ABSTRACT
HIV-1 (HIV) infects CD4+ T cells, the gradual depletion of which can lead to AIDS in the absence of antiretroviral therapy (ART). Some cells, however, survive HIV infection and persist as part of the latently infected reservoir that causes recurrent viremia after ART cessation. Improved understanding of the mechanisms of HIV-mediated cell death could lead to a way to clear the latent reservoir. Death induced by survival gene elimination (DISE), an RNA interference (RNAi)-based mechanism, kills cells through short RNAs (sRNAs) with toxic 6-mer seeds (positions 2 to 7 of sRNA). These toxic seeds target the 3′ untranslated region (UTR) of mRNAs, decreasing the expression of hundreds of genes critical for cell survival. In most cells under normal conditions, highly expressed cell-encoded nontoxic microRNAs (miRNAs) block access of toxic sRNAs to the RNA-induced silencing complex (RISC) that mediates RNAi, promoting cell survival. HIV has been shown to inhibit the biogenesis of host miRNAs in multiple ways. We now report that HIV infection of cells deficient in miRNA expression or function results in enhanced RISC loading of an HIV-encoded miRNA HIV-miR-TAR-3p, which can kill cells by DISE through a noncanonical (positions 3 to 8) 6-mer seed. In addition, cellular RISC-bound sRNAs shift to lower seed viability. This also occurs after latent HIV provirus reactivation in J-Lat cells, suggesting independence of permissiveness of cells to viral infection. More precise targeting of the balance between protective and cytotoxic sRNAs could provide new avenues to explore novel cell death mechanisms that could be used to kill latent HIV.
IMPORTANCE Several mechanisms by which initial HIV infection is cytotoxic to infected cells have been reported and involve various forms of cell death. Characterizing the mechanisms underlying the long-term survival of certain T cells that become persistent provirus reservoirs is critical to developing a cure. We recently discovered death induced by survival gene elimination (DISE), an RNAi-based mechanism of cell death whereby toxic short RNAs (sRNAs) containing 6-mer seed sequences (exerting 6-mer seed toxicity) targeting essential survival genes are loaded into RNA-induced silencing complex (RISC) complexes, resulting in inescapable cell death. We now report that HIV infection in cells with low miRNA expression causes a shift of mostly cellular RISC-bound sRNAs to more toxic seeds. This could prime cells to DISE and is further enhanced by the viral microRNA (miRNA) HIV-miR-TAR-3p, which carries a toxic noncanonical 6-mer seed. Our data provide multiple new avenues to explore novel cell death mechanisms that could be used to kill latent HIV.
KEYWORDS: AIDS, RISC, RNA toxicity, RNAi, cell death
INTRODUCTION
Without effective treatment, HIV-1 (HIV) infection results in the widespread loss of CD4+ T cells and the onset of AIDS. Current combination antiretroviral therapy (ART) inhibits viral replication and rescues CD4+ T cell levels. However, ART is unable to clear the infection due to the persistence of the provirus in cellular reservoirs. The field is still searching for a cure for AIDS. Current avenues to achieve this include development of gene therapy, “block and lock,” specific immunotherapy, posttreatment controllers, and “shock-and-kill” strategies (reviewed in reference 1). The latter aims at clearing the persistent provirus reservoir through forced reactivation of silent proviruses and induction of viral- or immune-mediated cytopathicity of the virus-producing cells. These efforts, however, have been unsuccessful thus far. This is partly due to the resistance of CD4+ T cells that reactivate HIV to virus-induced cell death and to CD8+ T cell-mediated killing (2–6). Some mechanisms of resistance to immune cell-mediated killing have been suggested (7–10), but mechanisms of why these cells are resistant to death mediated by HIV replication/antigen expression remain under study. Several mechanisms by which initial HIV infection is cytotoxic to infected and/or bystander cells have been reported (11–16) and involve many viral genes (17–19) and various forms of cell death (20–25). Characterizing the mechanisms underlying the long-term survival of certain T cells that become persistent provirus reservoirs is critical to developing a cure.
RNA interference (RNAi) is a form of posttranscriptional regulation exerted by 19- to 22-nucleotide (nt)-long double-stranded RNAs (dsRNAs) that negatively regulate gene expression at the mRNA level. One of the two strands of the short dsRNA, the active guide strand, incorporates into the RNA-induced silencing complex (RISC) (26), and the other strand (the inactive passenger strand) is degraded (27). RNAi is used as an endogenous form of gene regulation largely through the expression of microRNAs (miRNAs), short noncoding RNAs that coordinate silencing across hundreds of gene targets. This form of silencing is mainly based on only a very short region of complete complementarity, the “seed,” at positions 2 to 7 or 8 of the guide strand (28, 29). Matching complementary regions (seed matches), predominantly located in the 3′ untranslated region (UTR) of mRNAs, are targeted (30, 31), and, in the case of miRNAs, nucleotides outside the seed also contribute to gene silencing (32). However, RNAi can be initiated with as little as a 6-nt base pairing between a 6-mer seed in the guide RNA and the targeted mRNA (28, 29). miRNA biogenesis begins in the nucleus with transcription of a primary miRNA precursor (33). They are first processed by the Drosha/DGCR8 microprocessor complex into premiRNAs (34), which are then exported from the nucleus to the cytoplasm by exportin 5 (XPO5) (35). Once in the cytoplasm, Dicer/TAR RNA-binding protein (TRBP) processes them further (36, 37), and these mature dsRNA duplexes are then loaded onto Argonaute (Ago) proteins to form the RISC (26).
We previously discovered death induced by survival gene elimination (DISE), a novel, tissue-independent, RNAi-based mechanism of cell death whereby toxic, 19-nt-long short RNAs (sRNAs) containing 6-mer seed sequences (exerting 6-mer seed toxicity) targeting essential survival genes are loaded into RISC complexes, resulting in inescapable cell death (38, 39). The most toxic seeds were identified in arrayed viability screens of all possible 4,096 6-mer seeds in a double-stranded shared sRNA backbone on three human and three mouse cell lines, and the most toxic 6-mer seeds were identified as being G rich (38, 40). G-rich 6-mer seeds were found to be present in a number of tumor-suppressive miRNAs, including miR-34a,c/449-5p and miR-15a,b/16-5p, and most of their cytotoxic activity could be attributed to 6-mer seed toxicity (38, 40). We provided evidence that the kill code is ancient (38), suggesting that viruses may also have adopted it. DISE includes hallmarks of many different cell death pathways, including accumulation of DNA damage, activation of caspases, and mitochondrial stress (41). We previously tested 215 miRNAs encoded by 17 human-pathogenic viruses, including HIV (42), and provided evidence that many virus-encoded miRNAs (v-miRNAs) have the capacity to kill cells through 6-mer seed toxicity (42). This was done by comparing the toxic activity of each of these v-miRNAs with just their 6-mer seed embedded in the 19-nt-long double-stranded RNA backbone used in the large screens. The toxicities of these pairs were highly similar. Many of them were predicted to target mRNAs essential for cell survival using a noncanonical 6-mer seed located at positions 3 to 8 of the RISC-bound sRNA (R-sRNA).
HIV has been shown to encode four v-miRNAs (as deposited in miRBase 22.1). Hiv1-miR-N367 has been reported to suppress the HIV gene nef (43). Hiv1-miR-H1 can suppress cellular coding genes and miRNAs involved in the regulation of apoptosis (i.e., Bcl-2) and RNAi (i.e., Dicer) (44). Finally, the HIV TAR element can give rise to two mature miRNAs: hiv1-miR-TAR-3p and hiv1-miR-TAR-5p (45). These miRNAs were found to be loaded into the RISC, and miR-TAR-3p targets a number of apoptosis-regulating host genes, including caspase-8 (46). Of note, caspase-8 is also suppressed in J-Lat cells, a derivative of Jurkat T cells that carry a transcriptionally latent HIV provirus and are often used to study latency reactivation (47). These v-miRNAs could therefore be important for cell survival and provirus persistence.
We recently proposed four different mechanisms that ensure that normal cells do not die by DISE (48). Normal tissues may be protected by high expression of miRNAs with nontoxic 6-mer seeds. This protection likely evolved to prevent G/C-rich degradation products of highly abundant cellular RNAs (i.e., tRNA or rRNAs) from entering the RISC. This raised the question of what would happen if the number of protective miRNAs was reduced in cells. We showed that the deletion/downregulation of Drosha, Dicer, or XPO5 results in a general loss/reduction of miRNAs (38, 49) and renders cells hypersensitive to DISE (38, 39, 50–52).
HIV infection of both CD4+ T cells and macrophages has been shown to affect both miRNA biogenesis and RNAi in infected cells. HIV suppresses the polycistronic miRNA cluster miR-17/92 and members of the let-7 miRNA family, both highly abundant miRNA families that carry nontoxic seeds (53, 54). Viral gene products have been reported to affect miRNA processing. miR-210 and miR-222 are upregulated by HIV in the T cell line supT1 and silence Dicer expression (55). Dicer expression can also be suppressed via the viral gene Vpr (56). Monocytes in general do not express Dicer, and HIV suppresses Dicer expression in differentiating monocytes, resulting in a reduction of miRNAs being produced (56). Finally, HIV TAT binds TAR RNA-binding protein (TRBP), attenuating the RNAi machinery (57), and HIV-Tat and Rev can suppress TRBP and the protein activator of PKR (PACT), which are both Dicer-associated RNAi components (58). We therefore wondered what effects HIV infection can have on cells with low protective miRNA expression.
We now report that infection with HIV results in a shift of host RISC-bound sRNAs (R-sRNAs) to lower 6-mer seed viability, a phenomenon we recently described to contribute to therapy sensitivity of ovarian cancer (51). This is also aided by expression and uptake into the RISC of the HIV-encoded v-miRNA miR-TAR-3p, which we determined to be highly toxic through its use of a noncanonical 3 to 8 6-mer seed. Importantly, depleting cells of miRNAs renders them much more sensitive to HIV-induced death. This was observed with deletion of the miRNA biogenesis genes Drosha, Dicer, or XPO5 and also with deletion of the RISC component Ago2. These findings add to the characterization of mechanisms of HIV-induced cell death with implications for future improvement of “shock-and-kill” approaches to an HIV cure during suppressive ART.
RESULTS
Vesicular stomatitis virus glycoprotein (VSV-G) pseudotyped HIV kills HCT116 cells that lack expression of genes required to generate mature miRNAs more effectively.
We recently reported that the human Kaposi sarcoma-associated herpesvirus-encoded v-miRNA miR-K12-6-5p induces DISE through a 6-mer seed shared with miR-15/16-5p (42). Interestingly, this shared seed match was not located at positions 2 to 7 but at positions 3 to 8 of the guide stand of the mature v-miRNA. This was validated by comparing the targeting of the mature miRNA with that of the sRNA backbone used in the viability screens containing exclusively either the canonical (2 to 7) or the noncanonical (3 to 8) 6-mer seed. Comparing the DISE-inducing activity of 215 v-miRNAs with those of their 6-mer seed-containing sRNAs revealed a high degree of correlation for the v-miRNAs in many viruses, suggesting that many v-miRNAs have the potential to kill cells by DISE, possibly using the noncanonical 3 to 8 seed (42). This analysis included the four mature v-miRNAs encoded by HIV deposited at miRBase. When comparing the viability of cells transfected with HIV v-miRNAs (Fig. 1A, x axis) with those of the sRNAs just containing the 6-mer seed of that miRNA (Fig. 1A, y axis), the ability of the four HIV-encoded v-miRNAs to affect cell viability showed a very high correlation with the activity of the four sRNAs just containing their noncanonical 6-mer seeds (r = 0.97; the analysis was based on the updated screening data on three human cell lines; https://www.6merdb.org). This analysis suggested that HIV-encoded v-miRNAs may have evolved to use 6-mer seed toxicity to affect cell fate and that they may be using a noncanonical 3 to 8 seed.
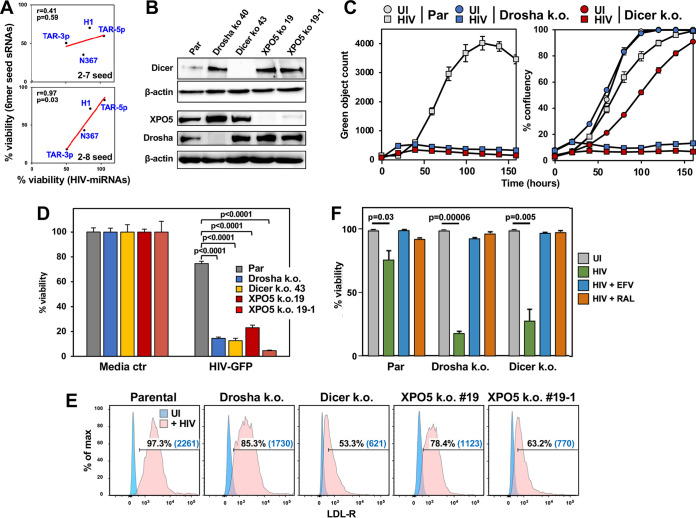
FIG 1.
HIV kills HCT116 cells lacking Drosha, Dicer, or XPO5 more effectively. (A) Regression analysis showing correlations between the toxicity of the four putative HIV miRNAs (x axis) and four sRNA duplexes carrying only the v-miRNA 6-mer seed in a shared backbone (y axis) as tested previously in three human cell lines (38, 40). The data were analyzed in two ways, comparing the full-length v-miRNAs (e.g., miR-TAR-3p with the sRNAs containing the canonical [top] or noncanonical 6-mer seed [bottom]). P values were calculated using Pearson correlation analysis. (B) Western blotting of parental (Par) HCT116 cells and Dicer-k.o., Drosha-k.o., or two XPO5-k.o. HCT116 clones. (C) Change in green object count (HIV-infected cells; left) and confluence (right) over time in HCT116 parental cells and Drosha-k.o. or Dicer-k.o. cells. Cells were infected with VSV-G pseudotyped HIV R9 ΔEnv iGFP virus (175 ng of p24/mL) (59); UI, uninfected. (D) Viability of the HCT116 parental and mutant cells determined 72 h after infection with 2.5% VSV-G pseudotyped HIV supernatant. Student’s t test P values are displayed; ctr, control. (E) Surface staining of LDL-R in all cell lines shown in B. Percent positivity and median fluorescence intensity (in parentheses) are shown. (F) Parental cells and Drosha-k.o. or Dicer-k.o. HCT116 cells were infected with VSV-G pseudotyped HIV R9 ΔEnv iGFP virus (175 ng of p24/mL) in the presence of either 10 μM efavirenz (EFA) or 10 μM raltegravir (RAL), as indicated. Seventy-two hours after challenge, cell death was quantified. Student’s t test P values are shown.
