Abstract
Heading date is a critical agronomic trait that determines crop yield. Although numerous genes associated with heading date have been identified in rice, the mechanisms involving Small Auxin Up RNA (SAUR) family have not been elucidated. In this study, the biological function of several SAUR genes was initially investigated using the CRISPR-Cas9 technology in the Japonica cultivar Zhonghua11 (ZH11) background. Further analysis revealed that the loss-of-function of OsSAUR56 affected heading date in both NLD (natural long-day) and ASD (artificial short-day). OsSAUR56 exhibited predominant expression in the anther, with its protein localized in both the cytoplasm and nucleus. OsSAUR56 regulated flowering time and heading date by modulating the expression of the clock gene OsGI, as well as two repressors Ghd7 and DTH8. Furthermore, haplotype-phenotype association analysis revealed a strong correlation between OsSAUR56 and heading date, suggesting its role in selection during the domestication of rice. In summary, these findings highlights the importance of OsSAUR56 in the regulation of heading date for further potential facilitating genetic engineering for flowering time during rice breeding.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11032-023-01409-w.
Keywords: Heading date, SAUR family, Natural variation, Rice
Introduction
Rice (Oryza sativa L.) is one of the most important cereal crops worldwide, serving as a staple food for over half of the global population (Foley et al. 2011). Heading date, also known as flowering time, is a crucial trait influencing regional adaptation and grain production in rice (Zhong et al. 2014). Cultivars with appropriate heading dates optimize grain yield by effectively utilizing light and temperature resources in their growing regions (Zhang et al. 2015). However, delayed heading can lead to incomplete flowering and reduced yield, particularly in regions with cold conditions. Conversely, early flowering is favored in environments with short or unpredictable growing seasons under optimal conditions (Roux et al. 2006). Photoperiod is the primary environmental factor affecting heading date in rice (Song et al. 2015). Extensive research has led to the cloning and functional characterization of numerous heading date-related genes in rice, revealing the complexity of its flowering pathways. Among them, Heading date 1 (Hd1), a homolog of Arabidopsis thaliana CONSTANS (CO), acts as an important regulator of photoperiod-sensitive heading (Yano et al. 2000). The tandem-duplicated genes Heading date 3a (Hd3a) and RICE FLOWERING LOCUS T1 (RFT1), orthologs to FLOWERING LOCUS T (FT) in Arabidopsis, serve as florigen genes in rice triggering the onset of flowering (Corbesier et al. 2007; Tamaki et al. 2007). The GI-CO-FT photoperiodic flowering pathway in Arabidopsis is partially conserved with the OsGI-Heading date 1 (Hd1)-Hd3a/RFT1 pathway in rice. OsGI activates the expression of CO ortholog Hd1, which promotes flowering under long day (LD) conditions, Hd1 generally promotes heading under short day (SD) conditions but delays heading under LD by up- and down-regulating Hd3a/RFT1 expression, respectively (Ryosuke et al. 2003; Yasue et al. 2016). The rice heading date gene Early heading date1 (Ehd1) encodes a B-type response regulator that promotes the expression of Hd3a and RFT1 (Jing et al. 2015). Under LD conditions, the expression of Ghd7 is enhanced, suppressing Ehd1 and delaying floral transition, suggesting its role as a flowering repressor (Xue et al. 2008). Moreover, Ghd7 interacts with Hd1 to inhibit flowering by repressing Hd3a and RFT1, indicating a relationship between these two pathways in photoperiodic flowering (Yasue et al. 2016). DTH8/Ghd8/Hd5 delays heading date under both LD and SD conditions (Roshi et al. 2014), and Ehd1 appears to function as a signal integrator for multiple regulatory pathways under LD conditions (Roshi et al. 2014). Although a preliminary gene regulatory network for heading date in rice has been proposed, further refinement is necessary.
The phytohormone auxin (Aux) plays an important role in plant flowering, with auxin response factors mediating pathway involved in floral regulation in various plants. For instance, manipulating the expression of Aux receptor genes OsTIR1 and OsAFB2 through OsmiR393 overexpression, resulted in early flowering in rice (Xia et al. 2012). Moreover, mutation of Aux signaling component IAA7/AXR2 has been found to influence floral transition under short-day light conditions by negatively regulating GA20 oxidase gene (Mai et al. 2011). ARFs such as strawberry FaARF4 promote flowering by activating floral meristem identity genes APETALA1 (AP1) and FRUITFULL (FUL) (Dong et al. 2021). The SAUR gene family, an early auxin-responsive gene family, is abundant in plants, consisting of 81 SAURs in Arabidopsis thaliana and 58 SAURs in rice, participating in diverse cellular, physiological, and developmental processes (Ren and Gray 2015). For example, the AtSAUR63 gene causes the elongation of hypocotyls, petals, and stamen filaments (Chae et al. 2012).
Recent studies have evidenced that OsSAUR39 acts as a negative regulator of auxin synthesis and transport; its overexpression in rice leads to phenotypic changes including reduced lateral root development, yield, and shoot and root lengths (Kant et al. 2009). Additionally, AtSAUR36 affects leaf senescence as a positive regulator (Hou et al. 2013), while SAUR15 acts downstream of auxin to promote lateral and adventitious root development (Copeland 2020). Moreover, SAUR transcript levels are also regulated in a circadian manner. For example, the sunflower SAUR50-like gene is highly expressed in the morning on the east side of the stem (Atamian et al. 2016), whereas many SAUR homologs play crucial roles in circadian floral opening and closure in waterlily (Zhang et al. 2020). Furthermore, different SAUR members display distinct responsiveness to the opening and closing of flowers. SAUR32, a homologous gene of OsSAUR56, mediates the flowering movement of water lilies by controlling the rapid expansion of flowering cells and the atrophy of closed flowering cells (Ke et al. 2018). AtSAUR62, a homologous of OsSAUR53 and OsSAUR55, plays a pivotal role in the morphogenesis of floral organs in Arabidopsis thaliana (He et al. 2018). SAUR41 is intricately involved in the hormonal regulation of flowering in orchids, ginkgo, and sweet cherries (Ahmad et al. 2021; Zhou et al. 2021; Villar et al. 2020). However, it remains unknown whether these genes regulate flowering in rice.
In this study, we employed CRISPR/Cas9 technology to precisely edit SAUR family genes in the Japonica rice cultivar ZH11. Surprisingly, we found that loss-of-function of OsSAUR56 significantly impacted heading date, suggesting its involvement in heading date regulation. Haplotype analysis of OsSAUR56 and its geographic distribution further indicated its relevance in rice domestication and differentiation. These findings provide valuable insights into the genetic basis and differentiation of photoperiodic flowering in rice and shed light on the molecular mechanisms underlying its regulation.
Materials and methods
Plant materials and growth conditions
The mutants of the target gene OsSAUR56 in the ZH11 background (Oryza sativa L.) were generated using the CRISPR/Cas9 system. The mutants of T1 and T2 generations were assayed consecutively for the target gene and hygromycin resistant gene. Three stable T3-generation mutants (ossaur56-1, ossaur56-2, and ossaur56-3) without hygromycin were selected for follow-up studies. The wild-type (WT) rice and three mutants were grown in the experimental field of South China Agricultural University (Guangzhou, Guangdong, China, 23.13° N, 113.27° E), in the normal rice-growing early season with natural LD (NLD) conditions (13.5–14.0 h day-lengths from mid-May to mid-July as photoperiod-sensing stage), in the late season with SD conditions (12.0–13.5 h day-lengths from mid-September to October), and in artificial SD conditions (ASD, with 11.0–11.5 h day-lengths by shading treatment in the late season), respectively. The WT and mutants without hygromycin were planted in a randomized block design. Each plot consisted of six rows with six plants per row at a planting interval of 20 cm × 20 cm. Ten and five plants in the middle of a plot were used to measure heading date and agronomic traits, respectively. In the early season of 2022, a set of 231 accessions (Table S1) from 16 countries were planted in the same field. Field management was in accordance with normal agricultural practices.
