Abstract
This study is aimed to optimise the preparation factors, such as sonication time (5–20 min), cholesterol to lecetin ratio (CHLR) (0.2–0.8), and essential oil content (0.1–0.3 g/100 g) in solvent evaporation method for formulation of liposomal nanocarriers containing garlic essential oil (GEO) in order to find the highest encapsulation efficiency and stability with strongest antioxidant and antimicrobial activity. The droplet size, zeta potential, encapsulation efficiency, turbidity, changes in turbidity after storage (as a measure of instability), antioxidant capacity, and antimicrobial activity were measured for all prepared samples of nanoliposome. The sonication time is recognised as the most effective factor on the droplet size, zeta potential, encapsulation efficiency, turbidity, and instability while CHLR was the most effective factor on zeta potential and instability. The content of GEO significantly affected the antioxidant and antimicrobial activity in particular against gram‐negative bacteria (Escherichia coli). The results of FTIR based on the identification of functional groups confirmed the presence of GEO in the spectra of the prepared nanoliposome and also it was not observed the interaction between the components of the nanoliposome. The overall optimum conditions were determined by response surface methodology (RSM) as the predicted values of the studied factors (sonication time: 18.99 min, CHLR: 0.59 and content of GEO: 0.3 g/100 g) based on obtaining the highest stability and efficiency as well as strongest antioxidant and antimicrobial activity.
Keywords: food technology, food safety, free radical reactions, nanobiotechnology, nano particles, nanotechnology
This study is aimed to optimise the preparation factors such as sonication time (5–20 min), cholesterol to lecetin ratio (CHLR) (0.2–0.8) and essential oil content (0.1–0.3 g/100 g) in solvent evaporation method for formulation of liposomal nanocarriers containing garlic essential oil (GEO) in order to find the highest encapsulation efficiency and stability with strongest antioxidant capacity and antimicrobial activity.

1. INTRODUCTION
Essential oils and extracts of plants, which are mainly used to flavour food beverages, are particularly well known for their antimicrobial and antifungal activities [1, 2]. There are many reports on the use of various essential oils such as thyme, oregano and eucalyptus in dairy beverages including Doogh [3]. Meanwhile, garlic (Allium sativum) has been introduced for centuries as an important food flavouring with unique medicinal properties [4, 5, 6]. Many studies have shown that garlic has strong antimicrobial activity against bacteria and fungi [7, 8]. The main compounds of garlic that are responsible for bioactive properties include allicin, ajoene, thiosulfinate, and other organicsulphurate compounds [9]. It has been found that allicin and thiosulfinates, which are the main cause of garlic's strong taste, can decompose under different temperature and pH conditions and form additional sulfur compounds, such as diallyl, methylallyl, and diethyl polysulfides and the vinyldithiins and also the antimicrobial compounds (E)‐ and (Z)‐ ajoene [10]. The sensitive nature and especially the pungent smell of garlic compounds limits its use in food products and only a few technological approaches have been introduced to solve this limitation [11, 12].
The use of nanocarrier systems such as liposomes is one of the ways that can protect various bioactive compounds against environmental and chemical changes, thus increasing their bioavailability and stability, thereby increasing their shelf life [13]. Besides the physical and chemical properties of the bioactive components, the encapsulation methods and the delivery system also depend on the characteristics of the final product. Encapsulated and delivered bioactive ingredients should not cause adverse changes in the appearance, texture, or mouthfeel of the final product. Delivery systems for bioactive components should be easily scalable, economical, and prepared from materials that are acceptable in foods [4, 14].
Liposomes are introduced as two spherical layers with an aqueous core inside. These vesicles are composed of phospholipid membranes with amphiphilic properties, and biodegradability, biocompatibility, and controlled release behaviour of water‐soluble compounds, fat‐soluble compounds, and amphiphilic compounds are among the advantages of this type of nanocarriers [15]. Encapsulation of garlic essential oil by nanoemulsion and investigation of physicochemical and antimicrobial properties and their stability have been done in our previous works (H [16]) However, in the present research, nanoliposome was chosen as an efficient system for encapsulation and controlled release of garlic's active compounds to investigate and optimise their encapsulation efficiency, inhibitory effect against bacteria, antioxidant properties, and instability.
2. MATERIALS AND METHOD
2.1. Materials
Phosphatidylcholine (lecithin) was provided from Merck (Germany). DPPH (2,2‐diphenyl‐1‐picrylhydrazyl) was supplied from Sigma‐Aldrich Company. The used microorganisms including Staphylococcus aureus (ATCC® Number: 25923) and Escherichia coli H7 0157 (ATCC® Number: 700728) were prepared from Iran Scientific and Industrial Research Organisation. GEO was purchased from Zardband Co.
2.2. Preparation of liposomes
First, phospholipid, cholesterol and a certain amount of garlic essential oil (according to the experimental design) are dissolved in an organic solvent containing chloroform and methanol with a ratio of 1:2 and the organic solvent is evaporated using a rotary evaporator under a temperature of 40°C and low pressure, then by forming a thin film of cholesterol and phospholipid at the bottom of the rotary evaporator, the removal of the organic solvent is finished. The created film containing the encapsulated substance was hydrated using double distilled water and its temperature was raised to a temperature above the Tm of phospholipid. Then, after the formation of liposomes, the samples were placed in a sonicator for 25 min to reduce the size of the liposomes after that, in order to create a homogeneous state, they were rested for 30 min at ambient temperature. Finally, the produced samples were kept in the refrigerator at a temperature of 2–4°C until the next tests [17].
