ABSTRACT
Foot-and-mouth disease (FMD) is an acute, highly contagious disease of cloven-hoofed animals caused by FMD virus (FMDV). Currently, the molecular pathogenesis of FMDV infection remains poorly understood. Here, we demonstrated that FMDV infection induced gasdermin E (GSDME)-mediated pyroptosis independent of caspase-3 activity. Further studies showed that FMDV 3Cpro cleaved porcine GSDME (pGSDME) at the Q271-G272 junction adjacent to the cleavage site (D268-A269) of porcine caspase-3 (pCASP3). The inhibition of enzyme activity of 3Cpro failed to cleave pGSDME and induce pyroptosis. Furthermore, overexpression of pCASP3 or 3Cpro-mediated cleavage fragment pGSDME-NT was sufficient to induce pyroptosis. Moreover, the knockdown of GSDME attenuated the pyroptosis caused by FMDV infection. Our study reveals a novel mechanism of pyroptosis induced by FMDV infection and might provide new insights into the pathogenesis of FMDV and the design of antiviral drugs.
IMPORTANCE Although FMDV is an important virulent infectious disease virus, few reports have addressed its relationship with pyroptosis or pyroptosis factors, and most studies focus on the immune escape mechanism of FMDV. GSDME (DFNA5) was initially identified as being associated with deafness disorders. Accumulating evidence indicates that GSDME is a key executioner for pyroptosis. Here, we first demonstrate that pGSDME is a novel cleavage substrate of FMDV 3Cpro and can induce pyroptosis. Thus, this study reveals a previously unrecognized novel mechanism of pyroptosis induced by FMDV infection and might provide new insights into the design of anti-FMDV therapies and the mechanisms of pyroptosis induced by other picornavirus infections.
KEYWORDS: foot-and-mouth disease virus, pyroptosis, porcine gasdermin E, 3Cpro, protease activity, cleavage
INTRODUCTION
Foot-and-mouth disease (FMD) is an acute, febrile, highly contagious infectious disease caused by foot-and-mouth disease virus (FMDV), which mainly affects artiodactyls (pigs, cattle, and sheep), occasionally in humans and other animals (1). Animals suffering from FMD develop blisters and ulcers on the oral mucosa, hooves, and breast skin, which seriously affects the productivity and quality of livestock and causes huge economic losses to the breeding industry (2). FMD is mainly divided into seven serotypes, including A, O, and C, South African Territories type 1 (SAT1), SAT2, SAT3, and Asian 1, of which O-type FMD is widely prevalent in the world (3). FMDV belongs to the genus Aphthovirus in the Picornaviridae family. The genome consists of a single positive-strand RNA of about 8.5 kb in length, encoding a large open reading frame (ORF), which is translated into a polyprotein precursor and then cleaved by viral proteases and processed into four structural proteins (VP4, VP2, VP3, and VP1) and eight nonstructural proteins (Lpro, 2A, 2B, 2C, 3A, 3B, 3Cpro, and 3D) (4).
FMDV 3C protease (3Cpro) belongs to the chymotrypsin-like cysteine protease family and is crucial in processing viral polyprotein precursors and viral replication. In addition, FMDV 3Cpro also plays an important role in the regulation of host innate immunity (5). Accumulating evidence has shown that 3Cpro inhibited multiple innate immune response signaling pathways, thereby escaping the host antiviral response. FMDV 3Cpro inhibited host protein synthesis by cleaving the translation initiation factors eIF4AI and eIF4G (6). Furthermore, 3Cpro promoted viral translation and replication by antagonizing the internal ribosomal entry site transacting factors (ITAFs) DDX23 and hnRNP K (7, 8). 3Cpro also escaped the innate immune response by degrading various RIG-I, MDA5, and NF-κB signaling molecules. Moreover, the proteolytic activity of 3Cpro is particularly important for inducing the degradation of these molecules, showing its strong potential as an antiviral target (6, 9, 10).
Programmed cell death is an important innate immune mechanism during the infection of host cells by pathogenic microorganisms, while excessive responses are also the main cause of its pathogenicity. Pyroptosis is a novel inflammation-associated programmed cell death characterized by the formation of membrane pores in the plasma membrane, cell swelling, and rupture of the plasma membrane, followed by the release of its cytoplasmic contents (including inflammatory cytokines) to the extracellular environment (11). The canonical pyroptosis pathway is mainly mediated by caspase-1. The inflammasome is assembled in response to danger signals, and caspase-1 is activated. Activated caspase-1 cleaves the pore-forming protein gasdermin D (GSDMD), eventually releasing GSDMD N-terminal fragments to bind membrane lipids and form membrane pores, resulting in cell swelling, dissolution, and death. Activated caspase-1 also cleaves pro-IL-1β and pro-IL-18 into mature interleukin-1β (IL-1β) and IL-18, which are then secreted to the extracellular environment and promote inflammatory responses (12–14).
Furthermore, it has been observed that both human caspase-4/5 and mouse caspase-11 exhibit binding affinity toward lipopolysaccharide (LPS), which subsequently triggers their activation and cleavage of GSDMD, ultimately resulting in the formation of membrane pores and induction of pyroptosis via the noncanonical pyroptosis pathway (15–17). Recent studies have shown that other members of the gasdermin family can also act as effector proteins to trigger pyroptosis (18–24). Caspase-8 activates GSDMC and converts apoptosis into pyroptosis (23). Similarly, caspase-3 activation drives pyroptosis by cleavage of GSDME and provides new insights into cancer chemotherapy (18). Furthermore, granzymes A and B derived from cytotoxic lymphocytes cleave GSDMB (19) and GSDME (20) to induce pyroptosis, enhancing antitumor immunity. Moreover, pyroptosis mediated by GSDME plays a crucial role in the pathogenesis of viral infection (21, 22).
Pyroptosis is a double-edged sword. On the one hand, it can effectively defend against pathogen infection. On the other hand, excessive inflammation will damage the immune system, which is conducive to the replication of viruses (25). Previous studies have shown that picornavirus infection can induce pyroptosis to facilitate viral replication and disrupt the host immune response (26, 27). However, the mechanism of pyroptosis induced by FMDV infection remains unclear.
This study demonstrated that FMDV infection induces GSDME-mediated pyroptosis independent of caspase-3 activity. In addition, FMDV 3Cpro cleaves pGSDME close to the cleavage site of pCASP3, and the protease activity of 3Cpro is required for pGSDME cleavage. Furthermore, we found that overexpression of pCASP3 or the 3Cpro-mediated cleavage fragment pGSDME-NT is sufficient to induce pyroptosis. Our study reveals a novel mechanism of pyroptosis induced by FMDV infection and might provide new insights into the pathogenesis of FMDV and the design of antiviral drugs.
RESULTS
FMDV infection induces GSDME-mediated pyroptosis in SK6 cells.
Programmed cell death caused by FMDV infection is an important defense mechanism against viral infection (11, 28, 29). Previous studies have reported that FMDV infection induces apoptosis in host cells (9). To investigate whether FMDV infection causes pyroptosis, the novel inflammation-associated programmed cell death, SK6 cells were infected with FMDV O/HN/CHA/93 strain at a multiplicity of infection (MOI) of 1 at the indicated time (Fig. 1). Morphological observation showed that SK6 cells infected with FMDV exhibited typical pyroptosis features. The cell morphology was similar to that of SK6 cells infected with the Seneca Valley virus (SVV) (26), characterized by swelling and rounding of the cells. The shape of fried eggs in the middle of the bulge (Fig. 1A). In addition, cell viability decreased significantly as the virus replicated (Fig. 1B). Lactate dehydrogenase (LDH), as a stable cytoplasmic enzyme, is released during cell lysis. To this end, we further measured the LDH release after FMDV infection in different cells. As shown in Fig. 1C, LDH release was dramatically increased at 4 and 8 h postinfection (hpi) in PK-15 and SK6 cells (porcine cells) and was significantly higher than that in BHK-21 (mouse-derived cell line) and HEK-293T cells (human cell lines), while there was no significant difference between PK-15 and SK6 cells. Propidium iodide (PI), a nucleic acid red fluorescent dye, can only stain necrotic cells with loss of cell membrane integrity and binds to nucleic acids to emit bright red fluorescence. To further characterize pyroptosis caused by FMDV infection in SK6 cells, the FMDV- or mock-infected cells were stained with PI. The results showed that a large amount of red fluorescence was observed in FMDV-infected cells, while no fluorescent signal was observed in mock-infected cells (Fig. 1D). Since pyroptosis is a kind of inflammatory cell death (11), we further detected the expression of inflammasome-associated genes. The results showed that the mRNA levels of IL-1β and IL-18 (Fig. 1E and F) and NLRP2 and NLRP3 (see Fig. S1 in the supplemental material) increased with virus infection, while the mRNA levels of ASC, GSDMD, and NLRP1 decreased significantly (see Fig. S1). Interestingly, the mRNA levels of caspase-1 slightly increased in the early stage of the virus infection and decreased in the late stage, although there was no significant difference throughout the infection (see Fig. S1).
