Abstract
The world has faced unprecedented disruptions like global quarantine and the COVID-19 pandemic due to SARS-CoV-2. To combat these unsettling situations, several effective vaccines have been developed and are currently being used. However, the emergence of new variants due to the high mutation rate of SARS-CoV-2 challenges the efficacy of existing vaccines and has highlighted the need for novel vaccines that will be effective against various SARS-CoV-2 variants. In this study, we exploited the four structural proteins of SARS-CoV-2 to execute a potential multi-epitope vaccine against SARS-CoV-2 and its variants. The vaccine was designed by utilizing the antigenic, non-toxic, and non-allergenic B-cell and T-cell epitopes, which were selected from conserved regions of viral proteins. To build a vaccine construct, epitopes were connected through different linkers and an adjuvant was also attached at the start of the construct to enhance the immunogenicity and specificity of the epitopes. The vaccine construct was then screened through the aforementioned filters and it scored 0.6019 against the threshold of 0.4 on VexiJen 2.0 which validates its antigenicity. Toll-like receptors (i.e., TLR2, TLR3, TLR4, TLR5, and TLR8) and vaccine construct were docked by Cluspro 2.0, and TLR8 showed strong interaction with construct having a maximum negative binding energy of − 1577.1 kCal/mole. C-IMMSIM's immune simulations over three doses of the vaccine and iMODS' molecular dynamic simulations were executed to assess the reliability of the docked complexes. The stability of the vaccine construct was evaluated through the physicochemical analyses and the findings suggested that the manufactured vaccine is stable under a wide range of circumstances and can trigger immune responses against various SARS-CoV-2 variants (due to conserved epitopes). However, to strengthen the formulation of the vaccine and assess its safety and effectiveness, additional investigations and studies are required to support the computational data of this research at in-vitro and in-vivo levels.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40203-023-00156-2.
Keywords: SARS-COV-2, Spike protein, Membrane protein, Nucleocapsid, Envelope protein, Immune simulation, Molecular docking, Multi-epitope
Introduction
COVID-19, a disease caused by the SARS-CoV-2 virus (Severe Acute Respiratory Syndrome Coronavirus 2), emerged as a result of a pneumonia outbreak in Wuhan, China. This extremely contagious disease quickly spread to 191 nations and affected over 75 million individuals, leading to more than 1.5 million fatalities. It was officially declared a pandemic and an international health crisis around the world (Abebe et al. 2020). Victims of COVID-19 who contracted the viral infection caused by SARS-CoV2 experienced a common cold as well as fever-like symptoms comparable to viral rhinitis (common cold); a small percentage of patients also developed a lethal type of pneumonia (Boopathi et al. 2021; Nguyen et al. 2020; Vankadari et al. 2020). Furthermore, COVID-19 patients admitted to the intensive care unit (ICU) were reported for abdominal pain, anorexia, and cerebrovascular and cardiovascular disease (Chan et al. 2020; Wang et al. 2020). Infected individuals play an important role in human-to-human transmission because they can spread the virus to others by sneezing, coughing, exhaling, and a variety of other mechanisms (Bhattacharya et al. 2020; Chan et al. 2020).
The + ve sense single-stranded RNA (+ + ssRNA) genome of SARS-CoV-2 is approximately 29.8–29.9 kb in size, with a 5′ end cap and a poly-A tail at the 3′ end. Its genomic sequence is 79.5% and 96% similar to bat coronavirus and SARS-CoV, respectively. Phylogenetic analysis revealed that it belongs to the Coronaviridae family, Nidovirales order, and member 2B group of β-coronavirus (Hui et al. 2020). The SARS-CoV-2 virus is made up of four structurally relevant proteins: spike (S), envelope (E), nucleocapsid (N), and membrane (M). These proteins are critical to viral structure and function. Through mass immunization, several vaccinations were made available to decrease the severity and impact of the COVID-19 pandemic. These clinically authorized vaccines were based on viruses that had been killed or inactivated, recombinant adenovirus, or mRNA of viral spike protein (Dong et al. 2020). The idea of an mRNA-based vaccination has been around for nearly two decades, and recent studies demonstrated its efficiency in both human and animal models against several viral diseases, including Zika, HIV-1, rabies, and influenza virus (Alberer et al. 2017; Bahl et al. 2017; Richner et al. 2017). Up to this point, only two vaccines based on mRNA targeting SARS-CoV2 were successfully created: BNT162b2 (Pfizer) and mRNA-1273 (Moderna) (Baden et al. 2021; Polack et al. 2020).
SARS-CoV-2, like other RNA viruses, has undergone evolutionary changes over time due to the occurrence of spontaneous mutations in multiple viral genes, leading to the emergence of diverse genetic variants. Natural selection has shaped this genetic variation, resulting in the evolution of viral variants with superior immune escape mechanisms, increased virulence, and enhanced transmission (Finkel et al. 2021). To emphasize the comprehensive worldwide monitoring and research efforts, the aforementioned genetic variations were classified as Variants of Interest (VOI) and subsequently as Variants of Concern (VOC). Despite ongoing efforts, the global alert level for COVID-19 remains elevated, primarily due to the emergence of new strains such as Delta, Gamma, and Lambda (Bian et al. 2021; Martin et al. 2021). The Omicron variant first surfaced in Botswana on November 11, 2021, and was swiftly disseminated to numerous other countries, ultimately being detected in more than 57 countries worldwide (Petersen et al. 2022).
There is no denying that the swift accumulation of mutations in SARS-CoV-2 strains will lead to the rapid development of resistance against existing vaccines. Consequently, there will be an annual necessity for new vaccines to safeguard against the constantly changing flu viruses (Kotey et al. 2019). Hence, the objective of this study was to develop a unified vaccine incorporating conserved immunogenic epitopes predicted from M-protein, E-protein, S-protein, and N-protein. This study also measured the physicochemical properties of B-cell and T-cell epitopes to characterize the vaccine and also screened it for any allergic and toxic effects. Molecular docking techniques were employed to examine the interaction between the construct and various Toll-Like Receptors (TLRs) and the stability of docked complexes was ensured by MD simulations or Normal Mode Analysis (NMA). In the final step immune simulations were performed to confirm the antigenicity of the fabricated vaccine by estimating the production of various immune molecules and cells involved in both innate and adaptive immune responses.
Methodology
Following is the complete methodology and details of tools that was applied in this research:
Retrieval of sequences viral proteins
The FASTA format sequences of SARS-COV-2’s structural proteins were retrieved from the UniProt database (available at https://www.uniprot.org/ and accessed on 25 September 2022). The fetched proteins were Spike glycoprotein (S), Envelope small membrane protein (E), Membrane protein (M), and Nucleocapsid or Nucleoprotein (N) (Satarker and Nampoothiri, 2020), each designated with distinct UniProt IDs-P0DTC5, P0DTC4, P0DTC2, and P0DTC9, correspondingly.
Predicting antigenicity of viral proteins
The VaxiJen v2.0 server was utilized to assess the antigenicity scores of the four selected proteins. This server employs an analysis of the physicochemical properties of proteins to predict their antigenicity (available at http://www.ddg-pharmfac.net/vaxijen/ and accessed on 25 September 2022). The recommended threshold value of 0.4 was adjusted to analyze the viral proteins, indicating that proteins with scores above this threshold are considered highly antigenic (Doytchinova and Flower 2007; Naveed et al. 2022).
Predicting physicochemical properties of viral proteins
To gain deeper insights into the physicochemical traits of the selected viral proteins, the ProtParam tool was used which is available on the ExPasy server (available at https://web.expasy.org/protparam/ and accessed on 25 September 2022) (Gasteiger et al. 2005). This tool computes the multiple physical and chemical characteristics of the proteins (i.e., amino acid composition, atomic composition, instability and aliphatic index, extinction coefficient, Grand Average of Hydropathicity (GRAVY), theoretical PI, and half-life), which are essential for understanding the molecular and structural properties of the protein (Dey et al. 2023).
Prediction of B-cell restricted epitopes of viral proteins
Linear B-cell restricted epitopes of selected viral proteins were predicted using the B-cell Antigen Sequence Properties tool of IEDB (available at http://tools.iedb.org/bcell/ and accessed on 26 September 2022). This online tool employed the Bepipred Linear Epitope Prediction 2.0 method, which is designed to predict potential epitope sites in protein sequences. The tool utilized a threshold of 0.500 and a center position of 4, which are the recommended settings for this method. The adjusted threshold is compatible with the Random Forest algorithm that screens protein sequences to predict B-cell confined sequences (Jespersen et al. 2017). This algorithm works in two steps by first determining the results from crystal structures and then reporting the sequential predictions, which can be subjected to several filters for further analysis. This tool helped us in the identification of potential linear B-cell epitopes within the viral proteins of SARS-COV-2.
T-cells-restricted epitopes prediction using IEDB tools
Two tools from IEDB were employed to predict T-cell-specific epitopes. The NetMHCpan EL 4.1 method, which is recommended for forecasting MHC-I binding epitopes, was utilized (available at http://tools.iedb.org/mhci/ and accessed on 26 September 2022) (Reynisson et al. 2020), and for predicting MHC-II binding epitopes IEDB Recommended 2.22 method was executed (available at http://tools.iedb.org/mhcii/ and accessed on 26 September 2022) (Jensen et al. 2018). To maintain consistency throughout the study, the same recommended sets of alleles and applied settings were utilized for both tools. This approach allowed us to predict potential T-cell-restricted epitopes from the selected viral proteins successfully.
Filtration and profiling of predicted epitopes
The most optimal epitopes for the final vaccine design were selected through a rigorous selection process involving four filters. The first filter was the Vaxijen v2.0 tool for predicting antigenicity (Doytchinova and Flower 2007), followed by the Toxinpred tool (available at https://webs.iiitd.edu.in/raghava/toxinpred/algo.php and accessed on 27 September 2022) for toxicity prediction (Sharma et al. 2022). The third filter was the AllerTOP tool for allergenicity prediction (available at https://www.ddg-pharmfac.net/AllerTOP/method.html and accessed on 29 September 2022) (Dimitrov et al. 2013) and finally, the Align tool of the UniProt portal available at https://www.uniprot.org/align (accessed on 29 September 2022) was applied to evaluate the conservancy of the epitopes (Coudert et al. 2022). Only those epitopes that passed through all four filters were selected to ensure the quality of the final vaccine design.
Percentage of filtered epitopes with population coverage
After filtering out the most suitable epitopes for the vaccine design, each epitope was analyzed by the population coverage tool which is integrated into the IEDB server and available at http://tools.iedb.org/population/ (accessed on 30 September 2022) (Bui et al. 2006). This tool allowed us to estimate the proportion of the world population that could potentially respond to each epitope by evaluating the interaction between the filtered epitopes and the maximum number of Major Histocompatibility complex (both type I and II) alleles provided to the tool (Kar et al. 2020).