To test how cells with low miRNA expression respond to HIV infection, we infected parental wild-type (Par), Drosha-knockout (k.o.), and Dicer-k.o. HCT116 cells, (Fig. 1B) with a VSV-G pseudotyped HIV R9 ΔEnv inverted green fluorescent protein (iGFP) reporter virus described previously (59). We chose HCT116 cells for these experiments as they provide a standardized way to test whether a cell death stimulus has a contribution of 6-mer seed toxicity, as used previously (38, 39, 50), and these are the only cells we have tested or generated that can tolerate a complete deletion of miRNA biogenesis enzymes. Tracking virus-infected (GFP+) cells in an IncuCyte Zoom microscope over 7 days showed a significant reduction in GFP positivity of the two mutant cell lines compared to in the parental cells (Fig. 1C, left). This was likely due to the observation that HIV-infected Drosha- and Dicer-k.o. cells succumbed to cell death much more quickly than parental cells (Fig. 1C, right). An increased sensitivity of these k.o. cells to HIV-induced cell death was confirmed in a cell death assay (Fig. 1D). Interestingly, this was also seen with HCT116 XPO5-deficient cells, which are also defective in producing most mature miRNAs (49); both XPO5-k.o. clones tested were hypersensitive to HIV-induced cell death. VSV-G pseudotyped HIV enters cells through the low-density lipoprotein receptor (LDL-R) (60), which could be regulated by cellular miRNAs (c-miRNAs), and, hence, in the absence of these miRNAs in the mutant cells, LDL-R could be upregulated, potentially explaining the increased effect of HIV on these cells. However, flow cytometry analysis showed that none of the mutant cells had upregulated surface LDL-R expression. By contrast, all the mutant cells showed reduced LDL-R relative to parental cells (Fig. 1E). To test whether the HIV-induced cytotoxicity is due to early viral replication events, we treated parental, Dicer- and Drosha-k.o. cells with either the reverse transcriptase (RT) inhibitor efavirenz (EFA) or the integrase inhibitor raltegravir (RAL). Cells were infected as before, and cell viability was monitored 3 days after infection (Fig. 1F). Both inhibitors completely prevented HIV-induced cell death. This indicates that postintegration events, rather than the viral RNA, RNA-DNA hybrid, or reverse-transcribed DNA, cause enhanced death of Drosha- and Dicer-k.o. cells. These results are consistent with a model wherein HIV induces cell death through its gene products, RNAs produced by the virus, or cellular changes in the infected cells. The fact that cells devoid of genes required for the generation of canonical miRNAs were hypersensitive to this form of cell death suggested that R-sRNAs, either virus or host cell derived, could be involved in regulating cell survival.
Dicer or Drosha deletion results in an increase in virus-derived sRNAs in the RISC.
To directly determine whether HIV-induced toxicity in cells with decreased miRNA biogenesis involved changes in R-sRNAs, we performed an Ago1 to 4-RNA precipitation combined with high-throughput sequencing (Ago-RP-Seq) of parental and Drosha- and Dicer-k.o. HCT116 cells infected with VSV-G pseudotyped HIV as previously described (38, 50, 51). Infection with HIV did not have a major effect on the total miRNA content in the parental cell line or in the two k.o. cell lines (Fig. 2A). As expected, the two k.o. cell lines had very low miRNA content, and many of the R-sRNAs were not c-miRNAs. Consistent with a previous analysis (61), only a few HIV-derived reads were found in the RISC of parental cells (Fig. 2B). By contrast, both Dicer- and Drosha-k.o. HCT116 cells took up greater than 5 to 10 times more HIV-derived reads into their RISC (Fig. 2C to E).
FIG 2.
Increase in virus-derived R-sRNAs in HIV-infected Drosha- or Dicer-k.o. cells. (A) Percentage of c-miRNAs bound to RISC of parental and Drosha-k.o. cells (left) or parental and Dicer-k.o. cells (right) uninfected (−) or infected (+) with VSV-G pseudotyped HIV. (B) Percentage of HIV-derived reads from miR-TAR-3p in the two experiments shown in A. (C) RawCounts of viral R-sRNAs in cells as described in A. (D and E) Whole HIV genome alignment of reads pulled down with Ago proteins in parental and Drosha-k.o. HCT116 cells (D) or in parental and Dicer-k.o. HCT116 cells (E) infected with HIV as in A. Each horizontal line represents one read. Reads from both replicates per condition are displayed. miR-TAR-3p-derived reads are shown in red. (F) Schematic of the HIV-1 genome. The location of the TAR loop is indicated in orange. Note, the ΔEnv virus still expresses robust amounts of ENV-derived RNA (see reads aligned to the ENV gene), as it carries a complete ENV gene with both a KDEL endoplasmic reticulum (ER) retention signal and a stop codon included (95). While the reads from the TAR loop aligned at both the 5′ and 3′ LTR, it was previously shown that the miR-TAR-derived reads are derived from the 5′ TAR loop (96).
Multiple HIV-derived miRNAs have been described (44, 46, 61, 62). In a comprehensive analysis of HIV-derived RNAs (63), the sequences of reads derived from the HIV TAR RNA element, which is the source of the putative HIV-derived miRNA miR-TAR, were provided (61) (Fig. S1A in the supplemental material). TAR-derived reads came from both arms of the TAR hairpin (Fig. S1B). In the hairpin, the two read clusters detected are juxtaposed with 3′ overhangs, which would have predicted processing by Dicer and loading into the RISC (64, 65), and, consequently, miR-TAR processing has been reported to be dependent on Dicer in infected 293T cells (45). The majority of the HIV-derived R-sRNAs in our analyses aligned with the HIV UTR and the TAR loop, the location of the putative v-miRNA miR-TAR (46) (Fig. 2D to F, red reads). Interestingly, while they constitute a smaller fraction of all HIV-derived R-sRNAs than the Drosha-k.o. cells, reads derived from the processed mature v-miRNA miR-TAR-3p were still loaded into the RISC in larger quantities in cells completely deficient of Dicer (49) (Fig. 2B), suggesting that they can be generated without the involvement of Dicer, at least in HCT116 cells.
HIV infection shifts the balance of R-sRNAs to lower seed viability in HCT116 cells.
We previously reported that the ratio of R-sRNAs with toxic versus nontoxic 6-mer seeds can prime cells for DISE (51). We therefore tested whether infection with HIV influenced the average seed viability of either all R-sRNAs or virus-derived R-sRNAs. We used SPOROS (Greek for seed), a recently developed bioinformatics pipeline that allows analysis of RNA sequencing (RNA-seq) data in a gene-agnostic way solely based on the predicted 6-mer seed viability of each read (66). One of the SPOROS outputs is a seed viability graph, which plots the read counts of all R-sRNAs (y axis) against the predicted cell viability based on our screen of all 4,096 6-mer seeds (x axis) (38, 40). We analyzed two independent experiments, one comparing parental cells with Drosha-k.o. cells (Fig. 3A) and the other comparing parental cells with Dicer-k.o. cells (Fig. 3B). As reported previously, in parental cells, greater than 95% of R-sRNAs were miRNAs with mostly nontoxic seeds (Fig. 3A and B, top left). By contrast, many sRNAs other than miRNAs (e.g., tRNA fragments) entered the RISC (labeled in black) in Dicer-k.o. cells (Fig. 3B, top right) and to a greater extent in Drosha-k.o. cells (Fig. 3A, top right).
FIG 3.
HIV infection results in displacement of nontoxic cellular R-sRNAs with toxic c-miRNAs and highly toxic v-miRNAs. (A and B) Seed viability graphs of all R-sRNAs in parental and Drosha-k.o. cells (A) or parental and Dicer-k.o. cells (B) uninfected (UI) or 28 h after infection with VSV-G pseudotyped HIV. miRNAs that contribute to peaks with greater than 5,000 reads are labeled. For each labeled peak, miRNAs are listed in the order of abundance. For both experiments, a seed viability graph is shown with only the v-miRNAs (in green). Three miR-TAR-3p isomiRs with their respective 2 to 7 seed are labeled in red. (C) Seed viability box plots showing the median seed viability (%) of R-sRNAs significantly up- or downregulated (>1.5×, P < 0.05) in parental, Drosha-k.o., or Dicer-k.o. cells after HIV infection. The median is highlighted.
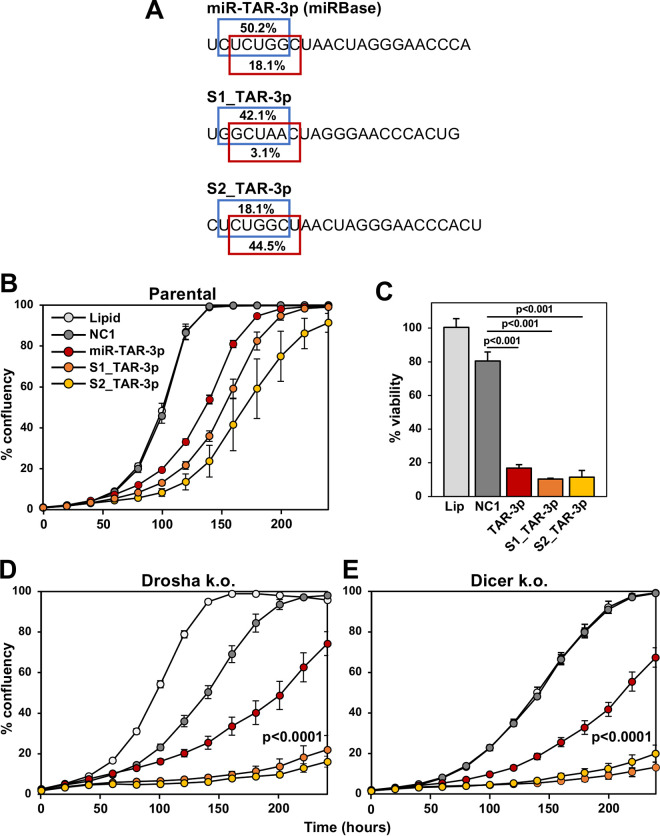
Most of the HIV-derived R-sRNAs in Drosha-k.o. cells were from miR-TAR, with the most abundant ones from the 3p arm of this v-miRNA (Fig. 3A, right bottom). Interestingly, three different species (isomiRs) were detected (labeled in red as miR-TAR-3p, S1_TAR-3p, and S2_TAR-3p). While the number of miR-TAR-derived reads was relatively small, we recently showed that very small numbers of CD95L-derived R-sRNAs with toxic seeds could kill HeyA8 ovarian cancer cells through RNAi (50). Interestingly, the relative amounts of miR-TAR-3p isomiRs detected in the RISC of Dicer-k.o. cells (Fig. 3B, bottom right) were different from the ones found in Drosha-k.o. cells (Fig. 3A, bottom right), suggesting that a nuclease other than Dicer could be generating these reads.
To determine overall changes in R-sRNAs with respect to their seed viability, we plotted the median seed viability of all R-sRNAs (a SPOROS output) in the different genotypes (Fig. 3C). HIV infection caused a general enrichment of R-sRNAs carrying toxic seeds versus nontoxic seeds (Fig. 3C). This was mainly due to a shift in abundant cellular R-sRNAs to more toxic ones. These findings were confirmed in an independent set of experiments directly comparing the response of parental cells and Drosha- and Dicer-k.o. cells (Fig. S2). In summary, these data are consistent with previous reports of HIV infection suppressing expression of c-miRNAs that we determined to carry nontoxic 6-mer seeds (53, 54). At the same time, virus-derived sRNAs appeared in the RISC (mostly miR-TAR-3p). Our data indicate that HIV infection can shift the balance from nontoxic to more toxic cellular R-sRNAs, which is most pronounced in cells with low miRNA expression.
HIV-miR-TAR-3p contains a highly toxic noncanonical 6-mer seed.
In our analysis of HIV-derived R-sRNAs in Drosha-k.o. cells, we found three different miR-TAR-3p isomiRs with different start sites and hence different predicted 6-mer seeds (Fig. 4A). For the two main isomiRs (the miRBase-annotated version and version S1), the noncanonical 6-mer seed (positions 3 to 8) was predicted to be more toxic than the canonical seed (positions 2 to 7; https://www.6merdb.org). Toxicity of the isomiRs was established by transfecting them at 1 nM (Fig. 4B) or at 10 nM (Fig. 4C) into HCT116 cells. As expected, both Dicer- and Drosha-k.o. cells with reduced amounts of c-miRNAs were more susceptible to this toxicity than parental cells (Fig. 4B, D, and E). Toxicity stemmed from the 6-mer seed, as shown by transfecting HCT116 cells with 1 nM sRNAs that only differed by their 2 to 7 or the more toxic 3 to 8 seeds present in the two main isomiRs (Fig. 5A to C). Again, the sRNAs were more potent when transfected into Drosha-k.o. cells (Fig. 5D). These data suggested that the main HIV-derived reads in the RISC of infected HCT116 cells were from the HIV miRNA miR-TAR-3p, which may therefore contribute to the cytotoxicity of HIV through 6-mer seed toxicity. This was also consistent with the observation that cells that lack most canonical c-miRNAs were hypersensitive to cell death induced by transfection with small amounts of miR-TAR-3p, implicating their contribution to toxicity in HIV-infected cells.
