Abstract
Purpose
The prognostic nutritional index (PNI), which is derived from the albumin concentration and absolute lymphocyte number, is an effective indicator of cancer patients’ nutritional and immunological status. According to multiple studies, PNI was strongly linked to the prognosis of patients with non-small cell lung cancer (NSCLC). The predictive value of PNI for survival outcomes in NSCLC patients receiving immune checkpoint inhibitors (ICIs) is still in dispute at present. This meta-analysis is devoted to fill this information gap and investigate the predictive ability of PNI in NSCLC patients treated with ICIs.
Methods
The PubMed, Embase, Cochrane Library databases, and conference proceedings were searched for eligible studies without language restriction. Overall survival (OS) and progression-free survival (PFS) were included. The predictive value of PNI was estimated using hazard ratios and their 95% confidence intervals.
Results
Thirteen relevant retrospective cohort studies were included and these studies included 1119 patients with stage III-IV NSCLC. Lower PNI status was found to be an independent risk factor for worse survival outcomes in patients with NSCLC (OS HR = 2.68; 95%CI: 1.76–4.06; P < 0.0001; PFS HR = 1.84; 95%CI: 1.39–2.42; P < 0.0001). According to the subgroup analysis, PNI was similarly connected to OS in most subgroups of NSCLC patients receiving ICIs, except for those receiving chemoimmunotherapy or first-line treatment, and those with a cut-off value < 45.
Conclusion
Our findings indicated that lower PNI was associated with poorer prognosis in NSCLC patients undergoing ICI therapy. Further prospective research with bigger patient groups is required.
Systematic Review Registration
International Prospective Register of Systematic Reviews (PROSPERO), identifier CRD42022327528.
Keywords: Prognostic nutritional index, Non-small cell lung cancer, Meta-analysis
1. Introduction
Lung cancer remains the leading cause of cancer-related deaths worldwide [1] and approximately 85% of lung cancer is non-small-cell lung cancer (NSCLC). Owing to the absence of clinical symptoms, the majority of patients with NSCLC are diagnosed at an advanced stage, resulting in a low 5-year survival rate [2]. Immune checkpoint inhibitors (ICIs), particularly anti-programmed death receptor-1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors, have completely changed the treatment paradigm for NSCLC patients [[3], [4], [5]]. Despite the fact that some patients respond to ICIs treatment, the majority of patients fail to experience any benefit. Generally, PD-1 inhibitor monotherapy produces a clinical response only in 20% of patients with NSCLC [3,4]. Therefore, it is essential to distinguish and validate predictive markers for screening patients who are most suitable for ICIs treatment.
The PD-L1 expression and tumor mutational burden (TMB) score are the two most clinically acknowledged and recognized biomarkers for ICIs efficacies [[6], [7], [8]]. The expression of PD-L1 as detected by immunohistochemistry (IHC) in tumor cells is the first FDA-approved biomarker for selecting beneficial patients with NSCLC receiving ICIs [9]. First-line pembrolizumab treatment for advanced NSCLC patients with high PD-L1 expression showed a substantial survival benefit [6,10]. However, in CheckMate-057, regardless of PD-L1 level, a prognostic advantage was reported for second-line nivolumab treatment [4]. Similarly, number of independent investigations indicated that TMB remained insufficiently predictive, due to its dynamic, heterogenous and methodology-affecting characteristics [11,12].
In addition to tumor features, the prognosis of patients with advanced NSCLC can also be predicted by patient-related parameters [[13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]]. The prognostic nutritional index (PNI) was created for the first time in 1980 to estimate perioperative risk for gastrointestinal surgeries [26]. In patients with early-stage NSCLC, PNI was shown to be useful in predicting postoperative recurrence and prognosis [27]. Moreover, it has been shown that pre-treatment PNI is strongly linked with progression free survival (PFS) and overall survival (OS) in patients with NSCLC who received chemotherapy or chemoradiotherapy [[28], [29], [30]]. However, there is no conclusive conclusion addressing the potential predictive values of PNI in NSCLC patients treated with ICIs. For the past few years, the ability of PNI to forecast the survival of NSCLC patients receiving ICIs has been investigated by several research. Therefore, it is necessary to integrate these studies to provide preliminary insight into this subject.
In this study, we included all pertinent retrospective cohort studies and performed a meta-analysis of them to clarify the predictive and clinical impact of PNI on NSCLC patients receiving ICIs treatment, thereby providing strong evidence for practical clinical decision-making.
