Abstract

Titanium dioxide (TiO2) is one of the most widely used photocatalysts due to its physical and chemical properties. In this study, hydrogen energy production using TiO2- and titanate-based photocatalysts is discussed along with the pros and cons. The mechanism of the photocatalysis has been elaborated to pinpoint the photocatalyst for better performance. The chief characteristics and limitations of the TiO2 photocatalysts have been assessed. Further, TiO2-based photocatalysts modified with a transition metal, transition metal oxide, noble metal, graphitic carbon nitride, graphene, etc. have been reviewed. This study will provide a basic understanding to beginners and detailed knowledge to experts in the field to optimize the TiO2-based photocatalysts for hydrogen production.
1. Introduction
Hydrogen is considered an ideal fuel and is highly preferred due to properties such as its life cycle, renewability, environmental friendliness, and cost-effectiveness. Hydrogen can be produced from both renewable and nonrenewable energy resources. There are two main sources of renewable energy: solar energy and wind energy. These two sources are very suitable for the production of clean hydrogen. The main problem associated with these renewable resources is that only 5% of hydrogen is derived from them and they also involve high cost. While about 95% of hydrogen can be produced from nonrenewable resources, scientists are focusing on the methods through which they can produce cost-effective hydrogen. Then, the concept of photovoltaic water electrolysis was developed in which semiconductor materials that have small band gaps are used. This technology produced hydrogen at low costs. Alternatively, for hydrogen production, photocatalytic water splitting using TiO2 as a photocatalyst through solar energy is very promising. This way of producing hydrogen was very clean, low-cost, and environmentally friendly.1−5
Catalytic hydrogen production using semiconductor materials as catalysts has attracted much attention because of the maximum utilization of solar energy. The apparatus for photocatalytic water splitting is shown in Figure 1. Photocatalytic reactions occur when semiconductor materials absorb photons with energy hυ equal to or greater than the band gap of the semiconductor. By absorbing this energy, electrons promote from the valence band to the conduction band and create an electron–hole pair. These photogenerated electrons promoted to conduction band and reduce H+ into H2, and holes on the semiconductor surface decompose H2O into O2 and H+.6 The behavior of these photogenerated carriers can have a significant influence on the performance of a semiconductor photocatalyst. Understanding and controlling the behavior of these carriers can lead to the evolution of efficient photocatalysts having a wide range of applications in environmental and energy-related fields. For example, the photogenerated charge carrier recombination can limit the photocatalyst efficiency. Similarly, controlling the transfer and migration of generated carriers can increase the efficiency of photocatalysts, such as the spatial separation of carriers, elongating their lifetime and thus increasing the photocatalytic performance. This behavior is explained in the following sections.
Figure 1.

Apparatus for photocatalytic water splitting. Reprinted with permission from ref (6). Copyright 2005 American Chemical Society.
The overall mechanism of water splitting and hydrogen production is explained in eqs 1–4.6−8
| 1 |
| 2 |
| 3 |
The overall reaction can be summarized as
| 4 |
Titanium(IV) oxide naturally exists in two phases, namely, rutile and anatase, with a tetrahedral shape and simple synthesis.5 Meanwhile, the other third phase is called brookite, which can be synthesized in laboratories and is rhombic in shape. The photocatalytic activity of TiO2 such as anatase and rutile is influenced by crystal structure, surface area, surface hydroxyl density, porosity, and size4,5,9 because all these factors affect the production of electrons and holes. These two forms have mostly been used in photocatalytic applications. Anatase is one of the most active phases. The enhancement of photocatalytic activity is related to the Fermi level of about 0.1 eV in the anatase phase, which was higher than that in the rutile phase.9,10 TiO2 is available commercially called Degussa P25, which is used in photo catalytic studies. The hydrolysis of TiCl4 in hot flame produces TiO2 with a surface area of about 50 m2/g, and it contains a 4:1 ratio of anatase and rutile phases.11
1.1. Catalytic Mechanism
In this process, induced charge carriers cause the oxidation of electrons (such as donor species) in the valence band (VB) and the reduction of electrons (such as acceptor species) in the conduction band (CB). In the photomodernizing reaction, organic substrates behaved as electron donors while H+ was used as an electron acceptor. In this reaction, radiation energy is converted into chemical energy because in the presence of photocatalyst it absorbs solar energy very efficiently. In recent years, many different metal-oxide-based semiconductors as catalysts have been reported.12 Some metal oxides such as SrTiO3, TiO2, BaTi4O9, ZrO2, and CeO2 have a reasonable ability to split water H2 and O2 under visible and ultraviolet light radiation.13,14 From all of these, TiO2 is favorable due to having a band gap of 3.2 eV in the anatase phase, high stability in the form of an aqueous solution under UV radiation, high reducing and oxidizing power, nontoxicity, and cost effectiveness.3,5
1.2. Limitations
There are some limitations while using TiO2, and the main problem facing TiO2 is its fast and undesirable electron–hole recombination reaction. In order to avoid this problem, sacrificial reagents can be used along with TiO2, which is also suitable to increase photo-efficiency. The main task of these sacrificial reagents is to keep separate the photoexcited electrons and holes from the recombination process or reversible process. Compounds such as methanol, EDTA (an ethylene diamine tetra acetic derivative), Na2SO4, ethanol, and Na2S and ions such as I–, IO–3, CN–, and Fe3+ are used as sacrificial reagents.6 Simply, we can say that the photocatalytic hydrogen efficiency obtained by simple TiO2 is low due to the following reasons: rapid recombination of photogenerated charge carriers, fast reverse reaction between H2 and O2, and large production of hydrogen, which becomes over potential.
