Abstract

Corn starch was gelatinized and treated with a metagenomic type 1 pullulanase (PulM), increasing the proportion of linear glucan chains. The debranched corn starch (DCS), containing amylose helices, was subjected to complexation with fatty acid molecules at moderate temperatures (50–60 °C). The amylose–lipid complexes prepared using saturated fatty acids, e.g., capric acid (CA) and lauric acid (LA), displayed higher CI values as compared to that of unsaturated fatty acid compounds, e.g., undecylenic acids (UAs) and oleic acid (OA). The DCS–fatty acid complex was estimated to contain about 14% of rapidly digested starch (RDS), 26% of slowly digested starch (SDS), and 60% of resistant starch V (RS-5). RS-5 samples exhibited high resistance toward digestive enzymatic hydrolysis. The surface microdetails of RS-5 were examined by scanning electron microscopy (SEM), depicting small spherulite-like structural aggregates. X-ray diffraction pattern analysis estimated about 46% of the crystallinity of RS-5. Thermal attributes of RS-5 were examined by thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) analysis, depicting the increase in melting enthalpies after the complexation of fatty acid molecules with debranched corn starch. Comparative DSC thermograms divulged a relatively higher stability of RS-5 as compared to that of RS-3. The findings advocated the potentiality of RS-5 (nondigestible DCS-LA complex) as a functional, valuable ingredient in the food industry.
1. Introduction
Starch, the most abundant plant polysaccharide, serves as the principal source of energy in the human diet. Primarily, it is composed of two types of polymers, amylose and amylopectin. Amylose is majorly the polymer of α-1,4-linked d-glucose units, with limited α-1,6 branching, while in amylopectin, α-1,4-d-glucan chain has a dense α-1,6-linked branching. The paramount plant resources of starch include corn, potato, wheat, rice, cassava, etc., possessing 20–30% of amylose and 70–80% of amylopectin therein.1 Starch is readily digested in the human gastrointestinal tract, followed by absorption of the hydrolytic product, d-glucose, exerting a significant glycemic response. The development of strategies to slacken the amylolytic hydrolysis and intestinal absorption of starch products has been a notable field of research.2 In view of the increasing incidences of obesity, overweight, insulin resistance, and diabetes, digestion-resistant and low-glycemic-carbohydrate products are in high demand.
The starch material that fails to get absorbed in the small intestine is consumed and fermented by colon microflora to produce short-chain fatty acid molecules of high-health benefits. Such modified starch products, with dense, recrystallized, and compact structures, are called resistant starch (RS).3 RS is majorly classified into five subclasses, i.e., RS-1, RS-2, RS-3, RS-4, and RS-5.4 RS-1 and RS-2 are native forms of starch, whereas RS-3, RS-4, and RS-5 are processed starch. RS-1 is naturally present in plant cells, the cell wall of which can resist hydrolysis by gastrointestinal enzymes and is thus physically inaccessible. However, the physical disruption of the cell wall makes type 1 starch available for gastrointestinal digestion. RS-2 is a native starch granule with compact and semicrystalline structures resistant to digestion. However, heat treatment often disrupts the complexity of RS-2 starch granules, enhancing their digestible proportion. Gelatinization and subsequent retrogradation of amylose glucan chains resulted in the preparation of RS-3 starch. The starch subjected to chemical modifications, e.g., cross-linking, etherification, and esterification, is known as RS-4. RS-5 refers to the amylose–lipid complexes.4 RS-3, RS-4, and RS-5 are thermally stable and digestion-resistant to a greater extent, exerting a prebiotic impact on human health.5 Many studies have demonstrated the physicochemical and functional properties of different resistant starch types.4
The preparation of resistant starch involves enzymatic, physical, and chemical modifications of native starch. Type I pullulanase is a critical enzyme, the treatment of which increases the amylose content in the starch by the cleavage of α-1,6-glycosidic linkages, releasing the linear, branched glucan chains.5 The linear glucan chains are prone to physical modifications, i.e., retrogradation and recrystallization. The combination treatment of α-1,6-hydrolyzing pullulanase (type 1) and autoclaving results in the preparation of heat-stable type III (RS-3) resistant starch.6,7 The complex of lipid hydrocarbon and helical amylose forms type-V (RS-5) resistant starch, as represented in Figure 1. The methylene group and glycosidic linkage make the helical cavity of the linear debranched starch strongly hydrophobic, facilitating the formation of inclusion networks and, subsequently, the complexation of amylose with the lipid molecules.8,9 These associations are primarily driven by hydrophobic interactions, which render it highly resistant to digestive enzymes and low-glycemic responsive features.10 The resistant amylose–lipid complex is a crucial macronutrient for people with hypercholesterolemia and metabolic syndromes like obesity, hypertension, and diabetes.11,12 Further, RS-5 is believed to regulate postprandial glycemic and insulinemic responses, and its consumption could prevent the development of colon cancer.13Figure 1 represents the positive impacts of RS-5 consumption on human health.
Figure 1.
Artwork demonstrating the health benefits of resistant starch.
In the food industry, resistant starch is a preferred ingredient that increases the dietary fiber content and lessens the glycemic response.14 A variety of parameters, such as the types of lipids, their molecular makeup, the lengths of hydrocarbon chains, the amount of amylose in the starch, and the reaction conditions, can affect the formation of type-V complexes among amylose and the guest lipid molecules.15,17 The quantity of linear amylose chains discharged from the swelled starch granules and the solubility and dispersibility of the lipid in gelatinized starch have been examined to be crucial in developing the V-type complex.16,17 The type of lipid molecules used in RS-5 preparation exerts a critical impact on the complexity index of the amylose–lipid inclusion network, which in turn affects crystallinity and resistance to enzymatic hydrolysis.18
The present investigation elaborates on a process for amylose–lipid complexation that leads to increased crystallinity, digestion resistivity, and reasonably good heat stability. The resistance of RS-5 toward digestive enzymes was assessed. The structural morphology, crystallinity, and thermogravimetric properties of RS-5 were examined using analytical techniques. Furthermore, the impact of saturated and unsaturated fatty acid molecules with shorter- and longer-chain lengths on the degree of starch–fatty acid complexation was examined.