Construction of vectors and rice transformation
For knock-out of OsSAUR56, the target sites were designed using the online tool of clustered regularly interspaced short palindromic repeats (CRISPR)-GE/targetDesign (http://skl.scau.edu.cn/) (Xie et al. 2017), and genome-targeting constructs were prepared using the pRGEB32 vector (Xie et al. 2015). The construct was transferred into ZH11 callus using Agrobacterium-mediated method. The target sites of T1- and T2-generation plants were sequenced and analyzed using CRISPR-GE/DSDecodeM (Liu et al. 2015; Xie et al. 2017). For subcellular localization assay, the OsSAUR56 coding sequence was amplified and cloned into the pAN580 vector to generate the fused construct of pAN580: OsSAUR56: GFP. All constructs were confirmed by sequencing through the Sanger method. The information of primers for construction of the vectors and amplifying the target site and hygromycin resistant gene are provided in Table S2.
DNA extraction and resequencing
Fifteen-day-old young samples (green leaves) were collected from all tested plants for DNA extraction by the CTAB (cetyltrimethylammonium bromide) method, and then, genomic DNA was stored in a refrigerator at 4 °C until use (Rogers and Bendich 1985). Qualified DNA was used for whole-genome resequencing of the collected rice accessions with an average coverage of approximately 10X on the Illumina HiSeq 2500 Sequencing Systems Platform.
Subcellular localization of OsSAUR56
The fused construct of p35S:OsSAUR56: EGFP was transiently expressed in the rice protoplast, and the GFP signal was observed using confocal microscopy (Zeiss Leica TCS SP5; Mannheim, Germany). The rice protoplast preparation and transfection followed previously described procedures (Zhang et al. 2011).
RNA extraction and analysis of gene expression
Sixty-day-old plants grown in ASD and NLD conditions were used for expression analysis of genes regulating flowering time. The top three leaves were collected every 4 h within a day (Zong et al. 2020). Total RNA of plant materials was extracted using TRIzol reagent (R401-01, Vazyme). The first-strand of cDNA was synthesized from 600 ng total RNA using a reverse transcription kit (R133-01, Vazyme). The qRT-PCR reaction was performed with ChamQ Universal SYBR qPCR Master Mix (Q711-03, Vazyme) on a CFX96 real-time PCR system according to the manufacturer’s instructions (Bio-Rad) (Huang et al. 2018). The ubiquitin (UBQ, Os03g13170) gene was used as an internal control. Normalized transcript levels were calculated using the comparative CT method (Livak and Schmittgen 2001). Three biological replicates were conducted. All the primers used for qRT-PCR were listed in Table S2.
Haplotype analysis
To identify the association between heading date and OsSAU56 at the haplotype level, the haplotype diversity analysis was conducted according to the genomic sequences of OsSAUR56 and its upstream and downstream noncoding regions using variant annotation files based on a genome-wide resequencing database of 231 rice varieties (unpublished) and 2832 accessions in the Rice Functional Genomics and Breeding (RFGB) public rice genetic/genomic database (http://www.rmbreeding.cn/Genotype/haplotype) (Wang et al. 2020).
Trait measurement
Heading date was individually measured as the days from sowing to the emergence of the first panicle in the plant. After maturity, only five plants in the middle of each plot were used to measure agronomic traits, including the plant height (PH), flag-leaf length (FL), flag-leaf width (FW), and panicles per plant (PP). The agronomic traits, such as grains per plant (GP), grain yield per plant (GYP), panicle length (PL), grain length (GL), grain width (GW), and 1000-grain weight (TGW) were measured by YTS-5D rice digital seed testing machine (Wuhan Gufeng Optoelectronic Technology Co., Ltd.).
Statistical analysis and data plotting
Experimental data were analyzed using the SAS software (Cary, NC, USA), and significant differences among samples were compared using Student’s t-test or analysis of variance (ANOVA) test. Graphs were drawn by GraphPad Prism 8.0 (GraphPad Software, Inc., La Jolla, CA, USA), and PhotoScape X (Pro version 4.0) was used for designing.
Results
Loss-of-function of OsSAUR56 affected heading date
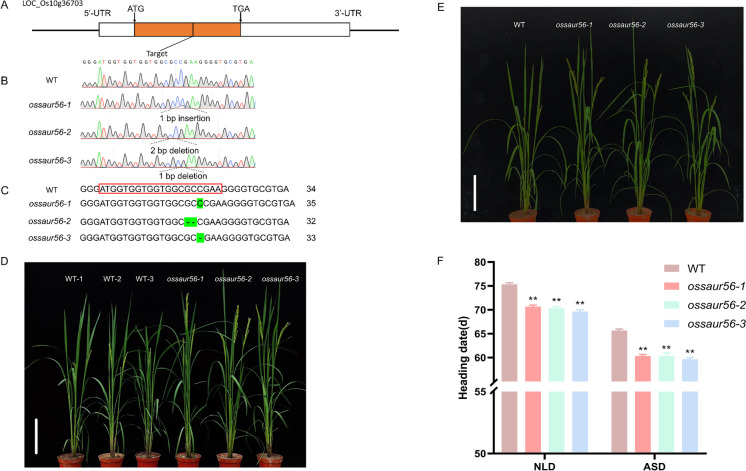
To investigate the potential role of SAUR family genes in regulating heading date in rice, we generated targeted mutants in the background of Japonica rice cultivar ZH11 using the CRISPR-Cas9 technology. The mutants of several SAUR genes, including OsSAUR55, OsSAUR41, and OsSAUR53, showed altered heading date (data not presented). Among these, the mutants of OsSAUR56 displayed the most remarkable change in heading date. Therefore, we focused on elucidating the functions and potential regulatory mechanisms of OsSAUR56 in photoperiodic flowering. We used three independent homozygous transgenic lines, free of hygromycin selection, which evidenced different editing effects (ossaur56-1 with 1 bp insertion, ossaur56-2 with 2 bp deletion and ossaur56-3 with 1 bp deletion) with the target gene (Fig. 1A–C). Compared to the wild type ZH11, all the OsSAUR56 mutants exhibited an approximately 5-day early heading phenotype under whether NLD (natural long-day) or ASD (artificial short-day) conditions in Guangzhou (Fig. 1D–F) and a significant increase in plant height (Fig. S1). No significant differences were observed in other agronomic traits, such as tillers number per panicle, 1000-grain weight, panicle length, and spikelets number per panicle, between the mutants and the wild type (Table S3). These results suggest that the knock-out of OsSAUR56 promotes an early heading date of approximately 5 days in rice without affecting other main agronomic traits.
Fig. 1.
CRISPR/Cas9-engineered mutations in OsSAUR56 caused early flowering under ASD and NLD conditions. A–C Gene structure diagram of OsSAUR56. The UTRs and CDS are indicated by black and orange rectangles, respectively; the black arrows indicate the start (ATG) and stop codon (TGA). The green background fonts indicate the mutation site of OsSAUR56 in the three mutants. Sequence length is shown on the right. D, E Phenotypes of WT, ossaur56-1, ossaur56-2, and ossaur56-3 at heading stage under ASD and NLD conditions (Guangzhou), respectively. Photographs were taken at 70 days after soaking. F Days to heading in WT, ossaur56-1, ossaur56-2, and ossaur56-3 under ASD and NLD conditions (Guangzhou). Values are shown as mean ± standard error (SEs) of heading date, n = 10. **P < 0.01, Student’s t-test
Expression patterns and subcellular localization of OsSAUR56
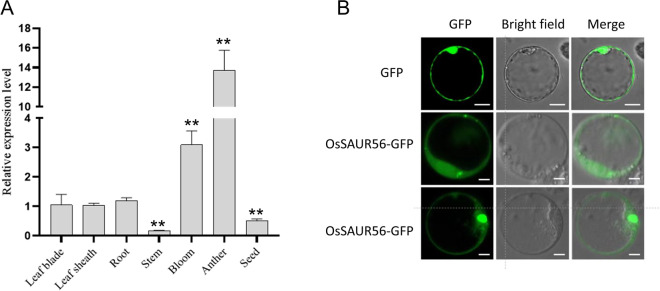
To further investigate the biological function of OsSAUR56, we analyzed its expression in various tissues at the developing stage and germinating seeds of rice using qRT-PCR. The results revealed that OsSAUR56 was constitutively expressed in the roots, stems, leaves, flowers, anthers, leaf sheaths, and seeds, with similar expression levels observed in the leaves, leaf sheaths, and roots (Fig. 2A). Notably, among these tissues, anthers exhibited the highest expression levels, followed by inflorescence, while the lowest expression level was observed in stems.
