Abstract
As vectors of numerous plant pathogens, herbivorous insects play a key role in the epidemiology of plant disease. But how phytopathogens impact the metabolism, physiology, and fitness of their insect vectors is often unexplored within these tripartite interactions. Here, we examine the diverse symbioses forged between insects and members of the ascomycete fungal genus Fusarium. While Fusarium features numerous plant pathogens that are causal to diseases such as wilts and rots, many of these microbes also engage in stable mutualisms across several insect clades. Matching a diversity in symbiont localization and transmission routes, we highlight the various roles fusaria fulfill towards their insect hosts, from upgrading their nutritional physiology to providing defense against natural enemies. But as the insect partner is consistently herbivorous, we emphasize the convergent benefit Fusarium derives in exchange: propagation to a novel host plant. Collectively, we point to the synergy arising between a phytopathogen and its insect vector, and the consequences inflicted on their shared plant.
1. Introduction
The genus Fusarium (Sordariomycetes: Hypocreales: Nectriaceae) represents one of the most ubiquitous plant-associated microbes [1]. Time-calibrated phylogenies indicate that fusaria originated approximately 91.3 Mya [2], coinciding with the radiation of flowering plants [3]. Members of the ascomycete genus are distributed worldwide and feature many plant pathogens impacting both managed and natural ecosystems [1,4,5]. By leveraging a remarkable arsenal of toxic secondary metabolites, fusaria cause several plant diseases, including wilts, blights, rots, and cankers [2]. But despite their known impact on plant health, members of the fungal genus are also increasingly recognized for their stable symbioses with insects (Fig 1), spanning a gradient of interactions outcomes. Here, we examine these associations and (1) highlight the repeated and independent origins of insect-fusarial symbioses, (2) outline the range of mutualistic benefits insects derive from these partnerships, and (3) emphasize the importance of the insect host in vectoring Fusarium to naïve plant populations, thereby expanding it transmission range.
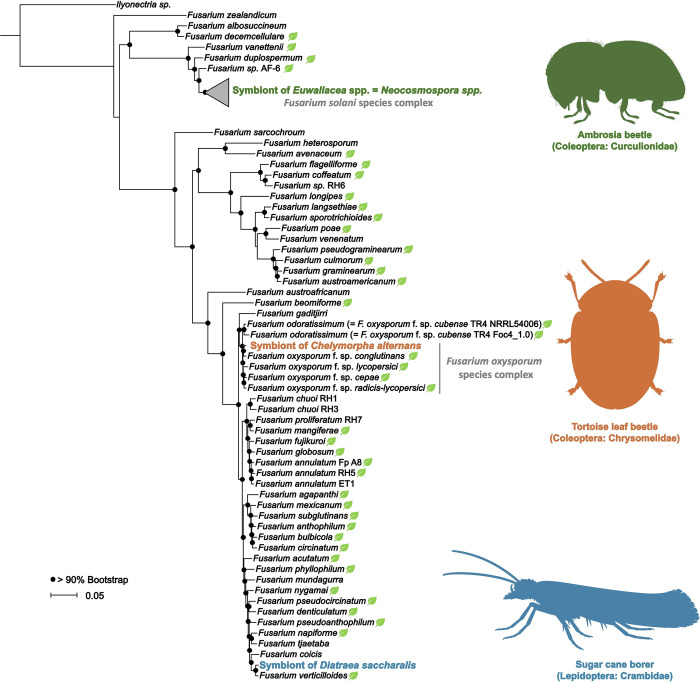
Fig 1. Independent origins of insect-fusarial symbioses.
Maximum Likelihood phylogeny based on a concatenated alignment of 1,087 BUSCO genes (Sordariomycete lineage) revealing the phylogenetic placement of insect-associated fusaria relative to other Fusarium strains. Node coloration reflects bootstrap support >90%. Leaf drawings depict phytopathogenic strains. Insect schematics top to bottom: ambrosia beetle (green), tortoise leaf beetle (orange) and sugar cane borer (blue). Insect schematics created with BioRender.com and not to scale.
