants and IRB approval was obtained by the Wayne State University IRB.
Fig. 1.
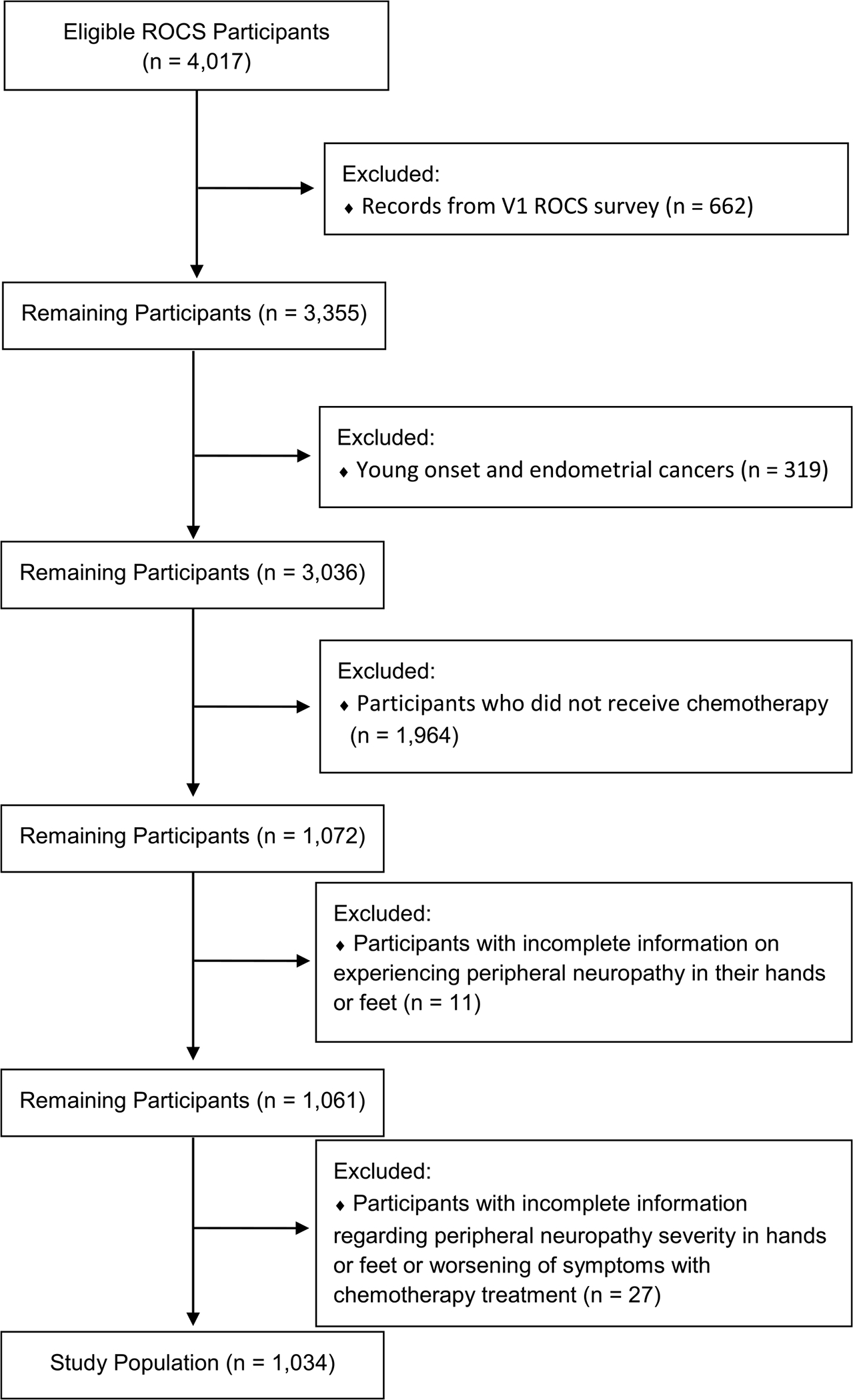
Consort diagram of participants in the study of chemotherapy-induced peripheral neuropathy in the Detroit Research on Cancer Survivors (ROCS) cohort
Assessment of CIPN
Five survey items evaluated the timing and severity of CIPN, and the presence of specific functional disabilities that participants attributed to CIPN. Functional assessments included gross motor skills (e.g., walking or lifting objects), and fine motor skills (e.g., writing, buttoning a shirt, or tying a shoe). Survey questions regarding CIPN severity were modeled in parallel to an objective validated instrument, the “FACT-GOG/Ntx” (version 4) subscale, previously used for analysis of CIPN in other cohorts including the WHI [15, 19, 20].