FIG 4.
HIV-miR-TAR-3p and its isomiRs can kill cells through DISE. (A) Three miR-TAR-3p isomiRs detected in the RISC of HIV-infected cells. Top, the canonical miR-TAR-3p as deposited in miRbase. Middle, S1_miR-TAR-3p, an isomiR with the 5′ end four nucleotides downstream from the canonical start site. Bottom, S2_miR-TAR-3p, an isomiR with the 5′ end one nucleotide downstream from the canonical start site. In each case, the predicted seed viability (in percent) of the canonical 2 to 7 (blue) and the noncanonical 3 to 8 (red) 6-mer seed is indicated. (B, D, and E) Confluency over time of HCT116 parental (B), Drosha-k.o. (D), or Dicer-k.o. (E) cells treated with lipid alone or transfected with 1 nM control miRNA NC1, HIV-miR-TAR-3p, S1_miR-TAR-3p, or S2_miR-TAR-3p. Samples were analyzed in quadruplicate, and standard error (SE) is shown. P values were calculated using a polynomial distribution test and support a difference between the effects of S1_TAR-3p in parental and Drosha- and Dicer-k.o. cells (versus NC1). (C) Cell viability (ATP assay) of parental HCT116 cells 72 h after transfection with the indicated v-miRNAs at a concentration of 10 nM; Lip, lipid-treated control. Student’s t test P values are shown.
FIG 5.
HIV-miR-TAR-3p isomiRs most effectively kill cells through a noncanonical 6-mer seed. (A) Structure of sRNAs containing the seeds (positions 2 to 7 or 3 to 8) of HIV-miR-TAR-3p and its isomer S1. Seeds are shown in bold. Nucleotides shown in blue are part of the shared double-stranded sRNA backbone in which all 6-mer seeds were tested in the screens of 4,096 6-mer seeds (38, 40). Underlined nucleotides are 2′-O-methylated to prevent loading of the sense strand into the RISC; sNT1, nontoxic control; s, sense; as, antisense. (B and D) Confluency over time of HCT116 parental (B) or Drosha-k.o. cells (D) treated with lipid alone or transfected with 1 nM sNT1, sTAR-3p (2 to 7 seed), sTAR-3p (3 to 8 seed), sS1_TAR-3p (2 to 7 seed), or sS1_TAR-3p (3 to 8 seed). Samples were analyzed in quadruplicate, and SE is shown. P values were calculated using a polynomial distribution test and support a difference between the effects of sTAR-3p (3 to 8) and sS1_TAR-3p (3 to 8) in parental and Drosha-k.o. cells (versus sNT1). (C) Cell viability (ATP assay) of parental HCT116 cells 96 h after transfection with the indicated sRNAs at a concentration of 1 nM; Lip, lipid-treated control. Student’s t test P values are shown. (E and F) Sylamer analysis (6-mers in E, 7-mers in F) for the list of open reading frames (ORFs; left) and 3′ UTRs (right) of mRNAs in cells treated with 10 nM miR-TAR-3p for 24 h ranked from most highly downregulated to most highly upregulated. Curves for the 10 most enriched seeds are shown. Enrichment of the GCCAGA sequence targeted by miR-TAR-3p through the noncanonical 3 to 8 6-mer seed is shown in red (E), the CCAGAG sequence targeted by miR-TAR-3p through the canonical 2 to 7 6-mer seed is shown in blue (E), and the GCCAGAG sequence targeted by miR-TAR-3p through the canonical 2 to 8 7-mer seed is shown in green (F). The stippled line corresponds to a P value threshold of 0.05 after Bonferroni correction for the number of words tested (4,096). Bonferroni-adjusted P values are shown.
Our comparison of the toxicity exerted by the four HIV miRNAs with the toxicity of just their 6-mer seeds embedded in a double-stranded sRNA backbone suggested that HIV miRNAs may target through a noncanonical 6-mer seed in positions 3 to 8 (Fig. 1A). This was consistent with our analysis of 215 v-miRNAs that suggested that many v-miRNAs may use this noncanonical seed (42). Because the two main species of miR-TAR-3p that we detected were predicted to be much more toxic when targeting through the 3 to 8 seed, we were wondering whether introduction of the authentic v-miRNA would target through a canonical 2 to 7 or a noncanonical 3 to 8 seed. To test this, we transfected HCT116 cells with either 10 nM control miRNA (NC1) or 10 nM miR-TAR-3p, and, 24 h after transfection, we subjected the RNA to an RNA-seq analysis. Enrichment of targeted 6-mer seed matches in the downregulated genes was determined using a Sylamer analysis (67).
This analysis detected a very strong enrichment of the noncanonical seed match GCCAGA in the 3′ UTR of downregulated genes (Fig. 5E, right, red line) and a much lower enrichment of the canonical CCAGAG seed match (Fig. 5E, blue line). A similar but less pronounced enrichment was also found in the open reading frame (ORF). Enrichment of the canonical 7-mer seed match was also found but was less pronounced (Fig. 5F, green lines). These data suggest that miR-TAR-3p targets predominantly through its much more toxic noncanonical 3 to 8 6-mer seed even more than through its canonical 7-mer seed. In contrast to most c-miRNAs, there was substantial targeting of miR-TAR-3p in both the 3′ UTR and the ORF. In summary, although the number of miR-TAR-3p-derived R-sRNAs is low, they are expected to have very high toxicity through 6-mer seed toxicity and noncanonical targeting.
Deletion of Drosha in Jurkat T cells renders them more sensitive to HIV-induced cytotoxicity.
To determine whether the lack of most miRNAs and/or Drosha expression could also affect susceptibility of T cell-derived lines to HIV-induced cell death, we generated and single-cell cloned Drosha-k.o. Jurkat T cells (Fig. 6A). Out of 100 clones analyzed, only 2 had substantially reduced Drosha expression (E11 and F6). Both clones had significantly reduced ability to produce mature miR-21 and miR-200b, but not miR-320, as detected by quantitative PCR (qPCR) (Fig. 6B); the latter is generated independent of Drosha (49). The reduction in mature miRNAs was not as pronounced as in Drosha-k.o. HCT116 cells (49), presumably due to the fact that we could not generate Jurkat clones that were completely devoid of Drosha, likely because Drosha is required for cell survival in this cell line. Both Drosha-k.o. Jurkat clones experienced a greater loss of viability following challenge with a VSV-G pseudotyped HIV ΔEnv iGFP reporter virus than the parental cells (Fig. 6C), suggesting that the extent of Drosha reduction in both clones was sufficient to see the enhanced HIV cytotoxicity as observed before in HCT116 cells. Consistent with the result in HCT116 cells, this higher amount of cell death was not due to an increased expression of the LDL-R entry receptor on the surface of the two k.o. clones, and LDL-R expression was instead decreased in these clones (Fig. 6D). Consequently, this did not lead to a higher permissiveness, as shown for the more sensitive clone F6 (Fig. 6E). A slightly higher, but not significant, amount of viral DNA was detected in these cells 5 h after infection. As seen with the results in HCT116 cells, seed viability plots of all R-sRNAs showed that in both Drosha-k.o. clones 24 h after infection with HIV, seed viability of R-sRNAs was reduced (Fig. 5F). Parental cells did not show this effect and were not killed by HIV at the concentration used when tested for viability after 72 h (Fig. 6C).
FIG 6.
HIV kills Jurkat T cells lacking Drosha more effectively. (A) Western blotting of parental Jurkat T cells or two Jurkat single-cell clones deficient in Drosha (Dro). Protein expression was quantified by densitometry relative to β-actin. (B) Real-time PCR analysis of miRNAs in parental Jurkat cells and single-cell clones (two wild type (wt) and two Drosha k.o.). miRNA expression was calculated relative to the expression of z30 RNA. Student’s t test P values are shown. (C) Cell viability (ATP assay) of parental Jurkat cells and wild-type or Drosha-k.o. clones determined 72 h after infection with 25 ng/mL VSV-G pseudotyped HIV. (D) Surface staining of LDL-R in parental Jurkat cells and the two Drosha-k.o. clones. Percent positivity and median fluorescence intensity (in parentheses) are displayed. (E) PCR quantification of viral DNA 5 h after infection of parental cells, a wild-type clone, and a Drosha-k.o. clone (F6) with 1% VSV-G pseudotyped HIV; ns, not significant. (F) Seed viability box plots showing the seed viability of R-sRNAs significantly enriched (up) or depleted (down) in HIV-infected Jurkat cells and clones. The median is highlighted. Student’s t test P values (B, C, and E) are shown. P values of deregulated seed viabilities (F) were determined using a Kruskal-Wallis median-rank test.
Infection with replication-proficient HIV or its reactivation shifts the balance of R-sRNAs to lower seed viability.
To test whether the increased susceptibility to cell death and the effect on the seed viability of R-sRNAs seen in HIV-infected cells was also found in a T cell line infected with T cell-tropic replication-proficient HIV, we infected parental Jurkat cells and the two Drosha-k.o. clones with HIV NL4-3 Nef:IRES:GFP virus. Similar to data from HCT116 cells, the Drosha-k.o. clone F6 was significantly more susceptible to HIV-induced cell death (Fig. 7A and B). Clone E11 was not efficiently infected by HIV, and this was likely due to substantial reduction of CD4 surface expression in these cells (Fig. 7C). By contrast, clone F6 had similar expression of both entry receptors for HIV infection (CXCR4 and CD4). Consistent with the level of cell death induction in clone F6, R-sRNAs enriched in infected Drosha-k.o. cells had a substantially reduced seed viability compared to R-sRNAs in infected parental cells (Fig. 7D). This was likely due to an effect on host cell sRNAs, as the amount of HIV-derived R-sRNAs was similar between genotypes (Fig. 7E).
FIG 7.
HIV infection or reactivation causes a reduction in seed viability of R-sRNAs in T cell lines. (A) Cell viability (ATP assay) of parental Jurkat cells or two Drosha-k.o. clones determined 8 days postinfection with replication-competent HIV NL4-3 Nef:IRES:GFP virus tested at MOIs of 2.5, 5, and 10. (B) GFP positivity of the cells in A infected with replication-competent HIV NL4-3 Nef:IRES:GFP virus tested at MOIs of 2.5, 5, and 10 8 days postinfection. (C) Surface staining of CD4 and CXCR4 in parental Jurkat cells and the two Drosha-k.o. clones. Percent positivity and median fluorescence intensity (in parentheses) are displayed. (D) Seed viability box plots showing the median seed viability of RISC R-sRNAs significantly enriched or depleted in replication-competent HIV-infected parental Jurkat cells (left) or Drosha-k.o. clone F6 (right). The median is highlighted. (E) Whole HIV genome alignment of reads pulled down with Ago proteins in HIV-infected parental Jurkat cells (top) or Drosha-k.o. clone F6 (middle). Each horizontal line represents one read. Reads from two replicates per condition are displayed. miR-TAR-3p-derived reads are shown in red. Bottom, schematic of the HIV-1 genome. The location of the TAR loop is indicated in orange. (F) Viability of J-Lat cells determined 48 and 96 h after the addition of PMA. (G) Total HIV-derived R-sRNAs in J-Lat cells treated with either DMSO control or PMA. The number of reads derived from HIV TAR are given in percent. The Student’s t test P value is shown. (H and I) Left, seed viability graph of total (H) or only differentially expressed (I) R-sRNAs in J-Lat cells with latent or induced HIV. Right, seed viability box plots showing the median seed viabilities of all R-sRNAs (H) or only the ones enriched or depleted in J-Lat cells after induction of virus (I). The median is highlighted. Student’s t test (A and G) or ANOVA (B) P values are shown. P values of deregulated seed viabilities (D, H, and I) were determined using a Kruskal-Wallis median-rank test.
To exclude that the effects of HIV on seed viability of R-sRNAs was caused by a difference in permissiveness of infected cells and given our data indicating that HIV-induced cytotoxicity was related to postintegration events, we next studied J-Lat cells, a derivative of Jurkat T cells that carry a transcriptionally latent HIV provirus and are often used to study latency reactivation (47). To induce viral transcription, cells were treated with phorbol myristate acetate (PMA). HIV induction caused profound cell death in these cells (Fig. 7F). The amount of RISC-bound v-miRNAs quadrupled in the PMA-induced cells (Fig. 7G), and specific changes in sRNAs occurred that resulted in a significant overall reduction in seed viability of all R-sRNAs (Fig. 7H) as well as a substantial drop in seed viability of R-sRNAs (Fig. 7I) despite the low absolute level of RISC-bound miR-TAR-3p.
Deletion of Ago-2 expression increases susceptibility of cells to HIV-induced toxicity.
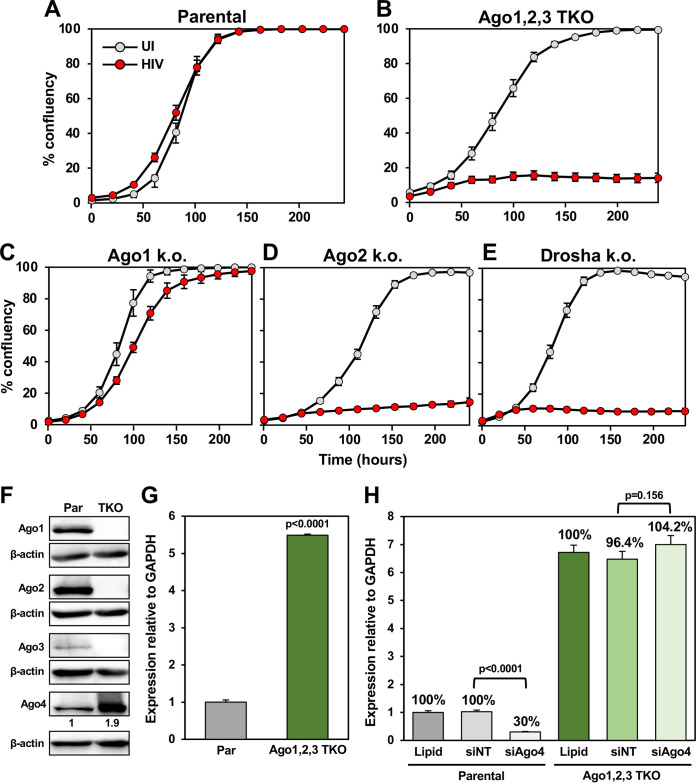
To test the role of the functionally essential RISC component Ago2 in HIV-induced cytotoxicity, we again used the only cells we have available that can tolerate a homozygous knockout of Argonaute proteins: HCT116 cells completely deficient in Ago1 alone, Ago2 alone, or the triple knockout (TKO) of Ago1, Ago2, and Ago3 (Ago1,2,3-TKO). Each of the Ago-deficient cell lines was infected with HIV (Fig. 8A to E). At the chosen HIV titer, we did not see much of an effect of infection on cell death in parental cells (Fig. 8A). By contrast, both Ago2-k.o. and Ago1,2,3-TKO cell lines, but not Ago1-k.o. cells, were hypersensitive to HIV-induced toxicity (Fig. 8B to D). This increase in sensitivity was similar to what we observed in Drosha-k.o. cells (Fig. 8E).
