Abstract
Background
Physical restraints (PR), such as bedrails and belts in chairs or beds, are commonly used for older people receiving long‐term care, despite clear evidence for the lack of effectiveness and safety, and widespread recommendations that their use should be avoided. This systematic review of the efficacy and safety of interventions to prevent and reduce the use of physical restraints outside hospital settings, i.e. in care homes and the community, updates our previous review published in 2011.
Objectives
To evaluate the effects of interventions to prevent and reduce the use of physical restraints for older people who require long‐term care (either at home or in residential care facilities)
Search methods
We searched ALOIS, the Cochrane Dementia and Cognitive Improvement Group's register, MEDLINE (Ovid Sp), Embase (Ovid SP), PsycINFO (Ovid SP), CINAHL (EBSCOhost), Web of Science Core Collection (ISI Web of Science), LILACS (BIREME), ClinicalTrials.gov and the World Health Organization's meta‐register, the International Clinical Trials Registry Portal, on 3 August 2022.
Selection criteria
We included randomised controlled trials (RCTs) and controlled clinical trials (CCTs) that investigated the effects of interventions intended to prevent or reduce the use of physical restraints in older people who require long‐term care. Studies conducted in residential care institutions or in the community, including patients' homes, were eligible for inclusion. We assigned all included interventions to categories based on their mechanisms and components.
Data collection and analysis
Two review authors independently selected the publications for inclusion, extracted study data, and assessed the risk of bias of all included studies. Primary outcomes were the number or proportion of people with at least one physical restraint, and serious adverse events related to PR use, such as death or serious injuries. We performed meta‐analyses if necessary data were available. If meta‐analyses were not feasible, we reported results narratively. We used GRADE methods to describe the certainty of the evidence.
Main results
We identified six new studies and included 11 studies with 19,003 participants in this review update. All studies were conducted in long‐term residential care facilities. Ten studies were RCTs and one study a CCT. All studies included people with dementia. The mean age of the participants was approximately 85 years.
Four studies investigated organisational interventions aiming to implement a least‐restraint policy; six studies investigated simple educational interventions; and one study tested an intervention that provided staff with information about residents' fall risk. The control groups received usual care only in most studies although, in two studies, additional information materials about physical restraint reduction were provided.
We judged the risk of selection bias to be high or unclear in eight studies. Risk of reporting bias was high in one study and unclear in eight studies.
The organisational interventions intended to promote a least‐restraint policy included a variety of components, such as education of staff, training of 'champions' of low‐restraint practice, and components which aimed to facilitate a change in institutional policies and culture of care. We found moderate‐certainty evidence that organisational interventions aimed at implementation of a least‐restraint policy probably lead to a reduction in the number of residents with at least one use of PR (RR 0.86, 95% CI 0.78 to 0.94; 3849 participants, 4 studies) and a large reduction in the number of residents with at least one use of a belt for restraint (RR 0.54, 95% CI 0.40 to 0.73; 2711 participants, 3 studies). No adverse events occurred in the one study which reported this outcome. There was evidence from one study that organisational interventions probably reduce the duration of physical restraint use. We found that the interventions may have little or no effect on the number of falls or fall‐related injuries (low‐certainty evidence) and probably have little or no effect on the number of prescribed psychotropic medications (moderate‐certainty evidence). One study found that organisational interventions result in little or no difference in quality of life (high‐certainty evidence) and another study found that they may make little or no difference to agitation (low‐certainty evidence).
The simple educational interventions were intended to increase knowledge and change staff attitudes towards PR. As well as providing education, some interventions included further components to support change, such as ward‐based guidance. We found pronounced between‐group baseline imbalances in PR prevalence in some of the studies, which might have occurred because of the small number of clusters in the intervention and control groups. One study did not assess bedrails, which is the most commonly used method of restraint in nursing homes. Regarding the number of residents with at least one restraint, the results were inconsistent. We found very‐low certainty evidence and we are uncertain about the effects of simple educational interventions on the number of residents with PR. None of the studies assessed or reported any serious adverse events. We found moderate‐certainty evidence that simple educational interventions probably result in little or no difference in restraint intensity and may have little or no effect on falls, fall‐related injuries, or agitation (low‐certainty evidence each). Based on very low‐certainty evidence we are uncertain about the effects of simple educational interventions on the number of participants with a prescription of at least one psychotropic medication.
One study investigated an intervention that provided information about residents' fall risk to the nursing staff. We found low‐certainty evidence that providing information about residents' fall risk may result in little or no difference in the mean number of PR or the number of falls. The study did not assess overall adverse events.
Authors' conclusions
Organisational interventions aimed to implement a least‐restraint policy probably reduce the number of residents with at least one PR and probably largely reduce the number of residents with at least one belt. We are uncertain whether simple educational interventions reduce the use of physical restraints, and interventions providing information about residents' fall risk may result in little to no difference in the use of physical restraints. These results apply to long‐term care institutions; we found no studies from community settings.
Keywords: Aged; Aged, 80 and over; Humans; Dementia; Dementia/prevention & control; Long-Term Care; Nursing Homes; Quality of Life; Restraint, Physical
Plain language summary
Interventions for preventing and reducing the use of physical restraints in all long‐term care settings
What was studied in this review?
Physical restraints (PR) are devices that prevent a person moving their body freely to a position of their choice. Examples are bedrails, belts and fixed tables, which prevent people from getting out of bed or a chair. PR use for older people who have dementia or who cannot walk well is used quite commonly when they are being looked after in care institutions or even in their own homes. The main reason given for using PR is to try to prevent accidental falls and fall‐related injuries, or to prevent people from walking into other people's rooms or generally walking around unobserved and putting themselves or others at risk.
It is questioned that PR use is an effective way of preventing falls or fall‐related injuries. In fact, by making people spend more time immobile, they may worsen walking problems and actually increase the risk of falling. They may also increase feelings of fear, anger and discomfort, and decrease well‐being. Other unintended consequences include an increased risk of pressure ulcers and incontinence, and injuries directly related to the use of PR. In some countries, the use of PR is illegal in most circumstances and guidelines recommend that its use should be reduced or stopped.
What did we want to find out?
We wanted to know which interventions are most effective for preventing or reducing the use of PR for older people receiving long‐term care either in care institutions or at home. Interventions for preventing and reducing the use of PR typically include education and training for nursing staff and may also include changes to policies and the way care is organised.
What did we do?
We updated a review that was last published in 2011. We searched for trials that investigated interventions intended to reduce or prevent the use of PR in older people receiving long‐term care. The trials had to include a comparison group of people who did not get the intervention (a control group). We included eleven studies. All of them were conducted in long‐term care facilities (residential and nursing homes). The average age of the people in the studies was about 85 years. In most studies, the intervention being tested was compared with treatment‐as‐usual although, in two studies, managers of nursing homes in the control group also received some additional information about PR.
Four studies tested organisational interventions, which aimed to change policy and practice so that nursing staff would use PR less often or not at all. An important part of these interventions was training 'champions' to support the rest of the staff in avoiding the use of PR. Six studies tested less complex interventions that offered education directly to nursing staff. One study provided nursing staff with specific assessments of the fall risk of individual residents.
What did we find?
Our main outcome of interest was the number of people who were restrained at least once during the period of the study. We found that organisational interventions probably lead to a reduction in the number of people restrained and a large reduction in the number of people restrained with a belt. One study reported whether the residents came to any harm during the study period and it reported no harmful events. We did not find any evidence that the interventions made a difference to the number of people with at least one fall or at least one fall‐related injury, or the number of people prescribed medication to modify behaviour. These studies were mainly well conducted and reported.
For simple educational interventions, the quality of the studies and how well they were reported varied, and this affected our confidence in the results. The results of the studies were inconsistent, so we could not draw any conclusion about the effect of this type of intervention on the use of PR. None of these studies reported harmful events. Again, we did not find any evidence that the interventions made a difference to the number of people with at least one fall or at least one fall‐related injury, and we could not be sure of the effect on prescription of medication.
Based on one study, informing nursing staff about residents' individual risk of falling may not lead to any reduction of PR use compared with the control group.
What is the conclusion?
Organisational interventions aimed at reducing use of PR through changing policy and practice in care homes are probably effective at reducing the number of people restrained overall and especially with belts. Reducing restraints did no lead to a higher number of people with falls. We are uncertain whether simple educational interventions reduce the use of PR, and interventions providing information about residents' fall risk may have little or no effect on the use of PR. All the evidence came from studies in institutions and it may not apply to care in people's own homes.
How up‐to‐date is this evidence?
The evidence is up‐to‐date to 4 August 2022.
Summary of findings
Summary of findings 1. Summary of findings table ‐ Organisational intervention compared to usual care for older people in all long‐term care settings.
| Organisational intervention compared to usual care for older people in all long‐term care settings | ||||||
| Patient or population: older people in all long‐term care settings Setting: long‐term care facilities Intervention: organisational intervention Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with organisational intervention | |||||
| Residents with at least one physical restraint follow‐up: range 3 months to 12 months | 274 per 1000 | 236 per 1000 (208 to 258) | RR 0.86 (0.76 to 0.94) | 3849 (4 RCTs) | ⊕⊕⊕⊝ Moderatea | |
| Residents with at least one belt follow‐up: range 6 months to 12 months | 19 per 1000 | 10 per 1000 (8 to 14) | RR 0.54 (0.40 to 0.73) | 12711 (3 RCTs) | ⊕⊕⊕⊝ Moderatea | |
| Serious adverse events related to the use of physical restraints | Only one study reported that no serious adverse events occurred | 8841 (1 RCT) | ⊕⊕⊕⊝ Moderateb | |||
| Duration of physical restraint use | One study found a greater reduction of the duration of PR use in the intervention group in comparison with the control group | 228 (1 RCT) | ⊕⊕⊕⊝ Moderatec | |||
| Residents with at least one fall follow‐up: range 3 months to 12 months | 293 per 1000 | 299 per 1000 (252 to 352) | RR 1.02 (0.86 to 1.20) | 17954 (4 RCTs) | ⊕⊕⊝⊝ Lowa,d | |
| Residents with at least one fall‐related fracture follow‐up: range 3 months to 12 months | 18 per 1000 | 19 per 1000 (14 to 26) | RR 1.05 (0.76 to 1.45) | 17954 (4 RCTs) | ⊕⊕⊝⊝ Lowa,e | |
| Residents with at least one psychotropic medication follow‐up: range 3 months to 12 months | 555 per 1000 | 555 per 1000 (528 to 589) | RR 1.00 (0.95 to 1.06) | 3452 (2 RCTs) | ⊕⊕⊕⊝ Moderatef | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_434560320644589669. | ||||||
a Downgraded one level for risk of bias: one study with a high risk and one study with unclear risk of selection bias, one study with high risk of reporting bias b Downgraded one level for imprecision: only one study with no events c Downgraded one level for imprecision: only one study with a small number of participants d Downgraded one level for inconsistency: I² = 77% e Downgraded one level for imprecision: confidence interval indicate a small effect of both the intervention and the control group f Downgraded one level for risk of bias: one study with a high risk of selection bias
Summary of findings 2. Summary of findings table ‐ Simple educational intervention compared to usual care for older people in all long‐term care settings.
| Simple educational intervention compared to usual care for older people in all long‐term care settings | ||||||
| Patient or population: older people in all long‐term care settings Setting: long‐term care facilities Intervention: simple educational intervention Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with simple educational intervention | |||||
| Residents with at least one physical restraint follow‐up: range 6 months to 12 months | In two studies, PR use decreased in the intervention groups and control groups; in two studies PR use decreased in the intervention groups, but not in the control groups; in one study, PR use increased in the intervention group and the control group, and in one study, PR use was nearly unchanged in the intervention group and increased in the control group. | 1483 (6 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | |||
| Serious adverse events ‐ not measured | ‐ | ‐ | ||||
| Restraint intensity follow‐up: 10 months | In one study, restraint intensity increased in both study groups during the study period, but there was no difference between the study groups at baseline and follow‐up. | 241 (1 RCT) | ⊕⊕⊕⊝ Moderated | |||
| Residents with at least one fall follow‐up: range 6 months to 12 months | In one study, the number of participants with at least on fall decreased in both study groups. In one other study, the number of participants with at least on fall increased in all study groups during the intervention period, with a higher increase in the control group in comparison with the two intervention groups. | 813 (2 RCTs) | ⊕⊕⊝⊝ Lowb,e | |||
| Residents with at least one fall‐related serious injury follow‐up: 12 months | In one study, the number of fall‐related serious injuries was small, no event occurred in one intervention group, 8 events occurred in the second intervention group, and 4 events occurred in the control group (463 participants). | 463 (1 RCT) | ⊕⊕⊝⊝ Lowd,f | |||
| Residents with at least one psychotropic medication follow‐up: range 6 months to 12 months | Different psychotropic medications were assessed in the studies and there were pronounced imbalances in the number of people with at least one psychotropic medication at baseline in some of the studies. We found inconclusive results, some studies found an effect in favour of the intervention groups, some studies found an effect in favour of the control group, and some studies found no difference between the study groups. | 1202 (5 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_434560411446552693. | ||||||
a Downgraded one level for risk of bias: high risk of selection bias in four studies b Downgraded one level for inconsistency: direction of effect differs between the studies c Downgraded one level for imprecision: several studies included a very small number of clusters and participants and pronounced baseline differences in some studies d Downgraded one level for imprecision: one study with a small number of participants e Downgraded one level for risk of bias: high risk of selection bias in all studies, unclear risk of detection bias and attrition bias in one study f Downgraded one level for risk of bias: high risk of selection bias and unclear risk of reporting bias
Summary of findings 3. Summary of findings table ‐ Providing information about the residents? fall risk compared to usual care for older people in all long‐term care settings.
| Providing information about the residents' fall risk compared to usual care for older people in all long‐term care settings | ||||||
| Patient or population: older people in all long‐term care settings Setting: long‐term care facilities Intervention: providing information about the residents' fall risk Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with providing information about the residents' fall risk | |||||
| Restraint use follow‐up: 8 months | The mean restraint use was 3.53 | MD 0.51 lower (1.72 lower to 0.7 higher) | ‐ | 98 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | |
| Serious adverse events related to the use of physical restraints ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Duration of physical restraint use ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Falls follow‐up: 8 months | The median number of falls per participant in both study groups at baseline and follow‐up was 1 | 98 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | |||
| Fall‐related injuries ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Residents with at least one psychotropic medication ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_437392448448914131. | ||||||
a Downgraded one level for risk of bias: high risk of selection bias and performance bias, unclear risk of detection bias b Downgraded one level for imprecision: one study with a small number of participants
Background
Description of the condition
Physical restraint (PR) of older people in different care settings occurs commonly in many countries (Foebel 2016; Lee 2021). A consensus statement developed by researchers and experts in the field defines physical restraints as "any action or procedure that prevents a person’s free body movement to a position of choice and/or normal access to his/her body by the use of any method, attached or adjacent to a person’s body that he/she cannot control or remove easily" (Bleijlevens 2016). Other types of restraint such as psychotropic medication, sometimes called "chemical" restraint, were not in the scope of this review.
There are pronounced differences in prevalence rates of PR use between studies, with a pooled prevalence of 33% in nursing homes (Lee 2021). Epidemiological studies also found pronounced variation amongst centres both between and within countries (De Vries 2004; Feng 2009; Meyer 2009a). In long‐term home care, prevalence rates of PR use ranged from 5% to 25% (Scheepmans 2018). Important determinants associated with PR use include cognitive impairment and aggressive behaviour or agitation, but it is unclear whether institutional characteristics, such as staffing and staff mix, significantly influence decisions on PR use (Heeren 2014; Hofmann 2014; Meyer 2009a; Pivodic 2020). It also remains unclear whether staff shortage has an impact on PR use since its use also requires resources, for example, for regular observation of people with PR use. The 'philosophy' of care (i.e. attitudes) and the beliefs of nursing staff are suspected to be powerful determinants of PR use as a routine measure (Goethals 2012; Meyer 2009a; Möhler 2014).
In older people who require long‐term care, the main reasons for using PR are safety issues, such as prevention of falls or fall‐related injuries or controlling specific behaviour, such as wandering or aggressive behaviour (Goethals 2012; Möhler 2014; Scheepmans 2018). However, PR is not an effective measure to reduce falls or fall‐related injuries (Sze 2012) and, on the contrary, may increase the risk of falling in older people (Fernández Ibáñez 2020; Köpke 2012). Several studies have shown that PR prevalence can be reduced without a significant increase in falls or fall‐related injuries (Abraham 2019; Gulpers 2011; Köpke 2012). There is also evidence from observational studies about adverse outcomes associated with use of PR use, for example, direct injuries, decreased mobility, and reduced psychological well‐being (Castle 2009; Engberg 2008; Fernández Ibáñez 2020; Freeman 2017).
Therefore, a restraint‐free nursing care environment has been recommended as the standard of care (Flaherty 2004) and the use of physical restraints is restricted by law in many countries (Castle 1998; Centers for Medicare and Medicaid Services 2008; RNAO 2012).
Description of the intervention
In recent decades, efforts have been made to reduce the use of PR. Programmes to reduce the use of PR with older people were first introduced in the US in the 1980s (Castle 1998). A number of studies have been conducted in nursing homes, but only a few studies investigated interventions to reduce PR in home care (Scheepmans 2018).
Educational interventions for preventing and reducing the use of PR in older people who require long‐term care are the most common approach. Simple educational interventions aim to increase nurses' knowledge about the lack of benefit and the adverse effects of PR use and sometimes include additional components offering advice about how to prevent or reduce PR in clinical practice. More complex organisational interventions typically involve several different components, including educational sessions aimed at changing nurses' knowledge and often uncritical attitudes towards PR use as well as components addressing the whole organisation, its culture of care, and policies. A further set of interventions provide technical devices that target common risk factors for PR use, such as a high risk of falling. Examples are sensor mats or low‐low beds, intended to reduce the risk of falling or fall‐related injuries, but such interventions are more common in general hospital settings (Abraham 2022).
All types of intervention to reduce PR use in older people who require long‐term care are directed primarily at health professionals, especially nurses (Möhler 2011; Möhler 2012; Scheepmans 2018; Vandervelde 2021), because these professions are primarily involved in the delivery of professional care in these settings. In the community, informal caregivers are also an important target group.
How the intervention might work
The use of PR represents a nonspecific reaction to specific behaviours of older people or to clinical situations which are experienced as particularly challenging or as threatening to a person's health. However, in many cases alternative, more specific interventions may be more helpful than PR use. Therefore, simple educational interventions or the educational components of organisational interventions are designed to inform nursing staff about the evidence concerning PR use and to address common barriers that hamper the reduction of PR. These barriers may include the belief that PR use can effectively reduce falls or fall‐related injuries, a lack of knowledge about alternative approaches and a lack of skills or resources needed to apply alternative interventions (Goethals 2012; Kong 2017; Möhler 2014). Because the organisational culture of care also seems to be an important predictor of PR use, organisational interventions have a stronger focus on the organisational level, i.e. leadership and institutional policies towards PR use.
Interventions that provide technical devices or enhanced information about risk factors for PR use aim to reduce the perceived need for PR. For example, instead of restraining someone with a high falls‐risk in bed, motion sensors may be applied to inform nursing staff if the person is getting out of bed. However, such position‐change alarm systems have also been classified as physical restraint by the US Centers for Medicare and Medicaid Services and the Department of Veterans Affairs. Related interventions may also provide information about a variety of devices, usually with instructions about their correct use. However, if devices such as motion sensors actually lead to restriction of a person's mobility, they can be seen as an adjunct to PR rather than as an alternative. Enhanced information about risk factors may come from, for example, the use of a structured assessment instrument.
