Abstract
Strabismus, deviation of the ocular alignment, can adversely affect quality of life and activities of daily living. Surgery was the prior standard of care for strabismus, but up to 40% of patients required additional surgeries. This need for more effective and less invasive treatment, along with the convergence of other events such as the development of electromyography, purification of botulinum toxin A, and the finding that injection of botulinum toxin type A could paralyze the hind limbs of chicks, led Dr. Alan Scott to investigate injection of his formulation for strabismus. The positive results of initial trials in monkeys segued to human trials with observations of alignment improvements and few adverse events. The success of botulinum toxin type A in the treatment of strabismus led to interest in its use to treat other skeletal muscles, particularly in blepharospasm, a type of focal dystonia involving eyelid spasms and involuntary eye closure that lacked an effective pharmacological treatment. Patient groups helped to increase awareness of this novel treatment, and results from clinical trials confirmed its effectiveness. Dr. Scott’s formulation, then known as Oculinum, received its first Food and Drug Administration approvals in 1989 for strabismus and blepharospasm. Allergan acquired Oculinum in 1991, renaming it Botox. These initial uses led to its application in a myriad of other indications as outlined in other articles of this supplement.
Keywords: botulinum toxin, neuromuscular agents
1. Overview of strabismus and blepharospasm
Strabismus is a deviation of ocular alignment in which the primary lines of sight deviate by at least 1 prism diopter.[1] It can be further categorized by direction of the deviation: inwards (esotropia), outwards (exotropia), upwards (hypertropia), downwards (hypotropia), and rotary (cyclotropia).[2] Prevalence is estimated to be 1.1–5% in the general population (from studies spanning 1992–2018) and is >40% in patients with cerebral palsy or Down syndrome.[1,3,4] Strabismus can range in severity, and can be corrected in some patients with lenses, prisms, and/or vision therapy.[1] Strabismus can be congenital or due to a brain motor control problem or an injury.[2] It usually develops in childhood but can develop at any age.[1] Ocular disturbances associated with strabismus include blurred vision, diplopia (double vision), and impaired depth perception.[2] Strabismus can also affect activities of daily living, quality of life (QOL), and work productivity.[1]
Blepharospasm is a form of focal dystonia characterized by spasms of the orbicularis oculi muscles of the eyelid, involuntary eye closures, and enhanced blinking, sometimes to a severity that renders a patient functionally blind.[5,6] Crude estimates of the prevalence of blepharospasm range from 16–133 per million.[7] Onset generally occurs in the fifth or sixth decade of life, and older age and female sex may be risk factors for blepharospasm development.[7] Though the etiology of primary blepharospasm is unclear, patients with blepharospasm had a higher frequency of prior eye diseases such blepharitis and keratoconjunctivitis than those without blepharospasm.[6,8] About one-quarter of patients with blepharospasm have at least one family member with dystonia.[6] Secondary blepharospasm, which is less common than idiopathic or primary blepharospasm, can develop after focal lesions in the brain or can develop in patients with parkinsonism, tardive dyskinesia, or conditions associated with lid weakness.
As in patients with other forms of dystonia, those with blepharospasm often employ a geste antagoniste, or sensory trick, that temporarily reduces dystonia. In one study, the frequency of gestes antagonistes in patients with blepharospasm was over 70%, which were most commonly stretching or rubbing the eyebrows or eyelids.[9] Blepharospasm can also have adverse effects on patient QOL.[5] Depression is more frequent in patients with blepharospasm compared with healthy controls. Many patients find difficulties in activities of daily living due to their condition, including social activities and work, and begin to withdraw from them.[10] Photophobia is common in blepharospasm,[11] and patients may have problems with driving, particularly night driving, and watching cinema, because the lights of the oncoming car and from the movie screen worsen the blepharospasm.
The following historical narrative was compiled based on review of the literature and interviews with the authors, and the quoted portions reflect the personal observations and reflections of the individuals who were interviewed. In some instances, this article describes uses for which Allergan has not sought and/or received regulatory approval in individual countries and are mentioned for historical context or background only.
2. Unmet need for the treatment of strabismus and blepharospasm
Prior to the use of botulinum toxin for strabismus, the standard of care was surgical treatment. However, many cases were not successfully treated.
Dr. Scott (Fig. 1), who was the director of the Smith-Kettlewell Eye Research Institute, where he performed the initial injections of botulinum toxin in animals and humans: In some categories [of strabismus] as many as 40% of patients needed re-operations, so we were looking for something new and different.
Figure 1.
. Drs. Alan Scott and Joseph Jankovic. Photo provided by Dr. Joseph Jankovic.
There was no effective pharmacological treatment for blepharospasm prior to botulinum toxin.[12,13] Medications to decrease muscle contraction were mainly ineffective and some conferred intolerable side effects.[6] Benztropine mesylate, clonazepam, pimozide, baclofen, lorazepam, amantadine hydrochloride, tridihexethyl hydrochloride, and trihexyphenidyl hydrochloride were used without much success.[14–16] In one study, two-thirds of patients with blepharospasm experienced improvement with clonazepam, but only 11% had a marked and lasting effect.[17]
Surgery to remove branches of the cranial nerve VII or part of the eyelid or brow muscles could result in long-term improvements in blepharospasm.[13,18] However, as with many other types of surgeries, general anesthesia, hospitalization, and weeks of convalescence are required.[14] In addition, scarring and other complications could occur.[13,14] The surgical treatment often left eye weakness, and as a consequence, sometimes people could not close their eyes adequately.