1.1. Fusarium the cultivar
Fungal agriculture evolved independently across 3 orders of insects: once in ants, once in termites, and at least 7 times in beetles [6]. Ambrosia beetles (Curculionidae: Scolytinae and Platypodinae) represent a polyphyletic group of arthropods that construct galleries in the woody tissue of trees, where they inoculate and cultivate fungal gardens [6]. The beetles obligately depend on their fungal partners for nutrition, development, and reproduction [7,8]. The most advanced ambrosia farmers are beetles of the Xyleborini tribe, which impact woody plants of economic importance, such as avocado and fig trees [6,9]. Their symbiotic fusaria belong to the Fusarium solani species complex and represent a monophyletic clade that contains at least 12 lineages (Fig 1). The Euwallacea-Fusarium mutualism dates to the Oligocene-Miocene boundary (19 to 24 Mya), which overlaps with the adaptive radiation of the Xyleborini tribe (21 Mya) [10,11].
Ambrosia beetles acquire spores of their fungal partner from the natal garden and carry them in specialized pockets, or mycangia. These structures are critical for the propagation of a fungal inoculum in order to establish a garden in a new host plant [7]. In Euwallacea, these mycangia are located near the oral cavity (Fig 2A–2C) [12]. However, cophylogenetic studies demonstrate incongruent trees across the Fusarium-Euwallacea mutualisms, suggesting multiple host shifts during evolution [11]. Most cultivars have convergently evolved traits or structures that accumulate nutrients and increase their value for their insect farmers. This is the case of gongylidia (enlarged hyphal tips) in ants, nodules in termites, and swollen cells in ambrosia beetles associating with Ophiostomatales or Microascales [6,13,14]. In the same manner, ambrosial fusaria have evolved nutrient rich, club-shaped macroconidia that are consumed by the beetles [11]. Reflecting the ecological success of fungiculture, no reversals to nonsymbiotic lifestyles have been observed in ants, termites, or ambrosia beetles [13–15].
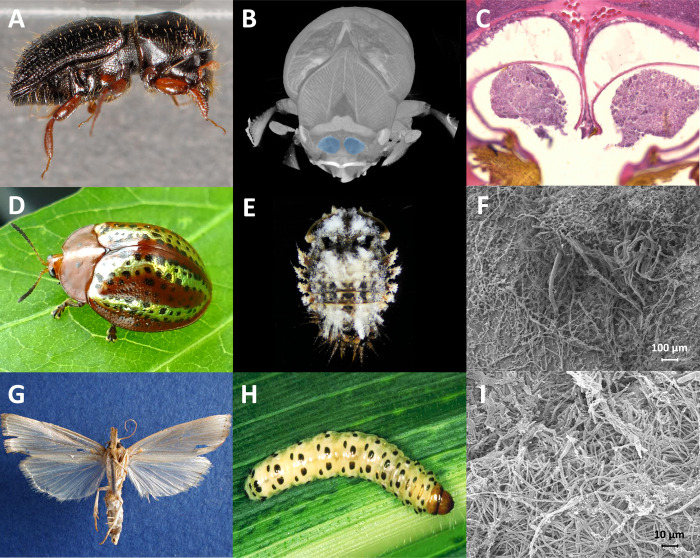
Fig 2. Fusarium localization in insects.
(A-C) Euwallacea validus beetles rely for nutrition on fusarioid fungi that they harbor in pocket-like structures near the oral cavity called “mycangia” [12]. (D-F) Chelymorpha alternans beetles associate with F. oxysporum, which grows on the surface of their pupae during metamorphosis, protecting pupae from predation [22]. (G-I) Diatraea saccharalis, the sugarcane borer partners with F. verticilloides, which colonizes the caterpillar gut [37] and indirectly protects the larvae against parasitoid attack.
