Abstract
Epilepsy is one common brain disorder, which is not well controlled by current pharmacotherapy. In this study we characterized the therapeutic potential of borneol, a plant-derived bicyclic monoterpene compound, in the treatment of epilepsy and elucidated the underlying mechanisms. The anti-seizure potency and properties of borneol were assessed in both acute and chronic mouse epilepsy models. Administration of (+)-borneol (10, 30, 100 mg/kg, i.p.) dose-dependently attenuated acute epileptic seizure in maximal-electroshock seizure (MES) and pentylenetetrazol (PTZ)-induced seizure models without obvious side-effect on motor function. Meanwhile, (+)-borneol administration retarded kindling-induced epileptogenesis and relieved fully kindled seizures. Importantly, (+)-borneol administration also showed therapeutic potential in kainic acid-induced chronic spontaneous seizure model, which was considered as a drug-resistant model. We compared the anti-seizure efficacy of 3 borneol enantiomers in the acute seizure models, and found (+)-borneol being the most satisfying one with long-term anti-seizure effect. In electrophysiological study conducted in mouse brain slices containing the subiculum region, we revealed that borneol enantiomers displayed different anti-seizure mechanisms, (+)-borneol (10 μM) markedly suppressed the high frequency burst firing of subicular neurons and decreased glutamatergic synaptic transmission. In vivo calcium fiber photometry analysis further verified that administration of (+)-borneol (100 mg/kg) inhibited the enhanced glutamatergic synaptic transmission in epilepsy mice. We conclude that (+)-borneol displays broad-spectrum anti-seizure potential in different experimental models via decreasing the glutamatergic synaptic transmission without obvious side-effect, suggesting (+)-borneol as a promising anti-seizure compound for pharmacotherapy in epilepsy.
Keywords: epilepsy, anti-seizure drug, (+)-borneol, valproate, subiculum, glutamatergic synaptic transmission
Introduction
Epilepsy, one of the most common and serious brain disorders, afflicting nearly 1% of the global population, manifests as recurrent episodic caused by abnormal discharge of neurons due to the imbalance between excitation and inhibition in the brain [1]. The pharmacotherapy strategy remains the primary choice currently. Although large numbers of antiseizure drugs (ASDs) have come into the market over the past few decades, ~30% of patients become drug-resistant, and the drug-resistant rate in temporal lobe epilepsy (TLE) patients is even more than 70% [2]. Moreover, current ASDs only control seizure rather than epileptogenesis, with undesirable reaction [3, 4], which produces heavy burden on both patients and society. This situation promotes us to look for novel antiepilepsy treatments.
Borneol, as bicyclic monoterpene alcohol separated from branches and leaves of the Cinnamomum camphora (L.) Presl, has a long history to be used as a supplementary agent in various Chinese herbal compound prescriptions [5, 6]. According to the relevant standards recorded in China Pharmacopoeia, (+)-borneol is obtained from fresh branches and leaves of natural Cinnamomum camphora (L.) Presl, and (-)-borneol is processed from the sublimate of the leaves of the Blumea balsamifera (L.) Dc., with isoborneol not exceeding 5% and no more than 10% camphor. The content of (+)-borneol in isoborneol is generally 55%, others include isoborneol and camphor. Borneol, the drug for inducing resuscitation, has the profile of ‘resuscitation with aromatics’ and ‘lifting the nature of the medicine’ based on traditional Chinese medicines (TCM) theory, promoting the absorption and the permeability of the blood-brain barrier [7, 8]. Modern studies have demonstrated that borneol, with potential molecular targets on GABAA receptor, TRPM8, TRPA1 and TRPV3 in the brain [9–12], generally displays analgesia, anesthesia, antibacterial and protection to cerebral ischemia, fear or anxiety [12–15]. Previous research also found the protective role of (-)-borneol against maximal-electroshock seizure (MES) and (+)-borneol against pentylenetetrazol (PTZ)-induced seizure in mice [16, 17]. However, whether borneol would show general anti-seizure potential in more epilepsy models is still unclear. In addition, borneol has three enantiomers that may have different activities in the brain. The anti-seizure feature and mechanism of borneol with the different enantiomers still need to be further investigated.
Thus, this study examined the anti-seizure potential of borneol in acute and chronic epilepsy models, as well as examined the anti-seizure mechanism of borneol that might regulate the excitatory glutamatergic synaptic transmission. This may provide promising insight for expanding the borneol indications and the novel pharmacotherapy strategies in epilepsy.
Materials and methods
Animals
Transgenic mouse strains were used and genotyped according to the protocols provided by Jackson Laboratories: Calcium/calmodulin-dependent protein kinase II alpha (CaMKIIα) Cre-recombinase mice (CaMKIIα-Cre, strain No. 005359, RRID: IMSR_JAX:005359). Wild-type (WT, RRID: IMSR_JAX:000664, male, weight 22–26 g, 6–8 weeks old) mice were purchased from SLAC Laboratory Animal Centre (Shanghai, China). All animals were raised in animal houses on a 12 h-light/dark circle with SPF standard conditions (22 ± 2 °C, relative humidity 50% ± 10%). Food and water were provided ad libitum. All procedures complied with the standards of the Institutional Animal Care and was approved by the ethical committee of Zhejiang Chinese Medical University (No.2021-1026). Animal studies are reported in compliance with ARRIE guidelines. All behavior tests were carried out by individuals blinded to the drug treatments.
Drugs
(+)-Borneol (C10H18O, Cat# 420247, Sigma, SL, USA), (-)-borneol (Cat# 139114, Sigma, SL, USA) and isoborneol (Cat# I13901, Sigma, SL, USA) were dissolved using 2.5% Tween-80 in 0.9% sterile saline. Pentylenetetrazole (PTZ, Cat# P6500, Sigma, SL, USA), valproate (VPA, Cat# P4543, Sigma, SL, USA) and kainic acid (KA, Cat# ab120100, Abcam, Cambridge, UK) were dissolved in 0.9% sterile saline. Vehicle animals all received the vehicle, which was the same solvent of the drug. All drug solutions were freshly made each day before administration.
MES model
The MES model was performed as our previous study [18]. Thirty minutes after administration of the tested drug, we clamped the ear of mice through a crocodile clip with saline and then a stimulus current was delivered. Electroshock seizures (maximal output voltage 750 V, 50 Hz sine wave pulse, 0.2 s stimulus duration) were generated via rodent shocker (HSE-HA, Hugo Sachs Elektronik, Freiburg, Germany). The initial stimulus current intensity was 6 mA and raised with 1 mA every 2 min. The threshold of MES was defined by the maximal current intensity at which the seizure severity reaches tonic-clonic seizures (an immediate severe tonic seizure with the maximal extension of the anterior and posterior legs occurs and the body becomes stiffened), the duration of tonic-clonic seizures and the survival rate of mice were also recorded. To detect the time-response effect of borneol, mice were tested at 0.5, 2, 4, 6, 8 and 12 h after 100 mg/kg (+)-borneol administration.
PTZ seizure model
PTZ model was performed as our previous study [19]. Mice were randomly assigned into five groups: vehicle (2.5% Tween-80 in 0.9% sterile saline), 200 mg/kg VPA, 10, 30 and 100 mg/kg (+)-borneol (i.p.). After administration of 30 min, mice were given 100 mg/kg PTZ to induce seizure, and then mice were observed for the occurrence of seizure activity and EEGs were recorded (PowerLab8/35, AD Instruments, Australia) for 30 min after PTZ administration. Two screws were placed over the cortex to record EEG and cerebellum served as the reference electrodes, respectively. The following behavior index was analyzed: latency to stage 2 (nodding), stage 4 (bilateral forelimb clonus and rearing), stage 6 (stiffened) and survival rate of mice. For detection of the difference in anti-seizure effect of borneol enantiomer, mice were tested at 0.5 h after drug administration.