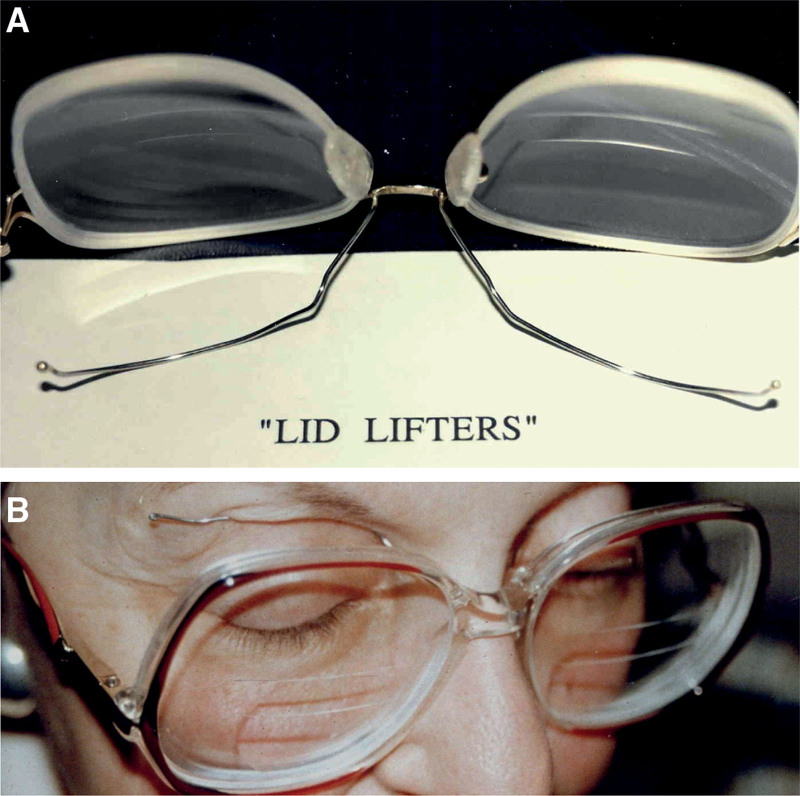
Dr. Brin, who was a fellow and faculty member at Columbia University when he first used botulinum toxin A to treat blepharospasm under the mentorship of Dr. Fahn: In mild cases [of blepharospasm] we also tried “lid lifters.” These were made by fashioning wire on eyeglasses, which would press against the eyelids to help keep them open [Fig. 2]. The mechanism of lid lifters is unknown, but it may be related to sensory feedback, such as occurs when blepharospasm patients reduce muscle spasm by touching their eyelids—a sensory trick.[19] The physical effect of lifting the lids may also be involved. But, in general, there was no good treatment for blepharospasm before botulinum toxin.
Figure 2.
Lid lifters designed for blepharospasm patients. (A) Photograph showing wire lifters fashioned on eyeglasses by the patient’s husband, who was a dentist. (B) Blepharospasm patient wearing lid lifters. Photos provided by Dr. Mitchell Brin.
3. Early use of botulinum toxin A as a treatment for strabismus and blepharospasm and its development program
Historically, the German physician Justinus Kerner gave the first detailed clinical description of “sausage poisoning,” now known as botulism, including weakness, dry mouth, and reduced body secretions.[20] Kerner was also the first to suggest a therapeutic use of botulinum toxin, particularly for conditions involving hyperexcitability of the nervous system such as hypersecretion of sweat or mucus and motor hyperkinesias.
As referenced in the first chapter of this supplement, a combination of ideas and events converged for the modern-day use of botulinum toxin A for therapeutic purposes.
Dr. Scott: There were 2 or 3 things that came together in the early 1970s. Another researcher, Dr. Carter Collins, and I had been working on muscle physiology and one of the techniques which we developed was electromyography to find the muscle. We developed the needle electrodes that allowed us to inject and localize in the muscle itself with substantial accuracy. The second thing that was going on at that time was that the outcomes from strabismus surgery were far from perfect. And then another thing happened about that time: Lance Simpson wrote a book on neuropoisons.[21] We’d been trying various things, injecting alcohol and various enzymes in the muscle to try to influence it but none of these had had a great effect. Simpson’s book had a chapter wherein Dr. Daniel Drachman described his experiments injecting the hind limb of chicks and showed that selectively with a small enough amount of botulinum toxin, you could interfere with the muscle.[22] Clearly, it seemed possible then that we could use very small amounts of toxin. And so we did what Drachman did; he got his toxin from Ed Schantz. I wrote to Ed and he sent us some toxin. [Dr. Edward Schantz at the University of Wisconsin developed a bulk purification method for botulinum toxin A.[23]] He supplied the scientific world with toxin, or the few people who were interested in it at the time, and sent it out freely and we started to use it in monkeys. First in some other animals, but then we rapidly moved to monkeys because we could very easily see the effects of tiny doses and work up until we found an appropriate dose. The miracle moment for me was injecting it in the monkey. I could immediately see that we really had something quite special, quite specific, not toxic to anything else, long-lasting. From that experience, you could tell that this was going to be very, very valuable.[24]
In the first published article on botulinum toxin A for injection, Dr. Scott and colleagues used electromyography (EMG) for guidance to inject botulinum toxin A into either the medial or lateral ocular rectus muscle in rhesus monkeys and observed a dose-dependent transient weakness from 2 weeks to at least 8 months without serious local or systemic adverse events (AEs).[25] The success of these experiments led to its testing in humans.
