Abstract
Background
Over seven decades, Brazil has made admirable progress in controlling schistosomiasis, and a frequent question about the explanation for this reduction refers to the effect of improving environmental factors in the country. This article seeks to identify factors related to the change in the epidemiological situation of schistosomiasis mansoni infection by analyzing three national prevalence surveys conducted since 1950.
Methodology/principal findings
This is an ecological study analyzing an unbalanced panel of data based on national surveys and considering the municipality as the unit of analysis. The sample consisted of 1,721 Brazilian municipalities, in which a total of 1,182,339 schoolchildren aged 7–14 were examined during the three periods corresponding to each survey (1947–1953, 1975–1979, and 2010–2015). The percentage of municipalities with zero cases of schistosomiasis was: 45.4%, 54.2% and 73.7%, respectively for those periods. A zero-inflated Poisson regression model, with fixed and random effects, was fitted to assess the association between candidate factors and disease prevalence using a significance level of 5%. There was a significant decrease in disease prevalence between the first and last periods analyzed (RR 0.214, CI 0.184–0.249), with a protective association with access to sanitation (RR 0.996, CI 0.994–0.998), urbanization (RR 0.991, CI 0.989–0.993), and living in own households (RR 0.986, CI 0.983–0.989); and an inverse association with piped water supply (RR 1.010, CI 1.008–1.011).
Conclusion
The findings of this study indicate a decrease in the prevalence of schistosomiasis over seven decades in schoolchildren from the analyzed Brazilian municipalities, associated with environmental factors and social conditions. The increased access to piped water in the municipalities apparently triggers other ways of contact with unsafe water bodies, generating new transmission routes and suggesting the need for a systemic approach concerning contact with water.
Author summary
Schistosomiasis mansoni is a neglected tropical disease caused by infection from parasitic worms of the species Schistosoma mansoni. Due to the complexity of the mechanism of transmission and maintenance of schistosomiasis, several preventive actions on diverse conditioning factors can promote disease control. Active search, timely treatment of cases, stool tests, and epidemiological investigations are the initial actions under programs for epidemiological surveillance of the disease. Thus, national surveys on prevalence of the disease covering a large time span can provide valuable information about its epidemiological pattern over the years. Our study addressed three national surveys with historical coverage (1947–1953, 1975–1979, and 2010–2015) that mapped the prevalence of the disease in children aged 7–14 for nearly seven decades. We also employed statistical models to investigate which environmental, economic, or demographic factors are associated with the disease at municipal level. The results showed that the decrease in schistosomiasis from the 1950s to the 2010s was statistically significant and suggests that improvements in water supply and sanitation require structured and systemic approaches for controlling the transmission of schistosomiasis.
Introduction
Over the last decades, several countries have tried to control neglected tropical diseases, including schistosomiasis, by establishing measures to intensify their management. Schistosomiasis is endemic in at least 52 countries [1], affecting approximately 240 million people worldwide. This disease is endemic in ten countries on the American continent. However, only Brazil and Venezuela needed to apply preventive chemotherapy for their population in 2020, including more than 2.2 million school-age children [2]. In addition to chemotherapy, which is not sufficient and accessible to all, the World Health Organization (WHO) recommends several strategies to control and eliminate the disease. These measures include access to safe drinking water, improvements in sanitation, health education, and hygiene, besides environmental and disease control management, even though considering that WaSH interventions (water, sanitation, and hygiene) are expected to provide modest benefits in limiting Schistosoma transmission” [3].
Prevalent in tropical and subtropical areas, especially in poor communities without access to drinking water and adequate sanitation, the disease caused by trematode helminths of the genus Schistosoma has epidemiological importance. The epidemiology of the disease is especially relevant in children since the absence of infection in this age group would mean the possible interruption of the transmission. On the other hand, eliminating the disease from the population, including adults, especially workers living in large endemic areas, requires improved household and environmental conditions. Among them, access to safe and continuous water and improved sanitary facilities that allow for better conditions dwelling can have an important role in breaking the disease cycle, interrupting the release of eggs in the environment and avoiding access to surface water for water supply [4].
In Brazil, the epidemiology of Schistosoma mansoni infection shows that social and, environmental conditions, drug treatment and access to health service contribute to a reduction in the prevalence rate [5]. Although the relationship between schistosomiasis infection and sanitary conditions has been showed in local or regional scales, nationwide and longitudinal studies can contribute to understanding disease dissemination as well as its explanatory factors throughout the Brazilian territory. Approaches to exploring and understanding the role of environmental, biological and medical interventions, as well as historical, socioeconomic, and cultural determinants, crucial for assessing this complex disease [6].
Brazil has an extensive experience in conducting surveys on the prevalence of schistosomiasis, covering a wide range of the country and an extended time of approximately seven decades. The first of these surveys was carried out in the 1950s [7,8]. Given the epidemiological and social impact of schistosomiasis on the population, other two national surveys were conducted: by the Special Schistosomiasis Control Program (PECE) (Programa Especial de Controle da Esquistosomose)in the 1970s [9] and the National Survey on the Prevalence of Schistosomiasis and Soil-Transmitted Helminth Infections (INPEG) (Inquérito Nacional de Esquistosomose e Geo-helmintose) in the 2010s. Throughout these seven decades, a reduction in prevalence could be observed [10]. However, these data demonstrate that schistosomiasis is still epidemiologically relevant [11] since, from the point of view of the infected patient and public health, there should be no acceptable level of morbidity due to this disease [3].
Hence, this study aimed to analyze the behavior of the prevalence of schistosomiasis and the impact on prevalence of access to water and sanitation services. The analysis is based on those three surveys conducted in Brazilian municipalities over seven decades.
Methods
Ethics statement
The current study used data from three national survey on prevalence schistosomiasis in schoolchildren. These data are anonymous and available for research purposes by the Brazilian government. Moreover, this study was conducted exclusively with secondary and aggregated data, publicly accessible and in accordance with resolutions of the National Health Council No. 466/2012 [12] and No. 510/2016 [13], exempt from evaluation by the Research Ethics Committee.
Study design
The epidemiological design of the research consists of a prospective study, covering three periods with observational ecological data. The outcome variable was the municipal prevalence of schistosomiasis in schoolchildren from seven to 14 years old.
Studied period and data source
Data were extracted from the three Brazilian surveys of schistosomiasis prevalence, as follows:
The National Helminthological Survey of Schoolchildren (IHE) (Inquérito Helmintológico Escolar) by Pellon & Teixeira, conducted from 1947–1953 in two phases [6,7]. The first phase included 11 states considered endemic for the disease, with a sampling plan that addressed locations of more than 1,500 inhabitants in which 440,786 schoolchildren were examined. In the second phase, locations of more than 1,250 inhabitants of five non-endemic states were included, and 174,192 schoolchildren were examined. In both phases, all regions of the country were sampled, except for the North region. In this way, 1,190 locations were surveyed, totaling 614,978 students examined.
Survey by the Special Schistosomiasis Control Program (PECE) (Programa Especial de Controle da Esquistosomose) conducted from 1975–1979 [9]. This survey consisted of a non-probabilistic sample of 327 municipalities in 18 states and areas that were disease-free or endemic, in which 447,779 schoolchildren aged 7–14 were examined. This survey took place in municipalities where the program had been implemented by the Ministry of Health and included all municipalities that adhered to PECE. The criteria for inclusion of schools and students were based on the decennial census and an active search in school classes [14,15].
INPEG conducted from 2010–2015 [10]. This survey also considered schoolchildren aged 7–14 by applying a cluster sampling plan, with areas categorized in three endemic levels (municipalities in non-endemic, low prevalence, and high prevalence areas) and four categories of population size (fewer than 20,000, between 20,000 and 150,000, between 150,000 and 500,000, and more than 500,000 inhabitants). Thus, samples were drawn from those stratums to determine the analyzed municipalities, elementary schools, and school classes. As a result, 521 municipalities representing all Brazilian states were analyzed. The amount of tests in each municipality ranged from 60% to 100% and in nine states it was higher than planned. In total, 197,564 schoolchildren aged 7–14 were examined.
