Abstract
Objective
To investigate the predictive value of preoperative neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic inflammation response index (SIRI), and systemic immune inflammation index (SII) for early recurrence after liver resection in patients with hepatitis B-related hepatocellular carcinoma.
Methods
A retrospective study was conducted on 162 patients who underwent hepatitis B-related hepatocellular carcinoma (HCC) resection between January 2013 and April 2016. The Youden index was utilized to calculate the optimal cut-off value. The Pearson Chi-square test was applied to analyze the relationship between inflammatory indexes and common clinical and pathological features. The Kaplan-Meier method and Log-Rank test were implemented to compare the recurrence-free survival rate within 2 years of the population. The Cox regression analysis was used to identify the risk factors for early postoperative recurrence.
Results
The best cut-off values of SIRI, PLR, NLR and SII were 0.785, 86.421, 2.231 and 353.64, respectively. Tumor diameter, degree of tumor differentiation, vascular invasion, SIRI>0.785, PLR>86.421, NLR>2.231 and SII>353.64 were risk factors for early recurrence. Combining the above seven risk factors to construct a joint index, the AUC of the joint prediction model was 0.804. The areas under the ROC curves of SIRI, PLR, NLR, and SII were 0.659, 0.725, 0.680, and 0.723, respectively. There was no significant difference in the predictive ability between the single inflammatory index models, but the predictive performance of the joint prediction model was significantly higher than that of the single inflammatory index models. The patients with lower SIRI, PLR, NLR, SII and joint index value had longer recurrence-free survival within 2 years.
Conclusion
The joint index CIP, constructed by combining preoperative SIRI, PLR, NLP and SII with pathological features, can better predict the early recurrence of HBV-related HCC patients after surgery, which is beneficial in identifying high-risk patients and assisting clinicians to make better clinical choices.
Keywords: inflammatory indexes, hepatocellular carcinoma, recurrence, predictive models, hepatitis B virus
1. Introduction
Primary liver cancer is one of the most malignant and influential cancers in the world. Its incidence is increasing year by year, but the treatment methods are extremely limited (1). Hepatocellular carcinoma (HCC) is the most common pathological type of primary liver cancer, accounting for about 75%-90% of all cases. The causes of HCC include hepatitis B virus (HBV) infection, hepatitis C virus infection, aflatoxin infection, alcohol consumption, and non-alcoholic fatty liver disease which has attracted much attention in recent years (2, 3). In China, HBV-related HCC accounts for the largest proportion. Although the infection rate of HBV is decreasing with the application of antiviral drugs, there is still a considerable base of HBV-related HCC in China (4).
The treatment methods for early and middle stage HCC include radiofrequency ablation, liver transplantation, and hepatectomy, among which hepatectomy is the most widely used. Tumor recurrence is a major complication after hepatectomy and a leading cause of cancer-related death. It is usually divided into early recurrence and late recurrence by 2 years (5, 6). Previous studies have shown that the early recurrence rate of HCC is as high as 30-50%, accounting for more than 70% of the total tumor recurrence (6–8). Timely identification of high-risk patients with early recurrence after surgery is very important for prolonging the survival time of patients and improving the quality of life after surgery. At present, there are many predictive models established in different medical centers based on risk factors related to early recurrence (such as male, large Tumor diameter, high serum AFP, vascular invasion, low tumor differentiation, etc.) and imaging features (9, 10). However, there is no consensus on the best tool for risk stratification.
The construction of clinical prediction models based on inflammation-related indicators is a research hotspot in recent years. Since the 20th century, the theory of cancer-related inflammation has been enriched and developed, and the role of inflammation in tumorigenesis, proliferation, invasion, and metastasis has been gradually elucidated (11, 12). Inflammatory indexes constructed based on peripheral blood neutrophil, lymphocyte, monocyte and platelet counts have been developed for cancer research due to their non-invasive, clinically readily available and low-cost nature. In a variety of malignant tumors, these inflammatory indexes have a good effect in predicting prognosis (13–15). In HCC, these inflammatory indicators such as neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic inflammatory response index (SIRI) and systemic immune inflammation index (SII) have also shown high application value in predicting HCC prognosis (16–18). However, previous studies only included 1-2 inflammatory markers. There is still a paucity of literature that combines these 4 important inflammatory markers to study early recurrence of HBV-related HCC after surgery. In this study, we compared the effects of four inflammatory markers models on the early recurrence of HBV-related HCC after surgery in the same population and constructed a common prediction model based on inflammatory markers and pathological characteristics. By comparing the predictive efficacy of each model, we found a more robust and accurate model that could predict the early recurrence of HBV-related HCC after surgery.
2. Materials and methods
2.1. Patient and clinical sample collection
We investigated 162 patients with HBV-related HCC who underwent hepatectomy at Shanxi Provincial Cancer Hospital from January 2013 to April 2016. Clinical and demographic data and detailed treatment information for all enrolled patients were extracted from the electronic medical record. The inclusion criteria were as follows: (1) HCC was diagnosed by postoperative pathology; (2) negative surgical margins; (3) liver reserve function Child-Pugh grade A or B; (4) patients over 18 years old; (5) complete clinical and pathological data were available. Exclusion criteria: (1) acute infection or high fever before surgery; (2) other malignant tumors, immune or hematological diseases; (3) preoperative anti-tumor therapy; (4) loss of critical data. This retrospective chart review study involving human participants complied with institutional and national research Council ethical standards and the 1964 Declaration of Helsinki.