2. Methods
2.1. Guidelines and registration
This meta-analysis was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) statement [31]. The International Prospective Register of Systematic Reviews (PROSPERO) received this meta-analysis for registration: number CRD42022327528.
2.2. Eligibility criteria
Studies were considered if the following criteria were met: 1) The research comprised patients with pathologically determined NSCLC; 2) Studies explored the predictive capability of PNI that was calculated by serum albumin levels and peripheral lymphocyte counts; 3) Retrospective or prospective studies contained the HR and accompanying 95% confidence interval (95%CI) for the OS or PFS; 4) Retrospective or prospective studies published before August 2022.
Studies meeting the following criteria were excluded: 1) reviews, conference abstracts, case reports, letters, or comments; 2) laboratory testing of clinical samples, cell lines, or animals; 3) inadequate data of PNI.
2.3. Data extraction
First author, year of publication, study design, region, sample size, biological sex, PNI cutoff value, treatment regimen, treatment lines and results with HRs and their associated95%CIs of high versus low PNI for OS and PFS were retrieved independently by two researchers from the eligible studies. Discussion and agreement were used to settle any disputes.
2.4. Study quality assessment
Newcastle-Ottawa Quality Assessment Scale (NOS) was used to assess the study's quality. Each study was given a number ranging from 0 (worst) to 9 (best). Studies receiving a score of 6 or less were deemed to be of low quality, while those receiving a score of 7 or above were deemed to be of excellent quality [32].
2.5. Statistical analysis
The present meta-analysis was assessed by R 4.1.3 software. HRs calculated from multivariate analyses were extracted preferentially where available. Univariate HRs were used instead if multivariate HRs were not available. According to heterogeneity, the random effect or fixed-effect model was merged with pooled HRs and 95% CIs. Q tests and I2 statistics were used to evaluate heterogeneity. P < 0.05 and/or I2>50% were defined as significant heterogeneity and random effect model was further to used. Otherwise, we chose the fixed-effect model. In accordance with the sample size, region, treatment regimen, treatment line and PNI cut-off value, subgroup analyses were performed. For subgroup analysis of cut-off value, the stratification was based on the median of the cut-off values used in the included studies [33,34]. Sensitivity analysis, in which one study was removed at a time, was performed to evaluate the stability of the results. Finally, publication bias was evaluated by funnel plots. If funnel plot asymmetry was suggested by a visual assessment, we would perform exploratory analyses (e.g. Rücker's arcsine test for dichotomous data) to further investigate funnel plot asymmetry [35,36].
3. Results
3.1. Study selection
Our database and manual searches retrieved a total of 173 publications, of which 38 were excluded due to duplication. We initially screened the abstracts and titles for eligibility. Of the 135 studies that were assessed for eligibility, 23 met our inclusion criteria. Then, by reading the full text, we further filter the selected studies. Ultimately, a total of 13 studies were included in the meta-analysis. Fig. 1 displays a flow chart of the studies’ selection process.
Fig. 1.
Flow chart of study selection and design.
3.2. Characteristics of the included studies
The key characteristics of the included studies are listed in Table 1. A total of 13 studies comprising 1119 patients were included for meta-analysis, all of which were retrospective cohort studies [[13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]]. Among all included studies, nine were conducted in Asia [[14], [15], [16],[18], [19], [20], [21], [22], [23]] and four in Europe [13,17,24,25]; patients with stage III-IV NSCLC were enrolled in each study. The sample size of these studies ranged from 24 to 237. The cut-off values of PNI varied from 40 to 50.
Table 1.
Summary of studies included in the present meta-analysis.
