Abstract
Matrix metalloproteinases (MMPs) are a host cell-derived proteolytic enzyme family which plays a major role in tissue-destructive inflammatory diseases such as periodontitis. The aim of the present study was to evaluate the inhibitory effect of chlorhexidine (CHX) on MMP-2 (gelatinase A), MMP-9 (gelatinase B), and MMP-8 (collagenase 2) activity. Heat-denatured type I collagen (gelatin) was incubated with pure human MMP-2 or -9 activated with p-aminophenylmercuric acetate (APMA), and the proteolytic degradation of gelatin was monitored by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie blue staining. The effect of CHX on MMP-8 activity was also studied with a cellular model addressing the ability of phorbol myristate acetate (PMA)-triggered human peripheral blood neutrophils (polymorphonuclear leukocytes [PMNs]) to degrade native type I collagen. CHX inhibited the activities of both gelatinases (A and B), but MMP-2 appeared to be more sensitive than MMP-9. Adding calcium chloride to the assay mixtures almost completely prevented the inhibition of MMP-9 activity by CHX, while the inhibition of MMP-2 activity could be reversed only when CHX was used at a low concentration. This observation suggests that CHX may act via a cation-chelating mechanism. CHX dose-dependently inhibited collagenolytic activity of MMP-8 released by PMA-triggered PMNs. MMP-8 without APMA activation was inhibited clearly more efficiently than APMA-activated MMP-8. Our study suggests that the direct inhibition of the MMPs’ activities by CHX may represent a new valuable effect of this antimicrobial agent and explains, at least in part, the beneficial effects of CHX in the treatment of periodontitis.
Periodontal diseases are inflammatory conditions which gradually lead to impairment and destruction of the tooth’s supportive tissues. The extracellular matrix components, including collagens, fibronectin, and proteoglycans, are the major tissue proteins responsible for the structural integrity of the tooth’s anchoring apparatus. Destruction of the supportive apparatus is characterized by a degradation of the extracellular matrix components, leading to irreversible loss of periodontal soft connective tissues and alveolar bone. Bacterial pathogens and their products are the primary etiologic agents that directly initiate periodontal disease (11). However, it is the host inflammatory and immune response, triggered by these pathogens and their virulence factors, that is mainly responsible for the tissue destruction observed during periodontitis (18). There is an increasing body of evidence that suggests that a family of host-derived proteolytic enzymes called matrix metalloproteinases (MMPs) plays a major role in this pathological process (3, 19, 23). Hence, MMP inhibition would be a valuable adjunct to any periodontal treatment. A wide variety of products are presently being investigated as MMP inhibitors (1). For instance, tetracyclines and their chemically modified derivatives have been shown to inhibit the activity of several MMPs (6–8). Interestingly, it has been demonstrated that low-dose doxycycline regimens inhibit pathologically elevated collagenase activity in subgingival sites and prevent the progression of periodontitis (5). Also, certain bisphosphonates can inhibit the activity of several human MMPs (28, 29).
Chlorhexidine (CHX) is a widely used antimicrobial agent that possesses a broad spectrum of activity against oral bacteria (27). This cationic bis-biguanide has a unique effect of dental plaque inhibition, mainly due to its substantivity and its antimicrobial property. It has been demonstrated that CHX mouth rinses are an efficient adjunct to periodontal therapy by controlling supragingival plaque and gingival inflammation (20). Other studies have confirmed that subgingival irrigation with CHX reduces periodontal inflammation, sulcate bleeding, periodontal pocket depth, and subgingival plaque (16, 34). Consequently, CHX mouth rinses are widely used in the prophylaxis and treatment of periodontal diseases.
Although numerous beneficial in vivo and in vitro effects of CHX have been reported, the mechanism of action of this compound continues to be the subject of numerous investigations as it has not been completely elucidated. Among its interesting properties, CHX has shown an antioxidative capacity (4) as well as an ability to significantly reduce the adherence of Porphyromonas gingivalis (9) and the proteolytic activity of a number of periodontal pathogens (2, 10). However, no data concerning the effect of CHX on host proteases present in affected periodontal sites are currently available. The aim of the present study was thus to evaluate the inhibitory effect of therapeutically attainable concentrations of CHX on the activities of MMP-2, -8, and -9, as a new beneficial effect in the treatment of periodontitis. These three MMPs were selected because they have been suggested to participate in tissue destruction during periodontitis (14, 15, 21, 24, 25).
