Abstract
Upper and lower limb spasticity (ULS, LLS) often occur following a stroke or in patients with other neurological disorders, leading to difficulties in mobility and daily living and decreased quality of life. Prior to the use of onabotulinumtoxinA, antispastic medications had limited efficacy and often caused sedation. Phenol injections were difficult for physicians to perform, painful, and led to tissue destruction. The success of onabotulinumtoxinA in treating cervical dystonia led to its use in spasticity. However, many challenges characterized the development of onabotulinumtoxinA for adult spasticity. The wide variability in the presentation of spasticity among patients rendered it difficult to determine which muscles to inject and how to measure improvement. Another challenge was the initial refusal of the Food and Drug Administration to accept the Ashworth Scale as a primary endpoint. Additional scales were designed to incorporate a goal-oriented, patient-centered approach that also accounted for the variability of spasticity presentations. Several randomized, double-blind, placebo-controlled trials of post-stroke spasticity of the elbow, wrist, and/or fingers showed significantly greater improvements in the modified Ashworth Scale and patient treatment goals and led to the approval of onabotulinumtoxinA for the treatment of ULS in adult patients. Lessons learned from the successful ULS trials were applied to design an LLS trial that led to approval for the latter indication. Additional observational trials mimicking real-world treatment have shown continued effectiveness and patient satisfaction. The use of onabotulinumtoxinA for spasticity has ushered in a more patient-centered treatment approach that has vastly improved patients’ quality of life.
Keywords: lower limb spasticity, onabotulinumtoxinA, stroke, upper limb spasticity
1. Overview of adult spasticity
Spasticity of the upper and/or lower limbs occurs following a stroke in about 40–60% of adult patients,[1,2] with an apparent increase in prevalence in the months after the stroke.[3] It can also occur in patients with multiple sclerosis (MS; 84%),[4] traumatic brain injury (23%),[5] spinal cord injury (65%),[6] or cerebral palsy (77%).[7] Spasticity adversely affects mobility, the ability to carry out activities of daily living, and quality of life.[8] Patients with post-stroke spasticity have a high incidence of pain, functional disability, and placement in nursing homes.[9] In addition, spasticity may lead to contractures and other complications that can prevent patients from benefiting from rehabilitation interventions.[10]
One of the challenges in spasticity is lack of a consensus on its definition.[11] The widely cited definition from James Lance in 1980 states that spasticity is “a motor disorder characterized by a velocity-dependent increase in tonic stretch reflexes (muscle tone) with exaggerated tendon jerks, resulting from hyperexcitability of the stretch reflex.”[12] However, other literature suggests that there is a low or no correlation of the tonic stretch reflex with tendon jerks,[13] that hypertonia is not always associated with reflex hyperexcitability,[14] and that muscles become spastic due to changes in their mechanical properties.[15]
The following historical narrative was compiled based on a review of the literature and interviews with the authors, and the quoted portions reflect the personal observations and reflections of the individuals who were interviewed. In some instances, this article describes uses for which Allergan has not sought and/or received regulatory approval in individual countries and are mentioned for historical context or background only.
2. Unmet need for the treatment of upper and lower limb spasticity in adults
Prior to the use of onabotulinumtoxinA, treatments for spasticity were not particularly effective. The antispastic medications baclofen, dantrolene, diazepam, and tizanidine were Food and Drug Administration (FDA)-approved but had many associated side effects.[16]
Dr. Jost, who has been an investigator on several clinical trials of onabotulinumtoxinA for adult spasticity and other indications: We had baclofen, we had muscle relaxants … but the whole story started with botulinum toxin.
Dr. Wein, who learned to inject onabotulinumtoxinA for spasticity in 1999 while a fellow at the University of Michigan: I was never quite sure this drug [baclofen] really had any significant impact or worked very well. In trying to get some clinical effect from it, we always had to titrate it up to higher dosages, sometimes above product monograph labeling, and it came with a problematic side effect of sedation in people who are already neurologically impaired. So, it increased their risk of falls, it made them less alert, and sometimes gave them cognitive deficits.
Phenol and ethanol injections directly into the muscles were also sometimes utilized but could be painful, were generally irreversible, and their mechanism of action involved non-selective tissue destruction (including muscle and nerve) and potential vascular damage.[17,18] Few physicians elected to perform this rather difficult injection procedure.
Physical therapy involved muscle stretching in an attempt to prevent shortening.[17] However, muscle stretching needed to be continuously performed for several hours a day in order to effectively prevent contractures.[17,19]
Dr. Wein: The drug [onabotulinumtoxinA] came around at an interesting time, at least for a stroke neurologist, where we were just starting to have evidence-based therapies for acute stroke as well as a myriad of therapies for stroke prevention. We [neurologists] really had nothing highly effective with acceptable tolerability for post-stroke spasticity until botulinum toxin came out.
3. Early use and development of onabotulinumtoxinA for upper and lower limb spasticity in adults
The first use of botulinum toxin A by Dr. Alan Scott for strabismus in the 1970s[20] led to its investigation and use in other conditions in which muscle weakening was beneficial, such as blepharospasm[21] and cervical dystonia[22,23] (also see related articles in this supplement, including the first one on the developmental history of onabotulinumtoxinA). From the experience of treating larger muscles in cervical dystonia, physicians had the idea of using botulinum toxin A to treat patients with spasticity.[24] The first double-blind, placebo-controlled trial of botulinum toxin for spasticity was conducted by Drs. Snow, Tsui, and colleagues in 9 patients with MS in Canada, where significant improvements both from baseline and compared with placebo in spasticity of leg adductors were observed 6 weeks following treatment with botulinum toxin.[25] A few years later, onabotulinumtoxinA was tested in post-stroke upper limb spasticity (ULS) in the United States, where clinically and statistically significant decreases in tone of elbow and wrist flexors were observed with no serious adverse events (AEs).[26]
Dr. Wein: As toxin became available and people thought of using selective paralysis of muscles to counteract the spasticity, it was like a brand-new field. It offered new hope for patients, for people not to have the sedating effects [of baclofen].
