Abstract
Purpose of Review
Racial/ethnic minority and socioeconomically disadvantaged individuals experience greater postpartum weight retention, which has been linked to the development of cardiovascular disease. This article reviews recent literature on behavioral interventions targeting postpartum weight retention in these populations.
Recent Findings
Seven randomized controlled trials published since 2010 were selected for this review. Four were successful in reducing or preventing postpartum weight retention. Recruitment primarily occurred in low-income urban areas. All interventions reported using the Social Cognitive Theory and targeted mostly individual-level behavior change focused on diet and physical activity. Four were technology-based, and most implemented strategies to increase cultural relevance of the intervention.
Summary
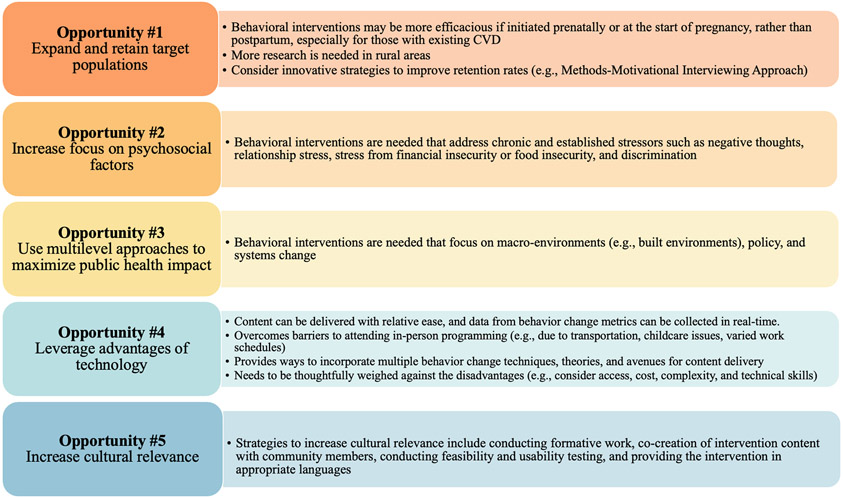
Opportunities for future interventions include expand target population to enroll individuals starting in pregnancy and address rural populations; incorporate empirically tested retention strategies; increase focus on psychosocial factors, particularly chronic stress; utilize multilevel approaches; continue to leverage technology; and maximize efforts to increase cultural relevancy.
Keywords: Postpartum weight retention, behavioral intervention, underserved, minority, low-income
INTRODUCTION
Pregnancy is a pivotal event in an individual’s life with critical implications for life-long cardiometabolic health. To support a growing fetus, an individual’s metabolism, blood composition, and vasculature adapt, in addition to gaining body weight during the 9-month period [1]. However, these factors may contribute to the increased risk for cardiovascular disease (CVD) [1]. Of particular concern is the postpartum retention of weight gained during pregnancy. A year after delivery, 60% of individuals who have given birth experience postpartum weight retention and many gain additional weight [2, 3]. When return to pre-pregnancy weight is not achieved after delivery, individuals face greater risk for obesity and the development of chronic diseases, including CVD [4-7]. Taken together, these findings highlight the importance of identifying and implementing strategies that reduce or prevent postpartum weight retention.
Intervening to prevent and reduce postpartum weight retention, particularly if applied to high-risk populations, is a necessary public health strategy that could mitigate CVD incidence. Underserved groups are at greater risk for retaining and gaining weight postpartum than those with social advantages [3]. Paralleling this, they are also at an increased risk of developing and dying from CVD [8]. This is in part due to higher incidence of high blood pressure, diabetes, and obesity in these populations [9-11]. Such factors also put a pregnant individual at a greater risk for excessive gestational weight gain during pregnancy, which is strongly associated with postpartum weight retention after delivery [12-14]. Indeed, epidemiologic investigations have found that minority and low-income individuals are at greater risk for excessive gestational weight gain, and as a result, inadequate postpartum weight loss is prevalent in these populations [3, 15].
Behavior and lifestyle change interventions show great promise for preventing and reducing postpartum weight retention, though very little work has focused solely on underserved populations [16, 17]. Interventions for general populations may not fully capture the different needs, barriers, motivators, and cultural considerations that racial and ethnic minorities and those from socioeconomically disadvantaged background possess. This review serves to provide a high-level narrative of the research landscape in this area. Thus, the purpose of this article is to present a literature review on behavioral interventions on postpartum weight retention specifically targeted towards low-income and racial or ethnic minority populations, and to provide recommendations for future work to continue advancing the cardiovascular health of underserved groups.
METHODOLOGY
A review of recent published literature was conducted to identify relevant behavioral interventions. Electronic databases including PubMed and Google Scholar were searched for intervention studies based on the following keywords: “low-income,” “postpartum,” “postpartum weight,” “postpartum health interventions,” and “minority.” Criteria for inclusion included interventions that 1) specifically targeted low-income and/or racial/ethnic minority individuals; 2) were a randomized controlled trial (RCT) with a primary outcome of reducing or preventing postpartum weight retention using an intention to treat (ITT) protocol; 3) reported weight change results; and 4) were published after 2010. These criteria were chosen to facilitate comparisons and identify successful features across recent interventions. Data extracted for each study included study design and objectives; characteristics at baseline, behavior change theories, description of trial groups, behaviors targeted, socioecological model level targeted, primary outcomes, trial retention, and any strategies used to increase cultural relevancy on the interventions. These data are presented in Table 1 and highlights are presented below. Findings were further organized into themes, emphasizing opportunities for future research in this area, and summarized in Figure 1.