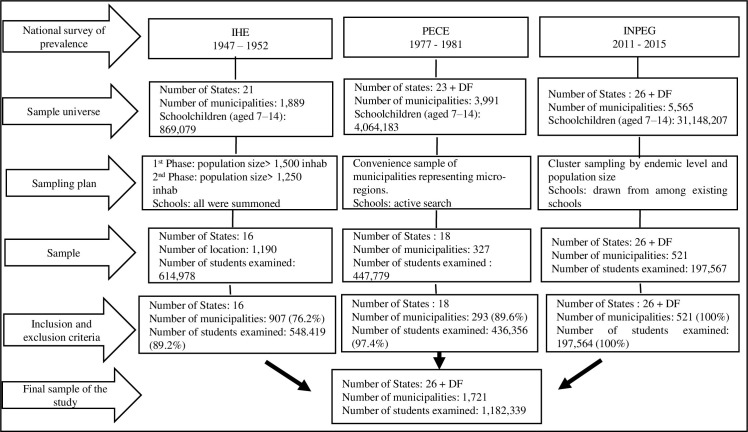
The broad extension of the Brazilian territory affected the implementation time of each of the three surveys. Therefore, the impossibility of collecting data in just one year led to the need for around five years of data gathering for each survey. Fig 1 describes the surveys, including their respective sampling strategies. S1 Note provides additional details on characteristics of each survey and their specific features.
Fig 1. Descriptive flowchart of the three national surveys on the prevalence of schistosomiasis mansoni in Brazil.
IHE: National Helminthological Survey of Schoolchildren. PECE: Special Schistosomiasis Control Program. INPEG: National Survey of Prevalence of Schistosomiasis and Soil-transmitted helminth infections. DF: Federal District.
For obtaining intercensal estimates, data related to the explanatory variables were collected from the 1950, 1960, 1970, 1980, 2000, and 2010 demographic censuses of the Brazilian Institute of Geography and Statistics (Instituto Brasileiro de Geografia e Estatística–IBGE) and from the Institute of Applied Economic Research (Instituto de Pesquisa Econômica Aplicada–IPEADATA) (Table 1).
Table 1. Description of evaluated outcome and explanatory variables, periods, and data source.
| Variable | Description | Source | Period | ||
|---|---|---|---|---|---|
| IHE | PECE | INPEG | |||
| Prevalence of schistosomiasis | Number of students with positive stool tests / Total number of students aged 7–14 examined | National surveys | 1947–1953a | 1975–1979a | 2010–2015a |
| % of water supply | Number of dwellings with internal piped water supply from the general distribution network / Total number of dwellings | IBGE Census | 1950 | 1970 and 1980b | 2000–2010c |
| % of sanitary sewerage | Number of dwellings with sanitary facilities with drainage connected to the general sewage networks/ Total number of dwellings | IBGE Census | 1950 and 1960d | 1970–1980b | 2000–2010c |
| % of urbanization | Number of inhabitants in the urban area / Total number of inhabitants | IBGE Census | 1950 | 1970 and 1980b | 2000–2010c |
| % literacy rate | Number of literate people aged 15 years old or older / Total population of the same age group | IBGE Census | 1950 | 1970–1980b | 2000–2010c |
| % Occupancy condition of households | Number of permanent households in occupancy and owning conditions / Total number of permanent households | IBGE Census | 1950 | 1970–1980b | 2000–2010c |
| Municipal GDP per capita | Municipal GDP at constant prices–R$ 1,000 per year 2000s/ total population in the municipality | IPEADATA | 1949 and 1959b | 1970–1975–1980b | 1999–2010c |
| Period | Variable with three categories corresponding to the periods of the surveys | – | 1950 (reference) | 1977 | 2013 |
a:Year interval used to define 1950, 1977, and 2013 midpoints for the collection and treatment of explanatory variables.
b: explanatory variables calculated by applying linear interpolation techniques.
c: explanatory variables calculated using linear and polynomial interpolation and extrapolation techniques.
d:values calculated by the population trend method or Apportionment Method (AiBi projection) [15]. National Helminthological Survey of Schoolchildren (Inquérito Helmintólogico Escolar–IHE). National Survey on the Prevalence of Schistosomiasis and Soil-transmitted helminth infections (INPEG). Brazilian Institute of Geography and Statistics (IBGE). Institute for Applied Economic Research (IPEADATA). Ministry of Health (MS). Special Schistosomiasis Control Program (PECE). Gross Domestic Product (GDP)
Inclusion and exclusion criteria
The intense evolution of the political-administrative organization of Brazilian states and municipalities over the decades, reflected in the number of currently existing municipalities, led to the adoption of inclusion and exclusion criteria for this study defined as: (i) sampled municipalities with territorial delimitation compatible with the demographic censuses of each analyzed period; (ii) sampled municipalities and/or municipal districts that, even incorporated to or emancipated from other municipalities or districts during the period of the three surveys (1947–1953, 1975–1979 and 2010–2015), had available legislative and historical information on their establishment or division process; (iii) sampled municipalities that met the criterion of quality of registration. Subsequently, according to the assumed criteria, the municipalities in which it was not possible to detail the evolution of their establishment, fusion, or incorporation, as well as those lacking enough records for the explanatory variables, were excluded. Fig 1 and S2 Note show the complete description of the methodological inclusion and exclusion criteria.
Outcome variable
The outcome variable of the study was the prevalence of infection with Schistosoma mansoni in samples of schoolchildren from 7 to 14 years old per municipality (Table 1).
Independent variables
The independent (explanatory) variables consisted of coverage of water supply and sewerage, and municipalities sociodemographic and socioeconomic variables such as population size, percentages of urbanization and literacy, per capita gross domestic product, and the survey period. These variables were defined considering the factors related to infection as indicated in the literature and the context and availability of data in the information systems for each period. Relevant factors related to the disease, such as family income, coverage of deworming treatment, water treatment for inactivating human schistosome cercariae or chemical molluscicide treatment, malacological surveys, and family or school hygiene practices, could not be included since there are not enough available data from all studied municipalities in the different periods, mainly in the 1950s and 1970s.
The reference year adopted for each of the survey periods were 1950, 1977, and 2013 to facilitate notation. Projection and/or interpolation techniques were used in cases where information about the explanatory variables was not available in the reference year. For 1950, we applied a projection by the population trend method—AiBi projection or Apportionment Method—for the sanitary sewage [16,17]; and a projection by interpolation for the municipal gross domestic product (GDP) per capita using data from 1949 and 1959. For explanatory variables in the 1977 period, estimates were made using linear interpolation techniques. For 2013, interpolation estimates were performed for 2000 and 2010, and then we extrapolated linear and geometric growth for 2011, 2012, and 2013. The projections for the years 1977 and 2013 were adopted because the Brazilian census information is collected every decade, therefore, in a non-annual series [18]. S2 Note provides additional details about the techniques used for each variable, explanations of the use of the AiBi technique and the number of observations involved.
Data analysis
As the national surveys were carried out in different periods and using different strategies, the sampled municipalities were not the same for all periods, resulting in an unbalanced data panel with different municipalities in each sampling period. Based on that, we conducted a prospective study covering three periods with observational ecological data to evaluate trends in the prevalence rates of the infection over time and their associations with economic, health, and social indicators for a total of 1,721 municipalities sampled during the periods represented by the reference years 1950, 1977, and 2013.
Descriptive analyses were performed for the municipal data in each period. In the inferential analyses, multilevel statistical models were fitted to estimate the prevalence of schistosomiasis considering data from the 1,721 sampled municipalities. According to the official territorial division proposed by the IBGE, the Brazilian political-administrative organization is divided into five macro-regions, which include 26 federal units (states) plus the Federal District and 5,570 municipalities. In order to consider this hierarchical characteristic of the data, we applied Generalized Linear Mixed Models (GLMMs) with random effects related to three levels: regions (Level 1,with 5 categories South, Southeast, North, Northeast and Midwest); states (Level 2, representing the 26 federative units plus the Federal District); and municipalities (Level 3, related to the 1,398 different municipalities included in the study with observation in at least one of the three surveys). These three hierarchical levels of data were incorporated into the random intercepts of the GLMMs to allow the joint modeling of data the three periods.
The GLMMs also included fixed effects related to the independent variables previously described. We considered the Poisson and Negative Binomial distributions in the analyses, with and without zero inflation for both cases [19–22]. Thus, we allowed the modeling of different data characteristics, such as over dispersion and zero inflation. Detailed discussion on models definition is provided in S3 Note. The count of the number of positive cases in each municipality was considered the outcome variable and the total number of examined students was used as an offset, responsible for controlling the number of cases per municipality. Logistic regression was used to adjust for the excess of zero.