CIPN was a patient-reported outcome (PRO) based on report of one or more of the following symptoms: numbness, pain or tingling in the hands or feet. PROs of severity level were supported by a descriptor of perceived interference with Independent Activities of Daily Living (IADLs). Participants were asked to categorize the severity of the numbness, pain or tingling in their hands or feet by selecting one of four possible responses: mild, moderate, moderate to severe, or severe. The “moderate to severe” choice indicated that symptoms interfered with IADLs and the “severe” choice indicated that the symptoms “almost completely impacted their ability to do activities of daily living”. Because only a few individuals ranked symptoms as “severe”, the “moderate to severe” and “severe” categories were combined to create a single “severe” category.
Assessment of CIPN also included the presence of PN symptoms prior to chemotherapy and whether PN symptoms worsened following treatment if they were previously present. Lastly, participants were asked to indicate the presence or absence of a comprehensive list of specific fine and gross motor functions that may have been compromised by the presence of CIPN symptoms (Supplemental Data: CIPN ROCS Survey Questions).
Outcome variables
Primary study outcomes included prevalent and current CIPN. CIPN prevalence was defined by the presence of self-reported PN symptoms (pain, numbness, or tingling in the hands or feet) at any time since starting chemotherapy or worsening of any prior PN symptoms after chemotherapy administration. We assumed no pre-existing PN if participants reported no PN symptoms prior to chemotherapy. Current CIPN was defined as CIPN occurring among individuals who experienced pain, numbness or tingling in hands or feet at the time of the survey.
Secondary study outcomes included severity of prevalent CIPN and severity of current CIPN. Severity was coded as mild, moderate or severe symptoms. Another secondary study outcome included persistent CIPN which was defined as symptoms among participants who no longer were receiving any cancer-directed therapy at study enrollment, and who had enrolled into ROCS at least 12 months following their cancer diagnosis.
Primary predictor variables
Primary predictor variables included age at diagnosis, educational attainment, marital status, primary cancer site, cancer stage, body mass index (BMI), personal history of arthritis, diabetes or thyroid disorders, co-morbidity count and past exposure to tobacco or alcohol use (in the past 4 weeks). Comorbidity count was based on self-report of ever being diagnosed with one or more of the following medical conditions: arthritis, emphysema or chronic obstructive pulmonary disease (COPD), smoking, alcohol intake, depression, diabetes, non-traumatic fracture (over age 50), cardiovascular disease (heart attack, congestive heart failure, atrial fibrillation, or coronary artery disease), hepatitis, high cholesterol, hypertension, stroke, or thyroid disease. Information on cancer stage was obtained from cancer registry data, and coded as local, regional or distant using the Surveillance, Epidemiology and End Results (SEER) system [21].
Statistical analysis
Statistical analyses were performed using Statistical Analysis Software, version 9.4 (Cary, NC). Chi-square tests were used to identify categorical predictors associated with the CIPN measures. When at least 25% of expected cell count values were < 5, Fisher’s exact test was used to identify categorical predictors. For ordinal variables, p-trends were calculated using the Cochran-Armitage trend test across CIPN status and the Mantel–Haenszel chi-square test across CIPN severity. A Wilcoxon rank-sum test was used to identify continuous predictors associated with CIPN. Odds ratios (OR) and 95% confidence intervals (CIs) of CIPN categories were calculated using logistic regressions adjusted for variables significant in univariate analysis. ORs among ordinal variables were evaluated as a trend interpreted as per one unit/level increase within category while nominal variables were elected as a reference group.
Results
Table 1 shows clinical and demographic characteristics of participants who reported receiving chemotherapy in the Detroit ROCS cohort stratified by whether they reported prevalent CIPN. Of the 704 (68%) individuals who experienced PN since starting chemotherapy, there were 124 who had these symptoms prior to chemotherapy, and who had worsening of sypmtoms after chemotherapy, and 580 who had these symptoms since chemotherapy, but not before. Average time from cancer diagnosis to survey completion was 23 months (range 2–84 months), with no differences in time to completion between individuals with or without CIPN. Average age at cancer diagnosis was 57.1 (SD 10.9), gender distribution included 77% women, and 91% had at least a high-school education. The distribution by cancer site included 58% with breast, 20% CRC, 17% lung and 5% prostate. The distribution by cancer stage included 33% with local disease, 48% regional and 18% distant. Average BMI was 30.3 (SD 7.2) and almost all participants reported at least one co-morbid condition (90%), with 22% reporting a history of diabetes mellitus. Almost half reported prior tobacco use, and approximately 40% reported consumption of alcohol. Only 37.6% reported any active cancer treatment at the time of the survey, whether chemotherapy, radiation, surgery, or any combination of modalities.
Table 1.