Fig. 2.
Expression analysis of OsSAUR56. A Expression pattern of OsSAUR56 in various tissues, including root, stem, leave, leaf sheath, inflorescence, anther, and seed of rice. Values = means ± SEs. B Subcellular localization of SAUR56. The construct of p35S:OsSAUR56:GFP was co-transformed into rice protoplasts and visualized by confocal microscopy. Scale bars = 10 μm
To determine the subcellular localization of OsSAUR56 protein, a vector containing OsSAUR56-GFP driven by the 35S promoter was transiently expressed in rice protoplasts. The green fluorescence signal accumulated in the cytoplasm and nucleus (Fig. 2B), indicating that OsSAUR56 was localized in the cytoplasm and nucleus.
Knock-out of OsSAUR56 affects the transcript levels of genes involved in the flowering network
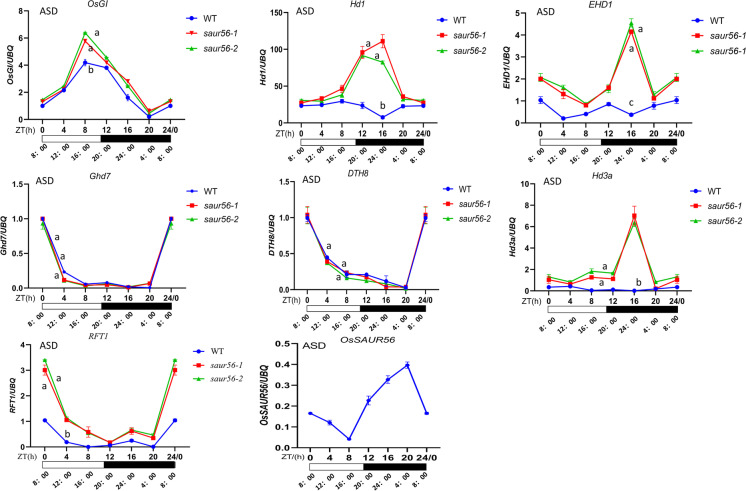
Two major photoperiodic flowering pathways have been reported in rice, and the expression patterns of genes involved in the flowering network differed under SD and LD conditions (Reina et al. 2009; Tsuji et al. 2010; Cai et al. 2019). To investigate the relationship between OsSAUR56 and previously identified flowering regulators, we examined the expression of OsGI, Hd1, Ehd1, Ghd7, DTH8, Hd3a, and RFT1 in the ossaur56 mutants and the WT plants grown under SDs or LDs. Sixty days after germination, the top three leaves were harvested every 4 h per day, and transcript levels were measured by qRT-PCR. Considering the important role of OsGI in controlling heading date under SD in rice, it was necessary to investigate if OsSAUR56 influences the expression of OsGI under artificial SD (ASD) condition. The transcript levels of OsGI were significantly increased in the two mutants, particularly at 8:00 AM, suggesting that knock-out of OsSAUR56 positively regulates OsGI (Fig. 3). Furthermore, OsGI can capture blue light signaling in the morning or regulating the photoperiod control of heading date by promoting the expression of Hd1 and Ehd1, which subsequently accelerates Hd3a to promote heading date under SD conditions (Ryosuke et al. 2003; Hironori et al. 2010). As expected, the mutants of OsSAUR56 up-regulated the expression level of Hd1 from 8 to 20, and increased the expression level of Ehd1 at multiple time points compared to the WT. However, the expression levels of Ghd7 and DTH8 were consistently low, and no significant differences were detected between the WT and mutants. The transcript levels of Hd3a and RFT1 significantly increased at multiple time points during the day in the mutants (Fig. 3), contributing to early flowering. The expression of OsSAUR56 under the daylight was notably lower than in the dark. These results demonstrated that the knock-out of OsSAUR56 may affect flowering under ASD, mainly by inducing the expression of OsGI, Hd1, Ehd1, Hd3a, and RFT1.
Fig. 3.
Diurnal expression of flowering genes under ASD (artificial short-day) condition in the WT and mutants. We normalized and set the values of WT as “1” at ZT of 0 h in each plot. Samples were collected every 4 h during light or dark conditions. White and black horizontal bars below the expression plots represent light and dark periods, respectively. Y-axis, relative transcript levels normalized with rice Ubiquitin, the internal control gene. WT, wild-type, representing ZH11. ZT, zeitgeber time. Error bars indicate standard deviations (n ≥ 3). Letters above the bars are ranked by Duncan test at P < 0.05; different letters indicate significant difference
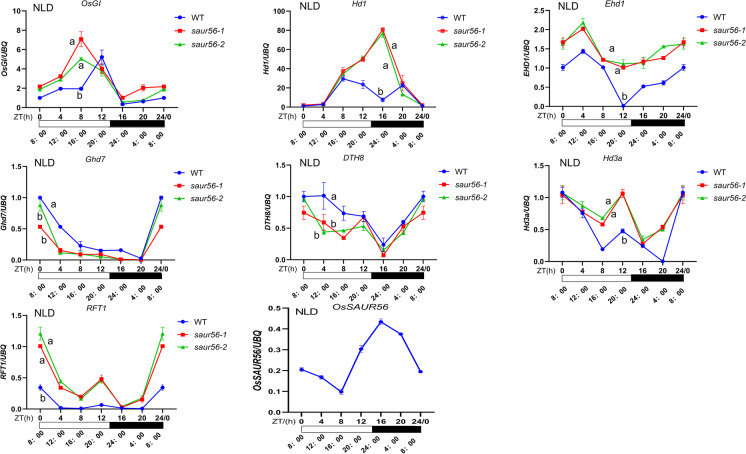
To further investigate the regulatory effect of OsSAUR56 on heading date, we examined the transcript levels of genes involved in the flowering network under natural LD condition. The transcript levels of OsGI and Hd1 were also higher in the mutants than in the wild-type plants (Fig. 4). However, OsGI has been reported to have a weak effect on flowering time under LD conditions because its expression is relatively low during the transitional state to flowering under LD (Takeshi et al. 2011; Lee and An 2015). The mutants exhibited higher expression levels of Ehd1 and lower expression levels of Ghd7 and DTH8 at multiple time points than the WT. Ghd7 and DTH8 inhibited the expression of Ehd1 and florigen genes under LD conditions (Xue et al. 2008; Wei et al. 2010). Down-regulation of Ghd7 and DTH8 may contribute to increased expression of Ehd1. Moreover, under LD conditions, Hd3a and RFT1 exhibited circadian rhythms in the WT plants, and both induced in ossaur56-1 and ossaur56-2 plants at multiple time points. Therefore, knock-out of OsSAUR56 may also result in the induced expression of Hd1, EHD1, Hd3a, and RFT1 under LD, altering the heading date. RT-qPCR analysis revealed that OsSAUR56 displayed diurnal expression patterns under LD and ASD conditions, with its transcript levels in the wild-type plants gradually decreasing in the morning, increased at 4:00 PM, and finally, peaked at 12:00 AM and 4:00 AM (Figs. 3 and 4).
Fig. 4.