2. Materials and Methods
2.1. Materials
Corn starch and corn amylopectin were procured from Central Drug House (P) Ltd., New Delhi, India, and MP Biomedicals, respectively. Amyloglucosidase (Aspergillus niger), porcine pancreatin (EC 232-468-9), and ethanol were obtained from Sigma-Aldrich (St. Louis, MO). K-GLUC d-glucose assay kit was purchased from Megazyme (Wicklow, Ireland). Lauric acid (LA) was obtained from HiMedia Mumbai, India. Capric acid (CA) and undecylenic acid (UA) were obtained from Central Drug House (P) Ltd., New Delhi, India. Olive oil (containing oleic acid (OA)) was procured from the local market.
2.2. Expression and Purification of Type 1 Pullulanase (PulM)
Type 1 pullulanase (PulM) was expressed in Escherichia coli BL21 cells, followed by extraction and purification of protein as described in our previous reports.5,7
2.3. Preparation of Debranched Corn Starch and DCS–Fatty Acid Complex
Corn starch (100 g) was suspended in hot water (1 L) and heated at 70 °C in a water bath for 20 min with constant stirring. The gelatinized starch was cooled at room temperature and then treated with the debranching enzyme, PulM (∼20 μg dissolved in 50 mM sodium acetate buffer (pH 6.0), containing 2 mM CaCl2), at 40 °C and 150 rpm for 6 h. Then, 500 mL of absolute ethanol was added, followed by incubation at room temperature for 20 min. The debranched corn starch (DCS) was precipitated by centrifugation at 3634g and dried at 40 °C for 24 h. Then, DCS was then crushed into a fine powder. Gelatinized corn starch (GCS) that was not treated with type 1 pullulanase was used as a control.
For the preparation of amylose–lipid complex, DCS powder was suspended in distilled water (1 g of DCS in 10 mL), followed by the addition of increasing concentrations (5–15% (w/w) of DCS) of lauric acid (LA), i.e., 50 mg (5%), 70 mg (7%), 100 mg (10%), and 150 mg (15%). The samples containing a mixture of DCS and fatty acids were incubated in boiling water for 30 min with a vortex after every 10 min. For complexing, samples were shifted in a shaker at 50 or 60 °C and 150 rpm for 60 min. To precipitate the DCS–fatty acid complex, absolute ethanol (5 mL) was added, followed by incubation at room temperature for 20 min and centrifugation at 3634g. In this way, the unbound fatty acid was removed, and the DCS–fatty acid complex was precipitated. The precipitate was air-dried at 40 °C for 24 h and crushed into powder for further investigation.
2.4. Complex Index Analysis
The complexing index (CI) of the DCS–fatty acid complex samples was determined by the iodine blue analysis method (Kaur and Singh, 2000).19 The DCS–fatty acid complex and GCS samples (100 mg each) were boiled in water (10 mL) for 15 min and cooled to room temperature. The sample was centrifuged at 1780g for 10 min. The supernatant (500 μL) was mixed with 4 mL of iodine solution (0.1% I2, w/w, and 2% KI, w/w, in deionized water). It was further diluted with distilled water (∼35 mL). The absorbance was measured at 690 nm. The following equation was used to determine the complexing index
| 1 |
2.5. Determination of the RS-5 Content
The DCS–LA complex and native corn starch (NCS) samples (100 mg) were enzymatically treated in 50 mM sodium acetate buffer (pH 6.0) by amyloglucosidase (300 U mL–1), α-amylase (40 U mL–1), and pancreatin (20 mg mL–1) in a water bath at 37 °C. After 20 and 120 min of enzymatic digestion, the aliquot of 200 μL of the reaction sample was taken and mixed with 800 μL of absolute ethanol, followed by centrifugation at 1308g for 3 min. The supernatant was taken for the glucose oxidase–peroxidase (GOPOD) assay to determine glucose concentration in the samples after enzymatic digestion. The GOPOD assay determined rapidly digestible starch (RDS) and slowly digestible (SDS) starch in the samples, which were subjected to enzymatic digestion for 20 and 120 min, respectively. The portion of starch left after 120 min of the DCS–fatty acid complex’s enzymatic digestion was considered RS-5.
2.6. Recovery of Resistant Starch
The DCS–fatty acid complex (500 mg) was subjected to enzymatic treatment in 50 mM sodium acetate buffer (pH 6.0), containing pancreatin (20 mg mL–1), amyloglucosidase (300 U mL–1), and human pancreatic-amylase (40 U mL–1), at 37 °C for 3 h with constant stirring. Then, an equal volume of absolute ethanol was added, followed by incubation for 20 min at room temperature and precipitation of RS-5 by centrifugation at 3634g for 10 min. RS-5 was washed with lukewarm water two times. The sample was dried at 40 °C and ground to make an RS-5 powder for further analysis.
2.7. Preparation of Resistant Starch-III (RS-3)
Debranched corn starch (DCS) was subjected to retrogradation, and RS-3 was recovered, as explained in our previous reports.5,7
2.8. Fourier Transform Infrared Spectroscopy (FTIR)
The infrared spectra of RS-5 and control samples were recorded in an FTIR spectrometer (CARY 660 FTIR) operated in the transmittance mode. The spectra were acquired at room temperature by performing scanning in the wavelength range of 4000–500 cm–1 at a 4 cm–1 resolution.
2.9. Scanning Electron Microscopy (SEM)
The samples were mounted on the metal stubs by using carbon tape that adhered to both sides. The samples were gold-coated with a small sputter coater. The surface strictures of the RS and control starch samples were analyzed in a scanning electron microscope (Nikon H600L) operated in a low vacuum mode with an accelerating voltage of 10 kV.