A backward selection procedure was used to identify the significant fixed effects, considering a 25% significance level for the removal of an explanatory variable. Thus, at each step of the analysis, the explanatory variable with the highest p-value, among those with a p-value>0.25, was removed from the model. After such a procedure, no further variable selection was necessary, as all variables retained were significant at the 5% significance level. The final regression model (Poisson or Negative Binomial distribution with or without zero inflation) was chosen according to the following criteria: (a) lower residual variance; (b) lower values of Akaike (AIC) and Bayesian (BIC) Information Criteria [23].
The software EPI INFO 7.1.1 and Microsoft Office Excel 2010 were used for database construction. Descriptive and inferential analyses were performed in the software R using the statistical package glmmTMB [24].
Results
Descriptive analysis
Table 2 shows the results of the descriptive analysis for the prevalence of schistosomiasis. Despite the large range, the mean prevalence of infection decreased between the three analyzed periods, with 8.3% for reference period 1950 (SD 17.2), 4.8% for 1977 (SD 12.4), and 0.8% for 2013 (SD 3.5). In addition, the median and amplitude of prevalence were 0.2 and 90.9 in 1950; 0.0 and 71.2 in 1977; and 0.0 and 50.0 in 2010–2015. The percentage of municipalities with zero cases of schistosomiasis were 45.4% for 1950, 54.2% for 1977 and 73.7% for 2015.
Table 2. Descriptive statistics on the prevalence of schistosomiasis per 100 students and independent variables per study period in the 1,721 sampled Brazilian municipalities.
| 1947–1953 (n = 907) | 1975–1979 (n = 293) | 2010–2015 (n = 521) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dependent variable | Mean | SD | Median | Range | Mean | SD | Median | Range | Mean | SD | Median | Range |
| Prevalence of schistosomiasis | 8.3 | 17.2 | 0.2 | 90.9 | 4.8 | 12.4 | 0.0 | 71.2 | 0.8 | 3.5 | 0.0 | 50.0 |
| Independent variables | Mean | SD | Median | Range | Mean | SD | Median | Range | Mean | SD | Median | Range |
| %Urbanization | 25.6 | 17.4 | 20.6 | 97.0 | 47.4 | 24.5 | 41.6 | 96.6 | 68.4 | 23.2 | 69.1 | 86.3 |
| %Literacy | 38.6 | 15.5 | 36.8 | 77.7 | 59.8 | 17.3 | 60.6 | 76.3 | 84.1 | 10.0 | 85.7 | 88.3 |
| %Water supply | 6.5 | 10.4 | 1.5 | 73.0 | 30.0 | 22.0 | 24.9 | 90.7 | 71.6 | 21.4 | 75.2 | 100.0 |
| %Sewerage | 2.6 | 4.7 | 0.0 | 28.8 | 8.6 | 16.2 | 0.0 | 73.1 | 30.6 | 30.8 | 20.5 | 98.7 |
| % Occupancy condition of the households | 54.9 | 21.1 | 55.4 | 91.5 | 66.2 | 15.1 | 66.3 | 85.4 | 76.3 | 9.1 | 76.7 | 51.9 |
| Municipal GDP per capita | 0.9 | 0.7 | 0.7 | 6.0 | 2.9 | 2.4 | 2.2 | 13.6 | 5.8 | 5.6 | 4.2 | 49.2 |
Range: difference between maximum and minimum values. SD: Standard Deviation. GDP: Gross Domestic Product, in 1,000 Brazilian Reais (BRL), adjusted to the base year of 2000. n = number of sampled municipalities.
Table 2 also shows descriptive statistics for the explanatory variables that composed the study. They all showed remarkable increasing values between 1947–1953 and 2010–2015, especially the sanitary variables related to water supply and sewerage coverages. On average, urbanization varied from 25.6% to 68.4% (a 2.6-fold increase); literacy from 38.6% to 84.1% (2.1-fold increase); coverage of water supply network from 6.5% to 71.6% (an 11-fold increase); coverage of sewerage from 2.6% to 31.0% (an 11.9-fold increase); condition of occupancy conditions of households from 54.9% to 76.3% (a 1.4-fold increase); and GDP from 0.91 to 5.77 (BRL) (a 6.3-fold increase). Additionally, for the 41 municipalities common to the three surveys, the percentage decrease in prevalence between the 1947–1953 survey and the 2010–2015 survey ranged from 0.1 percentage point (p.p) to 77.4 p.p., with only three municipalities presenting small positive percentage difference between 0.4 and 0.1 p.p.
Table 3 shows the hierarchical (multilevel) description adopted in the study, detailing the distribution of the number of municipalities according to regions and federative units state in each analyzed period. Regarding the distribution of the studied municipalities along the five geographical regions of the country, 758 (44.0%) are from to the Northeast, 506 (29.4%) from the Southeast, 206 (11.9%) from the South, 153 (8.9%) from the Midwest, and 98 (5.7%) from the North region. The Northeast region had the highest percentages of municipalities in each survey, following by the Southeast. In 1947–1953 (n = 907), the survey included 418 (46.0%) municipalities from the Northeast, 317 (35.0%) from the Southeast, 103 (11.4%) from the South, 69 (7.6%) from the Midwest, and no samples from the North region. For 1975–1979 (n = 293), 114 (38.9%) sampled municipalities belonged to the Northeast region, 73 (24.9%) to the Southeast, 50 (17.1%) to the South, 40 (13.7%) to the Midwest, and 16 (5.3%) to the North. Finally, in 2010–2015 (n = 521), 226 (43.4%) municipalities were in the Northeast, 116 (22.3%) in the Southeast, 82 (15.7%) in the North, 53 (10.2%) in the South, and 44 (8.4%) in the Midwest regions.
Table 3. Hierarchical levels and the distribution of the 1,721 sampled Brazilian municipalities (Level 1) included in the study according to state (Level 2) and region (Level 3) for each period.
| Level 3 | Level 2 | Level 1 | |||||
|---|---|---|---|---|---|---|---|
| Region | State | Municipalities | |||||
| IHE (1947–1953) | PECE (1975–1979) | INPEG (2010–2015) | |||||
| n | (%) | n | (%) | n | (%) | ||
| Northeast | Alagoas | 21 | 2.3 | 10 | 3.4 | 24 | 4.6 |
| Bahia | 123 | 13.6 | NA | – | 47 | 9.0 | |
| Ceará | 60 | 6.6 | 22 | 7.5 | 21 | 4.0 | |
| Maranhão | 29 | 3.2 | 16 | 5.5 | 23 | 4.4 | |
| Paraíba | 36 | 4.0 | 17 | 5.8 | 21 | 4.0 | |
| Pernambuco | 61 | 6.7 | 13 | 4.4 | 29 | 5.6 | |
| Piauí | 16 | 1.8 | 12 | 4.1 | 19 | 3.7 | |
| Rio Grande do Norte | 41 | 4.5 | 13 | 4.4 | 20 | 3.8 | |
| Sergipe | 31 | 3.4 | 11 | 3.7 | 22 | 4.2 | |
| Subtotal | 418 | 46.0 | 114 | 38.9 | 226 | 43.4 | |
| North | NA | Subtotal | NA | – | 10 | 1.9 | |
| Amapá | NA | Acre | NA | – | 5 | 1.0 | |
| Amazonas | NA | – | NA | – | 15 | 2.9 | |
| Pará | NA | – | 16 | 5.5 | 19 | 3.7 | |
| Rondônia | NA | – | NA | – | 13 | 2.5 | |
| Roraima | NA | – | NA | – | 7 | 1.3 | |
| Tocantins | NA | – | NA | – | 13 | 2.5 | |
| Subtotal | 0 | 0 | 16 | 5.5 | 82 | 15.7 | |
| Midwest | Distrito Federal | NA | – | NA | – | 1 | 0.2 |
| Goiás | 49 | 5.4 | 25 | 8.5 | 18 | 3.5 | |
| Mato Grosso | 20 | 2.2 | 7 | 2.4 | 12 | 2.3 | |
| Mato Grosso do Sul | NA | – | 8 | 2.7 | 13 | 2.5 | |
| Subtotal | 69 | 7.6 | 40 | 13.6 | 44 | 8.4 | |
| Southeast | Espírito Santo | 18 | 2.0 | 10 | 3.4 | 16 | 3.1 |
| Minas Gerais | 250 | 27.6 | 52 | 17.7 | 56 | 10.8 | |
| Rio de Janeiro | 49 | 5.4 | 11 | 3.7 | 21 | 4.0 | |
| São Paulo | NA | NA | – | 23 | 4.4 | ||
| Subtotal | 317 | 35.0 | 73 | 24.9 | 116 | 22.3 | |
| South | Paraná | 58 | 6.4 | 7 | 2.4 | 21 | 4.0 |
| Rio Grande do Sul | NA | – | 25 | 8.5 | 14 | 2.7 | |
| Santa Catarina | 45 | 5.0 | 18 | 6.1 | 18 | 3.5 | |
| Subtotal | 103 | 11.4 | 50 | 17.1 | 53 | 10.2 | |
| Total | 907 | 293 | 521 | ||||
NA: not analyzed. School Helminthological Survey (IHE). National Survey on the Prevalence of Schistosomiasis and Soil-transmitted helminth infections (INPEG). Special Schistosomiasis Control Program (PECE).