Table 1.
Summary of Findings
| Author and Yeara | Study Design and Objectives | Characteristics at Baselineb | Behavior Change Theory | Description of Trial Groups |
|---|---|---|---|---|
| Chang et. al., 2017 | RCT: To examine if promoting stress management, healthy eating and physical activity prevents postpartum weight gain in low-income mothers | I: N=387 C: N=182 Postpartum age (years, M ± SD): 1.71 ± 1.27; BMI: 32.03 ± 4.29; race/ethnicity: 79% Black, 21% White; low-income: 100% |
Social Cognitive Theory; Self-Determination Theory | Mothers in Motion intervention: 16 weeks (3-4 hours/week); included10 culturally sensitive DVD chapters and 10 peer support group teleconferences; participants created action plans via weekly worksheet to set and monitor 1-2 personal goals. Control group: received printed educational materials. Both groups received standard WIC care |
| Gilmore et. al., 2017 | Pilot RCT: To test the efficacy of a smart phone-based intervention to promote postpartum weight loss | I: N=19 C: N=16 Postpartum age: NR; BMI: 32 ± 3; race/ethnicity: 74% Black, 23% White, 3% Asian; low-income: 100% |
Learning Theory; Theory of Planned Behavior/Theory of Reasoned Action; Social Cognitive Theory | E-Moms intervention: 16 weeks; used "SmartLoss" smartphone app; measured daily weight and step data; included weekly tips (interactive activities, behavioral strategies, health information). Control group: received WIC standard care |
| Haire-Joshu et. al., 2019 * | RCT: To determine the efficacy of an intervention, provided both prenatally and after delivery, in minimizing body weight among socioeconomically disadvantaged African American women | I: N=92 C: N=93 Gestational age (weeks, M ± SD): 13.3 ± 1.6; BMI range: 25.0-45.0; race/ethnicity: 100% Black/African American; low-income: 100% |
Social Cognitive Theory | PAT+Lifestyle intervention: delivered from pregnancy through 12 months postpartum; used established Parents as Teachers (PAT) program to deliver the home-based lifestyle intervention; participants seen by intervention staff at bi-weekly visits during pregnancy and once a month after delivery. Control group: standard PAT alone |
| Herring et. al., 2014 * | Pilot RCT: To examine the feasibility, acceptability, and initial efficacy of a technology-based weight loss intervention for urban, low-income mothers | I: N=9 C: N=9 Postpartum age (months, M ± SD): 4.3 ± 3.9; BMI: 36.9 ± 6.1; race/ethnicity: 78% Black, 22% Hispanic; low-income: 100% |
Social Cognitive Theory | Healthy4Baby intervention: 14 weeks; technology-based using text-messages, health coach, and Facebook support group; participants received 15 min bi-weekly calls with health coach, daily text messages tailored to weight related strategy, self-monitoring texts 3-4 times per week, access to private Facebook group. Control group: received general postpartum care |
| Herring et. al., 2017 * | Pilot RCT: To determine whether a behavioral intervention implemented in early pregnancy through 6 months postpartum could increase the proportion of obese African American women who were at or below their early pregnancy weights | I: N=33 C: N=33 I: Gestational age (weeks, M ± SD): 7.9 ± 3.4; BMI: 33.5 ± 5.8; race/ethnicity: 100% Black/African American; low-income: NR C: Gestational age (weeks, M ± SD): 7.7 ± 4.0; BMI: 32.2 ± 5.4; race/ethnicity: 100% Black/African American; low-income: NR |
Social Cognitive Theory | Behavioral lifestyle intervention (adapted Healthy4Baby): delivered from pregnancy through 6 months postpartum; technology-based using text-messages, health coach, and Facebook support group; participants received 15 min bi-weekly calls with health coach, daily text messages tailored to weight related strategy, self-monitoring texts 3-4 times per week, access to private Facebook group. Control group: received general pregnancy and postpartum care |
| Palnati et. al., 2021 | RCT: To examine the effect of a lifestyle intervention on postpartum weight retention in Hispanic women with abnormal glucose tolerance in their current pregnancy | I: N=100 C: N=104 Gestational age (weeks, range): 24-28; BMI: 31 ± 8.1; race/ethnicity: 100% Hispanic |
Social Cognitive Theory; Transtheoretical Model | Lifestyle intervention: delivered from 24-28 weeks gestation through 12 months postpartum; culturally and linguistically modified, individually-tailored intervention; includes 4 in-person sessions and 13 telephone booster sessions by a health education. Control group: health and wellness intervention with mailed material and telephone calls |
| Phelan et. al., 2017 * | RCT: To test whether an internet-based weight loss program in addition to the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC program) for low-income postpartum women could produce greater weight loss than the WIC program alone | I: N=174 C: N=196 Postpartum age (months, M ± SD): 5.2 ± 3.2; BMI: 31.7 ± 5.1; race/ethnicity: 81.6% Hispanic/Latino; low-income: 100% |