FIG 8.
Analysis of the role of the four Argonaute proteins in HIV-induced cytotoxicity. (A to E) Confluency over time of HCT116 parental cells or k.o. clones uninfected or infected with 1% (A to C and E) or 2.5% (D) VSV-G pseudotyped HIV supernatant. The VSV-G pseudotyped HIV supernatant batch used in D had lower activity. (F) Western blotting of HCT116 parental and Ago1,2,3-TKO cells. Ago4 expression was quantified by densitometry relative to β-actin. (G) Real-time PCR analysis of Ago4 mRNA relative to GAPDH. (H) Real-time PCR analysis of Ago4 mRNA relative to GAPDH in HCT116 parental or Ago1,2,3-TKO cells 48 h after transfection with a control SmartPool (siNT) or a SmartPool specific for Ago4. Student’s t test P values are displayed.
There are four different Ago genes in the human genome. While Ago1,2,3-TKO cells were devoid of the three major Ago proteins, they could still express Ago4. In these cells, Ago4 was strongly increased both at the protein and mRNA levels (Fig. 8F and G). The contribution of Ago4 to the toxic effects of HIV could not be tested because Ago4 could not be knocked down in the TKO cells (Fig. 8H). This could be due to the fact that at least one Ago protein needs to be expressed for RNAi to function or due to the observation that no cell, not even an embryonic stem cell, can survive after deletion of all four Ago genes (68). We concluded that Ago1 and Ago2 do not play major roles in the cytotoxicity of HIV. While Ago4 protein is highly upregulated in Ago1,2,3-TKO cells, whether it contributes to HIV cytopathicity could not be assessed. In summary, our data suggest that the absence of Drosha, Dicer, or Ago2 allowed HIV to induce cell death much more efficiently, at least in part through the DISE mechanism; however, a general antiviral activity of these genes cannot be excluded.
DISCUSSION
When apoptosis was discovered and many of its players (e.g., the CD95 system, the apoptosome, mitochondria, and caspases), early reports indicated that HIV killed infected cells through apoptosis (12, 18). However, more detailed analyses revealed that apoptosis was not essential for HIV-mediated toxicity (69), and CD95 did not play a major role in infected patients (70). Since then, numerous different cell death pathways have been discovered and have been shown to be involved in the cytopathicity of HIV, and these pathways and cellular responses vary depending on the cell type and whether viral replication is productive or not (71). These include direct cytotoxicity of a number of soluble HIV proteins (e.g., Gp120, Tat, Nef, and Vpr) (17–19), activation-induced cell death driven by a chronically activated and hyperinflammatory immune state (11, 12), and cell death as a result of DNA-dependent protein kinase (DNA-PK) activation during viral integration (13). More recently, a series of studies (14–16) described how CD4+ T cells abortively infected with HIV die through caspase-1-dependent pyroptosis. Other forms of cell death implicated to occur in HIV-infected CD4+ T cells are autophagy (20, 21), necrosis (22, 23), necroptosis (24), and mitotic catastrophe (25). In Fig. 9, we summarize some of the cell death pathways that have been reported to contribute to the direct cytotoxicity of HIV in infected cells and how 6-mer seed toxicity fits into this model, particularly under conditions of impaired miRNA biogenesis, which HIV regulates in multiple ways (Fig. 9). Similar to other pathways, DISE will likely not be crucial to HIV-induced toxicity but will be part of a large redundant network of pathways that can lead to cells dying after infection with HIV.
FIG 9.
Scheme of the multiple ways HIV can be toxic to infected cells after integration. HIV has been shown to engage multiple cell death pathways after infecting cells and integration into the genome. They can all be blocked by treating cells with either an inhibitor of RT (i.e., EFA) or integrase (i.e., RAL). (A) The viral integration itself can activate DNA-dependent protein kinase (DNA-PK), resulting in phosphorylation of both p53 and H2AX and triggering cell death (13). (B) CD4+ T cells abortively infected with HIV-1 can die through caspase-1-dependent pyroptosis (14–16). (C) HIV protease cleaves the cellular protein procaspase-8 to generate a novel caspase-8 fragment, termed Casp8p41 (97, 98), which, after translocation to the mitochondria, induces depolarization of the mitochondrial outer membrane (MOMP), resulting in release of cytochrome c and activation of caspase-3 (99) in a Bax/Bak- and procaspase-9-dependent manner (100). (D) HIV-infected cells can undergo necrosis with an autophagic component (20, 21). This caspase-independent cell death involves lysosomal membrane permeabilization (LMP) and cathepsin release, resulting in MOMP. This requires upregulation of the damage-regulated autophagy modulator (DRAM) in a p53-dependent manner (21). (E) Infection with HIV can directly and indirectly affect the balance between RISC-bound sRNA with nontoxic seeds and those with toxic 6-mer seeds through multiple mechanisms. HIV suppresses the polycistronic miRNA cluster miR-17/92 and members of the let-7 miRNA family, both highly abundant miRNA families that carry nontoxic seeds (53, 54). Viral gene products have been reported to affect miRNA processing. miR-210 and miR-222 are upregulated by HIV in the T cell line supT1 and silence Dicer expression (55). Dicer expression can also be suppressed via the viral gene Vpr (56). Monocytes in general do not express Dicer, and HIV suppresses Dicer expression in differentiating monocytes, resulting in a drop in miRNA production (56). Finally, HIV TAT binds TAR RNA-binding protein (TRBP), attenuating the RNAi machinery (57), and HIV-Tat and Rev can suppress TRBP and the protein activator of PKR (PACT), both Dicer-associated RNAi components (58). The reduction of protective miRNAs can result in increased uptake of sRNA with toxic seeds into the RISC. These can be endogenous RNA degradation fragments or viral sRNAs, such as the TAR loop-derived miR-TAR-3p, which can be highly toxic when using its noncanonical 2 to 8 6-mer seed. This would be particularly relevant in HIV-infected cells with suppressed miRNA biogenesis machinery when the balance between R-sRNAs with toxic seeds and those with nontoxic seeds tips toward more toxic ones. Then, DISE induction can be the result; EFA, efavirenz; RAL, raltegravir; ssRNA, single-stranded RNA; dsRNA, double-stranded RNA; CASP, caspase; IFI16, interferon-γ inducible protein 16; XPO5, exportin 5.
DISE is a combination of various cell death pathways that are activated simultaneously due to targeting of multiple survival genes that abolish protection from different forms of cell death by sRNAs in a miRNA-like fashion. While each miRNA can often target hundreds of genes, the function of a miRNA is defined by the nature of the genes in its targeted network. We previously identified a kill code embedded in miRNAs that requires as little as 6 nt (the 6-mer seed) (38). When this 6-mer seed is G rich, then any sRNA that contains this seed and is loaded into the RISC can kill cells through DISE by targeting C-rich seed matches located in the 3′ UTR of genes whose expression is essential for cell survival (38, 40). We previously explored whether viruses could encode v-miRNAs carrying toxic seeds (42). We identified multiple v-miRNAs, including HIV-encoded v-miRNAs, as potentially affecting cell fate through DISE. Interestingly, we found evidence of use of a noncanonical (positions 3 to 8) 6-mer seed for a number of v-miRNAs.
In the context of HIV infection, miRNAs have been suggested to be relevant in two ways. HIV encodes at least four v-miRNAs (72). These have been shown to be involved in regulating viral replication, cell survival, host gene expression, and latency (46, 73, 74). However, the expression of these v-miRNAs is controversial (72). In addition, the expression of a number of c-miRNAs is affected by the virus, and some have antiviral activities (reviewed in reference 74). Of the v-miRNAs, the only v-miRNA we detected in the RISC of infected low-miRNA-expressing cells in significant numbers was miR-TAR-3p. We determined that, after transfection, miR-TAR-3p gains toxicity by using the noncanonical (positions 3 to 8) 6-mer seed.
The discovery of DISE allows a novel way of looking at any sRNA that can be taken up by the RISC and exert RNAi. In addition to the action of individual miRNAs on specific targets regulating various cellular functions, the study of 6-mer seed toxicity requires assessment of the entirety of all R-sRNAs with respect to their seed viability and changes in the average seed viability to contribute to cell fate regulation. To study the potency of R-sRNAs to kill cells, we recently developed SPOROS, a bioinformatics pipeline that allows analysis of all RISC-bound reads in a gene-agnostic and standardized fashion (66). Using SPOROS, we now provide evidence that HIV infection causes a shift of R-sRNAs to lower seed viability. Our data suggest both a possible involvement of a v-miRNA as well as a contribution of cellular R-sRNAs to HIV-induced cell death. Such a shift of cellular R-sRNAs to more toxic seeds may prime cells for DISE similar to other systems we have tested recently (51, 52, 75).
It is intriguing that deletion of multiple genes involved in either the maturation (Drosha, Dicer, or XPO5) or function of miRNAs (Ago2) caused an increase in susceptibility to DISE. Particularly, the results in cells deficient in Ago2 (but not Ago1) expression may argue against a contribution of the RISC in HIV-induced cytotoxicity. However, the data on Ago1,2,3-TKO cells do not exclude an involvement of the fourth Ago protein in the human genome, Ago4. Likely as a compensatory mechanism, Ago4 was found to be highly upregulated in the Ago1,2,3-TKO cells. While Ago4 was shown not to play a role in short interfering RNA (siRNA)-mediated activities, it can bind miRNAs and mediate their function (68). Interestingly, Ago4 has been shown to have an antiviral function, and this was, in part, due to loading of virus-derived siRNAs (vsiRNAs) (76). Unfortunately, we could not knockdown Ago4 to test its involvement as this requires the presence of Ago2, and knocking out Ago4 would also not be possible as any cell needs to express at least one Ago gene in order to survive (68).
The contribution of miR-TAR-3p and its isomiRs to HIV-induced toxicity via RISC function can therefore not be excluded at this point. Mechanistically, given the nature of DISE, which involves targeting a network of genes, it is difficult to perform rescue experiments. HIV has been shown to be cytopathic through the activity of the protein products of many of its genes, including Nef, Vpr, and Tat (17–19). DISE is therefore not the only mechanism mediating HIV cytopathicity (Fig. 9). It is therefore not to be expected that eliminating all RISC activity would completely block HIV-induced cytotoxicity.
Some c-miRNAs have been shown to have antiviral activities (reviewed in reference 74), and that would suggest a higher expression of HIV in cells lacking miRNA biogenesis enzymes or Ago2. We did detect a higher expression of HIV in all mutant cells (data not shown), and this could be due to altered miRNA levels/activity affecting expression levels of the HIV entry receptors. However, we can exclude this effect as a mode of action as we did not find increased expression of any of the entry receptors of the HIV constructs that we used in our study in the mutant cells. In addition, the large reduction in the seed viability of cellular R-sRNAs in J-Lat cells after induction of latent HIV expression suggests a substantial contribution of 6-mer seed toxicity to HIV-induced cell death irrespective of viral infection. Finally, the much higher sensitivity of Drosha- or Dicer-k.o. cells to the toxic effects of miR-TAR-3p or its seed-containing sRNAs also supports the increase in sensitivity of cells lacking c-miRNAs to the DISE-inducing properties of the v-miRNA.
Interestingly, Drosha, Dicer, XPO5, and Ago2 (but not Ago1) also function in the DNA damage response. Both Dicer and Drosha facilitate DNA repair through the generation of Dicer- and Drosha-dependent small RNAs (DDRNAs) (77), which mediate recruitment of repair complexes to the sites of DNA damage (78). Drosha also promotes nonhomologous end joining by interacting with the double-strand repair protein RAD50 (79). XPO5 has been reported to promote genomic stability by exporting premiRNAs from the nucleus that facilitate DNA repair (80). Finally, Ago2 is indispensable for its function in Rad51 recombinase recruitment and homologous recombination repair (81). HIV has been linked to DNA damage through its integration into the genome (82), and RNA viruses in general are known to interfere with the DNA damage response through multiple mechanisms (83).
In summary, our data suggest that HIV infection causes a shift of mostly cellular R-sRNA to lower seed viability. This could prime cells to DISE, which may be enhanced by the v-miRNA miR-TAR-3p, which carries a toxic 6-mer seed and could even gain higher toxicity through a noncanonical 3 to 8 seed. This is most pronounced under conditions of low miRNA expression. Many infected cells are resistant to HIV-induced cell death, and a better knowledge of how cells can die after HIV infection or reactivation of the virus may allow for the sensitization of cells to DISE, for instance by further lowering the amount of protective miRNAs through inhibition of Drosha or Dicer. Our data provide new avenues to explore novel cell death mechanisms that could be used to kill latent HIV.
MATERIALS AND METHODS
Cell lines.
