Abstract
Diseases associated with nicotine dependence in the form of habitual tobacco use are a major cause of premature death in the United States. The majority of tobacco smokers will relapse within the first month of attempted abstinence. Smoking cessation agents increase the likelihood that smokers can achieve long-term abstinence. Nevertheless, currently available smoking cessation agents have limited utility and fail to prevent relapse in the majority of smokers. Pharmacotherapy is therefore an effective strategy to aid smoking cessation efforts but considerable risk of relapse persists even when the most efficacious medications currently available are used. The past decade has seen major breakthroughs in our understanding of the molecular, cellular, and systems-level actions of nicotine in the brain that contribute to the development and maintenance of habitual tobacco use. In parallel, large-scale human genetics studies have revealed allelic variants that influence vulnerability to tobacco use disorder. These advances have revealed targets for the development of novel smoking cessation agents. Here, we summarize current efforts to develop smoking cessation therapeutics and highlight opportunities for future efforts.
1. Introduction
Tobacco smoking is a leading cause of preventable disease and premature death globally [1]. In the United States, over 480,000 deaths each year are attributable to smoking-related illnesses, such as chronic obstructive pulmonary disorder (COPD), lung cancer, cardiovascular disease, and diabetes [2]. In addition to the devastating health consequences, tobacco use disorder also has a staggering economic impact. More than $300 billion is spent in the United States each year on the treatment of smoking-related illnesses, accounting for approximately 12% of annual healthcare expenditures [3]. Evidence suggests that those who achieve long-term abstinence from tobacco use have a greatly reduced risk of developing lung cancer and other smoking-related illnesses than those who persist in their use tobacco products [4,5]. Despite the well-known negative health impact of tobacco use, and the clear benefits of quitting, the majority of smokers are often unsuccessful in their attempts to achieve long-term abstinence. Indeed, approximately 80% of smokers will relapse within the first month of a quit attempt [6]. Medications approved by the US Food and Drug Administration (FDA) as aids to smoking cessation include various forms of nicotine replacement therapy (NRT), such as gum, patch, spray, and lozenges, as well as Zyban (bupropion) and Chantix (varenicline). These medications demonstrate limited efficacy in the majority of smokers attempting to quit [6], and there remains a high residual risk of relapse despite the use of these medications. Use of bupropion and varenicline was thought to be associated with risk of serious mental health side effects in smokers [7], resulting in the inclusion of boxed warnings on their labeling. However, findings from a large-scale clinical trial indicated that these risks may in fact be lower than previously thought [8]. This prompted the FDA to remove boxed warnings from Chantix and Zyban drug labels. Nevertheless, negative perceptions about the safety and efficacy of these medications have limited their use [7,9]. Most recently, safety concerns related to high levels of an N-nitroso-varenicline impurity triggered a nationwide recall of Chantix and the approval of genetic formulations of varenicline. Collectively, this underscores the critical need to develop new safe and effective smoking cessation medications to mitigate the deleterious health consequences of tobacco dependence. Such medications are likely to derive from advances in our understanding of the neural mechanisms of tobacco dependence. Here, we briefly summarize the neurobiological mechanisms of tobacco use disorder, highlight ongoing efforts to develop new smoking cessation agents, and identify opportunities to leverage advances in our understanding of tobacco dependence from preclinical studies to establish new medications development programs for smoking cessation. While the focus of the article is on the mechanisms of tobacco use disorder within the context of pharmacological medications development for smoking cessation, ongoing work on other innovative approaches to treat tobacco dependence, including nicotine-based vaccines [10,11], are also briefly considered.
2. Clinical features of tobacco use disorder
Nicotine is considered the major psychoactive component of tobacco smoke that promotes the development and maintenance of tobacco use in those who suffer from tobacco use disorder [12]. According to the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), tobacco use disorder is characterized by three primary features: (1) a gradual increase in the amount of tobacco consumed over time; (2) the development of tolerance to the pharmacological actions of nicotine; and (3) the onset of withdrawal symptoms upon cessation of the use of nicotine-containing tobacco products. Thus, tobacco use disorder is also characterized by the consumption of progressively greater doses of nicotine in order to obtain the desired pharmacokinetic response; continued use despite adverse social, economic, or interpersonal consequences; and unsuccessful efforts to quit. The abrupt cessation or reduction in the amount of tobacco intake can precipitate an aversive nicotine withdrawal syndrome, which is characterized by physical, cognitive, and mood disturbances including difficulty concentrating, increased anxiety, and depression-like negative mood states. Avoidance and alleviation of the nicotine withdrawal syndrome is thought to contribute to the persistence of the tobacco habit and the high risk of relapse during periods of attempted abstinence [13]. Craving during abstinence has been included as a diagnostic criterion of tobacco use disorder in DSM-5. Craving in abstinent smokers reflects “a strong desire or urge to use tobacco” and is thought to be an important component of the withdrawal syndrome that contributes to the resumption of tobacco use. Indeed, the extent of craving during the early stages of abstinence is closely linked with the likelihood of relapsing to tobacco use [14–17]. This suggests that interventions that can attenuate craving and other components of the nicotine withdrawal syndrome in smokers during the early stages of abstinence may facilitate long-term abstinence.
Until recently, most efforts to develop smoking cessation medications were focused on pharmacological modulation of nicotinic acetylcholine receptors (nAChRs), which are the primary substrate for the actions of nicotine in the brains of smokers. Indeed, the efficacy of most of the currently available smoking cessation agents is thought to reflect their pharmacological actions at nAChRs in the brain [18,19]. Evidence has accumulated over recent years that signaling events beyond nAChR-mediated cascades contribute to the reinforcing properties of nicotine. For example, agents that modify the actions of endogenous opioids, hypocretin/orexin, and other neuropeptide systems, glutamatergic receptors, and γ-aminobutyric acid (GABA) receptors, have shown promise as smoking cessation pharmacotherapies (Table 1). Preclinical and clinical studies have been designed to test the effectiveness of novel compounds targeting these systems in regulating various behaviors thought to be important in maintaining tobacco use disorder, including the positive reinforcing actions of nicotine, craving-related behaviors, and aspects of the nicotine withdrawal syndrome that may contribute to negative reinforcing actions of nicotine.
Table 1.
Compounds that have been shown to alter behaviors relevant to nicotine seeking in laboratory animals.
| Compound | Mechanism of Action | Behavioral findings in animal models | Citation |
|---|---|---|---|
| Naltrexone/naloxone | MOR antagonist | Naloxone produced a conditioned place aversion in rats chronically treated with nicotine. | [20] |
| SB-334,867 | Hypocretin receptor 1 antagonist | SB-334,867 decreased nicotine self-administration in rats; pre-treatment with SB-334,867 blocked hypocretin-induced and cue-induced reinstatement of nicotine seeking behavior. | [21–23] |
| LY314582 | mGluR2 agonist | Infusion into the VTA of nicotine-dependent rats precipitated withdrawal-like increases in ICSS reward threshold. | [24–26] |
| AZD8418, AZD8529 | mGluR2 PAMs | Decreased nicotine self-administration and cue-induced reinstatement of nicotine-seeking behavior in rats. | [27,28] |
| MPEP | mGluR5 NAM | Decreased nicotine self-administration and reinstatement of nicotine-seeking behavior. | [29–31] |
| CGP44532 | GABAB receptor agonist | Decreased nicotine self-administration in rats. | [32,33] |
| CGP7930, BHF177, GS39783, KK-92A | GABAB receptor PAMs | Attenuated nicotine-self administration and cue-induced nicotine-seeking behavior. | [32,34,35] |
| Clofibrate | PPARα agonist | Clofibrate abolished nicotine self-administration in both naïve rats and those with a history of self-administering nicotine. | [36] |
| Pioglitazone | PPARγ agonist | Pioglitazone attenuated somatic and affective symptoms of nicotine withdrawal in rats and mice. | [37] |
3. Human genetics-based discovery of novel targets for smoking cessation
There is now considerable preclinical, clinical, and human genetics evidence implicating nAChRs located in the central nervous system (CNS) in regulating the abuse liability of nicotine-containing tobacco products [12,38,39] (Fig. 1). nAChRs are ligand-gated cationic channels that are widely distributed throughout the CNS [40]. nAChRs contain five membrane-spanning subunits (α and β subunits) [41,42]. Eight isoforms of the neuronal α subunit (α2–α7, α9, and α10) and three isoforms of the β subunit (β2–β4) have been identified in mammalian brain [43]. Within the CNS, combinations of α2–α6 and β2–β4 subunits can form functionally distinct subtypes of hetero-oligomeric nAChRs that display distinct pharmacological, kinetic, and other functional properties, whereas α7 subunits form homo-oligomeric receptor subtypes [44]. nAChRs containing the α4 and β2 subunits (denoted α4β2∗ nAChRs) are the most abundantly expressed subtype in the mammalian CNS [45]. and are thought to account for the majority of the high-affinity nicotine binding sites in the mammalian brain [46–49]. α4β2∗ nAChRs usually combine in a 2:3 stoichiometry, but can also combine in other stoichiometries (for example 3:2), and sometimes incorporate accessory subunits such the α5 nAChR subunit that influence the properties of the mature receptor complex [45]. β2∗ nAChRs are necessary for the positive reinforcing properties of nicotine in rodents, reflected by the fact that genetic deletion of the β2 subunit eliminates high-affinity nicotine binding sites in the brain and abolishes reward-related behavioral responses to nicotine in mice [46,50]. Furthermore, genetically modified mice that express a mutant version of the α4 subunit that renders α4∗ nAChRs hyper-responsive to nicotine demonstrate increased sensitivity to nicotine reward, even at “subthreshold” doses of nicotine that are too low to elicit reward-related behavioral responses in wild-type mice [51]. α4β2∗ nAChRs are thought to play an important role in the efficacy of currently available smoking cessation agents. Indeed, varenicline (Chantix) is a partial agonist at α4β2∗ nAChRs [52]. Varenicline is thought to compete with nicotine to bind to α4β2∗ nAChRs expressed by midbrain dopamine neurons, thereby attenuating the stimulatory effects of nicotine on mesoaccumbens dopamine transmission [53,54]. In this manner, varenicline is thought to attenuate the stimulatory actions of nicotine on dopamine transmission in brain reinforcement circuits that is considered critical for the establishment and maintenance of tobacco smoking behavior. Varenicline is a derivative of the nAChR partial agonist (−)-cytisine, a natural product derived from Cytisus laburnum and other plant species [53]. Cytisine has shown mixed results in human clinical trials assessing its efficacy as a smoking cessation agent [55–57]. This likely reflects the fact that cytisine has poorer absorption and brain penetration properties than varenicline [58,59]. Varenicline and related partial agonists exert low levels of α4β2∗ nAChR-mediated signaling in the brain during periods of nicotine abstinence and thereby at least partially substitute for the pharmacological actions of nicotine in smokers attempting to quit [53]. This action is thought to counteract the decrease in dopamine transmission and other reward-relevant signaling events in the brains of smokers during abstinence, the avoidance and alleviation of which contributes to relapse to tobacco use [53,60]. Thus, the clinical efficacy of NRT and nAChR partial agonists such as varenicline as smoking cessation agents is likely to reflect their ability to substitute for and/or block the actions of nicotine at α4β2∗ nAChRs [60,61]. Thus, next generation smoking cessation agents could be developed that more efficiently target the specific populations of α4β2∗ nAChRs involved in nicotine reinforcement and/or modify the activity patterns of these receptors in a manner that results in greater clinical efficacy. Bupropion is an atypical antidepressant that is clinically efficacious as a smoking cessation agent, which acts primarily by inhibiting the reuptake of dopamine and other monoamine neurotransmitters in the brain [62]. Intriguingly, bupropion is also an antagonist at α4β2∗ nAChRs in the brain [63–65]. Whether the direct actions of bupropion on nAChR signaling contributes to its efficacy as a smoking cessation agent is currently unknown.
Fig. 1.