2.10. X-ray Diffraction (XRD)
X-ray diffraction patterns of RS-5 and the control sample were studied on an X-ray diffractometer (Rigaku, Smart LAB SE). The diffraction spectra were recorded in the range of 4–35° (2θ), with a 2° min–1 scanning speed and a magnitude spacing of 0.02°. The XRD patterns were fitted by using Gaussian functions. The crystalline area under the peaks was computed by using Origin data analysis software (Origin 2022).
2.11. Thermogravimetric Analysis (TGA)
Thermal properties of RS-5 and control samples were studied in a TGA Analyzer (PerkinElmer model STA 8000). An empty aluminum pan was used as a reference. The runs were conducted in a nitrogen environment with a flow rate of 20 mL min–1 while applying a heating rate of 10 °C min–1 within the range of 30–800 °C. The loss in the weight of starch samples was estimated in relation to temperature and time.
2.12. Differential Scanning Calorimetry (DSC)
The thermodynamic profile of starch samples was examined in a differential scanning calorimeter (DSC 8000, PerkinElmer Co. Ltd.) by following the study of Liang.21 The starch sample (5 mg) was mounted on an aluminum pan with a nitrogen flow at a rate of 20 mL min–1. Samples were heated at a rate of 10 °C/min from 30 to 600 °C. The peak temperature (Tm) and the enthalpy change were determined using the peak area of the curve and maximum heat flow, respectively. A sealed empty pan was taken as a reference.
2.13. Statistical Analysis
To analyze the data, one-way analysis of variance (one-way ANOVA) was performed via Tukey multiple comparison tests using MiniTab 21.1 software. Statistically significant differences are indicated by the letters a–e. Graphs were generated by using OriginPro (OriginLab, Northampton, MA) and SigmaPlot 14.0.
3. Results and Discussion
3.1. Starch Debranching
Corn starch is rich in amylopectin, which has a branched structure. Branching limits the complexation ability of glucan chains with lipid molecules; therefore, starch debranching is an essential step in the preparation of resistant starch. Type 1 pullulanase is a potential biocatalyst that hydrolyzes α-16-linked branches in amylopectin, releasing linear glucan chains.5,7 The linear glucan chains are highly competent for complexation applications and thus can be used for making amylose–fatty acid complexes. In the present study, gelatinized corn starch (GCS) was treated with PulM type 1 pullulanase,5 which potentially catalyzed the debranching of starch, leading to a substantial increment (∼60%) in amylose proportion (Figure S1). The debranched corn starch (DCS), containing glucan chain helices, was subjected to fatty acid complexation.
3.2. Amylose–Lipid Complexation and Measurement of the Complex Index
Complex index (CI) is a measure of the degree of starch–fatty acid complexation, assessed through starch–iodine association.12 The formation of starch–fatty acid complexes causes a drop in the blue intensity of the supernatant’s blue intensity. The effect of fatty acid and debranched starch ratio on complexation was examined at 50 and 60 °C by measuring CI values. The temperature was observed to positively impact the starch–fatty acid complexation, with slightly higher CI values at 60 °C (Table 1). An increase in the CI value with the enhancement in complexation temperature could be attributed to elevated hydrophobic interactions among fatty acid (LA) and glucan helices with high mobility.23 The CI values progressively increased with the gradual increments in the level of LA from 5 to 10% of DCS at temperatures 50 and 60 °C (Table 1). The successive increments in CI values from 44 to 86% indicated the formation of amylose–fatty acid complexes.23 Nevertheless, further enhancement in the LA level did not lead to any noticeable increment in the CI value, which stipulated the failure of incorporation of fatty acid molecules into the amylose helices at a concentration beyond the threshold (i.e., 15% in this study). The results are in agreement with the previous observations.22,25
Table 1. Effect of Lauric Acid (LA) and Debranched Corn Starch (DCS) Ratio with Respect to Moderate Temperatures on Starch–Fatty Acid Complex Formationa.
| temperature | LA (w/w) | time (h) | Degree of complex (%) |
|---|---|---|---|
| 50 °C | 5% | 1 | 37 ± 1.8a |
| 7% | 1 | 61 ± 2.5b | |
| 10% | 1 | 72 ± 1.6c | |
| 15% | 1 | 71 ± 3.2d | |
| 60 °C | 5% | 1 | 44 ± 2.1a |
| 7% | 1 | 66 ± 2.8b | |
| 10% | 1 | 86 ± 1.89c | |
| 15% | 1 | 74 ± 2.9d |
The values are the means of three replications ± standard deviations. The mean values that do not share common alphabets (superscript) exhibit statistical differences at a P-value of <0.05.
The type of fatty acid molecule plays a critical role in the quality of complexation.21 To provide rationale between the use of short or higher carbon-chain forms of saturated and unsaturated fatty acids, in the present study, complexation reactions were conducted between DCS and different fatty acids (used at a concentration of 15% of DCS), e.g., capric acid (C10; saturated), lauric acid (C12; saturated), undecylenic acids (C10; unsaturated), and olive oil containing oleic acid (C18; unsaturated). The CI values of DCS–fatty acid complexes prepared with saturated fatty acids, LA and CA, were recorded to be higher as compared to that of unsaturated fatty acid compounds, UA and OA (Table 2). CA and UA have the same chain length, i.e., C10, but they significantly differ in CI values (Table 2), which could be attributed to the degree of saturation of the former. Unsaturated fatty acid contains double bonds that limit the number of carbon molecules involved in complex formation.21 Although the CI values of CA and LA were comparable, CA was noticed to be slightly better in complexation (Table 2). The findings suggest that saturated fatty acids with short carbon chains (C10–C12) are more competent in generating stable complexation with amylose helices. This is possibly because short-chain fatty acid exhibits relatively better dispersivity in gelatinized starch, leading to superior complex formation with glucan helices.17,23
Table 2. Effect of Saturated and Unsaturated Fatty Acids of Shorter- and Longer-Chain Lengths on CI Values of DCS–Fatty Acid Complexesa.
| samples | RS-5 (CA) | RS-5 (LA) | RS-5 (UA) | RS-5 (OA) |
|---|---|---|---|---|
| degree of complex (%) | 87 ± 2.8 | 86 ± 1.89 | 76 ± 3.2 | 72 ± 2.99 |
The values are the means of three replications ± standard deviations; capric acid (C10; saturated), lauric acid (C12; saturated), undecylenic acids (C10; unsaturated), and oleic acid (C18; unsaturated).