Constructing the vaccine design
The vaccine was designed to include several components that work together to elicit an immune response against SARS-CoV-2. The design consisted of a 6X histidine marker, an adjuvant, B-cell-specific epitopes, and T-cell-specific epitopes that were connected in a series using specific linkers. To connect the selected epitopes, we used various linkers, including EAAK for B-cell epitopes, GPGPG for MHC-I epitopes, and AAY for MHC-II epitopes (Naveed et al. 2022). The vaccine construct was designed, to begin with the inclusion of the adjuvant Human Beta Defensin 3 with UniProt ID: Q5U7J2, which has been shown to enhance the immune response to vaccines (Ferris et al. 2013). The use of specific linkers to connect the selected epitopes is an important aspect of the in-silico vaccine design as these linkers ensure that the epitopes are presented to the immune system in a way that maximizes their effectiveness (Gokhale and Khosla 2000; Parvizpour et al. 2020).
Profiling of vaccine construct
The newly developed vaccine underwent a thorough profiling process to evaluate its antigenic potential using the VaxiJen v2.0 tool (accessed on 3 October 2022), ensuring that the construct is enough antigenic to initiate a proper immune response against the viral particles (Doytchinova and Flower 2007). After the vaccine construct was created, it underwent a series of analyses to ensure its safety and efficacy. First, the AllerTop v2.0 (accessed on 3 October 2022) and ToxinPred tools (accessed on 3 October 2022) were used to assess the vaccine for any potential allergenicity and toxicity (Dimitrov et al. 2013; Sharma et al. 2022). These analyses are crucial in determining the safety of the vaccine and ensuring that it will not cause any harmful side effects in humans. Additionally, the physicochemical characteristics of the vaccine were analyzed using the ProtParam of the ExPasy server (accessed on 4 October 2022) to evaluate the stability, solubility, and other properties of the vaccine construct (Gasteiger et al. 2005). Finally, the vaccine underwent a secondary structure analysis using the PsiPred available at http://bioinf.cs.ucl.ac.uk/psipred/ (accessed on 4 October 2022) and SOPMA servers (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_sopma.html and accessed on 4 October 2022) to predict protein folding and structural stability (Geourjon and Deléage 1995; McGuffin et al. 2000). These analyses are necessary to ensure the vaccine is safe and enough effective to produce adequate immune responses against the SARS-COV-2 strains (Abraham Peele et al. 2021).
Three-dimensional (3D) modeling and validations
The three-dimensional arrangement of the vaccine construct was determined by utilizing the services of the I-TASSER server available at https://zhanggroup.org/I-TASSER/ and accessed on 6 October 2022 (Zhang 2008). The scrutinized vaccine's 3D model undergoes a series of validations and refinements process using PROCHECK v6.0 (available at https://saves.mbi.ucla.edu/ and accessed on 10 October 2022) and GalaxyRefine (available at http://galaxy.seoklab.org/cgi-bin/submit.cgi?type=REFINE and accessed on 10 October 2022), respectively. The former tool provided the Ramachandran plot for assessment while the latter one was used to adjust the bond angles in the 3D model of the vaccine (Heo et al. 2013; Wlodawer 2017). To augment PROCHECK's validation outcomes, the ProSA-web server available at https://prosa.services.came.sbg.ac.at/prosa.php (accessed on 10 October 2022) was also utilized (Wiederstein and Sippl 2007).
Disulfide engineering of vaccine construct
To evaluate the stability of the vaccine model, we employed the Disulfide tool on Design 2.0 (DbD2), which is available at http://cptweb.cpt.wayne.edu/DbD2/index.php (accessed on 12 October 2022) (Craig and Dombkowski 2013). This online server predicts potential disulfide linkages in the vaccine construct and presents the results in the form of a modified vaccine model (mutant model).
Molecular docking with toll-like receptors (TLRs)
The engagement of Toll-like receptors (TLRs) in the initiation of intracellular signaling pathways that culminate in the release of various immune compounds such as IRF3, NF-κB, inflammatory cytokines, and IL8 during the initial stages of viral infection has been well-established. To further explore this phenomenon, this study used the employed docking analysis of five TLRs, namely TLR2, TLR3, TLR4, TLR5, and TLR8, with 6NIG, 2A0Z, 4G8A, 3J0A, and 4R0A PDB IDs, respectively (Naveed et al. 2022). The Cluspro 2.0 server (available at https://cluspro.bu.edu/login.php?redir=/home.php and accessed on 13 October 2022) was utilized to carry out the docking (Kozakov et al. 2017) and the resulting docked complexes were then analyzed, and visualized using the PyMol software.
Molecular dynamics simulations
The iMODS tool is founded upon Normal Mode Analysis (NMA), a valuable technique for determining the range of conformational states available to a given macromolecule and examining protein stability and mobility on a large scale. In this study, the iMODS server (available at https://imods.iqfr.csic.es/ and accessed on 15 October 2022) was utilized, which render the docked complexes by modifying the force field over different time intervals (López-Blanco et al. 2014).
Codon optimization for virtual cloning
The process of codon optimization for the vaccine was initiated through the use of the Java Codon Adaptation Tool (JCAT) (available at http://www.jcat.de/ and accessed on 26 March 2023) (Grote et al. 2005). This JCAT server algorithmically determines the most appropriate synonymous codons based on specific features of the target organism (E-coli K12), such as codon usage bias, GC content, and other factors. Once the nucleotide sequence was optimized, it was integrated into the pET28a ( +) vector, which is commonly used for protein expression in E. coli, using SnapGene software (Drummond et al. 2022).
C-IMMSIM for immune simulation
The C-IMMSIM platform (available at https://kraken.iac.rm.cnr.it/C-IMMSIM/ and accessed on 29 October 2022) has been developed to anticipate and approximate the potential immune responses that may occur in the human body after the administration of the customized vaccine (Rapin et al. 2010). For this investigation, simulation steps were adjusted to 1050 to achieve the maximum elapsed time. The second modified parameter was the Time Steps of Injection for the second and third booster doses, which were adjusted to 84 and 170, respectively (Naveed et al. 2022).
Results
Physicochemical and antigenicity analysis of viral proteins
In this study, an analysis was conducted on four candidate proteins to evaluate their antigenicity scores, namely E-protein, M-protein, N-protein, and S-protein. The VaxiJen algorithm was used to predict the antigenic nature of the proteins, and the obtained scores were 0.6025, 0.5102, 0.5059, and 0.4646, respectively. The proteins were considered antigenic as their scores exceeded the adjusted threshold. Notably, E-protein exhibited the highest antigenicity score while S-protein had the lowest score among the selected viral proteins. In addition, the physicochemical properties of the proteins were analyzed using ProtParam. The results showed that all four proteins were stable based on their predicted instability index (II). However, the GRAVY scores indicated that S-protein and N-protein were hydrophilic while E-protein and M-protein were hydrophobic. Further details of the physicochemical attributes are provided in Table 1.
Table 1.
List of various physicochemical properties and VaxiJen scores with Corresponding UniProt IDs of selected viral proteins
| Protein | UniProt ID | VaxiJen score | Amino acids | Theoretical PI | Instability index (II) | GRAVY | Aliphatic index |
|---|---|---|---|---|---|---|---|
| Spike | P0DTC2 | 0.4646 | 1273 | 6.24 | 33.01 | − 0.079 | 84.67 |
| Envelope | P0DTC4 | 0.6025 | 75 | 8.57 | 38.68 | 1.128 | 144.00 |
| Membrane | P0DTC5 | 0.5102 | 222 | 9.51 | 39.14 | 0.446 | 120.86 |
| Nucleocapsid | P0DTC9 | 0.5059 | 419 | 10.07 | 55.09 | − 0.971 | 52.53 |
B-cell restricted epitopes predictions
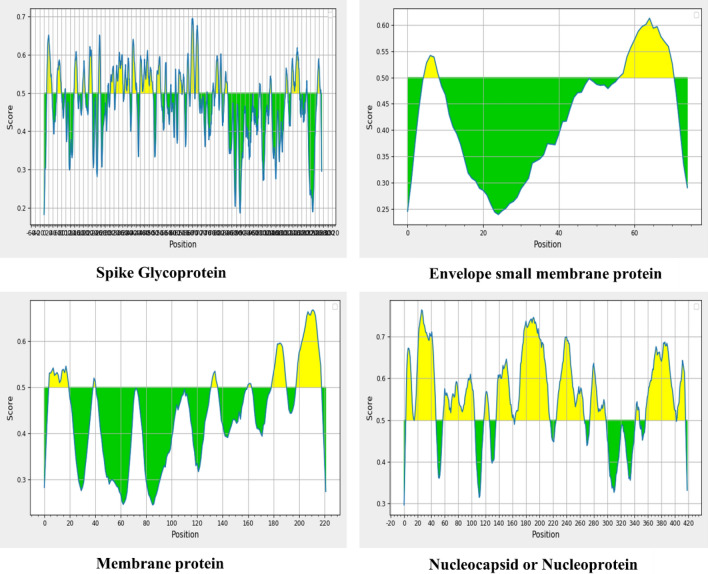
The aim of the B cell epitope prediction was to identify potential antigens capable of efficiently interacting with B lymphocytes, as B-cell epitopes possess modified features that enable B-cells to recognize and activate potent immune responses against viral infections, thereby initiating the production of antibodies from plasma cells (a differentiated form of B-cells). After conducting the analysis, a total of 34 B-cell-specific epitopes were identified for the S-protein, 2 for the E-protein, 6 for the M-protein, and 11 for the N-protein. However, to ensure that only the most promising epitopes were selected for further analysis, we narrowed our focus to those with a length between 9 and 50 amino acids. To facilitate the analysis of these predicted B-cell-restricted epitopes, we have listed all of them in Supplementary Tables S1a, S1b, S1c, and S1d. Additionally, the graphs in Fig. 1 were generated using the IEDB tool, which indicates the prediction of B-cell restricted epitopes based on threshold scores.
Fig. 1.
The graphs indicating the peptide regions (Yellow) that could become the part of epitope as their score is above the threshold (0.5), while the peptide regions below the threshold (Green) are not eligible to become a part of B-cell-restricted epitopes
Predicting the T-cell-specific epitopes
For the identification of potential epitopes that could be restricted to MHC-I and MHC-II of T-cells, the IEDB algorithm was employed. Numerous epitopes were identified for each viral protein, but we selected the top 30 based on their percentile scores. The final three epitopes from the predicted sets of MHC-I and MHC-II epitopes were filtered out for each viral protein. Using this approach, a total of 12 MHC-I restricted epitopes (three from each protein) and an equivalent number of MHC-II restricted epitopes were selected for the final vaccine construct. The epitopes were predicted based on the allele sets, which are provided in Supplementary Table S2a and S2b for both classes of MHCs (I & II), along with their respective peptide lengths.
