ABSTRACT
Pseudorabies virus (PRV), the causative pathogen of Aujeszky’s disease, is one of the most important pathogens threatening the global pig industry. Although vaccination has been used to prevent PRV infection, the virus cannot be eliminated in pigs. Thus, novel antiviral agents as complementary to vaccination are urgently needed. Cathelicidins (CATHs) are host defense peptides that play an important role in the host immune response against microbial infections. In the study, we found that the chemical synthesized chicken cathelicidin B1 (CATH-B1) could inhibit PRV regardless of whether CATH-B1 was added pre-, co-, or post-PRV infection in vitro and in vivo. Furthermore, coincubation of CATH-B1 with PRV directly inactivated virus infection by disrupting the virion structure of PRV and mainly inhibited virus binding and entry. Importantly, pretreatment of CATH-B1 markedly strengthened the host antiviral immunity, as indicated by the increased expression of basal interferon-β (IFN-β) and several IFN-stimulated genes (ISGs). Subsequently, we investigated the signaling pathway responsible for CATH-B1-induced IFN-β production. Our results showed that CATH-B1 induced phosphorylation of interferon regulatory transcription factor 3 (IRF3) and further led to production of IFN-β and reduction of PRV infection. Mechanistic studies revealed that the activation of Toll-like receptor 4 (TLR4), endosome acidification, and the following c-Jun N-terminal kinase (JNK) was responsible for CATH-B1-induced IRF3/IFN-β pathway activation. Collectively, CATH-B1 could markedly inhibit PRV infection via inhibiting virus binding and entry, direct inactivation, and regulating host antiviral response, which provided an important theoretical basis for the development of antimicrobial peptide drugs against PRV infection.
IMPORTANCE Although the antiviral activity of cathelicidins could be explained by direct interfering with the viral infection and regulating host antiviral response, the specific mechanism of cathelicidins regulating host antiviral response and interfering with pseudorabies virus (PRV) infection remains elusive. In this study, we investigated the multiple roles of cathelicidin CATH-B1 against PRV infection. Our study showed that CATH-B1 could suppress the binding and entry stages of PRV infection and direct disrupt PRV virions. Remarkably, CATH-B1 significantly increased basal interferon-β (IFN-β) and IFN-stimulated gene (ISG) expression levels. Furthermore, TLR4/c-Jun N-terminal kinase (JNK) signaling was activated and involved in IRF3/IFN-β activation in response to CATH-B1. In conclusion, we elucidate the mechanisms by which the cathelicidin peptide direct inactivates PRV infection and regulates host antiviral IFN-β signaling.
KEYWORDS: pseudorabies virus, CATH-B1, IFN-β, TLR4, JNK
INTRODUCTION
Pseudorabies virus (PRV) belongs to the Alphaherpesvirinae subfamily under the family Herpesviridae, which infects a broad host range of mammals, such as ruminants, carnivores, and rodents (1). PRV infection causes Aujeszky’s disease, characterized as respiratory disease and reproductive failure in adult pigs and neurological symptoms in piglets, respectively (2). Although the vaccination-differentiation of infected from vaccinated animals (DIVA) testing strategy has been well performed in the United States and several other countries, PRV remains an important pathogen in the pig industry in many countries (3). Particularly, the emerging PRV variants in China in 2011 caused huge economic losses in the pig industry, making it more difficult to eradicate PRV (4–6). In addition, PRV was recently isolated from acute encephalitis cases in humans (7), and a total of 25 cases of PRV infections in humans have been reported in China since 2017 (8), which suggests the great risk of PRV infection from pigs to humans and poses an increasing threat to public and human health.
Antimicrobial peptides (AMPs) are a group of short cationic peptides that constitute a crucial part of innate immunity in nearly all living organisms (9, 10). Nowadays, cathelicidins have been found in massive mammalian species, including chickens, rabbits, horses, pigs, rats, monkeys, cattle, and humans, but with a great variety of structure and size among these peptides. Cathelicidins are generally synthesized as inactive precursors of 15 to 18 kDa that contain an N-terminal signal sequence, a central cathelin domain, and a C-terminal mature peptide (11). The N-terminal domain (29 to 30 amino acids [aa]) is responsible for the release of biologically active peptides. The C-terminal domain (12 to 100 aa), as the mature peptide, exhibits broad bactericidal activity. However, the function of the cathelin domain (98 to 114 aa) is still unclear (12). In mammals, cathelicidins are one of the most abundant classes of AMPs, which have been shown to possess direct bactericidal activities and diverse immunomodulatory functions (13, 14).
Apart from the directly bactericidal activities, cathelicidins were recently shown to have broad antiviral activities (15). One of the most representative cathelicidins, the sole human cathelicidin LL-37, has been shown to inhibit a number of viral infections, including herpesviruses, vaccinia virus, HIV, West Nile virus, EV71, respiratory syncytial virus, and influenza A virus (IAV) (16–19). Similar to the killing effect on bacteria, cathelicidins LL-37 can directly disrupt viral envelope structures (16). Furthermore, LL-37 can regulate the host antiviral immune response, characterized by increased interferon-β (IFN-β) expression and interferon regulatory transcription factor 3 (IRF3) phosphorylation (18). However, the mechanism by which cathelicidins regulate type I IFNs activation remains unclear.
In addition to human cathelicidin LL-37, many cathelicidins derived from other species also exhibit antiviral activity. For instance, murine cathelicidin-related AMP (CRAMP) had a direct antiviral activity against vaccinia virus by destroying the viral envelope in a carpet-based mechanism (20). Bovine myeloid antimicrobial peptide-18 (BMAP-18) showed anti-HIV-1 activity (21), and chicken cathelicidin B1 (CATH-B1) possessed antiviral activity against IAV by binding to the viral particle and thereby blocking viral entry (22). Specifically, CATH-B1 is a newly identified chicken-derived peptide in the bursa of Fabricius (the immunological organ in chicken producing B-cells) (23), suggesting a potential role for CATH-B1 in the host immune system against microbial infections.
In this study, we examined antiviral activities of different cathelicidins derived from pigs, mice, and chickens against PRV. We found that CATH-B1 exhibited the most significant anti-PRV activity in vitro and some degree of antiviral effect in vivo. Furthermore, we observed the multiple roles of CATH-B1 in inhibition of PRV infection. On one side, CATH-B1 directly disrupted PRV virions and interfered with virus binding and entry. On the other side, CATH-B1 increased expression of IFN-β and several interferon-stimulated genes (ISGs). Remarkably, for the first time, Toll-like receptor 4 (TLR4) and c-Jun N-terminal kinase (JNK) signaling was found to be activated and involved in IRF3 phosphorylation and type I IFN activation in response to a cathelicidin peptide. Collectively, we elucidated that the mechanisms of CATH-B1 inhibiting PRV infection are through directly disrupting virus particles and upregulating antiviral IFN-β signaling via TLR4/JNK/IRF3 pathway.
RESULTS
Animal-derived cathelicidins show significant inhibitory effects on PRV infection in vitro.