Statistical models
Because of the larger amount of municipalities with zero cases of schistosomiasis (45.4% for 1947–1953 period; 54.2% for 1974–1979 period and 73.7% for 2010–2015 period), models with and without the adjustment for excess of zeros were employed in order to verify the robustness and consistency of the analyses.
The results between the goodness-of-fit measures for the adjusted models (Poisson and Negative Binomial with and without zero-inflation) can be verified in Table A in S3 Note. The Poisson models presented lower residual variance than Binomial Negative models, being the Poisson zero-inflated specification the model with the lowest AIC and BIC values. Table 4 shows the Rate Ratio (RR) estimates for schistosomiasis infection, and the respective 95% confidence intervals (CI) obtained from the zero-inflated Poisson multilevel regression model.
Table 4. Results from the zero-inflated Poisson multilevel regression model fitted to assess the prevalence of schistosomiasis mansoni in the sampled Brazilian schoolchildren.
| Coefficient | Poisson regression | |||
|---|---|---|---|---|
| RR | (CI 95%) | Estimate | P-value | |
| Model constant (intercept) | - | - | -5.488 | <0.001 |
| LN Population | 0.862 | (0.825–0.901) | -0.148 | <0.001 |
| %Urbanization | 0.991 | (0.989–0.993) | -0.009 | <0.001 |
| % Occupancy condition of the household | 0.986 | (0.983–0.989) | -0.014 | <0.001 |
| %Water supply | 1.010 | (1.008–1.011) | 0.010 | <0.001 |
| %Sewerage | 0.996 | (0.994–0.998) | -0.004 | <0.001 |
| Year: 1975–1979 | 1.352 | (1.256–1.454) | 0.301 | <0.001 |
| Year: 2010–2015 | 0.214 | (0.184–0.249) | -1.542 | <0.001 |
| Coefficient | Zero-inflation logistic regression | |||
| OR | (CI 95%) | Estimate | P-value | |
| Model constant (intercept) | - | - | -8.647 | <0.001 |
| %Urbanization | 0.976 | (0.961–0.991) | -0.025 | 0.002 |
Residuals Variance: 4,283.5. AIC: 11,162.2. BIC: 11,233.1. Reference year: 1950. CI: Confidence interval. LN: natural logarithm. RR: Rate Ratio. OR: odds ratio. Standard deviation of random effects: Municipality 2.136; State 2.066; Region 1.706.
The explanatory variables that remained in the model of the prevalence of schistosomiasis were the natural logarithm of population size, %Urbanization, %Occupancy condition of the domicile, %Water supply, %Sewerage, and the categorical variable related to the survey period (the 1947–1953 period was used as a reference for the analysis). For the zero-inflation logistic regression, only variable %Urbanization showed statistical significance.
A negative value in the estimate of the effect of a variable indicates that an increase in its value results in a decrease in the prevalence of the infection. This was the case for the variables natural logarithm of population size (-0.148; p-value <0.001), %Urbanization (-0.009; p-value <0.001), % Occupancy condition of the households (-0.014; p-value <0.001), and % Sewerage (-0.004; p-value 0.001) in the modeling of prevalence. Based on the associated RR, the increase of one unit in the numerical value of these variables causes a decrease of 13.8%, 0.9%, 1.4%, and 0.4% in the estimated mean for the prevalence, respectively. We highlight that one unit increase in the natural logarithm scale corresponds to an increase of approximately 2.718 times in the original variable scale. On the other hand, the results showed an inverse effect on the prevalence of infection for the water supply variable, with a positive value for its estimated effect (0.010; p-value <0.001; and RR corresponding to an increase of only 0,1% per one unit increase in the numerical value of the variable). Concerning the categorical variable representing the survey periods, in comparison with period 1947–1953 (taken as reference in the regression model) a positive regression effect was estimated for 1975–1979 (0.301; p-value <0.001) and a negative effect was estimated for 2013 (-1.542; p-value <0.001). Although this result seems to indicate an increase in prevalence from 1947–1953 to 1975–1979, contradicting the descriptive analysis shown in Table 2, it should be noted that the behavior of the other explanatory variables is quite different between those periods. In fact, an analysis of the municipal prevalence estimated by the model provided similar and consistent results with those observed in the data, corroborating the adequacy of the adjusted model (see Table B in S3 Note).
The use of GLMMS allowed the joint modeling of data from all municipalities in the three sampling periods. In order to evaluate the robustness of this approach, we performed a sensitivity analysis involving the data subset composed of the 41 common municipalities between the three sampling periods (see Table C in S3 Note). The zero-inflated Poisson was the best fitted model and composed of the same explanatory variables to explain the prevalence of schistosomiasis as for the multilevel zero-inflated Poisson model presented in Table 4. The estimates of the coefficients and Rate Ratio (RR) are similar. We also performed a statistical analysis comparing the distribution of the municipalities of the three surveys according to the endemicity classification used in the sampling procedure of the third survey (2010–2015). The results indicated that, although there are differences in the form of data collection regarding the selection of municipalities, the three samples are comparable in terms of the endemicity degree criterion used in the 2010–2015 survey.
Discussion
The analysis identified significant effects of environmental, economic, and demographic factors on the prevalence of schistosomiasis by evaluating its trend during the three national surveys. Hence, this study found significant associations between environmental factors and schistosomiasis. The descriptive analysis among the municipalities common to the three surveys indicated a decrease in the prevalence percentages for most of the analyzed municipalities (92.7%), when compared 1947–1953 and 2010–2015. The fitted statistical model also predicted a decreasing behavior in the prevalence among the three sampling surveys.
The results of the statistical model of this study showed that the environmental variables contributed significantly to the prevalence of schistosomiasis. The protective association between the expansion of sewerage coverage and the reduction of prevalence has been portrayed in epidemiological studies since the 1960s [25]. For instance, a significant association with the disease prevalence was found in households with any type of sewage disposal when compared to those using a safe sewage network (OR 1.8; CI 1.3–2.4) [26]. This result is in line with national and international studies, showing that improvement of sanitation was significantly associated with a decreased probability of infection [27,28]. Even when latrines were available, families’ preference for their use also reduced the occurrence of the disease [29], which was found when households lacked a functional toilet [30,31].
Therefore, although Brazil had sanitary sewage networks in only 60.3% of its municipalities in 2017 [32], the impact of this service in the interruption of the disease is evident, as a sanitary barrier to fecal contamination in water bodies containing intermediate hosts. The results of this study validate the importance of public policies promoting the implementation of sanitation solutions. According to these results, if municipalities with a coverage of 20% of the sewage system, a common situation in some areas of the country, reach 100% coverage, a 27.4% (value obtained from the equation: exp (-0.004*80) = 0,726) reduction in the average prevalence of schistosomiasis can be expected, which is an important outcome in terms of public health.
