Abstract
Aim
The study aimed to assess the anxiety and stress levels on acceptance of dental treatment in child patients approaching dental extraction procedures before and after nitrous oxide (N2O) inhalation sedation (IHS) by measuring serum amyloid A (SAA) and salivary cortisol (SC).
Materials and methods
A total of 32 children, ages ranging from 6 to 10 years, were randomly grouped as TI (before N2O IHS) and TII (after N2O IHS). Saliva samples were taken for biochemical evaluation of SAA before and after the procedure. Subjectively anxiety and stress levels were evaluated using modified child dental anxiety scale (MCDAS). Wilcoxon rank-sum test was used to compare the means of dental anxiety, SAA, and SC before and after N2O IHS. The Karl Pearson correlation coefficient was employed to determine the correlation between dental anxiety and SAA and SC before and after N2O IHS.
Results
There were significant differences in the dental anxiety level in child patients after administration of N2O IHS, and it also showed an increased rate of acceptance of dental treatment.
Conclusion
This study showed that N2O is a safe and effective method in reducing dental anxiety and increasing acceptance of dental treatment in child patients with improved behavior and with no adverse effects.
Clinical significance
Anxiety and stress will always hinder the acceptance of dental treatment in child patients, especially during extraction procedures. N2O IHS is a safe and effective technique to overcome anxiety and stress in child patients and as well as allows them to undergo dental treatment with improved behavior.
How to cite this article
Kunta S, Arora RV, Jain R, et al. The Effect of Anxiety and Stress on Acceptance of Dental Procedure before and after Inhalation Sedation in Pediatric Patients: An In Vivo Study. Int J Clin Pediatr Dent 2023;16(2):302-307.
Keywords: Dental anxiety, Nitrous oxide inhalation sedation, Salivary α-amylase, Salivary cortisol
Introduction
Dental anxiety and fear are widespread problems in the population of different countries, forming a barrier to oral health care. A child with a fear of pain is a precursor for dental anxiety. Characteristics of anxiety and fear depending on the age of a child, cognitive ability, and developmental stage. Like in preschool children, the fear lies in separation either from parents or favorite things, whereas children who are older than 8 years of age show fear of physical injury.1 Anxiety is considered an uncertain, unpleasant feeling accompanied by the premonition that something undesirable will happen. Dental fear and anxiety in a child can result in avoidance of treatment causing inappropriate oral health care with significate dental problems and behavior problems leading to stressful experiences for the child, parent, and dentist.2
Subjective assessment of dental anxiety and fear became an important tool in Pediatric dentistry. Rating of behavior during dental visits can be classified into three categories behavioral, direct self-report, and physiological measures. Studies have shown that simple direct self-reporting scales and physiological measures are reliable parameters for evaluating anxiety and fear.3,4
Physiologically, anxiety and fear trigger stimulation of the sympathetic nervous system, and hypothalamic pituitary adrenal axis (HPA), which releases epinephrine and norepinephrine from adrenal centers causing secretion of salivary α-amylase whereas activation of the HPA axis causes the release of corticotrophin-releasing hormone (CRH) and arginine vasopressin (AVP) which stimulates the adrenal cortex to synthesize glucocorticoids called cortisol. For the past 5 decades, SC and SAA have been used as a biomarker for both stress and anxiety.5 Salivary biomarkers received special attention over serum sampling.
The advantages are:
Saliva sampling is noninvasive.
Stress-free.
Easy sample collection when compared with subjective parameters.
Values are reliable.