Filtration and profiling of predicted epitopes
In order to improve the efficacy of the final vaccine construct, a set of four filters were employed to screen the predicted epitopes, ensuring their antigenicity, non-toxicity, non-allergenicity, conservancy, and length ranging from 9 to 50 amino acids, this length filter was only applied on B-cell-restricted linear epitopes. Among the 34 predicted B-cell epitopes for the spike protein, only four met the filtering criteria, while in the case of the envelope protein, only one out of two epitopes stand the test of filters. For the nucleoprotein, although 11 B-cell-restricted epitopes were identified, only three passed the filter tests and were chosen for further analysis and vaccine development. In contrast, all six B-cell-restricted linear epitopes predicted for the membrane protein were rejected by the filters, resulting in the exclusion of B-cell epitopes of this protein from the final vaccine construct. After applying these filters to the top 30 MHC-I and MHC-II restricted T-cell epitopes only three epitopes with elevated antigenicity scores were picked from each protein as the part of final vaccine construct. As a result, a total of 12 MHC-I restricted T-cell epitopes and 12 MHC-II restricted T-cell epitopes were included in the vaccine construct. Details of the epitope filtering process can be found in Tables 2, 3, and 4, and Supplementary Figures S1a, S1b, S1c, and S1d provide the conservancy analysis.
Table 2.
List of selected B-cell restricted epitopes and results of various applied filters
| Start | End | Peptide | Length | Vaxijen score | Antigenicity | Toxicity | Allergenicity | Conservancy |
|---|---|---|---|---|---|---|---|---|
| Spike glycoprotein | ||||||||
| 206 | 221 | KHTPINLVRDLPQGFS | 16 | 0.6403 | Antigen | Non-toxin | Non-allergen | Conserved |
| 369 | 393 | YNSASFSTFKCYGVSPTKLNDLCFT | 25 | 1.4031 | Antigen | Non-toxin | Non-allergen | Conserved |
| 404 | 426 | GDEVRQIAPGQTGKIADYNYKLP | 23 | 1.1017 | Antigen | Non-toxin | Non-allergen | Conserved |
| 656 | 666 | VNNSYECDIPI | 11 | 0.6124 | Antigen | Non-toxin | Non-allergen | Conserved |
| Envelope small membrane protein | ||||||||
| 57 | 71 | YVYSRVKNLNSSRVP | 15 | 0.4492 | Antigen | Non-toxin | Non-allergen | Conserved |
| Membrane protein | ||||||||
| N/A | N/A | – | – | – | – | – | – | – |
| Nucleoprotein | ||||||||
| 59 | 105 | HGKEDLKFPRGQGVPINTNSSPDDQIGYYRRATRRIRGGDGKMKDLS | 47 | 0.5773 | Antigen | Non-toxin | non-allergen | Conserved |
| 226 | 267 | RLNQLESKMSGKGQQQQGQTVTKKSAAEASKKPRQKRTATKA | 42 | 0.5627 | Antigen | Non-toxin | Non-allergen | Conserved |
| 276 | 299 | RRGPEQTQGNFGDQELIRQGTDYK | 24 | 0.6277 | Antigen | Non-toxin | Non-allergen | Conserved |
Table 3.
The results of various filters that were applied in the selection of 12-MHC-I epitopes
| Spike glycoprotein | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Allele | Start | End | Length | Peptide | IEDB score | Vaxijen score | Antigenicity | ToxinPred | AllerTOP | Conservancy |
| HLA-B*40:01 | 1016 | 1024 | 9 | AEIRASANL | 0.976223 | 0.7082 | Antigen | Non-toxin | Non-allergen | Conserved |
| HLA-B*15:01 | 1264 | 1272 | 9 | VLKGVKLHY | 0.943479 | 1.2378 | Antigen | Non-toxin | Non-allergen | Conserved |
| HLA-B*51:01 | 714 | 722 | 9 | IPTNFTISV | 0.942802 | 0.882 | Antigen | Non-toxin | Non-allergen | Conserved |
| Allele | Start | End | Length | Peptide | Score | Vaxijen score | Antigenicity | ToxinPred | AllerTOP | Conservancy |
|---|---|---|---|---|---|---|---|---|---|---|
| Envelope small membrane protein | ||||||||||
| HLA-A*31:01 | 61 | 69 | 9 | RVKNLNSSR | 0.939976 | 0.8998 | Antigen | Non-toxin | Non-allergen | Conserved |
| HLA-A*68:01 | 30 | 38 | 9 | TLAILTALR | 0.682278 | 0.7223 | Antigen | Non-toxin | Non-allergen | Conserved |
| HLA-A*30:02 | 49 | 57 | 9 | VSLVKPSFY | 0.652132 | 0.7476 | Antigen | Non-toxin | Non-allergen | Conserved |
| Membrane protein | ||||||||||
| HLA-A*68:01 | 137 | 146 | 10 | ELVIGAVILR | 0.977462 | 0.9998 | Antigen | Non-toxin | Non-allergen | Conserved |
| HLA-A*68:01 | 138 | 146 | 9 | LVIGAVILR | 0.948655 | 1.1027 | Antigen | Non-toxin | Non-allergen | Conserved |
| HLA-B*57:01 | 84 | 92 | 9 | MACLVGLMW | 0.935424 | 0.7889 | Antigen | Non-toxin | Non-allergen | Conserved |
| Nucleoprotein | ||||||||||
| HLA-A*03:01 | 361 | 370 | 10 | KTFPPTEPKK | 0.986352 | 0.7657 | Antigen | Non-toxin | Non-allergen | Conserved |
| HLA-B*15:01 | 305 | 314 | 10 | AQFAPSASAF | 0.986204 | 0.5986 | Antigen | Non-toxin | Non-allergen | Conserved |
| HLA-B*57:01 | 100 | 108 | 9 | KMKDLSPRW | 0.958352 | 1.7462 | Antigen | Non-toxin | Non-allergen | Conserved |
Table 4.
List of the 12 MHC-II epitopes along with results of various applied selection filters
| Allele | Start | End | Length | Peptide | Vaxijen score | Antigenicity | ToxinPred | AllerTOP | Conservancy |
|---|---|---|---|---|---|---|---|---|---|
| Spike glycoprotein | |||||||||
| HLA-DRB1*13:02 | 115 | 129 | 15 | QSLLIVNNATNVVIK | 0.4343 | Antigen | Non-toxin | Non-allergen | Conserved |
| HLA-DRB1*01:01 | 511 | 525 | 15 | VVLSFELLHAPATVC | 0.8618 | Antigen | Non-toxin | Non-allergen | Conserved |
| HLA-DRB1*01:01 | 510 | 524 | 15 | VVVLSFELLHAPATV | 0.8083 | Antigen | Non-toxin | Non-allergen | Conserved |
| Envelope small membrane protein | |||||||||
| HLA-DPA1*03:01/DPB1*04:02 | 18 | 32 | 15 | LLFLAFVVFLLVTLA | 0.8122 | Antigen | Non-toxin | Non-allergen | Conserved |
| HLA-DPA1*03:01/DPB1*04:02 | 19 | 33 | 15 | LFLAFVVFLLVTLAI | 0.7471 | Antigen | Non-toxin | Non-allergen | Conserved |
| HLA-DPA1*03:01/DPB1*04:02 | 21 | 35 | 15 | LAFVVFLLVTLAILT | 0.8229 | Antigen | Non-toxin | Non-allergen | Conserved |
| Membrane protein | |||||||||
| HLA-DQA1*01:01/DQB1*05:01 | 49 | 63 | 15 | IKLIFLWLLWPVTLA | 0.8704 | Antigen | Non-toxin | Non-allergen | Conserved |
| HLA-DPA1*01:03/DPB1*02:01 | 88 | 102 | 15 | VGLMWLSYFIASFRL | 0.6658 | Antigen | Non-toxin | Non-allergen | Conserved |
| HLA-DQA1*01:01/DQB1*05:01 | 50 | 64 | 15 | KLIFLWLLWPVTLAC | 0.7344 | Antigen | Non-toxin | Non-allergen | Conserved |
| Nucleoprotein | |||||||||
| HLA-DRB1*09:01 | 305 | 319 | 15 | AQFAPSASAFFGMSR | 0.5266 | Antigen | Non-toxin | Non-allergen | Conserved |
| HLA-DRB1*11:01 | 84 | 98 | 15 | IGYYRRATRRIRGGD | 0.6649 | Antigen | Non-toxin | Non-allergen | Conserved |
| HLA-DRB1*07:01 | 328 | 342 | 15 | GTWLTYTGAIKLDDK | 0.9934 | Antigen | Non-toxin | Non-allergen | Conserved |
Epitopes’ population-coverage analysis
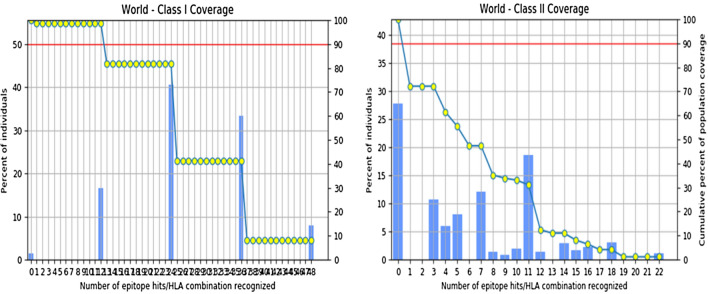
The cumulative population coverage of the 12 MHC-I restricted epitopes selected for the vaccine construct was found to be 98.55% for the global population. Similarly, for the 12 MHC-II restricted epitopes, the coverage was determined to be 72.18%. The population coverage scores were determined based on the selected alleles used in the analysis, which are provided in Supplementary Table S3a and S3b for individual epitopes. The cumulative population coverage for epitopes restricted to both MHC classes exceeded 50%, which indicates the potential effectiveness of the vaccine construct. The Fig. 2 illustrates the individual epitope coverage for both MHC classes. Additional information on individual epitope coverage scores and the percentage genotype frequencies of HLA alleles can be found in Supplementary Tables S4a, S4b, S4c, and S4d.
Fig. 2.
The graph on the left and right represent the cumulative world population coverage of selected MHC-I and MHC-II epitopes respectively in correspondence to the recognized number of HLA hits
Vaccine construct’s assembly
The vaccine components were systematically assembled in sequential order, commencing with the adjuvant sequence attached to the 6xHis tag, followed by the B-cell-restricted epitopes joined together with EAAK linkers. The MHC-I and MHC-II epitopes selected earlier were then attached to the chain using GPGPG and AYY linkers, respectively. To complete the chain, another 6 × His tag was added. The inclusion of these tags, one at the Amino (N) terminus and the other at the Carboxyl (C) terminus serves the purpose of identifying and purifying the cloned protein both in-vitro and in-vivo. Figure 3 shows a detailed representation of the vaccine construct.