Furthermore, in a simple aqueous system, pure TiO2 cannot split into H2 and O2. Therefore, to reduce these problems, many efforts have been made in recent years, such as the addition of sacrificial reagents, metal cation doping, carbon and nitrogen doping, and deposition of noble metals.15,16
Therefore, diverse techniques have been employed for the modification of TiO2 nanoparticles in order to attain the maximum possible hydrogen production rate. These methods involve the doping of transition metals, incorporation with other metal oxides, and surface modifications. Doping of transition metals in TiO2 surpasses the band gap of TiO2 by creating the quasi-static energy levels of dopants between the conduction and valence bands and also decreases the recombination rate of charge carriers.17 The reduction in the band gap of the TiO2 photocatalyst permits the material to harvest more photons during the reaction and thus produces more charge carriers. However, the incorporation of a metal oxide with TiO2 enhances its activity by transferring the photogenerated electrons to the conduction band in a lower position on the semiconductor while transferring holes to the less anodic valence band under the illumination of both semiconductors. This separation of charge carriers implies the reduction in the recombination rate and hence increases the photocatalytic performance.18 Therefore, it is claimed that TiO2 -based photocatalysts enhance the photocatalytic activity due to various factors such as an enhanced charge separation rate, a lower recombination rate, and the presence of oxygen vacancies.
As an example, a study affirmed the efficient hydrogen production rate of 23.5 mmol/g·h having an apparent quantum yield of 19% using the Ag-doped TiO2 photocatalyst. The observed efficient activity was attributed to the appearance of oxygen vacancies that enhanced the charge separation rate of (TiO2) responsible for the higher hydrogen production rate.19 Furthermore, a Ce3O4@C/TiO2 nanocomposite was fabricated by utilizing an incipient wet impregnation method for the photocatalytic production of hydrogen. The fabricated TiO2-based photocatalyst exhibited a hydrogen production rate of 11 400 μmol/g·h.20 These studies claimed the enhancement of the photocatalytic activity of TiO2 after its modification with different materials.
However, more fabricated modified TiO2-based photocatalysts are described in the proceeding sections.
2. Transition-Metal-Doped TiO2 Photocatalysts
2.1. Platinum (Pt)/TiO2 Photocatalyst
For the preparation of TiO2 nanosheets, a hydrothermal method was used. TiO2 nanosheets with exposed (001) faces were prepared in a mixed solution of Ti (OC4 H9)4–HF–H2O. Then, the deposition of Pt nanoparticles on the TiO2 nanosheets was carried out by a photochemical reduction method under xenon lamp radiation, and a Pt/TiO2 nanophotocatalyst is shown in Figure 2. The prepared sample was characterized by different characterization techniques such as scanning electron microscopy (SEM), photoluminescence spectroscopy (PS), X-ray diffraction (XRD), etc. Using coumarin as a probe material in photoluminescence (PL) spectroscopy, radicals of hydroxyl (OH•) were detected on the surface of the TiO2 nanosheets. However, the excited electrons, after migrating toward the conduction band of Pt nanoparticles, react with H+ ions to form the H2 molecule. In addition, the rates of photocatalytic H2 production were studied and discussed when Pt was loaded on the TiO2 nanosheets in an ethanol aqueous solution. It was shown by results that the loaded Pt on the TiO2 nanosheets enhanced the photocatalytic hydrogen production rates, and even 2 wt % deposited Pt showed the highest catalytic activity. Consequently, it was shown that, as compared to pure TiO2, fluorinated TiO2 nanosheets exhibited high photocatalytic activity. These Pt/TiO2 nanosheets have attracted too much interest in different fields such as solar cells, sensors, biomedical engineering, photonic devices, and catalysis.21,22
Figure 2.
Mechanism of photocatalytic oxidation under aerobic and anaerobic conditions. Reprinted with permission from ref (22). Copyright 2022 American Chemical Society.
The drawback of using Pt is that it is a rare and very expensive metal, so it has to be replaced, for which numerous efforts have been made.15 Moreover, when methanol was decomposed during the photocatalytic process on the Pt/TiO2 catalyst, carbon monoxide (CO), hydrogen, methane, and carbon dioxide were obtained. A concentration of about 2.7 vol % CO in hydrogen was observed. The concentration of CO in H2 was the main problem because a very small concentration of CO poisons the catalyst. In order to produce high amounts of and ultrapure hydrogen and for the reduction of the concentration of CO in H2, scientists are focusing on some other catalysts that could be more suitable and cost-effective as compared to Pt/TiO2.6,23
2.2. Gold (Au) /TiO2 Photocatalyst
For the production of H2, gold as a dopant over a TiO2 semiconductor can be selected as a catalyst in photocatalysis. The rate of H2 production is higher using a Au/TiO2 catalyst, as shown in Figure 3. H2 was produced during the photocatalytic decomposition of methanol on the Au/TiO2 catalyst using an ultralow concentration of CO. It was observed that when the size of the gold particles was decreased from 10 nm to smaller than 3 nm, the rate of H2 production was significantly increased. Additionally, it was perceived that the absorption of CO decreased when the size of the Au particles decreased. The suggested reason for reducing the concentration of CO was that when intermediate formic acid species form methanol through decomposition, mainly CO is produced. Because the size of the gold particles was reduced, the decomposition of formic acid (HCOOH) was stopped, which overturned the CO, and only H2 and CO2 were produced as byproducts.6,23
Figure 3.