Various approaches have been outlined for anxiety and behavior management, like oral pharmacological agents, which can reduce anxiety, but it shows relatively unpredictable effects and responses to various drugs in each child patient.6 Of all approaches, N2O/O2 IHS is considered as safest aid with minimum risk causing termination of a vicious cycle of dental anxiety and fear.2 American Academy of Pediatric Dentistry (AAPD) recognized nitrogen dioxide inhalation as a risk-free and efficacious technique in reducing pain and anxiety by enhancing the communication of a child with a dentist.4 N2O IHS not only reduces anxiety but also enhances positive behavior toward dental treatment by improving the stressful environment, which helps a child to attain trust and confidence.7
Nitrous oxide (N2O) is a colorless gas with an indefinite sweet smell and imperfectly soluble within a high minimum alveolar concentration showing the speedy onset of occurrence and thereby accompanied by a speedy rate of recovery. It causes central nervous system depression and euphoria and has little effect on the respiratory system. Since N2O is an insoluble gas and can easily excrete from lungs with minimal alveolar concentration, that allows rapid onset of recovery, that is, 2–3 minutes. It also causes minimum impairment of reflexes with minor depression in cardiac output and a slight increase in peripheral resistance and thereby maintaining blood pressure.8 Recommended concentrations of N2O vary from 30 to 50% and even can exceed 70–80%. Most children experience both subjective and objective symptoms. The common subjective experiences reported are tingling or warm sensations and objectively may appear with their hands open, legs limp, and trance-like expressions.9
Studies have been done to measure anxiety and fear in child patients using self-reporting behavior scales and physiological measures individually, but the comparative efficacy of measuring dental anxiety and its acceptance of treatment conjointly using a self-report scale and biochemical parameters under N2O IHS has not yet been reported.
Thus, the current study intended to compare and evaluate the dental anxiety and acceptance of dental treatment in child patients aged 6–10 years by using noninvasive biochemical investigation using salivary α-amylase which is considered to be a biomarker for stress and direct self-reporting behavioral questionnaires using MCDAS before and after N2O IHS.
Materials and Methods
After obtaining ethical clearance and parent consent, this in vivo cross-sectional research was carried out on 32 children between the ages of 6 and 10 who had deciduous teeth indicated for dental extraction were chosen. Patients who were willing to participate and who did not have any systemic or local disorders that affected salivary secretions were included in the study. Patients with a common cold, tonsillar adenoidal enlargement, patients requiring nasal airways, with chronic obstructive pulmonary disease, psychiatric diseases, patients with complex cardiac conditions, and who were uncooperative or unwilling to participate were excluded. The selected sample was separated into two groups—TI (before N2O IHS) and TII (after N2O IHS).
Evaluation of Participants
Presedation check-up—detailed patient's medical history and clinical examination were performed, and children who were satisfied with American Society of Anesthesiologists (ASA) grade I and grade II were selected.10 Purpose of using N2O IHS and its benefits were explained prior to the treatment, and also demonstrated the technique to parents and patients.
Airway check-up—detailed airway check-up was done for each child patient prior to the procedure to examine for adenotonsillar hypertrophy or any other airway abnormalities caused by anatomical features.
Fasting instructions—each child patient was instructed to fast prior appointment following the 2-4-6 rule of fasting (i.e., no intake of clear fluids for 2 hours, no breast milk for 4 hours, and no solids for 6 hours).11
Collection of saliva—group TI—first, unstimulated whole mixed saliva was collected from each child patient when they were waiting at reception before administration of N2O IHS using the draining method. For collecting the saliva samples, the floor of the mouth was maintained parallel to the ground, and the mouth was kept open such that the saliva was dripped passively from the lower lip into the sterile tube.12
Group TII—the second unstimulated saliva samples were collected from the same child patients after 10–15 minutes post administration of N2O IHS. Collected saliva samples were retained undisturbed at room temperature and sent to the laboratory to estimate salivary α-amylase and SC levels.
Estimation of salivary α-amylase levels was calculated by using a commercially obtainable diagnostic gear, ”salivary α-amylase kinetic enzyme assay kit,” and the sample was analyzed according to the instructions provided by the manufacturer.
Estimation of SC levels by commercially obtainable diagnostic gear ”cortisol enzyme-linked immunosorbent assay kit ARG81392” were used to determine SC levels, and the samples were analyzed according to the manufacturer's instructions provided in the kit.