Stereotaxic surgery for virus/drug injection and fiber/electrode implantation
Under isoflurane (3% induction and 1.5% maintenance, RWD Life science Co., Ltd, Shenzhen, China) anesthetization, mice were head-fixed in a stereotaxic frame (68043, RWD Life science Co., Ltd, Shenzhen, China). The coordinates were measured from Bregma according to the brain atlas of mice. Based on the Paxinos and Franklin (2007) atlas, the electrodes (795500, each 0.125 mm in diameter; A-M Systems, WA, USA) were implanted in the right ventral hippocampus (vCA3, AP −2.9 mm, ML −3.2 mm, DV −3.2 mm) for kindling and recording EEG. Two screws were placed over the cortex to record EEG and cerebellum served as the reference electrodes, respectively. Another two screws were placed over the right and left brain to fix and affixed with dental cement.
For fiber photometry recording, AAV-EF1a-DIO-axon-GCaMP6s (serotype: AAV2/9, viral titers: 5.38E+12 v.g./mL, 250 nL) was injected stereotactically into the dCA1 (AP −2.0 mm, ML −1.6 mm, DV −1.3 mm) of CaMKIIα-Cre mice. Then, a ceramic ferrule (200 μm, 0.37 NA, 6.0 mm, Inper Co. Ltd, Hangzhou, China) was implanted in the right dorsal subiculum (dSub, AP −3.4 mm, ML −2.0 mm, DV −1.8 mm) to record the Ca2+ activity. Mice were kept for at least 4 weeks before further experiments to induce the expression of virus. The virus was purchased from the BrainVTA Co., Ltd. (Wuhan, China) and stored at −80 °C until use.
Hippocampal kindling epilepsy model
The kindling model was performed as our previous study [20]. After 1-week surgery recovery, mice were randomly assigned into four groups: vehicle (2.5% Tween-80 in 0.9% sterile saline), 10, 30 and 100 mg/kg (+)-borneol (i.p.). To test the effect of borneol on kindling-induced epileptogenesis, test drugs were administered 30 min (daily) before the first kindling each day during the behavioral test. And then mice received kindling stimulations ten times each day (400 μA, 20 Hz, 2 s trains, 1 ms monophasic square wave pulses) with 30 min intervals continuous for 3–4 days until the mice were fully kindled. EEGs at the right ventral hippocampal CA3 were recorded with a digital amplifier (NuAmps, Neuroscan System, VA, USA). Behavioral seizure stages were scored according to the Racine (1972) methods [21]: stage 1 (ear and facial movement), stage 2 (nodding), stage 3 (unilateral forelimb clonus), stage 4 (bilateral forelimb clonus and rearing), stage 5 (falling and jumping with bilateral forelimb clonus). Stage 1–3 was defined as focal seizure (FS), and stage 4–5 was described as generalized seizure (GS). To test the effect of borneol on kindled seizure, fully kindled mice received kindling stimulations 30 min after drug administration. The seizure severity and EEGs were analyzed by an investigator blinded to the group allocation.
KA-induced chronic epilepsy model
KA-induced chronic epilepsy model was performed as our previous studies [22, 23]. Briefly, KA (0.6 ng/nL, 500 nL) was injected stereotactically into the dCA1 of WT mice. After the KA injection for 2 months (spontaneous seizure period), an electrode was implanted in the right vCA3 to record EEGs. Waiting for 7 days to recover from surgery, EEGs were continuously recorded in mice with 8 h/day for 3 days as baseline (Pre). Mice were consecutively treated with 100 mg/kg (+)-borneol (i.p.) once daily and EEGs were recorded for 3 days (Bor). EEGs were recorded for another 3 days after (+)-borneol withdrawal (Post). The spontaneous GS was defined as EEGs that lasted more than 30 s and has a period of post-inhibition, along with a tonic-clonic behavioral seizure. EEGs were recorded and the number of GS and the total time of GS were analyzed.
In vitro electrophysiological recordings
Brain slice preparation
Mice were deeply anesthetized with isoflurane and the brains were carefully extracted and transferred to a chamber filled with ice-cold oxygenated choline-based slicing solution containing (in mM): 110 Choline Chlorine, 25 NaHCO3, 20 D-Glucose, 2.5 KCl, 1.3 NaH2PO4, 0.5 CaCl2∙2H2O, 7 MgCl2, 1.2 Sodium ascorbate, 0.6 Sodium Pyruvate (pH 7.35–7.40 adjusted with HCl). Coronal sections (300 μm) were sectioned using a vibratome microtome (Leica VT1200S, Leica Biosystems, Wetzlar, Deutschland) and incubated at 34 °C for 25 min in artificial cerebrospinal fluid (ACSF) containing (in mM): 119 NaCl, 2.5 KCl, 2.5 CaCl2∙2H2O, 1.3 MgSO4, 1.25 NaH2PO4∙2H2O, 26.2 NaHCO3, 11 D-Glucose (pH 7.35–7.40 adjusted with HCl). Slices were maintained at room temperature until they were transferred to a recording chamber. The chamber was continuously superfused with ACSF at 2–3 mL/min using a pump (BT100-2J, Longer Precision Pump Co., Ltd., Hebei, China). All the solutions were constantly bubbled with 95% O2 and 5% CO2.
Whole-cell patch-clamp recordings
For current-clamp recording, pipettes (6–8 MΩ) were filled with an intracellular solution containing (in mM): 140 K gluconate, 5 NaCl, 10 HEPES, 0.2 EGTA, 2 Mg-ATP (pH 7.20 adjusted with KOH, 290–300 mOsm). Signals were amplified and recorded by a HEKA EPC10 amplifier (HEKA Instruments, Germany). To induce epileptic activity in dSub, slices were incubated in ACSF with 50 μM 4-AP (Cat# ab120122, Abcam, Cambridge, UK). Cells in dSub were clamped under the current clamp with 20 pA current injected to measure the firing activity. In trials of baseline recording, each cell was recorded for 1 min. In trials of borneol recording, 10 and 100 μM borneol for 5 min at a rate of 2–3 mL/min was applied by perfusing ACSF, and each cell was recorded for 1 min. The concentrations used in in vitro experiments here reflected somehow the doses used in vivo [9, 24]. In trials of washout recording, after ACSF washout (removing borneol) for 5 min, each cell was recorded for another 1 min.
For action potential (AP) properties, depolarizing currents were injected under the current clamp configuration in 20 pA increments from 0 pA to depolarizing 300 pA. Resting membrane potential was recorded immediately upon switching to the current-clamp mode. Rheobase was the minimum depolarizing current that elicits the first AP spike. For recording spontaneous synaptic currents, a low divalent ion ACSF (in mM): 125 NaCl, 3.5 KCl, 1.25 NaH2PO4, 0.5 MgCl2, 26 NaHCO3, 25 Dextrose, and 1 CaCl2 was used. Using patch pipettes (6–8 MΩ) contained with cesium-based internal fluid (in mM): 100 CsCH3SO3, 20 KCl, 10 HEPES, 4 Mg-ATP, 0.3 Tris-GTP, 7 Tris2-Phosphocreatine, and 3 QX-314 (pH 7.20 adjusted with CsOH, 290–300 mOsm). Spontaneous excitatory post-synaptic currents (sEPSCs) were recorded at a holding potential of −60 mV and spontaneous inhibitory post-synaptic currents (sIPSCs) were recorded at a holding potential of +10 mV for 2 min. (+)-borneol, (-)-borneol and iso-borneol (10 μM) were applied by perfusing ACSF to measure the frequency, amplitude, and charge transfer of sEPSCs and sIPSCs, respectively. Tetrodotoxin (1 μM) (Cat# ab120054, Abcam, Cambridge, UK) was added to block AP formation and its propagation for the recording of miniature EPSCs (mEPSCs) and miniature IPSCs (mIPSCs). Signals were amplified and recorded by a HEKA EPC10 amplifier (HEKA Instruments, Germany) with a sample rate of 10 kHz. Events were recorded and analyzed with Mini-analysis. All reagents used in electrophysiological recordings were purchased from Sigma (SL, USA).
In vivo fiber photometry recordings
Fiber photometry recording was performed as our previous study [25]. After dCA1 virus injection, mice were recovered 4 weeks for terminal virus expression, then a ceramic ferrule was implanted in the right dSub to record fiber photometry and an electrode was implanted in the right vCA3 to kindling and record EEGs. The fiber photometry system (QAXK-FPS-TC-MC-LED, ThinkerTech, Nanjing, China) emits 488 nm excitation light that is coupled into a 200 μm optical fiber, the light reaching the corresponding area excites green fluorescence signals. The GCaMP fluorescence signals were collected by a photomultiplier tube, then the current output was converted to voltage signals through an I-V amplifier, which was further filtered by a low-pass filter (40 Hz cut-off).