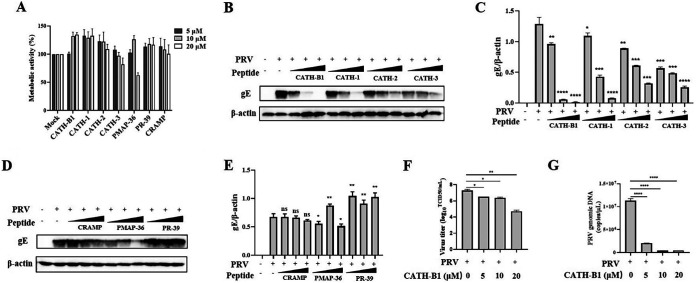
To determine whether cathelicidins have anti-PRV effects in vitro, we selected different animal-derived cathelicidins, including chicken-derived peptides (CATH-1, -2, -3, and -B1), murine-derived CRAMP, and porcine-derived PMAP-36 and PR-39. We examined the cytotoxicity of cathelicidins toward PK-15 cells by the WST-1 assay. The results showed that most of tested cathelicidins exhibited no significant cytotoxicity to PK-15 cells. Only PMAP-36 showed moderate toxicity at the highest dose (20 μM) with cell viability of approximately 50% (Fig. 1A). Next, anti-PRV effects of cathelicidins were evaluated by detecting expression of glycoprotein E (gE, a major envelope glycoprotein of PRV) in PK-15 cells at 24 h postinfection (hpi). The results showed that CATH-1, -2, -3, -B1, and PMAP-36 exhibited anti-PRV effects compared with the control group (Fig. 1B to E). Specifically, CATH-B1 showed the strongest inhibitory effect with almost complete inhibition of viral infectivity at concentrations of 10 and 20 μM (Fig. 1B and C). Furthermore, the results of the 50% tissue culture infective dose (TCID50) and quantitative PCR (qPCR) assays also indicated that CATH-B1 significantly inhibited PRV infection in PK-15 cells in a dose-dependent manner (Fig. 1F and G). In addition, the cytotoxicity of cathelicidins was also examined in BHK-21 cells. Unlike CATH-1, -2, and -3 and PMAP-36, CATH-B1 and the other two peptides (PR-39 and CRAMP) exhibited little to moderate cytotoxicity to BHK-21 cells (Fig. S1A). Among the peptides tested, CATH-B1 exhibited the most significant anti-PRV effect in BHK-21 cells by detecting expression of PRV gE protein (Fig. S1B).
FIG 1.
Screen of antimicrobial peptides derived from cathelicidins inhibiting pseudorabies (PRV) infection in PK-15 cells. PK-15 cells were treated with seven different peptides in different concentrations (5, 10, and 20 μM). After 2 h, the cultures were washed, and the cells were incubated in fresh medium for another 22 h. (A) Cell viability was determined by the WST-1 assay. The results are the averages ± SD of experiments performed in triplicate. PK-15 cells were infected with PRV at a multiplicity of infection (MOI) of 1 and then simultaneously treated with chicken-derived peptides CATH-B1 and CATH-1, -2, and -3 and peptides CRAMP, PMAP-36, and PR-39 derived from other animals at different concentrations (5, 10, and 20 μM), respectively. (B to E) After 24 h, PRV glycoprotein E (gE) protein levels were detected by Western blotting (B and D) and analyzed by ImageJ (C and E). In all cases, β-actin was used as a loading control, and the blots were representative of three independent experiments. (F, G) Meanwhile, the averages ± SD of virus titers in duplicate were determined by the 50% tissue culture infective dose (TCID50) assay (F) and that of viral genomic DNA in triplicate was calculated by the qPCR method (G). Student’s t tests were used for statistical analyses. ns, no significance; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, comparing the PRV-treated group to the PRV plus CATH-B1-treated groups. CRAMP, cathelicidin-related AMP.
CATH-B1 has both direct and indirect inhibitory effects on PRV infection in vitro and in vivo.
To further explore the anti-PRV mechanisms of CATH-B1 in vitro, we examined the anti-PRV effects of CATH-B1 under different treatments as designed in Fig. 2A. The results showed that coincubation with 20 μM CATH-B1 almost completely inhibited PRV infection in PK-15 cells as demonstrated by detection of virus titers and gE expression (Fig. 2B to D). Interestingly, pretreatment with 20 μM CATH-B1 also attenuated PRV infectivity with a significant decrease in virus titers and gE protein expression (Fig. 2B to D). Although post-treatment with CATH-B1 had no significant effect on virus titers (Fig. 2B), the gE expression was still significantly decreased (Fig. 2C and D). Likewise, the reduction of PRV infection in PK-15 cells treated with CATH-B1 in different ways was visualized by the immunofluorescence assay (IFA) (Fig. 2E). In addition, animal experiments showed that in vivo coinjection of CATH-B1 and PRV completely abrogated PRV infection in mice with no clinical symptoms and no deaths (Fig. 2F and G). Although pre- or postinjection of CATH-B1 did not reduce deaths of mice (Fig. 2G), the clinical symptoms of the infected mice in the two groups were found reduced at 66 and 72 hpi, respectively (Fig. 2F). These results suggested that CATH-B1 had the capacity of inhibiting PRV infection in vitro and in vivo and may act in both direct and indirect manners.
FIG 2.
Cathelicidin CATH-B1 suppressed PRV infection under different treatments in vitro and in vivo. (A) Schematic diagram of experimental setup of CATH-B1 (20 μM) and PRV (MOI = 1) treatments in PK-15 cells. (B to E) Virus titers in duplicate under different treatments were determined by the TCID50 assay (B), and PRV gE protein levels were detected by Western blotting (C), quantified by ImageJ (D), and simultaneously visualized by immunofluorescence assay (IFA) (magnification, ×200) (E). (F, G) The clinical score (F) and survival curve (G) of C57BL/6 mice (5 mice/group) in infection control, post-treatment, pretreatment, and coincubation groups were presented here, respectively. Student’s t tests were used for statistical analyses. Statistical significance is shown as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; and ns, no significance. DAPI, 4′,6-diamidino-2-phenylindole.
Coincubation with CATH-B1 prevents virus binding and entry and disrupts the virion structure of PRV.
To further understand the direct inhibition mechanism of CATH-B1 on PRV infection, PK-15 cells were treated with PRV-PBS-co, PRV-CATH-B1-co, and PRV-CATH-B1-sep as shown in Fig. 3A, and then virus titers and gE protein expression were measured by the TCID50 and Western blot assays, respectively. The results showed that coincubation of CATH-B1 and PRV for 2 h (PRV-CATH-B1-co) significantly reduced virus titers (Fig. 3B) and intracellular gE expression (Fig. 3C and D) in the virus-infected PK-15 cells compared to PRV-PBS-co group and PRV-CATH-B1-sep group, suggesting that coincubation with CATH-B1 directly renders the loss of virus infectivity. Furthermore, without preincubation (time = 0 h), CATH-B1 reduced virus titers by approximately 2 logs compared to the PRV-only treatment. With longer PRV+CATH-B1 incubation time, the inhibiting effect on viral infection increased to complete prevention at 2 h (Fig. 3E), indicating that the direct inhibition effect of CATH-B1 was dependent on incubation time with viruses. Meanwhile, the incubation temperature was also associated with the peptide inhibition effect, as indicated by the enhanced anti-PRV effect of CATH-B1 with increased incubation temperature (Fig. 3F). Next, we investigated whether CATH-B1 treatment inhibited the binding, entry, and release stages of PRV infection. The results of the TCID50 assay showed that PRV titers in CATH-B1 coincubation group (treated) were significantly reduced by about 1.5 and 1 log in the binding and entry experiments, respectively (Fig. 3G and H). Moreover, to exclude the possibility that CATH-B1 could alter virus binding by interfering with some receptors on the cell surface, the cell monolayers were also pretreated with the peptide for 2 h before PRV infection. The results showed that no significant difference in virus titers between untreated and CATH-B1-pretreated groups was observed in the binding and entry experiments (Fig. 3G and H). In addition, at the stage of virus release, coincubation of PRV-infected cells with CATH-B1 had no effect on viral titers in virus release stage, as indicated by the TCID50 assay (Fig. 3I). Furthermore, we investigated the potential capability of CATH-B1 to disrupt the structure of PRV virions via electron microscopy. Upon incubating PRV in the absence or presence of CATH-B1 for 1 h, we found that more PRV virions were perturbed in the PRV+CATH-B1 group compared to the PRV group (Fig. 3J). The results indicated that CATH-B1 can disrupt PRV virion structure, which may lead to the inactivation of virus infection. Altogether, our results suggested that CATH-B1 could directly disrupt PRV virions and inhibit virus binding and entry stages.