Although the use of onabotulinumtoxinA for adult spasticity revolutionized the field, development of the indication was fraught with several hurdles. One challenge was the heterogeneity of the patient population, as each patient’s muscle spasticity is complex and unique, making it difficult to formulate inclusion and exclusion criteria for clinical trials. A related challenge was identifying which muscles to treat with onabotulinumtoxinA. Dr. Esquenazi, who began using botulinum toxin in the late 1980s, and his colleague Dr. Nathaniel Mayer were the first to identify patterns of spasticity and link them to specific muscles (Fig. 1).[27] This work has continued today,[28] and has helped to determine the muscles to inject with onabotulinumtoxinA.
Figure 1.
(Left to right) Drs. Alberto Esquenazi, Alan Scott, and Nathaniel Mayer celebrating the 15th anniversary of Botox in 2004 in Newport Beach, CA. Photo provided by Dr. Esquenazi.
How to effectively measure the change in spasticity following onabotulinumtoxinA treatment was a corresponding issue. To this end, Allergan modified the Ashworth Scale, a 5-point scale to quantify muscle resistance during passive movements,[29] to a 9-point scale with 0.5-grade intervals to allow for finer grading of spasticity (Modified Ashworth Scale [MAS]).[30] In addition, Drs. Esquenazi, Mayer, Turkel (who was a senior director at Allergan for the clinical development of onabotulinumtoxinA for adult spasticity), and others assisted with the development of the Disability Assessment Scale (DAS), which was later shown to have good intra- and inter-rater reliability in post-stroke patients with ULS.[31] This scale addressed another of the challenging aspects in measuring spasticity outcomes: patients often have very different personal goals for treatment. For example, 1 patient may want to decrease pain and another may want to be able to dress themselves. The DAS allows the selection of functional outcomes that are important to individual patients and assesses the impact of treatment on those particular goals.
Along these lines, perhaps one of the biggest challenges for the development program was that the FDA initially rejected the use of the Ashworth Scale as a primary endpoint, instead insisting on establishment of a clinically meaningful functional change. However, restoration of function of a paralyzed limb was not consistently a realistic expectation, as onabotulinumtoxinA was not changing the underlying neurological disorder but rather managing the symptoms.
Dr. Turkel: There were some unique experiences that happened along the way that I think encouraged the organization. We decided to submit the adult spasticity data that had assessed improvements using the Ashworth Scale as the primary efficacy measure to the Switzerland regulatory authority. We thought this would help us get specific feedback as to what additional data might be required for approval. However, rather than getting feedback on additional data requirements, they approved the submission, and we got our first ULS registration. At about the same time, we had completed studies in the lower limb in Australia and identified a group of the more severely affected patients that had a favorable response on the Ashworth Scale. Combined with the upper limb data, the Australian authorities approved the product for both upper and lower limb spasticity. Two authorities approved the product on the basis of the Ashworth Scale, and clinicians kept telling us that this outcome variable is a reasonable assessment to determine whether this treatment that relaxes the muscle is indeed effective for this patient population.
Over time, the FDA recognized that the Ashworth Scale was an important primary assessment.
Dr. Dimitrova, who headed the developmental program that led to United States approval for spasticity: They [the FDA] accepted the data based on 2 outcomes. One was the demonstration that Botox alleviates spasticity based on the Ashworth Scale, and the second evidence was global treatment outcomes. Physicians felt that their patients were improved following Botox versus placebo.
The FDA ultimately approved onabotulinumtoxinA for the treatment of ULS in adult patients in March 2010.[32] Furthermore, the spasticity project team examined previously unsuccessful trials on lower limb spasticity (LLS) to determine why onabotulinumtoxinA had not demonstrated robust outcomes. The learnings from this assessment included a need for improved patient selection, higher doses, optimization of muscle selection, and treatment of a wider range of muscles contributing to a particular spastic presentation. These findings were used to design the registrational Botox for the Rehabilitation Treatment of Ankle Flexor Spasticity (REFLEX) study on LLS.[33] Investigators in this trial had the option to use higher doses than previously studied and to treat the plantar flexors together with other affected muscles of the knee or toes, thus allowing them to customize treatment to the clinical presentation of the individual patient to achieve a more favorable outcome. OnabotulinumtoxinA received FDA approval for the treatment of LLS in adults in January 2016, primarily on the basis of the results of the REFLEX trial.[32,33]
4. Efficacy and safety highlights
Several studies contributed to primary approvals of onabotulinumtoxinA for the treatment of adult spasticity as well as to the global evolution of label expansions. In addition, these trials contributed to recommendations from medical societies and others, some of which even preceded the US approvals of onabotulinumtoxinA for spasticity.[34–38] One of these trials tested up to 2 treatment cycles of onabotulinumtoxinA 90, 180, or 360U versus placebo in the upper limbs of patients in the US with post-stroke spasticity.[30] Significant, dose-dependent reductions in muscle tone via the MAS were observed at several post-injection time points in the wrist, elbow, and finger flexors in onabotulinumtoxinA-treated patients compared with placebo. Significantly greater physician global assessment scores were seen in patients who received onabotulinumtoxinA 180 or 360U compared with placebo.