Diurnal expression of flowering genes under the NLD (natural long-day) condition in the WT and mutants. We normalized and set the values of WT as “1” at ZT of 0 h in each plot. Samples were collected every 4 h during light or dark conditions. White and black horizontal bars below the expression plots represent light and dark periods, respectively. Y-axis, relative transcript levels normalized with rice Ubiquitin (Ubq), the internal control gene. WT, wild-type, representing ZH11. ZT, zeitgeber time. Error bars indicate standard deviations (n ≥ 3). Letters above the bars are ranked by Duncan test at P < 0.05; different letters indicate significant difference
In conclusion, knock-out of OsSAUR56 promoted flowering under ASD and natural NLD conditions, as indicated by the increased expression of Hd3a and RFT1 in the mutants. However, the regulatory mechanisms that induce the expression of Hd3a and RFT1 appears to be different.
Natural variation in OsSAUR56 is associated with heading date in rice
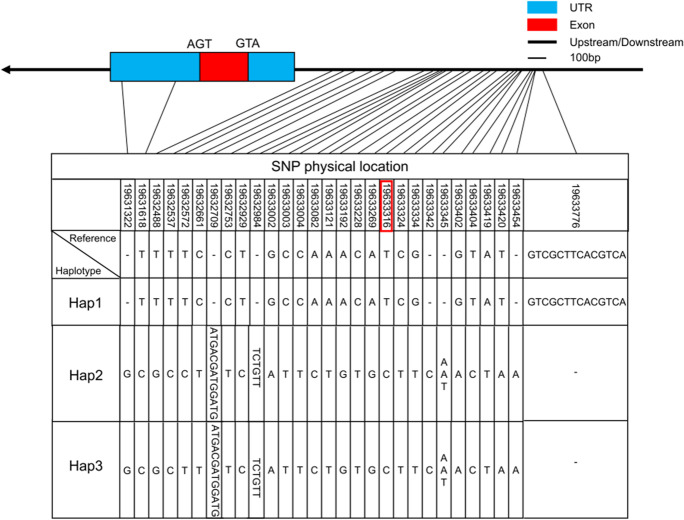
Given that OsSAUR56 controls heading date of rice regardless of day length, we investigated whether a specific haplotype of OsSAUR56 existing in natural accessions is associated with heading date. We performed haplotype diversity analysis using 231 rice accessions, examining the allele of OsSAUR56 and its up and downstream noncoding regions. A total of 29 variable sites, including 22 SNPs and 7 InDels, were identified within the promoter and downstream noncoding regions. No differences were found in the coding regions (Fig. 5). Three haplotypes were identified based on the 29 variable sites, and the SNP19 site (T/C) differentiated Hap 1 from Hap 2 and Hap 3 (Fig. 5). Haplotypes Hap 1 and Hap 3 were associated with the lowest and highest values of heading date, respectively, whereas Hap 2 represented an intermediate type.
Fig. 5.
Haplotype analysis of OsSAUR56 using the genomic region, upstream and downstream noncoding regions
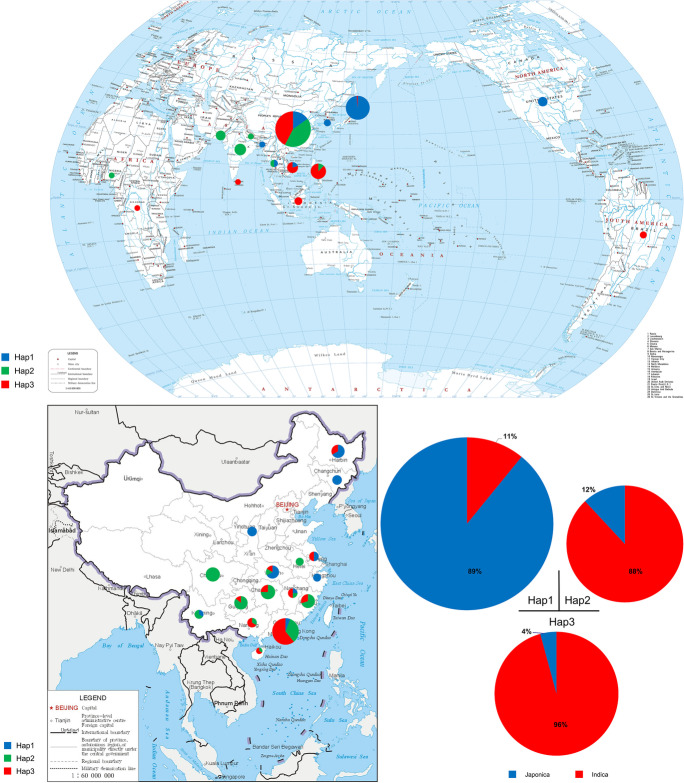
We also examined the subpopulation and geographical distribution of all 231 accessions in relation to the three haplotypes of OsSAUR56 (Fig. 6; Table S4). We observed that 89% of the accessions in the Hap 1 group belong to Japonica, whereas 88% of the accessions in the Hap 2 group belong to Indica. Moreover, 96% of the accessions in the Hap 3 group belonged to Indica, while only 4% belonged to Japonica (Fig. 6C). This phenomenon partially explains that Hap 2 exhibits an intermediate type in heading date. The accessions carrying Hap 2 and Hap 3 were mainly found in landraces growing in tropical and subtropical areas, whereas the Hap 1 was found mainly in Japonica landraces growing in temperate areas (Fig. 6A). Regarding accessions with Hap 3, we analyzed 98 from around the world and 133 from China. When conducting analysis using 2832 accessions from the rice 3 K database (RFGB, Rice Functional Genomics and Breeding public rice genetic/genomic database), we found that the proportion of different rice subgroups among Hap 1 and Hap 3 varied (Fig. 6D).
Fig. 6.
Geographical and subpopulation distribution of 231 accessions based on three haplotypes. Base map comes from the Ministry of Natural Resources, People’s Republic of China. Drawing review number: GS 5442 (2021) and GS 1652 (2019). A, B Geographic distribution of accessions with the three haplotypes on the world map and the map of China, respectively. The solid circles in different colors (blue, green, and red) represent accession numbers of the three haplotypes, respectively. C Proportions of 231 natural rice accessions according to the three haplotypes distributed in two subpopulations (Indica and Japonica). D Proportion of different subgroups in terms of site SNP19 (T/C) based on RFGB using 2832 accessions from the rice 3 K database. Aus, Aus population; Bas, Basmati population; GJ, Geng/Japonica population; XI, Xian/Indica population; Admix, admixed between any two or more of the XI, GJ, Aus, and Bas populations, respectively
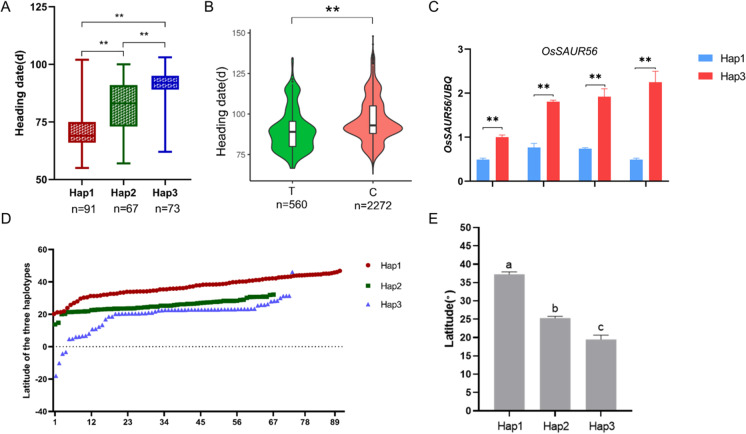
Multiple comparison test revealed significant differences in heading date among the three haplotypes (P < 0.05, Fig. 7A). Response elements in the promoter region were predicted using the PlantCare online system (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) (Magali et al. 2002). Interestingly, SNP19 (19633316) is located in the light response element (GGTTAA). To further validate whether SNP19 (T/C) was associated with HD, we performed a haplotype analysis of the locus using 2832 accessions from the rice 3 K database based on RFGB. Hap 1 (T) was significantly correlated with low DH, whereas Hap 2 and Hap 3 (C) exhibited a relatively high DH (Fig. 7B). The rice accessions with the T-base haplotype in SNP19 evidenced early heading date compared to those carrying the C-base haplotype, and the differences in HD between the two haplotypes were highly significant (Fig. 7A, B). Expression analysis of OsSAUR56 in Hap 1 and Hap 3 revealed that Hap 3 had a higher level of OsSAUR56 than Hap 1, and the difference between the two haplotypes reached a significant level (Fig. 7C). For the other SNPs in the promoter region, no significant differences in HD were detected. We assessed the latitudinal distribution of 231 accessions by drawing a scatter diagram according to their latitude of origins (Fig. 7D). The accessions with Hap 1 mainly came from higher latitude regions compared to the other two haplotypes accessions (P < 0.05; Fig. 7E).