The baseline clinical data of the patients were collected and analyzed, including gender, age, body mass index (BMI), diabetes mellitus, drinking history, family history of cancer, hepatitis B surface antigen (HBsAg), AJCC stage, tumor number, tumor diameter, tumor differentiation, vascular invasion, liver cirrhosis, postoperative treatment (including postoperative ablation or TACE), serum alpha-fetoprotein (AFP) and preoperative blood routine test. Routine blood samples were collected within 7 days before surgery. AJCC staging was performed according to the 8th edition of the classification of the Union for International Cancer Control and the American Joint Committee on Cancer (AJCC) (19).
Peripheral blood inflammation-related indicators refer to relevant indicators that can reflect systemic inflammation based on immune cells of the human circulatory system (13–18). The formula for the calculation of various inflammation-related indicators based on blood routine test was as follows: SIRI = neutrophil×monocyte/lymphocyte (13); PLR = platelet/lymphocyte (18); NLR= neutrophil/lymphocyte (18), SII = platelet ×neutrophil/lymphocyte (17).
2.2. Follow-up
Follow-up began after surgery and was performed every 3 months in the outpatient clinic for the first 2 years until recurrence or loss of follow-up. During follow-up, examinations included physical examination, AFP testing, and abdominal imaging scans. If there was no evidence of recurrence, chest X-ray and abdominal ultrasound were preferred. If abdominal ultrasound suggested recurrence, abdominal enhanced CT scan or magnetic resonance scan should be performed to confirm the diagnosis. In addition, chest CT enhancement should be used to rule out lung metastases, and positron emission tomography should be used to rule out metastases in other sites. Outpatient and inpatient medical record systems were combined with telephone follow-up. Recurrence-free survival (RFS) was defined as the time from the date of surgery to the first recurrence or loss to follow-up.
2.3. Statistical analysis
Taking the recurrence within 2 years as the outcome, ROC analysis was performed on the original values of inflammatory markers as variables, and the best cut-off value was found by the Youden index. The population was divided into high and low groups by cut-off value. The Pearson Chi-square test was used to evaluate the association between SIRI, PLR, NLR, SII and clinical and pathological data. The Kaplan-Meier method and Log-Rank test were used to analyze the differences between groups and recurrence-free survival rate within 2 years. The Cox regression analysis was used to identify the risk factors for early postoperative recurrence. According to the results, the regression coefficients of the included variables were obtained by binary logistic regression equation, and the joint predictors were obtained by the regression coefficients. The SPSS 24.0 and GraphPad Prism 9 were used for statistical analysis and drawing. The MedCalc(20.218) was used to conduct DeLong’s test. P<0.05 considered that the difference was statistically significant.
3. Results
3.1. Baseline data
A total of 162 HCC patients who underwent partial hepatectomy were enrolled. 58 patients had recurrence within 2 years, including 37 cases of intrahepatic recurrence, 7 cases of extrahepatic recurrence and 14 cases of both intrahepatic and extrahepatic recurrence, with a recurrence rate of 35.8%. ROC analysis showed that the optimal cut-off points of SIRI, PLR, NLR and SII were 0.785, 86.421, 2.231 and 353.64, respectively. HCC patients were divided into low SIRI group (≤0.785) and high SIRI group (>0.785), low PLR group (≤86.421), high PLR group (>86.421), low NLR group (≤2.231), high NLR group (>2.231), low SII group (≤353.64) and high SII group (>353.64). Table 1 detailed the association of different groups with the clinical and pathological features. Of the 162 patients, 125 (77.2%) were male and 37 (22.8%) were female. 107 cases (66.0%) were under 60 years, and 55 cases (34.0%) were over 60 years. There were 93 patients (57.4%) in the low SIRI group and 69 patients (42.6%) in the high SIRI group. There were 74 patients (45.7%) in the low PLR group and 88 patients (54.3%) in the high PLR group. There were 98 patients (60.5%) in the low NLR group and 69 patients (39.5%) in the high NLR group. There were 93 patients (57.4%) in the low SII group and 64 patients (42.6%) in the high SII group. The results showed that SIRI, PLR, NLR and SII were significantly correlated with tumor diameter (P< 0.05). PLR was significantly different in age and liver cirrhosis (P<0.05). NLR was associated with the type of tumor differentiation and the use of TACE/ablation after surgery (P< 0.05). SII had a statistically significant difference in HBsAg (+) (P< 0.05).
Table 1.
Baseline Characteristics of 162 Patients with HCC.
