Abstract
A monoclonal antibody (MAb; MAb CAP1) that was reactive with extracellular aspartic proteinase of Candida albicans (CAP) was produced. The MAb showed strong sensitivity and reactivity to CAP but not to the aspartic proteinases of Candida parapsilosis, Candida tropicalis, and Aspergillus fumigatus or to human cathepsin D or porcine pepsin. The epitope of the CAP recognized by the MAb was the proteinaseous part of CAP and the putative epitope of the MAb was located in the Asp77 to Gly103 sequence. This antibody could be useful for the characterization of CAP and would be a valuable probe for the detection of CAP antigen in the sera of patients with invasive candidiasis.
Among the medically important Candida species, Candida albicans is one of the most important pathogens causing severe candidiasis in immunocompromised patients (8, 13). The mortality rate among patients with systemic C. albicans infections is far higher than that among patients with bacterial septicemia (12, 35). This is due to difficulties in both diagnosis and treatment. Diagnosis of candidiasis is hampered by the fact that many patients with invasive candidiasis do not manifest any of the characteristic clinical features and the infection must be distinguished from other causes of a pyrexia that fail to respond to a broad spectrum of antibiotics. Therefore, numerous studies have been performed to develop reliable serological tests for the rapid diagnosis of invasive candidiasis. Investigators have made intensive attempts to detect circulating fungal antigens by biochemical and immunological techniques (4, 5, 18, 21, 25, 28, 29, 34, 38). One of those attempts involved the use of monoclonal antibody (MAb) against Candida antigen to develop a more specific and sensitive diagnostic method. Until now, many MAbs against Candida antigens have been produced, and most of them have been directed to cell wall components of the fungus (4, 7, 17, 19, 22, 23, 24, 32). However, none of these MAbs was useful for diagnosis of Candida infections because of their low specificities and sensitivities.
Aspartic proteinase is commonly secreted by the vast majority of C. albicans strains, as well as by other pathogenic Candida species such as C. tropicalis, C. parapsilosis, and C. stellatoidea, and it is a putative virulence factor that may have a high degree of potential for use in diagnosis. It was found that patients with candidiasis had high titers of antibodies to aspartic proteinase, and this antigen has been detected in the sera of patients with candidiasis (16, 20, 29). The advantage of using a pathogenic factor such as aspartic proteinase as a serodiagnostic marker for candidiasis lies in its potential to differentiate between simple colonization and invasive disease. However, serodiagnostic methods that use human sera and this enzyme have one problem in that it reacted not only with sera from patients with candidiasis but also with sera from patients infected with other fungi (20). Therefore, to use the aspartic proteinase of C. albicans (CAP) as a diagnostic antigen, more specific diagnostic materials must be produced. For use as diagnostic materials, MAbs against the enzyme were produced previously (1, 2). However, these MAbs were cross-reactive with other, related proteinases, such as those of C. tropicalis and C. parapsilosis, and with porcine pepsin. In this study, a highly specific MAb (MAb CAP1) against CAP was produced and characterized.
C. albicans KIT 1113, which was isolated from a clinical specimen from the Korean Institute of Tuberculosis in 1990, was used throughout the work described here. The other yeasts and fungi used in this study were also clinical isolates (Aspergillus fumigatus and C. parapsilosis) or were obtained from the American Type Culture Collection (ATCC) (C. albicans ATCC 10261, C. albicans ATCC 36802, and C. tropicalis ATCC 14056). C. albicans KIT 1113 was cultured under aerobic conditions in yeast nitrogen base (Difco Laboratories, Detroit, Mich.)–bovine serum albumin (BSA) broth supplemented with 2% glucose for 48 h at 30°C. The CAP antigen for immunization was purified from the culture supernatant as described previously (20).