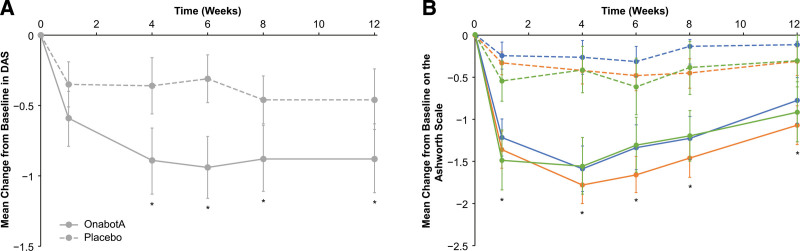
Another randomized, double-blind, placebo-controlled study tested the efficacy of 1 set of injections of onabotulinumtoxinA 200–240U in post-stroke focal spasticity of the wrist and fingers in the US.[39] In contrast to earlier trials, the primary outcome measure of this trial focused on function by having patients choose a principal target of treatment on the DAS (hygiene, dressing, limb position, or pain). Patients treated with onabotulinumtoxinA had significantly greater improvements in their chosen principal therapeutic target compared with placebo (Fig. 2A). In addition, significantly greater improvements were seen in onabotulinumtoxinA-treated patients versus placebo in mean scores for muscle tone of the wrist, finger, and thumb as measured on the Ashworth Scale (Fig. 2B) and in the physician’s global assessment. A 42-week open-label extension of this trial found that improvements on the Ashworth Scale and DAS were sustained with repeat treatments of onabotulinumtoxinA for ULS following stroke.[40]
Figure 2.
Mean changes from baseline on the chosen principal therapeutic target on (A) the Disability Assessment Scale and (B) the Ashworth Scale for wrist (orange), finger (blue), and thumb (green) flexors as measured in the trial published in Brashear A, Gordon MF, Elovic E, et al. Intramuscular injection of botulinum toxin for the treatment of wrist and finger spasticity after a stroke. N Engl J Med. 2002;347(6):395–400. Dashed lines indicate placebo, solid lines onabotulinumtoxinA, and error bars 95% confidence intervals. * P <.05. DAS = Disability Assessment Scale, onabotA = onabotulinumtoxinA.
The initial FDA approval of onabotulinumtoxinA for the treatment of ULS in adults was supported by the results of those 2 clinical trials.[30,39] A third study, conducted in Europe in a similar patient population, showed significant changes from baseline in muscle tone of the wrist, elbow, and finger flexors on the MAS at week 4 following treatment with onabotulinumtoxinA 360U.[32]
Two other trials in the US prescribing information for onabotulinumtoxinA, 1 conducted in Japan and the other in China, assessed the change in thumb flexor muscle tone, finding significant improvements on the MAS and physician global impression following treatment with onabotulinumtoxinA 40U compared with placebo.[32,41] One of these trials also confirmed the efficacy of onabotulinumtoxinA in the wrist and fingers on the MAS and principal therapeutic target of the DAS.[41]
Global labels expanded upon the results of trials that examined onabotulinumtoxinA treatment of additional muscles, including those in the lower limbs. The efficacy and safety of onabotulinumtoxinA for LLS in adults were documented in the results of the REFLEX trial, which supported FDA approval for this indication in 2016, as noted above.[32,33] Improvements in MAS, clinical global impression, and goal attainment scale with onabotulinumtoxinA treatment in the 12-week double-blind phase were maintained and further enhanced during the 48-week open-label phase, during which patients could receive an additional 3 onabotulinumtoxinA treatments.
For LLS, significant improvements with onabotulinumtoxinA treatment were seen in change from baseline to week 12 in MAS versus placebo in a large, randomized, double-blind trial of post-stroke patients in Japan.[42] Significant improvement in the clinician’s global impression of functional disability was observed with onabotulinumtoxinA treatment compared with placebo, though the differences were not significant for the patients’ global impression.
Over the years, in addition to Allergan-sponsored trials, investigators have studied multiple aspects of onabotulinumtoxinA treatment in addition to comparing with other therapies. A small, randomized, double-blind trial in the US found that onabotulinumtoxinA combined with physical therapy enhanced upper limb functional status to a greater degree than physical therapy alone in stroke subjects, with both groups showing significant decreases from their respective baselines in MAS scores.[43] Another randomized, double-blind, placebo-controlled trial in the US and United Kingdom compared onabotulinumtoxinA with oral tizanidine in treatment of ULS.[44] OnabotulinumtoxinA-treated patients showed significantly greater reductions in tone of the finger and wrist flexors as measured by the MAS compared with tizanidine and placebo. In contrast, tizanidine did not significantly improve tone versus placebo, and the tizanidine groups showed higher rates of AEs related to study treatment compared with onabotulinumtoxinA and placebo.