The modified child dental anxiety scale (MCDAS) questionnaires were given to child patients who were waiting at the reception area (group TI). The same MCDAS questionnaire was given to child patients after 10–15 minutes posttreatment under N2O IHS (group TII).
Results
Data were analyzed using Statistical Package for the Social Sciences version 21, IBM Inc. Descriptive data were reported for each variable. Descriptive statistics such as mean and standard deviation for continuous variables were calculated. Tests were performed to obtain the results.
The mean of dental anxiety among study subjects before N2O IHS was evaluated. Dental anxiety was found to be significantly lesser after N2O inhalation when compared using Wilcoxon's sum rank test as p < 0.05 with a standard deviation of 2.7779 before N2O IHS and 2.8504 after N2O IHS (Table 1).
Table 1.
Comparison of mean dental anxiety among study subjects before and after N2O IHS
| Mean | Standard deviation | Standard error mean | ||
|---|---|---|---|---|
| Dental anxiety | Before N2O IHS | 21.656 | 2.7779 | 0.4911 |
| After N2O IHS | 7.563 | 2.8504 | 0.5039 | |
| Difference | 14.09 | 3.25 | ||
| p-value | <0.0001* | |||
Wilcoxon sum-rank test; significant differences were seen in mean dental anxiety before and after N2O HIS with a standard deviation of 0.4911–0.5039. Dental anxiety was found to be significantly lesser after N2O inhalation when compared using the Wilcoxon sum-rank test/Mann–Whitney U test as p < 0.05
The mean of SAA (Table 2) and SC (Table 3) levels among study subjects were evaluated before N2O IHS. The results showed significantly reduced levels of SAA and SC levels after N2O inhalation when compared using Wilcoxon's sum rank test as p < 0.05 (Figs 1 and 2).
Table 2.
Comparison of mean salivary amylase levels among study subjects before and after N2O IHS
| Mean | Standard deviation | Standard error mean | ||
|---|---|---|---|---|
| Salivary Amylase levels | Before N2O IHS | 13.438 | 3.7238 | 0.6583 |
| After N2O IHS | 8.281 | 5.1508 | 0.9106 | |
| Difference | 5.1 | 5.2 | ||
| p-value | <0.0001* | |||
Wilcoxon sum-rank test; salivary amylase level significantly reduced after N2O inhalation when compared using the Wilcoxon sum-rank test as p < 0.05
Table 3.
Comparison of mean cortisol levels among study subjects before and after N2O IHS
| Mean | Standard deviation | Standard error mean | ||
|---|---|---|---|---|
| Cortisol | Before N2O IHS | 2.0525 | 1.34953 | 1.06179 |
| After N2O IHS | 0.84744 | 0.427305 | 0.075537 | |
| Difference | 1.21 | 0.862 | ||
| p-value | <0.0001* | |||
Wilcoxon sum-rank test; significant differences were seen in cortisol levels before and after N2O inhalation when compared using the Wilcoxon sum-rank test as p < 0.05
Fig. 1.

Dental anxiety
Fig. 2.

Nitrous oxide (N2O) sedation
A significant positive correlation was seen in dental anxiety, SSA, and SC levels before and after N2O inhalation when compared using the Karl Pearson correlation coefficient (Tables 4 and 5).
Table 4.
Correlation among dental anxiety, salivary amylase, and cortisol before N2O inhalation
| Amylase | Cortisol | ||
|---|---|---|---|
| Dental anxiety before N2O IHS | Pearson correlation | 0.361 | 0.236 |
| p-value | 0.042 | 0.058 | |
| N | 32 | 32 | |
Karl Pearson correlation; a significant positive correlation was seen in dental anxiety levels and salivary amylase and SC levels before N2O inhalation when compared using the Karl Pearson correlation coefficient
Table 5.