3.3. In Vitro Digestibility Profile
Digestibility profiling clearly indicated the release of the hydrolytic starch product in the case of native gelatinized corn starch (GCS). In the case of the DCS–LA complex, a limited release of the hydrolytic product was noted during 100 min of enzymatic digestion. Hardly any product was released during an extended reaction time for 8 h (Figure 2). RDS and SDS values were computed to be about 14 and 26%, respectively. In contrast, about a 60% proportion was estimated to be RS-5, which resisted enzymatic hydrolysis (Table 3). The amylose helices with lipid molecule complexation at a high CI level are less accessible to digestive enzymes, avoiding starch hydrolysis.26 Thus, reduced enzymatic hydrolysis of the modified starch validated the formation of the LA–amylose complexation. The type of fatty acid used for RS-5 preparation is critical in digestion resistance.10 The short carbon saturated fatty acid molecules exhibit low hydrolysis mainly after complexation with amylose helices.2 The digestible proportion of starch was removed by an extended period of enzymatic treatment, and RS-5 was recovered that was not digestible.
Figure 2.

Digestion profile of native gelatinized corn starch (GCS), debranched corn starch–lauric acid (DCS–LA) complex, and resistant starch V (RS-5).
Table 3. Contents of RDS, SDS, and RS in the DSC–LA Complexa.
| samples | RDS (%) | SDS (%) | RS-5 (%) |
|---|---|---|---|
| DCS-LA | 13.81 ± 2.3 | 25.97 ± 3.3 | 61.23 ± 2.5 |
The values are the means of three replications ± standard deviations.
3.4. Surface Microstructures
The effects of processing steps, e.g., gelatinization, debranching, and fatty acid complexation, on microstructural alterations in corn starch were investigated via SEM analysis. The native corn starch (NCS) granules were irregular in shape and size with a smooth surface (Figure 3a). This observation was consistent with previous reports.24,27,28 Gelatinization causes the melting of granules and disaggregation of glucan chains,29,30 disrupting the granular structure into a stretched structure (Figure 3b). Debranching and complexation with LA molecules transformed the starch into aggregates of small spherulite-like structure (Figure 3c,d). The intercalation of fatty acid molecules in the debranched glucan helices caused the appearance of aggregated spherulites representing amylose–lipid complexes. The surface micrographs of RS-5 were in line with previous studies.24,31,32
Figure 3.
Scanning electron micrographs (SEMs) of (a) native corn starch, (b) gelatinized corn starch, (c) debranched corn starch (DCS), and (d) resistant starch V (RS-5).
3.5. FTIR Assessment
FTIR is a potential method to analyze the structural changes in starch during its processing toward resistant starch.33 IR spectral variations were clearly visible between gelatinized starch and RS-5 samples (Figure 4). The inculcation of fatty acid molecules in glucan helices could be represented by the dominant peaks around 2917, 2850, 1018, and 720 cm–1.34,35 The peak near 1000 cm–1 indicates C–H bending. Further, the peaks around 1000 cm–1 could represent starch’s crystalline and amorphous characteristics.36 The distinctive peaks at 1155, 1080, and 1018 cm–1 could be attributed to the C–O stretching vibrations.37 The peak at 1345 cm–1 represents the O–H bending vibration.38 The predominant peak at around 1700 cm–1 in RS-5 is possibly accounted for the complexed fatty acid component.39
Figure 4.

FTIR spectra of native gelatinized corn starch (GCS) and resistant starch V (RS-5).
3.6. X-ray Diffraction Patterns
X-ray diffraction patterns of NCS, GCS, and RS-5 samples were compared to further validate the complexation of fatty acid molecules into glucan structures. The peaks at 2θ angles of 15, 17.1, 18, and 23° (Figure 5) represent characteristic peaks for the type A corn starch.11,20 In the case of RS-5, the diffraction peak near 15° was substantially weakened, whereas the peaks at 18 and 23° were disappeared. Diffraction spectra of RS-5 revealed dominant peaks at 2θ angles of 13, 17, 20, 21.6, and 23.9°. The diffraction patterns of RS-5 and corn starch are in good agreement with the previous studies.12,24,40 RS-5 diffraction represents typical V-type patterns, exhibiting strong peaks near 13 and 20° and weak intensity peaks near 7° (Figure 5). This is in agreement with the observations made in the previous reports.24,41,34 The additional peaks to a 2θ angle of 21.6 and 23.9° could represent the uncomplexed LA (Figure 5) in the resistant starch crystal.12 The region below the peaks is interpreted as the starch’s amorphous portion, whereas the areas under the peaks indicate crystalline proportion.42 The level of crystallinity for GCS and RS-5 was computed to be 16.67 ± 1.23 and 46.07 ± 2.13%, respectively. A marked increment in the diffraction intensity was observed in the case of the RS-5 (CA) sample as compared to that of RS-5 samples prepared with LA and UA (Figure S2). The results suggest that a shorter-chain length of saturated fatty acid molecules is favorable to achieving higher crystallinity of the V-form of resistant starch. Similar observations have been made in previous studies.2,22,45
Figure 5.