| Authors | Year | Study design | N | Gender (M/F) | Age | Region | Stage | Treatment | Line | Cut-off value | Outcomes | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lihong | 2019 | Retrospective | 102 | 87/15 | 62 | Asia | III-IV | Nivo, Pemb, Toripa, Sinti |
un-selected | 45 | OS,PFS | 7 |
| Taichi | 2020 | Retrospective | 24 | 7/17 | 64.5 (54.8–74.2) | Asia | III-IV | Atezo | >1 | 40 | OS | 5 |
| Cipriano | 2020 | Retrospective | 34 | 27/17 | 67 (34–79) | Europe | IV | Immunotherapy | un-selected | 50 | OS | 4 |
| Shi | 2021 | Retrospective | 103 | 68/35 | 66 | Asia | III-IV | Immunotherapy/Chemoimmunotherapy | un-selected | 45 | OS,PFS | 7 |
| Cinzia | 2021 | Retrospective | 44 | 26/18 | 70 (42–83) | Europe | III-IV | Pemb | 1 | 45.1 | PFS | 8 |
| Taisuke | 2021 | Retrospective | 36 | 31/5 | 68.5 | Asia | III-IV | Chemoimmunotherapy | 1 | 40 | OS,PFS | 6 |
| Junichi | 2021 | Retrospective | 73 | 52/21 | 70.9 (46–89) | Asia | III-IV | Nivo, Pemb,Atezo | un-selected | 43 | OS,PFS | 7 |
| Yuri | 2021 | Retrospective | 34 | 29/5 | 72 (55–81) | Asia | IV | Chemoimmunotherapy | 1 | 40 | OS,PFS | 6 |
| Na | 2021 | Retrospective | 123 | 98/25 | 59.9 (48.6–71.2) | Asia | IV | Nivo, Pemb, Sinti, Camre, Toripa |
un-selected | 46.05 | OS,PFS | 6 |
| Cipriano | 2021 | Retrospective | 52 | 42/10 | NR | Europe | IV | Immunotherapy | un-selected | 50 | OS,PFS | 4 |
| Stares | 2022 | Retrospective | 219 | 109/110 | 69 | Europe | III-IV | Pemb | 1 | 45 | OS,PFS | 8 |
| Satomi | 2022 | Retrospective | 237 | 187/187 | 69 (62–73) | Asia | IV | Chemoimmunotherapy | un-selected | 40.35 | OS,PFS | 7 |
| Naoki | 2022 | Retrospective | 38 | 30/8 | 75 (45–86) | Asia | III-IV | Pemb | 1 | 40 | PFS | 6 |
N, number; M, male; F, female; NR, not reported; OS, overall survival; PFS, progression-free survival; Nivo, Nivolumab; Pemb, Pembrolizumab; Toripa, Toripalimab; Sinti, Sintilimab; Camre, Camrelizumab; Atezo, Atezolizumab; NOS, Newcastle-Ottawa quality assessment Scale.
3.3. Correlation between PNI and survival in NSCLC
In a total of 11 studies, the OS andPFS outcomes were reported. The heterogeneity analyses indicated the presence of heterogeneity, hence the random effect model was employed. The results indicated that lower pretreatment PNI was associated with worse OS in NSCLC patients treated with ICIs (HR = 2.68; 95%CI: 1.76–4.06; P < 0.0001; I2 = 91.0%, P < 0.0001) (Fig. 2A). In addition, decreased pretreatment PNI was linked to poorer PFS as well (HR = 1.84; 95%CI: 1.39–2.42; P < 0.0001; I2 = 82.9%, P < 0.0001) (Fig. 2B).
Fig. 2.
Forest plot for the association between PNI and (A) overall survival (OS), (B) progression-free survival (PFS).
3.4. Subgroup analysis
In order to identify factors associated with heterogeneity, we performed subgroup analyses stratified by sample size, region, treatment regimen, treatment line, PNI cut-off value (stratified by median value as described in the Methods section) and NOS score. In the majority of stratified analyses, a lower PNI was associated with worse OS in NSCLC patients receiving ICIs treatment. However, this relationship was not statistically significant in the chemoimmunotherapy subgroup, the first-line treatment subgroup and the cut-off value < 45 subgroup (Table 2). Notably, the subgroup analysis based on cut-off values revealed a statistically significant difference between the two subgroups (P = 0.04). In the cut-off value ≥ 45 subgroup, pooled HR demonstrated that patients with low PNI had a worse OS compared to those with high PNI, (HR = 3.63; 95%CI: 2.47–5.31; P < 0.0001; I2 = 45.1%, P < 0.0001), whereas no difference was observed between patients with low and high PNI in the cut-off value < 45 subgroup (HR = 1.72; 95%CI: 0.91–3.24; P = 0.10; I2 = 84.6%, P < 0.0001).
Table 2.