Materials and methods. (i) Inhibition assay for MMP-2 (gelatinase A) and MMP-9 (gelatinase B) activity.
Two genetically distinct pure human gelatinases were used: MMP-2 (72-kDa gelatinase A isolated from human fibrosarcoma cells and purchased from Boehringer Mannheim, Montréal, Québec, Canada) and MMP-9 (human recombinant 92-kDa gelatinase B isolated from mammalian cells and purchased from Oncogene Research Products, Cambridge, Mass.). MMP-2 was used at a concentration of 15 U/ml in 50 mM NaCl–20 mM Tris-HCl, pH 8.3, whereas the MMP-9 was used at a concentration of 20 μg/ml in 5 mM Tris-HCl, pH 7.5, containing 0.1 mM CaCl2, 0.005% Brij 35, and 10% glycerol. Both gelatinases were activated by incubation at 37°C for 30 min in 0.5 mM p-aminophenylmercuric acetate (APMA) in a buffer containing 50 mM Tris-HCl, pH 7.5, and 50 mM NaCl (and 0.005% Triton X-100 for MMP-2). The effects of CHX on MMP-2 and -9 activity were tested as follows. Activated MMP-2 (3 μl) or -9 (5 μl) was incubated with 10 μl of the CHX solution and 5 μl of 50 mM Tris-HCl, pH 7.5, for 30 min at room temperature. Final concentrations of CHX diacetate salt (Sigma Chemical Co., St. Louis, Mo.) were 0.03, 0.015, 0.008, 0.004, 0.002, and 0.0001%, prepared in distilled water. Thereafter, 10 μl of gelatin at 0.5 mg/ml was added, and the assay mixtures were further incubated at 37°C for 18 h. Gelatin was prepared by treating type I collagen at 60°C for 30 min. The final pH of the assay mixtures was 7.5. EDTA at 10 mM was used as a control for inhibition of MMP-2 and -9 activity. Following incubation, the assay mixtures were heated at 60°C for 30 min in the presence of electrophoresis sample buffer and subjected to sodium dodecyl sulfate–7.5% polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were stained with Coomassie brilliant blue R-250. The inhibitory effect of CHX was also tested in the presence of 0.75 mM CaCl2 in the assay mixtures.
(ii) Inhibition assay for MMP-8 (collagenase 2) activity released by triggered human neutrophils.
Human neutrophils were used as a source of MMP-8. Human neutrophils (polymorphonuclear leukocytes [PMNs]) from peripheral blood were prepared according to the method of Konttinen et al. (17), adjusted to 1.5 × 107/ml in Hanks balanced salt solution, and treated for 30 min at 37°C with 50 ng of phorbol myristate acetate per ml. The cells were pelleted, and the supernatant containing MMP-8 activity was treated or not with either 0.005, 0.01, or 0.02% CHX and incubated with 1.5 μM native type I collagen at 3 mg/ml in 50 mM Tris-HCl (pH 7.4)–0.2 M NaCl–0.5 mM CaCl2 for 12 h at 22°C. Collagen cleavage products were analyzed by SDS–10% PAGE and stained by Coomassie brilliant blue R-250. These experiments were performed in the absence and presence of 1 mM APMA to activate latent MMP-8 activity.
Results and discussion.
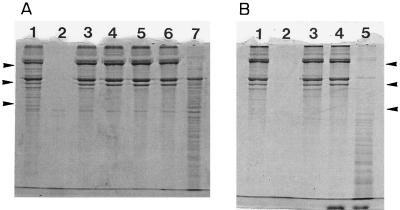
Under the assay conditions used, pure human MMP-2 and -9 completely degraded the gelatin, whereas no degradation occurred when the chelating agent EDTA was included during the incubation. At a concentration of 0.03%, CHX produced a complete inhibition of MMP-2 and -9 gelatinase activities (Fig. 1, lanes 4). The inhibitory effect of CHX was concentration dependent. The minimal concentration of CHX that led to a complete inhibition of MMP-9 activity was 0.002%, whereas MMP-2 activity was much more sensitive, being inhibited at a CHX concentration as low as 0.0001% (data not shown). Calcium chloride was added to the assay mixtures to verify whether a calcium-chelating mechanism might be involved in the inhibitory properties of CHX. Adding calcium chloride to the assay mixtures that contained 0.03% CHX almost completely prevented the inhibition of MMP-9 activity (Fig. 1B, lane 5) but had no effect on the inhibition of MMP-2 activity (Fig. 1A, lane 6). However, decreasing the concentration of CHX to 0.004% allowed partial reversibility of the inhibition of MMP-2 activity by addition of calcium chloride (Fig. 1A, lane 7).