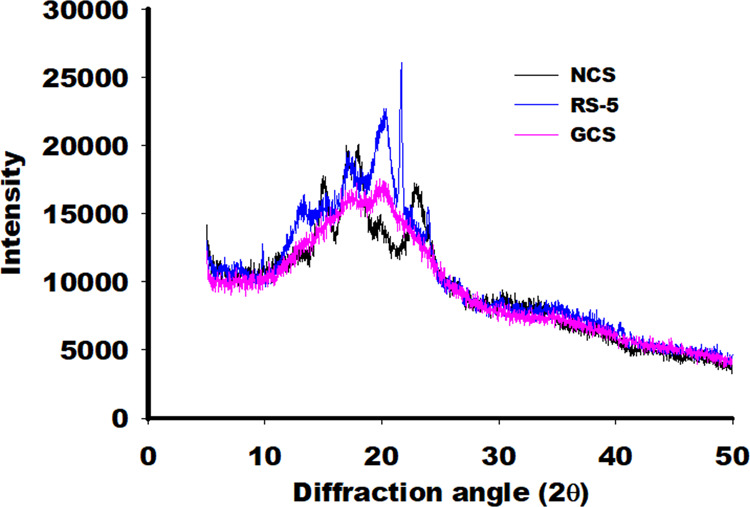
X-ray diffraction patterns of native corn starch (NCS), gelatinized corn starch (GCS), and resistant starch V (RS-5).
3.7. Thermal Attributes
The melting behavior of resistant starch is an essential feature of its industrial applications. The crystalline starch component with high complexity level should reflect increased physical stability at higher temperatures.37,43 Lauric acid (free version) component and GCS exhibited significant weight loss at 180–210 °C (Figure 6).
Figure 6.
TGA/DTG thermograms of lauric acid (LA), native gelatinized corn starch (GCS), and resistant starch V (RS-5).
In the case of gelatinized, the initial weight loss was noted at 210 °C that could be brought on by moisture evaporation.39 The RS-5 (LA) sample exhibited a slight loss in weight at nearly 200 °C, possibly representing the degradation of unbound LA in the sample. The second phase of weight loss in RS-5 was noted to be initiated at 270 °C. This could be linked to the oxidative disintegration of starch molecules.41 Contrary to this, RS-5 prepared by using CA and UA did not show any loss around 200 °C (Figure S3), which could indicate a relatively better adjustment of short-chain fatty acid molecules in amylose helices. However, substantial melting was recorded in all of the RS-5 samples at a temperature range of 300–340 °C (Figures 6 and S3). Overall, the complexation of lipid molecules with debranched corn starch helices led to the melting of RS-5 at relatively higher temperatures as compared with native gelatinized starch. TGA analysis depicted the RS-5 sample prepared with short fatty acid (CA) to be relatively more thermal-tolerant as compared to UA and LA (Figure S3), which is in agreement with a higher CI value and elevated crystallinity of RS-5 (CA). The results are consistent with the previous reports demonstrating enhanced thermal tolerance of RS-5 samples.17,22
3.8. Thermodynamics Study
DSC thermograms reveal the thermoanalytical perspective of heat’s influence on the sample’s chemical and physical properties. The thermal parameters of the processed starch samples are summarized in Table 4. The endothermic peaks recorded in the DSC thermograms of processed starch samples (Figure 7) could be associated with crystallization, decomposition, and oxidation processes.44,45
Table 4. Thermal Parameters of the Processed Starch Samplesa.
| peak 1 |
peak 2 |
peak
3 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| samples | TO (°C) | TP (°C) | TC (°C) | ΔH (J/g) | TO (°C) | TP (°C) | TC (°C) | ΔH (J/g) | TO (°C) | TP (°C) | TC (°C) | ΔH (J/g) |
| GCS | 40 | 47.19 | 53 | 7.05 | 170 | 177.83 | 190 | 4.87 | 195 | 205.65 | 210 | 2.00 |
| DCS | 60 | 68.46 | 80 | 22.16 | 273 | 283.49 | 310 | 32.41 | 312 | 318 | 325 | 25 |
| RS-5 (LA) | 235 | 275.34 | 309 | 183.66 | 310 | 318.92 | 335 | 11.15 | 380 | 420.09 | 470 | 99.45 |
| RS-5 (CA) | 291 | 308.39 | 325 | –22.91 | 410 | 420 | 440 | –10.8 | 554 | 564.89 | 580.2 | –10.31 |
| RS-5 (UA) | 260 | 284.56 | 310 | 29.86 | 555 | 565.57 | 575.2 | –7.66 | ||||
Tp: peak temperature; ΔH: enthalpy change; LA: lauric acid; CA: capric acid; UA: undecylenic acid; GCS: gelatinized (native) corn starch; DCS: debranched corn starch; and RS-5: resistant starch 5.
Figure 7.

DSC thermograms of native gelatinized corn starch (GCS) and resistant starch V (RS-5).
The temperature of RS-5 samples was higher than that of gelatinized corn starch (Table 4 and Figures 7 and S4), suggesting that the dispersivity of fatty acid molecules in amylose helices led to the formation of relatively compact and ordered structures.
Mostly, the phase transition temperature of type II complex peaks remains above 100 °C, while that of type I complex peaks has been found to be below 100 °C.41,43−45 This suggests that the RS-5 samples prepared in this study largely represent type II complexes. Higher enthalpy change (ΔH) designates the formation of a complex with increased melting temperature.46,47 DSC analysis revealed an increment in Tp and ΔH values after debranching of gelatinized corn starch (Table 4), depicting the positive impact of debranching on starch’s ability to form complex structures.23 This is in agreement with the increase in ΔH in high amylose starch.20 Higher peak temperatures of RS-5 complexes depict stronger intermolecular association through hydrogen bonding between the debranched starch and fatty acid molecules.47 Melting enthalpies of RS-5 starch samples prepared with CA, LA, and UA fatty acid molecules were in line with the crystallinity and CI values of the complexes.21,48
The comparative DSC thermograms of RS-5 and RS-3 revealed a relatively higher stability of RS-5 starch (Figure 8 and Table 5). The higher structural stability of RS-5 is a desirable feature for its use as an ingredient in processing applications in the food industry. The findings suggest that the interaction of fatty acid molecules with amylose helices facilitates the formation of a more complex structure than that of retrograded amylose chains.
Figure 8.

DSC thermograms of resistant starch-III (RS-3) and V (RS-5).