Production of MAb was carried out by immunization of BALB/c mice with three intraperitoneal injections, at 2-week intervals, of purified CAP. Purified CAP was emulsified in the same amount of Freund’s complete adjuvant (Difco) for the first injection and in Freund’s incomplete adjuvant (Difco) for the following two booster injections. Finally, 3 days before the fusion experiment, the antigen was injected intravenously without adjuvant. The protein concentration was measured by the method of Lowry et al. (15) with BSA as the standard. The fusion of murine spleen cells and myeloma cells (P3X63-Ag8-653; ATCC CRL 1580) was carried out as described previously (11). In brief, the immunized mouse was killed and the spleen was removed aseptically. The spleen cells were then mixed at a ratio of 5:1 with myeloma cells growing at the logarithmic phase. The cells were fused in the presence of 0.5% polyethylene glycol (PEG 1500; Boehringer Mannheim GmbH, Mannheim, Germany) while being maintained in a 37°C water bath. The fusion products were diluted in 40 ml of complete Dulbecco’s Modified Eagle medium containing 10% fetal bovine serum and were plated out at 100 μl per well in four 96-well plates. After 24 h of incubation, 100 μl of selective medium containing hypoxanthine, aminopterin, and thymidine (HAT) was added to each well. Two more HAT changes were made at 3-day intervals. After this the cells were grown in hypoxanthine and thymidine medium for the next 2 weeks with frequent changes of the same medium. Aliquots of medium from wells with growing hybridomas were screened for the production of antibodies against CAP by enzyme-linked immunosorbent assay (ELISA). Positive hybrids were subcloned by limiting dilution in 96-well plates, and one hybridoma was selected for further study.
The ELISA was performed by a modification of the method described previously (17). The 96-well microplates (Costar, Cambridge, Mass.) were coated with purified CAP (10 μg/ml) overnight at 4°C. The plates were blocked by the addition of 200 μl of 0.01 M phosphate-buffered saline (PBS; pH 7.4) containing 0.05% Tween 20 (PBST) supplemented with 1% BSA (fraction V; Sigma Chemical Co., St. Louis, Mo.) and incubation for 2 h at 37°C. The plates were washed three times with PBST, and 50 μl of undiluted hybridoma culture supernatants was added to each well. The plates were then incubated for 2 h at 37°C and washed three times with PBST. Peroxidase-conjugated goat anti-mouse immunoglobulin M (IgM) and IgG (Sigma) diluted 1:3,000 in 0.1% BSA–PBST was added to each well (100 μl was added to each well), and the plate was incubated for 2 h at 37°C. After three washings with PBST, the substrate mixture (100 μl of 0.05% ortho-phenylenediamine and 0.04% hydrogen peroxide in 0.1 M citrate buffer; pH 4.0) was added to each well and the plates were incubated in the dark at room temperature for 20 min. The reaction was stopped with 50 μl of 1 N H2SO4, and the optical densities at 490 nm were read with a Titertek Multiscan instrument.
MAb was purified from the hybridoma culture supernatant by precipitation with 50% saturated ammonium sulfate (pH 7.0), followed by dialysis against 0.04 M phosphate buffer (pH 6.8). The MAb was further purified with a Protein G column (Pharmacia, Uppsala, Sweden). The isotype of the MAb was IgG1(κ), as determined with the Mouse Hybridoma Isotyping Kit (Boehringer Mannheim). For inhibition of enzyme activity, purified CAP at concentrations ranging from 0.001 to 0.1 mM and purified MAb at concentrations ranging from 0.001 to 1 mM were slowly mixed at room temperature in 20 mM sodium phosphate buffer (pH 7.0) for 30 min. The enzyme assay was carried out with BSA as the substrate (20). CAP, which had been mixed with buffer instead of MAb solution, was used as a positive control. The reactivity of the MAb to various aspartic proteinases was studied by immunoblotting assay. The aspartic proteinases of C. albicans ATCC 10261 and C. albicans ATCC 36802 were purified by the methods described previously (16, 27). The aspartic proteinases of C. tropicalis ATCC 14056 and C. parapsilosis were purified by the method of Fusek et al. (6). The aspartic proteinase of A. fumigatus was purified by the method described previously (26). Human