Subgroup analyses based on a random effects model.
| N | Association |
Heterogeneity |
|||
|---|---|---|---|---|---|
| HR (95%CI) | p value | I2 (%) | p value | ||
| Sample size | |||||
| <100 | 6 | 1.94 [1.01; 3.71] | 0.04 | 72.80% | <0.01 |
| ≥100 | 5 | 3.48 [2.41; 5.04] | <0.01 | 53.00% | 0.07 |
| Region | |||||
| Asia | 8 | 2.52 [1.46; 4.35] | <0.01 | 91.90% | <0.01 |
| Europe | 3 | 2.87 [2.00; 4.13] | <0.01 | 0.00% | 0.55 |
| Treatment regimen | |||||
| Mono-Immunotherapy | 7 | 3.35 [2.11; 5.30] | <0.01 | 56.50% | 0.03 |
| Chemoimmunotherapy | 3 | 1.67 [0.77; 3.62] | 0.19 | 91.10% | <0.01 |
| Un-selected | 1 | 3.40 [1.42; 8.13] | – | – | – |
| Line | |||||
| >1 | 1 | 7.28 [0.92; 57.50] | – | – | – |
| 1 | 3 | 1.61 [0.78; 3.29] | 0.20 | 90.60% | <0.01 |
| Un-selected | 7 | 3.35 [2.25; 4.99] | <0.01 | 51.90% | 0.05 |
| Cut-off value | |||||
| <45 | 5 | 1.72 [0.91; 3.24] | 0.09 | 84.60% | <0.01 |
| ≥45 | 6 | 3.62 [2.47; 5.31] | <0.01 | 45.10% | 0.11 |
| Study quality | |||||
| <7 | 6 | 3.01 [1.39; 6.52] | <0.01 | 92.10% | <0.01 |
| ≥7 | 5 | 2.73 [2.11; 3.53] | <0.01 | 0.00% | 0.53 |
HR, hazard ratio; CI, confidence interval.
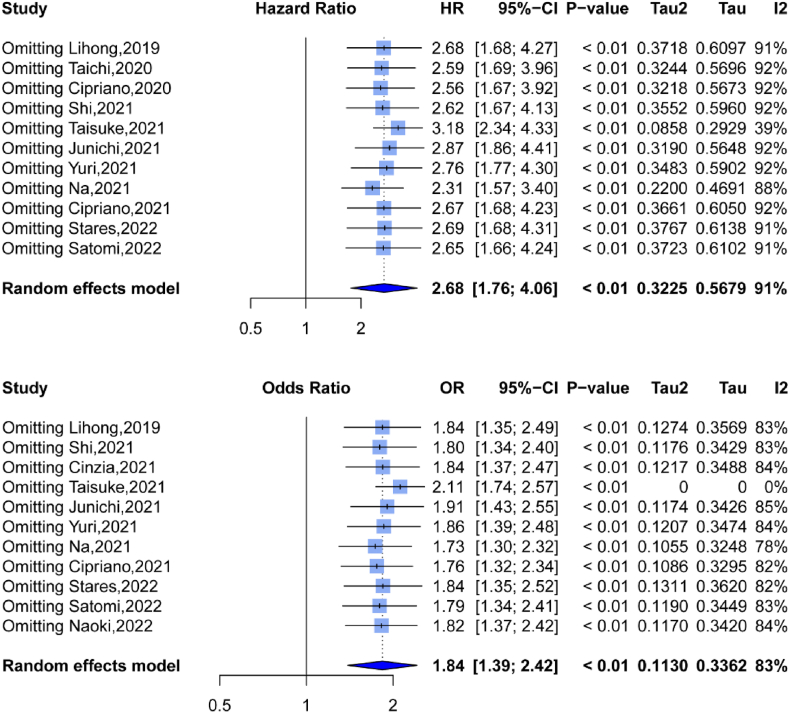
3.5. Sensitivity analysis
We conducted a sensitivity analysis by removing one study at a time and computed the combined HR. The pooled HRs and 95% CIs indicated that no research substantially influenced OS or PFS (Fig. 3A and B), demonstrating the stability and dependability of our findings in this meta-analysis.
Fig. 3.
Sensitivity analysis for the association between PNI and (A) overall survival (OS), (B) progression-free survival (PFS).