The human colorectal cancer cell line HCT116 and its mutant variants Drosha k.o. 40, Dicer k.o. 43, XPO5 k.o. 19 and 19-1 (49) were obtained from the Korean Collection for Type Cultures (KCTC) and cultured in McCoy’s 5A medium (ATCC, 30-2007) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Sigma-Aldrich, 14009C) and 1% penicillin/streptomycin (Corning, 30-002-CI). HCT116 Ago2-k.o. cells (a kind gift from Joshua T. Mendell, University of Texas Southwestern) (84), Ago1-k.o. cells, and Ago1,2,3-TKO cells (kindly provided by David Corey, University of Texas Southwestern) (85) were cultured in McCoy’s 5A medium (ATCC, 30-2007) supplemented with 10% FBS and 1% penicillin/streptomycin. The human embryonic kidney (HEK) cell line 293T was obtained from ATCC (CRL-3216) and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Corning, 10-013 CM) supplemented with 10% heat-inactivated FBS (Sigma-Aldrich, 14009C) and 1% penicillin/streptomycin (Corning, 30-002-CI). The human T lymphoblast cell line Jurkat, clone E6-1, was obtained from ATCC (TIB-152) and cultured in suspension in RPMI 1640 medium (Corning, 10-040 CM) supplemented with 10% heat-inactivated FBS (Sigma-Aldrich, 14009C) and 1% penicillin/streptomycin (Corning, 30-002-CI). The Jurkat-derived HIV-1 latency model cell line J-Lat (kindly provided by Judd Hultquist, Northwestern University) was cultured in suspension in RPMI 1640 medium (Corning, 10-040 CM) supplemented with 10% heat-inactivated FBS (Sigma-Aldrich, 14009C), 1% penicillin/streptomycin (Corning, 30-002-CI), and 10 μM antiretroviral cocktail. The Jurkat Drosha-k.o. cell pool was designed and engineered using the CRISPR-Cas9 system by Synthego. The single guide RNA (sgRNA) sequence 5′-CACAGAAUGUCGUUCCACCC-3′ targeting Drosha exon 4 was used for the generation of a Jurkat Drosha-k.o. pool, and editing efficiency of the sgRNA posttransfection and expansion of cells was determined to be 39% by Synthego, suggesting that Drosha is required for fitness in Jurkat cells. Jurkat Drosha-k.o. clonal cell lines E11 and F6 were isolated from the pool by limiting dilution and clonal expansion and were cultured in suspension in RPMI 1640 medium (Corning, 10-040 CM) supplemented with 10% heat-inactivated FBS (Sigma-Aldrich, 14009C) and 1% penicillin/streptomycin (Corning, 30-002-CI). Cell cultures were maintained at 37°C in a humidified incubator with 5% CO2 (Nuaire, NU-5510).
Production of HIV and viral infections.
HIV was produced by transient transfection of HEK293T cells using polyethyleneimine (PEI; 1 mg/mL, linear, molecular weight [MW] 25,000 Da; Polysciences, Inc.). Pseudotyping of GFP-HIV (HIV-1 NL4-3 ΔEnv inverted GFP [iGFP] reporter vector, a gift from Tom Hope, Northwestern University) by vesicular stomatitis virus glycoprotein (VSV-G) was performed by cotransfection with pCMV-VSV-G vector. Briefly, transfection mixtures were prepared using PEI (4 μL per 1 μg of DNA) with the GFP-HIV (6 μg) and VSV-G (4 μg) constructs in serum-free DMEM (Corning, 10-013-CM) and were added dropwise to HEK293T cells that were seeded onto 10-cm dishes a day before at a density of 5 to 6 × 106 cells in 10 mL of fresh DMEM with 10% heat-inactivated FBS (Sigma-Aldrich, 14009C). Eighteen hours after transfection, the medium was replaced with 10 mL of fresh DMEM supplemented with 10% heat-inactivated FBS (Sigma-Aldrich, 14009C). After 48 h, the supernatant containing VSV-G pseudotyped HIV was collected and clarified by centrifugation at 450 × g for 5 min and filtered through a sterile 0.45-μm-pore-size filter (MillexHV, Millipore). Viral supernatants were then used for infections or frozen in aliquots at −80°C.
For precipitation of the VSV-G pseudotyped HIV particles, 2.5 mL of sterile PEG-it solution (System Biosciences, LV810A-1) was added to 10 mL of filtered viral supernatant and stored at 4°C for 48 h. Following incubation at 4°C, the supernatant/PEG-it mixture was centrifuged at 1,500 × g for 30 min at 4°C. The supernatant was removed, and the vector particles that appeared as a pellet were resuspended in 1 mL of phosphate-buffered saline (PBS). Two hundred microliters of the sample was provided to the viral pathogenesis core (Third Coast CFAR) for a p24 enzyme-linked immunosorbent assay (ELISA) to quantitatively measure HIV-1 p24 protein. PEG-it concentrated virus was frozen in aliquots at −80°C.
To study the effects of HIV on cells, growth/viability assays and flow cytometry analyses were performed following infection of cells with either VSV-G pseudotyped HIV supernatant (1 to 5% of total media volume) or PEG-it concentrated virus (in ng of p24/mL). Additionally, the effect of HIV on cell types was also assessed in the presence of the nonnucleoside reverse transcriptase inhibitor (NNRTI) efavirenz (EFA; Sigma-Aldrich, SML0536) or the integrase strand transfer inhibitor raltegravir (RAL; Abcam, ab231360). Replication-competent HIV NL4-3 Nef:IRES:GFP (NIH AIDS Reagent Program, 11349) virus was produced, and cells were infected as previously described (86). J-Lat cells were activated to release viral particles using PMA (0.1 μM).
Analysis of HIV integration in cells.
For analysis of HIV integration in cells infected with the replication-competent HIV NL4-3 Nef:IRES:GFP virus, cells were seeded at a density of 1 × 105 in 200 μL of medium/well in 96-well, U-bottom plates and were infected with replication-competent HIV NL4-3 Nef:IRES:GFP at multiplicities of infection (MOIs) of 2, 5, and 10 in a volume of 250 μL. At a suitable time point following infection, 50 μL of infected cells from the original culture plate was transferred to a fresh plate containing 150 μL of 2% formaldehyde (prepared fresh in 1× PBS) to fix the cells. Fixed cells were analyzed for GFP positivity by flow cytometry.
Cell death/viability assays.
For the cell death assay shown in Fig. 1F, 1 × 106 cells were infected with VSV-G pseudotyped HIV R9 ΔEnv iGFP (175 ng of p24/mL) in the absence or presence of antivirals (10 μM EFA or 10 μM RAL). Seventy-two hours after infection, cells were harvested and stained using LIVE/DEAD fixable blue dead cell stain (Invitrogen, L23105), according to manufacturer’s instructions. HIV-infected cells and cell viability were both analyzed by flow cytometry (BD LSRFortessa) and quantified using FlowJo v10.
To monitor cell viability following HIV infection, adherent or suspended cells were seeded at a density of 4 to 5 × 103 cells in 100 μL of medium/well in a white-opaque, 96-well plate and infected with either supernatant or concentrated VSV-G pseudotyped HIV in a total volume of 200 μL of medium. Samples were assessed in quadruplicate. A cell viability assay involving the measurement of ATP within cells with or without HIV was performed 72 h following HIV infection unless otherwise indicated. Briefly on the day of cell viability measurement for adherent cells (ATP content), medium in each well was replaced with 70 μL of fresh medium and 70 μL of CellTiter-Glo reagent (Promega, G7570). For suspended cells, the plate was briefly centrifuged (1,500 rpm for 30 s) to ensure that all cells were settled at the bottom of the plate, followed by replacing 100 μL of culture medium in each well with 100 μL of CellTiter-Glo reagent (Promega, G7570). Plates were covered with aluminum foil and placed on an orbital shaker to induce cell lysis for 5 min, followed by incubation at room temperature for 10 min before quantifying luminescence on a BioTek Cytation 5 cell imaging multimode reader. The mean luminescence and standard deviation of the replicates for each condition were calculated in Microsoft Excel 2016 (Microsoft Corporation, USA) and are represented as a percentage of the mean luminescence of the uninfected controls designated as 100% viability. Percent viability was then plotted against the medium control or HIV infection and are represented in bar graph format.
Jurkat-derived cells were seeded at a density of 1 × 105 in 200 μL of medium/well in 96-well, U-bottom plates and were infected with replication-competent HIV NL4-3 Nef:IRES:GFP at MOIs of 2, 5, and 10 in 250 μL of medium. Cell viability (ATP content) following HIV infection was monitored at different time points (72 h, 6 days, and 8 days) by transferring 50 μL of infected cells from the original culture plate to a fresh white-opaque, 96-well plate containing 50 μL of culture medium. Each condition was assessed for cell viability in triplicate. One hundred microliters of CellTiter-Glo reagent (Promega, G7570) was then added to each well, and luminescence was measured as described above.
J-Lat cells were seeded at a density of 5 × 103 cells/well in a white-opaque, 96-well plate and were treated with either dimethyl sulfoxide (DMSO) control or PMA (0.1 μM) in a total volume of 200 μL of medium. Samples were assessed in triplicate. Cell viability (ATP content) was assessed 48 and 96 h following PMA treatment by brief centrifugation (1,500 rpm for 30 s) of the plate to ensure that all cells were settled at the bottom of the plate, followed by replacing 100 μL of culture medium in each well with 100 μL of CellTiter-Glo reagent (Promega, G7570). Luminescence was measured as described above.
Monitoring cell growth.
To monitor cell growth following HIV infection, adherent cells were seeded at a density of 3 × 103 cells in 100 μL of medium/well in a 96-well plate and infected with either supernatant (2.5%) or concentrated VSV-G pseudotyped HIV (25, 50, or 175 ng of p24/mL) in a total volume of 200 μL of medium. Samples were assessed in quadruplicate. Cell growth was then monitored (±HIV) at various time points for at least 160 h using the IncuCyte Zoom live-cell imaging system (Essen Bioscience, RRID:SCR_019874) with a 10× objective and data management software (version 2015A) to monitor cell proliferation. In each well, two phase-contrast images or GFP images were taken, and the mean ± standard error of the mean (SEM) percent confluence or green object integrated intensity was calculated using software processing definitions for the cell lines as recommended by the manufacturer.
To monitor cell growth following transfection with short oligonucleotides, 50 μL of Opti-MEM (Thermo Fisher Scientific, 31985070) mix containing 0.2 to 0.3 μL of RNAiMAX (optimized for HCT116-derived cells) and 1 or 10 nM miRNA or sRNAs was added per well of a 96-well plate. Three thousand cells in 200 μL of antibiotic-free medium were added to each well containing the transfection mixture. Each transfection condition, including test miRNA/sRNA, negative control, and RNAiMAX control, was performed in quadruplicate, and growth was monitored as described above in an IncuCyte Zoom live-cell imaging system (Essen Bioscience, RRID:SCR_019874).
The following viral miRNAs and negative controls were used: miR-TAR-3p (mirVana miRNA Mimic, mature sequence: 5'-UCUCUGGCUAACUAGGGAACCCA-3'; Ambion, 4464066), S1_TAR-3p (mirVana miRNA Mimic, mature sequence: 5'-UGGCUAACUAGGGAACCCACUG-3'; Ambion, 4464068), S2_TAR-3p (mirVana miRNA Mimic, mature sequence: 5'-CUCUGGCUAACUAGGGAACCCACU-3'; Ambion, 4464068), and NC1 (miRNA precursor negative control 1; Ambion, AM17110). The following sRNA oligonucleotides were designed as described in Putzbach et al. (39) and were obtained from Integrated DNA Technology and annealed as per the manufacturer’s instructions:
sNT1 sense: 5'-mUmGrGrUrUrUrArCrUrArCrArCrGrArCrUrArUTT-3';
sNT1 antisense: 5'-rArUrArGrUrCrGrUrGrUrArGrUrArArArCrCrAAA-3';
sTAR-3p (2 to 7) sense: 5'-mUmGrGrUrUrUrArCrArUrGrUrCrCrUrGrArGrATT-3';
sTAR-3p (2 to 7) antisense: 5'-rUrCrUrCrUrGrGrArCrArUrGrUrArArArCrCrAAA-3';
sTAR-3p (3 to 8) sense: 5'-mUmGrGrUrUrUrArCrArUrGrUrGrCrCrArGrArATT-3';
sTAR-3p (3 to 8) antisense: 5'-rUrUrCrUrGrGrCrArCrArUrGrUrArArArCrCrAAA-3';
sS1_TAR-3p (2 to 7) sense: 5'-mUmGrGrUrUrUrArCrArUrGrUrUrUrArGrCrCrATT-3';
sS1_TAR-3p (2 to 7) antisense: 5'-rUrGrGrCrUrArArArCrArUrGrUrArArArCrCrAAA-3';
sS1_TAR-3p (3 to 8) sense: 5'-mUmGrGrUrUrUrArCrArUrGrUrGrUrUrArGrCrATT-3';
sS1_TAR-3p (3 to 8) antisense: 5'-rUrGrCrUrArArCrArCrArUrGrUrArArArCrCrAAA-3'.
Cellular surface staining and flow cytometry analysis.
To study the expression of the surface receptors LDL-R, CD4, and CXCR4, cell surface staining was performed. Briefly 6 × 105 cells were pelleted and washed in 1× PBS and resuspended in 100 μL of fluorescence-activated cell sorting (FACS) buffer (PBS + 10% FBS + 0.1% sodium azide) in a microcentrifuge tube. Duplicates for each condition were set up, where 20 μL of isotype control antibody or surface receptor-specific primary labeled antibody was added to the 100 μL of cell suspension in FACS buffer and incubated on ice in the dark for 30 min. Following incubation, the cells were washed with 1 mL of FACS buffer three times by centrifugation at 1,500 rpm for 5 min and resuspended in 500 μL of ice-cold FACS buffer. The cell suspension was then transferred to FACS tubes, placed on ice in the dark, and immediately taken for analysis by flow cytometry (BD FACSymphony-A5). Percent surface-receptor-positive cells was quantified by FlowJo v10. The following antibodies were used: phycoerythrin (PE)-mouse anti-human LDL-R (BD Biosciences, 565653) and PE-mouse IgG2b, κ isotype control (BD Biosciences, 555058); PE-Cy5 mouse anti-human CD184 (BD Biosciences, 555975) and PE-Cy5 mouse IgG2a, κ isotype control (BD Biosciences, 555575); allophycocyanin (APC)-mouse anti-human CD4 (BD Biosciences, 561840) and APC-mouse IgG1, κ isotype control (BD Biosciences, 555571).
