Abstract
The main aimed of this study was to evaluate the physicochemical parameters, abundance and density of cyanobacteria, determine their blooms and the ecotoxicological risk of their cyanotoxins in fish ponds water. This study was conducted out in 20 fish farms in Rondônia state (Brazilian Amazon), samplings were carried out in the rainy and dry seasons. The experiment was developed in a completely randomized factorial design 20 × 3 x 3 (20 fish farms, 3 ponds and 3 replications). Regarding the composition of qualitative samples, horizontal and vertical hauls were carried out on the water surface, quantitative samples was obtained using a plankton net (50 μm mesh opening). Meanwhile, with the use of a multiparametric probe, physicochemical analyzes in fish ponds water were carried out. Furthermore, the cyanobacteria found were classified taxonomically and its blooms were recorded. Finally, blood was collected from 60 Colossoma macropomum. Concerning the higher averages in the rainy season 6.13 mg L⁻1 of dissolved oxygen, 40.02 cm of transparency, 0.35 NO31⁻ of nitrate, 0.15 NO21⁻ of nitrite, 44.55 mg L⁻1 CaCO3 of alkalinity and 50.10 mg L⁻1 CaCO3 of hardness, while higher averages of pH, phosphate and phosphorus were found in the dry season. A total of 15 families and 29 species of cyanobacteria were identified in the different seasons. The families that showed the highest densities (rainy and dry seasons) were Microcystaceae (356 and 760 cells mL⁻1), Leptolyngbyaceae (126 and 287 cells mL⁻1) and Microcoleaceae (111 and 405 cells mL⁻1). The species that showed the highest densities were Microcystis aeruginosa (356 and 697 cells mL⁻1), Planktolyngbya limnetica (98 and 257 cells mL⁻1) and Planktothrix sp. (111 and 239 cells mL⁻1). There were significant Pearson's correlations (r > 0.85; p < 0.05) between family abundances and cyanotoxin volume between physicochemical water variables and seasonality. A total of 20 cyanobacteria blooms were recorded, all of which in the dry season showed an ecotoxicological risk. Concerning the assessment mutagenicity in fish blood cells, a total of 78 abnormalities per slide were observed. In the dry season, the expected volume of cyanotoxins in the ponds from fish farms F1 and F4 were above the quantification limit (>QL). Abundance and density of cyanobacteria and their blooms and cyanotoxins can be used as bioindicators of eutrophication and/or water quality and ecotoxicological risk in fish ponds.
Keywords: Aquatic microbiology, Brazilian western amazon, Ecotoxicological risk, Environmental biomonitoring
Nomenclature
General abbreviations
- WHO
World Health Organization
- ANVISA
Agência Nacional de Vigilância Sanitária
- CONAMA
Conselho Nacional do Meio Ambiente
- PEIXE BR
Associação Brasileira da Piscicultura
- INPE
Instituto Nacional de Pesquisas Espaciais
- CPTEC
Centro de Previsão do Tempo e Estudos Climáticos
- UNIR
Universidade Federal de Rondônia
- USA
United States of America
- UK
United Kingdom
- SISBio
Remote service platform of the Chico Mendes Institute for Biodiversity Conservation
- CY
Cyanobacteria
- AQ
Average quantification of cyanotoxins (μg L−1)
- DA
Applied determination average
- MN
Micronucleus
- MNBC
MN test with cytokinesis blockade
- MEV
Minimum expected value of cyanobacteria blooms volume
- S
No ecotoxicological risk
- PR
Low ecotoxicological risk
- ELISA
Enzyme Linked ImmunonoSorbent Assay
- (d)
Margalef richness index
- (H′)
Shannon-Wiener diversity
- IBP
Dominance index
- (I)
Species similarity
- D
Simpson's index
- NR
Medium ecotoxicological risk
- RG
severe ecotoxicological risk
- QL
Quantification limit (μg L−1)
- F20
Fish farm number 20
- TSI
Trophic state index
Some physicochemical parameters
- pH
Hydrogenionic potential
- °C
Degree Celsius
- N–NH4
total ammonia
- NO31⁻
Nitrate
- NO21⁻
Nitrite
- PO₄³⁻
Phosphate
- P
Phosphorus
1. Introduction
Rondônia state is the largest farmer of native fish in Brazil, corresponding to a total of 57.2 thousand tons of fish produced in year 2022 [1] the main cultivated species being tambaqui (Colossoma macropomum). In fish farms, water quality is a constant concern, because when it is of poor quality, decreases in productive performance and fish mortality may occur [2], due to parasitic infestations and other environmental factors. These factors reduce the production and profitability of fish farmers [3]. Through this, fish farms perform the function of an artificial aquatic ecosystem, where the abiotic and biotic conditions can be partially manipulated [4], and also harbor a diverse biotic community, from primary producers to producers secondary and decomposers [2].
The phytoplankton community is described as a diverse group of photosynthetic or non-photosynthetic beings found in marine environments, freshwater, brackish water, soils, among others [5]. Phytoplankton plays an important role in primary production in the aquatic ecosystem, encompasses a diverse community at taxonomic, morphological and physiological levels, which have specific requirements and responses to physical and chemical variables, such as light, temperature, alkalinity, nutrient concentration, pH, oxygen etc. [6]. This community produces about 50–95% of the dissolved oxygen in the fish ponds, however, the plankton consumes about 50–80% of the dissolved oxygen in respiratory processes, during the night and in periods of reduced solar incidence. A balance between photosynthesis and respiration is a prerequisite for maintaining a constant chemical composition of freshwater [2].
The artificial enrichment of freshwater bodies promotes the excessive growth of microalgae and aquatic plants, systematically resulting in the death of fish, due to the deficit of dissolved oxygen in the water and the synthesis of cyanotoxins in cases of cyanobacteria blooms [7]. The assimilation of ammonia, nitrate and phosphorus by phytoplankton can lead to uncontrolled multiplication of cyanobacteria, which causes blooms in the aquatic environment, which if they are originated by certain species of cyanobacteria that are capable of releasing potentially toxic compounds in the water, can generate difficulties in the water treatment [8]. Generally, monitoring the physical, chemical and biological conditions is important, although the microalgae classifications, with their fluctuations in space and time, are essential for identifying favorable times for cyanobacteria blooms and the concentration of toxins in the freshwater [9].
Temporal and spatial analyzes of cyanobacteria are complex due to the various abiotic factors with which this community interacts. The physiological properties of planktonic species, associated with their qualitative and quantitative compositions, constitute fundamental information in the survey of aquatic communities, which is important in environmental diagnoses [10]. Due to their significant biodiversity, short life cycle, as well as the potential to respond to physical, chemical and biological variables in the environment, cyanobacteria can present a variation in abundance and species composition, both spatially and seasonally, and also, a coexistence of several taxa in the same water body [11].
The increase in nutrients, especially nitrogen and phosphorus, for example, fish farming surplus feed, or even organochlorines from pesticides, can lead to eutrophication processes and poor environmental conditions for biological communities [12]. Such conditions can favor cyanobacteria blooms, altering the smell, color, taste and dissolved oxygen content, which can also generate toxicity [13]. Dominance by cyanobacteria leads to a reduction in plankton biodiversity, and worse, it can lead to health problems in fish farming, such as free-living fungus blooms in the freshwater, due to the potential that these microorganisms can synthesize toxins [14]. Due to the degradation of water resources caused by human activity [15], especially due to the intensification of production (increased storage density) and agro-industrial activities [10,16]. Therefore, the management of water resources is essential to guide decision-making aimed at sustainability.
The scarcity of studies on the structure and dynamics of phytoplankton in artificial ecosystems such as fish ponds, highlights the need for research in this area of Science. In order to provide subsidies for actions of conservation and preservation of these environments. Basic knowledge about freshwater quality is essential for fish farmers, because the lack of information contributes to the decline in production quality. Consequently, the accumulation of organic and metabolic waste generated during the production process will be inevitable. Minimizing this accumulation of waste is a challenge, by successfully reducing the degradation of water quality, it is possible to obtain better fish performance and greater productivity. Given this overview, research focused on the dynamics of aquatic ecosystems and their water quality are of crucial importance for fish farming, since all factors act in an interconnected way. Therefore, the monitoring of cyanobacteria makes it possible to know in advance critical periods for cultivation. This information makes it possible to establish efficient management strategies, either by increasing or reducing the flow of water renewal, changing the crop supply and drainage system, use of mechanical aerators in periods of low and/or absence of photosynthesis, among others. Given the context presented, it is expected that the abundance and density of cyanobacteria and their blooms and cyanotoxins can be used as bioindicators of water eutrophication due to effluent pollution and ecotoxicological risk in fish ponds water.
Therefore, the aimed of this study aimed to evaluate the physicochemical parameters of fish farm water, abundance and density of cyanobacteria, determine their blooms and the ecotoxicological risk of their cyanotoxins in fish ponds water.
Prior to this study there was no justification for constant monitoring of cyanotoxins. In this way, this research serves to instigate future research on the risk of cyanotoxins for the health of aquatic organisms and the consumer (human being) and update of environmental legislations.
2. Material and methods
2.1. Study area and climate
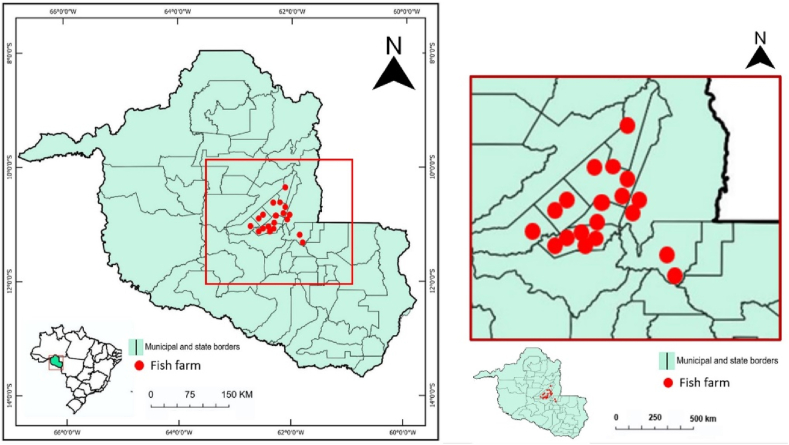
The study was conducted out in 20 fish farms in the municipalities of Ji-Paraná, Ouro Preto do Oeste, Presidente Médici, Urupá and Teixeirópolis, which belong to the Central-Eastern Microregion of the Rondônia state, Brazil (Fig. 1). More specifically, sample collections were carried out in the two Amazonian hydrological seasons, rainy (months from September 2021 to April 2022) and dry (months from May to August 2022). On average, the fish farms adopted a semi-intensive raised system (a maximum of 0.6 kg per m2 year−1 with an annual cycle), covered up to 3 ha of freshwater depth, distributed in semi-dug ponds, with individual areas that do not exceed 0.5 ha, with an average depth of 1.60 m, which are intended for farmed tambaqui (Colossoma macropomum Cuvier, 1818). It is worth mentioning that the fish farms showed their ponds built on the riverbed, that is, in the lower parts of the relief.
Fig. 1.
Geographic location of sampled fish farms in Rondônia state, Brazil.
Concerning the climate of Rondônia state is classified in the Köppen system as predominant type Am - Rainy Tropical Climate [17]. The rainfall data during the development in the current study were obtained at the Instituto Nacional de Pesquisas Espaciais (INPE) [18], at Centro de Previsão do Tempo e Estudos Climáticos (CPTEC), in Ouro Preto do Oeste city, Rondônia state, Brazil.
2.2. Data collects
Samples were collected at two hydrological seasonals, rainy and dry. Therefore, the 20 fish farms were visited twice, with phytoplankton collected in triplicate. The experiment was developed in a completely randomized factorial design 20 × 3 x 3 (20 fish farms, 3 ponds and 3 replications). Sampling was carried out at three points in each fish farm, in the pond further upstream of the river, in the drainage pipe (drainage) and in the water column in the middle of the other two fish ponds. The selection of sampling points, the supply of fish ponds was taken into account. In the current study, the suggestion by Costa et al. [19], the fish ponds, in all fish farms, supplied in an interconnected way, in which the supply dam supplies freshwater to the first fish pond and, from then on, the freshwater from one fish pond supplies the other. So the water contained in the last fish pond passed through all previous fish ponds. Based on this context, sampling took place in alternate ponds.
It was compared to qualitative samples, horizontal and vertical drags were carried out on the water surface. While each quantitative sample was obtained in a plankton net (50 μm mesh opening), with the aid of a graduated bucket. In the current study, the count focused on cyanobacteria, the samples were immediately stored in Polyethylene Terephthalate bottles, which were kept at 7 °C in cooler-type thermal boxes and were sent to the laboratory.
2.3. Physicochemical analysis in fish ponds
Physicochemical analyzes of the fish ponds water were carried out up to 40 cm deep and measured in situ. With a multiparametric probe model AT155 (Quality Analyser, USA), the variables hydrogenionic potential - pH, dissolved oxygen (mg L⁻1) and temperature (°C) were verified. While with the Secchi disk, the water transparency (cm) was verified. In the laboratory, total ammonia (N–NH4), nitrate (NO31⁻), nitrite (NO21⁻), phosphate (PO₄³⁻), phosphorus (P), alkalinity (mg L⁻1 CaCO3) and water hardness (mg L⁻1 CaCO3) were verified. For the framing of the analyzed parameters, CONAMA Resolution No. 357/2005 [20] was used for freshwater bodies intended for fish farming (Class 2).