| Variables | No.(%) | SIRI | χ² | P value | PLR | χ² | P value | NLR | χ² | P value | SII | χ² | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≼ | > | ≼ | > | ≼2.231 | >2.231 | ≼353.64 | >353.64 | ||||||||||||
| 0.785 | 0.785 | 86.421 | 86.421 | ||||||||||||||||
| All patients | 162(100.0) | 93 | 69 | 74 | 88 | 98 | 64 | 93 | 69 | ||||||||||
| Gender | |||||||||||||||||||
| Male | 125(77.2) | 71 | 54 | 0.083 | 0.774 | 61 | 64 | 2.148 | 0.143 | 78 | 47 | 0.832 | 0.362 | 73 | 52 | 0.221 | 0.639 | ||
| Female | 37(22.8) | 22 | 15 | 13 | 24 | 20 | 17 | 20 | 17 | ||||||||||
| Age | |||||||||||||||||||
| ≤60 | 107(66.0) | 64 | 43 | 0.746 | 0.388 | 55 | 52 | 4.160 | 0.041 | 65 | 42 | 0.008 | 0.927 | 63 | 44 | 0.279 | 0.597 | ||
| >60 | 55(34.0) | 29 | 26 | 19 | 36 | 33 | 22 | 30 | 25 | ||||||||||
| BMI | |||||||||||||||||||
| ≼28.0 | 150(92.5) | 80 | 63 | 1.068 | 0.301 | 67 | 76 | 0.677 | 0.410 | 86 | 57 | 0.064 | 0.800 | 84 | 59 | 0.887 | 0.346 | ||
| >28.0 | 12(7.5) | 13 | 6 | 7 | 12 | 12 | 7 | 9 | 10 | ||||||||||
| Diabetes | |||||||||||||||||||
| No | 151(93.2) | 86 | 65 | 0.187 | 0.665 | 70 | 81 | 0.413 | 0.521 | 91 | 60 | 0.049 | 0.825 | 87 | 64 | 0.040 | 0.842 | ||
| Yes | 11(6.8) | 7 | 4 | 4 | 7 | 7 | 4 | 6 | 5 | ||||||||||
| Alcohol | |||||||||||||||||||
| No | 130(80.2) | 74 | 56 | 0.063 | 0.802 | 60 | 70 | 0.060 | 0.807 | 79 | 51 | 0.021 | 0.885 | 76 | 54 | 0.299 | 0.584 | ||
| Yes | 32(19.8) | 19 | 13 | 14 | 18 | 19 | 13 | 17 | 15 | ||||||||||
| Family cancer history | |||||||||||||||||||
| No | 150(92.5) | 86 | 66 | 0.691 | 0.406 | 59 | 83 | 0.080 | 0.777 | 92 | 60 | 0.001 | 0.974 | 89 | 63 | 1.321 | 0.250 | ||
| Yes | 12(7.5) | 7 | 3 | 5 | 5 | 6 | 4 | 4 | 6 | ||||||||||
| HBsAg (+) | |||||||||||||||||||
| No | 27(16.7) | 15 | 12 | 0.045 | 0.831 | 8 | 19 | 3.363 | 0.067 | 15 | 12 | 0.331 | 0.565 | 9 | 18 | 7.680 | 0.006 | ||
| Yes | 135(83.3) | 78 | 57 | 66 | 69 | 83 | 52 | 83 | 51 | ||||||||||
| Tumor number | |||||||||||||||||||
| Multiple | 24(14.8) | 15 | 9 | 0.299 | 0.585 | 11 | 13 | 0.000 | 0.987 | 16 | 8 | 0.449 | 0.503 | 16 | 8 | 0.988 | 0.320 | ||
| Solitary | 138(85.2) | 78 | 60 | 63 | 75 | 82 | 56 | 77 | 61 | ||||||||||
| Tumor diameter | |||||||||||||||||||
| ≼5 | 101(62.3) | 69 | 32 | 13.056 | 0.000 | 55 | 46 | 8.326 | 0.004 | 69 | 32 | 6.869 | 0.009 | 70 | 31 | 15.533 | 0.000 | ||
| >5 | 61(37.7) | 24 | 37 | 19 | 42 | 29 | 32 | 23 | 38 | ||||||||||
| Differentiation | |||||||||||||||||||
| poor | 60(37.0) | 30 | 30 | 2.138 | 0.144 | 23 | 37 | 2.072 | 0.150 | 27 | 33 | 9.572 | 0.002 | 30 | 30 | 2.138 | 0.144 | ||
| Moderate and well | 102(63.0) | 63 | 39 | 51 | 51 | 71 | 31 | 63 | 39 | ||||||||||
| Vascular invasion | |||||||||||||||||||
| No | 147(90.7) | 87 | 60 | 2.049 | 0.152 | 70 | 77 | 2.408 | 0.121 | 91 | 56 | 1.322 | 0.250 | 86 | 61 | 0.780 | 0.377 | ||
| Yes | 15(9.3) | 6 | 9 | 4 | 11 | 7 | 8 | 7 | 8 | ||||||||||
| Liver cirrhosis | |||||||||||||||||||
| No | 26(16.0) | 14 | 12 | 0.161 | 0.689 | 6 | 20 | 6.376 | 0.012 | 16 | 10 | 0.014 | 0.905 | 13 | 13 | 0.695 | 0.404 | ||
| Yes | 136(84.0) | 79 | 57 | 68 | 68 | 82 | 54 | 80 | 56 | ||||||||||
| N | |||||||||||||||||||
| No | 157(97.0) | 90 | 67 | 0.014 | 0.905 | 73 | 84 | 1.371 | 0.242 | 95 | 62 | 0.001 | 0.982 | 91 | 66 | 0.639 | 0.424 | ||
| Yes | 5(3.0) | 3 | 2 | 1 | 4 | 3 | 2 | 2 | 3 | ||||||||||
| AJCC stage | |||||||||||||||||||
| I-II | 154(95.0) | 88 | 66 | 0.089 | 0.765 | 72 | 82 | 1.450 | 0.228 | 92 | 62 | 0.741 | 0.389 | 88 | 66 | 0.089 | 0.765 | ||
| III-IV | 8(5.0) | 5 | 3 | 2 | 6 | 6 | 2 | 5 | 3 | ||||||||||
| Postoperative treatment (Ablation or TACE) | |||||||||||||||||||
| No | 135(83.3) | 78 | 57 | 0.045 | 0.831 | 64 | 71 | 0.975 | 0.323 | 88 | 47 | 7.459 | 0.006 | 81 | 54 | 2.227 | 0.136 | ||
| Yes | 27(16.7) | 15 | 12 | 10 | 17 | 10 | 17 | 12 | 15 | ||||||||||
| AFP>400 | |||||||||||||||||||
| No | 112(69.1) | 65 | 47 | 0.059 | 0.809 | 70 | 42 | 0.611 | 0.434 | 65 | 47 | 0.059 | 0.809 | 73 | 39 | 0.152 | 0.697 | ||
| Yes | 50(30.9) | 28 | 22 | 28 | 22 | 28 | 22 | 31 | 19 | ||||||||||
| Child-Pugh grading | |||||||||||||||||||
| A | 154(95.0) | 89 | 65 | 0.189 | 0.664 | 72 | 82 | 1.450 | 0.228 | 94 | 60 | 0.388 | 0.533 | 90 | 64 | 1.364 | 0.240 | ||
| B | 8(5.0) | 4 | 4 | 2 | 6 | 4 | 4 | 3 | 5 | ||||||||||
SIRI, Systemic Inflammation Response Index; PLR, Platelet-to-lymphocyte ratio; NLR, Neutrophil-to-Lymphocyte Ratio; SII, Systemic Immune-Inflammation Index; BMI, Body mass index; N, Regional lymph node metastasis; AFP, alpha fetoprotein; TACE, transcatheter arterial chemoembolization.