1.2. Fusarium as a defensive symbiont
Predators, pathogens, and other natural enemies represent strong selective pressures for organisms to evolve effective defensive strategies [16]. Symbioses with beneficial microbes serve as a rich source of defensive adaptations [17], allowing insects to safeguard immature stages such as eggs [18] and larvae [19], as well as valuable resources such as cultivars [20]. Similar to other herbivorous insects in the tropics, the risk of parasitism and predation can be high for the tortoise leaf beetle, Chelymorpha alternans (Chrysomelidae, Cassidinae) (Fig 2D) [21]. Larvae counter these threats by deploying fecal shields to fend off ants and parasitoid wasps [21]. Pupae, however, lack that adaptation and instead rely on microbial protection during an immobile developmental stage [22]. In addition to its nutritional endosymbionts [23–25], C. alternans hosts a stable mycobiome predominantly composed of Fusarium oxysporum (Fig 1) [22]. While F. oxysporum is present throughout the life cycle of the insect and is faithfully transmitted, the symbiont rapidly and conspicuously proliferates at the onset of pupation where it coats the host during metamorphosis (Fig 2E and 2F). Symbiont elimination results in increased predation under natural field conditions relative to symbiont-bearing pupae, demonstrating the protective nature of the association [22]. Chromosome-scale genome sequencing and assembly revealed that the symbiont possesses a haploid genome that is relatively reduced in size (48.6 Mb) compared to close relatives within the F. oxysporum species complex [22]. Despite signatures of reductive genome evolution common among many symbionts, F. oxysporum retained the metabolic capacity to produce at least 42 secondary metabolites. Many of these compounds, such as beauvericin and bikaverin, are known for their insecticidal properties and likely underpin the defensive partnership with tortoise beetles [22].
1.3. Indirect benefits in moths
Similar to beetles, lepidopteran insects frequently harbor phytopathogenic fusaria (Figs 1 and 2G–2I). Caterpillars of the stalk borer (Eldana saccharina) and the European corn borer (Ostrinia nubilalis), for instance, partner with a diversity of Fusarium species, including F. verticilloides [26,27], which are responsible for a number of rot diseases. Sugarcane borer caterpillars (Diatraea saccharalis) are also demonstrated to host F. verticilloides in what was initially thought to be an opportunistic interaction for the fungus. It was assumed that F. verticilloides exploits openings caused by the borer’s larvae to penetrate the plant stem and initiate infection, causing red rot disease [28]. However, the phytopathogen also colonizes the caterpillar’s digestive system, inducing morphological changes in the intestinal wall such as increasing its thickness and the length of microvilli [29]. In response to herbivore damage, sugarcane plants emit volatiles that attract D. saccharalis enemies, such as Cotesia flavipes parasitoids. Strikingly, Fusarium-infected plants attract fewer parasitoids than noninfected sugarcanes [30], demonstrating an indirect benefit to D. saccharalis for vectoring the fungus [30].
1.4. Herbivorous insects are vehicles for phytopathogen transfer
Due to their ecological diversity and their herbivorous habits, insects are important vectors of many plant diseases [31]. Vector-borne pathogens are predicted to (i) increase their dissemination by manipulating insect behavior and (ii) evolve to be less virulent to its vector over evolutionary time [31]. While the benefits insects derive from their fusarial partners are diverse, spanning nutrition to defense, the benefit of associating with an insect for the microbial partner is conserved: propagation to naïve plants, thereby expanding their ecological range.
In the process of vertically transmitting their fusarial cultivars to establish new gardens, ambrosia beetles disseminate their mutualistic partners to a diversity of host plants such as avocado, hazelnut, and fig trees, where they are responsible for wilt and dieback disease [9]. While Fusarium cultivars can survive on trees for up to 2 years, they cannot spread beyond the galleries, highlighting their strict reliance on ambrosia beetles for dispersal via specialized mycangia [32,33].
Following metamorphosis, tortoise beetles shed their Fusarium-rich pupal skin with their tarsi [21]. This behavior favors the contamination of beetle appendages with fungal hyphae and spores that are subsequently propagated to naïve plants when beetles transition to new feeding sites [22]. Once on the vascular tissues of a new host plant, the phytopathogen initiates infection, causing yellow wilt disease in sweet potato plants [22]. Additionally, the fungus could manipulate beetle behavior to promote its own dissemination, as demonstrated in mealworm beetles (Tenebrio molitor), which prefers to consume wheat grains infected with F. proliferatum [34] and continues to excrete spores in their feces weeks after their ingestion.