Other trials examined treatment factors such as dosing and timing of treatment relative to onset of the brain lesion. One study in Italy for spastic foot showed that medium doses (mean 322U) resulted in higher and more prolonged responses in several physician- and patient-reported measures compared with lower doses (mean 167U), with fewer AEs than higher doses (mean 540U).[45] Results of a trial of patients with spastic pes equinovarus in Germany indicated that earlier treatment (within the first 3 months of brain lesion), rather than treatment after that time frame, was more effective in reducing spastic muscle tone.[46] A secondary analysis of a randomized trial of onabotulinumtoxinA treatment of plantar flexor spasticity found significant improvements in MAS and gait regardless of time since stroke, including those treated within the first 6 months following stroke.[47] In addition, a small, open-label trial reported significant improvements in ULS in a population limited to those experiencing a stroke within the last 3 years.[48] For LLS, a recent secondary analysis of the REFLEX trial found that patients treated with onabotulinumtoxinA ≤24 months since stroke exhibited greater improvements from baseline versus placebo in MAS, clinical global impression of change, and passive goal attainment scores compared with those >24 months since stroke.[49]
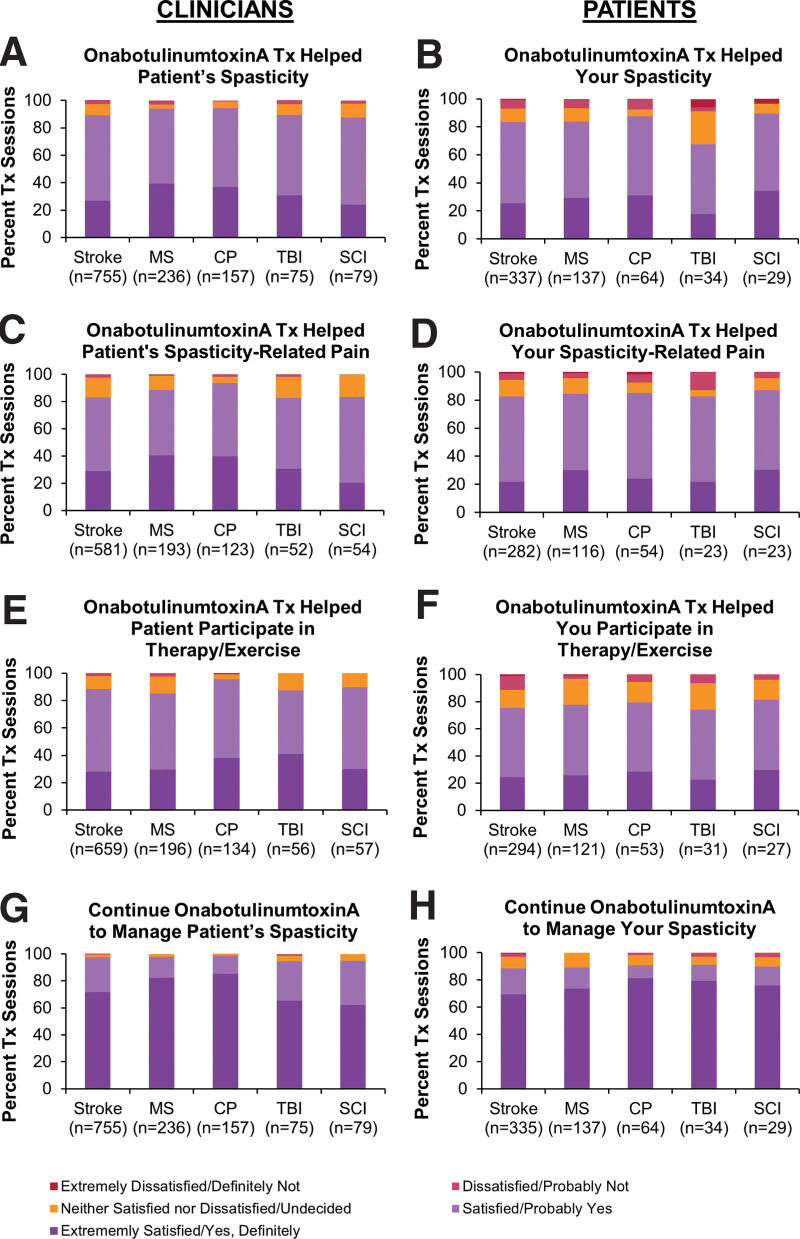
Additional trials have expanded on the idea of goal attainment and customization of treatment. The Botox Economic Spasticity Trial (BEST) aimed to treat patients in Europe and Canada with upper and/or lower limb post-stroke spasticity with onabotulinumtoxinA in a manner similar to clinical practice by using goal-oriented treatment (Goal Attainment Scaling).[50] While the primary endpoint of proportion of patients with active functional goal achievement was numerically but not significantly higher in the onabotulinumtoxinA-treated patients versus placebo, the proportion of patients with passive goal achievement (a secondary endpoint) was significant. Furthermore, both mean pain reduction from baseline and the proportion of patients with pain reduction at week 12 were significantly higher in the onabotulinumtoxinA-treated group compared with placebo, with pain reduction sustained through week 52 in the BEST study population.[51] The Adult Spasticity International Registry (ASPIRE) was based on the Patient Registry of Outcomes in Spasticity[52] and was a real-world study in the US, Europe, and Taiwan to examine onabotulinumtoxinA use in clinical practice of patients with spasticity from a variety of etiologies, generating data on dosing, target muscles, and injection guidance.[53] High rates of satisfaction of treatment of both upper and lower limbs were observed from both physicians and patients (Fig. 3).[54,55] MDs on Botox Utility (MOBILITY) was a multicenter, observational study in Canada that examined the health utility of onabotulinumtoxinA in a variety of therapeutic indications.[56] Patients with adult focal spasticity comprised over one-third of the patients, and health utility was maintained or trended toward improvement in both patients who were naïve to botulinum toxin at baseline or were receiving ongoing treatment.
Figure 3.