Fig. 3.
Graphical representation of vaccine assembly in sequential arrangements i.e., 6 × His tag, followed by adjuvant, B-cell restricted epitopes, MHC-I restricted, MHC-II restricted epitopes and end with another 6 × His tag, and the complete amino acid sequence of the designed chimeric vaccine
Quality check and physicochemical properties
The VexiJen 2.0 predicted that the construct had a 0.6019 score, which validated the antigenicity of the vaccine, similarly, the ToxinPred and AllerTop also showed that the construct is non-toxin and non-allergen in nature, respectively. These three tools validated the quality and safety of the vaccine as it can generate proper immune responses without the plausibility of side effects (i.e., toxicity and hypersensitivity or allergic reactions). The ProtParam results in Table 5 showed that the vaccine construct constituted 687 amino acids with a molecular weight of 73,725.68 Da. The Instability Index (II) score of 34.83 substantiated the stability of the construct in the test tube. The 87.87 computed Aliphatic Index score of the construct validated that the protein is thermostable which makes it a viable candidate to initiate an immunogenic response without any changes in the structural confirmation. As the immune response begins the body temperature changes due to the inflammation but the construct can withstand such changes without losing the structural integrity. The sturdy structural folding of the vaccine was corroborated through the theoretical PI of 9.90 and it will be helpful in the implementation of purification methods. The constructed vaccine is hydrophilic in nature as it has negative Grand Average of Hydropathicity (GRAVY) scores (− 0.028). This hydrophilicity of the construct will promote the immunogenic effects as it allowed the interaction of the construct with the intracellular and extracellular water residues of the host. The construct has a short half-life of 3.5 h and 30 h with and without 6 × His tag respectively in mammalian reticulocytes (in-vitro). This short half-life span illustrated that the antigens of the construct remained in the host only to develop immunity without acting as the pathogen. It also has a half-life of > 10 h in Escherichia coli (in-vivo) allowing enough time for the production and extraction of the vaccine during the cloning process.
Table 5.
Results of various physicochemical properties of vaccine construct along with VaxiJen score
| Protein | VaxiJen score | Amino acids | Molecular weight (Da) | Theoretical PI | Instability index (II) | GRAVY | Aliphatic index | Half-life (with 6 × His tag) | Half-life (without 6 × His tag) |
|---|---|---|---|---|---|---|---|---|---|
| Vaccine | 0.6019 | 687 | 73,725.68 | 9.90 | 34.83 | − 0.028 | 87.87 | 3.5 h | 30 h |
Secondary structure profiling
The secondary structure composition of the construct was analyzed using the SOPMA server, which revealed that the protein contained 264 residues (38.43%) of alpha helix, 127 residues (18.49%) of extended strands, and 296 residues (43.09%) of coils. The results obtained from the SOPMA server were validated by comparing them with the results from PsiPred, as presented in Fig. 4. According to the MEMSAT-SVM Schematics of PsiPred, the residues from 483 to 632 are the trans-membrane region of the construct while the residues from 483 to 513 are the part of the pore-lining of the plasma membrane of the host cells. The MEMSAT-SVM schematic of the construct is also depicted in Fig. 4.
Fig. 4.
Secondary structure analysis of the construct: a PsiPred results showing the distribution of various elements of secondary structure throughout the chain, b Represent the SOPMA analysis for the calculation of residues in various elements (alpha helix, beta turns, coils, etc.), and c Showed the MEMSAT-SVM schematic of the vaccine construct
Three-dimensional (3D) modeling and validations
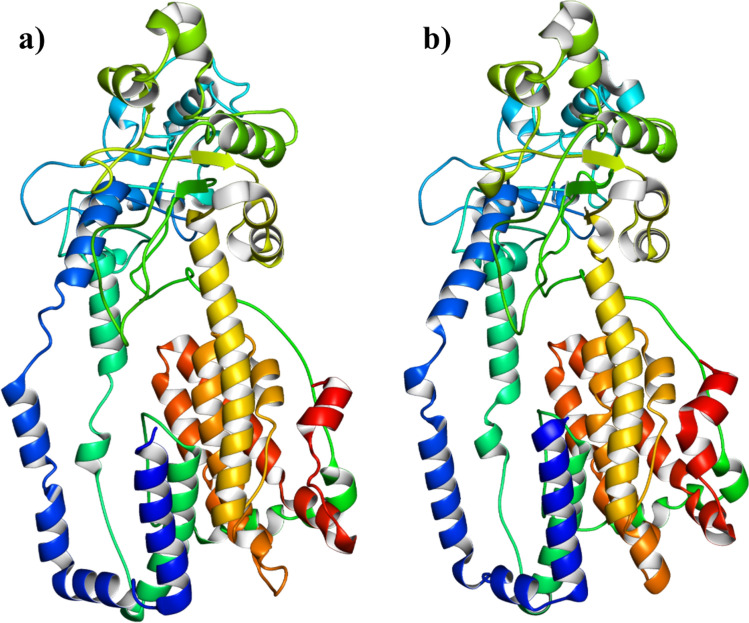
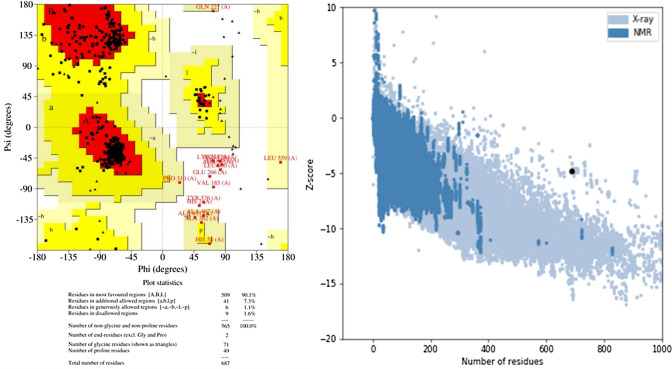
I-TASSAR predicted five 3D models of the construct out of them the model with the highest C-score (− 2.08) was selected for further analysis. The chosen model had TM-score and RMSD values of 0.47 ± 0.15 and 13.1 ± 4.1 Å, respectively, while Supplementary Table S5 contains the C-scores of all the predicted 3D models. The model was evaluated by PROCHECK for Ramachandran analysis, which revealed that the initial model had only 78.3% of the residues in the most favored region, which was not sufficient for the validation of the 3D model. Therefore, the model was refined by the GalaxyRefine server and the refined model has 90.1% residues in the most favored region, 7.3% residues in the additional allowed region, and 1.1% residues in the generously allowed region while only 1.6% residues were found in the disallowed region. The Ramachandran analysis with 90.1% residues in the most favored region validated the legitimacy of the model and it was also supported by the predicted -4.8 z-score of the ProSA-web evaluation of the model. The 3D structures of the raw and refined models are provided in Fig. 5, and the Ramachandran plot and ProSA-web evaluation charts are presented in Fig. 6.
Fig. 5.
Three-dimensional model of the vaccine construct: a Represent raw model with 78.3 residues in the most favored region, b Showed the refined model with 90.1% residues in the most favored region
Fig. 6.
The Ramachandran plot (left) with 90.1% of the residues in the most favored region, 7.3% of the residues in the additional allowed region, 1.1% of the residues in the generously allowed region, and 1.6% of the residues in the disallowed region and ProSA web analysis (right) chart with − 4.8 Z-score to support the Ramachandran analysis for the validation of the 3D model
Disulfide engineering for stability
The DbD2 server predicted 54 possible residue pairs that can form disulfide linkages in the tertiary structure of the construct but 7 pairs with > 2.20 kcal/mol binding energies were selected for disulfide engineering. This selection was based on the fact that 2.20 kcal/mol is the minimum energy required for the formation of a disulfide bond (Chatterjee et al. 2023). The DbD2 generated a mutated wireframe tertiary model of the construct with the disulfide bonds between the selected residues shown in yellow color in Supplementary Figure S2. The original wireframe structure is also included in Supplementary Figure S2, while Supplementary Table S6 provided a list of all predicted and selected residue pairs for disulfide engineering.
Toll-like receptors (TLRs) docked with vaccine
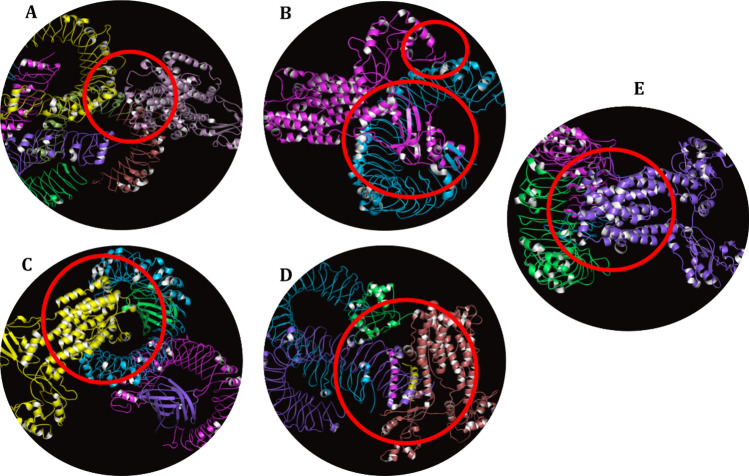
The underlying logic for conducting molecular docking between the vaccine and TLRs is based on the fact that these receptors play a vital role as the first-line immunogenic defense system for the activation of the innate immune response against viral infections. Figure 7 illustrates the selected docked complexes of TLRs with the vaccine model. For instance, Fig. 7A is depicting the complex of the TLR2 + vaccine model with the binding energy of − 1107.7 kCal/mole and 46 cluster members. The docked complex of TLR3 and vaccine model has − 1146.7 kCal/mole binding energy with 44 cluster members (Fig. 7B), and the docking of TLR4 + vaccine model involved 33 cluster members with the binding energy of − 1235.2 kCal/mole (Fig. 7C). Similarly, the TLR5 + vaccine complex represented in Fig. 7D has a binding energy of − 1497.2 kCal/mole with 42 cluster members while the binding energy of the TLR8 + vaccine complex is − 1577.1 kCal/mole with 77 cluster members is given in Fig. 7E. The models were selected based on two factors the first one is the highest negative binding energy as it indicates the stabilization of the reaction through tremendous binding affinities and mends the path toward the product. However, the second factor is the highest cluster members, which strengthen the intensity of binding affinities of the docked complex. The calculations in this research insinuated that the TLR8 has the highest negative binding energy and cluster members, enabling the receptor to become a predominant component of innate immune response followed by the activation of adaptive immune responses in case of infection. Figure 8 is showing the interacting residues between the TLR8 and vaccine construct within the range of 5 Å. The remaining TLRs also had the tendency to generate an appropriate immune response upon interacting with the non-self-epitopes. Supplementary Table S7 is demonstrating the lowest energies, center values, and cluster members of docked complexes obtained from the analysis by the ClusPro 2.0 server.