For recording the GCaMP fluorescence signals during seizure development, calcium signals were recorded in mice for 100 s as baseline, then another 100 s was recorded after 2 s kindling stimulation (400 μA, 20 Hz, 2 s trains, 1 ms monophasic square wave pulses). The event starts from the end of kindling stimulation, ‘base’ was defined as the last 20 s baseline.
For recording the GCaMP fluorescence signals after a single-pulse stimulation (400 μA, 500 ms pulse) in base and kindled mice, calcium signals were detected in mice for 20 s as baseline, then another 20 s was recorded after 0.5 s pulse stimulation. The event also starts from the end of pulse stimulation, ‘base’ was defined as the last 2 s baseline, post-stimulation activity for 5 s. Mice were treated with 100 mg/kg (+)-borneol 30 min before kindling. Quantification of responses to heatmap and peri-event. ΔF/F0 was calculated by the value of fluorescence change (F-F0)/F0.
Histology
Mice were deeply anesthetized with pentobarbital sodium and were perfused with cold 0.1 M phosphate-buffered saline (PBS, pH 7.4 ± 0.1, Cat# 243176, Biosharp, Anhui, China), followed by cold 4% (w/v) paraformaldehyde (PFA, Cat# P804536, Macklin, Shanghai, China) in PBS. The whole brain was dissected and post-fixed for 8 h in 4% PFA at 4 °C, then transferred to 30% (w/v) sucrose (Cat# S11055, Yuanye Bio-Technology Co., Ltd, Shanghai, China) until up to the bottom. Coronal brain sections (30 μm) were frozen in optimum cutting temperature compound (Cat# 6506, Epredia, USA) and cut with a cryostar NX70 (Thermo Fisher Scientific, CA, USA), then floated in PBS. Sections were blocked with 1% BSA (Cat# A8010, Solarbio, Beijing, China) and 5% donkey serum (Cat# 36116ES10, YEASEN, Shanghai, China) in 0.3% Triton X-100/PBS for 2 h at room temperature. Free-floating sections were then incubated overnight at 4 °C with the following primary antibodies: 1:500 NeuN rabbit monoclonal (Cat# MABN140, RRID: AB_2571567, Millipore, USA), 1:500 GFAP mouse monoclonal (Cat# 3670 T, RRID: AB_561049, Cell Signaling Technology, MA, USA), 1:100 Iba1/AIF-1 rabbit monoclonal (Cat# 17198 T, RRID: AB_2820254, Cell Signaling Technology, MA, USA). Sections were then washed with PBS three times and incubated with 1:000 Alexa Fluor 488-conjugated donkey anti-rabbit IgG (Cat# ab150073, RRID: AB_2636877, Abcam, Cambridge, UK) or 1:000 Alexa Fluor 488-conjugated donkey anti-mouse IgG (Cat# ab150105, RRID: AB_2732856, Abcam, Cambridge, UK) for 2 h at room temperature in the dark. DAPI Fluoromount-G™ (Cat# 36308ES20, RRID: AB_2636877, YEASEN, Shanghai, China) was used to stain the nucleus. A Leica TCS SP8 confocal laser scanning microscope (Leica Microsystems, Mannheim, Germany) captured the image using LAS X software (Version 3.7.1).
Assessment of acute side effects
Mice were transferred to the test room at least 2 h before the test and were randomly assigned into two groups: vehicle (2.5% Tween-80 in 0.9% sterile saline, i.p.) and (+)-borneol (100 mg/kg, i.p.).
Open field test
Thirty minutes after administration, mice were placed into test boxes (50 cm × 50 cm × 50 cm; length × width × height) and the locomotor activity was recorded and analyzed for 15 min by automatic video tracking (ANY-maze 7.0, Stoelting, USA). Locomotor activity was evaluated by the total distance, the immobility time and the time in the center area.
Rota-rod test
Motor performance was assessed using an accelerating Rota-rod (LE8205, Panlab Harvard Apparatus, Barcelona, Spain). Thirty minutes after administration, mice were placed on the accelerating rod (5–40 rpm with an acceleration time of 300 s period) and the maximum fall latency and speed for each animal were recorded. Each mouse was tested in eight consecutive trials at 20 min intervals [26].
Data processing and statistical analyses
Data are presented as means ± SEM. The number of experimental replicates (n) is indicated in each figure legend. Statistical comparisons were performed using GraphPad Prism (version 8.0, GraphPad Software, CA, USA) with appropriate methods indicated in figure legends. Photometry data were analyzed with MATLAB R2020b (The MathWorks Inc., MA, USA).
Results
(+)-Borneol dose-dependently attenuates acute epileptic seizure in MES- and PTZ-induced models
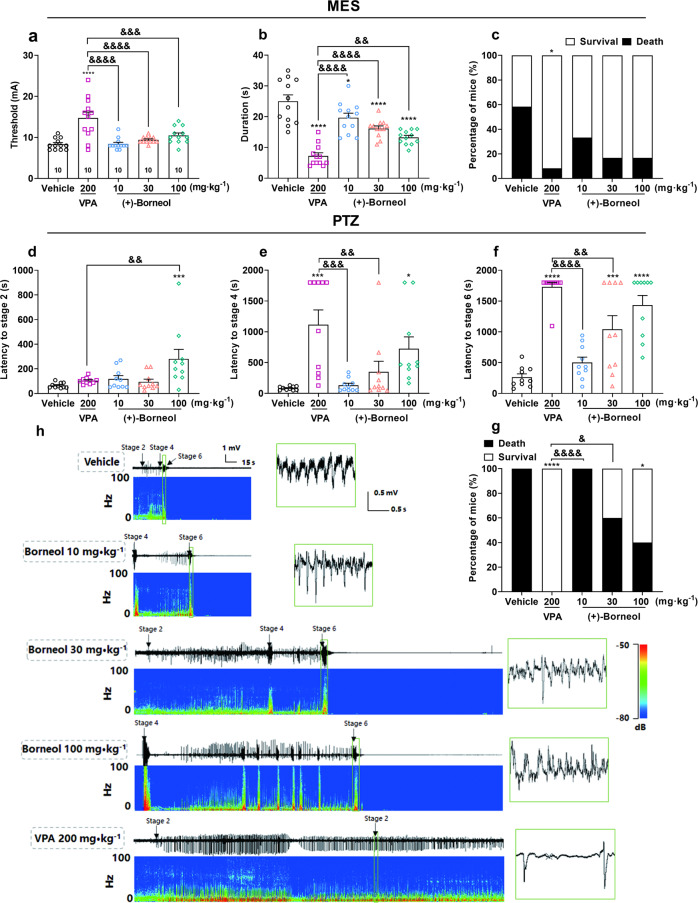
MES and PTZ tests are the classical models for screening pre-clinical anti-seizure drugs [27, 28], we first used these two models to test whether (+)-borneol shows anticonvulsant activity, as (+)-borneol is the essential component of the natural Cinnamomum camphora (L.) Presl, which is considered the most authentic between the different enantiomers. The MES model mimics typical focal to bilateral tonic-clonic seizure behaviors. We found that (+)-borneol increased the tonic-clonic threshold (Fig. 1a, F (4, 55) = 12.58, P < 0.0001), and significantly reduced the duration of the tonic-clonic seizure (Fig. 1b, F (4, 55) = 25.96, P < 0.0001) and mortality (Fig. 1c, VPA: χ2 = 4.69, P < 0.05) in a dose-dependent manner (10, 30 and 100 mg/kg, i.p. injection) compared to vehicle group. We included VPA as a positive control and found that it more effectively increased the tonic-clonic threshold and reduced the duration of the tonic-clonic seizure. However, no significant difference was observed between 200 mg/kg VPA and 10, 30 or 100 mg/kg borneol groups in mortality (P > 0.05).
