Abstract
Background
The benefits of vitamin E (VE) for multiple health outcomes have been well evaluated in many recent studies.
Objective
The purpose of this umbrella review was to conduct a systematic evaluation of the possible associations between VE intake and various health outcomes.
Methods
We systematically searched various databases, such as PubMed, Embase, and the Web of Science, to identify related meta-analyses of observational studies and randomized trials. We estimated the effect size of each association by using the random or fixed effects models and the 95% confidence intervals. We used standard approaches to evaluate the quality of the articles (AMSTAR) and classified the evidence into different levels of quality (GRADE).
Results
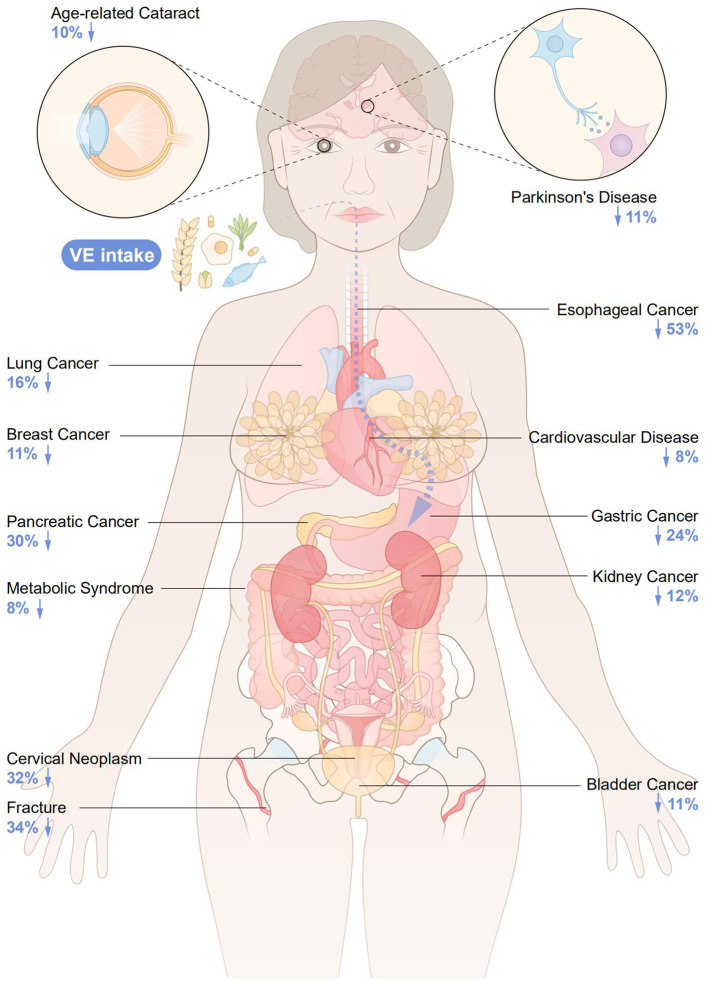
A total of 1,974 review articles were searched, and 27 articles with 28 health outcomes were yielded according to our exclusion criteria. The intake of VE was inversely associated with the risk of breast cancer, lung cancer, esophageal cancer, gastric cancer, pancreatic cancer, kidney cancer, bladder cancer, cervical neoplasms, cardiovascular disease, Parkinson's disease, depression, age-related cataracts, metabolic syndrome, and fracture. Overall, most of the quality of the evidence was low or very low. Three outcomes (stroke, age-related cataracts, obesity) were identified as having a “moderate” level of quality. The AMSTAR scores for all health outcomes ranged from 5 to 10.
Conclusion
Our study revealed that VE intake is beneficially related to multiple health outcomes. However, future studies on recommended doses and recommended populations of VE are also needed.
Systematic review registration
http://www.crd.york.ac.uk/PROSPERO/, identifier: CRD42022339571.
Keywords: vitamin E, health outcomes, umbrella review, meta-analyses, intake
1. Introduction
Vitamin E (VE), a fat-soluble antioxidant, is composed of tocopherol and tocotrienol α, β, δ, γ subtypes (1). Previous studies have found a potential link between VE and many diseases (2). Since the burden of morbidity and mortality from chronic diseases and cancer is increasing, VE has been widely studied as a potential preventive measure. Oxidative stress is considered a central mechanism of carcinogenesis and is an important process in many diseases, and VE generally helps prevent multiple diseases that are caused by oxidative damage (3, 4). The effects of VE on different types of cancers (such as breast, stomach, and bladder cancer), cardiovascular diseases, and neurological disorders may all be related to it. The possible mechanisms of carcinogenesis are as follows: (1) prevention of DNA damage through scavenging lipid hydrogen peroxide radicals; (2) protection of the nerves from free radical-mediated damage; (3) repression of the protein kinase C (PKC) pathway and enhancement of immune system function; (4) inhibition of cell cycle progression and cell proliferation via reduction of cyclin D1 and cyclin E; and (5) decrease in the expression of cyclooxygenase-2 and 8-hydroxydeoxyguanosine and type I insulin-like growth factor receptor to inhibit peroxidation and induce cell apoptosis, leading to suppression of cell proliferation (5–7). The association between VE and various health outcomes has been evaluated in a large cohort study, a case-control study, and randomized controlled trials. The results of these studies are summarized by systematic reviews and meta-analyses. However, a comprehensive review of the association between VE and multiple health outcomes (cancer and non-cancer outcomes) has been published. An umbrella review is a popular method for systematically assessing evidence from multiple sources and may be useful in assessing potential biases in the relationship between exposure and outcomes (2, 8, 9). Therefore, we conducted this study to provide a comprehensive review for investigating the relationship between VE and health outcomes reported in published systematic reviews and meta-analyses and to further assess the validity and quality of the available evidence.
2. Methods
2.1. Umbrella review methods
We systematically searched, organized, and evaluated existing evidence from numerous systematic reviews and meta-analyses on multiple health outcomes associated with VE intake (10). We included only those systematic reviews in our study that had incorporated meta-analyses. This umbrella review was registered in PROSPERO (CRD42022339571).
2.2. Literature search and eligibility criteria
We searched systematic reviews and meta-analyses of observational studies and randomized trials from PubMed, Embase, and Web of Science databases from inception to March 2022. The search strategy we used was as follows: ((((((vitamin E[Title/Abstract]) OR (Tocopherol[Title/Abstract])) OR (alpha-Tocopherol [Title/Abstract])) OR (beta-Tocopherol[Title/Abstract])) OR (gamma-Tocopherol[Title/Abstract])) OR (Tocotrienol[Title/Abstract])) AND (((systematic review[Title/Abstract]) OR (meta-analysis[Title/Abstract])) OR (systematic overview[Title/Abstract])). Meta-analyses and systematic reviews with meta-analyses of observational (cohort and case-control) and interventional (randomized and nonrandomized controlled trials) studies that evaluated VE intake and health outcomes in humans were included regardless of the race, gender, country, or region of participants. If two or more health outcomes existed in a single article, data for each outcome were extracted separately. If two or more meta-analyses revealed the same association, we chose the largest one to avoid duplicate assessments. Furthermore, articles reporting VE intake with therapeutic utilities were excluded only if nontherapeutic intake was also reported. Articles written in languages other than English and not involving humans were also excluded.