cathepsin D (from human liver) and porcine pepsin (from porcine stomach mucosa) were purchased from Sigma. Purified proteinases were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) with the discontinuous system of Laemmli (14). The separated proteins were electrophoretically transferred to a nitrocellulose membrane (pore size, 0.45 μm; Bio-Rad, Hercules, Calif.) essentially as described by Towbin et al. (33). After the transfer, the membrane was blocked in PBST containing 3% skim milk for 2 h at room temperature and was then incubated with MAb in PBST containing 3% skim milk for 2 h at room temperature. After three washings with PBST, the membrane was incubated with peroxidase-conjugated goat anti-mouse IgG (Sigma) for 2 h at room temperature. The conjugate was diluted 1:1,000 immediately before use in 3% skim milk in PBST. After an additional three washings with PBST, the membrane was incubated in a freshly prepared mixture of substrate solution (4 mg of diaminobenzidine per ml, 0.01% hydrogen peroxide in 0.1 M PBS [pH 7.2]) for 20 min at room temperature. The reaction was stopped by washing the membrane with distilled water several times. A slot blot for the detection of CAP was performed with a Bio-Slot microfiltration apparatus (Bio-Rad). A nitrocellulose membrane sheet (pore size, 0.45 μm; Bio-Rad) was wetted in PBS and was then placed in the apparatus. A total of 100 μl of PBS was added to each well. This was followed by the addition of 100 μl of twofold serially diluted purified CAP at concentrations ranging from 1 to 512 ng/ml. The liquids were allowed to drain through the nitrocellulose membrane under gravity control, after which 100 μl of PBS was added. The membrane was removed from the apparatus, and the subsequent steps were identical to those for immunoblotting described above.
To characterize the epitope of the CAP recognized by MAb CAP1, the effects of periodate oxidation and proteinase K treatment of CAP antigen on the binding of MAb were analyzed by ELISA. Periodate oxidation of antigen was performed as described by Woodward et al. (36). The CAP coated on the microtiter plates (10 μg/ml) was exposed to various concentrations of sodium m-periodate (0.039 to 10 mM; Sigma) and proteinase K (0.039 to 10 μg/ml; Sigma). The binding of MAb to the treated antigen was then determined by ELISA. Limited proteolytic digestion was performed by the method of Cleaveland et al. (3). The purified enzyme was heat denatured in SDS-PAGE sample buffer at 95°C for 3 min. After cooling, equal aliquots were placed in separate microcentrifuge tubes. Each protease dilution (alkaline protease, 0.1 μg; endoproteinase Glu-C, 0.1 μg; and endoproteinase Lys-C, 0.1 μg [Promega, Madison, Wis.]) was mixed with an aliquot of heat-denatured CAP (10 μg). The mixtures were immediately loaded into the wells of a 20% polyacrylamide gel. Following electrophoresis, the proteins were electrotransferred to a nitrocellulose membrane for immunoblotting and to a polyvinylidene difluoride membrane for N-terminal and C-terminal sequencing.
Yeast genomic DNA was isolated from spheroplasts as described previously (9). PCR for the amplification and sequencing of the full CAP gene was performed with isolated genomic DNA and oligonucleotide primers (predenaturation at 94°C for 4 min, denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 1 min for 30 cycles). The forward primer was 5′-CAAGCTGTCCCAGTGACTTTACAC-3′, and the reverse primer was 5′-GGTCAAGGCAGAAATACTGGAA-3′. The sequences of primers were designed on the basis of the N-terminal amino acid sequence of purified CAP and the nucleotide sequence of CAP (20, 37). The PCR product was cloned into the PCR 2.1 vector with the Original TA Cloning Kit (Invitrogen, San Diego, Calif.) and was transformed into INVα F′ competent cells (Invitrogen). White colonies were selected on plates containing ampicillin (50 μg/ml; Sigma) and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; 100 μg/ml). The plasmid was purified and sequenced by the dideoxynucleotide chain termination method (31). For the amplification of CAP gene fragments which were to be used for epitope mapping of CAP, three pairs of primers, PA1-PA2, PB1-PB2, and PC1-PC2, were designed on the basis of the full gene sequence (see Fig. 3). PCR was performed with isolated genomic DNA and oligonucleotide primers in a standard PCR buffer (10 mM Tris-HCl [pH 8.3], 1.5 mM MgCl2, 50 mM KCl, 0.1% gelatin, each deoxynucleoside triphosphate at a concentration of 200 μM, 2.5 U of Taq DNA polymerase). Amplification reactions were performed by 30 cycles of predenaturation at 94°C for 1 min, annealing at 53°C for 1 min, and extension at 72°C for 1 min. At the start of the first amplification cycle, predenaturation at 94°C for 4 min was performed, and at the end of the last amplification cycle, a final extension at 72°C for 5 min was performed. The amplified PCR products were electrophoresed in 1.5% agarose gels and were stained with ethidium bromide. The PCR products were purified from the gel with the Geneclean Kit (Bio 101, Inc., La Jolla, Calif.) and were directly subcloned into the Original TA vector (Invitrogen) by following the manufacturer’s instruction. The inserts in the subcloned TA vectors were purified, digested with EcoRI, and ligated into the pGEX-GLi expression vector (kindly provided by M. Y. Lim, GeneLab Technology, Redwood City, Calif.) that had been digested with EcoRI. The resulting plasmids were named pGEX-GLiA, pGEX-GLiB, and pGEX-GLiC. These plasmids were transformed into Escherichia coli XL1-Blue competent cells by standard methods, and the cells were spread onto plates containing 50 μg of ampicillin per ml (30). The transformed cells were selected, and the orientations of the inserts were confirmed by sequencing. The expression of fusion proteins was performed by the following method. A single colony of XL1-Blue/pGEX-GLiA, XL1-Blue/pGEX-GLiB, or XL1-Blue/pGEX-GLiC was inoculated in 5 ml of Luria-Bertani broth containing ampicillin (50 μg/ml), and the colony was allowed to grow at 37°C overnight. Five milliliters of this culture was inoculated into 25 ml of the same medium, and isopropyl-β-thiogalactopyranoside (Sigma) was added to a final concentration of 0.5 mM. The culture was grown at 37°C for 3 h. The cells were pelleted by centrifugation at 3,000 rpm for 5 min, washed with PBS (150 mM NaCl, 16 mM Na2HPO4, 4 mM KH2PO4 [pH 7.3]), and resuspended in 1 ml of PBS. The cells were lysed by vortexing vigorously with glass beads for 30 min, and the supernatants were collected by centrifugation at 10,000 rpm for 10 min. SDS-PAGE was carried out as described above. After SDS-PAGE, the proteins were transferred to a nitrocellulose membrane and were immunoblotted with an anti-glutathione-S-transferase (anti-GST) MAb (Sigma) or MAb CAP1.
FIG. 3.
Mapping of epitope of CAP recognized by MAb CAP1. The predicted amino acid sequence is numbered from the N-terminal amino acid residue of the CAP. Asterisks indicate two essential aspartic acid residues at positions 32 and 218. Bold underscores indicate the N-terminal and C-terminal amino acid sequences of peptides that were proteolytically digested to a limited extent. The black box indicates the overlapping region of the 16.2- and 18.7-kDa fragments. Arrows indicate the directions and locations of PCR primers PA1-PA2, PB1-PB2, and PC1-PC2.
MAb CAP1 reacted not only with native CAP but also with denatured conformations of the homologous CAP antigen. Since CAP undergoes irreversible conformational changes above neutral pH (20), it was not surprising that the MAb reacted with the denatured enzyme. This suggests that the epitope recognized by MAb CAP1 is not susceptible to denaturation. In fact, the denatured CAP in the alkaline pH condition was also detected by MAb CAP1 by immunoblotting. MAb CAP1 was not able to inhibit the enzyme activity of the CAP. Similar results have been reported previously (1, 2). This result may be explained by the fact that the relevant epitopes of the catalytic center of the fungal proteinase might be subjected to immunosuppression because the fungal proteinase is closely related to cathepsin D and renin, which are important enzymes in mammalian physiology (10). Antibodies directed against the substrate binding site or the catalytic center may be degraded by the enzyme. This possibility has been partially supported by the fact that CAP was not inhibited by alpha-2-macroglobulin, which acts as a general proteinase scavenger in mammalian plasma (28).