Fig. 1. (+)-Borneol dose-dependently attenuates acute epileptic seizure in MES- and PTZ-induced models.
a–c Effects of (+)-Borneol on (a) seizure threshold, (b) duration and (c) survival rate of mice in MES-induced generalized tonic-clonic seizures. d–g Effects of (+)-borneol on PTZ-induced tonic-clonic seizures, as described by the latency of (d) stage 2, (e) stage 4, (f) stage 6 and (g) survival rate of mice. h Representative cortical EEGs and corresponding power spectra during PTZ-induced seizure. Data are presented as means ± SEM. n = 10 in each group. *P < 0.05, ***P < 0.001 and ****P < 0.0001 versus vehicle group, &P < 0.05, &&P < 0.01, &&&P < 0.001 and &&&&P < 0.0001 versus VPA group. One-way ANOVA followed by post hoc Dunn’s test was used for (a, b, d–f) and Chi-square test was used for (c and g).
Meanwhile, PTZ, a gamma aminobutyric acid (GABA) receptor inhibitor, induces acute generalized myoclonic seizure behaviors by decreasing the inhibitory synaptic transmission to enhance neural excitability. We found that (+)-borneol dose-dependently extended the latency to seizure, among which 30 mg/kg borneol significantly increased the latency to stage 6 (Fig. 1f, P < 0.001), while 100 mg/kg borneol significantly increased the latency to stage 2 (Fig. 1d, F (4, 45) = 5.02, P < 0.01), stage 4 (Fig. 1e, F (4, 45) = 7.60, P < 0.0001) and stage 6 (Fig. 1f, F (4, 45) = 21.22, P < 0.0001) and decreased the mortality to 40% (Fig. 1g, VPA: χ2 = 16.20, P < 0.0001, 100 mg/kg borneol: χ2 = 5.95, P < 0.05). Furthermore, 100 mg/kg borneol demonstrated a similar magnitude as 200 mg/kg VPA in increasing the latency to stage 4 and stage 6 (P > 0.05). Interestingly, 100 mg/kg borneol also significantly delays the latency to FS (stage 2) (P < 0.001), which is not a typical therapeutic effect of VPA. Typical cortical EEGs during the seizure and corresponding power spectra were shown in Fig. 1h. These results demonstrated that (+)-borneol is efficacious in acute tonic-clonic seizures and myoclonic seizures.
(+)-Borneol attenuates epileptic seizures in the hippocampal-kindling epilepsy model
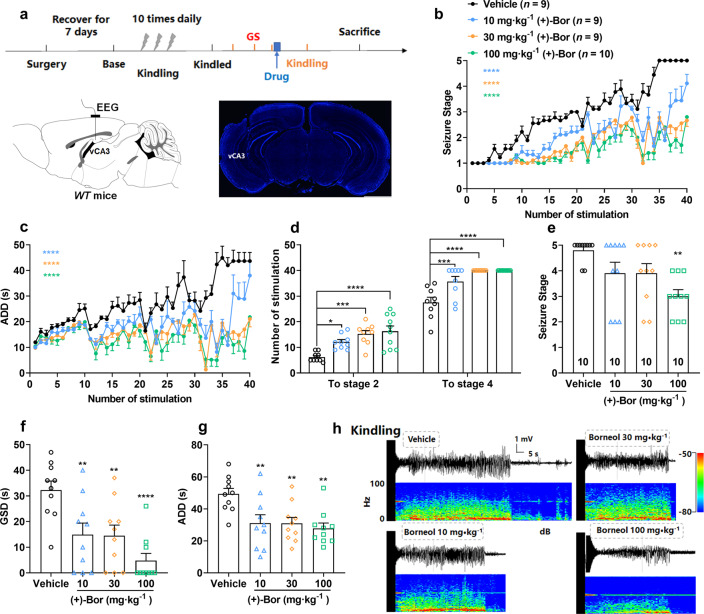
Next, we further tested the anti-seizure effect of borneol in the chronic epilepsy model, a hippocampal kindling model that resembles FSs with secondarily GSs. We found that (+)-borneol inhibited seizure development (Fig. 2b, F (117, 1320) = 4.61, P < 0.0001), decreased afterdischarge durations (ADDs) (Fig. 2c, F (117, 1320) = 3.50, P < 0.0001) and enhanced the number of stimulations required to reach seizure stage 2 (Fig. 2d, F (3, 33) = 10.39, P < 0.0001) and stage 4 (Fig. 2d, F (3, 33) = 17.94, P < 0.0001) in a dose-dependent manner during kindling-induced epileptogenesis, compared to the vehicle group, especially 100 mg/kg (+)-borneol markedly decreased ADDs within 40 stimuli from (43.67 ± 3.35 s) to (21.80 ± 0.76 s). Meanwhile, (+)-borneol significantly attenuated seizure severity in kindled seizure state, as indicated by the lowered seizure stage (Fig. 2e, F (3, 36) = 5.20, P < 0.01), shortened GS durations (GSDs) (Fig. 2f, F (3, 36) = 9.18, P < 0.0001), and ADDs (Fig. 2g, F (3, 36) = 5.89, P < 0.01). Typical hippocampal EEGs during the kindled seizure and corresponding power spectra were shown in Fig. 2h. These results demonstrated that (+)-borneol protects against not only kindling-induced epileptogenesis but also kindled seizures.
Fig. 2. (+)-Borneol attenuates epileptic seizures in the hippocampal-kindling model.
a Schematic illustration of the hippocampal kindling model and representative image of electrodes implanted in vCA3 (blue: DAPI). Scale bar: 1.5 mm. b–d (+)-Borneol dose-dependently retards the progression of (b) seizure stage, (c) ADDs and (d) the number of stimulations required to reach stage 2 or stage 4 in hippocampal kindling-induced epileptogenesis. e–g (+)-Borneol inhibits (e) seizure stage, (f) GSDs and (g) ADDs in hippocampal-kindled seizures. h Representative hippocampal EEGs and corresponding power spectra during kindled seizures. The number of mice used in each group is indicated in the figures. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 versus vehicle group. Two-way ANOVA followed by post hoc Dunn’s test was used for (b, c) and one-way ANOVA followed by post hoc Dunn’s test was used for (d–g).
(+)-Borneol enantiomer shows broad-spectrum and optimal anti-seizure effects
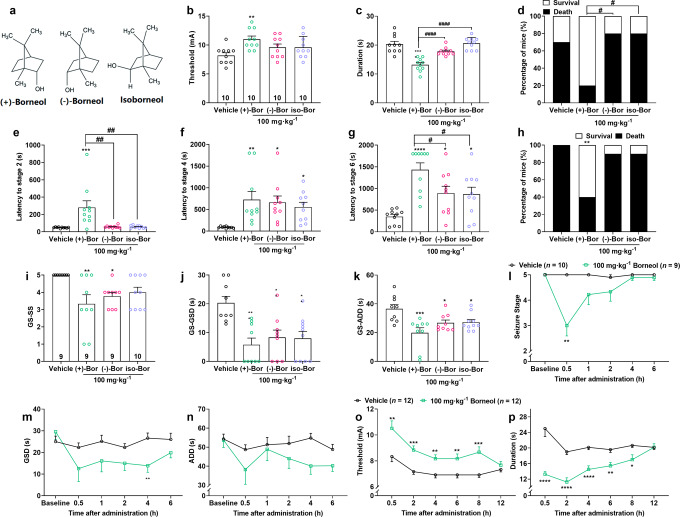
Given borneol has three different enantiomers (Fig. 3a), which were considered to be of different functions [13, 14]. To further make clear the optimal anti-seizure properties of borneol, we tested the anti-seizure activity of borneol with different enantiomers in epilepsy models. We found that three enantiomers of borneol, (+)-borneol, (-)-borneol and isoborneol, have different therapeutic efficacy against MES- and PTZ-induced acute seizure, and hippocampal-kindled seizures. In the MES seizure model, only (+)-borneol increased the seizure threshold (F (3, 36) = 4.26, P < 0.05), lowered seizure duration (F (3, 36) = 22.22, P < 0.0001) and improved survival rate (Fig. 3b–d, (+)-borneol: χ2 = 3.23, P = 0.07). In the PTZ seizure model, (+)-borneol delayed the latency to all seizure stages (stage 2: F (3, 36) = 8.54, P < 0.001) as well as improved survival rate (χ2 = 5.95, P < 0.05), while the other two enantiomers only increased latency to seizure stage 4 (F (3, 36) = 4.71, P < 0.01) and stage 6 (Fig. 3e–h, F (3, 36) = 9.81, P < 0.001). For comparison, (+)-borneol demonstrated a robust and statistically significant anticonvulsive effect than (-)-borneol and isoborneol in MES duration (F (2, 27) = 37.10, P < 0.0001), survival rate (χ2 = 5.00, P < 0.05) and the latency to PTZ-induced seizure stages 2 (F (2, 27) = 8.32, P < 0.001) or stage 6 (F (2, 27) = 4.01, P < 0.05).