Western blotting.
Protein lysates were prepared by lysing cells using killer RIPA lysis buffer (150 mM NaCl, 10 mM Tris-HCl [pH 7.2], 1% SDS, 1% Triton X-100, 1% deoxycholate, and 5 mM EDTA) supplemented with phenylmethylsulfonyl fluoride (PMSF; 1 mM) and protease inhibitor cocktail (Roche, 11836170001), followed by sonication of samples (25% amplitude for 10 s). A DC protein assay kit (Bio-Rad, Hercules, CA) was used to quantify protein concentration in each sample. Equal amounts of protein per sample (25 to 30 μg) were resolved on a 10% SDS-PAGE gel and transferred onto nitrocellulose membranes (Amersham Protran, 0.45-μm nitrocellulose, GE) at 90 V for 90 min. Membranes were incubated in blocking buffer (5% dry milk in 0.1% Tris-buffered saline [TBS]/Tween 20) for 1 h at room temperature. Appropriate dilutions of primary antibodies were prepared in blocking buffer, applied to membranes, and incubated overnight at 4°C. Membranes were washed three times in 0.1% TBS/Tween 20 for 10 min each. Secondary antibodies were diluted in blocking buffer and applied to membranes for 1 h at room temperature, followed by three additional washing steps with 0.1% TBS/Tween 20 for 10 min each. The protein-antibody complexes were detected using SuperSignal West Dura extended duration substrate (Thermo Fisher Scientific, 34076) and visualized using a G:BOX Chemi XT4 chemiluminescence imager (Syngene). The following primary antibodies used were diluted in blocking buffer: anti-Argonaute-2 (1:1,000; Abcam, 32381), anti-Argonaute-1 (1:1,000; Cell Signaling, 5053), anti-Argonaute-3 (1:1,000; Cell Signaling, 5054), anti-Argonaute-4 (1:1,000; Cell Signaling, 6913), anti-Dicer (1:1,000; Cell Signaling, D38E7), anti-Drosha (1:1,000; Cell Signaling, D28B1), anti-exportin 5 (1:1,000; Cell Signaling, 12565), and anti-β-actin (1:5,000; Santa Cruz, sc-47778). The secondary antibody goat anti-rabbit IgG-horseradish peroxidase (HRP; 1:5,000; Southern Biotech, 4030-05) was diluted in blocking buffer. Densitometry analysis of the blots was performed using GeneTools (Syngene) image analysis software, and protein bands were normalized to the loading control β-actin. All uncropped Western blots are shown in Fig. S3 in the supplemental material.
Ago pulldown and small RNA-sequencing (Ago-RP-Seq).
To determine the RISC content of cells infected with HIV, an Ago pulldown experiment followed by high-throughput sRNA sequencing was performed. All experiments involving HIV infection described above for 96-well plates were scaled up for 150-mm dishes. Briefly, Jurkat-derived cells were infected with 50 ng of p24/mL PEG-it concentrated VSV-G pseudotyped HIV, and HCT116-derived cells were infected with either 175 ng of p24/mL concentrated VSV-G pseudotyped HIV or 2.5% viral supernatant and harvested for pulldown 28 h following infection. Jurkat-derived cells infected with replication-competent HIV NL4-3 Nef:IRES:GFP at an MOI of 10 were harvested 12 days following infection, and J-Lat cells were harvested 36 h following induction with 0.1 μM PMA.
Harvested cells were washed with 1× PBS, and 106 cells were pelleted and frozen at −80°C until ready to be lysed with 1 mL of NP-40 lysis buffer (50 mmol/L Tris [pH 7.5], 150 mmol/L NaCl, 5 mmol/L EDTA, 0.5% NP-40, 10% [vol/vol] glycerol, and 1 mmol/L NaF supplemented with 1:200 EDTA-free protease inhibitors [Millipore, 539134] and 1:1,000 RNaisin Plus [Promega, N2615] before use). Lysates were incubated on ice for 15 min and vortexed briefly, followed by centrifugation at 20,000 × g for 20 min at 4°C. Lysates were transferred to siliconized microcentrifuge tubes (DNA LoBind, Eppendorf, 022431021), and 500 μg of Flag-GST-T6B peptide with high-affinity binding to Ago1 to 4 (87) was added coupled with 80 μL of anti-Flag M2 magnetic beads (Sigma, M8823) and incubated for 3 h on a rotor at 4°C. The beads were then washed three times with NP-40 lysis buffer, and, during the final wash, 10% of the beads were removed and incubated at 95°C for 5 min with 4× Laemmli sample buffer added. Ago pulldown efficiency was assessed by running these samples on a 10% SDS-PAGE gel, transferring onto a nitrocellulose membrane, and immunoblotting against Ago2 (Abcam, 32381). Five hundred microliters of TRIzol reagent was added to the remaining beads, and a phenol:chloroform RNA extraction was performed according to manufacturer’s instructions. The RNA pellet was dissolved in 20 μL of RNase-free water. Ten microliters of the RNA sample was dephosphorylated with 0.5 U/mL calf intestinal alkaline phosphatase (CIP) at 37°C for 15 min and radiolabeled with 0.5 mCi [γ-32P]ATP and 1 U/mL T4 polynucleotide kinase for 20 min at 37°C. RNA bound to Ago1 to 4 was visualized by 15% urea-PAGE. The remaining RNA sample was used for sRNA library preparation using Illumina primers (RRID:SCR_010233) as described previously (88). Briefly, RNA was first ligated with 3′-adenylated adapters and separated by 15% denaturing urea-PAGE. The RNA corresponding to an insert size of 19 to 35 nt was eluted from the gel using 32P-radiolabeled size markers, ethanol precipitated, and ligated with the 5′ adapter. The RNA samples were then separated by 12% urea-PAGE, followed by gel extraction and subsequent reverse transcription using Superscript III reverse transcriptase (Invitrogen, 18080–044). The cDNA was PCR amplified and sequenced on an Illumina Hi-Seq 4000. The sequences used for the RNA size marker, 3′ and 5′ adaptors, reverse transcription primers, and PCR primers were used as described previously (51).
6-mer seed viability analysis of R-sRNAs.
The SPOROS pipeline was used to analyze the abundance of RISC-bound sRNAs according to their predicted 6-mer seed viability and generate seed viability graphs and seed viability plots (66). In brief, the RISC-bound small RNA-seq data were trimmed to remove adaptor sequences and reads that were longer than 25 nt or shorter than 18 nt and were compiled into a counts table. Reads with fewer counts than the number of samples were removed, and the remaining reads were subjected to a BLAST search against a curated list of all mature human miRNAs or other short RNAs (RNAworld). Reads that matched any artificial sequences in the RNA data sets were also removed, and the subsequent raw read count table (RawCounts) was either normalized to 1 million reads to generate a normalized count table (normCounts) or normalized and used for differential expression analysis (differential). Each table was then annotated with 6-mer seed, predicted 6-mer seed viability (as determined from https://www.6merdb.org/), miRNA, and RNAworld matches. At this step, the percent miRNA/short RNA content in the RISC was determined from the annotated normCount table. Seed viability graphs were then generated by collapsing the table according to 6-mer seed and RNA type and aggregating all the rows according to the predicted 6-mer seed viability in 1%-sized bins and adding up all the seeds that had a specific toxicity with each 1% bin. Line graphs with smoothing enabled were then generated in Microsoft Excel, with 6-mer seed viability on the x axis and normalized read count on the y axis. The data file with the collapsed data was used to identify the most abundant miRNAs/short RNAs that make up each peak of the seed viability graph and was labeled if greater than 5,000 reads. Where a peak consisted of more than one abundant miRNA/short RNA (i.e., greater than 5,000 reads), RNAs were listed in order of abundance. To determine average seed viability of all reads in the data set, the counts for all individual reads were divided by a factor that reduces the number of the most abundant reads to less than 1,000 to ease computational complexity of the downstream analyses. The columns containing the 6-mer seed viability were then expanded according to the normalized read counts, and the resultant data column containing the frequency distribution of the average 6-mer seed viability for various groups was used to generate a basic box plot (seed viability plot) using StatPlus (v 7.7). Differential analysis between two groups was performed by taking significantly (P < 0.05) up- or downregulated reads and calculating the delta read counts for each row between groups (perturbed sample-control sample). Associated 6-mer seed viabilities were then multiplied based on the delta read counts to produce the seed viability graphs and seed viability plots as described above. While the effects seen on cell viability are likely caused by toxic seed-containing sRNAs, we express them as percent seed viability, where 0% seed viability equals 100% toxicity. Throughout this work, percent seed viability refers to the average viability score a seed received during arrayed screens of three human cell lines (38, 40).
Identification of HIV-derived reads in the RISC of HIV-infected cells.
Briefly, the reads from each sample were compiled as a BLAST database, and blastn was used to query the HIV-1 whole-genome sequence (NCBI reference sequence NC_001802.1) against reads from cells infected with HIV. Reads were considered matches if they had an E value of less than 0.05% and 100% identity across the entire length of the read. The filtered BLAST hits were converted to a bed formatted file describing the locations of reads relative to the HIV-1 whole genome and was used for subsequent analyses. Sample replicates were combined, and stack plots depicting the locations where RISC-bound HIV sRNAs map along the HIV-1 whole genome were generated using the R package Sushi. HIV sRNA reads containing the core region of the HIV-miR-TAR3p sequence (UGGCUAACUAGGGAAC) were highlighted in red.
Transfection of cells with miR-TAR-3p and large RNA-seq analysis.
HCT116 parental cells were seeded at a density of 2.5 ×105 cells/well in 6-well plates. The following day, cells were forward transfected with miR-TAR-3p (mirVana miRNA Mimic, mature sequence: 5'-UCUCUGGCUAACUAGGGAACCCA-3'; Ambion, 4464066) or NC1 (miRNA precursor negative control 1; Ambion, AM17110) in duplicate with 500 μL of Opti-MEM mix containing 3 μL of RNAiMAX, and miRNA mimic at 10 nM was added to 2 mL of antibiotic-free medium. Cells were harvested for RNA 24 h following transfection using Qiazol. An on-column DNase digestion step was included using an RNase-free DNase set (Qiagen, 79254), and total RNA was isolated using a miRNeasy minikit (Qiagen, 217004) according to manufacturer’s instructions. The quality of RNA was determined using an Agilent Bioanalyzer. RNA library preparation and subsequent sequencing on a NovaSeq SP PE150 was performed by the NU-Seq core at Northwestern University (Chicago). Paired-end RNA-seq libraries were prepared using an Illumina TruSeq total RNA library prep kit with a Ribo-Zero rRNA depletion step included. Reads were trimmed with Trimmomatic v0.33 (TRAILING:30 MINLEN:20) (89) and aligned to the hg38 human genome assembly with Tophat v 2.1 (90) or STAR (91). Exonic reads were assigned to genes using the Ensembl 78 version of the hg38 transcriptome and HTSeq v 0.6.1 (92). Differential expression analysis was performed using the edgeR package (93) to fit a negative binomial generalized log-linear model to the read counts for each gene. Sylamer analysis was performed as recently described (38). The 3′ UTRs or ORFs were used from Ensembl, version 76.
miRNA and viral DNA quantification.
Briefly, 25 ng of total RNA was used to make cDNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems, 4368814) and miRNA-specific primers. The cDNA was diluted 1:5, and a qPCR mixture composed of the diluted cDNA, target-specific TaqMan microRNA assay (Thermo Scientific, 4427975), and TaqMan universal PCR master mix (Applied Biosystems, 4324018) was prepared. Cycle threshold (CT) values were determined, as described previously, using an Applied Biosystems 7500 real-time PCR system. The ΔΔCT values between the miRNA of interest and the control were calculated to determine relative abundance of the miRNA. Each sample was run in triplicate, and z30 (Thermo Scientific, 001092) was used as an endogenous loading control. The following TaqMan microRNA assays (Thermo Scientific, 4427975) were used: miR-21 (assay ID 000397), miR-200b (assay ID 002251), and miR-320 (assay ID 002277).
For the viral DNA quantification shown in Fig. 6E, briefly, 1 × 105 cells were seeded in a 24-well plate, infected with 1% VSV-G pseudotyped HIV-1 viral supernatant, and harvested for DNA after 5 h. DNA was extracted using a DNeasy blood and tissue kit (Qiagen, 69504) according to the manufacturer’s instructions. The qPCR mixture composed of target-specific TaqMan primer-probe and the TaqMan universal PCR master mix (Applied Biosystems, 4324018) was prepared, and 5 ng of DNA was used per qPCR. CT values were determined using an Applied Biosystems 7500 real-time PCR system using predefined thermal cycling conditions (50°C for 2 min, 95°C for 10 min, and then 40 cycles of 95°C for 15 s and 60°C for 1 min). Each sample was run in triplicate, and GAPDH (Thermo Scientific, assay ID Hs02786624_g1) was used as an endogenous loading control.
Relative expression was calculated using the following equations:
Average CT values (avg CT): average of the CT values of the triplicates of each sample.
ΔCT: avg CTTarget – avg CTGAPDH; Relative expression value: 2−ΔCT
Positive and negative errors of relative expression were determined by calculating 2(−ΔCT + SD ΔCT) and 2(−ΔCT − SD ΔCT).
TaqMan primer-probe sets targeting the long terminal repeat (LTR) region of the HIV-1 genome (94) were custom designed and purchased from Thermo Scientific:
forward primer: 5'-TGTGTGCCCGTCTGTTGTGT-3';
reverse primer: 5'-GAGTCCTGCGTCGAGAGATC-3';
probe: 5'-CAGTGGCGCCCGAACAGGGA-3'.
