Abstract

Monosaccharides play a vital role in the human diet due to their interesting biological activity and functional properties. Conventionally, sugars are extracted using volatile organic solvents (VOCs). Deep eutectic solvents (DESs) have recently emerged as a new green alternative to VOCs. Nonetheless, the selection criterion of an appropriate DES for a specific application is a very difficult task due to the designer nature of these solvents and the theoretically infinite number of combinations of their constituents and compositions. This paper presents a framework for screening a large number of DES constituents for monosaccharide extraction application using COSMO-RS. The framework employs the activity coefficients at infinite dilution (γi∞) as a measure of glucose and fructose solubility. Moreover, the toxicity analysis of the constituents is considered to ensure that selected constituents are safe to work with. Finally, the obtained viscosity predictions were used to select DESs that are not transport-limited. To provide more insights into which functional groups are responsible for more effective monosaccharide extraction, a structure-solubility analysis was carried out. Based on an analysis of 212 DES constituents, the top-performing hydrogen bond acceptors were found to be carnitine, betaine, and choline chloride, while the top-performing hydrogen bond donors were oxalic acid, ethanolamine, and citric acid. A research initiative was presented in this paper to develop robust computational frameworks for selecting optimal DESs for a given application to develop an effective DES design strategy that can aid in the development of novel processes using DESs.
1. Introduction
The scientific community’s interest in developing sustainable solvents has grown rapidly since the establishment of the 12 green engineering principles by Anastas and Warner.1 These principles are considered grounds of recent research advancements and a guide to a future with chemical processes that do not generate or use any harmful solvents. Several breakthrough innovations have been achieved, including liquid polymers,2 switchable solvents,3 supercritical fluids,4,5 ionic liquids,6,7 and most recently, deep eutectic solvents (DESs).8,9 DESs, green solvents that were first coined by Abbott and co-workers in 2003,8 are emerging green solvents extensively studied for the extraction of various natural bioactive compounds, such as phenolic compounds, polysaccharides, proteins, and fatty acids.10
DESs are “mixtures of pure compounds for which the eutectic point temperature is far below that of an ideal liquid mixture”.11 They are generally formed by mixing one or more hydrogen bond acceptors (HBAs) and hydrogen bond donors (HBDs), resulting in a liquid solvent at room temperature.12 These solvents can be prepared to be biodegradable and sustainable by choosing the appropriate constituents.8 They also have attractive characteristics, such as thermal and chemical stability, nonflammability, low volatility, high conductivity, and good solubilization capacity for several organic compounds.9 These physical properties depend on the mixture’s constituents and can be easily tailored for specific applications.10 Attempts to understand the formation of naturally occurring DESs, such as honey, maple syrup, and sugar beet, led to the discovery of a new class of DESs called natural DESs (NADESs).13 NADESs were first reported by Dai et al.,13 where it was defined as supramolecular liquids composed of common metabolites in certain molar ratios, including water in some cases, which are characterized by extensive intermolecular interactions (hydrogen bonding). This class of DES has great potential as a green medium in many fields, such as food, nutraceutical, pharmaceutical, biomedical, and cosmetics due to the relatively low toxicity levels.14
Monosaccharides and sugars generally play a vital role in the human diet due to their interesting biological activity and functional properties.15 Currently, sugars are commonly extracted using conventional organic solvents, such as ethanol or methanol aqueous solutions. However, organic solvents are characterized by high volatility, inflammability, and toxicity levels, which negatively impact the environment. At the industrial scale, sugar is produced from sugarcane and sugar beet through a water-based extraction that is heat intensive and involves several chemical-based treatments.16 Thus, a green yet high-performing alternative solvent that can extract sugar and other heat-sensitive bioactive compounds under mild temperature conditions is needed. NADESs can provide excellent alternatives to water and other organic solvents. Recently, extraction of bioactive compounds using various techniques (ultrasound-assisted extraction, microwave-assisted extraction, pressurized liquid extraction, etc.) using green solvents such as NADES attracted the attention of researchers.17 For example, Gómez et al.(18) investigated several NADESs for the extraction of soluble sugars from ripe bananas. Among the NADESs studied, malic acid:beta-alanine:water (1:1:3) with an additional 30 g water/100 g solvent was found to be the most effective in extracting the soluble sugars from banana puree. The solvent achieved a sugar recovery of 106.9 g/100 g of solvent (25 °C, 30 min), resulting in a much more effective extraction than traditional benchmark solvents, ethanol (79.7 g/100 g), and water (71.5 g/100 g).18 Zhang and Wang19 studied the ultrasound-assisted extraction of polysaccharides from Dioscorea oppositaThunb using several DESs, and ChCl:1,4-butanediol proved to be suitable for the extraction.
Moreover, DESs and NADESs were utilized for food waste biomass pretreatment to enhance sugar production through an enzymatic hydrolysis process. These derived sugars can then be used for biofuel production.20 Although the results obtained in the literature seem to be very promising, there are some limitations that hinder the application of NADESs, such as their high viscosity and/or low polarity, which can lead to a reduction in the solute diffusivity into the extraction medium and a reduction in the solute dissolution capacity, resulting in a low yield. However, these issues could be altered by changing the constituents, composition, of DESs and the operating temperature.10 The controllable addition of water can also be another promising solution as it can help weaken the NADES interactions, causing a decrease in viscosity and increase in its polarizability. Dai et al.(21) proved that the addition of 50% (v/v) water caused the complete disappearance of hydrogen bonding interactions between the two components of NADESs. Other researchers reported the preservation of the molecular-level NADES structure under certain water content conditions of 50 wt % or below.18,22 Therefore, the dilution of NADES with water should be carefully controlled to avoid any adverse impact on NADES performance.
Despite the promising characteristics of NADES, research investigating
the extraction of monosaccharides using NADESs is very scarce and
limited. Therefore, more studies are needed to find an optimal NADES
capable of effectively replacing conventional solvents. However, considering
the high versatility and “designer” nature of NADES,
there is a theoretically infinite number of possible NADES mixtures
based on the choice of HBA, HBD, and their molar ratio. In that sense,
relying only on experimental measurements for designing NADES is ineffective,
and thus, using molecular-based modeling and simulation tools for
pre-screening the NADES constituents that are effective for specific
applications would save both investigation time and resources. The
Conductor-like Screening Model for Real Solvents (COSMO-RS) has been
successfully used in the literature to screen solvents for various
separation processes, such as fuel purification,23 CO2 capture,24 and
the valorization of volatile fatty acids from wastewater.25 The application of COSMO-RS screening for saccharide
extraction has only been reported in a paper by Mohan et al.,(26) where they screened 64 ionic liquids
(ILs) for their glucose, fructose, xylose, and galactose solubilities.
The results indicated that imidazolium [Im+]-, ammonium
[N+]-, and phosphonium [P+]-based cations, especially
those with lower chain lengths, showed promising performance. On the
other hand, the best investigated anions were thiocyanate [SCN–] and methylsulfate 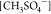 . According to the author’s knowledge,
a methodical COSMO-RS evaluation for the utilization of sugar extraction
via DESs or NADESs has not been reported up to this point.
. According to the author’s knowledge,
a methodical COSMO-RS evaluation for the utilization of sugar extraction
via DESs or NADESs has not been reported up to this point.
Therefore, herein, we present the screening of 212 DES constituents, including 89 HBAs and 123 HBDs, for the extraction of glucose and fructose using COSMO-RS. The screening framework initially considers the infinite dilution activity coefficients (γi∞) as a quantitative assessment of the monosaccharide’s solubility, and then a toxicity analysis of the best constituents is conducted to confirm that the selected components are non-toxic and appropriate for use in food applications. Finally, viscosity predictions are applied to select NADESs that are not transport- or diffusion-limited. Additionally, a structure-solubility analysis was also conducted to offer molecular insights into how the structure of the NADES influences their attraction toward monosaccharides. A schematic summarizing the applied approach is shown in Figure 1. The results of this work can be used as a molecular-based guide and database for the design of new NADESs that are non-toxic, having low viscosity, and with relatively high monosaccharide solubility, which can be used in a plethora of food applications, such as the extraction of sugars from fruits with high sugar content (such as dates) and from food bio-wastes.
Figure 1.
Schematic depicting the approach used in this study.
2. Computational Method
COSMO-RS is a predictive molecular modeling approach devised by Eckert and Klamt. It uses quantum chemistry and statistical mechanics to predict the thermodynamic behavior of pure substances and their combinations, based on their three-dimensional structure and screening charge density. This model can predict the attributes of traditional organic solvents, ILs, and has more recently been used to predict the properties of DESs. More comprehensive information about the theories and applications of COSMO-RS can be found in other sources.27,28
In this work, a list of DES systems that are commonly used in extraction was compiled based on an extensive literature survey. The list consisted of 89 HBAs and 123 HBDs. The three-dimensional structures of each molecule were initially created using Turbomole software29 (TmoleX19 4.5.1) by inputting the SMILES for each HBA and HBD as well as for glucose and fructose into the software. The density functional theory (DFT) optimizations were carried out using the def-TZVP “triple-zeta valence polarized” basis alongside the Becke–Perdew (BP86) exchange–correlation function.30 This method was previously applied in the literature for modeling DES components.31,32 The generated “.cosmo” files (depicted in Figure 2) are subsequently imported into the BIOVIA COSMOtherm (2022 version) for the execution of quantum chemical simulations.
Figure 2.

3D COSMO-RS structures of typical examples of cations, anions, and HBDs along with the monosaccharides (glucose and fructose) and the benchmark solvents (ethanol and water).
In COSMO-RS, DESs can be depicted using three main strategies: (a) the ion-pair strategy, (b) the meta file strategy, and (c) the electroneutral strategy. For this study, we utilized the electroneutral strategy, where the DESs are treated as three separate dissociated entities—the salt cation, the salt anion, and the HBD, considering their molar proportions. For instance, ChCl:ethanolamine (1:6) is viewed as a mole of choline cation, a mole of chloride anion, and 6 moles of ethanolamine. The advantage of using this approach is its flexibility in modeling any combination of DESs as it enables the cation, the anion, the HBD, or their molar ratios to be easily changed. Furthermore, by this method, it is plausible to study as many variations as possible of cation-anion-HBD-monosaccharide permutations that can occur in solution.
To screen the best DES constituents for the extraction of monosaccharides, the activity coefficient at infinite dilution (γi∞) method was used as an evaluation parameter, which is described as follows in COSMO-RS:33
| 1 |
where μS and μi are the COSMO-RS predicted chemical potentials of the DES component and the monosaccharide (glucose or fructose), respectively. The solubility of a certain compound in a solvent is inversely proportional to its activity coefficient in the system. Thus, COSMO-RS was used to predict the activity coefficient of reducing sugars, namely, fructose and glucose, in the 212 constituents of DESs at 298.2 K.
The liquid viscosities of the DESs in COSMO-RS were computed based on (1) the Grunberg–Nissan equation, (2) the surface area as read from the “.cosmo” file (ai), (3) the second σ-moment (Mi2), (4) the number of ring atoms (NiRing), and (5) the multiplication of temperature and entropy (TSi) that is calculated from the enthalpy (Hi) and the chemical potential [TSi = – (Hi – μi)].34
The COSMO-RS solubility predictions were made in the “Multiple Solvents” panel with the selected options of (i) absolute values and (ii) SLE (best quality, slow). The predictions require the melting temperature and the enthalpy of fusion of the solutes (glucose and fructose), which were obtained from the NIST Chemistry Database as Tm = 414 K; ΔHfus = 31.42 kJ/mol for glucose, and Tm = 376 K; ΔHfus = 30.30 kJ/mol for fructose. The IDs of glucose and fructose in the NIST Database are C50997 and C57487, respectively. The fusion entropy was also fed into the COSMO-RS software, which was determined as given below:
| 2 |
where ΔSfus is in units of kJ·K–1·mol–1 and is calculated to be 0.0759 and 0.0806 kj·K–1·mol–1 for glucose and fructose, respectively. Using the COSMO-RS SLE panel, predictions of the DES density and viscosity are also provided as an output, which are also reported in this work.
3. Results and Discussion
3.1. σ-Profiles: Their Practical Significance and Contribution to the Predictions
The predictions of COSMO-RS are based on two main factors, (i) the geometrically optimized DFT structures of the molecules and (ii) their σ-profiles Ps(σ). The σ-profile of a molecule is a probability distribution that denotes the relative likelihood of a molecular surface segment having a specific surface charge, σ, which could be positive, neutral, or negative.35 As a result, the position, height, and breadth of the peaks in the σ-profile carry the necessary structural and energy-related data required to forecast its dominant intermolecular interactions, such as hydrogen bonding, electrostatic, and polar inducing interactions. Figure 3 shows eight representative examples of the σ-profiles of two cations, two anions, two HBDs, and the monosaccharides (glucose and fructose). The σ-profiles of the rest of the molecules are available in Figures S1 and S2 of the Supporting Information. In Figure 3, the profile can be divided into three separate areas. Negative charge densities (σ < −0.008 e/Å2) indicate positive polarity surfaces with a hydrogen “donating” trait (shown by blue molecular surfaces), whereas positive charge densities (σ > +0.008 e/Å2) indicate negative polarity surfaces with a hydrogen “accepting” trait (represented by red molecular surfaces). Charge densities within the range of (−0.008 ≤ σ ≤ +0.008 e/Å2) signify neutral surfaces within the molecule.
Figure 3.