FIG 3.
Coincubation with CATH-B1 inhibited PRV infection by interfering with virus binding and entry but not release and disrupting virus structure. PRV (1 × 108 TCID50/mL) was coincubated with phosphate-buffered saline (PRV-PBS-co) or peptide (PRV-CATH-B1-co) with a final concentration of 20 μM at 37°C for 2 h, or PRV and peptide were separately incubated (PRV-CATH-B1-sep) at 37°C for 2 h, and then the PRV-PBS mixture, PRV-CATH-B1 mixture, or the separately incubated PRV and CATH-B1 mixture were added to PK-15 cells. (A) After incubation for 2 h, the culture supernatants were removed, the cells were washed three times with PBS, and fresh culture media were added to the cells for another 22 h. (B to D) Virus titers of the freeze-thaw supernatant of the virus-infected cells in duplicate were determined by the TCID50 assay (B), and PRV gE protein levels in virus-infected cells were examined by Western blotting (C) and further quantified by ImageJ (D). (E, F) Meanwhile, experiments of time- and temperature-dependent inhibition of PRV infection by CATH-B1 with a final concentration of 20 μM were performed, respectively. Briefly, PRV (1 × 108 TCID50/mL) were coincubated with CATH-B1 at 37°C for indicated time points or at different temperatures for 2 h. Then, the mixtures were added to PK-15 cells and incubated for 2 h, cell cultures were removed, and fresh media were added to the cells for another 22 h. Virus titers of the freeze-thaw supernatant of PRV-infected cells in duplicate were determined by the TCID50 assay. Then, the binding, entry, and release assays were performed. (G to I) Virus titers in the binding (G), entry (H), and release (I) assays are shown. Furthermore, the purified PRV virions were incubated for 1 h at 37°C in the presence or absence of CATH-B1. Then, virus suspensions were prepared for electron microscopy observation. Representative images of PRV virions under different experimental conditions were shown. (J) Yellow arrows indicated the complete virus particles, and red arrows indicated the disrupted virions. Student’s t tests were used for statistical analyses. Statistical significance is shown as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; and ns, no significance.
CATH-B1 markedly activates type I IFNs signaling and antiviral gene expression.
Type I IFN signaling plays a crucial role in restricting virus infection. Meanwhile, the virus has evolved different strategies to defend host type I IFN production in order to achieve successful infection in the host (24). Interestingly, herein we found that CATH-B1 in the absence of virus could induce IFN-β mRNA transcription in PK-15 cells and further enhance the IFN-β level induced by PRV (Fig. 4A). Additionally, ISGs (ISG15 and CH25H) mRNA expression levels were also upregulated 6- and 20-fold by CATH-B1 compared to the mock group, respectively (Fig. 4B and C). It has been reported that IRF3 phosphorylation directly modulates IFN-β expression and plays a critical role in anti-PRV immune response (18, 25, 26). Subsequently, we investigated the phosphorylation and nuclear translocation of IRF3 in PK-15 cells in response to CATH-B1 with and without PRV infection, respectively. The results showed that PRV infection (with or without CATH-B1) could not significantly affect the level of p-IRF3/IRF3 at 24 hpi (Fig. 4D; Fig. S2A). However, 20 μM CATH-B1 treatment could significantly increase the level of p-IRF3/IRF3 (Fig. 4D and E; Fig. S2). Additionally, we also detected the nuclear translocation of IRF3 upon PRV and CATH-B1 treatment. We found that CATH-B1 treatment significantly induced IRF3 nuclear translocation, while PRV infection could inhibit IRF3 nuclear translocation induced by CATH-B1 (Fig. 4F), which was consistent with the results in Fig. 4D. Further, we found that CATH-B1-induced activation of p-IRF3 was significantly reduced in PRV-infected cells compared to that in CATH-B1-treated cells in an infection time-dependent manner (Fig. 4G), suggesting that CATH-B1-induced IFN-β signaling could be inhibited by PRV infection, and therefore it could be reasonably concluded that the anti-PRV effect of CATH-B1-induced IFN-β signaling could be relatively limited.
FIG 4.
Cathelicidin CATH-B1 markedly induced type I interferon (IFN) signaling activation. PK-15 cells were infected with PRV (MOI = 1) for 24 h (PRV group) or incubated with CATH-B1 (20 μM) at 37°C for 2 h, then the cells were washed with PBS for three times, and fresh culture media were added for 24 h of incubation (CATH-B1 group). Alternatively, PK-15 cells were inoculated with CATH-B1 (20 μM) at 37°C for 2 h, and then cells were washed and incubated with PRV (MOI = 1) for 24 h (PRV+CATH-B1 group). (A to D) After incubation, IFN-β, ISG15, and CH25H RNA levels were determined by qPCR (A, B, and C), and the protein levels of IRF3 and p-IRF3 in whole-cell lysates were determined by Western blotting (D). (E) Then, the levels of IRF3 and p-IRF3 were also detected in PK-15 cells treated with different concentrations of CATH-B1. (F) Furthermore, the protein levels of IRF3 upon CATH-B1 (20 μM) and/or PRV treatments in the cytoplasmic and nuclear lysates were analyzed by Western blotting with α-tubulin and histone H3 as cytoplasmic fraction and nuclear fraction markers, respectively. (G) At different time points of each stimulus, the levels of IRF3 and p-IRF3 in the CATH-B1 (20 μM) and PRV+CATH-B1 (20 μM) groups were examined by Western blotting. Student’s t tests were used for statistical analyses. Statistical significance is shown as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; and ns, no significance.
TLR4/JNK signaling is responsible for CATH-B1-induced IFN-β activation.
TLRs are important components of the innate immune response that can recognize microorganisms and then lead to production of inflammatory cytokines or type I IFNs (27). Among the TLRs, TLR4 is a major molecule that can recognize both bacterial components and viral envelope proteins with a broad capacity to induce type I IFNs in many cell types (28). Herein, our study showed that blockage of TLR4 and related signaling pathways by their specific inhibitors TAK-242 (TLR4 inhibitor), chloroquine (endosomal acidification inhibitor), R406 (SyK inhibitor), and SP600125 (JNK inhibitor) significantly reduced CATH-B1-induced IRF3 phosphorylation, respectively (Fig. 5A and B), suggesting that the TLR4 and its related signalings might be involved in CATH-B1-induced type I IFN production. Furthermore, the increased TLR4 and p-JNK expression, perinuclear clustering of p-JNK, and staining of endosome were visualized by IFA assay in CATH-B1-treated PK-15 cells compared to that in mock-treated PK-15 cells (Fig. 5C).
FIG 5.
TLR4, JNK, and endosome were involved in the activation of p-IRF3 induced by CATH-B1. PK-15 cells were pretreated with 10 μM chloroquine (endosomal acidification inhibitor) or inhibitors specific for TLR4 (1 μM TAK-242), JNK (50 μM SP600125), SyK (5 μM R406), ERK (10 μM FR180204), p38 (10 μM SB203580), or phosphatidyl inositol 3-kinase (PI3K)/AKT (1 μM wortmannin) for 1 h and then treated with CATH-B1 (20 μM) for 12 h. (A, B) Then the levels of total IRF3 and p-IRF3 were determined by Western blotting (A) and analyzed by ImageJ (B). (C) Fluorescent labeling of TLR4, p-JNK, and endosome in PK-15 cells following CATH-B1 treatment. The expression and distribution of TLR4, p-JNK, and Texas Red-transferrin (an endocytic marker) in PK-15 cells in the presence or absence of CATH-B1 were determined by IFA (magnification, ×200). Student’s t tests were used for statistical analyses. Statistical significance is shown as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; and ns, no significance.