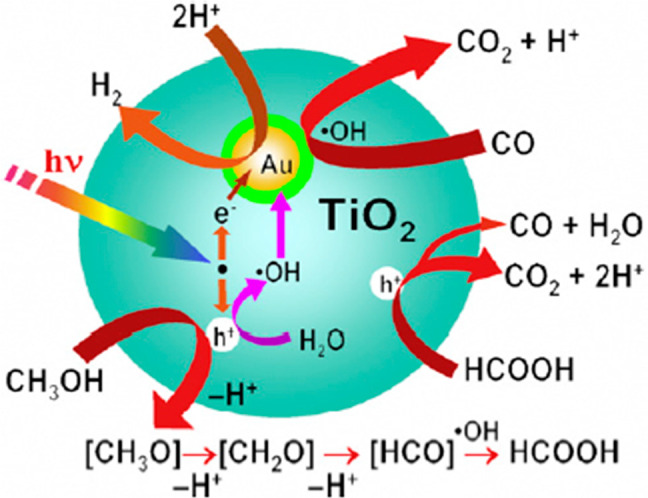
Schematic diagram of photocatalytic reaction on a Au/TiO2 catalyst. Reprinted with permission from ref (23). Copyright 2008 Elsevier.
Bamwenda and co-workers made an experiment in order to compare the catalytic activity of Au/TiO2 and Pt/TiO2 catalysts for H2 production. TiO2 powder in aqueous suspensions of H2PtCl6·6H2O or HAuCl4·4H2O was used for the deposition of Pt and Au particles through a chemical deposition method. The byproducts obtained during this reaction were acetaldehyde, carbon dioxide, hydrogen, and formic acid. It was revealed the performance activity of the Pt sample was 30% higher than that of the Au sample. This is because of Au, which is highly dependent on preparation method as compared to Pt. When both Au and Pt samples was calcined at 573 K in air, they showed high production of hydrogen at this temperature. However, it was observed that the production of hydrogen become lower when calcination temperature was increased from 573 K temperature.24
2.3. Copper (Cu)/TiO2 Photocatalyst
The photocatalyst of a Cu-doped TiO2 semiconductor material was used for the production of H2 under visible light. Complex precipitation and wet impregnation methods were used to prepare the Cu/TiO2 photocatalyst. The schematic diagram of the Cu/TiO2 photocatalyst is shown in Figure 4. The copper nitrate trihydrate was used as a starting material. The content of the copper dopant on the TiO2 semiconductor varied from 2% to 15%. The sample of the synthesized Cu/TiO2 photocatalyst was characterized by different characterization techniques, such as XRD, thermogravimetric analysis (TGA), SEM, and Fourier transform infrared spectroscopy (FITR). As mentioned above, the Cu/TiO2 photocatalyst was prepared by two synthesis methods under different concentrations of dopant and different calcination temperatures and showed different trends. The sample with 10% copper loading on TiO2 and a calcination temperature of 300 °C that was prepared by the complex precipitation method showed better photocatalytic activity than the other, which was prepared by the wet impregnation method. The size of the copper dopant particles varied from 20 to 110 nm. Its rate of H2 production was much higher than that of TiO2.25
Figure 4.

Schematic diagram of transfer and separation of charges of the Cu/TiO2 photocatalyst. Reprinted with permission from ref (25). Copyright 2009 Elsevier.
2.4. Iron (Fe)- and Chromium (Cr)-Doped TiO2 Photocatalyst
Transition metals like Cr, V, Fe, Cu, Ni, and Mn are mainly used as dopants of titania in order to enhance the photoelectrochemical and optical properties of TiO2.26 As compared to other transition metals, Mn, Cu, and Fe can be used for trapping both charge carriers, namely, holes and electrons. While on the other hand, Ni and Cr can trap only one type of charge carrier.1,27 Two methods were used to synthesize the Fe- and Cr-doped TiO2 photocatalysts, namely, sol gel and radio frequency magnetron sputtering. It was observed that the Fe-doped TiO2 catalyst has a high rate of hydrogen production (such as 15.5 μmol/h) as compared to the Cr-doped TiO2 catalyst (only 5.3 μmol/h). This is because of the trapping ability of Fe, which can trap both types of charge carriers and also avoid the recombination process. However, Cr only traps one type of carrier and also causes the recombination process, which lowers its ability to produce more hydrogen as compared to Fe.28
3. Transition-Metal-Oxide-Doped TiO2 Photocatalysts
3.1. Indium Tin Oxide/Cr-Doped TiO2 Photocatalyst
To enhance the hydrogen production rate of a Cr-doped TiO2 catalyst, a single bilayer of indium tin oxide (ITO) is used with a Cr-doped TiO2 catalyst. Negligible photocurrent was observed due to increased recombination of charge carriers. To reduce this recombination process, multiple bilayers of indium tin oxide were deposited over TiO2. Then, it was observed that the photocurrent increased as the number of bilayers increased. The rate of hydrogen production obtained was about 24.5 μmol/h, which was two times greater than that of the pure titania (12.5 μmol/h).29
3.2. Cadmium Selenide (CdS)/TiO2 and Cobalt Oxide (CoO)/CdS/TiO2 Photocatalysts
CdS/TiO2 bulk nanocomposite material can be used as a photocatalyst. It was synthesized by using two methods, namely, sol gel and precipitation methods. A high H2 production rate was observed for the CdS/TiO2 photocatalyst, as shown in Figure 5. However,, CdS has some limitations, such as being unstable against photocorrosion. To overcome this problem, sacrificial agents are used in solutions such as S2– and SO32–. In the presence of these sacrificial agents, CdS showed high H2 production activity under light irradiation.30
Figure 5.