Fig. 7.
Haplotypes of OsSAUR56 associated with heading date in rice. A Haplotype distribution in 231 natural rice accessions. B Distribution of HD of different SNP19 haplotypes (T/C) analyzed using 2832 accessions from the rice 3 k database. C Expression of OsSAUR56 in Hap 1 and Hap 3. **P < 0.01 (Student’s t-test). D Scatter diagram of the latitudes (sorted ascending) of the origins of accessions carrying the three haplotypes. E Comparison of latitude distributions among accessions carrying the three haplotypes. Letters above the bars are ranked by Duncan test at P < 0.05; different letters indicate significant difference. Values = means ± SEs
Discussion
Flowering is an important trait in plants, and the timing of flowering plays a crucial role in growth and successful sexual reproduction (Feng et al. 2014). Controlling the flowering time in rice is of great importance for improving rice yield. Previous studies highlighted the positive role of SAURs in promoting flowering in various plants, such as the mediation of the heliotropic movement in young sunflowers by SAUR50-like genes and the involvement of SAUR32, SAUR36, and SAUR72 genes in the flowering process of water lilies (Atamian et al. 2016; Ke et al. 2018; Zhang et al. 2020). However, the function of SAUR genes in rice flowering remains poorly understood. In this study, we found that the mutation of OsSAUR56 promoted flowering in rice. To date, only OsSAUR33, OsSAUR39, and OsSAUR45 have been identified in rice. OsSAUR33 regulated germination rate and seedling emergence uniformity (Zhao et al. 2021), OsSAUR39 regulated plant height and yield (Kant et al. 2009), and OsSAUR45 regulated plant height, and root number, and length (Xu et al. 2017). To our knowledge, this study represents the first report highlighting the involvement of a SAUR gene in the regulation of heading date in rice.
Rice exhibits two major photoperiodic flowering pathways: the Hd1- and Ehd1-mediated pathways (Tsuji et al. 2010). The Hd1-mediated pathway shares conservation with the CO-mediated pathway in Arabidopsis. OsGI promotes the expression of Hd1, which subsequently induces the transcription of Hd3a and RFT1 to promote flowering under SD conditions. However, Hd1 down-regulates the expression of Hd3a and RFT1 under LD conditions (Yano et al. 2000). Additionally, Hd1 also participates in the Ehd1-mediated pathway (Doi et al. 2004). Ghd7 interacts with Hd1 to form a complex that represses the transcription Ehd1 by binding to its promoter under LD conditions (Yasue et al. 2016). In the Ehd1-mediated pathway, Ghd7 and Ehd1 act as unique flowering regulators, with Ghd7 serving as a flowering suppressor and Ehd1 as a flowering activator (Doi et al. 2004). Additionally, DTH8 acts as a key floral repressor under LD conditions. Recent studies have demonstrated that DTH8 interacts with Hd1 to repress the expression of Hd3a by modulating H3K27me3 levels in the Hd3a promoter (Du et al. 2017). Ghd7 can also interact with DTH8. It has been reported that Ghd7, Hd1, and DTH8 form a protein complex that mediates photoperiodic heading (Cai et al. 2019).
Most SAUR genes exhibit circadian expression patterns and bind to clock genes (Van et al. 2017). The regulation of flowering time is influenced by clock-modulated photoperiodic pathways (Shim et al. 2017). In this study, loss-function of OsSAUR56 resulted in insensitivity to photoperiod and promoted heading regardless of day length. Previous studies have evidenced that the clock gene OsGI is a major positive flowering regulator under SD conditions and enhances the expression of several flowering regulatory genes, including Hd1, Ehd1, Hd3a, and RFT1 (Fig. 3) (Ryosuke et al. 2003; Hironori et al. 2010). This indicates that OsSAUR56 may induce flowering under SD conditions, mainly by activating OsGI.
Auxins regulate plant flowering through a comprehensive process involving biosynthesis, transport, and signal transduction (Dong et al. 2021; Li et al. 2015). As an early-response gene to auxin, OsSAUR56 could potentially influence the expression of OsGI and Hd1 through the auxin signal transduction pathway. Moreover, the expression level of Ghd7 is significantly lower in SD (Xue et al. 2008), which leads to the reduced formation of Hd1-Ghd7 and Hd1-DTH8 complexes. As a result, Hd1 promoted the heading date under SD conditions (Yasue et al. 2016; Zhang et al. 2017). The increased expression of OsGI, Hd1, and Ehd1 resulted in elevated expression of HD3a and RFT1, which may have affected the heading date in the mutants under ASD conditions. However, OsGI has been reported to have a weak effect on flowering time under LD conditions because its expression is relatively low during the transition to flowering (Lee and An 2015). Hd1 down-regulated Hd3a and RFT1 under LD conditions (Yano et al. 2000).
In this study, the transcription levels of OsGI and Hd1 were up-regulated in the mutants under LD conditions, whereas the transcription levels of Ehd1, Hd3a, and RFT1 did not decrease. This may have been caused by decreased expression of two major flowering repressors Ghd7 and DTH8 under LD conditions. Recent studies have revealed that both Ghd7 and DTH8 can interact with Hd1; DTH8 interacts with Ghd7. DTH8-Hd1 and Ghd7-Hd1 complexes are necessary for the floral inhibition of Hd1 in LD (Cai et al. 2019; Zong et al. 2020). Although Hd1 was up-regulated in the mutants under LD conditions in the present study, the decreased expression levels of Ghd7 and DTH8 weakened the inhibitory effect of Hd1 on EHD1, Hd3a, and RFT1, resulting in early flowering. Another finding was that OsGI promoted Ghd7 degradation in a 26S-dependent manner, promoting flowering (Zheng et al. 2019). Thus, the combined effect of increased OsGI and reduced Ghd7 and DTH8 may result in elevated Ehd1, which resulted in the up-regulation of HD3a and RFT1, facilitating early flowering under LD conditions.
Additionally, we found that the height of ossaur56 mutant plants increased significantly, which may result from the SAUR family genes mediating auxin synthesis and transportation (Xu et al. 2017). In the present study, we observed the absence of a diurnal peak in Ehd1 and Hd3a expression, which deviated from previous reports. Plausible explanations for this disparity revolve around two distinctive inhibitors of Ehd1, namely, Ghd7 and DTH8 (Zong et al. 2020; Du et al. 2017) exhibiting maximal activity during the early hours of the day (8:00 a.m.), followed by a gradual decline. Consequently, this temporal modulation may impede the timely increase in Ehd1 acme. Given its role as an influential instigator of Hd3a (Zhao et al. 2015; Doi et al. 2004), the postponed culmination of Ehd1 may cause a corresponding delay in the peak of Hd3a.