Furthermore, knockdown of TLR4 led to approximately 50% reduction of IFN-β expression, suggesting that TLR4 played a critical role in CATH-B1-induced type I IFN activation (Fig. 6A and B). Moreover, TLR4 knockdown significantly decreased CATH-B1-induced phosphorylation of JNK and IRF3 as indicated by the Western blot and ImageJ analyses (Fig. 6C to F). Similarly, inhibition of TLR4, endosomal acidification, and JNK by specific inhibitors resulted in 75, 38, and 57% reduction of IFN-β transcription, respectively (Fig. 6G). Phosphorylation of IRF3 and JNK induced by CATH-B1 was also abrogated by inhibitors of TLR4, endosomal acidification, and JNK (Fig. 6H to J); however, the slightly promoted TLR4 expression by CATH-B1 was basically unaffected by these inhibitors (Fig. 6H and K). These data suggested that TLR4, JNK, and endosomal acidification were crucial factors for CATH-B1-induced IRF3 activation. Ultimately, inhibitors of TLR4, endosomal acidification, and JNK were used to treat PK-15 before PRV infection or PRV+CATH-B1 stimuli, and virus titers at 24 hpi were measured by the TCID50 assay. The results showed that these inhibitors could slightly reduce PRV infection in PK-15 cells (Fig. 6L and M). Nonetheless, virus titers in PRV+CATH-B1 groups pretreated with these inhibitors were higher than that in the PRV+CATH-B1 group, and statistical analysis showed that the inhibitory effect of CATH-B1 on viral titers was significantly weakened after treatment with the related inhibitors (Fig. 6L and M), suggesting that the inhibitory effect of CATH-B1 on PRV infection in PK-15 cells could be alleviated by these inhibitors. In addition, confocal microscopy also provided evidences for colocalization of TLR4, endosomes, and JNK (Fig. 7A and B), and the use of fluorescein isothiocyanate (FITC)-labeled CATH-B1 further suggested that CATH-B1, TLR4, and endosomes may directly interact with one another (Fig. 7C). Taken together, these results showed that CATH-B1 activated IRF3/IFN-β signaling through a TLR4/endosomal acidification/JNK-dependent pathway.
FIG 6.
TLR4/JNK signaling contributed to IFN-β activation and antiviral effect induced by CATH-B1. PK-15 cells were transfected with control siRNA (si-NC) or TLR4 siRNA (si-TLR4) for 48 h and then treated with CATH-B1 for 12 h. (A, B) The mRNA levels of TLR4 (A) and IFN-β (B) were determined by qPCR assay. (C to F) The protein expression levels of TLR4, IRF3, p-IRF3, JNK, and p-JNK were determined by Western blotting (C) and analyzed by ImageJ (D to F). In addition, PK-15 cells were pretreated with 10 μM chloroquine (to block endosomal acidification) or inhibitors specific for TLR4 (1 μM TAK-242) or JNK (50 μM SP600125) for 1 h before treating with CATH-B1. (G, H) The cells were subjected to qRT-PCR (G) and Western blot (H) analyses for relative mRNA expression of IFN-β and protein expression of TLR4, JNK, p-JNK, IRF3, and p-IRF3, respectively. (I to K) Quantification of the ratios of p-IRF3 to total IRF3 (I), p-JNK to total JNK (J), and TLR4 to β-actin (K) from immunoblots were analyzed by ImageJ. (L, M) Then, PK-15 cells were pretreated with 10 μM chloroquine or inhibitors specific for TLR4 (1 μM TAK-242) or JNK (50 μM SP600125) for 1 h, and/or treated with CATH-B1 (20 μM) for 12 h, and/or subsequently infected with 1 MOI (L) or 0.1 MOI (M) PRV for 24 h, respectively. Virus titers of freeze-thaw cell lysates in duplicate were determined by the TCID50 assay. Student’s t tests were used for statistical analyses. Statistical significance is shown as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; and ns, no significance.
FIG 7.
The confocal microscopy images to illustrate the immunofluorescent staining of TLR4, JNK, endosome (Texas Red-transferrin), and fluorescein isothiocyanate (FITC)-labeled CATH-B1. First, PK-15 cells were treated with or without CATH-B1 (20 μM) at 37°C for 2 h, and then cells were washed and incubated for 10 h. (A, B) After incubation, the cellular localization of TLR4, JNK, and endosome were stained with anti-TLR4 antibody, anti-p-JNK antibody, and the internalized Texas Red-transferrin and then analyzed by confocal immunofluorescence microscopy. Furthermore, the FITC-labeled CATH-B1 (20 μM) were used to treat PK-15 cells. (C) After incubation, the cellular distribution of FITC-CATH-B1, TLR4, and endosome were stained with FITC, anti-TLR4 antibody, and the internalized Texas Red-transferrin and then analyzed by confocal immunofluorescence microscopy. For the colocalization analysis, the Pearson’s correlation coefficients (r) were calculated and are shown for each set of marked areas.
DISCUSSION
Vaccines against PRV have been successfully used for many years in the pig industry. Hence, Aujeszkey’s disease in pigs has been controlled well by the use of vaccination in the United States and many European countries (3). However, with the prevalence of PRV variants in China, it is suggested that the existing vaccines may be unable to provide complete protection against wild-type PRV infection (29). Recently, reports on human cases of PRV infection further indicated the potential risk of PRV transmission from pigs to humans (7). Therefore, the development of novel antiviral agents as complementary to vaccination is urgently needed for designing PRV control strategies.
Cathelicidins are a group of short cationic peptides that have inhibitory effects on several human and animal viruses. Previous studies have shown that several cathelicidins, including human-derived LL-37 and GF-17 (the truncated form of LL-37), bovine-derived BMAP-18, and snake-derived BF-30 can reduce virus infection mainly through two aspects: (i) directly inactivating viruses and (ii) strengthening the host antiviral immunity (10, 18, 30). In this study, we attempted to investigate the anti-PRV activity and mechanism of animal-derived cathelicidins. We found that several cathelicidins had remarkable anti-PRV infection activities in vitro without any significant cytotoxicity (Fig. 1). Among them, a chicken-derived peptide CATH-B1 showed the strongest inhibitory effect in vitro and a certain degree of anti-PRV activity in vivo (Fig. 1 and 2). Furthermore, coincubation of CATH-B1 with PRV nearly completely reduced PRV infection in PK-15 cells (Fig. 2B and C), while the inhibitory effects of pre- and post-treatments with CATH-B1 were much lower (Fig. 2B to E), indicating that the direct interaction between CATH-B1 and PRV may be the main antiviral mechanism adopted by CATH-B1. Additionally, we also found that coincubation of CATH-B1 inhibited PRV binding and entry stages instead of the release stage (Fig. 3G to I). Since cathelicidin LL-37 has been shown to inhibit the viral binding and entry stages in previous studies (31–33), which suggested that blocking virus binding and entry might be an important element of cathelicidins in inhibiting virus infection via direct interactions. We have previously demonstrated that CATH-B1 can direct bind to and aggregate IAV particles, which was likely involved in the anti-IAV mechanism of CATH-B1 (22). To further investigate the direct effects of CATH-B1 on PRV virions, we examined the PRV virions’ integrity by electron microscopy after exposure to CATH-B1 and observed more disrupted particles in the CATH-B1-treated PRV sample (Fig. 3J). This suggested that CATH-B1 adopted a different mechanism to directly disrupt PRV virions, which might lead to decreased virus binding and entry into host cells.