Social Cognitive Theory | Fit Moms/Mamás Activas intervention: 12 months; internet-based weight loss program including website with weekly lessons, web diary, instructional videos, computerized feedback, text messages, and monthly face to face groups at WIC clinics; physical activity and calorie goals; weekly text messages for motivation/feedback. Control group: WIC standard care, and newsletters every 2 months with wellness information |
| Author and Year | Behaviors Targeted | Socioecological Model Level | Weight Outcomes and Trial Retentionc |
Strategies for Increasing Cultural Relevance of Intervention |
| Chang et. al., 2017 | Stress; Diet; Physical Activity | Individual; Interpersonal |
Assessed at 7 months
(weight differences): I: 188.3 ± 10.6 lbs; C:187.7 ± 10.6 lbs (Partial ITT/powered) Trial Retention: 56.2% |
-Peers from target audience featured in DVD and participated at group meetings -A community advisory group (including a WIC coordinator and nutrition consultant) reviewed components and topics of intervention; planned and implemented recruitment, intervention and data collection; helped maintain progress of the intervention; and provided feedback on DVD to ensure cultural sensitivity -Peer advisory group (9 WIC mothers with overweight/obesity, 5 African American and 4 white) were recruited from a WIC program to review DVD |
| Gilmore et. al., 2017 | Stress; Diet; Physical Activity; Sleep | Individual |
Assessed at 16 weeks: I: −0.1 ± 0.9 kg; C: 1.8 ± 0.9 kg (ITT/powered) Trial Retention: 87.5% |
-Adapted "SmartLoss" application for WIC participants, e.g., incorporated WIC-approved foods |
| Haire-Joshu et. al., 2019 * | Diet; Physical Activity; Sleep; Breastfeeding | Individual; Interpersonal; Environmental (home) |
Assessed at 12 months: I: 2.5 ± 7.4 kg; C: 5.7 ± 8.8 kg (Modified ITT/powered) Trial Retention: 69.3% |
-Developed in partnership with PAT program to assure consistency with its organizational mission, format, practice, and funding requirements -Addressed barriers and facilitators for healthy gestational weight gain identified by African American women during the intervention development |
| Herring et. al., 2014 * | Diet; Physical Activity; Sleep | Individual; Interpersonal |
Assessed at 14 weeks: I: −2.8 ± 3.7 kg; C: 0.7 ± 2.7 kg (ITT/not powered) Trial Retention: 94.4% |
-Formative work on motivators and barriers to healthy eating in pregnancy for target population |
| Herring et. al., 2017 * | Diet; Physical Activity; Sleep | Individual; Interpersonal |
Assessed at 6 months postpartum
(% of participants at or below early pregnancy weight): I: 56%; C: 29% (Modified ITT/not powered) Trial Retention: 84.8% |
-Formative work on motivators and barriers to healthy eating in pregnancy for target population |
| Palnati et. al., 2021 | Diet; Physical Activity | Individual |
Assessed at 12 months: I: −1.61 kg (95% CI: −9.68, 6.46); C: 4.85 kg (1.36, 8.33) (ITT/powered) Trial Retention: 70% |
-Used culturally and linguistically modified, individually tailored intervention materials developed in prior RCTs -Took into account social, cultural, economic, and environmental resources/challenges relevant to Hispanic women |
| Phelan et. al., 2017 * | Diet; Physical Activity | Individual; Interpersonal |
Assessed at 12 months: I: −3.2 kg (95% CI: −4.1, −2.4); C: −0.9 kg (−1.7, −0.1) (ITT/Powered) Trial Retention: 82% |
-Program available in English and Spanish |
RCT= Randomized Controlled Trial; ITT= Intention to Treat; I=Intervention; C: Comparison Group; NR=Not Reported; BMI=Body mass index [reported as mean ± SD kg/m2]
Trials that found statistically significant weight differences between trial groups are denoted with as asterisk (*)
Baseline characteristics are reported as both trial arms combined unless the authors reported trial arms separately only.
Weight outcomes are reported the way authors originally presented them and are reported as weight change unless otherwise noted.
Figure 1.
Opportunities for future research for behavioral interventions targeting postpartum weight retention in underserved populations
HIGHLIGHTS OF FINDINGS
Target Populations
Seven behavioral intervention studies were identified that met inclusion criteria (Table 1). There was overlap between studies that targeted low-income individuals and those that targeted individuals from racial/ethnic minority backgrounds. Three studies had specific inclusion criteria based on race or ethnicity, two specifically for African American/Black mothers [18] and another for Hispanic mothers [19]. Four other studies targeted individuals from low-income backgrounds; most of these recruited a large proportion of racial/ethnic minorities, and from urban settings [20-23]. Recruitment often occurred through the Special Supplemental Nutrition Program for Women, Infants and Children (WIC) [21-23]. One study utilized the Parents as Teachers (PAT) program for participant recruitment and study intervention. PAT is a home visiting model that promotes the optimal early development, learning, and health of children by partnering with federally funded programs for low-income communities [18].
