Abstract
Background and Objective
In our outpatient pediatric and adult psychiatry centers, we reserve psychostimulants for predominantly inattentive attention deficit hyperactivity disorder (ADHD) due to the potential for appetite and growth suppression, insomnia, wear off, exacerbation of mood, anxiety, and tics, or misuse. We utilize extended-release (ER) alpha-2 agonists primarily for hyperactivity/impulsivity but find them less effective for inattention, and they can cause sedation and hypotension. Oftentimes, we need to combine an alpha-2 agonist for behavior with psychostimulants for inattention. We employ atomoxetine or viloxazine ER (VER) for combined ADHD. However, our patients’ insurers mandate a trial of generic atomoxetine prior to covering branded VER. The objective of this study was to determine whether pediatric and adult patients taking atomoxetine for DSM-5-TR ADHD combined type would experience improvement in ADHD symptoms following voluntary, open-label switch to VER.
Methods
50 patients (35 children) received mean doses of atomoxetine 60 mg (25–100 mg once daily) followed by VER 300 mg (100–600 mg once daily) after a 5-day atomoxetine washout. Both atomoxetine and VER were flexibly titrated according to US Food and Drug Administration (FDA) guidelines. The pediatric ADHD-Rating Scale-5 (ADHD-RS-5) and the Adult Investigator Symptom Rating Scale (AISRS) were completed prior to starting atomoxetine, and 4 weeks after treatment with atomoxetine or upon earlier response or discontinuation due to side effects, whichever occurred first; the same protocol was used after treatment with VER. We conducted a blinded, de-identified, retrospective review of charts from these 50 patients in the regular course of outpatient practice. Statistical analysis was performed using a within-subject, 2-tailed t-test with significance level of p < 0.05.
Results
From the baseline total ADHD-RS-5 mean score (40.3 ± 10.3), improvements were greater on VER (13.9 ± 10.2) than atomoxetine (33.1 ± 12.1; t = − 10.12, p < 0.00001) in inattention (t = − 8.57, p < 0.00001) and in hyperactivity/impulsivity (t = − 9.87, p < 0.00001). From the baseline total AISRS mean score (37.3 ± 11.8), improvements were greater on VER (11.9 ± 9.4) than atomoxetine (28.8 ± 14.9; t = − 4.18, p = 0.0009) in inattention (t = − 3.50, p < 0.004) and in hyperactivity/impulsivity (t = − 3.90, p < 0.002). Of patients on VER, 86% reported positive response by 2 weeks versus 14% on atomoxetine. A total of 36% discontinued atomoxetine for side effects, including gastrointestinal (GI) upset (6 patients), irritability (6), fatigue (5), and insomnia (1), versus 4% who discontinued VER due to fatigue. A total of 96% preferred VER over atomoxetine, with 85% (22 out of 26) choosing to taper psychostimulants following stabilization on VER.
Conclusions
Pediatric and adult ADHD patients who have experienced less than optimal response to atomoxetine demonstrate rapid improvement in inattention and in hyperactivity/impulsivity with greater tolerability on extended-release viloxazine.