(a–c). Schematic diagram of the CdS/TiO2 photocatalyst. Panels a and b reprinted with permission from ref (30). Copyright 2007 Elsevier. Panel c reprinted with permission from ref (31). Copyright 2017 Elsevier.
Recently, it was found that noble metal cobalt oxide (CoO) has been used as an electrocatalyst for proton reduction, and also it can be used as a photocatalyst for H2 production. CoO over TiO2/CdS was synthesized using a solvothermal method, as shown in Figure 6. These samples were characterized by TEM, XRD, and XPS in an aqueous solution (having sodium sulfite and sodium sulfide) as hole scavengers under visible light (λ > 400 nm). For a concentration of 2.1 wt % CoO, the hydrogen production rate was 660 μmol/g·h, which was seven times greater than that of the simple TiO2/CdS photocatalyst under the same conditions.32
Figure 6.

Schematic diagram of the CoO-loaded TiO2/CdS photocatalyst. Reprinted with permission from ref (32). Copyright 2014 Elsevier.
3.3. Fe2O3/TiO2 Photocatalyst
Metal oxide nanomaterials play a vital role in photocatalytic reactions to excite the electron to the CB and create a hole in the VB for the evolution of H2. These materials are interesting due to having high stability, low toxicity, and low-cost materials. A metal oxide such as TiO2 has a large band gap, which is suitable for photocatalytic fuel production but only absorbs UV radiation. For this reason, only 5% of energy is shown in the solar energy spectrum. Metal oxide nanomaterials such as Fe2O3 have suitable band gaps and are ineffective during photocatalytic reactions. A metal–organic framework (MOF) template is used to prepare the titania-based nanocomposite materials, as shown in Figure 7. Iron (Fe)-based MOFs are coated with titanium dioxide. Then, it was calcined to produce nanoparticles of composite Fe2O3/TiO2. This enables the composite to produce hydrogen when exposed to visible light radiation.33
Figure 7.

MOF template synthesis of Fe2O3/TiO2. Reprinted with permission from ref (33). Copyright 2012 John Wiley and Sons.
3.4. Ni(OH)2/TiO2 Photocatalyst
A Ni(OH)2 cluster over TiO2 was used to synthesize a nanocomposite catalyst (Ni(OH)2/TiO2) via the precipitation method. In this process, Ni(NO3)2 was used as the precursor while the supportive material was Degussa P25 TiO2, as shown in Figure 8. It was observed that the rate of H2 production increased when a cluster of Ni(OH)2 was used within an aqueous solution of methanol. It was revealed that applying a Ni(OH)2 cluster on TiO2 increased the photocatalytic activity. Moreover, when 0.23 mol % Ni(OH)2 cluster was used, the rate of H2 production was increased by 3056 μmol/g·h. It was observed that the prepared sample had a 223× greater quantum efficiency as compared to that of pure TiO2, which was obtained to be 12.4%. The main function of Ni0 is to separate charges and also for the reduction of water. The potential of Ni2+/Ni is more negative as compared to the H+/H2 potential, and it was also smaller than the CB of TiO2.34
Figure 8.

Schematic diagram of the transfer and separation of charges of the Ni(OH)2 cluster over modified TiO2. Reprinted with permission from ref (34). Copyright 2011 American Chemical Society.
4. Noble-Metal-Modified TiO2 Photocatalyst
TiO2 photocatalysts based on noble metals, such as Au, a Au alloy, and Pt, and Ag, can be synthesized by different methods, such as spray pyrolysis or deposition. When noble metals were deposited on TiO2, a good photocatalytic activity was observed. Moreover, by feeding methanol and water vapor on them, different byproducts were obtained, such as formaldehyde, carbon dioxide, methane, formic acid, dimethyl, methyl formate, ether, and acetaldehyde. These noble-metal-modified photocatalysts showed the best photocatalytic performance and produced a high H2 rate and low emission of CO. It was observed that Pt was best for a cocatalyst for the evolution of H2 as compared to other noble metals.12
5. Graphitic Carbon Nitride (g-C3N4)/TiO2 Photocatalyst
Another polymer semiconductor, namely, g-C3N4 over TiO2, can be used as a photocatalyst in photocatalytic water splitting under visible light (in the presence of sacrificial reagents), as shown in Figure 9. The benefit of using this polymer semiconductor material was its optical band gap, which is 2.7 eV. Moreover, due to strong covalent bonds between nitride and carbon atoms, it shows high stability in water, acid, and base solutions under light irradiation. However,, the catalytic activity of this composite TiO2-g-C3N4 is reasonably acceptable, meaning very low.35,36
Figure 9.

Schematic diagram of the transfer and separation of charges of composite TiO2 and g-C3N4. Reprinted with permission from ref (35). Copyright 2011 Elsevier.