In addition to coincubation, pretreatment with CATH-B1 showed a significant anti-PRV effect in vitro. Intriguingly, we found that CATH-B1 alone significantly induced phosphorylation and nuclear translocation of IRF3 and increased the expression levels of IFN-β and ISGs (Fig. 4), indicating that type I IFNs could be activated by the CATH-B1 peptide via phosphorylation and nuclear translocation of IRF3, which could further induce ISGs production through recognizing the IFN-α/β receptor (IFNAR). Previous studies also showed that human-derived LL-37 and GF-17 could promote type I IFNs activation in vitro (10, 18). Moreover, LL-37 and poly(I·C) synergistically enhanced IFN-β production in keratinocytes, and the IFN-β expression in response to poly(I·C) and LL-37 was dependent on TBK1-AKT-IRF3 and mitochondrial antiviral-signaling protein (MAVS) (34, 35). These studies supported that cathelicidins were capable of activating type I IFNs; however, the mechanism by which cathelicidins alone regulate type I IFNs activation remains to be determined. In addition, although CATH-B1 alone induced robust IRF3 phosphorylation and IFN-β expression, the CATH-B1-induced IRF3 activation was increasingly counteracted along with PRV infection and could not continue for a long period (Fig. 4G), suggesting that PRV infection had the capacity to counteract CATH-B1-induced IFN-β activation. Given that PRV has evolved many strategies to evade IFN-β responses (24), it will be interesting to identify the mechanism by which PRV counteracts the activation of type I IFNs induced by CATH-B1 in the future.
Different types of pathogen recognition receptors (PRRs), including Toll-like receptors, can induce the activation of IRF3 and the secretion of type I IFNs in response to different stimuli (36). In our study, we found that blockage of TLR4 and its related signaling pathways by their specific inhibitors significantly reduced CATH-B1-induced IRF3 phosphorylation (Fig. 5A and B); meanwhile, we first observed that CATH-B1 alone induced expression and aggregation of TLR4 and formation of endosome (Fig. 5C), and then the subsequent IRF3 phosphorylation and IFN-β expression can be abrogated by inhibition of TLR4 (TAK-242 or siTLR4) and endosomal acidification (chloroquine) (Fig. 6), suggesting that TLR4-associated endosome is important for IRF3 phosphorylation and type I IFN activation. Moreover, a growing number of kinases were shown to have a role in IRF3 activation. For example, mitogen-activated protein kinases (MAPKs), phosphatidyl inositol 3-kinase (PI3K)/AKT, and protein kinase C have been reported to participate in the modulation of IRF3 activation (37–39). Furthermore, previous studies have also suggested that MAPKs, especially JNK, can be activated by TLR4 signaling (40, 41) and also can mediate the activation of IRF3 and the induction of type I IFNs signaling (39). However, it is unknown whether the TLR4/JNK signaling is the critical pathway for CATH-B1-induced IRF3 activation. Herein, we found that increased expression and perinuclear aggregation of p-JNK were induced by CATH-B1 (Fig. 5C). Furthermore, either knockdown or inhibition of TLR4 remarkably inhibited JNK and IRF3 phosphorylation, and inhibition of JNK remarkably reduced IRF3 phosphorylation and IFN-β expression (Fig. 6). Importantly, colocalization of TLR4, endosomes, and JNK and direct interactions between CATH-B1 and TLR4 and endosomes were demonstrated by confocal microscopy (Fig. 7), which indicated that TLR4/JNK signaling could be responsible for CATH-B1-induced IFN-β activation. In addition, the blockage of TLR4, endosomal acidification, and JNK can alleviate the inhibition effect of CATH-B1 on PRV infectivity (Fig. 6L and M), suggesting that CATH-B1-induced type I IFNs pathway activation inhibited PRV infection in PK-15 cells to some extent. Taken together, these results suggested that cathelicidin CATH-B1 triggered type I IFNs activation through a TLR4/JNK/IRF3-dependent pathway (Fig. 8).
FIG 8.
Model of the main findings in this study. Chicken-derived cathelicidin CATH-B1 can directly interact with PRV and disrupt the virion structure, interfere with virus binding and entry stages, and even strengthen the host antiviral type I IFN response via TLR4/JNK/IRF3 pathway. ISG, IFN-stimulated gene; IFNAR, IFN-α/β receptor; JNK, c-Jun N-terminal kinase.
In summary, our findings provide new insights into how cathelicidin CATH-B1 inhibits pseudorabies virus infection via different ways and illustrate how the cathelicidin activates type I IFNs pathway. Here, we demonstrate that cathelicidin CATH-B1 is capable of inhibiting PRV infection via direct disruption, inhibiting virus binding and entry, and activating the type I IFNs antiviral response (Fig. 8), which provides a new idea for the development of antimicrobial peptide drug against PRV infection.
MATERIALS AND METHODS
Peptides.
All short peptides shown in Table 1 were synthesized by China Peptides (Shanghai, China) using 9-fluorenylmethoxy carbonyl (Fmoc) chemistry and purified by reverse-phase high-performance liquid chromatography to a purity >95%.
TABLE 1.
Characteristics of peptides used in this study
| Peptide | Source of animals | Amino acid sequence | Length (aa) |
|---|---|---|---|
| PMAP-36 | Porcine | GRFRRLRKKTRKRLKKIGKVLKWIPPIVGSIPLGCG | 36 |
| PR-39 | Porcine | RRRPRPPYLPRPRPPPFFPPRLPPRIPPGFPPRFPPRFP | 39 |
| CATH-1 | Chicken | RVKRVWPLVIRTVIAGYNLYRAIKKK | 26 |
| CATH-2 | Chicken | RFGRFLRKIRRFRPKVTITIQGSARF | 26 |
| CATH-3 | Chicken | RVKRFWPLVPVAINTVAAGINLYKAIRRK | 29 |
| CATH-B1 (FITC-CATH-B1) | Chicken | PIRNWWIRIWEWLNGIRKRLRQRSPFYVRGHLNVTSTPQP | 40 |
| CRAMP | Murine | GLLRKGGEKIGEKLKKIGQKIKNFFQKLVPQPEQ | 34 |
Antibodies.
The primary antibodies in this study were as follows: anti-PRV glycoprotein E (gE) monoclonal antibody (MAb) (preserved in our laboratory), anti-p-IRF3 antibody (Ab) (Cell Signaling Technology, USA), anti-p-JNK Ab used for IFA (Cell Signaling Technology, USA), anti-JNK Ab (Cell Signaling Technology, USA), anti-p-JNK Ab used for Western blot (Bioss, Beijing, China), anti-TLR4 Ab (Santa Cruz Biotechnology, USA), anti-IRF3 Ab (Beyotime, China), anti-β-actin Ab (Beyotime, China), anti-histone H3 Ab (Beyotime, China), and anti-α-tubulin Ab (ABclonal, China).
PRV strain and cell lines.
Porcine kidney (PK)-15, baby hamster kidney-21 (BHK-21), and African green monkey kidney (Vero) cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS). All cell cultures were maintained at 37°C in a humidified cell incubator containing 5% CO2. The PRV strain JS-2012 (kindly provided by professor Guangzhi Tong, Shanghai Veterinary Research Institute, Chinese Academy of Agricultural Sciences) was maintained in our laboratory and stored at −80°C (42).
The virus was propagated and titrated on Vero cells. Virus titers were determined by the 50% tissue culture infective dose (TCID50) assay using the Reed-Muench method and expressed as TCID50/mL.
Mice.
The wild-type C57BL/6 mice were purchased from Chongqing Academy of Chinese Materia Medical (Chongqing, China). All the mice were maintained in specific pathogen-free (SPF) conditions for being used at 6 to 8 weeks old. All of the animal experiments were approved by Institutional Animal Care and Use Committee of Southwest University, Chongqing, China (IACUC-20210415-03).
Cell viability.
Cell viability was determined using the WST-1 assay according to the previous study (22) with a minor modification. In brief, the cells were incubated with peptides for 2 h at 37°C; then peptides were washed away; and the cells were further incubated for 22 h at 37°C. After 24 h of incubation, 150 μL of 10% WST-1 reagent was added according to the manufacturer’s protocol. After 20 min incubation, absorbance was measured at 450 nm with a microplate reader (Bio-Rad, Japan). Untreated cells were set as a control. The percentage of cell viability (%) was calculated as: cell viability (%) = (OD450(sample)/OD450(control)) × 100%.
