Abstract
Background
An artificial intelligence (AI) algorithm has been developed for fully automated body composition assessment of lung cancer screening noncontrast low-dose CT of the chest (LDCT) scans, but the utility of these measurements in disease risk prediction models has not been assessed.
Purpose
To evaluate the added value of CT-based AI-derived body composition measurements in risk prediction of lung cancer incidence, lung cancer death, cardiovascular disease (CVD) death, and all-cause mortality in the National Lung Screening Trial (NLST).
Materials and Methods
In this secondary analysis of the NLST, body composition measurements, including area and attenuation attributes of skeletal muscle and subcutaneous adipose tissue, were derived from baseline LDCT examinations by using a previously developed AI algorithm. The added value of these measurements was assessed with sex- and cause-specific Cox proportional hazards models with and without the AI-derived body composition measurements for predicting lung cancer incidence, lung cancer death, CVD death, and all-cause mortality. Models were adjusted for confounding variables including age; body mass index; quantitative emphysema; coronary artery calcification; history of diabetes, heart disease, hypertension, and stroke; and other PLCOM2012 lung cancer risk factors. Goodness-of-fit improvements were assessed with the likelihood ratio test.
Results
Among 20 768 included participants (median age, 61 years [IQR, 57–65 years]; 12 317 men), 865 were diagnosed with lung cancer and 4180 died during follow-up. Including the AI-derived body composition measurements improved risk prediction for lung cancer death (male participants: χ2 = 23.09, P < .001; female participants: χ2 = 15.04, P = .002), CVD death (males: χ2 = 69.94, P < .001; females: χ2 = 16.60, P < .001), and all-cause mortality (males: χ2 = 248.13, P < .001; females: χ2 = 94.54, P < .001), but not for lung cancer incidence (male participants: χ2 = 2.53, P = .11; female participants: χ2 = 1.73, P = .19).
Conclusion
The body composition measurements automatically derived from baseline low-dose CT examinations added predictive value for lung cancer death, CVD death, and all-cause death, but not for lung cancer incidence in the NLST.
Clinical trial registration no. NCT00047385
© RSNA, 2023
Supplemental material is available for this article.
See also the editorial by Fintelmann in this issue.
Summary
CT body composition assessment using an artificial intelligence algorithm extended the predictive value of lung cancer screening noncontrast low-dose CT of the chest for morbidities beyond early detection of lung cancer.
Key Results
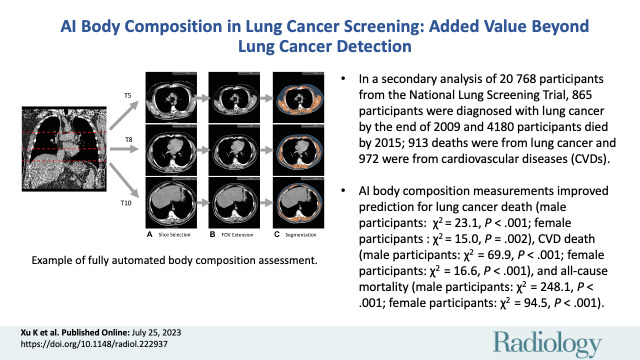
■ In a secondary analysis of 20 768 participants from the National Lung Screening Trial, 865 participants were diagnosed with lung cancer by the end of 2009, and 4180 participants died by 2015: 913 deaths were due to lung cancer and 972 due to cardiovascular diseases (CVDs).
■ Artificial intelligence body composition measurements improved prediction for lung cancer death (male participants: χ2 = 23.09, P < .001; female participants: χ2 = 15.04, P = .002), CVD death (male participants: χ2 = 69.94, P < .001; female participants: χ2 = 16.60, P < .001), and all-cause mortality (male participants: χ2 = 248.13, P < .001; female participants: χ2 = 94.54, P < .001).
Introduction
Body composition measurements capture the physical and constitutional characteristics of the human body. Abnormal body composition phenotypes, such as obesity and sarcopenia, are associated with several chronic health conditions, including metabolic disorders (1,2), chronic inflammation (3), and physical dysfunction (4). In lung cancer therapy, body composition has been shown to affect survival following chemotherapy, overall survival, quality of life, and other essential oncologic metrics (5). Studies have also demonstrated the risk stratification and prognostic value of body composition for cardiovascular disease (CVD) (6) and chronic obstructive pulmonary disease (7). Important sex-specific differences in body composition and its association with health outcomes have been reported in previous studies (8,9).
Noncontrast low-dose CT of the chest (LDCT) is the standard imaging protocol for lung cancer screening (10). In addition to the lung parenchyma, this imaging modality provides high-spatial-resolution depiction of the thoracic anatomy, which enables the opportunistic assessment of body composition (11). One prohibitive factor for its application is the restricted field of view, as conventional section-wise assessment is often insufficient when key body sections are truncated by scan boundaries (5). Indeed, a recent study (12) reported that 96.1% of LDCT images from the National Lung Screening Trial (NLST) (13) and 69.4% of LDCT images from an in-house lung screening program showed mild to severe body section truncation. Several studies have demonstrated the value in assessing regional tissues that are included in the field of view, such as pectoralis (7,14) or paraspinous muscles (15), as surrogate measurements for overall muscle assessments. However, regional tissue assessments may misrepresent the whole-body assessments, as previous studies have reported decreased correlation with reference measurements at the level of the third lumbar vertebra (5).