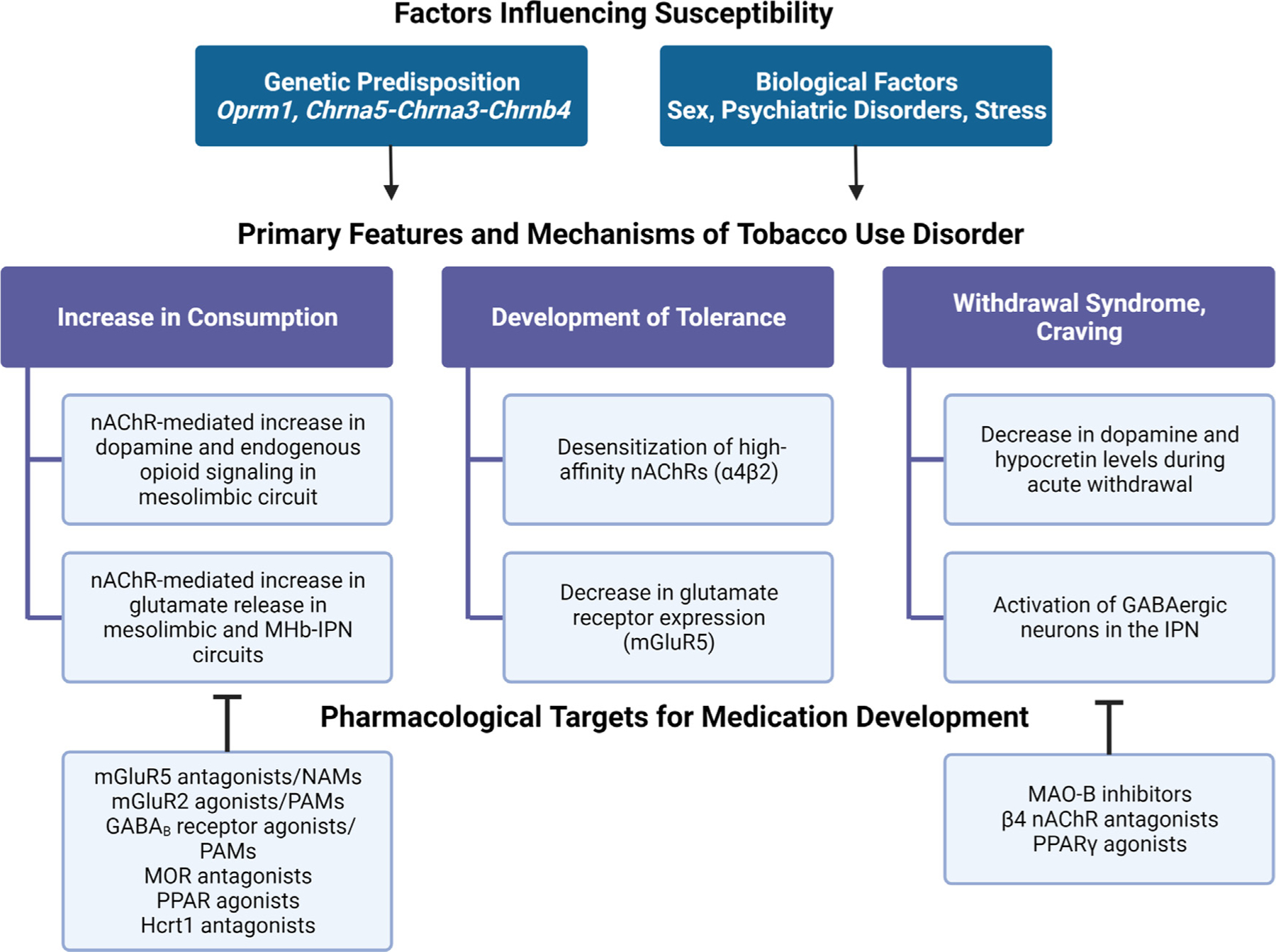
Summary of factors that influence vulnerability to tobacco use disorder and opportunities for new medications development. GABA, ϒ-aminobutyric acid; Hcrt1, hypocretin receptor 1; IPN, interpeduncular nucleus; MAO-B, monoamine oxidase B; mGluR; metabotropic glutamate receptor; MHb, medial habenula; MOR, mu opioid receptor; nAChR, nicotinic acetylcholine receptor; NAM, negative allosteric modulator; PAM, positive allosteric modulator; PPAR, peroxisome-proliferator-activated receptor.
In addition to α4 and β2 nAChR subunits, other lesser studied nAChR subunits have also been implicated in the addiction-related actions of nicotine [46]. Most notably, the CHRNA5-CHRNA3-CHRNB4 gene cluster, which encodes the α5, α3, and β4 nAChR subunits, respectively, has been heavily implicated in tobacco dependence by human genome-wide association studies (GWAS) [66–69]. Evidence from GWAS indicates that allelic variation in this gene cluster increases the risk of tobacco dependence and smoking-related diseases [66,70,71]. In animals, genetic manipulations that decrease the expression and/or function of α3, α5, or β4 subunits result in striking alterations in behavioral responses to nicotine [72–74]. For example, mice with a null mutation in the Chrna5 gene consume greater quantities of nicotine than wild-type mice, which is reversed by re-expressing α5 nAChR subunits in the medial habenula (MHb) - interpeduncular nucleus (IPN) circuit [75]. More broadly, the MHb-IPN circuit has been heavily linked to the regulation of nicotine intake [75]. RNA interference-mediated knockdown of Chrna5 gene transcripts in this region does not alter the rewarding properties of nicotine in rats but instead attenuates the aversive properties of nicotine that are thought to limit consumption of the drug [72–75]. α3∗ nAChRs in the brain are almost exclusively expressed in the MHb-IPN circuit [74]. Mice engineered to express constitutively low levels of α3∗ nAChRs are less sensitive to nicotine aversion and self-administer greater quantities of the drug than wild-type mice [74]. These findings suggest that nAChRs containing subunits derived from the CHRNA5-CHRNA3-CHRNB4 gene cluster influence tobacco dependence vulnerability by regulating the aversive properties of nicotine that limit consumption and protect against the establishment of regulate tobacco use [46]. Combinations of polymorphisms in the CHRNA5-CHRNA3-CHRNB4 region that tend to be inherited together, known as haplotypes, have been identified that contribute to different risk levels of nicotine dependence (low, intermediate, and high risk) [76]. A large-scale study of genetic factors that influence the efficacy of smoking cessation agents showed that individuals who carry the high-risk CHRNA5-CHRNA3-CHRNB4 haplotype were more sensitive to currently available smoking cessation therapeutics than those with the low-risk haplotype [76]. Smokers in the high-risk haplotype group that received placebo medication were also more likely to relapse to tobacco use compared with the low-risk haplotype group [76]. In a subsequent study it was reported that the efficacy of NRT was moderated by genetic variants in the CHRNA5 gene [77]. These data lend support to the idea that novel pharmacological agents that modulate the activity of nAChRs containing α5, α3, and/or β4 subunits may be effective smoking cessation agents, particularly in those individuals who carry CHRNA5-CHRNA3-CHRNB4 risk alleles.
Allelic variants in genes that encode the enzymes involved in nicotine metabolism, including cytochrome P450 2A6 (CYP2A6) and cytochrome P450 2B6 (CYP2B6), also influence risk of tobacco dependence and the efficacy of smoking cessation therapeutics [78–81]. Higher abstinence rates with bupropion have been reported in individuals carrying alleles that decrease CYP2A6 function resulting in slower nicotine metabolism [79]. In a randomized smoking cessation trial, an association between CYP2A6 genotype and abstinence was found in individuals receiving NRT, but not those receiving bupropion [82], consistent with altered nicotine but not bupropion metabolism in these individuals. Hence, agents that modify CYP2A6/CYP2B6 activity may have efficacy as smoking cessation medications. Identification of other gene variants that influence the risk of tobacco dependent or vulnerability to relapse may similarly yield novel targets for medications development and catalyze personalized medicine-based approaches to tobacco use disorder [71,83]. Hence, next-generation smoking cessation therapeutics are likely to be derived from advances in our understanding of the genetics of tobacco use disorder. Studies employing cultured human neurons that carry risk-associated gene variants are likely to shed important new light on the molecular mechanisms that influence vulnerability to tobacco use disorders [84,85]. Furthermore, advances in high-throughput screening of cultured human neurons may facilitate the identification of small molecule drugs that reverse the functional consequences of risk-associated gene variants, and thereby have utility as smoking cessation agents.
Finally, rates of tobacco use disorder and amounts of tobacco consumed are much higher in individuals suffering from major depressive disorder, anxiety disorders, schizophrenia, and other neuropsychiatric disorders relative to the general population [86]. Whether the onset of a psychiatric disorder increases the risk of developing tobacco dependence or whether tobacco use increases vulnerability to psychiatric abnormalities is unclear, but the available evidence suggests that both relationships are likely to exist [87,88]. This suggests that tobacco use disorder and neuropsychiatric abnormalities may share some overlap in their genetic underpinnings [86,89–92]. If so, insights into the genetic mechanisms of neuropsychiatric disorders may reveal novel treatment approaches for tobacco use disorders and vice versa. Notably, rates of tobacco use in the general population have steadily decreased in recent years [93,94], but have remained stubbornly high in individuals suffering from neuropsychiatric abnormalities [86,95]. This is important because currently available and newly developed smoking cessation therapies may demonstrate altered efficacy in those smokers who also suffer from comorbid psychiatric disorders [96,97].
4. Pharmacogenetics and personalized medicine in the context of smoking cessation
Accumulating evidence support an important link between genetic factors and vulnerability to nicotine dependence and the effectiveness of different smoking cessation agents. As described above, individuals who carried CHRNA5-CHRNA3-CHRNB4 risk alleles were more sensitive to currently available smoking cessation therapeutics than those with low-risk haplotypes [76,77,98]. The variation in treatment efficacy between smokers with different genetic phenotypes underscores the potential of personalized smoking cessation medications based on genotype to maximize treatment efficacy, while potentially minimizing potential side-effects [98]. Thus, identifying these genes and understanding how they combine to influence nicotine dependence is an important goal of current research that may be addressed through modern genetic approaches, including transcriptomic analysis in animal models and postmortem human brain tissue. Hopefully, these approaches will expand our understanding of the mechanisms of nicotine dependence, and ultimately facilitate the translation of these findings into more efficacious personalized clinical smoking cessation therapies.
5. Preclinical model-based discovery of novel targets for smoking cessation
Significant efforts have been directed toward the development of new medications for smoking cessation based on a growing understanding of the neurobiological mechanisms of nicotine addiction derived from preclinical models of aspects of tobacco use disorder. Indeed, novel targets have been identified in mouse and rat studies that show considerable promise for the treatment of nicotine dependence, leading to the development of new pharmacotherapies currently being evaluated for their efficacy in human clinical trials (Table 2). In this section, we review recent findings supporting the therapeutic potential of compounds targeting newly identified brain targets for smoking cessation, with a focus on endogenous peptide systems, receptors involved in excitatory and inhibitory neurotransmission, and peroxisome proliferator-activated receptors (PPARs). We will also highlight new pharmacological approaches for the treatment of nicotine dependence such as positive and negative allosteric modulators (PAM and NAM, respectively) of metabotropic glutamate and GABA receptors, which may possess fewer negative side-effects compared with full orthostatic agonists and antagonists at targeted receptors.
Table 2.
Novel compounds in clinical testing for smoking cessation.
| Compound | Mechanism of Action | Sponsor | Status | Phase |
|---|---|---|---|---|
| Suvorexant | Hcrt1/2 receptor antagonist | University of Texas Health Science Center | Recruiting | Phase 1 |
| Baclofen | GABAB receptor agonist | University of Pennsylvania | Completed | Phase 2 |
| Naltrexone | MOR antagonist | University of Chicago | Completed | Phase 2 |
| Naltrexone combined with Bupropion | MOR antagonist and antidepressant | Yale University | Completed | Phase 2 |
| Selegiline | MAO-B Inhibitor | NIDA | Completed | Phase 2 |
| AZD8529 | mGluR2 PAM | NIDA | Completed | Phase 2 |
| Gemfibrozil | PPARα agonist | University of Texas | Completed | Phase 2 |
| S-adenosyl-L-Methionine (SAMe) | Facilitates monoamine synthesis | Mayo Clinic | Completed | Phase 2/3 |
5.1. Endogenous opioid system
The endogenous opioid system in the brain is thought to play an important role in the actions of nicotine that contribute to tobacco dependence [99]. The endogenous opioid system consists of 3 families of peptides (β-endorphins, enkephalins, and dynorphin), and 3 families of opioid receptors (μ/MOR, δ/DOR, and κ/KOR) [100]. Opioid receptors are regionally distributed within brain sites involved in pain processing, reward/aversion, and the modulation of stress responses [100,101]. The influence of endogenous opioids in the addiction-related actions of nicotine has been supported in both clinical studies and animal models. Within the ventral tegmental area (VTA), a brain region known to mediate the facilitatory actions of nicotine on dopaminergic transmission, MORs are predominantly expressed on inhibitory GABAergic neurons and act to suppress the release of GABA [102]. Recruitment of the inhibitory action of MORs on GABAergic neurotransmission may therefore act to potentiate the excitatory effects of nicotine on mesoaccumbens dopamine release, and in turn, its reinforcing actions. Evidence from animal studies supports a role of endogenous opioids in nicotine reward. Nicotine has been shown to increase levels of endogenous opioids within the striatum [103–105], and the opioid receptor antagonist naloxone blocks nicotine-induced increases in food responding [106], nicotine-induced analgesia [107], and aversive behaviors associated with mecamylamine-precipitated withdrawal in nicotine-dependent animals [20]. Studies in MOR knockout mice have further demonstrated that this receptor is critical for nicotine reward [108,109]. Together, these findings suggest that the reward-related actions of nicotine may be mediated in part by MOR activation within the mesoaccumbens dopamine system [109].