Clinical and demographic characteristics of participants who reported receiving chemotherapy in the Detroit Research on Cancer Survivors (ROCS) cohort stratified by whether they reported prevalent chemotherapy-induced peripheral neuropathy
| Total |
Prevalent CIPN1 |
p-value* | ||||
|---|---|---|---|---|---|---|
| N | % | Yes | No | Row % | ||
|
| ||||||
| 1034 | 704 | 330 | 68% | |||
| Demographics | ||||||
| Gender | < .001 | |||||
| Male | 237 | 22.9 | 134 | 103 | 57% | |
| Female | 797 | 77.1 | 570 | 227 | 72% | |
| Age at Diagnosis | < .001 | |||||
| ≤50 | 255 | 24.7 | 198 | 57 | 78% | |
| 51–64 | 508 | 49.1 | 350 | 158 | 69% | |
| 65 + | 271 | 26.2 | 156 | 115 | 58% | |
| mean (std) | 57.1 (10.9) | 55.8 (10.8) | 59.9 (10.4) | |||
| median (range) | 58 (27–79) | 57 (27–79) | 61 (27–79) | |||
| Marital status | 0.011 | |||||
| Married or equivalent | 347 | 33.8 | 232 | 115 | 67% | |
| Widowed | 113 | 11.0 | 71 | 42 | 63% | |
| Divorced or separated | 278 | 27.1 | 211 | 67 | 76% | |
| Never married | 288 | 28.0 | 186 | 102 | 65% | |
| Educational attainment | 0.011 | |||||
| Less than High School | 89 | 8.7 | 52 | 37 | 58% | |
| High school/GED | 254 | 24.9 | 159 | 95 | 63% | |
| Some college/2-year degree | 403 | 39.5 | 281 | 122 | 70% | |
| Four-year college degree | 120 | 11.8 | 86 | 34 | 72% | |
| Graduate/professional degree | 154 | 15.1 | 117 | 37 | 76% | |
| Cancer characteristics | ||||||
| Cancer site | < .001 | |||||
| Breast | 599 | 57.9 | 444 | 155 | 74% | |
| Colorectal | 206 | 19.9 | 163 | 43 | 79% | |
| Lung | 177 | 17.1 | 79 | 98 | 45% | |
| Prostate | 52 | 5.0 | 18 | 34 | 35% | |
| SEER summary stage | 0.005 | |||||
| Local | 342 | 33.1 | 231 | 111 | 68% | |
| Regional | 498 | 48.2 | 358 | 140 | 72% | |
| Distant | 188 | 18.2 | 111 | 77 | 59% | |
| Medical History | ||||||
| BMI at enrollment | 0.003 | |||||
| Underweight/normal weight | 231 | 22.7 | 143 | 88 | 62% | |
| Overweight | 322 | 31.5 | 208 | 114 | 65% | |
| Obese | 469 | 45.9 | 344 | 125 | 73% | |
| Mean (std) | 30.3 (7.2) | 30.8 (7.4) | 29.2 (6.6) | |||
| Median (range) | 29.4 (14.6–66.2) | 29.9 (14.6–66.2) | 28.5 (15.8–51.6) | |||
| Comorbidity count | 0.946 | |||||
| None | 94 | 9.2 | 62 | 32 | 66% | |
| 1 | 206 | 20.3 | 145 | 61 | 70% | |
| 2 | 222 | 21.8 | 150 | 72 | 68% | |
| 3 | 170 | 16.7 | 115 | 55 | 68% | |
| 4 or more | 325 | 32.0 | 221 | 104 | 68% | |
| Diabetes mellitus | 0.377 | |||||
| Yes | 224 | 21.8 | 147 | 77 | 66% | |
| No | 806 | 78.3 | 554 | 252 | 69% | |
| Thyroid problem | 0.508 | |||||
| Yes | 113 | 11.0 | 80 | 33 | 71% | |
| No | 917 | 89.0 | 621 | 296 | 68% | |
| Arthritis | 0.571 | |||||
| Yes | 419 | 40.7 | 281 | 138 | 67% | |
| No | 611 | 59.3 | 420 | 191 | 69% | |
| Smoker (≥ 100 cigs lifetime) | 0.004 | |||||
| Yes | 513 | 49.9 | 328 | 185 | 64% | |
| No | 515 | 50.1 | 372 | 143 | 72% | |
| Alcohol Use (in the past 4 weeks) | 0.184 | |||||
| Yes | 395 | 38.4 | 278 | 117 | 70% | |
| No | 634 | 61.6 | 421 | 213 | 66% | |
| Diagnosis to enrollment time (months) | 0.289 | |||||
| Mean (std) | 23.0 (18.4) | 23.1 (18.2) | 22.8 (18.8) | |||
| Median (range) | 16 (2–84) | 16 (2–82) | 16 (2–84) | |||
p-values based on chi-square testing, Fisher’s exact test for small cell counts and p-trend for ordinal values
CIPN prevalence was defined by the presence of self-reported PN symptoms (pain, numbness, or tingling in the hands or feet) at any time since starting chemotherapy or worsening of any prior PN symptoms after chemotherapy administration
In univariate analysis (Table 1), several variables were significantly associated with a higher rate of CIPN prevalence, but after multivariable adjustment (Table 2), increased CIPN prevalence was associated with younger age at diagnosis (OR trend for advancing age: (0.66, 95% CI 0.53–0.83), primary cancer site (prostate referent) (breast cancer: OR 3.88, 95% CI 2.02–7.46), (CRC: OR 5.37, 95% CI 2.69–10.73) and divorced or separated martal status (never married referent) (OR 2.13, 95% CI 1.42–3.21).