σ-profiles of (A) bromide, chloride, tetrabutylphosphonium, choline, and (B) polyethylene glycol 200, and thymol as representative examples of anions, cations, and HBDs, respectively, with glucose and fructose.
When contrasting the ions in Figure 3A, it is apparent that cations are skewed to the left in their profile, whereas anions have a right-side skewness, reflecting their positive and negative charges, respectively. When different anions are compared, it can be observed that chloride is slightly more negative than bromide (more toward the right) because chloride has a higher electronegativity than bromide. However, bromide has a peak higher than chloride because the atomic weight of bromide is higher. In the same sense, it can also be observed that the tetrabutylphosphonium cation exhibits a peak (particularly in the neutral region) that is significantly higher than that of the choline cation as it encompasses more non-polar surfaces. Conversely, it can be observed that the choline cation possesses a more localized charge (to the left). This occurs because the impact of the N+ is more evident as fewer carbons are available for charge stabilization and due to the H+ part of the hydroxy functional group.36 Moving on to Figure 3B, it can be observed that tetraethylene glycol, glucose, and fructose showcase relatively large peaks in both the HBA and the HBD regions, suggesting that these molecules can function as both an HBA and an HBD. In addition, it can be observed that the sizes of the glucose and fructose profiles are very similar due to their similarity in nature and molecular weight. On the other hand, thymol has more pronounced peaks in the HBA region than in the HBD region. It can also be observed that the charges in thymol are spread over a wide peak, a characteristic resulting from its resonance structures and charge delocalization of the lone electron pairs from the oxygen through the aromatic ring.37
3.2. COSMO-RS Evaluation: Screening the Influence of HBAs and HBDs
For selecting the DES components with the highest capacity to extract glucose and fructose, the activity coefficient at infinite dilution (γi∞) of each DES constituent was determined at 298.2 K with COSMO-RS. The γi describes the behavior of the monosaccharide molecules when they are fully surrounded by the molecules of the DES constituent (i.e., xi ≈ 0). The lower the value of γi∞, the higher the solubility of the solute in the DES constituent (stronger interactions). The predicted averaged γi values of both solutes (fructose and glucose) are listed from lowest to highest in Table 1 and are visually represented in Figure 4 and compared to two commonly used benchmark solvents (ethanol and water) for extracting monosaccharides. The dashed lines in the figures correspond to four quadrants indicating the effectiveness of each DES component compared to ethanol, with Q1 corresponding to DES components performing worse than ethanol with respect to both monosaccharides, Q3 corresponds to DES constituents performing better than ethanol with respect to both monosaccharides, and Q2 and Q4 correspond to DES constituents that are better than ethanol for only fructose or glucose, respectively.
Table 1. Predicted Activity Coefficients at Infinite Dilution (γi∞) of the Targeted Monosaccharides in the 212 DES Constituents at 298.2 K and 1.01 Bar.
| # | constituent | ln(γ∞Fru) | ln(γ∞Glu) | # | constituent | ln(γ∞Fru) | ln(γ∞Glu) |
|---|---|---|---|---|---|---|---|
| hydrogen bond acceptors (HBAs) | D17 | hydroquinone38 | –3.534 | –1.812 | |||
| A1 | ammonium chloride | –76.380 | –76.020 | D18 | maleic acid39 | –3.165 | –2.097 |
| A2 | ammonium bromide | –68.513 | –66.997 | D19 | urea40 | –1.935 | –3.001 |
| A3 | tetramethylammonium chloride | –15.282 | –17.604 | D20 | phenol41 | –3.446 | –1.118 |
| A4 | carnitine42 | –13.710 | –16.525 | D21 | 3-salicylic acid43 | –2.978 | –1.486 |
| A5 | tetramethyl phosphonium chloride | –13.725 | –16.151 | D22 | tartaric acid44 | –2.672 | –1.692 |
| A6 | betaine42 | –12.899 | –15.610 | D23 | malonic acid45 | –2.565 | –1.765 |
| A7 | tetraethylammonium chloride | –12.540 | –15.047 | D24 | lidocaine46 | –1.549 | –2.761 |
| A8 | tetraethylphosphonium chloride | –12.514 | –15.030 | D25 | diethylene glycol47 | –1.566 | –2.727 |
| A9 | choline chloride8 | –11.912 | –14.189 | D26 | methanol48 | –1.460 | –2.700 |
| A10 | tetramethylammonium bromide | –11.796 | –13.654 | D27 | formic acid49 | –2.446 | –1.601 |
| A11 | tetrapropylammonium chloride | –11.001 | –13.526 | D28 | diethanolamine50 | –1.364 | –2.611 |
| A12 | tetrapropylphosphonium chloride | –10.838 | –13.354 | D29 | 1,4-butanediol19 | –1.152 | –2.522 |
| A13 | benzyltriethylammonium chloride51 | –10.543 | –12.955 | D30 | 2-salicylic acid52 | –2.637 | –0.669 |
| A14 | benzyltriethylphosphonium chloride51 | –10.466 | –12.882 | D31 | 1,3-propanediol53 | –0.985 | –2.150 |
| A15 | dimethylethylethanolammonium chloride | –10.472 | –12.833 | D32 | 3-cresol50 | –2.619 | –0.479 |
| A16 | diethylethanolammonium chloride | –10.393 | –12.849 | D33 | bisphenol Z54 | –2.499 | –0.452 |
| A17 | tetrabutylammonium chloride55 | –10.244 | –12.752 | D34 | 2-methylpentanediol56 | –0.793 | –2.142 |
| A18 | tetrabutylphosphonium chloride57 | –10.065 | –12.566 | D35 | 4-phenyl phenol38 | –2.741 | –0.058 |
| A19 | tetramethyl phosphonium bromide | –10.284 | –12.182 | D36 | ethanol58 | –0.785 | –1.948 |
| A20 | tetrapentylammonium chloride | –9.795 | –12.289 | D37 | 4-cresol50 | –2.400 | –0.157 |
| A21 | benzyl trimethylammonium chloride | –9.698 | –12.040 | D38 | 2-cresol50 | –2.510 | –0.033 |
| A22 | benzyl trimethyl phosphonium chloride | –9.689 | –12.039 | D39 | sesamol43 | –2.121 | –0.188 |
| A23 | tetrapentylphosphonium chloride | –9.510 | –11.995 | D40 | ethylene glycol53 | –0.649 | –1.644 |
| A24 | acetylcholine chloride | –9.435 | –11.704 | D41 | malic acid44 | –1.431 | –0.646 |
| A25 | tetrahexylammonium chloride | –9.248 | –11.730 | D42 | 4-ethylphenol59 | –2.183 | 0.121 |
| A26 | 1-butyl-3-methylimidazolium chloride52 | –9.183 | –11.654 | D43 | N-methyl diethanolamine60 | –0.465 | –1.366 |
| A27 | butyltriphenylphosphonium chloride | –9.146 | –11.499 | D44 | propylene glycol61 | –0.442 | –1.343 |
| A28 | tetra hexyl phosphonium chloride | –9.062 | –11.535 | D45 | 4-cyanophenol38 | –1.474 | 0.042 |
| A29 | tetraheptylammonium chloride62 | –8.826 | –11.297 | D46 | acetic acid63 | –0.884 | –0.515 |
| A30 | allyl triphenylphosphonium chloride | –8.826 | –11.145 | D47 | 4-propyl phenol59 | –1.876 | 0.484 |
| A31 | methyl triphenylphosphonium chloride | –8.743 | –11.058 | D48 | xylitol40 | –0.286 | –0.903 |
| A32 | tetraheptylphosphonium chloride62 | –8.624 | –11.085 | D49 | 1,6-hexanediol19 | –0.091 | –1.093 |
| A33 | tetraethylammonium bromide | –8.908 | –10.794 | D50 | glycerol64 | –0.211 | –0.950 |
| A34 | tetraethylphosphonium bromide | –8.849 | –10.713 | D51 | thiourea65 | –1.073 | –0.050 |
| A35 | tetraoctylammonium chloride66 | –8.506 | –10.968 | D52 | ascorbic acid67 | –0.806 | –0.221 |
| A36 | methyl triphenyl ammonium chloride | –8.546 | –10.866 | D53 | 1,2-butanediol53 | –0.065 | –0.831 |
| A37 | 1-hexyl-3-methylimidazolium chloride52 | –8.420 | –10.879 | D54 | lactic acid68 | –0.585 | –0.216 |
| A38 | choline bromide | –8.715 | –10.581 | D55 | levulinic acid69 | –0.426 | –0.363 |
| A39 | methyltrioctylphosphonium chloride70 | –8.418 | –10.870 | D56 | polyethylene glycol 20071 | –0.085 | –0.695 |
| A40 | benzyltriphenylphosphonium chloride72 | –8.464 | –10.750 | D57 | thymol56 | –1.754 | 0.998 |
| A41 | benzyltriphenylammonium chloride72 | –8.413 | –10.698 | D58 | 1-propanol53 | 0.096 | –0.850 |
| A42 | methyltrioctylammonium chloride70 | –8.299 | –10.753 | D59 | propionic acid73 | –0.570 | –0.028 |
| A43 | tetraoctylphosphonium chloride66 | –8.298 | –10.749 | D60 | glycolic acid74 | –0.414 | –0.157 |
| A44 | hexadecyltrimethylphosphonium chloride | –7.831 | –10.248 | D61 | mequinol59 | –1.143 | 0.602 |
| A45 | dodecyldimethylbenzylphosphonium chloride75 | –7.678 | –10.029 | D62 | 1,2-decanediol76 | 0.348 | –0.865 |
| A46 | dodecyldimethylbenzylammonium chloride75 | –7.595 | –9.948 | D63 | maltitol40 | 0.214 | –0.730 |