Table 5. Comparative Analysis of Thermal Characteristics of RS-3 and RS-5 Samplesa.
| peak 1 |
peak 2 |
peak
3 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| samples | TO (°C) | TP (°C) | TC (°C) | ΔH (J/g) | TO (°C) | TP (°C) | TC (°C) | ΔH (J/g) | TO (°C) | TP (°C) | TC (°C) | ΔH (J/g) |
| RS-3 | 67 | 80.08 | 110 | 63.75 | 197 | 218.72 | 235 | 48.32 | 340 | 364 | 395 | –23.87 |
| RS-5 | 235 | 275.34 | 309 | 183.66 | 310 | 318.92 | 335 | 11.15 | 380 | 420.09 | 470 | 99.45 |
Tp: peak temperature; ΔH: enthalpy change; gelatinized (native) corn starch; DCS: debranched corn starch; RS-5: resistant starch 5; and RS-3: resistant starch 3.
4. Conclusions
The present study investigated the debranching of corn starch, followed by fatty acid complexation of the glucan chain helices. The amylose–lipid complexation yielded about 60% starch resistant to digestion, i.e., RS-5. The CI values of amylose helices complexed with saturated fatty acids, e.g., CA and LA, were notably higher than that of complexes prepared with unsaturated lipid molecules, e.g., UA and OA. Analytical measurements were conducted to track the structural and thermal characteristics of RS-5 samples. The complexation of fatty acid molecules with debranched corn starch helices caused an increment in crystallinity and the melting enthalpies of RS-5 samples as compared to those of native corn starch. Thermal attributes of RS-5 were found to be even better than retrograded amylose helices, i.e., RS-3 sample. The crystalline amylose–lipid complex, with substantially good thermal stability and digestion resistivity, is a value-added functional ingredient for the food industry.
Acknowledgments
The authors acknowledge the Department of Biotechnology (DBT), Govt. of India, for financial support. M.T. acknowledges the CSIR-JRF/SRF fellowship and the Department of Biotechnology, Panjab University, Chandigarh, for Ph.D. registration.
Glossary
Abbreviations Used
- NCS
native corn starch
- GCS
gelatinized corn starch
- DCS
debranched corn starch
- LA
lauric acid
- CA
capric acid
- UA
undecylenic acid
- RS-5
resistant starch-V
- RS-3
resistant starch-III
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c01093.
Relative amylose content (%) in native starch and PulM-treated debranched starch; X-ray diffraction patterns, TGA thermograms, and DSC thermograms of RS-5 samples prepared by using capric acid (CA), lauric acid (LA), and undecylenic acids (UAs) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Schirmer M.; Höchstötter A.; Jekle M.; Arendt E.; Becker T. Physicochemical and morphological characterization of different starches with variable amylose/amylopectin ratio. Food Hydrocolloids 2013, 32, 52–63. 10.1016/j.foodhyd.2012.11.032. [DOI] [Google Scholar]
- Chen B.; Guo Z.; Miao S.; Zeng S.; Jia X.; Zhang Y.; Zheng B. Preparation and characterization of lotus seed starch-fatty acid complexes formed by microfluidization. J. Food Eng. 2018, 237, 52–59. 10.1016/j.jfoodeng.2018.05.020. [DOI] [Google Scholar]
- Chang R.; Jin Z.; Lu H.; Qiu L.; Sun C.; Tian Y. Type III resistant starch prepared from debranched starch: Structural changes under simulated saliva, gastric, and intestinal conditions and the impact on short-chain fatty acid production. J. Agric. Food Chem. 2021, 69 (8), 2595–2602. 10.1021/acs.jafc.0c07664. [DOI] [PubMed] [Google Scholar]
- Tekin T.; Dincer E. Effect of resistant starch types as a prebiotic. Appl. Microbiol. Biotechnol. 2023, 107, 491–515. 10.1007/s00253-022-12325-y. [DOI] [PubMed] [Google Scholar]
- Thakur M.; Sharma N.; Rai A. K.; Singh S. P. A novel cold-active type I pullulanase from a hot-spring metagenome for effective debranching and production of resistant starch. Bioresour. Technol. 2021, 320, 124288 10.1016/j.biortech.2020.124288. [DOI] [PubMed] [Google Scholar]
- Sun H.; Fan J.; Tian Z.; Ma L.; Meng Y.; Yang Z.; Zeng X.; Liu X.; Kang L.; Nan X. Effects of treatment methods on the formation of resistant starch in purple sweet potato. Food Chem. 2022, 367, 130580 10.1016/j.foodchem.2021.130580. [DOI] [PubMed] [Google Scholar]
- Thakur M.; Rai A. K.; Mishra B. B.; Singh S. P. Novel insight into valorization of potato peel biomass into type III resistant starch and maltooligosaccharide molecules. Environ. Technol. Innov. 2021, 24, 101827 10.1016/j.eti.2021.101827. [DOI] [Google Scholar]
- Liu K.; Chi C.; Huang X.; Li X.; Chen L. Synergistic effect of hydrothermal treatment and lauric acid complexation under different pressure on starch assembly and digestion behaviors. Food Chem. 2019, 278, 560–567. 10.1016/j.foodchem.2018.11.097. [DOI] [PubMed] [Google Scholar]
- He H.; Zheng B.; Wang H.; Li X.; Chen L. Insights into the multi-scale structure and in vitro digestibility changes of rice starch-oleic acid/linoleic acid complex induced by heat-moisture treatment. Food Res. Int. 2020, 137, 109612 10.1016/j.foodres.2020.109612. [DOI] [PubMed] [Google Scholar]