Clinical studies also support a link between tobacco smoking and the endogenous opioid system. Evidence from human studies indicates that smoking triggers an increase in plasma levels of endogenous opioids [104]. Numerous clinical studies have evaluated the efficacy of opioid receptor antagonists alone or in combination with NRT on smoking behavior. A systematic review and meta-analysis found no significant beneficial effects of naltrexone, a MOR antagonist, on short-term or long-term smoking abstinence [110]. However, a more recent smoking cessation clinical trial reported that naloxone, an inverse agonist of MORs, decreased the desire to smoke in comparison to placebo treatment [111], although this study had a relatively small sample size. In addition, there is evidence that polymorphisms in the OPRM1 gene encoding MOR in humans may increase the risk of tobacco dependence. The effectiveness of NRT or placebo was evaluated in participants harboring a single nucleotide polymorphism (SNP) in the OPRM1 gene, leading to a substitution of asparagine (A) for aspartate (G). Individuals with at least one copy of the G allele (GG or GA phenotype) on active NRT treatment were less likely to remain abstinent relative to placebo compared with individuals in the AA phenotype group [112]. Interestingly, these genotype-treatment interactions were found to be sexually dimorphic; males in the AA group were more likely to remain abstinent at long-term follow-up periods (up to 8 years), whereas the reverse was true for female participants [112]. In contrast to these findings, another study found that NRT was more effective in promoting abstinence in individuals with at least one copy of the G allele of the OPRM1 gene compared with those with two copies of the A allele [112]. Thus, further research is needed to elucidate genotype- and sex-specific influences of the endogenous opioid system in the efficacy of smoking cessation treatments.
5.2. Hypocretin/Orexin
The hypocretin (aka orexin) system comprises 2 neuropeptides (Hcrt1 and Hcrt1) that are produced almost exclusively by neurons located in the lateral hypothalamus and regulate sleep/wake cycles, arousal, and motivational states [113–115]. Hypocretins act at two receptors, Hcrt receptor 1 and 2 (Hcrtr1 and Hcrtr2, respectively) [114,115]. Accumulating evidence suggests that hypocretin plays an important role in the addiction-relevant actions of nicotine. Nicotine increases the expression of hypocretin and its receptors in the lateral hypothalamus [116], and the selective Hcrtr1 antagonist SB334867 decreases nicotine self-administration in rats [21]. Pre-treatment with SB334867 was also found to block the reinstatement of previously extinguished nicotine-seeking behavior by intracerebroventricular infusions of hypocretin [22] and cue-induced reinstatement of nicotine-seeking behavior [23]. A recent study [117] examining electrophysiological responses of hypocretin neurons in the lateral hypothalamus provided further insight into acetylcholine-hypocretin interactions. Acetylcholine stimulated an increase in the firing frequency of a population of hypocretin neurons in the lateral hypothalamus. Interestingly, these effects were also observed following application of the nAChR antagonist mecamylamine. The authors concluded that phasic acetylcholine release in the lateral hypothalamus enhances the firing of a subset of hypocretin neurons through postsynaptic nAChRs, while simultaneously disrupting presynaptic nAChR-mediated glutamatergic inputs to hypocretin neurons [117]. Hence, the regulation of presynaptic glutamatergic inputs to hypocretin neurons within the lateral hypothalamus by acetylcholine may explain the effects of nicotine exposure on the hypocretin system, and hence, the influence of hypocretin receptor antagonists on nicotine-seeking behaviors.
Promising evidence suggests that hypocretin may be associated with the extent of craving during smoking cessation and the risk for relapse in human smokers. In a large sample of smokers, participants who relapsed within the first 4 weeks exhibited a decrease in hypocretin levels at 24 h of abstinence [118]. Further, lower hypocretin levels during withdrawal predicted shorter time to relapse, even after accounting for potential confounding factors such as stress hormone levels. These findings suggest that a decrease in hypocretin levels during the initial withdrawal period promotes nicotine craving and potentiates the risk for relapse in abstinent smokers. Hence, hypocretin levels during early nicotine withdrawal may be useful as a biomarker of craving and relapse risk in smokers attempting to quit [118]. Notably, the dual Hcrtr1/2 receptor antagonist suvorexant was recently shown to attenuate craving in opioid use disorder (OUD) patients undergoing buprenorphine-facilitated abstinence [119]. A randomized phase 1 clinical trial to evaluate the effect of suvorexant on factors impacting the motivation to smoke is currently recruiting participants (Table 2). Recently completed phase 1 clinical trials sponsored by the NIH and AstraZeneca established that a selective Hcrt1 receptor antagonist (AZD4041) was safe for assessment as a novel smoking cessation agent (www.clinicaltrials.gov/ct2/show/NCT04076540). The findings from these human clinical studies will yield further insights into the therapeutic potential of medications targeting the hypocretin system for smoking cessation.
5.3. Glutamate - metabotropic glutamate receptors
Glutamate-mediated excitatory transmission plays an important role in the reinforcing actions of nicotine and the establishment of nicotine dependence [120]. Nicotine facilitates excitatory neurotransmission in brain circuits involved in reward and aversion, including the mesolimbic dopamine [121] and MHb-IPN circuits [75]. The positive reinforcing properties of nicotine are driven by nAChR-mediated increases in the activity of dopamine neurons in the VTA, thereby increasing their firing rate and enhancing dopamine release in the NAc [122–124]. The activity of mesolimbic dopamine neurons is further regulated by glutamatergic inputs, including from the prefrontal cortex and lateral hypothalamus [120]. Within the mesolimbic circuit, nicotine facilitates glutamate release from presynaptic terminals in both the VTA and NAc, which is believed to play a role in its reinforcing actions [120]. Evidence suggests that blocking glutamatergic transmission within these brain circuits attenuates nicotine reward-related behaviors in rodents [120,125,126]. Conversely, facilitation of inhibitory GABAergic transmission attenuates nicotine seeking behaviors in rodent models of tobacco dependence [120,127,32]. These actions are thought to be mediated through the regulation of dopaminergic transmission; inhibition of GABA release from local interneurons that provide inhibitory input to VTA dopaminergic neurons thereby facilitates nicotine-induced stimulation of dopamine release [120]. However, the specific receptors and circuit-level mechanisms underlying these effects have only recently been elucidated; these will be discussed in more detail in the next section. Ultimately, a better understanding of how glutamate and GABA mediate the reinforcing actions of nicotine will lead to improved pharmacotherapies targeting these neurotransmitter systems for the treatment of nicotine addiction.
The actions of glutamate are mediated through two separate categories of receptors: the fast-acting ionotropic receptors and the slower-acting G-protein coupled metabotropic receptors [120]. The ionotropic glutamate receptors include the amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA), N-methyl-D-aspartate (NMDA), and kainite-type glutamate receptors, which mediate fast excitatory glutamatergic neurotransmission. The metabotropic glutamate receptor family consists of 8 receptors (mGluR1–8) that are classified into three groups (I, II, and III) based on their functional properties. Group I receptors (mGluR1 and mGluR5) are primarily located postsynaptically, whereas Group II receptors (mGluR2 and mGluR3) are located presynaptically, and their activation inhibits glutamate release to maintain glutamate homeostasis. A growing body of evidence supports a role of mGluRs in nicotine dependence-related behaviors [120,24,29,128].
Preclinical and human studies support the therapeutic potential of mGluRs as targets for smoking cessation. In animals, compounds that decrease glutamatergic transmission either by inhibiting postsynaptic glutamate receptors (NMDARs, mGluR5) or by activating inhibitory presynaptic glutamate receptors (mGluR2/3) decrease nicotine self-administration and block the reinstatement of nicotine-seeking behaviors. In nicotine-dependent rats, infusion of the mGluR2 agonist LY314582 into the VTA precipitated withdrawal-like increases in intracranial self-stimulation (ICSS) reward thresholds, reflecting a deficit in reward function. This effect was attributed at least partly to reductions in glutamate-mediated excitatory transmission at postsynaptic AMPARs in the VTA [24]. Similar results have been observed with the use of antagonists that block mGluR5-mediated excitatory neurotransmission [29,30]. While the mGluR5 antagonist 6-methyl-2-[phenylethynyl]-pyridine (MPEP) did not affect ICSS reward thresholds in nicotine-dependent mice [24], it was found to decrease nicotine-self administration in rats and mice, without affecting the acquisition of food self-administration [29]. Studies in human subjects have also supported a link between mGluRs and nicotine dependence. A positron emission tomography (PET) imaging study reported a global reduction in mGluR5 distribution volume ratio (DVR) in gray matter, which was particularly evident in the medial orbitofrontal cortex. Further, there were significant differences in mGluR5 DVR between smokers and non-smokers, and between ex-smokers and non-smokers. The authors concluded that this decrease may have been an adaptation to chronic increases in glutamate caused by chronic nicotine use [129]. Thus, medications that opposite the stimulatory effects of nicotine on excitatory glutamatergic signaling in the VTA and other reward-relevant brain sites may represent novel smoking cessation agents.
5.4. GABAB receptors
GABAergic transmission in reward-relevant brain sites has also been heavily implicated in nicotine dependence, particularly in the nicotine withdrawal syndrome. In rodents, somatic withdrawal symptoms are characterized by behaviors such as scratching, head nods, and body shakes, and can be induced by administration of the nAChR antagonist mecamylamine in nicotine-dependent mice [130,131]. Mecamylamine-precipitated withdrawal has been shown to induce expression of the immediate-early gene product c-Fos in the GABAergic neurons located in the IPN, suggesting that nicotine withdrawal may enhance neural activity in the IPN [132]. Moreover, optogenetic activation of GABAergic neurons in the IPN was sufficient to trigger somatic withdrawal symptoms in mice [132]. Notably, infusion of the NMDA glutamatergic receptor antagonist AP5 directly into the IPN reduced GABAergic activation following mecamylamine, and AP5 infusions during spontaneous withdrawal decreased the expression of somatic withdrawal symptoms [132]. The same study also reported that nicotinic β3 and β4 receptors were upregulated in somatostatin-expressing GABAergic interneurons in the IPN during chronic nicotine exposure, and that blockade of β4 receptors activated somatostatin interneurons and triggered somatic withdrawal symptoms that were more dramatic in nicotine-dependent compared to nicotine-naive mice. These findings suggest that glutamate release in the IPN during nicotine withdrawal may drive increases in the activity of GABAergic neurons in the IPN that contribute to the expression of somatic withdrawal symptoms.
Similar to the glutamatergic system, the actions of GABA are mediated through binding to receptors that can be defined as fast-acting ionotropic receptors (GABAA) and slow-acting G-protein-coupled metabotropic receptors (GABAB) [133]. Evidence from animal studies has supported an important role of GABAB receptors in animal models of addiction and craving. Administration of GABAB receptor agonists or positive allosteric modulators has been shown to decease self-administration of various addictive drugs and inhibit cue-induced reinstatement of nicotine-seeking behavior [127,32,134], presumably by enhancing inhibitory neurotransmission in brain reward circuits and thereby opposing the stimulatory effects of nicotine on those sane circuits. Interestingly, prior studies have found that both agonists and antagonists of GABAB receptors potentiated somatic and anxiety-like responses to mecamylamine-precipitated nicotine withdrawal [135,34]. While it is unclear why the modulation of GABAB receptors in opposite directions would yield similar behavioral effects, the authors speculated that these results may reflect differential efficacies of the various GABAB receptor ligands at distinct receptor subtypes in brain regions involved in reward and aversion [34]. Thus, several lines of evidence suggest that GABAB receptors may modulate both the reinforcing aspects of nicotine as well as the aversive nicotine withdrawal effects in a complex manner. In human clinical trials, the GABAB receptor antagonist baclofen was shown to decrease cigarette consumption relative to placebo treatment [136], supporting the potential of the GABAB receptor as a suitable target for the development of pharmacotherapies for smoking cessation.
5.5. Peroxisome proliferator-activated receptors
PPARs have been proposed as therapeutic targets for the treatment of tobacco use disorder [37,137–140]. PPARs ligand-activated transcription factors that regulate the transcription of genes involved in metabolic function and energy hemostasis [141], and more recently have been implicated in addiction-related behaviors. The PPAR receptor family is composed of three subtypes (PPARα, PPARδ, and PPARγ) which differ in their expression patterns and physiological functions [141]. In laboratory animals, compounds that activate PPARs have been shown to reduce reinforcement-related physiological and behavioral responses to nicotine [37,137–140]. A class of drugs known as fibrates that activate PPARs have been widely used to reduce triglyceride levels and reduce the risk of heart disease [142]. A prior study utilizing nicotine self-administration in rats demonstrated that clofibrate, a type of fibrate drug, decreased nicotine consumption, suggesting that clofibrate blocks the reward-related actions of nicotine that motivate consumption of the drug [36]. Clofibrate also attenuated nicotine-induced responses in dopaminergic neurons in the VTA and dopamine release in the NAc [36]. PPARγ has been shown to have potential as a therapeutic target for the development of smoking cessation medications [37]. During acute withdrawal, the expression of PPARγ was found to be elevated in the hippocampus and amygdala of rodents and microinjections of the PPARγ agonist pioglitazone into these regions attenuated somatic and affective symptoms of nicotine withdrawal [37]. Several clinical studies have also evaluated the effects of PPAR agonists on tobacco dependence. The PPARα partial agonist Gemfibrozil did not modify reactivity to nicotine-related cues or the number of days of abstinence in smokers compared to the placebo group [143]. However, another study reported that the PPARγ agonist pioglitazone significantly reduced measures of nicotine craving in smokers [144], suggesting that medications targeting PPARγ may be effective in promoting abstinence in smokers attempting to quit.