FIG 1.
FMDV infection induces pyroptosis in SK6 cells. SK6 cells were infected with FMDV O/HN/CHA/93 strain at an MOI of 1 at the indicated times. (A) Morphological examination of SK6 cells with FMDV or mock infection. SVV infection was used as a positive control. The cell morphology was observed using an Olympus IX71 microscope (white arrows, pyroptotic cells). (B) FMDV inhibited SK6 cell viability. The cell viability of SK6 was determined by using a Cell Counting Kit-8. (C) SK6, PK-15, BHK-21, and HEK-293T cells were infected with FMDV O/HN/CHA/93 strain at an MOI of 1 at the indicated times. The release of LDH from FMDV-infected cells was measured with an LDH cytotoxicity assay kit. (D) PI staining of SK6 cells with FMDV or mock infection. (E and F) The relative expression levels of IL-1β and IL-18 were analyzed by quantitative RT-PCR (qRT-PCR) in SK6 cells, and the housekeeping gene GAPDH was used as the control. (G) FMDV infection induces GSDME-mediated pyroptosis in SK6 cells. Proteolytic cleavage of GSDMD or GSDME in SK6 cells with FMDV infection was determined by Western blotting. The abundance of caspase-3 (CASP3), IL-1β, MLKL, phospho-MLKL, FMDV VP1 protein, and α-tubulin as an internal control was also determined by Western blotting. Two lanes represent two biological replicates. (H and I) The release of IL-1β and IL-18 from FMDV-infected cells was measured with porcine IL-1β and IL-18 using an enzyme-linked immunosorbent assay (ELISA) kit. (J and K) SK6 cells were transfected with plasmids encoding pGSDME for 24 h and then infected with FMDV O/HN/CHA/93 strain at an MOI of 1 at the indicated times. (L and M) SK6 cells were infected with FMDV at various MOIs as indicated for 24 h. Proteolytic cleavage of GSDME in SK6 cells with FMDV infection was determined by Western blotting. The release of LDH from FMDV-infected cells was measured with LDH Cytotoxicity assay kit. The data in panels B, C, E, F, H, I, K, and M are presented as means ± the SD. Experiments were performed independently with at least two biological replicates. Student t test or one/two-way ANOVA was used for analysis. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Previous studies have shown that picornavirus infection-induced pyroptosis is executed through the caspase-1-GSDMD axis (11, 12, 28–31). However, our results showed that caspase-1 was not activated after FMDV infection. Therefore, FMDV infection might cause pyroptosis through other pathways. Western blotting revealed that consistent with mRNA levels, caspase-1 was not activated after FMDV infection, and GSDMD was degraded rather than cleaved (Fig. 1G). Excitingly, GSDME, another pyroptotic executive protein in the gasdermin family (18), was activated, and an obvious cleavage product appeared. Also, activation of caspase-3 and maturation of IL-1β was observed (Fig. 1G), suggesting that FMDV infection-induced pyroptosis might be executed through the caspase-3-GSDME axis. Furthermore, we examined whether MLKL (mixed-lineage kinase domain-like) protein, the executor in the necroptosis pathway, was phosphorylated (29, 32–35). The results showed that MLKL was phosphorylated after FMDV infection, indicating that FMDV infection causes multiple forms of cell death (pyroptosis, necroptosis, and apoptosis). In addition, the levels of proinflammatory cytokines IL-1β and IL-18 in the supernatant of SK6 cells infected with FMDV were significantly higher than those in the control group (Fig. 1H and I).
To verify the cleavage of pGSDME, SK6 cells were transfected with Flag-pGSDME, followed by FMDV infection at the indicated time. Western blotting found an obvious cleavage product of transfected pGSDME in FMDV-infected SK6 cells at 12 hpi (Fig. 1J). The release of LDH from FMDV-infected cells was dramatically increased compared to the mock-infected cells (Fig. 1K). In addition, similar results were observed when pGSDME-transfected cells were infected with different doses of FMDV (Fig. 1L and M). These data indicated that FMDV infection induces GSDME-mediated pyroptosis in SK6 cells.
FMDV infection induces pyroptosis independent of caspase-3 activity.
As the upstream initiator or executor of the programmed cell death pathway, caspases play a crucial role in pyroptosis (11). Since our results showed that FMDV infection might induce pyroptosis through the caspase-3/GSDME pathway, we investigated whether the caspase-3 activity is required for pyroptosis induced by FMDV infection. SK6 cells were infected with FMDV O/HN/CHA/93 strain at an MOI of 1 for 12 h in the presence or absence of the caspase-3-specific inhibitor Ac-DEVD-CHO (20 μM). As shown in Fig. 2A, the cells treated with Ac-DEVD-CHO still exhibited typical pyroptosis features. LDH release from Ac-DEVD-CHO-treated cells was not significantly different from that of DMSO-treated cells (Fig. 2B). PI staining also showed amounts of red fluorescence in Ac-DEVD-CHO-treated cells (Fig. 2C). Western blotting revealed that FMDV infection still caused pyroptosis in the presence of Ac-DEVD-CHO, which was manifested as the cleavage of pGSDME, activation of caspase-3, and maturation of IL-1β (Fig. 2D). As expected, there were no significant differences of the IL-1β and IL-18 levels in the FMDV-infected SK6 cell supernatant between the AC-DEVD-CHO- and DMSO-treated groups (Fig. 2E and F).
FIG 2.
FMDV infection induces pyroptosis in SK6 cells independent of caspase-3 activity. SK6 cells were infected with FMDV O/HN/CHA/93 strain at an MOI of 1 for 12 h in the presence or absence of Ac-DEVD-CHO (20 μM). (A) Morphological examination of SK6 cells with FMDV or mock infection. The cell morphology was observed using an Olympus IX71 microscope (white arrows, pyroptotic cells). (B) The release of LDH from FMDV-infected cells was measured with an LDH cytotoxicity assay kit. (C) PI staining of SK6 cells with FMDV or mock infection. (D) Proteolytic cleavage of GSDME in SK6 cells with FMDV infection was determined by Western blotting. The abundance of caspase-3 (CASP3), IL-1β, FMDV VP1 protein, and α-tubulin as an internal control was also determined by Western blotting. (E and F) The release of IL-1β and IL-18 from FMDV-infected cells was measured with porcine IL-1β and IL-18 using an ELISA kit. The data in panels B, E, and F are presented as means ± the SD. Experiments were performed independently with at least three biological replicates. Two-way ANOVA was used for analysis. ns, not significant.
To further exclude the role of caspase-3 in pyroptosis induced by FMDV infection, caspase-3 knockdown was performed using small interfering RNAs (siRNAs). Similar to the results of AC-DEVD-CHO treatment, caspase-3 knockdown had no significant effect on pyroptosis caused by FMDV infection (see Fig. S2). These results indicated that FMDV infection induces pyroptosis independent of caspase-3 activity.
FMDV 3Cpro induces pyroptosis in SK6 cells.