Why it is important to do this review
The first version of this review entitled "Interventions for preventing and reducing the use of physical restraints in long‐term geriatric care" was published in 2011 (Möhler 2011) and a first update including one additional study was published in 2012 (Möhler 2012). Both reviews found inconclusive evidence about the effects of educational interventions intended to reduce PR use in older people who require long‐term care. Since then several new studies on this topic have been published (Abraham 2019; Gulpers 2011; Köpke 2012; Testad 2016) and a review update is needed to investigate the available body of evidence about this important topic and to inform clinical practice.
Objectives
To evaluate the effects of interventions for preventing and reducing the use of physical restraints in older people in all long‐term care settings (either in the community or in residential care facilities).
To evaluate these complex interventions by retrieving detailed data on implementation.
To describe the quality and quantity of research evidence available and to set an agenda for future research.
Methods
Criteria for considering studies for this review
Types of studies
The first version of this review (Möhler 2011) and this review update were conducted based on the published review protocol (Meyer 2009b).
We included all individually randomised or cluster‐randomised controlled trials (RCTs) and controlled clinical trials (CCTs) investigating the effects of interventions intended to prevent or reduce the use of physical restraints in older people who require long‐term nursing care.
Types of participants
Older people requiring long‐term nursing care either in the community or in residential care facilities, irrespective of their cognitive status.
Types of interventions
We included all non‐pharmacological interventions intended to prevent or reduce the use of PR for older people in all long‐term care settings. The following different groups of interventions were anticipated:
Organisational interventions aimed at implementing a least‐restraint policy and changing the organisational culture of PR use. As well as components that target organisational policies and culture, these are likely to include educational components and may include additional components (e.g. technical devices that target risk factors for the use of PR or changes to the care environment);
Simple educational interventions offering information about PR and their adverse effects, and about alternative strategies or measures. Such interventions may also aim to change nurses' attitudes towards the use of physical restraints. They might include additional components offering advice how to prevent or reduce physical restraints in clinical practice, but they should not include components addressing the organisational culture, leadership or policy regarding the use of physical restraints;
Interventions that provide technical devices targeting common risk factors for PR use, such as sensors or low‐low beds to reduce the risk of falling or fall‐related injuries. Interventions that provide information about common risk factors for PR use, such as fall‐risk assessments, also fit in this category. The interventions might also comprise instructions about the correct use of devices or assessment tools.
We excluded interventions including pharmacological components, i.e. psychotropic medication, since there is no clear definition of chemical restraints and it is often unclear whether medication is used for therapeutic reasons or not.
Comparator: usual care (no intervention) or optimised usual care
Types of outcome measures
Primary outcomes
We defined the following primary outcomes:
number or proportion of participants with at least one physical restraint, assessed with validated methods (e.g. direct observation or from the documentation);
serious adverse events, e.g. death or serious injuries related to PR use (e.g. strangulation).
For the assessment of physical restraints, we counted measures that comply with the definition developed through expert consensus, defining physical restraints as "any action or procedure that prevents a person’s free body movement to a position of choice and/or normal access to his/her body by the use of any method, attached or adjacent to a person’s body that he/she cannot control or remove easily" (Bleijlevens 2016). We also included studies using a more narrow definition. We did not consider as PR forced care (assessed in the studies by Testad 2010 and Testad 2016) or involuntary treatment, which includes, beside PR, also psychotropic drugs and non‐consensual care (e.g. forced hygiene, hiding medication) (Mengelers 2022). However, studies investigating these broad concepts were eligible for inclusion if they presented separate data about the use of physical restraints as defined in this review.
Secondary outcomes
Duration of physical restraints
Number of falls or fall‐related injuries
Agitation, assessed by e.g. the Cohen‐Mansfield Agitation Inventory (CMAI)
Quality of life
Mobility
Incidence of pressure ulcers
Use of psychotropic medication
Caregiver‐related outcomes: caregiver burden (assessed by, e.g. the Zarit Burden scale), quality of life
Costs
Search methods for identification of studies
We did not apply any language restrictions.
Electronic searches
We searched the Cochrane Dementia and Cognitive Improvement Group’s Specialised Register. The Register is maintained by the Information Specialists of the Cochrane Dementia and Cognitive Improvement Group and contains studies in the areas of dementia (prevention and treatment), mild cognitive impairment and cognitive improvement. The studies are identified from:
1. Monthly searches of a number of major healthcare databases: MEDLINE, Embase, CINAHL, PsycINFO and LILACS;
2. Monthly searches of the trial registers: the WHO International Clinical Trials Registry Platform (which covers ClinicalTrials.gov, ISRCTN, the Chinese Clinical Trials Register, the German Clinical Trials Register, the Iranian Registry of Clinical Trials, and the Netherlands National Trials Register, plus others) and ClinicalTrials.gov;
3. Quarterly search of the Cochrane Library’s Central Register of Controlled Trials (CENTRAL);
4. Six‐monthly searches of a number of grey literature sources from ISI Web of Science Core Collection.
Details of the search strategies used for the retrieval of reports of trials from the healthcare databases, CENTRAL and conference proceedings can be viewed in the ‘Methods used in reviews’ section within the editorial information about the Dementia and Cognitive Improvement Group. We performed additional searches in many of the sources listed above, to cover the timeframe from the last searches performed for ALOIS to ensure that the search for the review was as up‐to‐date and as comprehensive as possible.
The search strategies used are described in Appendix 1. The most recent search was carried out on 3 August 2022.
Searching other resources
We screened reference lists and citations of all potentially eligible publications for additional trials. We also tried to contact relevant researcher to identify additional studies.
Data collection and analysis
Selection of studies
Two of three reviewers (RM, TR, CM) independently screened all titles and abstracts obtained from the search against the inclusion criteria. We resolved any disagreements with a third reviewer (GM). Authors who were involved in trials eligible for inclusion in this review did not perform study selection of these studies.
Data extraction and management
Two of three reviewers (RM, TR, CM) extracted data from the included studies using a standardised and piloted form. We resolved disagreements by discussion or, if necessary, we consulted a third reviewer to reach consensus (GM). Authors who were involved in trials included in this review did not extract study data from these studies. We extracted the following data: study registration number/published study protocol, study design, definition of PR, characteristics of participants, baseline data, length of follow‐up, outcome measures, and study results including adverse effects. In case of cluster‐randomised trials, we also extracted the intra‐cluster correlation coefficient (ICC).
For each intervention, we extracted characteristics relevant for complex interventions (Hoffmann 2014; Möhler 2015): theoretical basis of the intervention, information about a pilot test, characteristics of the intervention's components (e.g. duration and frequency), and information about implementation fidelity. We contacted the study authors to obtain missing information, if necessary.
Assessment of risk of bias in included studies
We followed the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). Two of three reviewers (RM, TR, CM) independently assessed risk of bias in the following domains: selection bias, performance bias, attrition bias, detection bias, and other bias. In case of disagreement, a third reviewer was consulted to reach consensus (GM). Authors who were involved in trials included in this review did not perform the risk of bias assessment for these studies. In case of missing information, we contacted the study authors.
Measures of treatment effect
For dichotomous data, we calculated risk ratios (RR) with 95% confidence intervals (CI) if possible. For some studies, it was not feasible to calculate the RR due to baseline imbalances of several factors, such as PR prevalence (Evans 1997; Testad 2010; Testad 2016). Two studies did not report the number of participants with PR use, and we calculated the numbers from the reported proportion of people with PR use (Gulpers 2011; Huizing 2009).
For continuous outcomes assessed with the same scale, we calculated the mean difference (MD). If studies used different rating scales for the same outcome, we planned to calculate the standardised mean difference (SMD), which is the absolute mean difference divided by the standard deviation (SD), but this was not necessary in this review.
One study presented results for participants with data both at baseline and follow‐up as well as for the complete study population (including participants admitted during the study follow‐up and those lost to follow‐up), and we used the latter results in our analysis (Pellfolk 2010).
Two studies assessed intervention costs for all intervention groups (Abraham 2019; Köpke 2012). We calculated costs per participant based on the number of participants that were included in the main analyses in both studies.
We performed all statistical analysis using RevMan Web (Review Manager 2022).
Unit of analysis issues
For the included cluster‐randomised trials, we checked for unit of analysis issues. With one exception (Dever Fitzgerald 2016), all included studies randomised clusters to the study groups, but assessed the outcomes on the individual level. Only two studies reported the ICC (Köpke 2012; Testad 2016), but Testad 2016 incorporated only a cluster effect greater than 5% in the analysis and, since the ICC was lower, the cluster effect was not included in the analyses. One study reported a cluster‐adjusted analysis of the likelihood of being restrained but no ICC (Pellfolk 2010). In the study by Abraham 2019, no ICC was available since the two intervention groups were compared separately with the control group, using a Bonferroni‐adjustment for two tests. It was not possible to combine the data of both intervention groups to calculate an ICC. Abraham 2019 and Köpke 2012 reported the number of events for each study group on participant levels (not adjusted for clustering) as well as cluster‐adjusted analyses, comparing mean prevalences between study groups. For meta‐analyses, we did not use cluster‐adjusted data, as the number of events per study group were the only data available across all studies. We applied the ICC reported by Köpke 2012 to all studies included in the meta‐analyses (Abraham 2019; Gulpers 2011; Koczy 2011; Köpke 2012) to re‐calculate the effective sample size using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We used this approach only for the number of participants with at least one physical restraint, but not for the analysis including belt use (since the number of events was very low) or the secondary outcomes.
We also did not use this approach for studies investigating simple educational interventions (Huizing 2009; Testad 2005; Testad 2010; Testad 2016), because a meta‐analysis for the primary outcome was not feasible (due to the reasons described above), and because we did not identify an adequate ICC. These studies were at risk of a unit of analysis error.
Dealing with missing data
We contacted authors of the included studies to obtain missing data. We used data from intention‐to‐treat analyses, if available.
Assessment of heterogeneity
We assessed the included studies for differences in the settings, participants, and comparators. Two authors (RM, TR) assigned the included interventions to the predefined groups described above (see: Types of interventions). If an intervention could be categorised in different groups, we selected the group which best fit the aims, theoretical approach, and components described. In case of disagreement, we consulted a third reviewer (GM) to reach consensus. We described the characteristics and components of all included interventions to assess differences in the aim and underlying mechanisms (Skivington 2021).
We assessed statistical heterogeneity by calculating the I2 and Chi2 statistics.
Assessment of reporting biases
We included studies in any language to minimise language bias. We planned to prepare funnel plots to estimate visually small study effects that may reflect reporting bias (Higgins 2019) if we included at least 10 studies per intervention group, but this was not the case.
Data synthesis
In the last version of this review, we did not perform meta‐analyses since we found pronounced clinical heterogeneity in terms of definitions of PR, as well as baseline imbalances in some of the included studies. We aimed to perform meta‐analyses with individual patient data, but we did not receive the necessary data.
In this update, we performed meta‐analyses using a random‐effects model (due to clinical diversity of the interventions and statistical heterogeneity) for the use of physical restraints and belt restraints in our comparison of organisational interventions aimed at implementing a least‐restraint policy with usual care.
For simple educational interventions, we did not perform meta‐analyses for most outcomes, because of the pronounced baseline imbalances in PR prevalence in some of the studies and the heterogeneity in the definitions of PR. We described the results for all outcomes without meta‐analysis narratively.
Subgroup analysis and investigation of heterogeneity
We did not perform subgroup analyses according to severity of cognitive impairment at baseline, as planned in the protocol, because the necessary data were not available.
Sensitivity analysis
We did not perform any sensitivity analyses.
Summary of findings and assessment of the certainty of the evidence
We assessed the certainty of evidence using the GRADE method. GRADE defines the certainty of evidence as the extent to which one can be confident that an estimate of effect is close to the true quantity of interest (Guyatt 2011). Two reviewers (RM, TR or CM) independently performed the GRADE assessment based on the risk of bias of included studies, inconsistency of study results, indirectness of the evidence, imprecision of the study results, and risk of publication bias. We resolved disagreements by discussion or, if necessary, by consulting a third reviewer (GM).
We created summary of findings tables for the different intervention categories including the following outcomes:
number of residents with at least one physical restraint;
number of residents with at least one belt (only for organisational interventions aimed at implementing a least‐restraint policy);
adverse events related to PR use;
number of residents with at least one fall;
number of residents with fall‐related injuries;
number of residents with a prescription of psychotropic medication;
quality of life.
Results
Description of studies
Results of the search
For this update, we screened a total of 1123 titles and abstracts and 15 publications in full text (see Figure 1). Six new studies met the inclusion criteria (Abraham 2019; Dever Fitzgerald 2016; Gulpers 2011; Koczy 2011; Köpke 2012; Testad 2016). Five studies were carried over from our earlier review (Evans 1997; Huizing 2009; Pellfolk 2010; Testad 2005; Testad 2010). Therefore, we included a total of 11 studies in this review update.
1.
Flow diagram
Included studies
Ten studies were randomised controlled trials (Abraham 2019; Dever Fitzgerald 2016; Evans 1997; Huizing 2009; Koczy 2011; Köpke 2012; Pellfolk 2010; Testad 2005; Testad 2010; Testad 2016) and one study was a controlled clinical trial (Gulpers 2011).
Setting and participants
All studies were conducted in long‐term care facilities. We did not find any eligible studies that were conducted in the community.
The studies were carried out in Germany (n = 3), Norway (n = 3), the Netherlands (n = 2), Canada (n = 1), Sweden (n = 1), and the United States (n = 1). Ten studies were conducted in nursing homes (Abraham 2019; Dever Fitzgerald 2016; Evans 1997; Gulpers 2011; Huizing 2009; Koczy 2011; Köpke 2012; Testad 2005; Testad 2010; Testad 2016) and one study in group dwelling units (Pellfolk 2010).
Most studies allocated clusters (long‐term care facilities or independent wards) to the intervention and control groups (Abraham 2019; Evans 1997; Gulpers 2011; Huizing 2009; Koczy 2011; Köpke 2012; Pellfolk 2010; Testad 2005; Testad 2010; Testad 2016), with only Dever Fitzgerald 2016 randomising individual participants. The number of clusters per intervention group ranged from 1 (Evans 1997) to 40 (Abraham 2019). Duration of follow‐up ranged from three months (Koczy 2011) to twelve months (Abraham 2019; Evans 1997). Two studies included two intervention groups and one control group (Abraham 2019; Evans 1997).
A total of 19,003 participants were included in the review across all included studies. The mean age of the participants was approximately 85 years in most of the studies. All studies included people with dementia, based on different diagnostic criteria. Only one study did not define such an inclusion criterion, but stated that it did include people with dementia (Testad 2016).
For further information about the included studies, see Characteristics of included studies.
Description of interventions
Four studies investigated organisational interventions aimed at implementing a least‐restraint policy (Abraham 2019; Gulpers 2011; Koczy 2011; Köpke 2012). Six studies investigated simple educational interventions (Evans 1997; Huizing 2009; Pellfolk 2010; Testad 2005; Testad 2010; Testad 2016). These interventions also involved some additional components, such as case discussions to improve the implementation of knowledge in clinical decision‐making, but these interventions did not involve any components addressing the local restraint policies. One study investigated an intervention that provided information about each resident's risk of falling to the nursing staff to reduce PR use (Dever Fitzgerald 2016). We classified this study under interventions providing technical devices or specific information targeting common risk factors for PR use.
Development and piloting of the interventions
Most of the studies referred to the absence of evidence about the effectiveness of PR use, ethical issues and risk of adverse effects. With this background, Evans 1997 conducted an RCT investigating the effect of education or education with additional consultation to reduce PR in nursing homes. This study strongly focused on nurses' knowledge and alternative strategies in clinical practice. A similar approach was also investigated by other studies (Huizing 2009; Pellfolk 2010; Testad 2005; Testad 2010) although, in these studies, nurses' attitudes towards the use of PR were also addressed in the educational programmes.
Since there was no clear evidence about the effects of these simple educational interventions (Möhler 2011; Möhler 2012), more complex interventions aimed at changing organisational culture and policy regarding PR use have been developed (Abraham 2019; Gulpers 2011; Koczy 2011; Köpke 2012). These interventions also address nurses' knowledge about and attitudes towards PR use, but include additional components to facilitate change at the organisational level, aiming to implement a least‐restraint culture of care. Two of the studies (Abraham 2019; Köpke 2012) used the theory of planned behaviour (Ajzen 1991) and an evidence‐based guideline (Köpke 2009; Köpke 2015) as the theoretical basis for the intervention development. Gulpers 2011 based their intervention on the available evidence about barriers to PR reduction and the influence of policy to reduce PR use, on the experience of an earlier study investigating a simple educational intervention (Huizing 2009) and a pilot study (Hamers 2009). Koczy 2011 also referred to evidence about PR use, but did not report further information about the theoretical basis of the intervention. Dever Fitzgerald 2016 also referred to the lack of effectiveness and the negative effects of PR use, but focused on the risk of falls as a major reason for using PR.
Five studies investigating a simple educational intervention did not provide any information about a pilot or feasibility study prior to the clinical trial (Evans 1997; Huizing 2009; Pellfolk 2010; Testad 2005; Testad 2010). Testad 2016 used the earlier studies of the research group (Testad 2005; Testad 2010) as pilot studies. Three studies investigating organisational interventions aimed at implementing a least‐restraint policy included a pilot phase or referred to a pilot study (Abraham 2019; Gulpers 2011; Köpke 2012). Koczy 2011 did not report any information about a pilot study. Dever Fitzgerald 2016 used an earlier study of the working group as a pilot study.
Components of the interventions
Organisational interventions aimed at implementing a least‐restraint policy
All organisational interventions comprised an educational component and the following additional components (Table 4).
1. Overview intervention components: organisational interventions aimed at implementing a least‐restraint policy.
| Study IG | Abraham 2019 | Gulpers 2011 | Koczy 2011 | Köpke 2012 | |
| IG 1 | IG 2 | ||||
| Intervention period | 12 months | 12 months | 7 months | 3 months | 6 months |
| Training and implementation of multipliers | 1.5‐day seminar | 1.5‐day seminar plus train‐the‐trainer module | – | One 6‐hour session, including the voluntary use of PR with one multiplier | 1.5‐day seminar |
| Education | Information sessions for all nurses (90 min, offered up to three times per cluster) | – | Weekly 3‐h sessions for 3 weeks; delivered by a nurse specialist (registered nurses with extensive experience in physical restraint reduction) | – | Information sessions for all nurses (90 min, offered up to three times per cluster) |
| Consultation | – | – | Delivered by a nurse specialist (which also delivered the education) on demand, at least 2 consultations per cluster (month 2 to month 8) | – | – |
| Support for multipliers | Monthly contacts (phone or personal) for three months | Monthly contacts (phone or personal) for three months | – | Telephone support for 3 months, 1 visit by a member of the research team on request | Monthly contacts (phone or personal) for three months |
| Organisational component | Support by leaders to implement a least‐restraint policy | Support by leaders to implement a least‐restraint policy | Institutional policy change | – | Support by leaders to implement a least‐restraint policy |
| Other components/ intervention materials | Full and shot version of the guideline, information brochures (for nurses, and residents, legal guardians and relatives), further material (poster, mugs and pencils with the guideline logo) | Full and shot version of the guideline, information brochures (for nurses, and residents, legal guardians and relatives), further material (poster, mugs and pencils with the guideline logo) | Provision of alternative measures (hip protectors, infrared alarm systems, balance training, exercise, special pillows, low‐low beds) | Provision of alternative measures, (hip protectors, antislip socks, sensor mats) | Full and shot version of the guideline, information brochures (for nurses, and residents, legal guardians and relatives), further material (poster, mugs and pencils with the guideline logo) |
IG = intervention group; PR = physical restraints
Training of champions to support implementation
Three interventions included training of 'champions' (sometimes referred to as 'key nurses' or 'multipliers') to foster the intended changes towards a least‐restraint policy (Abraham 2019; Koczy 2011; Köpke 2012). The champions were nominated by the participating nursing homes and received specific education and training. The following topics were addressed:
Information on PR, e.g. definition, legal aspects, lack of effectiveness to reduce falls and fall‐related injuries, adverse events, experiences of being restrained (Abraham 2019; Koczy 2011; Köpke 2012);
Management of challenging behaviour and adaptation of environmental and organisational factors to increase well‐being of people with dementia (Koczy 2011);
Information about relevant evidence‐based guideline and recommendations, and the corresponding implementation materials (Abraham 2019; Köpke 2012);
Nurses' attitudes to and experiences of PR use (Abraham 2019; Köpke 2012);
Alternatives to use of PR (Abraham 2019; Koczy 2011; Köpke 2012);
Discussions between nurses from different nursing homes about strategies to reduce PR (including the use of real cases or vignettes) and the development, presentation and documentation of nursing home‐specific agendas for PR reduction (Abraham 2019; Köpke 2012);
Discussions about the baseline prevalence of PR use and the effect of educational sessions with the champions of the respective nursing home on nurses’ knowledge and assessment of self‐efficacy (Köpke 2012).
All studies also offered structured support for the champions, by phone or personal visits (see Table 4 for details). In Koczy 2011, champions were encouraged to offer case consultations for residents with PR use.
Educational Component
Three studies offered education to all nurses in the participating clusters (Abraham 2019, intervention group 1; Gulpers 2011; Köpke 2012). In two studies, the champions' training included a module about delivering the educational content to the nursing staff. In addition, champions received training materials from the education component (Abraham 2019, intervention group 2; Koczy 2011).
Educational programmes covered the following topics:
Information on PR, e.g. definition, legal aspects, lack of effectiveness to reduce falls and fall‐related injuries adverse events, experiences of being restrained (Abraham 2019; Gulpers 2011; Köpke 2012);
Information about relevant evidence‐based guidelines, i.e. development and recommendations (Abraham 2019; Köpke 2012);
Nurses' attitudes to and experiences of PR use (Abraham 2019; Gulpers 2011; Köpke 2012);
Alternatives to use of PR (Abraham 2019; Gulpers 2011; Köpke 2012);
Falls and fall prevention (Gulpers 2011).
Consultation
In Gulpers 2011, two nurse specialists (registered nurse level), who delivered the educational component, offered consultation on challenges of PR reduction for six months. The nurse specialists were available on demand and all clusters received at least two consultations. A nurse from each of the intervention wards and one of the nurse specialists analysed specific resident cases and discussed possible solutions for reducing PR use.
Organisational component
Three interventions included an organisational component addressing a policy‐change towards the reduction of PR use (Abraham 2019; Gulpers 2011; Köpke 2012). Gulpers 2011 implemented an institutional policy change comprising the prohibition of belts in bed or chair for newly admitted residents or for residents without a prior use of belts, and an overall reduction of belts. During the first four months of the study, the policy change was announced to all staff members, residents’ relatives and legal representatives in the nursing home (via written and oral communication by the nursing home managers, letter and announcements in internal newspapers and in group meetings). The policy was also presented as part of the educational component. Abraham 2019 and Köpke 2012 aimed to implement a least‐restraint policy, which was the key message of the evidence‐based guidelines. In addition to the educational components for champions and nursing staff, the nursing home leader in the intervention groups signed a policy statement supporting the least‐restraint policy and the aim of the intervention (to reduce PR use).
Other components
Two studies offered measures that might be used as an alternative to PR use in the intervention groups (Gulpers 2011; Koczy 2011). In the study by Gulpers 2011, nursing home managers in the intervention group provided hip protectors, infrared alarm systems, balance training, exercise, special pillows, and adjustable low‐height beds. The measures were not provided by the study team. In Koczy 2011, the study team offered up to three hip protectors and five pairs of antislip socks for each resident, and each cluster received at least one pressure sensor mat.
Simple educational interventions
All included studies in this category of intervention provided education for nursing staff aimed at changing clinical practice by improving nurses' knowledge about PR use and changing nurses' attitudes regarding the use of PR. Further components to foster the change in clinical practice were offered: consultation in clinical practice (Evans 1997; Huizing 2009) and guidance session (Testad 2005; Testad 2010; Testad 2016).
An overview of the interventions and components is displayed in Figure 2.
2.
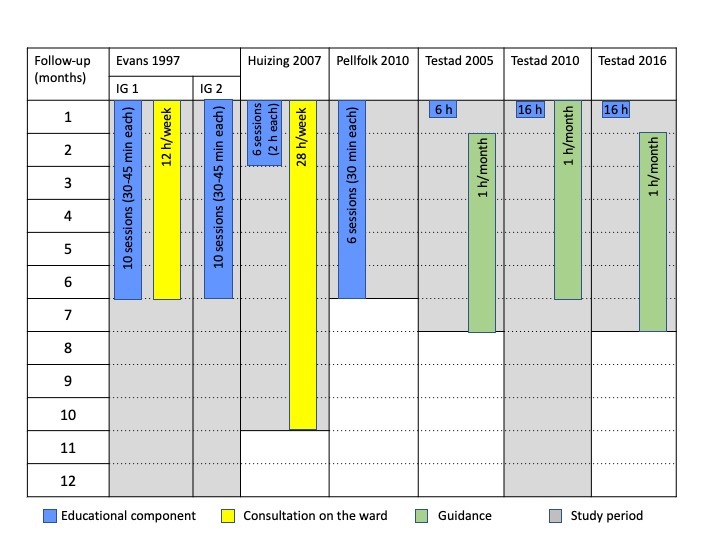
Overview: simple educational interventions
Educational component
All interventions comprised an educational component. The educational programme by Huizing 2009 was developed on the basis of a previous educational programme for restraint reduction in hospitals (Dielis‐van Houts 2004). Pellfolk 2010 based the educational programme on previous research and the clinical experience of experts in geriatric medicine and nursing. Testad 2010 and Testad 2016 used the ‘practical framework for staff to reduce agitation and use of restraint in the interaction with residents with dementia’, which has been developed based on clinical practice. Two studies (Evans 1997; Testad 2005) did not provide further information about the theoretical basis of their intervention.
The total amount of education ranged from 6 to 16 hours, but the number, duration, and frequency of the educational sessions varied (Figure 2).
The educational sessions covered the following topics (Testad 2010 did not report details about the content of the educational sessions):
Information on dementia, aggression and challenging behaviour (Pellfolk 2010; Testad 2005);
Strategies for analysing and handling aggression or challenging behaviour (Evans 1997; Huizing 2009; Pellfolk 2010; Testad 2016);
Information on PR, e.g. legal aspects, adverse events, experiences of being restrained, correct use (Evans 1997; Huizing 2009; Pellfolk 2010);
Decision‐making processes and alternatives to use of PR (Huizing 2009; Pellfolk 2010; Testad 2005);
Information about the Norwegian legislation on restraint and best practice for person‐centred care (Testad 2016);
Falls and fall prevention (Evans 1997; Pellfolk 2010).
The educational sessions were offered to all members of the nursing staff in four studies (Evans 1997; Testad 2005; Testad 2010; Testad 2016). Huizing 2009 offered five 2‐hour sessions for selected staff (24%‐39% of the total nursing staff of each ward attended the sessions) and afterwards one 90‐min session for all nursing staff. In Pellfolk 2010, one volunteer staff member from each cluster attended the complete educational programme delivered in a 2‐day seminar and other staff members received six 30‐minute sessions with videotaped lectures (three sessions included a clinical vignette).
In Huizing 2009, the educational component was delivered by a nurse specialist (RN level). The other studies did not report information about the education or professional background of the staff delivering the educational component.
The studies by Testad 2005, Testad 2010, and Testad 2016 used a manual to standardise the content delivered during the educational sessions. Pellfolk 2010 used videotaped lectures. Evans 1997 audiotaped and reviewed randomly selected educational sessions in order to assess standardised administration.
Consultation
Two interventions included a ward‐based consultation delivered by a nurse specialist at registered nurse level (Huizing 2009) or a master’s‐prepared gerontological nurse specialist (Evans 1997). Evans offered six months' consultation for intervention group 1, and Huizing 2009 eight months. Consultation included discussions about residents with challenging behaviour or history of multiple falls (Evans 1997) and multidisciplinary meetings, evaluating the use of physical restraints on individual residents, discussing difficulties in achieving PR‐free care and stimulating the use of PR alternatives or less restrictive measures (Huizing 2009).
Guidance
Three studies (Testad 2005; Testad 2010; Testad 2016) offered a monthly one‐hour guidance session for six months after the single educational session. The aim of the guidance sessions was to develop care plans for individual participants, taking into account the content of the educational session and case‐specific information (Testad 2005). In Testad 2010 and Testad 2016, the guidance sessions also aimed to support the implementation of knowledge from the educational sessions and the reinforcement of new skills. None of the studies reported information about the education of staff delivering guidance.
Interventions providing technical devices or information on common risk factors for PR use
Only the study by Dever Fitzgerald 2016 was allocated to this category. A member of the study team (licenced physiotherapist) assessed the fall risk of each participant and grouped each participant in a risk category of low, medium or high fall‐risk. For each participant, a case conference was conducted including all nurses who were involved in caring for the respective participant, the physiotherapist and a clinical psychology graduate student to present the results of the fall‐risk assessment.
Implementation fidelity
Organisational interventions aimed at implementing a least‐restraint policy
Three studies assessed implementation fidelity (Abraham 2019; Gulpers 2011; Köpke 2012).
In Abraham 2019 and Köpke 2012, trained staff (at least a Master's degree, with experiences in reducing physical restraints) delivered the intervention based on a standardised presentation. Data about the duration, content and deviations from the protocol of the educational component, the champion training, and the support for champions were collected. Furthermore, it was checked whether the champions received the study materials as planned and whether the organisational component was implemented. Awareness of staff about the intervention was investigated using a short survey with all champions and three randomly selected nurses per cluster, as well as in focus groups including relatives, legal guardians, and members of the board of residents. Barriers and facilitators were investigated using focus groups with champions. In Gulpers 2011, trained nurse specialists delivered the intervention based on a manual. Delivery was documented, and the primary investigator supervised the delivery of the intervention components. In monthly meetings, nurse specialists received feedback and discussed strategies to improve the diffusion of the interventions.
Simple educational interventions
In the study by Evans 1997, 81% of the nursing staff in intervention group 1 and 78% in intervention group 2 attended at least one out of ten educational sessions, and 42% (intervention group 1) and 39% (intervention group 2), respectively, attended five or more sessions. In Huizing 2009, 90% of the staff attended at least four out of five educational sessions. In Pellfolk 2010, 83.2% of the nurses watched the videotaped lectures about physical restraints and 73.0% to 96.4% of the staff members watched the other lectures (median number of lectures 5). In Testad 2010, all the nursing staff attended all the educational and guidance sessions and, in Testad 2016, over 90% of all nurses attended the 2‐day seminar. Testad 2005 did not report any information about implementation fidelity.
Attrition rates of nursing staff were reported in two studies. In Testad 2010, 56 staff members (53.8%) in the intervention group and 53 (57.0%) in the control group were still employed at the end of the follow‐up period. Reasons for attrition included retirement, pregnancy, long‐term sick leave, and moving or changing job. In Testad 2005, nursing staff attrition was only presented as the number of nurses who left the study, without reporting the corresponding proportion.
Characteristics of the control groups
In most studies, the control group did not receive any intervention beyond usual care (Dever Fitzgerald 2016; Evans 1997; Gulpers 2011; Huizing 2009; Koczy 2011; Pellfolk 2010; Testad 2005; Testad 2010; Testad 2016). In two studies, the control group also received written information about PR reduction (Abraham 2019; Köpke 2012). Details about usual care were not reported in any of the studies.
Outcomes and methods of data collection
An overview of the outcomes assessed in the included studies is displayed in Table 5.
2. Overview ‐ outcomes.
| Outcomes | Abraham 2019 | Dever Fitzgerald 2016 | Evans 1997 | Gulpers 2011 | Huizing 2009 | Koczy 2011 | Köpke 2012 | Pellfolk 2010 | Testad 2005 | Testad 2010 | Testad 2016 |
| Use of restraints | X | X | X | X | X | X | X | X | X | X | X |
| Restraint intensity | ‐ | ‐ | ‐ | ‐ | X | X | ‐ | ‐ | ‐ | ‐ | ‐ |
| Falls | X | ‐ | X | X | ‐ | X | X | X | ‐ | ‐ | ‐ |
| Fall‐risk | ‐ | X | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Fall‐related injuries | X | ‐ | X | X | ‐ | X | X | ‐ | ‐ | ‐ | ‐ |
| Quality of life | X | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Psychotropic medications | ‐ | ‐ | X | X | ‐ | X | X | X | ‐ | ‐ | ‐ |
| Behavioural symptoms | ‐ | ‐ | ‐ | ‐ | ‐ | X | ‐ | ‐ | X | X | X |
Primary outcome
Use of physical restraints
All studies gave a formal definition of PR or mentioned examples of the devices assessed as physical restraints. One study did not assess the use of bedrails (Koczy 2011), which are the most commonly used restrictive devices. Two studies (Testad 2010; Testad 2016) assessed both physical restraints and forced care (in the studies, defined as structural and interactional restraints). We only included the results regarding physical restraints from these studies. An overview of the assessed devices is presented in Table 6.
3. Definitions of physical restraints.
| Definition of physical restraints | Abraham 2019 | Dever Fitzgerald 2016 | Evans 1997 | Gulpers 2011 | Huizing 2009 | Koczy 2011 | Köpke 2012 | Pellfolk 2010 | Testad 2005 | Testad 2010 | Testad 2016 |
| Full‐enclosure bedrails | X | ‐ | ‐ | X | X | ‐ | X | ‐ | X | X | X |
| Belts | X | X | X | X | X | X | X | X | X | X | X |
| Chairs with fixed tables | X | ? | X | X | X | X | X | X | X | X | X |
| Restrictive clothes, sleep suits | X | ? | ‐ | X | X | ‐ | X | ‐ | X | X | ? |
| Electronic devices | X | ? | ‐ | ‐ | X | ‐ | X | ‐ | X | X | X |
Six studies assessed PR use by direct observation (Abraham 2019; Dever Fitzgerald 2016; Evans 1997; Gulpers 2011; Huizing 2009; Köpke 2012). In Abraham 2019 and Köpke 2012, PR use was observed by trained raters twice a day (morning and evening, Abraham 2019) or three times a day (morning, noon, evening, Köpke 2012), respectively. Observers were accompanied by a nurse and only the cluster's head nurse was informed in advance about the day and time of the visits. In Dever Fitzgerald 2016, PR use was assessed by direct observation and the observers also checked the nursing documentation. Observations were randomly timed in order to cover different time points and each participant was observed eight times at baseline and follow‐up. In the study by Evans 1997, trained observers visited all residents 18 times within 72 hours. The visits covered all three shifts and the order of visits was randomised. In Gulpers 2011 and Huizing 2009, a single trained observer assessed PR use four times a day (morning, afternoon, evening, and night) and the units were not informed about the day and time of the observations.
Three studies (Testad 2005; Testad 2010; Testad 2016) used a standardised interview with the residents’ nurse in charge covering the use of PR during the previous seven days (Kirkevold 2004). In Koczy 2011 and Pellfolk 2010, nursing staff documented PR using a special documentation sheet.
Serious adverse events
None of the studies described serious adverse events as an outcome, but one study reported that no adverse events were observed (Abraham 2019). Several studies mentioned falls and fall‐related injuries as potential adverse events of PR reduction (see secondary outcomes).
Secondary outcomes
Restraint intensity
Three studies assessed the intensity or duration of PR use (Evans 1997; Huizing 2009; Koczy 2011).
Agitation
Four studies assessed agitation. Three studies used a version of the Cohen‐Mansfield Agitation Inventory (CMAI) (Koczy 2011; Testad 2010; Testad 2016). The number of items differed, but higher scores indicate more severe behaviours. Testad 2016 also used the Neuropsychiatric Inventory (NPI) and calculated the total score and the score for the agitation subscale. Higher scores indicate more severe symptoms. Testad 2005 used the Brief Agitation Rating Scale (BARS); higher scores indicate more severe agitation.
Falls and fall‐related injuries
Six studies assessed the number of residents with at least one fall (Abraham 2019; Evans 1997; Gulpers 2011; Koczy 2011; Köpke 2012; Pellfolk 2010) from the nursing record or incident reports.
Five studies assessed fall‐related injuries from the nursing record or incident reports. Three studies assessed fall‐related fractures (Abraham 2019; Koczy 2011; Köpke 2012). Two studies assessed fall‐related injuries, defined as fracture and other injuries resulting in medical attention or bedrest for at least two days (Evans 1997), and as haematomas, bruises, lacerations, joint dislocations, and fractures (Gulpers 2011).
Two studies assessed residents' fall risk. Dever Fitzgerald 2016 used the Tinetti‐Performance‐Oriented Mobility Assessment (POMA) including two subscales (balance and gait). Scores ranged from 0 to 28 and a score below 19 indicates high fall‐risk; a score between 19 and 24 moderate fall‐risk. Pellfolk 2010 used a 100‐mm visual analogue scale (range: 0 to 100, higher scores indicate a higher fall risk).
Psychotropic medications
Most studies assessed the use of psychotropic medication from the medical records (Evans 1997; Gulpers 2011; Koczy 2011; Köpke 2012; Pellfolk 2010; Testad 2005; Testad 2010; Testad 2016).
Quality of life
Abraham 2019 assessed quality of life in a randomly selected subsample (10% of residents per cluster) by proxy‐rating (nurses with direct contact with the residents) using the German version of the validated Quality of Life‐Alzheimer’s Disease (QoL‐AD) instrument (13 Items, range 13 to 52; higher scores indicate better quality of life).
Costs
Abraham 2019 and Köpke 2012 collected data about the intervention costs, considering the salary for personnel delivering the intervention components and for the participating nursing staff, and the materials. Abraham 2019 planned a health economic evaluation from a German social insurance perspective, but since there was no statistically significant difference in the primary outcome between the study groups, this analysis was not performed.
Implementation fidelity
Three studies performed a process‐evaluation as part of the evaluation study using a mixed‐methods design (Abraham 2019; Gulpers 2011; Köpke 2012). Information about the implementation process and implementation fidelity as well as barriers to and facilitators of the implementation were assessed.
Excluded studies
We excluded a majority of the studies because the intervention or the study design did not meet the inclusion criteria.
Risk of bias in included studies
In the first version of this review, we had contacted the authors of all included studies and asked for missing information. All authors responded to our requests. We also had contacted the study authors of one of the newly included studies for an earlier update of this review (Möhler 2012) and asked for missing information, but the authors did not provide the requested information (Koczy 2011). For this update, we contacted the authors of all newly included studies asking for missing information and received additional information from authors of three studies (Abraham 2019; Dever Fitzgerald 2016; Köpke 2012).
All studies were at high risk of bias in at least one domain. Detailed information about the risk of bias of the included studies is presented in the Characteristics of included studies table, and an overview is provided in Figure 3 and Figure 4.
3.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies
4.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study
Allocation
Sequence generation was adequate in six studies (Abraham 2019; Dever Fitzgerald 2016; Huizing 2009; Köpke 2012; Pellfolk 2010; Testad 2005), unclear in four studies (Evans 1997; Koczy 2011; Testad 2010; Testad 2016), and one study did not allocate the clusters at random (Gulpers 2011).
Allocation of clusters was adequately concealed in three studies (Abraham 2019; Huizing 2009; Köpke 2012) and unclear in three studies (Koczy 2011; Testad 2010; Testad 2016). We found pronounced baseline differences in the prevalence of PR in two studies (Evans 1997; Testad 2010), but these differences might have occurred by chance because of the small number of clusters per group.
We judged overall risk of selection bias to be low in three studies (Abraham 2019; Huizing 2009; Köpke 2012), unclear in two studies (Koczy 2011; Testad 2010), and high in the other studies (Dever Fitzgerald 2016; Evans 1997; Pellfolk 2010; Testad 2005; Testad 2016).
Blinding
Most studies did not provide information about whether the residents were informed about the study, but the intervention was delivered to the nursing staff rather than the residents.
We judged blinding of personnel (nursing staff and staff delivering the intervention) not possible due to the nature of the interventions. Most studies allocated nursing homes or independent wards to the study groups, and we judged risk of contamination of the clusters in the control group to be low (Abraham 2019; Evans 1997; Gulpers 2011; Huizing 2009; Koczy 2011; Köpke 2012; Pellfolk 2010; Testad 2005; Testad 2010; Testad 2016). In Dever Fitzgerald 2016, individual participants were allocated to the study groups, and we judged risk of performance bias to be high since the same staff cared for participants in the intervention and the control groups.
Outcome assessors were blinded to group allocation in eight studies (Abraham 2019; Evans 1997; Gulpers 2011; Huizing 2009; Köpke 2012; Testad 2005; Testad 2010; Testad 2016). In three studies, outcome assessors were not blinded to group allocation. Although the presence of most devices used as PR seems not to be prone to detection bias (e.g. bedrails or belts in bed or chair), some measures required a judgement from the outcome assessors (e.g. whether a fixed table on a wheelchair or a half‐length bedrail was used as a restrictive measure), and we judged risk of detection bias to be unclear for these studies (Dever Fitzgerald 2016; Koczy 2011; Pellfolk 2010).
Incomplete outcome data
In most of the cluster‐randomised trials, all clusters completed the study. However, in Huizing 2009, one cluster was lost‐to follow‐up, leading to an unbalanced attrition rate in the study groups. In Pellfolk 2010, attrition rates also differed slightly between the study groups. Pellfolk 2010 and Testad 2016 did not report the reasons for attrition. In Testad 2010, the attrition rate was approximately 43%.
We judged risk of attrition bias to be unclear in four studies (Huizing 2009; Pellfolk 2010; Testad 2010; Testad 2016).
Selective reporting
Two studies were prospectively registered, and all outcomes were reported as planned (Abraham 2019; Köpke 2012). In one study, the primary outcome differed between the study protocol and the final publication, and we judged risk of reporting bias to be high (Koczy 2011). The other studies were retrospectively or not registered, and we judged risk of reporting bias to be unclear (Dever Fitzgerald 2016; Evans 1997; Gulpers 2011; Huizing 2009; Pellfolk 2010; Testad 2005; Testad 2010; Testad 2016).
Other potential sources of bias
We did not identify any other sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Organisational interventions aimed at implementing a least‐restraint policy
We included four studies investigating organisational interventions aimed at implementing a least‐restraint policy (Abraham 2019; Gulpers 2011; Koczy 2011; Köpke 2012).
See: Table 1
Primary outcomes
Number of participants with at least one physical restraint
We performed a meta‐analysis for the number of participants with at least one PR which included all four studies, and a meta‐analysis for the number of participants with at least one belt which included three studies (Abraham 2019; Gulpers 2011; Köpke 2012). The Koczy 2011 study reported PR use only for a subgroup of participants with at least one PR at baseline and did not include bedrails.
For the number of participants with at least one PR, we found moderate‐certainty evidence (downgraded one level for risk of bias) that organisational interventions probably reduce the number of people with at least one PR (RR 0.86, 95% CI 0.78 to 0.94, I² = 25%, 3849 participants; 4 studies; Analysis 1.1; Figure 5).
1.1. Analysis.