6. Corticostriatal circuits in tobacco dependence
The stimulatory action of nicotine on dopamine transmission in brain reinforcement circuits is thought to play a crucial role in the establishment and maintenance of the tobacco smoking habit [46]. As previously mentioned, nicotine stimulates nAChRs located on the terminals of excitatory inputs to the VTA and also nAChRs located directly on dopamine neurons to facilitate VTA-derived dopaminergic signaling in the NAc, which supports the positive reinforcing properties of the drug [121]. Conversely, avoidance and alleviation of the deficits in dopamine transmission in the NAc and other corticolimbic brain sites during periods of nicotine withdrawal is thought to provide an important source of negative reinforcement that contributes to relapse [145]. α6 nAChR subunits are highly localized to midbrain and hindbrain regions containing dopamine-producing and norepinephrine-producing neurons, respectively [122–124]. α6 nAChR subunits are likely incorporated along with β3 nAChRs subunits into subtypes of α4β2∗ nAChRs, which play particularly important roles in regulating the stimulatory effects of nicotine on mesoaccumbens dopamine transmission [45]. Indeed, the α6∗ nAChR antagonist α-conotoxin-MII suppresses nicotine-evoked dopamine release in the NAc and decreases nicotine self-administration behavior in rodents [124]. Hence, pharmacological agents that target the nAChR subtypes involved in modulating dopamine transmission may have therapeutic utility as smoking cessation therapeutics. Haloperidol, a dopamine D2 receptor antagonist, was found to reduce cigarette smoking in a clinical study [146]. However, haloperidol and other neuroleptic agents have marked adverse effects and it is unclear whether the effects of such compounds on smoking behavior are secondary to such adverse effects. Concerningly, neuroleptics have been reported to increase tobacco smoking behavior [147,148], which may reflect compensatory increases in tobacco consumption to overcome the inhibitory effects of neuroleptics on D2 dopamine receptor-mediated signaling. A clinical study evaluating the effectiveness of S-adenosyl-L-methionine (SAMe), a methyl group donor that stimulates the synthesis of catecholamines including dopamine, failed to identify difference in abstinence rates or withdrawal symptoms between SAMe and placebo treatment groups [149]. Thus, it is presently unclear whether interventions designed to modulate mesoaccumbens dopamine transmission will yield clinically efficacious smoking cessation agents, except perhaps in certain populations of smokers that may be particularly sensitive to beneficial effects of such compounds.
Nicotine-enhanced glutamatergic transmission in the NAc, derived from the higher-order cortical inputs, is thought to contribute to relapse vulnerability during periods of abstinence [126]. In the cue-induced reinstatement model of relapse, nicotine-seeking behavior was associated with an increase in glutamate release in the NAc core of rats [126]. Furthermore, cue-induced reinstatement led to an increase in spine diameter along with an increase in AMPA/NMDA glutamatergic receptor ratio in medium spiny neurons in the NAc core, indicative of increased synaptic plasticity. These changes were associated with reductions in the expression of the GluN2A and GluN2B subunits, and pharmacological inhibition of GluN2A or GluN2B in the NAc core prevented nicotine reinstatement [126]. It was also demonstrated that the mGlu2/3 receptor agonist LY379268 decreased cue-induced reinstatement of nicotine-seeking responses in rats after infusion into the VTA or NAc [25]. As mGlu2/3 receptors act as presynaptic autoreceptors, these manipulations would be expected to decrease phasic increases in glutamatergic transmission in the NAc derived from the cortex and other reward-relevant brain sites [25]. An mGlu2 receptor positive allosteric modulator, AZD8529, similarly decreased nicotine self-administration and cue-induced reinstatement of nicotine-seeking responses in rats [27] and squirrel monkeys [150]. AZD8529 advanced to a phase 2 clinical trial to test whether it facilitates smoking cessation in tobacco users [151]. Reports of an efficacious response to AZD8529 have not appeared in the published literature and its development as a novel smoking cessation agent appear to have ceased. However, other pharmacological interventions that attenuate cortically derived glutamatergic transmission in the mesoaccumbens dopamine system may represent novel treatment strategies to prevent relapse to tobacco use.
7. Habenular circuits in tobacco dependence
Evidence has accumulated over the past 15 years implicating the MHb-IPN circuit in the motivational properties of nicotine and other addictive drugs. The habenula is a diencephalic structure that is comprised of two anatomically and functionally distinct medial (MHb) and lateral (LHb) subnuclei [152]. The LHb receives prominent inputs from limbic, basal ganglia, midbrain, and cortical regions, and sends reciprocal projections to many of these same regions. In contrast, the MHb receives input almost exclusively from just a few regions of the septum, and projects almost exclusively to the IPN [152]. The MHb contains a large population of acetylcholine-producing neurons and contains some of the highest densities of nAChRs in the brain [153]. In particular, the expression of α3, α5, and β4 subunits are highly enriched in the MHb-IPN circuit [154]. As mentioned above, RNA interference-mediated knockdown of α3 or α5 nAChR subunits in the MHb-IPN circuit attenuated the aversive effects of nicotine and increased nicotine self-administration behavior in rats [75]. Evidence suggests that the vesicular transporters for acetylcholine and glutamate are co-localized within synaptic vesicles of MHb axonal terminals in the IPN, and that acetylcholine and glutamate are co-released in the IPN [155]. Notably, the elimination of acetylcholine in MHb neurons by the conditional deletion of the acetylcholine-synthesizing enzyme choline acetyltransferase (ChAT) led to a reduction in the amplitude of miniature excitatory post-synaptic potentials (mEP-SCs) and deceased the vesicular content of glutamate but did not affect nicotine-evoked responses in IPN neurons [155], reflecting a reduction in acetylcholine-glutamate co-release. Moreover, nicotine-induced increases in mEPSC frequency were absent in ChAT-KO mice, indicating a decrease in the function of presynaptic nAChRs [155]. Together, these findings demonstrate that acetylcholine facilitates the packaging of glutamate into synaptic vesicles and glutamate transmitter release through the activation of presynaptic nAChRs in the MHb. These acetylcholine-dependent actions may explain the reduction of nicotine dependence in ChAT-KO mice [155]. These findings support that in addition to glutamate neurotransmission within the mesolimbic circuit, glutamate neurotransmission in the MHb-IPN circuit is also an important mediator of the reinforcement-related actions of nicotine. Hence, pharmacological agents that modulate MHb-derived cholinergic and/or glutamatergic transmission in the IPN may represent novel classes of smoking cessation agents. Specifically, pharmacological agents that modify the activity of the MHb-IPN circuit could promote avoidance of nicotine contained in tobacco smoke and thereby facilitate smoking cessation efforts. Therefore, identification of other “druggable” receptors that are expressed by neurons in the MHb-IPN circuit could serve as targets for development of smoking cessation agents. Using microarray-based RNA profiling to characterize gene expression it was reported that neurons in the MHb densely express the orphan G-protein–coupled receptor (GPCR) GPR151 [156]. Subsequently, Ibanez-Tallon and colleagues used Translating Ribosome Affinity Purification (TRAP) to profile the transcriptomes of MHb cholinergic neurons and confirmed that GPR151 is very densely and almost exclusively expressed MHb cholinergic neurons in rodent and human brain [157], with modest expression also detected in the dorsal root ganglion [158]. The concentrated expression of GPR151 in the MHb has been confirmed by other groups [159,160]. Using electron microscopy, it was reported that GPR151 accumulates at the presynaptic terminals of MHb neurons in the IPN in close proximity to the synaptic “active zones”, thereby positioning GPR151 to regulate MHb-derived neurotransmitter release into the IPN [157]. It was also shown that GPR151 colocalizes nAChRs on the terminals of MHb neurons in the IPN [157]. Most importantly, GPR151 knockout mice were shown to self-administer greater quantities of nicotine than their wild-type littermates [157]. Hence, pharmacological agents that enhance the activity of GPR151 in the MHb-IPN circuit may decrease the motivational properties of nicotine and thereby have clinical utility for the treatment of tobacco use disorder.
8. Immune-based therapeutics for smoking cessation
A novel approach to treating tobacco dependence is to recruit the immune system to sequester or degrade nicotine before it can exert its pharmacological actions in the brains of smokers [161]. Vaccines have been developed by several groups that stimulate the immune system to produce antibodies that recognize and bind to nicotine; for example, see [162–164]. Several vaccines and have shown promise as smoking cessation in rodent studies of nicotine reinforcement [162–167]. However, the development of a safe and effective vaccine for smoking cessation in humans has proven to be challenging. For example, NicVAX is a nicotine vaccine developed by Nabi Biopharmaceuticals that attenuated many addiction-related physiological and behavioral responses to nicotine in rodents but failed to meet its primary endpoint in a phase III clinical trial of its utility as a smoking cessation agent [168–170]. There is continued interest in developing a vaccine for smoking cessation, and several new approaches are being explored including the use adjuvants to enhance the immune response to a vaccine [171–173]. Novel non-vaccine strategies are also being pursued to sequester and/or metabolize nicotine at sufficiently high levels to prevent behaviorally relevant amounts of the drug from reaching the brain of human smokers. For example, skin grafts harbouring cells that have been gene-edited using CRISPR to secrete enzymes that rapidly metabolize drugs of abuse such as cocaine have been developed [174]. Skin grafts that secrete agents that reduce the motivation to consume nicotine and other drugs of abuse, such as glucagon-like peptide 1 (GLP-1), have also been developed [175,176]. These skin grafts have shown promise as therapeutics strategies for the treatment of substance use disorders in laboratory rodents [174]. The work points to the exciting possibility of developing cutaneous gene-therapy strategies as safe and effective treatments for tobacco use disorder [177].
9. Future approaches for the identification of therapeutic targets for smoking cessation
In addition to the targets highlighted in this article, which have compounds that are in development or clinical assessment as smoking cessation therapeutics, there is on-going research into discovering additional mechanisms that may be beneficial for the treatment of tobacco use disorder and other substance use disorders. There is clear evidence for a robust genetic component to tobacco dependence, although the specific genes mediating risk have not yet been fully characterized. Nevertheless, findings from GWAS over the past decade have begun to elucidate potential genes associated with the risk for nicotine addiction, although the identified genes typically contribute only a small amount to the overall risk, highlighting the complexity of the phenotype [178]. Indeed, the genetic basis of addiction may be influenced by several hundred genes comprising the risk for addiction. Genetic variations in addiction-relevant genes can also influence the effectiveness of smoking cessation treatments. Thus, identifying risk-associated genes and understanding how they influence vulnerability to tobacco use disorder using animal models, postmortem human brain tissue, and neurons and brain-like organoids derived from human induced pluripotent stem (IPS) cells will likely yield new strategies for medications development. Innovative techniques such as single cell RNA sequencing and spatial transcriptomics, which can provide transcriptional information with unprecedented cellular and brain regions resolution, will enable future studies to identify the genes involved in nicotine dependence-related neuroplasticity and thereby influence risk of tobacco use disorder. Hence, a better understanding of the genetic and transcriptional mechanisms involved in nicotine dependence will be crucial for the identification of novel targets for smoking cessation therapies.
In addition to genetic mechanisms of drug addiction, promising research has implicated a role of microRNAs (miRNA) and other classes of noncoding RNAs that can regulate the expression and/or translation of protein-coding gene transcripts in the mechanism underlying substance use disorders. Previous studies have established a role of miRNAs in the development of dependence on cocaine and alcohol [179]. The contributions of miRNA to nicotine dependence have been less well studied, but a few reports have demonstrated that miR-140–5P regulates the expression of specific genes encoding proteins and receptors important in mediating addiction-related responses to nicotine, including the dopamine D1 receptor and Dynamin-1, a protein that binds to the nAChR β2 subunit. Moreover, neuron-specific miRNAs have been shown to be secreted into the bloodstream in response to tobacco. Several human studies have also reported differences in the expression of specific miRNAs between smokers and nonsmokers. Thus, miRNAs may be useful as biomarkers for addiction in the future. Another example of a novel direction for smoking cessation treatments is targeting neuroimmune mechanisms. Although changes in neural function induced by drug exposure have historically been the focus of substance use disorder research, increasing evidence indicates that glial cells, including microglia and astrocytes, are also impacted by exposure to addictive drugs and may play a role in the behavioral consequences of substance use. There is strong evidence to suggest that addictive compounds such as opioids can lead to increases in surface markers indicative of glial activation and increase the expression of neuroimmune signaling molecules including pro-inflammatory cytokines [180]. Recent evidence also indicates that nicotine may alter astrocyte morphology through nAChRs [180]. These glial responses to nicotine and other drugs of abuse could influence neuronal function through a number of mechanisms, including structural remodeling or through cytokine and chemokine signaling [181]. Thus, there is a significant need for future studies to investigate the role of microglia and astrocytes in drug self-administration paradigms, which may identify new avenues of therapeutic approaches to treating nicotine addiction as well as other substance use disorders.
10. Conclusions
Despite considerable research efforts over the past two decades, tobacco dependence remains a significant public health concern. Evidence suggests that pharmacotherapy is an effective approach to facilitate smoking cessation, although there is a pressing need to develop new medications with improved efficacy, tolerability, and side effect profiles. Importantly, findings from preclinical animal models and human clinical studies highlight that for tobacco use disorder is complex and multifaceted and influenced by a plethora of variables including genetic factors, sex of the subject, history of drug use, and comorbid neuropsychiatric conditions. Hence, it is unlikely that a “silver bullet” approach exists for the treatment of tobacco use disorder. Accumulating evidence supports the benefits of personalized medicine, wherein smoking cessation treatments are tailored to the specific symptoms and characteristics of the individual seeking treatment. Further research into identifying potential biomarkers of relapse risk, such as hypocretin levels, will be useful for mitigating relapse rates in smokers attempting to quit. Moreover, a better understanding of the interactions between specific genes that contribute to the risk for nicotine addiction and the efficacy of smoking cessation treatments could allow for more effective treatment approaches targeted to individuals with certain genetic phenotypes (e.g., OPRM1 gene haplotypes).