| A47 | tetrapropylammonium bromide | –7.586 | –9.414 | D64 | maltose61 | –0.034 | –0.450 |
| A48 | hexadecyltrimethylammonium chloride | –7.304 | –9.695 | D65 | mandelic acid73 | –0.780 | 0.442 |
| A49 | tetrapropylphosphonium bromide | –7.456 | –9.272 | D66 | pyruvic acid68 | –0.804 | 0.570 |
| A50 | dimethylethylethanolammonium bromide | –7.395 | –9.289 | D67 | 1,8-octanediol77 | 0.355 | –0.572 |
| A51 | diethylethanolammonium bromide | –7.048 | –8.952 | D68 | diclofenac78 | –1.321 | 1.212 |
| A52 | benzyltriethylammonium bromide51 | –7.107 | –8.871 | D69 | sorbitol40 | 0.087 | –0.171 |
| A53 | benzyltriethylphosphonium bromide51 | –7.054 | –8.806 | D70 | 1-butanol53 | 0.475 | –0.450 |
| A54 | tetrabutylammonium bromide55 | –6.864 | –8.647 | D71 | atropine76 | 0.354 | –0.299 |
| A55 | tetrabutylphosphonium bromide57 | –6.721 | –8.495 | D72 | benzoic acid52 | –0.619 | 0.766 |
| A56 | benzethonium chloride | –6.482 | –8.613 | D73 | cyclohexanol53 | 0.584 | –0.424 |
| A57 | benzyl trimethyl phosphonium bromide | –6.602 | –8.363 | D74 | trioctylphosphine79 | 1.058 | –0.725 |
| A58 | benzyl trimethylammonium bromide | –6.536 | –8.305 | D75 | phenylacetic acid73 | –0.456 | 0.848 |
| A59 | tetrapentylammonium bromide | –6.385 | –8.137 | D76 | trifluoroacetamide80 | –0.323 | 0.832 |
| A60 | acetylcholine bromide | –6.370 | –8.139 | D77 | 1,10-decanediol75 | 0.800 | –0.043 |
| A61 | tetrapentylphosphonium bromide | –6.168 | –7.912 | D78 | anise alcohol81 | 0.454 | 0.371 |
| A62 | 1-butyl-3-methylimidazolium bromide52 | –5.904 | –7.755 | D79 | sobrerol82 | 0.655 | 0.248 |
| A63 | tetrahexylammonium bromide | –5.843 | –7.574 | D80 | ethyl paraben83 | –0.026 | 1.132 |
| A64 | butyltriphenylphosphonium bromide | –5.757 | –7.408 | D81 | allantoic acid | 0.808 | 0.575 |
| A65 | tetrahexylphosphonium bromide | –5.703 | –7.425 | D82 | 2-phenylethanol84 | 0.716 | 0.675 |
| A66 | allyl triphenylphosphonium bromide | –5.544 | –7.178 | D83 | trimethyl-1,3-pentanediol85 | 1.073 | 0.340 |
| A67 | methyl triphenylphosphonium bromide86 | –5.510 | –7.151 | D84 | camphor82 | 0.982 | 0.627 |
| A68 | tetraheptylammonium bromide62 | –5.407 | –7.119 | D85 | hexanoic acid87 | 0.529 | 1.303 |
| A69 | 1-hexyl-3-methylimidazolium bromide52 | –5.271 | –7.079 | D86 | phenyl salicylate88 | 0.562 | 1.450 |
| A70 | methyl triphenyl ammonium bromide | –5.336 | –6.992 | D87 | 1-hexanol53 | 1.421 | 0.688 |
| A71 | tetraheptylphosphonium bromide62 | –5.264 | –6.967 | D88 | coumarin76 | 1.013 | 1.257 |
| A72 | methyltrioctylphosphonium bromide70 | –5.163 | –6.868 | D89 | ketoprofen89 | 0.613 | 1.660 |
| A73 | benzyltriphenylphosphonium bromide72 | –5.193 | –6.785 | D90 | heptanoic acid90 | 0.779 | 1.659 |
| A74 | benzyltriphenylammonium bromide72 | –5.155 | –6.752 | D91 | ibuprofen91 | 0.438 | 2.069 |
| A75 | methyltrioctylammonium bromide70 | –5.080 | –6.788 | D92 | 1-heptanol90 | 1.748 | 1.082 |
| A76 | tetraoctylammonium bromide66 | –5.053 | –6.750 | D93 | water92−94 | 2.128 | 0.856 |
| A77 | tetraoctylphosphonium bromide66 | –4.910 | –6.598 | D94 | octanoic acid95 | 1.026 | 1.969 |
| A78 | hexadecyltrimethylphosphonium bromide | –4.889 | –6.591 | D95 | alpha-terpineol96 | 1.755 | 1.272 |
| A79 | dodecyldimethylbenzylphosphonium bromide75 | –4.798 | –6.443 | D96 | glycine97 | 2.830 | 0.403 |
| A80 | dodecyldimethylbenzylammonium bromide75 | –4.697 | –6.347 | D97 | nonanoic acid95 | 1.228 | 2.248 |
| A81 | hexadecyltrimethylammonium bromide | –4.490 | –6.181 | D98 | 2-dodecanol72 | 2.208 | 1.273 |
| A82 | beta-alanine18 | –4.068 | –6.547 | D99 | 1-octanol53 | 2.045 | 1.444 |
| A83 | benzethonium bromide | –3.859 | –5.310 | D100 | 10-undecenoic acid98 | 1.161 | 2.371 |
| A84 | butylammonium chloride | –2.788 | –5.550 | D101 | carvacrol99 | 0.807 | 2.771 |
| A85 | ethyl ammonium chloride | –2.723 | –5.591 | D102 | decanoic acid70 | 1.444 | 2.496 |
| A86 | propylammonium chloride | –2.483 | –5.312 | D103 | 1-nonanol100 | 2.286 | 1.731 |
| A87 | butylammonium bromide | –0.806 | –2.874 | D104 | menthol101 | 2.302 | 1.789 |
| A88 | propylammonium bromide | –0.173 | –2.333 | D105 | undecanoic acid102 | 1.595 | 2.748 |
| A89 | ethyl ammonium bromide | 0.563 | –1.744 | D106 | 1-decanol53 | 2.513 | 2.027 |
| hydrogen bond donors (HBDs) | D107 | ricinoleic acid103 | 2.185 | 2.385 | |||
| D1 | perfluorodecanoic acid104 | –11.536 | –9.043 | D108 | dodecanoic acid68 | 1.791 | 2.970 |
| D2 | trioctylphosphine oxide79 | –6.611 | –9.218 | D109 | borneol82 | 2.463 | 2.308 |
| D3 | hexafluoroisopropanol42 | –7.599 | –4.814 | D110 | 1-undecanol105 | 2.697 | 2.239 |
| D4 | dodecylmethylsulfoxide54 | –5.123 | –7.257 | D111 | carveol76 | 1.712 | 3.331 |
| D5 | oxalic acid61 | –6.874 | –5.407 | D112 | 1,3-dihexylthiourea54 | 2.192 | 3.124 |
| D6 | 5-sulfosalicylic acid106 | –6.455 | –5.022 | D113 | 1-dodecanol53 | 2.911 | 2.527 |
| D7 | ethanolamine50 | –4.586 | –6.403 | D114 | tetradecanoic acid107 | 2.075 | 3.367 |
| D8 | polyethylene glycol 40071 | –4.632 | –6.327 | D115 | oleic acid91 | 2.240 | 3.731 |
| D9 | 3,5-di-tert-butylcatechol54 | –6.254 | –3.195 | D116 | hexadecenoic acid108 | 2.326 | 3.691 |
| D10 | p-toluenesulfonic acid109 | –4.734 | –2.832 | D117 | 1-tetradecanol53 | 3.200 | 2.869 |
| D11 | gallic acid110 | –4.497 | –3.023 | D118 | octadecanoic acid107 | 2.560 | 4.012 |
| D12 | 4-chlorophenol59 | –4.711 | –2.094 | D119 | 1-hexadecanol54 | 3.456 | 3.188 |
| D13 | citric acid40 | –3.926 | –2.866 | D120 | oleyl alcohol111 | 3.492 | 3.504 |
| D14 | 1-naphthol76 | –4.768 | –1.824 | D121 | triphenyl phosphate79 | 3.737 | 5.175 |
| D15 | triethylene glycol112 | –2.517 | –3.766 | D122 | benzoyltrifluoroacetone79 | 3.775 | 6.082 |
| D16 | triethanolamine50 | –2.140 | –3.543 | D123 | thenoyltrifluoroacetone79 | 3.236 | 5.180 |
Figure 4.
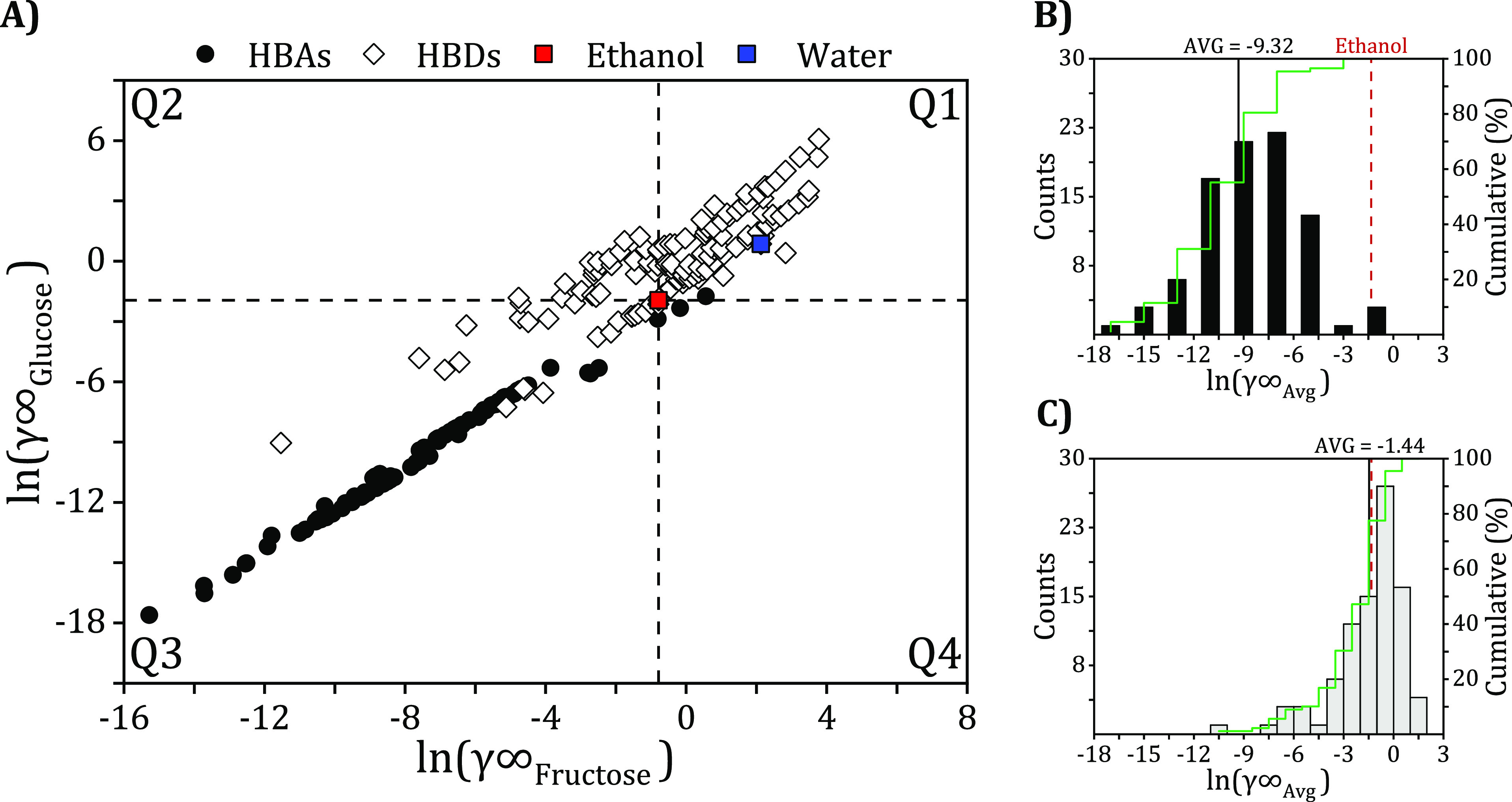
(A) Predicted activity coefficients (γi∞) of glucose versus those of fructose and the histogram distributions of the averaged γi of (B) HBAs and (C) HBDs. The average γi∞ of all HBAs and all HBDs is shown as a black solid line, and the cumulative count is in green according to the right axis.
3.2.1. Effect of Varying HBA
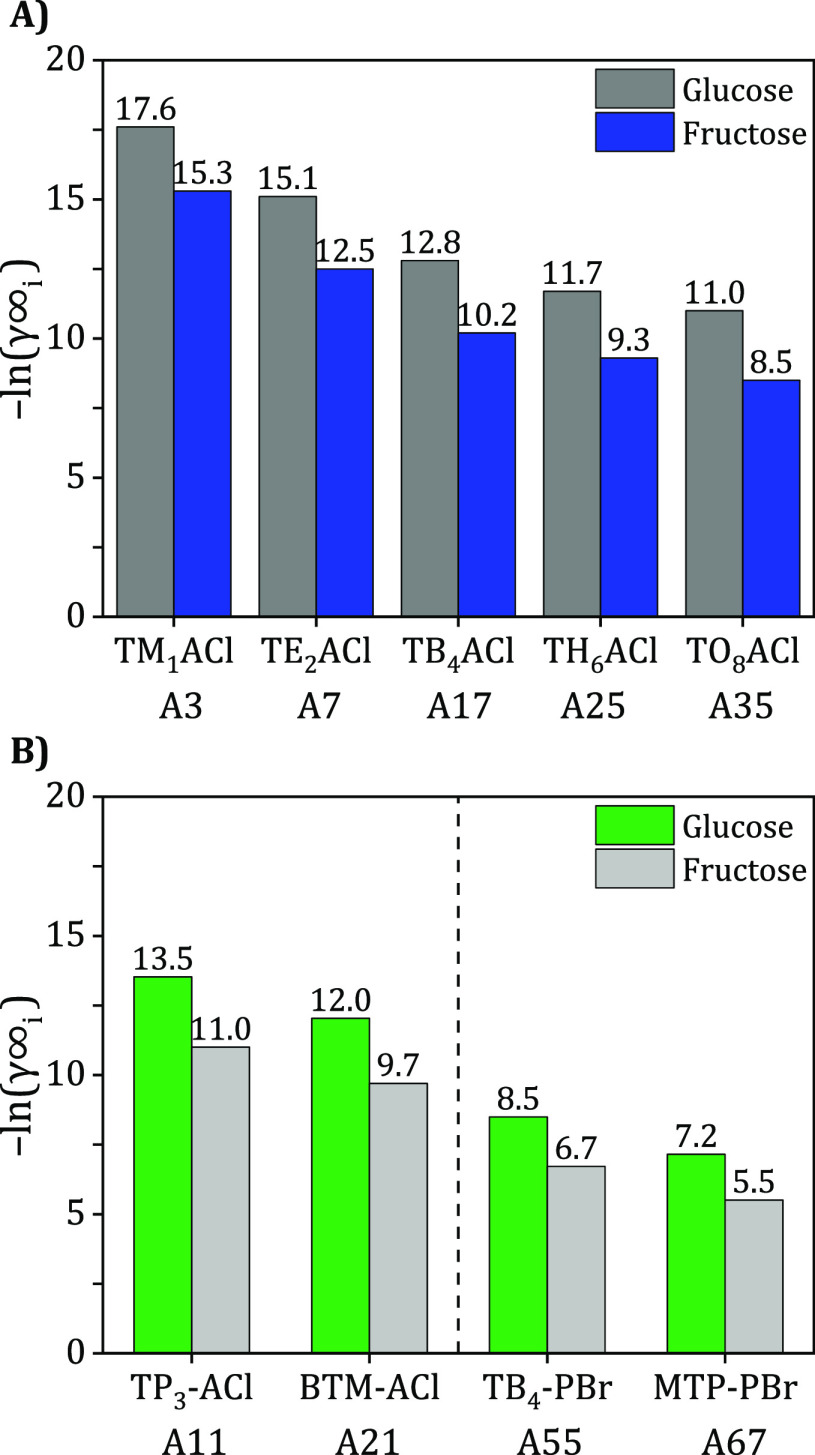
As can be seen in Table 1, hundreds of DES constituents have been reported in the literature. However, a systematic analysis that determines which types of DES constituents have high potential to extract monosaccharides has not yet been reported. In Figure 4, it can be observed that the performance of HBAs is generally better than the performance of HBDs as they correspond to lower γi∞ of glucose and fructose. Focusing specifically on HBAs, it can be deduced that chloride anions result in much higher solubility compared to bromide anions, as shown in Figure 5A [e.g., TBACl (A17) > TBABr (A54) with a 49% increase in ln(γGlu)]. This is likely due to chloride’s smaller anionic volume and greater electronegativity in comparison to bromide. Therefore, chloride anions are more likely to form stronger supramolecular clusters with cations,113,114 which may facilitate interactions between the HBA and the extracted monosaccharides due to the formation of an even stronger hydrogen bonding network. Additionally, it can also be noticed that HBAs with oxygen anions (carboxylate groups) tend to perform very well, such as carnitine (A4) and betaine (A6). This can be specifically noticed when comparing betaine (A6) and choline chloride (A9) in Figure 5B as both HBAs have a very similar structure (trimethylethylammonium core) and are mainly different due to their ethanoate group versus the ethanol-chloride group. This is also consistent with the previous results since oxygen anions also have a smaller volume and higher electronegativity compared to Cl– and Br–. On the other hand, when comparing the central cationic cores of ammonium and phosphonium, it can be seen that the difference is minuscule. However, ammonium-based HBAs were slightly outperforming phosphonium-based HBAs [e.g., TBACl (A17) > TBPCl (A18)]. Despite the fact that both HBAs have the same electric charge, the HBAs that contain a nitrogen core (smaller volume and higher polarity) tend to attract monosaccharides slightly better than a phosphorous core (larger with lower polarity).