Since FMDV infection induces pyroptosis in SK6 cells independent of caspase-3 activity, we speculated that viral proteins might be involved. SK6 cells were cotransfected with plasmids encoding pGSDME and viral proteins to identify the key proteins for 24 h. The results showed that only 3Cpro had a cleavage effect on pGSDME (Fig. 3A), suggesting that 3Cpro might be involved in the pyroptosis induced by FMDV infection. Furthermore, typical pyroptotic morphology was found in cells overexpressing 3Cpro (Fig. 3B). Moreover, significantly increased LDH release was observed in 3Cpro-transfected cells (Fig. 3C). To verify the cleavage effect of 3Cpro on pGSDME, SK6 cells were cotransfected with plasmids encoding 3Cpro and pGSDME for 24 h. As indicated in Fig. 3D, a distinct cleavage product was observed in the cells cotransfected with 3Cpro and pGSDME. These results indicated that FMDV 3Cpro induces pyroptosis in SK6 cells.
FIG 3.
FMDV 3Cpro induces pyroptosis in SK6 cells. (A) Proteolytic cleavage of pGSDME in SK6 cells by viral proteins. SK6 cells were cotransfected with plasmids encoding pGSDME and viral proteins for 24 h. Proteolytic cleavage of pGSDME in SK6 cells by viral proteins was determined by Western blotting. The red asterisk indicates the pGSDME N-terminal cleavage product. (B) SK6 cells were transfected with recombinant plasmids encoding 3Cpro. The cell morphology was observed using an Olympus IX71 microscope (white arrows, pyroptotic cells). (C) The release of LDH from 3Cpro-transfected cells was measured using an LDH cytotoxicity assay kit. (D) Proteolytic cleavage of pGSDME in SK6 cells by 3Cpro. SK6 cells were cotransfected with plasmids encoding 3Cpro and pGSDME for 24 h. Proteolytic cleavage of pGSDME in SK6 cells by 3Cpro was determined by Western blotting. Two lanes represent two biological replicates. The data in panel C are presented as means ± the SD. Experiments were performed independently with at least three biological replicates. A Student t test was used for analysis. *, P < 0.05.
Catalytic residues in the active sites of 3Cpro are essential for the pGSDME cleavage induced by 3Cpro.
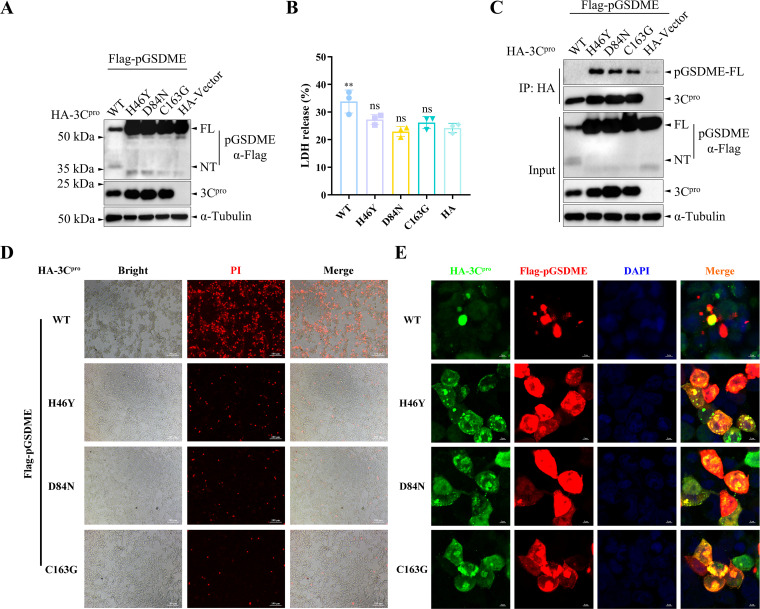
The catalytic triad of H46, D84, and C163 in FMDV 3Cpro has been identified as critical sites that play important roles in its enzyme activity (9, 10, 36). To determine whether the enzyme activity of 3Cpro is necessary for pGSDME cleavage, three 3Cpro mutants (HA-3Cpro-H46Y, HA-3Cpro-D84N, and HA-3Cpro-C163G) that eliminated the protease activity of 3Cpro were constructed. HEK-293T cells were cotransfected with plasmids encoding pGSDME and 3Cpro or its mutants for 12 h. The results showed that the elimination of the enzyme activity of 3Cpro abolished its cleavage effect on pGSDME, indicating that the enzyme activity of 3Cpro was necessary for the cleavage of pGSDME (Fig. 4A). LDH release from 3Cpro mutant-transfected cells was suppressed compared to wild-type HA-3Cpro (HA-3Cpro-WT) (Fig. 4B). To determine whether 3Cpro interacts with pGSDME for cleavage, we performed coimmunoprecipitation analysis. The results showed that 3Cpro interacts with and cleaves pGSDME (Fig. 4C). Due to the strong degradation effect of 3Cpro-WT on pGSDME, we did not detect pGSDME in the immunoprecipitation samples. As expected, we detected the cleavage product of pGSDME in the input samples (Fig. 4C). In addition, with the elimination of the enzyme activity of 3Cpro, the number of dead cells decreased significantly (Fig. 4D). Consistent with the results of coimmunoprecipitation, 3Cpro mutants and pGSDME had good intracellular colocalization. In contrast, only a small amount of colocalization was observed in the WT group due to the degradation of pGSDME by 3Cpro-WT (Fig. 4E). Collectively, these results suggested that catalytic residues in the active sites of 3Cpro are essential for the pGSDME cleavage induced by 3Cpro.
FIG 4.
Catalytic residues in the active sites of 3Cpro are essential for the pGSDME cleavage induced by 3Cpro. (A) The effects of catalytic residues of the 3Cpro active sites on 3Cpro-induced pGSDME cleavage. HEK-293T cells were cotransfected with plasmids encoding pGSDME and 3Cpro or its mutants for 12 h. Proteolytic cleavage of pGSDME in HEK-293T cells by 3Cpro or its mutants was determined by Western blotting. (B) The release of LDH from pGSDMD and 3Cpro-transfected cells was measured with an LDH cytotoxicity assay kit. (C) FMDV 3Cpro interacts with and cleaves pGSDME. HEK-293T cells were cotransfected with plasmids encoding pGSDME and 3Cpro or its mutants for 12 h. The interaction between 3Cpro and pGSDME was analyzed by using coimmunoprecipitation. (D) PI staining of the transfected cells. (E) The interaction between 3Cpro and pGSDME was analyzed by using confocal immunofluorescence. The data in panel B are presented as means ± the SD. Experiments were performed independently with at least three biological replicates. One-way ANOVA was used for analysis. **, P < 0.01.
Rupintrivir inhibits FMDV-induced pyroptosis in SK6 cells.
Rupintrivir (AG7088) (Fig. 5A) is a potent inhibitor of picornavirus 3Cpro and has been applied in many studies against picornaviruses (37–39). Since the enzyme activity of 3Cpro is critical for pGSDME cleavage, we used the 3Cpro inhibitor rupintrivir to further investigate its important role in pyroptosis induced by FMDV infection. First, we performed molecular docking analysis to evaluate the affinity of rupintrivir for FMDV 3Cpro. Results showed that rupintrivir could effectively bind to 3Cpro. In addition, the hydrophobic pocket containing the catalytic residues (H46 and D84) of 3Cpro was occupied successfully by rupintrivir (Fig. 5B). Next, we assessed the inhibitory effect of rupintrivir on the enzyme activity of 3Cpro. HEK-293T cells were cotransfected with plasmids encoding 3Cpro and pGSDME for 12 h in the presence or absence of rupintrivir. We found that rupintrivir inhibits the enzyme activity of 3Cpro in a dose-dependent manner (Fig. 5C). Then, SK6 cells were infected with FMDV at an MOI of 1 for 12 h in the presence or absence of rupintrivir (8 μM). PI staining and LDH release assays revealed significant inhibition of FMDV proliferation in rupintrivir-treated cells (Fig. 5D and E). As expected, the rupintrivir treatment inhibited: (i) the cleavage of pGSDME, (ii) the activation of caspase-3, and (iii) the maturation of IL-1β. (Fig. 5F). Moreover, rupintrivir significantly inhibited the secretion of proinflammatory cytokines IL-1β and IL-18 in FMDV-infected SK6 cells (Fig. 5G and H).