Key Points
| Compared with atomoxetine, viloxazine ER seems to produce greater improvement in total ADHD symptoms than atomoxetine in both children and adults. |
| Viloxazine ER also seems to produce greater improvement in both inattention and in hyperactivity/impulsivity and to work more rapidly than atomoxetine and was better tolerated. |
Introduction
This study compares the effectiveness and tolerability of viloxazine ER (VER) to atomoxetine in the treatment of pediatric and adult attention deficit hyperactivity disorder (ADHD). In our outpatient pediatric and adult psychiatry centers, we reserve psychostimulants for predominantly inattentive ADHD due to the potential for appetite and growth suppression, insomnia, wear off, exacerbation of mood, anxiety, and tics, or misuse. We utilize extended-release (ER) alpha-2 agonists primarily for hyperactivity/impulsivity but find them less effective for inattention, and they can cause sedation and hypotension. Oftentimes, we need to combine an alpha-2 agonist for behavior with psychostimulants for inattention. We employ atomoxetine or VER for combined ADHD. However, challenges we have encountered with atomoxetine are that it is often only mildly effective, takes several weeks to work, and requires dosage adjustment for poor cytochrome P450 (CYP) 2D6 metabolizers, and capsules cannot be opened for young children. Viloxazine was marketed as an antidepressant in Europe for 30 years [1], and it was recently reformulated as an ER and approved by the US Food and Drug Administration (FDA) for pediatric and adult ADHD in the USA. Potential advantages of VER are improvements in ADHD symptoms by 1 week in children and by 2 weeks in adults, no adjustment required for CYP2D6 (it is a CYP1A2 inhibitor), and the ability to open capsules [2, 3]. Although both medications are norepinephrine reuptake inhibitors (NRIs), viloxazine demonstrates less inhibition of norepinephrine (NE) reuptake (Ki = 2300 nM) than atomoxetine (Ki = 3.4 nM), negligible serotonin (5-HT) reuptake inhibition (Ki > 10,000 nM) versus atomoxetine (Ki = 390 nM), and no dopamine (DA) reuptake inhibition versus atomoxetine (Ki = 1750 nM) [4]. In contrast, viloxazine is a 5-HT2B antagonist, 5-HT2C partial agonist, and 5-HT7 antagonist associated with increases in prefrontal cortex 5-HT, NE, and DA levels in vivo [5]. Our patients’ insurers mandate a trial of generic atomoxetine prior to covering branded VER. We wanted to know whether patients taking atomoxetine for DSM-5-TR ADHD combined type would experience improvement in ADHD symptoms following voluntary, open-label switch to VER.
Participants and Methods
A total of 50 patients (35 children; Table 1) who presented to our centers with a chief complaint and primary diagnosis of ADHD combined type according to DSM-5-TR criteria and had no other concurrent psychiatric, medical, or substance-related comorbidities as per clinical diagnostic interview or prior exposure to either atomoxetine or VER received mean doses of atomoxetine 60 mg (25–100 mg once daily) followed by VER 300 mg (100–600 mg once daily) after a 5-day atomoxetine washout. All patients were allowed to stay on stable doses of concomitant psychostimulant medication throughout both treatments. All patients received “a non-stimulant ADHD medication for inattentiveness and hyperactivity/impulsivity called atomoxetine” prior to receiving VER as per their insurance prior authorization requirement. Following up to a 4-week trial of atomoxetine, as tolerated, all 50 patients voluntarily opted for a trial of a “similar, non-stimulant ADHD medication called viloxazine ER” either due to side effects or insufficient response. Both atomoxetine [6] and VER [7] were flexibly titrated and administered at either daytime or nighttime, as tolerated, according to the following FDA guidelines: For atomoxetine, children 6 years and older and < 70 kg received 0.5 mg/day for 3 days, increased to a maximum of 1.2 mg/kg/day after at least 2 weeks, and children 6 years and older and > 70 kg and adults received 40 mg/day for 3 days, increased to a maximum of 100 mg/day after at least 2 weeks. For VER, children ages 6–11 years received 100 mg/day for 1 week, increasing by 100 mg/day each week to a maximum of 400 mg/day; children ages 12 years and older received 200 mg/day for 1 week, increasing to a maximum of 400 mg/day; and adults received 200 mg/day for 1 week increasing by 200 mg/day per week to a maximum of 600 mg/day. Patients were seen weekly for titration and monitoring. The pediatric ADHD-Rating Scale-5 (ADHD-RS-5) [8] and the Adult Investigator Symptom Rating Scale (AISRS) [9] were completed prior to starting atomoxetine; 4 weeks after treatment with atomoxetine or upon earlier response or discontinuation due to side effects, whichever occurred first; and 5 days after discontinuing atomoxetine, which re-established baseline ADHD scores; the same protocol was used after treatment with VER. As per our clinical protocol, a maximum time frame of a 4-week trial on each treatment to observe response was chosen to balance the time required for treatments to take effect with the natural urgency for both children and adults to experience some relief of ADHD symptoms that are impairing their daily functioning. We obtained informed consent from participants and Institutional Review Board (IRB) approval prior to conducting a blinded, de-identified, retrospective review of charts from these 50 patients in the regular course of outpatient practice between 1 July and 30 October 2022. Statistical analysis was performed using a within-subject, 2-tailed t-test with significance level of p < 0.05. Power analysis indicated that a sample size of 35 children per treatment group with an estimated Cohen’s d effect size between the VER and the atomoxetine groups of 0.819, which we calculated from this sample, yielded a statistical power of 92.3%. Power analysis indicated that a sample size of 15 adults per treatment group with an estimated Cohen’s d effect size between the VER and the atomoxetine group of 1.36 that we calculated from this sample yielded a statistical power of 94.7%.