Fig. 3. (+)-Borneol enantiomers show broad-spectrum and optimal anti-seizure effects.
a The molecular structure of (+)-borneol, (−)-borneol and iso-borneol enantiomers. b–d Effects of borneol enantiomers on (b) seizure threshold, (c) duration and (d) survival rate of mice in MES-induced seizures. e–h Effects of borneol enantiomers on PTZ-induced seizures, as described by the latency to (e) stage 2, (f) stage 4, (g) stage 6 and (h) survival rate. The data in the (+)-borneol group were from Fig. 1 as a comparison with the other enantiomers. i–k Effects of borneol enantiomers on hippocampal-kindled seizure, as described by (i) seizure stage, (j) GSDs and (k) ADDs. l–n The anti-seizure effect of (+)-borneol at different time points post-drug administration in the hippocampal kindling model, also as described by (l) seizure stage, (m) GSDs and (n) ADDs. o, p The antiseizure effect of (+)-borneol at different time points post-drug administration in the MES model, is described as the (o) threshold and (p) duration. Data are presented as means ± SEM. The number of mice used in each group is indicated in figures. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 versus vehicle group. #P < 0.05, ##P < 0.01 and ####P < 0.0001 versus (+)-borneol group. One-way ANOVA followed by post hoc Dunn’s test was used for (b, c, e–g, i–k), Chi-square test was used for (h), and an unpaired t-test was used for (l–p).
Whereas in hippocampal-kindled seizure, all three enantiomers inhibited seizure stage (F (3, 33) = 4.68, P < 0.01), shortened GSDs (F (3, 33) = 7.43, P < 0.001) and ADDs (Fig. 3i–k, F (3, 33) = 6.50, P < 0.01), although (+)-borneol, that is we explored in the whole study, possessed the most significant difference in anti-seizure efficacy. These results indicated that borneol has the anti-seizure efficacy in its three enantiomers, among which (+)-borneol shows the most satisfying anti-seizure action in different types of seizure models.
Then, we also tested the anti-seizure time window of (+)-borneol. First, we did MES tests at different time points after the treatment of 100 mg/kg (+)-borneol. We found that (+)-borneol continuously increased the threshold of the tonic-clonic seizure and reduced the seizure duration to 8 h (Fig. 3o, p). Similarly, 100 mg/kg (+)-borneol also lowered seizure stage (F (5, 85) = 8.02, P < 0.0001), decreased GSDs (F (5, 85) = 1.82) and ADDs (F (5, 85) = 1.01) up to 6 h in the hippocampal kindling epilepsy model (Fig. 3l–n). These data suggested that (+)-borneol may have an optimal anti-seizure activity among its enantiomers with a lasting therapeutic action.
(+)-Borneol attenuates epileptic seizures in the KA-induced chronic epilepsy model
Further, we also examined the epileptic activity of (+)-borneol on the KA-induced chronic spontaneous model, which was the most similar to clinical chronic epilepsy syndrome and does not respond to various ASDs [29]. We verified that a large number of neurons loss, astrocyte activation and microgliosis in dCA1 2 months after KA injection as shown with NeuN, GFAP and Iba1-staining (Fig. 4c, Supplementary Fig. S1), which recapitulates the major neuropathological features of TLE. Here, we recorded hippocampal EEG for 8 h daily for 3 days as the baseline, and then we treated epileptic mice with 100 mg/kg (+)-borneol for 3 days and finally withdrew the drug to record EEG for another 3 days as post-stage. We found that (+)-borneol greatly attenuated the spontaneous epileptic seizure (Fig. 4d–h), reduced the number (Fig. 4f, g, P < 0.01) and total duration (Fig. 4h, P < 0.01) of GS. The seizure severity could go back to the degree of baseline after the drug is withdrawn. In addition, borneol alleviated neuronal loss and microgliosis indicated by NeuN and Iba1 staining, but seems to have no effects on GFAP staining (Supplementary Fig. S1). Together, these results demonstrated that (+)-borneol attenuates seizure severity in both hippocampal kindling-induced and KA-induced chronic epilepsy models.
Fig. 4. (+)-Borneol attenuates spontaneous seizures in the KA-induced chronic epilepsy model.
a Schematic for the sites of KA injection and EEG recording electrode. b The time course for the treatment of borneol in KA-induced chronic epilepsy model. c Representative images showing NeuN (green) staining in dCA1 after KA (bottom) and vehicle (upper) treatment. Scale bar: 300 μm. d Heatmap of the number of spontaneous generalized seizures (GS) recorded each day. e The typical EEG of GS. f–h Statistic of (g) the number and (h) the total duration of GS detected at each day. Data are presented as means ± SEM. n = 10. For (g and h), **P < 0.01 versus pre group, ##P < 0.01 versus Bor group, Wilcoxon test.
To test the adverse effect of (+)-borneol in the brain, open-field and Rota-rod tests were used to examine whether the (+)-borneol would lead to a sedative effect and affect motor balance, respectively. Firstly, we tested the effect of 100 mg/kg (+)-borneol in normal mice on those paradigms. The results showed that 100 mg/kg (+)-borneol did not change the overall locomotor activity (Supplementary Fig. S2a–d, P > 0.05) and the balance ability on rota-rod (Supplementary Fig. S2e,f, P > 0.05). Then, we tested the effect of (+)-borneol in KA mice on those paradigms. In the open-field test, as shown with the move traces, KA mice showed markedly increased locomotor activities (Supplementary Fig. S2b, P < 0.001), reduced immobility time (Supplementary Fig. S2c, P < 0.001), or the time in the centre area (Supplementary Fig. S2d), indicating neural hyperactivity. In the Rota-rod test, KA mice also had decreased the latency and speed (Supplementary Fig. S2e, f, P < 0.01) to fall off from a rotating drum, indicating the impaired ability of motor learning. While (+)-borneol treatment at the dose of 100 mg/kg reversed impaired motor function. These results suggested that (+)-borneol does not produce any obvious side effects on motor function at the dosage in this study and it even protects against impaired motor function in chronic epilepsy.
(+)-Borneol decreases the excitability of glutamatergic transmission
To explore the anti-seizure mechanism of (+)-borneol, we evaluated the effect of (+)-borneol on neuronal excitability with in vitro electrophysiological recording of glutamatergic neurons in the subiculum, a critical brain region that mediates seizure generation and spread [23, 30]. To elicit hyperexcitatory activity in vitro, brain slices containing the subiculum region were perfused with ACSF containing 50 μM 4-AP, a potassium channel inhibitor. We found that both 100 μM (Fig. 5a, F (2, 21) = 85.35, P < 0.0001) and 10 μM (+)-borneol (Fig. 5b, F (2, 31) = 36.37, P < 0.0001) greatly reduced the frequency of AP firing in the subicular glutamatergic neuron. This action was partially restored to some extent after the drug washout.