The promoter is located upstream of the gene coding region and contains many cis-acting elements (CRE), and transcriptional regulation is mainly determined by the promoter CREs (Zou et al. 2011). Haplotype analysis based on the promoter sequence showed that SNP19 (T) at the position of 19633316 bp on chromosome 10 may account for the decreased expression of OsSAUR56 in ZH11. The SNP19 (T/C) is located in the GGTTAA motif structure in the upstream promoter of OsSAUR56. The TGTCACA motif is the core sequence of the light responsive element GT1 that is critical for GT-1-binding activity, which is involved in the regulation of light-dependent target genes. Changes in the TGTCACA motif may reduce the photoperiod sensitivity of plants, leading to early heading of rice under both SD and LD conditions (Lee and Hahn 2002); how light responsive elements regulate the expression of OsSAUR56 remains to be validated. Most (93% and 89%) of the rice varieties carrying the T-base haplotype in SNP19 were Japonica, whereas most (73% and 96%) varieties carrying the C-base haplotype in SNP19 were Indica in 2832 accessions from RFGB and 231 accessions in this study (Fig. 6C, D). These ratios suggested that SNP19 (T/C) may be a key locus for Indica-Japonica differentiation in rice.
During the evolution of rice, the regional adaptability of cultivated rice was affected by its response to daylight duration (Takeshi 2007). The heading date is determined by various internal and external signals, including light time, temperature, and hormones (Luan et al. 2009). The sensitivity of rice to photoperiod and temperature differs, causing rice lines to vary greatly in different areas (Yuan et al. 2022). Many rice lines in tropical and subtropical regions have a strong photoperiod sensitivity to flowering. This strong photoperiod sensitivity completely inhibited heading under long day conditions, allowing heading to be induced only under short daylight conditions (Zong et al. 2020). From the haplotype analysis, we also found that the latitudinal distribution of rice germplasms and the haplotypes of OsSAUR56 were associated with the heading date.
In the present study, we assessed the geographical distribution of 231 accessions, which were divided into 2–5 subsets according to their origins (Fig. 6A, B). Most of the accessions in Hap 2 and Hap 3 mainly fell into Indica subset coming from lower latitude regions, including several major rice growing belts, whereas Hap 1 comprised more Japonica rice accessions, mainly distributed at high latitudes. The differential expression of OsSAUR56 in Hap 1 and Hap 3 indicated that OsSAUR56 may be an important factor to distinguish Hap 1 and Hap 3 and control heading date (Fig. 7C). There were obvious differences in latitude distribution among them (Figs. 6 and 7), indicating that the accessions with Hap 1 may be less sensitive to the photoperiod and have expanded more greatly than the other rice haplotypes. During long-term evolution, rice may have evolved adaptability to different environments and latitudes. These data suggest that, during the extension of rice cultivation to higher latitude regions, the Hap 1 of OsSAUR56 should be selected as much as possible for growth adaptation under LD conditions. Adaptation of rice to northern regions is associated with the attenuation or loss of function of several LD-specific repressor genes for flowering, such as Ghd7 (Xue et al. 2008), DTH8/Ghd8/Hd5 (Wei et al. 2010; Yan et al. 2011; Kenji et al. 2013), OsPRR37/Ghd7.1 (Koo et al. 2013; Yan et al. 2013), and Hd1 (Yano et al. 2000; Takeshi 2007). Longer day-length conditions greatly enhanced the activities of LD-specific repressor genes, leading to stronger suppression in the expression of Ehd1 and Hd3a (Zhao et al. 2015). Therefore, the loss or weakening of functional OsSAUR56, which may down-regulate repressor genes under LD conditions, is essential for promoting floral transition for rice cultivation in northern regions. We speculated that the heading date of Indica rice could be shortened by introducing Hap 1 from Japonica into Indica rice accessions.
Conclusion
In the present study, we constructed mutants of OsSAUR56 by CRISPR/Cas9 editing technology and found an earlier flowering phenotype, with no negative effects on yield under NLD and ASD conditions. Further analysis revealed that OsSAUR56 may control the flowering time by affecting the expression of the clock gene OsGI and two repressors, Ghd7 and DTH8. In addition, we identified an excellent early heading haplotype that could be used for breeding. Our study provides a valuable genetic resource aimed at reducing the heading date in rice.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
Z.Z. and T.C. designed the research. Z.Z. and J.Y. performed the experiments. L.L., T.G., M.H., and W.X. provided lab support. N.P. and J.L. analyzed the data. Z.Z. and T.C. wrote the manuscript. All authors read and approved the manuscript.
Funding
This research was supported by the Seed Industry Revitalization Project of Guangdong Provincial Rural Revitalization Strategy Special Fund (2022-NPY-00–001) and the Heyuan Branch, Guangdong Laboratory for Lingnan Modern Agriculture Project (DT20220012 and DT20220002) of China.
Data availability
Not applicable.
Declarations
Ethics approval
All authors approved the submission.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhe Zhao and Tengkui Chen contributed equally to this work.
Contributor Information
Tao Guo, Email: guoguo@scau.edu.cn.
Wuming Xiao, Email: heredity24@126.com.
References
- Ahmad S, Lu CQ, Wei YL, Gao J, Jin JP, Zheng CY, Zhu GF, Yang FX. Stage specificity, the dynamic regulators and the unique orchid Arundina graminifolia. Int J Mol Sci. 2021;22(20):10935. doi: 10.3390/ijms222010935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atamian HS, Creux NM, Brown EA, Garner AG, Blackman BK, Harmer SL. Circadian regulation of sunflower heliotropism, floral orientation, and pollinator visits. Science. 2016;353(6299):587–590. doi: 10.1126/science.aaf9793. [DOI] [PubMed] [Google Scholar]
- Cai MH, Chen SH, Wu MM, Zheng TH, Zhou L, Li CN, Zha H, Wang JC, Xu XY, Chai JT. Early heading 7 interacts with DTH8, and regulates flowering time in rice. Plant Cell Rep. 2019;38(5):521–532. doi: 10.1007/s00299-019-02380-7. [DOI] [PubMed] [Google Scholar]
- Chae K, Isaacs Cameron G, Reeves Paul H, Maloney Gregory S, Muday Gloria K, Nagpal P, Reed Jason W. Arabidopsis small auxin up RNA63 promotes hypocotyl and stamen filament elongation. Plant J. 2012;71(4):684–697. doi: 10.1111/j.1365-313X.2012.05024.x. [DOI] [PubMed] [Google Scholar]
- Copeland C. SAUR15 connects auxin perception to lateral and adventitious root formation. Plant Physiol. 2020;184(2):558–559. doi: 10.1104/pp.20.01089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, Coupland G. Ft protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science. 2007;316(5827):1030–1033. doi: 10.1126/science.1141752. [DOI] [PubMed] [Google Scholar]