Table 1.
Demographic and baseline characteristics
| Variables | Pediatric | Adult |
|---|---|---|
| Population, N | 35 | 15 |
| Age, years | ||
| Mean ± SD | 11.9 ± 2.9 | 29.3 ± 9.0 |
| Median (min, max) | 12 (6, 17) | 28 (20, 51) |
| Sex, n (%) | ||
| Male | 33 (94.3%) | 11 (73.3%) |
| Female | 2 (5.7%) | 4 (26.7%) |
| Race, n (%) | ||
| White | 33 (94.3%) | 14 (93.3%) |
| Non-white | 2 (5.7%) | 1 (6.7%) |
| Hispanic/Latino | 1 (2.9%) | 1 (6.7%) |
| Black | 1 (2.9%) | 0 (0%) |
| Asian | 0 (0%) | 0 (0%) |
| Concurrent stimulant, n (%) | 15 (42.9%) | 11 (73.3%) |
Max maximum, min minimum, n number of subjects, N number of subjects in the population, SD standard deviation
Results
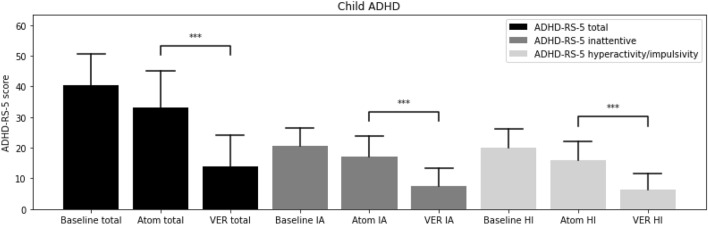
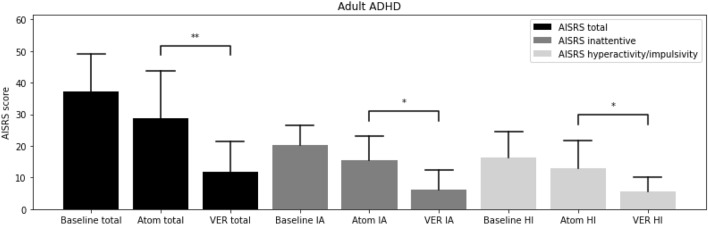
From the baseline total ADHD-RS-5 mean score (40.3 ± 10.3), improvements were greater on VER (13.9 ± 10.2) than atomoxetine (33.1 ± 12.1; t = − 10.12, p < 0.00001) in inattention (t = − 8.57, p < 0.00001) and in hyperactivity/impulsivity (t = − 9.87, p < 0.00001; Fig. 1). From the baseline total AISRS mean score (37.3 ± 11.8), improvements were greater on VER (11.9 ± 9.4) than atomoxetine (28.8 ± 14.9; t = − 4.18, p = 0.0009) in inattention (t = − 3.50, p < 0.004) and in hyperactivity/impulsivity (t = − 3.90, p < 0.002; Fig. 2). Of the children on VER, 89% reported positive response by 2 weeks versus 14% on atomoxetine (Fig. 3). Of the adults on VER, 87% reported positive response by 2 weeks versus 13% on atomoxetine (Fig. 4). No patients discontinued treatment prior to 4 weeks due to lack of response. A total of 36% discontinued atomoxetine for side effects, including gastrointestinal (GI) upset (6 patients), irritability (6), fatigue (5), and insomnia (1), versus 4% who discontinued VER due to fatigue. A total of 96% preferred VER over atomoxetine, with 85% (22 out of 26) choosing to taper psychostimulants following stabilization on VER, specifically 100% (15 out of 15) of children and 64% (7 out of 11) of adults.