Dr. Scott, on the very first patient he injected with botulinum toxin, a man with strabismus: The first injection patient was a man who had a retinal detachment operation that had scarred his muscles and pulled his eye out of line. He did not have good functional visual return from his retinal detachment. Our chance of creating a serious problem for him was pretty low. He wasn’t really seeing double because his vision was poor. Though I’d done many injections behind the eye, here we were doing something new and different, always an exciting experience in clinical research.
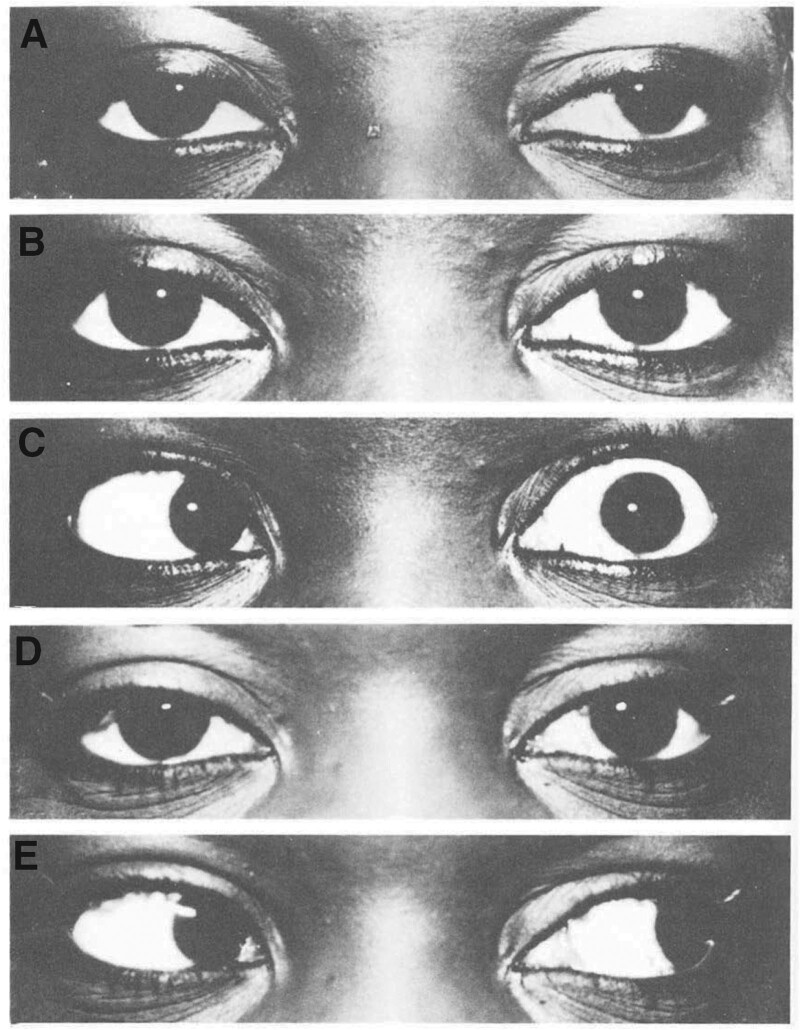
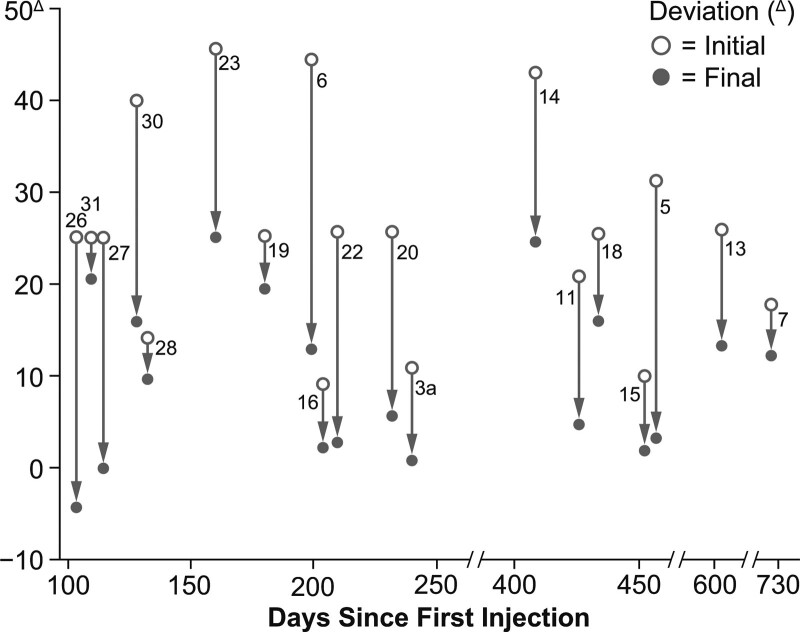
A case series of botulinum toxin A injections using EMG guidance to correct strabismus documented that 19 patients had been treated with 67 doses, with no systemic effects.[26] A maximum correction of 40 diopters and duration of effect of at least 6 months were observed. Example photos before and after botulinum toxin A treatment are shown in Fig. 3, with resultant decreases in deviation in Fig. 4.
Figure 3.