Our understanding of the mechanisms of nicotine addiction has improved significantly over the past decade. We now have strong evidence that several signaling systems including hypocretin, endogenous opioids, glutamate, and GABA all contribute to the addictive actions of nicotine. These findings have led to the development of novel compounds that have shown to be effective in regulating nicotine-relevant behaviors. A promising new approach to pharmacological treatment for nicotine addiction is the use of allosteric modulators at addiction-relevant targets, such as mGluR2/5 and GABAB receptors, which display better target specificity and fewer adverse side effects compared with agonists and antagonists. These novel compounds have shown promise in decreasing nicotine self-administration and relapse to nicotine-seeking behaviors in animal models and are now beginning to be evaluated for their efficacy in clinical trials. The findings from these clinical studies will confirm whether these compounds may be a viable approach to facilitate smoking cessation. Thus, these recent advances in our knowledge of the neural mechanisms of nicotine addiction will hopefully translate into new and more effective smoking cessation medications.
Footnotes
Disclosures
P.J.K. is a co-founder of Eolas Therapeutics Inc., which has a licensing agreement with AstraZeneca to develop small molecule treatments for substance use disorders.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Data availability
No data was used for the research described in the article.
References
- [1].Jha P, Avoidable global cancer deaths and total deaths from smoking, Nat. Rev. Cancer 9 (2009) 655–664. [DOI] [PubMed] [Google Scholar]
- [2].Alberg AJ, Shopland DR, Cummings KM, The 2014 surgeon general’s report: commemorating the 50th Anniversary of the 1964 report of the advisory committee to the US Surgeon General and updating the evidence on the health consequences of cigarette smoking, Am. J. Epidemiol 179 (2014) 403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Xu X, Shrestha SS, Trivers KF, Neff L, Armour BS, BA.U.S. King, healthcare spending attributable to cigarette smoking in 2014, Prev. Med 150 (2021) 106529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R, Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies, BMJ 321 (2000) 323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, McAfee T, Peto R, 21st-century hazards of smoking and benefits of cessation in the United States, N. Engl. J. Med 368 (2013) 341–350. [DOI] [PubMed] [Google Scholar]
- [6].Benowitz NL, Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics, Annu. Rev. Pharmacol. Toxicol 49 (2009) 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hays JT, Ebbert JO, Adverse effects and tolerability of medications for the treatment of tobacco use and dependence, Drugs 70 (2010) 2357–2372. [DOI] [PubMed] [Google Scholar]
- [8].Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, Ascher J, Russ C, Krishen A, Evins AE, Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial, Lancet 387 (2016) 2507–2520. [DOI] [PubMed] [Google Scholar]
- [9].Oncken C, Gonzales D, Nides M, Rennard S, Watsky E, Billing CB, Anziano R, Reeves K, Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation, Arch. Intern. Med 166 (2006) 1571–1577. [DOI] [PubMed] [Google Scholar]
- [10].Goniewicz ML, Delijewski M, Nicotine vaccines to treat tobacco dependence, Hum. Vaccines Immunother 9 (2013) 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Desai RI, Bergman J, Nicotine-targeting nano-vaccines for smoking cessation, Neuropsychopharmacology 41 (2016) 377–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Stolerman IP, Jarvis MJ, The scientific case that nicotine is addictive, Psychopharmacology 117 (1995) 2–10 (Berl)discussion 14–20. [DOI] [PubMed] [Google Scholar]
- [13].Robinson JD, Li L, Chen M, Lerman C, Tyndale RF, Schnoll RA, Hawk LW, George TP, Benowitz NL, Cinciripini PM, Evaluating the temporal relationships between withdrawal symptoms and smoking relapse, Psychol. Addict. Behav 33 (2019) 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shiffman S, Engberg JB, Paty JA, Perz WG, Gnys M, Kassel JD, Hickcox M, A day at a time: predicting smoking lapse from daily urge, J. Abnorm. Psychol 106 (1997) 104–116. [DOI] [PubMed] [Google Scholar]
- [15].Killen JD, Fortmann SP, Craving is associated with smoking relapse: findings from three prospective studies, Exp. Clin. Psychopharmacol 5 (1997) 137–142. [DOI] [PubMed] [Google Scholar]
- [16].al’Absi M, Hatsukami D, Davis GL, Wittmers LE, Prospective examination of effects of smoking abstinence on cortisol and withdrawal symptoms as predictors of early smoking relapse, Drug Alcohol Depend 73 (2004) 267–278. [DOI] [PubMed] [Google Scholar]
- [17].Nakajima M, Al’Absi M, Predictors of risk for smoking relapse in men and women: a prospective examination, Psychol. Addict. Behav 26 (2012) 633–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Simon JA, Duncan C, Carmody TP, Hudes ES, Bupropion for smoking cessation: a randomized trial, Arch. Intern. Med 164 (2004) 1797–1803. [DOI] [PubMed] [Google Scholar]
- [19].Aubin HJ, Bobak A, Britton JR, Oncken C, Billing CB Jr., Gong J, Williams KE, Reeves KR, Varenicline versus transdermal nicotine patch for smoking cessation: results from a randomised open-label trial, Thorax 63 (2008) 717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ise Y, Narita M, Nagase H, Suzuki T, Modulation of opioidergic system on mecamylamine-precipitated nicotine-withdrawal aversion in rats, Psychopharmacology 151 (2000) 49–54 (Berl). [DOI] [PubMed] [Google Scholar]
- [21].Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ, Insular hypocretin transmission regulates nicotine reward, Proc. Natl. Acad. Sci. USA 105 (2008) 19480–19485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Plaza-Zabala A, Martin-Garcia E, de Lecea L, Maldonado R, Berrendero F, Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior, J. Neurosci 30 (2010) 2300–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Plaza-Zabala A, Flores A, Martin-Garcia E, Saravia R, Maldonado R, Berrendero F, A role for hypocretin/orexin receptor-1 in cue-induced reinstatement of nicotine-seeking behavior, Neuropsychopharmacology 38 (2013) 1724–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kenny PJ, Gasparini F, Markou A, Group II metabotropic and alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate glutamate receptors regulate the deficit in brain reward function associated with nicotine withdrawal in rats, J. Pharmacol. Exp. Ther 306 (2003) 1068–1076. [DOI] [PubMed] [Google Scholar]
- [25].Liechti ME, Lhuillier L, Kaupmann K, Markou A, Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence, J. Neurosci 27 (2007) 9077–9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liechti ME, Markou A, Metabotropic glutamate 2/3 receptor activation induced reward deficits but did not aggravate brain reward deficits associated with spontaneous nicotine withdrawal in rats, Biochem. Pharmacol 74 (2007) 1299–1307. [DOI] [PubMed] [Google Scholar]
- [27].Li X, D’Souza MS, Nino AM, Doherty J, Cross A, Markou A, Attenuation of nicotine-taking and nicotine-seeking behavior by the mGlu2 receptor positive allosteric modulators AZD8418 and AZD8529 in rats, Psychopharmacology 233 (2016) 1801–1814 (Berl). [DOI] [PubMed] [Google Scholar]
- [28].Justinova Z, Le Foll B, Redhi GH, Markou A, Goldberg SR, Differential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on nicotine versus cocaine self-administration and relapse in squirrel monkeys, Psychopharmacology 233 (2016) 1791–1800 (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kenny PJ, Paterson NE, Boutrel B, Semenova S, Harrison AA, Gasparini F, Koob GF, Skoubis PD, Markou A, Metabotropic glutamate 5 receptor antagonist MPEP decreased nicotine and cocaine self-administration but not nicotine and cocaine-induced facilitation of brain reward function in rats, Ann. NY Acad. Sci 1003 (2003) 415–418. [DOI] [PubMed] [Google Scholar]
- [30].Tronci V, Balfour DJ, The effects of the mGluR5 receptor antagonist 6-methyl-2-(phenylethynyl)-pyridine (MPEP) on the stimulation of dopamine release evoked by nicotine in the rat brain, Behav. Brain Res 219 (2011) 354–357. [DOI] [PubMed] [Google Scholar]
- [31].Tronci V, Vronskaya S, Montgomery N, Mura D, Balfour DJ, The effects of the mGluR5 receptor antagonist 6-methyl-2-(phenylethynyl)-pyridine (MPEP) on behavioural responses to nicotine, Psychopharmacology 211 (2010) 33–42 (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Paterson NE, Vlachou S, Guery S, Kaupmann K, Froestl W, Markou A, Positive modulation of GABA(B) receptors decreased nicotine self-administration and counteracted nicotine-induced enhancement of brain reward function in rats, J. Pharmacol. Exp. Ther 326 (2008) 306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Paterson NE, Froestl W, Markou A, The GABAB receptor agonists baclofen and CGP44532 decreased nicotine self-administration in the rat, Psychopharmacology 172 (2004) 179–186 (Berl). [DOI] [PubMed] [Google Scholar]
- [34].Vlachou S, Paterson NE, Guery S, Kaupmann K, Froestl W, Banerjee D, Finn MG, Markou A, Both GABA(B) receptor activation and blockade exacerbated anhedonic aspects of nicotine withdrawal in rats, Eur. J. Pharmacol 655 (2011) 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Li X, Sturchler E, Kaczanowska K, Cameron M, Finn MG, Griffin P, McDonald P, Markou A, KK-92A, a novel GABA(B) receptor positive allosteric modulator, attenuates nicotine self-administration and cue-induced nicotine seeking in rats, Psychopharmacology 234 (2017) 1633–1644 (Berl). [DOI] [PubMed] [Google Scholar]
- [36].Panlilio LV, Justinova Z, Mascia P, Pistis M, Luchicchi A, Lecca S, Barnes C, Redhi GH, Adair J, Heishman SJ, Yasar S, Aliczki M, Haller J, Goldberg SR, Novel use of a lipid-lowering fibrate medication to prevent nicotine reward and relapse: preclinical findings, Neuropsychopharmacology 37 (2012) 1838–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Domi E, Caputi FF, Romualdi P, Domi A, Scuppa G, Candeletti S, Atkins A, Heilig M, Demopulos G, Gaitanaris G, Ciccocioppo R, Ubaldi M, Activation of PPARgamma attenuates the expression of physical and affective nicotine withdrawal symptoms through mechanisms involving amygdala and hippocampus neurotransmission, J. Neurosci 39 (2019) 9864–9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].El-Boraie A, Tyndale RF, The role of pharmacogenetics in smoking, Clin. Pharmacol. Ther 110 (2021) 599–606. [DOI] [PubMed] [Google Scholar]
- [39].Picciotto MR, Kenny PJ, Mechanisms of nicotine addiction, Cold Spring Harb. Perspect. Med 11 (2021) a039610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wittenberg RE, Wolfman SL, De Biasi M, Dani JA, Nicotinic acetylcholine receptors and nicotine addiction: a brief introduction, Neuropharmacology 177 (2020) 108256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hogg RC, Raggenbass M, Bertrand D, Nicotinic acetylcholine receptors: from structure to brain function, Rev. Physiol. Biochem. Pharmacol 147 (2003) 1–46. [DOI] [PubMed] [Google Scholar]
- [42].Fowler CD, Arends MA, Kenny PJ, Subtypes of nicotinic acetylcholine receptors in nicotine reward, dependence, and withdrawal: evidence from genetically modified mice, Behav. Pharmacol 19 (2008) 461–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Albuquerque EX, Pereira EF, Alkondon M, Rogers SW, Mammalian nicotinic acetylcholine receptors: from structure to function, Physiol. Rev 89 (2009) 73–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Dani JA, Neuronal nicotinic acetylcholine receptor structure and function and response to nicotine, Int. Rev. Neurobiol 124 (2015) 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wills L, Ables JL, Braunscheidel KM, Caligiuri SPB, Elayouby KS, Fillinger C, Ishikawa M, Moen JK, Kenny PJ, Neurobiological mechanisms of nicotine reward and aversion, Pharmacol. Rev 74 (2022) 271–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wills L, Kenny PJ, Addiction-related neuroadaptations following chronic nicotine exposure, J. Neurochem 157 (2021) 1652–1673. [DOI] [PubMed] [Google Scholar]