In addition, the treatment and supply of safe drinking water have been considered another environmental variable as an effective and lasting measure to prevent disease [26,33–35]. Some studies, in convergence with this research, found no significant association between drinking water supply and reduced prevalence of schistosomiasis [36–39]. Although schistosomiasis is not a waterborne disease, adequate water supply is expected to be positively associated with its control, by avoiding the need for individuals to have contact with surface water in order to fetch water for household supply. Thus, it is reasonable to assume that the presence of piped water should not pose a risk of transmission. However, although the results of this study indicate a controversial finding, three possible explanations can be put forward.
Firstly, the infrastructure for piped water supply has expanded over the decades, but this expansion has not guaranteed uninterrupted supply, or the quality of water supplied. Even in a more recent period, in 2006, the irregularity in water supply from the public network can affect about 80% of Brazilian municipalities in certain regions, like the state of Bahia, where schistosomiasis is endemic [40]. Moreover, the Northeast and Southeast regions, which presented the highest prevalence of the disease, exhibited the highest frequencies of systematic interruptions in the water supply in 2020, reaching 66.1% and 46.5%, respectively [41]. This intermittence can lead users to depend on contact with unsafe water sources, contacts that may even increase at day times of high schistosomiasis transmission. Consequently, even in municipalities with households supplied with piped water, there could be a high probability of infection by the disease. Intermittent water supply can disrupt family dynamics, a situation directly related to obligations that often still fall on women. In a society and economy marked by the sexual division of labor, this dynamic leads to the penalization mainly of women and their children, who end up accompanying their mothers [42] in using unsafe water sources, a risk factor in the dynamics of schistosomiasis transmission, reported since the 1980s [43].
In addition, discontinuity of water supply produces other adverse effects, such as disruption of water networks designed for continuous supply, leading to leaks and deterioration of the water quality. Consequently, users adapt to meet adversities, highlighting the inequality and vulnerability to shortages to which a city or region is exposed [44]. An intervention study showed that the positive impact of piped water occurred only when the amount of water available was higher than 1,000 liters per person per year, i.e., the use of unsafe water can continue if only a small amount of water is provided or if there are interruptions due to precarious distribution systems [45].
The second explanation regarding disease prevalence despite the availability of piped water is related to a supply insufficiency for some households to eliminate other contact forms with surface water for domestic, leisure, behavioral, or labor use, such as fishing and irrigation. Eventually, the presence of piped water supply may free up more time for residents to perform these activities more frequently, increasing the risk of contamination. When disassociated from facilities for other home uses, such as laundry, sink, and shower, piped water supply can contribute to the continuity or increase of the behavior of accessing transmission sites [46,47]. Another aspect related to the water contact practices was demonstrated by a spatial community study verifying that the public water supply could potentially decrease dependence on surface water. However, this relationship was modified by the quality of the water from the sources of public supply, which was considered poor by domestic users [48,49].
Thirdly, an aspect probably not strongly related to our results although worthy of analysis, is the effect of the technology used in surface uptake, adduction, and water treatment on dermal contact and survival of infectious forms of the schistosome. Filtration and chlorination are widely used methods for water treatment in conventional and simplified treatment plants all over the country [50,51]. These processes are credited as likely to produce waters free of contamination from cercariae, depending on storage time, exposure temperature, chlorine concentration, or filtration rates, besides the concentration of cercariae itself [51]. However, there are no current guidelines for the specific care related to water treatment and its respective technical and operational infrastructure in endemic schistosomiasis regions, as demonstrated by other systematic reviews [47,51].
Therefore, operational deficiencies such as lack of water treatment have been observed despite Brazil have enhanced access to water supply networks and infrastructure since the 1940s. In 1948, shortly before implementation of the first survey included in this study, only 9% of municipalities received treated water, a deficiency even more prominent in rural areas [52]. Incomplete water treatment and deficient distribution systems are still a reality since 11.7% of Brazilian municipalities still lacked operative water treatment plants, either conventional or simplified, in 2017 [32,53].
Other conditions different from environmental factors contributed to the decrease in disease prevalence, such as the condition of household occupation, degree of urbanization, and population size. Low socioeconomic status is a known risk factor for diseases caused by parasitic infections such as schistosomiasis [27,54]. In this study, residents’ housing conditions, such as acquired households and owned rather than rented dwellings, were used as a proxy for socioeconomic status. A similar conclusion was obtained in studies in Pakistan, Bangladesh, and Thailand, with families living in rented houses at increased risk of developing infectious diseases or their symptoms, including parasitic bowel diseases, compared with families who owned their housing [55–57]. Thus, the condition of home ownership was associated as a protective factor against disease, demonstrating that socioeconomic structure can produce and condition the distribution of schistosomiasis in the population.
Regarding the degree of urbanization, recent outbreaks of schistosomiasis have been prevalent in urban and peri-urban environments due to unplanned urbanization [58–62]. On the other hand, rapid urbanization has implications for infectious diseases usually described in rural areas and reduces the risk of exposure to infection in previously endemic areas [62]. Thus, the effect on epidemiological patterns of the relationship between demographic events of inter and intra-regional migratory flows with economic cycles of retraction and expansion of agricultural and industrial activities experienced by the country is undeniable. This relationship generated the model of capitalist expansion and economic growth, sometimes excluding but also enabling the last seven decades of educational, sanitary, economic, and infrastructure improvements that also resulted in changes to epidemiological patterns of infectious diseases [63,64]. Therefore, urbanization is assumed as a protective trait against the disease, which could be reverberate in the institutional feasibility of increasing and expanding public health and sanitary policies. The establishment of the Brazilian Unified Health System (SUS), including an alternative model focused on the promotion and prevention of health from the decentralization of strategies and programs for the control of schistosomiasis, is an example of these health and sanitary polices [65,66]. Other public policies, which could correspond to the changes that have occurred over the decades, are water and sanitation services, such as the National Sanitation Plan (Plano Nacional de Saneamento–Planasa), established in 1971 and abolished at the end of the following decade, and the current federal basic sanitation policy from Law No. 11.445/2007 and No. 14.026/2020. Although increased access to public services was considered deeply discriminatory in the 1970s regarding demographic and social criteria and currently poses risks concerning the universal access to services and human rights, they were essential instruments for expanding public water supply and sanitary sewage networks in the country [67,68].
Regarding the parasitological tests used in the surveys, although two different methods were used, the comparability between them is possible. Firstly, during the 1947–1953 IHE Brazil presented a high prevalence of schistosomiasis and a high intensity of infection, implying that the application of less sensitive diagnostic methods, such as the technique of spontaneous sedimentation in water (Hoffman, Pons et al. Janer, or HPJ technique) [69], leads to a low number of false-negative [70]. Secondly, the results obtained in the two last surveys (PECE and INPEG), which used the Kato-Katz method, could identify a greater number of true positive, with a low detection of false-negatives due to superior sensitivity of the method. It is well known that the Kato-Katz method is currently the gold standard method recommended by the WHO [3,71].
The applied statistical analysis is supported for the structure of the data and allowed revealing important results not yet studied in the country, considering the representativeness of a national sample and with historical temporality. The option of using GLMMs is based on the fact that it allowed to use the information from all municipalities of the three surveys (n = 1721) in the analysis and improving the estimation of the parameters of the model, respective standard deviations and p-values. It is well known that multilevel models (with random effects) provide better inference from grouped data (in the case of the presented study, students are grouped in municipalities which are grouped in states which are grouped in regions) since the coefficient and variance error for each explanatory variable are better estimated, avoiding the problem of underestimation of coefficients and overstatement of their significance that occur when clustering effect is not taken into account [19]. Summary statistics for the prevalence estimated by the model were consistent with those observed in the data. Sensitivity analysis shown that results obtained using the zero-inflated Poisson GLMM are consistent with those found in the restricted analysis of samples common to the three surveys.
In general, the findings of this research show that the reduction in the prevalence of schistosomiasis in Brazil over seven decades can be explained by the combination of community, demographic, socioeconomic, and specific environmental factors. The ecological design of the study, with the municipality as the unit of analysis, impairs including behavioral and other individual variables in the model, likely associated with infection. The mechanism of schistosomiasis transmission is complex and includes several conditioning factors [72]. Thus, disease control depends on preventive measures, such as early diagnosis and timely treatment, health education, surveillance and control of intermediate hosts, and basic sanitation. It is also noteworthy that the Brazilian regions differ in how their governments administer the promotion of disease control policies, especially among states that differ in aspects like location, territorial extension, and environmental and socioeconomic conditions that could interfere with the disease cycle. In line with findings of other studies, differences between forms of access and exposure to water and sanitation relate to variations in disease infection rates over time and in different regions, suggesting that the impact of access to water and sanitation is mediated by other social, behavioral, and environmental factors [73].