FIG 5.
Rupintrivir inhibits FMDV-induced pyroptosis in SK6 cells. SK6 cells were infected with FMDV O/HN/CHA/93 strain at an MOI of 1 for 12 h in the presence or absence of rupintrivir (8 μM). (A) Chemical formula of rupintrivir (AG7088). (B) Binding mode of rupintrivir to FMDV 3Cpro. Molecular docking studies were performed by Autodock Vina 1.2.2 (http://autodock.scripps.edu/) and visualized using the PyMOL 2.5 software (DeLano Scientific LLC), with PDB ID 5hm2 (FMDV 3Cpro) and 4ght (EV71 3Cpro-AG7088 complex) used as the templates. The yellow structure represents rupintrivir, and the pink residues represent His46 (H46) and Asp84 (D84). (C) Rupintrivir inhibits the activity of 3Cpro in a dose-dependent manner. HEK-293T cells were cotransfected with plasmids encoding 3Cpro and pGSDME for 12 h in the presence or absence of rupintrivir. Proteolytic cleavage of pGSDME in HEK-293T cells by 3Cpro was determined by Western blotting. (D) PI staining of SK6 cells with FMDV or mock infection. (E) The release of LDH from FMDV-infected cells was measured with an LDH cytotoxicity assay kit. (F) Proteolytic cleavage of GSDME in SK6 cells with FMDV infection was determined by Western blotting. The abundance of caspase-3 (CASP3), IL-1β, FMDV VP1 protein, and α-tubulin as an internal control was also determined by Western blotting. (G and H) The release of IL-1β and IL-18 from FMDV-infected cells was measured with porcine IL-1β and IL-18 using an ELISA kit. The data in panels E, G, and H are presented as means ± the SD. Experiments were performed independently with at least three biological replicates. Two-way ANOVA was used for analysis. ****, P < 0.0001.
FMDV 3Cpro cleaves pGSDME adjacent to the cleavage site of pCASP3.
Previous studies have shown that human GSDME (hGSDME) has a cleavage motif (267DMPD270) that can be recognized by caspase-3 (18, 21). However, in porcine GSDME (pGSDME), the cleavage site by porcine caspase-3 (pCASP3) remains ambiguous. To address this question, we first aligned the amino acid sequences of pGSDME, hGSDME, and mouse GSDME (mGSDME) and revealed that there might be a similar cleavage motif (265DMTD268) in pGSDME to those in hGSDME (Fig. 6A and B). We performed a cleavage experiment using the D268A mutant to verify this site. HEK-293T cells were cotransfected with plasmids encoding pCASP3 and pGSDME or its mutants for 24 h. Meanwhile, tumor necrosis factor alpha (TNF-α; 50 ng/mL) was added to activate pCASP3 (18). As shown in Fig. 6C, the D268A mutation appears to remove the cleavage effect of pCASP3 on pGSDME, suggesting that pCASP3 might cleave pGSDME at D268-A269. Intriguingly, there is a clear decrease in pCASP3 cleavage of pGSDME-Q271A mutant, indicating that this mutation also interferes with pCASP3 processing of pGSDME. Since 3Cpro can also cleave pGSDME independently, we further explored the cleavage site of 3Cpro on pGSDME. Based on the cleavage preference of picornavirus 3Cpro (Q-G or E-Q) (26, 27) and the cleavage fragment size of pGSDME, we constructed a panel of potential pGSDME mutants. We found that the pGSDME-Q271A mutant was resistant to 3Cpro-induced cleavage (Fig. 6D; see also Fig. S3 in the supplemental material), indicating that 3Cpro probably cleaves pGSDME at Q271-G272. Collectively, FMDV 3Cpro cleaves pGSDME adjacent to the cleavage site of pCASP3.
FIG 6.
FMDV 3Cpro cleaves pGSDME adjacent to the cleavage site of pCASP3. (A) Sequence alignment of GSDME in pigs, humans, and mice. (B) Logo analysis of the cleavage sites predicted from the polyprotein cleavage of pCaspase-3 (pCASP3) or 3Cpro. (C) Cleavage sites of pGSDME induced by pCASP3. HEK-293T cells were cotransfected with plasmids encoding pCASP3 and pGSDME or its mutants for 24 h in the presence or absence of TNF-α (50 ng/mL). (D) Cleavage sites of pGSDME induced by 3Cpro. HEK-293T cells were cotransfected with plasmids encoding 3Cpro and pGSDME or its mutants for 12 h. Proteolytic cleavage of pGSDME or its mutants in HEK-293T cells by pCASP3 or 3Cpro was determined by Western blotting.
pCASP3 or 3Cpro-mediated cleavage fragment pGSDME-NT localizes to the cell membrane and triggers pyroptosis.
The gasdermin-N domain oligomerizes and targets the cell membrane to form membrane pores, thus triggering pyroptosis (11, 26, 27, 31, 40–42), which provoked us to wonder whether overexpression of pCASP3 or 3Cpro-mediated cleavage fragment pGSDME-NT was sufficient to induce pyroptosis. HEK-293T cells were transfected with recombinant plasmids encoding pGSDME or its cleavage fragments for 48 h (Fig. 7A and B). Confocal immunofluorescence analysis revealed that the N-terminal fragments of pGSDME cleaved by pCASP3 and 3Cpro was localized to the cell membrane, whereas the full-length and C-terminal fragments of pGSDME were found throughout the cytoplasm (Fig. 7C). Furthermore, the LDH release of pGSDME-NT1-268- and pGSDME-NT1-271-transfected cells was significantly higher than that of other transfected cells (Fig. 7D). The PI uptake in N-terminal transfected cells increased dramatically (Fig. 7E). As expected, the N-terminal transfected cells exhibited typical pyroptotic morphology (Fig. 7F). Taken together, these results indicated that pCASP3- or 3Cpro-mediated cleavage fragment pGSDME-NT localizes to the cell membrane and triggers pyroptosis.
FIG 7.
pCASP3 or 3Cpro -mediated cleavage fragment pGSDME-NT localizes to the cell membrane and triggers pyroptosis. (A) Schematic diagram of pGSDME and its cleavage fragments induced by pCaspase-3 (pCASP3) or 3Cpro. HEK-293T cells were transfected with recombinant plasmids encoding pGSDME or its cleavage fragments for 48 h. (B) The expression of pGSDME and its cleavage fragments was determined by Western blotting. (C) The localization of pGSDME or its cleavage fragments was analyzed by confocal immunofluorescence. (D) The release of LDH from the transfected cells was measured with an LDH cytotoxicity assay kit. (E) PI staining of the transfected cells. (F) The cell morphology was observed using an Olympus IX71 microscope (white arrows, pyroptotic cells). The data in panel D are presented as means ± the SD. Experiments were performed independently with at least three biological replicates. One-way ANOVA was used for analysis. ****, P < 0.0001.
pGSDME knockdown attenuates FMDV-induced pyroptosis in SK6 cells.
To further confirm the crucial role of GSDME in FMDV infection-induced pyroptosis, pGSDME knockdown was performed using short hairpin RNAs (shRNAs). First, we verified the knockdown efficiency of the three shRNAs, and the results showed that shRNA3 had the highest efficiency (Fig. 8A). Therefore, we chose it for subsequent experiments. We then determined that the knockdown of pGSDME did not affect FMDV replication (see Fig. S4). SK6 cells were transduced with shRNA lentivirus targeting pGSDME (sh-pGSDME) or negative control (sh-NC) for 24 h, and then the cells were infected with FMDV at an MOI of 1 for 12 h. Morphological examination showed that knockdown of pGSDME reduced cell death caused by FMDV infection (Fig. 8B). LDH release assays and PI staining also revealed that pGSDME knockdown attenuated cell death induced by FMDV infection (Fig. 8C and D). In addition, pGSDME knockdown suppressed the cleavage of pGSDME and maturation of IL-1β (Fig. 8E). Moreover, pGSDME knockdown significantly diminished the secretion of proinflammatory cytokines IL-1β and IL-18 in FMDV-infected SK6 cells (Fig. 8F and G). These results indicated that pGSDME knockdown attenuates FMDV-induced pyroptosis in SK6 cells.