2.4. Quantification and qualification of cyanobacteria
Cyanobacteria were identified directly, immediately after collection. These analyzes were performed with the aid of a Trinocular Stereoscopic Microscope (Sigma, USA) with 10 × magnification and equipped with a digital camera. A total of 9 slides were read per sample (a drop of sedimented material placed between slide and coverslip). The visualized cyanobacteria were compared with illustrations and descriptions from the specialized bibliography that indicates the taxonomic keying of freshwater microalgae [[21], [22], [23], [24]]. From the identifications, abundance was calculated and the relative abundance expressed in the frequency (F%) of occurrence [25]. In addition, the classification categories constant (>50%), accessory (25 ≥ 50%) and rare (≤25%) taxa were considered [26]. Finally, based on the identifications, they were also expressed in density (cells mL−1) and calculated according to the formula described in Weber [27]. CONAMA Resolution No. 357/2005 [20] establishes the maximum density of 50 × 103 cells mL−1 for aquatic environments intended for aquaculture and fishing activities.
Biological indices were determined, such as the Margalef richness index (d) [28], Shannon-Wiener diversity (H′) [29], dominance (IBP) and similarity (I) [30]. Data were processed using the PAST program, version 2.14. Species richness index was calculated using the following Equation (1). Species diversity index was calculated using the Shannon-Weaver index [31]. This index assumes that each individual is randomly collected from an infinitely larger population and that all species are represented in the sample [31].
| R (S-1)/log N | (1) |
Where, R = species richness, S = number of species, N = number of individuals.
Equitability index or the uniformity in the distribution of individuals among the species was verified through the value of the Pielou equitability index (J), calculated from the Shannon index, given by Krienitz et al. [29] formula, Equation (2) [29].
| E = H'/ H'max | (2) |
Where, E = Evenness; H' = Shannon-Wiener diversity and H'max = maximum H’.
Simpson's dominance index adapted by Oksanen et al. [32] assigns greater importance to common species and is given by Ref. [32] Equation (3).
| D = Σ[ni(ni-1)/N(N-1)] | (3) |
Where, D = Simpson's index; ni = number of individuals of species i in the sample; N = total number of individuals.
2.5. Records of cyanobacteria blooms and potential ecotoxicological risk
As suggested by Brasil et al. [22], every count >300 cells of the same cyanophyte species on the same slide can be considered as a case of cyanobacteria blooms. The blooms were considered in the two hydrological seasons, and the analyzes carried out from suspects, water with a very greenish, bluish and red-brown color. Numerical data from records and species identifications were tabulated and class intervals were defined in the evaluation of cyanobacterial (cells mL−1), according to the methodology adopted by Fonseca et al. [33]. Based on the counts, the formula by Aimi et al. [34] for cases of cyanobacteria blooms [34], Equation (4)
| MEV = 33.33 (CY)/10 × 103 | (4) |
Where, MEV is the minimum expected volume; CY is the total number of identified cells mL−1 of cyanobacteria.
The ecotoxicological risk classification was suggested by Merel et al. [35], <1 g L⁻1 no risk (s), 1.0–1.9 g L⁻1 low risk (PR), >2 g L⁻1 medium risk (NR) e > 3 2 g L⁻1 severe risk (RG), for aquatic organisms (fish and aquatic invertebrates) and serious risk for mammals (including humans).
To further support the ecotoxicological risk, the micronucleus test (MN) and other abnormalities associated with blood cells, was carried out in the peripheral blood of the farmed fish, for conditions with and without cyanobacteria blooms, in addition to seasonal factors, rainy and dry. A total of 3 specimens of tambaqui was fished with rod and hook per fish ponds sampled in the plankton collection. In total, the blood cells of 60 tambaqui were analyzed. For this procedure, the proposal was first submitted to the Instituto Chico Mendes de Conservação da Biodiversidade (SisBio platform), and received approval with protocol No. 84820–1. The study was also submitted to the Ethics in Animal Use Committee, of the Universidade Federal de Rondônia, Brazil. This proposal was approved, under protocol No. 84820–1/UNIR/2021.
Immediately after capture, the tambaqui were immobilized with the use of hands and with nitrile gloves and on a wet tray with water, 10 μL of peripheral blood was collected through puncture of the caudal vein (at 45° on its ventral side). The blood was dripped directly onto the slides, the smears were conducted in duplicate for each animal. Immediately, after blood collection, the tambaqui were returned to the fish ponds. The biological material was sent to the laboratory, to a period of 12 h at room temperature. Then, the slides were hydrated for 5 min in distilled water and subsequently stained using the Quick Panotic LB kit. This kit consists of three containers, 0.1% Triarylmethane, 0.1% Xanthenes and 0.1% Thiazines, the slides were immersed 30 times in each container with submersion of 1 s in duration in the sequence described above according to Meneguetti et al. [36]. Subsequently, the slides were washed in deionized water at pH 7.0 and dried at room temperature as described by Meneguetti et al. [36].
On each slide, 2 thousands erythrocytes per fish were counted, and the frequencies of erythrocytes with abnormalities were recorded. The frequencies of MN, cells undergoing apoptosis, pyknosis, karyorrhexis, necrosis, nucleoplasmic bridge, binucleated cells - were in mitosis, cells in interphase cell budding, according to the methodology of Silva et al. [37]. These records were performed using a Trinocular Stereoscopic Microscope (Sigma, USA) through a 100× objective in oil immersion.
2.6. Maximum allowed value of cyanotoxins in fish ponds
The World Health Organization (WHO), after several opinions with researchers from different countries, decided to edit a specific “Guideline” for cyanotoxins in public supply freshwater, in which a quantification limit (QL) of 1.0 μg L⁻1 was established, as the maximum acceptable concentration for daily human oral consumption. While Ordinance No. 518 of March 25, 2004/ANVISA [38], presents a rule that provides for procedures and responsibilities inherent in the control and surveillance of the water quality for human consumption, establishes its potability standard and provides other measures, makes several references and recommendations regarding total cyanotoxins, such as a sum of microcystin toxins, cylindrospermopsin, saxitoxins and others.
Adopting the quantification methodology of Fonseca et al. [33] adapting to the MEV suggested by García-Neto et al. [39], which uses the MEV formula relating to spatial and seasonal factors to find the determined average quantification (AQ) of cyanotoxins (μg L⁻1), the limit was applied determination average (DA) of 1.0 μg L⁻1 for the recorded cyanobacteria blooms.
2.7. Experimental design, database and statistical analysis
To determine the variation in observation of cyanobacteria in the stereomicroscope, the counts were performed twice, in different hydrological seasons (rainy and dry), by the same individual observer.
The data obtained were stored and organized in the Epi info™ software, version 3.5.3–2011 (OS: MS-Windows, C Sharp programming language). These data were submitted to descriptive statistical analysis, averages (μ) and standard deviation ± SD (σ) in addition to variance analysis averages.
The factors water quality, relative abundance of cyanobacteria families, seasonal variation, spatial variation and ecototoxicological risk (potential for cyanotoxin synthesis) were statistically analyzed using Pearson's correlation test, only correlations of p < 0.05 were considered significant. To compare the means of water quality variables and the MN test and associated abnormalities in the conditions with and without cyanobacteria blooms, in addition to seasonal factors, rainy and dry, Student's t-test was applied, with 5% significance.
All statistical analysis were performed using RStudio Development Core Team, version 3.5.3.
3. Results
3.1. Climatic conditions and water quality
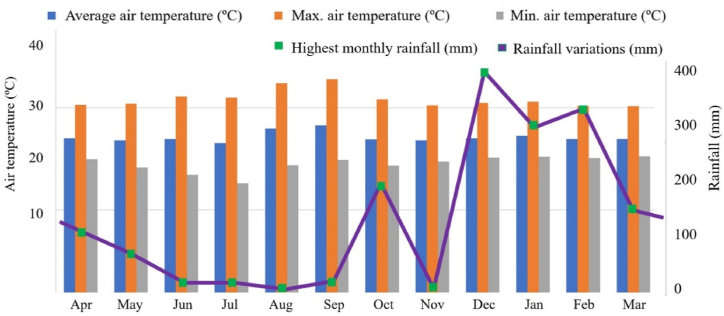
The climatological average of the air temperature, during the coldest month, exceeds 18 °C (megathermal) and a well-defined dry season, when there is a moderate water deficit with rainfall indices below 50 mm month⁻1. The average annual rainfall varied between 1400 and 2600 mm year⁻1, while the monthly average air temperature varied between 24 and 26 °C (Fig. 2). Regarding meteorological data, the months of October 2021 (200 mm), December 2021 (189.50 mm), January 2022 (336.05 mm) and February 2022 (359.66 mm), showed the highest precipitation averages. While the months of August and September 2021 showed the highest average temperatures, >35 °C, respectively. There were no significant variations in the other months (Fig. 2).
Fig. 2.
Monthly averages of rainfall (mm) and air temperature in the interior of Rondônia state, in the different hydrological seasons (rainy and dry), in the years 2021 and 2022.
Regarding the parameters of water quality, with cyanobacteria blooms, there was a statistical difference (p < 0.05) between rainy and dry seasons. Higher averages of dissolved oxygen (6.13 mg L⁻1), water transparency (40.02 cm), nitrate (0.35 NO31⁻), nitrite (0.15 NO21⁻), alkalinity (44.55 mg L⁻1 CaCO3) and water hardness (50.10 mg L⁻1 CaCO3) in the rainy season. While higher averages of phosphate and phosphorus were found in the dry season. Concerning water quality parameters, without cyanobacteria blooms, there was statistical difference between rainy and dry seasons. Higher averages of dissolved oxygen, transparency, nitrate, nitrite, alkalinity and hardness were found in the rainy season. While higher averages of pH and phosphorus were found in the dry season (Table 1).
Table 1.
Water quality averages in fish ponds with and without cyanobacteria blooms at different hydrological seasons, rainy and dry.
| Variables | With blooms |
Without blooms |
General averages |
p value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Rainy | Dry | p value | Rainy | Dry | p value | Rainy | Dry | ||
| Temperature (°C) in situ | 27.17 ± 1.36a | 29.33 ± 1.47a | *ns | 27.60 ± 1.38a | 29.03 ± 1.45a | *ns | 27.39 ± 1.37a | 29.18 ± 1.46a | *ns |
| pH | 6.67 ± 0.45a | 7.01 ± 0.47a | *ns | 6.02 ± 0.40b | 7.33 ± 0.49a | 0.0345 | 6.35 ± 0.43a | 7.17 ± 0.48a | *ns |
| Dissolved oxygen (mg L⁻1) | 6.13 ± 0.44a | 3.00 ± 0.22b | 0.0140 | 6.00 ± 0.43a | 3.10 ± 0.22b | 0.0125 | 6.07 ± 0.44a | 3.05 ± 0.22b | 0.0120 |
| Water transparency (cm) | 40.02 ± 5.60a | 32.29 ± 4.52b | 0.2550 | 49.40 ± 6.92a | 36.67 ± 5.13b | 0.0275 | 44.71 ± 6.26a | 34.48 ± 4.83a | *ns |
| Nitrate (NO31⁻) | 0.35 ± 0.08a | 0.15 ± 0.03b | 0.0330 | 0.30 ± 0.07a | 0.15 ± 0.03b | 0.3565 | 0.33 ± 0.07a | 0.15 ± 0.03b | 0.03825 |
| Nitrite (NO21⁻) | 0.15 ± 0.04a | 0.05 ± 0.01b | 0.0440 | 0.10 ± 0.03a | 0.05 ± 0.01b | 0.0422 | 0.13 ± 0.03a | 0.05 ± 0.01b | 0.04334 |
| Total ammonia (mg L−N-NH4) | 0.10 ± 0.03a | 0.13 ± 0.04a | *ns | 0.10 ± 0.03a | 0.13 ± 0.04a | *ns | 0.10 ± 0.03a | 0.13 ± 0.04a | *ns |
| Phosphate (PO₄³⁻) | 1.00 ± 0.20b | 2.26 ± 0.45a | 0.0095 | 0.60 ± 0.12b | 1.55 ± 0.31a | 0.0099 | 0.80 ± 0.16b | 1.91 ± 0.38a | 0.0745 |
| Phosphorus (P) | 0.41 ± 0.10b | 0.90 ± 0.22a | 0.0388 | 0.22 ± 0.05b | 0.66 ± 0.16a | 0.0410 | 0.32 ± 0.08b | 0.78 ± 0.19a | 0.0444 |
| Alkalinity (mg L⁻1 CaCO3) | 44.55 ± 6.24a | 31.43 ± 4.40b | 0.0234 | 48.10 ± 6.73a | 37.64 ± 5.27b | 0.0287 | 46.33 ± 6.49a | 34.54 ± 4.83b | 0.0356 |
| Water hardness (mg L⁻1 CaCO3) | 50.10 ± 6.01a | 28.57 ± 3.43b | 0.0195 | 54.20 ± 6.50a | 34.85 ± 4.66b | 0.0208 | 52.15 ± 6.26a | 31.71 ± 3.80b | 0.0169 |
If there are different letters (a,b) between the columns, it is different (p < 0.05) by Student's t-test; *ns – not significant (p > 0.05).
Regarding the average total values of the rainy and dry seasons, there was a statistical difference (p < 0.05) between rainy and dry seasons. Higher averages were found in the rainy season (p < 0.05) of dissolved oxygen (6.07 mg L⁻1), nitrate (0.33 NO31⁻), nitrite (0.13 NO21⁻), alkalinity (46.33 mg L⁻1 CaCO3) and water hardness (52.15 mg L⁻1 CaCO3). While in the dry season, higher averages of phosphate (1.91 PO₄³⁻) and phosphorus (0.78 PO₄³⁻) were found (Table 1).
