Abstract
Prolonged survival in brain metastasis patients increases recurrence rates and places added importance on salvage therapies. Research examining carmustine polymer wafers as an adjuvant therapy for brain metastasis is limited. We present a single institution retrospective series documenting the use of BCNU wafers placed in the cavity of resected recurrent brain metastases that had failed prior stereotactic radiosurgery (SRS). Between February 2002 and April 2013, a total of 31 patients with brain metastases failed SRS and underwent resection with intracavitary placement of carmustine wafers. Clinical outcomes including local control, survival, cause of death, and toxicity were determined from electronic medical records. Kaplan–Meier analysis was performed to assess local control and survival. Imaging features were reviewed and described for patients with serial post-operative follow-up imaging examinations over time. Overall survival at 6 months and 12 months was 63% and 36%, respectively. Fourteen of 31 patients (45%) died from neurologic causes. Local control within the resection cavity was 87% and 70% at 6 and 12 months, respectively. Five patients (16%) underwent further salvage therapy following carmustine wafer placement after local failure. Resection cavities of all six patients with follow-up imaging showed linear peripheral enhancement. Pericavity and wafer enhancement was present as early as the same day as surgery and persisted in all cases to 6 months or longer. Carmustine polymer wafers are an effective salvage treatment following resection of a brain metastasis that has failed prior SRS. For patients with successful local control after wafer implantation, linear enhancement at the cavity is common.
Keywords: BCNU, Brain metastases, Carmustine polymer wafers, Gliadel, Stereotactic radiosurgery
1. Introduction
Patients with brain metastases are experiencing increased survival times because of improved therapies for extracranial disease [1], earlier detection of brain metastases [2], and more effective therapies for brain metastases [3]. This improvement in survival places increased importance on salvage therapies for brain metastases, since local recurrence occurs at a higher rate for patients with prolonged survival. While surgery can be performed in the salvage setting after failure of radiosurgery or whole brain radiotherapy, the local recurrence rate after resection alone for a brain metastasis is 19–46% [4]. Recurrence of the brain metastasis even after gross resection is thought to be the result of microscopic tumor cells that lie just outside the resection cavity or have infiltrated normal-appearing brain tissue. For this reason, radiosurgery and whole brain radiotherapy have commonly been used as adjuvant therapy after resection of a brain metastasis in order to decrease the likelihood of failing within the resection cavity.
A disadvantage of further radiotherapy as adjuvant therapy after resection of a recurrent brain metastasis is the limited lifetime tolerance of brain tissue to radiation, which results in a cumulative risk of radiation necrosis. An adjuvant treatment option that does not require radiotherapy is the application of carmustine (1,3-bis[2chloroethyl]-1-nitrosourea or BCNU) polymer wafers. The advantage of these wafers is that they deliver a high concentration of chemotherapy at the predominant location of treatment failure. Several studies have demonstrated the efficacy of carmustine polymer wafers on primary malignant brain tumors [5,6], although the scientific literature describing the use of carmustine wafers for adjuvant treatment of brain metastases is limited [7].
We present a single institution retrospective series documenting the use of BCNU wafers placed at the resection cavity of resected recurrent brain metastases that had failed prior stereotactic radiosurgery. The purpose of this study is to examine the effectiveness of carmustine wafers in maintaining local control within the cavity. Additional outcomes of interest, which are descriptively summarized, include survival, likelihood of neurologic death, and patterns of failure of recurrent brain metastases treated with this modality.
2. Materials and methods
2.1. Data acquisition
This retrospective study was approved by the Wake Forest University Institutional Review Board. The Wake Forest University Department of Radiation Oncology Gamma Knife Tumor Registry was searched for all patients who received radiosurgical treatment for a brain metastasis and later underwent craniotomy with carmustine wafer placement (Gliadel Wafer, MGI Pharma, Bloomington, MN, USA). Between February 2002 and April 2013, a total of 31 consecutive patients with brain metastases who failed radiosurgery and underwent surgical resection with intracavitary placement of carmustine polymer wafers were identified. Patients were considered to have failed locally if failure was pathologically proven or if there was evidence of enlargement in volume by at least 25% of the enhancing nodularity at the cavity. Electronic medical records were reviewed to determine patient characteristics including age, sex, race, date of diagnosis, prior whole brain radiotherapy, date of first brain metastasis, date of radiosurgery, size of metastasis at radiosurgery, marginal dose, date of treatment failure, and date of craniotomy. Outcomes such as local control, toxicity, development of leptomeningeal disease and cause of death were also determined from electronic medical records. Table 1 summarizes the patient characteristics in this study.