Postpartum Weight Retention Outcomes and Trial Retention
Four of the interventions identified in this review were successful in significantly reducing or preventing postpartum weight retention: Fit Moms/Mamás Activas conducted by Phelan et.al. [22]; Healthy4Baby conducted by Herring et. al. [20]; Herring and colleagues’ secondary trial which this review will refer to as “adapted Healthy4Baby” [24] and PAT+Lifestyle conducted by Haire-Joshu et. al., which was conducted as part of the Lifestyle Interventions for Expectant Moms (LIFE-Moms) consortium [18]. Of these, the Fit Moms/Mamás Activas and PAT+Lifestyle trials were appropriately powered to detect significant differences [18, 22]. The Fit Moms/Mamás Activas trial found significant weight loss as compared to the usual care group (2.3 kg weight difference between groups), while the PAT+Lifestyle trial was able to significantly prevent weight gain in their intervention group (3.2 kg weight difference between groups). Both trials achieved this by 12 months postpartum. The Healthy4Baby and adapted Healthy4Baby trials, both pilot RCTs not reported to be powered, also reported significant outcomes. Healthy4Baby found significant weight loss in the intervention group (3.5 kg weight difference), immediately after the 14-week intervention [20]. Adapted Healthy4Baby found a significantly greater percentage of participants in the intervention group that were at or below their early pregnancy weight at six months postpartum (56% vs. 29%) [24].
Behavior Change Strategies Used and Behaviors Targeted
The reporting of behavior change strategies (i.e., the use of theories, models and behavior change techniques) is essential to understand how an intervention works and, thus, can be replicated. All trials reported using a behavioral theory, described either in the trial outcomes paper or formative work. In addition, all used the Social Cognitive Theory, either alone or in combination with other behavioral theories. Across trials, common behavior change techniques [25] included goal setting, self-monitoring, and feedback on both behaviors (e.g., dietary intake) and the outcome of behaviors (i.e., weight). All seven interventions in this review targeted diet and physical activity behaviors. Six trials targeted additional behaviors, including stress (3 trials), sleep (4 trials), and breastfeeding (1 trial).
OPPORTUNITIES AND RECOMMENDATIONS FOR FUTURE WORK
Opportunity #1: Expand and Retain Target Populations
This review found that four studies initiated intervention during the postpartum period [20-23] while three began during pregnancy and extended to the postpartum period [18, 19, 24]. Postpartum weight retention is influenced by gestational weight gain [26] and pre-pregnancy body mass index (BMI), with over 60% of individuals having a pre-pregnancy BMI in the overweight or obese range [27, 28]. For those individuals with pre-existing CVD, obesity increases the risk of maternal cardiovascular complications [29]. Taking this evidence together, behavioral interventions may be more efficacious if initiated prenatally or at the start of pregnancy, rather than postpartum, especially for those with existing CVD [30, 31]. Additionally, pregnant individuals may have increased awareness of healthy behaviors to support the growing fetus, while also having increased access to healthcare providers and health insurance. These factors lend themselves to create an opportune moment to intervene and promote long-lasting lifestyle changes [32].
This review found no interventions conducted in the rural United States (U.S.). Rural areas in the U.S. have limited access to healthcare services during pregnancy and childbirth and have higher rates of maternal morbidity and mortality, putting them under the umbrella of underserved populations [33]. Research comparing postpartum weight retention and its risk factors in rural vs. urban populations is scarce. One study conducted in rural Pennsylvania found that 74% of pregnant individuals gained above recommended amounts [34]. Given that gestational weight gain is a strong predictor of postpartum weight retention [12-14], conducting behavioral interventions in rural areas may prove to be an effective strategy to improve postpartum health, though considerably more research is needed to understand rural postpartum weight retention trends and outcomes.
Trial retention for studies found in this review ranged from 56-100%. Successful retention and engagement of participants is known to be one of the most challenging issues when conducting RCTs [35]. Inadequate participant retention affects the ability to draw conclusive results therefore it is vital to ensure that strategies are implemented early to preemptively maximize retention. From the studies found in this review, the Mothers in Motion trial had the largest sample size (N=569), yet also had the lowest (56.2%) and differential retention (53.2% intervention vs 62.6% control) across trial arms [21]. The authors state that one reason for trial inefficacy may have been related to the mismatch of recruitment messaging and the expectations of the target population. One way to address this in future studies might be the use of the Methods-Motivational Interviewing Approach, which was found to be successful in improving retention rates [36]. In this approach, participants to have the opportunity to understand if the trial is a good fit for them, be fully informed of what they can expect from the trial and know what the trial expects from them. Such strategies may further increase the perceived trustworthiness of researchers who seek to engage members of historically disadvantaged communities. In addition to application of this strategy, more research is needed to empirically test retention strategies in underserved populations.
Opportunity #2: Increase Focus on Psychosocial Factors
Weight-related interventions primarily rely on diet and/or physical activity to create a negative energy balance and ultimately produce weight loss, and these strategies should be part of postpartum weight retention interventions [37]. However, other factors that tend to change during the postpartum period, like stress, sleep, and depression, may also play a role in energy balance and subsequent weight loss.