2.3. Data extraction
The following information was extracted independently by two investigators: (1) name of the first author, (2) cancer outcomes and non-cancer outcomes, (3) year of publication, (4) category of exposure (dietary and supplement VE), (5) the number of included studies, (6) the number of events and total participants in each study, (7) study design [case-control, cohort, randomized controlled trial (RCT)], (8) type of comparisons (highest v lowest dose reduction of any dietary and supplement VE), (9) the estimated summary effect (RR, relative risk; OR, odds ratio; SMD, standard mean difference) and corresponding 95% confidence intervals (CIs), (10) type of effect model (fixed or random model), and (11) P-value and publication bias by Egger's test. Any difference was resolved by the third investigator.
2.4. Quality of included studies and quality of evidence
The AMSTAR items (a reliable strategy for evaluating the quality of system reviews and meta-analyses) were used to evaluate the quality of the included articles (11). In our umbrella review, the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach was used to assess the strength of the evidence and categorize it to grade different levels of quality (“high,” “moderate,” “low,” and “very low”) (12).
2.5. Data analysis
A summary effect size was presented with a 95% confidence interval through the fixed or random effects models reported in the meta-analysis, if available. If both the cohort and case-control studies existed in the same article, the data were extracted separately. Publication bias was assessed. I2 statistics and Cochran's Q test were used to estimate the heterogeneity between studies (13, 14). The results of Egger's and heterogeneity tests were significantly higher than those of the control group when the p < 0.10. For other tests, a p < 0.05 was considered significant.
3. Results
3.1. Characteristics of the included meta-analysis
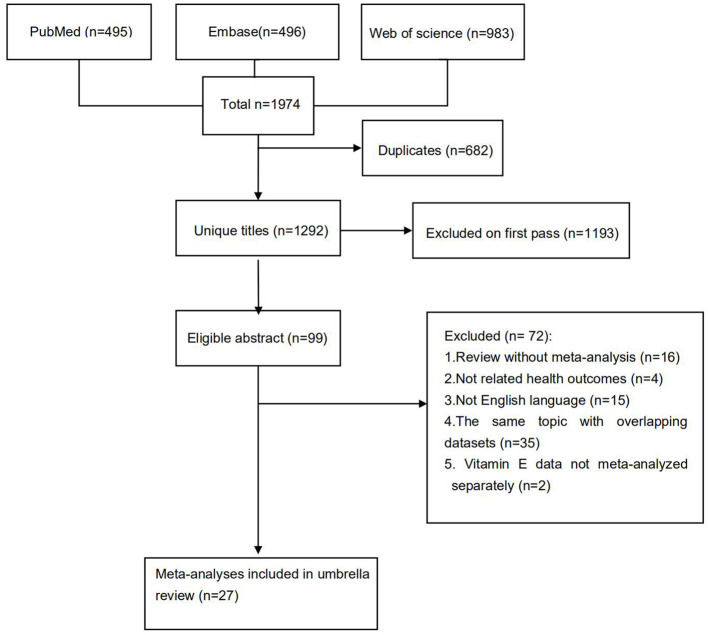
The flowchart of the detailed selection process is presented in Figure 1. Overall, after a systematic search, a total of 1,974 articles were identified, and 27 meta-analyses with 28 health outcomes (including 13 cancer-related and 16 non-cancer-related outcomes) were enrolled according to our exclusion criteria. The associations between VE and multiple health outcomes are shown in Figure 2, and more details are presented in Table 1 (non-cancer outcomes) and Table 2 (cancer outcomes). The assessments of AMSTAR scores and GRADE classification are shown in Table 3.
Figure 1.
Flowchart of the systematic search and selection process.
Figure 2.
The associations between VE and multiple health outcomes.
Table 1.
Associations between Vitamin E intake and non-cancer outcomes.
| Outcome | Category | References | No. of cases/ total | No. of studies | Cohort | Case-control | RCT | Meta metric | Effects model | Estimates | 95%CI | I2; Q test P-value | Egger's test P- value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Significant associations | |||||||||||||
| Cardiovascular Disease | Vitamin E supplements | Han et al. (15) | 7,852/ 233,310 | 10 | 0 | 0 | 10 | RR | Random | 0.92 | 0.46,0.95 | 55.5%; 0.244 | NA |
| Parkinson's Disease | Dietary vitamin E | Talebi et al. (16) | 3,444/ 316,405 | 7 | 7 | 0 | 0 | RR | Random | 0.84 | 0.71,0.99 | 51.9%; 0.244 | NA |
| Depression | Dietary vitamin E | Lee et al. (17) | 187/ 354 | 7 | 0 | 0 | 7 | SMD | Random | −0.88 | −1.54,−0.21 | 87.0%; < 0.001 | NA |
| Age-related Cataract | Dietary vitamin E | Jiang et al. (18) | NA/ 42,147 | 6 | 6 | 0 | 0 | RR | Fixed | 0.90 | 0.80,1.00 | 25.4%; 0.244 | 0.016 |
| Metabolic Syndrome | Dietary vitamin E | Zhang et al. (19) | NA/ 51,276 | 10 | NA | NA | NA | RR | Random | 0.92 | 0.85,1.00 | 67.1%; < 0.001 | NA |
| Fracture | Dietary vitamin E | Zhou et al. (20) |
14,738/ 62,571 | 1 | 1 | 0 | 0 | RR | Random | 0.66 | 0.46,0.95 | 94.2%; 0.00 | 0.447 |
| Non-significant associations | |||||||||||||
| Stroke | Vitamin E supplements | Loh et al. (21) | 74,000/148,016 | 18 | 0 | 0 | 18 | RR | Random | 0.98 | 0.92,1.04 | 0.0%; 0.390 | 0.251 |
| Parkinson's Disease | Dietary vitamin E | Talebi et al. (16) | 1,024 / 2,604 | 5 | 0 | 5 | 0 | RR | Random | 0.80 | 0.57,1.12 | 23.4%; 0.262 | NA |
| Alzheimer's Disease | Vitamin E supplements | Wang et al. (22) | 1,313/ 13,311 | 5 | 5 | 0 | 0 | RR | Random | 0.81 | 0.53,1.33 | 69.2%; 0.012 | 0.659 |
| Anxiety | Dietary vitamin E | Lee et al. (17) | 153/ 306 | 5 | 0 | 0 | 5 | SMD | Random | −0.86 | −2.11,0.40 | 94.1%; < 0.001 | NA |
| Glaucoma | Dietary vitamin E | Han and Fu (23) | 1,262/ 244,254 | 5 | 5 | 0 | 0 | OR | Fixed | 0.91 | 0.71,1.16 | 25.0%; 0.250 | NA |
| Age-related cataract | Dietary vitamin E | Jiang et al. (18) | NA/ 92,243 | 6 | 0 | 0 | 6 | RR | Fixed | 0.97 | 0.91,1.03 | 0.0%; 0.937 | 0.016 |
| Obesity | Vitamin E supplements | Emami (24) | NA/ 1,129 | 21 | 0 | 0 | 21 | WMD | Random | 0.04 | −0.29,0.37 | 0.0%; 0.999 | 0.384 |
| All-Cause Mortality | Dietary vitamin E | Jayedi et al. (25) | 22,823/386,854 | 11 | 9 | 0 | 2 | RR | Random | 0.95 | 0.90,1.01 | 48.8%; 0.030 | 0.460 |
CI, confidence interval; RCT, randomized controlled trial; RR, relative risk; OR, odds ratio; SMD, standardized mean difference; MD, mean differences; WMD, weighted mean difference; NA, not available.