Table 1.
Patient characteristics
| Number (%) | |
|---|---|
|
| |
| Patients | 31 |
| Median age, years | 55 (range 29–75) |
| Sex | |
| Female | 16 (52%) |
| Male | 15 (48%) |
| Primary disease site | |
| Non-small cell lung | 13 (42%) |
| Small cell lung | 3 (10%) |
| Melanoma | 4 (13%) |
| Breast | 8 (26%) |
| GI | 2 (6%) |
| Thyroid | 1 (3%) |
| Median tumor volume prior to resection | 4.9 cc (range 0.2–53) |
| Treatment history | |
| Prior whole brain radiotherapy | 8 (26%) |
| Prior median Gamma Knifea margin dose | 18 Gy (range 10–24) |
GI = gastrointestinal.
Elekta AB, Stockholm, Sweden.
2.2. Radiosurgery technique
Prior to treatment failure, all patients had been treated with Gamma Knife radiosurgery (Leksell Model C unit prior to May 2009, Leksell Gamma Knife Perfexion unit after May 2009; Elekta AB, Stockholm, Sweden). Prior to radiosurgery, patients underwent a high-resolution contrast-enhanced stereotactic MRI study of the brain. Treatment planning was performed using the Leksell GammaPlan Treatment Planning System (Elekta AB). Dose prescription was determined based on size and volume of each metastasis, generally following the guidelines published by Shaw et al. for single fraction radiosurgical treatment of brain metastases [8]. The median marginal dose of the previously treated lesion was 18 Gy (range 10– 24 Gy) and the median resected volume at the time of stereotactic radiosurgery failure was 4.9 cc (range 0.2–53 cc).
2.3. Carmustine wafer placement
Carmustine wafer was placed at the time of salvage craniotomy if the frozen section was consistent with recurrent brain metastasis. Three of 31 patients underwent carmustine wafer placement after frozen section suggested recurrent metastasis but had a final pathology consistent with radiation necrosis. Carmustine wafer placement was not performed if there was a gross communication between the resection cavity and the ventricular system, or if the resection cavity was of insufficient size to fit a carmustine wafer. The cavity was lined with a single layer of carmustine wafers. A maximum of eight wafers were used per patient; fewer were used if the cavity was of insufficient size to accommodate eight wafers. Wafers were placed equidistant from each other within the cavity. If wafers appeared mobile at time of implantation, they were covered with a single layer of surgical cellulose to hold them in place.
2.4. Patient follow-up, response assessment, and salvage therapy
Patients were followed with a repeat MRI of the brain approximately 6 weeks after craniotomy and then approximately every 3 months thereafter. Local failure was defined as either a pathologically-proven recurrence within the resection cavity, growing nodular enhancement outside of the expected region of carmustine penetration, or by clinical characteristics of local treatment failure. Local failures were treated with further surgical excision, or whole brain irradiation. Neurological death was defined as had been reported by Patchell et al. [4].
2.5. Prospective imaging review
Imaging was prospectively reviewed by consensus between a neuroradiology fellow and a neuroradiologist with 17 years of experience. Both physicians were blinded to patient outcomes and tumor pathology. Imaging follow-up of this patient population is complicated by the variable appearances of the surgical cavity (pericavity infarct and/or blood products/local reaction to a residua of carmustine wafers); concurrent or preceding radiotherapy, surgery, and chemotherapy; various imaging intervals (which were appropriately tailored to patient condition); and lack of tissue confirmation in the majority of cavities. Imaging features were thus described only for patients who had serial post-operative follow-up imaging examinations over time. As the goal of prospective imaging review was to describe treatment-related changes over time, patients were required to have 6 months of imaging follow-up in order to be included in the imaging review.