Fig. 1.
ADHD-Rating Scale-5 (ADHD-RS-5) at baseline, on atomoxetine (Atom), on extended-release viloxazine (VER). ***p < 0.00001. ADHD attention-deficit hyperactivity disorder, Atom atomoxetine, HI hyperactivity/impulsivity, IA inattentive, RS rating scale, VER viloxazine extended-release
Fig. 2.
Adult Investigator Symptom Rating Scale (AISRS) at baseline, on atomoxetine (Atom), on extended-release viloxazine (VER). *p < 0.005. **p < 0.001. ADHD attention-deficit hyperactivity disorder, AISRS Adult Investigator Symptom Rating Scale, Atom atomoxetine, HI hyperactivity/impulsivity, IA inattentive, VER viloxazine extended-release
Fig. 3.
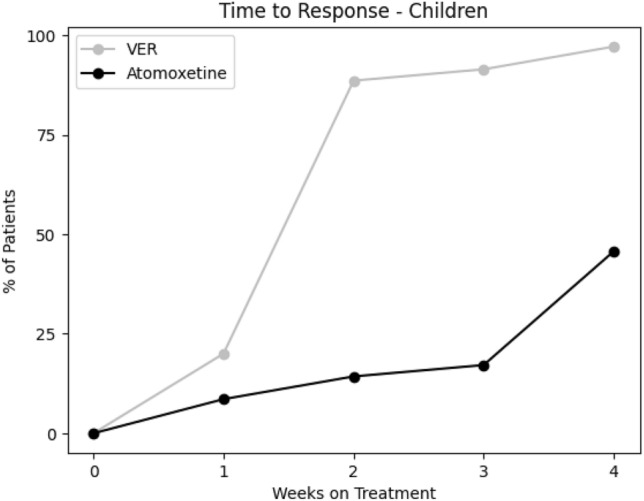
Cumulative percent of children with positive response per week to viloxazine ER (VER) compared with atomoxetine. VER viloxazine extended-release
Fig. 4.
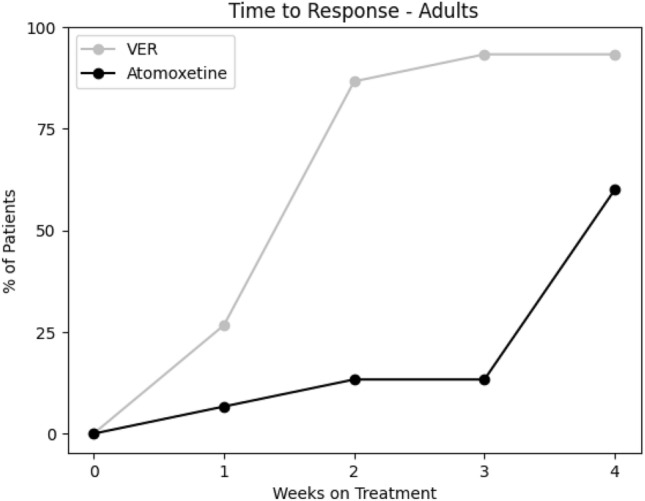
Cumulative percent of adults with positive response per week to viloxazine ER (VER) compared with atomoxetine. VER viloxazine extended-release
Discussion
Pediatric and adult ADHD diagnoses have been rising over the past decade [10] alongside increasing numbers of prescriptions for psychostimulant medications [11]. While psychostimulants have demonstrated superiority to non-stimulants in rapidly improving inattention [12], risk for abuse and side effects, such as insomnia, appetite suppression, wear off, and potential exacerbation of mood, anxiety, and tics [13], give clinicians cause to consider prescribing non-stimulants either as monotherapy or in combination with psychostimulants to enhance efficacy and/or mitigate side effects of psychostimulants [14]. While the alpha-2 agonists, ER clonidine and ER guanfacine, can help manage symptoms of hyperactivity/impulsivity, they are less effective for inattentive symptoms compared with psychostimulants [15]. As an NRI, atomoxetine can potentially address the full spectrum of inattentive and hyperactive/impulsivity symptoms, albeit more modestly compared with psychostimulants for inattention [16] and ER alpha-2-agonists for hyperactivity/impulsivity [17]. When VER was introduced to the US market for ADHD in 2021, it was classified as another NRI and equated with atomoxetine in terms of its mechanism of action and corresponding mild efficacy in ADHD [18]. However, additional serotonin receptor targets have been more recently identified that may better explain its primary mechanism of action beyond the NE reuptake inhibition that distinguishes it from atomoxetine pharmacologically [5]. Using a within-subject, crossover design from atomoxetine to VER, this study is important in differentiating the clinical utility, speed of onset, and tolerability of VER from atomoxetine. Compared with baseline total ADHD-RS-5 and AISRS scores, these participants who were initially moderately to severely impaired by their ADHD symptoms were much to very much improved after 2 weeks on VER. Over half of study participants had been previously exposed to stable doses of psychostimulants, and the vast majority were able to reduce or discontinue their psychostimulant use as the addition of VER became effective after 2 weeks. Furthermore, the common aforementioned side effects found with psychostimulants did not present causes for discontinuation of VER. Likewise, the most common reason for discontinuation of atomoxetine, gastrointestinal upset, was also