Ago4 knockdown.
HCT116 cells (2.5 × 105) and Ago1,2,3-TKO cells were reverse transfected with 40 nM siNT (Dharmacon ON-TARGETplus nontargeting pool, D-001810-10-05) and siAgo4 (Dharmacon ON-TARGETplus human AGO4 siRNA, L-004641-01-0005) in a 6-well plate with 3 μL of RNAiMAX. A mock condition with RNAiMAX was also set up for each cell type. Cells were harvested for RNA 48 h following transfection using an miRNeasy minikit (Qiagen, 217004) according to the manufacturer’s instructions, and 200 ng of RNA was used to make cDNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems, 4368814). The cDNA was diluted 1:5, and a qPCR mixture composed of the diluted cDNA or Ago4-specific (Thermo Scientific, assay ID Hs01059739_m1) or GAPDH-specific (Thermo Scientific, assay ID Hs00266705_g1) TaqMan primer-probe and TaqMan universal PCR master mix (Applied Biosystems, 4324018) was prepared. CT values were determined as described previously using an Applied Biosystems 7500 real-time PCR system. Each sample was run in triplicate, and the ΔΔCT values between the gene of interest and control were calculated to determine relative abundance of the gene. Samples were normalized to GAPDH.
Statistical analysis.
Statistical analysis was performed using Student’s t tests. P values for statistical significance between the groups of deregulated sRNAs (in seed viability plots) were determined using a Kruskal-Wallis median-rank test. Two-way analysis of variance (ANOVA) was performed using STATA1C software for evaluating GFP positivity over increasing HIV concentrations, choosing one component for the treatment condition as primary interest and a second component for the drug concentration as a categorical variable. STATA was also used for comparing differences in polynomial distributions, as described previously (38).
Data availability.
RNA-seq data are available at the Gene Expression Omnibus under accession number GSE218195.
ACKNOWLEDGMENTS
We are grateful to David Corey and Joshua Mendell for providing the Ago1-k.o. and Ago1,2,3-TKO and Ago2-k.o. HCT116 cells, respectively. This work was supported by NIH grant R21 AI150910 to M.E.P.
Footnotes
Supplemental material is available online only.
Contributor Information
Marcus E. Peter, Email: m-peter@northwestern.edu.
Guido Silvestri, Emory University.
REFERENCES
- 1.Moranguinho I, Valente ST. 2020. Block-and-lock: new horizons for a cure for HIV-1. Viruses 12:1443. doi: 10.3390/v12121443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA, Fauci AS. 1997. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA 94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD, Siliciano RF. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 4.Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang HC, Zhang H, Margolick JB, Blankson JN, Siliciano RF. 2012. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker B, McMichael A. 2012. The T-cell response to HIV. Cold Spring Harb Perspect Med 2:a007054. doi: 10.1101/cshperspect.a007054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang SH, Ren Y, Thomas AS, Chan D, Mueller S, Ward AR, Patel S, Bollard CM, Cruz CR, Karandish S, Truong R, Macedo AB, Bosque A, Kovacs C, Benko E, Piechocka-Trocha A, Wong H, Jeng E, Nixon DF, Ho YC, Siliciano RF, Walker BD, Jones RB. 2018. Latent HIV reservoirs exhibit inherent resistance to elimination by CD8+ T cells. J Clin Invest 128:876–889. doi: 10.1172/JCI97555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez Larrosa PN, Croci DO, Riva DA, Bibini M, Luzzi R, Saracco M, Mersich SE, Rabinovich GA, Martinez Peralta L. 2008. Apoptosis resistance in HIV-1 persistently-infected cells is independent of active viral replication and involves modulation of the apoptotic mitochondrial pathway. Retrovirology 5:19. doi: 10.1186/1742-4690-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clayton KL, Collins DR, Lengieza J, Ghebremichael M, Dotiwala F, Lieberman J, Walker BD. 2018. Resistance of HIV-infected macrophages to CD8+ T lymphocyte-mediated killing drives activation of the immune system. Nat Immunol 19:475–486. doi: 10.1038/s41590-018-0085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D. 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 10.Altfeld M, Gale M, Jr. 2015. Innate immunity against HIV-1 infection. Nat Immunol 16:554–562. doi: 10.1038/ni.3157. [DOI] [PubMed] [Google Scholar]
- 11.Peter ME, Ehret A, Berndt C, Krammer PH. 1997. AIDS and the death receptors. Br Med Bull 53:604–616. doi: 10.1093/oxfordjournals.bmb.a011633. [DOI] [PubMed] [Google Scholar]
- 12.Alimonti JB, Ball TB, Fowke KR. 2003. Mechanisms of CD4+ T lymphocyte cell death in human immunodeficiency virus infection and AIDS. J Gen Virol 84:1649–1661. doi: 10.1099/vir.0.19110-0. [DOI] [PubMed] [Google Scholar]
- 13.Cooper A, Garcia M, Petrovas C, Yamamoto T, Koup RA, Nabel GJ. 2013. HIV-1 causes CD4 cell death through DNA-dependent protein kinase during viral integration. Nature 498:376–379. doi: 10.1038/nature12274. [DOI] [PubMed] [Google Scholar]
- 14.Doitsh G, Cavrois M, Lassen KG, Zepeda O, Yang Z, Santiago ML, Hebbeler AM, Greene WC. 2010. Abortive HIV infection mediates CD4 T cell depletion and inflammation in human lymphoid tissue. Cell 143:789–801. doi: 10.1016/j.cell.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, Hunt PW, Hatano H, Sowinski S, Munoz-Arias I, Greene WC. 2014. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 505:509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monroe KM, Yang Z, Johnson JR, Geng X, Doitsh G, Krogan NJ, Greene WC. 2014. IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science 343:428–432. doi: 10.1126/science.1243640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schindler M, Munch J, Kutsch O, Li H, Santiago ML, Bibollet-Ruche F, Muller-Trutwin MC, Novembre FJ, Peeters M, Courgnaud V, Bailes E, Roques P, Sodora DL, Silvestri G, Sharp PM, Hahn BH, Kirchhoff F. 2006. Nef-mediated suppression of T cell activation was lost in a lentiviral lineage that gave rise to HIV-1. Cell 125:1055–1067. doi: 10.1016/j.cell.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 18.Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin KM, Krammer PH. 1995. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 19.Finkel TH, Tudor-Williams G, Banda NK, Cotton MF, Curiel T, Monks C, Baba TW, Ruprecht RM, Kupfer A. 1995. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med 1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Gao Y, Tan J, Devadas K, Ragupathy V, Takeda K, Zhao J, Hewlett I. 2012. HIV-1 and HIV-2 infections induce autophagy in Jurkat and CD4+ T cells. Cell Signal 24:1414–1419. doi: 10.1016/j.cellsig.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Laforge M, Limou S, Harper F, Casartelli N, Rodrigues V, Silvestre R, Haloui H, Zagury JF, Senik A, Estaquier J. 2013. DRAM triggers lysosomal membrane permeabilization and cell death in CD4+ T cells infected with HIV. PLoS Pathog 9:e1003328. doi: 10.1371/journal.ppat.1003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plymale DR, Tang DS, Comardelle AM, Fermin CD, Lewis DE, Garry RF. 1999. Both necrosis and apoptosis contribute to HIV-1-induced killing of CD4 cells. AIDS 13:1827–1839. doi: 10.1097/00002030-199910010-00004. [DOI] [PubMed] [Google Scholar]
- 23.Petit F, Arnoult D, Lelievre JD, Moutouh-de Parseval L, Hance AJ, Schneider P, Corbeil J, Ameisen JC, Estaquier J. 2002. Productive HIV-1 infection of primary CD4+ T cells induces mitochondrial membrane permeabilization leading to a caspase-independent cell death. J Biol Chem 277:1477–1487. doi: 10.1074/jbc.M102671200. [DOI] [PubMed] [Google Scholar]
- 24.Pan T, Wu S, He X, Luo H, Zhang Y, Fan M, Geng G, Ruiz VC, Zhang J, Mills L, Bai C, Zhang H. 2014. Necroptosis takes place in human immunodeficiency virus type-1 (HIV-1)-infected CD4+ T lymphocytes. PLoS One 9:e93944. doi: 10.1371/journal.pone.0093944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castedo M, Perfettini JL, Roumier T, Kroemer G. 2002. Cyclin-dependent kinase-1: linking apoptosis to cell cycle and mitotic catastrophe. Cell Death Differ 9:1287–1293. doi: 10.1038/sj.cdd.4401130. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Sheng G, Juranek S, Tuschl T, Patel DJ. 2008. Structure of the guide-strand-containing Argonaute silencing complex. Nature 456:209–213. doi: 10.1038/nature07315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leuschner PJ, Ameres SL, Kueng S, Martinez J. 2006. Cleavage of the siRNA passenger strand during RISC assembly in human cells. EMBO Rep 7:314–320. doi: 10.1038/sj.embor.7400637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. 2003. Prediction of mammalian microRNA targets. Cell 115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 29.Lai EC. 2002. Micro RNAs are complementary to 3′ UTR sequence motifs that mediate negative post-transcriptional regulation. Nat Genet 30:363–364. doi: 10.1038/ng865. [DOI] [PubMed] [Google Scholar]
- 30.Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. 2008. Widespread changes in protein synthesis induced by microRNAs. Nature 455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 31.Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. 2008. The impact of microRNAs on protein output. Nature 455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eulalio A, Huntzinger E, Izaurralde E. 2008. GW182 interaction with Argonaute is essential for miRNA-mediated translational repression and mRNA decay. Nat Struct Mol Biol 15:346–353. doi: 10.1038/nsmb.1405. [DOI] [PubMed] [Google Scholar]
- 33.Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN. 2004. MicroRNA genes are transcribed by RNA polymerase II. EMBO J 23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han J, Lee Y, Yeom KH, Kim YK, Jin H, Kim VN. 2004. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev 18:3016–3027. doi: 10.1101/gad.1262504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yi R, Qin Y, Macara IG, Cullen BR. 2003. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev 17:3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. 2001. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 37.Hutvagner G, McLachlan J, Pasquinelli AE, Balint E, Tuschl T, Zamore PD. 2001. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 293:834–838. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- 38.Gao QQ, Putzbach WE, Murmann AE, Chen S, Ambrosini G, Peter JM, Bartom E, Peter ME. 2018. 6mer seed toxicity in tumor suppressive miRNAs. Nature Comm 9:4504. doi: 10.1038/s41467-018-06526-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Putzbach W, Gao QQ, Patel M, van Dongen S, Haluck-Kangas A, Sarshad AA, Bartom ET, Kim K-Y, Scholtens DM, Hafner M, Zhao JC, Murmann AE, Peter ME. 2017. Many si/shRNAs can kill cancer cells by targeting multiple survival genes through an off-target mechanism. eLife 6:e29702. doi: 10.7554/eLife.29702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel M, Bartom ET, Paudel B, Kocherginsky M, O'Shea KL, Murmann AE, Peter ME. 2022. Identification of the toxic 6mer seed consensus in human cancer cells. Sci Rep 12:5130. doi: 10.1038/s41598-022-09051-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hadji A, Ceppi P, Murmann AE, Brockway S, Pattanayak A, Bhinder B, Hau A, De Chant S, Parimi V, Kolesza P, Richards JS, Chandel N, Djaballah H, Peter ME. 2014. Death induced by CD95 or CD95 ligand elimination. Cell Rep 7:208–222. doi: 10.1016/j.celrep.2014.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murmann AE, Bartom ET, Schipma MJ, Vilker J, Chen S, Peter ME. 2020. 6mer seed toxicity in viral microRNAs. iScience 23:100737. doi: 10.1016/j.isci.2019.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Omoto S, Ito M, Tsutsumi Y, Ichikawa Y, Okuyama H, Brisibe EA, Saksena NK, Fujii YR. 2004. HIV-1 nef suppression by virally encoded microRNA. Retrovirology 1:44. doi: 10.1186/1742-4690-1-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaul D, Ahlawat A, Gupta SD. 2009. HIV-1 genome-encoded hiv1-mir-H1 impairs cellular responses to infection. Mol Cell Biochem 323:143–148. doi: 10.1007/s11010-008-9973-4. [DOI] [PubMed] [Google Scholar]
- 45.Ouellet DL, Plante I, Landry P, Barat C, Janelle ME, Flamand L, Tremblay MJ, Provost P. 2008. Identification of functional microRNAs released through asymmetrical processing of HIV-1 TAR element. Nucleic Acids Res 36:2353–2365. doi: 10.1093/nar/gkn076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ouellet DL, Vigneault-Edwards J, Letourneau K, Gobeil LA, Plante I, Burnett JC, Rossi JJ, Provost P. 2013. Regulation of host gene expression by HIV-1 TAR microRNAs. Retrovirology 10:86. doi: 10.1186/1742-4690-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jordan A, Bisgrove D, Verdin E. 2003. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J 22:1868–1877. doi: 10.1093/emboj/cdg188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haluck-Kangas A, Patel M, Paudel B, Vaidyanathan A, Murmann AE, Peter MP. 2021. DISE/6mer seed toxicity—a powerful anti-cancer mechanism with implications for other diseases. J Exp Clin Cancer Res 40:389. doi: 10.1186/s13046-021-02177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim YK, Kim B, Kim VN. 2016. Re-evaluation of the roles of Drosha, exportin 5, and Dicer in microRNA biogenesis. Proc Natl Acad Sci USA 113:E1881–E1889. doi: 10.1073/pnas.1602532113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Putzbach W, Haluck-Kangas A, Gao QQ, Sarshad AA, Bartom ET, Stults A, Qadir AS, Hafner M, Peter ME. 2018. CD95/Fas ligand mRNA is toxic to cells. eLife 7:e38621. doi: 10.7554/eLife.38621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel M, Wang Y, Bartom ET, Dhir R, Nephew KP, Matei D, Murmann AE, Lengyel E, Peter ME. 2021. The ratio of toxic-to-nontoxic microRNAs predicts platinum sensitivity in ovarian cancer. Cancer Res 81:3985–4000. doi: 10.1158/0008-5472.CAN-21-0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paudel B, Jeong SY, Pena Martinez C, Rickman A, Haluck-Kangas A, Be T, Frederiksen K, Affaneh A, Kessler JA, Mazzulli JR, Murmann AE, Ferreira A, Heckmann BL, Green DR, Sadleir KR, Vassar R, Peter ME. 2023. A contribution of death induced by survival gene elimination (DISE) to the neurotoxicity in Alzheimer’s disease. bioRxiv. https://www.biorxiv.org/content/10.1101/2022.09.08.507157v2. [DOI] [PMC free article] [PubMed]