2.3. Droplet size and zeta potential
The droplet size, polydispersity Index (PDI), and zeta potential of nanoliposomes containing GEO were derermined by dynamic light scattering (DLS) and zetasizer (Malvern Instruments, U.K).
2.4. Encapsulation efficiency
Encapsulation efficiency (EE) value of GEO loaded nanoliposomes was determined by centrifugation method [18]. An aliquot of the produced nanoliposome solution was placed in the upper chamber of a Millipore Amicon® Ultra filtration tube (100 kDa cutoff) and centrifuged (3k‐30, Sigma, Germany) at 900 rpm for 4 min. The amount of GEO was calculated (Ultrospec2000, Pharmacia Biotech, England) by recording the absorbance at λmax = 217 nm and using the calibration curve by spectrophotometer. Finally, the following equation was used for calculating the encapsulation efficiency of produced nanoliposoms [19]:
3.
Droplet size (average Z) and polydispersity index (PDI) of GEO nanoliposomes were measured using dynamic light scattering (DLS) method (Zetasizer Nano ZS, Malvern Instruments, UK) [19].
3.1. Free radical scavenging activity
The antioxidant capacity of GEO nanoliposomes was measured by DPPH method [20]. 1 ml of methanolic DPPH solution (0.4 mM) was mixed with 1 ml of samples and kept in the dark place at room temperature for 1 h and finally the absorbance was recorded at 517 nm via a UV–Vis spectrophotometer [19].
3.2. Antibacterial activity of GEO nanoliposomes
The microdilution method was used to determine the minimum inhibitory concentration (MIC). For this purpose, a fresh culture of bacteria was prepared. The studied bacteria including Staphylococcus aureus (ATCC 25923) and Escherichia coli H7 O157 (ATCC 700728) were inoculated in 15 mL Falcon tubes containing liquid Mullerhinton culture medium and placed in a greenhouse at 37°C for 24 h. After 24 h of incubation, the grown bacteria in the liquid environment caused turbidity, so in order to obtain the bacterial sediment, Falcon tubes were centrifuged at 3000 rpm for 15 min. The bacterial sediment was washed twice with sterile physiological serum and in the fourth step, the 0.5 McFarland dilution was prepared by McFarland standard tubes and sterile physiological serum. Tryptic soy broth (TSB) culture medium was prepared separately and added to 7 wells of the plate in the amount of 100 μL.
Then, different dilutions of nanoliposomes were prepared in this culture medium inside the wells of the 96‐well plate (first, 100 μL of nanoemulsion was added to 100 μL of TSB culture medium in the first well and 1 ml of this mixture was added to the second well and this process is repeated from the second well to the third well until the sixth well). In fact, 1/2 to 1/64 dilutions were prepared from liposomes. In the next step, 20 μL of the prepared dilution from bacteria were inoculated in the wells containing culture medium and liposomes. In the seventh well, only 100 μL of culture medium was added to the prepared dilution from bacteria which this cell is considered as the control cell. The dilution prepared from bacteria was 0.5 McFarland (approximate number of 1.5 × 108), but it was also diluted by sterile physiological serum to prepare an approximate number of 5 × 105 in order to obtain more correct results. Finally, the plates of 96‐well plates were placed in an incubator at 37°C for 18 h, then the MIC level was determined by comparison of turbidity in treated and control wells.
3.3. FT‐IR analysis
Infrared spectra were obtained using potassium bromide (KBr) method by Fourier transform infrared spectrophotometer (Nexus‐670, Thermo Nicolet, USA). First, 2 mg of samples were mixed with 50 mg of KBr to fill the pellet. Identification of functional groups in the spectral region of 4000‐500 cm−1 was performed with 64 scans recorded at a resolution of 4 cm−1.
3.4. Statistical analysis
In this research, the response surface methodology (RSM) and specifically the central composite design (CCD) have been used to optimise the encapsulation efficiency of the bioactive compound by the nano‐carriers. Design of experiments was carried out for the three factors of sonication time (5–25 min), the amount of essential oil used in the range (0.1–0.3 g/100g) and the ratio of cholesterol to lecithin (CHLR) (0.25–1) by Design Expert software version 13. and analysis of variance (ANOVA) and optimisation were done numerically in the same software.
4. RESULTS AND DISCUSSION
4.1. Droplet size, PDI, and zeta potential
DLS results showed that the sonication time was the most effective factor on the size of the droplets in the produced nanocarriers, so that the droplet size decreased from about 300 nm to about 100 nm with an increase of the sonication time from 10 to 20 min (Figure 1a & Figure 2). It should be noted that the interaction effect of sonication time and the concentration of essential oil used in the nanocarrier was also significant, which means that the effect of sonication time was different on the droplet size in different concentrations of essential oil. As shown in Figure 1a, unlike low concentrations of essential oil, with increasing sonication time from 10 to 20 min, the droplet size has decreased to only about 200 nm in the high concentrations of essential oil.
FIGURE 1.

Counterplot to show the interaction effect of GEO amount and sonication time (a) and the interaction effect of CHLR and sonication time (b) on the droplet size.
FIGURE 2.

The droplet size of the typical nanoliposome sample.
Similar results was obtained by other researchers, for instance, the little increase in droplet size was reported by increasing the cardamom essential oil in nanostructured lipid carrier formulation [21]. In contrast, Lacatusu et al [22] reported the non‐significant differences in droplet size of nanostructured lipid carrier prepared with different ratios of Hempseed oil and Amaranth oil.