. Before and after photos of botulinum toxin A treatment for strabismus. (A) Prior to injection with botulinum toxin A. (B) Primary position gaze 2 days after injection with 1.56 × 10-3 μg botulinum toxin A. (C) Left gaze 2 days after injection, with absence of abduction due to lateral rectus paralysis. (D) Primary position gaze at 3 months after injection, with reduction of exotropia. (E) Left gaze 3 months after injection, with full return of abduction. Reprinted from Ophthalmology, Vol 87, Scott AB. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Pages 1044–9, Copyright 1980, with permission from Elsevier.
Figure 4.
Decreases in deviation of ocular alignment by patients following injection of botulinum toxin A for the treatment of strabismus. Data are included for patients (numbered) who received at least the therapeutic dose threshold of 3.12 x 10-4 μg botulinum toxin A, did not receive eyelid injections, and were followed for at least 100 days after injection. Patients with paralytic strabismus or neuromyotonia were excluded. Reprinted with permission of the American Ophthalmological Society from Scott AB. Botulinum toxin injection of eye muscles to correct strabismus. Trans Am Ophthalmol Soc. 1981;79:734–70.
The success of botulinum toxin A in the treatment of strabismus led to its use in other disorders such as blepharospasm and those involving skeletal muscles, as predicted in the early paper on results of its use in animals.[25] Patient support and education groups also began forming, which assisted in communicating new therapies to patients.
Dr. Scott: As an ophthalmologist-clinician looking at the problem of blepharospasm, it appeared to be an extraordinarily rare disorder described in books but for which you almost never saw a case. There really aren’t a lot of cases around—there’s a few thousand—but many of those people were off the streets. They were confined, they couldn’t get out. They’d been told by a number of doctors that there was no effective treatment really for it and we didn’t see the patients. So, when I first started to see blepharospasm patients, it was new to me. It only took studying 2 to 3 patients to develop a reasonable protocol for how we should inject: multiple injections around the eye in the affected muscles. By that time, 1982, Mattie Lou [Koster], had made herself known. She was featured in a Wall Street Journal article, and she developed a patient help foundation [the Benign Essential Blepharospasm Research Foundation]. The foundation provided a place for patients to find out information, to get moral support for their disorder, and receive guidance on treatment. Once Oculinum or the idea of Oculinum (later called Botox), got out there, it spread like wildfire through that community and those patients knew quite a lot more than most of the doctors very, very quickly and came asking for it.
Dr. Fahn, who is the H. Houston Merritt Professor of Neurology and Director Emeritus of the Center for Parkinson’s Disease and Other Movement Disorders at Columbia University, had been studying focal and generalized dystonia at Columbia University Medical Center, evaluating medications to alleviate the excessive muscle contractions. After he became aware of the Wall Street Journal article, he contacted Mattie Lou Koster to learn more about her blepharospasm foundation. In their subsequent correspondence, he encouraged her to utilize the foundation to raise money for blepharospasm research, and she informed him of the early results of botulinum toxin A injections by Dr. Scott. Dr. Fahn flew to Iowa City, Iowa, to meet Dr. Scott, who was invited by Dr. Richard Anderson at the University of Iowa to demonstrate how to inject botulinum toxin into the orbicularis oculi muscles to treat blepharospasm. When Dr. Fahn returned to New York, he prepared a protocol for a clinical trial to study botulinum toxin injections for the treatment of blepharospasm and other forms of focal dystonia. However, a hurdle at Columbia University was obtaining institutional review board (IRB) approval of the research protocol.
Dr. Fahn: One of the stumbling blocks was [that] the IRB thought it was too dangerous. Basically, the review board members insisted that the study investigators be inoculated with botulinum toxoid to protect ourselves in case we stuck ourselves with a needle and got botulinum toxin into us. It seemed a little strange to me, but I went and talked to our group… and we agreed that this was too much for us to accept. We felt that if we eventually got blepharospasm or some other focal dystonia and that botulinum toxin would be a good treatment for it, we might be immune to it and would not get the clinical benefit from this approach. So, it ended up that we agreed not to be inoculated with botulinum toxoid, but we offered to sign a waiver to Columbia University and the medical center that we would not hold them liable if we accidentally got botulism symptoms. And that was satisfactory to the IRB, giving us the green light to go ahead with our study.
Once work could begin in this area at Columbia University, open-label trials were begun. With that success, the first double-blind trial testing botulinum toxin A for blepharospasm was conducted.[27] Eight subjects were injected; 5 were injected unilaterally, with the other eye receiving saline as a control. Despite the double-blind protocol, the 5 patients and the investigators were able to detect the eye that received botulinum toxin owing to the less forceful contractions and shorter times of eyelid closure. Evoked motor potentials, contractions at rest, and maximally forced contractions were all significantly reduced.
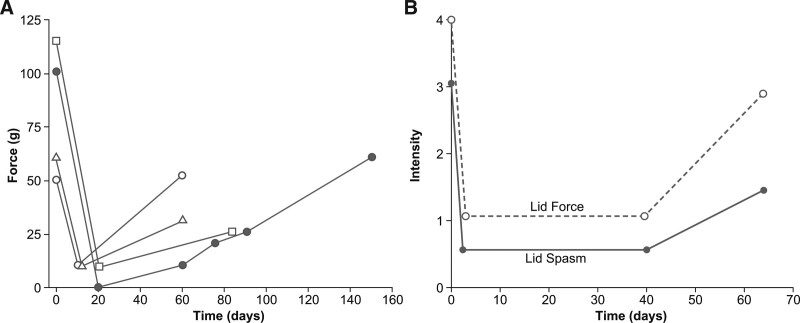
An additional Columbia study tested injections of botulinum toxin type A in patients with a variety of focal dystonias, including 49 with blepharospasm.[28] Motor symptom relief was observed in 69% of blepharospasm patients, with a mean of 2.7 days to onset and an 11.4 week duration of effect. Ptosis (19%), conjunctivitis (7%), and entropion (2%) were reported. Similarly, in another open-label study, 39 patients with blepharospasm were injected with a mean of 21.3U botulinum toxin A per eye over 124 treatment visits with a mean between-treatment interval of 9.9 weeks.[29] Eyelid closure force decreased following treatment (Fig. 5). In the patients who had not had previous surgery, an 8.5% rate of AEs was seen, with ptosis the most common AE.