Western blot.
Differentiated cells were prepared in 12-well plates and treated with peptides and/or small interfering RNA (siRNA)/PRV/the corresponding inhibitors. After each treatment, the total proteins were extracted with radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime, China) supplemented with phenylmethylsulfonyl fluoride (PMSF) protease inhibitor (Beyotime, China) and PhosSTOP phosphatase inhibitor (Roche, USA). When indicated, nuclear and cytoplasmic proteins were separated by nuclear and cytoplasmic protein extraction kit (Beyotime, China). The protein samples were then subjected to 12% SDS-PAGE and subsequently transferred onto a polyvinylidene difluoride (PVDF) membrane by electroblotting. The membranes were blocked with TBST containing 5% nonfat dried milk/bovine serum albumin (BSA). After washing with TBST, membranes were incubated overnight at 4°C with the corresponding antibodies. Subsequently, the corresponding membranes were incubated with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 h at room temperature following five washes with TBST. After washing with TBST, the distinct protein bands were detected by ECL detection reagent (Biosharp, China).
Immunofluorescence microscopy.
After each treatment, PK-15 cells in 48-well plates were fixed with 4% paraformaldehyde (Sango Biotech, Shanghai, China) for 30 min and then permeabilized with 0.1% Triton X-100 for 5 min at room temperature. Next, the cells were blocked with 5% BSA for 1 h at room temperature. After washing steps, the primary antibodies (anti-PRV gE Ab, anti-TLR4 Ab, and anti-p-JNK Ab) were respectively added and incubated overnight at 4°C. Subsequently, goat anti-mouse IgG H&L (Alexa Fluor 488) Ab (Abcam, United Kingdom), goat anti-rabbit IgG H&L (Alexa Fluor 488) Ab (Bioss, Beijing, China), and goat anti-mouse IgG H&L (Alexa Fluor 594) Ab (Bioss, Beijing, China) were added at room temperature for 1 h after washing with phosphate-buffered saline (PBS), respectively. In addition, Texas Red-transferrin was internalized in the dark for 15 min to label the endosome. 4′,6-diamidino-2-phenylindole (DAPI) (Beyotime, China) was added and incubated in the dark for 5 min to stain the nucleus. Finally, anti-fluorescence attenuation mounting tablets (Solarbio, China) were used, and fluorescence images of cells were observed with an inverted fluorescence microscope (Olympus, Japan) or an Olympus FV3000 confocal microscope (Olympus, Japan). For the colocalization analysis, the Pearson’s correlation coefficient was calculated via the use of JACoP plugin from ImageJ.
Virus binding, entry, and release assay.
(i) Virus binding assay. First, PK-15 cells were infected with PRV at a multiplicity of infection (MOI) = 1 for 2 h at 4°C, following the mock treatment (untreated: no CATH-B1 was added), cotreatment (treated: CATH-B1 was simultaneously added with PRV), or pretreatment (pretreated: CATH-B1 was added 2 h before PRV infection and washed with PBS) with CATH-B1. Next, the cells were washed with ice-cold PBS three times to remove unbound virus, and samples to measure bound virus were harvested for viral titers.
(ii) Virus entry assay. PK-15 cells were challenged with PRV at an MOI = 5 for 2 h at 4°C, following the mock treatment (untreated: no CATH-B1 was added), cotreatment (treated: CATH-B1 was added 2 h after virus binding), or pretreatment (pretreated: CATH-B1 was added 2 h before virus infection and washed with PBS) with CATH-B1. After washing, the cells were incubated for 2 h at 37°C. Next, the cells were treated with 0.05% trypsin and washed to remove surface bound virus. Then, the samples were harvested for the measurement of viral titers.
(iii) Virus release assay. PK-15 cells were challenged with PRV at an MOI = 0.1/0.01 for 24 h at 37°C. After washing with PBS three times, the medium was replaced with DMEM of 2% FBS and treated with CATH-B1 for another 4 h in 5% CO2 at 37°C. Then, the supernatants were harvested for viral titer measurement.
Electron microscopy.
A total of four to six T-75 flasks of Vero cells were prepared for PRV infection. Then, PRV was used to infect these Vero cells at an MOI of 5 when cells are 95% confluent. After 48 hpi, the PRV-infected cells were moved to −80°C, and the samples were frozen and thawed three times. The cell supernatants were collected and centrifuged at 8,000 × g for 10 min, and the new supernatants were recollected and centrifuged in a 100-kDa ultrafiltration tube at 5,000 × g at 4°C and concentrated to 10 mL. Then, the relatively purified PRV virions in the concentrated supernatant were obtained by 5 and 45% glycerol density gradient centrifugation at 30,000 × g for 2 h.
For electron microscopy observation, the virus suspensions in sterile PBS were diluted in Dulbecco’s PBS (DPBS) in ratios of 1:100 and then visualized by 1% phosphotungstic acid negative staining. About 10 μL virus suspensions were added to grids containing a carbon-coated Formvar supporting film for 5 min. The virus solutions were soaked off with filter paper, and the samples were stained for 5 min and air-dried. The samples were visualized using electron microscope (JEM-1400 FLASH, Japan) at 80 kV. The images were taken with a digital camera (EMSIS, Germany).
Quantitative PCR analysis.
The RNA transcription levels of IFN-β, TLR4, ISG15, and CH25H in PK-15 cells from different groups were determined by quantitative reverse transcription (RT)-PCR, respectively. The total RNAs were extracted using the TRIzol reagent (Invitrogen, USA) according to the manufacturer’s instruction, and cDNAs were synthesized from the above RNA using PrimeScript RT reagent kit (Perfect Real Time) (TaKaRa, Japan). Subsequently, the quantitative real-time PCR was performed using 2× Universal SYBR green fast qPCR mix reagent (ABclonal, China) with the indicated primers for target genes (Table 2). The relative mRNA expression levels were calibrated to the level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and calculated using the 2−ΔΔCt method. In addition, the primers gB-forward and gB-reverse in Table 2 were used in a specific absolute qPCR method for detection of PRV genome copies/μL as described in our previous study (43).
TABLE 2.
Primer sequences for quantitative PCR
| Gene | Sequence (5′ to 3′) | |
|---|---|---|
| GAPDH | Forward | GAAGGTCGGAGTGAACGGATTT |
| Reverse | TGGGTGGAATCATACTGGAACA | |
| IFN-β | Forward | GGCTGGAATGAAACCGTCAT |
| Reverse | TCCAGGATTGTCTCCAGGTCA | |
| gB | Forward | ACTACGAGGACTACAGCTACGTGCG |
| Reverse | GTCACCCGCGTGCTGATC | |
| TLR4 | Forward | GCGTGCAGGTGGTTCCTA |
| Reverse | AGCACCTGCAGTTCTGGAAA | |
| ISG15 | Forward | GATCGGTGTGCCTGCCTTC |
| Reverse | CGTTGCTGCGACCCTTGT | |
| CH25H | Forward | TGGGACCACCTGAAGACCTG |
| Reverse | TCGGGAACACGAACACCACA | |
RNA interference.
PK-15 cells were transfected using Lipofectamine RNAiMAX (Thermo Fisher Scientific, USA) with 80 nM TLR4 siRNA (sense, 5′-GGAAUGAACUGGUAAAGAATT-3′; antisense, 5′-UUCUUUACCAGUUCAUUCCTT-3′) or 80 nM control siRNA (sense, 5′-UUCUCCGAACGUGUCACGUTT-3′; antisense, 5′-ACGUGACACGUUCGGAGAATT-3′) for 48 h before treatment with CATH-B1 and then were treated with CATH-B1 for 12 h. Finally, cell lysates were collected for RT-qPCR and Western blot, respectively. The relative TLR4 gene expression in mRNA and protein levels were normalized against the mRNA level of GAPDH and the protein level of β-actin, respectively.