Figure 5.
(A) Effect of Cl– and Br– on γGlu∞ for similar cationic cores [A17, A54, A31, A67, A84, and A87]. (B) Comparison of the γGlu and γFru∞ on betaine (with O–) and choline (with Cl–/Br–) [A6, A9, and A38].
Furthermore, the effect the alkyl chain length (n) of HBA on monosaccharide solubilities is shown in Figure 6A, where solubility increases as the alkyl chain length decreases [TM1ACl (A3) > TE2ACl (A7) > TP3ACl (A11) > TB4ACl (A17) > TP5ACl (A20) > TH6ACl (A25) > TH7ACl (A29) > TO8ACl (A35)]. This may be attributed to the rise in hydrophobicity of the molecule as a result of the increase in the chain length of the HBA, which causes a decrease in the solubility of the monosaccharides as they become highly different in nature (similarity–intermiscibility theory). Additionally, for further investigation, the γi∞ of n = 0 that corresponds to ammonium chloride [NH4Cl (A1)] has also been determined, and it can be seen that the activity coefficients are extremely low at values of ln(γi)around–76. However, to our knowledge, no DESs have previously been reported using NH4Cl as an HBA. Therefore, according to the results obtained, further investigations using ammonium chloride as an HBA for the formation of DES (or diluted within a particular solvent system) could be a useful route to study for some specific applications. In addition to cations containing linear alkyl chains, the performance of HBAs containing benzyl and phenyl groups, such as alkyltriphenyl, benzyltrialkyl, and benzyltriphenyl cores, has been investigated. Notably, it can be observed that the ring-containing HBAs have lower ability to dissolve sugars than those of alkyl-containing HBAs. For example, when comparing HBAs with similar molecular weights (Figure 6B), it can be seen that TB4-PBr (A55) outperforms MTP-PBr (A67). Similar results were found when comparing TP3-ACl (A11) and BTM-ACl (A21). These results are noteworthy because it was initially hypothesized that ring-based HBAs would be better in extracting monosaccharides (as they are also ring-structured); however, the opposite was true. This is presumably due to the high steric effects that could hinder the interactions between the cations and the monosaccharide molecules. Furthermore, the results showed that the ethanol groups attached to the cations tend to perform well, which is the case in choline chloride (A9), dimethylethylethanolammonium chloride (A15), and diethylethanolammonium chloride (A16). This could be due to the hydrogen bonding between the monosaccharide molecules and the cationic species. Finally, according to the aforementioned results, it can be concluded that an HBA with a (1) small central cation, such as nitrogen, (2) short side chain lengths, (3) a chloride or a carboxylate anion, and (4) a hydroxy group are considered optimal for monosaccharide extraction.
Figure 6.
(A) Effect of cationic alkyl chain length (n) on the γ∞ of glucose and fructose [A3, A7, A17, A25, and A35]. (B) Comparison of the γi∞ of cations with linear chains and cations with rings [A11 & A21, A55 & A67].
3.2.2. Effect of Varying HBD
Compared to the benchmark solvents (ethanol and water), it was found that most HBAs had much better performance than the benchmarks, while for HBDs, their performance was variable: some HBDs showed similar performance, others better, and in some cases even lower than ethanol, as shown in Figure 4. Among all the HBDs, ethanol was ranked as #36 in Table 1, while water was ranked as #93. To find optimal HBDs, special interest was given to the common solvents used in biomass valorization, such as glycerol, ethylene glycol, levulinic acid, lactic acid, and glycolic acid, due to their availability and generally environmentally benign nature. These solvents demonstrated good performance; however, they were all ranked lower than the benchmark ethanol (D36) in the following order: D50, D40, D55, D54, and D60. Among the top 20 ranked HBDs, a few other promising natural candidates were also given special attention, such as oxalic acid (D5), ethanolamine (D7), citric acid (D13), triethylene glycol (D15), triethanolamine (D16), maleic acid (D18), and urea (D19). These constituents, coupled with a suitable HBA, could be utilized as a starting point to develop highly effective NADES to extract sugars.
By conducting additional analysis, it can be seen that the HBDs with the highest predicted performance are perfluorodecanoic acid (D1), trioctylphosphine oxide (D2), hexafluoroisopropanol (D3), and dodecyl-methyl sulfoxide (D4). From D1 and D3, it can be deduced that fluorinated HBDs have a tremendous ability to attract glucose and fructose. However, it should be noted that these fluorine-containing solvents tend to be corrosive and toxic and therefore would not be applicable in most food-based applications.115 From D2 and D4, it can be seen that the functional groups of phosphine oxide (P=O) and sulfoxide (S=O) perform very well, especially when comparing D2 with trioctylphosphine (D74), which are very similar in structure, the only difference being the P=O group. Oxalic acid (D5), gallic acid (D11), and citric acid (D13) showed similar results, indicating that the carbonyl group (C=O) increases the affinity toward fructose and glucose. The acidic functional group −SOOOH group in 5-sulfosalicylic acid (D6) and p-toluenesulfonic acid (D10) showed similar results. One can conclude that these oxygen-based double bonds (X=O) have excellent capacity and affinity for monosaccharides, presumably due to their π–π bonding and hydrogen bonding interactions. On the other hand, alkanolamines (D7 and D16), phenolic (D9, D12, D14, D17, and D20), and some glycols (D8 and D15) were also promising. At the other end of the spectrum, the least performing HBDs (D97–D123) are quite hydrophobic, which agrees with the fact that as the HBD chain length increases, the solubility decreases (hexanoic acid D85/hexanol D87 > heptanoic acid D90/heptanol D92 > octanoic acid D94/octanol D99). This is probably due to the large number of hydroxy groups on glucose and fructose, which makes it hard for these monosaccharides to dissolve in these non-polar HBDs. This result is also consistent with the results from HBAs, where the more hydrophobic HBAs with larger chain lengths tended to have lower affinities with the monosaccharides. Furthermore, similar to the results obtained for HBAs, the addition of aromatic rings led to a reduction in performance as can be observed when comparing acetic acid (D46) vs phenylacetic acid (D75) and glycolic acid (D60) vs mandelic acid (D65). As for mono-, di-, and tri-molecules such as ethanolamines, it can be observed that the trend is as follows: mono (D7) > tri (D16) > di (D28). This implies that HBDs with alkyl chains on the unexposed (inside) and functional groups surrounding the molecule (outside) are better for monosaccharide extraction.
3.3. Multiselection Criteria of Potential DESs for Monosaccharide Extraction
To identify the best DESs as green solvents for the extraction of monosaccharides (fructose and glucose), certain properties that are important for food applications were evaluated, namely, toxicity, viscosity, density, and solubility.
3.3.1. Toxicity of DES Constituents
Toxicity data are vital information in the extraction application of bioactive compounds. However, unlike ionic liquids, DESs are poorly studied at the toxicological level due to their recent emergence. Given these challenges and limitations, a toxicity analysis was conducted using PubChem Database116 for the 15 top-performing HBAs and HBDs. The complete list of all 30 constituents is available in Table S1 of the Supporting Information, whereas a summarized version of the least toxic constituents is shown in Table 2. Choline chloride and betaine can be observed to be the least toxic HBAs, with LD50 values of 3900 and 10,800 mg/kg, respectively. These chosen HBAs have been commonly used in DES synthesis for various applications, including extraction, due to their environmentally benign nature. Both selected HBAs are approved by the US Food and Drug Administration (FDA) and are used as food additives at the industrial level.17 For HBDs, ethanolamine, triethylene glycol, oxalic acid, gallic acid, and citric acid were selected because they are FDA-approved and used as a fruit-washing ingredient, nutrient supplement, and flavoring agent. To consider the synergistic effects of the DES constituents, the cytotoxicities of the ChCl-based DESs were studied by Radošević et al.,117 and their results showed that the ChCl-DESs exhibited low cytotoxicity levels. On the other hand, the toxicity levels of the betaine-based DESs were not reported in the literature; hence, further research is needed in this area. Nonetheless, because DESs are mixtures, if the toxicity of the constituents is low, then the toxicity of the overall solvent can also generally be considered low in most cases. This behavior is different than that of ILs because ILs are synthesized using chemical reactions and thus their properties are not similar to the reactants.118
Table 2. Reported Toxicities and Typical Uses of the Selected HBAs and HBDsb.
| # | constituent | toxicity (LD50: mg/kg)a | typical uses |
|---|---|---|---|
| A6 | betaine | 10,800 | flavoring agents |
| A9 | choline chloride | 3900 | flavoring agents and agrochemicals |
| D5 | oxalic acid | 2268 | indirect food additive |
| D7 | ethanolamine | 700 | agricultural chemicals, lubricants, surface-treating agents, solvents |
| D11 | gallic acid | 5000 | flavoring agents, drugs |
| D13 | citric acid | 7280 | flavoring agents, food additives, cosmetics, drugs |
| D15 | triethyleneglycol | 20,000 | cleaning products, household care, microbicides |
50% of lethal dose.
Data were obtained from the PubChem Database.116
Based on the following selection of two HBAs and five HBDs, 10 binary combinations of DESs can be obtained. The eutectic molar ratios of the resulting combinations were surveyed in the literature and are shown in Table 3. Based on the literature survey, DES1 (betaine:ethanolamine) and DES4 (betaine:gallic acid) were not reported, and therefore, they were excluded from further investigation. The remaining eight DES combinations out of the 10 rationally chosen constituents are further considered for the thermophysical property prediction, namely, viscosity, density, and solubility.
Table 3. Reported Molar Ratios of Pre-screened DES Combinations in the Literature.
| # | HBA | HBD 1 | molar ratios | ref |
|---|---|---|---|---|
| DES#01 | betaine | ethanolamine | N.R. | |
| DES#02 | betaine | oxalic acid | 2:1 | (17,119,120) |
| DES#03 | betaine | citric acid | 1:1 | (13,17,121−123) |
| DES#04 | betaine | gallic acid | N.R. | |
| DES#05 | betaine | triethylene glycol | 1:4 | (124,125) |
| DES#06 | choline chloride | ethanolamine | 1:6 | (60,126,127) |
| DES#07 | choline chloride | oxalic acid | 1:1 | (17,128,129) |
| DES#08 | choline chloride | citric acid | 2:1 | (17,130−132) |
| DES#09 | choline chloride | gallic acid | 2:1 | (110) |
| DES#10 | choline chloride | triethylene glycol | 1:3 | (133) |
3.3.2. Predicted Viscosities and Densities of the DESs
Viscosity is one of the primary features of desired solvents because it directly affects the mass transfer kinetics and ultimately the extraction efficiency. Among the selected DESs, acid-based DESs have been reported to have high viscosities, ≫1000 mPa·s, at room temperature due to the strong hydrogen bonding between their components.130 The dilution of DES with water causes a significant reduction in its viscosity, leading to enhanced extraction efficiency. However, a rational DES to water ratio must be chosen to prevent reduction in DES’s extraction capacity due to possible interference of water molecules and breakage of the hydrogen bond framework of DES.21 In the literature, 20–40 wt % water addition was considered ideal to reduce the overall viscosities of the acid-based DESs to a level where it is close to a liquid viscosity of 100 mPa·s that is considered manageable at room temperature.125,134 Particularly for the extraction of sugars, Gómez et al.(18) reported that the addition of 30 wt % of water (after DES synthesis) to acid-based DESs was found to be the best compromise between extraction efficiency and viscosity. Therefore, following the reported threshold value, the selected acid-DESs in this work (DESs 2, 3, 7, 8, and 9) were adjusted to include an additional 30 wt % of water. The predicted viscosities of the DESs at the chosen molar ratios and 298.2 K are presented in Table 4. It can be observed that the predicted viscosities for all selected DESs are all considered manageable, even for the acid-DESs. For example, the predicted viscosities for ChCl:CA (2:1) and ChCl:GA (2:1) before the addition of water were 10250.49 and 5760.78 mPa·s, respectively, and the addition of water led to a large reduction in viscosities, 6.45 and 6.13, respectively. These predictions are in agreement with the reported experimental trends in the literature, showing that the addition of 30 wt % of water to acid-DESs is necessary to reduce the viscosity.18,22 Furthermore, the densities of the eight selected DESs were also predicted at 298.2 K (Table 4), and it can be observed that the predicted densities of all DESs were higher than those of water and ethanol.