- Okumus B. N.; Tacer-Caba Z.; Kahraman K.; Nilufer-Erdil D. Resistant starch type V formation in brown lentil (Lens culinaris Medikus) starch with different lipids/fatty acids. Food Chem. 2018, 240, 550–558. 10.1016/j.foodchem.2017.07.157. [DOI] [PubMed] [Google Scholar]
- Gutiérrez T. J.; Tovar J. Update of the concept of type 5 resistant starch (RS-5): Self-assembled starch V-type complexes. Trends Food Sci. Technol. 2021, 109, 711–724. 10.1016/j.tifs.2021.01.078. [DOI] [Google Scholar]
- Lee H. S.; Kim K. H.; Park S. H.; Hur S. W.; Auh J. H. Amylose-lipid complex as a fat replacement in the preparation of low-fat white pan bread. Foods 2020, 9 (2), 194. 10.3390/foods9020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.; Wang P.; Xiao Z. Resistant starch prevents tumorigenesis of dimethylhydrazine-induced colon tumors via regulation of an ER stress-mediated mitochondrial apoptosis pathway. Int. J. Mol. Med. 2018, 41 (4), 1887–1898. 10.3892/ijmm.2018.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyoo A. E.; Emmambux M. N. Amylose-lipid complex production and potential health benefits: A mini-review. Starch-Starke 2017, 69 (7–8), 1600203 10.1002/star.201600203. [DOI] [Google Scholar]
- Mariscal-Moreno R. M.; Figueroa-Cárdenas J. D.; Santiago-Ramos D.; Rayas-Duarte P. Amylose lipid complexes formation as an alternative to reduce amylopectin retrogradation and staling of stored tortillas. Int. J. Food Sci. Technol. 2019, 54 (5), 1651–1657. 10.1111/ijfs.14040. [DOI] [Google Scholar]
- Kim S. R. B.; Choi Y. G.; Kim J. Y.; Lim S. T. Improvement of water solubility and humidity stability of tapioca starch film by incorporating various gums. LWT--Food Sci. Technol. 2015, 64 (1), 475–482. 10.1016/j.lwt.2015.05.009. [DOI] [Google Scholar]
- Kang X.; Liu P.; Gao W.; Wu Z.; Yu B.; Wang R.; Cui B.; Qiu L.; Sun C. Preparation of starch-lipid complex by ultrasonication and its film forming capacity. Food Hydrocolloids 2020, 99, 105340 10.1016/j.foodhyd.2019.105340. [DOI] [Google Scholar]
- Tang M. C.; Copeland L. Analysis of complexes between lipids and wheat starch. Carbohydr. Polym. 2007, 67 (1), 80–85. 10.1016/j.carbpol.2006.04.016. [DOI] [Google Scholar]
- Singh N.; Kaur K.; Singh H.; Singh H. Effect of starch-lipids inclusion complex formation on functional properties of flour in tandoori roti. Food Chem. 2000, 69 (2), 129–133. 10.1016/S0308-8146(99)00229-0. [DOI] [Google Scholar]
- Ai Y.; Hasjim J.; Jane J.-L. Effects of lipids on enzymatic hydrolysis and physical properties of starch. Carbohydr. Polym. 2013, 92 (1), 120–127. 10.1016/j.carbpol.2012.08.092. [DOI] [PubMed] [Google Scholar]
- Liang Q.; Chen X.; Ren X.; Yang X.; Raza H.; Ma H. Effects of ultrasound-assisted enzymolysis on the physicochemical properties and structure of arrowhead-derived resistant starch. LWT 2021, 147, 111616 10.1016/j.lwt.2021.111616. [DOI] [Google Scholar]
- Kawai K.; Takato S.; Sasaki T.; Kajiwara K. Complex formation, thermal properties, and in-vitro digestibility of gelatinized potato starch–fatty acid mixtures. Food Hydrocolloids 2012, 27 (1), 228–234. 10.1016/j.foodhyd.2011.07.003. [DOI] [Google Scholar]
- Tufvesson F.; Wahlgren M.; Eliasson A. C. Formation of amylose-lipid complexes and effects of temperature treatment. Part 2. Fatty acids. Starch-Stärke 2003, 55 (3–4), 138–149. 10.1002/star.200390028. [DOI] [Google Scholar]
- Wang Y. S.; Liu W. H.; Zhang X.; Chen H. H. Preparation of VII-type normal cornstarch-lauric acid complexes with high yield and stability using a combination treatment of debranching and different complexation temperatures. Int. J. Biol. Macromol. 2020, 154, 456–465. 10.1016/j.ijbiomac.2020.03.142. [DOI] [PubMed] [Google Scholar]
- Meng S.; Ma Y.; Sun D.-W.; Wang L.; Liu T. Properties of starch-palmitic acid complexes prepared by high pressure homogenization. J. Cereal Sci. 2014, 59 (1), 25–32. 10.1016/j.jcs.2013.10.012. [DOI] [Google Scholar]
- Chao C.; Yu J.; Wang S.; Copeland L.; Wang S. Mechanisms underlying the formation of complexes between maize starch and lipids. J. Agric. Food Chem. 2018, 66 (1), 272–278. 10.1021/acs.jafc.7b05025. [DOI] [PubMed] [Google Scholar]
- Liu H.; Liang R.; Antoniou J.; Liu F.; Shoemaker C. F.; Li Y.; Zhong F. The effect of high moisture heat-acid treatment on the structure and digestion property of normal maize starch. Food Chem. 2014, 159, 222–229. 10.1016/j.foodchem.2014.02.162. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Zhou X.; Wang T.; He J.; Yue M.; Luo X.; Wang L.; Wang R.; Chen Z. Enzymatically modified waxy corn starch with amylosucrase: The effect of branch chain elongation on structural and physicochemical properties. Food Hydrocolloids 2017, 63, 518–524. 10.1016/j.foodhyd.2016.09.043. [DOI] [Google Scholar]
- Hill R. D.; Dronzek B. L. Scanning electron microscopy studies of wheat, potato and corn starch during gelatinization. Starch-Starke 1973, 25 (11), 367–372. 10.1002/star.19730251104. [DOI] [Google Scholar]