In Figure 1b, the effect of CHLR on droplet size at different sonication times is shown. In general, Figure 1 shows that the effect of other factors was not remarkable at higher sonication times, but the droplet size decreased with increasing CLHR at lower sonication times. It is probably the reason for the formation of fewer nanocarrier vesicles in high CHLR, which basically contain less amount of lecithin to form vesicles. In consistent with results, the using of cholesterol in liposome formulation was presented as a reducing factor in droplet size. The authors associated the effect of cholesterol on droplet size with the method of nanoliposome production and the type of phospholipids [23]. In contrast, Pezeshky [24] reported no considerable effect on the particle size by increasing the level of cholesterol (from 0 to 30 mg) in Vitamin A palmitate‐bearing nanoliposome formulations. According to the review of similar researches, cholesterol is more effective on the stability in comparison with vesicle size of the produced nanoliposomes and [24].
The surface charge of a suspension or dispersion is defined as the zeta potential. Zeta potential can affect the electrostatic stability of droplets, the ability of nanoliposomes to bind to the membrane of target cells, the strength of the bond between the carrier material and the bioactive material, as well as the release kinetics of bioactive material. Lipid composition may also affect the electrophoretic mobility of vesicles. Lipophilic bioactive substances encapsulated in a bilayer membrane can neutralise the phospholipid charge of the carrier [25]. High values of electronegative zeta potential, which is the result of increasing electrostatic repulsion between liposomes, can lead to good stability of liposomal suspensions (lower aggregation and higher size stability) [8, 26]. Figure 3 shows the results of the zeta potential for prepared nanocarriers under the influence of the studied factors. Among the studied factors, sonication time and especially CHLR had a significant effect on zeta potential. As shown in Figure 3a, with increasing sonication time from 10 to 20 min, the zeta potential reaches from −5 to −10 in medium concentrations of essential oil. Essential oil concentration had a little effect on zeta potential only at low sonication times but it was not statistically significant (p = 0.1). Figure 3b shows the interaction of sonication time and CHLR as two influencing factors in zeta potential. The highest amount of zeta potential was obtained at higher sonication times and CLHR ratios, which could be due to the creation of smaller and more stable vesicles by more sonication and higher amounts of cholesterol. Cholesterol is mainly used to promote liposome layering and leads to improvement of membrane fluidity, stability of layers and reduction of permeability of water‐soluble molecules through membranes [27]. Cholesterol also increases stability and produces rigid membranes with similar liquid properties in lipid bilayers [28].
FIGURE 3.

Counterplot to show the interaction effect of GEO amount and sonication time (a) and the interaction effect of CHLR and sonication time (b) on the zeta potential.
According to the obtained results, the surface charge of all samples was lower than the expected values recorded for nanospherical systems made with SPC (about −26 mV). Allicin (the main component of GEO with positive charge of S groups) may interact with the negative charge of phosphatidylcholine phosphate group and produce less negative charge for the formed nanoliposomes. The physical stability of developed nanophytosomes should be focused in food to ensure the successful performance of nanoparticles during the storage period [19].
It is generally reported that zeta values of ±30 mV can protect nanovesicles from aggregation and reduce the electrostatic repulsion between nanocarriers, which can cause the formation of large masses of nanocarrier vesicles [19, 29]. Size increase has been reported as the main disadvantage of vesicular systems during storage time [30]. However, sometimes the size increase was still in the acceptable nano range for successful antibacterial effect. For example, in one study, the size of phytosomes containing quercetin increased approximately 6‐fold possibly due to high initial dispersion and lower zeta potential after 7 days of storage [31].
4.2. Encapsulation efficiency
Encapsulation efficiency is a vital factor in nano‐based delivery systems to study on the ability of various nanocarriers in delivery of bioactive components [32]. The results of RSM showed that the quadratic statistical model was significant for predicting the changes of the studied factors to optimise the encapsulation efficiency (p < 0.001). Among these factors, the concentration of the essential oil had no significant effect on the encapsulation efficiency (p = 0.579) while the sonication time and the CHLR had a significant effect on the encapsulation efficiency (p < 0.01). When the main effects, interaction effects and square effects of the studied factors were significant in the quadratic model, the interpretation is ambiguous and in this cases it is possible to interpret and investigate the interaction effects of the factors in food studies [33].
As shown in Figure 4a, there was no significant change in the encapsulation efficiency with an increase in the essential oil concentration while encapsulation efficiency decreases from nearly 100% to 80% with an increase in the CHLR (from 0.75/0.25 to 0.25/0.75). Lecithin, which simultaneously has a hydrophobic and hydrophilic head in its structure, plays the main role in trapping hydrophobic or non‐polar bioactive compounds, so it is expected that the encapsulation efficiency will increase with raising the lecithin content in nano‐carrier formulation. On the other hand, cholesterol which is added into the lipid bilayer membrane to decrease the permeability of the lipid membrane, stiffening the membrane and improves the stability. Therefore, cholesterol in liposome membrane maybe prevents from disruption and alters in liposome membrane which inhibits the incorporation of essential oil into the liposomes in turn [24]. In consistent with previous works, Wu, et al. [32] also reported an increase in encapsulation efficiency with the increasing lecithin to cholesterol ratio. Also, Mohammadi, et al [34], demonstrated that the sterol made liposomes for vitamin D entrapment recorded the higher encapsulation efficiency and lower size stability than sterol‐free liposomes.
FIGURE 4.

Counterplot to show the interaction effect of GEO amount and CHLR (a) and the interaction effect of GEO amount and sonication time (b) on the encapsulation efficiency.