FIG. 1.
Effect of CHX on MMP-2 and -9 activity. MMPs activated with APMA, CHX, and gelatin were incubated for 18 h at 37°C. Gelatin degradation was monitored by SDS–7.5% PAGE and Coomassie blue staining. (A) Lanes: 1, gelatin alone; 2, MMP-2 and gelatin; 3, MMP-2, gelatin, and 10 mM EDTA; 4, MMP-2, gelatin, and 0.03% CHX; 5, MMP-2, gelatin, and 0.004% CHX; 6, MMP-2, gelatin, 0.03% CHX, and 0.75 mM calcium chloride; 7, MMP-2, gelatin, 0.004% CHX, and 0.75 mM calcium chloride. (B) Lanes: 1, gelatin alone; 2, MMP-9 and gelatin; 3, MMP-9, gelatin, and 10 mM EDTA; 4, MMP-9, gelatin, and 0.03% CHX; 5, MMP-9, gelatin, 0.03% CHX, and 0.75 mM calcium chloride. Molecular mass markers were, from top to bottom, myosin (200 kDa), phosphorylase b (97.4 kDa), and bovine serum albumin (68 kDa).
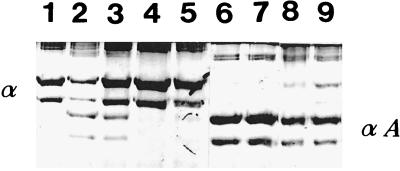
The activity of MMP-8 released by phorbol myristate acetate-triggered human neutrophils and without the APMA activation treatment was completely inhibited by CHX at concentrations of 0.02 and 0.01% (Fig. 2). The MMP-8 collagenase activity present in the PMN supernatant and activated by APMA was only partially inhibited by the same concentrations of CHX.
FIG. 2.
Effect of CHX on autoactivation of MMP-8 activity. Human neutrophil supernatant, CHX, and collagen were incubated for 12 h at 22°C. The assays were carried out in the absence or presence of 1 mM APMA to activate the MMP-8 (collagenase 2) activity. Collagen degradation was monitored by SDS–10% PAGE and Coomassie blue staining. α, intact α-chains of type I collagen; αA, characteristic three to four cleavage products resulting from collagenase action. Lanes: 1, type I control collagen; 2, type I collagen and neutrophil supernatant; 3, type I collagen, neutrophil supernatant, and 0.005% CHX; 4, type I collagen, neutrophil supernatant, and 0.01% CHX; 5, type I collagen, neutrophil supernatant, and 0.02% CHX; 6 to 9, same as lanes 2 to 5, respectively, except that 1 mM APMA was present.
Considerable evidence supports the importance of MMPs as key host cell-derived mediators of the pathological tissue destruction observed during periodontitis (3, 19, 23). More particularly, MMP-2, -8 and -9 in active forms have been found at pathologically elevated levels in saliva, gingival crevicular fluid, and gingival tissues of periodontitis patients (7, 14, 15, 21, 25). The potential cellular sources of MMP-2 and -9 include resident gingival and periodontal ligament fibroblasts, as well as endothelial and epithelial cells. In addition, MMP-9 in periodontal inflammation can originate from triggered neutrophils, which are also major sources of MMP-8. Furthermore, recent studies have shown that certain non-PMN lineage cells, such as gingival and periodontal ligament fibroblasts and endothelial cells, can express MMP-8 (12, 25). Overall, multiple resident and inflammatory host cellular sources may be the origin of the MMPs studied for the inhibitory effect of CHX. Based on these observations, MMP inhibitors are being investigated as a new class of potential therapeutic agents in the treatment of periodontitis. As a matter of fact, inhibition of MMP activity by tetracyclines and their chemically modified derivatives has shown promising results (5, 7, 25).