FIG 8.
pGSDME knockdown attenuates FMDV-induced pyroptosis in SK6 cells. SK6 cells were transduced with lentivirus targeting pGSDME (sh-pGSDME) or negative control (sh-NC) for 24 h, and then the cells were infected with FMDV O/HN/CHA/93 strain at an MOI of 1 for 12 h. (A) The knockdown efficiency of pGSDME was determined by Western blotting. (B) Morphological examination of SK6 cells with FMDV or mock infection. The cell morphology was observed using an Olympus IX71 microscope (white arrows, pyroptotic cells). (C) The release of LDH from FMDV-infected cells was measured with an LDH cytotoxicity assay kit. (D) PI staining of SK6 cells with FMDV or mock infection. (E) Proteolytic cleavage of GSDME in SK6 cells with FMDV infection was determined by Western blotting. The abundance of caspase-3, IL-1β, FMDV VP1 protein, and α-tubulin as an internal control was also determined by Western blotting. (F and G) The release of IL-1β and IL-18 from FMDV-infected cells was measured with porcine IL-1β and IL-18 using an ELISA kit. The data in panels C, F, and G are presented as means ± the SD. Experiments were performed independently with at least three biological replicates. Two-way ANOVA was used for analysis. *, P < 0.05; ***, P < 0.001.
DISCUSSION
FMD is listed as a class A infectious disease by the World Organization for Animal Health (OIE) due to its wide prevalence, multiple transmission routes, and fast spread speed. In addition, it always threatens the safety of livestock and the trade of livestock products in countries and regions without FMD (4). Thus, detailed research is required on its pathogenic mechanisms to provide better guidance for preventing and controlling FMD. In the present study, we revealed that FMDV infection induces GSDME-mediated pyroptosis independent of caspase-3 activity. In addition, FMDV 3Cpro induces pyroptosis by cleaving pGSDME adjacent to the cleavage site of pCaspase-3, and the protease activity of 3Cpro is required for pGSDME cleavage (Fig. 9).
FIG 9.
Schematic diagram showing the proposed mechanism of FMDV-induced GSDME-mediated pyroptosis. FMDV infection induces GSDME-mediated pyroptosis independent of caspase-3 activity. In addition, FMDV 3Cpro induces pyroptosis by cleaving pGSDME adjacent to the cleavage site of pCaspase-3, and the protease activity of 3Cpro is required for pGSDME cleavage.
Several pore-forming proteins in the gasdermin family are involved in various cellular processes, such as inflammation and cell death (43). Previous studies have shown that GSDMD, a pore-forming protein, is critical in pyroptosis induced by pathogen infection (11, 26, 30, 31, 40, 44–47). Gram-negative bacterial infection activates mouse caspase-11 through LPS and induces GSDMD-mediated noncanonical pyroptosis (46). SARS-CoV-2 NSP6 triggers NLRP3-dependent pyroptosis by targeting ATP6AP1 (47). SVV 3Cpro induces pyroptosis in picornaviruses by directly cleaving porcine GSDMD (26). Recent studies have shown that other members of the gasdermin family also play key roles in pyroptosis. GSDME was initially identified as being associated with deafness disorders (11). Accumulating evidence indicates that GSDME switches caspase-3-mediated apoptosis to pyroptosis (18, 20). Furthermore, GSDME is required to induce pyroptosis and severe disease during EV71 infection (21). Moreover, ZIKV causes placental pyroptosis and associated adverse fetal outcomes through the activation of GSDME (22). In this study, initial morphological observations indicated that FMDV infection of SK6 cells could induce pyroptosis (swelling and rounding of the cells and the shape of fried eggs in the middle of the bulge) (Fig. 1A). To further confirm the pyroptosis caused by FMDV infection, we detected the key proteins in the pyroptosis pathway (activation of CASP1/CASP3 and cleavage of GSDMD/GSDME) by Western blotting, and the results showed that the activation of CASP3 and the cleavage of GSDME were detected (Fig. 1G). In addition, we also detected some other common features of cell death, such as cell viability, LDH and inflammatory cytokines (IL-1β and IL-18) levels in the cell culture supernatant, and PI staining of the cell nucleus. In summary, these results demonstrated that GSDME mediates FMDV infection-induced pyroptosis. Therefore, our study might reveal a previously unrecognized novel mechanism of pyroptosis induced by FMDV infection.
FMDV 3Cpro affects antiviral innate immune responses by degrading or cleaving various host proteins (6–10). Here, we revealed for the first time that pGSDME is a novel cleavage substrate of FMDV 3Cpro and can induce pyroptosis. In addition, we found that FMDV 3Cpro cleaved pGSDME at a putative cleavage site (Q271-G272), which is similar to other picornaviruses (26, 27). However, FMDV 3Cpro had only one cleavage site at the N-terminal of pGSDME, which was close to the cleavage site of pCaspase-3, while in other picornaviruses, 3Cpro had two cleavage sites at the N-terminal of pGSDMD and inhibited pyroptosis by cleavage of the pGSDMD-NT fragment (26, 27). Our results suggest that 3Cpro has different cleavage modes for pyroptotic executive proteins. The protease activity of 3Cpro is crucial for its function, and mutation of the active sites of 3Cpro will abolish the cleavage function (10, 38). Hence, 3Cpro is a potential FMDV antiviral therapy target (37–39). In this study, 3Cpro lost the ability to cleave GSDME after mutating the catalytic triad. In addition, we further demonstrated the importance of the protease activity of 3Cpro by using the 3Cpro inhibitor rupintrivir (AG7088). Molecular docking analysis showed that rupintrivir inhibited protease activity, possibly by binding to the enzyme active site of 3Cpro. Small molecule anti-FMDV drugs with rupintrivir analogs will be of interest.
Pyroptosis is a double-edged sword. On the one hand, pyroptosis is beneficial to the elimination of pathogens. On the other hand, pyroptosis activates massive inflammatory factors to induce the inflammatory response, which may be the main cause of clinical symptoms such as blisters and ulcers in pigs caused by FMDV infection. Previous studies have shown that GSDME deficiency inhibits pyroptosis and alleviates pathological symptoms caused by EV71 infection in mice (21). In addition, adverse fetal outcomes associated with placental pyroptosis caused by ZIKV infection were effectively attenuated in GSDME-deficient mice (22). In our study, the knockdown of GSDME attenuated the pyroptosis caused by FMDV infection, but it was insufficient to abolish the cell death. The possible reason is that there might be multiple forms of cell death (pyroptosis, necroptosis, apoptosis, or ferroptosis) caused by FMDV infection (34, 35, 48). Given the current difficulties and limitations in developing and applying FMD vaccines, the design and comprehensive application of antiviral drugs targeting 3Cpro might be a more feasible strategy for preventing and controlling the disease.