Fig. 5. (+)-Borneol decreases the excitability of glutamatergic transmission.
a and b Representative traces and the corresponding quantification show the action potential in subicular glutamatergic neurons incubated with (a) 100 μM or (b) 10 μM (+)-borneol. n (cells) = 9 (100 μM (+)-bor) and 12 (10 μM (+)-bor). Data are presented as means ± SEM. ****P < 0.0001 versus baseline group, ####P < 0.0001 versus 100 μM Bor group and ##P < 0.01 versus 10 μM Bor group, one-way ANOVA followed by post hoc Dunn’s test. Representative traces showing the (c) sEPSCs and (g) sIPSCs recorded in the subicular glutamatergic neuron incubated with 10 μM (+)-borneol. d–f Quantification of sEPSCs (d) frequency, (e) amplitude, and (f) charge transfer. h–j Quantification of sIPSCs (h) frequency, (i) amplitude, (j) charge transfer and (k) sEPSCs/sIPSCs charge transfer ratios incubated with 10 μM (+)-borneol. *P < 0.05 and ***P < 0.001, Wilcoxon test.
Then, to test the effect of borneol on the intrinsic excitability of the neuron, we further monitored the AP properties of subicular glutamatergic neurons after treatment with borneol enantiomers. There was no difference in RPM between the vehicle and borneol enantiomers groups (Supplementary Fig. S3a, F (3, 48) = 2.42, P > 0.05). Surprisingly, we observed that (-)-borneol and isoborneol, but not (+)-borneol, effectively increased the minimum current intensity to elicit an AP (Rheobase) (Supplementary Fig. S3b, F (3, 48) = 5.39, P < 0.01) and reduced the number of AP spikes under the same injection currents (Supplementary Fig. S3c, F (45, 768) = 1.13).
Next, we evaluated the effect of (+)-borneol on synaptic transmission by recording the spontaneous synaptic currents in the subicular glutamatergic neuron. We found that (+)-borneol decreased the frequency (P < 0.05) and reduced the charge transfer ratio of sEPSCs from (0.36 ± 0.14) to (0.14 ± 0.06) (P < 0.001), but did not change the amplitude of sEPSCs (Fig. 5c–f, P > 0.05). Whereas, (+)-borneol did not affect any index of sIPSCs (Fig. 5g–k, P > 0.05). As the frequency of sEPSCs is usually considered as the presynaptic mechanisms correlated with either the release probability or the number of synapses. Meanwhile, we also test the effect of (+)-borneol on the spontaneous miniature synaptic currents in the subicular glutamatergic neuron and found that it decreased all the index of mEPSCs (Supplementary Fig. S4b–d, h, P < 0.01), but did not influence the mIPSCs (Supplementary Fig. S4e–g, P > 0.05). This suggests that (+)-borneol induces a mechanism of weakened glutamatergic neurotransmission that may cause the anti-seizure effect.
As a comparison, we also quantified the effects of (-)-borneol and isoborneol on synaptic transmission. Isoborneol had on significant inference on either sEPSCs or sIPSCs (Supplementary Fig. S5j–r, P > 0.05), while (-)-borneol even increased the sEPSCs (Supplementary Fig. S5a–d, amplitude, P < 0.01 and the charge transfer ratio of sEPSCs, P < 0.05). These experiments highlight different anti-seizure mechanisms underlying borneol enantiomers, among which (+)-borneol may produce an anti-seizure effect via the modulation of the mechanism of weakened glutamatergic neurotransmission.
(+)-Borneol decreases the enhanced glutamatergic transmission in epileptic mice
Finally, we evaluated the effect of borneol on synaptic transmission in epilepsy mice. We used in vivo fiber photometry recording of calcium activity of the synaptic terminal to reflect the release of the synaptic transmitter. As the mainly glutamatergic projection of dSub is from the CA1 region, we injected the AAV-EF1a-DIO-axon-GCaMP6s, an axon-target calcium activity indicator [31], into the dCA1 and we recorded the calcium fluorescence signals of glutamatergic fiber in the subiculum during kindling-induced epilepsy model. We found that Ca2+ signals (ΔF/F0) were slightly increased during the FS (P < 0.05), while it progressively increased during seizure development with the largest extends during GS onset (Fig. 6b–h, P < 0.01). In a fully kindled seizure state, treatment with 100 mg/kg (+)-borneol in kindled mice decreased the seizure severity as previously mentioned, which was accompanied by the reduction of Ca2+ activity of the synaptic terminus (Fig. 6i, j, P < 0.05).
Fig. 6. (+)-Borneol decreases the enhanced glutamatergic transmission in epileptic mice.
a Schematic illustration of the fiber photometry for recording the dCA1-dSub glutamatergic projection in hippocampal kindling model. AAV-EF1a-DIO-axon-GCaMP6s is injected into the dCA1 of CaMKIIα-Cre mice and the representative image showing GCaMP6s (green) expression in the dSub. Scale bar: 100 μm. b Representative EEG (top) and fiber photometry of Ca2+ activities in dSub (bottom) of a focal seizure (FS) in hippocampal kindling model. c Heatmap and (d) quantification of responses of the calcium signal of each trial during FS. ‘Base’ represents the average delta F/F0 during 20 s before kindling stimulation, and ‘FS’ is the average delta F/F0 during the seizure. *P < 0.05 versus Base group, Wilcoxon test. e Representative EEG (top) and fiber photometry of Ca2+ activities in dSub (bottom) of a generalized seizure (GS) in hippocampal kindling model. f Heatmap and (g) quantification of responses of the calcium signal of each trial during GS. ‘Base’ represents the average delta F/F0 during 20 s before kindling stimulation and the ‘GS’ is the average delta F/F0 during the generalized seizure (GSD). **P < 0.01 versus pre group, Wilcoxon test. h Heatmap of Ca2+ activities of different seizure stages in dSub. i EEG (top) and peri-event plots (bottom) of the Ca2+ signals in GS treated with 100 mg/kg borneol. j Quantification of responses corresponding to (i). *P < 0.05 versus GS group, Wilcoxon test. k and l Borneol relieves the increase of delta F/F0 in an instantaneous stimulation in both (k) base or (l) kindled states. m Quantification of responses corresponding to (k) and (l). n = 9. **P < 0.01 versus vehicle group, Wilcoxon test.
To directly test the effect of (+)-borneol on the strength of synaptic transmission, we used a transient pulse electrical stimulation (400 μA, 500 ms) to evoke the Ca2+ activity of the synaptic terminal in the base condition (without seizure) in both normal and kindled mice. The calcium fluorescence signals in the kindled state were significantly increased compared with those in the base state, suggesting the enhanced glutamatergic transmission in epileptic mice. Importantly, administration of (+)-borneol reversed the increased Ca2+ activity of the synaptic terminals (Fig. 6k–m, P < 0.01), which is consistent with the results of Ca2+ activity of kindling-induced seizure. These results collectively suggest that (+)-borneol attenuates seizure severity by decreasing the enhanced excitability of glutamatergic transmission in epilepsy.
Discussion
Epilepsy is not well controlled by current pharmacotherapy [1], and natural compound is an important resource for the discovery of new ASD. Although previous research has demonstrated borneol is anticonvulsive in acute seizure models [16, 17, 32], this study further examined the pharmacological effects of borneol in various epilepsy models and its potential anti-seizure mechanisms.
We found that (+)-borneol was does-dependently efficacious in suppressing the MES- and PTZ-induced seizures, which correlate well with generalized tonic-clonic seizures and generalized clonic seizures [33]. Intriguingly, (+)-borneol at a dose of 100 mg/kg demonstrated comparable efficacy to 200 mg/kg VPA, the first-line ASD for the treatment of epilepsy, in the various seizure assays and also delayed the latency to PTZ-induced myoclonic jerking seizure while VPA did not, suggesting much more broad-spectrum anti-seizure effect of (+)-borneol. More importantly, (+)-borneol treatment plays a significant protective role in seizure development and GS expression in the hippocampal kindling model (FS with secondarily GS) and chronic spontaneous seizure in the KA-induced epilepsy model, which often does not respond to various ASD, further supporting its broad-spectrum anti-seizure effects in various epilepsy models. In addition, (+)-borneol did not induce apparent motor ataxia under the doses studied here. As borneol is approved by the FDA to be used only as a flavoring substance or adjuvant in food, this suggests the safety of the use of borneol. Together, our results strongly indicate the broad-spectrum anti-seizure action of (+)-borneol in different epileptic seizures, even in drug-resistant epilepsy.