3.2. Differences in RFS between groups
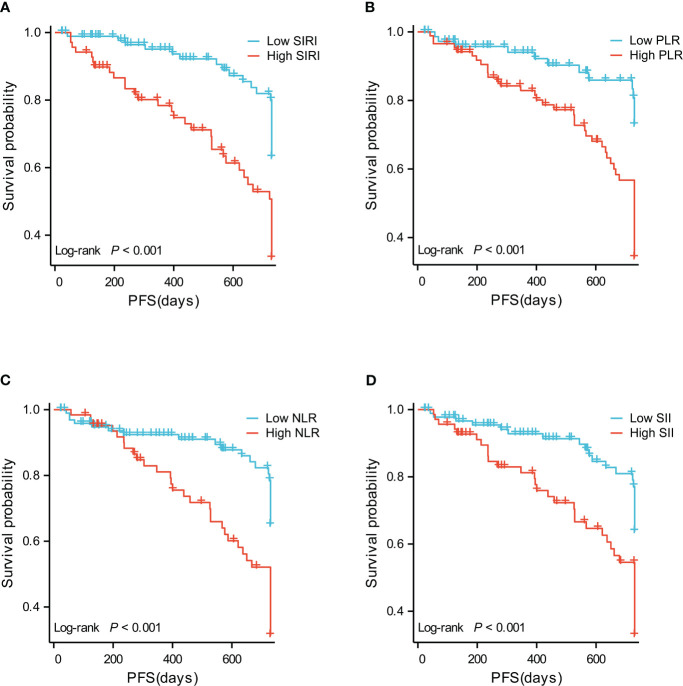
The difference between SIRI ≤ 0.785 group and SIRI>0.785 groups was tested by Log-Rank test, and P<0.001 ( Figure 1A ), indicating that there was a significant difference between groups in terms of recurrence-free survival rate. The 2-year recurrence-free survival rate of the SIRI ≤ 0.785 group was 76.3%, the other was 47.8%.
Figure 1.
(A) Recurrence-free survival curve of patients with high SIRI and low SIRI. (B) Recurrence-free survival curve of patients with high PLR and low PLR. (C) Recurrence-free survival curve of patients with high NLR and low NLR. (D) Recurrence-free survival curves of patients with high SII and low SII.
The difference between PLR ≤ 86.421 group and PLR>86.421 group was tested by Log-Rank test, and P<0.001 ( Figure 1B ), indicating that there was a significant difference between groups in terms of recurrence-free survival rate. The 2-year recurrence-free survival rate of the PLR ≤ 86.421 group was 82.7%, the other was 48.2%.
The difference between NLR ≤ 2.231 group and NLR>2.231 group was tested by Log-Rank test, and P<0.001 ( Figure 1C ), indicating that there was a significant difference between groups in terms of recurrence-free survival rate. The 2-year recurrence-free survival rate of the NLR ≤ 2.231 group was 77.8%, the other was 42.9%.
The difference between SII ≤ 353.64 group and SII>353.64 group was tested by Log-Rank test, and P<0.001 ( Figure 1D ), indicating that there was a significant difference between groups in terms of recurrence-free survival rate. The 2-year recurrence-free survival rate of the SII ≤ 353.64 group was 76.3%, the other was 47.8%.
3.3. Univariate and multivariate COX regression analysis
Cox univariate analysis of tumor diameter (>5cm vs ≤5cm: HR=1.700, 95%CI: 1.015-2.845, P=0.044), degree of differentiation (poorly differentiated vs moderately or well differentiated: HR=2.438, 95%CI: 1.449-4.103, P<0.001), vascular invasion (invasion vs no invasion: HR=3.053, 95%CI: 1.519-6.136, P=0.002), SIRI (>0.785 vs ≤0.785: HR=2.738, 95%CI:1.609-4.660, P<0.001), PLR(>86.421 vs ≤86.421, HR=3.060, 95%CI: 1.649-5.676, P< 0.001), NLR(>2.231 vs ≤2.231, HR=2.613, 95%CI: 1.536-4.443, P< 0.001), SII(>353.64 vs ≤353.64, HR=2.547, 95%CI: 1.497-4.334, P<0.001) ( Table 2 ). Multivariate analysis showed that only tumor differentiation (poor differentiation vs moderate-high differentiation: HR=2.043, 95%CI: 1.152-3.623, P=0.014) was an independent risk factor for early recurrence within 2 years ( Table 2 ).