Table 2.
Associations between Vitamin E intake and cancer outcomes.
| Outcome | Category | References | No. of cases/ total | No. of studies | Cohort | Case-control | RCT | Meta metric | Effects model | Estimates | 95%CI | I2; Q test P value | Egger test P value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Significant associations | |||||||||||||
| Breast Cancer | total intake vitamin E | Fulan et al. (26) | NA/ NA | 43 | 14 | 26 | 3 | OR | Random | 0.89 | 0.81,0.97 | 68.3%; NA | 0.180 |
| Breast Cancer | dietary vitamin E | Fulan et al. (26) | NA/ NA | 29 | 9 | 20 | 0 | OR | Random | 0.82 | 0.73,0.91 | 72.1%; NA | 0.150 |
| Lung Cancer | dietary vitamin E | Zhu et al. (27) | 4,164/ 435,532 | 9 | 9 | 0 | 0 | RR | Fixed | 0.84 | 0.76,0.93 | 41.1%; 0.075 | 0.246 |
| Esophageal Cancer | dietary vitamin E | Cui et al. (28) | 3,013/ 11,384 | 12 | 1 | 11 | 0 | RR | Random | 0.47 | 0.36,0.60 | 67.5%; < 0.001 | 0.008 |
| Gastric Cancer | dietary vitamin E | Kong et al. (29) | 3,299/ 634,667 | 8 | 3 | 5 | 0 | RR | Random | 0.76 | 0.67,0.85 | 43.0%; 0.090 | 0.254 |
| Pancreatic Cancer | dietary vitamin E | Chen et al. (30) | 3,070/ 230,206 | 11 | 4 | 7 | 0 | OR | Fixed | 0.70 | 0.62,0.81 | 0.0%; 0.455 | 0.596 |
| Kidney Cancer | dietary vitamin E | Shen et al. (31) | 1,213/ 450,463 | 6 | 6 | 0 | 0 | RR | NA | 0.88 | 0.72,1.08 | 49.2%; 0.023 | 0.928 |
| Bladder Cancer | dietary vitamin E | Lin et al. (32) | 3,265/ 575,601 | 11 | 8 | 0 | 3 | RR | Fixed | 0.89 | 0.78,1.00 | 19.9%; 0.254 | 0.707 |
| Cervical Neoplasm | total intake vitamin E | Hu et al. (33) | NA/ 5,301 | 6 | 0 | 6 | 0 | OR | Random | 0.68 | 0.49,0.94 | 70.0%; 0.005 | 0.530 |
| Non-significant associations | |||||||||||||
| Glioma | dietary vitamin E | Qin et al. (34) | 3180/ NA | 12 | 2 | 10 | 0 | RR | Random | 0.88 | 0.69,1.12 | 64.9%; 0.001 | NA |
| Thyroid Cancer | dietary vitamin E | Zhang et al. (35) | 1,021/ 15,005 | 4 | 0 | 3 | 1 | OR | NA | 1.50 | 0.90,2.60 | NA; NA | NA |
| Breast Cancer | vitamin E supplements | Fulan et al. (26) | NA | 12 | NA | NA | NA | OR | Random | 0.98 | 0.92,1.04 | 0.0%; NA | 0.010 |
| Breast Cancer | combined intake vitamin E | Fulan et al. (26) | NA | 14 | NA | NA | NA | OR | Random | 0.82 | 0.73,0.91 | 44.2%; NA | 1.000 |
| Colorectal Cancer | dietary vitamin E | Liu (36) | NA | 13 | NA | NA | NA | RR | Random | 0.94 | 0.82,1.07 | 10.3%; NA | 0.018 |
| Kidney Cancer | dietary vitamin E | Shen et al. (31) | 5,731/ 20,543 | 7 | 0 | 7 | 0 | RR | NA | 0.78 | 0.62,0.97 | 49.2%; 0.023 | 0.928 |
| Endometrial Cancer | dietary vitamin E | Bandera et al. (37) | 2,800/ 3,873 | 6 | 0 | 6 | 0 | OR | Random | 0.91 | 0.84,0.99 | 0.0%; 0.450 | NA |
| Ovarian Cancer | total intake vitamin E | Leng et al. (38) | 4,597/ 440,532 | 14 | 5 | 9 | 0 | RR | Random | 0.95 | 0.78,1.16 | 53.2%; 0.019 | NA |
| NHL | total intake vitamin E | Psaltopoulou et al. (39) | 3,840/ 189,522 | 4 | 4 | 0 | 0 | RR | Random | 0.94 | 0.65,1.36 | 0.0%; 0.889 | NA |
| Total Cancer | dietary vitamin E | Aune et al. (40) | 5,718/ 169,236 | 5 | NA | NA | NA | RR | Random | 0.97 | 0.93,1.02 | 61.0 %; 0.030 | 0.070 |
| Cancer Mortality | dietary vitamin E | Schwingshackl et al. (41) | 3,605/ 279,666 | 3 | NA | NA | NA | RR | Fixed | 1.00 | 0.79,1.28 | 45.0%; NA | NA |
CI, confidence interval; RCT, randomized controlled trial; RR, relative risk; OR, odds ratio; NA, not available; NHL, non-Hodgkin lymphoma.
Table 3.