- [47].McGranahan TM, Patzlaff NE, Grady SR, Heinemann SF, Booker TK, alpha4beta2 nicotinic acetylcholine receptors on dopaminergic neurons mediate nicotine reward and anxiety relief, J. Neurosci 31 (2011) 10891–10902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ, A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment, Mol. Pharmacol 41 (1992) 31–37. [PubMed] [Google Scholar]
- [49].Buisson B, Bertrand D, Nicotine addiction: the possible role of functional upregulation, Trends Pharmacol. Sci 23 (2002) 130–136. [DOI] [PubMed] [Google Scholar]
- [50].Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP, Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine, Nature 391 (1998) 173–177. [DOI] [PubMed] [Google Scholar]
- [51].Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA, Nicotine activation of alpha4∗ receptors: sufficient for reward, tolerance, and sensitization, Science 306 (2004) 1029–1032. [DOI] [PubMed] [Google Scholar]
- [52].Lerman C, LeSage MG, Perkins KA, O’Malley SS, Siegel SJ, Benowitz NL, Corrigall WA, Translational research in medication development for nicotine dependence, Nat. Rev. Drug Discov 6 (2007) 746–762. [DOI] [PubMed] [Google Scholar]
- [53].Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD 3rd, O’Neill BT, Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation, J. Med. Chem 48 (2005) 3474–3477. [DOI] [PubMed] [Google Scholar]
- [54].Reperant C, Pons S, Dufour E, Rollema H, Gardier AM, Maskos U, Effect of the alpha4beta2∗ nicotinic acetylcholine receptor partial agonist varenicline on dopamine release in beta2 knock-out mice with selective re-expression of the beta2 subunit in the ventral tegmental area, Neuropharmacology 58 (2010) 346–350. [DOI] [PubMed] [Google Scholar]
- [55].Scharfenberg G, Benndorf S, Kempe G, [Cytisine (Tabex) as a pharmaceutical aid in stopping smoking], Dtsch. Gesundh 26 (1971) 463–465. [PubMed] [Google Scholar]
- [56].Benndorf S, Scharfenberg G, Kempe G, Wendekamm R, Winkelvoss E, [Smoking withdrawal treatment with Cytisin (Tabex). Results of a semi-annual survey of former smokers after 4 weeks of therapy], Dtsch. Gesundheitsw 24 (1970) 774–776. [PubMed] [Google Scholar]
- [57].Etter JF, Cytisine for smoking cessation: a literature review and a meta-analysis, Arch. Intern. Med 166 (2006) 1553–1559. [DOI] [PubMed] [Google Scholar]
- [58].Barlow RB, McLeod LJ, Some studies on cytisine and its methylated derivatives, Br. J. Pharmacol 35 (1969) 161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Reavill C, Walther B, Stolerman IP, Testa B, Behavioural and pharmacokinetic studies on nicotine, cytisine and lobeline, Neuropharmacology 29 (1990) 619–624. [DOI] [PubMed] [Google Scholar]
- [60].Kenny PJ, Markou A, Neurobiology of the nicotine withdrawal syndrome, Pharmacol. Biochem. Behav 70 (2001) 531–549. [DOI] [PubMed] [Google Scholar]
- [61].Wadgave U, Nagesh L, Nicotine replacement therapy: an overview, Int. J. Health Sci 10 (2016) 425–435 (Qassim). [PMC free article] [PubMed] [Google Scholar]
- [62].Patel K, Allen S, Haque MN, Angelescu I, Baumeister D, Tracy DK, Bupropion: a systematic review and meta-analysis of effectiveness as an antidepressant, Ther. Adv. Psychopharmacol 6 (2016) 99–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Slemmer JE, Martin BR, Damaj MI, Bupropion is a nicotinic antagonist, J. Pharmacol. Exp. Ther 295 (2000) 321–327. [PubMed] [Google Scholar]
- [64].Pandhare A, Hamouda AK, Staggs B, Aggarwal S, Duddempudi PK, Lever JR, Lapinsky DJ, Jansen M, Cohen JB, Blanton MP, Bupropion binds to two sites in the Torpedo nicotinic acetylcholine receptor transmembrane domain: a photoaffinity labeling study with the bupropion analogue [(125)I]-SADU-3–72, Biochemistry 51 (2012) 2425–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Damaj MI, Grabus SD, Navarro HA, Vann RE, Warner JA, King LS, Wiley JL, Blough BE, Lukas RJ, Carroll FI, Effects of hydroxymetabolites of bupropion on nicotine dependence behavior in mice, J. Pharmacol. Exp. Ther 334 (2010) 1087–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, Waterworth D, Muglia P, Mooser V, Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking, Mol. Psychiatry 13 (2008) 368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, Lissowska J, Rudnai P, Fabianova E, Mates D, Bencko V, Foretova L, Janout V, Chen C, Goodman G, Field JK, Liloglou T, Xinarianos G, Cassidy A, McLaughlin J, Liu G, Narod S, Krokan HE, Skorpen F, Elvestad MB, Hveem K, Vatten L, Linseisen J, Clavel-Chapelon F, Vineis P, Bueno-de-Mesquita HB, Lund E, Martinez C, Bingham S, Rasmuson T, Hainaut P, Riboli E, Ahrens W, Benhamou S, Lagiou P, Trichopoulos D, Holcatova I, Merletti F, Kjaerheim K, Agudo A, Macfarlane G, Talamini R, Simonato L, Lowry R, Conway DI, Znaor A, Healy C, Zelenika D, Boland A, Delepine M, Foglio M, Lechner D, Matsuda F, Blanche H, Gut I, Heath S, Lathrop M, Brennan P, A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25, Nature 452 (2008) 633–637. [DOI] [PubMed] [Google Scholar]
- [68].Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, Manolescu A, Thorleifsson G, Stefansson H, Ingason A, Stacey SN, Bergthorsson JT, Thorlacius S, Gudmundsson J, Jonsson T, Jakobsdottir M, Saemundsdottir J, Olafsdottir O, Gudmundsson LJ, Bjornsdottir G, Kristjansson K, Skuladottir H, Isaksson HJ, Gudbjartsson T, Jones GT, Mueller T, Gottsater A, Flex A, Aben KKH, de Vegt F, Mulders PFA, Isla D, Vidal MJ, Asin L, Saez B, Murillo L, Blondal T, Kolbeinsson H, Stefansson JG, Hansdottir I, Runarsdottir V, Pola R, Lindblad B, van Rij AM, Dieplinger B, Haltmayer M, Mayordomo JI, Kiemeney LA, Matthiasson SE, Oskarsson H, Tyrfingsson T, Gudbjartsson DF, Gulcher JR, Jonsson S, Thorsteinsdottir U, Kong A, Stefansson K, A variant associated with nicotine dependence, lung cancer and peripheral arterial disease, Nature 452 (2008) 638–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, Saccone NL, Saccone SF, Bertelsen S, Fox L, Horton WJ, Breslau N, Budde J, Cloninger CR, Dick DM, Foroud T, Hatsukami D, Hesselbrock V, Johnson EO, Kramer J, Kuperman S, Madden PA, Mayo K, Nurnberger J Jr., Pomerleau O, Porjesz B, Reyes O, Schuckit M, Swan G, Tischfield JA, Edenberg HJ, Rice JP, Goate AM, Variants in nicotinic receptors and risk for nicotine dependence, Am. J. Psychiatry 165 (2008) 1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau O, Swan GE, Goate AM, Rutter J, Bertelsen S, Fox L, Fugman D, Martin NG, Montgomery GW, Wang JC, Ballinger DG, Rice JP, Bierut LJ, Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs, Hum. Mol. Genet 16 (2007) 36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Chen LS, Bierut LJ, Genomics and personalized medicine: CHRNA5-CHRNA3-CHRNB4 and smoking cessation treatment, J. Food Drug Anal 21 (2013) S87–S90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Muldoon PP, Jackson KJ, Perez E, Harenza JL, Molas S, Rais B, Anwar H, Zaveri NT, Maldonado R, Maskos U, McIntosh JM, Dierssen M, Miles MF, Chen X, De Biasi M, Damaj MI, The alpha3beta4∗ nicotinic ACh receptor subtype mediates physical dependence to morphine: mouse and human studies, Br. J. Pharmacol 171 (2014) 3845–3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Semenova S, Contet C, Roberts AJ, Markou A, Mice lacking the beta4 subunit of the nicotinic acetylcholine receptor show memory deficits, altered anxiety- and depression-like behavior, and diminished nicotine-induced analgesia, Nicotine Tob. Res 14 (2012) 1346–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Elayouby KS, Ishikawa M, Dukes AJ, Smith ACW, Lu Q, Fowler CD, Kenny PJ, alpha3∗ nicotinic acetylcholine receptors in the habenula-interpeduncular nucleus circuit regulate nicotine intake, J. Neurosci 41 (2021) 1779–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ, Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake, Nature 471 (2011) 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Chen LS, Baker TB, Piper ME, Breslau N, Cannon DS, Doheny KF, Gogarten SM, Johnson EO, Saccone NL, Wang JC, Weiss RB, Goate AM, Bierut LJ, Interplay of genetic risk factors (CHRNA5-CHRNA3-CHRNB4) and cessation treatments in smoking cessation success, Am. J. Psychiatry 169 (2012) 735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Bergen AW, Javitz HS, Krasnow R, Nishita D, Michel M, Conti DV, Liu J, Lee W, Edlund CK, Hall S, Kwok PY, Benowitz NL, Baker TB, Tyndale RF, Lerman C, Swan GE, Nicotinic acetylcholine receptor variation and response to smoking cessation therapies, Pharmacogenet. Genom 23 (2013) 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Thorgeirsson TE, Gudbjartsson DF, Surakka I, Vink JM, Amin N, Geller F, Sulem P, Rafnar T, Esko T, Walter S, Gieger C, Rawal R, Mangino M, Prokopenko I, Magi R, Keskitalo K, Gudjonsdottir IH, Gretarsdottir S, Stefansson H, Thompson JR, Aulchenko YS, Nelis M, Aben KK, den Heijer M, Dirksen A, Ashraf H, Soranzo N, Valdes AM, Steves C, Uitterlinden AG, Hofman A, Tonjes A, Kovacs P, Hottenga JJ, Willemsen G, Vogelzangs N, Doring A, Dahmen N, Nitz B, Pergadia ML, Saez B, De Diego V, Lezcano V, Garcia-Prats MD, Ripatti S, Perola M, Kettunen J, Hartikainen AL, Pouta A, Laitinen J, Isohanni M, Huei-Yi S, Allen M, Krestyaninova M, Hall AS, Jones GT, van Rij AM, Mueller T, Dieplinger B, Haltmayer M, Jonsson S, Matthiasson SE, Oskarsson H, Tyrfingsson T, Kiemeney LA, Mayordomo JI, Lindholt JS, Pedersen JH, Franklin WA, Wolf H, Montgomery GW, Heath AC, Martin NG, Madden PA, Giegling I, Rujescu D, Jarvelin MR, Salomaa V, Stumvoll M, Spector TD, Wichmann HE, Metspalu A, Samani NJ, Penninx BW, Oostra BA, Boomsma DI, Tiemeier H, van Duijn CM, Kaprio J, Gulcher JR, Consortium E, McCarthy MI, Peltonen L, Thorsteinsdottir U, Stefansson K, Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior, Nat. Genet 42 (2010) 448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lee AM, Jepson C, Hoffmann E, Epstein L, Hawk LW, Lerman C, Tyndale RF, CYP2B6 genotype alters abstinence rates in a bupropion smoking cessation trial, Biol. Psychiatry 62 (2007) 635–641. [DOI] [PubMed] [Google Scholar]
- [80].Tyndale RF, Sellers EM, Genetic variation in CYP2A6-mediated nicotine metabolism alters smoking behavior, Ther. Drug Monit 24 (2002) 163–171. [DOI] [PubMed] [Google Scholar]
- [81].King DP, Paciga S, Pickering E, Benowitz NL, Bierut LJ, Conti DV, Kaprio J, Lerman C, Park PW, Smoking cessation pharmacogenetics: analysis of varenicline and bupropion in placebo-controlled clinical trials, Neuropsychopharmacology 37 (2012) 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Chen LS, Bloom AJ, Baker TB, Smith SS, Piper ME, Martinez M, Saccone N, Hatsukami D, Goate A, Bierut L, Pharmacotherapy effects on smoking cessation vary with nicotine metabolism gene (CYP2A6), Addiction 109 (2014) 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Saunders GRB, Wang X, Chen F, Jang SK, Liu M, Wang C, Gao S, Jiang Y, Khunsriraksakul C, Otto JM, Addison C, Akiyama M, Albert CM, Aliev F, Alonso A, Arnett DK, Ashley-Koch AE, Ashrani AA, Barnes KC, Barr RG, Bartz TM, Becker DM, Bielak LF, Benjamin EJ, Bis JC, Bjornsdottir G, Blangero J, Bleecker ER, Boardman JD, Boerwinkle E, Boomsma DI, Boorgula MP, Bowden DW, Brody JA, Cade BE, Chasman DI, Chavan S, Chen YI, Chen Z, Cheng I, Cho MH, Choquet H, Cole JW, Cornelis MC, Cucca F, Curran JE, de Andrade M, Dick DM, Docherty AR, Duggirala R, Eaton CB, Ehringer MA, Esko T, Faul JD, Fernandes Silva L, Fiorillo E, Fornage M, Freedman BI, Gabrielsen ME, Garrett ME, Gharib SA, Gieger C, Gillespie N, Glahn DC, Gordon SD, Gu CC, Gu D, Gudbjartsson DF, Guo X, Haessler J, Hall ME, Haller T, Harris KM, He J, Herd P, Hewitt JK, Hickie I, Hidalgo B, Hokanson JE, Hopfer C, Hottenga J, Hou L, Huang H, Hung YJ, Hunter DJ, Hveem K, Hwang SJ, Hwu CM, Iacono W, Irvin MR, Jee YH, Johnson EO, Joo YY, Jorgenson E, Justice AE, Kamatani Y, Kaplan RC, Kaprio J, Kardia SLR, Keller MC, Kelly TN, Kooperberg C, Korhonen T, Kraft P, Krauter K, Kuusisto J, Laakso M, Lasky-Su J, Lee WJ, Lee JJ, Levy D, Li L, Li K, Li Y, Lin K, Lind PA, Liu C, Lloyd-Jones DM, Lutz SM, Ma J, Magi R, Manichaikul A, Martin NG, Mathur R, Matoba N, McArdle PF, McGue M, McQueen MB, Medland SE, Metspalu A, Meyers DA, Millwood IY, Mitchell BD, Mohlke KL, Moll M, Montasser ME, Morrison AC, Mulas A, Nielsen JB, North KE, Oelsner EC, Okada Y, Orru V, Palmer ND, Palviainen T, Pandit A, Park SL, Peters U, Peters A, Peyser PA, Polderman TJC, Rafaels N, Redline S, Reed RM, Reiner AP, Rice JP, Rich SS, Richmond NE, Roan C, Rotter JI, Rueschman MN, Runarsdottir V, Saccone NL, Schwartz DA, Shadyab AH, Shi J, Shringarpure SS, Sicinski K, Skogholt AH, Smith JA, Smith NL, Sotoodehnia N, Stallings MC, Stefansson H, Stefansson K, Stitzel JA, Sun X, Syed M, Tal-Singer R, Taylor AE, Taylor KD, Telen MJ, Thai KK, Tiwari H, Turman C, Tyrfingsson T, Wall TL, Walters RG, Weir DR, Weiss ST, White WB, Whitfield JB, Wiggins KL, Willemsen G, Willer CJ, Winsvold BS, Xu H, Yanek LR, Yin J, Young KL, Young KA, Yu B, Zhao W, Zhou W, Zollner S, Zuccolo L, andMe Research T, Biobank Japan P, Batini C, Bergen AW, Bierut LJ, David SP, Gagliano Taliun SA, Hancock DB, Jiang B, Munafo MR, Thorgeirsson TE, Liu DJ, Vrieze S, Genetic diversity fuels gene discovery for tobacco and alcohol use, Nature 612 (2022) 720–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Scholze P, Huck S, The alpha5 nicotinic acetylcholine receptor subunit differentially modulates alpha4beta2(∗) and alpha3beta4(∗) receptors, Front. Synaptic Neurosci 12 (2020) 607959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Oni EN, Halikere A, Li G, Toro-Ramos AJ, Swerdel MR, Verpeut JL, Moore JC, Bello NT, Bierut LJ, Goate A, Tischfield JA, Pang ZP, Hart RP, Increased nicotine response in iPSC-derived human neurons carrying the CHRNA5 N398 allele, Sci. Rep 6 (2016) 34341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Markou A, Kenny PJ, Neuroadaptations to chronic exposure to drugs of abuse: relevance to depressive symptomatology seen across psychiatric diagnostic categories, Neurotox. Res 4 (2002) 297–313. [DOI] [PubMed] [Google Scholar]