Table 2.
Univariate and multivariate analysis of RFS within 2 years.
| Characteristics | Total (N) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Gender | 162 | ||||
| Female | 37 | Reference | |||
| Male | 125 | 1.154 (0.611-2.179) | 0.658 | ||
| Age | 162 | ||||
| ≤60 | 107 | Reference | |||
| >60 | 55 | 1.104 (0.642-1.898) | 0.720 | ||
| BMI>28 | 162 | ||||
| ≤28 | 143 | Reference | |||
| >28 | 19 | 1.848 (0.873-3.916) | 0.109 | ||
| Diabetes | 162 | ||||
| No | 151 | Reference | |||
| Yes | 11 | 0.485 (0.151-1.552) | 0.222 | ||
| Drinking history | 162 | ||||
| No | 130 | Reference | |||
| Yes | 32 | 1.432 (0.795-2.578) | 0.232 | ||
| HBSAg(+) | 162 | ||||
| No | 27 | Reference | |||
| Yes | 135 | 0.748 (0.387-1.443) | 0.386 | ||
| Tumor number | 162 | ||||
| Solitary | 138 | Reference | |||
| Multiple | 24 | 0.887 (0.420-1.872) | 0.754 | ||
| Tumor diameter (5cm) | 162 | ||||
| ≤5 | 101 | Reference | |||
| >5 | 61 | 1.700 (1.015-2.845) | 0.044 | 1.338 (0.763-2.343) | 0.309 |
| Differentiation | 162 | ||||
| Moderate and well | 102 | Reference | |||
| poor | 60 | 2.438 (1.449-4.103) | <0.001 | 2.043 (1.152-3.623) | 0.014 |
| Vascular invasion | 162 | ||||
| No | 147 | Reference | |||
| Yes | 15 | 3.053 (1.519-6.136) | 0.002 | 1.959 (0.936-4.097) | 0.074 |
| Cirrhosis | 162 | ||||
| No | 26 | Reference | |||
| Yes | 136 | 0.574 (0.304-1.085) | 0.087 | ||
| AJCC Staging | 162 | ||||
| I-II | 154 | Reference | |||
| III-IV | 8 | 1.609 (0.581-4.456) | 0.360 | ||
| Postoperative treatment (Ablation or TACE) | 162 | ||||
| No | 135 | Reference | |||
| Yes | 27 | 1.274 (0.687-2.364) | 0.442 | ||
| SIRI | |||||
| ≤0.785 | 93 | Reference | |||
| >0.785 | 69 | 2.738 (1.609-4.660) | <0.001 | 1.690 (0.848-3.368) | 0.136 |
| PLR | 162 | ||||
| ≤86.421 | 74 | Reference | |||
| >86.421 | 88 | 3.060 (1.649-5.676) | <0.001 | 1.758 (0.859-3.600) | 0.123 |
| NLR | 162 | ||||
| ≤2.231 | 98 | Reference | |||
| >2.231 | 64 | 2.613 (1.536-4.443) | <0.001 | 1.113 (0.554-2.238) | 0.763 |
| SII | 162 | ||||
| ≤353.64 | 93 | Reference | |||
| >353.64 | 69 | 2.547 (1.497-4.334) | <0.001 | 1.319 (0.674-2.581) | 0.419 |
| AFP (ng/ml) | 162 | ||||
| ≤400 | 112 | Reference | |||
| >400 | 50 | 1.124 (0.649-1.946) | 0.676 | ||
SIRI, Systemic Inflammation Response Index; PLR, Platelet-to-Lymphocyte Ratio; NLR, Neutrophil-to-Lymphocyte Ratio; SII, Systemic Immune-Inflammation Index; BMI, Body mass index; N, Regional lymph node metastasis; AFP, alpha fetoprotein.