3.2. Biodiversity of cyanobacteria
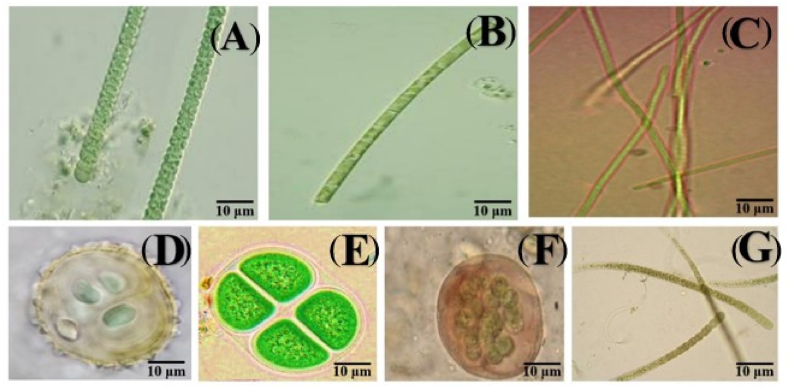
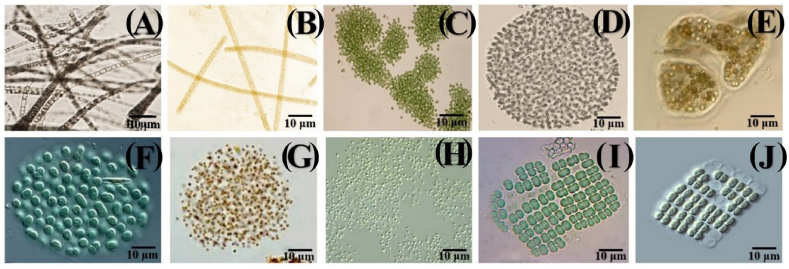
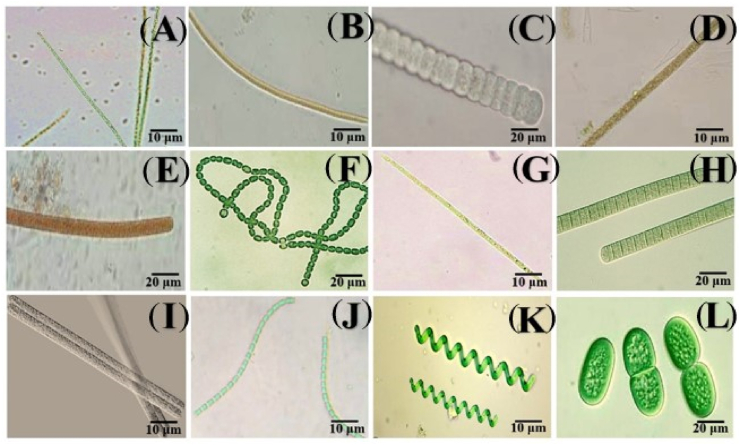
As illustrated in Fig. 3A – G, Fig. 4A – J and Fig. 5(A – L), a total of 15 families and 29 species of cyanobacteria were identified in the different hydrological seasons (rainy and dry). During the study, 1/29 (3.45%) constant, 7/29 (24.14%) common and 21/29 (72.41%) rare taxa were recorded. The species Microcystis aeruginosa was the constant taxon. Of the common taxa, the main species corresponding to the two seasons were Planktolyngbya limnetica, Planktothrix sp., Alkalinema sp., Microcystis flos-aquae, Oscillatoria sp. And Anabaena sp. As for the rare taxa, the corresponding species were Nodularia sp., Aulacoseira sp., Asterocapsa aerophytica, Gloeotrichia sp., M. wesenbergii, Aphanocapsa sp., A. planctonica, A. elachista, Merismopedia sp., M. punctata, Planktothrix planctonica, P. isothrix, P. rubescens, Cylindrospermopsis raciborskii, Tychonema bornetii, Pseudoanabaena mucicola, Spirulina sp. And Synechococcus sp. (Table 2).
Fig. 3.
Photomicrographs of cyanobacterial species of the families Aphanizomenonaceae: Nodularia sp. (A); Aulacoseiraceae: Aulacoseira sp. (B); Coleofasciculaceae: Geitlerinema sp. (W); Chroococcaceae: Asterocapsa submersa (D), Chroococcus turgidus (E), A. aerophytica (F); Gloeotrichiaceae: Gloeotricia sp. (G).
Fig. 4.
Photomicrographs of cyanobacterial species of the families Leptolyngbyaceae: Planktolyngbya limnetica (A), Alkalinema sp. (B); Microcystaceae: Microcystis aeruginosa (C), M. flos-aquae (D), M. wesenbergii (E), Merismopediaceae: Aphanocapsa sp. (F), A. planctonica (G), A. elachista (H), Merismopedia punctata (I) and Merismopedia sp. (J).
Fig. 5.
Photomicrographs of cyanobacterial species of the families Microcoleaceae: Planktothrix sp. (A), P. agardhii (B), P. planctonica (C), Planktothrix isothrix (D), P. rubescens (E); Nostocaceae: Anabaena sp. (F), Cylindrospermopsis raciborskii (G); Oscillatoriaceae: Oscillatoria sp. (H); Phormidiaceae: Tychonema bornetii (I); Pseudanabaenaceae: Pseudoanabaena mucicola (J); Spirulinaceae: Spirulina sp. (K); Synechococcaceae: Synechococcus sp. (L).
Table 2.
Inventorya of cyanobacteria and their respective abundances and densities (cells mL−1) in the different hydrological seasons (rainy and dry) in fish ponds water.
| Families | Species | Abundance |
Density |
||
|---|---|---|---|---|---|
| Rainy | Dry | Rainy | Dry | ||
| Apanizomenonaceae | Nodularia sp. (Mertens, 1822) | 0 | 2 | 0 | 1 |
| Aulacoseiraceae | Aulacoseira sp. (G.HK Thwaites, 1848) | 0 | 2 | 0 | 1 |
| Coleofasciculaceae | Geitlerinema sp. (K. Anagnostidis, 1989) | 0 | 95 | 0 | 64 |
| Chroococcaceae | Asterocapsa submersa (Komárek, 1993) | 4 | 5 | 3 | 3 |
| Chroococcus turgidus (Kützing) Nägeli, 1849 | 2 | 3 | 1 | 2 | |
| Asterocapsa aerophytica Lederer | 13 | 0 | 9 | 0 | |
| Gloeotrichiaceae | Gloeotrichia sp. (J.Agardh ex Bornet & Flahault, 1886). | 3 | 0 | 2 | 0 |
| Leptolyngbyaceae | Planktolyngbya limnetica (Lemmermann) Komarková-Legnerová et Cronberg | 146 | 383 | 98 | 257 |
| Alkalinema sp. (M. G. M. V. Vaz, 2015) | 42 | 5 | 28 | 30 | |
| Microcystaceae | Microcystis aeruginosa (Kützing, 1846) | 531 | 1041 | 356 | 697 |
| Microcystis wesenbergii (Komárek) Komárek ex Komárek, 2006 | 0 | 2 | 0 | 1 | |
| Microcystis flos-aquae (Wittrock) Kirchner, 1898 | 0 | 93 | 0 | 62 | |
| Merismopediaceae | Aphanocapsa sp. Nägeli | 0 | 13 | 0 | 9 |
| Aphanocapsa planctonica (G.M.Sm.) Komárek & Anagn | 5 | 0 | 3 | 0 | |
| Aphanocapsa elachista (West & G.S.West 1894) | 0 | 10 | 0 | 7 | |
| Merismopedia punctata (Meyen, 1839) | 4 | 0 | 3 | 0 | |
| Merismopedia sp. (Meyen, 1839) | 0 | 2 | 0 | 1 | |
| Microcoleaceae | Planktothrix sp. (Anagnostidis & Komárek, 1988) | 165 | 356 | 111 | 239 |
| Planktothrix agardhii (Gomont) Anagnostidis & Komárek | 0 | 237 | 0 | 159 | |
| Planktothrix planctonica (Elenkin) Anagnostidis & Komárek | 0 | 2 | 0 | 1 | |
| Planktothrix isothrix (Skuja) Komárek & Komárková | 0 | 7 | 0 | 5 | |
| Planktothrix rubescens (De Candolle ex Gomont) Anagnostidis & Komárek 1988 | 0 | 2 | 0 | 1 | |
| Nostocaceae | Anabaena sp. Bory de Saint-Vincent ex Bornet & Flahault, 1886 | 48 | 14 | 32 | 9 |
| Cylindrospermopsis raciborskii (Woloszynska) Seenaya & Subbaraju | 2 | 0 | 1 | 0 | |
| Oscillatoriaceae | Oscillatoria sp. (Vaucher ex Gomont, 1822) | 11 | 50 | 7 | 34 |
| Phormidiaceae | Tychonema bornetii (Zukal) Anagn. & Komárek Name | 7 | 0 | 5 | 0 |
| Pseudanabaenaceae | Pseudoanabaena mucicola (Naumann & Huber-Pestalozzi) Bourrelly, 1970 | 0 | 8 | 0 | 5 |
| Spirulinaceae | Spirulina sp. (Turpin ex Gomont, 1892) | 3 | 7 | 2 | 5 |
| Synechococcaceae | Synechococcus sp. (Nägeli, 1849) | 0 | 2 | 0 | 1 |
The families that showed the highest abundances were Microcystaceae, Leptolyngbyaceae and Microcoleaceae. The species that showed the highest abundances were Microcystis aeruginosa, Planktolyngbya limnetica, Planktothrix sp. And P. agardhii. The families that showed the highest densities (rainy and dry seasons) were Microcystaceae (356 and 760 cells mL⁻1), Leptolyngbyaceae (126 and 287 cells mL⁻1), and Microcoleaceae (111 and 405 cells mL⁻1). The species that showed the highest densities were M. aeruginosa (356 and 697 cells mL⁻1), P. limnetica (98 and 257 cell mL⁻1), Planktothrix sp. (111 and 239 cells mL⁻1), and P. agardhii (0 and 159 cells mL⁻1) (Table 2).
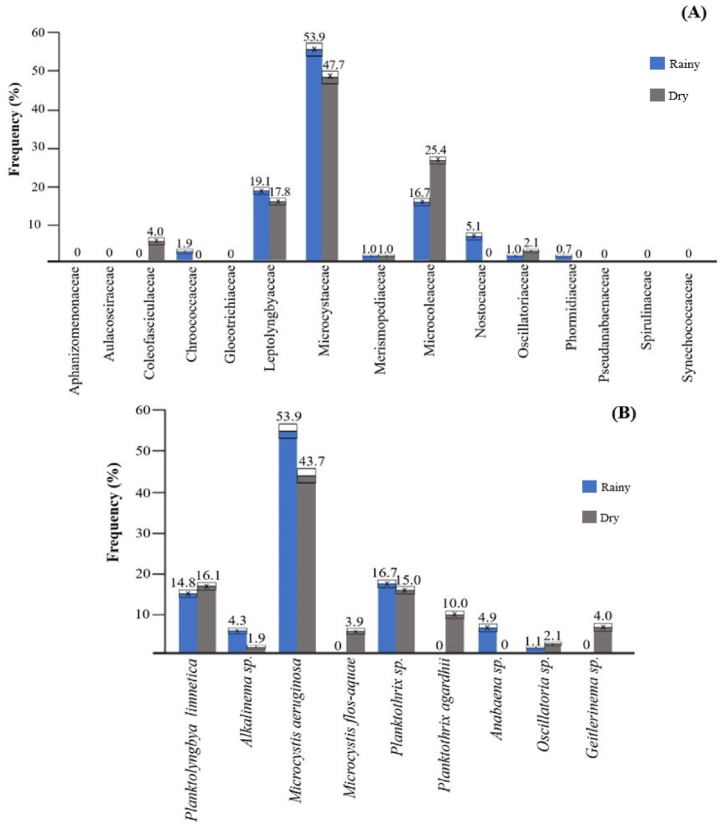
As shown in Fig. 6A, the families Microcystaceae (53.9 and 47.7%), Leptolyngbyaceae (19.1 and 17.8%) and Microcoleaceae (16.7 and 25.4%) contributed most to the relative abundance of cyanobacteria at the seasons. Species were highlighted: M. aeruginosa (53.9 and 43.7%) (Microcystaceae), Planktothrix sp. (16.7 and 15.0%) (Microcoleaceae) and P. limnetica (14.8 and 16.1%) (Leptolyngbyaceae). It is important to point out that the species P. agardhii was not recorded in the rainy season, although was found with 10.0% frequency in the dry season (Fig. 6B).
Fig. 6.
Relative abundance expressed in frequency (F%) of families (A) and species (B) of cyanobacteria.
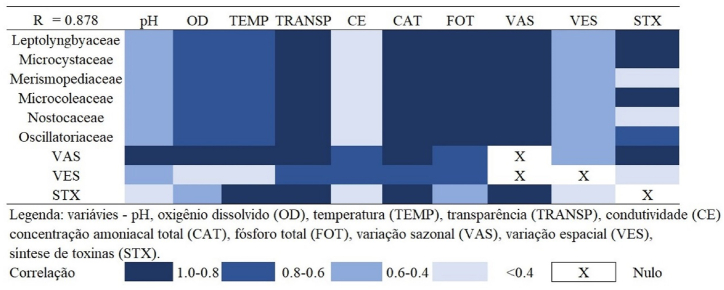
There was a highly significant correlation between the relative abundance (F%) of the cyanobacterial families and the variables dissolved oxygen, temperature, water transparency, total ammonia and total phosphorus. Seasonal variation expressed a highly significant correlation with physicochemical variables. Seasonal variation expressed a highly significant correlation with cyanobacterial families, as well as for cyanotoxin synthesis. While the spatial variation expressed highly significant correlation with water transparency, conductivity, total ammonia and total phosphorus. Finally, cyanotoxin synthesis expressed a highly significant correlation with temperature, water transparency, total ammonia and seasonal variation (Fig. 7).
Fig. 7.
Pearson's correlation coefficients of physicochemical variables, relative abundance of cyanobacteria families, seasonal variation, spatial variation and cyanototoxin synthesis.
There was no significant correlation between conductivity and cyanobacterial families, just as there was no significant correlation between spatial variation and dissolved oxygen and water temperature. Also, the potential for cyanotoxin synthesis did not express significant correlation with conductivity and spatial variation (Fig. 7).