- [87].Taylor GM, Lindson N, Farley A, Leinberger-Jabari A, Sawyer K, Te Water Naude R, Theodoulou A, King N, Burke C, Aveyard P, Smoking cessation for improving mental health, Cochrane Database Syst. Rev 3 (2021) CD013522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Stevenson J, Miller CL, Martin K, Mohammadi L, Lawn S, Investigating the reciprocal temporal relationships between tobacco consumption and psychological disorders for youth: an international review, BMJ Open 12 (2022) e055499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kendler KS, Lonn SL, Sundquist J, Sundquist K, Smoking and schizophrenia in population cohorts of Swedish women and men: a prospective co-relative control study, Am. J. Psychiatry 172 (2015) 1092–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Barkhuizen W, Taylor MJ, Freeman D, Ronald A, A twin study on the association between psychotic experiences and tobacco use during adolescence, J. Am. Acad. Child Adolesc. Psychiatry 58 (2019) 267–276 e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ohi K, Kuwata A, Shimada T, Kataoka Y, Yasuyama T, Uehara T, Kawasaki Y, Genome-wide variants shared between smoking quantity and schizophrenia on 15q25 Are associated with CHRNA5 expression in the brain, Schizophr. Bull 45 (2019) 813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Hartz SM, Horton AC, Hancock DB, Baker TB, Caporaso NE, Chen LS, Hokanson JE, Lutz SM, Marazita ML, McNeil DW, Pato CN, Pato MT, Johnson EO, Bierut LJ, Genetic correlation between smoking behaviors and schizophrenia, Schizophr. Res 194 (2018) 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Services UDoHaH: Smoking Cessation. A Report of the Surgeon General. in National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. Edited by US Dept of Health and Human Services CfDCaP, 2020.
- [94].Hunt LJ, Covinsky KE, Cenzer I, Espejo E, Boscardin WJ, Leutwyler H, Lee AK, Cataldo J, The epidemiology of smoking in older adults: a national cohort study, J. Gen. Intern. Med (2022). [DOI] [PMC free article] [PubMed]
- [95].Han B, Volkow ND, Blanco C, Tipperman D, Einstein EB, Compton WM, Trends in prevalence of cigarette smoking among US adults with major depression or substance use disorders, 2006–2019, JAMA 327 (2022) 1566–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Evins AE, Cather C, Laffer A, Treatment of tobacco use disorders in smokers with serious mental illness: toward clinical best practices, Harv. Rev. Psychiatry 23 (2015) 90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Evins AE, West R, Benowitz NL, Russ C, Lawrence D, McRae T, Maravic MC, Heffner JL, Anthenelli RM, Efficacy and safety of pharmacotherapeutic smoking cessation aids in schizophrenia spectrum disorders: subgroup analysis of EAGLES, Psychiatr. Serv 72 (2021) 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Chen LS, Horton A, Bierut L, Pathways to precision medicine in smoking cessation treatments, Neurosci. Lett 669 (2018) 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Xue Y, Domino EF, Tobacco/nicotine and endogenous brain opioids, Prog. Neuropsychopharmacol. Biol. Psychiatry 32 (2008) 1131–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Holden JE, Jeong Y, Forrest JM, The endogenous opioid system and clinical pain management, AACN Clin. Issues 16 (2005) 291–301. [DOI] [PubMed] [Google Scholar]
- [101].Toubia T, Khalife T, The endogenous opioid system: role and dysfunction caused by opioid therapy, Clin. Obstet. Gynecol 62 (2019) 3–10. [DOI] [PubMed] [Google Scholar]
- [102].Kudo T, Konno K, Uchigashima M, Yanagawa Y, Sora I, Minami M, Watanabe M, GABAergic neurons in the ventral tegmental area receive dual GABA/enkephalin-mediated inhibitory inputs from the bed nucleus of the stria terminalis, Eur. J. Neurosci 39 (2014) 1796–1809. [DOI] [PubMed] [Google Scholar]
- [103].Davenport KE, Houdi AA, Van Loon GR, Nicotine protects against mu-opioid receptor antagonism by beta-funaltrexamine: evidence for nicotine-induced release of endogenous opioids in brain, Neurosci. Lett 113 (1990) 40–46. [DOI] [PubMed] [Google Scholar]
- [104].Pomerleau OF, Fertig JB, Seyler LE, Jaffe J, Neuroendocrine reactivity to nicotine in smokers, Psychopharmacology 81 (1983) 61–67 (Berl). [DOI] [PubMed] [Google Scholar]
- [105].Dhatt RK, Gudehithlu KP, Wemlinger TA, Tejwani GA, Neff NH, Hadjiconstantinou M, Preproenkephalin mRNA and methionine-enkephalin content are increased in mouse striatum after treatment with nicotine, J. Neurochem 64 (1995) 1878–1883. [DOI] [PubMed] [Google Scholar]
- [106].Corrigall WA, Herling S, Coen KM, Evidence for opioid mechanisms in the behavioral effects of nicotine, Psychopharmacology 96 (1988) 29–35 (Berl). [DOI] [PubMed] [Google Scholar]
- [107].Aceto MD, Scates SM, Ji Z, Bowman ER, Nicotine’s opioid and anti-opioid interactions: proposed role in smoking behavior, Eur. J. Pharmacol 248 (1993) 333–335. [DOI] [PubMed] [Google Scholar]
- [108].Berrendero F, Kieffer BL, Maldonado R, Attenuation of nicotine-induced antinociception, rewarding effects, and dependence in mu-opioid receptor knock--out mice, J. Neurosci 22 (2002) 10935–10940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Walters CL, Cleck JN, Kuo YC, Blendy JA, Mu-opioid receptor and CREB activation are required for nicotine reward, Neuron 46 (2005) 933–943. [DOI] [PubMed] [Google Scholar]
- [110].David SP, Chu IM, Lancaster T, Stead LF, Evins AE, Prochaska JJ, Systematic review and meta-analysis of opioid antagonists for smoking cessation, BMJ Open 4 (2014) e004393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Krause D, Warnecke M, Schuetz CG, Soyka M, Manz KM, Proebstl L, Kamp F, Chrobok AI, Pogarell O, Koller G, The impact of the opioid antagonist naloxone on experimentally induced craving in nicotine-dependent individuals, Eur. Addict. Res 24 (2018) 255–265. [DOI] [PubMed] [Google Scholar]
- [112].Lerman C, Wileyto EP, Patterson F, Rukstalis M, Audrain-McGovern J, Restine S, Shields PG, Kaufmann V, Redden D, Benowitz N, Berrettini WH, The functional mu opioid receptor (OPRM1) Asn40Asp variant predicts short-term response to nicotine replacement therapy in a clinical trial, Pharmacogenom. J 4 (2004) 184–192. [DOI] [PubMed] [Google Scholar]
- [113].Ebrahim IO, Howard RS, Kopelman MD, Sharief MK, Williams AJ, The hypocretin/orexin system, J. R. Soc. Med 95 (2002) 227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG, The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity, Proc. Natl. Acad. Sci. USA 95 (1998) 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M, Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior, Cell 92 (1998) 573–585. [DOI] [PubMed] [Google Scholar]
- [116].Kane JK, Parker SL, Matta SG, Fu Y, Sharp BM, Li MD, Nicotine up-regulates expression of orexin and its receptors in rat brain, Endocrinology 141 (2000) 3623–3629. [DOI] [PubMed] [Google Scholar]
- [117].Zhou WL, Gao XB, Picciotto MR, Acetylcholine acts through nicotinic receptors to enhance the firing rate of a subset of hypocretin neurons in the mouse hypothalamus through distinct presynaptic and postsynaptic mechanisms, eNeuro 2 (2015) ENEURO.0052–14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Al’Absi M, Lemieux A, Hodges JS, Allen S, Circulating orexin changes during withdrawal are associated with nicotine craving and risk for smoking relapse, Addict. Biol 24 (2019) 743–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Huhn AS, Finan PH, Gamaldo CE, Hammond AS, Umbricht A, Bergeria CL, Strain EC, Dunn KE, Suvorexant ameliorated sleep disturbance, opioid withdrawal, and craving during a buprenorphine taper, Sci. Transl. Med 14 (2022) eabn8238. [DOI] [PubMed] [Google Scholar]
- [120].D’Souza MS, Markou A, The “stop” and “go” of nicotine dependence: role of GABA and glutamate, Cold Spring Harb. Perspect. Med 3 (2013) a012146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].D’Souza MS, Markou A, Neuronal mechanisms underlying development of nicotine dependence: implications for novel smoking-cessation treatments, Addict. Sci. Clin. Pract 6 (2011) 4–16. [PMC free article] [PubMed] [Google Scholar]
- [122].Liu L, Zhao-Shea R, McIntosh JM, Gardner PD, Tapper AR, Nicotine persistently activates ventral tegmental area dopaminergic neurons via nicotinic acetylcholine receptors containing alpha4 and alpha6 subunits, Mol. Pharmacol 81 (2012) 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ, Alpha6-containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens, Neuropsychopharmacology 33 (2008) 2158–2166. [DOI] [PubMed] [Google Scholar]
- [124].Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, Moretti M, Zanardi A, Rimondini R, Mugnaini M, Clementi F, Chiamulera C, Zoli M, Nicotinic acetylcholine receptors in the mesolimbic pathway: primary role of ventral tegmental area alpha6beta2∗ receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement, J. Neurosci 30 (2010) 5311–5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Souter EA, Chen YC, Zell V, Lallai V, Steinkellner T, Conrad WS, Wisden W, Harris KD, Fowler CD, Hnasko TS, Disruption of VGLUT1 in cholinergic medial habenula projections increases nicotine self-administration, eNeuro 9 (2022) ENEURO.0481–21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, Kalivas PW, Reinstatement of nicotine seeking is mediated by glutamatergic plasticity, Proc. Natl. Acad. Sci. USA 110 (2013) 9124–9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Li X, Sturchler E, Kaczanowska K, Cameron M, Finn MG, Griffin P, McDonald P, Markou A, KK-92A, a novel GABAB receptor positive allosteric modulator, attenuates nicotine self-administration and cue-induced nicotine seeking in rats, Psychopharmacology 234 (2017) 1633–1644 (Berl). [DOI] [PubMed] [Google Scholar]
- [128].Cross AJ, Anthenelli R, Li X, Metabotropic glutamate receptors 2 and 3 as targets for treating nicotine addiction, Biol. Psychiatry 83 (2018) 947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Akkus F, Ametamey SM, Treyer V, Burger C, Johayem A, Umbricht D, Gomez Mancilla B, Sovago J, Buck A, Hasler G, Marked global reduction in mGluR5 receptor binding in smokers and ex-smokers determined by [11C]ABP688 positron emission tomography, Proc. Natl. Acad. Sci. USA 110 (2013) 737–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Salas R, Pieri F, De Biasi M, Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit, J. Neurosci 24 (2004) 10035–10039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Damaj MI, Kao W, Martin BR, Characterization of spontaneous and precipitated nicotine withdrawal in the mouse, J. Pharmacol. Exp. Ther 307 (2003) 526–534. [DOI] [PubMed] [Google Scholar]