Table 2.
Multivariable analysis of predictors of chemotherapy-induced peripheral neuropathy prevalence 1 in the Detroit Research on Cancer Survivors (ROCS) Cohort
| aOR* | Lower CI | Upper CI | |
|---|---|---|---|
|
| |||
| Demographics | |||
| Age at Diagnosis (per age group) | 0.66 | 0.53 | 0.83 |
| Marital Status | |||
| Married or equivalent | 1.24 | 0.86 | 1.79 |
| Widowed | 1.34 | 0.80 | 2.26 |
| Divorced or separated | 2.13 | 1.42 | 3.21 |
| Never married | ref | ||
| Educational attainment (per category) | 1.10 | 0.97 | 1.26 |
| Cancer characteristics | |||
| Cancer Site | |||
| Breast | 3.88 | 2.02 | 7.46 |
| Colorectal | 5.37 | 2.69 | 10.73 |
| Lung | 1.15 | 0.58 | 2.28 |
| Prostate | ref | ||
| SEER summary stage | |||
| Local | 0.79 | 0.50 | 1.25 |
| Regional | 1.01 | 0.67 | 1.53 |
| Distant | ref | ||
| Trend | 1.15 | 0.92 | 1.45 |
| Medical history | |||
| BMI at enrollment (per category) | 1.14 | 0.94 | 1.38 |
| Comorbidity count | |||
| None | ref | ||
| 1 | 1.46 | 0.81 | 2.62 |
| 2 | 1.51 | 0.84 | 2.73 |
| 3 | 1.45 | 0.78 | 2.73 |
| 4 or more | 1.76 | 0.95 | 3.25 |
| Trend | 1.10 | 0.97 | 1.24 |
| Diabetes mellitus | |||
| Yes | 0.88 | 0.62 | 1.24 |
| No | ref | ||
| Thyroid problem | |||
| Yes | 1.00 | 0.63 | 1.59 |
| No | ref | ||
| Arthritis | |||
| Yes | 1.05 | 0.78 | 1.42 |
| No | ref | ||
| Smoker (≥ 100 cigs lifetime) | |||
| Yes | 1.20 | 0.87 | 1.65 |
| No | ref | ||
| Alcohol use (in the past 4 weeks) | |||
| Yes | 1.10 | 0.81 | 1.49 |
| No | ref | ||
Multivariable adjustment for age at diagnosis, marital status, education, cancer site, SEER summary stage, body mass index at enrollment, and smoking
CIPN prevalence was defined by the presence of self-reported PN symptoms (pain, numbness, or tingling in the hands or feet) at any time since starting chemotherapy or worsening of any prior PN symptoms after chemotherapy administration
Bold values denote statistical signicance at p<0.05
The distribution of CIPN severity for prevalent CIPN included 224 (31.8%) with mild symptoms, 217, 30.8% moderate and 263, 37.4%) severe. Factors associated with increased CIPN severity in this group, included younger age at diagnosis (age >/ 65 referent), (OR trend with advancing age: 0.70, 95% CI 0.55–0.89), lower levels of educational attainment (OR trend with increasing education: 0.84, 95% CI 0.73–0.97), and history of arthritis (OR, 1.55, 95% CI 1.06–2.26) (Table 3).
Table 3.