not a cause for discontinuation of VER. During the 5-day washout period from atomoxetine, side effects from atomoxetine abated, and participants reverted back to their baseline ADHD symptoms. Given the superior efficacy for both inattentive and hyperactivity/impulsivity symptoms, speed of onset, and tolerability of VER over atomoxetine, we recommend considering VER as a first-line non-stimulant option for addressing the full spectrum of ADHD symptoms, either as a monotherapy in patients for whom psychostimulants are not ideal or as an adjunct to psychostimulants. Limitations of this study were that it was an unblinded, open-label, single arm, retrospective analysis of a relatively small, heterogeneous sample of children and adults, without a comparison group or placebo control. Placebo effect, period effect, or carry-over effect could not be excluded. Future double-blind studies with larger sample sizes, greater representation of females and minority groups, and weekly ADHD-RS-5 and AISRS administered over longer periods of time are warranted. A more rigorous study would include two parallel arms with random groups, one starting with atomoxetine prior to use of VER and one starting with VER prior to use of atomoxetine to take into account the improvements of each first starting treatment, where the baseline of each group would be the reference to investigate improvements. However, this was not possible in our real-world clinical practice setting where patients’ insurance mandated a trial of atomoxetine prior to providing coverage for VER, and all patients previously taking atomoxetine voluntarily chose to switch to VER.
Conclusions
Pediatric and adult ADHD patients who have experienced less-than-optimal response to atomoxetine demonstrate rapid improvement in inattention and in hyperactivity/impulsivity with greater tolerability on extended-release viloxazine.
Declarations
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The open access fee was funded by the investigators: Maxwell Z. Price and Richard L. Price.
Conflicts of Interest
Maxwell Z. Price certifies that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript. Richard L. Price has received honoraria from AbbVie, Alkermes, Idorsia, Intra-Cellular Therapies, Janssen, Jazz, Lundbeck, Neuronetics, Otsuka, and Supernus, and discloses no other conflicts of interest in this work.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
This research study was conducted retrospectively from data obtained for clinical purposes and performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. We consulted extensively with WCG IRB, who determined that our study did not need ethical approval. An IRB official waiver of ethical approval (#1-1597751-1) was granted from WCG IRB.
Consent to Participate
Informed consent was obtained from all individual participants included in the study (or their parent or legal guardian in the case of children under 16 years).
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Authors’ Contributions
Both authors substantially contributed to the conception and design of the work, drafting the work, and approving the final version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy of any part of the work are appropriately investigated and resolved. Both authors read and approved the final version of the manuscript and agree to be accountable for the work.