Comparison 1: Organisational interventions, Outcome 1: Residents with at least one physical restraint
5.

Forest plot (1.1 Residents with at least one physical restraint)
Three studies assessed the use of belt restraints, and we found moderate‐certainty evidence (downgraded one level for risk of bias) that organisational interventions probably result in a large reduction of people with at least one belt (RR 0.54, 95% CI 0.40 to 0.73; 2711 participants; 3 studies; Analysis 1.2; Figure 6).
1.2. Analysis.

Comparison 1: Organisational interventions, Outcome 2: Residents with at least one belt
6.

Forest plot (1.2 Residents with at least one belt)
Serious adverse events
One of the studies reported that no adverse events related to PR use occurred (Abraham 2019), and none of the other studies reported any information about adverse events. We considered this to be moderate‐certainty evidence (downgraded for imprecision) that organisational interventions intended to reduce PR use probably result in little to no difference in adverse events.
Secondary outcomes
Duration of PR use
Koczy 2011 assessed the duration of restraint use and reported this outcome as the proportion of participants with a relative reduction of time with PR use. The proportion of participants with a reduced duration of at least 75% was 21.6% in the intervention group and 10.4% in the control group; the proportion with a reduction between 50% and 75% was 26.9% in the intervention group and 14.4% in the control group; the proportion with a reduction between 25% and 50% was 33.2% in the intervention group and 21.6% in the control group.
We considered this to be moderate‐certainty evidence (downgraded one level for imprecision) that organisational interventions probably reduce the duration of PR use.
Number of falls or fall‐related injuries
We performed meta‐analyses for the number of participants with at least one fall and the number of participants with at least one fall‐related fracture, including four studies in each meta‐analysis (Abraham 2019; Gulpers 2011; Koczy 2011; Köpke 2012).
We found low‐certainty evidence (downgraded one level for each of risk of bias and inconsistency) that organisational interventions aimed at implementing a least‐restraint policy may result in little to no difference in the number of participants with at least one fall (RR 1.02, 95% CI 0.86 to 1.20, I² = 77%, 4 studies, 17,954 participants; Analysis 1.3; Figure 7).
1.3. Analysis.

Comparison 1: Organisational interventions, Outcome 3: Residents with at least one fall
7.

Forest plot (1.3 Residents with at least one fall)
We found low‐certainty evidence (downgraded one level for each of risk of bias and imprecision) that organisational interventions aimed at implementing a least‐restraint policy may result in little to no difference in the number of participants with at least one fall‐related fracture (RR 1.05, 95% CI 0.76 to 1.45, I² = 22%, 4 studies, 17,954 participants; Analysis 1.4; Figure 8).
1.4. Analysis.

Comparison 1: Organisational interventions, Outcome 4: Residents with at least one fall‐related fracture
8.

Forest plot (1.4 Residents with at least one fall‐related fracture)
Agitation
Only one study assessed agitation in a subgroup of participants with PR use at baseline (Koczy 2011). Agitation was nearly unchanged in both intervention groups with no clear between‐group difference (MD ‐0.44, 95% CI ‐1.94 to 1.06 for agitated and inappropriate behaviour; ‐0.57, 95% CI ‐1.67 to 0.54 for verbally agitated behaviour; ‐0.03, 95% CI ‐0.99 to 0.93 for aggressive behaviour).
We considered this low‐certainty evidence (downgraded one level for each of risk of bias and imprecision) that organisational interventions aimed at implementing a least‐restraint policy may result in little to no difference in agitation.
Quality of life
Only one study investigated quality of life (Abraham 2019). We found high‐certainty evidence that organisational interventions aimed at implementing a least‐restraint policy make little to no difference to residents' quality of life (MD 0.04, 95% CI ‐1.17 to 1.24; 951 participants; Analysis 1.6).
1.6. Analysis.

Comparison 1: Organisational interventions, Outcome 6: Quality of life
Use of psychotropic medication
We performed a meta‐analysis for the number of participants with at least one psychotropic medication which included two studies (Gulpers 2011; Köpke 2012). We found moderate‐certainty evidence (downgraded one level for risk of bias) that organisational interventions aimed at implementing a least‐restraint policy probably result in little to no difference in the number of participants with at least one psychotropic medication (RR 1.0, 95% CI 0.95 to 1.06, 3452 participants; Analysis 1.5; Figure 9).
1.5. Analysis.

Comparison 1: Organisational interventions, Outcome 5: Residents with at least one psychotropic medication
9.

Forest plot (1.5 Residents with at least one psychotropic medication)
In the study that was not included in the meta‐analysis, the mean number of psychotropic medications was nearly unchanged in both study groups and there was no difference between the study groups (MD ‐0.04, 95% CI ‐0.2 to 0.11, 333 participants; Koczy 2011).
Costs
Two studies assessed intervention costs (Abraham 2019; Köpke 2012) and reported the total costs of the intervention, including the salary of the staff (champions and nurses), research staff, and study materials. In Köpke 2012, intervention costs per participant were 11.95 Euros. No information about the costs of the study materials offered to the control group was reported. In Abraham 2019, intervention costs per participant for intervention 1 (updated version of the intervention tested by Köpke 2012) were 9.22 Euros and 4.75 Euros for intervention 2. The study materials offered to the control group incurred almost no costs (less than one Euro cent per participant).
Other secondary outcomes
None of the included studies assessed mobility, incidence of pressure ulcers, or caregiver‐related outcomes.
Barriers and facilitators to implementation
Three studies included a process‐evaluation alongside the clinical trial (Abraham 2019; Gulpers 2011; Köpke 2012). In all studies, the interventions were predominantly implemented as planned, i.e. no deviations from the study protocol were observed regarding the implementation of the components (e.g. dose delivered and dose received). The participants' satisfaction with the intervention itself and the intervention components was described as high. Two studies described that, after the educational sessions, most participants had good knowledge concerning the aim of the intervention and the content of the educational sessions. Key nurses also showed rather critical attitudes about PR use, but other nurses were partly less critical (Abraham 2019; Köpke 2012).
All studies identified several barriers to and facilitators of implementation. Gulpers 2011 identified mainly organisational barriers, e.g. the short time period between the recruitment and the delivery of the intervention; the ratio between lectures and case discussions during the education and training of the champions; the challenge of balancing the number of participants and attempts to reduce PR use with the available staff resources; and that sometimes devices, like low‐low beds or alarms, were not available in time. Köpke 2012 identified as facilitators support from head nurses and nursing home leaders; quality circles with case discussions; and information materials for relatives, legal guardians, and physicians. Barriers included negative experiences of nurses with PR reduction; concerns of relatives and legal guardians regarding PR reduction; and organisational challenges (such as staff fluctuation). In Abraham 2019, some champions reported that the intended policy change was not, or partly not, implemented as planned. One important reason was that some nurses still believed that physical restraints were effective measures to prevent falls and fall‐related injuries. Uncritical attitudes regarding PR use were described amongst relatives and legal guardians and this also hampered the reduction of PR use. Other barriers included lack of devices such as low‐low beds and limited time resources (Abraham 2021).
Simple educational interventions
Six studies investigated simple educational interventions (Evans 1997; Huizing 2009; Pellfolk 2010; Testad 2005; Testad 2010; Testad 2016).
See: Table 2
Primary outcomes
Number of participants with at least one physical restraint
All studies assessed PR use, but we did not perform meta‐analyses because we found pronounced imbalances in the baseline PR prevalence in some studies and some heterogeneity in the definition of PR between studies, i.e. two studies did not include bedrails, which are the most commonly used devices. We reported the study results narratively and give an overview of the intervention components in Figure 2.
The study by Evans 1997 included three study groups (463 participants). The baseline prevalence of people with at least one PR use differed between the three groups (intervention group 1: 34%, intervention group 2: 28% and control group: 45%). PR use decreased to some extent in all study groups after 12 months (intervention group 1 (educational programme and guidance) from 34% to 16%; intervention group 2 (educational programme only) from 28% to 19%; control group: PR from 45% to 42%).
The intervention group in Huizing 2009 received an educational programme plus consultation. Prevalence of PR use increased in both study groups after ten months (intervention group from 54% to 64%, control group from 49% to 60%), but there was no difference between the study groups at follow‐up (RR 1.07, 95 % CI 0.88 to 1.31, 241 participants; Analysis 2.1).
2.1. Analysis.

Comparison 2: Simple educational interventions, Outcome 1: Residents with at least one physical restraint
Three studies investigated an educational programme plus guidance. Testad 2005 (142 participants) found a decrease in the mean number of PRs per resident per week in the intervention group after seven months (baseline: 3.3, follow‐up: 1.5), and a small increase in the control group (baseline: 3.1, follow‐up: 3.7). In Testad 2010 (90 participants), the number of participants with at least one PR decreased in the intervention group after 12 months (baseline 60%, follow‐up 18%), and was nearly unchanged in the control group, but the baseline prevalence was much lower (baseline and follow‐up both 13%). In Testad 2016 (197 participants), the number of participants with at least one PR decreased in both study groups after seven months (intervention group: 14.5% to 10.5%; control group 10.5% to 6.1%).
Pellfolk 2010 included participants from group‐dwelling units and offered only an educational component. The number of participants with at least one PR was nearly unchanged in the intervention group (baseline 21.5%, follow‐up 20.1%) and increased in the control group (baseline 20.1%, follow‐up 38.1%). The RR was 0.50 (95% CI 0.34 to 0.74, 350 participants; Analysis 2.1).
We considered this to be very low‐certainty evidence (downgraded one level for each of risk of bias, inconsistency, and imprecision) and consequently we are uncertain about the effects of simple educational interventions on the number of residents with PR use in older people who require long‐term care.
Serious adverse events
None of the studies assessed adverse events related to PR use (e.g. direct injuries) or reported such events.
Secondary outcomes
Restraint intensity
One study assessed restraint intensity, i.e. the number of observations per resident with PR observed in 24 hours, and multiple restraints (Huizing 2009).
Restraint intensity did not differ between the study groups at baseline. The mean number of observations with PR use was 1.36 ± 1.62 (17% of the residents were restrained at one observation, 6% at two observations, 7% at three observations and 22% at four observations in a 24‐hour period). Restraint intensity increased in both study groups during the study period, but there was no difference between the study groups at follow‐up (241 participants).
Multiple restraints did not differ between the study groups at baseline (in 23% of the participants, one type of PR was used, in 17% of the participants two different types were used, in 10% three, and in 2% four different types of PR were used within 24 hours). The mean number of PR measures per resident was 0.93 ± 1.10 at baseline. Use of multiple restraints increased in both study groups and there was no difference between the study groups at follow‐up (241 participants).
We considered this to be moderate‐certainty evidence (downgraded one level for imprecision) that simple educational interventions probably result in little or no difference in restraint intensity.
Falls and fall‐related injuries
Two studies assessed the number of residents with at least one fall (Evans 1997; Pellfolk 2010). Evans 1997 also assessed serious fall‐related injuries.
In Evans 1997, the proportion of residents with at least one fall in a 90‐day period before the intervention period was 33.1% in intervention group 1, 37.7% in intervention group 2, and 20.1% in the control group. In the first three months of the study period, 42.5% of participants in intervention group 1 experienced at least one fall, 41.5% in intervention group 2, and 64.7% in the control group. In months three to six post‐intervention, 37.8% of participants in intervention group 1 experienced at least one fall, 32.2% in intervention group 2, and 53.3% in the control group. In the study by Pellfolk 2010, the number of participants with at least one fall during a one‐month period before and after the intervention period decreased in both groups (intervention group baseline 12.0%, follow‐up 9.1%; control group baseline 14.7%, follow‐up 13.3%). We considered this to be low‐certainty evidence (downgraded one level for each of risk of bias and inconsistency) that simple educational interventions may result in little or no difference in the number of residents with at least one fall (813 participants).
In Evans 1997, nine serious fall‐related injuries occurred in a 90‐day period before the intervention was implemented (reportedly no difference between the study groups, but no information about the number of injuries per study group). In the post‐intervention period (months 6 to 12), no fall‐related serious injury occurred in intervention group 1, 8 fall‐related injuries (5.3%) were documented in intervention group 2 and 4 (2.2%) in the control group. We considered this to be low‐certainty evidence (downgraded one level for each of risk of bias and imprecision) that simple educational interventions may result in little or no difference in the number of residents with serious fall‐related injuries (463 participants).
Use of psychotropic medication
We did not perform a meta‐analysis due to baseline imbalances between the intervention and control groups in most of the studies.
Evans 1997 assessed neuroleptics and benzodiazepines. The proportion of participants with at least one neuroleptic was nearly unchanged in intervention group 1 (baseline 18.2%, follow‐up 19.0%), slightly increased in intervention groups 2 (baseline 13.5%, follow‐up 15.5%) and decreased in the control group (baseline 18.6%, follow‐up 11.3%). The number of residents with at least one benzodiazepine decreased in all study groups (intervention group 1: baseline 22.3%, follow‐up 18.2 %; intervention group 2: baseline 37.2%, follow‐up 27.0%; control group: baseline 32.8%, follow‐up 26.6%; 446 participants).
In Pellfolk 2010, the number of residents with benzodiazepines decreased in both study groups (intervention group baseline 34.1%, follow‐up 31.8%; control group baseline 31.5%, follow‐up 23.6%). The number of participants with neuroleptics was nearly unchanged in the intervention group (baseline 50.5%, follow‐up 49.7%) and decreased in the control group (baseline 43.2%, follow‐up 39.2%) (327 participants).
In Testad 2005, the number of residents with at least one psychotropic medication decreased in both study groups (intervention group: baseline 71%, follow‐up 55%; control group: baseline 61%, follow‐up 52%; 142 participants). In Testad 2010, the number of residents with at least one psychotropic medication increased slightly in the intervention group (baseline 28%, follow‐up 31.8%) and was nearly unchanged in the control group (baseline 8.6%, follow‐up 8.7%) (90 participants). In Testad 2016, the number of residents with at least one antipsychotic and antidepressant increased slightly in both study groups (no further information reported; 197 participants).
We considered this very low‐certainty evidence (downgraded one level for each of risk of bias, inconsistency and imprecision) and consequently we are uncertain about the effects of simple educational interventions on the number of participants with at least one psychotropic medication (1202 participants).
Agitation
Three studies assessed agitation (Testad 2005; Testad 2010; Testad 2016) and we performed a meta‐analysis including two studies which used the CMAI as a measure of agitation (Testad 2010; Testad 2016). There was a small but uncertain difference in favour of the intervention groups (CMAI, MD ‐2.33, 95% CI ‐8.39 to 3.74; I² = 78%, 287 participants; Analysis 2.2; Figure 10).
2.2. Analysis.

Comparison 2: Simple educational interventions, Outcome 2: Agitation
10.

Forest plot (2.2 Agitation)
Testad 2016 also assessed agitation using the NPI. There was nearly no change in both study groups on the NPI behaviour subscale and no clear between‐group difference (MD ‐0.40, 95% CI ‐3.68 to 2.88; 287 participants; Analysis 2.3), while there was a small but uncertain difference in favour of the control group on the NPI total score (MD 3.9, 95% CI ‐1.83 to 9.63; 287 participants; Analysis 2.3).
2.3. Analysis.

Comparison 2: Simple educational interventions, Outcome 3: Behaviour (NPI)
In Testad 2005, which was not included in the meta‐analysis, agitation increased in the intervention group and was unchanged in the control group (intervention group baseline mean score 16.8, follow‐up 21.2; control group baseline mean score 17.3, follow‐up 17.4; 142 participants).
We considered this to be low‐certainty evidence (downgraded one level for each of risk of bias and imprecision) that simple educational interventions may result in little or no difference in agitation.
Other secondary outcomes
None of the included studies assessed mobility, incidence of pressure ulcers, caregiver‐related outcomes, or costs.
Barriers and facilitators to implementation
One study assessed some barriers and facilitators and found that the involvement of nursing home leaders was an important facilitator of successful implementation of the intervention (Testad 2016).
Interventions providing technical devices or information for common risk factors for PR use
We included one study in this category (Dever Fitzgerald 2016). See: Table 3
Primary outcomes
Number of participants with at least one physical restraint
In Dever Fitzgerald 2016, the mean number of PRs assessed by observation slightly increased in both study groups, but there was no difference between the study groups (MD ‐0.51, 95% CI ‐1.72 to 0.70; 98 participants; Analysis 3.1; Figure 11). We found low‐certainty evidence (downgraded one level for risk of bias and one level for imprecision) that an intervention providing information about the fall risk of residents may result in little to no difference in the mean number of PRs.
3.1. Analysis.

Comparison 3: Interventions providing information about the residents' fall risk, Outcome 1: Restraint use
11.