Figure 5.
. Eyelid closure force before and after botulinum toxin A treatment for blepharospasm (A) measured with a modified calibrated spring-loaded speculum in four patients, and (B) mean eyelid closure force and eyelid spasm scores from 27 patients, each measured on scales ranging from 0 to 4. For force, 0 indicated minimal effort for eyelid separation and 4 an inability to separate the eyelids with the thumbs. For spasm, 0 indicated no spasm, with 4 indicating severe, incapacitating spasm. Panel A: Reproduced with permission from Arch Ophthalmol. 1985;103(3):347–50. Copyright (1985) American Medical Association. All rights reserved. Panel B: Figure 2 from "Treatment of blepharospasm with medication, surgery and type A botulinum toxin" by Arthurs B, Flanders M, Codere F, et al published in the Canadian Journal of Ophthalmology. 1987;22(1):24–8 is used under a CC BY-ND 4.0 license.
Early research supply was disrupted in 1985, when the insurance carried by Smith-Kettlewell for the work on botulinum toxin A was canceled. As a result of this loss of insurance, Dr. Scott established a company, Oculinum, so that work could continue.
Dr. Scott: I was personally not very worried about being sued, whereas the directors of the lab had this concern for the laboratory facility more generally. If I were to be sued, and the laboratory had no insurance, the magnitude of impact on the laboratory was unknown, and that might take the institution down. So, they had a responsibility to say, “We can’t be doing this without insurance.” This is when I established Oculinum Inc. That was a kind of a construct word. “Oculo”: eyeball and “linum”: line-them-up with the Latinate ending “-um,” which was euphonic. So “Oculinum.”
In both strabismus and blepharospasm, injection of botulinum toxin into the muscles around the affected eye blocks presynaptic release of acetylcholine at the neuromuscular junction and reduces muscle contraction. In the case of blepharospasm, injections of botulinum toxin into the eyelid and brow muscles block neural signals to the orbicularis oculi and associated periocular muscles, thus reducing eye spasm. For strabismus, injections into the extraocular muscle inhibit contractions in the deviated eye, allowing the opposing muscles of the same eye to assume a greater movement force and reposition the eye into a straighter alignment.[2]
4. Efficacy and safety highlights
The first indications for which any botulinum toxin (at the time, Oculinum) received Food and Drug Administration (FDA) approval were for the treatment of strabismus and blepharospasm associated with dystonia, both for patients aged ≥12 years, on December 29, 1989.[30]
The package insert consolidated much of the early research performed for both strabismus and blepharospasm. The dataset supporting the strabismus approval included Dr. Scott’s multicenter open-label trial in which patients (N=677) received one or more injections of Oculinum.[30] Improvements in alignment of ≤10 prism diopters were seen in 55% of patients at evaluations ≥6 months following treatment. A subsequent study that reported health-related QOL assessments showed improved reading function following treatment with onabotulinumtoxinA.[31]
AEs from the treatment with botulinum toxin A for strabismus were local to the injection area. Those affecting extraocular muscles occurred at a rate of 17% in a population of 2058 adults who received 3650 injections. Ptosis varied by the location of the injected muscles, with incidences of 38% following injections into the superior rectus, 16% in the horizontal rectus, and 1% in the inferior rectus. Retrobulbar hemorrhage occurred at a rate of 0.3% in a series of 5587 injections.[30]
Oculinum was the first botulinum toxin approved for blepharospasm, and botulinum toxin type A is now considered to be the first-line treatment for this condition.[32] Due to the dramatic results from the open-label trials, few randomized, controlled trials have been undertaken for blepharospasm.[12,27,32] In one of the trials submitted to the FDA for approval, 27 patients with persistent moderate to severe blepharospasm, of which 26 had not responded to previous drug treatments, were treated with 2U of Oculinum in 6 sites per side (total of 12U per eye).[14] Of these, 25 patients reported an improvement in eyelid force and eyelid spasm within 48 hours, with a mean duration of effect of 8.1 weeks (range: 2–17 weeks). Ptosis was the most frequent AE, occurring in 22.2% (6/27) patients; all cases resolved. No systemic effects related to treatment were identified.
In a small (N=11), double-blind, placebo-controlled study, patients with blepharospasm received 6.25U injected subcutaneously, medially, and laterally (25U per eye).[33] All patients significantly improved after treatment with Oculinum. Of 68 injections (performed in controlled and open-label phases), AEs observed in the Oculinum-treated patients included blurred vision (n=6), tearing (n=5), ecchymosis (n=3), ptosis (n=2), and diplopia (n=1).