Fig. 7.
The docked complexes between the vaccine construct and various TLRs (TLR2, TLR3, TLR4, TLR5, and TLR8) obtained from the ClusPro docking analysis
Fig. 8.
The interacting residues between the TLR8 and vaccine construct within 5 Å, visualized by the analysis of TLR8 + Vaccine docked complex on PyMol software
Molecular dynamic (MD) simulations
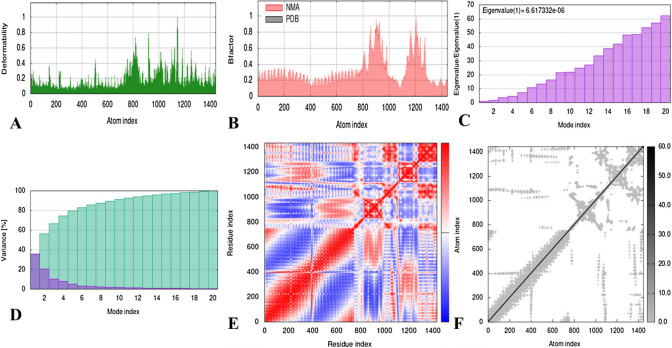
The stability and mobility of the docked complexes were determined by molecular dynamics (MD) simulations, which investigated the conformational states of the macromolecules in a virtual biomolecular system upon perturbation. To render the docked complex, the iMODS server adjusted the force field at various time intervals and calculated several properties including individual and cumulative variance, deformability, eigenvalues, B-factor, covariance map, and elastic network depicted in Fig. 9 (TLR8 + vaccine). Figure 9A shows the deformability of the docked complex at individual residue levels, which is dependent on the distortion of C-α atoms. The average root-mean-square fluctuation (RMSF) of each residue, calculated in correspondence to the NMA mobility factor 8π2, is presented in the B-factor value graph of the stable docked complex in Fig. 9B. The eigenvalues, which demonstrate the motion stiffness of the complex and are directly linked with the energy required for structure deformation, are shown in Fig. 9C. The binding interaction between the protein and the ligand was stabilized and made flexible by a low eigenvalue of 6.6173e−06. In Fig. 9D, the eigenvalue and variance, which are inversely related, are illustrated using red and green colors to represent them individually and cumulatively. Using the C-α Cartesian coordinates, the covariance map was generated to demonstrate the concatenation between the residues. Figure 9E visually presents the motion analysis of coupled residues by using blue, red, and white colors to represent anti-correlated, correlated, and uncorrelated motions, respectively. The spring network model delineated in Fig. 9F demonstrates that the C-α atoms in the docked molecules are connected by springs of varying strengths, represented by dots. The strength of each dot (spring) is determined by the darkness of the color in the graph, as lighter grays indicate less stiffness while darker colors represent the higher stiffness of the springs. The iMODS graphs of the remaining complexes are provided in Supplementary Figures S3a, S3b, S3c, and S3d respectively, while Supplementary Table S8 presents the eigenvalue of all the docked complexes.
Fig. 9.
Graphical representation of various Normal mode Analyses (NMA) generated by iMODS for the docked complex (Vaccine + TLR8). A represents the deformability of the docked complex, B illustrates the B-factors and NMA predicted mobilities, C demonstrates the Eigenvalues plot evidences the relative modal stiffness, D displays the variance associated to the modes indicates their relative contribution to the equilibrium motions, E shows the covariance matrix indicates which parts of the macromolecule move in a correlated, uncorrelated or anti-correlated fashion, and F is representing the elastic network model illustrating the linking matrix
Codon adaptation and in-silico cloning
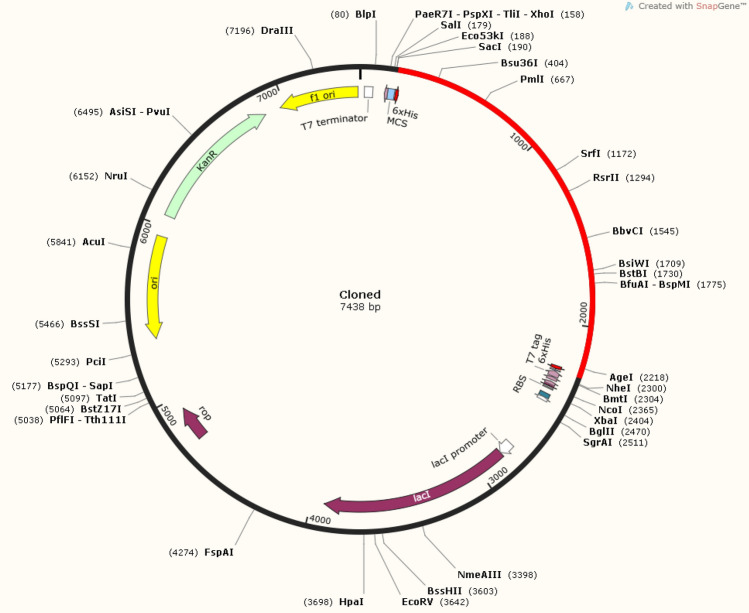
The codon of the vaccine construct was optimized for expression in E. coli (K-12 strain) and the refined series consisted of 2061 nucleotides with optimized CAI = 0.97 and GC content = 54.1% which are in the optimal range, thus increasing the probability of protein expression. In Fig. 10, the graph is illustrating the optimized CAI values, while the optimized nucleotide sequence is shown in Supplementary Figure S4. Adaptors in the form of EcoR1 (GAATTC) and BamH1 (GGATCC) restricted endonucleases were added to the beginning and end of the optimized nucleotide sequence, respectively, to facilitate its integration into the pET28a ( +) plasmid. In-silico cloning was carried out using SnapGene software, resulting in a cloned recombinant plasmid with a length of 7.438 kb illustrated in Fig. 11.
Fig. 10.
Codon adaption/optimization results generated by JCAT for the vaccine construct, Codon Adaptation Index (CAI) was 0.97, and the GC content was 54.1% optimized for the expression system E. coli K-12 strain
Fig. 11.
The cloned vector generated by SnapGene containing the vaccine sequence inserted (represented in red color) in pET28a ( +) plasmid. The vaccine was connected to the plasmid by introducing the EcoR1 (GAATTC) and BamH1 (GGATCC) adaptors (restriction sites) at the start and end of the construct respectively
Immune simulation by C-IMMSIM
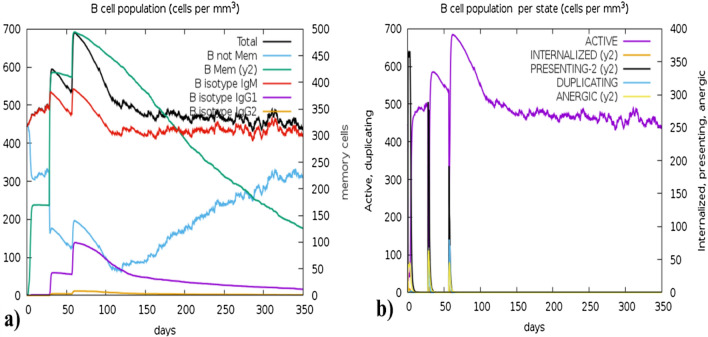
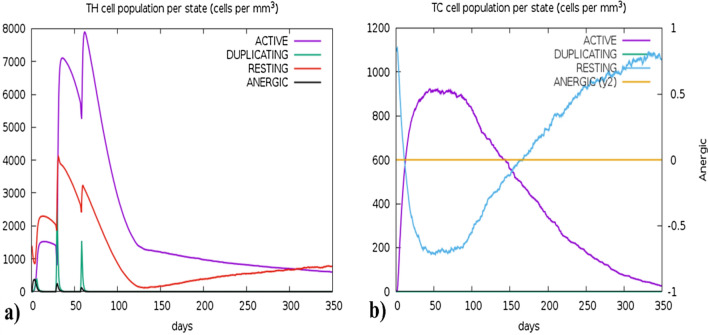
The C-IMMSIM algorithm computed the host's immune responses upon the administration of the vaccine. The simulation predicted elapsed time of 280 days against 1050 simulation steps for 3 doses at 4-week intervals. Figure 12a presents the significant production of cytokines, interleukins, and Simpson index (D) levels (depicted in a sub-graph Fig. 12a). The adaptive immune response is essential for the development of long-term immunity which depends on the T-cell and B-cell generation upon the interaction with the vaccine and Figs. 13 and 14 described their surge. However, Figs. 13a and b demonstrate the memory B-cell population and B-cell population per state (cells/mm3) respectively, while Figs. 14a and b illustrate the sudden rise of Helper T-cells (TH-cells) and cytotoxic T-cells (TC- cells), correspondingly. The vaccine also exhibited significant efficacy in producing various types of antibodies, including IgM, IgA, IgG1 + IgG2, IgM + IgG, IgG1, and IgG2, as presented in Fig. 12b. Supplementary Figures S5 and S6 provide the production levels of other immune cells such as natural killer cells and dendritic cells.
Fig. 12.
Immune simulation results: a The sudden rise in the production of various cytokines can be observed while the sub-graph of the straight Simpson index Danger (D) line ensures the safety of the vaccine, while b represents the production of various types of antibodies
Fig. 13.
The immune simulation results, a present the production of B-cells isotypes (mm−3) in various forms, which are involved in the production of different antibodies (i.e., IgM, IgG1, and IgG2). Three different peaks can be seen representing three dosages of vaccine, while b represents the production B-cell population in various states (cells/mm3) including active, internalized, presenting-2, duplicating and anergic
Fig. 14.
The immune simulation predictions by C-IMMSIMS, a illustrate the production of helper T-cells per state (cells/mm3), these states include Active, Duplicating, Resting, and Anergic TH-cells, the purple peak is the highest representing the boost in the production of active helper T-cells upon inoculation, and b showed the Tc cell per state (cells/mm3), the curve of Active Cytotoxic T-cells (Purple) is rising at the start while the curve of Resting T-cells (Blue) is reducing which represents that upon inoculation the vaccine has triggered the production active TC-cells
Discussion
Vaccines have played a crucial role in the history of public health, providing an effective means of immunization against newly emerging pathogens. However, traditional methods of vaccine design often involve the use of entire organisms or large proteins, which can trigger adverse and unwanted hypersensitive reactions (Jorge and Dellagostin 2017). With the progress in bioinformatics, the process of vaccine design has also undergone remarkable advancements, making it a much faster and more efficient process. In recent times, bioinformatics tools have been great contributors to the identification and analysis of potential epitopes associated with different pathogens, leading to the development of novel vaccine designs and strategies (Arya and Bhatt 2021). Therefore, in this study, we employed a promising field of immunoinformatics to create a multi-epitope vaccine that targets various structural proteins of SARS-CoV-2, the pathogen behind the COVID-19 pandemic. To effectuate this, a combination of computational tools and algorithms was employed to detect the immunogenic/antigenic epitopes in the viral proteins and subsequently create a chimeric peptide that could generate a dynamic and precise immune reaction against SARS-CoV-2 while reducing the possibilities of adverse effects.