Table 4. Predicted Viscosities and Densities Using COSMO-RS for the Selected DESs and Ethanol at 298.2 K and 1.01 Bar.
| DES | μpred (mPa·s) | ρpred (g/mL) |
|---|---|---|
| DES#02: Bet:OxA (2:1 30 wt % H2O) | 2.65 | 1.1385 |
| DES#03: Bet:CA (1:1 30 wt % H2O) | 3.83 | 1.1862 |
| DES#05: Bet:TEG (1:4) | 53.76 | 1.0969 |
| DES#06: ChCl EA (1:6) | 24.46 | 1.0267 |
| DES#07: ChCl:OxA (1:1 30 wt % H2O) | 4.54 | 1.1997 |
| DES#08: ChCl:CA (2:1 30 wt % H2O) | 6.45 | 1.1786 |
| DES#09: ChCl:GA (2:1 30 wt % H2O) | 6.13 | 1.1916 |
| DES#10: ChCl:TEG (1:3) | 53.76 | 1.1152 |
| ethanol | 2.84 | 0.7769 |
| water | 0.89 | 0.9967 |
3.3.3. Predicted Solubilities in DESs
The predicted glucose and fructose solubilities (g sugar/100 g solvent) in the eight chosen DESs compared to the benchmark solvent (ethanol) between 298 and 353 K are shown in Figure 7. The dashed lines indicate the betaine-based DESs, whereas the solid lines indicate the choline chloride-based DESs. First, it can be observed that the overall solubility increased considerably with increasing temperature for all DESs and the benchmark solvents, which is the general trend reported in the literature attributed to increased kinetic energy of the mixture that results in an enhanced solubilities.135,136 It can also be observed that ChCl:EA (1:6) was predicted to have the highest solubility for both monosaccharides as compared to the rest of DESs and the benchmark. On the other hand, Bet:CA (1:1 30 wt % H2O) showed the lowest solubility. Nonetheless, it can be observed that the predicted solubility in the majority of the selected DESs is higher than >50 g/100 g solvent at 303.2 K, which was chosen as an optimal operating condition to prevent Maillard reactions and browning effects on the extracted sugars that are reported to occur at elevated temperatures.137
Figure 7.
COSMO-RS predicted solubilities (g sugar/100 g solvent) for (A) glucose and (B) fructose.
4. Conclusions
Selecting an appropriate DES for a particular application is a very daunting task due to the designer nature of DESs and their theoretically infinite combinations of constituents and compositions. In this work, COSMO-RS screening of 212 DES constituents was conducted including 89 hydrogen bond acceptors (HBAs) and 123 hydrogen bond donors (HBDs) for predicting the solubility of glucose and fructose. The effects of HBA and HBD structures were carefully mapped to assess the impact of each functional group on the solubility of monosaccharides. The results showed that both DES constituents play a vital role due to their affinities toward glucose and fructose. It was concluded that the solubility can be specifically improved by an HBA with a small central cation (N+), short side chains, and a small anionic volume with high electronegativity (Cl– and O–). As for HBDs, it was found that HBDs with alkyl chains on the inner side (unexposed) and with functional groups surrounding the molecule are more suitable for monosaccharide extraction. Thus, the predictions indicate that alkanolamines such as ethanolamine and di-/tri-carboxylic acids such as oxalic acid and citric acid are highly performing and are considered environmentally benign. In addition, the potential NADES combinations for the extraction of reducing sugars were selected based on criteria considering toxicity, viscosity, density, and solubility. It was found that eight of the shortlisted NADESs had high predicted solubilities, low predicted viscosities, and low toxicities. This work provides a better understanding of the roles of the DES constituents in the extraction of sugars. The results can be used as a molecular guide and database for the design of novel NADES that can be used in a variety of food applications, such as the valorization of sugars from fruit bio-wastes.
Acknowledgments
The authors deeply express their sincere gratitude for the generous support and contributions from Khalifa University of Science and Technology in Abu Dhabi, United Arab Emirates (UAE). This work is supported by the project grant CIRA-2019-028 under the Competitive Internal Research Award scheme of Khalifa University, UAE, the Center for Membrane and Advanced Water Technology (CMAT) under grant RC2-2018-009, Khalifa University, UAE.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c03326.
(Figure S1) Determined σ-profiles of ionic HBA constituents, (Figure S2) determined σ-profiles of neutral HBD constituents, and (Table S1) reported toxicities and typical uses of the top 15 HBAs and top 15 HBDs (PDF)
Author Contributions
# J.A. and A.S.D. share first authorship.
The authors declare no competing financial interest.
Supplementary Material
References
- Anastas P. T.; Warner J. C.. Green Chemistry: Theory and Practice; Oxford University Press: UK, 1998. [Google Scholar]
- Chen J.; Spear S. K.; Huddleston J. G.; Rogers R. D. Polyethylene Glycol and Solutions of Polyethylene Glycol as Green Reaction Media. Green Chem. 2005, 7, 64–82. 10.1039/b413546f. [DOI] [Google Scholar]
- Herrero M.; Mendiola J. A.; Ibáñez E. Gas Expanded Liquids and Switchable Solvents. Curr. Opin. Green Sustainable Chem. 2017, 5, 24–30. 10.1016/j.cogsc.2017.03.008. [DOI] [Google Scholar]
- Eckert C. A.; Knutson B. L.; Debenedetti P. G. Supercritical Fluids as Solvents for Chemical and Materials Processing. Nature 1996, 313–318. 10.1038/383313a0. [DOI] [Google Scholar]
- Brunner G. Applications of Supercritical Fluids. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 321–342. 10.1146/annurev-chembioeng-073009-101311. [DOI] [PubMed] [Google Scholar]
- Petkovic M.; Seddon K. R.; Rebelo L. P. N.; Pereira C. S. Ionic Liquids: A Pathway to Environmental Acceptability. Chem. Soc. Rev. 2011, 40, 1383–1403. 10.1039/c004968a. [DOI] [PubMed] [Google Scholar]
- Wilkes J. S. A Short History of Ionic Liquids - From Molten Salts to Neoteric Solvents. Green Chem. 2002, 4, 73–80. 10.1039/b110838g. [DOI] [Google Scholar]
- Abbott A. P.; Capper G.; Davies D. L.; Rasheed R. K.; Tambyrajah V.. Novel Solvent Properties of Choline Chloride / Urea Mixtures †. 2003, 70–71, 10.1039/B210714G. [DOI] [PubMed]
- Smith E. L.; Abbott A. P.; Ryder K. S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. 10.1021/cr300162p. [DOI] [PubMed] [Google Scholar]
- Diego J. R.; Gabriela G.. Deep Eutectic Solvents: Synthesis, Properties, and Applications Edited; 2019.
- Martins M. A. R.; Pinho S. P.; Coutinho J. A. P. Insights into the Nature of Eutectic and Deep Eutectic Mixtures. J. Solution Chem. 2019, 48, 962–982. 10.1007/s10953-018-0793-1. [DOI] [Google Scholar]
- Tang X.; Zuo M.; Li Z.; Liu H.; Xiong C.; Zeng X.; Sun Y.; Hu L.; Liu S.; Lei T.; Lin L. Green Processing of Lignocellulosic Biomass and Its Derivatives in Deep Eutectic Solvents. ChemSusChem 2017, 10, 2696–2706. 10.1002/cssc.201700457. [DOI] [PubMed] [Google Scholar]
- Dai Y.; van Spronsen J.; Witkamp G. J.; Verpoorte R.; Choi Y. H. Natural Deep Eutectic Solvents as New Potential Media for Green Technology. Anal. Chim. Acta 2013, 766, 61–68. 10.1016/j.aca.2012.12.019. [DOI] [PubMed] [Google Scholar]
- Hayyan M.; Mbous Y. P.; Looi C. Y.; Wong W. F.; Hayyan A.; Salleh Z.; Mohd-Ali O. Natural Deep Eutectic Solvents: Cytotoxic Profile. Springerplus 2016, 5, 1. 10.1186/s40064-016-2575-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krylov V. B.; Ustyuzhanina N. E.; Nifantiev N. E. Synthesis of Low-Molecular-Weight Carbohydrate Mimetics of Heparin. Russ. J. Bioorg. Chem. 2011, 37, 672–706. 10.1134/S1068162011060100. [DOI] [Google Scholar]
- Tomasik P.Chemical and Functional Properties of Food Saccharides; CRC press: London, 2003, 10.1201/9780203495728. [DOI] [Google Scholar]
- Huang J.; Guo X.; Xu T.; Fan L.; Zhou X.; Wu S. Ionic Deep Eutectic Solvents for the Extraction and Separation of Natural Products. J. Chromatogr. A 2019, 1598, 1–19. 10.1016/j.chroma.2019.03.046. [DOI] [PubMed] [Google Scholar]
- Gómez A. V.; Tadini C. C.; Biswas A.; Buttrum M.; Kim S.; Boddu V. M.; Cheng H. N. Microwave-Assisted Extraction of Soluble Sugars from Banana Puree with Natural Deep Eutectic Solvents (NADES). Lwt 2019, 107, 79–88. 10.1016/j.lwt.2019.02.052. [DOI] [Google Scholar]
- Zhang L.; Wang M. Optimization of Deep Eutectic Solvent-Based Ultrasound-Assisted Extraction of Polysaccharides from Dioscorea Opposita Thunb. Int. J. Biol. Macromol. 2017, 95, 675–681. 10.1016/j.ijbiomac.2016.11.096. [DOI] [PubMed] [Google Scholar]
- Procentese A.; Raganati F.; Olivieri G.; Russo M. E.; Rehmann L.; Marzocchella A. Deep Eutectic Solvents Pretreatment of Agro-Industrial Food Waste. Biotechnol. Biofuels 2018, 11, 1–12. 10.1186/s13068-018-1034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y.; Witkamp G. J.; Verpoorte R.; Choi Y. H. Tailoring Properties of Natural Deep Eutectic Solvents with Water to Facilitate Their Applications. Food Chem. 2015, 187, 14–19. 10.1016/j.foodchem.2015.03.123. [DOI] [PubMed] [Google Scholar]
- Gutiérrez M. C.; Ferrer M. L.; Mateo C. R.; Del Monte F. Freeze-Drying of Aqueous Solutions of Deep Eutectic Solvents: A Suitable Approach to Deep Eutectic Suspensions of Self-Assembled Structures. Langmuir 2009, 25, 5509–5515. 10.1021/la900552b. [DOI] [PubMed] [Google Scholar]
- Hizaddin H. F.; Hadj-Kali M. K.; Ramalingam A.; Ali Hashim M. Extractive Denitrogenation of Diesel Fuel Using Ammonium- and Phosphonium-Based Deep Eutectic Solvents. J. Chem. Thermodyn. 2016, 95, 164–173. 10.1016/j.jct.2015.12.009. [DOI] [Google Scholar]
- Liu Y.; Yu H.; Sun Y.; Zeng S.; Zhang X.; Nie Y.; Zhang S.; Ji X. Screening Deep Eutectic Solvents for CO2 Capture With COSMO-RS. Front. Chem. 2020, 8, 1–11. 10.3389/fchem.2020.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Llorente D.; Bengoa A.; Pascual-Muñoz G.; Navarro P.; Águeda V. I.; Delgado J. A.; Álvarez-Torrellas S.; García J.; Larriba M. Sustainable Recovery of Volatile Fatty Acids from Aqueous Solutions Using Terpenoids and Eutectic Solvents. ACS Sustainable Chem. Eng. 2019, 7, 16786–16794. 10.1021/acssuschemeng.9b04290. [DOI] [Google Scholar]
- Mohan M.; Goud V. V.; Banerjee T. Solubility of Glucose, Xylose, Fructose and Galactose in Ionic Liquids: Experimental and Theoretical Studies Using a Continuum Solvation Model. Fluid Phase Equilib. 2015, 395, 33–43. 10.1016/j.fluid.2015.03.020. [DOI] [Google Scholar]