Particularly important to highlight in this target population is stress. Stress has been hypothesized to be an explanatory factor for disparities in health, related to the varied exposure to acute and chronic stressors experienced by racial/ethnic minorities and low-income individuals [38, 39]. Compared to advantaged populations (i.e., white and higher income individuals), underserved populations may experience higher stress related to discrimination, poverty, poor housing and crime, fostering fewer opportunities to engage in healthy behaviors [40]. Acute and chronic stress have also been linked to increased weight and obesity, potentially mediated by increases in hormonal cortisol levels, and to CVD [41, 42]. While some studies found in this review addressed stress, the focus tended to be on everyday stressors. For example, the Mothers in Motion trial addressed hassles such as “handling large laundry piles” and “not being a perfect mom,” with little attention to significant parenting stress and interpersonal conflict [21]. No studies found in this review addressed chronic and established stressors such as negative thoughts, relationship stress, stress from financial insecurity or food insecurity, or handling discrimination, all of which have implications for health and engaging in healthy lifestyle behaviors. Future intervention work could consider a greater emphasis on targeting these social determinants of health and helping individuals manage chronic stressors.
Opportunity #3: Use Multilevel Approaches to Maximize Public Health Impact
The socioecological model has been successfully applied in obesity prevention efforts [43]. The effectiveness of targeting multiple spheres of influence within this model for maximum public health impact have been documented [44]. Findings from this literature review in the postpartum health field demonstrate that current research focuses primarily on individual behavior change. The majority of these trials aimed to change the diet and physical activity behaviors of pregnant or postpartum individuals through increasing knowledge, self-efficacy, using motivational interviewing, or other individual level behavioral strategies. Some interventions used interpersonal strategies such as providing peer support [21], and one intervention explicitly reported efforts to change the individual’s home environment to be supportive of behavior change [18].
While individual-level interventions are important for facilitating behavior change, there is a clear need for more postpartum weight retention interventions that expand into the larger environmental levels, with a focus on macro-environments (e.g., built environments), policy and systems change [45]. Multilevel approaches—behavioral interventions that target individual factors such as diet and exercise, while also addressing the broader environment—may be essential for sustainable, positive outcomes [45]. However, interventions that act on multiple levels are challenging to develop, implement, and eventually scale-up. One potential solution to this is to engage in participatory based research methods for the co-creation of behavioral interventions for postpartum weight retention. This includes engaging with the organizations (e.g., WIC), policy makers, and individuals the intervention will target, and inviting them to be equal or greater partners in the decision-making processes for intervention development and delivery. For example, the efficacious PAT+Lifestyle trial was developed in partnership with the established organization (PAT), to ensure that the intervention was consistent with the organizational mission, format, practice, and funding requirements [18]. Attention to community resources and strengths and obtaining their buy-in can maximize not only effectiveness but sustainability, particularly in communities that have been marginalized and are socially disadvantaged [46].
Opportunity #4: Leverage Advantages of Technology
Out of the seven trials found for this review, four were technology-based [20, 22-24]. Three of these, the Healthy4Baby trials and Fit Moms/Mamás Activas trials, were efficacious [20, 22, 24]. Using technology in behavioral interventions provides many benefits that should continue to be leveraged in future work.
Unlike in-person interventions that may only make contact with individuals periodically, technology-based intervention content can be delivered with relative ease, and data from behavior change related metrics (including engagement) can be collected in real-time. For example, in the successful Healthy4Baby and Fit Moms/Mamás Activas trials, text messaging was leveraged to track behaviors quickly and provide motivation, support, and feedback. Using text messaging as part of behavioral interventions is a cost-effective and scalable strategy [20, 22]. When working with organizations that serve primarily low-income and/or minority ethnic groups, such as WIC, their existing technology infrastructure could be leveraged to deliver intervention content.
Technology-based interventions may particularly be useful for underserved populations. A PEW report of mobile phone usage has found that minorities and low-income households have a high use of mobile phones and are more likely to rely solely on their phones for internet access [47]. Additionally, for populations with barriers to attending in-person programming (e.g., due to transportation, childcare issues, varied work schedules), technology can be an effective way to meet them where they are.
Technology provides ways to incorporate multiple behavior change techniques, theories, and avenues for content delivery. For example, in the E-Moms trial, the mobile application used to deliver the intervention applied Social Cognitive Theory (e.g., reinforcing behavior change, fostering personal agency, promoting clear outcome expectations, and goal setting) as well as learning theory, Theory of Planned Behavior, and Theory of Reasoned Action (e.g., clearly defining behavioral goals, promoting self-efficacy, and promoting the use of objective data to regulate behavior) [23]. Similarly, the successful Fit Moms/Mamás Activas trial was grounded in Social Cognitive Theory targeting constructs such as self-efficacy, goal-setting and self-regulation [22]. This intervention included a website with weekly lessons, web diary, instructional videos, computerized feedback, text messages, and monthly face to face groups at WIC clinics. Of note, a disadvantage to this complex approach is that it provides little clarity about which specific intervention components may be producing the intended effect and which, if any, are lessening it. Research frameworks like the Multiphase Optimization Strategy (MOST) provide an alternative way of building theory-driven, technology-based interventions: individual intervention components can be tested systematically and once identified as efficacious, can be assembled into future multi-component interventions [48].