Clinician (A, C, E, G)- and patient (B, D, F, H)-reported satisfaction with the previous onabotulinumtoxinA treatment for upper and/or lower limb spasticity. Questions were (A) “How satisfied, on average, are you that the most recent Botox treatment has helped to manage your patient’s spasticity symptoms?” (B) “How satisfied are you that your most recent Botox treatment has helped your spasticity?” (C) “How satisfied are you that the most recent Botox treatment has helped to manage the pain associated with your patient’s spasticity?” (D) “How satisfied are you that your most recent Botox treatment has helped your spasticity-related pain?” (E) “How satisfied are you that the most recent Botox treatment has helped with your patient’s ability to participate in physiotherapy, occupational therapy (PT/OT) and/or an exercise program?” (F) “How satisfied are you that your most recent Botox treatment has helped with your ability to participate in physiotherapy, occupation therapy (PT/OT), and/or home exercise?” (G) “Taking everything into consideration, would you continue to use Botox to manage your patient’s spasticity?” (H) “Taking everything into consideration, would you continue to use Botox to treat your spasticity?” Respondents were given a choice of 5 answers ranging from “extremely satisfied” to “extremely dissatisfied” for A to F and from “yes, definitely” to “definitely not” for G and H. Figure is reprinted with permission from “High clinician- and patient-reported satisfaction with individualized onabotulinumtoxinA treatment for spasticity across several etiologies from the ASPIRE study” by Francisco GE, licensed under CC BY-NC-ND 4.0. CP = cerebral palsy, MS = multiple sclerosis, n = number of treatment sessions, OT = occupational therapy; PT = physical therapy; SCI = spinal cord injury; TBI = traumatic brain injury, Tx = treatment session.
OnabotulinumtoxinA is generally well tolerated for the treatment of both ULS and LLS.[30,33,39,41,50,55,57] As per the US label, the most frequently reported AEs for ULS were pain in extremity, nausea, fatigue, and muscular weakness (rates of 2–9%) and in LLS, arthralgia, back pain, myalgia, upper respiratory tract infection, and injection site pain (rates of 2–3%).[32] Region-specific information regarding safety and efficacy can be found in local labeling. A pooled analysis of 9 trials conducted as part of Allergan’s registration program for ULS and LLS found that the most frequent treatment-related AEs were hypertonia, peripheral edema, and injection site pain (rates of 3–5%), with injection site pain reported at higher rates in the placebo group.[57] The effect of onabotulinumtoxinA treatment for predominantly ULS in patients with decreased pulmonary function at baseline (mean baseline forced vital capacity [FVC] 71–78%) was analyzed in 2 randomized, placebo-controlled trials.[58] Small, subclinical but statistically significant decreases in FVC or forced expiratory volume in one second (FEV1) were observed but were not correlated with pulmonary function-related AEs.
Due to the need for repeated injections over time, patients can develop neutralizing antibodies (nAbs) against onabotulinumtoxinA. However, at the recommended doses and treatment intervals, neutralizing antibody formation is infrequent: a meta-analysis showed that 0.3% (1/317) of patients were nAb positive (as measured by the mouse protection assay) at any visit in post-stroke spasticity trials of onabotulinumtoxinA (200-600U).[59] This conversion rate is comparable to the 0.5% rate for onabotulinumtoxinA obtained across 5 licensed indications.[59]
OnabotulinumtoxinA is recommended by the American Academy of Neurology and several other societies and medical groups as a level A treatment for both ULS and LLS.[34–38] Due to differences in manufacturing, formulation, and assays used to determine activity, onabotulinumtoxinA dosing is not interchangeable with that of other botulinum toxin products.[32,60]
5. Lessons learned, the clinical and scientific impact, and questions for the future in onabotulinumtoxinA for adult spasticity
OnabotulinumtoxinA has had a significant impact on adult spasticity management and has led to the generation of much useful information for therapy over the past decades.
Dr. Jost: I think that Botox was very important for any therapy of spasticity … It was the first step in a new era of spasticity therapy, to see spasticity not only as a defect but as a kind of movement disorder.
A notable aspect of the research on spasticity was the inclusion of the goal-oriented approach to treatment, a patient-centric approach that also incorporates the variability of spasticity presentations and may change over time based on age, functionality, spasticity presentation, and caregiver situation.
Dr. Wein: It’s a new aspect of medicine that’s been incorporated into the field of spasticity research and rehab: to have a goal-oriented approach to people but that also emphasizes the variability of deficiencies that developed because of the spasticity … that’s a different aspect [than] what we traditionally did in medicine. We try to treat a blood pressure number and abnormal blood value, but here we really have a therapy that’s trying to change quality of life as a whole for patients, which we don’t really encounter with very many other medications that I can think of.
Expanded availability of treatment has been another positive outcome of the use of onabotulinumtoxinA for adult spasticity. Although most physiatrists learned how to inject phenol or alcohol during residency, most did not continue to offer that treatment because it was a complex procedure and could be associated with significant treatment-related AEs. The approval of onabotulinumtoxinA, the relative ease of its injection, and acceptable safety profile led to a greater number of physicians who could treat patients with spasticity.
Dr. Esquenazi: The availability of Botox really enlarged the number of clinicians that were willing and available to treat patients with spasticity. So, the patients who used to really suffer because of the lack of available interventions suddenly found a more willing and better-able-to-provide-care community of clinicians.
Determination of optimal treatment remains a long-standing initiative. The understanding of functional anatomy progressed rapidly through the use of onabotulinumtoxinA for spasticity, though identifying which muscles to inject remains one of the biggest challenges. Nevertheless, onabotulinumtoxinA has made a tremendous impact on the care of patients with spasticity.
Dr. Wein: That’s still one of the biggest hurdles today: having a good comprehension of functional anatomy and identifying which muscles to inject. I have so many patients I see as reinjections from other colleagues who “failed therapy.” The question to pose is: “Why did therapy fail to provide adequate results?” Reasons for treatment failure include: lack of clear-cut goal setting or unrealistic goal setting, subtherapeutic dosing, possible poor anatomic localization, or the need to consider injecting additional muscles using functional anatomy to assess the spastic posture. In addition, many patients do not even receive treatment due to misconceptions that only people with visible active function may receive or deserve treatment. It is important to remember that sometimes spasticity masks active function, and treatment with Botox may produce some astonishing results.
Dr. Esquenazi:[With onabotulinumtoxinA treatment] patients have found a way to what they term their “new normal” in life.