- Doi K, Izawa T, Fuse T, Yamanouchi U, Kubo T, Shimatani Z, Yano M, Yoshimura A. Ehd1, a B-type response regulator in rice, confers short-day promotion of flowering and controls FT-like gene expression independently of Hd1. Gene Dev. 2004;18(8):926–936. doi: 10.1101/gad.1189604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XX, Li YJ, Guan YH, Wang SX, Luo H, Li XM, Li H, Zhang ZH. Auxin-induced AUXIN RESPONSE FACTOR4 activates APETALA1 and FRUITFULL to promote flowering in woodland strawberry. Hortic Res. 2021;8:115. doi: 10.1038/s41438-021-00550-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du A, Tian W, Wei M, Yan W, He H, Zhou D, Huang X, Li S, Ouyang X. The DTH8-Hd1 module mediates day-length-dependent regulation of rice flowering. Mol Plant. 2017;10(7):948–961. doi: 10.1016/j.molp.2017.05.006. [DOI] [PubMed] [Google Scholar]
- Feng ZM, Zhang L, Yang CY, Wu T, Lv J, Chen YL, Liu X, Liu SJ, Jiang L, Wan JM. EF8 is involved in photoperiodic flowering pathway and chlorophyll biogenesis in rice. Plant Cell Rep. 2014;33:2003–2014. doi: 10.1007/s00299-014-1674-8. [DOI] [PubMed] [Google Scholar]
- Foley JA, Ramankutty N, Brauman KA, Cassidy ES, Gerber JS, Johnston M, Mueller ND, O’Connell C, Ray DK, West PC. Solutions for a cultivated planet. Nature. 2011;478(7369):337–342. doi: 10.1038/nature10452. [DOI] [PubMed] [Google Scholar]
- He SL, Hsieh HL, Jauh GY. SMALL AUXIN UP RNA62/75 are required for the translation of transcripts essential for pollen tube growth. Plant Physiol. 2018;178(2):626–640. doi: 10.1104/pp.18.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hironori I, Yasunori N, Masahiro Y, Takeshi I. A pair of floral regulators sets critical day length for Hd3a florigen expression in rice. Nat Genet. 2010;42(7):635–638. doi: 10.1038/ng.606. [DOI] [PubMed] [Google Scholar]
- Hou K, Wu W, Gan SS. SAUR36, a small auxin up RNA gene, is involved in the promotion of leaf senescence in Arabidopsis. Plant Physiol. 2013;161(2):1002–1009. doi: 10.1104/pp.112.212787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Wang Y, Wang PR, Li CM, Xiao FL, Chen NG, Li N, Li CX, Sun CH, Li LH, Chen RJ, Xu ZJ, Zhu JQ, Deng XJ. A single nucleotide mutation of IspF gene involved in the MEP pathway for isoprenoid biosynthesis causes yellow-green leaf phenotype in rice. Plant Mol Biol. 2018;96:5–16. doi: 10.1007/s11103-017-0668-7. [DOI] [PubMed] [Google Scholar]
- Kant S, Bi YM, Zhu T, Rothstein SJ. SAUR39, a small auxin-up RNA gene, acts as a negative regulator of auxin synthesis and transport in rice. Plant Physiol. 2009;151(2):691–701. doi: 10.1104/pp.109.143875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke M, Gao Z, Chen J, Qiu Y, Zhang L, Chen X. Auxin controls circadian flower opening and closure in the waterlily. BMC Plant Biol. 2018;18(1):1–21. doi: 10.1186/s12870-018-1357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenji F, Utako Y, Masahiro Y. Roles of the Hd5 gene controlling heading date for adaptation to the northern limits of rice cultivation. Theor Appl Genet. 2013;126:611–618. doi: 10.1007/s00122-012-2005-5. [DOI] [PubMed] [Google Scholar]
- Koo B, Yoo S, Park J, Kwon C, Lee B, An G, Zhang Z, Li J, Li Z, Paek N. Natural variation in OsPRR37 regulates heading date and contributes to rice cultivation at a wide range of latitudes. Mol Plant. 2013;6(6):1877–1888. doi: 10.1093/mp/sst088. [DOI] [PubMed] [Google Scholar]
- Lee Y, An G. OsGI controls flowering time by modulating rhythmic flowering time regulators preferentially under short day in rice. J Plant Biol. 2015;58:137–145. doi: 10.1007/s12374-015-0007-y. [DOI] [Google Scholar]
- Lee S, Hahn T. Two light-responsive elements of pea chloroplastic fructose-1,6-bisphosphatase gene involved in the red-light-specific gene expression in transgenic tobaccos. BBA-Gene Struc Exp. 2002;1579(1):8–17. doi: 10.1016/S0167-4781(02)00498-0. [DOI] [PubMed] [Google Scholar]
- Liu W, Xie X, Ma X, Li J, Chen J, Liu Y. Dsdecode: a web-based tool for decoding of sequencing chromatograms for genotyping of targeted mutations. Mol Plant. 2015;8(9):1431–1433. doi: 10.1016/j.molp.2015.05.009. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luan WJ, Chen HZ, Fu YP, Si HM, Peng W, Song SS, Liu WZ, Hu GC, Sun ZX, Xie DX. The effect of the crosstalk between photoperiod and temperature on the heading-date in rice. Plos One. 2009;4:e5891. doi: 10.1371/journal.pone.0005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magali L, Patrice D, Gert T, Kathleen M, Yves M, de Peer V, Yves PR, Stephane R. PlantCare, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30(1):325–327. doi: 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai YX, Wang L, Yang HQ. A gain-of-function mutation in IAA7/AXR2 confers late flowering under short-day light in Arabidopsis F. J Integr Plant Biol. 2011;53(6):480–492. doi: 10.1111/j.1744-7909.2011.01050.x. [DOI] [PubMed] [Google Scholar]
- Reina K, Shuji Y, Ko S. A gene network for long-day flowering activates RFT1 encoding a mobile flowering signal in rice. Deve (cambridge, England) 2009;136(20):3443–3450. doi: 10.1242/dev.040170. [DOI] [PubMed] [Google Scholar]
- Ren H, Gray WM. SAUR proteins as effectors of hormonal and environmental signals in plant growth. Mol Plant. 2015;8(8):1153–1164. doi: 10.1016/j.molp.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SO, Bendich AJ. Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues. Plant Mol Biol. 1985;5:69–76. doi: 10.1007/BF00020088. [DOI] [PubMed] [Google Scholar]
- Roshi S, Jorge G, Vittoria B, Fabio F. Molecular control of seasonal flowering in rice. Arabidopsis Temperate Cereals Ann Bot. 2014;114(7):1445–1458. doi: 10.1093/aob/mcu032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux F, Touzet P, Cuguen J, Le Corre V. How to be early flowering: an evolutionary perspective. Trends Plant Sci. 2006;11(8):375–381. doi: 10.1016/j.tplants.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Ryosuke H, Shuji Y, Shojiro T, Masahiro Y, Ko S. Adaptation of photoperiodic control pathways produces short-day flowering in rice. Nature. 2003;422(6933):719–722. doi: 10.1038/nature01549. [DOI] [PubMed] [Google Scholar]
- Shim JS, Kubota A, Imaizumi T. Circadian clock and photoperiodic flowering in Arabidopsis: CONSTANS is a hub for signal integration. Plant Physiol. 2017;173(1):5–15. doi: 10.1104/pp.16.01327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YH, Shim JS, Kinmonth-Schultz HA, Imaizumi T. Photoperiodic flowering: time measurement mechanisms in leaves. Annu Rev of Plant Biol. 2015;66:441–464. doi: 10.1146/annurev-arplant-043014-115555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshi I. Adaptation of flowering-time by natural and artificial selection in Arabidopsis and rice. J ExpBot. 2007;58(12):3091–3097. doi: 10.1093/jxb/erm159. [DOI] [PubMed] [Google Scholar]
- Takeshi I, Motohiro M, Yuji S, Meenu G, Hironori I, Nagano AJ, Ritsuko M, Yuji S, Masahiro Y, Yokota HM, Amane M, Yoshiaki N. Os-GIGANTEA confers robust diurnal rhythms on the global transcriptome of rice in the field. Plant Cell. 2011;23(5):1741–1755. doi: 10.1105/tpc.111.083238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K. Hd3a protein is a mobile flowering signal in rice. Science. 2007;316(5827):1033–1036. doi: 10.1126/science.1141753. [DOI] [PubMed] [Google Scholar]