While research to improve postpartum weight retention in underserved population is signaling that technology-based interventions can be effectively implemented [20, 22, 24], it is important to note that these advantages exist alongside several population specific, technology-related challenges. Behavioral researchers need to ensure not only that participants have access to the required technology, but that they have reliable and constant access to that device and internet service (e.g., individuals are not canceling services or changing phone numbers). While large strides have been made in closing the “digital divide” (i.e., the division between people who have and do not have access to and use of technology), there remains inequity in underserved populations who do not have access to internet (e.g., rural areas), ownership of technological devices (e.g., smartphones, wearable activity trackers), and skills to interact with technology, also referred to as digital health literacy [49, 50]. Additionally, designing and implementing technology-based interventions may be quite complex. Therefore, communities and researchers would be required to partner with technological companies or already possess the level of expertise needed for technological behavioral interventions [51]. These factors are important considerations for future work.
Opportunity #5: Increase Cultural Relevance
Creating culturally relevant interventions is essential to meet the needs of diverse populations. Reviews of nutrition and exercise-based interventions have found tailoring interventions to be a promising strategy that meet the needs of the target population [52, 53]. Strategies to increase cultural relevance include conducting formative work, co-creation of intervention content with community members, conducting feasibility and usability testing, and providing the intervention in appropriate languages. Most studies included in the present narrative review implemented approaches to increase cultural relevancy. For example, both the efficacious PAT+Lifestyle and Healthy4Baby trials conducted formative work with their target population to understand motivators, facilitators, and barriers to health behaviors that were targeted in the intervention [18, 20]. In addition, the PAT+Lifestyle trial engaged in co-creation of the intervention with community partners. Although not efficacious, the Mothers in Motion trial utilized two advisory groups, a community and a peer advisory group, to help inform, modify, and implement their intervention [21]. While the E-moms trial provided a strong theoretical rationale, strategies or preliminary work to increase cultural relevance to the WIC population were not reported [23].
Conclusions
High-quality trials investigating behavioral interventions to mitigate postpartum weight retention should be a priority for improving cardiometabolic health in underserved populations. Recommendations for future work include expanding target populations to encompass individuals from pregnancy through postpartum and incorporating empirically tested strategies for greater trial retention. Behavioral intervention research is also needed in rural areas. Furthermore, interventions are needed that target psychosocial factors and intervene on multiple levels, including the environment, policies, and systemic change to facilitate health behaviors. Interventions should continue to leverage technology to reach underserved populations with theory-driven content and strive to increase cultural relevancy.
Acknowledgments
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R01HL142996. The authors also acknowledge support from the National Institute of Diabetes and Digestive and Kidney Diseases under Award Numbers R25DK118763 and P30DK092924. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Maryam Yuhas, Department of Nutrition and Food Studies, Syracuse University.
Caroline Fletcher Moore, Department of Nutrition and Food Studies, Syracuse University.
Jessica Garay, Department of Nutrition and Food Studies, Syracuse University.
Susan D. Brown, Department of Internal Medicine, University of California, Davis School of Medicine.
References:
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Ramlakhan KP, Johnson MR, Roos-Hesselink JW. Pregnancy and cardiovascular disease. Nat Rev Cardiol. 2020;17(11):718–31. [DOI] [PubMed] [Google Scholar]
- 2.Olson C, Strawderman M, Hinton P, Pearson T. Gestational weight gain and postpartum behaviors associated with weight change from early pregnancy to 1 y postpartum. Int J Obes. 2003;27(1):117–27. [DOI] [PubMed] [Google Scholar]
- 3.Siega-Riz AM, Herring AH, Carrier K, Evenson KR, Dole N, Deierlein A. Sociodemographic, perinatal, behavioral, and psychosocial predictors of weight retention at 3 and 12 months postpartum. Obesity. 2010;18(10):1996–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Endres LK, Straub H, McKinney C, Plunkett B, Minkovitz CS, Schetter CD, et al. Postpartum weight retention risk factors and relationship to obesity at one year. Obstet Gynecol. 2015;125(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linné Y, Dye L, Barkeling B, Rössner S. Long-term weight development in women: a 15-year follow-up of the effects of pregnancy. Obes Res. 2004;12(7):1166–78. [DOI] [PubMed] [Google Scholar]
- 6.Kirkegaard H, Bliddal M, Støvring H, Rasmussen KM, Gunderson EP, Køber L, Sørensen TI, Nøhr EA. Maternal weight change from prepregnancy to 18 months postpartum and subsequent risk of hypertension and cardiovascular disease in Danish women: A cohort study. PLoS medicine. 2021. Apr 2;18(4):e1003486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger AA, Peragallo-Urrutia R, Nicholson WK. Systematic review of the effect of individual and combined nutrition and exercise interventions on weight, adiposity and metabolic outcomes after delivery: evidence for developing behavioral guidelines for post-partum weight control. BMC Pregnancy Childb. 2014;14(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy SL, Xu J, Kochanek KD, Arias E, Tejada-Vera B. Deaths: Final data for 2018. National Vital Statistics Reports; vol 69 no 13. Hyattsville, MD: National Center for Health Statistics. 2021. [PubMed] [Google Scholar]