2.6. Statistical analysis
Time to event data were summarized using Kaplan–Meier plots. Primary endpoints included time to local failure and time to death. All analyses were done using SAS version 9.2 (SAS Institute, Cary, NC, USA).
3. Results
3.1. Survival
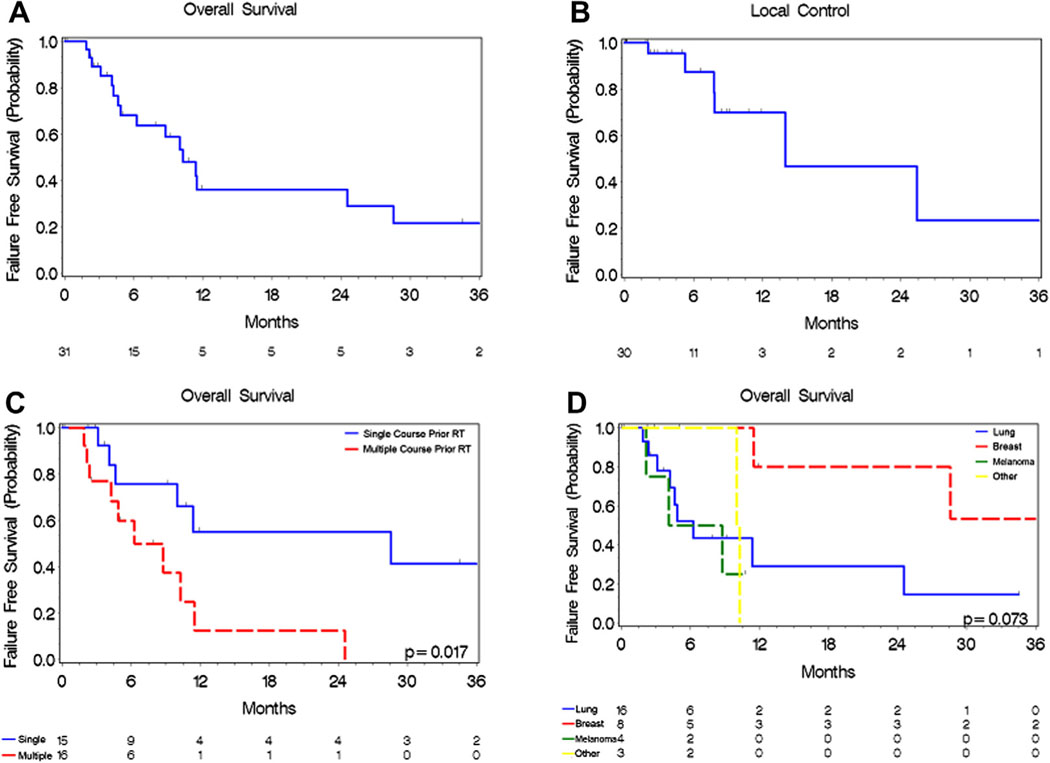
Overall survival at 6 and 12 months from time of carmustine wafer placement was 63% and 36%, respectively (Fig. 1A). Median survival time was 10 months. Fourteen of 31 patients (45%) died from neurologic causes. Overall survival stratified by tumor histology is presented in Figure 1D. The only factor that increased the hazard for death was having had multiple courses of prior radiotherapy (hazard ratio 3.512, 95% confidence interval 1.18–10.49, p = 0.025). Results of univariate analysis for survival are presented in Table 2.
Fig. 1.
Kaplan–Meier analysis of patient outcomes post-carmustine wafer placement. (A) Overall survival of patients following carmustine wafer placement. (B) Local tumor control following carmustine wafer placement. (C) Overall survival of patients receiving single (solid blue line) and multiple courses (dashed red line) of radiotherapy. (D) Overall survival stratified by tumor histology: Breast (red line), Lung (blue line), Melanoma (green line), Other (yellow line).
Table 2.