Correlation among dental anxiety, salivary amylase, and cortisol after N2O inhalation
| Amylase | Cortisol | ||
|---|---|---|---|
| Dental anxiety after N2O IHS | Pearson correlation | 0.169 | 0.168 |
| p-value | 0.354 | 0.357 | |
Karl Pearson correlation; no significant correlation was seen in dental anxiety levels and salivary amylase levels and cortisol after N2O inhalation when compared using the Karl Pearson correlation coefficient
Discussion
Dental anxiety is a generalized, unpleasant emotion triggered by objective situations like the sight of needles, the sound of a high-speed drill, etc.13 Even a cooperative child gets scared of specific procedures, which results in negative outcomes where parents consistently fail to take their child to a dentist, which leads to avoidance of treatment.14–16 There are many ways to manage dental anxiety in children, for instance, the application of N2O IHS, anxiety-related medications, and also the use of general anesthesia.
In accordance with the literature, N2O IHS helps in obtaining anxiolytic and analgesic effects to accomplish dental procedures in younger age groups.17,18 Studies emphasize that N2O IHS is the most efficient and safest aid in managing child patients, even in long dental procedures, with increased acceptance by patients and parents.2,19–21 In the present study, we used N2O IHS to manage dental anxiety in children.
Initial visits to the dentist and waiting in the reception area, followed by situations like dental operatory procedures, mostly extractions, provoke anxiety and stress in children.22 According to behavior guidance of pediatric dental patients by AAPD (2020), the use of N2O IHS is indicated for anxious, fearful, special healthcare child needs, children with gag reflexes, and also for patients who are undergoing lengthy dental procedures.6 Guidelines proposed by American Dental Association in selecting child patients for sedation must belong to ASA I and ASA II according to ASA patient physical status classification.23 In this present study, we selected child patients visiting the Department of Pediatric and Preventive Dentistry for the first time, ages ranging from 6 to 10 years, and were classified as ASA I and ASA II.
The N2O IHS delivery system is always a straight forward where N2O/O2 is given in stepwise concentration.24,25 In this present study, Monitored dial mixer Quantiflex machine with a color-coded cylinder system was used. This machine automatically adjusts the flow rate of N2O and oxygen (O2) gases. The efficacy of N2O IHS can be variable and sometimes may lead to a failure due to the factors such as the equipment discharge of gases due to imperfectly fitting nasal hood, dead space, mouth breathing, and ventilator status of the patients.26 In our study, we used a comfortable and suitable nasal hood mask size (small) and did not observe any discrepancies. According to the studies, the minimum and maximum concentration of N2O concentrations ranges from 40 to 70%. Studies show that above 50% concentration of N2O can cause nausea and vomiting.5,8 We administered N2O at 40–50% concentrations to achieve anxiolysis and analgesic effects.
According to AAPD guidelines (2020), the use of a pulse oximeter is recommended throughout the procedure to measure physiological parameters.21 In the present study, we used a pulse oximeter throughout the procedure and observed no adverse changes in physiological parameters.
Only 10% of studies show that individuals experience diffuse hypoxia post N2O IHS.27 To overcome this effect, we administered 100% O2 for 5–10 minutes posttreatment and did not see any adverse effects after treatment under N2O IHS. This is in accordance with the studies.2,8 Many studies have demonstrated that the use of N2O IHS showed a 90% of success rate in treating dental anxiety and allowing child patients to accept treatment with improved behavior by responding to verbal commands. In our study, every child patient obtained a 100% analgesic effect after administration of N2O IHS and allowed the dentist to perform dental procedures without any disruptions.
Various approaches have been introduced to diagnose dental anxiety and fear levels in children. Studies showed that out of many scales of behavior measures, the self-reporting behavior scale using facial images is simple and straightforward with numerous advantages for a dental team, service provider, and dentist to assess the subjective form of dental anxiety, which can be completed by the child himself.
According to the studies,28,29 MCDAS with facial images is the most definitive and trouble-free method to assess dental anxiety in children aged from 5 to 15 years. The present study used an MCDAS scale with eight questions and five faces ranging from very happy to very sad with scores of 1–5. For the sake of convenience, we have demonstrated the MCDAS questionnaire in Hindi to parents and patients.