Dr. Scott’s large multicenter (N=1684), open-label trial conducted with multiple investigators showed that patients with blepharospasm experienced clinical improvements in eyelid force and lid spasm intensity that lasted for a mean of 12 weeks before needing retreatment.[30] As noted in the package insert, there have been no reports of definitive serious AEs associated with distant spread of onabotulinumtoxinA at the recommended dose for blepharospasm (≤30U).[30] The most frequent AEs with blepharospasm are ptosis (21%), superficial punctate keratitis (6%), and eye dryness (6%).[30] As physicians have refined the injection techniques over time, ptosis rates have decreased, with a 2007 review reporting a mean ptosis rate of 13%[34] and several studies reporting significantly lower ptosis rates for blepharospasm and hemifacial spasm following injection into the pretarsal rather than preseptal orbicularis oculi (eg, 13% pretarsal vs 16% preseptal[35] and 0% pretarsal vs 7.5% preseptal).[36] Importantly, unit doses are not interchangeable among different botulinum toxin products, each of which has its own dosing guidelines and clinical profile.[30,37]
Dr. Brin: With our first patient, the remarkable thing is when she came for a follow-up at Columbia University Medical Center in upper Manhattan, she mentioned that after one of her visits to us, she walked all the way down to the bottom of Manhattan, to Bowery and Canal Street. When I asked her why, she said, “Because I now could.”
Subsequent studies captured QOL improvements relative to baseline. These were observed with repeat treatments of onabotulinumtoxinA, particularly in patients who were previously naïve to treatment.[38] A retrospective analysis found that 92% (33/36) and 90% (18/20) of patients with blepharospasm experienced substantial benefit at 2 and 5 years of treatment, respectively, with AEs occurring in 10.1% of patients over 398 treatment cycles, with ptosis and dry eye the most common AEs.[39] Another retrospective analysis of 73 patients with blepharospasm with at least 10 years of follow-up after a mean of 8.7 onabotulinumtoxinA treatments found a mean duration of effect of 18.2 weeks and increases in doses after the third or fourth treatments.[40] Ptosis, ecchymosis, and diplopia occurred in 19.2%, 8.2%, and 5.4% of patients, respectively. Region-specific information regarding safety and efficacy can be found in local labeling.
5. Impact of onabotulinumtoxinA on patients and the broader biomedical community
The development of botulinum toxin A (Oculinum) for therapeutic use has had a profound effect on patients with a variety of conditions as well as on the biomedical community. In 1991, Allergan purchased the manufacturing and licensing rights to Oculinum and changed the name to Botox (non-proprietary name: onabotulinumtoxinA). Since that time, it has received FDA approval for multiple additional indications.
Dr. Brin: Patients rallied around the treatment for which they saw hope and they felt that there was a light at the end of the tunnel in terms of making progress in their disease. Encouraged by their patients, movement disorder clinicians explored this treatment off-label, and professional societies moved quickly to develop guidelines. Stanley van der Noort was the chair of the American Academy of Neurology’s Therapeutics and Technology Assessment (TTA) subcommittee. He asked me to convene [a] team of experts to assess botulinum toxin therapy, and the TTA subcommittee issued the report on the clinical use of botulinum toxin type A in neurology in 1990.[41] In addition, Mark Hallett at the National Institutes of Health chaired the Consensus Development Conference in November 1990, followed by a published report in 1991.[42] The TTA subcommittee published the AAN Training Guidelines in 1994.[43]
Dr. Scott: After the word got out [about Botox], there was a rapid expansion in use because a large number of these conditions didn’t have any adequate treatment at this time. We mentioned in the very first paper that we wrote in 1973[25] that we expected its use would go beyond just the ocular and extraocular muscles and indeed, that’s where it went.
Dr. Fahn: It’s rather shocking that a potent food poison can actually be something good. It’s interesting that if you take the dose down, it’s a helpful agent for the right condition. It’s been remarkable to see how well this compound has done in helping so many people, and it’s not just our specialty of movement disorders. So much of the rest of medicine has been helped. I was always amazed when I heard it was helpful in migraine, for example, so you’re always learning something new.
In addition, training physicians who went on to bring the new therapy to their home countries had an especially important impact on the medical community.
Dr. Fahn: We were getting more and more patients referred to us, and eventually I stopped doing the injections myself because everybody else in our team wanted to do them, and it was very gratifying to see so many patients getting better and everybody learning how to do it. I remember some of the foreign fellows going back to their home countries and being the first ones to do botulinum toxin injections. For example, Andrzej Friedman had injected with us, and he went back and started injecting in Poland. We trained Nir Giladi from Israel who went back and started injecting patients there; he was the first in Israel to use botulinum toxin. Nearly all my trainees, from both the US and other countries, learned about botulinum toxin treatments. Training people and spreading the word was very, very important to us.
Physicians who trained with Dr. Fahn within the first 10 years of testing experimental botulinum toxin: US: Susan Bressman, Mitchell Brin, Robert Burke, Arif Dalvi, Rolando Diaz-Olivo (Puerto Rico), Enrico Fazzini, Blair Ford, Paul E. Greene, Ann Hunt, Un Jung Kang, Elan Louis, Giselle Petzinger, Seth Pullman, John Rogers, Miran Salgado, Rachel Saunders-Pullman, Heidi Shale, Marie-Helene St. Hilaire, Daniel Togasaki, Richard Trosch, Daniel Truong; Outside the US: Sylvain Chouinard (Montreal, Canada), Oren Cohen (Tel Aviv, Israel), Andrej Friedman (Warsaw, Poland), Nir Giladi (Tel Aviv, Israel), Zygmunt Jamrozic (Warsaw, Poland), Beom Jeon (Seoul, South Korea), Vladimir Kostic (Belgrade, Serbia), Timothy Lynch (Dublin, Ireland), Uday Muthane (Bangalore, India), Gianni Pezzoli (Milan, Italy), Samer Tabbal (Beirut, Lebanon), Dominic Thyagarajan (Melbourne, Australia).