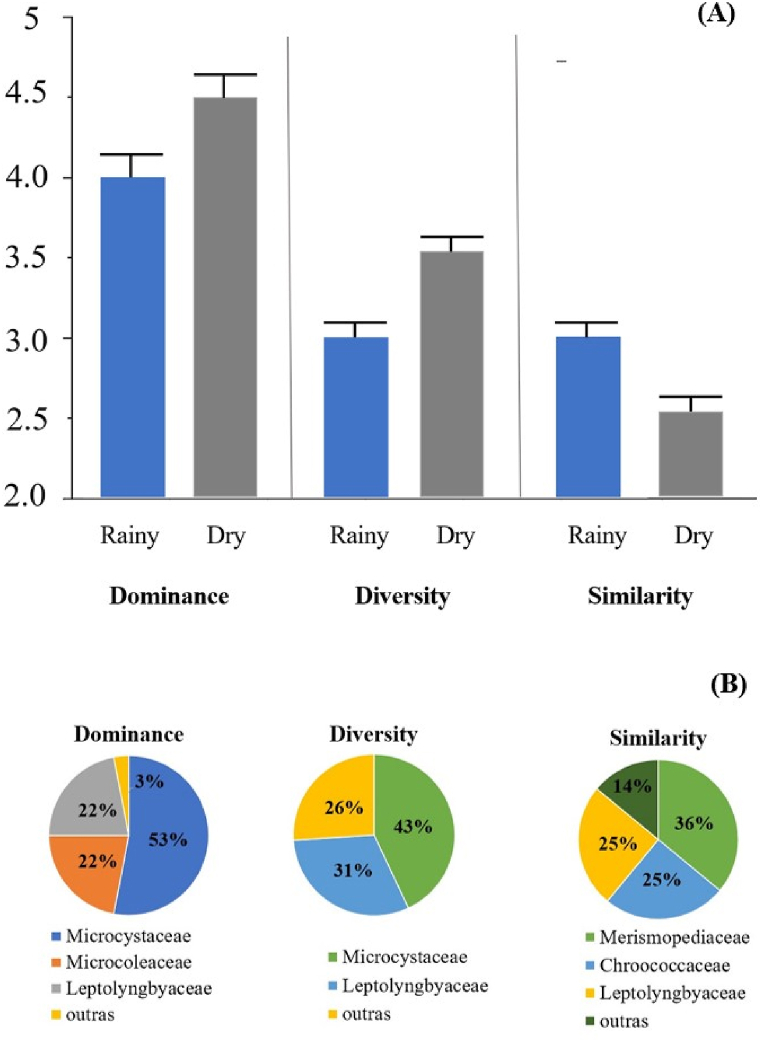
The cyanobacterial community showed higher levels of richness, diversity and dominance right after the rainy season, allowing the species coexistence. Consequently, there was less species similarity in the dry season (Fig. 8A). More specifically, the Microcystaceae family represented greater diversity (43%) and also dominance (53%). While the Merismopediaceae family represented greater similarity (36%) (Fig. 8B).
Fig. 8.
Biological indices diversity, dominance and similarity (A) and percentages per family of cyanobacteria (B).
3.3. Cyanobacteria blooms
During the study, cyanobacteria blooms of 6 species Microcystis aeruginosa, M. flos-aquae, Planktothrix sp., P. agardhii, Planktolyngbya limnetica and Oscillatoria sp. A total of 20 cyanobacteria blooms were recorded, 18 blooms in the dry season and only 2 blooms in the rainy season. The 2 species that bloomed the most, M. aeruginosa (8 blooms in the dry season and 1 bloom in the rainy), M. flos-aquae (6 blooms in the dry season) and a blooms of Oscillatoria sp. in the rainy season, in fish farm F20. Fish farm F1 recorded the highest number of species that bloomed simultaneously. In the dry season, at least one bloom of 4 different species was recorded, M. aeruginosa, M. flos-aquae, Planktothrix sp. And Planktolyngbya limnetica simultaneously (Table 3).
Table 3.
Cyanobacteria blooms recorded in ponds from fish farms water in different hydrological seasons, rainy and dry.
| Species | Fish farms |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | F11 | F12 | F13 | F14 | F15 | F16 | F17 | F18 | F19 | F20 | |
| Microcystis aeruginosa | D | D | D | D | D | D | D | R | D | |||||||||||
| Microcystis flos-aquae | D | D | D | D | D | D | ||||||||||||||
| Planktothrix sp. | D | D | ||||||||||||||||||
| Planktothrix agardhii | D | |||||||||||||||||||
| Planktolyngbya limnetica | D | |||||||||||||||||||
| Oscillatoria sp. | R | |||||||||||||||||||
Subtitle: R-cyanobacteria blooms recorded in the rainy season; D-cyanobacteria blooms recorded in the dry season.
Table 3, Table 4 showes cases of simultaneous cyanobacteria blooms and their respective densities, in which fish farms F1 and F4 exceeded the cyanobacteria density limit established by CONAMA Resolution No. 357/2005 by more than 10 times. The other fish farms recorded density below the maximum established for human consumption.
Table 4.
Density (cells mL−1) of cyanobacteria blooms in ponds from fish farms water.
| Espécies | Fish farms |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F4 | F5 | F6 | F8 | F10 | F13 | F15 | F16 | F19 | ||
| Microcystis aeruginosa | 297.33 × 103 | 480.00 × 103 | 12.00 × 103 | 12.00 × 103 | 0 | 4.00 × 103 | 28.00 × 103 | 30.67 × 103 | 0 | 0 | |
| Microcystis flos-aquae | 132.00 × 103 | 16.00 × 103 | 18.67 × 103 | 12.00 × 103 | 0 | 0 | 0 | 0 | 29.33 × 103 | 8.00 × 103 | |
| Planktothrix sp. | 49.32 × 103 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Planktothrix agardhii | 0 | 0 | 0 | 0 | 4.00 × 103 | 0 | 0 | 0 | 0 | 0 | |
| Planktolyngbya limnetica | 52.00 × 103 | 0 | 0 | 0 | 4.00 × 103 | 0 | 0 | 0 | 0 | 0 | |
| Oscillatoria sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total sum* | 530.65 × 103 | 496.00103 | 30.67103 | 24.00103 | 8.00103 | 4.00103 | 28.00103 | 30.67103 | 29.33103 | 8.00103 | |
| CV (%)** | 6.50 | 5.99 | 17.10 | 4.40 | 4.80 | 5.00 | 5.40 | 8.30 | 9.60 | 8.00 | |
Subtitle: Cyanobacteria density limit established by CONAMA Resolution No. 357/2005, 50.0 × 103 cells mL−1; *Total sum per fish farming unit (which in turn is the sum of the 3 fish ponds); **coefficient of variation between densities per fish ponds.
When analyzing the cyanotoxin synthesis potential of the two rainy season blooms, none showed an ecotoxicological risk. However, all dry season blooms showed some significant ecotoxicological risk. More specifically, the cyanobacteria blooms of Microcystis aeruginosa, M. flos-aquae, Planktothrix sp in fish farms F1 and F4, expressed potential toxicological risk even for mammals (including humans) (Table 5; Fig. 9).
Table 5.
Potential ecotoxicological risk in relation to the synthesis of total cyanotoxins from cyanobacteria blooms.
| Species | Fish farms |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F4 | F5 | F6 | F8 | F10 | F13 | F15 | F16 | F17 | F19 | F20 | |
| Microcystis aeruginosa | 22.3RG*# | 36.0RG*# | 0.9S | 0.9S | 0 | 0.3S | 2.1NR* | 2.3NR* | 0 | 0.7S | 0 | 0.3S |
| Microcystis flos-aquae | 9.9RG*# | 1.2PR* | 1.4PR* | 0.9S | 0 | 0 | 0 | 0 | 2.2NR* | 0 | 0.6S | 0 |
| Planktothrix sp. | 3.7RG*# | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Planktothrix agardhii | 0 | 0 | 0 | 0 | 0.3S | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Planktolyngbya limnetica | 3.9RG*# | 0 | 0 | 0 | 0.3S | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Oscillatoria sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.2S |
Subtitle: 0 = there was no cyanobacteria blooms; <1 g L−1 no risk (s), 1.0–1.9 g L−1 low risk (PR), >2 g L−1 medium risk (NR) e > 3 2 g L−1 severe risk (RG); *represents a risk to aquatic organisms (fish and aquatic invertebrates); #represents that there is a risk to the health of mammals (human beings); Blue coloring represents the rainy season; Green coloring represents dry season.
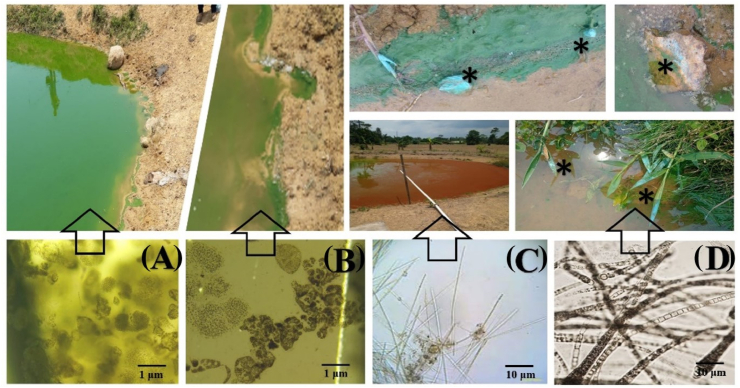
Fig. 9.
Cyanobacteria blooms in fish ponds water, Microcystis flors-aquae (A), M. aeruginosa (B), Planktothrix sp. (C) And Planktolyngbya limnetica (D). Caption: arrows indicate fish ponds with recorded cyanobacteria blooms and *asterisk indicates bluish slime characteristic of P. limnetica.
3.4. Micronucleus test (MN) and other associated abnormalities in fish blood
Concerning the biological analysis performed using the MN test as a way to assess mutagenicity in blood cells, Fig. 10 showes some types of abnormalities, such as the presence of MN (A), cells undergoing apoptosis (B) and pyknosis (C) and cells with nucleoplasmic bridge (D), for conditions with and without cyanobacteria blooms, in addition to seasonal factors, rainy and dry.
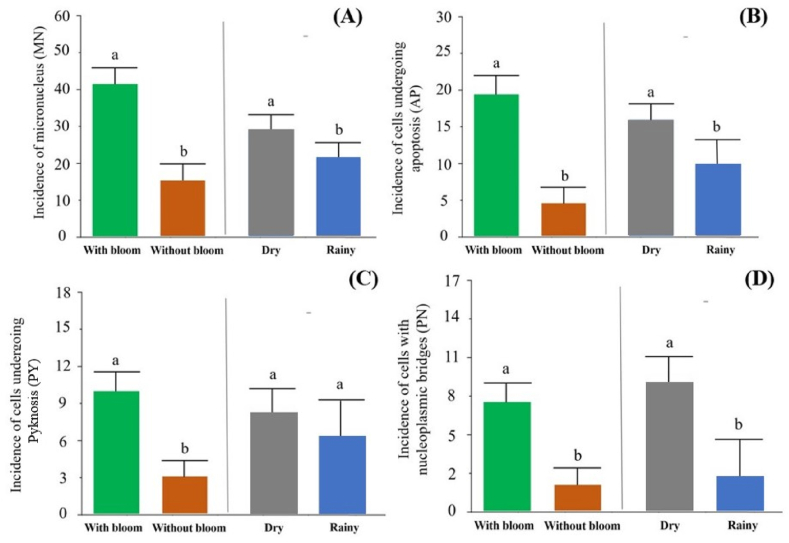
Fig. 10.
Incidences of abnormality in tambaqui (Colossoma macropomum) blood cells, micronucleus (MN) (A), cells undergoing apoptosis (B) and pyknosis (C) and cells with nucleoplasmic bridge (D) (Statistical comparison, Student's t-test, p < 0.05).
The incidence of MN was higher (43 abnormal cells per slide) in fish ponds water with cyanobacteria blooms, just as it was higher (31 abnormal cells per slide) in the dry season (p < 0.05) (Fig. 10A). The incidence of cells undergoing apoptosis was higher (18 abnormal cells per slide) in freshwater from ponds with cyanobacteria bloom, as well as it was higher (16 abnormal cells per slide) in the dry season (p < 0.05) (Fig. 10B). While the incidence of cells undergoing apoptosis was higher (10 abnormal cells per slide) in fish ponds water with cyanobacteria bloom, there was no difference (p > 0.05) between the rainy and dry seasons (8 and 6 abnormal cells per slide, respectively) (Fig. 10C). Finally, the incidence cells with nucleoplasmic bridges was higher (7 abnormal cells per slide) in from fish ponds water with cyanobacteria blooms, as well as higher (10 abnormal cells per slide) in the dry season (p < 0.05) (Fig. 10D).