Multivariable analysis of predictors of severity of prevalent chemotherapy-induced peripheral neuropathy in the Detroit Research on Cancer Survivors (ROCS) cohort
| aOR* | Lower CI | Upper CI | |
|---|---|---|---|
|
| |||
| Demographics Age at Diagnosis | |||
| < 50 | 2.11 | 1.30 | 3.43 |
| 51–64 | 1.84 | 1.21 | 2.81 |
| 65 + | Ref | ||
| Trend | 0.70 | 0.55 | 0.89 |
| Marital status | |||
| Married or equivalent | 1.18 | 0.77 | 1.80 |
| Widowed | 0.93 | 0.49 | 1.78 |
| Divorced or separated | 1.34 | 0.86 | 2.07 |
| Never married | Ref | ||
| Educational attainment | 0.84 | 0.73 | 0.97 |
| Cancer characteristics | |||
| Cancer Site | |||
| Breast | 0.52 | 0.19 | 1.44 |
| Colorectal | 0.57 | 0.20 | 1.61 |
| Lung | 0.35 | 0.12 | 1.03 |
| Prostate | ref | ||
| SEER summary stage | |||
| Local | ref | ||
| Regional | 1.29 | 0.90 | 1.85 |
| Distant | 1.43 | 0.87 | 2.34 |
| Trend | 1.21 | 0.95 | 1.54 |
| Medical history | |||
| Body Mass Index (BMI) at Enrollment (per category) | 1.06 | 0.85 | 1.32 |
| Comorbidity Count | |||
| None | ref | ||
| 1 | 1.53 | 0.77 | 3.05 |
| 2 | 1.46 | 0.73 | 2.94 |
| 3 | 1.80 | 0.86 | 3.76 |
| 4 or more | 1.98 | 0.97 | 4.07 |
| Trend | 1.14 | 0.99 | 1.31 |
| Diabetes mellitus | |||
| Yes | 0.83 | 0.54 | 1.29 |
| No | ref | ||
| Thyroid problem | |||
| Yes | 1.01 | 0.61 | 1.70 |
| No | ref | ||
| Arthritis | |||
| Yes | 1.55 | 1.06 | 2.26 |
| No | ref | ||
| Smoker (≥ 100 cigs lifetime) | |||
| Yes | 1.07 | 0.76 | 1.50 |
| No | ref | ||
| Alcohol use (in the past 4 weeks) | |||
| Yes | 1.00 | 0.72 | 1.38 |
| No | ref | ||
Multivariable adjustment for age at diagnosis, education, comorbidity count, arthritis
Severity of chemotherapy-induced peripheral neuropathy (CIPN) was coded as mild, moderate or severe symptoms. Comparisons were for symptoms ranked as high or severe vs. low (mild or moderate). The results in this table are based on 704 individuals with prevalent CIPN of which 224 had mild severity and 480 had moderate or severe CIPN Bold values denote statistical significance at p<0.05
A smaller proportion of participants N = 552 (53.4%) reported current CIPN (Table 4). Predictors of current CIPN included younger age (OR trend with advancing age: 0.77, 95% CI 0.62–0.96), primary cancer site (breast cancer: OR 3.09, 95% CI 1.59–6.03; CRC: OR 3.62, 95% CI 1.80–7.29) and divorced/separated status (OR 1.90, 95% CI 1.32–2.75). For current CIPN, later stage at diagnosis (distant referent) (OR trend 1.34, 95% CI 1.08–1.66), and increased comorbidity count (none referent) (OR trend 1.23, 95% CI 1.09–1.40) were also associated with increased current CIPN. Younger age and lower educational achievement were associated with more severe current CIPN (OR trend for advancing age: 0.71, 95% CI 0.54–0.93) and (OR trend for increasing educational attainment: 0.84, 95% CI 0.72–0.98) (data not shown).
Table 4.
Multivariable analysis of factors related to current 1chemotherapy-induced peripheral neuropathy in the Detroit Research on Cancer Survivors (ROCS) cohort
| aOR* | Lower CI | Upper CI | |
|---|---|---|---|
|
| |||
| Demographics | |||
| Age at Diagnosis (per age group) Marital Status | 0.77 | 0.62 | 0.96 |
| Married or equivalent | 1.31 | 0.94 | 1.84 |
| Widowed | 1.31 | 0.80 | 2.15 |
| Divorced or separated | 1.90 | 1.32 | 2.75 |
| Never married | ref | ||
| Educational Attainment (per category) | 0.97 | 0.86 | 1.10 |
| Cancer Characteristics | |||
| Cancer Site | |||
| Breast | 3.09 | 1.59 | 6.03 |
| Colorectal | 3.62 | 1.80 | 7.29 |
| Lung | 0.94 | 0.46 | 1.93 |
| Prostate | ref | ||
| SEER Summary Stage | |||
| Local | 0.57 | 0.37 | 0.89 |
| Regional | 0.78 | 0.53 | 1.16 |
| Distant | ref | ||
| Trend | 1.34 | 1.08 | 1.66 |
| Medical history | |||
| Body Mass Index (BMI) at Enrollment (per category) | 0.99 | 0.82 | 1.20 |
| Comorbidity count | |||
| None | ref | ||
| 1 | 1.07 | 0.63 | 1.82 |
| 2 | 1.16 | 0.68 | 1.98 |
| 3 | 1.66 | 0.92 | 2.99 |
| 4 or more | 2.08 | 1.15 | 3.76 |
| Trend | 1.23 | 1.09 | 1.40 |
| Diabetes mellitus | |||
| Yes | 1.03 | 0.72 | 1.48 |
| No | ref | ||
| Thyroid problem | |||
| Yes | 0.93 | 0.60 | 1.45 |
| No | ref | ||
| Arthritis | |||
| Yes | 1.09 | 0.80 | 1.49 |
| No | ref | ||
| Smoker (≥ 100 cigs lifetime) | |||
| Yes | 1.23 | 0.92 | 1.64 |
| No | ref | ||
| Alcohol use (in the past 4 weeks) | |||
| Yes | 0.92 | 0.70 | 1.21 |
| No | ref | ||
Multivariable adjustment for age at diagnosis, marital status, cancer site, BMI at enrollment, comorbidity count, and arthritis
Current CIPN was defined as CIPN occurring among individuals who experienced pain, numbness or tingling in hands or feet at the time of the survey. This analysis was based on 552 (53.4%) with current peripheral neuropathy compared to 482 individuals who did not report current peripheral neuropathy
Bold values denote statistical significance at p<0.05
Of the 494 participants who enrolled in the cohort more than 12 months following their cancer diagnosis, 255 (51.6%) had persistent CIPN (Supplemental Table 1). Significant predictors of persistent CIPN included younger age (OR trend for advancing age: 0.72, 95% CI 0.54–0.96), more advanced stage (OR trend 1.64, 95% CI 1.15–2.33), higher co-morbidity count (OR trend 1.29, 95% CI 1.09–1.53), and divorced/separated marital status (OR 1.93, 95% CI 1.11–3.34). For severity of persistent CIPN, BMI was significantly associated with worse severity (OR trend 1.58, 95% 1.07–2.35) (data not shown).