Assessments of AMSTAR scores and GRADE classification.
| Outcome | Category | Author | Year | AMSTAR | GRADE |
|---|---|---|---|---|---|
| Non-cancer outcomes | |||||
| Significant associations | |||||
| Cardiovascular disease | Vitamin E supplements | Han | 2020 | 10 | Low |
| Parkinson's Disease | Dietary vitamin E | Talebi | 2022 | 8 | Very low |
| Depression | Dietary vitamin E | Lee | 2022 | 8 | Very low |
| Anxiety | Dietary vitamin E | Lee | 2022 | 8 | Very low |
| Age-related Cataract | Dietary vitamin E | Jiang | 2019 | 9 | Very low |
| Metabolic Syndrome | Dietary vitamin E | Zhang | 2021 | 9 | Very low |
| Fracture | Dietary vitamin E | Zhou | 2020 | 9 | Low |
| Non-significant associations | |||||
| Stroke | Vitamin E supplements | Loh | 2021 | 9 | Moderate |
| Parkinson's Disease | Dietary vitamin E | Talebi | 2022 | 8 | Very low |
| Alzheimer's Disease | Vitamin E supplements | Wang | 2021 | 9 | Very low |
| Obesity | Vitamin E supplements | Mohammad | 2021 | 10 | Moderate |
| Glaucoma | Dietary vitamin E | Han | 2022 | 7 | Very low |
| Age-related Cataract | Dietary vitamin E | Jiang | 2019 | 9 | Moderate |
| All-Cause Mortality | Dietary vitamin E | Jayedi | 2018 | 7 | Low |
| Cancer outcomes | |||||
| Significant associations | |||||
| Breast Cancer | Total intake vitamin E | Fulan | 2011 | 10 | Low |
| Breast Cancer | Dietary vitamin E | Fulan | 2011 | 10 | Low |
| Lung Cancer | Dietary vitamin E | Zhu | 2017 | 7 | Low |
| Esophageal Cancer | Dietary vitamin E | Cui | 2018 | 7 | Very low |
| Gastric Cancer | Dietary vitamin E | Kong | 2014 | 8 | Low |
| Pancreatic Cancer | Dietary vitamin E | Chen | 2016 | 9 | Low |
| Kidney Cancer | Dietary vitamin E | Shen | 2015 | 8 | Low |
| Bladder Cancer | Dietary vitamin E | Lin | 2019 | 8 | Low |
| Cervical Neoplasm | Total intake vitamin E | Hu | 2017 | 8 | Very low |
| Non-significant associations | |||||
| Glioma | Dietary vitamin E | Qin | 2014 | 9 | Very low |
| Thyroid Cancer | Dietary vitamin E | Zhang | 2013 | 6 | Very low |
| Breast Cancer | Combined intake vitamin E | Fulan | 2011 | 10 | Low |
| Breast Cancer | Combined intake vitamin E | Fulan | 2011 | 10 | Low |
| Colorectal Cancer | Dietary vitamin E | Liu | 2015 | 9 | Very low |
| Kidney Cancer | Dietary vitamin E | Shen | 2015 | 8 | Low |
| Endometrial Cancer | Dietary vitamin E | Elisa | 2008 | 6 | Low |
| Ovarian Cancer | Total intake vitamin E | Leng | 2019 | 9 | Very low |
| NHL | Total intake vitamin E | Psaltopoulou | 2018 | 5 | Very low |
| Total Cancer | Dietary vitamin E | Aune | 2018 | 7 | Low |
| Cancer Mortality | Dietary vitamin E | Schwingshackl | 2017 | 8 | Very low |
AMSTAR, a measurement tool to assess systematic reviews; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; NHL, non-Hodgkin lymphoma.
3.2. Associations between VE intake and cancer outcomes
Comparing “the highest” with “the lowest” intake, total intake of VE and dietary intake of VE significantly reduced the risk of breast cancer by 11% (OR = 0.89, 95% CI: 0.81,0.97) and 18% (OR = 0.82, 95% CI:0.73,0.91), respectively (26). The highest dietary VE intake was significantly associated with a decreased risk of lung cancer (RR = 0.84, 95% CI = 0.76,0.93). Subgroup analysis by geographic location showed significant negative associations between dietary VE intake and the risk of lung cancer for the American and European populations (RR = 0.85, 95% CI = 0.75,0.95) but not for the Asian population. Note that there exists a linear relationship between dietary VE intake and lung cancer risk: a daily dietary intake of 2 mg of VE reduces the risk by 5% (27).
Higher dietary VE was also related to lower esophageal cancer risk (OR = 0.47, 95% CI: 0.36, 0.60), especially for esophageal squamous cell carcinoma (ESCC) (OR = 0.29, 95% CI: 0.18, 0.44). A slightly linear dose–response relationship was detected between a 3 mg/day increment of dietary VE and the risk of esophageal cancer (OR = 0.78; 95% CI: 0.57, 1.06) (28). Furthermore, a dose–response relationship was detected between 10 mg/day of dietary VE intake and gastric cancer risk (RR=0.76, 95% CI: 0.67, 0.85) (29). A significant association was found between VE intake and pancreatic cancer risk for only case-control studies (pooled OR=0.63, 95% CI 0.53, 0.75). Meanwhile, a subgroup analysis based on the geographic area found that the intake of VE was not significantly associated with pancreatic cancer risk in European countries, while the inverse association was found in other geographic areas (30).
An analysis of the highest vs. lowest VE intake revealed that the intake of VE played a protective role in bladder cancer progression (RR=0.89; 95% CI: 0.78,1.00). Moreover, a potential linear association was also detected between VE intake and bladder cancer risk (32). A significant negative association between VE intake and kidney cancer risk was found only for cohort studies (RR=0.88, 95% CI 0.72,1.08) (31). In addition, VE intake also has a significant inverse association with the risk of cervical neoplasia (OR=0.68; 95% CI: 0.49, 0.94) (33).
No significant association was observed between VE consumption and the risks of glioma (34), thyroid cancer (35), colorectal cancer (36), endometrial cancer (37), ovarian cancer (38), and non-Hodgkin lymphoma (NHL) (39). Furthermore, the association between VE and the risk of overall cancer mortality (41) or total cancer (40) is not significant. Additionally, no significant association was detected between the combined intake of VE or VE supplements and breast cancer. Additionally, when we performed a subgroup analysis based on the type of study, the relationship between VE intake and breast cancer or pancreatic cancer was not significant (26, 30). For kidney cancer, the association was non-significant in case-control studies (31).