Acknowledgments
Writing and editorial assistance was provided to the authors by Jennifer L. Giel, PhD, on behalf of Evidence Scientific Solutions and was funded by Allergan, an AbbVie company, Dublin, Ireland. All authors met the ICMJE authorship criteria. Neither honoraria nor other forms of payments were made for authorship.
Author contributions
Conceptualization: Rozalina Dimitrova.
Funding acquisition: Rozalina Dimitrova.
Supervision: Rozalina Dimitrova.
Writing – review & editing: Alberto Esquenazi, Wolfgang H. Jost, Catherine Turkel, Theodore Wein, Rozalina Dimitrova.
Abbreviations:
- AE
- adverse event
- ASPIRE
- Adult Spasticity International Registry
- BEST
- Botox Economic Spasticity Trial
- DAS
- Disability Assessment Scale
- FDA
- Food and Drug Administration
- FVC
- forced vital capacity
- LLS
- lower limb spasticity
- MAS
- Modified Ashworth Scale
- MOBILITY
- MDs on Botox Utility
- MS
- multiple sclerosis
- REFLEX
- Botox for the Rehabilitation Treatment of Ankle Flexor Spasticity
- ULS
- upper limb spasticity
This manuscript was funded by AbbVie. AbbVie was involved in the manuscript concept and participated in writing, reviewing, and approval of the final version. No honoraria or payments were made for authorship.
A Esquenazi has received research funds from Allergan, an AbbVie Company, and Ipsen and has served as a consultant for Allergan, an AbbVie Company, Ipsen, and Merz. WH Jost is an advisor and speaker for Allergan, an AbbVie Company, Ipsen, and Merz. CC Turkel was previously employed by Allergan plc from September 1998 until June 2015 and during that period received salary and Allergan plc stock/stock options. Currently she receives no salary and has no stock or any relationship with Allergan plc. T Wein has received research funds from Allergan, an AbbVie Company, and Ipsen and has served as a consultant for Allergan, an AbbVie Company, and Ipsen. R Dimitrova is a full-time employee of Allergan, an AbbVie Company, and may hold AbbVie stock.
How to cite this article: Esquenazi A, Jost WH, Turkel CC, Wein T, Dimitrova R. Treatment of adult spasticity with Botox (onabotulinumtoxinA): Development, insights, and impact. Medicine 2022;102:S1(e32376).
Correspondence: Alberto Esquenazi, Chair and Professor of PM&R, MossRehab, 60 Township Line Rd, Elkins Park, PA 19027, USA (e-mail: aesquena@einstein.edu).
Contributor Information
Wolfgang H. Jost, Email: w.jost@parkinson-klinik.de.
Catherine C. Turkel, Email: catherineturkel@outlook.com.
Theodore Wein, Email: twein@videotron.ca.
Rozalina Dimitrova, Email: Dimitrova_Rozalina@allergan.com.
References
- [1].National Stroke Association. National Stroke Association Stroke Perceptions Study. Russell Research. 2006. Available at: https://www.strokesolutions.co.uk/wp-content/uploads/2014/07/StrokePerceptions_FinalSurveyResults_2006.pdf. [accessed July 22, 2019].
- [2].Urban PP, Wolf T, Uebele M, et al. Occurence and clinical predictors of spasticity after ischemic stroke. Stroke. 2010;41:2016–20. [DOI] [PubMed] [Google Scholar]
- [3].Wissel J, Manack A, Brainin M. Toward an epidemiology of poststroke spasticity. Neurology. 2013;80(3 Suppl 2):S13–9. [DOI] [PubMed] [Google Scholar]
- [4].Rizzo MA, Hadjimichael OC, Preiningerova J, et al. Prevalence and treatment of spasticity reported by multiple sclerosis patients. Mult Scler. 2004;10:589–95. [DOI] [PubMed] [Google Scholar]
- [5].Hibbard MR, Uysal S, Sliwinski M, et al. Undiagnosed health issues in individuals with traumatic brain injury living in the community. J Head Trauma Rehabil. 1998;13:47–57. [DOI] [PubMed] [Google Scholar]
- [6].Sköld C, Levi R, Seiger Å. Spasticity after traumatic spinal cord injury: nature, severity, and location. Arch Phys Med Rehabil. 1999;80:1548–57. [DOI] [PubMed] [Google Scholar]
- [7].Andersson C, Mattsson E. Adults with cerebral palsy: a survey describing problems, needs, and resources, with special emphasis on locomotion. Dev Med Child Neurol. 2001;43:76–82. [DOI] [PubMed] [Google Scholar]
- [8].Gillard PJ, Sucharew H, Kleindorfer D, et al. The negative impact of spasticity on the health-related quality of life of stroke survivors: a longitudinal cohort study. Health Qual Life Outcomes. 2015;13:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wissel J, Schelosky LD, Scott J, et al. Early development of spasticity following stroke: a prospective, observational trial. J Neurol. 2010;257:1067–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ada L, O’Dwyer N, O’Neill E. Relation between spasticity, weakness and contracture of the elbow flexors and upper limb activity after stroke: an observational study. Disabil Rehabil. 2006;28:891–7. [DOI] [PubMed] [Google Scholar]
- [11].Ibuki A, Bernhardt J. What is spasticity? The discussion continues. Int J Ther Rehabil. 2007;14:391–4. [Google Scholar]
- [12].Lance JW. Symposium synopsis. In: Feldman RG, Young RR, Koella WP, eds. Spasticity: Disordered Motor Control. Chicago, IL: Year Book Medical Publishers; 1980:485–494. [Google Scholar]