Further, given the enantiomers of Chinese herbal medicine plays great distinction in different diseases, we investigated the difference in anti-seizure action among the three enantiomers of borneol. Our results showed that (+)-borneol, but not the other two enantiomers, showed anti-seizure effect in the MES model. While in the PTZ seizure model, (+)-borneol delayed the latency to all seizure stages as well as improved survival rate, while the other two enantiomers only delayed the latency to generalized tonic-clonic seizures, indicating the different pharmacological effects of borneol enantiomers. Interestingly, in hippocampal-kindled seizure, there is no obvious distinction between three enantiomers of borneol, although (+)-borneol, that is we explored in the whole study, possessed the most significant difference in anti-seizure efficacy. Accordingly, it is rational to assume that borneol enantiomers might be expected to possess different anti-seizure actions. Thus, borneol with different enantiomers may be promising anti-seizure compounds suitable for different types of seizures. Notably, (+)-borneol shows broad-spectrum anti-seizure efficacy on epileptic seizures in different models, which may be optimally suitable for the treatment of epilepsy with different seizure types.
Considering the anti-seizure mechanism, (+)-borneol is efficacious for acute seizures, suggesting that it may have other rapid-action mechanisms. Electrophysiological data further revealed that the (+)-borneol mainly inhibited mEPSCs or sEPSCs, but not mIPSCs or sIPSCs, indicating that (+)-borneol weakened glutamatergic transmission. Unlike (+)-borneol, there was no decrease in spontaneous synaptic currents by the treatment of (−)-borneol or isoborneol. While, both (−)-borneol and isoborneol decreased the intrinsic excitability of the neuron, indicated by the increased minimum current intensity to elicit an AP (Rheobase) and the reduced number of AP spikes under the same injection currents, highlighting different anti-seizure mechanisms underlying borneol enantiomers. Furthermore, epileptogenesis refers to multiple processes in molecular signalling, one of the significant hypotheses is that repeated seizures can lead to the release of a large number of glutamates, strengthening the long-term synaptic plasticity at the neural circuit level [34]. Moreover, the in vivo fiber photometry recording of calcium activity of the synaptic terminal reflected the enhanced release of the glutamatergic transmitter in epileptic mice, and (+)-borneol treatment in kindled mice decreased the seizure severity as well as reduced Ca2+ activity of the synaptic terminal. Notably, (+)-borneol even stabilized the baseline noise levels of glutamatergic transmission, as the mice received an administration of borneol 30 min early to maintain the high level of drug concentration, which was consistent with the decrease of excitatory synaptic transmission in dSub in vitro. These results collectively suggested that (+)-borneol might attenuate seizure severity by decreasing the enhanced excitability of glutamatergic transmission in epilepsy. This mechanism is similar to that of levetiracetam, leading (+)-borneol to be a promising anti-seizure compound with a novel mechanism. Currently, further mechanisms about how (+)-borneol modulates presynaptic glutamate release remain to be explored. One possible explanation is that borneol can activate TRPM8 (EC50 = 65 μM) [15] and this TRPM8 activation would produce anti-seizure effects [35], which may be related to the modulation of glutamate transmission [36].
Previous studies have regarded borneol at a large dose (>1.5 mM) as an efficacious positive modulator of GABA action, directly activating the alpha 1 GABAA receptor [9], and (+)-borneol is a selective positive allosteric modulator of alpha 2/3 GABAA receptor and is insensitive to alpha 1 GABAA receptor in the spinal cord dorsal horn [12]. Interestingly, at least the doses (10 μM) studied here have little potency for inhibitory transmission. This mechanistic diversity might reflect the differential modulation of borneol between the glutamatergic and the GABAergic transmission system in different disease states and brain areas. In addition, previous studies also have indicated that borneol suppresses neuroinflammatory and oxidative stress to play protection in the PTZ-induced kindling model [16]. In patients with TLE, there are typical pathological changes in the brain, including neuronal cell death, astrocyte activation and microgliosis in the hippocampus, and neuroinflammation [37, 38]. Interestingly, (+)-borneol treatment also markedly ameliorated neural loss and the activation of microglia, but did not affect astrogliosis. This indicates that (+)-borneol might also show disease-modifying antiepileptic effect by microglia-related signalling pathway or excitatory transmission between microglia and neuron. As (+)-borneol may regulate neuroinflammation, glutamatergic and the GABAergic transmission system in various pathways, it may have a beneficial effect on epilepsy comorbidities, such as severe intellectual or behavioral disabilities, which deserve further investigation.
Overall, the present study demonstrated the broad-spectrum anti-seizure potential of (+)-borneol in epilepsy by inhibiting the excitability of glutamatergic synaptic transmission. This may expand our understanding of (+)-borneol’s therapeutic potency and suggest (+)-borneol as a novel promising therapeutic drug for epilepsy.
Supplementary information
Appendix A. Supplementary Figure Legends
Acknowledgements
This project was supported by grants from the National Key R&D Program of China (2021ZD0202803 and 2020YFA0803902), the National Natural Science Foundation of China (82022071), the Natural Science Foundation of Zhejiang Province (LD22H310003) and the Research Project of Zhejiang Chinese Medical University (2022JKJNTZ13).
Author contributions
ZC, YiW, and YuW designed the research. YuW, XYQ, JYL, FW, and MJS conducted the experiments. YuW, XYQ, and YiW conducted the data analysis. BT, XHJ, XMJ, and CLX provided technical guidance and contributed to the data discussion. YuW and YiW wrote the paper. YiW and ZC supervised all aspects of the work.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Yu Wang, Xiao-yun Qiu, Jia-ying Liu
Contributor Information
Yi Wang, Email: wang-yi@zju.edu.cn.
Zhong Chen, Email: chenzhong@zju.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-023-01075-w.
References
- 1.Wang Y, Chen Z. An update for epilepsy research and antiepileptic drug development: toward precise circuit therapy. Pharmacol Ther. 2019;201:77–93. doi: 10.1016/j.pharmthera.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Thijs RD, Surges R, O’Brien TJ, Sander JW. Epilepsy in adults. Lancet. 2019;393:689–701. doi: 10.1016/S0140-6736(18)32596-0. [DOI] [PubMed] [Google Scholar]
- 3.Pierre-Louis SJ, Brannegan RT, Evans AT. Seizure control and side-effect profile after switching adult epileptic patients from standard to extended-release divalproex sodium. Clin Neurol Neurosurg. 2009;111:437–41. doi: 10.1016/j.clineuro.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Smith MC, Centorrino F, Welge JA, Collins MA. Clinical comparison of extended-release divalproex versus delayed-release divalproex: pooled data analyses from nine trials. Epilepsy Behav. 2004;5:746–51. doi: 10.1016/j.yebeh.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Fan Q, Liu Y, Rao J, Zhang Z, Xiao W, Zhu T, et al. Anti-atherosclerosis effect of angong niuhuang pill via regulating Th17/Treg immune balance and inhibiting chronic inflammatory on ApoE(-/-) mice model of early and mid-term atherosclerosis. Front Pharmacol. 2019;10:1584. doi: 10.3389/fphar.2019.01584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, Fan X, Chen Y, Liang X, Shen W, Zhang Y. Efficacy and safety of Xingnaojing injection for emergency treatment of acute ischemic stroke: a systematic review and meta-analysis. Front Pharmacol. 2022;13:839305. doi: 10.3389/fphar.2022.839305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang QL, Fu BM, Zhang ZJ. Borneol, a novel agent that improves central nervous system drug delivery by enhancing blood-brain barrier permeability. Drug Deliv. 