It was observed that before N2O IHS, there were increased levels of dental anxiety and a low acceptance rate towards dental treatment in group TI (before N2O IHS) when compared with group TII (after N2O IHS).
The highest levels of dental anxiety were seen in patients approaching extraction procedures rather than undergoing regular dental check-ups, prophylaxis, or restorations; this was confined to the studies.30 Thus, in our study, we considered patients approaching the dental extraction procedure.
Most of the children are afraid to explore new things due to a lack of understanding; likewise, they are afraid of going to the dentist for less obvious reasons.1,2 Fear and anxiety put the human body to experience physical and emotional changes. This triggers stress and thereby stimulates the sympathetic nervous system and HPA axis, which releases epinephrine and norepinephrine from adrenal centers while CRH, AVP, from the adrenal cortex. It helps in the secretion of salivary biomarkers like SAA and glucocorticoids called SC.3
Salivary α-amylase is a digestive enzyme found in saliva that breaks down starch and glycogen into glucose and maltose. It is synthesized in parotid glands and accounts for 40–50% of total salivary proteins. Stimulation of the sympathetic nervous system causes a decreased flow of saliva containing amylase in high concentration.
The study used SAA as one of the biomarkers to evaluate dental anxiety in children. Saliva samples were collected twice, that is, first, when the patient was waiting at the reception area and second 10–15 minutes posttreatment. It was observed that there was an increase in levels of SAA in group TI (before N2O IHS) when compared to group TII (after N2O IHS). The reason for decreased levels of salivary α-amylase in group TII could be attributed to a reduction in dental anxiety.
Salivary α-amylase levels were used as an objective tool in measuring anxiety. A previous study had advocated the use of SAA in relation to pain and collected saliva samples three times, that is, during the initial examination, immediately before dental treatment, and 15 minutes after the end of the treatment. And concluded that increased levels of SAA were seen even after the end of the treatment. This may be due to the presence of persistent pain stimulus after dental treatment.31–33 Thus, in the present study, we used N2O IHS during dental extraction treatment and correlated anxiety levels with salivary α-amylase.
Cortisol is released from the stimulation of the HPA system. Cortisol acts upon several physiological processes showing effects on moods and behavior. There are several methods to evaluate cortisol levels; they are a 24-hour urine collection, plasma/serum, saliva, and cerebrospinal fluid. Cortisol levels in serum show binding to ”cortisol-binding globulin,” leaving 10% of cortisol in the free state and biologically inactive, whereas cortisol entering into saliva by intracellular mechanism shows completely in free bioactive (unbound proteins) state.
Salivary cortisol (SC) is another reliable stress biomarker in determining anxiety levels. In our study, we considered morning SC as the second biomarker to determine anxiety levels. This is attributed to the reason that the concentration of free cortisol levels in saliva is higher in the morning time than compared to those in the evening.32,33
The current study found that there was an increase in levels of SC in group TI (before N2O IHS) when compared to group TII (after N2O IHS). This change in SC levels could be attributed to an increase in dental anxiety at the sight of local anesthetic injections and the sounds of the dental operator. Henceforth to reduce the anxiety, the study used N2O IHS during dental extraction treatment and correlated dental anxiety with SC levels and observed a significant reduction in levels of SC after N2O IHS.
The results of our study observed that there were no significant differences between age and sex distribution with the levels of SAA and SC in relation to dental anxiety (Figs 3 and 4). This might be attributed to unequal sample size distribution where the number of boys who participated was more as compared to girls. Also, sex distribution was unequal because of the different age groups. This is in accordance with earlier studies.22
Fig. 3.