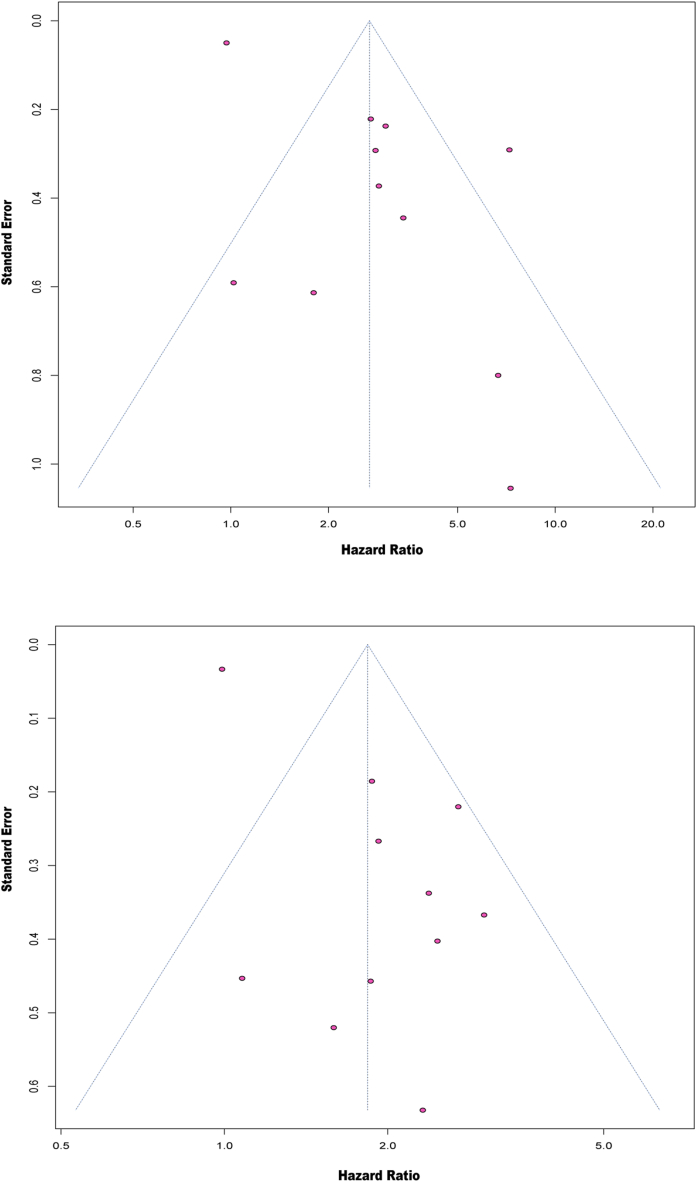
3.6. Publication bias
The funnel plots for publication bias showed some asymmetry (Fig. 4A and B). Arcsine tests were therefore carried out to better examine the publication bias. The P values of the Arcsine tests indicated that there were no discernible biases in the pooled HRs for OS and PFS (P = 0.2576 for OS; P = 0.1544 for PFS).
Fig. 4.
Funnel plot for (A) overall survival (OS) and (B) progression-free survival (PFS).
4. Discussion
This systematic review and meta-analysis provided first-of-its kind evidence for the association between PNI and the prognosis of patients with NSCLC receiving ICIs. The PFS and OS data gathered from the included studies were pooled for statistical analysis. Collectively, the findings of the present research suggested that a lower PNI was associated with poorer outcomes (OS and PFS) in NSCLC patients receiving ICIs treatment. In the subgroup analyses, it was revealed that lower PNI remained a risk factor for worse OS in certain subgroups (i.e., region, sample size, tumor stage, histology, and study quality). The subgroup analysis in the chemoimmunotherapy and first-line therapy scenario was based solely on data from three studies. The results of this analysis should be treated with caution due to the small number of research included. In light of the limited number of included trials, this stratified result should be interpreted with some caution. Notably, there is currently no gold standard to define the optimal cut-off value for PNI [13,14]. Nevertheless, our results indicated that the cut-off value of 45 or above was more valuable for predicting the prognosis of NSCLC patients undergoing ICI therapy.
As an effective immune-nutritional marker, PNI is determined based on serum albumin and lymphocyte count and reflects both immunological and nutritional status. Several potential mechanisms may account for the observed association between increased PNI and worse prognosis in NSCLC patients treated with ICIs. On the one hand, serum albumin has been proven to be a reliable indicator of patients’ nutritional condition [37]. Malnutrition is one of the main causes for immunodeficiency and profoundly affects the anti-tumor immune responses [38,39]. Hypoalbuminemia implies poor nutritional status, impairs immunological function, including humoral and cell-mediated immunity as well as antigen-presenting cell activities, thus correlating with poor prognosis for cancer patients [40]. On the other hand, lymphocytes play a fundamental role in suppressing tumor growth and progression via direct effects on cancer cells or indirect effects on the tumor microenvironment [41]. Numerous studies have shown that lymphocytopenia is related with impaired anti-tumor immune response, and lymphocyte count level can be utilized as an indicator to predict the overall treatment outcomes in cancer patients [42,43]. In a single-center retrospective study of 268 patients with advanced NSCLC, those with lymphopenia (absolute lymphocyte count<1000 cells/mm3) had poor performance status and extensive disease when receiving immunotherapy. In addition, the results from multivariate analysis demonstrated that lymphopenia was associated with unfavorable prognosis and poor response to ICIs [44]. Overall, as a potential biomarker, PNI has excellent application prospects in the field of antitumor immunotherapy.