- Tsuji H, Taoka K, Shimamoto K. Regulation of flowering in rice: two florigen genes, a complex gene network, and natural variation. Curr Opin Plant Biol. 2010;14(1):45–52. doi: 10.1016/j.pbi.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Van MH, Van D, Stortenbeker N, Angenent GC, Bemer M. Divergent regulation of Arabidopsis SAUR genes: a focus on the SAUR10-clade. BMC Plant Biol. 2017;17:245. doi: 10.1186/s12870-017-1210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar L, Lienqueo I, Llanes A, Rojas P, Perez J, Correa F, Sagredo B, Masciarelli O, Luna V, Almada R. Comparative transcriptomic analysis reveals novel roles of transcription factors and hormones during the flowering induction and floral bud differentiation in sweet cherry trees. PLOS ONE. 2020;15(3):e0230110. doi: 10.1371/journal.pone.0230110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CC, Yu H, Huang J, Wnag WS, Faruquee M, Zhang F, Zhao XQ, Fu BY, Chen K, Zhang HL, Tai SS, Wei CC, McNally KL, Alexandrov N, Gao XY, Li JY, Li ZK, Xu JL, Zheng TQ. Towards a deeper haplotype mining of complex traits in rice with RFGB v2.0. Plant Biotechnol J. 2020;18(1):114. doi: 10.1111/pbi.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei XJ, Xu JF, Guo HN, Jiang L, Chen SH, Yu CY, Zhou ZL, Hu PS, Zhai HQ, Wan JM. DTH8 suppresses flowering in rice, influencing plant height and yield potential simultaneously. Plant Physiol. 2010;153(4):1747–1758. doi: 10.1104/pp.110.156943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia K, Wang R, Ou XJ, Fang ZM, Tian CE, Duan J, Wang YQ, Zhang MY. OsTIR1 and OsAFB2 downregulation via OsmiR393 overexpression leads to more tillers, early flowering and less tolerance to salt and drought in rice. PloS one. 2012;7(1):e30039. doi: 10.1371/journal.pone.0030039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie K, Minkenberg B, Yang Y (2015) Boosting CRISPR/Cas9 multiplex editing capability with the endogenous tRNA-processing system . Proc Natl Acad Sci 112(11):3570–3575. 10.1073/pnas.1420294112 [DOI] [PMC free article] [PubMed]
- Xie X, Ma X, Zhu Q, Zeng D, Li G, Liu Y. CRISPR-GE: a convenient software toolkit for CRISPR-based genome editing. Mol Plant. 2017;10(9):1246–1249. doi: 10.1016/j.molp.2017.06.004. [DOI] [PubMed] [Google Scholar]
- Xu YX, Xiao MZ, Liu Y, Fu JL, He Y, Jiang DA. The small auxin-up RNA OsSAUR45 affects auxin synthesis and transport in rice. Plant Mol Biol. 2017;94:97–107. doi: 10.1007/s11103-017-0595-7. [DOI] [PubMed] [Google Scholar]
- Xue WY, Xing YZ, Weng XY, Zhao Y, Tang WJ, Wang L, Zhou HJ, Yu SB, Xu CG, Li XH. Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet. 2008;40(6):761–767. doi: 10.1038/ng.143. [DOI] [PubMed] [Google Scholar]
- Yan W, Wang P, Chen H, Zhou H, Li Q, Wang C, Ding Z, Zhang Y, Yu S, Xing Y, Zhang Q. A major QTL, Ghd8, plays pleiotropic roles in regulating grain productivity, plant height, and heading date in rice. Mol Plant. 2011;4(2):319–330. doi: 10.1093/mp/ssq070. [DOI] [PubMed] [Google Scholar]
- Yan WH, Liu HY, Zhou XC, Li QP, Zhang J, Lu L, Liu TM, Liu HJ, Zhang CJ, Zhang ZY, Shen GJ, Yao W, Chen HX, Yu SB, Xie WB, Xing YZ. Natural variation in Ghd7.1 plays an important role in grain yield and adaptation in rice. Cell Res. 2013;23(7):969–971. doi: 10.1038/cr.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M, Katayose Y, Ashikari M, Yamanouchi U, Lisa M, Fuse T, Baba T, Yamamoto K, Umehara Y, Nagamura Y. Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene constans. Plant Cell. 2000;12(12):2473–2483. doi: 10.1105/tpc.12.12.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasue N, Yasunori N, Masahiro Y, Takeshi I. Hd1, a CONSTANS ortholog in rice, functions as an Ehd1 repressor through interaction with monocot-specific CCT-domain protein Ghd7. Plant J. 2016;86(3):221–233. doi: 10.1111/tpj.13168. [DOI] [PubMed] [Google Scholar]
- Yuan HR, Wang RH, Cheng MX, Wei X, Wang W, Fan FF, Zhang LC, Wang ZK, Tian ZH, Li SQ. Natural variation of OsHd8 regulates heading date in rice. Agronomy. 2022;12(10):2260. doi: 10.3390/agronomy12102260. [DOI] [Google Scholar]
- Zhang Y, Su JB, Duan S, Ao Y, Dai JR, Liu J, Wang P, Li YG, Liu B, Feng DR, Wang JF, Wang HB (2011) A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes . Plant Methods 7(1):1–14. 10.1186/1746-4811-7-30 [DOI] [PMC free article] [PubMed]
- Zhang J, Zhou XC, Yan WH, Zhang ZY, Lu L, Han ZM, Zhao H, Liu HY, Song P, Hu Y. Combinations of the Ghd7, Ghd8 and Hd1 genes largely define the ecogeographical adaptation and yield potential of cultivated rice. New Phytol. 2015;208(4):1056–1066. doi: 10.1111/nph.13538. [DOI] [PubMed] [Google Scholar]
- Zhang ZY, Hu W, Shen GJ, Liu HY, Hu Y, Zhou XC, Liu TM, Xing YZ. Alternative functions of Hd1 in repressing or promoting heading are determined by Ghd7 status under long-day conditions. Sci Rep. 2017;7(5388):1–11. doi: 10.1038/s41598-017-05873-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LS, Chen F, Zhang XT, Li Z, Zhao YY, Lohaus R, Chang XJ, Dong W, Ho SYW, Liu X. The water lily genome and the early evolution of flowering plants. Nature. 2020;577(7788):79–84. doi: 10.1038/s41586-019-1852-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Chen HG, Ren D, Tang HW, Qiu R, Feng JL, Long YM, Niu BX, Chen DP, Zhong TY, Liu YG, Guo JG. Genetic interactions between diverged alleles of Early heading date 1 (Ehd1) and Heading date 3a (Hd3a)/ RICE FLOWERING LOCUS T1 (RFT1) control differential heading and contribute to regional adaptation in rice (Oryza sativa) New Phytol. 2015;208(3):936–948. doi: 10.1111/nph.13503. [DOI] [PubMed] [Google Scholar]
- Zhao J, Li WJ, Sun S, Peng Li L, Huang ZB, He YQ, Wang ZF. The rice small auxin-up RNA gene OsSAUR33 regulates seed vigor via sugar pathway during early seed germination. Int J Mol Sci. 2021;22(4):1562. doi: 10.3390/ijms22041562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng TH, Sun J, Zhou SR, Chen SH, Lu J, Cui S, Tian YL, Zhang H, Cai MH, Zhu SS, Wu MM, Wang YH, Jiang L, Zhai HQ, Wang HY, Wan JM. Post-transcriptional regulation of Ghd7 protein stability by phytochrome and OsGI in photoperiodic control of flowering in rice. New Phytol. 2019;224(1):306–320. doi: 10.1111/nph.16010. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Wu W, Wang H, Chen L, Liu L, Wang C, Zhao Z, Lu G, Gao H, Wei X, Yu C, Chen M, Shen Y, Zhang X, Cheng Z, Wang J, Jiang L, Wan J. Fine mapping of a minor-effect QTL, DTH12, controlling heading date in rice by up-regulation of florigen genes under long-day conditions. Mol Breeding. 2014;34:311–322. doi: 10.1007/s11032-014-0035-1. [DOI] [Google Scholar]
- Zhou X, Wang LL, Yan JP, Ye JB, Cheng SY, Xu F, Wang GY, Zhang WW, Liao YL, Liu XM. Functional characterization of the EMBRYONIC FLOWER 2 gene involved in flowering in Ginkgo biloba. Front Plant Sci. 2021;12:681166. doi: 10.3389/fpls.2021.681166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong WB, Ren D, Huang MH, Sun KL, Feng JL, Zhao J, Xiao DD, Xie WH, Liu SQ, Zhang H. Strong photoperiod sensitivity is controlled by cooperation and competition among Hd1, Ghd7 and DTH8 in rice heading. New Phytol. 2020;229(3):1635–1649. doi: 10.1111/nph.16946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou C, Sun K, Mackaluso JD, Shiu SH. Cis-regulatory code of stress-responsive transcription in Arabidopsis thaliana. Proc Natl Acad Sci. 2011;108(36):14992–14997. doi: 10.1073/pnas.1103202108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.