Sonication time is another factor that had a significant effect on the encapsulation efficiency of the produced nanocarriers. Figure 4b shows the simultaneous effect of sonication time and essential oil concentration on the encapsulation efficiency, in which the significant increasing effect of the sonication time on the encapsulation efficiency is clearly observed, so that this trend is the same for all essential oil levels. The application of more sonication time could produce much smaller vesicles with transparent suspension, in which the lower turbidity would provide higher encapsulation efficiency and stability [19]. In a similar study, Nazari, et al [19] also reported that the simultaneous use of an ultrasound probe and a homogeniser resulted in a higher encapsulation efficiency compared to the ultrasound probe alone due to the creation of smaller lipid vesicles.
4.3. Turbidity measurement
It should be noted that the turbidity is an index of block level of suspended solids vesicles in water to light which the lower turbidity could led to the better quality of food stuff [35]. It was stated that the size of lipid vesicles was effected by preparation method and highly correlated with the turbidity of liposome [36]. Analysis variance of turbidity shows that the concentration of garlic essential oil had no significant effect while the sonication time and CHLR had a significant effect on turbidity. Figure 5 shows the interaction effect of garlic essential oil concentration and CHLR, in which in lower CHLR, there was no significant change in the amount of turbidity at all levels of garlic essential oil. In contrast, in higher ratios of CHL, due to the decrease of lecithin, with the increase of essential oil concentration, the fewer vesicles are formed and it leads to lower turbidity. Also, with the increase of CHLR, the amount of turbidity decreased from 600 to 100. In the other word, the higher the ratio of CHLR, the lower the amount of lecithin, and this can lead to the formation of fewer vesicles so less light deviation occurs, and this reduces the turbidity in turn. Finally, another factor that more than others affects the turbidity of produced liposomes is the sonication time. It is clear that with increasing sonication time, the formed vesicles are more broken and produce small particles, which causes less light deviation and more transparency of the produced nanocarrier in turn.
FIGURE 5.
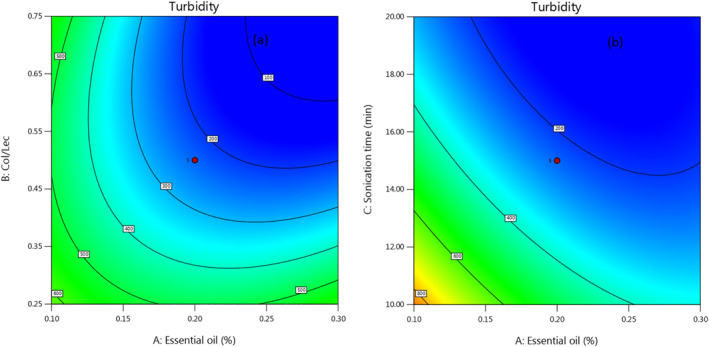
Counterplot to show the interaction effect of GEO amount and CHLR (a) and the interaction effect of GEO amount and sonication time (b) on the turbidity.
4.4. Storage instability
Liposomes must have good storage stability to become a commercial product, meaning they must remain intact during the product's shelf life [37]. Although the mechanism of instability is still unclear, it may be due to aggregation and leakage of nanoliposomes and chemical degradation of bioactive substances in nanoliposome solutions, leading to discolouration or precipitation at high storage temperatures [38]. In general, unmodified conventional liposomes are thermodynamically unstable systems that tend to aggregate, melt, dissociate, or hydrolyse, leading to leakage of loaded compounds [37]. In relation to stability during storage, it can be mentioned that the lipid nanoparticles are thermodynamically unstable due to their heterogeneous systems and are generally prone to losing their physical stability during storage, which is considered as an important feature of the product. Physical instability can be measured by microscopy, spectroscopy, turbidity and particle size analysis. The main destabilisation mechanisms that affect the homogeneity of dispersions are: particle migration that is, creaming or sedimentation, and particle size change or aggregation that is, coalescence and flocculation [21].
The percentage of changes in turbidity after 1 month of storage at refrigerator temperature is recorded as percentage of instability. The results of turbidity changes after 1 month of storage showed that the sonication time has the greatest effect on the instability of the produced nanoliposomes, so that by changing the sonication time from 10 to 20 min, the percentage of instability (changes in turbidity) has decreased by 50% (Figure 6a). Another important factor that has a significant effect on the instability of produced nanocarriers is CHLR, which means that as the ratio of cholesterol increases and lecithin decreases, lecithin molecules do not have enough ability to surround the entrapped essential oil molecules and therefore the droplets formed more easily break apart or merge together and form larger droplets, increasing the turbidity and the instability of the nanocarrier in turn. In consistent with this result, the highest stability of nanoliposomes containing vitamin C after 20 days was recorded in the ratio of 75:25 (phospholipids and campesterol) [28]. As shown in Figure 6b, the amount of instability increases with increasing the content of essential oil surrounded in the nanocarrier, especially at lower sonication times. At the similar attempt, the storage stability of the nanoliposomes containing Naringenin was evaluated by measuring changes in their mean droplet size, polydispersity index and zeta‐potentials and reported that Naringenin‐loaded nanoliposomes are more hydrophobic and probably accelerate aggregation, oxidation, leakage, and other chemical reactions during storage, which precipitate Naringenin crystals and reduce stability [39]. In another research, the stability of anthocyanin compound loaded nanoliposomes was evaluated at 37°C and no significant difference was detected in the parameters of polydispersity index and zeta‐potentials after 30 days of storage while the encapsulation efficiency and droplet size had a significant decreasing and increasing trend respectively [40].
FIGURE 6.