Animal experiments.
The mice were injected intraperitoneally with 100 μL CATH-B1 (10 mg/kg) either together with PRV infection (coincubation), 2 h preinfection (pretreatment), or 2 h postinfection (post-treatment). Then, PRV JS-2012 (1 × 105 TCID50) in a volume of 100 μL was injected intraperitoneally into the corresponding mice. The mice injected intraperitoneally with PRV JS-2012 alone were as infection control. The clinical signs and mortality of mice were observed and recorded every 6 h. For clinical signs evaluation, the mice were scored as previously described with a minor revision (44). Symptoms were scored with the following three-point system: 0 = posture normal, no clinical symptoms were present; 1 = isolation and lethargy were observed; 2 = constant tremors, attempt to scratch, scratching of the abdomen, lack of weight bearing; and 3 = same as score 2 but with biting and bleeding of the abdomen. The mice were euthanized when scored as 3.
Statistical analysis.
All of the data are presented as averages ± SD of three independent experiments unless specified otherwise and analyzed by Student’s t tests for two-group comparisons. Statistical significance is shown as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; and ns, no significance. All the graphs were made by GraphPad Prism software.
ACKNOWLEDGMENTS
We thank Sha Jiang and Jianing Chen for reagents and advice. We thank Edwin J.A. Veldhuizen for critical review of the manuscript.
This study was supported by grant 2021YFD1800800 from the National Key Research and Development Program of China, grant SWU-KT22016 from the Fundamental Research Funds for the Central Universities, grant 32102684 from the National Natural Science Foundation of China, grant NCTIP-XD/C17 from the National Center of Technology Innovation for Pigs, grant 20211105 from the Chongqing Pig Industry Technology System, and grant CXQT20004 from the Foundation for Innovation Research Group in Chongqing Universities. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
C.Y., C.W., and J.C. contributed to the acquisition, analysis, and interpretation of the data and writing of the manuscript; C.Y., C.W., G.L., Y.L., Q.T., L.P., and R.F. contributed to the study design and data interpretation and participated in performing the experiments; J.C. and Y.W. contributed to the data analysis and interpretation.
We declare no conflict of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Chao Ye, Email: yechao123@swu.edu.cn.
Lianci Peng, Email: penglianci@swu.edu.cn.
Rendong Fang, Email: rdfang@swu.edu.cn.
Jae U. Jung, Lerner Research Institute, Cleveland Clinic
REFERENCES
- 1.Mettenleiter TC. 2000. Aujeszky’s disease (pseudorabies) virus: the virus and molecular pathogenesis-state of the art, June 1999. Vet Res 31:99–115. doi: 10.1051/vetres:2000110. [DOI] [PubMed] [Google Scholar]
- 2.Pomeranz LE, Reynolds AE, Hengartner CJ. 2005. Molecular biology of pseudorabies virus: impact on neurovirology and veterinary medicine. Microbiol Mol Biol Rev 69:462–500. doi: 10.1128/MMBR.69.3.462-500.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Müller T, Hahn EC, Tottewitz F, Kramer M, Klupp BG, Mettenleiter TC, Freuling C. 2011. Pseudorabies virus in wild swine: a global perspective. Arch Virol 156:1691–1705. doi: 10.1007/s00705-011-1080-2. [DOI] [PubMed] [Google Scholar]
- 4.An TQ, Peng JM, Tian ZJ, Zhao HY, Li N, Liu YM, Chen JZ, Leng CL, Sun Y, Chang D, Tong GZ. 2013. Pseudorabies virus variant in Bartha-K61-vaccinated pigs, China, 2012. Emerg Infect Dis 19:1749–1755. doi: 10.3201/eid1911.130177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun Y, Luo Y, Wang CH, Yuan J, Li N, Song K, Qiu HJ. 2016. Control of swine pseudorabies in China: opportunities and limitations. Vet Microbiol 183:119–124. doi: 10.1016/j.vetmic.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Yu X, Zhou Z, Hu D, Zhang Q, Han T, Li X, Gu X, Yuan L, Zhang S, Wang B, Qu P, Liu J, Zhai X, Tian K. 2014. Pathogenic pseudorabies virus, China, 2012. Emerg Infect Dis 20:102–104. doi: 10.3201/eid2001.130531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Q, Wang X, Xie C, Ding S, Yang H, Guo S, Li J, Qin L, Ban F, Wang D, Wang C, Feng L, Ma H, Wu B, Zhang L, Dong C, Xing L, Zhang J, Chen H, Yan R, Wang X, Li W. 2021. A novel human acute encephalitis caused by pseudorabies virus variant strain. Clin Infect Dis 73:e3690–e3700. doi: 10.1093/cid/ciaa987. [DOI] [PubMed] [Google Scholar]
- 8.Liu Q, Kuang Y, Li Y, Guo H, Zhou C, Guo S, Tan C, Wu B, Chen H, Wang X. 2022. The epidemiology and variation in pseudorabies virus: a continuing challenge to pigs and humans. Viruses 14:1463. doi: 10.3390/v14071463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zasloff M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 10.He M, Zhang H, Li Y, Wang G, Tang B, Zhao J, Huang Y, Zheng J. 2018. Cathelicidin-derived antimicrobial peptides inhibit Zika virus through direct inactivation and interferon pathway. Front Immunol 9:722. doi: 10.3389/fimmu.2018.00722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei L, Yang J, He X, Mo G, Hong J, Yan X, Lin D, Lai R. 2013. Structure and function of a potent lipopolysaccharide-binding antimicrobial and anti-inflammatory peptide. J Med Chem 56:3546–3556. doi: 10.1021/jm4004158. [DOI] [PubMed] [Google Scholar]
- 12.Agier J, Efenberger M, Brzezińska-Błaszczyk E. 2015. Cathelicidin impact on inflammatory cells. Cent Eur J Immunol 40:225–235. doi: 10.5114/ceji.2015.51359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Harten RM, van Woudenbergh E, van Dijk A, Haagsman HP. 2018. Cathelicidins: immunomodulatory antimicrobials. Vaccines 6:63. doi: 10.3390/vaccines6030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Travis SM, Anderson NN, Forsyth WR, Espiritu C, Conway BD, Greenberg EP, McCray PB, Jr, Lehrer RI, Welsh MJ, Tack BF. 2000. Bactericidal activity of mammalian cathelicidin-derived peptides. Infect Immun 68:2748–2755. doi: 10.1128/IAI.68.5.2748-2755.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barlow PG, Findlay EG, Currie SM, Davidson DJ. 2014. Antiviral potential of cathelicidins. Future Microbiol 9:55–73. doi: 10.2217/fmb.13.135. [DOI] [PubMed] [Google Scholar]
- 16.Brice DC, Diamond G. 2020. Antiviral activities of human host defense peptides. Curr Med Chem 27:1420–1443. doi: 10.2174/0929867326666190805151654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chessa C, Bodet C, Jousselin C, Larivière A, Damour A, Garnier J, Lévêque N, Garcia M. 2022. Antiviral effect of hBD-3 and LL-37 during human primary keratinocyte infection with West Nile virus. Viruses 14:1552. doi: 10.3390/v14071552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu J, Dai Y, Fu Y, Wang K, Yang Y, Li M, Xu W, Wei L. 2021. Cathelicidin antimicrobial peptides suppress EV71 infection via regulating antiviral response and inhibiting viral binding. Antiviral Res 187:105021. doi: 10.1016/j.antiviral.2021.105021. [DOI] [PubMed] [Google Scholar]