- [132].Zhao-Shea R, Liu L, Pang X, Gardner PD, Tapper AR, Activation of GABAergic neurons in the interpeduncular nucleus triggers physical nicotine withdrawal symptoms, Curr. Biol 23 (2013) 2327–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Sigel E, Steinmann ME, Structure, function, and modulation of GABA(A) receptors, J. Biol. Chem 287 (2012) 40224–40231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Vlachou S, Guery S, Froestl W, Banerjee D, Benedict J, Finn MG, Markou A, Repeated administration of the GABAB receptor positive modulator BHF177 decreased nicotine self-administration, and acute administration decreased cue-induced reinstatement of nicotine seeking in rats, Psychopharmacology 215 (2011) 117–128 (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Varani AP, Pedron VT, Aon AJ, Canero EM, Balerio GN, GABAB receptors blockage modulates somatic and aversive manifestations induced by nicotine withdrawal, Biomed. Pharmacother 140 (2021) 111786. [DOI] [PubMed] [Google Scholar]
- [136].Franklin TR, Harper D, Kampman K, Kildea-McCrea S, Jens W, Lynch KG, O’Brien CP, Childress AR, The GABA B agonist baclofen reduces cigarette consumption in a preliminary double-blind placebo-controlled smoking reduction study, Drug Alcohol Depend 103 (2009) 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Melis M, Scheggi S, Carta G, Madeddu C, Lecca S, Luchicchi A, Cadeddu F, Frau R, Fattore L, Fadda P, Ennas MG, Castelli MP, Fratta W, Schilstrom B, Banni S, De Montis MG, Pistis M, PPARalpha regulates cholinergic-driven activity of midbrain dopamine neurons via a novel mechanism involving alpha7 nicotinic acetylcholine receptors, J. Neurosci 33 (2013) 6203–6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Mascia P, Pistis M, Justinova Z, Panlilio LV, Luchicchi A, Lecca S, Scherma M, Fratta W, Fadda P, Barnes C, Redhi GH, Yasar S, Le Foll B, Tanda G, Piomelli D, R Goldberg S, Blockade of nicotine reward and reinstatement by activation of alpha-type peroxisome proliferator-activated receptors, Biol. Psychiatry 69 (2011) 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Melis M, Carta S, Fattore L, Tolu S, Yasar S, Goldberg SR, Fratta W, Maskos U, Pistis M, Peroxisome proliferator-activated receptors-alpha modulate dopamine cell activity through nicotinic receptors, Biol. Psychiatry 68 (2010) 256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Matheson J, Le Foll B, Therapeutic potential of peroxisome proliferator-activated receptor (PPAR) agonists in substance use disorders: a synthesis of preclinical and human evidence, Cells 9 (2020) 1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Tyagi S, Gupta P, Saini AS, Kaushal C, Sharma S, The peroxisome proliferator-activated receptor: a family of nuclear receptors role in various diseases, J. Adv. Pharm. Technol. Res 2 (2011) 236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC, Mechanism of action of fibrates on lipid and lipoprotein metabolism, Circulation 98 (1998) 2088–2093. [DOI] [PubMed] [Google Scholar]
- [143].Gendy MNS, Di Ciano P, Kowalczyk WJ, Barrett SP, George TP, Heishman S, Le Foll B, Testing the PPAR hypothesis of tobacco use disorder in humans: a randomized trial of the impact of gemfibrozil (a partial PPARalpha agonist) in smokers, PLoS ONE 13 (2018) e0201512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Jones JD, Comer SD, Metz VE, Manubay JM, Mogali S, Ciccocioppo R, Martinez S, Mumtaz M, Bisaga A, Pioglitazone, a PPARgamma agonist, reduces nicotine craving in humans, with marginal effects on abuse potential, Pharmacol. Biochem. Behav 163 (2017) 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Zhang L, Dong Y, Doyon WM, Dani JA, Withdrawal from chronic nicotine exposure alters dopamine signaling dynamics in the nucleus accumbens, Biol. Psychiatry 71 (2012) 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Brauer LH, Cramblett MJ, Paxton DA, Rose JE, Haloperidol reduces smoking of both nicotine-containing and denicotinized cigarettes, Psychopharmacology 159 (2001) 31–37 (Berl). [DOI] [PubMed] [Google Scholar]
- [147].McEvoy JP, Freudenreich O, Levin ED, Rose JE, Haloperidol increases smoking in patients with schizophrenia, Psychopharmacology 119 (1995) 124–126 (Berl). [DOI] [PubMed] [Google Scholar]
- [148].Dawe S, Gerada C, Russell MA, Gray JA, Nicotine intake in smokers increases following a single dose of haloperidol, Psychopharmacology 117 (1995) 110–115 (Berl). [DOI] [PubMed] [Google Scholar]
- [149].Sood A, Prasad K, Croghan IT, Schroeder DR, Ehlers SL, Ebbert JO, S-adenosyl-L-methionine (SAMe) for smoking abstinence: a randomized clinical trial, J. Altern. Complement. Med. 18 (2012) 854–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Justinova Z, Panlilio LV, Secci ME, Redhi GH, Schindler CW, Cross AJ, Mrzljak L, Medd A, Shaham Y, Goldberg SR, The novel metabotropic glutamate receptor 2 positive allosteric modulator, AZD8529, decreases nicotine self-administration and relapse in squirrel monkeys, Biol. Psychiatry 78 (2015) 452–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Trabanco AA, Bartolome JM, Cid JM, mGluR2 positive allosteric modulators: an updated patent review (2013–2018), Expert Opin. Ther. Pat 29 (2019) 497–507. [DOI] [PubMed] [Google Scholar]
- [152].Hikosaka O, The habenula: from stress evasion to value-based decision-making, Nat. Rev. Neurosci 11 (2010) 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Lee HW, Yang SH, Kim JY, Kim H, The role of the medial habenula cholinergic system in addiction and emotion-associated behaviors, Front. Psychiatry 10 (2019) 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].De Biasi M, Salas R, Influence of neuronal nicotinic receptors over nicotine addiction and withdrawal, Exp. Biol. Med 233 (2008) 917–929 (Maywood). [DOI] [PubMed] [Google Scholar]
- [155].Frahm S, Antolin-Fontes B, Gorlich A, Zander JF, Ahnert-Hilger G, Ibanez–Tallon I, An essential role of acetylcholine-glutamate synergy at habenular synapses in nicotine dependence, eLife 4 (2015) e11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Quina LA, Wang S, Ng L, Turner EE, Brn3a and Nurr1 mediate a gene regulatory pathway for habenula development, J. Neurosci 29 (2009) 14309–14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Gorlich A, Antolin-Fontes B, Ables JL, Frahm S, Slimak MA, Dougherty JD, Ibanez-Tallon I, Reexposure to nicotine during withdrawal increases the pacemaking activity of cholinergic habenular neurons, Proc. Natl. Acad. Sci. USA 110 (2013) 17077–17082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Holmes FE, Kerr N, Chen YJ, Vanderplank P, McArdle CA, Wynick D, Targeted disruption of the orphan receptor Gpr151 does not alter pain-related behaviour despite a strong induction in dorsal root ganglion expression in a model of neuropathic pain, Mol. Cell. Neurosci 78 (2017) 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Wagner F, Bernard R, Derst C, French L, Veh RW, Microarray analysis of transcripts with elevated expressions in the rat medial or lateral habenula suggest fast GABAergic excitation in the medial habenula and habenular involvement in the regulation of feeding and energy balance, Brain Struct. Funct 221 (2016) 4663–4689. [DOI] [PubMed] [Google Scholar]
- [160].Broms J, Antolin-Fontes B, Tingstrom A, Ibanez-Tallon I, Conserved expression of the GPR151 receptor in habenular axonal projections of vertebrates, J. Comp. Neurol 523 (2015) 359–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Scendoni R, Bury E, Ribeiro ILA, Cameriere R, Cingolani M, Vaccines as a preventive tool for substance use disorder: a systematic review including a meta-analysis on nicotine vaccines’ immunogenicity, Hum. Vaccines Immunother 18 (2022) 2140552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Moreno AY, Azar MR, Warren NA, Dickerson TJ, Koob GF, Janda KD, A critical evaluation of a nicotine vaccine within a self-administration behavioral model, Mol. Pharm 7 (2010) 431–441. [DOI] [PubMed] [Google Scholar]
- [163].Kallupi M, Xue S, Zhou B, Janda KD, George O, An enzymatic approach reverses nicotine dependence, decreases compulsive-like intake, and prevents relapse, Sci. Adv 4 (2018) eaat4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Raleigh MD, Beltraminelli N, Fallot S, LeSage MG, Saykao A, Pentel PR, Fuller S, Thisted T, Biesova Z, Horrigan S, Sampey D, Zhou B, Kalnik MW, Attenuating nicotine’s effects with high affinity human anti-nicotine monoclonal antibodies, PLoS ONE 16 (2021) e0254247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Alzhrani RF, Xu H, Valdes SA, Cui Z, Intranasal delivery of a nicotine vaccine candidate induces antibodies in mouse blood and lung mucosal secretions that specifically neutralize nicotine, Drug Dev. Ind. Pharm 46 (2020) 1656–1664. [DOI] [PubMed] [Google Scholar]
- [166].Fraleigh NL, Lewicky JD, Martel AL, Diaz-Mitoma F, Le HT, Assessing neutralized nicotine distribution using mice vaccinated with the mucosal conjugate nicotine vaccine, Vaccines (Basel) 9 (2021) 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Hu Y, Zhao Z, Ehrich M, Zhang C, Formulation of nanovaccines toward an extended immunity against nicotine, ACS Appl. Mater. Interfaces 13 (2021) 27972–27982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Hartmann-Boyce J, Cahill K, Hatsukami D, Cornuz J, Nicotine vaccines for smoking cessation, Cochrane Database Syst. Rev 2012 (2012) CD007072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Hoogsteder PH, Kotz D, van Spiegel PI, Viechtbauer W, van Schayck OC, Efficacy of the nicotine vaccine 3′-AmNic-rEPA (NicVAX) co-administered with varenicline and counselling for smoking cessation: a randomized placebo-controlled trial, Addiction 109 (2014) 1252–1259. [DOI] [PubMed] [Google Scholar]
- [170].Raupach T, Hoogsteder PH, Onno van Schayck CP, Nicotine vaccines to assist with smoking cessation: current status of research, Drugs 72 (2012) e1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Hu Y, Smith D, Frazier E, Zhao Z, Zhang C, Toll-like receptor 9 agonists as adjuvants for nanoparticle-based nicotine vaccine, Mol. Pharm 18 (2021) 1293–1304. [DOI] [PubMed] [Google Scholar]
- [172].Nouri-Shirazi M, Guinet E, TLR3 and TLR7/8 agonists improve immunization outcome in nicotine exposed mice through different mechanisms, Immunol. Lett 246 (2022) 18–26. [DOI] [PubMed] [Google Scholar]
- [173].Celik M, Fuehrlein B, A review of immunotherapeutic approaches for substance use disorders: current status and future prospects, Immunotargets Ther 11 (2022) 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Li Y, Kong Q, Yue J, Gou X, Xu M, Wu X, Genome-edited skin epidermal stem cells protect mice from cocaine-seeking behaviour and cocaine overdose, Nat. Biomed. Eng 3 (2019) 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Tuesta LM, Chen Z, Duncan A, Fowler CD, Ishikawa M, Lee BR, Liu XA, Lu Q, Cameron M, Hayes MR, Kamenecka TM, Pletcher M, Kenny PJ, GLP-1 acts on habenular avoidance circuits to control nicotine intake, Nat. Neurosci 20 (2017) 708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Yue J, Gou X, Li Y, Wicksteed B, Wu X, Engineered epidermal progenitor cells can correct diet-induced obesity and diabetes, Cell Stem Cell 21 (2017) 256–263 e254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Liu XA, Kenny PJ, Cocaine-metabolizing skin grafts, Nat. Biomed. Eng 3 (2019) 81–82. [DOI] [PubMed] [Google Scholar]
- [178].Walker DM, Nestler EJ, Neuroepigenetics and addiction, Handb. Clin. Neurol 148 (2018) 747–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Smith ACW, Kenny PJ, MicroRNAs regulate synaptic plasticity underlying drug addiction, Genes Brain Behav 17 (2018) e12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Aryal SP, Fu X, Sandin JN, Neupane KR, Lakes JE, Grady ME, Richards CI, Nicotine induces morphological and functional changes in astrocytes via nicotinic receptor activity, Glia 69 (2021) 2037–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Lacagnina MJ, Rivera PD, Bilbo SD, Glial and neuroimmune mechanisms as critical modulators of drug use and abuse, Neuropsychopharmacology 42 (2017) 156–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.