Univariate analysis of overall survival
| HR | 95% CI | p value | ||
|---|---|---|---|---|
|
| ||||
| Sex | ||||
| Male vs. Female | 0.803 | 0.286 | 2.255 | 0.6775 |
| Primary | ||||
| Lung vs. Other | 0.859 | 0.173 | 4.255 | 0.8519 |
| Breast vs. Other | 0.154 | 0.019 | 1.236 | 0.0783 |
| Melanoma vs. Other | 1.41 | 0.233 | 8.521 | 0.7081 |
| SRS dose, Gy | 0.903 | 0.781 | 1.044 | 0.1683 |
| Volume, cc | 1.02 | 0.986 | 1.054 | 0.2508 |
| Prior radiotherapy course(s) | ||||
| Multiple vs. Single | 3.512 | 1.175 | 10.492 | 0.0245 |
| Time to second radiotherapy, days | 0.946 | 0.852 | 1.05 | 0.2981 |
| Time to craniotomy, days | 0.983 | 0.927 | 1.043 | 0.5715 |
| Craniotomy pathology | ||||
| Necrosis vs. Tumor | 0.517 | 0.064 | 4.163 | 0.5353 |
| Mixed vs. Tumor | 1.806 | 0.603 | 5.41 | 0.2909 |
| Post-craniotomy WBRT | 1.71 | 0.546 | 5.354 | 0.3573 |
| Resection cavity diameter, cm | 1.02 | 0.963 | 1.082 | 0.4815 |
CI = confidence interval, HR = hazard ratio, SRS = stereotactic radiosurgery, WBRT = whole brain radiotherapy.
3.2. Local control and patterns of failure
Local control within the resection cavity at 6 and 12 months was 87% and 70%, respectively (Fig. 1B). Five patients (16%) underwent further local salvage therapy for local recurrence within the resection cavity. Two patients (6%) experienced leptomeningeal carcinomatosis after carmustine wafer placement.
3.3. Imaging characteristics at follow-up
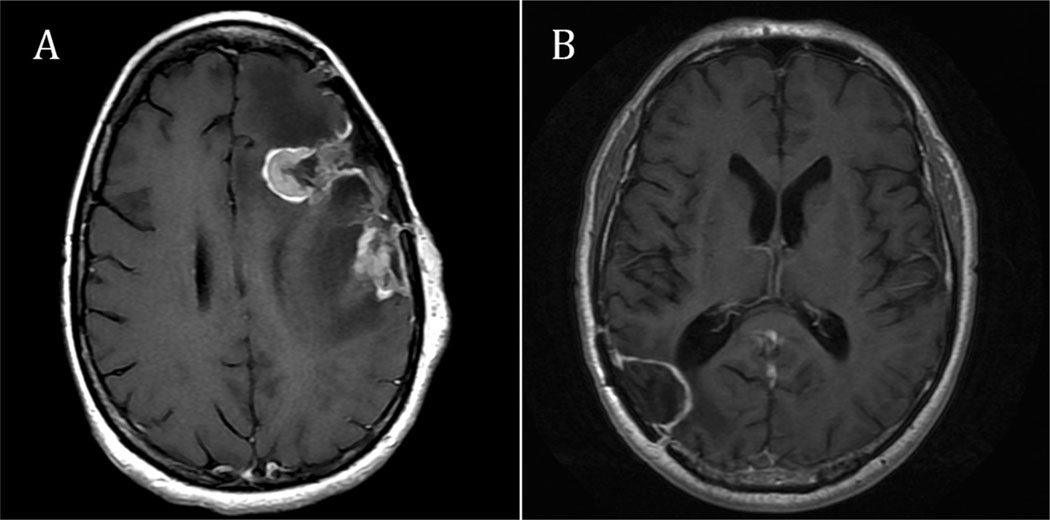
Four of five patients deemed to have local failure had serial follow-up imaging sufficient for prospective review. Medical imaging for a single patient was no longer available in the imaging archive. When a recurrence was first seen on imaging in these four patients, imaging revealed nodular, rather than linear or circumferential, enhancement on serial scan (Fig. 2A). The diameter of the enhancing area ranged from 6 to 21 mm, with a mean of 15 mm. In each of these four patients, the enhancing areas showed restricted diffusion. Edema (surrounding fluid attenuated inversion recovery hyperintensity) increased from the prior study in 75% of these local failure patients. MRI perfusion scans, either arterial spin labeled or dynamic susceptibility contrast, were available in three of these patients: perfusion was positive in one, whereas susceptibility artifact obscured the lesion in the other two.