3.4. Joint index construction
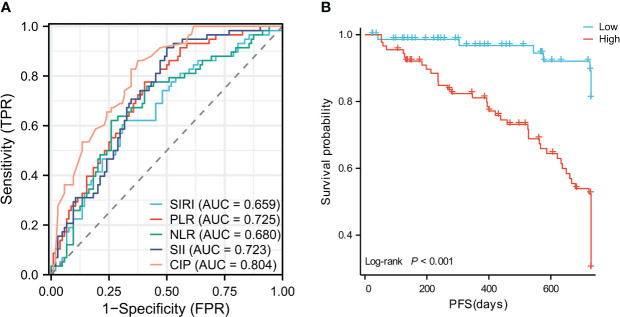
According to the results of univariate and multivariate analysis, based on the available clinical and pathological parameters, the 4 inflammatory indicators combined with tumor diameter, degree of differentiation and vascular invasion were used to construct a combined inflammation and pathology model, referred to as CIP. Scoring criteria: tumor diameter (>5cm=1,≤5cm=0), degree of differentiation (undifferentiated and poorly differentiated=1, moderately and well differentiated=0), vascular invasion (with invasion=1, no invasion=0), SIRI (>0.785 = 1,≤0.785 = 0), PLR (>86.421 = 1,≤86.421 = 0), NLR(>2.231 = 1,≤2.231 = 0), SII(>353.64 = 1,≤353.64 = 0). According to the regression coefficient of binary logistic equation, the joint index calculation formula was as follows: CIP=0.331×Tumor diameter +1.141×degree of differentiation +0.970×vascular invasion+0.469×SIRI+1.152×PLR+0.630×NLR+0.031×SII. The area under the ROC curve of joint index CIP was 0.804 (the best cut-off value was 1.48), which was higher than that of other single indexes (SIRI=0.659, PLR=0.725, NLR=0.680, SII=0.723) ( Figure 2A ). DeLong’s test was performed using MedCalc (20.218), and it was found that there was no significant difference between the single inflammation indexes, and the joint index CIP was significantly different from the single inflammation models (P<0.05) ( Table 3 ). The KM curve showed that the group with a lower joint index had a longer RFS (P<0.001) ( Figure 2B ). To avoid the interaction between variables, univariate and multivariate cox analysis were performed again after removing the factors included in the combined index. The joint index was an independent risk factor for early recurrence and had good predictive value ( Table 4 ).
Figure 2.
(A) Receiver operating characteristic analysis of SIRI, PLR, NLR, SII,CIP. (B) Recurrence-free survival curves of patients with high CIP and low CIP.
Table 3.
DeLong’s test between models.
| Model 1 | Model 2 | P value |
|---|---|---|
| SIRI | PLR | 0.191 |
| SIRI | NLR | 0.5528 |
| SIRI | SII | 0.062 |
| SIRI | CIP | 0.0006 |
| PLR | NLR | 0.3621 |
| PLR | SII | 0.9536 |
| PLR | CIP | 0.0115 |
| NLR | SII | 0.2426 |
| NLR | CIP | 0.0012 |
| SII | CIP | 0.0091 |
SIRI, Systemic Inflammation Response Index; PLR, Platelet-to-lymphocyte ratio; NLR, Neutrophil-to-Lymphocyte Ratio; SII, Systemic Immune-Inflammation Index; CIP, combined inflammation and pathology index.
Table 4.
Univariate and multivariate analysis of RFS within 2 years.
| Characteristics | Total (N) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Gender | 162 | ||||
| Female | 37 | Reference | |||
| Male | 125 | 1.154 (0.611-2.179) | 0.658 | ||
| Age | 162 | ||||
| ≤60 | 107 | Reference | |||
| >60 | 55 | 1.104 (0.642-1.898) | 0.720 | ||
| BMI>28 | 162 | ||||
| ≤28 | 143 | Reference | |||
| >28 | 19 | 1.848 (0.873-3.916) | 0.109 | ||
| Diabetes | 162 | ||||
| No | 151 | Reference | |||
| Yes | 11 | 0.485 (0.151-1.552) | 0.222 | ||
| Drinking history | 162 | ||||
| No | 130 | Reference | |||
| Yes | 32 | 1.432 (0.795-2.578) | 0.232 | ||
| HBSAg (+) | 162 | ||||
| No | 27 | Reference | |||
| Yes | 135 | 0.748 (0.387-1.443) | 0.386 | ||
| Tumor number | 162 | ||||
| Solitary | 138 | Reference | |||
| Multiple | 24 | 0.887 (0.420-1.872) | 0.754 | ||
| Cirrhosis | 162 | ||||
| No | 26 | Reference | |||
| Yes | 136 | 0.574 (0.304-1.085) | 0.087 | ||
| AJCC Staging | 162 | ||||
| I-II | 154 | Reference | |||
| III-IV | 8 | 1.609 (0.581-4.456) | 0.360 | ||
| Postoperative treatment (Ablation or TACE) | 162 | ||||
| No | 135 | Reference | |||
| Yes | 27 | 1.274 (0.687-2.364) | 0.442 | ||
| AFP (ng/ml) | 162 | ||||
| ≤400 | 112 | Reference | |||
| >400 | 50 | 1.124 (0.649-1.946) | 0.676 | ||
| CIP | |||||
| ≤1.48 | 73 | Reference | |||
| >1.48 | 89 | 5.851 (2.770 - 12.360) | < 0.001 | 5.851 (2.770 - 12.360) | < 0.001 |
SIRI, Systemic Inflammation Response Index; PLR, Platelet-to-Lymphocyte Ratio; NLR, Neutrophil-to-Lymphocyte Ratio; SII, Systemic Immune-Inflammation Index; BMI, Body mass index; N, Regional lymph node metastasis; AFP, alpha fetoprotein.
4. Discussion
Cancer-associated inflammation can be divided into two categories: local inflammation and systemic inflammation. Local inflammation is mainly related to the immune response in the tumor microenvironment, which usually occurs before the appearance of tumors. Systemic inflammation is a continuous response to malignant tumors mediated by cytokines, inflammatory proteins, and immune cells (20). HBV-related HCC is an inflammation-driven tumor, which mostly occurs based on chronic hepatitis, and its development, proliferation and metastasis are seriously affected by the inflammatory environment (21). Currently, there is no consensus on the time point of early recurrence, which ranges from 6 months to 2 years in most studies (22–24). It is generally accepted that recurrences within 2 years represent “true recurrences,” whereas after this period, “recurrences” are thought to be largely caused by “de novo” tumors (6). Our study used 2 years as the cutoff point. Whether 2 years is the best cut-off point to determine early recurrence needs further study.