Since the emergence of SARS-CoV-2, numerous studies have been conducted to develop effective vaccines against the virus. Several of these studies have focused on developing multi-epitope vaccines that target either the S-protein (Kar et al. 2020), M-protein (Bashir et al. 2021), E-protein (Adam 2021), or N-protein (Kumar et al. 2021; Oliveira et al. 2020). This study had a different approach compared to others that aimed to develop multi-epitope vaccines focusing on specific proteins of the SARS-CoV-2. Instead, the study designed a vaccine that would target all the structural proteins virus to achieve a comprehensive immunization against the viral infection. The vaccine construct is composed of 12 MHC class-I epitopes specified to cytotoxic T-leukocytes (CTLs), 12 MHC class-II epitopes specified/restricted to Helper T-leukocytes (HTLs), and 8 linear B-cell-specified epitopes, which were selected carefully from each protein through the rigorous barrier of filters that ensured the occurrence of both adaptive and innate immune responses. The goal was to activate the immune system by targeting all the structural proteins, resulting in the production of a diverse range of epitope-specific antibodies against different SARS-COV-2 strains. Notably, the inclusion of MHC-I and MHC-II restricted T-cell epitopes plays a crucial role in activating cell-mediated immune responses, which can target infected cells and eliminate viral particles (Broere and van Eden, 2019). In addition, the presence of linear epitopes restricted to B-cells enhances the humoral immune response by inducing the production of neutralizing antibodies (Cancro and Tomayko, 2021).
Creating effective vaccines against viruses has always been a major hurdle, primarily due to the rapid mutation rates displayed by viruses. As viruses evolve, they tend to develop new variants that can evade the immune response generated by the existing vaccines, thereby rendering them less effective (Wang et al. 2021). The characteristic of viruses to mutate rapidly makes them resistant to vaccines through antigenic shift or drift, thus necessitating the need for new vaccines each year against different viral infections such as swine flu (H1N1) which require refurbished vaccine almost every year (Bellino et al. 2019; Treanor 2004). Similarly, the SARS-CoV-2 virus has existed in several variants since the outbreak of the pandemic, posing a significant challenge to vaccine development efforts (Harvey et al. 2021). In this research, we aimed to address this challenge by selecting epitopes from the conserved regions of the viral proteins. This approach ensured the longevity of the vaccine construct, as the conserved regions are less prone to mutation and reduced the risk of resistance against the vaccine. The final vaccine construct developed in this study is a hybrid of conserved B-cell and T-cell epitopes from all the structural proteins. This design aimed to generate a cascade of immune responses while ensuring the safety of the vaccine, as it is non-allergen, non-toxin, and has a short half-life. The use of conserved epitopes in the vaccine design also ensures that the vaccine can provide protection against multiple variants of the virus which can reduce the need for frequent updates or refurbishments in the vaccine over time.
The efficacy and safety of epitope-based vaccines rely on several crucial factors, such as their ability to elicit a robust immune response while remaining safe for the recipient (Dutta and Langenburg 2023). With these considerations in mind, the selected epitopes for this vaccine experienced a rigorous screening process to ensure the quality and safety of the final construct. Existing vaccines against SARS-COV-2 have reported various side effects, including anaphylaxis, mild headache, tiredness, and skin rashes (Hosseini and Askari 2023). Therefore, each epitope included in the final construct was screened for any potential allergenic and toxic reaction in the living systems. Only those epitopes that passed all criteria were selected for inclusion in the final construct of the vaccine, while those that did not meet the required standards were completely rejected. This careful selection process helped us to minimize the risk of adverse reactions in vaccinated individuals and promote the safety and effectiveness of the vaccine.
The stability of the vaccine construct is a critical factor in ensuring its efficacy and longevity in the patient's body (Kim et al. 2022). Several physicochemical analyses were performed to assess the construct's stability, indicating that the protein is inherently stable (with instability index = 34.83). The high aliphatic index (87.87) suggests that it can withstand temperature fluctuations in the body of the host upon inflammatory reaction (Devi et al. 2021). Similarly, disulfide engineering was also employed to enhance and maintain the stability of tertiary structure with at least 7 identified disulfide linkages in the mutant model. In addition, 3-D modeling was used to validate the structure of the vaccine construct, with the Ramachandran plot demonstrating that over 90% of the residues were in the most favorable region and only a small percentage of residues (1.6%) were found in the disallowed region.
In the case of viral infections, TLRs are members of the first-line immunogenic defense system that detect the conserved pathogen-associated molecular patterns (PAMP) to initiate the immune response through a wide range of signaling pathways (Kaur et al. 2022). This research involved analyzing the binding energies and cluster members of various docked complexes between TLRs and the vaccine model. The results indicated that TLR8 had the highest negative binding energy and cluster members. Moreover, this study also utilized the normal mode analysis (NMA) of the iMODS server to examine the stability and dynamic properties of the docked complex formed by our designed vaccine and TLRs. By analyzing the data, we observed that the proteins in the complex showed minimal deformation at each residue. Additionally, eigenvalues were calculated for the docked complex (vaccine + TLR8), which exhibited a value of 6.6173e−06, thus validating the reliability of our in-silico model for the vaccine (Ezzemani et al. 2022). Such a comprehensive NMA analysis is a crucial step for the development of an effective vaccine candidate, as it can ensure the stability and functionality of the model within the dynamics of the host system in real-time (Kirar et al. 2022).
The primary objectives of vaccines are to generate a vigorous immune response and provide durable immunity against specific viral infections (Moss 2022). In this study, the antigenicity of the vaccine construct was determined by the VaxiJen score of 0.6019, which exceeded the threshold of 0.4 indicating the antigenic nature of the vaccine construct. Nevertheless, to further enhance the potency and longevity of the immune response against the viral particles, an adjuvant, Human Beta Defensin 3, was incorporated at the beginning of the construct to magnify the effects of selected epitopes. As we know the adjuvants are known to modulate the immunogenicity of epitopes while reducing the need for a large number of antigens to induce the same response (Naveed et al. 2022). The antigenicity results were confirmed by immune simulations, which demonstrated the generation of immunogenic cells and molecules that are required for the establishment of long-term immunity against various SARS-COV-2 variants.
Study limitation
As this study is based on computational algorithms and in-silico data, which means it may not accurately reflect the real-world experimental validations, so, it is essential to validate the algorithmic prediction through in-vitro and in-vivo experiments. Secondly, even though there are advancements in computational technologies but still risks are there that some important epitopes may have been missed in the process due to the limitation of tools to understand the dynamic nature of pathogens. The binding affinity between an epitope and the major histocompatibility complex (MHC) molecule is a critical step in antigen presentation and T-cell activation. So, accurate prediction about this binding affinity is a big challenge, which may impact the efficacy of the vaccine construct designed in this study.
Conclusion
This study demonstrates that the designed vaccine has the potential to overcome the perpetual damage of SARS-CoV-2 and its variants. Our computationally designed hybrid vaccine has epitopes from conserved regions of all structural proteins which makes it effective against the emerging strains of SARS-CoV-2. The results elicit that this vaccine tends to stimulate a powerful immune response and provide persistent immunization against the SARS-CoV-2 infection. We concur that our vaccine can impede COVID-19 from igniting unending havoc. Despite efforts that were previously made to prevent the spread of the pandemic, the appearance of new strains emphasizes the continuous threat of COVID-19 and demands new approaches to tackle this threat. The world has witnessed tremendous pain and disruption during the pandemic but we anticipate that our findings will provide the groundwork for future study in this area, and eventually contribute to better world health accomplishments. However, the work is based on computational analyses which required in-vitro and in-vivo data for confirmational application of the vaccine construct in real-world settings.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Figure S1a: Conservancy analysis of Spike protein for the filtration of epitopes; Figure S2b: Conservancy analysis of Envelope protein for the filtration of epitopes; Figure S1c: Conservancy analysis of Membrane protein for the filtration of epitopes; Figure S1d: Conservancy analysis of Nucleocapsid protein for the filtration of epitopes; Figure S2: The wireframe model of original and mutant model after the disulfide engineering: The yellow colored rods represents the disulfide bond formation after disulfide engineering; Figure S3a: Graphical representation of various Normal mode Analyses (NMA) generated by iMODS for the docked complex (Vaccine + TLR2); Figure S3b: Graphical representation of various Normal mode Analyses (NMA) generated by iMODS for the docked complex (Vaccine + TLR3); Figure S3c: Graphical representation of various Normal mode Analyses (NMA) generated by iMODS for the docked complex (Vaccine + TLR4); Figure S3d: Graphical representation of various Normal mode Analyses (NMA) generated by iMODS for the docked complex (Vaccine + TLR5); Figure S4: Immune simulation analysis: a) Represents the production of Dendritic cells per state (mm-3) while b) showed the generation of MA population per state (mm-3); Figure S5: Immune simulation analysis: a) Represents the production of EP population per state (mm-3) while b) showed the generation of NK cell population per state (mm-3); Table S1a: List of B-cell restricted epitopes predicted for Spike (S) protein; Table S1b: The list of B-cell-specific epitopes predicted from Envelope (E) protein; Table S1c: List of all the B-cell restricted protein predicted from Membrane (M) protein; Table S1d: The list of linear B-cell restricted epitope predicted from Nucleocapsid (N); Table S2a: List of reference HLA alleles used for the prediction of MHC-I restricted epitopes; Table S2b: The selected HLA alleles as reference for the prediction of MHC-II restricted epitopes; Table S3a: The set alleles for individual MHC-I epitopes used in Population Coverage Analysis; Table S3b: The set alleles for individual MHC-II epitopes used in Population Coverage Analysis; Table S4a: Cumulative population coverage against individual MHC-I restricted epitopes and HLA hits; Table S4b: Percentage genotype frequencies of each HLA allele against individual MHC-I restricted epitopes; Table S4c: Cumulative population coverage against individual MHC-II restricted epitopes and HLA hits; Table S4d: Percentage genotype frequencies of each HLA allele against individual MHC-II restricted epitopes; Table S5: C-score for the designed 3D model by I-TASSAR; Table S6: List of predicted (black) and selected (Green) pairs for Disulfide engineering; Table S7: List of cluster members and lowest energies obtained from Cluspro docking analysis against various TLRs and vaccine construct; Table S8: The eigenvalue of various docked complexes that were obtained from ClusPro analysis. File named Tools and access dates contained the links and access dates of all the software, servers, and tools used in this study (RAR 19896 KB)
Author contributions
Conceptualization, A.R.Y.; methodology, A.R.Y., and M.S.; software, A.R.Y., D.M.K., and M.S.; validation, A.R.Y., and M.S.; formal analysis, A.R.Y., A.S.Q., and I.A.; investigation, A.R.Y., and M.S.; resources, A.R.Y., and M.S.; data curation, A.R.Y., A.A., and M.S.; writing—original draft preparation, A.R.Y., and M.S.; writing—review and editing A.R.Y., M.S., A.S.Q., A.A., I.A., and D.M.K. supervision, A.R.Y., and M.S., project administration, A.R.Y.; prepared figures 1-12, A.S.Q., A.A., I.A., and D.M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data availability
More data related to this study can be accessed by sending a rea-sonable email to 123allah.rakha@gmail.com.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Allah Rakha Yaseen, Email: 123allah.rakha@gmail.com.