- [13].Fellows SJ, Ross HF, Thilmann AF. The limitations of the tendon jerk as a marker of pathological stretch reflex activity in human spasticity. J Neurol Neurosurg Psychiatry. 1993;56:531–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].O’Dwyer NJ, Ada L, Neilson PD. Spasticity and muscle contracture following stroke. Brain. 1996;119(Pt 5):1737–49. [DOI] [PubMed] [Google Scholar]
- [15].Dietz V, Trippel M, Berger W. Reflex activity and muscle tone during elbow movements in patients with spastic paresis. Ann Neurol. 1991;30:767–79. [DOI] [PubMed] [Google Scholar]
- [16].Gracies JM, Nance P, Elovic E, et al. Traditional pharmacological treatments for spasticity. Part II: general and regional treatments. Muscle Nerve Suppl. 1997;6:S92–120. [PubMed] [Google Scholar]
- [17].Gracies JM, Elovic E, McGuire J, et al. Traditional pharmacological treatments for spasticity. Part I: local treatments. Muscle Nerve Suppl. 1997;6:S61–91. [PubMed] [Google Scholar]
- [18].Gonnade N, Lokhande V, Ajij M, et al. Phenol versus botulinum toxin A injection in ambulatory cerebral palsy spastic diplegia: a comparative study. J Pediatr Neurosci. 2017;12:338–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tardieu C, Lespargot A, Tabary C, et al. For how long must the soleus muscle be stretched each day to prevent contracture? Dev Med Child Neurol. 1988;30:3–10. [DOI] [PubMed] [Google Scholar]
- [20].Scott AB. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Ophthalmology. 1980;87:1044–9. [DOI] [PubMed] [Google Scholar]
- [21].Frueh BR, Felt DP, Wojno TH, et al. Treatment of blepharospasm with botulinum toxin. A preliminary report. Arch Ophthalmol. 1984;102:1464–8. [DOI] [PubMed] [Google Scholar]
- [22].Jankovic J, Orman J. Botulinum A toxin for cranial-cervical dystonia: a double-blind, placebo-controlled study. Neurology. 1987;37:616–23. [DOI] [PubMed] [Google Scholar]
- [23].Tsui JK, Eisen A, Mak E, et al. A pilot study on the use of botulinum toxin in spasmodic torticollis. Can J Neurol Sci. 1985;12:314–6. [DOI] [PubMed] [Google Scholar]
- [24].Scott AB. Development of botulinum toxin therapy. Dermatol Clin. 2004;22:131–3. [DOI] [PubMed] [Google Scholar]
- [25].Snow BJ, Tsui JK, Bhatt MH, et al. Treatment of spasticity with botulinum toxin: a double-blind study. Ann Neurol. 1990;28:512–5. [DOI] [PubMed] [Google Scholar]
- [26].Simpson DM, Alexander DN, O’Brien CF, et al. Botulinum toxin type A in the treatment of upper extremity spasticity: a randomized, double-blind, placebo-controlled trial. Neurology. 1996;46:1306–10. [DOI] [PubMed] [Google Scholar]
- [27].Mayer NH, Esquenazi A, Childers MK. Common patterns of clinical motor dysfunction. Muscle Nerve Suppl. 1997;6:S21–35. [PubMed] [Google Scholar]
- [28].Esquenazi A, Wein TH, Ward AB, et al. Optimal muscle selection for onabotulinumtoxinA injections in poststroke lower-limb spasticity: a randomized trial. Am J Phys Med Rehabil. 2019;98:360–8. [DOI] [PubMed] [Google Scholar]
- [29].Ashworth B. Preliminary trial of carisoprodol in multiple sclerosis. Practitioner. 1964;192:540–2. [PubMed] [Google Scholar]
- [30].Childers MK, Brashear A, Jozefczyk P, et al. Dose-dependent response to intramuscular botulinum toxin type A for upper-limb spasticity in patients after a stroke. Arch Phys Med Rehabil. 2004;85:1063–9. [DOI] [PubMed] [Google Scholar]
- [31].Brashear A, Zafonte R, Corcoran M, et al. Inter- and intrarater reliability of the Ashworth Scale and the Disability Assessment Scale in patients with upper-limb poststroke spasticity. Arch Phys Med Rehabil. 2002;83:1349–54. [DOI] [PubMed] [Google Scholar]
- [32].Botox (onabotulinumtoxinA) [prescribing information]. Irvine, CA: Allergan Pharmaceuticals Ireland, a subsidiary of Allergan, Inc.; 2020. [Google Scholar]
- [33].Wein T, Esquenazi A, Jost WH, et al. OnabotulinumtoxinA for the treatment of poststroke distal lower limb spasticity: a randomized trial. PM R. 2018;10:693–703. [DOI] [PubMed] [Google Scholar]
- [34].Simpson DM, Hallett M, Ashman EJ, et al. Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2016;86:1818–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Deltombe T, Wautier D, De Cloedt P, et al. Assessment and treatment of spastic equinovarus foot after stroke: guidance from the Mont-Godinne interdisciplinary group. J Rehabil Med. 2017;49:461–8. [DOI] [PubMed] [Google Scholar]
- [36].Royal College of Physicians, British Society of Rehabilitation Medicine, The Chartered Society of Physiotherapy, Association of Chartered Physiotherapists in Neurology, Royal College of Occupational Therapists. Spasticity in adults: management using botulinum toxin. National guidelines 2018. London, UK: RCP; 2018. [Google Scholar]
- [37].Winstein CJ, Stein J, Arena R, et al. American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47:e98–e169. [DOI] [PubMed] [Google Scholar]
- [38].Intercollegiate Stroke Working Party. National clinical guideline for stroke. London, UK: Royal College of Physicians; 2016. [Google Scholar]
- [39].Brashear A, Gordon MF, Elovic E, et al. Botox Post-Stroke Spasticity Study Group. Intramuscular injection of botulinum toxin for the treatment of wrist and finger spasticity after a stroke. N Engl J Med. 2002;347:395–400. [DOI] [PubMed] [Google Scholar]