Forest plot (3.1 Restraint use)
Serious adverse events
This study did not assess adverse events.
Secondary outcomes
Falls
Dever Fitzgerald 2016 found no difference in the median number of falls between baseline and follow‐up in either study group (median in both study groups at baseline and follow‐up was 1). We found low‐certainty evidence (downgraded one level for risk of bias and one level for imprecision) that an intervention providing information about the fall risk of residents may result in little or no difference in the median number of falls (150 participants).
Nurses' fear of participants falling slightly increased in the intervention group and was stable in the control group (intervention group baseline 10.72 ± 4.03, follow‐up 12.30 ± 2.92; control group baseline 10.59 ± 4.59, follow‐up 10.97 ± 4.12; 128 participants (nursing staff)). We found low‐certainty evidence (downgraded one level for risk of bias and one level for imprecision) that an intervention providing information about the fall risk of residents may result in little or no difference in nurses' fear of participants falling.
Other secondary outcomes
This study did not assess any other secondary outcome of this review.
Discussion
Summary of main results
We included 11 studies in this updated review, which investigated interventions aiming to reduce PR use in older people who require long‐term care. Four studies, all of which were newly included in this update, investigated organisational interventions aiming to implement a least‐restraint policy. Six studies, one of these newly included, investigated simple educational interventions, and one newly included study investigated an intervention that provided nursing staff with information about residents' risk of falling. All studies were conducted in long‐term care institutions, predominantly in nursing homes. We did not identify any studies that were conducted in a community setting.
All organisational interventions aiming to implement a least‐restraint policy used intervention champions and some type of policy change in the care facilities to address staff attitudes and support a least‐restraint culture of care. The interventions seemed comparable regarding intervention approach, content of the education component and delivery. The study by Gulpers 2011 comprised a strong policy change that prohibited the use of new belts, while the organisational components in the three other studies were less strict (Abraham 2019; Koczy 2011; Köpke 2012), since prohibiting the use of a specific restrictive measure was judged not to be feasible in Germany. The results of the process evaluation indicated good implementation fidelity. We found moderate‐certainty evidence that organisational interventions aiming to implement a least‐restraint policy probably lead to a reduction in the number of residents with at least one PR and a large reduction in the number of residents with at least one belt (moderate‐certainty evidence each) (it is worth noting here that the latter result was not heavily reliant on the Gulpers 2011 study with its strong prohibition of belts). Only one study reported any information about serious adverse events, but none occurred. There was evidence from one study that organisational interventions probably reduce the duration of PR use. We found no evidence of any effect on the number of residents experiencing falls or fall‐related injuries (low‐certainty evidence each), but the reduction of PR use seems not to be associated with an increase of the number of people with at least one fall. We also found no evidence of any effect on the number of prescribed psychotropic medications (moderate‐certainty evidence). One study found that organisational interventions result in little or no difference in quality of life (high‐certainty evidence) and another study found that they may lead to little or no difference in agitation amongst participants (low‐certainty evidence).
The simple educational interventions aiming to change staff attitudes towards PR included primarily an educational component but also further components in some studies, such as ward‐based guidance. Regarding the number of residents with at least one restraint, the results are inconsistent. Some studies found a decrease of PR use in both study groups, some a decrease only in the educational intervention group, and one study found an increase of PR in both study groups. We found pronounced baseline imbalances in PR prevalence in some of the studies, which might have occurred because of the small number of clusters per study group. Most of the studies did not consider cluster effects in the analysis and were prone to a unit of analysis error. One study did not assess bedrails, which is the most commonly used measure in nursing homes. We found very low‐certainty evidence, and we are uncertain about the effects of simple educational interventions on the number of residents with PR. None of the studies assessed or reported adverse events. We found moderate‐certainty evidence that simple educational interventions probably result in little or no difference in restraint intensity. There was no evidence about an increase or decrease of falls, fall‐related injuries, or agitation (low‐certainty evidence each). Based on very low‐certainty evidence, we are uncertain about the effects of simple educational interventions on the number of participants with at least one psychotropic medication.
One study investigated an intervention that provided nursing staff with information about residents' fall‐risk. We found low‐certainty evidence that providing information about residents' fall‐risk may result in little or no difference in the mean number of PRs or the number of falls. The study did not assess adverse events.
In summary, organisational interventions aiming to implement a least‐restraint policy can effectively reduce PR use in general and specifically the use of belts in nursing home residents. The evidence about simple educational interventions is still inconclusive (as we found in the earlier version of this review (Möhler 2011)). Providing information about residents' fall‐risk did not reduce PR use in one study. We found no evidence that less PR use is associated with an increase of falls, fall‐related injuries or the prescription of psychotropic medication.
Overall completeness and applicability of evidence
Although the number of studies investigating organisational interventions aiming to implement a least‐restraint policy was small, we found no important statistical heterogeneity. However, Abraham 2019 and Köpke 2012 found pronounced centre differences in PR reduction at follow‐up between intervention clusters. In the pragmatic trial by Abraham 2019, which included a large sample of nursing homes in four regions in Germany and did not apply specific inclusion criteria, PR prevalence was nearly unchanged or even increased in several clusters in both interventions groups during the study period. A descriptive subgroup analysis of the process‐evaluation data from clusters with a pronounced decrease of PR use (responders) and clusters with a pronounced increase of PR use (non‐responders) did not result in meaningful results (Abraham 2021). We have insufficient information about specific contextual factors or organisational characteristics that might facilitate or hamper the implementation of the intervention or a successful policy change regarding PR use. Although some data from the process‐evaluation indicated that nurses' knowledge and attitude supported the goal to reduce PR use in general, it remains unclear whether this is a good predictor of PR use in clinical practice (Möhler 2014).
De‐implementation, that is stopping ineffective or harmful interventions (low‐value care), is challenging because such interventions are often part of the daily routine and are not questioned. Important contextual factors to be addressed in de‐implementation of low‐value care are characteristics of the inappropriate intervention, patients or residents, health professionals, and the organisation (Norton 2020). One recent study investigated the de‐implemention of position‐change alarm systems, such as bed alarms, a measure that was classified as physical restraint by the US Centers for Medicare and Medicaid Services and the Department of Veterans Affairs. In this study, clear evidence about harms and the lack of effectiveness of the intervention, and the support of local leadership and people with decision‐making authority were identified as important facilitators for de‐implementing bed alarms (Hartmann 2021). Organisational interventions which aim to implement a least‐restraint policy include components specifically addressing these factors, e.g. the champion approach and the organisational components. In contrast, simple educational interventions which do not address these factors seem to be ineffective in changing attitudes and the culture of care regarding PR use.
Three studies investigating organisational interventions were conducted in Germany and one in the Netherlands. Although the intervention approach seems applicable in different healthcare systems and contexts, further studies in other countries are needed (see Implications for research).
We did not find any eligible studies conducted in the community. One of the excluded studies reported the development and pilot‐test of a guideline‐based intervention aimed at reducing PR use during home care (Vandervelde 2021), but no evaluation study has been conducted yet. The results of this review may not be applicable to people receiving long‐term care in the community.
Quality of the evidence
The certainty of evidence differed for the different intervention groups. For organisational interventions aiming to implement a least‐restraint policy, we found predominantly moderate‐certainty evidence. The methodological quality of included studies was mainly good, although one non‐randomised trial in this group had a high risk of selection bias, and another study had a high risk of reporting bias. The results of the studies with low risk of bias and the study with a high risk of bias were comparable.
For simple educational interventions, we found very low‐ or low‐certainty evidence for most of the outcomes. We found high risk of selection bias for four out of six studies and there were pronounced baseline imbalances in PR prevalence in some of the studies. Several studies had also an unclear risk of attrition bias. Further uncertainties derived from different definitions of PR and the small number of clusters and participants in some of the studies; three studies included only one (Evans 1997) or two clusters (Testad 2005; Testad 2010) in each study group. There was a tendency to find larger effect sizes in studies with a higher risk of bias.
Most of the included studies randomised clusters to the study groups, but only three studies included cluster effects in the analysis (Abraham 2019; Köpke 2012; Pellfolk 2010). We performed a cluster‐adjusted meta‐analysis for the main outcome (the number of participants with at least one physical restraint) for organisational interventions aiming to implement a least‐restraint policy using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). The analysis for simple educational interventions was not cluster‐adjusted in this way, because meta‐analysis was not feasible. It remains unclear to what extent cluster effects have influenced the results and the results are prone to a unit‐of‐analysis error.
The results about PR use were assessed by direct observation in six studies (Abraham 2019; Dever Fitzgerald 2016; Evans 1997; Gulpers 2011; Huizing 2009; Köpke 2012), three studies used a standardised interview covering the use of PR during one week retrospectively (Testad 2005; Testad 2010; Testad 2016) and, in two studies, PR use was prospectively documented by nursing staff using a special sheet (Koczy 2011; Pellfolk 2010). There is some evidence that direct observation is the most valid method to assess PR use, but interviews with nursing staff seem to be an adequately valid method (Laurin 2004). Meyer 2009a used both direct observation and prospective documentation by nurses and found comparable results.
Potential biases in the review process
We followed the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019) to reduce bias in the review process. The search strategy was developed in co‐operation with the Cochrane Dementia and Cognitive Improvement Group's Information Specialist, and we included different sources in the literature search (databases, trial registers, citation tracking). Two review authors performed the study selection, data extraction and quality assessment independently. We contacted study authors for missing data and included additional information delivered by the study authors in the review update. We were not able to assess the risk of publication bias, because the number of studies per intervention group was small.
Agreements and disagreements with other studies or reviews
Two systematic reviews about educational interventions for reducing PR use have been published. As in our review, both reviews found positive effects in favour of the interventions, but the effect sizes seem to be overestimated because of several methodological limitations of the reviews, e.g. different publications of the same studies were included separately in the meta‐analyses, baseline imbalances, and the lack of cluster‐adjusted analysis were not taken into account (Brugnolli 2020; Lan 2017).
Authors' conclusions
Implications for practice.
Long‐term care facilities should introduce organisational interventions aiming to implement a least‐restraint policy as these constitute a promising approach to reducing PR use in older people who require long‐term care. Such interventions comprise education for policy 'champions' and other components to facilitate a change in the culture of care to make it less restrictive. We found no evidence that a reduction of physical restraints is associated with an increase of falls or fall‐related injuries. Educational interventions without an organisational approach seem ineffective.
No conclusions for community settings can be drawn, since we did not include any study from this setting.
Implications for research.
Although we found moderate‐certainty evidence about an effect in favour of organisational interventions which aim to implement a least‐restraint policy, more research is needed about contextual factors facilitating a change in the culture of care towards less restrictive practice, and about the requirements of an organisational component aimed to support such a policy change. Specifically, characteristics of effective organisational policies and leadership need to be explored in more detail. Also, further insights into and strategies to overcome barriers to PR reduction, such as myths of positive effects of PR in reducing falls or fall‐related injuries (Kong 2017; Möhler 2014), should be investigated.
Studies should include all restrictive measures covered by the consensus definition of PR (Bleijlevens 2016). In particular, bedrails should be included, since these are the most commonly used restrictive measures.
Further studies should adhere to good clinical practice of clinical trials, i.e. conduct prospectively registered, adequately powered, multicentre, cluster‐randomised controlled trials, including blinded outcome assessment and statistical methods to reduce unit‐of‐analysis error. Alongside such trials, a comprehensive process evaluation of implementation fidelity is required (Skivington 2021). Reporting should adhere to the established guidelines for complex interventions (e.g. Hoffmann 2014; Möhler 2015) and the study designs used, e.g. the CONSORT (Consolidated Standards of Reporting Trials) statement for randomised controlled trials (EQUATOR Network 2022).
What's new
| Date | Event | Description |
|---|---|---|
| 27 July 2023 | New citation required and conclusions have changed | New search performed, new studies included. Conclusions changed |
| 27 July 2023 | New search has been performed | The most recent search for this review was performed on 3 August 2022. New studies added, conclusions changed |
History
Protocol first published: Issue 1, 2009 Review first published: Issue 2, 2011
Acknowledgements
We acknowledge the contributions of the consumer editors Johanna Waßmuß (Nurse Manager, Max‐Brauer‐Nursing Home, Hamburg, Germany) and Helga Schneider‐Schelte (German Alzheimer Society, Berlin, Germany) to the development of the protocol. We also thank Pia von Lützau for her contributions to the development process of the first review, in particular for handsearching the abstract books and data preparation. We also thank Christina Manietta (CM) for her contributions to the review update.
We would like to acknowledge the Cochrane Dementia and Cognitive Improvement review group for their support.
Appendices
Appendix 1. Sources searched and search strategies post‐2009
| Source | Search strategy | Hits retrieved |
| Dementia Register (CRSO) [Date of most recent search: 3 August 2022] |
"physical restraint" OR "physical restraints" OR bedrail OR bedrails OR bedchair OR bedchairs OR "containment measure" OR "containment measures" | Feb 2017: 2 Mar 2018: 0 Dec 2018: 0 Nov 2019: 3 Oct 2020: 4 Oct 2021: 11 Aug 2022: 9 |
| 1. CENTRAL (The Cochrane Library) http://crso.cochrane.org/SearchSimple.php [Date of most recent search: 3 August 2022] |
#1 “physical restraint*” #2 bedrail* #3 bedchair* #4 “containment measure*” #5¬ #1 or #2 or #3 or #4 #6 elderly #7 “old people” #8 geriatric* #9 aged #10 “nursing home*” #11 “care home*” #12 “geriatric care” #13 “residential facit*” #14¬ #6 or #7 or #8 or #9 or #10 or #12 or #13 #15¬ #5 and #14 #16¬ #15 [clinical trials] |
Feb 2017: 27 Mar 2018: 7 Dec 2018: 4 Nov 2019: 29 Oct 2020: 18 Oct 2021: 17 Aug 2022: 17 |
| 2. MEDLINE (Ovid SP) Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily, Ovid MEDLINE and Versions(R) [Date of most recent search: 3 August 2022] |
1 physical restraint*.mp. 2 (bedrail* or "bed rail*").mp. 3 (bedchair* or "bed chair*").mp. 4 "containment measure*".mp. 5 exp Restraint, Physical/ 6 Education, Nursing/ 7 6 or 4 or 1 or 3 or 2 or 5 8 elderly.mp. 9 ("old people" or "old person*").mp. 10 geriatric*.mp. 11 aged.mp. 12 ("nursing home*" or nursinghome).mp. 13 "care home*".mp. 14 ("residential home*" or "residential facilit*").mp. 15 Aged/ 16 Residential Facilities/ 17 11 or 9 or 12 or 15 or 14 or 8 or 16 or 10 or 13 18 7 and 17 19 randomized controlled trial.pt. 20 controlled clinical trial.pt. 21 randomi?ed.ab. 22 randomly.ab. 23 trial.ab. 24 groups.ab. 25 22 or 21 or 24 or 23 or 19 or 20 26 (animals not (humans and animals)).sh. 27 25 not 26 28 27 and 18 |
Feb 2017: 44 Mar 2018: 35 Dec 2018: 31 Nov 2019: 72 Oct 2020: 64 Oct 2021: 79 Aug 2022: 72 |
| 3. EMBASE via OVID 1974 to present [Date of most recent search: 3 August 2022] |
1 Physical restraint*.mp. 2 bedrail*.mp. 3 bedchair*.mp. 4 Containment measure*.mp. 5 1 or 2 or 3 or 4 6 elderly.mp. 7 old people.mp. 8 geriatric*.mp. 9 aged.mp. 10 nursing home.mp. 11 care home.mp. 12 geriatric care.mp. 13 residential facility*.mp. 14 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 15 randomized controlled trial.mp. 16 controlled clinical trial.mp. 17 randomized.mp. 18 groups.mp. 19 15 or 16 or 17 or 18 20 5 and 14 and 19 |
Feb 2017: 10 Mar 2018: 19 Dec 2018: 13 Nov 2019: 21 Oct 2020: 26 Oct 2021: 67 Aug 2022: 86 |
| 4. PsycINFO via OVID 1806 to present [Date of most recent search: 3 August 2022] |
1 Physical restraint*.mp. 2 bedrail*.mp. 3 "bed rail*".mp. 4 (bedchair* or "bed chair*").mp. 5 Containment measure*.mp. 6 4 or 1 or 3 or 2 or 5 7 exp Physical Restraint/ 8 6 or 7 9 elderly.mp. 10 ("old people" or "old person*").mp. 11 geriatric*.mp. 12 aged.mp. 13 ("nursing home*" or nursinghome).mp. 14 "care home*".mp. 15 ("residential home*" or "residential facilit*").mp. 16 exp Residential Care Institutions/ 17 11 or 9 or 12 or 15 or 14 or 10 or 13 or 16 18 exp Clinical Trials/ 19 randomized controlled trial.mp. 20 controlled clinical trial.mp. 21 randomized.mp. 22 groups.mp. 23 22 or 21 or 18 or 19 or 20 24 8 and 17 and 23 |
Feb 2017: 30 Mar 2018: 3 Dec 2018: 2 Nov 2019: 7 Oct 2020: 13 Oct 2021: 14 Aug 2022: 11 |
| 5. CINAHL (EBSCOhost) [Date of most recent search: 3 August 2022] |
S1¬ TX physical restraint* S2¬ TX bedrail* S3¬ TX bedchair* S4¬ TX “containment measure*” S5¬ S1 OR S2 OR S3 OR S4 S6¬ TX elderly S7¬ TX “old people” or “old person*” S8¬ TX geriatric* S9¬ TX aged S10¬ TX “nursing home*” or nursinghome S11¬ TX “care home*” S12¬ TX “residential home*” or “residential facility*” S13¬ S6 or S7 or S8 or S9 or S10 or S11 or S12 S14¬ S5 and S13 S15¬ TX “randomized controlled trial” S16¬ TX “controlled clinical trial” S17¬ AB random* S18¬ AB trial S19¬ AB groups S20¬ S15 or S16 or S17 or S18 or S19 S21 S14 and S20 |
Feb 2017: 67 Mar 2018: 14 Dec 2018: 16 Nov 2019: 26 Oct 2020: 22 Oct 2021: 13 Aug 2022: 10 |
| 6. Clarivate Web of Science – all databases [includes: Web of Science (1945‐present); BIOSIS Previews (1926‐present); MEDLINE (1950‐present); Journal Citation Reports] [Date of most recent search: 3 August 2022] |
TOPIC: ("physical restraint*" OR bedrail* OR bedchair* OR "containment measure*") AND TOPIC: (elderly OR "Old people" OR geriatric* OR aged OR "nursing home" Or "care home" OR "geriatric care" Or "residential facilit*") AND TOPIC: ("randomized controlled trial" OR "controlled clinical trial" OR randomized OR groups) | Feb 2017: 129 Mar 2018: 23 Dec 2018: 17 Nov 2019: 29 Oct 2020: 30 Oct 2021: 71 Aug 2022: 119 |
| 7. LILACS (BIREME) [Date of most recent search: 3 August 2022] |
(“physical restraint*” AND (elderly OR geriatr$)) | Feb 2017: 0 Mar 2018: 0 Dec 2018: 0 Nov 2019: 10 Oct 2020: 6 Oct 2021: 1 Aug 2022: 1 |
| 8. ClinicalTrials.gov (www.clinicaltrials.gov) [Date of most recent search: 3 August 2022] |
elderly OR geriatr$ | “physical restraint*” | | Feb 2017: 2 Mar 2018:0 Dec 2018: 6 Nov 2019: 7 Oct 2020: 1 Oct 2021: 1 Aug 2022: 0 |
| 9. ICTRP [Date of most recent search: 3 August 2022] |
elderly OR geriatr* AND physical restraint* | Feb 2017: 0 Mar 2018: 0 Dec 2018: 0 Nov 2019: 0 Oct 2020: 0 Oct 2021: 0 Aug 2022: 0 |
| TOTAL before de‐duplication | Feb 2017: 307 Mar 2018: 101 Dec 2018: 89 Nov 2019: 204 Oct 2020: 184 Oct 2021: 274 Aug 2022: 325 TOTAL: 1484 |
|
| TOTAL after de‐duplication | Feb 2017: 258 Mar 2018: 74 Dec 2018: 64 Nov 2019: 150 Oct 2020: 138 Oct 2021: 206 Aug 2022: 233 TOTAL: 1123 |
|
| TOTAL after first assessment by Cochrane Information Specialist | Feb 2017: 44 Mar 2018: 12 Dec 2018: 20 TOTAL: 76 |
|
Appendix 2. Sources searched and search strategies used: September 2009
| Source | Date Searched | Hits Retrieved |
| MEDLINE (Pubmed) | Searched 7 September 2009 | 68 |