Revealing a more intimate tripartite interaction than previously envisioned, sugarcane plants up-regulate the production of defensive proteins upon D. saccharalis attack that do not negatively affect the insect but leads to fungal apoptosis [35,36]. Despite the deployment of antifungal plant defenses induced by the herbivore, F. verticilloides overall benefits from its interaction with the sugarcane borer by manipulating both its plant host and the insect to enhance its own transmission [37]. On the one hand, F. verticilloides emits volatile compounds that are attractive for the insect. But on the other hand, the fungus alters female oviposition preference. While females that are not colonized by Fusarium prefer to lay eggs on fungus-infected plants, females carrying the phytopathogen prefer to lay eggs on fungus-free plants. In both cases, the fungus increases its own dissemination [37].
1.5. Concluding remarks
Herbivorous insects are increasingly recognized for their role in the epidemiology of numerous plant pathogens, including diverse Fusarium species. While the physiological consequences of pathogen infection are well-characterized for the host plant, how these microbes affect the behavior, development, and overall fitness of their insect vectors remains underexplored in most cases. Given their tractability, insect–Fusarium interactions serve as emerging experimental model systems to improve our understanding of the ecology and evolution of plant–pathogen–vector associations.
Acknowledgments
We thank Teiya Kijimoto and Marcio de Castro Silva Filho for insect photographs. We also thank Rebecca Graham from the Department of Agriculture Western Australia for the D. saccharalis image, shared under a Creative Commons Attribution 3.0 Australia license, and Steve Scholnick for the E. validus picture, shared under the Creative Commons Attribution 4.0 International license.
Funding Statement
We gratefully acknowledge funding from the Max Planck Society (HS, SJ), and the German Research Foundation (Deutsche Forschungsgemeinschaft) under Germany’s Excellence Strategy [EXC 2124 – 390838134] (AB) and project SA 3105/2-1 (HS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Summerell BA. Resolving Fusarium: Current status of the genus. Annu Rev Phytopathol. 2019;57:323–339. [DOI] [PubMed] [Google Scholar]
- 2.Ma L-J, Geiser DM, Proctor RH, Rooney AP, O’Donnell K, Trail F, et al. Fusarium pathogenomics. Annu Rev Microbiol. 2013;67:399–416. [DOI] [PubMed] [Google Scholar]
- 3.Zeng L, Zhang N, Zhang Q, Endress PK, Huang J, Ma H. Resolution of deep eudicot phylogeny and their temporal diversification using nuclear genes from transcriptomic and genomic datasets. New Phytol. 2017;214:1338–1354. doi: 10.1111/nph.14503 [DOI] [PubMed] [Google Scholar]
- 4.Hill R, Buggs RJA, Vu DT, Gaya E. Lifestyle transitions in fusarioid fungi are frequent and lack clear genomic signatures. Mol Biol Evol. 2022:39. doi: 10.1093/molbev/msac085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nesic K, Ivanovic S, Nesic V. Fusarial toxins: secondary metabolites of Fusarium fungi. Rev Environ Contam Toxicol. 2014;228:101–120. [DOI] [PubMed] [Google Scholar]
- 6.Mueller UG, Gerardo NM, Aanen DK, Six DL, Schultz TR. The evolution of agriculture in insects. Annu Rev Ecol Evol Syst. 2005;36:563–595. [Google Scholar]
- 7.Mayers CG, Harrington TC, Biedermann PHW. Mycangia define the diverse ambrosia beetle-fungus symbiosis. In: Schultz TR, Gawne R, Peregrine PN, editors. The Convergent Evolution of Agriculture in Humans and Insects. The MIT Press; 2022. [Google Scholar]