There have been many studies on the effects of inflammatory indexes on RFS and overall survival of HCC after surgery. However, there are few studies on the effect of inflammatory indexes on early recurrence (within 2 years) of liver cancer after surgery. In 2021, Wu et al. proposed that inflammatory indexes could be combined with clinical risk factors to construct a more effective prediction model for early recurrence (25). Our study focused on patients with HBV-related HCC, explored the predictive value of SIRI, PLR, NLR, and SII for early recurrence, and constructed a joint index CIP combining inflammatory indicators and pathological features. Compared with the model constructed by single inflammatory indexes, the CIP model had better predictive ability (AUC=0.804). It is worthy of further research and promotion.
Studies showed that all 4 inflammatory indexes were associated with tumor diameter and high SIRI, PLR, NLR and SII were associated with shorter RFS within 2 years. This may be due to the role of immune cells that constitute the inflammatory markers. Elevated levels of circulating neutrophils have been linked to the stimulation of tumor-derived cytokines, such as granulocyte colony-stimulating factor, platelet-derived growth factor, and Interleukin-8, which mobilize bone marrow-derived cells and splenocytes and lead to their circulation and migration to organs (26). Neutrophils are thought to drive tumor progression through immunosuppression, direct enhancement of tumor cell survival, invasiveness, and metastatic ability, extracellular matrix remodeling, and angiogenesis (27). Lymphocytes control tumor growth by inducing cytotoxic cell death and secreting cytokines, and decreased levels of lymphocytes can impair host immune function and accelerate tumor progression (28). Monocytes can infiltrate tumors and further differentiate into Tumor-associated macrophages, which can induce apoptosis of CD8+ T cells with anticancer activity and promote tumor growth, invasion, and migration (29). It has been suggested that macrophage populations, as indicated by peripheral blood mononuclear cell counts, are correlated with tumor burden. In this study, it was found that platelet-related parameters such as PLR and SII had better predictive performance. It has been proposed that platelets can promote tumor growth and metastasis through the release of mediators such as vascular endothelial growth factor and platelet-derived growth factor. Additionally, platelets may also protect tumor cells from natural killer cells and promote epithelial-mesenchymal transition. It is suggested that high platelet counts may be associated with a poor prognosis in HCC.
Tumor markers such as alpha-fetoprotein and abnormal prothrombin are effective indicators to judge the prognosis of HCC, but many patients have normal tumor markers when they are diagnosed with HCC. Therefore, it is essential to find more prognostic biomarkers for clinical decision-making. Peripheral blood inflammatory markers are highly generalizable due to their non-invasive and easy availability. Future prospective multicenter studies with larger sample sizes and studies targeting other HCC etiologies are necessary to verify the results of this study.
5. Conclusion
The joint index CIP constructed by combining preoperative SIRI, PLR, NLP and SII with pathological features, can better predict the early recurrence of HBV-related HCC patients after surgery, which is helpful to identify high-risk patients and assist clinicians to make better clinical decisions.
Data availability statement
The datasets presented in this article are not readily available because hepatocellular carcinoma. Requests to access the datasets should be directed to 953168503@qq.com
Ethics statement
The studies involving human participants were reviewed and approved by Shanxi Provincial Cancer Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
GW is the main writer. LY, MJ and WB collected the data. LY also organized the data and participated in writing the paper. LLB analyzed the data. NZ and NY reviewed and revised the manuscript. LLX designed the overall topic and guiding the paper. All the authors contributed to the original draft preparation and reviewing and editing of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet (2022) 400(10360):1345–62. doi: 10.1016/S0140-6736(22)01200-4 [DOI] [PubMed] [Google Scholar]
- 3. Toh MR, Wong EYT, Wong SH, Ng AWT, Loo LH, Chow PK, et al. Global epidemiology and genetics of hepatocellular carcinoma. Gastroenterology (2023) 164(5):766–82. doi: 10.1053/j.gastro.2023.01.033 [DOI] [PubMed] [Google Scholar]
- 4. Liu Z, Mao X, Jiang Y, Cai N, Jin L, Zhang T, et al. Changing trends in the disease burden of primary liver cancer caused by specific etiologies in China. Cancer Med (2019) 8(12):5787–99. doi: 10.1002/cam4.2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. European Association for Study of Liver. European Organisation for Research and Treatment of Cancer . EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur J Cancer (2012) 48(5):599–641. doi: 10.1016/j.jhep.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 6. Chan AWH, Zhong J, Berhane S, Toyoda H, Cucchetti A, Shi K, et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol (2018) 69(6):1284–93. doi: 10.1016/j.jhep.2018.08.027 [DOI] [PubMed] [Google Scholar]
- 7. Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg (2015) 261(5):947–55. doi: 10.1097/SLA.0000000000000710 [DOI] [PubMed] [Google Scholar]