- 53.Triboulet R, Mari B, Lin YL, Chable-Bessia C, Bennasser Y, Lebrigand K, Cardinaud B, Maurin T, Barbry P, Baillat V, Reynes J, Corbeau P, Jeang KT, Benkirane M. 2007. Suppression of microRNA-silencing pathway by HIV-1 during virus replication. Science 315:1579–1582. doi: 10.1126/science.1136319. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, Yin Y, Zhang S, Luo H, Zhang H. 2016. HIV-1 infection-induced suppression of the let-7i/IL-2 axis contributes to CD4+ T cell death. Sci Rep 6:25341. doi: 10.1038/srep25341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Modai S, Farberov L, Herzig E, Isakov O, Hizi A, Shomron N. 2019. HIV-1 infection increases microRNAs that inhibit Dicer1, HRB and HIV-EP2, thereby reducing viral replication. PLoS One 14:e0211111. doi: 10.1371/journal.pone.0211111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coley W, Van Duyne R, Carpio L, Guendel I, Kehn-Hall K, Chevalier S, Narayanan A, Luu T, Lee N, Klase Z, Kashanchi F. 2010. Absence of Dicer in monocytes and its regulation by HIV-1. J Biol Chem 285:31930–31943. doi: 10.1074/jbc.M110.101709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bennasser Y, Yeung ML, Jeang KT. 2006. HIV-1 TAR RNA subverts RNA interference in transfected cells through sequestration of TAR RNA-binding protein, TRBP. J Biol Chem 281:27674–27678. doi: 10.1074/jbc.C600072200. [DOI] [PubMed] [Google Scholar]
- 58.Heyam A, Lagos D, Plevin M. 2015. Dissecting the roles of TRBP and PACT in double-stranded RNA recognition and processing of noncoding RNAs. Wiley Interdiscip Rev RNA 6:271–289. doi: 10.1002/wrna.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang W, Zhou J, Halambage UD, Jurado KA, Jamin AV, Wang Y, Engelman AN, Aiken C. 2017. Inhibition of HIV-1 maturation via small-molecule targeting of the amino-terminal domain in the viral capsid protein. J Virol 91:e02155-16. doi: 10.1128/JVI.02155-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nikolic J, Belot L, Raux H, Legrand P, Gaudin Y, Albertini AA. 2018. Structural basis for the recognition of LDL-receptor family members by VSV glycoprotein. Nat Commun 9:1029. doi: 10.1038/s41467-018-03432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whisnant AW, Bogerd HP, Flores O, Ho P, Powers JG, Sharova N, Stevenson M, Chen CH, Cullen BR. 2013. In-depth analysis of the interaction of HIV-1 with cellular microRNA biogenesis and effector mechanisms. mBio 4:e000193. doi: 10.1128/mBio.00193-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klase Z, Kale P, Winograd R, Gupta MV, Heydarian M, Berro R, McCaffrey T, Kashanchi F. 2007. HIV-1 TAR element is processed by Dicer to yield a viral micro-RNA involved in chromatin remodeling of the viral LTR. BMC Mol Biol 8:63. doi: 10.1186/1471-2199-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li L, Feng H, Da Q, Jiang H, Chen L, Xie L, Huang Q, Xiong H, Luo F, Kang L, Zeng Y, Hu H, Hou W, Feng Y. 2016. Expression of HIV-encoded microRNA-TAR and its inhibitory effect on viral replication in human primary macrophages. Arch Virol 161:1115–1123. doi: 10.1007/s00705-016-2755-5. [DOI] [PubMed] [Google Scholar]
- 64.Macrae IJ, Zhou K, Li F, Repic A, Brooks AN, Cande WZ, Adams PD, Doudna JA. 2006. Structural basis for double-stranded RNA processing by Dicer. Science 311:195–198. doi: 10.1126/science.1121638. [DOI] [PubMed] [Google Scholar]
- 65.Ma JB, Ye K, Patel DJ. 2004. Structural basis for overhang-specific small interfering RNA recognition by the PAZ domain. Nature 429:318–322. doi: 10.1038/nature02519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bartom ET, Kocherginsky M, Paudel B, Vaidyanathan A, Haluck-Kangas A, Patel M, O’Shea KL, Murmann AE, Peter ME. 2022. SPOROS: a pipeline to analyze DISE/6mer seed toxicity. PLoS Comput Biol 18:e1010022. doi: 10.1371/journal.pcbi.1010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Dongen S, Abreu-Goodger C, Enright AJ. 2008. Detecting microRNA binding and siRNA off-target effects from expression data. Nat Methods 5:1023–1025. doi: 10.1038/nmeth.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Su H, Trombly MI, Chen J, Wang X. 2009. Essential and overlapping functions for mammalian Argonautes in microRNA silencing. Genes Dev 23:304–317. doi: 10.1101/gad.1749809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bolton DL, Hahn BI, Park EA, Lehnhoff LL, Hornung F, Lenardo MJ. 2002. Death of CD4+ T-cell lines caused by human immunodeficiency virus type 1 does not depend on caspases or apoptosis. J Virol 76:5094–5107. doi: 10.1128/jvi.76.10.5094-5107.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gandhi RT, Chen BK, Straus SE, Dale JK, Lenardo MJ, Baltimore D. 1998. HIV-1 directly kills CD4+ T cells by a Fas-independent mechanism. J Exp Med 187:1113–1122. doi: 10.1084/jem.187.7.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Doitsh G, Greene WC. 2016. Dissecting how CD4 T cells are lost during HIV infection. Cell Host Microbe 19:280–291. doi: 10.1016/j.chom.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Swaminathan G, Navas-Martin S, Martin-Garcia J. 2014. MicroRNAs and HIV-1 infection: antiviral activities and beyond. J Mol Biol 426:1178–1197. doi: 10.1016/j.jmb.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 73.Bukhari MMM, Mir I, Idrees M, Afzal S, Shahid M. 2020. Role of microRNAs in establishing latency of human immunodeficiency virus. Crit Rev Eukaryot Gene Expr 30:337–348. doi: 10.1615/CritRevEukaryotGeneExpr.2020034571. [DOI] [PubMed] [Google Scholar]
- 74.Joshi N, Chandane Tak M, Mukherjee A. 2022. The involvement of microRNAs in HCV and HIV infection. Ther Adv Vaccines Immunother 10:25151355221106104. doi: 10.1177/25151355221106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haluck-Kangas A, Fink M, Bartom ET, Peter ME. 2023. CD95/Fas ligand mRNA is toxic to cells through more than one mechanism. Mol Biomed 4:11. doi: 10.1186/s43556-023-00119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adiliaghdam F, Basavappa M, Saunders TL, Harjanto D, Prior JT, Cronkite DA, Papavasiliou N, Jeffrey KL. 2020. A requirement for Argonaute 4 in mammalian antiviral defense. Cell Rep 30:1690–1701. doi: 10.1016/j.celrep.2020.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Francia S, Michelini F, Saxena A, Tang D, de Hoon M, Anelli V, Mione M, Carninci P, d'Adda di Fagagna F. 2012. Site-specific Dicer and Drosha RNA products control the DNA-damage response. Nature 488:231–235. doi: 10.1038/nature11179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei W, Ba Z, Gao M, Wu Y, Ma Y, Amiard S, White CI, Rendtlew Danielsen JM, Yang YG, Qi Y. 2012. A role for small RNAs in DNA double-strand break repair. Cell 149:101–112. doi: 10.1016/j.cell.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 79.Cabrini M, Roncador M, Galbiati A, Cipolla L, Maffia A, Iannelli F, Sabbioneda S, d'Adda di Fagagna F, Francia S. 2021. Drosha is recruited to DNA damage sites by the MRN complex to promote non-homologous end joining. J Cell Sci 134:jcs249706. doi: 10.1242/jcs.249706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wan G, Zhang X, Langley RR, Liu Y, Hu X, Han C, Peng G, Ellis LM, Jones SN, Lu X. 2013. DNA-damage-induced nuclear export of precursor microRNAs is regulated by the ATM-AKT pathway. Cell Rep 3:2100–2112. doi: 10.1016/j.celrep.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gao M, Wei W, Li MM, Wu YS, Ba Z, Jin KX, Li MM, Liao YQ, Adhikari S, Chong Z, Zhang T, Guo CX, Tang TS, Zhu BT, Xu XZ, Mailand N, Yang YG, Qi Y, Rendtlew Danielsen JM. 2014. Ago2 facilitates Rad51 recruitment and DNA double-strand break repair by homologous recombination. Cell Res 24:532–541. doi: 10.1038/cr.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Daniel R, Kao G, Taganov K, Greger JG, Favorova O, Merkel G, Yen TJ, Katz RA, Skalka AM. 2003. Evidence that the retroviral DNA integration process triggers an ATR-dependent DNA damage response. Proc Natl Acad Sci USA 100:4778–4783. doi: 10.1073/pnas.0730887100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ryan EL, Hollingworth R, Grand RJ. 2016. Activation of the DNA damage response by RNA viruses. Biomolecules 6:2. doi: 10.3390/biom6010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Golden RJ, Chen B, Li T, Braun J, Manjunath H, Chen X, Wu J, Schmid V, Chang T-C, Kopp F, Ramirez-Martinez A, Tagliabracci VS, Chen ZJ, Xie Y, Mendell JT. 2017. An Argonaute phosphorylation cycle promotes microRNA-mediated silencing. Nature 542:197–202. doi: 10.1038/nature21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chu Y, Kilikevicius A, Liu J, Johnson KC, Yokota S, Corey DR. 2020. Argonaute binding within 3′-untranslated regions poorly predicts gene repression. Nucleic Acids Res 48:7439–7453. doi: 10.1093/nar/gkaa478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hultquist JF, Hiatt J, Schumann K, McGregor MJ, Roth TL, Haas P, Doudna JA, Marson A, Krogan NJ. 2019. CRISPR-Cas9 genome engineering of primary CD4+ T cells for the interrogation of HIV-host factor interactions. Nat Protoc 14:1–27. doi: 10.1038/s41596-018-0069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hauptmann J, Schraivogel D, Bruckmann A, Manickavel S, Jakob L, Eichner N, Pfaff J, Urban M, Sprunck S, Hafner M, Tuschl T, Deutzmann R, Meister G. 2015. Biochemical isolation of Argonaute protein complexes by Ago-APP. Proc Natl Acad Sci USA 112:11841–11845. doi: 10.1073/pnas.1506116112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hafner M, Renwick N, Farazi TA, Mihailović A, Pena JTG, Tuschl T. 2012. Barcoded cDNA library preparation for small RNA profiling by next-generation sequencing. Methods 58:164–170. doi: 10.1016/j.ymeth.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. 2013. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. 2013. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Anders S, Pyl PT, Huber W. 2015. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Robinson MD, McCarthy DJ, Smyth GK. 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mbisa JL, Delviks-Frankenberry KA, Thomas JA, Gorelick RJ, Pathak VK. 2009. Real-time PCR analysis of HIV-1 replication post-entry events, p 55–72. In Prasad VK, Kalpana GV (ed), HIV protocols, 2nd ed. Humana Press, Totowa, NJ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang H, Zhou Y, Alcock C, Kiefer T, Monie D, Siliciano J, Li Q, Pham P, Cofrancesco J, Persaud D, Siliciano RF. 2004. Novel single-cell-level phenotypic assay for residual drug susceptibility and reduced replication capacity of drug-resistant human immunodeficiency virus type 1. J Virol 78:1718–1729. doi: 10.1128/jvi.78.4.1718-1729.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Harwig A, Jongejan A, van Kampen AH, Berkhout B, Das AT. 2016. Tat-dependent production of an HIV-1 TAR-encoded miRNA-like small RNA. Nucleic Acids Res 44:4340–4353. doi: 10.1093/nar/gkw167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nie Z, Phenix BN, Lum JJ, Alam A, Lynch DH, Beckett B, Krammer PH, Sekaly RP, Badley AD. 2002. HIV-1 protease processes procaspase 8 to cause mitochondrial release of cytochrome c, caspase cleavage and nuclear fragmentation. Cell Death Differ 9:1172–1184. doi: 10.1038/sj.cdd.4401094. [DOI] [PubMed] [Google Scholar]
- 98.Nie Z, Bren GD, Rizza SA, Badley AD. 2008. HIV protease cleavage of procaspase 8 is necessary for death of HIV-infected cells. Open Virol J 2:1–7. doi: 10.2174/1874357900802010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Algeciras-Schimnich A, Belzacq-Casagrande AS, Bren GD, Nie Z, Taylor JA, Rizza SA, Brenner C, Badley AD. 2007. Analysis of HIV protease killing through caspase 8 reveals a novel interaction between caspase 8 and mitochondria. Open Virol J 1:39–46. doi: 10.2174/1874357900701010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sainski AM, Natesampillai S, Cummins NW, Bren GD, Taylor J, Saenz DT, Poeschla EM, Badley AD. 2011. The HIV-1-specific protein Casp8p41 induces death of infected cells through Bax/Bak. J Virol 85:7965–7975. doi: 10.1128/JVI.02515-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S3. Download jvi.00652-23-s0001.pdf, PDF file, 2.8 MB (2.8MB, pdf)
Data Availability Statement
RNA-seq data are available at the Gene Expression Omnibus under accession number GSE218195.