Counterplot to show the interaction effect of GEO amount and sonication time (a) and the interaction effect of CHLR and sonication time (b) on the storage instability.
4.5. Antioxidant and antimicrobial activity
The results of DPPH method showed that the sonication time and different CHLR had no significant effect on the antioxidant capacity of the produced nanocarriers while the concentration of the essential oil significantly affected the antioxidant properties so that the antioxidant capacity has almost doubled by increasing the concentration of essential oil from 0.1 to 0.3 g/100 g (Figure 7). However, researchers including Ha et al. [41] related the antioxidant property of emulsion nanocarriers loaded‐tomato extract to the particle size, while in the present study, the antioxidant property was more influenced by the garlic essential oil. In general, the antioxidant property in food is more related to polyphenol substances that exist in plant extracts, while essential oils are rich in terpenoid compounds and their antimicrobial properties are more prominent. In the present research, more focus has been applied on the antimicrobial properties of nanocarriers loaded‐GEO.
FIGURE 7.

Counterplot to show the interaction effect of GEO amount and CHLR (a) and the interaction effect of GEO amount and sonication time (b) on the antioxidant capacity.
The MIC and MBC results of the produced nanocarriers for a gram‐negative bacterium (E. coli) and a gram‐positive bacterium (S. aureus) showed that, in general, these nanocarriers are only in high concentrations of essential oils are able to prevent the growth of the studied bacteria and practically did not show antimicrobial properties in low concentrations (lower than 0.3 g/100 g of nanocarrier) (Figure 8). It should be noted that this effect is different for two bacteria, E. coli and S. aureus, so that the produced nanocarriers have a greater effect on E. coli and stop growth at concentrations of 0.3 g/100 g of nanocarrier, while they were able to prevent growth only at concentrations of 0.3 g/100 g of nanocarrier for S. aureus bacteria. The reason for the most antimicrobial activity of GEO is allicin and its metabolites in which they perform their antimicrobial activities by specifically inhibiting the enzyme acetyl coenzyme A‐Synthetase and inhibiting the synthesis of lipids and fatty acids, which causes damage to the cell in turn. Also, one of the most remarkable characteristics for organic sulfur compounds of garlic is permeability and passing through membrane phospholipids. Microbial cells are more sensitive to GEO than human cells because they do not have sufficient amounts of intracellular thiols to neutralise or balance the oxidation of thiols caused by organosulfur derivatives and allicin [11].
FIGURE 8.

The effect of GEO amount on the antimicrobial activity against E. coli (a) and against S. aureus (b).
These results can be due to the cell wall structure and characteristics of gram‐negative and gram‐positive bacteria. A bilayer‐like cell membrane surrounds the surface of gram‐negative bacteria, while the cell wall of gram‐positive bacteria consists of a thick layer of peptidoglycan. Vesicular nanoparticles (for example, liposomes) tend to intermingle with similar bilayer structures, which could explain the stronger antimicrobial effect of GEO‐nanocarriers against gram‐negative compared to gram‐positive bacteria [19, 42]. In the most similar research, based on the MIC results of GEO loaded nanocarriers, the inhibitory effect of GEO loaded nanophytosomes was reported more against E. coli than S. aureus (MIC = 3.75 and 7.5 mg/ml respectively) [19]. In another research, the most and least effectiveness is reported on E. coli and S. aureus, respectively for nanostructured lipid carriers containing cardamom essential oil [21].
4.6. FT‐IR analysis
In this work essential oil compounds is trapped by surfactants interactions, so FT‐IR spectroscopy has been used for the characterisation of the samples functional groups. Figure 9 presents the FT‐IR spectra of cholesterol, tween 80, lecithin, GEO and prepared nanoliposoms (25% and 5%). The spectrum of cholesterol is shown in Figure 9a.The peak at 3430 cm−1 corresponding to hydroxyl group (O‐H bond vibration), and 2920‐ 2848 cm−1 is related to methyl groups (asymmetric vibrations of C‐H bonds). Furthermore, characteristic bonds appeared at 1464 cm−1 and 1375 cm−1 refers to C=C bending and stretching vibrations of O‐H and C‐O‐H respectively. The peaks at 1247 cm−1, 1130 cm−1, 1055 cm−1 and 1020 cm−1 are due to C–O esters stretching vibrations and the peaks at 955 cm−1, 835 cm−1, 730 cm−1 and 600 cm−1 are related to aromatic substitution. In the spectrum of tween 80 (is used as an emulsifier) in Figure 9B, absorbance peak in 3431 cm−1 is attributed to hydroxyl group vibrations. The absorbance peak in the regions of 2847–2945 cm−1 refers to symmetrical and asymmetrical aliphatic ‐CH vibrations. The C=O ester vibrations is shown at 1730 cm−1 and C=C vibrations is appeared in the region of 1604 cm−1. The bending vibrations of methyl and ‐CH2 groups and the absorbance peak of C‐O‐C group are appeared at 1455 cm−1 and 1109 cm−1 regions respectively. In the FTIR spectra of lecithin in Figure 9c, the strong peak at 3390 cm−1 corresponds to the O‐H stretching of alcoholic esters. In addition, the absorbance peaks at 2920‐2849 cm−1 and 1730 cm−1 were due to stretching