MATERIALS AND METHODS
Materials and reagents.
jetPRIME in vitro DNA and siRNA transfection reagent was purchased from Polyplus-Transfection Inc. (New York, NY). A Pierce BCA protein assay kit and Alexa Fluor 555-goat anti-mouse antibody were purchased from Thermo Fisher Scientific (Waltham, MA). Dulbecco modified Eagle medium (DMEM), fetal bovine serum (FBS), and Pen-Strep antibiotics (10,000 U/mL penicillin and 10,000 µg/mL streptomycin) were purchased from Gen-View Scientific, Inc. (Jacksonville, FL). Mouse anti-His monoclonal antibody (MAb), anti-Flag MAb, anti-HA MAb, rabbit anti-HA MAb, and horseradish peroxidase-conjugated goat anti-mouse or rabbit IgG were obtained from Medical & Biological Laboratories Co., Ltd. (Nagoya, Japan). GSDMD rabbit MAb, GSDME rabbit polyclonal antibody (pAb), IL-1β rabbit pAb, IL-18 rabbit pAb, MLKL rabbit pAb, Phospho-MLKL-T357, S358, S360 rabbit pAb, and caspase-1 rabbit pAb were purchased from ABclonal Technology Co., Ltd. (Wuhan, China). Caspase-3 rabbit pAb was purchased from Proteintech (Wuhan, China). An enhanced chemiluminescence (ECL) system was purchased from UElandy, Inc. (Suzhou, China). A Cell Counting Kit-8 and rupintrivir (AG7088) were obtained from MedChemExpress (Shanghai, China). TRIpure reagent was obtained from Aidlab Biotechnologies Co., Ltd. (Beijing, China). Hifair first-strand cDNA synthesis SuperMix and Hieff UNICON Universal Blue qPCR SYBR green Master Mix were purchased from Yeasen Biotechnology Co., Ltd. (Shanghai, China). An LDH cytotoxicity assay kit, PI, caspase inhibitor Z-VAD-FMK, caspase-3 inhibitor Ac-DEVD-CHO, dimethyl sulfoxide (DMSO), puromycin, protein A+G agarose (beads), and DAPI (4′,6′-diamidino-2-phenylindole) were purchased from Beyotime Biotechnology (Shanghai, China). FMDV VP1 rabbit polyclonal antibody was prepared and maintained in our laboratory.
Swine Kidney-6 (SK6) cells, Porcine Kidney-15 (PK-15) cells, Baby Hamster Syrian Kidney (BHK-21) cells, and human embryonic kidney (HEK-293T) cells were cultured in DMEM supplemented with 10% FBS and 1% Pen-Strep antibiotics at 37°C with 5% CO2.
FMDV O/HN/CHA/93 strain was maintained in the National Foot-and-Mouth Disease Reference Laboratory, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences. All virus experiments were conducted at the Biosafety Level 3 Laboratory, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences.
Plasmid construction and transfection.
All the eukaryotic expression plasmids of FMDV viral proteins used in this study were constructed and cloned into pCAGGS-HA, pEBG-GST, or pEGFP-C1 vector using the primers listed in Table S1 in the supplemental material. Three 3Cpro mutants (HA-3Cpro-H46Y, HA-3Cpro-D84N, and HA-3Cpro-C163G) that eliminated the protease activity of 3Cpro were constructed and cloned into the pCAGGS-HA vector. The porcine GSDME (pGSDME) gene (GenBank accession no. XM_003134855.4) was amplified from SK6 cells and cloned into pcDNA3.1(+)3×Flag vector, and the mutants and cleavage fragments of pGSDME were also cloned into pcDNA3.1(+)3×Flag vector. Porcine caspase-3 (GenBank accession no. NM_214131.1) was synthesized by Sangon Biotech (Shanghai) Co., Ltd., and cloned into pcDNA3.1(+)3×Flag vector, and the 3×Flag tag was substituted with 6×His tag. Cell transfection was conducted using the jetPRIME in vitro DNA and siRNA transfection reagent according to the manufacturer’s instructions.
Drug treatment.
In caspase-3 activity inhibition experiments, caspase-3 inhibitor Ac-DEVD-CHO or DMSO was added to the medium 2 h before virus infection. For 3Cpro enzyme activity inhibition tests, rupintrivir (AG7088) was added to the medium for 4 h after plasmid transfection or virus infection.
Knockdown of pCASP3 or pGSDME.
pCASP3 knockdown was performed using small interfering RNAs (siRNAs). SK6 cells were transfected with siRNAs targeting pCASP3 for 48 h. The sequences (5′–3′) of siRNAs were as follows: siRNA-1 (CAGCGCUGAAACAGUAUGU and ACAUACUGUUUCAGCGCUG), siRNA-2 (CUGCUGUAGAACUCUAACU and AGUUAGAGUUCUACAGCAG), and siRNA-3 (CCGAAAGGUAGCAGUAGAA and UUCUACUGCUACCUUUCGG). Three shRNAs targeting pGSDME were cloned into the lentiviral vector pLKO.1. For lentivirus generation, 0.7 μg of psPAX2, 0.3 μg of pMD2.G, and 1 μg of shRNA constructs were cotransfected into HEK-293T cells. After 48 h of transfection, the lentivirus was harvested and further transduced into SK6 cells. The shRNA-expressing cells were selected with 1.5 μg/mL of puromycin and then plated into 12-well plates for further experiments. The sequences (5′–3′) of shRNAs were as follows: shRNA-1, GGCTCTTGAAGAGGAACATCC; shRNA-2, GCTGACATTAACCTGGAAATG; and shRNA-3, GGAGAACAATCGATCTGAAGA.
Western blotting.
Cell samples were harvested, lysed, and quantified by using a Pierce BCA protein assay kit. Then, the lysate samples were mixed with 5× loading buffer, boiled at 95°C for 10 min, subjected to 12% SDS-PAGE, and transferred onto polyvinylidene difluoride membranes. The membranes were blocked, washed, and incubated with the indicated primary and secondary antibodies. After intensive washing, protein bands were detected using an ECL system and analyzed with Image Lab software 4.0.1 (Bio-Rad, Hercules, CA).
Cytotoxicity assay.
Cell death was assessed by measuring cell viability or LDH release with a Cell Counting Kit-8 and an LDH cytotoxicity assay kit, respectively.
Propidium iodide staining.
Cell membrane integrity was assessed by observing the cells’ PI uptake. The infected or transfected cells were stained with PI at the indicated times. The fluorescence signal was observed by using an inverted fluorescence microscope (Ti-U-Nikon, Tokyo, Japan).
Quantitative reverse transcription-PCR.
Cell samples were collected, and total RNA was extracted with TRIpure Reagent, followed by reverse transcription with Hifair first-strand cDNA synthesis SuperMix. All qPCR assays were performed with Hieff UNICON Universal Blue qPCR SYBR green Master Mix on a ViiA 7 real-time PCR system (Applied Biosystems, Grand Island, NY) according to the manufacturer’s instructions.
Determination of TCID50.
BHK-21 cells were cultured in DMEM supplemented with 10% FBS and 1% Pen-Strep antibiotics at 37°C with 5% CO2 and seeded into 96-well plates. Then, the diluted virus was incubated with the cells for 3 days. The cytopathic effect was observed daily, and the virus titers were calculated according to the Reed-Muench method (49).
Coimmunoprecipitation assay.
HEK-293T cells were cultured in DMEM supplemented with 10% FBS and 1% Pen-Strep antibiotics at 37°C with 5% CO2 and seeded into 12-well plates. The cells were transfected with the indicated plasmids for 12 h. Then, the cell samples were harvested, lysed, and subjected to Western blotting and immunoprecipitation analysis. Briefly, the lysates were centrifuged at 4°C for 10 min, and the supernatants were incubated with anti-HA binding beads at 4°C for 4 h. Subsequently, the binding beads were washed with PBST six times and then denatured in 1× SDS-PAGE loading buffer for 10 min. Finally, the supernatants were analyzed by Western blotting.
Confocal immunofluorescence assay.
HEK-293T cells were seeded on coverslips in a 24-well plate and transfected with the indicated plasmids. After 12 h of transfection, cells were washed with phosphate-buffered saline (PBS) three times, fixed with 4% paraformaldehyde for 30 min at room temperature, permeabilized with 0.5% Triton X-100 for 20 min at –20°C, and further blocked with 2% bovine serum albumin for 2 h at room temperature. Subsequently, the blocked cells were incubated with the primary antibodies (rabbit anti-HA MAb or mouse anti-Flag MAb) for 2 h at 37°C. The cells were then washed with PBST three times and incubated with the secondary antibodies (Alexa Fluor 488-goat anti-rabbit antibody or Alexa Fluor 555-goat anti-mouse antibody) for 1 h at 37°C. Nuclei were stained with DAPI. After intensive washing, the fluorescence signal was analyzed using a laser scanning confocal microscope (LSM 510; Zeiss, USA).
Molecular docking analysis.