- 8.Dzurenko M, Hulcr J. Ambrosia beetles. Curr Biol. 2022;32:R61–R62. doi: 10.1016/j.cub.2021.11.043 [DOI] [PubMed] [Google Scholar]
- 9.Eskalen A, Stouthamer R, Lynch SC, Rugman-Jones PF, Twizeyimana M, Gonzalez A, et al. Host range of fusarium dieback and its ambrosia beetle (Coleoptera: Scolytinae) vector in Southern California. Plant Dis. 2013;97:938–951. doi: 10.1094/PDIS-11-12-1026-RE [DOI] [PubMed] [Google Scholar]
- 10.Kasson MT, O’Donnell K, Rooney AP, Sink S, Ploetz RC, Ploetz JN, et al. An inordinate fondness for Fusarium: phylogenetic diversity of fusaria cultivated by ambrosia beetles in the genus Euwallacea on avocado and other plant hosts. Fungal Genet Biol. 2013;56:147–157. [DOI] [PubMed] [Google Scholar]
- 11.O’Donnell K, Sink S, Libeskind-Hadas R, Hulcr J, Kasson MT, Ploetz RC, et al. Discordant phylogenies suggest repeated host shifts in the Fusarium-Euwallacea ambrosia beetle mutualism. Fungal Genet Biol. 2015;82:277–290. [DOI] [PubMed] [Google Scholar]
- 12.Spahr E, Kasson MT, Kijimoto T. Micro-computed tomography permits enhanced visualization of mycangia across development and between sexes in Euwallacea ambrosia beetles. PLoS ONE. 2020;15:e0236653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schultz TR, Brady SG. Major evolutionary transitions in ant agriculture. Proc Natl Acad Sci. 2008;105:5435–5440. doi: 10.1073/pnas.0711024105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massoumi Alamouti S, Tsui CKM, Breuil C. Multigene phylogeny of filamentous ambrosia fungi associated with ambrosia and bark beetles. Mycol Res. 2009;113:822–835. doi: 10.1016/j.mycres.2009.03.003 [DOI] [PubMed] [Google Scholar]
- 15.Aanen DK, Eggleton P, Rouland-Lefevre C, Guldberg-Froslev T, Rosendahl S, Boomsma JJ. The evolution of fungus-growing termites and their mutualistic fungal symbionts. Proc Natl Acad Sci U S A. 2002;99:14887–14892. doi: 10.1073/pnas.222313099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hembry DH, Weber MG. Ecological interactions and macroevolution: A new field with old roots. Annu Rev Ecol Evol Syst. 2020;51:215–243. [Google Scholar]
- 17.Flórez LV, Biedermann PHW, Engl T, Kaltenpoth M. Defensive symbioses of animals with prokaryotic and eukaryotic microorganisms. Nat Prod Rep. 2015;32:904–936. doi: 10.1039/c5np00010f [DOI] [PubMed] [Google Scholar]
- 18.Flórez LV, Scherlach K, Gaube P, Ross C, Sitte E, Hermes C, et al. Antibiotic-producing symbionts dynamically transition between plant pathogenicity and insect-defensive mutualism. Nat Commun. 2017;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piel J. A polyketide synthase-peptide synthetase gene cluster from an uncultured bacterial symbiont of Paederus beetles. Proc Natl Acad Sci U S A. 2002;99:14002–14007. doi: 10.1073/pnas.222481399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Currie CR, Scott JA, Summerbell RC, Malloch D. Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature. 1999;398:701–704. [Google Scholar]
- 21.Morrison CR, Windsor DM. The life history of Chelymorpha alternans (Coleoptera: Chrysomelidae: Cassidinae) in Panamá. Ann Entomol Soc Am. 2017;111:31–41. [Google Scholar]
- 22.Berasategui A, Breitenbach N, García-Lozano M, Pons I, Sailer B, Lanz C, et al. The leaf beetle Chelymorpha alternans propagates a plant pathogen in exchange for pupal protection. Curr Biol. 2022;4114–4127. [DOI] [PubMed] [Google Scholar]