Discussion
We evaluated rates and severity of CIPN in 1,034 African American cancer survivors enrolled in the Detroit ROCS cancer survivorship cohort and only included in our analysis individuals who were diagnosed with common cancers and who had received chemotherapy for breast, colorectal, lung or prostate cancer. As suspected the rates of CIPN reported in this cohort were high: nearly 70% reported either new or worsening CIPN (CIPN prevalence) and 53% reporting CIPN at the time of the survey (current CIPN). Also remarkable was the high proportion (52%) who reported persistent CIPN 12 or more months post-completion of therapy. The CIPN experience of participants in the ROCS cohort was non-trivial, with more than 2/3 of participants ranking CIPN as moderate to severe (37% severe), indicating that these symptoms interfered with IADLs.
In the current analysis, we expand on data from our earlier ROCS report where only current CIPN was reported [16]. In another medical record review of breast cancer survivors, of the 123 women treated with taxanes for early-stage breast cancer, 49 (40%) required a chemotherapy dose reduction, and within that subgroup, 21 (43%) required a dose reduction due to CIPN. Factors that predicted a greater likelihood of dose reduction included AA race, use of paclitaxel and history of diabetes mellitus [22]. In another study looking at content validity of a 16-item Quality of Life Questionnaire pertaining to CIPN, the investigators suggested that patients may be less likely to report CIPN symptoms for fear of dose reduction of potentially life-saving chemotherapy [23].
In the WHI’s LILAC cohort, only 17% of women with breast cancer reported CIPN, however 74% of those with CIPN reported symptoms at 6.5 years after diagnosis suggesting that CIPN is a persistent problem in cancer survivors [15]. In contrast to our cohort where all patients reported chemotherapy use, only 26% of women in the LILAC study received chemotherapy, likely contributing to the overall lower rate of CIPN compared to ROCS, however the rates of persistent CIPN were much higher in LILAC likely due to longer follow-up.
In other studies, rates of CIPN were comparable to our findings including a report of 70% with CIPN among individuals with several types of cancer, surveyed at least 3 months post-completion of platinum- or taxane-based chemotherapy [24]. Similarly in other studies, among women with breast cancer and gynecological cancers, mean CIPN rates at 6-years post-diagnosis were 58% and 47%, respectively [25, 26]. Consistent among several reports is the relatively high rates of persistent CIPN, suggesting a major impact on cancer survivorship. A meta-analysis of 27 studies including 9,853 patients showed a 45% rate of persistent CIPN at a median of 12 months after completing chemotherapy, and a relative reduction in CIPN symptom prevalence of 26% annually [27]. All of these findings lend credence to the generalizability of our results when compared to other studies which had lower rates of inclusion of AA participants.
We also identified several predictors of CIPN including younger age at diagnosis (all CIPN measures), less education (severity for both prevalent and current CIPN), and being divorced/separated (prevalent and current CIPN). The relationship between higher risk of CIPN by cancer site for breast and colon (prevalence, current) was likely due to the more predominant use of taxanes and platinum agents for those tumor types. Various medical conditions were also associated with CIPN including arthritis (severity of prevalent CIPN) and number of co-morbid medical conditions (current and persistent CIPN). Advanced stage was also associated with current and persistent CIPN and higher BMI with persistent CIPN.
In the WHI, treatment with paclitaxel was a very strong predictor of PN. [15] as was shown in the meta-analysis [14]. From our analysis it is difficult to disentangle the reason why different predictor variables were more or less likely to predict risk of the different measures of CIPN. It is possible that different socioeconomic and clinical influences impact risk of toxicity from therapy and special attention should be placed towards reducing predisposing factors including obesity and uncontrolled co-morbid medical conditions. It is also possible that chronic symptoms from other medical conditions may enhance an individual’s perception of CIPN symptoms, which has been reported by another group [28]. In our analysis, history of diabetes mellitus was not associated with an increased risk of any CIPN measure that we evaluated, and similar findings were elicited in 9 of 13 other studies as cited by Timmins et al. as part of a systematic review [29]. Younger age of onset appears to be is an important marker of vulnerability to CIPN which should be considered carefully in the clinical setting. This relationship may be due in part to a tendency to use more aggressive treatment regimens for younger patients and possibly higher doses or duration of therapy [30–32].