To analyze the binding affinities and modes of interaction between rupintrivir and FMDV 3Cpro, AutodockVina 1.2.2, a silico protein-ligand docking software, was employed (50, 51). The molecular structure of rupintrivir (AG7088) was retrieved from the EV71 3Cpro-AG7088 complex (PDB ID 4ght). The three-dimensional coordinate of FMDV 3Cpro (PDB ID 5hm2) was downloaded from the PDB (http://www.rcsb.org/pdb/home/home.do). For docking analysis, all protein and molecular files were converted into PDBQT format with all water molecules excluded and polar hydrogen atoms added. The grid box covered each protein’s domain and accommodated free molecular movement. Molecular docking studies were performed by Autodock Vina 1.2.2 (http://autodock.scripps.edu/) and visualized using the PyMOL 2.5 software (DeLano Scientific LLC).
Sequence alignment and logo analysis.
Porcine GSDME (GenBank accession no. XM_003134855.4), human GSDME (GenBank accession no. NM_004403.3), and mouse GSDME (GenBank accession no. NM_018769.4) amino acid sequences were obtained from GenBank (https://www.ncbi.nlm.nih.gov/nuccore/). Sequence alignment was performed by CLUSTALW (https://www.genome.jp/tools-bin/clustalw) and visualized with BioEdit v7.2.5 (http://www.mbio.ncsu.edu/BioEdit/bioedit.html). Logo analysis of the cleavage sites predicted from the polyprotein cleavage of pCaspase-3 or 3Cpro was generated by WebLogo (https://weblogo.threeplusone.com/).
Statistical analysis.
All data were analyzed using Prism 8.0 software (GraphPad Software, Inc., La Jolla, CA) and are presented as means ± the standard deviations (SD) of three independent experiments. Comparisons were performed through one- or two-way analysis of variance (ANOVA), followed by Tukey’s test. A P value of <0.05 was considered statistically significant.
ACKNOWLEDGMENTS
This study was supported by the National Program on Key Research Project of China (2021YFD1800300) and the National Natural Science Foundation of China (32072841).
P.Q., X.L., and Z.L. designed the experiments. X.R., M.Y., Q.Z., Z.Z., and H.W. performed the experiments. X.R. analyzed the data. X.R. wrote the paper. P.Q., X.L., and Z.L. proofed the manuscript.
We declare there are no conflicts of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Zengjun Lu, Email: luzengjun@caas.cn.
Xiangmin Li, Email: lixiangmin@mail.hzau.edu.cn.
Ping Qian, Email: qianp@mail.hzau.edu.cn.
Rebecca Ellis Dutch, University of Kentucky College of Medicine.
REFERENCES
- 1.David W, Brown G. 2001. Foot and mouth disease in human beings. Lancet 357:1463. doi: 10.1016/S0140-6736(00)04670-5. [DOI] [PubMed] [Google Scholar]
- 2.Thomson GR, Vosloo W, Bastos AD. 2003. Foot and mouth disease in wildlife. Virus Res 91:145–161. doi: 10.1016/s0168-1702(02)00263-0. [DOI] [PubMed] [Google Scholar]
- 3.Sangare O, Bastos AD, Marquardt O, Venter EH, Vosloo W, Thomson GR. 2001. Molecular epidemiology of serotype O foot-and-mouth disease virus with emphasis on West and South Africa. Virus Genes 22:345–351. doi: 10.1023/a:1011178626292. [DOI] [PubMed] [Google Scholar]
- 4.Grubman MJ, Baxt B. 2004. Foot-and-mouth disease. Clin Microbiol Rev 17:465–493. doi: 10.1128/CMR.17.2.465-493.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curry S, Roqué-Rosell N, Zunszain PA, Leatherbarrow RJ. 2007. Foot-and-mouth disease virus 3C protease: recent structural and functional insights into an antiviral target. Int J Biochem Cell Biol 39:1–6. doi: 10.1016/j.biocel.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ekanayaka P, Shin SH, Weeratunga P, Lee H, Kim TH, Chathuranga K, Subasinghe A, Park JH, Lee JS. 2021. Foot-and-mouth disease virus 3C protease antagonizes interferon signaling and C142T substitution attenuates the FMD virus. Front Microbiol 12:737031. doi: 10.3389/fmicb.2021.737031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdullah SW, Han S, Wu J, Zhang Y, Bai M, Jin Y, Zhi X, Guan J, Sun S, Guo H. 2020. The DDX23 negatively regulates translation and replication of foot-and-mouth disease virus and is degraded by 3C proteinase. Viruses 12. doi: 10.3390/v12121348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu W, Yang D, Sun C, Wang H, Zhao B, Zhou G, Yu L. 2020. hnRNP K is a novel internal ribosomal entry site-transacting factor that negatively regulates foot-and-mouth disease virus translation and replication and is antagonized by viral 3C protease. J Virol 94:e00803-20. doi: 10.1128/JVI.00803-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yi J, Peng J, Ren J, Zhu G, Ru Y, Tian H, Li D, Zheng H. 2021. Degradation of host proteins and apoptosis induced by foot-and-mouth disease virus 3C protease. Pathogens 10. doi: 10.3390/pathogens10121566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim H, Kim AY, Choi J, Park SY, Park SH, Kim JS, Lee SI, Park JH, Park CK, Ko YJ. 2021. Foot-and-mouth disease virus evades innate immune response by 3C-targeting of MDA5. Cells 10. doi: 10.3390/cells10020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi J, Gao W, Shao F. 2017. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci 42:245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 12.He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, Han J. 2015. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res 25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali MF, Dasari H, Van Keulen VP, Carmona EM. 2017. Canonical stimulation of the NLRP3 inflammasome by fungal antigens links innate and adaptive B-lymphocyte responses by modulating IL-1β and IgM production. Front Immunol 8:1504. doi: 10.3389/fimmu.2017.01504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Błażejewski AJ, Thiemann S, Schenk A, Pils MC, Gálvez EJC, Roy U, Heise U, de Zoete MR, Flavell RA, Strowig T. 2017. Microbiota normalization reveals that canonical caspase-1 activation exacerbates chemically induced intestinal inflammation. Cell Rep 19:2319–2330. doi: 10.1016/j.celrep.2017.05.058. [DOI] [PubMed] [Google Scholar]
- 15.Yokoyama S, Cai Y, Murata M, Tomita T, Yoneda M, Xu L, Pilon AL, Cachau RE, Kimura S. 2018. A novel pathway of LPS uptake through syndecan-1 leading to pyroptotic cell death. Elife 7. doi: 10.7554/eLife.37854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casson CN, Yu J, Reyes VM, Taschuk FO, Yadav A, Copenhaver AM, Nguyen HT, Collman RG, Shin S. 2015. Human caspase-4 mediates noncanonical inflammasome activation against Gram-negative bacterial pathogens. Proc Natl Acad Sci U S A 112:6688–6693. doi: 10.1073/pnas.1421699112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viganò E, Diamond CE, Spreafico R, Balachander A, Sobota RM, Mortellaro A. 2015. Human caspase-4 and caspase-5 regulate the one-step non-canonical inflammasome activation in monocytes. Nat Commun 6:8761. doi: 10.1038/ncomms9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Gao W, Shi X, Ding J, Liu W, He H, Wang K, Shao F. 2017. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547:99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Z, He H, Wang K, Shi X, Wang Y, Su Y, Wang Y, Li D, Liu W, Zhang Y, Shen L, Han W, Shen L, Ding J, Shao F. 2020. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science 368. doi: 10.1126/science.aaz7548. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, Zhang Y, Xia S, Kong Q, Li S, Liu X, Junqueira C, Meza-Sosa KF, Mok TMY, Ansara J, Sengupta S, Yao Y, Wu H, Lieberman J. 2020. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 579:415–420. doi: 10.1038/s41586-020-2071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong S, Shi Y, Dong X, Xiao X, Qi J, Ren L, Xiang Z, Zhou Z, Wang J, Lei X. 2022. Gasdermin E is required for induction of pyroptosis and severe disease during enterovirus 71 infection. J Biol Chem 298:101850. doi: 10.1016/j.jbc.2022.101850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Z, Li Q, Ashraf U, Yang M, Zhu W, Gu J, Chen Z, Gu C, Si Y, Cao S, Ye J. 2022. Zika virus causes placental pyroptosis and associated adverse fetal outcomes by activating GSDME. Elife 11. doi: 10.7554/eLife.73792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou J, Zhao R, Xia W, Chang CW, You Y, Hsu JM, Nie L, Chen Y, Wang YC, Liu C, Wang WJ, Wu Y, Ke B, Hsu JL, Huang K, Ye Z, Yang Y, Xia X, Li Y, Li CW, Shao B, Tainer JA, Hung MC. 2020. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat Cell Biol 22:1396. (Author correction.) doi: 10.1038/s41556-020-00599-1. [DOI] [PubMed] [Google Scholar]