Exploring biomarkers of responses to ICIs will facilitate the development of precise or personalized treatment strategies, which will improve the efficacy of ICIs. The expression of PD-L1 level is the most common clinically used and approved biomarker for ICIs treatment [6,9]. Certain hematological parameters can also reflect immune status of different cancers, and thus have the predictive potential for ICIs effectiveness. PNI has been found to have possible correlations with PD-L1 expression. For example, high expression of PD-L1 and malnutrition are both associated with immunosuppression [45,46]. Riki and his colleagues found a negative correlation between PD-L1 expression and PNI [47], with one possible explanation being that both inflammatory factors and nutritional status regulate the metabolism and function of immune cells [[48], [49], [50]]. A number of studies have elucidated that hematological markers, such as C-reactive protein (CRP), neutrophil-to-lymphocyte ratio (NLR) and PNI have high predictive values for the efficacy of ICIs treatment in patients with NSCLC [[51], [52], [53], [54]]. These markers have the advantages of being reliable, inexpensive, and easy to obtain. Additionally, researchers have reported that PNI is a more useful biomarker than CRP or NLR by comparing the AUC value, sensitivity and specificity [55]. Therefore, PNI exhibits substantial clinical potential as a biomarker to predict the prognosis for NSCLC patients receiving ICIs treatment.
Several limitations, however, must be taken into account when interpreting our findings. First, the majority of included studies had a retrospective design, which is a potential source of bias; Second, we observed considerable heterogeneity across studies. Although subgroup and sensitivity analyses were performed, the main source of heterogeneity was not identified. That may be attributed to the fact that different cut-off values were employed in the analyzed studies. Third, our subgroup analysis demonstrated that setting the cutoff value of PNI at 45 or above might have a greater predictive effect for the efficacy of ICIs treatment in patients with NSCLC. However, considering that the range for 45 and above is still broad, it is necessary to conduct large-scale clinical studies to obtain more precise cut-off value for achieving optimal predictive effects.
5. Conclusion
In conclusion, our study provides evidence that a reduced PNI level is associated with worse survival outcomes in NSCLC patients undergoing ICIs treatment, which may help clinicians to predict the prognosis of NSCLC patients and to choose the optimal treatments. More comprehensive, better designed, and prospective studies are needed to verify our results.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
Data included in article/supp. material/referenced in article.
Additional information
This work was supported by grants from the National Natural Science Foundation of China (No. 81874044), the Shandong Provincial Natural Science Foundation (No. ZR2020MH236 and No. ZR2019MH050) and Jinan Science and Technology Bureau Principal Investigator Studio Program (No. 202228122).
Declaration of competing interest
All authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Contributor Information
Xiuwen Wang, Email: xiuwenwang12@sdu.edu.cn.
Yanguo Liu, Email: liuyanguo@sdu.edu.cn.
References
- 1.Sung H., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Arbour K.C., Riely G.J. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: a review. JAMA. 2019;322(8):764–774. doi: 10.1001/jama.2019.11058. [DOI] [PubMed] [Google Scholar]
- 3.Garon E.B., et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 4.Borghaei H., et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fehrenbacher L., et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 6.Reck M., et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 7.Herbst R.S., Morgensztern D., Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–454. doi: 10.1038/nature25183. [DOI] [PubMed] [Google Scholar]
- 8.Yarchoan M., Hopkins A., Jaffee E.M. Tumor mutational burden and response rate to PD-1 inhibition. N. Engl. J. Med. 2017;377(25):2500–2501. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel S.P., Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol. Cancer Therapeut. 2015;14(4):847–856. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 10.Mok T.S.K., et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 11.Chan T.A., et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann. Oncol. 2019;30(1):44–56. doi: 10.1093/annonc/mdy495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Passaro A., Stenzinger A., Peters S. Tumor mutational burden as a pan-cancer biomarker for immunotherapy: the limits and potential for convergence. Cancer Cell. 2020;38(5):624–625. doi: 10.1016/j.ccell.2020.