3.3. Associations between VE intake and non-cancer outcomes
A higher intake of VE supplements was associated with a significant reduction in the risk of cardiovascular disease (RR=0.92; 95% CI: 0.46, 0.95) (15). Compared with the lowest category of dietary VE intake, the highest dietary VE intake was significantly associated with a 16% lower risk of Parkinson's disease in the analysis of cohort studies (RR= 0.84; 95% CI: 0.71, 0.99). A linear dose–response meta-analysis suggested that each 5 mg/day increment in VE intake was associated with a 16% lower risk of Parkinson's disease (16). In adults who are at risk of or clinically diagnosed with depression, the positive effect of VE supplements on mood outcomes was observed (SMD = −0.88; 95% CI: −1.54, −0.21) (17). A 10% reduction in the risk of age-related cataracts for individuals was found in the highest categories of VE supplements for cohort studies (RR = 0.90; 95% CI: 0.80, 1.00) (18). In addition, dietary VE intake was inversely associated with a lower risk of metabolic syndrome (MetS) for high versus low consumption (SMD = −0.08; 95% CI: −0.14, −0.02) (19). In terms of obesity, subgroup analysis for baseline body mass index (BMI) suggested that VE supplements had a significant effect on increasing BMI in participants with normal baseline BMI (18.5–24.9) (WMD=0.636; 95% CI: 0.01, 1.26) (24). Furthermore, the risk of fracture at all sites was significantly reduced with higher VE intake (RR = 0.66; 95% CI: 0.46,0.95), especially for men (20).
Dietary VE intake was not associated with the risk of all-cause mortality while comparing the highest group with the lowest group (RR= 0.95; 95% CI: 0.90, 1.01), even in the further subgroup analysis (25). Higher intake of VE supplements did not show a significant association with stroke (21), anxiety (17), or Alzheimer's disease (22). Dietary VE intake was also associated with a lower risk of glaucoma (23), but it was not statistically significant. In addition, the subgroup analysis did not present a significant association between dietary VE intake and the risk of Parkinson's disease in case-control studies (16), and VE supplements had no significant effect on age-related cataract risk in RCTs (18).
3.4. Heterogeneity and publication bias of included meta-analyses
Among the 28 non-overlapping meta-analyses, 13 meta-analyses reported a Q test P < 0.10. One meta-analysis did not report the I2 statistic. A very high level of heterogeneity (I2>70%) was observed in three meta-analyses, and eight meta-analyses reported moderate-to-high levels of heterogeneity (I2 50%−70%). Sixteen meta-analyses reported low levels of heterogeneity (I2 < 50%).
3.5. AMSTAR and GRADE evaluation of included meta-analyses
The quality of the evidence by GRADE was low (47.1%) or very low (44.1%) (Table 3). Three outcomes (stroke, age-related cataracts, and obesity) were identified as having a “moderate” level of quality. The AMSTAR scores of all health outcomes ranged from 5 to 10, with a median score of 8 (IQR 7-9) (Table 3). More details are presented in Supplementary Tables S1, S2.
4. Discussion
The associations between VE and multiple health outcomes have been reported by a large number of studies and integrated into many meta-analyses. Overall, 27 meta-analyses involving 28 unique outcomes of the correlation between VE intake and multiple health outcomes were included in this umbrella review. The results indicated that the intake of VE was related to a lower risk of subsequent cancer outcomes (breast cancer, lung cancer, esophageal cancer, gastric cancer, pancreatic cancer, kidney cancer, bladder cancer, and cervical neoplasms) and non-cancer outcomes (cardiovascular disease, Parkinson's disease, depression, age-related cataract, metabolic syndrome, and fracture). Given that most of the evidence was from observational studies, compelling evidence for VE and multiple health outcomes does not seem to exist.
There is some discrepancy in subgroup analyses based on the study type of several outcomes (breast cancer, pancreatic cancer, kidney cancer, Parkinson's disease, and age-related cataract). Some of the potential reasons are mentioned below. First, the development of some diseases, such as age-related cataracts, is a long process for healthy individuals, and it may take a decade to manifest the protective effect of VE intake (42). The lengths of follow-up in RCTs may not be long enough to observe the effects of VE supplementation on the risks of disease (43). Second, the dose of VE intake in RCTs, cohort studies, and case-control studies was different (44). Third, due to the different levels of difficulty associated with conducting different studies, there are generally more case-control studies than cohort studies and RCTs. However, for the retrospective design of the case–control studies, there was more selection and recall bias for VE intake measurement in the case-control studies (30). There were also discrepant findings in subgroup analyses of the type of VE intake. Most VE supplements were synthetic and only contained the α-tocopherol form of the eight isoforms found in natural VE. Thus, the natural form, taken in dietary form, shows a more pronounced protective effect than the synthetic form (45). This may explain the non-significant association between VE supplements or the combined intake of VE and breast cancer (26). Another possible reason may be that VE supplements are generally used by those who are more concerned about their health condition than others, which means that they are more likely to tend to adopt a healthier lifestyle (45), which may also lead to discrepancies in the results.
The results of this study suggested that higher VE intake is negatively associated with the risk of cancer outcomes (breast cancer, gastric cancer, pancreatic cancer) and non-cancer outcomes (cardiovascular disease, Parkinson's disease, depression, age-related cataract). The protective effect of higher VE intake on these outcomes may be explained by the oxidative properties of VE. A possible mechanism might be that, as an antioxidant, VE can prevent DNA from being damaged by scavenging lipid hydrogen peroxide radicals (44, 46, 47). Moreover, VE could activate apoptosis by repressing the protein kinase C (PKC) pathway, enhancing immune system function, and inhibiting cancer cell growth by decreasing the phosphoinositide 3-kinase pathway (48). Recent research found that dietary VE could inhibit the dendritic cell checkpoint SHP1 from boosting antigen presentation, strengthening antitumor T-cell immunity, and enhancing immunotherapy (49). In addition, inhibiting cell cycle progression and cell proliferation via the reduction of cyclin D1 and cyclin E could be a feasible explanation for the protective effect of VE against lung cancer and bladder cancer (50, 51). The functions of inhibiting peroxidation and inducing cell apoptosis and, in turn, leading to the suppression of cell proliferation by effectively decreasing the expression of cyclooxygenase-2 and 8-hydroxydeoxyguanosine and type I insulin-like growth factor receptors may play an important role in the significant inverse association of VE intake and kidney cancer risk (52). In previous studies, VE also displayed neuroprotective functions against free radical-mediated injury. This is exemplified by its protection of neurons in the locus coeruleus (the main site of norepinephrine synthesis) from death in an early model of Parkinson's disease, preventing the toxin-induced damage of striatal dopaminergic terminals, and controlling the functions of antioxidant defenses, such as glutathione and superoxide dismutase (SOD) (53–55). Furthermore, to maintain the integrity of proteins and membranes and mediate the function of the lens, VE plays a vital role in blocking the excessive activation of oxidative stress (56–58). These findings can also explain the reverse association, which is a dynamic and interactive process, between dietary VE intake and metabolic syndrome (MetS) (59, 60). Although the exact mechanism of VE supplements' effects on body mass indices (BMI) has not yet been detected, the role of VE on the activation of peroxisome proliferator-activated receptor gamma (PPARg), which could lead to the upregulation of adiponectin gene expression, could be a possible pathway. Moreover, improving insulin sensitivity and suppressing HMG-CoA reductase could also be possible mechanisms affecting body composition indicators (61–63).