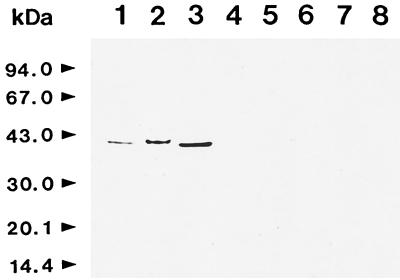
MAb CAP1 showed strong reactivity only to proteinases of different strains of C. albicans but not to other related proteinases such as those of C. parapsilosis, C. tropicalis, and A. fumigatus or to human cathepsin D or porcine pepsin (Fig. 1). MAb CAP1 was able to detect 2 and 16 ng of CAP antigen per ml by ELISA and slot blotting, respectively. The specificity and sensitivity of MAb CAP1 strongly suggested that this antibody might be useful as a diagnostic material for the detection of CAP antigen in the sera of patients with candidiasis.
FIG. 1.
Cross-reactivity of MAb CAP1 with various aspartic proteinases. The purified or partially purified aspartic proteinases from various sources were analyzed by SDS-PAGE and immunoblotted with MAb CAP1. Lane 1, proteinase of C. albicans KIT 1113; lane 2, proteinase of C. albicans ATCC 10261; lane 3, proteinase of C. albicans ATCC 36802; lane 4, proteinase of C. tropicalis ATCC 14056; lane 5, proteinase of C. parapsilosis; lane 6, proteinase of A. fumigatus; lane 7, human cathepsin D; lane 8, porcine pepsin.
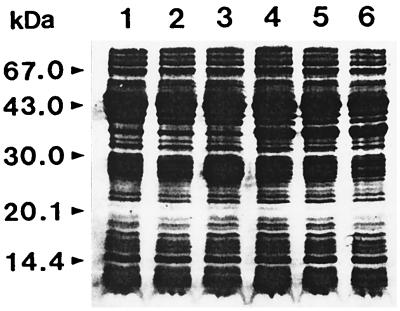
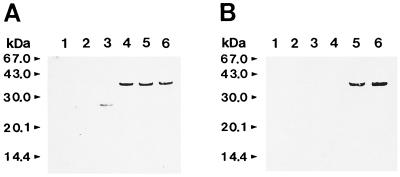
The epitope recognized by the MAb was susceptible to treatment with proteinase K at concentrations of ≥1.25 μg/ml and was resistant to oxidation with sodium m-periodate at concentrations of up to 10 mM (Table 1). This indicated that the epitope of MAb CAP1 was the proteinaceous part of CAP. In fact, the deglycosylated CAP produced by treatment with endoglycosidase was detected by MAb CAP1 by immunoblotting. For further analysis of potential epitopes, the limited proteolytic digestion of CAP with proteinases, alkaline protease, endoproteinase Glu-C, and endoproteinase Lys-C and immunoblotting with MAb CAP1 were performed. MAb CAP1 recognized various proteolytically degraded fragments of CAP of different sizes. Among the immunoreactive fragments, the 16.2- and 18.7-kDa fragments were the most reactive and were the smallest fragments detected. They resulted from endoproteinase Glu-C digestion and endoproteinase Lys-C digestion, respectively (Fig. 2). After alkaline protease digestion, CAP was so thoroughly digested that it was impossible to obtain any fragment that could be applied to this study. The N-terminal amino acid sequences of the two fragments were QAVPVTLHNE for the 16.2-kDa fragment and LNVIVDTG for the 18.2-kDa fragment. The C-terminal amino acid sequence of the 16.2-kDa fragment was LGVGYKTNE, and that of the 18.7-kDa fragment was FGGVDNAK. On the basis of the results of amino acid sequence analyses, the 16.2-kDa fragment corresponded to Gln1 to Glu132 and the 18.7-kDa fragment corresponded to Leu27 to Lys178 (Fig. 3). This suggests that the potential epitope of MAb CAP1 is located in the overlapping sequence from Leu27 to Glu132. For the fine mapping of the epitope of MAb CAP1, the putative epitope region from Leu27 to Glu132 was divided into three overlapping regions, and each region was cloned and expressed. As a result, three GST-fusion proteins, namely, CAP-A, CAP-B, and CAP-C, whose molecular masses were approximately 34.5, 34.2, and 35.3 kDa, respectively, were obtained (Fig. 4). This coincided with the predicted molecular mass of each fusion protein. By immunoblot analysis, MAb CAP1 reacted to CAP-B and CAP-C but not to CAP-A (Fig. 5). This suggested that the epitope of MAb CAP1 is located in the overlapping region of CAP-B and CAP-C, which is the sequence of CAP from Asp77 to Gly103.