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Stares M., et al. Biomarkers of systemic inflammation predict survival with first-line immune checkpoint inhibitors in non-small-cell lung cancer. ESMO Open. 2022;7(2) doi: 10.1016/j.esmoop.2022.100445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka S., et al. Prognostic nutritional index and lung immune prognostic index as prognostic predictors for combination therapies of immune checkpoint inhibitors and cytotoxic anticancer chemotherapy for patients with advanced non-small cell lung cancer. Diagnostics. 2022;12(2) doi: 10.3390/diagnostics12020423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shijubou N., et al. Immunological and nutritional predictive factors in patients receiving pembrolizumab for the first-line treatment of non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2022;148(8):1893–1901. doi: 10.1007/s00432-022-03941-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Y., et al. Correlations between peripheral blood biomarkers and clinical outcomes in advanced non-small cell lung cancer patients who received immunotherapy-based treatments. Transl. Lung Cancer Res. 2021;10(12):4477–4493. doi: 10.21037/tlcr-21-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baldessari C., et al. Body composition and inflammation impact in non-small-cell lung cancer patients treated by first-line immunotherapy. Immunotherapy. 2021;13(18):1501–1519. doi: 10.2217/imt-2021-0038. [DOI] [PubMed] [Google Scholar]
- 18.Araki T., et al. Prognostic implication of erector spinae muscles in non-small-cell lung cancer patients treated with immuno-oncology combinatorial chemotherapy. Thorac. Canc. 2021;12(21):2857–2864. doi: 10.1111/1759-7714.14142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaitsu J., et al. Systemic inflammatory score predicts response and prognosis in patients with lung cancer treated with immunotherapy. Anticancer Res. 2021;41(7):3673–3682. doi: 10.21873/anticanres.15158. [DOI] [PubMed] [Google Scholar]
- 20.Ogura Y., et al. Predictors of survival among Japanese patients receiving first-line chemoimmunotherapy for advanced non-small cell lung cancer. Thorac. Canc. 2021;12(1):97–105. doi: 10.1111/1759-7714.13720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsubara T., et al. The impact of immune-inflammation-nutritional parameters on the prognosis of non-small cell lung cancer patients treated with atezolizumab. J. Thorac. Dis. 2020;12(4):1520–1528. doi: 10.21037/jtd.2020.02.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu N., et al. Prognostic nutritional index identifies risk of early progression and survival outcomes in advanced non-small cell lung cancer patients treated with PD-1 inhibitors. J. Cancer. 2021;12(10):2960–2967. doi: 10.7150/jca.55936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng L., et al. Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunology. Immunotherapy. 2020;69(9):1813–1822. doi: 10.1007/s00262-020-02585-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cipriano É., et al. The prognostic nutritional index and neutrophil-to-lymphocyte ratio as prognostic factors in advanced non-small cell lung cancer patients treated with immunotherapy. Ann. Oncol. 2019;30:xi6. [Google Scholar]
- 25.Cipriano É., Magalhães H., Estevinho F. P02.21 the impact of inflammatory serum biomarkers in non-small cell lung cancer patients treated with immune checkpoint inhibitors. J. Thorac. Oncol. 2021;16(3):S255. [Google Scholar]
- 26.Buzby G.P., et al. Prognostic nutritional index in gastrointestinal surgery. Am. J. Surg. 1980;139(1):160–167. doi: 10.1016/0002-9610(80)90246-9. [DOI] [PubMed] [Google Scholar]
- 27.Shoji F. Clinical impact of preoperative immunonutritional status in patients undergoing surgical resection of lung cancer. J. Thorac. Dis. 2019;11(Suppl 3):S408–S412. doi: 10.21037/jtd.2018.11.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bozkaya Y., et al. Is the prognostic nutritional index a prognostic and predictive factor in metastatic non-small cell lung cancer patients treated with first-line chemotherapy? Support. Care Cancer. 2020;28(5):2273–2282. doi: 10.1007/s00520-019-05055-x. [DOI] [PubMed] [Google Scholar]
- 29.Ozdemir Y., et al. Low prognostic nutritional index predicts poor clinical outcomes in patients with stage IIIB non-small-cell lung carcinoma undergoing chemoradiotherapy. Cancer Manag. Res. 2020;12:1959–1967. doi: 10.2147/CMAR.S248034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J., et al. The prognostic value of prognostic nutritional index (PNI) and neutrophil to lymphocyte ratio (NLR) for advanced non-small cell lung cancer treated with platinum-based chemotherapeutics. Ann. Palliat. Med. 2020;9(3):967–978. doi: 10.21037/apm.2020.04.31. [DOI] [PubMed] [Google Scholar]