- 23.Salem H, Bauer E, Kirsch R, Berasategui A, Cripps M, Weiss B, et al. Drastic genome reduction in an herbivore’s pectinolytic symbiont. Cell. 2017;171:1520–1531.e13. doi: 10.1016/j.cell.2017.10.029 [DOI] [PubMed] [Google Scholar]
- 24.Salem H, Kirsch R, Pauchet Y, Berasategui A, Fukumori K, Moriyama M, et al. Symbiont digestive range reflects host plant breadth in herbivorous beetles. Curr Biol. 2020;30:2875–2886.e4. doi: 10.1016/j.cub.2020.05.043 [DOI] [PubMed] [Google Scholar]
- 25.Pons I, González Porras MÁ, Breitenbach N, Berger J, Hipp K, Salem H. For the road: calibrated maternal investment in light of extracellular symbiont transmission. Proc Biol Sci. 2022;289:20220386. doi: 10.1098/rspb.2022.0386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McFarlane SA, Govender P, Rutherford RS. Interactions between Fusarium species from sugarcane and the stalk borer, Eldana saccharina (Lepidoptera: Pyralidae). Ann Appl Biol. 2009;155:349–359. [Google Scholar]
- 27.Gatch EW, Munkvold GP. Fungal species composition in maize stalks in relation to European corn borer injury and transgenic insect protection. Plant Dis. 2002;86:1156–1162. doi: 10.1094/PDIS.2002.86.10.1156 [DOI] [PubMed] [Google Scholar]
- 28.Ogunwolu EO, Reagan TE, Flynn JL, Hensley SD. Effects of Diatraea saccharalis (F.) (Lepidoptera: Pyralidae) damage and stalk rot fungi on sugarcane yield in Louisiana. Crop Prot. 1991;10:57–61. [Google Scholar]
- 29.Gallan DZ, Henrique MO, Silva-Filho MC. The phytopathogen Fusarium verticillioides modifies the intestinal morphology of the sugarcane borer. Pathogens. 2023:12. doi: 10.3390/pathogens12030443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peñaflor MFGV, Bento JMS. Red-rot infection in sugarcane attenuates the attractiveness of sugarcane borer-induced plant volatiles to parasitoid. Arthropod Plant Interact. 2019;13:117–125. [Google Scholar]
- 31.Santiago MFM, King KC, Drew GC. Interactions between insect vectors and plant pathogens span the parasitism–mutualism continuum. Biol Lett. 2023;19:20220453. doi: 10.1098/rsbl.2022.0453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freeman S, Miller G, Protasov A, Maymon M, Elazar M, David-Schwartz R, et al. Aposymbiotic interactions of three ambrosia beetle fungi with avocado trees. Fungal Ecol. 2019;39:117–130. [Google Scholar]
- 33.Mayers CG, Harrington TC, Biedermann PHW. Mycangia define the diverse ambrosia beetle–fungus symbioses. The Convergent Evolution of Agriculture in Humans and Insects. The MIT Press; 2022. [Google Scholar]
- 34.Guo Z, Pfohl K, Karlovsky P, Dehne H-W, Altincicek B. Dissemination of Fusarium proliferatum by mealworm beetle Tenebrio molitor. PLoS ONE. 2018;13:e0204602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franco FP, Santiago AC, Henrique-Silva F, de Castro PA, Goldman GH, Moura DS, et al. The sugarcane defense protein SUGARWIN2 causes cell death in Colletotrichum falcatum but not in non-pathogenic fungi. PLoS ONE. 2014;9:e91159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Medeiros AH, Franco FP, Matos JL, de Castro PA, Santos-Silva LK, Henrique-Silva F, et al. Sugarwin: a sugarcane insect-induced gene with antipathogenic activity. Mol Plant Microbe Interact. 2012;25:613–624. doi: 10.1094/MPMI-09-11-0254 [DOI] [PubMed] [Google Scholar]
- 37.Franco FP, Túler AC, Gallan DZ, Gonçalves FG, Favaris AP, Peñaflor MFGV, et al. Fungal phytopathogen modulates plant and insect responses to promote its dissemination. ISME J. 2021;15:3522–3533. doi: 10.1038/s41396-021-01010-z [DOI] [PMC free article] [PubMed] [Google Scholar]