The composite g-C3N4/TiO2 catalyst was prepared and characterized by XRD and FTIR. It was shown that this composite material consisted of peaks of g-C3N4 and TiO2. The FTIR spectrum showed that this composite material has a stronger absorbance band intensity as compared to C3N4 alone. The hydrogen production rate under visible light was remarkably increased by coupling TiO2 with g-C3N4.35
5.1. g-C3 N4/SrTiO3-Based Photocatalyst
A g-C3N4-loaded SrTiO3 photocatalyst was synthesized by the decomposition of urea at 400 °C. g-C3N4 is a molecular photocatalyst in nature and has many advantages, such as a small band gap, facile absorption of visible light, and a high negative conduction band of about −1.12 eV, which causes facile transfer of photoelectrons to other components and is prepared by a simple and cheap method. In contrast, SrTiO3 has a conduction band level about −0.5 eV and a forbidden band gap about 3.2 eV; this property provides a close interfacial area to combine the g-C3N4 and SrTiO3. The rate of H2 production was observed to be nearly 440 μmol/g·h, which was larger than that of the simple anion-doped SrTiO3 photocatalyst.37
6. Graphene-Based GO/TiO2 Photocatalyst
Many researchers have expressed interest in rational designs of graphene for the performance of photocatalysts. A graphene-based photocatalyst converts solar energy into chemical energy in order to increase the H2 production, as shown in Figure 10. Graphene has different properties such as sp2 hybridization; due to this, it shows high thermal conductivity, which is about 5000 W/m·k. Graphene also offers excellent mobility of about 200·000 cm2/v· at room temperature, and its surface area is about 2600 m2·g.38 For these properties, graphene becomes more interesting as a photocatalyst because it has the ability to increase the transfer and separation of charge carriers. It enhances the efficiency in the following terms: reduces the recombination of electrons and holes, tunes the band gap of a semiconductor material, provides support to adsorption and catalytic sites, and acts as cocatalyst for producing hydrogen.39,40
Figure 10.
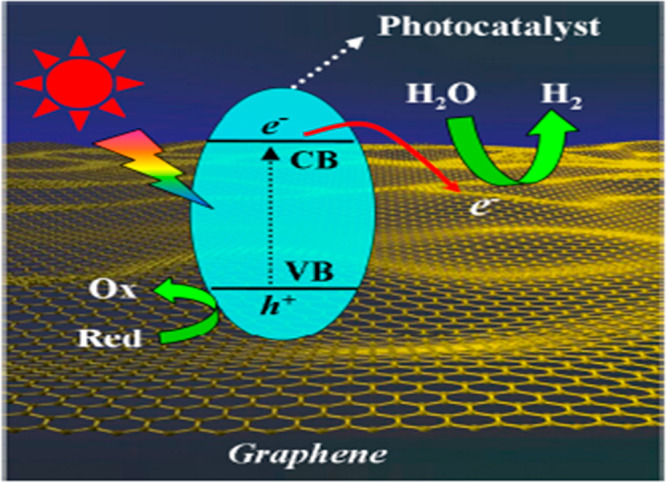
Proposed mechanism of graphene photocatalyst to enhance the photocatalytic performance. Reprinted with permission from ref (39). Copyright 2013 American Chemical Society.
To increase the surface area of the photocatalyst, graphene provides two dimensional (2D) support and enhances its electrical and redox properties.41 The main problem was observed in using graphene when photocatalyst nanoparticles were applied on graphene sheets, and it was shown that a very small number of particles have direct contact with the graphene sheet, which causes a delay in the transfer of electrons in the photocatalytic reaction and creates weak interaction. To provide the larger specific surface area and strong interaction, the new structure of graphene was found in the form of nanosized graphene oxide with titania (GO/TiO2), as shown in Figure 11. This new developed structure of graphene has a self-assembled core and shell structure that shows a high rate of production of H2.39,42
Figure 11.
Schematic diagram of the preparation procedure of GO/TiO2 and TiO2/GO. Reprinted with permission from ref (39). Copyright 2013 American Chemical Society.
6.1. Reduced Graphene Oxide (RGO)/TiO2 Photocatalyst
For the production of H2, RGO loaded over TiO2 can also be used as a photocatalyst in an alcohol solution under UV radiation, as shown in Figure 12. It was synthesized through the hydrothermal method, which showed the best photocatalytic activity and best performance. RGO/TiO2 contents in a ratio of 1:0.2 were used. The titanium dioxide (P25)–RGO was very stable and could be used as a recycle and catalyst for the evolution of H2. It was observed that when nanoparticles of P25 about 20–30 nm in size were loaded on RGO sheet, there was strong interaction between RGO and TiO2, which suppresses the recombination process and enhances the photocatalytic performance.43
Figure 12.
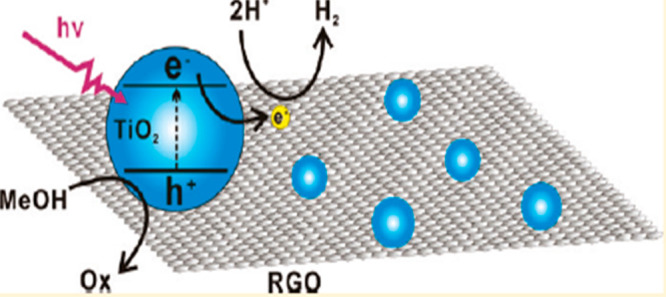
Schematic diagram of the RGO/TiO2 photocatalyst. Reprinted with permission from ref (43). Copyright 2011 American Chemical Society.