For the safety of VE supplements, healthy individuals should not use more than 1,000 mg of VE per day. A daily intake of VE of up to 800 IU appears safe and beneficial (64). When the intake of VE reaches between 400 and 800 IU, healthy individuals appear to have a decreased risk of several diseases, such as CVDs (65). However, the intake of VE could promote the degradation of essential medications for conditions such as cancer, cardiovascular diseases, hypertension, or diabetes. Although a study conducted by Podszun and Frank did not find evidence for the interaction between tocopherols or tocotrienols in the body, it has been suggested that exceeding dosages of 300 mg/day may interfere with some xenobiotics, such as tamoxifen, cyclosporine A, aspirin, or warfarin (66). Under these special circumstances, it is necessary to seek the opinion of a specialist regarding the need and appropriate dosage of vitamin E supplementation. In short, to date, many studies have found potential benefits of VE for multiple disease risks, but no study suggests a specific dose or appropriate population. For healthy individuals, 1,000 mg a day is the upper limit, but more research is needed to determine whether regular supplements are recommended and how much they should be given to specific populations, such as those at high risk for disease and cancer. Prophylactic use of large doses of vitamin E supplements (>1,000 mg per day) is not recommended. When some drugs are being used, the dose of VE should be more carefully designed.
Notably, this is the first comprehensive review of available evidence on VE intake and multiple health outcomes. Standard tools were used to assess the quality of the methods in the included literature (AMSTAR) and the strength of the evidence (GRADE). To avoid possible selection bias, the study was conducted by two researchers. However, our study has several limitations. First, most of the meta-analyses included were based on retrospective studies, and the overall GRADE quality was low. Second, considering that the most common source of VE is dietary intake, it is difficult to obtain VE as the only antioxidant or key nutrient. Third, the results of the study have a large deviation, and there are more confounding factors. Micronutrient combinations have also been frequently used in studies where the interaction between multivitamins is not further elucidated, and not all meta-analyses have a subgroup analysis of these factors. Finally, the definitions of maximum and minimum intakes were not clearly and uniformly quantified, making it difficult to determine the magnitude of the correlation and the impact of standardized baselines, and dose–response analyses were performed in less than half of the included meta-analyses.
5. Conclusion
In conclusion, we concluded that the intake of VE was related to a lower risk of multiple types of cancer and other diseases of diverse systems. Thus, VE intake at a safe dose is recommended to gain protect against certain diseases. However, further high-quality studies on recommended doses and recommended populations of VE are also needed. For specific populations (such as patients with high blood pressure, diabetes, and cancer) who are taking medication, additional vitamin E supplementation needs to be evaluated by a specialist before use.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JA and TZ conceived the project and drafted the manuscript. TZ, XY, JL, and XZ performed the screening and extraction. DL and HX performed the statistical analysis. JA revised the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (82070784 and 81702536) to JA and a grant from the Science and Technology Department of Sichuan Province, China (2022JDRC0040) to JA.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1035674/full#supplementary-material
References
- 1.Jiang Q. Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med. (2014) 72:76–90. 10.1016/j.freeradbiomed.2014.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brigelius-Flohé R. Vitamin E research: Past, now and future. Free Radic Biol Med. (2021) 177:381–90. 10.1016/j.freeradbiomed.2021.10.029 [DOI] [PubMed] [Google Scholar]
- 3.Abraham A, Kattoor AJ, Saldeen T, Mehta JL. Vitamin E and its anticancer effects. Crit Rev Food Sci Nutr. (2019) 59:2831–8. 10.1080/10408398.2018.1474169 [DOI] [PubMed] [Google Scholar]
- 4.Kelleher J. Vitamin E and the immune response. Proc Nutr Soc. (1991) 50:245–9. 10.1079/PNS19910034 [DOI] [PubMed] [Google Scholar]
- 5.Lunec J, Halligan E, Mistry N, Karakoula K. Effect of vitamin E on gene expression changes in diet-related carcinogenesis. Ann N Y Acad Sci. (2004) 1031:169–83. 10.1196/annals.1331.016 [DOI] [PubMed] [Google Scholar]
- 6.Chiera F, Meccia E, Degan P, Aquilina G, Pietraforte D, Minetti M, et al. Overexpression of human NOX1 complex induces genome instability in mammalian cells. Free Radic Biol Med. (2008) 44:332–42. 10.1016/j.freeradbiomed.2007.09.018 [DOI] [PubMed] [Google Scholar]
- 7.Yang H, Jia X, Chen X, Yang CS Li N. Time-selective chemoprevention of vitamin E and selenium on esophageal carcinogenesis in rats: the possible role of nuclear factor kappaB signaling pathway. Int J Cancer. (2012) 131:1517–27. 10.1002/ijc.27423 [DOI] [PubMed] [Google Scholar]
- 8.Ioannidis JPA. Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. CMAJ. (2009) 181:488–93. 10.1503/cmaj.081086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gianfredi V, Nucci D, Amerio A, Signorelli C, Odone A, Dinu M. What can we expect from an umbrella review? Adv Nutr. (2022) 13:684–5. 10.1093/advances/nmab150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antonio MG, Petrovskaya O, Lau F. The state of evidence in patient portals: umbrella review. J Med Internet Res. [2020] 22:e23851. 10.2196/23851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. (2009) 62:1013–20. 10.1016/j.jclinepi.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 12.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clini Epidemiol. (2011) 64:383–94. 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 13.Ioannidis JPA, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ (Clinical Research ed). (2007) 335:914–6. 10.1136/bmj.39343.408449.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Research ed). (1997) 315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han J, Zhao C, Cai J, Liang Y. Comparative efficacy of vitamin supplements on prevention of major cardiovascular disease: systematic review with network meta-analysis. Complement Ther Clin Pract. (2020) 39:101142. 10.1016/j.ctcp.2020.101142 [DOI] [PubMed] [Google Scholar]