- 24.Zeng CY, Li CG, Shu JX, Xu LH, Ouyang DY, Mai FY, Zeng QZ, Zhang CC, Li RM, He XH. 2019. ATP induces caspase-3/gasdermin E-mediated pyroptosis in NLRP3 pathway-blocked murine macrophages. Apoptosis 24:703–717. doi: 10.1007/s10495-019-01551-x. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Yu H, Zhuang W, Chen J, Jiang Y, Guo Z, Huang X, Liu Q. 2022. Cell pyroptosis in picornavirus and its potential for treating viral infection. J Med Virol 94:3570–3580. doi: 10.1002/jmv.27813. [DOI] [PubMed] [Google Scholar]
- 26.Wen W, Li X, Wang H, Zhao Q, Yin M, Liu W, Chen H, Qian P. 2021. Seneca valley virus 3C protease induces pyroptosis by directly cleaving porcine gasdermin D. J Immunol 207:189–199. doi: 10.4049/jimmunol.2001030. [DOI] [PubMed] [Google Scholar]
- 27.Lei X, Zhang Z, Xiao X, Qi J, He B, Wang J. 2017. Enterovirus 71 inhibits pyroptosis through cleavage of gasdermin D. J Virol 91:e01069-17. doi: 10.1128/JVI.01069-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taabazuing CY, Okondo MC, Bachovchin DA. 2017. Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages. Cell Chem Biol 24:507–514.e4. doi: 10.1016/j.chembiol.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallach D, Kang TB, Dillon CP, Green DR. 2016. Programmed necrosis in inflammation: toward identification of the effector molecules. Science 352:aaf2154. doi: 10.1126/science.aaf2154. [DOI] [PubMed] [Google Scholar]
- 30.Man SM, Kanneganti TD. 2015. Gasdermin D: the long-awaited executioner of pyroptosis. Cell Res 25:1183–1184. doi: 10.1038/cr.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. 2015. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 32.Place DE, Lee S, Kanneganti TD. 2021. PANoptosis in microbial infection. Curr Opin Microbiol 59:42–49. doi: 10.1016/j.mib.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samir P, Malireddi RKS, Kanneganti TD. 2020. The PANoptosome: a deadly protein complex driving Pyroptosis, Apoptosis, and Necroptosis (PANoptosis). Front Cell Infect Microbiol 10:238. doi: 10.3389/fcimb.2020.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertheloot D, Latz E, Franklin BS. 2021. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell Mol Immunol 18:1106–1121. doi: 10.1038/s41423-020-00630-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang R, Xu J, Zhang B, Liu J, Liang C, Hua J, Meng Q, Yu X, Shi S. 2020. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J Hematol Oncol 13:110. doi: 10.1186/s13045-020-00946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grubman MJ, Zellner M, Bablanian G, Mason PW, Piccone ME. 1995. Identification of the active-site residues of the 3C proteinase of foot-and-mouth disease virus. Virology 213:581–589. doi: 10.1006/viro.1995.0030. [DOI] [PubMed] [Google Scholar]
- 37.Ramajayam R, Tan KP, Liang PH. 2011. Recent development of 3C and 3CL protease inhibitors for anti-coronavirus and anti-picornavirus drug discovery. Biochem Soc Trans 39:1371–1375. doi: 10.1042/BST0391371. [DOI] [PubMed] [Google Scholar]
- 38.Jung E, Lee JY, Kim HJ, Ryu CK, Lee KI, Kim M, Lee CK, Go YY. 2018. Identification of quinone analogues as potential inhibitors of picornavirus 3C protease in vitro. Bioorg Med Chem Lett 28:2533–2538. doi: 10.1016/j.bmcl.2018.05.046. [DOI] [PubMed] [Google Scholar]
- 39.van der Linden L, Ulferts R, Nabuurs SB, Kusov Y, Liu H, George S, Lacroix C, Goris N, Lefebvre D, Lanke KH, De Clercq K, Hilgenfeld R, Neyts J, van Kuppeveld FJ. 2014. Application of a cell-based protease assay for testing inhibitors of picornavirus 3C proteases. Antiviral Res 103:17–24. doi: 10.1016/j.antiviral.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aglietti RA, Dueber EC. 2017. Recent insights into the molecular mechanisms underlying pyroptosis and gasdermin family functions. Trends Immunol 38:261–271. doi: 10.1016/j.it.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Shi F, Lv Q, Wang T, Xu J, Xu W, Shi Y, Fu X, Yang T, Yang Y, Zhuang L, Fang W, Gu J, Li X. 2022. Coronaviruses Nsp5 antagonizes porcine gasdermin D-mediated pyroptosis by cleaving pore-forming p30 fragment. mBio 13:e02739-21. doi: 10.1128/mbio.02739-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao G, Li T, Liu X, Zhang T, Zhang Z, Kang L, Song J, Zhou S, Chen X, Wang X, Li J, Huang L, Li C, Bu Z, Zheng J, Weng C. 2022. African swine fever virus cysteine protease pS273R inhibits pyroptosis by noncanonically cleaving gasdermin D. J Biol Chem 298:101480. doi: 10.1016/j.jbc.2021.101480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang C, Ruan J. 2023. An ancient defense mechanism: conservation of gasdermin-mediated pyroptosis. PLoS Biol 21:e3002103. doi: 10.1371/journal.pbio.3002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kovacs SB, Miao EA. 2017. Gasdermins: effectors of pyroptosis. Trends Cell Biol 27:673–684. doi: 10.1016/j.tcb.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiu S, Liu J, Xing F. 2017. “Hints” in the killer protein gasdermin D: unveiling the secrets of gasdermins driving cell death. Cell Death Differ 24:588–596. doi: 10.1038/cdd.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM. 2015. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 47.Sun X, Liu Y, Huang Z, Xu W, Hu W, Yi L, Liu Z, Chan H, Zeng J, Liu X, Chen H, Yu J, Chan FKL, Ng SC, Wong SH, Wang MH, Gin T, Joynt GM, Hui DSC, Zou X, Shu Y, Cheng CHK, Fang S, Luo H, Lu J, Chan MTV, Zhang L, Wu WKK. 2022. SARS-CoV-2 nonstructural protein 6 triggers NLRP3-dependent pyroptosis by targeting ATP6AP1. Cell Death Differ 29:1240–1254. doi: 10.1038/s41418-021-00916-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao J, Wang Q, Tang YD, Zhai J, Hu W, Zheng C. 2023. When ferroptosis meets pathogenic infections. Trends Microbiol 31:468–479. doi: 10.1016/j.tim.2022.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Lei C, Yang J, Hu J, Sun X. 2021. On the calculation of TCID50 for quantitation of virus infectivity. Virol Sin 36:141–144. doi: 10.1007/s12250-020-00230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eberhardt J, Santos-Martins D, Tillack AF, Forli S. 2021. AutoDock Vina 1.2.0: new docking methods, expanded force field, and Python bindings. J Chem Infect Model 61:3891–3898. doi: 10.1021/acs.jcim.1c00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trott O, Olson AJ. 2010. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S4 and Table S1. Download jvi.00686-23-s0001.docx, DOCX file, 2.3 MB (2.3MB, docx)