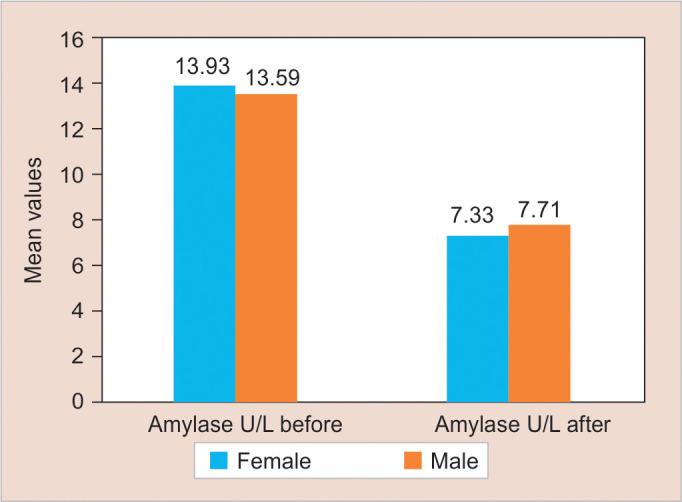
Salivary α-amylase
Fig. 4.

salivary cortisol (SC)
Our study findings were correlated with the study confined to Sinem Yıldırım, where they compared pre and postdental anxiety levels with the levels of SAA, SC, and chromogranin A before and after general anesthesia (GA) by showing decreased levels of dental anxiety in posttreatment.34 However, the use of GA has many drawbacks, such as it requires laboratory investigations, specially trained personnel, and requires pre and postoperative care. Moreover, GA cannot be used for simple dental procedures like tooth restorations and single-tooth extractions. To overcome this drawback, N2O IHS was opted for in our study.
Thus, this present study was done to compare the efficacy of measuring dental anxiety and acceptance of treatment by conjointly using self-reporting scale MCDAS questionnaires and biochemical parameters, that is, salivary α-amylase and SC under N2O IHS. It was observed that there was a greater change in dental anxiety and acceptance of treatment before and after N2O IHS with improved behavior in children.
Conclusion
Based on the data obtained, the final conclusion can be drawn as:
The N2O IHS helps reduce anxiety in a child patient and produces an analgesic effect which enhances a child patient to accept the dental treatment with improved behavior.
Salivary α-amylase and SC can be used as reliable salivary biomarkers to evaluate dental anxiety. However, further studies are needed with a larger sample size to evaluate the reliability and validity of salivary biomarkers.
Modified child dental anxiety scale with facial images can help the child and dental team to evaluate anxiety levels and acceptance towards the dental treatment.
Clinical Significance
Using N2O IHS is a safe and straightforward method of reducing anxiety and stress in child patients approaching dental treatment. An assessment of salivary α-amylase and SC can be easy, noninvasive, and reliable salivary biomarkers for measuring anxiety in the dental operatory.
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Adolphs R. The biology of fear. Curr Biol. 2013;23(2):R79–R93. doi: 10.1016/j.cub.2012.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hallonsten AL, Koch G, Schröder U. Nitrous oxide-oxygen sedation in dental care. Community Dent Oral Epidemiol. 1983;11(6):347–355. doi: 10.1111/j.1600-0528.1983.tb01390.x. [DOI] [PubMed] [Google Scholar]
- 3.Manepalli S, Nuvvula S, Kamatham R, et al. Comparative efficacy of a self-report scale and physiological measures in dental anxiety of children. J Investig Clin Dent. 2014;5(4):301–306. doi: 10.1111/jicd.12046. [DOI] [PubMed] [Google Scholar]
- 4.Swetah CSV, Ramakrishnan M. Dental anxiety among children regarding different dental treatment-modified child dental anxiety scale (MCDAS)—a cross sectional study. Indian J Public Health Res Dev. 2019;10(11):3668. doi: 10.5958/0976-5506.2019.04159.7. [DOI] [Google Scholar]
- 5.Polat C, Düzer S, Ayyıldız H, et al. Association between anxiety, depression, and salivary cortisol levels in patients with recurrent aphthous stomatitis. Turk Arch Otorhinolaryngol. 2018;56(3):166, 169. doi: 10.5152/tao.2018.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.https://www.aapd.org/globalassets/media/policies_guidelines/bp_useofnitrous.pdf https://www.aapd.org/globalassets/media/policies_guidelines/bp_useofnitrous.pdf