TABLE 1.
Effects of treatment of CAP with sodium m-periodate (oxidation) and proteinase K on the binding of MAb CAP1 as assayed by ELISA
| Periodate
oxidation
|
Proteinase K treatment
|
||
|---|---|---|---|
| Periodate concn (mM) | ELISA result (A490) | Proteinase K concn (μg/ml) | ELISA result (A490) |
| 0 | 2.325 | 0 | 2.325 |
| 0.039 | 2.309 | 0.039 | 2.362 |
| 0.078 | 2.322 | 0.078 | 2.299 |
| 0.156 | 2.410 | 0.156 | 2.299 |
| 0.313 | 2.461 | 0.313 | 2.286 |
| 0.625 | 2.363 | 0.625 | 2.229 |
| 1.250 | 2.397 | 1.250 | 2.080 |
| 2.500 | 2.362 | 2.500 | 1.428 |
| 5.000 | 2.332 | 5.000 | 0.247 |
| 10.000 | 2.351 | 10.000 | 0.029 |
FIG. 2.
Western blot analysis of limited proteolytically digested CAP fragments with MAb CAP1. CAP was partially digested with proteinases, resulting in the production of peptides that had molecular masses of 16.2 and 18.7 kDa and that were reactive with MAb CAP1. Lane 1, CAP control; lane 2, alkaline protease; lane 3, endoproteinase Glu-C; lane 4, endoproteinase Lys-C.
FIG. 4.
Analysis of fusion proteins expressed in E. coli XL1-Blue. Samples were electrophoresed in SDS-polyacrylamide gels and stained with Coomassie blue. Lane 1, cell lysate of E. coli XL1-Blue; lane 2, cell lysate of E. coli XL1-Blue/pGEX-GLi; lane 3, cell lysate of induced E. coli XL1-Blue/pGEX-GLi; lane 4, cell lysate of induced E. coli XL1-Blue/pGEX-GLiA; lane 5, cell lysate of induced E. coli XL1-Blue/pGEX-GLiB; lane 6, cell lysate of induced E. coli XL1-Blue/pGEX-GLiC.
FIG. 5.
Western blot analysis of expressed fusion proteins. (A) Western blot analysis with anti-GST antibody. (B) Western blot analysis with MAb CAP1. Lane 1, cell lysate of E. coli XL1-Blue; lane 2, cell lysate of E. coli XL1-Blue/pGEX-GLi; lane 3, cell lysate of induced E. coli XL1-Blue/pGEX-GLi; lane 4, cell lysate of induced E. coli XL1-Blue/pGEX-GLiA; lane 5, cell lysate of induced E. coli XL1-Blue/pGEX-GLiB; lane 6, cell lysate of induced E. coli XL1-Blue/pGEX-GLiC.
In conclusion, a mouse MAb with a high degree of specificity and sensitivity for the detection of CAP was produced in this study. Since the epitope recognized by the MAb was the proteinaceous part of CAP, this MAb might be useful for the further characterization of CAP. Furthermore, the MAb may be of value in the development of a more specific and sensitive test for the diagnosis of invasive candidiasis. Application of the MAb to the serodiagnosis of candidiasis is being investigated in our laboratory.
Acknowledgments
This work was supported by a grant from Chung-Ang University in 1997.