- [40].Gordon MF, Brashear A, Elovic E, et al. Botox Poststroke Spasticity Study Group. Repeated dosing of botulinum toxin type A for upper limb spasticity following stroke. Neurology. 2004;63:1971–3. [DOI] [PubMed] [Google Scholar]
- [41].Kaji R, Osako Y, Suyama K, et al. GSK1358820 Spasticity Study Group. Botulinum toxin type A in post-stroke upper limb spasticity. Curr Med Res Opin. 2010;26:1983–92. [DOI] [PubMed] [Google Scholar]
- [42].Kaji R, Osako Y, Suyama K, et al. GSK1358820 Spasticity Study Group. Botulinum toxin type A in post-stroke lower limb spasticity: a multicenter, double-blind, placebo-controlled trial. J Neurol. 2010;257:1330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Meythaler JM, Vogtle L, Brunner RC. A preliminary assessment of the benefits of the addition of botulinum toxin A to a conventional therapy program on the function of people with longstanding stroke. Arch Phys Med Rehabil. 2009;90:1453–61. [DOI] [PubMed] [Google Scholar]
- [44].Simpson DM, Gracies JM, Yablon SA, et al. BoNT/TZD Study Team. Botulinum neurotoxin versus tizanidine in upper limb spasticity: a placebo-controlled study. J Neurol Neurosurg Psychiatry. 2009;80:380–5. [DOI] [PubMed] [Google Scholar]
- [45].Mancini F, Sandrini G, Moglia A, et al. A randomised, double-blind, dose-ranging study to evaluate efficacy and safety of three doses of botulinum toxin type A (Botox) for the treatment of spastic foot. Neurol Sci. 2005;26:26–31. [DOI] [PubMed] [Google Scholar]
- [46].Fietzek UM, Kossmehl P, Schelosky L, et al. Early botulinum toxin treatment for spastic pes equinovarus–a randomized double-blind placebo-controlled study. Eur J Neurol. 2014;21:1089–95. [DOI] [PubMed] [Google Scholar]
- [47].Oh HM, Park GY, Choi YM, et al. The effects of botulinum toxin injections on plantar flexor spasticity in different phases after stroke: a secondary analysis from a double-blind, randomized trial. PM R. 2018;10:789–97. [DOI] [PubMed] [Google Scholar]
- [48].Slawek J, Bogucki A, Reclawowicz D. Botulinum toxin type A for upper limb spasticity following stroke: an open-label study with individualised, flexible injection regimens. Neurol Sci. 2005;26:32–9. [DOI] [PubMed] [Google Scholar]
- [49].Patel AT, Ward AB, Geis C, et al. Impact of early intervention with onabotulinumtoxinA treatment in adult patients with post-stroke lower limb spasticity: results from the double-blind, placebo-controlled, phase 3 REFLEX study. J Neural Transm (Vienna). 2020;127:1619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ward AB, Wissel J, Borg J, et al. BEST Study Group. Functional goal achievement in post-stroke spasticity patients: the Botox® Economic Spasticity Trial (BEST). J Rehabil Med. 2014;46:504–13. [DOI] [PubMed] [Google Scholar]
- [51].Wissel J, Ganapathy V, Ward AB, et al. OnabotulinumtoxinA improves pain in patients with post-stroke spasticity: findings from a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage. 2016;52:17–26. [DOI] [PubMed] [Google Scholar]
- [52].Esquenazi A, Mayer N, Lee S, et al. PROS Study Group. Patient registry of outcomes in spasticity care. Am J Phys Med Rehabil. 2012;91:729–46. [DOI] [PubMed] [Google Scholar]
- [53].Francisco GE, Bandari DS, Bavikatte G, et al. Adult Spasticity International Registry Study: methodology and baseline patient, healthcare provider, and caregiver characteristics. J Rehabil Med. 2017;49:659–66. [DOI] [PubMed] [Google Scholar]
- [54].Francisco GE, Bandari DS, Bavikatte G, et al. High clinician- and patient-reported satisfaction with individualized onabotulinumtoxinA treatment for spasticity across several etiologies from the ASPIRE study. Toxicon X. 2020;7:100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Francisco GE, Jost WH, Bavikatte G, et al. Individualized onabotulinumtoxinA treatment for upper limb spasticity resulted in high clinician- and patient-reported satisfaction: long-term observational results from the ASPIRE study. PM R. 2020;12:1120–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Jog M, Wein T, Bhogal M, et al. Real-world, long-term quality of life following therapeutic onabotulinumtoxinA treatment. Can J Neurol Sci. 2016;43:687–96. [DOI] [PubMed] [Google Scholar]
- [57].Turkel CC, Bowen B, Liu J, et al. Pooled analysis of the safety of botulinum toxin type A in the treatment of poststroke spasticity. Arch Phys Med Rehabil. 2006;87:786–92. [DOI] [PubMed] [Google Scholar]
- [58].Ayyoub Z, Brashear A, Banach M, et al. Safety and stability of pulmonary function in patients with decreased respiratory function treated for spasticity with onabotulinumtoxinA. Toxins (Basel). 2020;12:661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Naumann M, Carruthers A, Carruthers J, et al. Meta-analysis of neutralizing antibody conversion with onabotulinumtoxinA (Botox®) across multiple indications. Mov Disord. 2010;25:2211–8. [DOI] [PubMed] [Google Scholar]
- [60].Brin MF, James C, Maltman J. Botulinum toxin type A products are not interchangeable: a review of the evidence. Biologics. 2014;8:227–41. [DOI] [PMC free article] [PubMed] [Google Scholar]