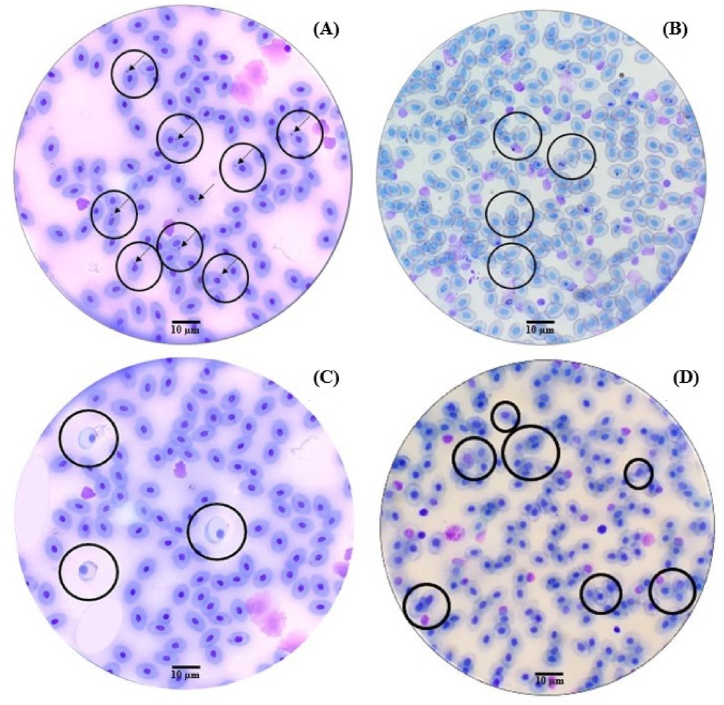
In total, averages of 78 abnormalities per slide were observed in fish ponds water with cyanobacteria blooms, 23 abnormalities in fish ponds without blooms, 65 abnormalities in the dry season, and 39 abnormalities in the rainy season. Fig. 11 illustrates through photomicrographs the mutagenic effects on tambaqui blood cells. Some types of abnormalities found are illustrated, such as the presence of MN (A), cells undergoing apoptosis (B) and pyknosis (C) and cells with nucleoplasmic bridge (D).
Fig. 11.
Photomicrographs of abnormalities observed in tambaqui (Colossoma macropomum) blood cells, micronucleated erythrocytes (A), cells undergoing apoptosis (B) and pyknosis (C) and cells with nucleoplasmic bridge (D).
3.5. Quantification limit (QL) of cyanotoxins
In the dry season, the expected volume of cyanotoxins in the ponds from fish farms F1 and F4 were above the quantification limit (>QL) established by Brazilian law. However, in the other fish farms the quantification limit was not exceeded (<QL) (Table 6). In the rainy season, none of the fish farms showed an expected volume of cyanotoxins above the quantification limit (<QL) established by Brazilian law (Table 7).
Table 6.
Expected volume for cyanotoxins and quantification limits in fish ponds with cyanobacteria blooms in the dry season.
| Fish farms* | DA** | QL*** |
|---|---|---|
| F1 | 13.27 ± 1.77 | >QL |
| F4 | 12.48 ± 1.42 | >QL |
| F5 | 0.76 ± 0.08 | <QL |
| F6 | 0.61 ± 0.07 | <QL |
| F8 | 0.20 ± 0.03 | <QL |
| F10 | 0.10 ± 0.01 | <QL |
| F13 | 0.70 ± 0.08 | <QL |
| F15 | 0.76 ± 0.08 | <QL |
| F16 | 0.73 ± 0.08 | <QL |
| F19 | 0.20 ± 0.03 | <QL |
| F20 | 0.17 ± 0.03 | <QL |
*Fish farms with records of cyanobacteria blooms (check Table 5) in the dry season.
**Determined average amount of cyanotoxins.
***quantification limit of <1.0 μg L−1 in relation to the average volume allowed by law, Ordinance No. 518 of March 25, 2004/ANVISA.
Table 7.
Expected volume for cyanotoxins and quantification limits in fish ponds with cyanobacteria blooms in the rainy season.
| Fish farms* | DA** | QL*** |
|---|---|---|
| F17 | <10−1 | <QL |
| F20 | <10−1 | <QL |
*Fish farms with records of cyanobacteria blooms (check Table 5) in the rainy season.
**Determined average amount of cyanotoxins.
***quantification limit of <1.0 μg L−1 in relation to the average volume allowed by law, Ordinance No. 518 of March 25, 2004/ANVISA.
4. Discussion
The abundance and density of cyanobacteria and their blooms can be used as bioindicators of water eutrophication due to pollution of industrial, agricultural and domestic effluents and ecotoxicological risk in fish ponds. Therefore, it is necessary to quantify cyanotoxins in fish ponds water constantly, to check the levels of toxicity, not only to check the water quality, although also the well-being of the farmed fish and whether they are contaminated by these cyanotoxins.
Despite the cyanobacterial blooms having influenced the physicochemical parameters of the water, even under blooming conditions, all remained suitable for the fish raised [40,41]. Therefore, the ecotoxicological risk showed in this study is not caused by water quality variables, confirming that the problem is caused by microbiological agents, that is, cyanobacteria and their cyanotoxins.
Regarding the cyanobacterial population is interrelated with physical, chemical and biological factors, in addition to being related to spatial and seasonal variation, as in the study by Pessoa et al. [26]. These factors are correlated with the action of hydrological pulses produced in the local water system, which may have a natural origin, such as precipitation, wind or anthropogenic origin, mainly due to the contribution of nutrients and freshwater drainage due to its various uses. Therefore, cyanobacterial communities have a potential for compositional change that can result in the addition, replacement, or removal of planktonic species. Changes in species richness or diversity, coexistence and dominance of certain planktonic species are direct results of variability in abiotic factors, long-term climate trends or short-term weathering variations, as well as downward control by zooplankton [42].
During the conduction of this study, there were not enough climatological pulses to cause resuspension of the sediment in the fish ponds, thermal stratification, as well as they were not enough for abrupt variations in the physicochemical parameters in freshwarer from fish ponds, nor to cause fish mortality. The recorded oscillations were expected for the two Amazonian hydrological seasons, which are very marked. In light of this, cyanobacteria blooms were attributed to their biodegradation and purification functions in the aquatic ecosystem, as they participate in the carbon and nitrogen cycle. That is, the cyanobacteria blooms occurred due to the introduction of xenobiotic pollutants. For example, fish ponds close to coffee and corn crops, common in the interior of Rondônia state [42], receive from the rainy through leaching, polluting agents from agriculture, such as residues of pesticides and drugs (medicines for use veterinarian, antibiotics, fungicides, acaricides, etc) [[43], [44]]. The conditions of fish ponds close to pig feedlots and chicken farms, also common in Rondônia state, can also be exemplified [[45], [46]], due to the same leaching effect mentioned above. Mainly runoff carries loads of significantly concentrated nutrients [47].
In addition to the examples cited above, there are several other contaminating factors, from industrial and domestic effluents close to fish farms. These factors are aggravated by the low rates of sewage in the urban areas of Rondônia state, Brazil [48]. Or an equally serious factor, are the environmental impacts caused by the mining areas, and many fish farms in Rondônia state are close to these mining areas, mainly in the Jamari Valley region, Rondônia state.
Analyzes of the physicochemical factors of the fresh water in fish ponds showed that it was suitable for farmed tambaqui (Colossoma macropomum) in excavated fish ponds [49]. Some authors corroborate how the results of the variables fluctuated seasonally, except for temperature. As expected, the highest critical variations were recorded in the dry season, especially for dissolved oxygen, transparency and phosphorus [50].
It is important to point out that almost all fish ponds were at some level of eutrophication, propitious environments for an increase in the density of cyanobacteria, the species Microcystis aeruginosa, M. flos-aquae, Planktolyngbya limnetica and Planktothrix sp. found deserve attention, with the number of cells varying (rainy and dry) between 531 and 1041, 0 and 93, 146 and 383, 165 and 356 cells mL−1, respectively, here in cases that were not considered cyanobacteria blooms (Table 5). They stand out for the considerable increase in the dry season, and with the beginning of the rains there is a reduction in the number of cells, and already in the beginning of the drought this density rises again. These cyanobacteria are common in eutrophic to hypereutrophic aquatic environments, often becoming dominant in relation to phytoplankton, in these cases they can synthesize microcystins and other cyanotoxins that affect the liver of aquatic organisms, which can cause acute intoxication [14]. At first, all cyanobacteria are considered toxic, and may cause intoxication to aquatic organisms and humans in cases of proven bioactive blooms. The predominance of toxic strains is related to several environmental factors, such as the competitive interrelationships between populations and the release of toxins related to the presence of some environmental stressor [51]. In view of this, the absence of specialized centers in the detection of cyanobacteria blooms makes it difficult to quantify the occurrence of blooms in fish farm waters, this set of factors compromises the quality of the fish grown and, consequently, human health.
CONAMA Resolution No. 357/2005 – Class II Water: Reservoirs, establishes maximum values for cyanobacteria density 50 × 103 cell mL−1. In relation to this, in the current study, only 10% of the fish farms were above the established in the dry season, although with a high ecotoxicological risk potential. The low percentage of density can be explained by the fact that fish farms are a lotic environment, due to the continuous renewal of freshwater in the ponds. The low residence time favors the dispersion and discrimination of the permanence of cyanobacteria [14]. The complexity of the emergence and establishment of cyanobacteria is still not fully understood. This phenomenon is influenced by several factors and one of the conditions that favor cyanobacteria blooms are high temperatures, caused by global warming [52]. Therefore, high levels of cyanobacteria density indicate the continuation of the eutrophic state and represent an important parameter for assessing water quality [49]. High density values of cyanobacteria were found by Messineo et al. [53], varying between 106 and 200 × 109 cells mL−1, and other studies in the Northeast region of Brazil, by Molica et al. [54], Bouvy et al. [55] and Vasconcelos et al. [56]. Almeida et al. [57] described in seven reservoirs in Southeast and Northeast Brazil, found 10 cyanobacterial genus with potential to synthesize cyanotoxins, including seven microcystin producers and the highest microcystin value was 1.032 μg L−1, which was above the limit established by law. Based on this information, Krienitz et al. [58] described Microcystis sp. And Planktothrix sp. As the main potential producers of cyanotoxins, which was confirmed in the current study.
Cyanobacteria are efficient in moving through the water column, as they have gaseous vacuoles or pseudovacuoles and a mucilage layer, which reduce their density, pushing them to surface layers, which optimizes the absorption of sunlight. These microorganisms are efficient in obtaining carbon dioxide for the photosynthetic process; and some species have the ability to fix molecular nitrogen and store phosphorus in the form of polyphosphate granules, optimizing the use of nutrients [59]. All these factors give a great competitive advantage in relation to other photoplanktonic groups. Cyanobacteria constitute one of the most important phytoplanktonic groups in eutrophic waters because they form blooms, which in most cases can be toxic [60].
Cyanobacteria blooms are enhanced by the dominance of the Planktolyngbya limnetica species. This occurs when the aquatic environment has alkaline pH and extreme temperature (very low 15 °C or very high above 30 °C), high concentration of nutrients (Nitrogen and Phosphorus, mainly) and water circulation [22]. Granéli et al. [61] and Vidal and Kruk [62] observed P. limnetica blooms in shallow and eutrophic reservoirs, in a tropical climate, with similar morphometry, low transparency and high concentration of nutrients. In all cases, the blooms provided blue mud on the due of the reservoirs, as found in the current study (Fig. 8). Granéli et al. [61] also state that P. limnetica blooms occur after heavy rains at night, the next day the blue mud appears. This characteristic slime is synthesized from the toxic bloom accompanied by Lyngbyatoxins B and C [35].
In Brazil and worldwide, cyanotoxins have been studied in reservoir water and for human consumption, mainly microcystins and saxitoxins. However, in fish farming water reports are scarce. Fonseca et al. [33] cite several studies in Brazil, reporting cyanotoxins at ecotoxicological risk levels in freshwater reservoirs. According to data collected by the same authors, the main species of cyanobacteria found were Planktothrix agardhii, Microcystis spp., Cilindrospermopsis raciborskii, Aphanizomenon gracile and Anabaena spp. The occurrence of microcystins has also been reported in several countries, such as Spain in reservoirs with levels between 0.055 and 1.032 μg L−1 [63]. Pawlik-Skowrońska et al. [64] described a high value of microcystin corresponding to 22.2 μg L−1 in a reservoir located in Lublin (Poland) and, Bláha et al. [65] described microcystin levels in 36.9 μg L−1 reservoir freshwater in Czechia. In other South American countries, on the La Plata River (Uruguay), Pirez et al. [66] detected a high microcystin value of 65 μg L−1.
The increasing eutrophication and dominance of cyanobacteria is reported in public freshwater supply reservoirs in Pernambuco [52], Paraíba [55] and Rio Grande do Norte states (Brazil) [66]. CONAMA Resolution No. 357/2005 limits total phosphorus to 30 μg L−1 for Class II lentic water ponds (reservoirs) - Brazilian classification of water bodies for human supply established by CONAMA. The permanent eutrophic condition and the frequent events of intense cyanobacteria blooms of potentially toxic are associated with multiple water uses, low water transparency, frequent sediment resuspension, high solar incidence in the water layer, long residence time and good availability of phosphorus and nitrogen [67]. In the current study, there was dominance of potentially toxic cyanobacteria, with bloom events. The limit allowed by law for the concentration of cyanotoxins is 1.0 μg L−1, however, in the dry season in two fish farms, values well above the allowed, of 13.27 and 12.48 μg L−1 (Table 6). However, in the rainy season no fish farming above the limit was recorded (Table 7). Vasconcelos et al. [55] reported the presence of microcystins above 1.0 μgL−1 in 55% of reservoirs in Paraíba state (Brazil) during the dry season and 20% during the rainy season, 15% of which showed concentrations below 1.0 μg L−1. Microcystin levels, by the ELISA method, were also found at levels above 1.0 μg L−1 by Spyros and Nikos [68] between 3.9 and 108 μg L−1, by Pírez et al. [66] 65.0 μg L−1 and by Sotero-Santos et al. [69] between 28.0 and 45.0 μg L−1 and all samples together with the co-occurrence of microcystins and saxitoxins Spyros and Nikos [68] and Costa et al. [70], in the São Paulo, Paraíba and Pernambuco states (Brazil).
Cyanobacteria blooms are strongly correlated with water quality and climatic conditions (seasonality) (Table 2). Therefore, the growth rate of these microalgae increases as temperature increases, surface light and nutrients increase due to higher rainfall [70]. Due to the greater amount of cyanobacteria, competition may be greater between phytoplanktonic species, a factor that can promote the appearance of fungal toxins and influence the increase in ecotoxicity [71,72]. Considering the influence of environmental variables on the distribution of microcystins, temperature may represent an important factor that can influence the production of cyanotoxins [73].