- Huang J.; Wei M.; Ren R.; Li H.; Liu S.; Yang D. Morphological changes of blocklets during the gelatinization process of tapioca starch. Carbohydr. Polym. 2017, 163, 324–329. 10.1016/j.carbpol.2017.01.083. [DOI] [PubMed] [Google Scholar]
- Hasjim J.; Lee S.-O.; Hendrich S.; Setiawan S.; Ai Y.; Jane J.-L. Characterization of a novel resistant-starch and its effects on postprandial plasma-glucose and insulin responses. Cereal Chem. 2010, 87 (4), 257–262. 10.1094/CCHEM-87-4-0257. [DOI] [Google Scholar]
- Wongprayoon S.; Tran T.; Gibert O.; Dubreucq E.; Piyachomkwan K.; Sriroth K. Pullulanase debranching of various starches upgrades the crystalline structure and thermostability of starch-lauric acid complexes. Starch/Stärke 2018, 70 (7–8), 1700351 10.1002/star.201700351. [DOI] [Google Scholar]
- Zhang C.; Qiu M.; Wang T.; Luo L.; Xu W.; Wu J.; Zhao F.; Liu K.; Zhang Y.; Wang X. Preparation, structure characterization, and specific gut microbiota properties related to anti-hyperlipidemic action of type 3 resistant starch from Canna edulis. Food Chem. 2021, 351, 129340 10.1016/j.foodchem.2021.129340. [DOI] [PubMed] [Google Scholar]
- Cheng W.; Luo Z.; Li L.; Fu X. Preparation and characterization of debranched-starch/phosphatidylcholine inclusion complexes. J. Agric. Food Chem. 2015, 63 (2), 634–641. 10.1021/jf504133c. [DOI] [PubMed] [Google Scholar]
- Wang S.; Wu T.; Cui W.; Liu M.; Wu Y.; Zhao C.; et al. Structure and in vitro digestibility on complex of corn starch with soy isoflavone. Food Sci. Nutr. 2020, 8 (11), 6061–6068. 10.1002/fsn3.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindaraju I.; Zhuo G. Y.; Chakraborty I.; Melanthota S. K.; Mal S. S.; Sarmah B.; Baruah V. J.; Mahato K. K.; Mazumder N. Investigation of structural and physico-chemical properties of rice starch with varied amylose content: A combined microscopy, spectroscopy, and thermal study. Food Hydrocolloids 2022, 122, 107093 10.1016/j.foodhyd.2021.107093. [DOI] [Google Scholar]
- Gao S.; Liu H.; Sun L.; Cao J.; Yang J.; Lu M.; Wang M. Rheological, thermal and in vitro digestibility properties on complex of plasma modified Tartary buckwheat starches with quercetin. Food Hydrocolloids 2021, 110, 106209 10.1016/j.foodhyd.2020.106209. [DOI] [Google Scholar]
- Rokhade A. P.; Patil S. A.; Aminabhavi T. M. Synthesis and characterization of semi-interpenetrating polymer network microspheres of acrylamide grafted dextran and chitosan for controlled release of acyclovir. Carbohydr. Polym. 2007, 67 (4), 605–613. 10.1016/j.carbpol.2006.07.001. [DOI] [Google Scholar]
- Marinopoulou A.; Papastergiadis E.; Raphaelides S. N. An investigation into the structure, morphology and thermal properties of amylomaize starch-fatty acid complexes prepared at different temperatures. Food Res. Int. 2016, 90, 111–120. 10.1016/j.foodres.2016.10.035. [DOI] [PubMed] [Google Scholar]
- Luo F.; Huang Q.; Fu X.; Zhang L.; Yu S. Preparation and characterization of crosslinked waxy potato starch. Food Chem. 2009, 115 (2), 563–568. 10.1016/j.foodchem.2008.12.052. [DOI] [Google Scholar]
- Zhang B.; Huang Q.; Luo F. X.; Fu X. Structural characterizations and digestibility of debranched high-amylose maize starch complexed with lauric acid. Food Hydrocolloids 2012, 28 (1), 174–181. 10.1016/j.foodhyd.2011.12.020. [DOI] [Google Scholar]
- Ferraz C. A.; Fontes R. L.; Fontes-Sant’Ana G. C.; Calado V.; López E. O.; Rocha-Leão M. H. Extraction, Modification, and Chemical, Thermal and Morphological Characterization of Starch from the Agro-Industrial Residue of Mango (Mangifera indica L) var. Ubá. Starch-Stärke 2019, 71 (1–2), 1800023 10.1002/star.201800023. [DOI] [Google Scholar]
- Zhang B.; Huang Q.; Luo F. X.; Fu X.; Jiang H.; Jane J. L. Effects of octenylsuccinylation on the structure and properties of high-amylose maize starch. Carbohydr. Polym. 2011, 84 (4), 1276–1281. 10.1016/j.carbpol.2011.01.020. [DOI] [Google Scholar]
- Dong D.; Cui B. Fabrication, characterization and emulsifying properties of potato starch/soy protein complexes in acidic conditions. Food Hydrocolloids 2021, 115, 106600 10.1016/j.foodhyd.2021.106600. [DOI] [Google Scholar]
- Putseys J. A.; Lamberts L.; Delcour A. J. Amylose-inclusion complexes: Formation, identity and physico-chemical properties. J. Cereal Sci. 2010, 51 (3), 238–247. 10.1016/j.jcs.2010.01.011. [DOI] [Google Scholar]
- Arijaje E. O.; Wang Y. J. Effects of enzymatic modifications and botanical source on starch–stearic acid complex formation. Starch-Stärke 2016, 68 (7–8), 700–708. 10.1002/star.201500249. [DOI] [Google Scholar]
- Gutiérrez T. J.; Tovar J. Update of the concept of type 5 resistant starch (RS-5): Self-assembled starch V-type complexes. Trends Food Sci.Technol. 2021, 109, 711–724. 10.1016/j.tifs.2021.01.078. [DOI] [Google Scholar]
- Reddy C. K.; Choi S. M.; Lee D.-J.; Lim S.-T. Complex formation between starch and stearic acid: Effect of enzymatic debranching for starch. Food Chem. 2018, 244, 136–142. 10.1016/j.foodchem.2017.10.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