CH2 alkane groups and vibration of C=O groups, respectively and absorbance peaks of P‐O stretching are appeared at 577 cm–1. In Figure 9d which refers to FT‐IR spectrum of GEO the absorbance peaks of aromatic and aliphatic (‐CH) are appeared in the regions of 3078 cm−1 and 2900–2950 cm−1 respectively. The peaks in 1634 cm−1 and 1422 cm−1 regions are related to C=C group and the bending vibrations of ‐CH3 group respectively. The characteristic bonds at 1285 cm−1 and 1078–1092 cm−1 attributed to O=S=O vibrations and thiosulphate groups respectively. The absorbance peaks in 1075 cm−1 and 922–990 cm−1 regions demonstrated stretching vibrations of the C‐(OS)‐C and diallyl trisulfide groups respectively. Stretching vibrations of C‐S groups is exhibited in the region of 723 cm−1 and the absorbance peaks of 480–495 cm−1 refers to the S=S group. The presence of Lecithin, tween 80 and GEO in prepared nano‐liposomes is confirmed by FTIR spectra in Figures 9e and f which they have a similar spectrum. The only difference between them is the absorbance intensity in some regions, which confirms change of ingredients in their formulation. In this case the following spectral regions were revealed: ‐NH and hydroxyl–OH asymmetric stretching vibration (1620–1665 cm−1), carbonyl stretching mode C=O (1685–1800 cm−1) and symmetric CH2 stretching vibration (2850–2860 cm−1), phosphate asymmetric stretching vibration PO2‐(1220–1260 cm−1). According to the Figure 9F, the change of intensity and location of some absorbance peaks in FTIR spectrum of GEO loaded nanoliposome can indicate the loading of GEO into structure of nanoliposome. Also, the peaks correspond to P‐O group region shifted towards higher transmittance (%T) which can be related to higher degree of hydration at the result of hydrogen bonding with water molecules. The presence of peak at 1730 cm−1 can be corresponded to carbonyl (C=O) groups of lecithin structure in nanoliposome. As can be seen in these spectra, by increasing the GEO percentage in the nanoliposome formulation the peak intensity of aromatic (‐CH) vibrations was increased. Moreover, the characteristic peaks of S = S and C‐S groups and diallyl disulfide which are related to GEO components have more intensity in 0.3 g/100g nanoliposome in comparison to the 0.1% nanoliposome. Finally, it can be concluded that the identification of functional groups by FT‐IR confirmed the presence of GEO in spectra of the prepared nanoliposome and also it was not observed interaction between the components of the nanoliposome.
FIGURE 9.

FT‐IR spectroscopy of Cholesterol (a), Tween 80 (b), Lecithin (c), GEO (d) nanoliposome containing 0.1 g/100g GEO (e) and nanoliposome containing 0.3 g/100g GEO (f).
4.7. Optimization
In order to optimize the production conditions of nanoliposomes loaded‐GEO by solvent evaporation method, factors such as sonication time, concentration of GEO and CHLR in their normal range were evaluated numerically using the RSM. The optimum values of studied factors and responses were determined. The mentioned factors have been optimised based on the best desired results to obtain the lowest turbidity, instability, and droplet size, as well as the highest encapsulation efficiency, antioxidant capacity, antimicrobial properties, and zeta potential, and the following values have been obtained with desirability = 0.697. The values of lowest turbidity (59.45), instability (5.37), and droplet size (159.8), as well as the highest encapsulation efficiency (95%), antioxidant capacity (29.22), antimicrobial properties (0.84 and 0.99), and zeta potential (−10.97) were obtained by adjusting the production factors on sonication time = 20 min, CHL = 0.58 and GEO concentration = 0.3 g/100g. Finally, a confirmation test was performed for the optimal sample and the relevant responses were measured again in this research, and all the responses had a correlation higher than 80% with the values predicted by the model.
5. CONCLUSION
Considering that the limitations of using garlic essential oil, including unpleasant smell, high volatility, sensitivity to oxidation and lack of solubility in the aqueous phase, are mentioned in several literature. Various delivery systems have recently been applied to improve the properties of garlic essential oil and remove its limitations. In this study, the method of nanoliposomes loaded‐garlic essential oil has been optimised under the influence of important factors. The results of FTIR based on the identification of functional groups confirmed the presence of garlic essential oil in the spectra of the prepared nanoliposome and also it was not observed interaction between the components of the nanoliposome. The results of the optimisation showed that by controlling the process, it is possible to produce nanoliposomes loaded‐garlic essential oil with acceptable stability and encapsulation efficiency, as well as having antioxidant and antimicrobial properties, especially against gram‐negative bacteria. All of these can be promising the possibility of using nanoliposomes loaded‐garlic essential oil to enrich different foods, including dairy products and beverages.
AUTHOR CONTRIBUTION
Salar Ali Ahmed: Investigation; Methodology. Mahmood Fadhil Saleem: Formal analysis; Writing – review & editing. Hamed Hassanzadeh: Supervision; Writing – original draft.
CONFLICT OF INTEREST STATEMENT
All authors declare that they have no conflict of interest.