| Embase (Ovid SP) | Searched 7 September 2009 | 34 |
| PSYCINFO (Ovid SP) | Searched 7 September 2009 | 7 |
| CINAHL (Ovid SP) | Searched 7 September 2009 | 11 |
| Lilacs (Bireme) | Searched 7 September 2009 | 0 |
| CDCIG SR | Searched 7 September 2009 | 71 |
| CENTRAL (The Cochrane Library) | Issue 4 2009 | 34 |
| ISTP Conference Proceedings http://portal.isiknowledge.com/portal.cgi | Searched 7 September 2009 | 4 |
| Australian Digital Theses Program http://adt.caul.edu.au/ |
Searched 7 September 2009 | 0 |
| Canadian Theses and Dissertations http://www.collectionscanada.ca/thesescanada/index-e.html |
Searched 7 September 2009 | 2 |
| DATAD http://www.aau.org/datad/backgrd.htm |
Searched 7 September 2009 | 0 |
| WHO trials register http://www.who.int/ictrp/search/en/ | Searched 7 September 2009 | 9 |
| Current Controlled trials: Meta Register of Controlled trials (mRCT) http://www.controlled-trials.com/ |
Searched 7 September 2009 | 6 |
| ISRCTN Register | Searched 7 September 2009 together with mRCT | 0 |
| Nederlands Trial Register http://www.trialregister.nl/trialreg/index.asp | Searched 7 September 2009 | 1 |
| ClinicalTrials.gov http://www.ClinicalTrials.gov |
Searched 7 September 2009 with mRCT | 0 |
| IPFMA Clinical Trials Register www.ifpma.org/clinicaltrials.html |
Searched 7 September 2009 | 0 |
| UMIN Japan Trial Register http://www.umin.ac.jp/ctr/ |
Searched 7 September 2009 | 0 |
| ISI Web of Knowledge | Searched 7 September 2009 | 49 |
| TOTAL before de‐duplication | Sept 2009: 296 | |
| TOTAL after de‐duplication | Sept 2009: 160 | |
| TOTAL after first assessment | Sept 2009: 27 | |
Appendix 3. Items for quality assessment of included studies
| Item | Evans 1997 | Testad 2005 | Huizing 2009 | Pellfolk 2010 | Testad 2010 |
| METHOD | |||||
| Allocation sequence adequately generated | Yes* | Yes* | Yes* | Yes* | Unclear* |
| Allocation adequately concealed | No* | No* | Yes* | No | Unclear* |
| No evidence for cluster imbalance | No | Yes | Yes | Yes | No |
| Clusters lost to follow‐up | 0/3 | 0/4 | 1/15 | 0/40 | 0/4 |
| Participants identified before randomisation | Yes | No* | Yes | No* | No* |
| If no: no evidence for biassed selection of participants | ‐‐‐ | Unclear | ‐‐‐ | Unclear | Unclear |
| PARTICIPANTS | |||||
| Inclusion/exclusion criteria for participants clearly defined | Yes* | Yes* | Yes* | Yes* | Yes |
| Inclusion/exclusion criteria for clusters clearly defined | Unclear* | Yes* | Yes* | Yes* | Yes* |
| Sample size calculation | Yes* | No* | No* | Yes | Yes* |
| Adequate sample size calculation using methods for cluster randomisation | No* | No* | No* | No* | No* |
| No relevant differences between groups after randomisation | No | Yes | Yes | Unclear | No |
| Loss to follow‐up less 5% of participants | Unclear | Yes | No | Unclear | No |
| Were incomplete data adequately explained | Yes* | Yes* | Yes | No* | Yes |
| INTERVENTIONS | |||||
| All groups treated equally, except of intervention or control | Yes | Yes | Yes | Yes | Yes |
| OUTCOMES | |||||
| Primary outcome clearly stated? | Yes | Yes | Yes | Yes | Yes |
| Method of primary outcome assessment adequate | Yes | Yes | Yes | Yes | Yes |
| Outcome assessors blinded to group allocation | Yes | Yes | Yes | No | Yes |
| Data collection started immediately after randomisation | No* | Unclear* | No* | No* | Yes* |
| RESULTS | |||||
| Intention‐to‐treat analysis | Yes | Yes | Yes | Yes | No* |
| Complete reporting of outcome (as scheduled) | Yes | Yes | Yes | Yes | Yes |
| Methods of analysis adequate for cluster‐randomised trials | No | No | No | Yes (partial)* | No* |
| Coefficient of intra‐cluster correlation reported | No | No | No | No* | No* |
| MISCELLANEOUS | |||||
| No evidence for interpretation bias | Yes | Yes | Yes | Yes | Yes |
| Conflicts of interest mentioned | No | No | No | No | Yes |
| Requests to authors required | Yes | Yes | Yes | Yes | Yes |
| * Items marked with an asterisk have been answered by the study authors following personal request. | |||||
Data and analyses
Comparison 1. Organisational interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Residents with at least one physical restraint | 4 | 3849 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.78, 0.94] |
| 1.2 Residents with at least one belt | 3 | 12711 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.40, 0.73] |
| 1.3 Residents with at least one fall | 4 | 17954 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.86, 1.20] |
| 1.4 Residents with at least one fall‐related fracture | 4 | 17954 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.76, 1.45] |
| 1.5 Residents with at least one psychotropic medication | 2 | 3452 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.95, 1.06] |
| 1.6 Quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 2. Simple educational interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Residents with at least one physical restraint | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.2 Agitation | 2 | 287 | Mean Difference (IV, Random, 95% CI) | ‐2.33 [‐8.39, 3.74] |
| 2.3 Behaviour (NPI) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 3. Interventions providing information about the residents' fall risk.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Restraint use | 1 | 98 | Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.72, 0.70] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abraham 2019.
| Study characteristics | ||
| Methods |
Study design: cluster‐randomised controlled trial (NCT02341898) Intervention period: 12 months Duration of follow‐up: 12 months (follow‐up data were assessed after the study period) Study period: February 2015‐February 2017 |
|
| Participants |
Country: Germany Setting: nursing homes randomly selected from publicly available registers Participants/clusters:
Baseline characteristics:
|
|
| Interventions |
Intervention 1: guideline‐based multi‐component intervention Intervention 2: concise version of the guideline‐based multi‐component intervention Control: optimised usual care (written study materials) |
|
| Outcomes |
Primary: number of residents with at least one physical restraint Secondary: number of falls and fall‐related fractures, quality of life |
|
| Notes | Funding: grant from the German Federal Ministry of Education and Research within the Nursing Research Network Northern Germany (grant 01GT0606 and 01GT0608) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Clusters were randomly assigned to study groups (...) using a computer‐generated randomization list stratified by region with blocks of six, nine, and twelve nursing homes (generated by an independent external biometrician)". |
| Allocation concealment (selection bias) | Low risk | "Clusters were randomly assigned to study groups by a person affiliated to the study center in Hamburg, but not involved in the study (...)." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of personnel was not possible due to the nature of the study. Clusters were allocated to the different study groups and there was no evidence for an increased risk of contamination of clusters in the control group. Residents were not informed about the study, but might be aware of the study. The intervention was delivered to the nursing staff rather than the residents. We judged the risk for a performance bias to be low. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Physical restraint use was assessed through direct observation (...) by raters blinded to group allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates were comparable between the study groups and reasons for attrition were reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as planned |
Dever Fitzgerald 2016.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial (not registered, no study protocol published) Intervention period: not clearly reported Duration of follow‐up: 8 months (4 months baseline period, follow‐up data were collected 4 months after the end of the baseline period) Study period: not reported |
|
| Participants |
Country: Canada Setting: recruited from 26 different nursing homes (no further information reported) Participants:
Baseline characteristics:
|
|
| Interventions |
Intervention: fall risk assessment (providing information about residents' fall risk to nursing staff) Control: usual care |
|
| Outcomes | No primary outcome defined
|
|
| Notes | Funding: partly funded through a grant from the Saskatchewan Health Research Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly assigned to the control or experimental groups". No further information provided Personal communication with study authors: "Each participant was assigned to the control vs. experimental group via coin toss." |
| Allocation concealment (selection bias) | High risk | No information reported Personal communication with study authors: "No specific method/effort to conceal allocation was used." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of personnel was not possible as the same nurses cared for participants in both study groups and there was a risk of contamination. Although no pressure sensors were available for the participants allocated to the control group, a performance bias might be present. Personal communication with study authors: The participants were informed about the study, but the intervention was delivered to the nursing staff. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors were not blinded to group allocation. We judged risk of bias to be unclear since the assessment of some measures included a judgement of the outcome assessors not blinded to group allocation (e.g. whether a fixed table at the wheelchair was used as a restrictive measure or not). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Overall, our sample had an attrition rate of 17% (i.e. 22 participants passed away before the follow‐up period was completed and four discontinued for other reasons)." Attrition rates were comparable between studies and the reasons were reported. |
| Selective reporting (reporting bias) | Unclear risk | Not registered; no study protocol available. We had insufficient information to permit a judgement of ‘low risk’ or ‘high risk'. |
Evans 1997.
| Study characteristics | ||
| Methods |
Study design: cluster‐randomised controlled trial (not registered, no study protocol published) Intervention period: 6 months Duration of follow‐up: 12 months (follow‐up data were assessed six months after the intervention period) Study period: not reported |
|
| Participants |
Country: USA Setting: three nursing homes (180 to 269 beds) in an urban region in the area of Philadelphia, geographically distant, comparable to the national profile in resident demographics and functional status, with comparable restraint policies Participants/clusters:
Baseline characteristics:
|
|
| Interventions |
Intervention 1: educational intervention plus consultation Intervention 2: educational intervention Control: usual care |
|
| Outcomes | No primary outcome defined
|
|
| Notes | Funding: grants from the National Institute on Aging (R01‐AG08324), the Alzheimer's Association, and the University of Pennsylvania Research Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Following baseline data collection, interventions were randomized to site using the sealed envelope technique". Important differences between groups (intervention group 1 showed a statistically significant lower level of care dependency, control group had a statistically significant higher level of physical restraints at baseline), mainly due to chance since only one cluster was randomised to each group. |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of personnel was not possible due to the nature of the study. Nursing homes were allocated to the study groups and there was no evidence for an increased risk of contamination of clusters in the control group. No information about blinding of the participants was reported, but the intervention was delivered to the nursing staff. We judged the risk for a performance bias to be low. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Observer nurses were unaware of the exact study design, interventions, and nursing home's group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "The sites did not differ in the sample proportion that survived (P = 0.14). Attrition (average 28%) mainly by death, reflects the populations advanced age and frailty." |
| Selective reporting (reporting bias) | Unclear risk | Not registered; no study protocol available. We had insufficient information to permit judgement of ‘low risk’ or ‘high risk'. |
Gulpers 2011.
| Study characteristics | ||
| Methods |
Study design: clustered non‐randomised controlled clinical trial (NL2023, original registration number NTR2140, retrospectively registered) Intervention period: 7 months (starting one month after baseline assessment) Duration of follow‐up: 8 months follow‐up (follow‐up data were assessed after the intervention period) Study period: not reported |
|
| Participants |
Country: The Netherlands Setting: nursing homes from four nursing home associations located in three regions in the Netherlands Participants/clusters:
Baseline characteristics:
|
|
| Interventions |
Intervention: promotion of institutional policy change that discourages use of belt restraint, nursing home staff education, consultation by a nurse specialist aimed at nursing home staff, and availability of alternative devices Control: receiving care as usual |
|
| Outcomes |
Primary: Participants with at least one belt restraint Secondary:
|
|
| Notes |
Funding: Netherlands Organization for Health Research and Development Grant (No. 8140.0006) Cluster effect was not incorporated in the analysis (risk of unit‐of‐analysis error). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Since no randomization took place, allocation was based on avoidance of contamination bias. Overlap of nursing home staff between the intervention and control wards was averted. In addition, based on the geographical location of the participating wards, wards from each of the four nursing associations that were situated closely together were allocated to the same group." |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of personnel was not possible due to the nature of the study. Clusters from different regions were allocated to the study groups and there was no evidence for an increased risk of contamination of clusters in the control group. No information about blinding of the participants was reported, but the intervention was delivered to the nursing staff. We judged the risk for a performance bias to be low. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "A single trained observer, blinded to group assignment, recorded belt use as present or absent four times during a 24‐hour period (morning, afternoon, evening, and night). The day and timing of measurements was unannounced to prevent any temporary removal of belts." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates were comparable between the study groups and reasons for attrition were reported. |
| Selective reporting (reporting bias) | Unclear risk | The study was retrospectively registered; the study protocol was published after the trial was completed. We had insufficient information to permit judgement of ‘low risk’ or ‘high risk'. |
Huizing 2009.
| Study characteristics | ||
| Methods |
Study design: cluster‐randomised controlled trial (not registered) Intervention period: 10 months Duration of follow‐up: 10 months (follow‐up data were assessed after the intervention period) Study period: not reported |
|
| Participants |
Country: The Netherlands Setting: 14 gerontopsychiatric nursing home wards from seven nursing homes; region: Kerkrade, Landgraaf and Bocholtz Participants/clusters:
Baseline characteristics:
|
|
| Interventions |
Intervention: educational intervention plus consultation Control: usual care |
|
| Outcomes |
Primary outcome: participants with at least one PR use Secondary outcome: restraint intensity |
|
| Notes |
Funding: MeanderGroep Zuid‐Limburg, the Province of Limburg and Maastricht University Cluster effect was not incorporated in the analysis (risk of unit‐of‐analysis error). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The 15 psychogeriatric wards were assigned at random to educational intervention (8 experimental wards) or control status (7 control wards). To avoid 'cross contaminating' the intervention, information for nursing staff about the study’s aim and design was initially limited." Additional information from the study authors: "Cards representing all 15 wards were put in a bag. An independent person took out the cards (first a card for the experimental group, second a card for the control group, third a card for the experimental group, and so on)." |
| Allocation concealment (selection bias) | Low risk | No information reported. Additional information from the study authors: "The randomization was performed by an independent person (a colleague not involved in the study). Furthermore, the experimental wards were informed about their status and requested to be careful with this information with regard to the control wards." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of personnel was not possible due to the nature of the study. Clusters were allocated to the study groups and there was no evidence for an increased risk of contamination of clusters in the control group. No information about blinding of the participants was reported, but the intervention was delivered to the nursing staff. We judged the risk for a performance bias to be low. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Trained observers (n = 11) blinded to the experimental and control conditions measured the use of physical restraints on four separate occasions (morning, afternoon, evening, and night) over 24 hours. The same observer made observations for each ward; visiting dates remained unannounced to discourage artificial removal of restraints by staff." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | One cluster out of 15 clusters was lost to follow‐up, leading to a higher attrition rate in the intervention group. Reasons for attrition were reported and comparable between groups. |
| Selective reporting (reporting bias) | Unclear risk | Not registered; no study protocol available. We had insufficient information to permit judgement of ‘low risk’ or ‘high risk'. |
Koczy 2011.
| Study characteristics | ||
| Methods |
Study design: cluster‐randomised controlled trial (not registered, study protocol published in German) Intervention period: 3 months Duration of follow‐up: 3 months (follow‐up data were assessed after the intervention period) Study period: 2004‐2006 |
|
| Participants |
Country: Germany Setting: nursing homes in different regions (no further information reported) Participants/clusters:
Baseline characteristics: (participants with physical restraint use at baseline that completed the study):
|
|
| Interventions |
Intervention group: Educational intervention plus support and provision of technical aids Control group: usual care |
|
| Outcomes |
Primary:
Secondary:
|
|
| Notes |
Funding: The study was funded by the German Ministry of Family, Seniors, Women and Youth. Two researcher (DB, KR) received a personal Grant from the Robert Bosch Foundation, Germany. Several industry companies provided material such as hip protectors (Rölke Pharma, Germany), sensor mats (WinkerTec, Germany), antislip socks (Vitaness, Germany), and a bed for practical exercise (Völker, Germany). They had no role in the planning or conduct of the study. Cluster effect was not incorporated in the analysis (risk of unit‐of‐analysis error). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "An independent organization performed randomization according to [the] nursing home after baseline assessment of all restrained residents." Information from study authors: "Randomisation was conducted by the Institute of Biometry and Medical Documentation, Ulm University. They used computer aided random numbers". |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment was reported or delivered by the study authors. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of personnel was not possible due to the nature of the study. Clusters were allocated to the study groups and there was no evidence for an increased risk of contamination of clusters in the control group. No information about blinding of the participants was reported, but the intervention was delivered to the nursing staff. We judged the risk for a performance bias to be low. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "A staff member in the participating homes completed the daily documentation for each resident." Outcome assessors were not blinded to group allocation. We judged risk of bias to be unclear since the assessment of some measures included a judgement of the outcome assessors not blinded to group allocation (e.g. whether a fixed table at the wheelchair was used as a restrictive measure or not). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates of the participants with physical restraint use at baseline were comparable between the study groups and reasons for attrition were reported. |
| Selective reporting (reporting bias) | High risk | The primary outcome defined in the study protocol (published in German) was changed in the publication reporting the study results. |
Köpke 2012.
| Study characteristics | ||
| Methods |
Study design: cluster‐randomised controlled trial (ISRCTN34974819) Intervention period: 6 months Duration of follow‐up: 6 months (follow‐up data were assessed after the intervention period) Study period: February 2009 to April 2010 |
|
| Participants |
Country: Germany Setting: nursing homes in Hamburg (northern Germany) and the region of Witten (Western Germany) Participants/clusters:
Baseline characteristics:
|
|
| Interventions |
Intervention: multi‐component intervention (addressed main components: attitudes, subjective norms, and perceived behavioural control), full and concise versions of the guideline, education for all nursing staff, explicit endorsement of nursing home leaders, education and structured support of key nurses in each cluster, and support material Control: head nurses received written information about the use of physical restraints and methods to avoid physical restraints, using three 12‐ to 24‐page brochures; also the topic of physical restraints was discussed during a short presentation by one of the researchers. |
|
| Outcomes |
Primary: percentage of residents with at least 1 physical restraint Secondary:
|
|