The present study has shown that CHX directly inhibits MMP-2, -8, and -9 activity. MMP-2 appeared to be more sensitive than MMP-8 and -9. A chelating mechanism might be involved in the inhibition of MMP-2 and -9, since adding calcium chloride to the assay mixture prevented the inhibition of these enzymes by CHX. However, the effect of an excess of calcium chloride was not identical for MMP-2 and -9; the inhibition of MMP-2 activity was reversed only when CHX was used at a low concentration (0.004%). It is likely that at high concentrations of CHX, MMP-2 is inactivated by protein denaturation (13) rather than by the chelation of cations. It has been previously proposed that tetracyclines inhibit MMP activity by their cation-binding properties (6), since MMPs require metal ions, namely, calcium and zinc, for their catalytic activity. Dichloromethylene biphosphonate (clodronate), which has been shown to inhibit MMP-1 and -8, is also thought to act as a cation chelator (28, 29).
Additionally, it was found that CHX (0.01 and 0.02%) completely inhibited autoactivated MMP-8, in comparison to a poor inhibition of APMA-activated MMP-8, released by triggered human PMNs. APMA, an organomercurial MMP activator capable of attacking protein sulfhydryl groups or inducing cysteine switch reactions involved in both the activation and inhibition of MMP-8 (30–32), was found to prevent the CHX inhibition of MMP-8. This suggests that CHX may interact with the essential sulfhydryl groups and/or cysteine present in the active site of MMPs. Regarding the molecular pathogenesis of periodontitis, it is worthy of note that CHX efficiently inhibited impure endogenously activated MMP-8 in the presence of other biologically active molecules and inflammatory mediators released by triggered PMNs (33), since it has been frequently shown that in active periodontitis lesions, i.e., gingival tissues and adjacent crevicular fluid (19, 22), MMP-8 is converted from a latent form to an active form (25).
CHX is a valuable agent for the treatment of periodontal diseases and can be used in mouth rinses or other local delivery methods (26). The inhibition of MMPs by CHX demonstrates new beneficial antiproteolytic properties which, in addition to the known antimicrobial properties of this substance, are useful in the treatment of periodontitis. Clinical trials are now in progress to further evaluate these new anti-MMP properties in vivo.
Acknowledgments
This work was supported by the Fonds Emile-Beaulieu.
REFERENCES
- 1.Beckett R P, Davidson A H, Drummond A H, Huxley P, Whittaker M. Recent advances in matrix metalloproteinase inhibitor research. Drug Discov Today. 1996;1:16–26. [Google Scholar]
- 2.Beighton D, Decker J, Homer K A. Effects of chlorhexidine on enzyme activities of dental plaque bacteria. J Clin Periodontol. 1991;18:85–89. doi: 10.1111/j.1600-051x.1991.tb01693.x. [DOI] [PubMed] [Google Scholar]
- 3.Birkedal-Hansen H. Role of matrix metalloproteinases in human periodontal diseases. J Periodontol. 1993;64:474–484. doi: 10.1902/jop.1993.64.5s.474. [DOI] [PubMed] [Google Scholar]
- 4.Charon J, Thomas T, Joachim F, Lempereur G, Capron A. Métabolisme oxydatif et chlorhexidine. J Parodontol. 1985;4:377–382. [PubMed] [Google Scholar]
- 5.Crout R J, Lee H M, Schroeder K, Crout H, Ramamurthy N S, Wiener M, Golub L M. The cyclic regimen of low-dose doxycycline for adult periodontitis: a preliminary study. J Periodontol. 1996;67:506–514. doi: 10.1902/jop.1996.67.5.506. [DOI] [PubMed] [Google Scholar]