Fig. 2.
Post-gadolinium axial T1-weighted MRI showing different outcomes following Gliadel carmustine wafer placement (MGI Pharma, Bloomington, MN, USA). (A) Recurrence in a patient 2 months after tumor resection and Gliadel carmustine wafer placement. Two thick, nodular foci of enhancing tumor are present around the anterior and posterior margins of the surgical cavity. (B) Local control in a patient with stable resection cavity 6 months post-operatively. Thin, linear enhancement is present around the margins of the surgical cavity.
A single patient had a lesion that was shown pathologically on re-resection to represent radionecrosis rather than malignancy. In this patient, serial imaging showed increased nodular enhancement, up to 9 mm in diameter; however, the enhancing area did not show restricted diffusion or increased blood volume on dynamic susceptibility contrast imaging.
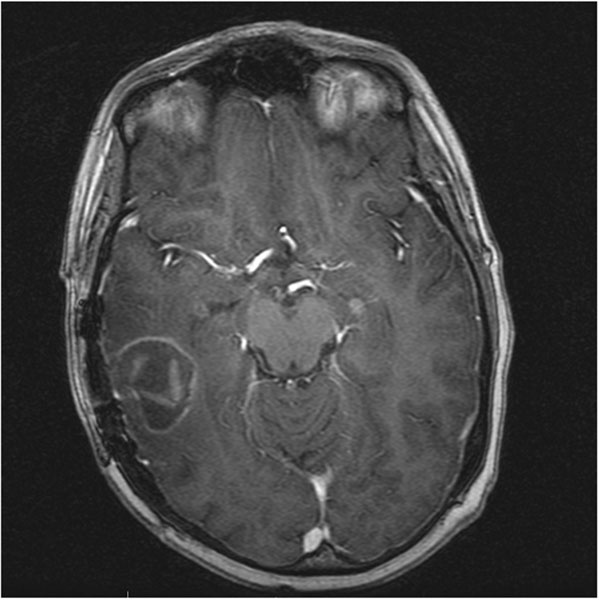
Six of 25 patients without local failure at the site of carmustine wafer placement had follow-up imaging at 6 months. Among these patients without local failure, all of the resection cavities showed occasionally discontinuous linear peripheral enhancement (Fig. 2B). One of six patients showed nodular enhancement, which was likely subacute pericavity infarct based on the evolution of the diffusion and enhancement abnormalities as well as eventual development of encephalomalacia. Pericavity and wafer enhancement was present as early as the same day as surgery and persisted in all patients to 6 months or longer (Fig. 3). Pericavity enhancement mainly ranged from 1 to 5 mm, with the maximum thickness measuring 11 mm at 3 months. At 6 months, 50% of cavities showed decreased enhancement compared to the maximum enhancement for that patient. The other 50% showed stable or minimally increased enhancement (no more than 1 mm) at 6 months as compared to that patient’s previous imaging. Aside from pericavity infarct, diffusion restriction was not seen in these patients. Perfusion imaging was either unavailable or non-contributory in these patients due to susceptibility artifact in the area of the lesion.
Fig. 3.
Gliadel carmustine wafers (MGI Pharma, Bloomington, MN, USA) are present and enhancing at 1 month post-tumor resection on post-gadolinium axial T1-weighted MRI.
3.4. Toxicity
Three patients (11%) developed hydrocephalus requiring shunt placement after carmustine wafer placement. Three patients (11%) reported post-operative headaches. One patient (4%) experienced a cerebrospinal fluid leak post-operatively. One patient (4%) suffered post-operative methicillin-resistant Staphylococcus aureus surgical site infection.