- 16.Talebi S, Ghoreishy SM, Jayedi A, Travica N, Mohammadi H. Dietary antioxidants and risk of parkinson's disease: a systematic review and dose-response meta-analysis of observational studies. Adv Nutr. (2022). 10.1093/advances/nmac001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee ARYB, Tariq A, Lau G, Tok NWK, Tam WWS, Ho CSH. Vitamin E, alpha-tocopherol, and its effects on depression and anxiety: a systematic review and meta-analysis. Nutrients. (2022) 14:3. 10.3390/nu14030656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang H, Yin Y, Wu CR, Liu Y, Guo F, Li M, et al. Dietary vitamin and carotenoid intake and risk of age-related cataract. Am J Clin Nutr. (2019) 109:43–54. 10.1093/ajcn/nqy270 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Ding J, Guo H, Liu Z, Liu Q, Li Y, et al. Associations of dietary and circulating vitamin E level with metabolic syndrome. a meta-analysis of observational studies. Front Nutr. (2021) 8:783990. 10.3389/fnut.2021.783990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou P, Shao R, Wang H, Miao J, Wang X. Dietary vitamin A, C, and E intake and subsequent fracture risk at various sites A meta-analysis of prospective cohort studies. Medicine. (2020) 99:35. 10.1097/MD.0000000000020841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loh HC, Lim R, Lee KW, Ooi CY, Chuan DR, Looi I, et al. Effects of vitamin E on stroke: a systematic review with meta-analysis and trial sequential analysis. Stroke Vasc Neurol. (2021) 6:109–20. 10.1136/svn-2020-000519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W, Li J, Zhang H, Wang X, Zhang X. Effects of vitamin E supplementation on the risk and progression of AD: a systematic review and meta-analysis. Nutr Neurosci. (2021) 24:13–22. 10.1080/1028415X.2019.1585506 [DOI] [PubMed] [Google Scholar]
- 23.Han FF, Fu XX. Vitamin intake and glaucoma risk: a systematic review and meta-analysis. J francais d'ophtalmologie. (2022) 45:519–28. 10.1016/j.jfo.2021.10.010 [DOI] [PubMed] [Google Scholar]
- 24.Emami MR, Jamshidi S, Zarezadeh M, Khorshidi M, Olang B, Sajadi Hezaveh Z, et al. Can vitamin E supplementation affect obesity indices? A systematic review and meta-analysis of twenty-four randomized controlled trials. Clin Nutr. (2021) 40:3201–9. 10.1016/j.clnu.2021.02.002 [DOI] [PubMed] [Google Scholar]
- 25.Jayedi A, Rashidy-Pour A, Parohan M, Zargar MS, Shab-Bidar S. Dietary antioxidants, circulating antioxidant concentrations, total antioxidant capacity, and risk of all-cause mortality: a systematic review and dose-response meta-analysis of prospective observational studies. Adv Nutr. (2018) 9:701–16. 10.1093/advances/nmy040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fulan H, Changxing J, Baina WY, Wencui Z, Chunqing L, Fan W, et al. Retinol, vitamins A, C, and E and breast cancer risk: a meta-analysis and meta-regression. Cancer Causes Control. (2011) 22:1383–96. 10.1007/s10552-011-9811-y [DOI] [PubMed] [Google Scholar]
- 27.Zhu Y-J, Bo Y-C, Liu X-X, Qiu C-G. Association of dietary vitamin E intake with risk of lung cancer: a dose-response meta-analysis. Asia Pac J Clin Nutr. (2017) 26:271–7. 10.6133/apjcn.032016.04 [DOI] [PubMed] [Google Scholar]
- 28.Cui L, Li L, Tian Y, Xu F, Qiao T. Association between dietary vitamin E intake and esophageal cancer risk: an updated meta-analysis. Nutrients. (2018) 10:7. 10.3390/nu10070801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong P, Cai Q, Geng Q, Wang J, Lan Y, Zhan Y, et al. Vitamin intake reduce the risk of gastric cancer: meta-analysis and systematic review of randomized and observational studies. PLoS ONE. (2014) 9:12. 10.1371/journal.pone.0116060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J, Jiang W, Shao L, Zhong D, Wu Y, Cai J. Association between intake of antioxidants and pancreatic cancer risk: a meta-analysis. Int J Food Sci Nutr. (2016) 67:744–53. 10.1080/09637486.2016.1197892 [DOI] [PubMed] [Google Scholar]
- 31.Shen C, Huang Y, Yi S, Fang Z, Li L. Association of vitamin E intake with reduced risk of kidney cancer: a meta-analysis of observational studies. Med Sci Monitor. (2015) 21:3420–6. 10.12659/MSM.896018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin JH, Chen SJ, Liu H, Yan Y, Zheng JH. Vitamin E consumption and the risk of bladder cancer. Int J Vitam Nutr Res. (2019) 89:168–75. 10.1024/0300-9831/a000553 [DOI] [PubMed] [Google Scholar]
- 33.Hu X, Li S, Zhou L, Zhao M, Zhu X. Effect of vitamin E supplementation on uterine cervical neoplasm: a meta-analysis of case-control studies. PLoS ONE. (2017) 12:8. 10.1371/journal.pone.0183395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin S, Wang M, Zhang T, Zhang S. Vitamin E intake is not associated with glioma risk: evidence from a meta-analysis. Neuroepidemiology. (2014) 43:253–8. 10.1159/000369345 [DOI] [PubMed] [Google Scholar]
- 35.Zhang LR, Sawka AM, Adams L, Hatfield N, Hung RJ. Vitamin and mineral supplements and thyroid cancer: a systematic review. Eur J Cancer Prevent. (2013) 22:158–68. 10.1097/CEJ.0b013e32835849b0 [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Yu Q, Zhu Z, Zhang J, Chen M, Tang P, et al. Vitamin and multiple-vitamin supplement intake and incidence of colorectal cancer: a meta-analysis of cohort studies. Med Oncol. (2015) 32:434. 10.1007/s12032-014-0434-5 [DOI] [PubMed] [Google Scholar]
- 37.Bandera EV, Gifkins DM, Moore DF, McCullough ML, Kushi LH. Antioxidant vitamins and the risk of endometrial cancer: a dose-response meta-analysis. Cancer Causes & Control. (2009) 20:699–711. 10.1007/s10552-008-9283-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leng Y, Zhou H, Meng F, Tian T, Xu J, Yan F. Association of vitamin E on the risk of ovarian cancer: a meta-analysis. Biosci Reports. (2019) 39:BSR20193311. 10.1042/BSR20193311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Psaltopoulou T, Ntanasis-Stathopoulos I, Tsilimigras DI, Tzanninis IG, Gavriatopoulou M, Sergentanis TN. Micronutrient intake and risk of hematological malignancies in adults: a systematic review and meta-analysis of cohort studies. Nutr Cancer. (2018) 70:821–39. 10.1080/01635581.2018.1490444 [DOI] [PubMed] [Google Scholar]