- Lemaoui T.; Abu Hatab F.; Darwish A. S.; Attoui A.; Hammoudi N. E. H.; Almustafa G.; Benaicha M.; Benguerba Y.; Alnashef I. M. Molecular-Based Guide to Predict the PH of Eutectic Solvents: Promoting an Efficient Design Approach for New Green Solvents. ACS Sustainable Chem. Eng. 2021, 9, 5783–5808. 10.1021/acssuschemeng.0c07367. [DOI] [Google Scholar]
- Lemaoui T.; Darwish A. S.; Attoui A.; Hatab F. A.; El N.; Hammoudi H.; Benguerba Y.; Vega L. F.; Alnashef I. M. Predicting the Density and Viscosity of Hydrophobic Eutectic Solvents : Towards the Development of Sustainable Solvents †. Green Chem. 2020, 15–8530. 10.1039/d0gc03077e. [DOI] [Google Scholar]
- Allouche A. Software News and Updates Gabedit — A Graphical User Interface for Computational Chemistry Softwares. J. Comput. Chem. 2011, 32, 174–182. 10.1002/jcc. [DOI] [PubMed] [Google Scholar]
- Bououden W.; Benguerba Y.; Darwish A. S.; Attoui A.; Lemaoui T.; Balsamo M.; Erto A.; Alnashef I. M. Surface Adsorption of Crizotinib on Carbon and Boron Nitride Nanotubes as Anti-Cancer Drug Carriers: COSMO-RS and DFT Molecular Insights. J. Mol. Liq. 2021, 338, 116666 10.1016/j.molliq.2021.116666. [DOI] [Google Scholar]
- Hadj-Kali M. K.; Mulyono S.; Hizaddin H. F.; Wazeer I.; El-Blidi L.; Ali E.; Hashim M. A.; AlNashef I. M. Removal of Thiophene from Mixtures with N-Heptane by Selective Extraction Using Deep Eutectic Solvents. Ind. Eng. Chem. Res. 2016, 55, 8415–8423. 10.1021/acs.iecr.6b01654. [DOI] [Google Scholar]
- Adeyemi I.; Sulaiman R.; Almazroui M.; Al-Hammadi A.; AlNashef I. M. Removal of Chlorophenols from Aqueous Media with Hydrophobic Deep Eutectic Solvents: Experimental Study and COSMO RS Evaluation. J. Mol. Liq. 2020, 311, 113180 10.1016/j.molliq.2020.113180. [DOI] [Google Scholar]
- Diedenhofen M.; Eckert F.; Klamt A.; Gmbh C.; Kg C.; Strasse B.; Leverkusen D. Compounds in Ionic Liquids Using COSMO-RS. Engineering 2003, 475–479. [Google Scholar]
- Benabid S.; Benguerba Y.; AlNashef I. M.; Haddaoui N. Theoretical Study of Physicochemical Properties of Selected Ammonium Salt-Based Deep Eutectic Solvents. J. Mol. Liq. 2019, 285, 38–46. 10.1016/j.molliq.2019.04.052. [DOI] [Google Scholar]
- Lemaoui T.; Darwish A. S.; Hammoudi N. E. H.; Abu Hatab F.; Attoui A.; Alnashef I. M.; Benguerba Y. Prediction of Electrical Conductivity of Deep Eutectic Solvents Using COSMO-RS Sigma Profiles as Molecular Descriptors: A Quantitative Structure–Property Relationship Study. Ind. Eng. Chem. Res. 2020, 59, 13343–13354. 10.1021/acs.iecr.0c02542. [DOI] [Google Scholar]
- Lemaoui T.; Darwish A. S.; Almustafa G.; Boublia A.; Sarika P. R.; Jabbar N. A.; Ibrahim T.; Nancarrow P.; Yadav K. K.; Fallatah A. M.; Abbas M.; Algethami J. S.; Benguerba Y.; Jeon B. H.; Banat F.; AlNashef I. M. Machine Learning Approach to Map the Thermal Conductivity of over 2,000 Neoteric Solvents for Green Energy Storage Applications. Energy Storage Mater. 2023, 59, 102795 10.1016/j.ensm.2023.102795. [DOI] [Google Scholar]
- Abranches D. O.; Martins M. A. R.; Silva L. P.; Schaeffer N.; Pinho S. P.; Coutinho J. A. P. Phenolic Hydrogen Bond Donors in the Formation of Non-Ionic Deep Eutectic Solvents: The Quest for Type v Des. Chem. Commun. 2019, 55, 10253–10256. 10.1039/c9cc04846d. [DOI] [PubMed] [Google Scholar]
- Zhang K.; Liu C.; Li S.; Fan J. A Hydrophobic Deep Eutectic Solvent Based Vortex-Assisted Liquid-Liquid Microextraction for the Determination of Formaldehyde from Biological and Indoor Air Samples by High Performance Liquid Chromatography. J. Chromatogr. A 2019, 1589, 39–46. 10.1016/j.chroma.2018.12.063. [DOI] [PubMed] [Google Scholar]
- Biswas A.; Shogren R. L.; Stevenson D. G.; Willett J. L.; Bhowmik P. K. Ionic Liquids as Solvents for Biopolymers: Acylation of Starch and Zein Protein. Carbohydr. Polym. 2006, 66, 546–550. 10.1016/j.carbpol.2006.04.005. [DOI] [Google Scholar]
- Min K.; Ko J.; Zhao J.; Jin Y.; Eun D.; Young S.; Lee J. Multi-Functioning Deep Eutectic Solvents as Extraction and Storage Media for Bioactive Natural Products That Are Readily Applicable to Cosmetic Products. J. Cleaner Prod. 2017, 151, 87–95. 10.1016/j.jclepro.2017.03.038. [DOI] [Google Scholar]
- Hosseini A.; Haghbakhsh R.; Raeissi S. Experimental Investigation of Liquid-Liquid Extraction of Toluene + Heptane or Toluene + Hexane Using Deep Eutectic Solvents. J. Chem. Eng. Data 2019, 64, 3811–3820. 10.1021/acs.jced.9b00237. [DOI] [Google Scholar]
- Deng W.; Yu L.; Li X.; Chen J.; Wang X.; Deng Z.; Xiao Y.. Hexafluoroisopropanol-Based Hydrophobic Deep Eutectic Solvents for Dispersive Liquid-Liquid Microextraction of Pyrethroids in Tea Beverages and Fruit Juices; 2019; Vol. 274, 10.1016/j.foodchem.2018.09.048. [DOI] [PubMed] [Google Scholar]
- Mat Hussin S. A.; Varanusupakul P.; Shahabuddin S.; Yih Hui B.; Mohamad S. Synthesis and Characterization of Green Menthol-Based Low Transition Temperature Mixture with Tunable Thermophysical Properties as Hydrophobic Low Viscosity Solvent. J. Mol. Liq. 2020, 308, 113015 10.1016/j.molliq.2020.113015. [DOI] [Google Scholar]
- Aslan Türker D.; Doğan M. Application of Deep Eutectic Solvents as a Green and Biodegradable Media for Extraction of Anthocyanin from Black Carrots. Lwt 2021, 138, 110775 10.1016/j.lwt.2020.110775. [DOI] [Google Scholar]
- Hu H. C.; Liu Y. H.; Le Li B.; Cui Z. S.; Zhang Z. H. Deep Eutectic Solvent Based on Choline Chloride and Malonic Acid as an Efficient and Reusable Catalytic System for One-Pot Synthesis of Functionalized Pyrroles. RSC Adv. 2015, 5, 7720–7728. 10.1039/c4ra13577f. [DOI] [Google Scholar]
- Dietz C. H. J. T.; Kroon M. C.; Stefano D. Selective Separation of Furfural and Hydroxymethylfurfural from an Aqueous Solution Using a Supported Hydrophobic Deep Eutectic Solvent Liquid Membrane. Faraday Discuss. 2017, 00, 1–16. 10.1039/C7FD00152E. [DOI] [PubMed] [Google Scholar]
- Ghaedi H.; Ayoub M.; Sufian S.; Lal B.; Uemura Y. Thermal Stability and FT-IR Analysis of Phosphonium-Based Deep Eutectic Solvents with Different Hydrogen Bond Donors. J. Mol. Liq. 2017, 242, 395–403. 10.1016/j.molliq.2017.07.016. [DOI] [Google Scholar]
- Yu Q.; Song Z.; Chen X.; Fan J.; Clark J. H.; Wang Z.; Sun Y.; Yuan Z. A Methanol-Choline Chloride Based Deep Eutectic Solvent Enhances the Catalytic Oxidation of Lignin into Acetovanillone and Acetic Acid. Green Chem. 2020, 22, 6415–6423. 10.1039/d0gc02189j. [DOI] [Google Scholar]
- Shishov A.; Gerasimov A.; Nechaeva D.; Volodina N.; Bessonova E.; Bulatov A. An Effervescence-Assisted Dispersive Liquid–Liquid Microextraction Based on Deep Eutectic Solvent Decomposition: Determination of Ketoprofen and Diclofenac in Liver. Microchem. J. 2020, 156, 104837 10.1016/j.microc.2020.104837. [DOI] [Google Scholar]
- Cai C.; Wang Y.; Yu W.; Wang C.; Li F.; Tan Z. Temperature-Responsive Deep Eutectic Solvents as Green and Recyclable Media for the Efficient Extraction of Polysaccharides from Ganoderma Lucidum. J. Cleaner Prod. 2020, 274, 123047 10.1016/j.jclepro.2020.123047. [DOI] [Google Scholar]
- Faraji M. Determination of Some Red Dyes in Food Samples Using a Hydrophobic Deep Eutectic Solvent-Based Vortex Assisted Dispersive Liquid-Liquid Microextraction Coupled with High Performance Liquid Chromatography. J. Chromatogr. A 2019, 1591, 15–23. 10.1016/j.chroma.2019.01.022. [DOI] [PubMed] [Google Scholar]
- Sadeghi S.; Davami A. A Rapid Dispersive Liquid-Liquid Microextraction Based on Hydrophobic Deep Eutectic Solvent for Selective and Sensitive Preconcentration of Thorium in Water and Rock Samples: A Multivariate Study. J. Mol. Liq. 2019, 291, 111242 10.1016/j.molliq.2019.111242. [DOI] [Google Scholar]
- Cao J.; Yang M.; Cao F.; Wang J.; Su E. Well-Designed Hydrophobic Deep Eutectic Solvents As Green and Efficient Media for the Extraction of Artemisinin from Artemisia Annua Leaves. ACS Sustainable Chem. Eng. 2017, 5, 3270–3278. 10.1021/acssuschemeng.6b03092. [DOI] [Google Scholar]
- van den Bruinhorst A.; Raes S.; Maesara S. A.; Kroon M. C.; Esteves A. C. C.; Meuldijk J. Hydrophobic Eutectic Mixtures as Volatile Fatty Acid Extractants. Sep. Purif. Technol. 2019, 216, 147–157. 10.1016/j.seppur.2018.12.087. [DOI] [Google Scholar]
- Mulyono S.; Hizaddin H. F.; Alnashef I. M.; Hashim M. A.; Fakeeha A. H.; Hadj-Kali M. K. Separation of BTEX Aromatics from N-Octane Using a (Tetrabutylammonium Bromide + Sulfolane) Deep Eutectic Solvent-Experiments and COSMO-RS Prediction. RSC Adv. 2014, 4, 17597–17606. 10.1039/c4ra01081g. [DOI] [Google Scholar]
- Almustafa G.; Sulaiman R.; Kumar M.; Adeyemi I.; Arafat H. A.; AlNashef I. Boron Extraction from Aqueous Medium Using Novel Hydrophobic Deep Eutectic Solvents. Chem. Eng. J. 2020, 395, 125173 10.1016/j.cej.2020.125173. [DOI] [Google Scholar]
- Kareem M. A.; Mjalli F. S.; Hashim M. A.; Hadj-Kali M. K. O.; Bagh F. S. G.; Alnashef I. M. Phase Equilibria of Toluene/Heptane with Tetrabutylphosphonium Bromide Based Deep Eutectic Solvents for the Potential Use in the Separation of Aromatics from Naphtha. Fluid Phase Equilib. 2012, 333, 47–54. 10.1016/j.fluid.2012.07.020. [DOI] [Google Scholar]
- Haghbakhsh R.; Raeissi S. A Study of Non-Ideal Mixtures of Ethanol and the (1 Choline Chloride +2 Ethylene Glycol) Deep Eutectic Solvent for Their Volumetric Behaviour. J. Chem. Thermodyn. 2020, 150, 106219 10.1016/j.jct.2020.106219. [DOI] [Google Scholar]
- Xiong D.; Zhang Q.; Ma W.; Wang Y.; Wan W.; Shi Y.; Wang J. Temperature-Switchable Deep Eutectic Solvents for Selective Separation of Aromatic Amino Acids in Water. Sep. Purif. Technol. 2021, 265, 118479 10.1016/j.seppur.2021.118479. [DOI] [Google Scholar]
- Adeyemi I.; Abu-Zahra M. R. M.; AlNashef I. M. Physicochemical Properties of Alkanolamine-Choline Chloride Deep Eutectic Solvents: Measurements, Group Contribution and Artificial Intelligence Prediction Techniques. J. Mol. Liq. 2018, 256, 581–590. 10.1016/j.molliq.2018.02.085. [DOI] [Google Scholar]
- Alañón M. E.; Ivanović M.; Pimentel-Mora S.; Borrás-Linares I.; Arráez-Román D.; Segura-Carretero A. A Novel Sustainable Approach for the Extraction of Value-Added Compounds from Hibiscus Sabdariffa L. Calyces by Natural Deep Eutectic Solvents. Food Res. Int. 2020, 137, 109646 10.1016/j.foodres.2020.109646. [DOI] [PubMed] [Google Scholar]
- Elik A.; Bingöl D.; Altunay N. Ionic Hydrophobic Deep Eutectic Solvents in Developing Air-Assisted Liquid-Phase Microextraction Based on Experimental Design: Application to Flame Atomic Absorption Spectrometry Determination of Cobalt in Liquid and Solid Samples. Food Chem. 2021, 350, 129237 10.1016/j.foodchem.2021.129237. [DOI] [PubMed] [Google Scholar]