| Notes | Funding: grant from the German Federal Ministry of Education and Research within the Nursing Research Network Northern Germany (projects 01GT0606 and 01GT0608) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomization lists were used for allocation of clusters in blocks of 4, 6, and 8 nursing homes. Randomization was stratified by region, i.e. Hamburg and Witten." |
| Allocation concealment (selection bias) | Low risk | "Allocation of clusters was performed by an external person not involved in the study, who informed cluster representatives about group assignment." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of personnel was not possible due to the nature of the study. Clusters were allocated to the study groups and there was no evidence for an increased risk of contamination of clusters in the control group. No information about blinding of the participants was reported, but the intervention was delivered to the nursing staff. We judged the risk for a performance bias to be low. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Data on the prevalence of physical restraint use at baseline were obtained by trained external investigators before randomization through direct observation at 3 time points during 1 day (morning, noon, evening)." "Data on prevalence of physical restraint use at the 3‐ and 6‐month follow‐ups were assessed similarly to baseline by external investigators blinded to cluster group allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates were comparable between the study groups and reasons for attrition were reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as planned. |
Pellfolk 2010.
| Study characteristics | ||
| Methods |
Study design: cluster‐randomised controlled trial (ISRCTN36604462, retrospectively registered) Intervention period: 6 months Duration of follow‐up: 6 months (follow‐up data were assessed after the intervention period) Study period: not reported |
|
| Participants |
Country: Sweden Setting: group dwelling units for people with dementia, designed as home‐like environments (six to eight residents with dementia), including private rooms and communal dining and living rooms. The units were generally locked. Organised as single or multiple units, or integrated into nursing homes. Staffing levels were higher than in other long‐term care facilities (staff resident ratio was 0.78 ± 0.12 in the intervention and 0.83 ± 0.18 in the control group). Participants/clusters:
Baseline characteristics:
|
|
| Interventions |
Intervention: education programme for nursing staff (registered nurses, licenced practical nurses, and nurse’s aides) Control: usual care |
|
| Outcomes | No primary outcome defined (prevalence of physical restraints was used in the sample size calculation)
|
|
| Notes |
Funding: grants from the Lions Research Foundation for Age‐related Diseases, King Gustaf V’s and Queen Victoria’s Freemason Foundation, the Field Research Center for the Elderly in Västerbotten, and the Swedish Research Council (Grant K2005‐27‐VX‐15357‐01A) Cluster effect was only incorporated in the analysis of the primary outcome; risk of unit‐of‐analysis error for other outcomes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization was based on a lottery system using identification codes. When more than one unit was located in the same facility, all were allocated to the same group to avoid contamination between units." |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of personnel was not possible due to the nature of the study. Clusters were allocated to the study groups and there was no evidence for an increased risk of contamination of clusters in the control group. No information about blinding of the participants was reported, but the intervention was delivered to the nursing staff. We judged the risk for a performance bias to be low. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Nursing staff registered the type of restraint, the reason for its use, and time spent in restraint daily on a form for 3 weeks before and after the intervention." Outcome data were assessed by unblinded nursing staff. We judged risk of bias to be unclear since the assessment of some of the measures included a judgement of the outcome assessors not blinded to group allocation (e.g. whether a fixed table at the wheelchair was used as a restrictive measure or not). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No cluster was lost to follow‐up, attrition rate differed between the study groups (22% in the intervention group and 14% in the control group) and no reasons for attrition were reported. |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered; no published study protocol available. We had insufficient information to permit judgement of ‘low risk’ or ‘high risk'. |
Testad 2005.
| Study characteristics | ||
| Methods |
Study design: cluster‐randomised controlled trial (not registered, no study protocol published) Intervention period: 6 months Duration of follow‐up: 6 months (follow‐up data were assessed after the intervention period) Study period: not reported |
|
| Participants |
Country: Norway Setting: four public nursing and residential homes in Stavanger. Additional information from the study authors: nursing homes were representative of all Norwegian nursing homes in terms of size and organisation. Participants/clusters:
Baseline characteristics:
|
|
| Interventions |
Intervention: educational intervention plus guidance Control: usual care |
|
| Outcomes | Both outcomes were defined as primary outcomes by the authors.
|
|
| Notes |
Funding: Norwegian Research Council Cluster effect was not incorporated in the analysis (risk of unit‐of‐analysis error). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The nursing homes were randomly assigned to the treatment intervention or control condition, two in each group, after stratification for size." "The two groups were similar with respect to age, CDR and gender distribution and proportion of subjects using medication for physical disease." Information provided by the study authors: sealed envelopes were used. |
| Allocation concealment (selection bias) | High risk | Not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of personnel was not possible due to the nature of the study. Clusters were allocated to the study groups and there was no evidence for an increased risk of contamination of clusters in the control group. No information about blinding of the participants was reported, but the intervention was delivered to the nursing staff. We judged the risk for a performance bias to be low. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Data were collected immediately before and after the intervention period by a trained rater who was blind to the study hypothesis and to treatment allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All patients in the intervention group and 87 in the control group (nine had died or moved to another facility) were assessed at follow‐up." It is unlikely that the higher attrition rate in the control group was associated with the intervention, so we judged risk of bias to be low. |
| Selective reporting (reporting bias) | Unclear risk | Not registered; no published study protocol available. We had insufficient information to permit judgement of ‘low risk’ or ‘high risk'. |
Testad 2010.
| Study characteristics | ||
| Methods |
Study design: cluster‐randomised controlled trial (not registered, no study protocol published) Intervention period: 6 months Duration of follow‐up: 12 months (follow‐up data were assessed 6 months after the intervention period) Study period: 2003 to 2004 |
|
| Participants |
Country: Norway Setting: four nursing homes, region Rogaland; all seven nursing homes in the region were invited and four agreed to participate (two small facilities (17 and 21 residents) and two larger facilities (81 and 92 residents)). Participants/clusters:
Baseline characteristics:
|
|
| Interventions |
Intervention: educational intervention plus guidance Control: usual care |
|
| Outcomes |
|
|
| Notes |
Funding: Norwegian Research Council Cluster effect was not incorporated in the analysis (risk of unit‐of‐analysis error). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "(...) we randomly assigned subjects on home level. One small and one larger home were randomly allocated to either intervention or control condition (...)." No further information reported or given by the study authors on request. There were statistically significant differences between the study groups at baseline (proportion of participants with physical restraints, challenging behaviour, proportion of participants with antipsychotics). However, these differences may have been occurred by chance, since the number of clusters per group was small. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of personnel was not possible due to the nature of the study. Clusters were allocated to the study groups and there was no evidence for an increased risk of contamination of clusters in the control group. No information about blinding of the participants was reported, but the intervention was delivered to the nursing staff. We judged the risk for a performance bias to be low. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "(...) rater‐blinded randomised‐controlled trial (...)"; "(...) blinded assessment procedure (...)". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition rates were comparable between the study groups, but the attrition rate was approximately 43%. Reasons for attrition were reported. |
| Selective reporting (reporting bias) | Unclear risk | Not registered; no published study protocol available. We had insufficient information to permit judgement of ‘low risk’ or ‘high risk'. |
Testad 2016.
| Study characteristics | ||
| Methods |
Study design: cluster‐randomised controlled trial (NCT01715506, retrospectively registered) Intervention period: 7 months Duration of follow‐up: 7 months (follow‐up data were assessed after the intervention period) Study duration: January 2011 and May 2013 |
|
| Participants |
Country: Norway Setting: 24 care homes within the Western Norway Regional Health Authority. The Western Norway Regional Health Authority consists of three counties and four health trusts, with a total of 83 care homes. All homes in the geographical area were invited to participate following a list in randomised order. Recruitment continued until six care homes were included from each of the four health trusts. Participants/clusters:
Baseline characteristics:
|
|
| Interventions |
Intervention: "Trust Before Restraint"‐Programme Control: usual care |
|
| Outcomes |
Primary: use of restraint Secondary: agitation, use of psychotropic drugs |
|
| Notes |
Funding: Norwegian Research Council "The effect of clustering was taken into account and adjusted for [when] if the ICC had a value greater than 5%." ICC was lower than 5% and the analysis was not adjusted for clustering (risk of unit‐of‐analysis error). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Care homes were randomized after recruitment to a 7‐month educational intervention or treatment as usual." Method not mentioned There were statistically significant differences between the study groups at baseline (ADL score, challenging behaviour, NPI sum score) and some differences in the prevalence of physical restraint use indicating inadequate randomisation and/or allocation concealment. We had insufficient information to permit judgement of ‘high risk'. |
| Allocation concealment (selection bias) | Unclear risk | No information reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Treatment allocation was revealed to the facilitating teams by the principal investigator, when baseline was completed." Blinding of personnel was not possible due to the nature of the study. Clusters were allocated to the study groups and there was no evidence for an increased risk of contamination of clusters in the control group. No information about blinding of the participants was reported, but the intervention was delivered to the nursing staff. We judged the risk for a performance bias to be low. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All data in the 24 care homes were collected within 1 week by research assistants blind to the study." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No cluster was lost to follow‐up; attrition rate differed slightly between the study groups (30% in the intervention group and 26% in the control group) and no reasons for attrition were reported. |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered; no published study protocol available. We had insufficient information to permit judgement of ‘low risk’ or ‘high risk'. |
BARS = Brief Agitation Rating Scale; CDR = Clinical Dementia Rating Scale; FAST = Functional Assessment Staging; ICC = intracluster correlation coefficient; MMSE = Mini‐Mental State Examination; PR = physical restraints; SD = standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Branitzki 2005 | Wrong study design |
| Capezuti 1998 | Primary outcome not physical restraints |
| Capezuti 2007 | Wrong study design |
| Chan 2022 | Wrong study design |
| Chang 2016 | Wrong study design |
| Choi 2009 | Wrong study design |
| Dewey 2000 | Wrong study design |
| Ejaz 1994 | Wrong study design |
| Enns 2014 | Wrong setting |
| Evans 2002 | Wrong study design |
| Frank 1996 | Wrong study design |
| Healey 2008 | Wrong study design |
| Kong 2017 | Wrong outcome (use of physical restraint was not assessed) |
| Kotynia‐English 2005 | Wrong intervention |
| Levine 1995 | Wrong study design |
| Levine 2000 | Wrong study design |
| McCallion 1999 | Wrong intervention |
| Mengelers 2022 | Wrong study design |
| Moretz 1995 | Wrong study design |
| Patterson 1995 | Wrong study design |
| Ralphs‐Thibodeau 2006 | Wrong study design |
| Ramos Cordero 2015 | Wrong study design |
| Rovner 1996 | Wrong intervention |
| Schnelle 1992 | Wrong study design |
| Si 1999 | Wrong setting |
| Steinert 2009 | Wrong study design |
| Toseland 1997 | Wrong intervention |
| Vandervelde 2021 | Wrong study design |
| Verbeek 2014 | Wrong intervention |
| Williams 2011 | Wrong study design |
| Woods 2005 | Wrong intervention |
| Zwijsen 2014 | Wrong intervention |
Differences between protocol and review
With the first update, the title of the review was changed from "Interventions for preventing and reducing the use of physical restraints in long‐term geriatric care" to "Interventions for preventing and reducing the use of physical restraints for older people in all long‐term care settings".
We added the number of falls or fall‐related injuries as a secondary outcome; in the protocol, these outcomes were included as adverse events, but not mentioned in the list of outcomes.
In the protocol, no statistical model for the meta‐analysis was defined. We used a random‐effects model since we found clinical diversity of the interventions and statistical heterogeneity.
Contributions of authors
GM and SK initially planned the study; RM, SK, and GM wrote the study protocol. In the first review, RM and TR selected studies for inclusion/exclusion, evaluated the methodological quality of included trials, and extracted data. RM and GM interpreted the study data. RM corresponded with the study authors and wrote the drafts of the review with major contributions by GM. RM and GM started collecting individual patient data from the study authors. All authors contributed to all drafts of the review.
In the review update, RM and TR selected studies for inclusion/exclusion; RM and CM evaluated the methodological quality of included trials and extracted data. RM and GM interpreted the study data. RM corresponded with the study authors and wrote the drafts of the review. All authors contributed to all drafts of the review.
Sources of support
Internal sources
No sources of support provided
External sources
-
NIHR, UK
This update was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health
Declarations of interest
Some authors (RM, SK, and GM) were involved in trials that were included in this review. All trials were funded by national grant agencies. No review author was involved in the data extraction of any trials in which they were or are involved.
Ralph Möhler: none known
Tabja Richter: none known
Sascha Köpke: none known
Gabriele Meyer: none known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Abraham 2019 {published data only}
- Abraham J, Bake M, Berger-Höger B, Köpke S, Kupfer R, et al. Process evaluation of a multicomponent intervention to prevent physical restraints in nursing homes (IMPRINT): A mixed methods study. Journal of Advanced Nursing 2021;77(3):1465-77. [DOI] [PubMed] [Google Scholar]
- Abraham J, Kupfer R, Behncke A, Berger-Höger B, Icks A, Haastert B, et al. Implementation of a multicomponent intervention to prevent physical restraints in nursing homes (IMPRINT): A pragmatic cluster randomized controlled trial. International Journal of Nursing Studies 2019;96:27-34. [DOI] [PubMed] [Google Scholar]
- Abraham J, Möhler R, Henkel A, Kupfer R, Icks A, et al. Implementation of a multicomponent intervention to prevent physical restraints in nursing home residents (IMPRINT): study protocol for a cluster-randomised controlled trial. BMC Geriatrics 2015;15:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02341898. Implementation of a multicomponent intervention to prevent physical restraints in nursing home residents (IMPRINT). https://www.clinicaltrials.gov/ct2/show/NCT02341898 (first received 19 January 2015). [DOI] [PMC free article] [PubMed]
- Nordhausen T, Abraham J, Kupfer R, Köpke S, Meyer G, Möhler R. Physical restraints from the perspective of advocates of nursing home residents - a qualitative Study [Freiheitseinschränkung aus Sicht der Interessenvertretungen von Pflegeheimbewohnerinnen und -bewohnern − eine qualitative Studie]. Pflege 2019;32(2):147-56. [DOI] [PubMed] [Google Scholar]
Dever Fitzgerald 2016 {published and unpublished data}
- Denver Fitzgerald TG, Hadjistavropoulos T, MacNab YC. Caregiver fear of falling and functional ability among seniors residing in long-term care facilities. Gerontology 2009;55(4):460-7. [DOI] [PubMed] [Google Scholar]
- Dever Fitzgerald T, Hadjistavropoulos T, Williams J, Lix L, Zahir S, Alfano D, Scudds R. The impact of fall risk assessment on nurse fears, patient falls, and functional ability in long-term care. Disability and Rehabilitation 2016;38(11):1041-52. [DOI] [PubMed] [Google Scholar]
Evans 1997 {published and unpublished data}
- Evans LK, Strumpf NE, Allen-Taylor SL, Capezuti E, Maislin G, Jacobsen B. A clinical trial to reduce restraints in nursing homes. Journal of the American Geriatrics Society 1997;45(6):675-81. [DOI] [PubMed] [Google Scholar]
- Siegler EL, Capezuti E, Maislin G, Baumgarten M, Evans L, Strumpf N. Effects of a restraint reduction intervention and OBRA '87 regulations on psychoactive drug use in nursing homes. Journal of the American Geriatrics Society 1997;45(7):791-6. [DOI] [PubMed] [Google Scholar]
Gulpers 2011 {published data only}
- Bleijlevens MH, Gulpers MJ, Capezuti E, Rossum E, Hamers JP. Process evaluation of a multicomponent intervention program (EXBELT) to reduce belt restraints in nursing homes. Journal of the American Medical Directors Association 2013;14(8):599-604. [DOI] [PubMed] [Google Scholar]
- Gulpers MJ, Bleijlevens MH, Ambergen T, Capezuti E, Rossum E, Hamers JP. Belt restraint reduction in nursing homes: effects of a multicomponent intervention program. Journal of the American Geriatrics Society 2011;59(11):2029-36. [DOI] [PubMed] [Google Scholar]
- Gulpers MJ, Bleijlevens MH, Ambergen T, Capezuti E, Rossum E, Hamers JP. Reduction of belt restraint use: long-term effects of the EXBELT intervention. Journal of the American Geriatrics Society 2013;61(1):107-12. [DOI] [PubMed] [Google Scholar]
- Gulpers MJ, Bleijlevens MH, Capezuti E, Rossum E, Ambergen T, Hamers JP. Preventing belt restraint use in newly admitted residents in nursing homes: a quasi-experimental study. International Journal of Nursing Studies 2012;49(12):1473-9. [DOI] [PubMed] [Google Scholar]
- Gulpers MJ, Bleijlevens MH, Rossum E, Capezuti E, Hamers JP. Belt restraint reduction in nursing homes: design of a quasi-experimental study. BMC Geriatrics 2010;10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamers JP, Gulpers MJ. Reducing physical restraints in nursing homes:results of a pilot study. Journal of Nutrition, Health & Aging 2009;13(Suppl 13):S17. [Google Scholar]
- NTR2140. Belt restraint reduction in nursing homes: effects of a multicomponent intervention program. https://trialsearch.who.int/Trial2.aspx?TrialID=NTR2140 (first received 11 December 2009). [DOI] [PubMed]
Huizing 2009 {published and unpublished data}
- Huizing A, Hamers J, Gulpers M, Berger M. A cluster-randomized trial of an educational intervention to reduce the use of physical restraints with psychogeriatric nursing home residents. Journal of the American Geriatrics Society 2009;57(7):1138-48. [DOI] [PubMed] [Google Scholar]
- Huizing A, Hamers J, Gulpers M, Berger M. Preventing the use of physical restraints on residents newly admitted to psycho-geriatric nursing home wards. International Journal of Nursing Studies 2009;46(4):459-69. [DOI] [PubMed] [Google Scholar]
- Huizing A, Hamers J, Gulpers M, Berger M. Short-term effects of an educational intervention on physical restraint use: a cluster randomized trial. BMC Geriatrics 2006;26:6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koczy 2011 {published data only}
- Koczy P, Becker C, Rapp K, Klie T, Beische D, Büchele G, Kleiner A, Guerra V, Rissmann U, Kurrle S, Bredthauer D. Effectiveness of a Multifactorial Intervention to Reduce Physical Restraints in Nursing Homes Residents. Journal of the American Geriatrics Society 2011;59(2):333-9. [DOI] [PubMed] [Google Scholar]
- Koczy P, Klie T, Kron M, Bredthauer D, Rissmann U, Branitzki S, Guerra V, Klein A, Pfundstein T, Nikolaus T, Sander S, Becker C. Effectiveness of a multifactorial intervention to reduce physical restraints in nursing home residents with dementia [Effektivität einer multifaktoriellen Intervention zur Reduktion von körpernaher Fixierung bei demenzerkrankten Heimbewohnern]. Zeitschrift für Gerontologie und Geriatrie 2005;38(1):33-9. [DOI] [PubMed] [Google Scholar]
Köpke 2012 {published data only}
- Gerlach A, Köpke S, Haut A, Meyer G. Process evaluation of the implementation of a guideline-based complex Intervention in nursing homes [Prozessevaluation der Implementierung einer Leitlinien-gestützten komplexen Intervention in Alten- und Pflegeheim]. In: 13th Annual Meeting of the German Network for Evidence-Based Medicine March 15th- 17th, Hamburg. 2012.