4. Discussion
Placement of carmustine wafers in the resection cavity of high grade glioma has been studied in randomized, controlled trials [5,9]. A disadvantage of carmustine wafers for high grade gliomas is that these tumors infiltrate well beyond the resection cavity, and carmustine may not penetrate sufficiently to such a distance.
Brain metastases, on the other hand, usually have relatively little infiltration into the normal brain parenchyma. A surgical pathology study of 45 patients showed that only melanoma and small cell lung cancer demonstrated greater than 1 mm infiltration beyond the metastasis boundary [10]. We found only one additional published series documenting the use of carmustine wafers in the resection cavity of patients with a resected brain metastasis. In this series, 25 patients were treated with the combination of surgery, radiotherapy and carmustine wafer placement in a multi-institutional study [7]. There were no local failures within the cavity. The major difference between this series and the current one is that the current series is assessing local control following carmustine wafer placement without any further radiotherapy, and in the setting of prior radiosurgical failure.
Durable local control for radiosurgery relates to the volume of the lesion at the time of radiosurgery [11] and to the competing risk of death from extracranial disease [12]. However, for the subset of patients with well-controlled extracranial disease and larger volume brain metastasis, patients can go on to have single site multiple recurrent intracranial disease. Multiple salvage options are available to such patients, including surgery, laser interstitial thermal therapy, and whole brain radiotherapy. However, in patients who have already received whole brain radiotherapy and radiosurgery, additional salvage radiation would likely breech their lifetime threshold for safe radiotherapy. It is in this population that a local adjuvant salvage option to surgery alone is likely to be most useful because surgery in the absence of adjuvant therapy for a brain metastasis has a local failure rate of 19–46% [4].
A potential drawback of craniotomy and carmustine wafer placement is the increased risk of post-operative infection. Surgical site infection rates following carmustine placement at different institutions ranges from 5–28% [13,14]. In this series, a single patient experienced a post-operative surgical site infection and was hospitalized. The risk of post-operative infection should be weighed against the risk of toxicity of alternative adjuvant options such as brachytherapy [15] and brain radiotherapy [16].
Imaging findings of linear and sometimes discontinuous enhancement were seen in all patients who met the criteria to be included in the prospective imaging review, and these were found to persist to 6 months or longer. Enhancement either stabilized or decreased after 3 months in all evaluable patients with local control. In cases of local recurrence after carmustine wafer implantation, the typical imaging appearance was a nodular focus of new or increasing enhancement that was a mean of 15 mm in diameter and that showed restricted diffusion. Perfusion imaging may be helpful in individual cases if the area in question is not obscured by post-surgical susceptibility artifact. The imaging portion of our study represents, to our knowledge, the first attempt at prospective and detailed imaging assessment after intracavitary carmustine wafer placement for brain metastases. A previous series by Colen et al. of eight patients with recurrent glioblastoma treated with surgery and carmustine wafer placement, demonstrated that five patients developed a subsequent progressive increase in enhancement in the resection cavity [17]. Our series confirms that this phenomenon also occurs in patients treated for brain metastases, and also suggests that the mechanism of the new enhancement is less likely to be due to tumor pseudoprogression, as this phenomenon is not as common in brain metastases as with glioblastoma.
There are several limitations to this study. As a retrospective review, it is subject to patient selection bias. A well-designed prospective study would be better equipped to determine factors that predict treatment success or failure. Furthermore, the sample size makes it difficult to generalize conclusions, especially since there was a diversity of cancer histologies treated with carmustine wafers in the current series. Stratification analysis in this study did not show improved survival or response to treatment of any one tumor histology over other types. Prior animal series have suggested that melanoma and renal cell metastases respond better to carmustine wafers than lung cancer histologies. In spite of its limitations, to our knowledge this study is the largest series looking at the role of carmustine wafer placement at the site of surgical salvage after radiosurgical failure. As such, this series proposes a clinically useful indication for intracavitary carmustine wafers, showing that local control can be achieved in the absence of further radiotherapy.