There are few more recent studies reported that describe the peculiar behavior of modified TiO2 photocatalysts for the enhancement of hydrogen production rate. A study claimed the production of red phosphorus-modified TiO2 hollow spheres achieved the highest hydrogen production rate of about 215.5 μmol/g·h. It was revealed that the heterostructure incorporated photoinduced charge separation that enhanced the hydrogen production activity.44 Moreover, Bi/CdS/TiO2 nanocomposites were prepared via the successive ionic layer adsorption and reaction method. This nanocomposite manifested 673.81 μmol/h·cm2.45
Another study reported the preparation of a NiSe2 nanoparticle as a cocatalyst over TiO2 using a supercritical fluid process. The fabricated photocatalyst was investigated for hydrogen production and was revealed to possess a 219.2 mmol/g·h hydrogen production rate.46 Furthermore, a 2584.9 μmol/g· hydrogen production rate was revealed using the O-ZIS/TiO2–x heterojunction.47
7. Conclusion
Hydrogen energy has become an emerging renewable energy resource because it is environmentally friendly, cost-effective, and has a stable life cycle. The most promising technique for hydrogen production is photocatalysis. The TiO2 photocatalyst is the most widely used in photocatalytic water splitting and hydrogen production. The noncommercial boundaries of the process are due to the catalyst limitation and are analyzed briefly in this Review. The process can be improved by doping and modifying the catalyst with transition metals, noble metals, graphite, and graphene and has been reviewed deliberately. This Review will provide insight into the TiO2 and TiO2-based photocatalysts to choose the best and optimized photocatalyst for water splitting and hydrogen production. There are various factors that must be focused on and considered to achieve the maximum hydrogen production activity, such as effective preparation methods, sacrificial reagents, photocatalyst stability and most importantly, hydrogen transport and storage as a future perspective.
However, it can be summarized that photocatalytic generation of hydrogen utilizing a TiO2 based photocatalyst is an economical and effective way to acquire sustainable energy. Doping of various nonmetal and metal materials can increase the performance of the photocatalyst by carefully tuning the band gap of the nanomaterials. Moreover, an optimum amount of the photocatalyst should be employed to achieve maximum activity because a high concentration of photocatalyst can reduce the efficiency of the photocatalyst.
Acknowledgments
The authors express their appreciation to the Deanship of Scientific Research at King Khalid University, Saudi Arabia, for funding this work through a research group program under Grant RGP-2/370/44.
The authors declare no competing financial interest.
References
- Ni M.; et al. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renewable and Sustainable Energy Reviews 2007, 11 (3), 401–425. 10.1016/j.rser.2005.01.009. [DOI] [Google Scholar]
- Moustafa H. M.; et al. Co–TiO2 supported on reduced graphene oxide as a highly active and stable photocatalyst for hydrogen generation. Fuel 2023, 338, 127232. 10.1016/j.fuel.2022.127232. [DOI] [Google Scholar]
- Nabi G.; et al. Green synthesis of TiO2 nanoparticles using lemon peel extract: their optical and photocatalytic properties. Int. J. Environ. Anal. Chem. 2022, 102, 434–442. 10.1080/03067319.2020.1722816. [DOI] [Google Scholar]
- Tahir M. B.; et al. Photocatalytic performance of hybrid WO3/TiO2 nanomaterials for the degradation of methylene blue under visible light irradiation. Int. J. Environ. Anal. Chem. 2021, 101, 1448–1460. 10.1080/03067319.2019.1685093. [DOI] [Google Scholar]
- Tahir M. B.; et al. Development of Sol Gel Derived Nanocrystalline TiO 2 Thin Films via Indigenous Spin Coating Method. Journal of Inorganic and Organometallic Polymers and Materials 2018, 28 (1), 1–8. 10.1007/s10904-017-0690-x. [DOI] [Google Scholar]
- Galińska A.; Walendziewski J. Photocatalytic water splitting over Pt– TiO2 in the presence of sacrificial reagents. Energy Fuels 2005, 19 (3), 1143–1147. 10.1021/ef0400619. [DOI] [Google Scholar]
- Wafi A.; et al. Coumarin-based quantification of hydroxyl radicals and other reactive species generated on excited nitrogen-doped TiO2. J. Photochem. Photobiol., A 2021, 404, 112913. 10.1016/j.jphotochem.2020.112913. [DOI] [Google Scholar]