Limitations of our analysis include the possibility for recall bias as well as the lack of availability of information on type of chemotherapy or duration of treatment. It is also possible that CIPN is under-reported by patients to maintain a sense of “social desirability” to their providers, and may therefore bias answers on surveys to prevent perceived potential consequences such as loss of driving privileges or attenuation of necessary cancer treatments [23]. Also, in the design of the current study, there were no objective measures or medical record information to confirm the presence of CIPN [33].
Strengths of our study include the use of a unique AA survivorship cohort, and detailed clinical and sociodemographic data to represent a broad spectrum of individuals with the four most common types of cancer. The ROCS survey also focused specifically on defining and evaluating numbness and tingling for patients, as those two symptoms were previously most highly correlated (r = 0.69) as onset or sentinel symptoms of CIPN compared to other symptoms such as shooting pain or burning [34].
The recognition and evaluation of CIPN faces several challenges and it is paramount that providers and patients are able to identify symptoms to decrease the burden patients face from CIPN [35].
Conclusion
In summary, CIPN is a common and persistent side effect from chemotherapy which represents an important aspect of survivorship after cancer. As the cancer survivorship population grows over time, it is increasingly important to identify predictors of CIPN and to evaluate and intervene to limit CIPN as a long-term complication of cancer survivorship. Further analyses specifically geared toward underrepresented cohorts such as AA survivors are important and must be done to formulate applicable solutions for prevalent complications seen in all groups of cancer survivors.
Supplementary Material
Funding
This work was supported by the National Cancer Institute of the National Institutes of Health (U01 CA199240) and by the Epidemiology Research Core and the National Cancer Institute Center Grant (P30CA022453) awarded to the Karmanos Cancer Institute at Wayne State University.
Footnotes
Conflicts of interest Mark K. Greenwald: Honoraria for Consulting/Speaker—Indivior Inc. All other authors have no conflicts of interest to disclose.
Ethical approval This study was approved by the Institutional Review Board (IRB) at Wayne State University.
The data in this manuscript was presented as a poster presentation at the American Society of Clinical Oncology (ASCO) meeting in June of 2021.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s10552-023-01676-0.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.American Cancer Society (2019) Cancer Facts & Figures 2019. American Cancer Society, Atlanta [Google Scholar]
- 2.Hayat MJ et al. (2007) Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist 12(1):20–37 [DOI] [PubMed] [Google Scholar]
- 3.Arndt V et al. (2017) Quality of life in long-term and very long-term cancer survivors versus population controls in Germany. Acta Oncol 56(2):190–197 [DOI] [PubMed] [Google Scholar]
- 4.Mols F et al. (2014) Chemotherapy-induced peripheral neuropathy and its association with quality of life: a systematic review. Support Care Cancer 22(8):2261–2269 [DOI] [PubMed] [Google Scholar]
- 5.Hsu HT et al. (2020) Emotional distress and quality of life during folinic acid, fluorouracil, and oxaliplatin in colorectal cancer patients with and without chemotherapy-induced peripheral neuropathy: a cross-sectional study. Medicine (Baltimore) 99(6):e19029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zanville NR et al. (2016) Evaluating the impact of chemotherapy-induced peripheral neuropathy symptoms (CIPN-sx) on perceived ability to work in breast cancer survivors during the first year post-treatment. Support Care Cancer 24(11):4779–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gradishar WJ et al. (2020) Breast Cancer, Version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 18(4):452–478 [DOI] [PubMed] [Google Scholar]
- 8.Swain SM, Arezzo JC (2008) Neuropathy associated with microtubule inhibitors: diagnosis, incidence, and management. Clin Adv Hematol Oncol 6(6):455–467 [PubMed] [Google Scholar]
- 9.Bae EH et al. (2021) Chemotherapy-induced peripheral neuropathy: mechanisms and therapeutic avenues. Neurotherapeutics 18(4):2384–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LaPointe NE et al. (2013) Effects of eribulin, vincristine, paclitaxel and ixabepilone on fast axonal transport and kinesin-1 driven microtubule gliding: implications for chemotherapy-induced peripheral neuropathy. Neurotoxicology 37:231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gradishar WJ (2012) Taxanes for the treatment of metastatic breast cancer. Breast Cancer (Auckl) 6:159–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seretny M et al. (2014) Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain 155(12):2461–2470 [DOI] [PubMed] [Google Scholar]