Limitations
Although the results obtained in this study came from different municipalities and in different periods, consisting of non-serial temporal trend surveys, the analysis of municipalities common to the three surveys supported the other findings (see S3 Note). Other limitations must be considered when interpreting these results. The variables were collected in different periods, and such practice of collecting old census data required a process of harmonization between variables to allow comparisons. Another limitation inherent to census data includes the availability of access-restricted information on public service facilities and not the quality and availability of WaSH services. Further exploration of other data is necessary to understand the positive association between the prevalence of schistosomiasis and the availability of drinking water networks, including the effects of supply interruptions and changes in use based on water quality or behavioral and occupational habits. Finally, we cannot make conclusions on the causality of this association due to a limitation of ecological design.
To the best of our knowledge, this is the first study that used a longitudinal epidemiological design to analyze data from national prevalence surveys covering a large period of many decades. The results showed that the prevalence of schistosomiasis infection in schoolchildren in Brazilian municipalities decreased significantly over the decades. This decrease in prevalence of infection may be associated with environmental factors, urbanization, and housing conditions, which have improved over the decades. It is noteworthy that the association with water supply should be carefully interpreted and focused on other possible factors not evaluated here, confirming the need for a systemic approach. In addition, safe sanitation sewage should be widely provided to the population at the household level and other spheres of life, such as workplaces, health centers and school environments. Other national prevalence surveys and research should be conducted more continuously to monitor the disease prevalence and its determinants over time.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank the task teams responsible for organizing and operationalizing the research field in all surveys. We are immensely grateful to the researcher Prof. Dr. Naftale Katz, from Instituto René Rachou/Fiocruz Minas, who assisted in making this research feasible by guiding us to the source and acquisition of data and for sharing with us his experience in conducting surveys.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was carried out with the support of the Coordination for the Improvement of Higher Education Personnel – Brazil (CAPES) – Financing Code 001 (https://www.gov.br/capes/pt-br), which granted financial aid in the form of a scholarship granted to MCSS. The financier did not participate in the study design, data collection and analysis, publication decision or manuscript preparation.
References
- 1.World Health Organization (WHO). Schistosomiasis: progress report 2001–2011, strategic plan 2012–2020. Geneva: WHO Library Cataloguing-in-Publication; 2013. Available from: https://apps.who.int/iris/handle/10665/78074. [Google Scholar]
- 2.WHO. Schistosomiasis and soiltransmitted helminthiases: progress report, 2021. 2022. Available from: https://www.eliminateschisto.org/resources/who-wer-9648-schistosomiasis-and-soil-transmitted-helminthiases-progress-report-2020.
- 3.WHO. World Health Organization. GUIDELINE on control and elimination of human schistosomiasis. Geneva: World Intellectual Property Organization; 2022. Available from: https://www.who.int/publications/i/item/9789240041608. [PubMed] [Google Scholar]
- 4.Cairncross S, Feachem R. Environmental Health Engineering in the Tropics: Water, Sanitation and Disease Control. 3o. UK, NY: Routledge; 2019. Available from: https://www.crcpress.com/Environmental-Health-Engineering-in-the-Tropics-Water-Sanitation-and-Disease/Cairncross-Feachem/p/book/9781844071913.
- 5.Casavechia MTG, Melo G de AN de, Fernandes ACBDS, Castro KRD, Pedroso RB, Santos TDS, et al. Systematic review and meta-analysis on Schistosoma mansoni infection prevalence, and associated risk factors in Brazil. Parasitology. 2018;145: 1000–1014. doi: 10.1017/S0031182017002268 [DOI] [PubMed] [Google Scholar]
- 6.Barbosa C, Carvalho O, Coelho P. In: Schitosoma mansoni e esquistossomose: uma visão multidisciplinar. Epidemiologia e controle da Esquistossomose mansoni. Rio de Janeiro: Fiocruz; 2008. pp. 964–1008. [Google Scholar]
- 7.Pellon AB, Teixeira I. Distribuição da esquistossomose mansônica no Brasil. Divisão de Organização Sanitária do Ministério da Saúde. Rio de Janeiro: MS; 1950. [Google Scholar]
- 8.Pellon AB, Teixeira I. O Inquérito helmintológico escolar em cinco Estados das regiões: leste, sul e centro-oeste. Divisão de Organização Sanitária do Ministério da Saúde. Rio de Janeiro: MS; 1953. [Google Scholar]
- 9.Brasil. Ministério da Saúde. Levantamento Nacional de Prevalência da esquisstossomose mansoni, 1975–1979. Programa Especial de Controle da Esquistossomose. Brasília; 1981.
- 10.Katz N. Inquérito Nacional de Prevalência da Esquistossomose mansoni e Geo-helmintoses. Belo Horizonte: CPqRR; 2018. Available from: http://tabnet.datasus.gov.br/cgi/sinan/inpeg/RelatorioINPEG.pdf.
- 11.Brasil. Ministério da Saúde. Doenças tropicais negligenciadas 30 de janeiro–Dia mundial de combate às Doenças tropicais negligenciadas. Secretaria de Vigilância em Saúde; 2021. Available from: https://www.gov.br/saude/pt-br/media/pdf/2021/marco/3/boletim_especial_doencas_negligenciadas.pdf.
- 12.Brasil. Conselho Nacional de Saúde (CNS). 1. Sect. 3, Resolução n.o 466, de 12 de dezembro de 2012. Dec 12, 2012 p. 12. [Google Scholar]
- 13.Brasil. Conselho Nacional de Saúde (CNS). 1. Sect. 3, RESOLUÇÃO No 510 2016. p. 10. [Google Scholar]
- 14.Barbosa CS, Favre TC, Amaral RS, Pieri OS. Epidemiologia e controle da Esquistossomose mansoni. CARVALHO, OS., COELHO, PMZ., and LENZI, HL., orgs. Schitosoma mansoni e esquistossomose: uma visão multidisciplinar. CARVALHO, OS., COELHO, PMZ., and LENZI, HL., orgs. Rio de Janeiro: Fiocruz; 2008. pp. 964–1008.