- Eidsvåg H.; et al. TiO2 as a photocatalyst for water splitting—An experimental and theoretical review. Molecules 2021, 26 (6), 1687. 10.3390/molecules26061687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafique M.; et al. Investigation of Photocatalytic and Seed Germination Effects of TiO 2 Nanoparticles Synthesized by Melia azedarach L. Leaf Extract. Journal of Inorganic and Organometallic Polymers and Materials 2019, 29 (6), 2133–2144. 10.1007/s10904-019-01173-5. [DOI] [Google Scholar]
- Tahir M. B.; et al. Optical, microstructural and electrical studies on sol gel derived TiO2 thin films. Indian J. Pure Appl. Phys. 2017, 55 (01), 81–85. 10.56042/ijpap.v55i01.12169. [DOI] [Google Scholar]
- Wold A. Photocatalytic properties of titanium dioxide (TiO2). Chem. Mater. 1993, 5 (3), 280–283. 10.1021/cm00027a008. [DOI] [Google Scholar]
- Chiarello G. L.; Aguirre M. H.; Selli E. Hydrogen production by photocatalytic steam reforming of methanol on noble metal-modified TiO2. J. Catal. 2010, 273 (2), 182–190. 10.1016/j.jcat.2010.05.012. [DOI] [Google Scholar]
- López-Vásquez A.; Delgado-Niño P.; Salas-Siado D. Photocatalytic hydrogen production by strontium titanate-based perovskite doped europium (Sr 0.97 Eu 0.02 Zr 0.1 Ti 0.9 O 3). Environmental Science and Pollution Research 2019, 26, 4202–4214. 10.1007/s11356-018-3116-6. [DOI] [PubMed] [Google Scholar]
- Karthik K.; et al. Barium titanate nanostructures for photocatalytic hydrogen generation and photodegradation of chemical pollutants. Journal of Materials Science: Materials in Electronics 2019, 30, 20646–20653. 10.1007/s10854-019-02430-6. [DOI] [Google Scholar]
- Jin Z.; et al. 5.1% Apparent quantum efficiency for stable hydrogen generation over eosin-sensitized CuO/TiO2 photocatalyst under visible light irradiation. Catal. Commun. 2007, 8 (8), 1267–1273. 10.1016/j.catcom.2006.11.019. [DOI] [Google Scholar]
- Suhag M. H.; et al. Application of Rh/TiO2 Nanotube Array in Photocatalytic Hydrogen Production from Formic Acid Solution. Journal of Composites Science 2022, 6 (11), 327. 10.3390/jcs6110327. [DOI] [Google Scholar]
- Singh P.; et al. Review on various strategies for enhancing photocatalytic activity of graphene based nanocomposites for water purification. Arabian Journal of Chemistry 2020, 13 (1), 3498–3520. 10.1016/j.arabjc.2018.12.001. [DOI] [Google Scholar]
- López U.; et al. Synthesis and Characterization of ZnO-ZrO2 Nanocomposites for Photocatalytic Degradation and Mineralization of Phenol. J. Nanomater. 2019, 1015876. 10.1155/2019/1015876. [DOI] [Google Scholar]
- Gogoi D.; et al. Ag-doped TiO2 photocatalysts with effective charge transfer for highly efficient hydrogen production through water splitting. Int. J. Hydrogen Energy 2020, 45 (4), 2729–2744. 10.1016/j.ijhydene.2019.11.127. [DOI] [Google Scholar]
- El-Bery H. M.; Abdelhamid H. N. Photocatalytic hydrogen generation via water splitting using ZIF-67 derived Co3O4@ C/TiO2. Journal of Environmental Chemical Engineering 2021, 9 (4), 105702. 10.1016/j.jece.2021.105702. [DOI] [Google Scholar]
- Yu J.; Qi L.; Jaroniec M. Hydrogen production by photocatalytic water splitting over Pt/TiO2 nanosheets with exposed (001) facets. J. Phys. Chem. C 2010, 114 (30), 13118–13125. 10.1021/jp104488b. [DOI] [Google Scholar]
- Chiarello G. L.; Bernareggi M.; Selli E. Redox Dynamics of Pt and Cu Nanoparticles on TiO2 during the Photocatalytic Oxidation of Methanol under Aerobic and Anaerobic Conditions Studied by In Situ Modulated Excitation X-ray Absorption Spectroscopy. ACS Catal. 2022, 12 (20), 12879–12889. 10.1021/acscatal.2c03025. [DOI] [Google Scholar]
- Wu G.; et al. H2 production with ultra-low CO selectivity via photocatalytic reforming of methanol on Au/TiO2 catalyst. Int. J. Hydrogen Energy 2008, 33 (4), 1243–1251. 10.1016/j.ijhydene.2007.12.020. [DOI] [Google Scholar]
- Bamwenda G. R.; et al. Photoassisted hydrogen production from a water-ethanol solution: a comparison of activities of Au— TiO2 and Pt— TiO2. J. Photochem. Photobiol., A 1995, 89 (2), 177–189. 10.1016/1010-6030(95)04039-I. [DOI] [Google Scholar]
- Yoong L.; Chong F. K.; Dutta B. K. Development of copper-doped TiO2 photocatalyst for hydrogen production under visible light. Energy 2009, 34 (10), 1652–1661. 10.1016/j.energy.2009.07.024. [DOI] [Google Scholar]
- Choi W.; Termin A.; Hoffmann M. R. The role of metal ion dopants in quantum-sized TiO2: correlation between photoreactivity and charge carrier recombination dynamics. J. Phys. Chem. 1994, 98 (51), 13669–13679. 10.1021/j100102a038. [DOI] [Google Scholar]
- Litter M.; Navio J. A. Photocatalytic properties of iron-doped titania semiconductors. J. Photochem. Photobiol., A 1996, 98 (3), 171–181. 10.1016/1010-6030(96)04343-2. [DOI] [Google Scholar]