Muhammad Suleman, Email: msulemanmughal123@gmail.com.
Abdul Salam Qadri, Email: a_salam98@hotmail.com.
Ali Asghar, Email: Aliasghar7004@gmail.com.
Iram Arshad, Email: Iramarshad5577@gmail.com.
Daulat Munaza Khan, Email: daulatmunazakhan@gmail.com.
References
- Abebe EC, Dejenie TA, Shiferaw MY, T.J.V.j. Malik. The newly emerged COVID-19 disease: a systemic review. Virol J. 2020;17:1–8. doi: 10.1186/s12985-020-01363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham Peele K, Srihansa T, Krupanidhi S, Ayyagari VS, Venkateswarulu TJJOBS, Dynamics. Design of multi-epitope vaccine candidate against SARS-CoV-2: a in-silico study. J Biomole Struc Dynam. 2021;39:3793–3801. doi: 10.1080/07391102.2020.1770127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam KM. Immunoinformatics approach for multi-epitope vaccine design against structural proteins and ORF1a polyprotein of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) Trop Dis Travel Med Vacc. 2021;7:22. doi: 10.1186/s40794-021-00147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberer M, Gnad-Vogt U, Hong HS, Mehr KT, Backert L, Finak G, Gottardo R, Bica MA, Garofano A, Koch SD, Fotin-Mleczek M, Hoerr I, Clemens R, von Sonnenburg F. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet. 2017;390:1511–1520. doi: 10.1016/S0140-6736(17)31665-3. [DOI] [PubMed] [Google Scholar]
- Arya H, Bhatt TK. Chapter 20 - role of bioinformatics in subunit vaccine design. In: Coumar MS, editor. Molecular docking for computer-aided drug design. UK: Academic Press; 2021. pp. 425–439. [Google Scholar]
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl K, Senn JJ, Yuzhakov O, Bulychev A, Brito LA, Hassett KJ, Laska ME, Smith M, Almarsson Ö, Thompson J, Ribeiro A, Watson M, Zaks T, Ciaramella G. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses. Mol Ther. 2017;25:1316–1327. doi: 10.1016/j.ymthe.2017.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir Z, Ahmad SU, Kiani BH, Jan Z, Khan N, Khan U, Haq I, Zahir F, Qadus A, Mahmood T. Immunoinformatics approaches to explore B and T cell epitope-based vaccine designing for SARS-CoV-2 Virus. Pak J Pharm Sci. 2021;34:345–352. [PubMed] [Google Scholar]
- Bellino S, Bella A, Puzelli S, Di Martino A, Facchini M, Punzo O, Pezzotti P, Castrucci MR, The InfluNet Study Moderate influenza vaccine effectiveness against A(H1N1) pdm09 virus, and low effectiveness against A(H3N2) subtype, 2018/19 season in Italy. Expert Rev Vaccines. 2019;18:1201–1209. doi: 10.1080/14760584.2019.1688151. [DOI] [PubMed] [Google Scholar]
- Bhattacharya M, Sharma AR, Patra P, Ghosh P, Sharma G, Patra BC, Lee SS, Chakraborty C. Development of epitope-based peptide vaccine against novel coronavirus 2019 (SARS-COV-2): Immunoinformatics approach. J Med Virol. 2020;92:618–631. doi: 10.1002/jmv.25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian L, Gao Q, Gao F, Wang Q, He Q, Wu X, Mao Q, Xu M, Liang Z. Impact of the delta variant on vaccine efficacy and response strategies. Expert Rev Vaccines. 2021;20:1201–1209. doi: 10.1080/14760584.2021.1976153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boopathi S, Poma AB, P.J.J.o.B.S. Kolandaivel, and Dynamics. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J Biomole Struc Dynm. 2021;39:3409–3418. doi: 10.1080/07391102.2020.1758788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broere F, van Eden W. T cell subsets and T cell-mediated immunity. In: Parnham MJ, Nijkamp FP, Rossi AG, editors. Nijkamp and Parnham's principles of immunopharmacology. Cham: Springer International Publishing; 2019. pp. 23–35. [Google Scholar]
- Bui HH, Sidney J, Dinh K, Southwood S, Newman MJ, Sette A. Predicting population coverage of T-cell epitope-based diagnostics and vaccines. BMC Bioinformatics. 2006;7:153. doi: 10.1186/1471-2105-7-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancro MP, Tomayko MM. Memory B cells and plasma cells: the differentiative continuum of humoral immunity. Immunol Rev. 2021;303:72–822. doi: 10.1111/imr.13016. [DOI] [PubMed] [Google Scholar]
- Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, Xing F, Liu J, Yip CC, Poon RW, Tsoi HW, Lo SK, Chan KH, Poon VK, Chan WM, Ip JD, Cai JP, Cheng VC, Chen H, Hui CK, Yuen KY. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet (london, England) 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee R, Mahapatra SR, Dey J, Raj Takur K, Raina V, Misra N, Suar M. An immunoinformatics and structural vaccinology study to design a multi-epitope vaccine against Staphylococcus aureus infection. J Molecular Recognit. 2023;36:e3007. doi: 10.1002/jmr.3007. [DOI] [PubMed] [Google Scholar]
- Coudert E, Gehant S, de Castro E, Pozzato M, Baratin D, Neto T, Sigrist CJA, Redaschi N, Bridge A, T.U. Consortium Annotation of biologically relevant ligands in UniProtKB using ChEBI. Bioinformatics. 2022;39:b793. doi: 10.1093/bioinformatics/btac793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig DB, Dombkowski AA. Disulfide by design 2.0: a web-based tool for disulfide engineering in proteins. BMC Bioinf. 2013;14:346. doi: 10.1186/1471-2105-14-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi A, Chaitanya NSJJOBS, Dynamics. In silico designing of multi-epitope vaccine construct against human coronavirus infections. J Biomole Struct Dyn. 2021;39:6903–6917. doi: 10.1080/07391102.2020.1804460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey J, Mahapatra SR, Singh PK, Prabhuswamimath SC, Misra N, Suar M. Designing of multi-epitope peptide vaccine against Acinetobacter baumannii through combined immunoinformatics and protein interaction-based approaches. Immunol Res. 2023 doi: 10.1007/s12026-023-09374-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov I, Flower DR, Doytchinova I. AllerTOP—a server for in silico prediction of allergens. BMC Bioinform. 2013;14:S4. doi: 10.1186/1471-2105-14-S6-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Dai T, Wei Y, Zhang L, Zheng M, Zhou F. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct Target Ther. 2020;5:237. doi: 10.1038/s41392-020-00352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doytchinova IA, Flower DR. VaxiJen: a server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinform. 2007;8:4. doi: 10.1186/1471-2105-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond E, Kavanagh T, Pires G, Marta-Ariza M, Kanshin E, Nayak S, Faustin A, Berdah V, Ueberheide B, Wisniewski T. The amyloid plaque proteome in early onset Alzheimer’s disease and down syndrome. Acta Neuropathol Commun. 2022;10:53. doi: 10.1186/s40478-022-01356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta SK, Langenburg T. A perspective on current flavivirus vaccine development: a brief review. Viruses. 2023 doi: 10.3390/v15040860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzemani W, Kettani A, Sappati S, Kondaka K, El Ossmani H, Tsukiyama-Kohara K, Altawalah H, Saile R, Kohara M, Benjelloun SJJOBS. Reverse vaccinology-based prediction of a multi-epitope SARS-CoV-2 vaccine and its tailoring to new coronavirus variants. J Biomol Struct Dyn. 2022 doi: 10.1080/07391102.2022.2075468. [DOI] [PubMed] [Google Scholar]
- Ferris LK, Mburu YK, Mathers AR, Fluharty ER, Larregina AT, Ferris RL, Falo LD., Jr Human beta-defensin 3 induces maturation of human langerhans cell-like dendritic cells: an antimicrobial peptide that functions as an endogenous adjuvant. J Invest Dermatol. 2013;133:460–468. doi: 10.1038/jid.2012.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel Y, Mizrahi O, Nachshon A, Weingarten-Gabbay S, Morgenstern D, Yahalom-Ronen Y, Tamir H, Achdout H, Stein D, Israeli O, Beth-Din A, Melamed S, Weiss S, Israely T, Paran N, Schwartz M, Stern-Ginossar N. The coding capacity of SARS-CoV-2. Nature. 2021;589:125–130. doi: 10.1038/s41586-020-2739-1. [DOI] [PubMed] [Google Scholar]
- Gasteiger E, Hoogland C, Gattiker A, S.e. Duvaud, M.R. Wilkins, R.D. Appel, and A. Bairoch. Protein identification and analysis tools on the ExPASy Server. In: Walker JM, editor. The proteomics protocols handbook. Totowa, NJ: Humana Press; 2005. pp. 571–607. [Google Scholar]
- Geourjon C, Deléage G. SOPMA: significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Comp Appl Biosci. 1995;11:681–684. doi: 10.1093/bioinformatics/11.6.681. [DOI] [PubMed] [Google Scholar]
- Gokhale RS, Khosla CJCOICB. Role of linkers in communication between protein modules. Curr Opin Chem Biol. 2000;4:22–27. doi: 10.1016/S1367-5931(99)00046-0. [DOI] [PubMed] [Google Scholar]
- Grote A, Hiller K, Scheer M, Münch R, Nörtemann B, Hempel DC, Jahn D. JCat: a novel tool to adapt codon usage of a target gene to its potential expression host. Nucleic Acids Res. 2005;33:W526–531. doi: 10.1093/nar/gki376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, Ludden C, Reeve R, Rambaut A, Peacock SJ, Robertson DL, C.-G.U. Consortium SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19:409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo L, Park H, Seok C. GalaxyRefine: protein structure refinement driven by side-chain repacking. Nucleic Acids Res. 2013;41:W384–388. doi: 10.1093/nar/gkt458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini R, Askari N. A review of neurological side effects of COVID-19 vaccination. Eur J Med Res. 2023;28:102. doi: 10.1186/s40001-023-00992-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui DS, Madani IAETA, Ntoumi F, Kock R, Dar O, Ippolito G, McHugh TD, Memish ZA, Drosten C, Zumla A, Petersen E. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KK, Andreatta M, Marcatili P, Buus S, Greenbaum JA, Yan Z, Sette A, Peters B, Nielsen M. Improved methods for predicting peptide binding affinity to MHC class II molecules. Immunology. 2018;154:394–406. doi: 10.1111/imm.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen MC, Peters B, Nielsen M, Marcatili P. BepiPred-2.0: improving sequence-based B-cell epitope prediction using conformational epitopes. Nucleic Acids Res. 2017;45:W24–w29. doi: 10.1093/nar/gkx346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge S, Dellagostin OA. The development of veterinary vaccines: a review of traditional methods and modern biotechnology approaches. Biotechnol Res Innovat. 2017;1:6–13. doi: 10.1016/j.biori.2017.10.001. [DOI] [Google Scholar]