- 6.Golub L, Lee H M, Lehrer G, Nemiroff A, McNamara T F, Kaplan R, Ramamurthy N. Minocycline reduces gingival collagenolytic activity during diabetes: preliminary observations and a proposed new mechanism of action. J Periodontal Res. 1983;18:516–526. doi: 10.1111/j.1600-0765.1983.tb00388.x. [DOI] [PubMed] [Google Scholar]
- 7.Golub L M, Sorsa T, Lee H M, Ciancio S, Sorbi D, Ramamurthy N S, Gruber B, Salo T, Konttinen Y T. Doxycycline inhibits neutrophil (PMN)-type matrix metalloproteinases in human adult periodontitis gingiva. J Clin Periodontol. 1995;22:100–109. doi: 10.1111/j.1600-051x.1995.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 8.Greenwald R A, Golub L M, Lavietes B, Ramamurthy N S, Gruber B, Laskin R S, McNamara T F. Tetracyclines inhibit human synovial collagenase in vivo and in vitro. J Rheumatol. 1987;14:28–32. [PubMed] [Google Scholar]
- 9.Grenier D. Effect of chlorhexidine on the adherence properties of Porphyromonas gingivalis. J Clin Periodontol. 1996;23:140–142. doi: 10.1111/j.1600-051x.1996.tb00547.x. [DOI] [PubMed] [Google Scholar]
- 10.Grenier D. Reduction of proteolytic degradation by chlorhexidine. J Dent Res. 1993;72:630–633. doi: 10.1177/00220345930720031301. [DOI] [PubMed] [Google Scholar]
- 11.Haffajee A D, Socransky S S. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 12.Hanemaaijer R, Sorsa T, Konttinen Y T, Ding Y, Sutinen M, Visser H, van Hinsbergh V W M, Helaakoski T, Kainulainen T, Ronka H, Tschesche H, Salo T. Matrix metalloproteinase-8 is expressed in rheumatoid synovial fibroblasts and endothelial cells. Regulation by tumor necrosis factor-alpha and doxycycline. J Biol Chem. 1997;272:31504–31509. doi: 10.1074/jbc.272.50.31504. [DOI] [PubMed] [Google Scholar]
- 13.Hjeljord L G, Rolla G, Bonesvoll P. Chlorhexidine-protein interactions. J Periodontal Res. 1973;8:11–16. doi: 10.1111/j.1600-0765.1973.tb02158.x. [DOI] [PubMed] [Google Scholar]
- 14.Ingman T, Sorsa T, Lindy O, Koski H, Konttinen Y T. Multiple forms of gelatinases/type IV collagenases in saliva and gingival crevicular fluid of periodontitis patients. J Clin Periodontol. 1994;21:26–31. doi: 10.1111/j.1600-051x.1994.tb00272.x. [DOI] [PubMed] [Google Scholar]
- 15.Ingman T, Tervahartiala T, Ding Y, Tschesche H, Haerian A, Kinane D F, Konttinen Y T. Matrix metalloproteinases and their inhibitors in gingival crevicular fluid and saliva of periodontitis patients. J Clin Periodontol. 1996;23:1127–1132. doi: 10.1111/j.1600-051x.1996.tb01814.x. [DOI] [PubMed] [Google Scholar]
- 16.Jolkovsky D L, Waki M Y, Newman M G, Otomo-Corgel J, Madison M, Flemming T F, Nachnani S, Nowzari H. Clinical and microbiological effects of subgingival and gingival marginal irrigation with chlorhexidine gluconate. J Periodontol. 1990;61:663–669. doi: 10.1902/jop.1990.61.11.663. [DOI] [PubMed] [Google Scholar]
- 17.Konttinen Y T, Lindy O, Kemppinen P, Saari H, Suomalainen K, Vauhkonen M, Lindy S, Sorsa T. Collagenase reserves in polymorphonuclear neutrophil leukocytes from synovial fluid and peripheral blood of patients with rheumatoid arthritis. Matrix. 1991;11:296–301. doi: 10.1016/s0934-8832(11)80238-6. [DOI] [PubMed] [Google Scholar]
- 18.Kornman K S, Page R C, Tonetti M S. The host response to the microbial challenge in periodontitis: assembling the players. Periodontol 2000. 1997;14:33–53. doi: 10.1111/j.1600-0757.1997.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee W, Aitken S, Sodek J, McCulloch C A G. Evidence of a direct relationship between neutrophil collagenase activity and periodontal tissue destruction in vivo: a role of active enzyme in human periodontitis. J Periodontal Res. 1995;30:23–33. doi: 10.1111/j.1600-0765.1995.tb01249.x. [DOI] [PubMed] [Google Scholar]