- 19.Tripathi S, Wang G, White M, Qi L, Taubenberger J, Hartshorn KL. 2015. Antiviral activity of the human cathelicidin, LL-37, and derived peptides on seasonal and pandemic influenza a viruses. PLoS One 10:e0124706. doi: 10.1371/journal.pone.0124706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dean RE, O’Brien LM, Thwaite JE, Fox MA, Atkins H, Ulaeto DO. 2010. A carpet-based mechanism for direct antimicrobial peptide activity against vaccinia virus membranes. Peptides 31:1966–1972. doi: 10.1016/j.peptides.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 21.Wang G, Watson KM, Buckheit RW, Jr. 2008. Anti-human immunodeficiency virus type 1 activities of antimicrobial peptides derived from human and bovine cathelicidins. Antimicrob Agents Chemother 52:3438–3440. doi: 10.1128/AAC.00452-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng L, Du W, Balhuizen MD, Haagsman HP, de Haan CAM, Veldhuizen EJA. 2020. Antiviral activity of chicken cathelicidin B1 against influenza A virus. Front Microbiol 11:426. doi: 10.3389/fmicb.2020.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goitsuka R, Chen CL, Benyon L, Asano Y, Kitamura D, Cooper MD. 2007. Chicken cathelicidin-B1, an antimicrobial guardian at the mucosal M cell gateway. Proc Natl Acad Sci USA 104:15063–15068. doi: 10.1073/pnas.0707037104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang R, Tang J. 2021. Evasion of I interferon-mediated innate immunity by pseudorabies virus. Front Microbiol 12:801257. doi: 10.3389/fmicb.2021.801257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu H, Li C, He W, Chen J, Yang G, Chen L, Chang H. 2022. Free ISG15 inhibits pseudorabies virus infection by positively regulating type I IFN signaling. PLoS Pathog 18:e1010921. doi: 10.1371/journal.ppat.1010921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong Z, Yin H, Wang F, Liu Z, Luan X, Sun L, Liu W, Shang Y. 2022. Pseudorabies virus tegument protein UL13 recruits RNF5 to inhibit STING-mediated antiviral immunity. PLoS Pathog 18:e1010544. doi: 10.1371/journal.ppat.1010544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeda K, Kaisho T, Akira S. 2003. Toll-like receptors. Annu Rev Immunol 21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 28.Monroe KM, McWhirter SM, Vance RE. 2010. Induction of type I interferons by bacteria. Cell Microbiol 12:881–890. doi: 10.1111/j.1462-5822.2010.01478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu ZQ, Tong W, Zheng H, Li LW, Li GX, Gao F, Wang T, Liang C, Ye C, Wu JQ, Huang Q, Tong GZ. 2017. Variations in glycoprotein B contribute to immunogenic difference between PRV variant JS-2012 and Bartha-K61. Vet Microbiol 208:97–105. doi: 10.1016/j.vetmic.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 30.Xing M, Ji M, Hu J, Zhu T, Chen Y, Bai X, Mwangi J, Mo G, Lai R, Jin L. 2020. Snake cathelicidin derived peptide inhibits Zika virus infection. Front Microbiol 11:1871. doi: 10.3389/fmicb.2020.01871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brice DC, Toth Z, Diamond G. 2018. LL-37 disrupts the Kaposi’s sarcoma-associated herpesvirus envelope and inhibits infection in oral epithelial cells. Antiviral Res 158:25–33. doi: 10.1016/j.antiviral.2018.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Currie SM, Gwyer Findlay E, McFarlane AJ, Fitch PM, Böttcher B, Colegrave N, Paras A, Jozwik A, Chiu C, Schwarze J, Davidson DJ. 2016. Cathelicidins have direct antiviral activity against respiratory syncytial virus in vitro and protective function in vivo in mice and humans. J Immunol 196:2699–2710. doi: 10.4049/jimmunol.1502478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee CJ, Buznyk O, Kuffova L, Rajendran V, Forrester JV, Phopase J, Islam MM, Skog M, Ahlqvist J, Griffith M. 2014. Cathelicidin LL-37 and HSV-1 corneal infection: peptide versus gene therapy. Transl Vis Sci Technol 3:4. doi: 10.1167/tvst.3.3.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takiguchi T, Morizane S, Yamamoto T, Kajita A, Ikeda K, Iwatsuki K. 2014. Cathelicidin antimicrobial peptide LL-37 augments interferon-β expression and antiviral activity induced by double-stranded RNA in keratinocytes. Br J Dermatol 171:492–498. doi: 10.1111/bjd.12942. [DOI] [PubMed] [Google Scholar]
- 35.Zhang LJ, Sen GL, Ward NL, Johnston A, Chun K, Chen Y, Adase C, Sanford JA, Gao N, Chensee M, Sato E, Fritz Y, Baliwag J, Williams MR, Hata T, Gallo RL. 2016. Antimicrobial peptide LL37 and MAVS signaling drive interferon-β production by epidermal keratinocytes during skin injury. Immunity 45:119–130. doi: 10.1016/j.immuni.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amarante-Mendes GP, Adjemian S, Branco LM, Zanetti LC, Weinlich R, Bortoluci KR. 2018. Pattern recognition receptors and the host cell death molecular machinery. Front Immunol 9:2379. doi: 10.3389/fimmu.2018.02379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarkar SN, Peters KL, Elco CP, Sakamoto S, Pal S, Sen GC. 2004. Novel roles of TLR3 tyrosine phosphorylation and PI3 kinase in double-stranded RNA signaling. Nat Struct Mol Biol 11:1060–1067. doi: 10.1038/nsmb847. [DOI] [PubMed] [Google Scholar]
- 38.Johnson J, Albarani V, Nguyen M, Goldman M, Willems F, Aksoy E. 2007. Protein kinase Cα is involved in interferon regulatory factor 3 activation and type I interferon-beta synthesis. J Biol Chem 282:15022–15032. doi: 10.1074/jbc.M700421200. [DOI] [PubMed] [Google Scholar]
- 39.Zhang B, Li M, Chen L, Yang K, Shan Y, Zhu L, Sun S, Li L, Wang C. 2009. The TAK1-JNK cascade is required for IRF3 function in the innate immune response. Cell Res 19:412–428. doi: 10.1038/cr.2009.8. [DOI] [PubMed] [Google Scholar]
- 40.Seimon TA, Obstfeld A, Moore KJ, Golenbock DT, Tabas I. 2006. Combinatorial pattern recognition receptor signaling alters the balance of life and death in macrophages. Proc Natl Acad Sci USA 103:19794–19799. doi: 10.1073/pnas.0609671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H, Luo HY, Liu Q, Xiao Y, Tang L, Zhong F, Huang G, Xu JM, Xu AM, Zhou ZG, Dai RP. 2018. Intermittent high glucose exacerbates A-FABP activation and inflammatory response through TLR4-JNK signaling in THP-1 cells. J Immunol Res 2018:1319272. doi: 10.1155/2018/1319272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye C, Huang Q, Jiang J, Li G, Xu D, Zeng Z, Peng L, Peng Y, Fang R. 2021. ATP-dependent activation of NLRP3 inflammasome in primary murine macrophages infected by pseudorabies virus. Vet Microbiol 259:109130. doi: 10.1016/j.vetmic.2021.109130. [DOI] [PubMed] [Google Scholar]
- 43.Ye C, Chen J, Cheng X, Zhou S, Jiang S, Xu J, Zheng H, Tong W, Li G, Tong G. 2019. Functional analysis of the UL24 protein of suid herpesvirus 1. Virus Genes 55:76–86. doi: 10.1007/s11262-018-1619-3. [DOI] [PubMed] [Google Scholar]
- 44.Laval K, Vernejoul JB, Van Cleemput J, Koyuncu OO, Enquist LW. 2018. Virulent pseudorabies virus infection induces a specific and lethal systemic inflammatory response in mice. J Virol 92:e01614-18. doi: 10.1128/JVI.01614-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Download jvi.00706-23-s0001.docx, DOCX file, 0.2 MB (230.4KB, docx)
Fig. S2. Download jvi.00706-23-s0002.docx, DOCX file, 0.1 MB (127.4KB, docx)