Regarding the physicochemical variables, in the current study there was a positive correlation between the variables of water quality and the levels of cyanotoxins, going against what was found by Fonseca et al. [33]. Because these authors found a positive correlation only for temperature and cyanotoxin levels. However, Asencio [63] found no correlation between environmental parameters (including water temperature, dissolved oxygen, conductivity and pH) with microcystin values or between high nutrient values (Phosphorus and Nitrogen) with microcystin values. According to Rigosi et al. [74], there is a probable synergistic effect between global warming and eutrophication, promoting increased production of cyanotoxins and cyanobacteria. Ekvall et al. [75] described, through an experiment, that water temperature and color (Humic acid and other nutrients) increase microcystin production, but when water color and temperature were acting alone, there was no increase in microcystin. Mowe et al. [76] demonstrate that high temperature together with high levels of Phosphorus often influence the rate of production of toxic strains rather than non-toxic strains of the genus Microcystis.
Among the species of greatest health concern found in this study were Microcystis aeruginosa, M. flos-aquae, Planktolyngbya limnetica and Planktothrix sp. Despite the prominent dominance of these species, the potential toxin-producing cyanobacteria coexisted in almost all analyzed periods, somehow allowing the overlapping of niches. One of the factors that most affects the reproduction and metabolic rate of cyanobacteria is the water temperature, which are generally favored by high incidence of light and temperatures above 20 °C. In the freshwater from fish ponds studied, temperatures remained high throughout the hydrological period, ranging 28.2° to 32.60 °C, conditions favorable to the occurrence of cyanobacteria blooms (Table 2, Table 4). Nitrogenous compounds as well as temperature are indispensable for the cyanobacteria blooms, which despite registering close to neutral (6–7.5) in all seasons studied, ammonia concentrations were not high (Table 2). Another important compound is Phosphorus, a limiting macronutrient for the cyanobacteria blooms, in the fish ponds the concentrations ranged 0.29–2.35 mg L−1, values well above those established by the CONAMA Resolution 357/2005, there was a prominent increase in the rainy season (1.61–2.35 mg L−1), favored by the natural leaching of the soil that occurs in the period, carrying phosphoric compounds that are deposited in the soil into the fish ponds (Table 2).
In the freshwater from fish ponds studied, the reproductive rates of cyanobacteria remained constant in the seasonal periods, with a small decrease in the reproductive process during the rainy, however not enough to comply with CONAMA Resolution No. 357/2005. Some studies in other regions of the world confirm this, Jardim et al. [77], Góis and Oliveira [78] among others. Another important piece of information, 80% (48/60) of ponds were classified by the trophic state index (TSI) as hypereutrophic, according to the classification of Cigagna et al. [79], whose TSI values were greater than 60, hypereutrophic environments are characterized by high concentration of nutrients, associated with algal and cyanobacteria blooms, with the presence of constant blooms. It should be noted that studies carried out in hypereutrophic ponds [80], resulted in suspension of fish farming and fish consumption in places with high concentrations of cyanobacteria, studies carried out in Rio de Janeiro city, it was found the presence of cyanotoxins found in fish meat higher than those recommended for human consumption according to the World Health Organization, which is an alarming and emergency data for public health.
Another component that can influence the rate of microcystin production is the intensity of sunlight. Typically, low levels of cyanotoxins are commonly found at low light intensities. However, high intensities of sunlight influence the transcription of genes associated with the synthesis of cyanotoxins [81]. Zilliges et al. [82] described a function of microcystins as binding peptides to intracellular proteins that act to protect against high levels of UVR, this factor can cause a negative effect of oxidative stress. In the current study, the highest cyanotoxin values, including the highest cyanobacteria blooms, occurred in the dry season. This information is confirmed by Lehman et al. [83], the authors described, in tropical reservoirs, cyanobacteria blooms with potential ecotoxicological risk, are more frequent in the dry season rather than the wet season (rainfall). These authors defined that the concentration of cyanotoxins increased when the abundance of the genus Microcystis increased in years with a more vigorous dry season. They also identified a seasonal threshold that was responsible for Micrcystis dominance, due to higher than normal water temperature, or even higher temperature variations.
According to Wörmer et al. [84], when higher levels of cyanotoxins occur in the rainy season, this corresponds to an annual cycle of lower rainfall. According to the same authors, it is very common not to have cyanotoxic blooms during the rainy season, due to the dilution caused by the abundant precipitation. However, another hypothesis is that it may have occurred in the freshwater, an accumulation of cyanobacteria only in the fish ponds sediment. However, sedimentation of cyanobacteria may represent a greater ecotoxicological risk, especially when these cells are resuspended and cyanotoxins are released. Furthermore, when cyanobacteria blooms occur, toxic strains can replace non-toxic strains and conversely the same thing happens [84]. Rinta-Kanto [85] described a high variability of cyanotoxic strains in populations of the genus Planktotrix and Microcystis ranging 0.01–100% and this may also influence the decrease in cyanotoxin levels, causing low levels of toxic strains in both Planktotrix and Microcystis [57].
As previously mentioned, cyanobacteria are photosynthetic prokaryotic microorganisms that have the ability to produce secondary metabolites with a highly toxic effect at a mutagenic level for living beings, called cyanotoxins. The presence of these microalgae in aquatic environments is initially due to the excess of nutrients, mainly derived from the excessive disposal of urban and industrial effluents and pollutants from agricultural activities [14]. Cyanobacteria produce endotoxin-type cyanotoxins, whose toxicity usually occurs when the toxin is released into the extracellular environment, as a result of cell lysis, due to aging or death of cyanobacteria [86]. Farmed fish such as tambaqui, the most farmed species in Rondônia state, are predisposed to cyanotoxins, because they are filter feeders that are confined in the freshwater of the fish ponds and feed on phytoplankton, a group to which cyanobacteria also belong.
Recently, there has been greater concern in relation to cyanobacteria blooms in fish farms, is the fact that these microalgae are potentially producers of toxins that can cause serious damage to mammals and various organisms of the aquatic biota. The most common cyanotoxins, the so-called microcystins [87], have the liver as their main target organ in vertebrates, since hepatocytes are able to capture them through Bile acid transporters [88]. However, there is still a lack of long-term toxicological studies of this toxin at sublethal doses in farmed fish.
Pesnya et al. [89] conducted a study to evaluate the cytotoxic, genotoxic and mutagenic effects of microcystin purified in the laboratory, from environmental samples collected in environments with recurrent by cyanobacteria blooms, with biomass of the species Microcystis aeruginosa. It was observed in the tests carried out with the strain, that in the concentrations and conditions to which the cells were exposed, the purified microcystin has mutagenic and genotoxic action and that its action may be related to the inhibition of the cell repair system and with a clastogenic on DNA. The same authors concluded that by using the micronucleus test with cytokinesis blockade (MNBC), performed with HepG2 and ZFL cell lines, a high rate of mutagenicity and genotoxicity could be observed, however, no significant results of genotoxicity were observed by the assay of comet. This information raises the alert for the need to create specific legislation to improve the control of water quality in fish farms, including the monitoring of cyanotoxins, with Brazil being the first country to establish such a measure. However, there is no legislation for cyanotoxin residues in fish meat. Therefore, experimental toxicological studies are an important tool in assessing the risks of these toxins for the human population.
According to the results in the current study and the classifications by Aimi et al. [34], on the targets of cyanotoxins. The species Microcystis aeruginosa, M. flos-aquae, Planktothrix sp. And P. agardhii blooms synthesize microcystins, classified as cyclic peptides, with the liver being the main target of the animal organism. While Planktolyngbya limnetica bloom synthesizes Lyngbyatoxin A, classified as an alkaloid, with the integument and gastrointestinal tract being the main targets of the animal organism. And finally, Oscillatoria sp. bloom synthesizes Aplisiotoxin, classified as an alkaloid, the tegument being the main target of the animal organism (Table 8).
Table 8.
Cyanotoxins targets by classification and by species.
| Species | Cyanotoxins | Classification | Target |
|---|---|---|---|
| Microcystis aeruginosa | Microcystins | Cyclic peptides | Liver |
| Microcystis flos-aquae | |||
| Planktothrix sp. | |||
| Planktothrix agardhii | |||
| Planktolyngbya limnetica | Lyngbyatoxin A | Alkaloids | Integument, gastrointestinal tract |
| Oscillatoria sp. | Aplisiotoxin | Integument |
The evidenced information confirms how adapted the cyanobacteria inventoried in the studied fish farms are, it is important to point out that the permanence of the observed populations occurs due to the ecological conditions of the system and also due to the few mitigating measures necessary for the functioning balance of these environments [90], which can compromise the quality of the fish that reaches the consumer's table. Amorim et al. [52] concluded that the increase in harmful cyanobacteria blooms in reservoirs and tropical production units can have serious impacts on biodiversity and the functioning of the aquatic ecosystem. This increase in cyanobacteria blooms is caused by a number of factors, such as global warming, longer droughts, eutrophication (freshwater pollution), etc. Amorim and Moura [91] indicate a potential control of cyanobacteria blooms to the biomanipulation of submerged aquatic macrophytes. Because they contribute to the structuring of aquatic ecosystems. The same applies to the zooplanktonic fauna, whose group is capable of controlling the population of cyanobacteria. Given the reality showed, mitigation measures need to be adopted to increase the efficiency and conservation of freshwater resources in reservoirs and fish farms, ensuring quality water [34].
Ordinance Ministério da Saúde of Brazil No. 2914, published on December 12, 2011, is the current legislation to meet the requirements of effective monitoring of cyanobacteria and cyanotoxins in water for human consumption. The ordinance in force requires that those responsible for controlling the water quality in supply systems maintained by surface springs monitor cyanobacteria at the water collection point on a monthly basis, when the number of cyanobacteria cells must not exceed 50 × 103 cells mL−1. As for maintaining the integrity of aquatic ecosystems, CONAMA Resolution No.357/2005, of the Ministério do Meio Ambiente of Brazil, provides for the classification of fresh, brackish and saline waters. Class 2 of this legislation regulates the supply after conventional treatment of aquaculture and fishing. The legislation establishes the conditions and standards for releasing effluents, with the aim of preserving the balance of environments and aquatic communities. Even though analyzes of the number of cyanobacterial cells and chlorophyll a are required in this Resolution, there is no indication of the implementation of systematic monitoring points, nor of the frequencies to be adopted to evaluate chlorophyll a, cyanobacterial cells and their cyanotoxins. As can be seen, there is no legislation regulating the safe quantification limit of cyanotoxins in fish meat. In this case, the Brazilian population may be consuming fish with cyanotoxins. Therefore, the population may be exposed to risk without the knowledge of public health authorities.
5. Conclusion
Abundance and density of cyanobacteria and their blooms can be used as bioindicators of eutrophication and/or water quality and ecotoxicological risk in fish ponds. The results obtained in this study reinforce the need for constant monitoring of cyanotoxins. In the current study may instigate future research to detect cyanotoxins in system different fish ponds, as well as to detect and quantify cyanotoxins in fish meat, not just in fish ponds water.
The rainfall oscillations influenced the water quality in the fish ponds, the abundance and cyanobacteria blooms in fish farms. A total of 15 families and 29 species of cyanobacteria were identified. The families that showed the highest abundances were Microcystaceae, Leptolyngbyaceae and Microcoleaceae. Concerning the species that showed the highest abundances were Microcystis aeruginosa, Planktolyngbya limnetica and Planktothrix sp. The cyanobacterial population and ecotoxicological risk varied according to water quality and climatic conditions as well as seasonality. Regarding the cyanobacterial community showed higher levels of abundance, diversity and dominance right after the rainy season, allowing the coexistence of the species. Consequently, there was less species similarity in the dry season. Cyanobacteria blooms of species Microcystis aeruginosa, M. flos-aquae, Planktothrix sp., P. agardhii, Planktolyngbya limnetica and Oscillatoria sp. A total of 20 blooms were found, 18 blooms in the dry season and only 2 blooms in the rainy season. Some fish farms exceeded the cyanobacteria density limit established by Brazilian legislation by more than 10 times.
The micronucleus test (MN) and other anomalies associated with fish blood showed a higher incidence of mutations in conditions of cyanobacteria bloom, as well as being higher in the dry season. In the dry season, the expected volume of cyanotoxins in fish pond water from some fish farms was above the quantification limit established by Brazilian law.
In addition to there being no indication of the implementation of systematic monitoring points for cyanobacteria in fish farms, there is no legislation limiting the quantification of cyanotoxins in fish meat. That's why, this study will continue categorizing and quantifying cyanotoxins using the ELISA method, with the purpose of suggesting updating of Brazilian legislation. It is expected to find in this future research the cyanotoxin classes Microcystins, Lyngbyatoxin, Saxitoxin etc. As well as, carry out an anatomohistopathological study of farmed fish in ponds under ecotoxicological risk.
Author contribution statement
Maria Mirtes de Lima Pinheiro, MSc.; Bruna Lucieny Temponi Santos, MSc.; Jerônimo Vieira Dantas Filho, PhD: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Vinícius Perez Pedroti, MSc.: Performed the experiments. materils. analysis tools or data.
Jucilene Cavali, PhD; Raphael Brito dos Santos, PhD; Ana Claudia Oliveira Carreira Nishiyama, PhD: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Elica Amara Cecilia Guedes, PhD.: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Sandro de Vargas Schons, PhD.: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
The research was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) through by Fundação Rondônia de Amparo ao Desenvolvimento das Ações Científicas e Tecnológicas e à Pesquisa do Estado de Rondônia (FAPERO), awarded a postdoctoral scholarship to Jerônimo Vieira Dantas Filho [167879/2022-7].