- Lemaoui T.; Benguerba Y.; Darwish A. S.; Hatab F. A.; Warrag S. E. E.; Kroon M. C.; Alnashef I. M. Simultaneous Dearomatization, Desulfurization, and Denitrogenation of Diesel Fuels Using Acidic Deep Eutectic Solvents as Extractive Agents: A Parametric Study. Sep. Purif. Technol. 2021, 256, 117861 10.1016/j.seppur.2020.117861. [DOI] [Google Scholar]
- Warrag S. E. E.; Darwish A. S.; Adeyemi I. A.; Hadj-Kali M. K.; Kroon M. C.; Alnashef I. M. Extraction of Pyridine from N-Alkane Mixtures Using Methyltriphenylphosphonium Bromide-Based Deep Eutectic Solvents as Extractive Denitrogenation Agents. Fluid Phase Equilib. 2020, 517, 112622 10.1016/j.fluid.2020.112622. [DOI] [Google Scholar]
- Gautam R. K.; Seth D. Thermal Conductivity of Deep Eutectic Solvents. J. Therm. Anal. Calorim. 2020, 140, 2633–2640. 10.1007/s10973-019-09000-2. [DOI] [Google Scholar]
- Dietz C. H. J. T.; Kroon M. C.; Van Sint Annaland M.; Gallucci F. Thermophysical Properties and Solubility of Different Sugar-Derived Molecules in Deep Eutectic Solvents. J. Chem. Eng. Data 2017, 62, 3633–3641. 10.1021/acs.jced.7b00184. [DOI] [Google Scholar]
- Maneffa A. J.; Harrison A. B.; Radford S. J.; Whitehouse A. S.; Clark J. H.; Matharu A. S. Deep Eutectic Solvents Based on Natural Ascorbic Acid Analogues and Choline Chloride. ChemistryOpen 2020, 9, 559–567. 10.1002/open.202000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro B. D.; Florindo C.; Iff L. C.; Coelho M. A. Z.; Marrucho I. M. Menthol-Based Eutectic Mixtures: Hydrophobic Low Viscosity Solvents. ACS Sustainable Chem. Eng. 2015, 3, 2469–2477. 10.1021/acssuschemeng.5b00532. [DOI] [Google Scholar]
- Hatab F. A.; Darwish A. S.; Lemaoui T.; Warrag S. E. E.; Benguerba Y.; Kroon M. C.; Alnashef I. M. Extraction of Thiophene , Pyridine , and Toluene from n - Decane as a Diesel Model Using Betaine-Based Natural Deep Eutectic Solvents. J. Chem. Eng. Data 2020, 65, 5443–5457. 10.1021/acs.jced.0c00579. [DOI] [Google Scholar]
- Van Osch D. J. G. P.; Zubeir L. F.; Van Den Bruinhorst A.; Rocha M. A. A.; Kroon M. C. Hydrophobic Deep Eutectic Solvents as Water-Immiscible Extractants. Green Chem. 2015, 17, 4518–4521. 10.1039/c5gc01451d. [DOI] [Google Scholar]
- Chen Y.; Fu L.; Liu Z.; Dai F.; Dong Z.; Li D.; Liu H.; Zhao D.; Lou Y. Surface Tension and Surface Thermodynamic Properties of PEG-Based Deep Eutectic Solvents. J. Mol. Liq. 2020, 318, 1–9. 10.1016/j.molliq.2020.114042. [DOI] [Google Scholar]
- Mostafavi B.; Feizbakhsh A.; Konoz E.; Faraji H. Hydrophobic Deep Eutectic Solvent Based on Centrifugation-Free Dispersive Liquid–Liquid Microextraction for Speciation of Selenium in Aqueous Samples: One Step Closer to Green Analytical Chemistry. Microchem. J. 2019, 148, 582–590. 10.1016/j.microc.2019.05.021. [DOI] [Google Scholar]
- Křížek T.; Bursová M.; Horsley R.; Kuchař M.; Tůma P.; Čabala R.; Hložek T. Menthol-Based Hydrophobic Deep Eutectic Solvents: Towards Greener and Efficient Extraction of Phytocannabinoids. J. Cleaner Prod. 2018, 193, 391–396. 10.1016/j.jclepro.2018.05.080. [DOI] [Google Scholar]
- Vázquez-González M.; Fernández-Prior Á.; Bermúdez Oria A.; Rodríguez-Juan E. M.; Pérez-Rubio A. G.; Fernández-Bolaños J.; Rodríguez-Gutiérrez G. Utilization of Strawberry and Raspberry Waste for the Extraction of Bioactive Compounds by Deep Eutectic Solvents. Lwt 2020, 130, 109645 10.1016/j.lwt.2020.109645. [DOI] [Google Scholar]
- Benabid S.; Haddaoui N.; Lemaoui T.; Darwish A. S.; Benguerba Y.; Alnashef I. M. Computational Modeling of Polydecanediol-Co-Citrate Using Benzalkonium Chloride-Based Hydrophobic Eutectic Solvents: COSMO-RS, Reactivity, and Compatibility Insights. J. Mol. Liq. 2021, 339, 116674 10.1016/j.molliq.2021.116674. [DOI] [Google Scholar]
- Gallucci F.; Annaland M. V. S.; Tuinier R. A Search for Natural Hydrophobic Deep Eutectic Solvents Based on Natural Components. ACS Sustainable Chem. Eng. 2019, 7, 2933–2942. 10.1021/acssuschemeng.8b03520. [DOI] [Google Scholar]
- García-Argüelles S.; Serrano M. C.; Gutiérrez M. C.; Ferrer M. L.; Yuste L.; Rojo F.; Del Monte F. Deep Eutectic Solvent-Assisted Synthesis of Biodegradable Polyesters with Antibacterial Properties. Langmuir 2013, 29, 9525–9534. 10.1021/la401353r. [DOI] [PubMed] [Google Scholar]
- Kurtulbaş E.; Pekel A. G.; Toprakçı İ.; Özçelik G.; Bilgin M.; Şahin S. Hydrophobic Carboxylic Acid Based Deep Eutectic Solvent for the Removal of Diclofenac. Biomass Convers. Biorefin. 2022, 1. 10.1007/s13399-020-00721-1. [DOI] [Google Scholar]
- Hanada T.; Goto M. Synergistic Deep Eutectic Solvents for Lithium Extraction. ACS Sustainable Chem. Eng. 2021, 9, 2152–2160. 10.1021/acssuschemeng.0c07606. [DOI] [Google Scholar]
- Kareem M. A.; Mjalli F. S.; Hashim M. A.; Alnashef I. M. Phosphonium-Based Ionic Liquids Analogues and Their Physical Properties. J. Chem. Eng. Data 2010, 4632. 10.1021/je100104v. [DOI] [Google Scholar]
- Fan Y.; Wu H.; Cai D.; Yang T.; Yang L. Effective Extraction of Harmine by Menthol/Anise Alcohol-Based Natural Deep Eutectic Solvents. Sep. Purif. Technol. 2020, 250, 117211 10.1016/j.seppur.2020.117211. [DOI] [Google Scholar]
- Martins M. A. R.; Silva L. P.; Schaeffer N.; Abranches D. O.; Maximo G. J.; Pinho S. P.; Coutinho J. A. P. Greener Terpene-Terpene Eutectic Mixtures as Hydrophobic Solvents. ACS Sustainable Chem. Eng. 2019, 7, 17414–17423. 10.1021/acssuschemeng.9b04614. [DOI] [Google Scholar]
- Shi Y.; Li X.; Shang Y.; Li T.; Zhang K.; Fan J. Effective Extraction of Fluorescent Brightener 52 from Foods by in Situ Formation of Hydrophobic Deep Eutectic Solvent. Food Chem. 2020, 311, 125870 10.1016/j.foodchem.2019.125870. [DOI] [PubMed] [Google Scholar]
- Rajabi M.; Ghassab N.; Hemmati M.; Asghari A. Emulsification Microextraction of Amphetamine and Methamphetamine in Complex Matrices Using an Up-to-Date Generation of Eco-Friendly and Relatively Hydrophobic Deep Eutectic Solvent. J. Chromatogr. A 2018, 1576, 1–9. 10.1016/j.chroma.2018.07.040. [DOI] [PubMed] [Google Scholar]
- Almustafa G.; Darwish A. S.; Lemaoui T.; O’Conner M. J.; Amin S.; Arafat H. A.; AlNashef I. Liquification of 2,2,4-Trimethyl-1,3-Pentanediol into Hydrophobic Eutectic Mixtures: A Multi-Criteria Design for Eco-Efficient Boron Recovery. Chem. Eng. J. 2021, 426, 131342 10.1016/j.cej.2021.131342. [DOI] [Google Scholar]
- Darwish A. S.; Hatab F. A.; Lemaoui T.; Ibrahim O. A.; Almustafa G.; Zhuman B.; Warrag S. E.; Hadj-Kali M. K.; Benguerba Y.; Alnashef I. M. Multicomponent Extraction of Aromatics and Heteroaromatics from Diesel Using Acidic Eutectic Solvents: Experimental and COSMO-RS Predictions. J. Mol. Liq. 2021, 336, 116575 10.1016/j.molliq.2021.116575. [DOI] [Google Scholar]
- Malik A.; Kashyap H. K. Heterogeneity in Hydrophobic Deep Eutectic Solvents: SAXS Prepeak and Local Environments. Phys. Chem. Chem. Phys. 2021, 23, 3915–3924. 10.1039/d0cp05407k. [DOI] [PubMed] [Google Scholar]
- Abdi K.; Ezoddin M.; Pirooznia N. Temperature-Controlled Liquid–Liquid Microextraction Using a Biocompatible Hydrophobic Deep Eutectic Solvent for Microextraction of Palladium from Catalytic Converter and Road Dust Samples Prior to ETAAS Determination. Microchem. J. 2020, 157, 104999 10.1016/j.microc.2020.104999. [DOI] [Google Scholar]
- Ghorbani Ravandi M.; Fat’Hi M. R. Green Effervescence Assisted Dispersive Liquid-Liquid Microextraction Based on a Hydrophobic Deep Eutectic Solvent for Determination of Sunset Yellow and Brilliant Blue FCF in Food Samples. New J. Chem. 2018, 42, 14901–14908. 10.1039/c8nj00782a. [DOI] [Google Scholar]
- Momotko M.; Łuczak J.; Przyjazny A.; Boczkaj G. First Deep Eutectic Solvent-Based (DES) Stationary Phase for Gas Chromatography and Future Perspectives for DES Application in Separation Techniques. J. Chromatogr. A 2021, 1635, 461701 10.1016/j.chroma.2020.461701. [DOI] [PubMed] [Google Scholar]
- Tereshatov E. E.; Boltoeva M. Y.; Folden C. M. First Evidence of Metal Transfer into Hydrophobic Deep Eutectic and Low-Transition-Temperature Mixtures: Indium Extraction from Hydrochloric and Oxalic Acids. Green Chem. 2016, 18, 4616–4622. 10.1039/c5gc03080c. [DOI] [Google Scholar]
- Zhang H.; Ferrer M. L.; Rolda J.; Monte F. Brillouin Spectroscopy as a Suitable Technique for the Determination of the Eutectic Composition in Mixtures of Choline Chloride and Water. J. Phys. Chem. B 2020, 124, 4002–4009. 10.1021/acs.jpcb.0c01919. [DOI] [PubMed] [Google Scholar]
- Rahman S.; Raynie D. E. Thermal Behavior , Solvatochromic Parameters , and Metal Halide Solvation of the Novel Water-Based Deep Eutectic Solvents. J. Mol. Liq. 2021, 324, 114779 10.1016/j.molliq.2020.114779. [DOI] [Google Scholar]
- Triolo A.; Lo F.; Brehm M.; Di V.; Russina O. Liquid Structure of a Choline Chloride-Water Natural Deep Eutectic Solvent : A Molecular Dynamics Characterization. J. Mol. Liq. 2021, 331, 115750 10.1016/j.molliq.2021.115750. [DOI] [Google Scholar]
- Florindo C.; Romero L.; Rintoul I.; Branco L. C.; Marrucho I. M. From Phase Change Materials to Green Solvents: Hydrophobic Low Viscous Fatty Acid-Based Deep Eutectic Solvents. ACS Sustainable Chem. Eng. 2018, 6, 3888–3895. 10.1021/acssuschemeng.7b04235. [DOI] [Google Scholar]
- Jouyban A.; Ali Farajzadeh M.; Afshar Mogaddam M. R.; Khodadadeian F.; Nemati M.; Khoubnasabjafari M. In-Situ Formation of a Hydrophobic Deep Eutectic Solvent Based on Alpha Terpineol and Its Application in Liquid-Liquid Microextraction of Three β-Blockers from Plasma Samples. Microchem. J. 2021, 170, 106687 10.1016/j.microc.2021.106687. [DOI] [Google Scholar]
- Georgantzi C.; Lioliou A. E.; Paterakis N.; Makris D. P. Combination of Lactic Acid-Based Deep Eutectic Solvents (DES) with β-Cyclodextrin: Performance Screening Using Ultrasound-Assisted Extraction of Polyphenols from Selected Native Greek Medicinal Plants. Agronomy 2017, 7, 54. 10.3390/agronomy7030054. [DOI] [Google Scholar]
- Makoś P.; Przyjazny A.; Boczkaj G. Hydrophobic Deep Eutectic Solvents as “Green” Extraction Media for Polycyclic Aromatic Hydrocarbons in Aqueous Samples. J. Chromatogr. A 2018, 1570, 28–37. 10.1016/j.chroma.2018.07.070. [DOI] [PubMed] [Google Scholar]
- Fan C.; Sebbah T.; Liu Y.; Cao X. Terpenoid-Capric Acid Based Natural Deep Eutectic Solvent: Insight into the Nature of Low Viscosity. Clean. Eng. Technol. 2021, 3, 100116 10.1016/j.clet.2021.100116. [DOI] [Google Scholar]