Acknowledgments
Writing and editorial assistance was provided to the authors by Jennifer L. Giel, PhD, on behalf of Evidence Scientific Solutions, Inc, and was funded by Allergan, an AbbVie company. All authors met the ICMJE authorship criteria. Neither honoraria nor other form of payments were made for authorship.
Author contributions
Conceptualization: Mitchell F. Brin.
Funding acquisition: Mitchell F. Brin.
Supervision: Mitchell F. Brin.
Writing – review & editing: Alan B. Scott, Stanley Fahn, Mitchell F. Brin.
Abbreviations:
- AEs
- adverse events
- EMG
- electromyography
- FDA
- Food and Drug Administration
- IRB
- institutional review board
- QOL
- quality of life
This manuscript was funded by AbbVie. AbbVie was involved in the manuscript concept and participated in writing, reviewing, and approval of the final version. No honoraria or payments were made for authorship.
AB Scott and S Fahn have nothing to disclose. MF Brin is a full-time employee of AbbVie and holds stock in the company.
How to cite this article: Scott AB, Fahn S, Brin MF. Treatment of strabismus and blepharospasm with Botox (onabotulinumtoxinA): Development, insights, and impact. Medicine 2022;102:S1(e32374).
Correspondence: Mitchell F. Brin, Senior Vice President, Chief Scientific Officer, Botox & Neurotoxins, Allergan, an AbbVie Company, 2525 Dupont DriveT2-3, Irvine, CA 92623-9534, USA (e-mail: mitchell.brin@abbvie.com).
References
- [1].Rutstein RP, Cogen MS, Cotter SA, et al. Optometric clinical practice guideline: care of the patient with strabismus: esotropia and exotropia. 2010. Available at: https://www.aoa.org/documents/optometrists/CPG-12.pdf.
- [2].Rowe FJ, Noonan CP. Botulinum toxin for the treatment of strabismus. Cochrane Database Syst Rev. 2017;3:Cd006499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hashemi H, Pakzad R, Heydarian S, et al. Global and regional prevalence of strabismus: a comprehensive systematic review and meta-analysis. Strabismus. 2019;27:54–65. [DOI] [PubMed] [Google Scholar]
- [4].Hultman O, Beth Høeg T, Munch IC, et al. The Danish Rural Eye Study: prevalence of strabismus among 3785 Danish adults – a population-based cross-sectional study. Acta Ophthalmol. 2019;97:784–92. [DOI] [PubMed] [Google Scholar]
- [5].Valls-Sole J, Defazio G. Blepharospasm: update on epidemiology, clinical aspects, and pathophysiology. Front Neurol. 2016;7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Defazio G, Hallett M, Jinnah HA, et al. Blepharospasm 40 years later. Mov Disord. 2017;32:498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Defazio G, Livrea P. Epidemiology of primary blepharospasm. Mov Disord. 2002;17:7–12. [DOI] [PubMed] [Google Scholar]
- [8].Defazio G, Berardelli A, Abbruzzese G, et al. Possible risk factors for primary adult onset dystonia: a case-control investigation by the Italian Movement Disorders Study Group. J Neurol Neurosurg Psychiatry. 1998;64:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Martino D, Liuzzi D, Macerollo A, et al. The phenomenology of the geste antagoniste in primary blepharospasm and cervical dystonia. Mov Disord. 2010;25:407–12. [DOI] [PubMed] [Google Scholar]
- [10].Henderson JW. Essential blepharospasm. Trans Am Ophthalmol Soc. 1956;54:453–520. [PMC free article] [PubMed] [Google Scholar]
- [11].Judd RA, Digre KB, Warner JEA, et al. Shedding light on blepharospasm: a patient–researcher partnership approach to assessment of photophobia and impact on activities of daily living. Neuro-Ophthalmol. 2007;31:49–54. [Google Scholar]
- [12].Simpson DM, Blitzer A, Brashear A, et al. Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Assessment: botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): report of the therapeutics and technology assessment subcommittee of the American academy of neurology. Neurology. 2008;70:1699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Waller RR, Kennedy RH, Henderson JW, et al. Management of blepharospasm. Trans Am Ophthalmol Soc. 1985;83:367–86. [PMC free article] [PubMed] [Google Scholar]
- [14].Arthurs B, Flanders M, Codère F, et al. Treatment of blepharospasm with medication, surgery and type A botulinum toxin. Can J Ophthalmol. 1987;22:24–8. [PubMed] [Google Scholar]
- [15].Nutt JG, Hammerstad JP, deGarmo P, et al. Cranial dystonia: double-blind crossover study of anticholinergics. Neurology. 1984;34:215–7. [DOI] [PubMed] [Google Scholar]
- [16].Fahn S, Hening WA, Bressman S, et al. Long term usefulness of baclofen in the treatment of essential blepharospasm. Adv Ophthal Plastic Reconst Surg. 1985;4:219–26. [Google Scholar]
- [17].Jankovic J, Ford J. Blepharospasm and orofacial-cervical dystonia: clinical and pharmacological findings in 100 patients. Ann Neurol. 1983;13:402–11. [DOI] [PubMed] [Google Scholar]