- 40.Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, et al. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: a systematic review and dose-response meta-analysis of prospective studies. Am J Clinical Nutr. (2018) 108:1069–91. 10.1093/ajcn/nqy097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwingshackl L, Boeing H, Stelmach-Mardas M, Gottschald M, Dietrich S, Hoffmann G, et al. Dietary supplements and risk of cause-specific death, cardiovascular disease, and cancer: a systematic review and meta-analysis of primary prevention trials. Adv Nutr. (2017) 8:27–39. 10.3945/an.116.013516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma L. Hao Z-x, Liu R-r, Yu R-b, Shi Q, Pan J-p. A dose-response meta-analysis of dietary lutein and zeaxanthin intake in relation to risk of age-related cataract. Graefes Arch Clin Exp Ophthalmol. (2014) 252:63–70. 10.1007/s00417-013-2492-3 [DOI] [PubMed] [Google Scholar]
- 43.Choi Y-H, Miller JM, Tucker KL, Hu H, Park SK. Antioxidant vitamins and magnesium and the risk of hearing loss in the US general population. Am J Clin Nutr. (2014) 99:148–55. 10.3945/ajcn.113.068437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christen WG, Liu S, Glynn RJ, Gaziano JM, Buring JE. Dietary carotenoids, vitamins C and E, and risk of cataract in women: a prospective study. Arch Ophthalmol. (2008) 126:102–9. 10.1001/archopht.126.1.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gray SL, Anderson ML, Crane PK, Breitner JCS, McCormick W, Bowen JD, et al. Antioxidant vitamin supplement use and risk of dementia or Alzheimer's disease in older adults. J Am Geriatr Soc. (2008) 56:291–5. 10.1111/j.1532-5415.2007.01531.x [DOI] [PubMed] [Google Scholar]
- 46.Ricciarelli R, Zingg J-M, Azzi A. The 80th anniversary of vitamin E: beyond its antioxidant properties. Biol Chem. (2002) 383:457–65. 10.1515/BC.2002.048 [DOI] [PubMed] [Google Scholar]
- 47.van Poppel G, van den Berg H. Vitamins and cancer. Cancer Lett. (1997) 114:195–202. 10.1016/S0304-3835(97)04662-4 [DOI] [PubMed] [Google Scholar]
- 48.Kontek R, Jakubczak M, Matlawska-Wasowska K. The antioxidants, vitamin A and E but not vitamin C and melatonin enhance the proapoptotic effects of irinotecan in cancer cells in vitro. Toxicol In Vitro. (2014) 28:282–91. 10.1016/j.tiv.2013.11.007 [DOI] [PubMed] [Google Scholar]
- 49.Yuan X, Duan Y, Xiao Y, Sun K, Qi Y, Zhang Y, et al. Vitamin E enhances cancer immunotherapy by reinvigorating dendritic cells via targeting checkpoint SHP1. Cancer Discov. (2022). 10.1158/2159-8290.22541254.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quin J, Engle D, Litwiller A, Peralta E, Grasch A, Boley T, et al. Vitamin E succinate decreases lung cancer tumor growth in mice. J Surg Res. (2005) 127:139–43. 10.1016/j.jss.2005.01.014 [DOI] [PubMed] [Google Scholar]
- 51.Gysin R, Azzi A, Visarius T. Gamma-tocopherol inhibits human cancer cell cycle progression and cell proliferation by down-regulation of cyclins. FASEB J. (2002) 16:1952–4. 10.1096/fj.02-0362fje [DOI] [PubMed] [Google Scholar]
- 52.Zhang D, Okada S, Yu Y, Zheng P, Yamaguchi R, Kasai H. Vitamin E inhibits apoptosis, DNA modification, and cancer incidence induced by iron-mediated peroxidation in Wistar rat kidney. Cancer Res. (1997) 57:2410–4. [PubMed] [Google Scholar]
- 53.Pasbakhsh P, Omidi N, Mehrannia K, Sobhani AG, Ragerdi Kashani I, Abbasi M, et al. The protective effect of vitamin E on locus coeruleus in early model of Parkinson's disease in rat: immunoreactivity evidence. Iran Biomed J. (2008) 12:217–22. 10.1016/S1353-8020(08)70267-0 [DOI] [PubMed] [Google Scholar]
- 54.Perumal AS, Gopal VB, Tordzro WK, Cooper TB, Cadet JL. Vitamin E attenuates the toxic effects of 6-hydroxydopamine on free radical scavenging systems in rat brain. Brain Res Bull. (1992) 29:699–701. 10.1016/0361-9230(92)90142-K [DOI] [PubMed] [Google Scholar]
- 55.Cadet JL, Katz M, Jackson-Lewis V, Fahn S. Vitamin E attenuates the toxic effects of intrastriatal injection of 6-hydroxydopamine (6-OHDA) in rats: behavioral and biochemical evidence. Brain Res. (1989) 476:10–5. 10.1016/0006-8993(89)91530-8 [DOI] [PubMed] [Google Scholar]
- 56.Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem. (2015) 97:55–74. 10.1016/j.ejmech.2015.04.040 [DOI] [PubMed] [Google Scholar]
- 57.Selin JZ, Lindblad BE, Rautiainen S, Michaëlsson K, Morgenstern R, Bottai M, et al. Are increased levels of systemic oxidative stress and inflammation associated with age-related cataract? Antioxid Redox Signal. (2014) 21:700–4. 10.1089/ars.2014.5853 [DOI] [PubMed] [Google Scholar]
- 58.Christen WG, Glynn RJ, Chew EY, Buring JE. Vitamin E and age-related cataract in a randomized trial of women. Ophthalmology. (2008) 115:5. 10.1016/j.ophtha.2007.06.040 [DOI] [PubMed] [Google Scholar]
- 59.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. (2015) 4:180–3. 10.1016/j.redox.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seo J-A, Song S-W, Han K, Lee K-J, Kim H-N. The associations between serum zinc levels and metabolic syndrome in the Korean population: findings from the 2010 Korean National Health and Nutrition Examination Survey. PLoS ONE. (2014) 9:e105990. 10.1371/journal.pone.0105990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Landrier J-F, Gouranton E, El Yazidi C, Malezet C, Balaguer P, Borel P, et al. Adiponectin expression is induced by vitamin E via a peroxisome proliferator-activated receptor gamma-dependent mechanism. Endocrinology. (2009) 150:5318–25. 10.1210/en.2009-0506 [DOI] [PubMed] [Google Scholar]
- 62.Faure P, Rossini E, Lafond JL, Richard MJ, Favier A, Halimi S. Vitamin E improves the free radical defense system potential and insulin sensitivity of rats fed high fructose diets. J Nutr. (1997) 127:103–7. 10.1093/jn/127.1.103 [DOI] [PubMed] [Google Scholar]
- 63.Parker RA, Pearce BC, Clark RW, Gordon DA, Wright JJ. Tocotrienols regulate cholesterol production in mammalian cells by post-transcriptional suppression of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Biol Chem. (1993) 268:11230–8. 10.1016/S0021-9258(18)82115-9 [DOI] [PubMed] [Google Scholar]
- 64.Bell SJ, Grochoski GT. How safe is vitamin E supplementation? Crit Rev Food Sci Nutr. (2008) 48:760–74. 10.1080/10408390701719355 [DOI] [PubMed] [Google Scholar]
- 65.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. (2005) 294:56–65. 10.1001/jama.294.1.56 [DOI] [PubMed] [Google Scholar]
- 66.Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. (2005) 142:37–46. 10.7326/0003-4819-142-1-200501040-00110 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.