- 15.Schall VT, Massara CL, Diniz MCP. Educação em saúde no controle da esquistossomose. Fundação Oswaldo Cruz; 2008. Available from: https://www.arca.fiocruz.br/handle/icict/40285. [Google Scholar]
- 16.Jannuzzi PM. Projeções populacionais para pequenas áreas: método e aplicações. Escola Nacional de Ciências Estatísticas Rio de Janeiro ISSN 1677-7093. 2006Textos para discussão: 67. [Google Scholar]
- 17.Libânio M, Neto M, Prince A, Sperling M, Heller L. Consumo de Água. In: Heller L, Pádua VL, editors. Abastecimento de água para consumo humano. 3rd ed. Belo Horizonte: UFMG; 2016. [Google Scholar]
- 18.Santos J, Gibim G. Cálculo numérico. In: Unidade 3: interpolação. Londrina: Editora e Distribuidora Educacional S.A; 2015. [Google Scholar]
- 19.Lambert D. Zero-Inflated Poisson Regression, with an Application to Defects in Manufacturing. Technometrics. 1992;34: 1–14. doi: 10.2307/1269547 [DOI] [Google Scholar]
- 20.Hilbe JM. Negative Binomial Regression. 2nd ed. New York, EUA: Cambridge University Press; 2011. [Google Scholar]
- 21.Bolker B. Linear and Generalized Linear Mixed Models. In: Fox E by GA, Negrete-Yankelevich S, Sosa and VJ, editors. Ecological Statistics: Oxford, New York: Oxford University Press; 2015. pp. 378–379. [Google Scholar]
- 22.Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, et al. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol. 2009;24: 127–135. doi: 10.1016/j.tree.2008.10.008 [DOI] [PubMed] [Google Scholar]
- 23.Chakrabarti A, Ghosh JK. AIC, BIC and Recent Advances in Model Selection. In: Bandyopadhyay PS, Forster MR, editors. Philosophy of Statistics. Amsterdam: North-Holland; 2011. pp. 583–605. doi: 10.1016/B978-0-444-51862-0.50018-6 [DOI] [Google Scholar]
- 24.Brooks ME, Kristensen K, Benthem KJ van, Magnusson A, Berg CW, Nielsen A, et al. glmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modeling. The R Journal. 2017;9: 378–400. [Google Scholar]
- 25.Farooq M, Nielsen J, Samaan SA, Mallah MB, Allam AA. The epidemiology of Schistosoma haematobium and S. mansoni infections in the Egypt-49 project area. 2. Prevalence of bilharziasis in relation to personal attributes and habits. Bull World Health Organ. 1966;35: 293–318. [PMC free article] [PubMed] [Google Scholar]
- 26.Barreto ML. Geographical and socioeconomic factors relating to the distribution of Schistosoma mansoni infection in an urban area of north-east Brazil. Bull World Health Organ. 1991;69: 93–102. [PMC free article] [PubMed] [Google Scholar]
- 27.Ximenes R, Southgate B, Smith PG, Guimarães Neto L. Socioeconomic determinants of schistosomiasis in an urban area in the Northeast of Brazil. Rev Panam Salud Publica. 2003;14: 409–421. doi: 10.1590/s1020-49892003001100006 [DOI] [PubMed] [Google Scholar]
- 28.Kabatereine NB, Standley CJ, Sousa-Figueiredo JC, Fleming FM, Stothard JR, Talisuna A, et al. Integrated prevalence mapping of schistosomiasis, soil-transmitted helminthiasis and malaria in lakeside and island communities in Lake Victoria, Uganda. Parasites & Vectors. 2011;4: 232. doi: 10.1186/1756-3305-4-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abou-Zeid AHA, Abkar TA, Mohamed RO. Schistosomiasis and soil-transmitted helminths among an adult population in a war affected area, Southern Kordofan state, Sudan. Parasit Vectors. 2012;5: 133. doi: 10.1186/1756-3305-5-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sady H, Al-Mekhlafi HM, Mahdy MAK, Lim YAL, Mahmud R, Surin J. Prevalence and Associated Factors of Schistosomiasis among Children in Yemen: Implications for an Effective Control Programme. PLoS Negl Trop Dis. 2013;7: e2377. doi: 10.1371/journal.pntd.0002377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ugbomoiko US, Dalumo V, Danladi YK, Heukelbach J, Ofoezie IE. Concurrent urinary and intestinal schistosomiasis and intestinal helminthic infections in schoolchildren in Ilobu, South-western Nigeria. Acta Trop. 2012;123: 16–21. doi: 10.1016/j.actatropica.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 32.Brasil. Pesquisa Nacional de Saneamento Básico 2017: abastecimento de água e esgotamento sanitário. Instituto Brasileiro de Geografia e Estatística; 2020. Available from: https://biblioteca.ibge.gov.br/visualizacao/livros/liv101734.pdf.
- 33.Assefa A, Dejenie T, Tomass Z. Infection prevalence of Schistosoma mansoni and associated risk factors among schoolchildren in suburbs of Mekelle city, Tigray, Northern Ethiopia. Momona Ethiopian Journal of Science. 2013;5: 174–188. doi: 10.4314/mejs.v5i1.85339 [DOI] [Google Scholar]
- 34.Enk MJ, Lima ACL, Barros H da S, Massara CL, Coelho PMZ, Schall VT. Factors related to transmission of and infection with Schistosoma mansoni in a village in the South-eastern Region of Brazil. Memórias do Instituto Oswaldo Cruz. 2010;105: 570–577. doi: 10.1590/s0074-02762010000400037 [DOI] [PubMed] [Google Scholar]
- 35.Coura-Filho P, Rocha RS, Lamartine S da S, Farah MW, de Resende DF, Costa JO, et al. Control of schistosomiasis mansoni in Ravena (Sabará, state of Minas Gerais, Brazil) through water supply and quadrennial treatments. Mem Inst Oswaldo Cruz. 1996;91: 659–664. doi: 10.1590/s0074-02761996000600001 [DOI] [PubMed] [Google Scholar]
- 36.Guimarães MDC, Costa MFF de L e, Lima LB de, Moreira MA. Clinical-epidemiological study of schistosomiasis mansoni in school children of Ilha, Arcos County, Minas Gerais, Brazil, 1983. Rev Saúde Pública. 1985;19: 8–17. doi: 10.1590/S0034-89101985000100002 [DOI] [PubMed] [Google Scholar]
- 37.Palmeira DCC, Carvalho AG de, Rodrigues K, Couto JLA. Prevalência da infecção pelo Schistosoma mansoni em dois municípios do Estado de Alagoas. Rev Soc Bras Med Trop. 2010;43: 313–317. doi: 10.1590/S0037-86822010000300020 [DOI] [PubMed] [Google Scholar]
- 38.Lima e Costa MFF, Rocha RS, Leite MLC, Carneiro RG, Colley D, Gazzinelli G, et al. A multivariate analysis of socio-demographic factors, water contact patterns and Schistosoma mansoni infection in an endemic area in Brazil. Rev Inst Med trop S Paulo. 1991;33: 58–63. doi: 10.1590/s0036-46651991000100011 [DOI] [PubMed] [Google Scholar]
- 39.Firmo JO, Lima Costa MF, Guerra HL, Rocha RS. Urban schistosomiasis: morbidity, sociodemographic characteristics and water contact patterns predictive of infection. Int J Epidemiol. 1996;25: 1292–1300. doi: 10.1093/ije/25.6.1292 [DOI] [PubMed] [Google Scholar]
- 40.Filho SSA, Borja PC, Moraes LRS, Souza DN. Desigualdade no acesso à água de consumo humano: uma proposta de indicadores. Brazilian Journal of Environmental Sciences (Online). 2010; 43–55. [Google Scholar]
- 41.SNIS. Sistema Nacional de Informações sobre Saneamento. Diagnóstico Temático Serviços de Água e Esgoto. Brasília, DF: Ministério do Desenvolvimento Regional Secretaria Nacional de Saneamento; 2021 p. 91. Report No.: 1. Available: http://www.snis.gov.br/downloads/diagnosticos/ae/2020/DIAGNOSTICO_TEMATICO_VISAO_GERAL_AE_SNIS_2021.pdf
- 42.Teixeira J. Saneamento rural no Brasil. In: Heller, L.; Moraes, L.R.S.; Britto, A.L; Borja, P.C.; Rezende, S.C.. Panorama do Saneamento Básico no Brasil. Ministério das Cidades: Secretaria Nacional de Saneamento Ambiental; 2014. Report No.: 7. Available from: https://www.gov.br/mdr/pt-br/assuntos/saneamento/plansab/panorama_vol_07.pdf.
- 43.Coura-Filho P. Uso do paradigma de risco para a esquistossomose em áreas endêmicas no Brasil. Cadernos de Saúde Pública. 1994;10: 464–472. doi: 10.1590/S0102-311X1994000400006 [DOI] [PubMed] [Google Scholar]
- 44.Diniz TG, Grande MHD, Galvão C de O. Vulnerabilidade domiciliar em situação de intermitência no abastecimento de água. Eng Sanit Ambient. 2021;26: 535–543. doi: 10.1590/S1413-415220190038 [DOI] [Google Scholar]
- 45.Noda S, Shimada M, Muhoho ND, Sato K, Kiliku FBM, Gatika SM, et al. Effect of Piped Water Supply on Human Water Contact Patterns in a Schistosoma haematobium-Endemic Area in Coast Province, Kenya. 1997. [cited 24 May 2022]. Available from: https://core.ac.uk/display/58751059?utm_source=linkout. [DOI] [PubMed] [Google Scholar]
- 46.Atalabi TE, Lawal U, Ipinlaye SJ. Prevalence and intensity of genito-urinary schistosomiasis and associated risk factors among junior high school students in two local government areas around Zobe Dam in Katsina State, Nigeria. Parasit Vectors. 2016;9: 388. doi: 10.1186/s13071-016-1672-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braun L, Grimes JET, Templeton MR. The effectiveness of water treatment processes against schistosome cercariae: A systematic review. PLoS Negl Trop Dis. 2018;12. doi: 10.1371/journal.pntd.0006364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kulinkina AV, Kosinski KC, Plummer JD, Durant JL, Bosompem KM, Adjei MN, et al. Indicators of improved water access in the context of schistosomiasis transmission in rural Eastern Region, Ghana. Sci Total Environ. 2017;579: 1745–1755. doi: 10.1016/j.scitotenv.2016.11.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cruz JIN, Salazar G de O, Corte RL, Cruz JIN, Salazar G de O, Corte RL. Retrocesso do Programa de Controle da Esquistossomose no estado de maior prevalência da doença no Brasil. Revista Pan-Amazônica de Saúde. 2020;11. doi: 10.5123/s2176-6223202000567 [DOI] [Google Scholar]
- 50.Nascimento RS do, Curi RC, Curi WF, Oliveira R de, Santana CFD de, Meira CMBS. Simulação de alterações numa ETA convencional de porte médio para a produção de água segura. RBRH. 2016;21: 439–450. doi: 10.21168/rbrh.v21n2.p439-450 [DOI] [Google Scholar]
- 51.Braun L, Sylivester YD, Zerefa MD, Maru M, Allan F, Zewge F, et al. Chlorination of Schistosoma mansoni cercariae. PLoS Negl Trop Dis. 2020;14: e0008665. doi: 10.1371/journal.pntd.0008665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.IBGE. Instituto Brasileiro de Geografia e Estatística. Anuário Estatístico do Brasil (AEB). Fundação Instituto Brasileiro de Geografia e Estatística. Rio de Janeiro: INSTITUTO BRASILEIRO DE GEOGRAFIA E ESTATISTICA; 1951 p. 583. Report No.: XI.