5. Conclusion
Carmustine polymer wafers offer an adjuvant treatment option after resection of a brain metastasis that has failed prior stereotactic radiosurgery. For patients with successful local control after wafer implantation, linear enhancement at the cavity is commonly seen.
Footnotes
Conflicts of Interest/Disclosures
The authors declare that they have no financial or other conflicts of interest in relation to this research and its publication.
References
- [1].Cochran DC, Chan MD, Aklilu M, et al. The effect of targeted agents on outcomes in patients with brain metastases from renal cell carcinoma treated with Gamma Knife surgery. J Neurosurg 2012;116:978–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Loganathan AG, Chan MD, Alphonse N, et al. Clinical outcomes of brain metastases treated with Gamma Knife radiosurgery with 3.0 T versus 1.5 T MRI-based treatment planning: have we finally optimised detection of occult brain metastases? J Med Imaging Radiat Oncol 2012;56:554–60. [DOI] [PubMed] [Google Scholar]
- [3].Jensen CA, Chan MD, McCoy TP, et al. Cavity-directed radiosurgery as adjuvant therapy after resection of a brain metastasis. J Neurosurg 2011;114:1585–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 1998;280:1485–9. [DOI] [PubMed] [Google Scholar]
- [5].Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol 2003;5:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Reithmeier T, Graf E, Piroth T, et al. BCNU for recurrent glioblastoma multiforme: efficacy, toxicity and prognostic factors. BMC Cancer 2010;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ewend MG, Brem S, Gilbert M, et al. Treatment of single brain metastasis with resection, intracavity carmustine polymer wafers, and radiation therapy is safe and provides excellent local control. Clin Cancer Res 2007;13:3637–41. [DOI] [PubMed] [Google Scholar]
- [8].Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90–05. Int J Radiat Oncol Biol Phys 2000;47:291–8. [DOI] [PubMed] [Google Scholar]
- [9].Brem H, Piantadosi S, Burger PC, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet 1995;345:1008–12. [DOI] [PubMed] [Google Scholar]
- [10].Baumert BG, Rutten I, Dehing-Oberije C, et al. A pathology-based substrate for target definition in radiosurgery of brain metastases. Int J Radiat Oncol Biol Phys 2006;66:187–94. [DOI] [PubMed] [Google Scholar]
- [11].Shiau CY, Sneed PK, Shu HK, et al. Radiosurgery for brain metastases: relationship of dose and pattern of enhancement to local control. Int J Radiat Oncol Biol Phys 1997;37:375–83. [DOI] [PubMed] [Google Scholar]
- [12].Kuremsky JG, Urbanic JJ, Petty WJ, et al. Tumor histology predicts patterns of failure and survival in patients with brain metastases from lung cancer treated with gamma knife radiosurgery. Neurosurgery 2013;73:641–7 [discussion 647]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].McGovern PC, Lautenbach E, Brennan PJ, et al. Risk factors for postcraniotomy surgical site infection after 1,3-bis (2-chloroethyl)-1-nitrosourea (Gliadel) wafer placement. Clin Infect Dis 2003;36:759–65. [DOI] [PubMed] [Google Scholar]
- [14].Subach BR, Witham TF, Kondziolka D, et al. Morbidity and survival after 1,3bis(2-chloroethyl)-1-nitrosourea wafer implantation for recurrent glioblastoma: a retrospective case-matched cohort series. Neurosurgery 1999;45:17–22 [discussion 22–3]. [DOI] [PubMed] [Google Scholar]
- [15].Gutin PH, Phillips TL, Wara WM, et al. Brachytherapy of recurrent malignant brain tumors with removable high-activity iodine-125 sources. J Neurosurg 1984;60:61–8. [DOI] [PubMed] [Google Scholar]
- [16].Greene-Schloesser D, Robbins ME, Peiffer AM, et al. Radiation-induced brain injury: A review. Front Oncol 2012;2:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Colen RR, Zinn PO, Hazany S, et al. Magnetic resonance imaging appearance and changes on intracavitary Gliadel wafer placement: a pilot study. World J Radiol 2011;3:266–72. [DOI] [PMC free article] [PubMed] [Google Scholar]