REFERENCES
- 1.Borg M, Watters D, Reich B, Rüchel R. Production and characterization of monoclonal antibodies against secretory proteinase of Candida albicans CBS 2730. Zentbl Bakteriol Parasitenkd Infektionskr Hyg Abt 1 Orig Reihe. 1988;268:62–73. doi: 10.1016/s0176-6724(88)80116-0. [DOI] [PubMed] [Google Scholar]
- 2.Borg-von Zepelin M, Grüess V. Characterization of two monoclonal antibodies against secretory proteinase of Candida tropicalis DSM 4238. J Med Vet Mycol. 1993;31:1–15. [PubMed] [Google Scholar]
- 3.Cleaveland D W, Fischer S G, Kirschner M W, Laemmli U K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977;252:1102–1106. [PubMed] [Google Scholar]
- 4.De Bernardis F, Girmenia C, Boccanera M, Adriani D, Martino P, Cassone A. Use of a monoclonal antibody in a dot immunobinding assay for detection of a circulating mannoprotein of Candida spp. in neutropenic patients with invasive candidiasis. J Clin Microbiol. 1993;31:3142–3146. doi: 10.1128/jcm.31.12.3142-3146.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferreira R P, Yu B, Niki Y, Armstrong D. Detection of Candida antigenemia in disseminated candidiasis by immunoblotting. J Clin Microbiol. 1990;28:1075–1078. doi: 10.1128/jcm.28.5.1075-1078.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fusek M, Smith E A, Mond M, Dunn B M, Foundling S I. Extracellular aspartic proteinases from Candida albicans, Candida tropicalis, and Candida parapsilosis yeasts differ substantially in their specificities. Biochemistry. 1994;33:9791–9799. doi: 10.1021/bi00198a051. [DOI] [PubMed] [Google Scholar]
- 7.Hopwood V, Poulain D, Fortier B, Evans G, Vernes A. A monoclonal antibody to a cell component of Candida albicans. Infect Immun. 1986;54:222–227. doi: 10.1128/iai.54.1.222-227.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horn R, Wong B, Kiehn T E, Armstrong D. Fungemia in a cancer hospital: changing frequency, earlier onset, and results of therapy. Rev Infect Dis. 1985;7:646–654. doi: 10.1093/clinids/7.5.646. [DOI] [PubMed] [Google Scholar]
- 9.Johnston J R. Procedures for isolating yeast DNA for different purposes. In: Johnston J R, editor. Molecular genetics of yeast: a practical approach. New York, N.Y: Oxford University Press Inc; 1994. pp. 1–16. [Google Scholar]
- 10.Kay J. Aspartic proteinases and their inhibitors. Biochem Soc Trans. 1985;13:1027–1029. doi: 10.1042/bst0131027. [DOI] [PubMed] [Google Scholar]
- 11.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature (London) 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 12.Komshian S V, Uwaydah A K, Sobel J D, Crane L R. Fungemia caused by Candida species and Torulopsis glabrata in the hospitalized patient. Rev Infect Dis. 1989;11:379–390. doi: 10.1093/clinids/11.3.379. [DOI] [PubMed] [Google Scholar]
- 13.Kwon-Chung K J, Bennett J E. Candidiasis. In: Kwon-Chung K J, Bennett J E, editors. Medical mycology. Philadelphia, Pa: Lea & Febiger; 1992. pp. 280–286. [Google Scholar]
- 14.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 16.MacDonald F, Odds F C. Inducible proteinases of Candida albicans in diagnostic serology and the pathogenesis of systemic candidosis. J Med Microbiol. 1980;13:423–435. doi: 10.1099/00222615-13-3-423. [DOI] [PubMed] [Google Scholar]
- 17.Markus W O, Calderone R A. A monoclonal antibody that defines a surface antigen on Candida albicans hyphae cross-reacts with yeast cell protoplasts. Infect Immun. 1990;58:625–631. doi: 10.1128/iai.58.3.625-631.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews R C. Early diagnosis of systemic candidal infection. J Antimicrob Chemother. 1993;31:809–812. doi: 10.1093/jac/31.6.809. [DOI] [PubMed] [Google Scholar]
- 19.Miyakawa Y, Kagaya K, Fukazawa Y, Soe G. Production and characterization of agglutinating monoclonal antibody against predominant antigenic factors for Candida albicans. J Clin Microbiol. 1986;23:881–886. doi: 10.1128/jcm.23.5.881-886.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Na B K, Lee S I, Kim S O, Park Y K, Bai G H, Kim S J, Song C Y. Purification and characterization of extracellular aspartic proteinase of Candida albicans. J Microbiol. 1997;35:109–116. [Google Scholar]