- 7.Kupietzky A, Emmanouil D. Nitrous Oxide/Oxygen Inhalation Sedation in Children. Wright's Behavior Management in Dentistry for Children. 2021 Sep 10;:189–203. [Google Scholar]
- 8.Paterson SA, Tahmassebi JF. Paediatric dentistry in the new millennium: 3. Use ofinhalation sedation in paediatric dentistry. Dent Update. 2003;30(7):350–358. doi: 10.12968/denu.2003.30.7.350. [DOI] [PubMed] [Google Scholar]
- 9.Houpt MI, Limb R, Livingston RL. Clinical effects of nitrous oxide conscious sedation in children. Pediatr Dent. 2004;26(1):29–36. [PubMed] [Google Scholar]
- 10.Harbuz DK, O’Halloran M. Techniques to administer oral, inhalational, and IV sedation in dentistry. Australas Med J. 2016;9(2):25–32. doi: 10.4066/AMJ.2015.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapur A, Kapur V. Conscious sedation in dentistry. Ann Maxillofac Surg. 2018;8(2):320–323. doi: 10.4103/ams.ams_191_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Priya KY, Prathibha KM. Methods of collection of saliva-a review. Int J Oral Health Dent. 2017;3(3):149–153. doi: 10.18231/2395-499X.2017.0032. [DOI] [Google Scholar]
- 13.Viswanath D, Kumar M, Prabhuji MLV. Dental anxiety, fear and phobia in children. Int J Dent Res Dev. 2014;4(1):1–14. [Google Scholar]
- 14.Newton JT, Buck DJ. Anxiety and pain measures in dentistry: a guide to their quality and application. J Am Dent Assoc. 2000;131(10):1449–1457. doi: 10.14219/jada.archive.2000.0056. [DOI] [PubMed] [Google Scholar]
- 15.Taani DQ, El, -, Qaderi SS, Abu Alhaija ES. Dental anxiety in children and its relationship to dental caries and gingival condition. Int J Dent Hyg. 2005;3(2):83–87. doi: 10.1111/j.1601-5037.2005.00127.x. [DOI] [PubMed] [Google Scholar]
- 16.Klein U, Manangkil R, DeWitt P. Parents’ ability to assess dental fear in their six-to 10-year-old children. Pediatr Dent. 2015;37(5):436–441. [PubMed] [Google Scholar]
- 17.Alexopoulos E, Hope A, Clark SL, et al. A report on dental anxiety levels in children undergoing nitrous oxide inhalation sedation and propofol target controlled infusion intravenous sedation. Eur Arch Paediatr Dent. 2007;8(2):82–86. [PubMed] [Google Scholar]
- 18.Prud'homme T, Allio A, Dajean-Trutaud S, et al. Assessment of an equimolar mixture of oxygen and nitrous oxide: effects in pediatric dentistry. Int J Clin Pediatr Dent. 2019;12(5):429–436. doi: 10.5005/jp-journals-10005-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arch LM, Humphris GM, Lee GT. Children choosing between general anaesthesia orinhalation sedation for dental extractions: the effect on dental anxiety. Int J Paediatr Dent. 2001;11(1):41–48. doi: 10.1046/j.1365-263x.2001.00238.x. [DOI] [PubMed] [Google Scholar]
- 20.Veerkamp JS, van Amerongen WE, Hoogstraten J, et al. Dental treatment of fearful children, using nitrous oxide. Part I: treatment times. ASDC J Dent Child. 1991;58(6):453–457. [PubMed] [Google Scholar]
- 21.Kashyap V, Suneja B, Kaur J, et al. Comparison of patient and parental preference between nitrous oxide sedation and conventional behavior management technique for dental treatment: a randomized crossover study. Int J Appl Dent Sci. 2021;7:398–401. doi: 10.22271/oral.2021.v7.i1f.1159. [DOI] [Google Scholar]