Ahmed, S.A. , Saleem, M.F. , Hassanzadeh, H. : Optimization of solvent evaporation method in liposomal nanocarriers loaded‐garlic essential oil (Allium sativum): based on the encapsulation efficiency, antioxidant capacity, and instability. IET Nanobiotechnol. 17(5), 438–449 (2023). 10.1049/nbt2.12142
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Zolfaghari, A. , Ansari, S. : Physicochemical and microbiological properties of Chaerophyllum, Oliveria and Zataria essential oils and their effects on the sensory properties of a fermented dairy drink,‘doogh. Int. J. Food Prop. 23(1), 1540–1555 (2020). 10.1080/10942912.2020.1818777 [DOI] [Google Scholar]
- 2. Nanakali, N.M. : Fabrication of Nano‐encapsulated Angelica (Heracleum Persicum) Essential Oil for Enriching Dairy Dessert: Physicochemical, Rheological and Sensorial Properties. IET nanobiotechnology; (2023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ziaolhagh, S. , Jalali, H. : Physicochemical properties and survivability of probiotics in bio‐doogh containing wild thyme essence and xanthan gum. Int. Food Res. J. 24(4), 1805 (2017) [Google Scholar]
- 4. Habibvand, M. , et al.: Formulation of Nanoemulsion Carriers Containing Pennyroyal (Mentha Pulegium) and Gijavash (Froriepia Subpinnata) Essential Oils for Enriching Doogh (Iranian Dairy Drink). IET nanobiotechnology; (2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hassanzadeh, H. , et al.: Garlic essential oil‐based nanoemulsion carrier: release and stability kinetics of volatile components. Food Sci. Nutr. 10(5), 1613–1625 (2022). 10.1002/fsn3.2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martins, N. , Petropoulos, S. , Ferreira, I.C. : Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre‐and post‐harvest conditions: a review. Food Chem. 211, 41–50 (2016). 10.1016/j.foodchem.2016.05.029 [DOI] [PubMed] [Google Scholar]
- 7. Hasanzadeh, H. , Alizadeh, M. , Rezazad Bari, M. : Production and assessment of physicochemical characteristics and encapsulation efficiency of garlic essential oil nanoemulsions. J. Food Res. 27(4), 159–170 (2017) [Google Scholar]
- 8. Pinilla, C.M.B. , Noreña, C.P.Z. , Brandelli, A. : Development and characterization of phosphatidylcholine nanovesicles, containing garlic extract, with antilisterial activity in milk. Food Chem. 220, 470–476 (2017). 10.1016/j.foodchem.2016.10.027 [DOI] [PubMed] [Google Scholar]
- 9. Ledezma, E. , Apitz‐Castro, R. : Ajoene the main active compound of garlic (Allium sativum): a new antifungal agent. Rev. Iberoam. De. Micol. 23(2), 75–80 (2006). 10.1016/s1130-1406(06)70017-1 [DOI] [PubMed] [Google Scholar]
- 10. Rose, P. , Moore, P.K. , Zhu, Y.‐Z. : Garlic and gaseous mediators. Trends Pharmacol. Sci. 39(7), 624–634 (2018). 10.1016/j.tips.2018.03.009 [DOI] [PubMed] [Google Scholar]
- 11. Hasssanzadeh, H. , Alizadeh, M. , Rezazad Bari, M. : Formulation of garlic oil‐in‐water nanoemulsion: antimicrobial and physicochemical aspects. IET Nanobiotechnol. 12(5), 647–652 (2018). 10.1049/iet-nbt.2017.0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang, Y.‐F. , et al.: Study of allicin microcapsules in β‐cyclodextrin and porous starch mixture. Food Res. Int. 49(2), 641–647 (2012). 10.1016/j.foodres.2012.09.033 [DOI] [Google Scholar]
- 13. Reza Mozafari, M. , et al.: Nanoliposomes and their applications in food nanotechnology. J. Liposome Res. 18(4), 309–327 (2008). 10.1080/08982100802465941 [DOI] [PubMed] [Google Scholar]
- 14. Gruskiene, R. , Bockuviene, A. , Sereikaite, J. : Microencapsulation of bioactive ingredients for their delivery into fermented milk products: a review. Molecules 26(15), 4601 (2021). 10.3390/molecules26154601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brandelli, A. , Pinilla, C.M.B. , Lopes, N.A. : Nanoliposomes as a platform for delivery of antimicrobials. Nanotechnology Applied To Pharmaceutical Technology, 55–90 (2017). 10.1007/978-3-319-70299-5_3 [DOI] [Google Scholar]
- 16. Hassanzadeh, H. , Alizadeh, M. , Rezazad, B. : Nano‐encapsulation of garlic extract by water‐in‐oil emulsion: physicochemical and antimicrobial characteristics. Iranian Food Science & Technology 15(84), 337–347 (2019) [Google Scholar]
- 17. Fang, J.‐Y. , et al.: Effect of liposome encapsulation of tea catechins on their accumulation in basal cell carcinomas. J. Dermatol. Sci. 42(2), 101–109 (2006). 10.1016/j.jdermsci.2005.12.010 [DOI] [PubMed] [Google Scholar]
- 18. Amjadi, S. , et al.: Improvement in the stability of betanin by liposomal nanocarriers: its application in gummy candy as a food model. Food Chem. 256, 156–162 (2018). 10.1016/j.foodchem.2018.02.114 [DOI] [PubMed] [Google Scholar]
- 19. Nazari, M. , et al.: Garlic essential oil nanophytosomes as a natural food preservative: its application in yogurt as food model. Colloid and Interface Science Communications 30, 100176 (2019). 10.1016/j.colcom.2019.100176 [DOI] [Google Scholar]
- 20. Niu, Y. , et al.: Temperature‐dependent stability and DPPH scavenging activity of liposomal curcumin at pH 7.0. Food Chem. 135(3), 1377–1382 (2012). 10.1016/j.foodchem.2012.06.018 [DOI] [PubMed] [Google Scholar]