- 9.Ostchega Y, Fryar CD, Nwankwo T, Nguyen DT. Hypertension prevalence among adults aged 18 and over: United States, 2017–2018. NCHS data brief, no 3642020. Hyattsville, MD: National Center for Health Statistics. 2020. [PubMed] [Google Scholar]
- 10.Clark AM, DesMeules M, Luo W, Duncan AS, Wielgosz A. Socioeconomic status and cardiovascular disease: risks and implications for care. Nat Rev Cardiol. 2009;6(11):712–22. [DOI] [PubMed] [Google Scholar]
- 11.Conway BN, Han X, Munro HM, Gross AL, Shu X-O, Hargreaves MK, et al. The obesity epidemic and rising diabetes incidence in a low-income racially diverse southern US cohort. PloS one. 2018;13(1):e0190993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deputy NP, Sharma AJ, Kim SY. Gestational weight gain—United States, 2012 and 2013. MMWR Morbidity and mortality weekly report. 2015;64(43):1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haugen M, Brantsæter AL, Winkvist A, Lissner L, Alexander J, Oftedal B, et al. Associations of pre-pregnancy body mass index and gestational weight gain with pregnancy outcome and postpartum weight retention: a prospective observational cohort study. BMC Pregnancy Childb. 2014;14(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nehring I, Schmoll S, Beyerlein A, Hauner H, von Kries R. Gestational weight gain and long-term postpartum weight retention: a meta-analysis. Am J Clin Nutr. 2011;94(5):1225–31. [DOI] [PubMed] [Google Scholar]
- 15.Rothberg BEG, Magriples U, Kershaw TS, Rising SS, Ickovics JR. Gestational weight gain and subsequent postpartum weight loss among young, low-income, ethnic minority women. Am J Obstet Gynecol. 2011;204(1):52. e1–. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.**. Michel S, Raab R, Drabsch T, Guenther J, Stecher L, Hauner H. Do lifestyle interventions during pregnancy have the potential to reduce long-term postpartum weight retention? A systematic review and meta-analysis. Obes Rev. 2019;20(4):527–42. Findings from this systematic review and meta-analysis highlight the current state of behavioral interventions for postpartum weight retention and indicate that underserved groups are not adequately represented in such studies.
- 17.Van der Pligt P, Willcox J, Hesketh K, Ball K, Wilkinson S, Crawford D, et al. Systematic review of lifestyle interventions to limit postpartum weight retention: implications for future opportunities to prevent maternal overweight and obesity following childbirth. Obes Rev. 2013;14(10):792–805. [DOI] [PubMed] [Google Scholar]
- 18.*. Haire-Joshu D, Cahill AG, Stein RI, Cade WT, Woolfolk CL, Moley K, et al. Randomized Controlled Trial of Home-Based Lifestyle Therapy on Postpartum Weight in Underserved Women with Overweight or Obesity. Obesity. 2019;27(4):535–41. This study is an exemplary trial for postpartum weight retention trials for underserved women.
- 19.Palnati M, Marcus BH, Pekow P, Rosal MC, Manson JE, Chasan-Taber L. The impact of a lifestyle intervention on postpartum weight retention among at-risk Hispanic women. Am J Prev Med. 2021;61(1):44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herring SJ, Cruice JF, Bennett GG, Davey A, Foster GD. Using technology to promote postpartum weight loss in urban, low-income mothers: a pilot randomized controlled trial. J Nutr Educ Behav. 2014;46(6):610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang M-W, Brown R, Nitzke S. Results and lessons learned from a prevention of weight gain program for low-income overweight and obese young mothers: mothers in motion. BMC Public Health. 2017;17(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phelan S, Hagobian T, Brannen A, Hatley KE, Schaffner A, Muñoz-Christian K, et al. Effect of an internet-based program on weight loss for low-income postpartum women: a randomized clinical trial. JAMA. 2017;317(23):2381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilmore LA, Klempel MC, Martin CK, Myers CA, Burton JH, Sutton EF, et al. Personalized mobile health intervention for health and weight loss in postpartum women receiving women, infants, and children benefit: a randomized controlled pilot study. J Women Health. 2017;26(7):719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herring SJ, Cruice JF, Bennett GG, Darden N, Wallen JJ, Rose MZ, et al. Intervening during and after pregnancy to prevent weight retention among African American women. Prev Med Rep. 2017;7:119–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46(1):81–95. [DOI] [PubMed] [Google Scholar]
- 26.Hedderson MM, Brown SD, Ehrlich SF, Tsai AL, Zhu Y, Quesenberry CP, et al. A tailored letter based on electronic health record data improves gestational weight gain among women with gestational diabetes: The Gestational Diabetes' Effects on Moms (GEM) cluster-randomized controlled trial. Diab Care. 2018;41(7):1370–7. doi: 10.2337/dc17-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Institute of Medicine (IOM), National Research Council (NRC). Weight Gain During Pregnancy: Reexamining the Guidelines. Washington (DC): National Academies Press (US); 2009. [PubMed] [Google Scholar]
- 28.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. J Amer Med Assoc. 2010;303(3):235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 29.Pfaller B, Siu SC, D'Souza R, Wichert-Schmitt B, Kumar Nair GK, Haberer K, Maxwell C, Silversides CK. Impact of obesity on outcomes of pregnancy in women with heart disease. J Am Coll Cardiol. 2021;77(10):1317–26. [DOI] [PubMed] [Google Scholar]