- 53.Formiga-Johnsson RM, Britto AL. Segurança hídrica, abastecimento metropolitano e mudanças climáticas: considerações sobre o caso do Rio de Janeiro. Ambient soc. 2020;23. doi: 10.1590/1809-4422asoc20190207r1vu2020L6TD [DOI] [Google Scholar]
- 54.Gazzinelli A, Velasquez-Melendez G, Crawford SB, LoVerde PT, Correa-Oliveira R, Kloos H. Socioeconomic determinants of schistosomiasis in a poor rural area in Brazil. Acta Trop. 2006;99: 260–271. doi: 10.1016/j.actatropica.2006.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mehraj V, Hatcher J, Akhtar S, Rafique G, Beg MA. Prevalence and Factors Associated with Intestinal Parasitic Infection among Children in an Urban Slum of Karachi. PLOS ONE. 2008;3: e3680. doi: 10.1371/journal.pone.0003680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chowdhury F, Khan IA, Patel S, Siddiq AU, Saha NC, Khan AI, et al. Diarrheal Illness and Healthcare Seeking Behavior among a Population at High Risk for Diarrhea in Dhaka, Bangladesh. PLoS One. 2015;10: e0130105. doi: 10.1371/journal.pone.0130105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chompook P, Todd J, Wheeler JG, Seidlein L von, Clemens J, Chaicumpa W. Risk factors for shigellosis in Thailand. International Journal of Infectious Diseases. 2006;10: 425–433. doi: 10.1016/j.ijid.2006.05.011 [DOI] [PubMed] [Google Scholar]
- 58.Gomes EC de S, Mesquita MC da S, Rehn VNC, Nascimento WRC do, Loyo R, Barbosa CS, et al. Transmissão urbana da esquistossomose: novo cenário epidemiológico na Zona da Mata de Pernambuco. Revista Brasileira de Epidemiologia. 2016;19: 822–834. doi: 10.1590/1980-5497201600040012 [DOI] [PubMed] [Google Scholar]
- 59.Tefera A, Belay T, Bajiro M. Epidemiology of Schistosoma mansoni infection and associated risk factors among school children attending primary schools nearby rivers in Jimma town, an urban setting, Southwest Ethiopia. PLoS One. 2020;15: e0228007. doi: 10.1371/journal.pone.0228007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barbosa CS, Silva CB da, Barbosa FS. Esquistossomose: reprodução e expansão da endemia no Estado de Pernambuco no Brasil. Rev Saúde Pública. 1996;30: 609–616. doi: 10.1590/S0034-89101996000600016 [DOI] [PubMed] [Google Scholar]
- 61.Klohe K, Koudou BG, Fenwick A, Fleming F, Garba A, Gouvras A, et al. A systematic literature review of schistosomiasis in urban and peri-urban settings. PLoS Negl Trop Dis. 2021;15: e0008995. doi: 10.1371/journal.pntd.0008995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buchwald AG, Grover E, Van Dyke J, Kechris K, Lu D, Liu Y, et al. Human Mobility Associated With Risk of Schistosoma japonicum Infection in Sichuan, China. Am J Epidemiol. 2021;190: 1243–1252. doi: 10.1093/aje/kwaa292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schramm JM de A, Oliveira AF de, Leite I da C, Valente JG, Gadelha ÂMJ, Portela MC, et al. Transição epidemiológica e o estudo de carga de doença no Brasil. Ciênc saúde coletiva. 2004;9: 897–908. doi: 10.1590/S1413-81232004000400011 [DOI] [Google Scholar]
- 64.Carvalho EMF de, Acioli MD, Branco MAF, Costa AM, Cesse EAP, Andrade AG de, et al. Evolução da esquistossomose na Zona da Mata Sul de Pernambuco. Epidemiologia e situação atual: controle ou descontrole? Cad Saúde Pública. 1998;14: 787–795. doi: 10.1590/S0102-311X1998000400020 [DOI] [PubMed] [Google Scholar]
- 65.Coura-Filho P. Participação popular no controle da esquistossomose através do Sistema Único de Saúde (SUS), em Taquaraçu de Minas, (Minas Gerais, Brasil), entre 1985–1995: construção de um modelo alternativo. Cad Saúde Pública. 1998;14: S111–S122. doi: 10.1590/S0102-311X1998000600010 [DOI] [PubMed] [Google Scholar]
- 66.ONU. Organização das Nações Unidas. Direitos humanos e a privatização dos serviços de água e esgotamento sanitário. Septuagésima quinta sessão: Organização das Nações Unidas; 2020 Jul p. 23. Report No.: A/75/208.
- 67.Brasil. Lei do Saneamento Básico. Estabelece diretrizes nacionais para o saneamento básico. Sect. Brasília, DF, Lei no 11.445 Jan 5, 2007.
- 68.Brasil. LEI No 14.026. Atualiza o marco legal do saneamento básico. Sect. Brasília, DF, LEI No 14.026 Jul 15, 2020.
- 69.Hoffman WA, Pons JA, Janer JL. The sedimentation concentration method in Schistosomiasis mansoni. J Publ Health and Trop Med. 19349: 283–298. [Google Scholar]
- 70.Xavier de Carvalho GL, Moreira LE, Pena JL, Marinho CC, Bahia MT, Lins Machado-Coelho GL. A comparative study of the TF-Test (R), Kato-Katz, Hoffman-Pons-Janer, Willis and Baermann-Moraes coprologic methods for the detection of human parasitosis. Mem Inst Oswaldo Cruz. 2012;107: 80–84. doi: 10.1590/S0074-02762012000100011 [DOI] [PubMed] [Google Scholar]
- 71.Chaves A, Alcantara OS de, Carvalho O dos S, Santos JS dos. Estudo comparativo dos métodos coprológicos de Lutz, Kato-Katz e Faust modificado. Rev Saúde Pública. 1979;13: 348–352. doi: 10.1590/S0034-89101979000400010 [DOI] [PubMed] [Google Scholar]
- 72.Poague KIHM, Mingoti SA, Heller L. Water, sanitation and schistosomiasis mansoni: a study based on the Brazilian National Prevalence Survey (2011–2015). Ciência & Saúde Coletiva. 2022. Available from: https://cienciaesaudecoletiva.com.br/artigos/water-sanitation-and-schistosomiasis-mansoni-a-study-based-on-the-brazilian-national-prevalence-survey-2011-2015/18447?id=18447. [DOI] [PubMed]
- 73.Grimes JET, Croll D, Harrison WE, Utzinger J, Freeman MC, Templeton MR. The Relationship between Water, Sanitation and Schistosomiasis: A Systematic Review and Meta-analysis. PLoS Negl Trop Dis. 2014;8. doi: 10.1371/journal.pntd.0003296 [DOI] [PMC free article] [PubMed] [Google Scholar]