- 8. Marasco G, Colecchia A, Colli A, Ravaioli F, Casazza G, Bacchi Reggiani ML, et al. Role of liver and spleen stiffness in predicting the recurrence of hepatocellular carcinoma after resection. J Hepatol (2019) 70(3):440–8. doi: 10.1016/j.jhep.2018.10.022 [DOI] [PubMed] [Google Scholar]
- 9. Li WF, Yen YH, Liu YW, Wang CC, Yong CC, Lin CC, et al. Preoperative predictors of early recurrence after resection for hepatocellular carcinoma. Am J Surg (2022) 223(5):945–50. doi: 10.1016/j.amjsurg.2021.08.012 [DOI] [PubMed] [Google Scholar]
- 10. Zeng J, Zeng J, Lin K, Lin H, Wu Q, Guo P, et al. Development of a machine learning model to predict early recurrence for hepatocellular carcinoma after curative resection. Hepatobiliary Surg Nutr (2022) 11(2):176–87. doi: 10.21037/hbsn-20-466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Balkwill F, Mantovani A. Inflammation and cancer: back to virchow? Lancet (2001) 357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0 [DOI] [PubMed] [Google Scholar]
- 12. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature (2008) 454(7203):436–44. doi: 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 13. Zhou Q, Su S, You W, Wang T, Ren T, Zhu L. Systemic inflammation response index as a prognostic marker in cancer patients: a systematic review and meta-analysis of 38 cohorts. Dose Response (2021) 19(4):15593258211064744. doi: 10.1177/15593258211064744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cao W, Yu H, Zhu S, Lei X, Li T, Ren F, et al. Clinical significance of preoperative neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in the prognosis of resected early-stage patients with non-small cell lung cancer: a meta-analysis. Cancer Med (2023) 12(6):7065–7076. doi: 10.1002/cam4.5505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Savioli F, Morrow ES, Dolan RD, Romics L, Lannigan A, Edwards J, et al. Prognostic role of preoperative circulating systemic inflammatory response markers in primary breast cancer: meta-analysis. Br J Surg (2022) 109(12):1206–15. doi: 10.1093/bjs/znac319 [DOI] [PubMed] [Google Scholar]
- 16. Mao S, Yu X, Sun J, Yang Y, Shan Y, Sun J, et al. Development of nomogram models of inflammatory markers based on clinical database to predict prognosis for hepatocellular carcinoma after surgical resection. BMC Cancer (2022) 22(1):249. doi: 10.1186/s12885-022-09345-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang J, Bao Y, Chen W, Duan Y, Sun D. Nomogram based on systemic immune inflammation index and prognostic nutrition index predicts recurrence of hepatocellular carcinoma after surgery. Front Oncol (2020) 10:551668. doi: 10.3389/fonc.2020.551668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zheng Z, Guan R, Zou Y, Jian Z, Lin Y, Guo R, et al. Nomogram based on inflammatory biomarkers to predict the recurrence of hepatocellular carcinoma-a multicentre experience. J Inflammation Res (2022) 15:5089–102. doi: 10.2147/JIR.S378099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Amin MB, Edge SB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin (2017) 67(2):93–99. doi: 10.3322/caac.21388 [DOI] [PubMed] [Google Scholar]
- 20. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol (2014) 15(11):e493–503. doi: 10.1016/S1470-2045(14)70263-3 [DOI] [PubMed] [Google Scholar]
- 21. Qu LS, Zhou GX. Significance of viral status on occurrence of hepatitis b-related hepatocellular carcinoma. World J Gastroenterol (2014) 20(20):5999–6005. doi: 10.3748/wjg.v20.i20.5999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li Z, Lei Z, Xia Y, Li J, Wang K, Zhang H, et al. Association of preoperative antiviral treatment with incidences of microvascular invasion and early tumor recurrence in hepatitis b virus-related hepatocellular carcinoma. JAMA Surg (2018) 153(10):e182721. doi: 10.1001/jamasurg.2018.2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xing H, Sun LY, Yan WT, Quan B, Liang L, Li C, et al. Repeat hepatectomy for patients with early and late recurrence of hepatocellular carcinoma: a multicenter propensity score matching analysis. Surgery (2021) 169(4):911–20. doi: 10.1016/j.surg.2019.11.005 [DOI] [PubMed] [Google Scholar]
- 24. Wei T, Zhang XF, Bagante F, Ratti F, Marques HP, Silva S, et al. Early versus late recurrence of hepatocellular carcinoma after surgical resection based on post-recurrence survival: an international multi-institutional analysis. J Gastrointest Surg (2021) 25(1):125–33. doi: 10.1007/s11605-020-04553-2 [DOI] [PubMed] [Google Scholar]
- 25. Wu Y, Tu C, Shao C. Inflammatory indexes in preoperative blood routine to predict early recurrence of hepatocellular carcinoma after curative hepatectomy. BMC Surg (2021) 21(1):178. doi: 10.1186/s12893-021-01180-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol (2012) 56(3):704–13. doi: 10.1016/j.jhep.2011.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chan SL, Chan AW, Chan AK, Jian P, Mo F, Chan CM, et al. Systematic evaluation of circulating inflammatory markers for hepatocellular carcinoma. Liver Int (2017) 37:280–9. doi: 10.1111/liv.13218 [DOI] [PubMed] [Google Scholar]
- 28. Geh D, Leslie J, Rumney R, Reeves HL, Bird TG, Mann DA. Neutrophils as potential therapeutic targets in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol (2022) 19(4):257–73. doi: 10.1038/s41575-021-00568-5 [DOI] [PubMed] [Google Scholar]
- 29. Chew V, Chen J, Lee D, Loh E, Lee J, Lim KH, et al. Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut (2012) 61(3):427–38. doi: 10.1136/gutjnl-2011-300509 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this article are not readily available because hepatocellular carcinoma. Requests to access the datasets should be directed to 953168503@qq.com