2017;24:1037–44. doi: 10.1080/10717544.2017.1346002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng Q, Chen ZX, Xu MB, Zhou XL, Huang YY, Zheng GQ, et al. Borneol, a messenger agent, improves central nervous system drug delivery through enhancing blood-brain barrier permeability: a preclinical systematic review and meta-analysis. Drug Deliv. 2018;25:1617–33. doi: 10.1080/10717544.2018.1486471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Granger RE, Campbell EL, Johnston GA. (+)- And (-)-borneol: efficacious positive modulators of GABA action at human recombinant alpha1beta2gamma2L GABA(A) receptors. Biochem Pharmacol. 2005;69:1101–11. doi: 10.1016/j.bcp.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Lai H, Liu C, Hou L, Lin W, Chen T, Hong A. TRPM8-regulated calcium mobilization plays a critical role in synergistic chemosensitization of borneol on doxorubicin. Theranostics. 2020;10:10154–70. doi: 10.7150/thno.45861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogt-Eisele AK, Weber K, Sherkheli MA, Vielhaber G, Panten J, Gisselmann G, et al. Monoterpenoid agonists of TRPV3. Br J Pharmacol. 2007;151:530–40. doi: 10.1038/sj.bjp.0707245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Zhang L, Xu C, Shen YY, Lin YH, Zhang Y, et al. A pain killer without analgesic tolerance designed by co-targeting PSD-95-nNOS interaction and alpha2-containning GABAARs. Theranostics. 2021;11:5970–85. doi: 10.7150/thno.58364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang L, Yin CY, Wu HY, Tian BB, Zhu Y, Luo CX, et al. (+)-Borneol is neuroprotective against permanent cerebral ischemia in rats by suppressing production of proinflammatory cytokines. J Biomed Res. 2017;31:306–14. doi: 10.7555/JBR.31.20160138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao B, Ni HY, Li J, Zhou Y, Bian XL, Tao Y, et al. (+)-Borneol suppresses conditioned fear recall and anxiety-like behaviors in mice. Biochem Biophys Res Commun. 2018;495:1588–93. doi: 10.1016/j.bbrc.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 15.Wang S, Zhang D, Hu J, Jia Q, Xu W, Su D, et al. A clinical and mechanistic study of topical borneol-induced analgesia. EMBO Mol Med. 2017;9:802–15. doi: 10.15252/emmm.201607300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tambe R, Jain P, Patil S, Ghumatkar P, Sathaye S. Antiepileptogenic effects of borneol in pentylenetetrazole-induced kindling in mice. Naunyn Schmiedebergs Arch Pharmacol. 2016;389:467–75. doi: 10.1007/s00210-016-1220-z. [DOI] [PubMed] [Google Scholar]
- 17.Quintans-Júnior LJ, Guimarães AG, Araújo BES, Oliveira GF, Santana MT, Moreira FV, et al. Carvacrol, (-)-borneol and citral reduce convulsant activity in rodents. Afr J Biotechnol. 2010;9:6566–72. [Google Scholar]
- 18.Tang Y, Feng B, Wang Y, Sun H, You Y, Yu J, et al. Structure-based discovery of CZL80, a caspase-1 inhibitor with therapeutic potential for febrile seizures and later enhanced epileptogenic susceptibility. Br J Pharmacol. 2020;177:3519–34. doi: 10.1111/bph.15076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Ying X, Chen L, Liu Y, Wang Y, Liang J, et al. Electroresponsive nanoparticles improve antiseizure effect of phenytoin in generalized tonic-clonic seizures. Neurotherapeutics. 2016;13:603–13. doi: 10.1007/s13311-016-0431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Wang Y, Xu C, Wang S, Tan N, Chen C, et al. Direct septum-hippocampus cholinergic circuit attenuates seizure through driving somatostatin inhibition. Biol Psychiatry. 2020;87:843–56. doi: 10.1016/j.biopsych.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Racine R, Okujava V, Chipashvili S. Modification of seizure activity by electrical stimulation. 3. Mechanisms. Electroencephalogr Clin Neurophysiol. 1972;32:295–9. doi: 10.1016/0013-4694(72)90178-2. [DOI] [PubMed] [Google Scholar]
- 22.Chen B, Xu C, Wang Y, Lin W, Wang Y, Chen L, et al. A disinhibitory nigra-parafascicular pathway amplifies seizure in temporal lobe epilepsy. Nat Commun. 2020;11:923. doi: 10.1038/s41467-020-14648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fei F, Wang X, Xu C, Shi J, Gong Y, Cheng H, et al. Discrete subicular circuits control generalization of hippocampal seizures. Nat Commun. 2022;13:5010. doi: 10.1038/s41467-022-32742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park TJ, Park YS, Lee TG, Ha H, Kim KT. Inhibition of acetylcholine-mediated effects by borneol. Biochem Pharmacol. 2003;65:83–90. doi: 10.1016/S0006-2952(02)01444-2. [DOI] [PubMed] [Google Scholar]
- 25.Qi Y, Cheng H, Lou Q, Wang X, Lai N, Gao C, et al. Paradoxical effects of posterior intralaminar thalamic calretinin neurons on hippocampal seizure via distinct downstream circuits. iScience. 2022;25:104218. doi: 10.1016/j.isci.2022.104218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng L, Xu C, Wang L, An D, Jiang L, Zheng Y, et al. Histamine H1 receptor deletion in cholinergic neurons induces sensorimotor gating ability deficit and social impairments in mice. Nat Commun. 2021;12:1142. doi: 10.1038/s41467-021-21476-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castel-Branco MM, Alves GL, Figueiredo IV, Falcao AC, Caramona MM. The maximal electroshock seizure (MES) model in the preclinical assessment of potential new antiepileptic drugs. Methods Find Exp Clin Pharmacol. 2009;31:101–6. doi: 10.1358/mf.2009.31.2.1338414. [DOI] [PubMed] [Google Scholar]
- 28.Dhir A. Pentylenetetrazol (PTZ) kindling model of epilepsy. Curr Protoc Neurosci. 2012;Chapter 9:Unit9 37. doi: 10.1002/0471142301.ns0937s58. [DOI] [PubMed] [Google Scholar]
- 29.Klein S, Bankstahl M, Loscher W. Inter-individual variation in the effect of antiepileptic drugs in the intrahippocampal kainate model of mesial temporal lobe epilepsy in mice. Neuropharmacology. 2015;90:53–62. doi: 10.1016/j.neuropharm.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Xu C, Xu Z, Ji C, Liang J, Wang Y, et al. Depolarized GABAergic signaling in subicular microcircuits mediates generalized seizure in temporal lobe epilepsy. Neuron. 2017;95:92–105.e105. doi: 10.1016/j.neuron.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Broussard GJ, Liang Y, Fridman M, Unger EK, Meng G, Xiao X, et al. In vivo measurement of afferent activity with axon-specific calcium imaging. Nat Neurosci. 2018;21:1272–80. doi: 10.1038/s41593-018-0211-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.da Fonseca DV, da Silva Maia Bezerra Filho C, Lima TC, de Almeida RN, de Sousa DP. Anticonvulsant essential oils and their relationship with oxidative stress in epilepsy. Biomolecules. 2019;9:835. [DOI] [PMC free article] [PubMed]
- 33.White HS, Johnson M, Wolf HH, Kupferberg HJ. The early identification of anticonvulsant activity: role of the maximal electroshock and subcutaneous pentylenetetrazol seizure models. Ital J Neuro Sci. 1995;16:73–7. doi: 10.1007/BF02229077. [DOI] [PubMed] [Google Scholar]
- 34.Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol. 2004;73:1–60. doi: 10.1016/j.pneurobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Moriyama H, Nomura S, Imoto H, Inoue T, Fujiyama Y, Haji K, et al. Suppressive effects of transient receptor potential melastatin 8 agonist on epileptiform discharges and epileptic seizures. Front Pharmacol. 2021;12:766782. doi: 10.3389/fphar.2021.766782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wrigley PJ, Jeong HJ, Vaughan CW. Primary afferents with TRPM8 and TRPA1 profiles target distinct subpopulations of rat superficial dorsal horn neurones. Br J Pharmacol. 2009;157:371–80. doi: 10.1111/j.1476-5381.2009.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das A, Wallace GCT, Holmes C, McDowell ML, Smith JA, Marshall JD, et al. Hippocampal tissue of patients with refractory temporal lobe epilepsy is associated with astrocyte activation, inflammation, and altered expression of channels and receptors. Neuroscience. 2012;220:237–46. doi: 10.1016/j.neuroscience.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao J, Sun J, Zheng Y, Zheng Y, Shao Y, Li Y, et al. Activated astrocytes attenuate neocortical seizures in rodent models through driving Na+-K+-ATPase. Nat Commun. 2022;13:7136. doi: 10.1038/s41467-022-34662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A. Supplementary Figure Legends