- 31.Liberati A., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 33.Byberg S., et al. Metabolic effects of dopamine agonists in patients with prolactinomas: a systematic review and meta-analysis. Endocr. Connect. 2019;8(10):1395–1404. doi: 10.1530/EC-19-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y., et al. Cryotherapy is associated with improved clinical outcomes of Sorafenib therapy for advanced hepatocellular carcinoma. Cell Biochem. Biophys. 2012;63(2):159–169. doi: 10.1007/s12013-012-9353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sterne J.A., et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 36.Rucker G., Schwarzer G., Carpenter J. Arcsine test for publication bias in meta-analyses with binary outcomes. Stat. Med. 2008;27(5):746–763. doi: 10.1002/sim.2971. [DOI] [PubMed] [Google Scholar]
- 37.Wang W.G., et al. Use of Clavien-Dindo classification in evaluating complications following pancreaticoduodenectomy in 1,056 cases: a retrospective analysis from one single institution. Oncol. Lett. 2018;16(2):2023–2029. doi: 10.3892/ol.2018.8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tilg H., Moschen A.R. Food, immunity, and the microbiome. Gastroenterology. 2015;148(6):1107–1119. doi: 10.1053/j.gastro.2014.12.036. [DOI] [PubMed] [Google Scholar]
- 39.Veldhoen M., Veiga-Fernandes H. Feeding immunity: skepticism, delicacies and delights. Nat. Immunol. 2015;16(3):215–219. doi: 10.1038/ni.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roxburgh C.S., McMillan D.C. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6(1):149–163. doi: 10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- 41.Mantovani A., et al. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 42.Yu P., Fu Y.X. Tumor-infiltrating T lymphocytes: friends or foes? Lab. Invest. 2006;86(3):231–245. doi: 10.1038/labinvest.3700389. [DOI] [PubMed] [Google Scholar]
- 43.Kinoshita T., et al. Prognostic value of tumor-infiltrating lymphocytes differs depending on histological type and smoking habit in completely resected non-small-cell lung cancer. Ann. Oncol. 2016;27(11):2117–2123. doi: 10.1093/annonc/mdw319. [DOI] [PubMed] [Google Scholar]
- 44.Cho Y., et al. Impact of treatment-related lymphopenia on immunotherapy for advanced non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019;105(5):1065–1073. doi: 10.1016/j.ijrobp.2019.08.047. [DOI] [PubMed] [Google Scholar]
- 45.McLaughlin J., et al. Quantitative assessment of the heterogeneity of PD-L1 expression in non-small-cell lung cancer. JAMA Oncol. 2016;2(1):46–54. doi: 10.1001/jamaoncol.2015.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rathmell J.C. Metabolism and autophagy in the immune system: immunometabolism comes of age. Immunol. Rev. 2012;249(1):5–13. doi: 10.1111/j.1600-065X.2012.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okita R., et al. Preoperative neutrophil-to-lymphocyte ratio correlates with PD-L1 expression in immune cells of patients with malignant pleural mesothelioma and predicts prognosis. Sci. Rep. 2023;13(1):5263. doi: 10.1038/s41598-023-31448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dong H., et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 49.Alwarawrah Y., Kiernan K., MacIver N.J. Changes in nutritional status impact immune cell metabolism and function. Front. Immunol. 2018;9:1055. doi: 10.3389/fimmu.2018.01055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen S., Danzaki K., MacIver N.J. Nutritional effects on T-cell immunometabolism. Eur. J. Immunol. 2017;47(2):225–235. doi: 10.1002/eji.201646423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li M., et al. Change in neutrophil to lymphocyte ratio during immunotherapy treatment is a non-linear predictor of patient outcomes in advanced cancers. J. Cancer Res. Clin. Oncol. 2019;145(10):2541–2546. doi: 10.1007/s00432-019-02982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McMillan D.C. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39(5):534–540. doi: 10.1016/j.ctrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 53.Shimizu K., et al. Preoperative neutrophil/lymphocyte ratio and prognostic nutritional index predict survival in patients with non-small cell lung cancer. World J. Surg. Oncol. 2015;13:291. doi: 10.1186/s12957-015-0710-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang H., et al. Prognostic significance of combination of preoperative platelet count and neutrophil-lymphocyte ratio (COP-NLR) in patients with non-small cell lung cancer: based on a large cohort study. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0126496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shoji F., et al. Pretreatment prognostic nutritional index as a novel biomarker in non-small cell lung cancer patients treated with immune checkpoint inhibitors. Lung Cancer. 2019;136:45–51. doi: 10.1016/j.lungcan.2019.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.