- Haut A, Köpke S, Gerlach A, Mühlhauser I, Haastert B, Meyer G. Evaluation of an evidence-based guidance on the reduction of physical restraints in nursing homes: a cluster-randomised controlled trial. BMC Geriatrics 2009;9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köpke S, Mühlhauser I, Gerlach A, Haut A, Haastert B, Möhler R, Meyer G. Effect of a guideline-based multicomponent intervention on use of physical restraints in nursing homes: a randomized controlled trial. Journal of the American Medical Association 2012;307(20):2177-84. [DOI] [PubMed] [Google Scholar]
Pellfolk 2010 {published data only}
- ISRCTN36604462. Effects of a restraint minimization educational programme on physical restraints use, staff knowledge, attitudes, and perceived job strain. https://www.isrctn.com/ISRCTN36604462 (first received 18 April 2008).
- Pellfolk T, Gustafson Y, Bucht G, Karlsson S. Effects of a Restraint Minimization Program on Staff Knowledge, Attitudes, and Practice: A Cluster Randomized Trial. Journal of the American Geriatrics Society 2010;58:62-9. [DOI] [PubMed] [Google Scholar]
Testad 2005 {published and unpublished data}
- Testad I, Aasland A, Aarsland D. The effect of staff training on the use of restraint in dementia: a single-blind randomised controlled trial. International Journal of Geriatric Psychiatry 2005;20:587-90. [DOI] [PubMed] [Google Scholar]
Testad 2010 {published and unpublished data}
- Testad I, Ballard C, Brønnick K, Aarsland D. The effect of staff training on agitation and use of restraint in nursing home residents with dementia: a single-blind, randomized controlled trial. Journal of Clinical Psychiatry 2010;71(1):80-6. [DOI] [PubMed] [Google Scholar]
Testad 2016 {published data only}
- Jacobsen FF, Mekki TE, Førland O, Folkestad B, Kirkevold Ø, Skår R, et al. A mixed method study of an education intervention to reduce use of restraint and implement person-centered dementia care in nursing homes. BMC Nursing 2017;16:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekki TE, Øye C, Kristensen B, Dahl H, Haaland A, Nordin KA, et al. The inter-play between facilitation and context in the promoting action on research implementation in health services framework: A qualitative exploratory implementation study embedded in a cluster randomized controlled trial to reduce restraint in nursing homes.. 2017 Journal of Advanced Nursing;73(11):2622-32. [DOI] [PubMed] [Google Scholar]
- NCT01715506. Continuing education in nursing home dementia care (MEDCED). https://clinicaltrials.gov/ct2/show/NCT01715506 (first received 29 October 2012).
- Testad I, Mekki TE, Førland O, Øye C, Tveit EM, Jacobsen F, Kirkevold Ø. Modeling and evaluating evidence-based continuing education program in nursing home dementia care (MEDCED)--training of care home staff to reduce use of restraint in care home residents with dementia. A cluster randomized controlled trial. International Journal of Geriatric Psychiatry 2016;31(1):24-32. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Branitzki 2005 {published data only}
- Branitzki S, Koczy P. [ReduFix- a study of reducing physical restraint: preventing risk of injury]. Pflege Zeitschrift 2005;58(5):310-3. [PubMed] [Google Scholar]
Capezuti 1998 {published data only}
- Capezuti E, Strumpf NE, Evans LK, Grisso JA, Maislin G. The relationship between physical restraint removal and falls and injuries among nursing home residents. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 1998;53(1):M47-52. [DOI] [PubMed] [Google Scholar]
Capezuti 2007 {published data only}
- Capezuti E, Wagner L, Brush B, Boltz M, Renz S, Talerico K. Consequences of an intervention to reduce restrictive side rail use in nursing homes. Journal of the American Geriatrics Society 2007;55:334-41. [DOI] [PubMed] [Google Scholar]
Chan 2022 {published data only}
- Chan HY, Ho FK, Chui KC, Wong BP, Chui MY, Zhao Y, et al. Evaluation of a multicomponent restraint reduction intervention in care homes. Collegian 2022 July 6 [Epub ahead of print]. [DOI: 10.1016/j.colegn.2022.06.009] [DOI]
Chang 2016 {published data only}
- Chang YY, Yu HH, Loh el-W, Chang LY. The Efficacy of an In-Service Education Program Designed to Enhance the Effectiveness of Physical Restraints. Journal of Nursing Research 2016;24(1):79-86. [DOI] [PubMed] [Google Scholar]
Choi 2009 {published data only}
- Choi K, Kim J. Effects of an educational program for the reduction of physical restraint use by caregivers in geriatric hospitals. Journal of Korean Academy of Nursing 2009;39(6):769-80. [DOI] [PubMed] [Google Scholar]
Dewey 2000 {published data only}
- Dewey K, Brill C. Decrease in restraint use in a study of a geropsychiatric unit. Journal of Psychosocial Nursing and Mental Health Services 2000;38(10):14-8, 44-5. [DOI] [PubMed] [Google Scholar]
Ejaz 1994 {published data only}
- Ejaz FK, Jones JA, Rose MS. Falls among nursing home residents: an examination of incident reports before and after restraint reduction programs. Journal of the American Geriatrics Society 1994;42(9):960-4. [DOI] [PubMed] [Google Scholar]
Enns 2014 {published data only}
- Enns E, Rhemtulla R, Ewa V, Fruetel K, Holroyd-Leduc JM. A controlled quality improvement trial to reduce the use of physical restraints in older hospitalized adults. Journal of the American Geriatrics Society 2014;62(3):541-5. [DOI] [PubMed] [Google Scholar]
Evans 2002 {published data only}
- Evans D, Wood J, Lambert L. A review of physical restraint minimization in the acute and residential care settings. Journal of Advanced Nursing 2002;40:616-25. [DOI] [PubMed] [Google Scholar]
Frank 1996 {published data only}
- Frank C, Hodgetts G, Puxty J. Safety and efficacy of physical restraints for the elderly. Review of the evidence. Canadian Family Physician 1996;42:2402-9. [PMC free article] [PubMed] [Google Scholar]
Healey 2008 {published data only}
- Healey F, Oliver D, Milne A, Connelly J. The effect of bedrails on falls and injury: a systematic review of clinical studies. Age and Ageing 2008;37:368-78. [DOI] [PubMed] [Google Scholar]
Kong 2017 {published data only}
- Kong EH, Song E, Evans LK. Effects of a Multicomponent Restraint Reduction Program for Korean Nursing Home Staff. Journal of Nursing Scholarship 2017;49(3):325-35. [DOI] [PubMed] [Google Scholar]
Kotynia‐English 2005 {published data only}
- Kotynia-English R, McGowan H, Almeida OP. A randomized trial of early psychiatric intervention in residential care: impact on health outcomes. International Psychogeriatrics 2005;17(3):475-85. [DOI] [PubMed] [Google Scholar]
Levine 1995 {published data only}
- Levine JM, Marchello V, Totolos E. Progress toward a restraint-free environment in a large academic nursing facility. Journal of the American Geriatrics Society 1995;43(8):914-8. [DOI] [PubMed] [Google Scholar]
Levine 2000 {published data only}
- Levine JM, Hammond M, Marchello V, Breuer B. Changes in bedrail prevalence during a bedrails-reduction initiative. Journal of the American Medical Directors Association 2000;1(1):34-6. [PubMed] [Google Scholar]
McCallion 1999 {published data only}
- McCallion P, Toseland RW, Freeman K. An evaluation of a family visit education program. Journal of the American Geriatrics Society 1999;47(2):203-14. [DOI] [PubMed] [Google Scholar]
Mengelers 2022 {published data only}
- Mengelers AM, Bleijlevens MH, Verbeek H, Capezuti E, Hamers JP. A quasi-experimental study on prevention and reductionof involuntary treatment at home (PRITAH) in people with dementia. Journal of Clinical Nursing 2022;31(21-22):3250-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengelers AMHJ, Bleijlevens MHC, Verbeek H, Moermans VRA, Capezuti E, Hamers JPH. Prevention and reduction of involuntary treatment at home: A feasibility study of the PRITAH intervention. Geriatric Nursing 2020;41(5):536-43. [DOI] [PubMed] [Google Scholar]
Moretz 1995 {published data only}
- Moretz C, Dommel A, Deluca K. Untied: a safe alternative to restraints. Medsurg Nursing 1995;4(2):128-32. [PubMed] [Google Scholar]
Patterson 1995 {published data only}
- Patterson JE, Strumpf NE, Evans LK. Nursing consultation to reduce restraints in a nursing home. Clinical Nurse Specialist 1995;9(4):231-5. [DOI] [PubMed] [Google Scholar]
Ralphs‐Thibodeau 2006 {published data only}
- Ralphs-Thibodeau S, Knoefel F, Benjamin K, Leclerc A, Pisterman S, Sohmer J, et al. Patient choice: an influencing factor on policy-related research to decrease bedrail use as physical restraint. Worldviews Evid Based Nurs 2006;3(1):31-9. [DOI] [PubMed] [Google Scholar]
Ramos Cordero 2015 {published data only}
- Ramos Cordero P, Lopez Trigo JA, Maillo Pedraz H, Paz Rubio JM, Interdisciplinary Committee on Restraints Spanish Geriatrics and Gerontology Society. Physical and pharmacological restraints in geriatric and gerontology services and centers [Sujeciones mecánicas y farmacológicas en servicios y centrosgeriátricos y gerontológicos]. Revista Española de Geriatría y Gerontología 2015;50(1):35-8. [DOI] [PubMed] [Google Scholar]
Rovner 1996 {published data only}
- Rovner BW, Steele CD, Shmuely Y, Folstein MF. A randomized trial of dementia care in nursing homes. Journal of the American Geriatrics Society 1996;44(1):7-13. [DOI] [PubMed] [Google Scholar]
Schnelle 1992 {published data only}
- Schnelle JF, Newman DR, White M, Volner TR, Burnett J, Cronqvist A, et al. Reducing and managing restraints in long-term-care facilities. Journal of the American Geriatrics Society 1992;40(4):381-5. [DOI] [PubMed] [Google Scholar]
Si 1999 {published data only}
- Si M, Neufeld RR, Dunbar J. Removal of bedrails on a short-term nursing home rehabilitation unit. Gerontologist 1999;39(5):611-4. [DOI] [PubMed] [Google Scholar]
Steinert 2009 {published data only}
- Steinert T, Bohnet U, Flammer E, Lüchtenberg D, Eisele F. Effects of a training of power and balance on the use of mechanical restraint among in-patients with dementia [Effekte eines Kraft- und Bewegungstrainings auf die Fixierungshäufigkeit bei Demenzpatienten in der stationären gerontopsychiatrischen Versorgung]. Psychiatrische Praxis 2009;36(6):273-8. [DOI] [PubMed] [Google Scholar]
Toseland 1997 {published data only}
- Toseland RW, Diehl M, Freeman K, Manzanares T, Naleppa M, McCallion P. The impact of validation group therapy on nursing home residents with dementia. Journal of Applied Gerontology 1997;16(1):31-50. [Google Scholar]
Vandervelde 2021 {published data only}
- Vandervelde S, Scheepmans K, Milisen K, Achterberg T, Vlaeyen E, et al. Reducing the use of physical restraints in home care: development and feasibility testing of a multicomponent program to support the implementation of a guideline. BMC Geriatrics 2021;21(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verbeek 2014 {published data only}
- Verbeek H, Zwakhalen SM, Rossum E, Ambergen T, Kempen GI, Hamers JP. Effects of small-scale, home-like facilities in dementia care on residents' behavior, and use of physical restraints and psychotropic drugs: a quasi-experimental study. International Psychogeriatrics 2014;26(4):657-68. [DOI] [PubMed] [Google Scholar]
Williams 2011 {published data only}
- Williams DE, Grossett DL. Reduction of restraint of people with intellectual disabilities: an organizational behavior management (OBM) approach. Research in Developmental Disabilities 2011;32(6):2336-9. [DOI] [PubMed] [Google Scholar]
Woods 2005 {published data only}
- Woods DL, Craven RF, Whitney J. The effect of therapeutic touch on behavioral symptoms of persons with dementia. Alternative Therapies in Health and Medicine 2005;11(1):66-74. [PubMed] [Google Scholar]
Zwijsen 2014 {published data only}
- Zwijsen SA, Smalbrugge M, Eefsting JA, Twisk JW, Gerritsen DL, Pot AM, Hertogh CM. Coming to grips with challenging behavior: a cluster randomized controlled trial on the effects of a multidisciplinary care program for challenging behavior in dementia. Journal of the American Medical Directors Association 2014;15(7):5314.e1-10. [DOI] [PubMed] [Google Scholar]
Additional references
Abraham 2021
- Abraham J, Bake M, Berger-Höger B, Köpke S, Kupfer R, et al. Process evaluation of a multicomponent intervention to prevent physical restraints in nursing homes (IMPRINT): A mixed methods study. Journal of Advanced Nursing 2021;77(3):1465-77. [DOI] [PubMed] [Google Scholar]
Abraham 2022
- Abraham J, Hirt J, Richter C, Köpke S, Meyer G, Möhler R. Interventions for preventing and reducing the use of physicalrestraints of older people in general hospital settings. Cochrane Database of Systematic Reviews 2022, Issue 8. Art. No: CD012476. [DOI: 10.1002/14651858.CD012476.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ajzen 1991
- Ajzen I. The theory of planned behavior. Organizational Behavior and Human Decision Processes 1991;50(2):179-211. [Google Scholar]
Bleijlevens 2016
- Bleijlevens MH, Wagner LM, Capezuti E, Hamers JP, International Physical Restraint Workgroup. Physical restraints: consensus of a research definition using a modified delphi technique. Journal of the American Geriatrics Society 2016;64(11):2307-10. [DOI] [PubMed] [Google Scholar]
Brugnolli 2020
- Brugnolli A, Canzan F, Mortari L, Saiani L, Ambrosi E, Debiasi M. The effectiveness of educational training or multicomponent programs to prevent the use of physical restraints in nursing home settings: a systematic review and meta-analysis of experimental studies. International Journal of Environmental Research and Public Health 2020;17(18):6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castle 1998
- Castle N, Mor V. Physical restraint in nursing homes: a review of the literature since the nursing home reform act of 1987. Medical Care Research and Review 1998;55:139-70. [DOI] [PubMed] [Google Scholar]
Castle 2009
- Castle NG, Engberg J. The health consequences of using physical restraints in nursing homes. Medical Care 2009;47(11):1164-73. [DOI] [PubMed] [Google Scholar]
Centers for Medicare and Medicaid Services 2008
- U S Department of Health and Human Services. Freedom from unnecessary physical restraints: two decades of national progress in nursing home care. Washington DC: U. S. Department of Health and Human Services 2008.
De Vries 2004
- De Vries O, Ligthart G, Nikolaus T, on behalf of the participants of the European Academy of Medicine of Ageing-Course III. Differences in period prevalence of the use of physical restraints in elderly inpatients of European hospitals and nursing homes. Journals of Gerontology A, Biological Science Medical Science 2004;59:M922-3. [DOI] [PubMed] [Google Scholar]
Dielis‐van Houts 2004
- Dielis-van Houts A, Schuurmans M. The balance between safety, freedom of movement and use of restraints [Een balans tussen: Veiligheid, Vrijheid en Vrijheidsbeperking]. UMC-Utrecht, Utrecht 2004.
Engberg 2008
- Engberg J, Castle N, McCaffrey D. Physical restraint initiation in nursing homes and subsequent resident health. Gerontologist 2008;48:442-52. [DOI] [PubMed] [Google Scholar]
EQUATOR Network 2022
- EQUATOR Network. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. www.equator-network.org/reporting-guidelines/consort (accessed 1 December 2022).
Feng 2009
- Feng Z, Hirdes JP, Smith TF, Finne-Soveri H, Chi I, Du Pasquier JN, et al. Use of physical restraints and antipsychotic medications in nursing homes: a cross-national study. International Journal of Geriatric Psychiatry 2009;24(10):1110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernández Ibáñez 2020
- Fernández Ibáñez JM, Morales Ballesteros MD, Montiel Moreno M, Mora Sánchez E, Arias Arias Á, Redondo González O. Physical restraint use in relation to falls risk in a nursing home [Uso de sujeciones físicas en relación con el riesgo de caídas en una residencia de ancianos ]. Revista Española de Geriatría y Gerontología 2020;55(1):3-10. [DOI] [PubMed] [Google Scholar]
Flaherty 2004
- Flaherty J. Zero tolerance for physical restraints: difficult but not impossible. Journal of Gerontology 2004;59A:919-20. [DOI] [PubMed] [Google Scholar]
Foebel 2016
- Foebel AD, Onder G, Finne-Soveri H, Lukas A, Denkinger MD, Carfi A, et al. Physical restraint and antipsychotic medication use among nursing home residents with dementia. Journal of the American Medical Directors Association 2016;17(2):184.e9-14. [DOI] [PubMed] [Google Scholar]
Freeman 2017
- Freeman S, Spirgiene L, Martin-Khan M, Hirdes JP. Relationship between restraint use, engagement in social activity, and decline in cognitive status among residents newly admitted to long-term care facilities. Geriatrics & Gerontology International 2017;17(2):246-55. [DOI] [PubMed] [Google Scholar]
Goethals 2012
- Goethals S, Dierckx de Casterlé B, Gastmans C. Nurses' decision-making in cases of physical restraint: A synthesis of qualitative evidence. Journal of Advanced Nursing 2012;68(6):1198–210. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, BrozekJ, et al. GRADE guidelines: 1. Introduction-GRADEevidence profiles and summary of findings tables. Journal ofClinical Epidemiology 2011;64(4):383-94. [DOI] [PubMed] [Google Scholar]
Hamers 2009
- Hamers JP, Gulpers MJ. Reducing physical restraints in nursing homes: results of a pilot study. Journal of Nutrition, Health & Aging 2009;12(Suppl 13):S17. [Google Scholar]
Hartmann 2021
- Hartmann CW, Gillespie C, Sayre GG, Snow AL. De-implementing and sustaining an intervention to eliminate nursing home resident bed and chair alarms: interviews on leadership and staff perspectives. Implementation Science Communications 2021;24(2):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heeren 2014
- Heeren P, Van de Water G, De Paepe L, Boonen S, Vleugels A, Milisen K. Staffing levels and the use of physical restraints in nursing homes: a multicenter study. Journal of Gerontological Nursing 2014;40(12):48-54. [DOI] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
Hofmann 2014
- Hofmann H, Hahn S. Characteristics of nursing home residents and physical restraint: a systematic literature review. Journal of Clinical Nursing 2014;23(12-22):3012-24. [DOI] [PubMed] [Google Scholar]
Kirkevold 2004
- Kirkevold Ø, Engedal K. Prevalence of patients subjected to constraint in norwegian nursing homes. Scandinavian Journal of Caring Sciences 2004;18(3):281-6. [DOI] [PubMed] [Google Scholar]
Kong 2017
- Kong EH, Choi H, Evans LK. Staff perceptions of barriers to physical restraint-reduction in long-term care: a meta-synthesis. Journal of Clinical Nursing 2017;26(1-2):49-60. [DOI] [PubMed] [Google Scholar]
Köpke 2009
- Köpke S, Gerlach A, Möhler R, Haut A, Meyer G. Evidence-based practice guideline. Avoidance of physical restraints in long-term geriatric care [Leitlinie FEM–Evidenzbasierte Praxisleitlinie. Vermeidung von freiheitseinschränkenden Maßnahmen in der beruflichen Altenpflege]. University of Hamburg and Witten/Herdecke University, 2009. [Google Scholar]
Köpke 2015
- Köpke S, Möhler R, Abraham J, Henkel A, Kupfer R, Meyer G. Evidence-based practice guideline. Avoidance of physical restraints in long-term geriatric care [Leitlinie FEM-Evidenzbasierte Praxisleitlinie Vermeidung von freiheitseinschränkenden Maßnahmen in der beruflichen Altenpflege]. 2nd edition. University of Lübeck and Martin-Luther University Halle-Wittenberg. Available from http://www.leitlinie-fem.de/materialien/ leitlinie, 2015. [Google Scholar]
Lan 2017
- Lan SH, Lu LC, Lan SJ, Chen JC, Wu W J, Chang, SP, et al. Educational intervention on physical restraint use in long-term care facilities–systematic review and meta-analysis. Kaohsiung Journal of Medical Sciences 2017;33(8):411-21. [DOI] [PubMed] [Google Scholar]
Laurin 2004
- Laurin D, Voyer P, Verreault R, Durand PJ. Physical restraint use among nursing home residents: A comparison of two data collection methods. BMC Nursing 2004;3(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2021
- Lee DA, Robins LM, Bell JS, Srikanth V, Möhler R, Hill KD, et al. Prevalence and variability in use of physical and chemical restraints in residential aged care facilities: A systematic review and meta-analysis. International Journal of Nursing Studies 2021;117:103856. [DOI] [PubMed] [Google Scholar]
Meyer 2009a
- Meyer G, Köpke S, Haastert B, Mühlhauser I. Restraint use among nursing home residents: cross-sectional study and prospective cohort study. Journal of Clinical Nursing 2009;18:981-90. [DOI] [PubMed] [Google Scholar]
Möhler 2014
- Möhler R, Meyer G. Attitudes of nurses towards the use of physical restraints in geriatric care: a systematic review of qualitative and quantitative studies.. International Journal of Nursing Studies 2014;51(2):274-88. [DOI] [PubMed] [Google Scholar]
Möhler 2015
- Möhler R, Köpke S, Meyer G. Criteria for Reporting the Development and Evaluation of Complex Interventions in healthcare: revised guideline (CReDECI 2). Trials 2015;16:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Norton 2020
- Norton WE, Chambers DA. Unpacking the complexities of de-implementing inappropriate health interventions. Implementation Science 2020;15(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pivodic 2020
- Pivodic L, Smets T, Gambassi G, Kylänen M, Pasman HR, Payne S, et al. Physical restraining of nursing home residents in the last week of life: An epidemiological study in six European countries. International Journal of Nursing Studies 2020;104:103511. [DOI] [PubMed] [Google Scholar]
Review Manager 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
RNAO 2012
- Registered Nurses’ Association of Ontario. Promoting safety: alternative approaches to the use of restraints; 2012. Available at rnao.ca/sites/rnao-ca/files/Promoting_Safety_-_Alternative_Approaches_to_the_Use_of_Restraints_0.pdf.
Scheepmans 2018
- Scheepmans K, Dierckx de Casterlé B, Paquay L, Milisen K. Restraint use in older adults in home care: A systematic review. International Journal of Nursing Studies 2018;79:122-36. [DOI] [PubMed] [Google Scholar]
Skivington 2021
- Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ 2021;374:n2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sze 2012
- Sze TW, Leng CY, Lin SK. The effectiveness of physical restraints in reducing falls among adults in acute care hospitals and nursing homes: a systematic review. JBI Library of Systematic Reviews 2012;10(5):307-351. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Meyer 2009b
- Meyer G, Möhler R, Köpke S. Interventions for preventing and reducing the use of physical restraints in long‐term geriatric care. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: CD007546. [DOI: 10.1002/14651858.CD007546] [DOI] [PMC free article] [PubMed] [Google Scholar]
Möhler 2011
- Möhler R, Richter T, Köpke S, Meyer G. Interventions for preventing and reducing the use of physical restraints in long-term geriatric care. Cochrane Database of Systematic Reviews 2011, Issue 2. Art. No: CD007546. [DOI: 10.1002/14651858.CD007546.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Möhler 2012
- Möhler R, Richter T, Köpke S, Meyer G. Interventions for preventing and reducing the use of physical restraints in long-term geriatric care - a Cochrane review. Journal of Clinical Nursing 2012;21(21-22):3070-81. [DOI] [PubMed] [Google Scholar]