- [18].Gillum WN, Anderson RL. Blepharospasm surgery. An anatomical approach. Arch Ophthalmol. 1981;99:1056–62. [DOI] [PubMed] [Google Scholar]
- [19].Poisson A, Krack P, Thobois S, et al. History of the “geste antagoniste” sign in cervical dystonia. J Neurol. 2012;259:1580–4. [DOI] [PubMed] [Google Scholar]
- [20].Erbguth FJ. From poison to remedy: the chequered history of botulinum toxin. J Neural Transm (Vienna). 2008;115:559–65. [DOI] [PubMed] [Google Scholar]
- [21].Simpson LL, ed. Neuropoisons: their pathophysiological actions. Boston, MA: Springer. 1971. [Google Scholar]
- [22].Drachman DB. Botulinum toxin as a tool for research on the nervous system. In: Simpson LL, (ed). Neuropoisons: their pathophysiological actions. Boston, MA, USA: Springer; 1971:325–347. [Google Scholar]
- [23].Schantz EJ, Johnson EA. Properties and use of botulinum toxin and other microbial neurotoxins in medicine. Microbiol Rev. 1992;56:80–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Scott AB. Botulinum toxin injection of eye muscles to correct strabismus. Trans Am Ophthalmol Soc. 1981;79:734–70. [PMC free article] [PubMed] [Google Scholar]
- [25].Scott AB, Rosenbaum A, Collins CC. Pharmacologic weakening of extraocular muscles. Invest Ophthalmol. 1973;12:924–7. [PubMed] [Google Scholar]
- [26].Scott AB. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Ophthalmology. 1980;87:1044–9. [DOI] [PubMed] [Google Scholar]
- [27].Fahn S, List T, Moslowitz C, et al. Double-blind controlled study of botulinum toxin for blepharospasm. Neurology. 1985;35(4 Suppl 1):271–2. [Google Scholar]
- [28].Brin MF, Fahn S, Moskowitz C, et al. Localized injections of botulinum toxin for the treatment of focal dystonia and hemifacial spasm. Mov Disord. 1987;2:237–54. [DOI] [PubMed] [Google Scholar]
- [29].Scott AB, Kennedy RA, Stubbs HA. Botulinum A toxin injection as a treatment for blepharospasm. Arch Ophthalmol. 1985;103:347–50. [DOI] [PubMed] [Google Scholar]
- [30].Botox (onabotulinumtoxinA) [prescribing information]. Irvine, CA: Allergan Pharmaceuticals Ireland, a subsidiary of Allergan, Inc. 2019. [Google Scholar]
- [31].Saunte JP, Holmes JM. Sustained improvement of reading symptoms following botulinum toxin A injection for convergence insufficiency. Strabismus. 2014;22:95–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hallett M, Albanese A, Dressler D, et al. Evidence-based review and assessment of botulinum neurotoxin for the treatment of movement disorders. Toxicon. 2013;67:94–114. [DOI] [PubMed] [Google Scholar]
- [33].Jankovic J, Orman J. Botulinum A toxin for cranial-cervical dystonia: a double-blind, placebo-controlled study. Neurology. 1987;37:616–23. [DOI] [PubMed] [Google Scholar]
- [34].Dutton JJ, Fowler AM. Botulinum toxin in ophthalmology. Surv Ophthalmol. 2007;52:13–31. [DOI] [PubMed] [Google Scholar]
- [35].Çakmur R, Ozturk V, Uzunel F, et al. Comparison of preseptal and pretarsal injections of botulinum toxin in the treatment of blepharospasm and hemifacial spasm. J Neurol. 2002;249:64–8. [DOI] [PubMed] [Google Scholar]
- [36].Lolekha P, Choolam A, Kulkantrakorn K. A comparative crossover study on the treatment of hemifacial spasm and blepharospasm: preseptal and pretarsal botulinum toxin injection techniques. Neurol Sci. 2017;38:2031–6. [DOI] [PubMed] [Google Scholar]
- [37].Brin MF, James C, Maltman J. Botulinum toxin type A products are not interchangeable: a review of the evidence. Biologics. 2014;8:227–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Jog M, Wein T, Bhogal M, et al. Real-world, long-term quality of life following therapeutic onabotulinumtoxinA treatment. Can J Neurol Sci. 2016;43:687–96. [DOI] [PubMed] [Google Scholar]
- [39].Hsiung GY, Das SK, Ranawaya R, et al. Long-term efficacy of botulinum toxin A in treatment of various movement disorders over a 10-year period. Mov Disord. 2002;17:1288–93. [DOI] [PubMed] [Google Scholar]
- [40].Cillino S, Raimondi G, Guépratte N, et al. Long-term efficacy of botulinum toxin A for treatment of blepharospasm, hemifacial spasm, and spastic entropion: a multicentre study using two drug-dose escalation indexes. Eye (Lond). 2010;24:600–7. [DOI] [PubMed] [Google Scholar]
- [41].Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Assessment: the clinical usefulness of botulinum toxin-A in treating neurologic disorders. Report of the Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 1990;40:1332–6. [DOI] [PubMed] [Google Scholar]
- [42].Clinical use of botulinum toxin. Institutes of Health Consensus Development Conference Statement, November 12-14, 1990. Arch Neurol. 1991;48:1294–8. [PubMed] [Google Scholar]
- [43].Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology, Training guidelines for the use of botulinum toxin for the treatment of neurologic disorders. Neurology. 1994;44:2401–3. [DOI] [PubMed] [Google Scholar]