- 21.Nakamura A, Ishikawa N, Suzuki H. Diagnosis of invasive candidiasis by detection of mannan antigen by using the avidin-biotin enzyme immunoassay. J Clin Microbiol. 1991;29:2363–2367. doi: 10.1128/jcm.29.11.2363-2367.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ollert H W, Calderone R A. A monoclonal antibody that defined a surface antigen on Candida hyphae cross-reacts with yeast cell protoplasts. Infect Immun. 1990;58:625–631. doi: 10.1128/iai.58.3.625-631.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polonelli L, Morace G. Specific and common antigenic determinants of Candida albicans isolates detected by monoclonal antibody. J Clin Microbiol. 1986;23:366–368. doi: 10.1128/jcm.23.2.366-368.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ponton J, Leblond A M, Ezkurra P A, Barturen B, Robert R, Senet J M. Characterization of Candida albicans cell wall antigens with monoclonal antibodies. Infect Immun. 1993;61:4842–4847. doi: 10.1128/iai.61.11.4842-4847.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reboli A C. Diagnosis of invasive candidiasis by a dot immunobinding assay for Candida antigen detection. J Clin Microbiol. 1993;31:518–523. doi: 10.1128/jcm.31.3.518-523.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reichard U, Eiffert H, Rüchel R. Purification and characterization of an extracellular aspartic proteinase from Aspergillus fumigatus. J Med Vet Mycol. 1995;32:427–436. doi: 10.1080/02681219480000581. [DOI] [PubMed] [Google Scholar]
- 27.Rüchel R. Properties of a purified proteinase from the yeast Candida albicans. Biochim Biophys Acta. 1981;659:99–113. doi: 10.1016/0005-2744(81)90274-6. [DOI] [PubMed] [Google Scholar]
- 28.Rüchel R, Böning B. Detection of Candida proteinase by enzyme immunoassay and interaction of the enzyme with alpha-2-macroglobulin. J Immunol Methods. 1983;61:107–116. doi: 10.1016/0022-1759(83)90014-5. [DOI] [PubMed] [Google Scholar]
- 29.Rüchel R, Böning-Stutzer B, Mari A. A synoptical approach to the diagnosis of candidosis, relying on serological antigen and antibody tests, on culture, and on evaluation of clinical data. Mycoses. 1988;31:87–106. [PubMed] [Google Scholar]
- 30.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 31.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen H D, Choo K B, Yu K W, Ling W L, Chang F C, Han S H. Characterization of a monoclonal antibody (RJ5) against the immuno-dominant 41-kDa antigen of Candida albicans. Int Arch Allergy Appl Immunol. 1991;96:142–148. doi: 10.1159/000235485. [DOI] [PubMed] [Google Scholar]
- 33.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walsh T J, Hathorn J W, Sobel J D, Merz W G, Sanchez V, Maret S M, Buckley H R, Pfaller M A, Schaufele R, Silva C, Navarro E, Lecciones J, Chandrasekar P, Lee J, Pizzo P A. Detection of circulating Candida enolase by immunoassay in patients with cancer and invasive candidiasis. N Engl J Med. 1991;324:1026–1031. doi: 10.1056/NEJM199104113241504. [DOI] [PubMed] [Google Scholar]
- 35.Wey S B, Mori M, Pfaller M A, Woolson R F, Wenzel R P. Hospital-acquired candidemia: the attributable mortality and excess length of stay. Arch Intern Med. 1988;148:2642–2645. doi: 10.1001/archinte.148.12.2642. [DOI] [PubMed] [Google Scholar]
- 36.Woodward M P, Young W W, Jr, Bloodgood R A. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985;78:143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]
- 37.Wright R J, Carne A, Hieber A D, Lamont I L, Emerson G W, Sullivan P A. A second gene for a secreted aspartate proteinase in Candida albicans. J Bacteriol. 1992;174:7848–7853. doi: 10.1128/jb.174.23.7848-7853.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zoller L, Kramer I, Kappe R, Sonntag H G. Enzyme immunoassays for invasive Candida infections: reactivity of somatic antigens of Candida albicans. J Clin Microbiol. 1991;29:860–867. doi: 10.1128/jcm.29.9.1860-1867.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]