References
- 1.Findling RL, Candler SA, Nasser AF, et al. Viloxazine in the management of CNS disorders: a historical overview and current status. CNS Drugs. 2021;35(6):643–653. doi: 10.1007/s40263-021-00825-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nasser A, Liranso T, Adewole T, et al. A phase III, randomized, placebo-controlled trial to assess the efficacy and safety of once-daily SPN-812 (viloxazine extended-release) in the treatment of attention-deficit/hyperactivity disorder in school-age children. Clin Ther. 2020;42(8):1452–1466. doi: 10.1016/j.clinthera.2020.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of viloxazine extended-release capsules in adults with attention-deficit/hyperactivity disorder. CNS Drugs. 2022;36(8):897–915. doi: 10.1007/s40263-022-00938-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu C, Garcia-Olivares J, Candler S, et al. New insights into the mechanism of action of viloxazine: serotonin and norepinephrine modulating properties. J Exp Pharmacol. 2020;12:285–300. doi: 10.2147/jep.s256586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ACNP 61st Annual meeting: Poster abstracts P271-P540. Neuropsychopharmacol. 2022;47(S1):220-370. 10.1038/s41386-022-01485-0. [DOI] [PMC free article] [PubMed]
- 6.Strattera—Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021411s004s012s013s015s021lbl.pdf. Accessed 24 Oct 2022.
- 7.Qelbree—Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/211964s003lbl.pdf. Accessed 24 Oct 2022.
- 8.DuPaul GJ, Power TJ, Anastopoulos AD, et al. ADHD Rating Scale—5 for children and adolescents: checklists, norms, and clinical interpretation. New York: The Guilford Press; 2016. pp. 1–6. [Google Scholar]
- 9.Spencer TJ, Adler LA, Qiao M, et al. Validation of the adult ADHD Investigator Symptom Rating Scale (AISRS) J Atten Disord. 2009;14(1):57–68. doi: 10.1177/1087054709347435. [DOI] [PubMed] [Google Scholar]
- 10.Chung W, Jiang S-F, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019 doi: 10.1001/jamanetworkopen.2019.14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Board AR, Guy G, Jones CM, et al. Trends in stimulant dispensing by age, sex, state of residence, and prescriber specialty—United States, 2014–2019. Drug Alcohol Depend. 2020;217:108297. doi: 10.1016/j.drugalcdep.2020.108297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faraone SV, Glatt SJ. A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. J Clin Psychiat. 2009;71(06):754–763. doi: 10.4088/jcp.08m04902pur. [DOI] [PubMed] [Google Scholar]
- 13.Charach A, Ickowicz A, Schachar R. Stimulant treatment over five years: adherence, effectiveness, and adverse effects. J Am Acad Child Psy. 2004;43(5):559–567. doi: 10.1097/00004583-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Pohl GM, Van Brunt DL, Ye W, et al. A retrospective claims analysis of combination therapy in the treatment of adult attention-deficit/hyperactivity disorder (ADHD) BMC Health Serv Res. 2009 doi: 10.1186/1472-6963-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harstad E, Shults J, Barbaresi W, et al. Α2-adrenergic agonists or stimulants for preschool-age children with attention-deficit/hyperactivity disorder. JAMA. 2021;325(20):2067. doi: 10.1001/jama.2021.6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Childress A. A critical appraisal of atomoxetine in the management of ADHD. Ther Clin Risk Manag. 2015 doi: 10.2147/tcrm.s59270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sikirica V, Findling RL, Signorovitch J, et al. Comparative efficacy of guanfacine extended-release versus atomoxetine for the treatment of attention-deficit/hyperactivity disorder in children and adolescents: applying matching-adjusted indirect comparison methodology. CNS Drugs. 2013;27(11):943–953. doi: 10.1007/s40263-013-0102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas Jordan MD. Viloxazine for ADHD. CARLAT PUBLISHING RSS. https://www.thecarlatreport.com/articles/3575-viloxazine-for-adhd. Published October 6, 2022. Accessed 5 Feb 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.