- 30.Mannan M, Doi SA, Mamun AA. Association between weight gain during pregnancy and postpartum weight retention and obesity: a bias-adjusted meta-analysis. Nutr Rev. 2013;71(6):343–52. doi: 10.1111/nure.12034. [DOI] [PubMed] [Google Scholar]
- 31.Nehring I, Schmoll S, Beyerlein A, Hauner H, von Kries R. Gestational weight gain and long-term postpartum weight retention: a meta-analysis. Am J Clin Nutr. 2011;94(5):1225–31. doi: 10.3945/ajcn.111.015289. [DOI] [PubMed] [Google Scholar]
- 32.Phelan S. Pregnancy: a “teachable moment” for weight control and obesity prevention. Am J Obstet Gynecol. 2010;202(2):135. e1–. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozhimannil KB, Interrante JD, Henning-Smith C, Admon LK. Rural-urban differences in severe maternal morbidity and mortality in the US, 2007–15. Health Affairs. 2019;38(12):2077–85. [DOI] [PubMed] [Google Scholar]
- 34.Power ML, Lott ML, Mackeen AD, DiBari J, Schulkin J. A retrospective study of gestational weight gain in relation to the Institute of Medicine’s recommendations by maternal body mass index in rural Pennsylvania from 2006 to 2015. BMC Pregnancy Childb. 2018;18(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDonald AM, Knight RC, Campbell MK, Entwistle VA, Grant AM, Cook JA, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials. 2006;7(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.*. Jake-Schoffman DE, Brown SD, Baiocchi M, Bibeau JL, Daubenmier J, Ferrara A, et al. Methods-Motivational Interviewing Approach for Enhanced Retention and Attendance. Am J Prev Med. 2021;61(4):606–17. This study is of importance to enhancing postpartum weight retention related behavioral interventions. Findings from this study provide support for a technique to enhance retention and engagement in studies, a key challenge when working with underserved populations.
- 37.Stiegler P, Cunliffe A. The role of diet and exercise for the maintenance of fat-free mass and resting metabolic rate during weight loss. Sports Med. 2006;36(3):239–62. [DOI] [PubMed] [Google Scholar]
- 38.Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96(5):826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schulz A, Israel B, Williams D, Parker E, Becker A, James S. Social inequalities, stressors and self reported health status among African American and white women in the Detroit metropolitan area. Soc Sci Med. 2000;51(11):1639–53. [DOI] [PubMed] [Google Scholar]
- 40.Jackson JS, Knight KM, Rafferty JA. Race and unhealthy behaviors: chronic stress, the HPA axis, and physical and mental health disparities over the life course. Am J Public Health. 2010;100(5):933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van der Valk ES, Savas M, van Rossum EF. Stress and obesity: are there more susceptible individuals? Curr Obes Rep. 2018;7(2):193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merz CNB, Dwyer J, Nordstrom CK, Walton KG, Salerno JW, Schneider RH. Psychosocial stress and cardiovascular disease: pathophysiological links. Behav Med. 2002;27(4):141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bronfenbrenner U. Toward an experimental ecology of human development. Am Psychol. 1977;32(7):513. [Google Scholar]
- 44.Bagnall A-M, Radley D, Jones R, Gately P, Nobles J, Van Dijk M, et al. Whole systems approaches to obesity and other complex public health challenges: a systematic review. BMC Public Health. 2019;19(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.*. Hill B. Expanding our understanding and use of the ecological systems theory model for the prevention of maternal obesity: A new socioecological framework. Obes Rev. 2021;22(3):e13147. This framework is of importance to enhancing postpartum weight retention related behavioral interventions. This framework considers multi-level influences on postpartum weight retention behaviors and provides strategies to improve future work.
- 46.Duran B, Oetzel J, Magarati M, Parker M, Zhou C, Roubideaux Y, et al. Toward health equity: A national study of promising practices in community-based participatory research. Progress in community health partnerships: research, education, and action. Johns Hopkins Univesity. 2019;13(4):337–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mobile Fact Sheet. Pew Research Center, Washington, D.C. https://www.pewresearch.org/internet/fact-sheet/mobile/?menuItem=d40cde3f-c455-4f0e-9be0-0aefcdaeee00 (2021). Accessed February 11th 2022. [Google Scholar]
- 48.Collins LM. Optimization of behavioral, biobehavioral, and biomedical interventions: The multiphase optimization strategy (MOST). Springer; 2018. [Google Scholar]
- 49.Lythreatis S, Singh SK, El-Kassar A-N. The digital divide: A review and future research agenda. Technol Forecast Soc. 2021:121359. [Google Scholar]
- 50.Smith B, Magnani JW. New technologies, new disparities: the intersection of electronic health and digital health literacy. Int J Cardiol. 2019;292:280–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arigo D, Jake-Schoffman DE, Wolin K, Beckjord E, Hekler EB, Pagoto SL. The history and future of digital health in the field of behavioral medicine. J Behav Med. 2019;42(1):67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mier N, Ory MG, Medina AA. Anatomy of culturally sensitive interventions promoting nutrition and exercise in Hispanics: A critical examination of existing literature. Health Promot Pract. 2010;11(4):541–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eyles HC, Mhurchu CN. Does tailoring make a difference? A systematic review of the long-term effectiveness of tailored nutrition education for adults. Nutr Rev. 2009;67(8):464–80. [DOI] [PubMed] [Google Scholar]