- Omar K. A.; Sadeghi R. Novel Nonanol-Based Deep Eutectic Solvents: Thermophysical Properties and Their Applications in Liquid-Liquid Extraction and Amino Acid Detection. J. Mol. Liq. 2021, 336, 116359 10.1016/j.molliq.2021.116359. [DOI] [Google Scholar]
- Darwish A. S.; Warrag S. E. E.; Lemaoui T.; Alseiari M. K.; Hatab F. A.; Rafay R.; Alnashef I.; Rodríguez J.; Alamoodi N. Green Extraction of Volatile Fatty Acids from Fermented Wastewater Using Hydrophobic Deep Eutectic Solvents. Fermentation 2021, 7, 226. 10.3390/fermentation7040226. [DOI] [Google Scholar]
- Wang X. L.; Lu Y.; Shi L.; Yang D.; Yang Y. Novel Low Viscous Hydrophobic Deep Eutectic Solvents Liquid-Liquid Microextraction Combined with Acid Base Induction for the Determination of Phthalate Esters in the Packed Milk Samples. Microchem. J. 2020, 159, 105332 10.1016/j.microc.2020.105332. [DOI] [Google Scholar]
- Cao J.; Yang M.; Cao F.; Wang J.; Su E. Tailor-Made Hydrophobic Deep Eutectic Solvents for Cleaner Extraction of Polyprenyl Acetates from Ginkgo Biloba Leaves. J. Cleaner Prod. 2017, 152, 399–405. 10.1016/j.jclepro.2017.03.140. [DOI] [Google Scholar]
- Dietz C. H. J. T.; van Osch D. J. G. P.; Kroon M. C.; Sadowski G.; van Sint Annaland M.; Gallucci F.; Zubeir L. F.; Held C. PC-SAFT Modeling of CO2 Solubilities in Hydrophobic Deep Eutectic Solvents. Fluid Phase Equilib. 2017, 448, 94–98. 10.1016/j.fluid.2017.03.028. [DOI] [Google Scholar]
- Liu X.; Bian Y.; Zhao J.; Wang Y.; Zhao L. Menthol-Based Deep Eutectic Solvent in Dispersive Liquid-Liquid Microextraction Followed by Solidification of Floating Organic Droplet for the Determination of Three Bisphenols with UPLC-MS/MS. Microchem. J. 2020, 159, 105438 10.1016/j.microc.2020.105438. [DOI] [Google Scholar]
- Zinov’eva I. V.; Fedorov A. Y.; Milevskii N. A.; Zakhodyaeva Y. A.; Voshkin A. A. A Deep Eutectic Solvent Based on Choline Chloride and Sulfosalicylic Acid: Properties and Applications. Theor. Found. Chem. Eng. 2021, 55, 371–379. 10.1134/S0040579521030246. [DOI] [Google Scholar]
- Martins M. A. R.; Crespo E. A.; Pontes P. V. A.; Silva L. P.; Bülow M.; Maximo G. J.; Batista E. A. C.; Held C.; Pinho S. P.; Coutinho J. A. P. Tunable Hydrophobic Eutectic Solvents Based on Terpenes and Monocarboxylic Acids. ACS Sustainable Chem. Eng. 2018, 6, 8836–8846. 10.1021/acssuschemeng.8b01203. [DOI] [Google Scholar]
- Haider M. B.; Dwivedi M.; Jha D.; Kumar R.; Marriyappan Sivagnanam B. Azeotropic Separation of Isopropanol-Water Using Natural Hydrophobic Deep Eutectic Solvents. J. Environ. Chem. Eng. 2021, 9, 104786 10.1016/j.jece.2020.104786. [DOI] [Google Scholar]
- Rodriguez Rodriguez N.; MacHiels L.; Binnemans K. P-Toluenesulfonic Acid-Based Deep-Eutectic Solvents for Solubilizing Metal Oxides. ACS Sustainable Chem. Eng. 2019, 7, 3940–3948. 10.1021/acssuschemeng.8b05072. [DOI] [Google Scholar]
- Song Y.; Shi X.; Ma S.; Yang X.; Zhang X. A Novel Aqueous Gallic Acid-Based Natural Deep Eutectic Solvent for Delignification of Hybrid Poplar and Enhanced Enzymatic Hydrolysis of Treated Pulp. Cellulose 2020, 27, 8301–8315. 10.1007/s10570-020-03342-z. [DOI] [Google Scholar]
- Dehury P.; Chaudhary R. K.; Banerjee T.; Dalal A. Evaluation of Thermophysical Properties of Menthol-Based Deep Eutectic Solvent as a Thermal Fluid: Forced Convection and Numerical Studies. Ind. Eng. Chem. Res. 2019, 58, 20125–20133. 10.1021/acs.iecr.9b01836. [DOI] [Google Scholar]
- Warrag S. E. E.; Darwish A. S.; Abuhatab F. O. S.; Adeyemi I. A.; Kroon M. C.; Alnashef I. M. Combined Extractive Dearomatization, Desulfurization, and Denitrogenation of Oil Fuels Using Deep Eutectic Solvents: A Parametric Study. Ind. Eng. Chem. Res. 2020, 59, 11723–11733. 10.1021/acs.iecr.0c01360. [DOI] [Google Scholar]
- Warrag S. E. E.; Rodriguez N. R.; Nashef I. M.; van Sint Annaland M.; Siepmann J. I.; Kroon M. C.; Peters C. J. Separation of Thiophene from Aliphatic Hydrocarbons Using Tetrahexylammonium-Based Deep Eutectic Solvents as Extracting Agents. J. Chem. Eng. Data 2017, acs.jced.7b00168 10.1021/acs.jced.7b00168. [DOI] [Google Scholar]
- Cassol C. C.; Umpierre A. P.; Ebeling G.; Ferrera B.; Chiaro S. S. X.; Dupont J. On the Extraction of Aromatic Compounds from Hydrocarbons by Imidazolium Ionic Liquids. Int. J. Mol. Sci. 2007, 8, 593–605. 10.3390/i8070593. [DOI] [Google Scholar]
- Quijano G.; Couvert A.; Amrane A.; Darracq G.; Couriol C.; Le Cloirec P.; Paquin L.; Carrié D. Toxicity and Biodegradability of Ionic Liquids: New Perspectives towards Whole-Cell Biotechnological Applications. Chem. Eng. J. 2011, 174, 27–32. 10.1016/j.cej.2011.07.055. [DOI] [Google Scholar]
- Kim S.; Chen J.; Cheng T.; Gindulyte A.; He J.; He S.; Li Q.; Shoemaker B. A.; Thiessen P. A.; Yu B.; Zaslavsky L.; Zhang J.; Bolton E. E. PubChem in 2021: New Data Content and Improved Web Interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. 10.1093/nar/gkaa971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radošević K.; Železnjak J.; Cvjetko Bubalo M.; Radojčić Redovniković I.; Slivac I.; Gaurina Srček V. Comparative in Vitro Study of Cholinium-Based Ionic Liquids and Deep Eutectic Solvents toward Fish Cell Line. Ecotoxicol. Environ. Saf. 2016, 131, 30–36. 10.1016/j.ecoenv.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Hayyan M.; Ali M.; Hayyan A.; Al-saadi M. A.; Alnashef I. M.; Mirghani M. E. S.; Kola O. Chemosphere Are Deep Eutectic Solvents Benign or Toxic ?. Chemosphere 2013, 90, 2193–2195. 10.1016/j.chemosphere.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Siani G.; Tiecco M.; Di Profio P.; Guernelli S.; Fontana A.; Ciulla M.; Canale V. Physical Absorption of CO2 in Betaine/Carboxylic Acid-Based Natural Deep Eutectic Solvents. J. Mol. Liq. 2020, 315, 113708. 10.1016/j.molliq.2020.113708. [DOI] [Google Scholar]
- Jiang Z. M.; Wang L. J.; Gao Z.; Zhuang B.; Yin Q.; Liu E. H. Green and Efficient Extraction of Different Types of Bioactive Alkaloids Using Deep Eutectic Solvents. Microchem. J. 2019, 145, 345–353. 10.1016/j.microc.2018.10.057. [DOI] [Google Scholar]
- Sánchez P. B.; González B.; Salgado J.; José Parajó J.; Domínguez Á. Physical Properties of Seven Deep Eutectic Solvents Based on L-Proline or Betaine. J. Chem. Thermodyn. 2019, 131, 517–523. 10.1016/j.jct.2018.12.017. [DOI] [Google Scholar]
- Cardellini F.; Tiecco M.; Germani R.; Cardinali G.; Corte L.; Roscini L.; Spreti N. Novel Zwitterionic Deep Eutectic Solvents from Trimethylglycine and Carboxylic Acids: Characterization of Their Properties and Their Toxicity. RSC Adv. 2014, 4, 55990–56002. 10.1039/c4ra10628h. [DOI] [Google Scholar]
- Aroso I. M.; Paiva A.; Reis R. L.; Duarte A. R. C. Natural Deep Eutectic Solvents from Choline Chloride and Betaine – Physicochemical Properties. J. Mol. Liq. 2017, 241, 654–661. 10.1016/j.molliq.2017.06.051. [DOI] [Google Scholar]
- Fanali C.; Della Posta S.; Dugo L.; Gentili A.; Mondello L.; De Gara L. Choline-Chloride and Betaine-Based Deep Eutectic Solvents for Green Extraction of Nutraceutical Compounds from Spent Coffee Ground. J. Pharm. Biomed. Anal. 2020, 189, 113421 10.1016/j.jpba.2020.113421. [DOI] [PubMed] [Google Scholar]
- Hsieh Y. H.; Li Y.; Pan Z.; Chen Z.; Lu J.; Yuan J.; Zhu Z.; Zhang J. Ultrasonication-Assisted Synthesis of Alcohol-Based Deep Eutectic Solvents for Extraction of Active Compounds from Ginger. Ultrason. Sonochem. 2020, 63, 104915 10.1016/j.ultsonch.2019.104915. [DOI] [PubMed] [Google Scholar]
- Sarmad S.; Nikjoo D.; Mikkola J. P. Amine Functionalized Deep Eutectic Solvent for CO2 Capture: Measurements and Modeling. J. Mol. Liq. 2020, 309, 113159 10.1016/j.molliq.2020.113159. [DOI] [Google Scholar]
- Mohammadi B.; Shekaari H.; Zafarani-Moattar M. T. Selective Separation of α-Tocopherol Using Eco-Friendly Choline Chloride – Based Deep Eutectic Solvents (DESs) via Liquid-Liquid Extraction. Colloids Surf., A 2021, 617, 126317 10.1016/j.colsurfa.2021.126317. [DOI] [Google Scholar]
- Gontrani L.; Plechkova N. V.; Bonomo M. In-Depth Physico-Chemical and Structural Investigation of a Dicarboxylic Acid/Choline Chloride Natural Deep Eutectic Solvent (NADES): A Spotlight on the Importance of a Rigorous Preparation Procedure. ACS Sustainable Chem. Eng. 2019, 7, 12536–12543. 10.1021/acssuschemeng.9b02402. [DOI] [Google Scholar]
- Bai C.; Wei Q.; Ren X. Selective Extraction of Collagen Peptides with High Purity from Cod Skins by Deep Eutectic Solvents. ACS Sustainable Chem. Eng. 2017, 5, 7220–7227. 10.1021/acssuschemeng.7b01439. [DOI] [Google Scholar]
- Abbott A. P.; Boothby D.; Capper G.; Davies D. L.; Rasheed R. K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. 10.1021/ja048266j. [DOI] [PubMed] [Google Scholar]
- Zhao B. Y.; Xu P.; Yang F. X.; Wu H.; Zong M. H.; Lou W. Y. Biocompatible Deep Eutectic Solvents Based on Choline Chloride: Characterization and Application to the Extraction of Rutin from Sophora Japonica. ACS Sustainable Chem. Eng. 2015, 3, 2746–2755. 10.1021/acssuschemeng.5b00619. [DOI] [Google Scholar]
- Sert M.; Arslanoğlu A.; Ballice L. Conversion of Sunflower Stalk Based Cellulose to the Valuable Products Using Choline Chloride Based Deep Eutectic Solvents. Renewable Energy 2018, 118, 993–1000. 10.1016/j.renene.2017.10.083. [DOI] [Google Scholar]
- Hayyan M.; Aissaoui T.; Hashim M. A.; AlSaadi M. A.; Hayyan A. Triethylene Glycol Based Deep Eutectic Solvents and Their Physical Properties. J. Taiwan Inst. Chem. Eng. 2015, 50, 24–30. 10.1016/j.jtice.2015.03.001. [DOI] [Google Scholar]
- van Osch D. J. G. P.; Dietz C. H. J. T.; Warrag S. E. E.; Kroon M. C. The Curious Case of Hydrophobic Deep Eutectic Solvents: A Story on the Discovery, Design and Applications. ACS Sustainable Chem. Eng. 2020, 10591. 10.1021/acssuschemeng.0c00559. [DOI] [Google Scholar]
- Macedo E.; Peres A. Thermodynamics of Ternary Mixtures Containing Sugars. SLE of D-Fructose in Pure and Mixed Solvents. Comparison between Modified UNIQUAC and Modified UNIFAC. Ind. Eng. Chem. Res. 2001, 40, 4633–4640. 10.1021/ie0102596. [DOI] [Google Scholar]
- Xingchu G.; Shanshan W.; Haibin Q. U. Solid-Liquid Equilibria of D-Glucose, D-Fructose and Sucrose in the Mixture of Ethanol and Water from 273.2 K to 293.2 K. Chinese J. Chem. Eng. 2011, 19, 217–222. 10.1016/S1004-9541(11)60157-2. [DOI] [Google Scholar]
- Jaeger H.; Janositz A.; Knorr D. The Maillard Reaction and Its Control during Food Processing. The Potential of Emerging Technologies. Pathol. Biol. 2010, 58, 207–213. 10.1016/j.patbio.2009.09.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.