- 22.Rayen R, Muthu MS, Sivakumar N, et al. Evaluation of physiological and behavioral measures in relation to dental anxiety during sequential dental visits in children. Indian J Dent Res. 2006;17(1):27–34. doi: 10.4103/0970-9290.29895. [DOI] [PubMed] [Google Scholar]
- 23.Emmanuel BJ, Raja J, Packiaraj I, et al. Nitrous oxide and special healthcare children in dentistry: a review article. J Adv Clin Res Insights. 2021;8:3–5. doi: 10.15713/ins.jcri.319. [DOI] [Google Scholar]
- 24.Holroyd I, Roberts GJ. Inhalation sedation with nitrous oxide: a review. Dent Update. 2000;27(3):141–146. doi: 10.12968/denu.2000.27.3.141. [DOI] [PubMed] [Google Scholar]
- 25.Mohan R, Asir VD, Dakir A, et al. Nitrousoxide as a conscious sedative in minor oral surgical procedure. J Pharm Bioallied Sci. 2015;7(Suppl 1):S248–S250. doi: 10.4103/0975-7406.155939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becker DE, Rosenberg M. Nitrous oxide and the inhalation anesthetics. Anesth Prog. 2008;55(4):124–130. doi: 10.2344/0003-3006-55.4.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samir PV, Namineni S, Sarada P. Assessment of hypoxia, sedation level, and adverse events occurring duringinhalation sedation using preadjusted mix of 30% nitrous oxide+ 70% oxygen. J Indian Soc Pedod Prev Dent. 2017;35(4):338–345. doi: 10.4103/JISPPD.JISPPD_15_17. [DOI] [PubMed] [Google Scholar]
- 28.Javadinejad S, Farajzadegan Z, Madahain M. Iranian version of a face version of the modified child dental anxiety scale: transcultural adaptation and reliability analysis. J Res Med Sci. 2011;16(7):872–877. [PMC free article] [PubMed] [Google Scholar]
- 29.Madouh M, Tahmassebi JF. Utilising a paediatric version of the indicator of sedation need for children's dental care: a pilot study. Eur Arch Paediatr Dent. 2016;17(4):265–270. doi: 10.1007/s40368-016-0238-8. [DOI] [PubMed] [Google Scholar]
- 30.Padmanabhan V, Rai K, Hegde A. Salivary cortisol changes in children during dental extractions. J Evol Med Dent Sci. 2014;3(4):811–814. doi: 10.14260/jemds/2014/1907. [DOI] [Google Scholar]
- 31.Rashkova MR, Ribagin LS, Toneva NG. Correlation between salivary [alpha]-amylase and stress-related anxiety. Folia Medic (Plovdiv) 2012;54(2):46–51. doi: 10.2478/v10153-011-0088-4. [DOI] [PubMed] [Google Scholar]
- 32.Vreeburg SA, Zitman FG, van Pelt J, et al. Salivary cortisol levels in persons with and without different anxiety disorders. Psychosom Med. 2010;72(4):340–347. doi: 10.1097/PSY.0b013e3181d2f0c8. [DOI] [PubMed] [Google Scholar]
- 33.Chaturvedi Y, Chaturvedy S, Marwah N, et al. Salivary cortisol and alpha-amylase—biomarkers of stress in children undergoing extraction: an in vivo study. Int J Clin Pediatr Dent. 2018;11(3):214–218. doi: 10.5005/jp-journals-10005-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yıldırım S, Bakkal M, Bulut H, et al. Quantitative evaluation of dental anxiety indicators in the serum and saliva samples of children treated under general anesthesia. Clin Oral Investig. 2018;22(6):2373–2380. doi: 10.1007/s00784-018-2340-2. [DOI] [PubMed] [Google Scholar]