- 20.Löe H, Schiott C R. The effect of mouthrinses and topical applications of chlorhexidine on the development of dental plaque and gingivitis in man. J Periodontal Res. 1970;5:79–83. doi: 10.1111/j.1600-0765.1970.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 21.Mäkela M, Salo T, Uitto V-J, Larjava H. Matrix metalloproteinases (MMP-2 and MMP-9) of the oral cavity: cellular origin and relationship to periodontal status. J Dent Res. 1994;73:1387–1406. doi: 10.1177/00220345940730080201. [DOI] [PubMed] [Google Scholar]
- 22.McCulloch C A G. Collagenolytic enzymes in gingival crevicular fluid as diagnostic indicators of periodontitis. Ann N Y Acad Sci. 1994;732:152–164. doi: 10.1111/j.1749-6632.1994.tb24732.x. [DOI] [PubMed] [Google Scholar]
- 23.Rifkin B R, Vernillo A T, Golub L M. Blocking periodontal disease progression by inhibiting tissue-destructive enzymes: a potential therapeutic role for tetracyclines and their chemically-modified analogs. J Periodontol. 1993;64:819–827. doi: 10.1902/jop.1993.64.8s.819. [DOI] [PubMed] [Google Scholar]
- 24.Sorsa T, Ding Y-L, Ingman T, Salo T, Westerlund U, Haapasalo M, Tschesche H, Konttinen Y T. Cellular source, activation and inhibition of dental plaque collagenase. J Clin Periodontol. 1995;22:709–717. doi: 10.1111/j.1600-051x.1995.tb00831.x. [DOI] [PubMed] [Google Scholar]
- 25.Sorsa T, Lukinmaa P L, Westerlund U, Ingman T, Ding Y, Tschesche H, Konttinen Y T, Helaakoski T, Salo T. The expression, activation and chemotherapeutic inhibition of matrix metalloproteinase-8 (neutrophil collagenase/collagenase-2) in inflammation. In: Davidovitch Z, Norton L A, editors. Biological mechanisms of tooth movement and craniofacial adaptation. Boston, Mass: Harvard Society for the Advancement of Orthodontics; 1996. pp. 317–323. [Google Scholar]
- 26.Southard G L, Godowski K C. Subgingival controlled release of antimicrobial agents in the treatment of periodontal disease. Int J Antimicrob Agents. 1998;9:239–253. doi: 10.1016/s0924-8579(98)00004-1. [DOI] [PubMed] [Google Scholar]
- 27.Stanley A, Wilson M, Newman H N. The in vitro effects of chlorhexidine on subgingival plaque bacteria. J Clin Periodontol. 1989;16:259–264. doi: 10.1111/j.1600-051x.1989.tb01651.x. [DOI] [PubMed] [Google Scholar]
- 28.Teronen O, Konttinen Y T, Lindqvist C, Salo T, Ingman T, Lauhio A, Ding Y, Santavirta S, Valleala H, Sorsa T. Inhibition of matrix metalloproteinase-1 by dichloromethylene bisphosphonate (clodronate) Calcif Tissue Int. 1997;61:59–61. doi: 10.1007/s002239900295. [DOI] [PubMed] [Google Scholar]
- 29.Teronen O, Konttinen Y T, Salo T, Ingman T, Lauhio A, Ding Y, Santavirta S, Sorsa T. Human neutrophil collagenase MMP-8 in peri-implant sulcus fluid and its inhibition by clodronate. J Dent Res. 1997;76:1529–1537. doi: 10.1177/00220345970760090401. [DOI] [PubMed] [Google Scholar]
- 30.Uitto V-J, Turio H, Huttunen A, Lindy S, Uitto J. Activation of human leukocyte collagenase by compounds reacting with sulfhydryl groups. Biochim Biophys Acta. 1980;613:168–177. doi: 10.1016/0005-2744(80)90203-x. [DOI] [PubMed] [Google Scholar]
- 31.Uitto V-J, Suomalainen K, Sorsa T. Salivary collagenase: origin, characteristics and relationship to periodontal health. J Periodontal Res. 1990;25:135–142. doi: 10.1111/j.1600-0765.1990.tb01035.x. [DOI] [PubMed] [Google Scholar]
- 32.Van Wart H E, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci USA. 1990;87:5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiss S J. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 34.Wieder S G, Newman H N, Strahan J D. Stannous fluoride and subgingival chlorhexidine irrigation in the control of plaque and chronic periodontitis. J Clin Periodontol. 1983;10:172–181. doi: 10.1111/j.1600-051x.1983.tb02205.x. [DOI] [PubMed] [Google Scholar]