- 13.Bandos H et al. (2018) Long-term peripheral neuropathy in breast cancer patients treated with adjuvant chemotherapy: NRG Oncology/NSABP B-30. J Natl Cancer Inst. 10.1093/jnci/djx162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hershman DL et al. (2016) Comorbidities and risk of chemotherapy-induced peripheral neuropathy among participants 65 years or older in Southwest Oncology Group Clinical Trials. J Clin Oncol 34(25):3014–3022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamgar M et al. (2021) Prevalence and predictors of peripheral neuropathy after breast cancer treatment. Cancer Med 10:6666–6676. 10.1002/cam4.4202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trendowski MR et al. (2021) Chemotherapy-induced peripheral neuropathy in African American cancer survivors: risk factors and quality of life outcomes. Cancer Med 10(22):8151–8161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knoerl R et al. (2019) Characterizing patient-clinician chemotherapy-induced peripheral neuropathy assessment and management communication approaches. Patient Educ Couns 102(9):1636–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beebe-Dimmer JL et al. (2019) The Detroit Research on Cancer Survivors (ROCS) pilot study: a focus on outcomes after cancer in a racially diverse patient population. Cancer Epidemiol Biomarkers Prev 28(4):666–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng HL et al. (2020) Psychometric testing of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity (FACT/GOG-Ntx) subscale in a longitudinal study of cancer patients treated with chemotherapy. Health Qual Life Outcomes 18(1):246–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon-Williams R, Farquhar-Smith P (2020) Recent advances in understanding chemotherapy-induced peripheral neuropathy. F1000Res. 10.12688/f1000research.21625.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Training.seer.cancer.gov. 2022. Summary Staging | SEER Training. [online] Available at: <https://training.seer.cancer.gov/staging/systems/summary/> [Google Scholar]
- 22.Bhatnagar B et al. (2014) Chemotherapy dose reduction due to chemotherapy induced peripheral neuropathy in breast cancer patients receiving chemotherapy in the neoadjuvant or adjuvant settings: a single-center experience. Springerplus 3:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavoie Smith EM et al. (2017) The content validity of a chemotherapy-induced peripheral neuropathy patient-reported outcome measure. Oncol Nurs Forum 44(5):580–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miaskowski C et al. (2018) Impact of chemotherapy-induced neurotoxicities on adult cancer survivors’ symptom burden and quality of life. J Cancer Surviv 12(2):234–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winters-Stone KM et al. (2017) Falls, functioning, and disability among women with persistent symptoms of chemotherapy-induced peripheral neuropathy. J Clin Oncol 35(23):2604–2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bao T et al. (2016) Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: prevalence, risk factors, and fall risk. Breast Cancer Res Treat 159(2):327–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teng C et al. (2021) Systematic review of long-term chemotherapy-induced peripheral neuropathy (CIPN) following adjuvant oxaliplatin for colorectal cancer. Support Care Cancer 30(1):33–47 [DOI] [PubMed] [Google Scholar]
- 28.Nyrop KA et al. (2019) Patient-reported and clinician-reported chemotherapy-induced peripheral neuropathy in patients with early breast cancer: current clinical practice. Cancer 125(17):2945–2954 [DOI] [PubMed] [Google Scholar]
- 29.Timmins HC et al. (2021) Metabolic and lifestyle risk factors for chemotherapy-induced peripheral neuropathy in taxane and platinum-treated patients: a systematic review. J Cancer Surviv. 10.1007/s11764-021-00988-x [DOI] [PubMed] [Google Scholar]
- 30.Khattak MA, Townsend AR, Beeke C, Karapetis CS, Luke C, Padbury R, Maddern G, Roder D, Price TJ (2012) Impact of age on choice of chemotherapy and outcome in advanced colorectal cancer. Eur J Cancer 48(9):1293–1298. 10.1016/j.ejca.2011.09.029 [DOI] [PubMed] [Google Scholar]
- 31.Barthélémy P, Heitz D, Mathelin C, Polesi H, Asmane I, Litique V, Rob L, Bergerat JP, Kurtz JE (2011) Adjuvant chemotherapy in elderly patients with early breast cancer. Impact of age and comprehensive geriatric assessment on tumor board proposals. Crit Rev Oncol Hematol 79(2):196–204 [DOI] [PubMed] [Google Scholar]
- 32.Meresse M, Bouhnik AD, Bendiane MK, Retornaz F, Rousseau F, Rey D, Giorgi R (2017) Chemotherapy in old women with breast cancer: is age still a predictor for under treatment? Breast J 23(3):256–266. 10.1111/tbj.12726 [DOI] [PubMed] [Google Scholar]
- 33.Zahiri M et al. (2019) Using wearables to screen motor performance deterioration because of cancer and chemotherapy-induced peripheral neuropathy (CIPN) in adults—Toward an early diagnosis of CIPN. J Geriatr Oncol 10(6):960–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolf SL et al. (2012) The relationship between numbness, tingling, and shooting/burning pain in patients with chemotherapy-induced peripheral neuropathy (CIPN) as measured by the EORTC QLQ-CIPN20 instrument, N06CA. Support Care Cancer 20(3):625–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cavaletti G et al. (2019) Patients’ and physicians’ interpretation of chemotherapy-induced peripheral neurotoxicity. J Peripher Nerv Syst 24(1):111–119 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