- 21. Nahr, F.K. , et al.: Food grade nanostructured lipid carrier for cardamom essential oil: preparation, characterization and antimicrobial activity. J. Funct.Foods 40, 1–8 (2018). 10.1016/j.jff.2017.09.028 [DOI] [Google Scholar]
- 22. Lacatusu, I. , et al.: Lipid nanocarriers based on natural compounds: an evolving role in plant extract delivery. Eur. J. Lipid Sci. Technol. 116(12), 1708–1717 (2014). 10.1002/ejlt.201300488 [DOI] [Google Scholar]
- 23. Ruktanonchai, U. , et al.: Effect of lipid types on physicochemical characteristics, stability and antioxidant activity of gamma‐oryzanol‐loaded lipid nanoparticles. J. Microencapsul. 26(7), 614–626 (2009). 10.3109/02652040802586571 [DOI] [PubMed] [Google Scholar]
- 24. Pezeshky, A. , et al.: Vitamin A palmitate‐bearing nanoliposomes: preparation and characterization. Food Biosci. 13, 49–55 (2016). 10.1016/j.fbio.2015.12.002 [DOI] [Google Scholar]
- 25. Nahr, F.K. , et al.: Investigation of physicochemical properties of essential oil loaded nanoliposome for enrichment purposes. Lebensm. Wiss. Technol. 105, 282–289 (2019). 10.1016/j.lwt.2019.02.010 [DOI] [Google Scholar]
- 26. Bashiri, S. , et al.: Preparation and characterization of chitosan‐coated nanostructured lipid carriers (CH‐NLC) containing cinnamon essential oil for enriching milk and anti‐oxidant activity. Lebensm. Wiss. Technol. 119, 108836 (2020). 10.1016/j.lwt.2019.108836 [DOI] [Google Scholar]
- 27. Laouini, A. , et al.: Preparation, characterization and applications of liposomes: state of the art. Journal of colloid Science and Biotechnology 1(2), 147–168 (2012). 10.1166/jcsb.2012.1020 [DOI] [Google Scholar]
- 28. Amiri, S. , et al.: New formulation of vitamin C encapsulation by nanoliposomes: production and evaluation of particle size, stability and control release. Food Sci. Biotechnol. 28(2), 423–432 (2019). 10.1007/s10068-018-0493-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu, R. : Progress in nanoparticles characterization: sizing and zeta potential measurement. Particuology 6(2), 112–115 (2008). 10.1016/j.partic.2007.12.002 [DOI] [Google Scholar]
- 30. Sahari, M.A. , et al.: Improved physical stability of docosahexaenoic acid and eicosapentaenoic acid encapsulated using nanoliposome containing α‐tocopherol. IJFST (Int. J. Food Sci. Technol.) 51(5), 1075–1086 (2016). 10.1111/ijfs.13068 [DOI] [Google Scholar]
- 31. Rasaee, S. , et al.: Nano phytosomes of quercetin: a promising formulation for fortification of food products with antioxidants. Pharmaceut. Sci. 20(3), 96–101 (2014) [Google Scholar]
- 32. Wu, J. , et al.: The preparation, characterization, antimicrobial stability and in vitro release evaluation of fish gelatin films incorporated with cinnamon essential oil nanoliposomes. Food Hydrocolloids 43, 427–435 (2015). 10.1016/j.foodhyd.2014.06.017 [DOI] [Google Scholar]
- 33. Hubbard, M.R. : Statistical Quality Control for the Food Industry. Springer Science & Business Media; (2012) [Google Scholar]
- 34. Mohammadi, M. , Ghanbarzadeh, B. , Hamishehkar, H. : Formulation of nanoliposomal vitamin D3 for potential application in beverage fortification. Adv. Pharmaceut. Bull. 4((Suppl 2)), 569 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cui, H. , Zhao, C. , Lin, L. : The specific antibacterial activity of liposome‐encapsulated Clove oil and its application in tofu. Food Control 56, 128–134 (2015). 10.1016/j.foodcont.2015.03.026 [DOI] [Google Scholar]
- 36. Patil, Y.P. , Jadhav, S. : Novel methods for liposome preparation. Chem. Phys. Lipids 177, 8–18 (2014). 10.1016/j.chemphyslip.2013.10.011 [DOI] [PubMed] [Google Scholar]
- 37. Chen, M. , et al.: Encapsulation of hydrophobic and low‐soluble polyphenols into nanoliposomes by pH‐driven method: Naringenin and naringin as model compounds. Foods 10(5), 963 (2021). 10.3390/foods10050963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li, R. , et al.: Fabrication of pea protein‐tannic acid complexes: impact on formation, stability, and digestion of flaxseed oil emulsions. Food Chem. 310, 125828 (2020). 10.1016/j.foodchem.2019.125828 [DOI] [PubMed] [Google Scholar]
- 39. Zhan, X. , et al.: Entrapment of curcumin in whey protein isolate and zein composite nanoparticles using pH‐driven method. Food Hydrocolloids 106, 105839 (2020). 10.1016/j.foodhyd.2020.105839 [DOI] [Google Scholar]
- 40. Homayoonfal, M. , et al.: Encapsulation of berberis vulgaris anthocyanins into nanoliposome composed of rapeseed lecithin: a comprehensive study on physicochemical characteristics and biocompatibility. Foods 10(3), 492 (2021). 10.3390/foods10030492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ha, T.V.A. , et al.: Antioxidant activity and bioaccessibility of size‐different nanoemulsions for lycopene‐enriched tomato extract. Food Chem. 178, 115–121 (2015). 10.1016/j.foodchem.2015.01.048 [DOI] [PubMed] [Google Scholar]
- 42. Mbah, C.C. , Attama, A.A. : Vesicular Carriers as Innovative Nanodrug Delivery Formulations. In: Organic Materials as Smart Nanocarriers for Drug Delivery, pp. 519–559. Elsevier; (2018) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