Data availability statement
Data will be made available on request.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Associação Brasileira da Piscicultura . 2022. Anuário da Peixe BR de 2022 Peixe BR, Pinheiros, SP. [Google Scholar]
- 2.Costa R.L., Figueiredo F.M., Bay M., Queiroz C.B., Bay-Hurtado F. Qualitative analysis of phytoplankton in a fish farming of Alvorada d′Oeste, Rondônia state, Brazil. Acta Agronómica. 2016;64:260–267. doi: 10.15446/acag.v64n3.45113. [DOI] [Google Scholar]
- 3.Sant'Ana F.J.F., Oliveira S.L., Rabelo R.E., Vulcani V.A.S., Silva S.M.G., Ferreira-Junior J.A. Outbreaks of Piscinoodinium pillulare and henneguya spp. infection in intensively raised Piaractus mesopotamicus in southwestern goiás, Brazil. Braz. J. Vet. Res. 2012;32:121–125. doi: 10.1590/S0100-736X2012000200005. [DOI] [Google Scholar]
- 4.Machado L.S., Santos L.G., López-Doval J.C., Pompêo M.L.M., Moschini V.C. Environmental factors related to the occurrence of potentially toxic cyanobacteria in Guarapiranga Reservoir, SP, Brazil, Ambiente & Água - an Interdisciplinary. J. Appl. Sci. 2016;11:810–818. doi: 10.4136/ambi-agua.1941. [DOI] [Google Scholar]
- 5.Corbel S., Mougin C., Bouaicha N. Cyanobacterial toxins: modes of actions, fate in aquatic and soil ecosystems, phytotoxicity and bioaccumulation in agricultural crops. Chemosphere. 2014;96:1–15. doi: 10.1016/j.chemosphere.2013.07.0561. [DOI] [PubMed] [Google Scholar]
- 6.Acevedo-Trejos E., Brandt G., Bruggeman J., Merico A. Mechanisms shaping size structure and functional diversity of phytoplankton communities in the ocean. Sci. Rep. 2015;5:8918. doi: 10.1038/srep08918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brasil J., Attayde J.L., Vasconcelos F.R., Dantas D.D.F., Huszar V.L.M. Drought-induced water-level reduction favors cyanobacteria blooms in tropical shallow lakes. Hydrobiologia. 2016;770:145–164. doi: 10.1007/s10750-015-2578-5. [DOI] [Google Scholar]
- 8.Boyd C.E., Tucker C.S., Somridhivej B. Alkalinity and hardness: critical but elusive concepts in Aquaculture. J. World Aquacult. Soc. 2016;47:6–41. doi: 10.1111/jwas.12241. [DOI] [Google Scholar]
- 9.Zohdi E., Abbaspour M. Harmful algal blooms (red tide): a review of causes, impacts and approaches to monitoring and prediction. Int. J. Environ. Sci. Technol. 2019;16:1789–1806. doi: 10.1007/s13762-018-2108-x. [DOI] [Google Scholar]
- 10.Brandão I.L.S., Mannaerts C.M., Saraiva A.C.F. Seasonal variation of phytoplankton indicates small impacts of anthropic activities in a Brazilian Amazonian reserve. Ecohydrol. Hydrobiol. 2017;17:217–226. doi: 10.1016/j.ecohyd.2017.04.001. [DOI] [Google Scholar]
- 11.Sánchez-Baracaldo P., Bianchini G., Wilson J.D., Knoll A.H. Cyanobacteria and biogeochemical cycles through Earth history. Trends Microbiol. 2021 doi: 10.1016/j.tim.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Ferrão-Filho A.S., Silva D.A.C. Saxitoxin-producing Raphidiopsis raciborskii (cyanobacteria) inhibits swimming and physiological parameters in Daphnia similis. Sci. Total Environ. 2019 doi: 10.1016/j.scitotenv.2019.135751. [DOI] [PubMed] [Google Scholar]
- 13.Ouattara M., Zongo F., Zongo B. Species diversity of Cyanobacteria and Desmids of a drinking water source under anthropogenic pressure, and their implication in toxin production and water quality in Aub-Saharan Africa (Burkina Faso, Western Africa) J. Water Resour. Protect. 2021;13:1000–1023. doi: 10.4236/jwarp.2021.1312054. [DOI] [Google Scholar]
- 14.Costa R.L., Todeschini T., Ribeiro M.J.P., Oliveira M.T. Potentially toxic cyanobacteria blooms in fish farms in the Center-South region of the Mato Grosso state. Biodiversidade. 2017;16:33–46. [Google Scholar]
- 15.Dantas-Filho J.V., Pedroti V.P., Santos B.L.T., Mira A.B., Silva F.C., Silva E.C.S., Schons S.V. First evidence of microplastics in freshwater from fish farms in Rondônia state, Brazil. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e15066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mokarram M., Saber A., Sheykh V. Effects of heavy metal contamination on river water quality due to the release of industrial effluents. J. Clean. Prod. 2020;277 doi: 10.1016/j.jclepro.2020.123380. [DOI] [Google Scholar]
- 17.Alves C.A., Stape P.C., Sentelhas J.L.M., Gonçalves G. Sparovek Koppen's climate classification map for Brazil. Meteorologische Zeitschrisft. 2013;22:711–728. doi: 10.1127/0941-2948/2013/0507. [DOI] [Google Scholar]
- 18.NPE . 2022. Instituto Nacional de Pesquisas Espaciais Centro de Previsão de Tempo e Estudos Climáticos (CPTEC). Estação meteorológica de Ouro Preto do Oeste – RO CPTEC. [Google Scholar]
- 19.Costa A.L., Lima J.M., Oliveira V.S., Silva C.A., Regitano R.N.O. Tiametoxam sorption and leaching in sewage sludge and swine wastewater treated water. Amazonian J. Agric. Environ. Sci. 2015;58:208–216. doi: 10.4322/rca.1717. [DOI] [Google Scholar]
- 20.Brasil Ministério do Meio Ambiente. Conselho Nacional do Meio Ambiente - CONAMA. Resolução n° 357, de 17 de março de 2005. Dispõe sobre a classificação dos corpos de água e diretrizes ambientais para o seu enquadramento, bem como estabelece as condições e padrões de lançamento de efluentes, e dá outras providências. Diário Oficial da União. 2005;53:58–63. Seção 1. [Google Scholar]
- 21.Dantas E.W., Moura A.N., Bittencourt-Oliveira C. Cyanobacterial blooms in stratified and destratified eutrophic reservoirs in semi-arid region of Brazil. An Acad. Bras Ciências. 2011;83:1327–1338. doi: 10.1590/S0001-37652011000400019. [DOI] [PubMed] [Google Scholar]
- 22.Brasil J., Attayde J.L., Vasconcelos F.R., Dantas D.D.F., Huszar V.L.M. Drought-induced water-level reduction favors cyanobacteria blooms in tropical shallow lakes. Hydrobiologia. 2015;770:145–164. doi: 10.1007/s10750-015-2578-5. [DOI] [Google Scholar]
- 23.Fonseca J.R., Vieira P.C.S., Kujibida P., Costa I.A.S. Cyanobacterial occurrence and detection of microcystins and saxitoxins in reservoirs of the Brazilian semi-arid. Acta Limnol. Bras. 2015;27:78–92. doi: 10.1590/S2179-975X2814. [DOI] [Google Scholar]
- 24.Oliveira M.C., Bassim I.D., Cammarota M.C. Microalgae and cyanobacteria biomass pretreatment methods: a comparative analysis of chemical and thermochemical pretreatment methods aimed at methane production. Fermentation. 2022;8:497. doi: 10.3390/fermentation8100497. [DOI] [Google Scholar]
- 25.Dajoz R. second ed. Editora da Universidade de São Paulo; São Paulo: 1973. Ecologia Geral. [Google Scholar]
- 26.Pessoa E.K.R., Lima P.L.S.C., Nascimento W.S., Chellappa S., Chellappa N.T. Temporal variation of limnological parameters, frequent groups and biological indices of phytoplankton community of Santa Cruz reservoir, Rio Grande do Norte, Brazil. Biota Amazôn. 2017;7:59–64. doi: 10.18561/2179-5746/biotaamazonia.v7n2p59-64. [DOI] [Google Scholar]
- 27.Weber C.I. National Environmental Research Center, Office of Research & Development, U.S. Environmental Protection Agency; Cincinnati, OH: 1973. Biological Field and Laboratory Methods for Mensuring the Quality of Surface Waters and Efluentes. EPA-670/4-73-001. [Google Scholar]
- 28.Ludwig A., Reynolds F. John Wiley and Sons; New York: 1988. Statistical Ecology: A Primer on Methods and Computing; p. 337. [Google Scholar]
- 29.Krienitz L., Dadheech P.K., Fastner J., Kotut K. The rise of potentially toxin producing cyanobacteria in lake naivasha, great african rift valley, Kenya. Harmful Algae. 2013;27:42–51. doi: 10.1016/j.hal.2013.04.005. [DOI] [Google Scholar]
- 30.Pielou E.C. Akademie-Verlag; Berlin: 1989. Mathematical Ecology; p. 181. [Google Scholar]
- 31.Shannon C.E., Weaver W. University of Illinois Press; Urbana: 1949. The Mathematic Theory of Communication. [Google Scholar]
- 32.Oksanen J., Blanchet F.G., Kindt R., Legendre P., Minchin P.R.…O’hara R.B. Package ‘vegan’. Community Ecolo. Package. 2013;2 [Google Scholar]
- 33.Fonseca J.R., Vieira P.C.S., KujBida P., Costa I.A.S. Cyanobacterial occurrence and detection of microcystins and saxitoxins in reservoirs of the Brazilian semi-arid. Acta Limnol. Bras. 2015;27:78–92. doi: 10.1590/S2179-975X2814. [DOI] [Google Scholar]
- 34.Aimi N., Odaka H., Sakai S., Fujiki H., Suganuma M., Moore R.E., Patterson G.M.L. Lyngbyatoxins B and C, two new irritants from Lyngbya majuscula. J. Nat. Prod. 1990;53:1593–1596. doi: 10.1021/np50072a035. [DOI] [PubMed] [Google Scholar]
- 35.Merel S., Walker D., Chicana R., Snyder S., Baurès E., Thomas O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013;59:303–327. doi: 10.1016/j.envint.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 36.Meneguetti D.U.O., Silva F.C., Zan R.A., Ramos L.J. Adaptation of the micronucleus technique in allium cepa, for mutagenicity analysis of the Jamari river valley, western Amazon, Brazil. J. Environ. Anal. Toxicol. 2012;2 doi: 10.4172/2161-0525.1000127. [DOI] [Google Scholar]
- 37.Silva F.D., Silva C.L., Portes R.G.R., Hoffman E., Oliveira R.S., Martins M., Silva C.O., Silva F.C. Ecotoxicological evaluation of water from the Ouro Preto stream using the bioindicator Leptodactylus petersii. South Am. J. Basic Educ. Techn. Technol. 2018;5:69–87. [Google Scholar]
- 38.Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária – ANVISA. Portaria No. 2914, de 12 de dezembro de 2011 Dispõe sobre os procedimentos de controle e de vigilância da qualidade da água para consumo humano e seu padrão de potabilidade. Diário Oficial da União. 2011;26:266. Seção 1. [Google Scholar]
- 39.García Nieto P.J., García-Gonzalo E., Alonso Fernández J.R., Díaz Muñiz C. A hybrid wavelet kernel SVM-based method using artificial bee colony algorithm for predicting the cyanotoxin content from experimental cyanobacteria concentrations in the Trasona reservoir (Northern Spain) J. Comput. Appl. Math. 2017;309:587–602. doi: 10.1016/j.cam.2016.01.045. [DOI] [Google Scholar]
- 40.Cavali J., Dantas-Filho J.V., Nóbrega B.A., Andrade L.H.V., Pontuschka R.B., Gasparotto P.H.G.…Porto M.O. Benefits of adding virginiamycin to Arapaima gigas (schinz, 1822) diet cultivated in the Brazilian Amazon. Sci. Tech. Rep. 2020 doi: 10.1155/2020/5953720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silva A.D.R., Santos R.B., Bruno A.M.S.S., Soares E.C. Tambaqui farming in irrigation channels under different fish densities. Acta Amazonica. 2013;43:517–524. doi: 10.1590/S0044-59672013000400014. [DOI] [Google Scholar]
- 42.Câmara F.R.A., Rocha O., Pessoa E.K.R., Chellappa S., Chellappa N.T. Morphofunctional changes of phytoplankton during pluvial anomaly in a tropical reservoir. Braz. J. Biol. 2015;75:628–637. doi: 10.1590/1519-6984.19513. [DOI] [PubMed] [Google Scholar]
- 43.Torres I.A., Silva T.M.F.E., Rodrigues L.S., Silva I.J., Costa T.A., Soto-Blanco B., Melo M.M. Physicochemical evaluation of water, sediment and riparian forest samples from a fish farm located in an agro-industrial area on the edge of Ribeirão da Mata (MG) Sanitary Environ. Engin. 2017;22:773–780. doi: 10.1590/s1413-41522017110861. [DOI] [Google Scholar]
- 44.Lopes C.V.A., Cavalcanti G.S., Albuquerque G.S.C. Pesticides and their impacts on human and environmental health: a systematic review. Saúde Debate. 2018;42:518–534. doi: 10.1590/0103-1104201811714. [DOI] [Google Scholar]
- 45.Camargo F.A.O., Silva L.S., Merten G.H., Carlos F.S., Baveye P.C., Triplett E.W. Brazilian agriculture in perspective. Adv. Agron. 2017;141:53–114. doi: 10.1016/bs.agron.2016.10.003. [DOI] [Google Scholar]
- 46.Costa A.L., Lima J.M., Oliveira V.S., Silva C.A., Regitano R.N.O. Tiametoxam sorption and leaching in sewage sludge and swine wastewater treated water. Amazonian J. Agric. Environ. Sci. 2015;58:208–216. doi: 10.4322/rca.1717. [DOI] [Google Scholar]