- Dholam R.; et al. Hydrogen production by photocatalytic water-splitting using Cr-or Fe-doped TiO2 composite thin films photocatalyst. Int. J. Hydrogen Energy 2009, 34 (13), 5337–5346. 10.1016/j.ijhydene.2009.05.011. [DOI] [Google Scholar]
- Dholam R.; et al. Efficient indium tin oxide/Cr-doped-TiO2 multilayer thin films for H2 production by photocatalytic water-splitting. international journal of hydrogen energy 2010, 35 (18), 9581–9590. 10.1016/j.ijhydene.2010.06.097. [DOI] [Google Scholar]
- Jang J. S.; et al. Optimization of CdS/TiO2 nano-bulk composite photocatalysts for hydrogen production from Na2S/Na2SO3 aqueous electrolyte solution under visible light (λ≥ 420 nm). J. Photochem. Photobiol., A 2007, 188 (1), 112–119. 10.1016/j.jphotochem.2006.11.027. [DOI] [Google Scholar]
- Meng A.; et al. Direct Z-scheme TiO2/CdS hierarchical photocatalyst for enhanced photocatalytic H2-production activity. Appl. Surf. Sci. 2017, 422, 518–527. 10.1016/j.apsusc.2017.06.028. [DOI] [Google Scholar]
- Yan Z.; et al. Noble metal-free cobalt oxide (CoOx) nanoparticles loaded on titanium dioxide/cadmium sulfide composite for enhanced photocatalytic hydrogen production from water. Int. J. Hydrogen Energy 2014, 39 (25), 13353–13360. 10.1016/j.ijhydene.2014.04.121. [DOI] [Google Scholar]
- Dekrafft K. E.; Wang C.; Lin W. Metal-Organic Framework Templated Synthesis of Fe2O3/TiO2 Nanocomposite for Hydrogen Production. Advanced materials 2012, 24 (15), 2014–2018. 10.1002/adma.201200330. [DOI] [PubMed] [Google Scholar]
- Yu J.; Hai Y.; Cheng B. Enhanced photocatalytic H2-production activity of TiO2 by Ni (OH) 2 cluster modification. J. Phys. Chem. C 2011, 115 (11), 4953–4958. 10.1021/jp111562d. [DOI] [Google Scholar]
- Yan H.; Yang H. TiO2–g-C3N4 composite materials for photocatalytic H2 evolution under visible light irradiation. Journal of alloys and compounds 2011, 509 (4), L26–L29. 10.1016/j.jallcom.2010.09.201. [DOI] [Google Scholar]
- Lin T.-H.; et al. Nanoscale Multidimensional Pd/TiO2/g-C3N4 Catalyst for Efficient Solar-Driven Photocatalytic Hydrogen Production. Catalysts 2021, 11 (1), 59. 10.3390/catal11010059. [DOI] [Google Scholar]
- Xu X.; et al. g-C3N4 coated SrTiO3 as an efficient photocatalyst for H2 production in aqueous solution under visible light irradiation. international journal of hydrogen energy 2011, 36 (21), 13501–13507. 10.1016/j.ijhydene.2011.08.052. [DOI] [Google Scholar]
- Xiang Q.; Yu J.; Jaroniec M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41 (2), 782–796. 10.1039/C1CS15172J. [DOI] [PubMed] [Google Scholar]
- Xiang Q.; Yu J. Graphene-based photocatalysts for hydrogen generation. J. Phys. Chem. Lett. 2013, 4 (5), 753–759. 10.1021/jz302048d. [DOI] [PubMed] [Google Scholar]
- Reza M. S.; et al. Hydrogen Production from Water Splitting through Photocatalytic Activity of Carbon-Based Materials. Chem. Eng. Technol. 2023, 46 (3), 420–434. 10.1002/ceat.202100513. [DOI] [Google Scholar]
- Kamat P. V. Graphene-based nanoarchitectures. Anchoring semiconductor and metal nanoparticles on a two-dimensional carbon support. J. Phys. Chem. Lett. 2010, 1 (2), 520–527. 10.1021/jz900265j. [DOI] [Google Scholar]
- Kim H.-i.; et al. Solar photoconversion using graphene/TiO2 composites: nanographene shell on TiO2 core versus TiO2 nanoparticles on graphene sheet. J. Phys. Chem. C 2012, 116 (1), 1535–1543. 10.1021/jp209035e. [DOI] [Google Scholar]
- Fan W.; et al. Nanocomposites of TiO2 and reduced graphene oxide as efficient photocatalysts for hydrogen evolution. J. Phys. Chem. C 2011, 115 (21), 10694–10701. 10.1021/jp2008804. [DOI] [Google Scholar]
- Huang G.; et al. Hierarchical red phosphorus incorporated TiO2 hollow sphere heterojunctions toward superior photocatalytic hydrogen production. Journal of Materials Science & Technology 2022, 108, 18–25. 10.1016/j.jmst.2021.09.026. [DOI] [Google Scholar]
- Wang Q.; et al. Construction of Bi-assisted modified CdS/TiO2 nanotube arrays with ternary S-scheme heterojunction for photocatalytic wastewater treatment and hydrogen production. Fuel 2022, 322, 124163. 10.1016/j.fuel.2022.124163. [DOI] [Google Scholar]
- Jayachitra S.; et al. Highly conductive NiSe2 nanoparticle as a co-catalyst over TiO2 for enhanced photocatalytic hydrogen production. Applied Catalysis B: Environmental 2022, 307, 121159. 10.1016/j.apcatb.2022.121159. [DOI] [Google Scholar]
- Liu J.; et al. Synergistic effect of oxygen defect and doping engineering on S-scheme O-ZnIn2S4/TiO2-x heterojunction for effective photocatalytic hydrogen production by water reduction coupled with oxidative dehydrogenation. Chemical Engineering Journal 2022, 430, 133125. 10.1016/j.cej.2021.133125. [DOI] [Google Scholar]