- Kar T, Narsaria U, Basak S, Deb D, Castiglione F, Mueller DM, Srivastava AP. A candidate multi-epitope vaccine against SARS-CoV-2. Sci Rep. 2020;10:10895. doi: 10.1038/s41598-020-67749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur A, Baldwin J, Brar D, Salunke DB, Petrovsky N. Toll-like receptor (TLR) agonists as a driving force behind next-generation vaccine adjuvants and cancer therapeutics. Curr Opin Chem Biol. 2022;70:102172. doi: 10.1016/j.cbpa.2022.102172. [DOI] [PubMed] [Google Scholar]
- Kim SC, Sekhon SS, Shin W-R, Ahn G, Cho B-K, Ahn J-Y, Kim Y-H. Modifications of mRNA vaccine structural elements for improving mRNA stability and translation efficiency. Mol Cell Toxicol. 2022;18:1–8. doi: 10.1007/s13273-021-00171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirar M, Singh H, Sehrawat N. Virtual screening and molecular dynamics simulation study of plant protease inhibitors against SARS-CoV-2 envelope protein. Inf Med Unlocked. 2022;30:100909. doi: 10.1016/j.imu.2022.100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotey E, Lukosaityte D, Quaye O, Ampofo W, Awandare G, Iqbal M. Current and novel approaches in influenza management. Vaccines. 2019;7:2–53. doi: 10.3390/vaccines7020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozakov D, Hall DR, Xia B, Porter KA, Padhorny D, Yueh C, Beglov D, Vajda S. The ClusPro web server for protein-protein docking. Nat Protoc. 2017;12:255–278. doi: 10.1038/nprot.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar J, Qureshi R, Sagurthi SR, Qureshi IA. Designing of nucleocapsid protein based novel multi-epitope vaccine against SARS-COV-2 using immunoinformatics approach. Int J Pept Res Ther. 2021;27:941–956. doi: 10.1007/s10989-020-10140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Blanco JR, Aliaga JI, Quintana-Ortí ES, Chacón P. iMODS: internal coordinates normal mode analysis server. Nucleic Acids Res. 2014;42:W271–276. doi: 10.1093/nar/gku339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DP, Weaver S, Tegally H, San EJ, Shank SD, Wilkinson E, Lucaci AG, Giandhari J, Naidoo S, Pillay Y, Singh L, Lessells RJ, Gupta RK, Wertheim JO, Nekturenko A, Murrell B, Harkins GW, Lemey P, MacLean OA, Robertson DL, de Oliveira T, Kosakovsky Pond SL. The emergence and ongoing convergent evolution of the N501Y lineages coincides with a major global shift in the SARS-CoV-2 selective landscape. medRxiv. 2021;1:33. doi: 10.1016/j.cell.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuffin LJ, Bryson K, Jones DT. The PSIPRED protein structure prediction server. Bioinformatics. 2000;16:404–405. doi: 10.1093/bioinformatics/16.4.404. [DOI] [PubMed] [Google Scholar]
- Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol. 2022;23:186–193. doi: 10.1038/s41590-021-01122-w. [DOI] [PubMed] [Google Scholar]
- Naveed M, Yaseen AR, Khalid H, Ali U, Rabaan AA, Garout M, Halwani MA, Al Mutair A, Alhumaid S, Al Alawi Z, Alhashem YN, Ahmed N, Yean CY. Execution and design of an Anti HPIV-1 vaccine with multiple epitopes triggering innate and adaptive immune responses: an immunoinformatic approach. Vaccines. 2022;10:869. doi: 10.3390/vaccines10060869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LH, Drew DA, Graham MS, Joshi AD, Guo C-G, Ma W, Mehta RS, Warner ET, Sikavi DR, Lo C-HJTLPH. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5:e475483. doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira SC, de Magalhães MTQ, Homan EJ. Immunoinformatic Analysis of SARS-CoV-2 Nucleocapsid Protein and Identification of COVID-19 Vaccine Targets. Front Immunol. 2020 doi: 10.3389/fimmu.2020.587615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvizpour S, Pourseif MM, Razmara J, Rafi MA, Omidi Y. Epitope-based vaccine design: a comprehensive overview of bioinformatics approaches. Drug Discovery Today. 2020;25:1034–1042. doi: 10.1016/j.drudis.2020.03.006. [DOI] [PubMed] [Google Scholar]
- Petersen E, Ntoumi F, Hui DS, Abubakar A, Kramer LD, Obiero C, Tambyah PA, Blumberg L, Yapi R, Al-Abri SJIJOID. Emergence of new SARS-CoV-2 variant of concern omicron (B 11 529)-highlights Africa's research capabilities, but exposes major knowledge gaps, inequities of vaccine distribution, inadequacies in global COVID-19 response and control efforts. J Inf Dis. 2022;114:268–272. doi: 10.1016/j.ijid.2021.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapin N, Lund O, Bernaschi M, Castiglione F. Computational immunology meets bioinformatics: the use of prediction tools for molecular binding in the simulation of the immune system. PLoS ONE. 2010;5:e9862. doi: 10.1371/journal.pone.0009862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynisson B, Alvarez B, Paul S, Peters B, Nielsen M. NetMHCpan-4.1 and NetMHCIIpan-4.0: improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Res. 2020;48:W449–w454. doi: 10.1093/nar/gkaa379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM, Julander JG, Tang WW, Shresta S, Pierson TC, Ciaramella G, Diamond MS. Modified mRNA vaccines protect against Zika virus infection. Cell. 2017;168:1114–1125.e1110. doi: 10.1016/j.cell.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satarker S, Nampoothiri M. Structural proteins in severe acute respiratory syndrome coronavirus-2. Arch Med Res. 2020;51:482–491. doi: 10.1016/j.arcmed.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Naorem LD, Jain S, Raghava GPS. ToxinPred2: an improved method for predicting toxicity of proteins. Brief Bioinform. 2022 doi: 10.1093/bib/bbac174. [DOI] [PubMed] [Google Scholar]
- Treanor J. Influenza vaccine–outmaneuvering antigenic shift and drift. N Engl J Med. 2004;350:218–220. doi: 10.1056/NEJMp038238. [DOI] [PubMed] [Google Scholar]
- Vankadari N, Wilce JAJEM. Emerging COVID-19 coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microb Infect. 2020;9:601–604. doi: 10.1080/22221751.2020.1739565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet (london, England) 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Chen J, Wei G-W. Mechanisms of SARS-CoV-2 evolution revealing vaccine-resistant mutations in Europe and America. The Journal of Physical Chemistry Letters. 2021;12:11850–11857. doi: 10.1021/acs.jpclett.1c03380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederstein M, Sippl MJ. ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007;35:W407–410. doi: 10.1093/nar/gkm290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodawer A. Stereochemistry and validation of macromolecular structures. Methods Mol Biol. 2017;1607:595–610. doi: 10.1007/978-1-4939-7000-1_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinform. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Figure S1a: Conservancy analysis of Spike protein for the filtration of epitopes; Figure S2b: Conservancy analysis of Envelope protein for the filtration of epitopes; Figure S1c: Conservancy analysis of Membrane protein for the filtration of epitopes; Figure S1d: Conservancy analysis of Nucleocapsid protein for the filtration of epitopes; Figure S2: The wireframe model of original and mutant model after the disulfide engineering: The yellow colored rods represents the disulfide bond formation after disulfide engineering; Figure S3a: Graphical representation of various Normal mode Analyses (NMA) generated by iMODS for the docked complex (Vaccine + TLR2); Figure S3b: Graphical representation of various Normal mode Analyses (NMA) generated by iMODS for the docked complex (Vaccine + TLR3); Figure S3c: Graphical representation of various Normal mode Analyses (NMA) generated by iMODS for the docked complex (Vaccine + TLR4); Figure S3d: Graphical representation of various Normal mode Analyses (NMA) generated by iMODS for the docked complex (Vaccine + TLR5); Figure S4: Immune simulation analysis: a) Represents the production of Dendritic cells per state (mm-3) while b) showed the generation of MA population per state (mm-3); Figure S5: Immune simulation analysis: a) Represents the production of EP population per state (mm-3) while b) showed the generation of NK cell population per state (mm-3); Table S1a: List of B-cell restricted epitopes predicted for Spike (S) protein; Table S1b: The list of B-cell-specific epitopes predicted from Envelope (E) protein; Table S1c: List of all the B-cell restricted protein predicted from Membrane (M) protein; Table S1d: The list of linear B-cell restricted epitope predicted from Nucleocapsid (N); Table S2a: List of reference HLA alleles used for the prediction of MHC-I restricted epitopes; Table S2b: The selected HLA alleles as reference for the prediction of MHC-II restricted epitopes; Table S3a: The set alleles for individual MHC-I epitopes used in Population Coverage Analysis; Table S3b: The set alleles for individual MHC-II epitopes used in Population Coverage Analysis; Table S4a: Cumulative population coverage against individual MHC-I restricted epitopes and HLA hits; Table S4b: Percentage genotype frequencies of each HLA allele against individual MHC-I restricted epitopes; Table S4c: Cumulative population coverage against individual MHC-II restricted epitopes and HLA hits; Table S4d: Percentage genotype frequencies of each HLA allele against individual MHC-II restricted epitopes; Table S5: C-score for the designed 3D model by I-TASSAR; Table S6: List of predicted (black) and selected (Green) pairs for Disulfide engineering; Table S7: List of cluster members and lowest energies obtained from Cluspro docking analysis against various TLRs and vaccine construct; Table S8: The eigenvalue of various docked complexes that were obtained from ClusPro analysis. File named Tools and access dates contained the links and access dates of all the software, servers, and tools used in this study (RAR 19896 KB)
Data Availability Statement
More data related to this study can be accessed by sending a rea-sonable email to 123allah.rakha@gmail.com.