- 47.Souza N.F.C., Andrade N.L.R., Ribeiro J.G.S., Gomes J.C., Orozco M.M.D., Pereira E.S. Practices in Environmental Education aimed at the implementation of the sanitary sewage system in Presidente Médici (RO) Brazilian J. Environ. Educ. 2019;14:275–294. doi: 10.34024/revbea.2019.v14.2604. [DOI] [Google Scholar]
- 48.Barroncas M.F., Pereira-Filho M., Gomes L.C., Roubach R., Ono E.A. Effects of water exchange on zootechnical indices and effluent quality in intensive rearing of tambaqui (Colossoma macropomum) in excavated fish ponds. Brazilian Journal of Fishing Engineering. 2015;7:55–75. [Google Scholar]
- 49.Arimoro F.O., Olisa H.E., Keke U.N., Ayanwale A.V., Chukwuemeka V.I. Exploring spatio-temporal patterns of plankton diversity and community structure as correlates of water quality in a tropical stream. Acta Ecol. Sin. 2018;38:216–223. doi: 10.1016/j.chnaes.2017.10.002. [DOI] [Google Scholar]
- 50.Moreira C., Vasconcelos V., Antunes A. Cyanobacteria blooms: current knowledge and new perspectives. Earth. 2022;3:127–135. doi: 10.3390/earth3010010. [DOI] [Google Scholar]
- 51.Yan X., Xu X., Wang M., Wang G., Wu S., Li Z., Yang Y. Climate warming and cyanobacteria blooms: looks at their relationships from a new perspective. Water Res. 2017;125:449–457. doi: 10.1016/j.watres.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 52.Amorim C.A., Dantas Ê.W., Nascimento-Moura A. Modeling cyanobacterial blooms in tropical reservoirs: the role of physicochemical variables and trophic interactions. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.140659. [DOI] [PubMed] [Google Scholar]
- 53.Messineo V.S., Bogialli S., Melchiorre N., Sechi A., Lugliè P., Casiddu M.A., Bruno M. Cyanobacterial toxins in Italian freshwaters. Limnologica-Ecology and management of Inland. Water S A (Pretoria) 2009;39:95–106. doi: 10.1016/j.limno.2008.09.001. [DOI] [Google Scholar]
- 54.Molica R.J., Oliveira E.J., Carvalho P.V., Costa N.A., Cunha M.C., Melo G.L., Azevedo S. Occurrence of saxitoxin and an anatoxin-a(s)-like anticholinesterase in a Brazilian drinking waters supply. Harmful Algae. 2005;4:743–753. [Google Scholar]
- 55.Bouvy M., Nascimento S.M., Molica R.J.R., Ferreira A., Huszar V., Azevedo S.M.F.O. Limnological features in Tapacurá reservoir (northeast Brazil) during a severe drought. Hydrobiologia. 2003;493:115–130. doi: 10.1023/A:1025405817350. [DOI] [Google Scholar]
- 56.Vasconcelos J.F., Barbosa J.E.L., Diniz C.R., Ceballos B.S.O. Cyanobacteria in reservoirs in the State of Paraíba: occurrence, toxicity and regulatory factors. Boletim da Sociedade Brasileira de Limnologia. 2011;39:1–20. [Google Scholar]
- 57.Almeida V., Dantas Ê., Melo-Júnior M., Bittencourt-Oliveira M., Moura A. Zooplanktonic community of six reservoirs in northeast Brazil. Braz. J. Biol. 2009;69:57–65. doi: 10.1590/s1519-69842009000100007. [DOI] [PubMed] [Google Scholar]
- 58.Krienitz L., Dadheech P.K., Fastner J., Kotut K. The rise of potentially toxin producing cyanobacteria in lake naivasha, great african rift valley, Kenya. Harmful Algae. 2013;27:42–51. doi: 10.1016/j.hal.2013.04.005. [DOI] [Google Scholar]
- 59.Su Y. Revisiting carbon, nitrogen, and phosphorus metabolisms in microalgae for wastewater treatment. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.144590. [DOI] [PubMed] [Google Scholar]
- 60.Paerl H.W., Xu H., Hall N.S., Rossignol K.L., Joyner A.R., Zhu G., Qin B. Nutrient limitation dynamics examined on a multi-annual scale in Lake Taihu, China: implications for controlling eutrophication and harmful algal blooms. J. Freshw. Ecol. 2015;30:5–24. doi: 10.1080/02705060.2014.994047. [DOI] [Google Scholar]
- 61.Granéli E., Weberg M., Salomon P.S. Harmful algal blooms of allelopathic microalgal species: the role of eutrophication. Harmful Algae. 2008;8:94–102. doi: 10.1016/j.hal.2008.08.011. [DOI] [Google Scholar]
- 62.Vidal L., Kruk C. Cylindrospermopsis raciborskii (Cyanobacteria) extends its distribution to Latitude 34°53’S’: taxonomical and ecological features in Uruguayan eutrophic lakes. Pan Am. J. Aquat. Sci. 2008;3:142–151. [Google Scholar]
- 63.Asencio A.D. Determination of microcystins in reservoirs of different basins in a semiarid area. J. Appl. Phycol. 2013;25:1753–1762. doi: 10.1007/s10811-013-0025-4. [DOI] [Google Scholar]
- 64.Pawlik-Skowronska B., Kalinowska R., Skowronski T. Cyanotoxin diversity and food web bioaccumulation in a reservoir with decreasing phosphorus concentrations and perennial cyanobacterial blooms. Harmful Algae. 2013;28:118–125. [Google Scholar]
- 65.Bláha, L., Bláhová, L., Kohoutek, J., Adamovsky, O., Babica, P., Marsálek, B., Temporal and spatial variability of cyanobacterial toxins microcystins in three interconnected freshwater reservoirs, J. Serb. Chem. Soc. 75 (201) 1303-1312, 10.2298/%20JSC100113106B. [DOI]
- 66.Pírez M., González-Sapienza G., Sienra D., Ferrari G., Last M., Last J.A., Brena B.M. Limited analytical capacity for cyanotoxins in developing countries may hide serious environmental health problems: simple and affordable methods may be the answer. J. Environ. Manag. 2013;114:63–71. doi: 10.1016/j.jenvman.2012.10.052. [DOI] [PubMed] [Google Scholar]
- 67.Silva L.A.P., Araujo F., Panosso R., Camacho F., Costa I.A.S. The green waters of Rio Grande do Norte Reservoirs: the problem of cyanobacteria and cyanotoxins. Ablimno Bulletin. 2011;2:1–10. [Google Scholar]
- 68.Spyros G., Nikos Z. Cyanotoxin occurrence and potentially toxin producing cyanobacteria in freshwaters of Greece: a multi-disciplinary approach. Toxicon. 2014;78:1–9. doi: 10.1016/j.toxicon.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 69.Sotero-Santos R.B., Carvalho E.G., Dellamano-Oliveira M.J., Rocha O. Occurrence and toxicity of an Anabaena bloom in a tropical reservoir (Southeast Brazil) Harmful Algae. 2008;7:590–598. [Google Scholar]
- 70.Costa I.A.S., Azevedo S.M.F.O., Senna P.A.C., Bernardo R.R., Costa S.M., Chellappa N.T. Occurrence of toxin-producing cyanobacteria blooms in a Brazilian semiarid reservoir. Braz. J. Biol. 2006;66:211–219. doi: 10.1590/s1519-69842006000200005. [DOI] [PubMed] [Google Scholar]
- 71.Schmidt J.J., Gagnon G.A., Jamieson R.C. Microalgae growth and phosphorus uptake in wastewater under simulated cold region conditions. Ecol. Eng. 2016;95:588–593. doi: 10.1016/j.ecoleng.2016.06.114. [DOI] [Google Scholar]
- 72.Nunes L.P.S., Cardoso-Filho F.C., Costa A.P.R., Muratori M.C.S. Monitoring of mycotoxigenic fungi in the cultivated fish, in the water and in the substrate from fish ponds in farms. Acta Veterinaria Brasilica. 2015;9:199–204. [Google Scholar]
- 73.Harke M.J., Steffen M.M., Gobler C.J., Otten T.G., Wilhelm S.W., Wood S.A., Paerl H.W. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae. 2016;54:4–20. doi: 10.1016/j.hal.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 74.Rigosi A., Carey C.C., Ibelings B.W., Brookes J.D. The interaction between climate warming and eutrophication to promote cyanobacteria is dependent on trophic state and varies among taxa. Limnol. Oceanogr. 2014;59:99–114. doi: 10.4319/lo.2014.59.1.0099. [DOI] [Google Scholar]
- 75.Ekvall M.K., Calle M.J., Faassen E.J., Gustafsson S., Lurling M., Hansson L.A. Synergistic and species‐specific effects of climate change and water colour on cyanobacterial toxicity and bloom formation. Freshw. Biol. 2013;58:2414–2422. doi: 10.1111/fwb.12220. [DOI] [Google Scholar]
- 76.Mowe M.A.D., Mitrovic S.M., Lim R.P., Furey A., Yeo D.C.J. Tropical cyanobacterial blooms: a review of prevalence, problem taxa, toxins and influencing environmental factors. J. Limnol. 2015;74:205–224. doi: 10.4081/jlimnol.2014.1005. [DOI] [Google Scholar]
- 77.Jardim F.A., Von Sperling E., Jardim B.F.M., Almeida K.C.B. Determining factors of cyanobacteria blooms in Rio doce water, minas gerais, Brazil. J. Sanitary and Environ. Engin. 2014;19:207–218. doi: 10.1590/S1413-41522014019000001026. [DOI] [Google Scholar]
- 78.Góis J.S., Oliveira F.H. Seasonal variation of cyanobacteria as a parameter for analyzing the water quality of the Mororó reservoir, in the municipality of Pedra/PE. Brazilian J. Phy. Geogr. 2014;7:960–968. doi: 10.26848/rbgf.v7.5.p960-968. [DOI] [Google Scholar]
- 79.Cigagna C., Bonotto D.M., Camargo A.F.M., Sturaro J.R. Trophic state index (TSI) and physico-chemical characteristics of a shallow reservoir in southeast Brazil. Environ. Earth Sci. 2016;75:102. doi: 10.1007/s12665-015-4951-0. [DOI] [Google Scholar]
- 80.Liames J.S., Salis W.B., Mehaffey M.H., Nash M.S., Christensen J.R., Schaeffer B.A. Modeling anthropogenic and environmental influences on freshwater harmful algal bloom development detected by MERIS over the Central United States. Water Resour. Res. 2021;57 doi: 10.1029/2020WR028946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Neilan B.A., Pearson L.A., Muenchhoff J., Moffitt M.C., Dittmann E. Environmental conditions that influence toxin biosynthesis in cyanobacteria. Environ. Microbiol. 2012;15:1239–1253. doi: 10.1111/j.1462-2920.2012.02729.x. [DOI] [PubMed] [Google Scholar]
- 82.Zilliges Y., Kehr J.C., Meissner S., Ishida K., Mikkat S., Hagemann M., Kaplan A., Borner T., Dittmann E. The cyanobacterial hepatotoxin microcystin binds to proteins and increases the fitness of microcystis under oxidative stress conditions. PLoS One. 2011;6 doi: 10.1371/journal.pone.0017615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lehman P., Marr K., Boyer G., Acuna S., Teh S. Long-term trends and causal factors associated with Microcystis abundance and toxicity in San Francisco Estuary and implications for climate change impacts. Hydrobiologia - The Int. J. Aquatic Sciences. 2013;718:141–158. doi: 10.1007/s10750-013-1612-8. [DOI] [Google Scholar]
- 84.Wormer L., Cirés S., Quesada A. Importance of natural sedimentation in the fate of microcystins. Chemosphere. 2011;82:1141–1146. doi: 10.1016/j.chemosphere.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 85.Rinta-Kanto J.M., Konopko E.A., DeBruyn J.M., Bourbonniere R.A., Boyer G.L., Wilhelm S.W. Lake Erie Microcystis: relationship between microcystin production, dynamics of genotypes and environmental parameters in a large lake. Harmful Algae. 2009;8:665–673. doi: 10.1016/j.hal.2008.12.004. [DOI] [Google Scholar]
- 86.Corbel S., Mougin C., Bouaicha N. Cyanobacterial toxins: modes of actions, fate in aquatic and soil ecosystems, phytotoxicity and bioaccumulation in agricultural crops. Chemosphere. 2014;96:1–15. doi: 10.1016/j.chemosphere.2013.07.0561. [DOI] [PubMed] [Google Scholar]
- 87.Cai T., Park S.Y., Li Y. Nutrient recovery from wastewater streams by microalgae: status and prospects. Renew. Sustain. Energy Rev. 2013;19:360–369. doi: 10.1016/j.rser.2012.11.030. [DOI] [Google Scholar]
- 88.Cao L., Massey I.Y., Feng H., Yang F. A review of cardiovascular toxicity of Microcystins. Toxins. 2019;11:507. doi: 10.3390/toxins11090507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pesnya D.S., Kurbatova S.A., Sharov A.N., Chernova E.N., Yershov I.Y., Shurganova G.V., Vodeneeva E.L. Genotoxicity of natural water during the mass development of cyanobacteria evaluated by the Allium Test Method: a model experiment with Microcosms. Toxins. 2022;14:359. doi: 10.3390/toxins14050359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Falcone-Dias M.F., Rodrigues M.V., Nielsen J.L., Jonge N., Jorgensen N.O.G., Alonso D.P., Araújo-Junior J.P. Occurrence of Cyanobacteria and microcystins in hydroelectric reservoirs used for fish farming. J. Water Health. 2020;18:983–994. doi: 10.2166/wh.2020.089. [DOI] [PubMed] [Google Scholar]
- 91.Amorim C.A., Moura A.N. Ecological impacts of freshwater algal blooms on water quality, plankton biodiversity, structure, and ecosystem functioning. Sci. Total Environ. 2021;758 doi: 10.1016/j.scitotenv.2020.143605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.