In previous research, an artificial intelligence (AI) algorithm to derive body composition measurements from lung screening LDCT was developed and tested (12,16). Unlike prior regional tissue assessment approaches, the algorithm automatically extends the field of view to recover truncated body sections, reconstructing synthetic CT sections with complete body anatomy approximating the untruncated versions. This innovation enables approximated section-wise assessment of body composition with use of all body sections included in the field of view while keeping the results “explainable,” where the assessment quality can be visually inspected. The purpose of this study was to assess the added value of the body composition measurements derived by this algorithm, which included the area and attenuation attributes of subcutaneous adipose tissue (SAT) and skeletal muscle (SM), for sex- and cause-specific risk prediction of lung cancer incidence, lung cancer death, CVD death, and all-cause mortality in the NLST cohort.
Materials and Methods
Study Design and Participants
This study was a secondary analysis of the NLST (ClinicalTrials.gov identifier NCT00047385), a Health Insurance Portability and Accountability Act–compliant multicenter randomized controlled trial comparing LDCT with chest radiography for lung cancer screening (13,17). The inclusion and exclusion criteria of the NLST have been previously detailed (13). Briefly, the NLST selected individuals aged 55–74 years with a cigarette smoking history of at least 30 pack-years who either smoked currently or had quit smoking within 15 years. Participants were invited for three LDCT screening examinations at 1-year intervals, with the baseline screening examinations performed soon after the randomization (13). The trial was approved by the institutional review board at each of the 33 participating medical institutions and took place from September 18, 2002, through December 31, 2009. All NLST participants provided written informed consent at participation. The authors obtained permission from the Cancer Data Access System of National Cancer Institute (18) to access and analyze the de-identified data collected by the NLST researchers.
Low-Dose CT Image Data
Among the 26 722 participants who were randomized to the CT arm of the NLST study, we obtained LDCT data from 25 139 participants. LDCT images were acquired following manufacturer-specific acquisition protocols in the form of web-distributed technique charts (13, 17). For this study, images were considered valid if the section thickness was less than or equal to 2.5 mm, scan length was longer than 200 mm, and the reconstruction kernel was among the soft-tissue kernels listed in the NLST protocol mandated by American College of Radiology Imaging Network (17) (Table S1).
Clinical Characteristics and Ascertainment of End Points
As mandated by the NLST study protocol, demographic characteristics—including age, sex, race and ethnic group, education level, smoking history and status, and disease history—were obtained from a questionnaire completed by participants at the time of randomization to one of the two arms of the trial (13). The categorization of race or ethnic group and education level considered in this study is listed in Table S2.
Quantitative emphysema was measured on baseline LDCT images as the percentage of the lungs with intensity less than −950 HU (19). Coronary artery calcification severity was automatically derived with a publicly available AI algorithm previously validated using NLST data (20), where each LDCT image was classified into very low, low, moderate, or high coronary artery calcification severity. Fat-free mass (in kilograms) and fat mass (in kilograms) were determined with an anthropometric approach (7,21). Details on the assessment of these clinical attributes are provided in Appendix S1.
The primary end point in the NLST was lung cancer mortality. Lung cancer incidence and overall mortality were secondary end points (13). We considered CVD-related death as an additional end point. Following reference 13, a death was ascertained as CVD-related death if the International Classification of Diseases 10th Revision diagnosis code of the underlying cause of the death from the death certificate was in the range of I00 to I99. We used the end of 2009 as the end of follow-up for lung cancer incidence and the end of 2015 as end of follow-up for mortality end points. Details on how the follow-up end dates were determined are given in Appendix S1.
Fully Automated CT-based Body Composition Assessment
In previous publications (12,16), an AI algorithm was developed to assess the SM and SAT at the levels corresponding to the fifth, eighth, and 10th thoracic vertebral bodies in lung screening LDCT. The systematic field-of-view limitation was addressed by the automated extension of the field of view and imputation of missing body sections. Derived body composition measurements included the area index (denoted “index”), which was defined as the summed area (in square centimeters) across three levels divided by the participant height squared (in square meters), and the attenuation (Hounsfield units) and SD (Hounsfield units), which were defined as the average and SD of image intensity in the delineated image regions. These measurements were obtained for both SAT and SM. Figure 1 shows an example of body composition assessment in a 57-year-old male participant. The developed AI algorithm is publicly available in the form of a Docker image (22). Documents for its use are maintained at https://github.com/MASILab/S-EFOV (commit b2ab4db9cf17a9ebe4b13b195491341ac687636b).
Figure 1:
Example of fully automated body composition assessment in the lung cancer screening noncontrast low-dose chest CT scan in a 57-year-old male participant. (A) CT axial plane levels corresponding to the fifth (T5), eighth (T8), and 10th (T10) vertebral bodies were predicted. Corresponding axial CT sections were selected for body composition assessment. (B) The field of view (FOV) of each CT section with body section truncation was extended with missing body section imputation. (C) Areas of subcutaneous adipose tissue (SAT) (blue) and skeletal muscle (SM) (orange) were segmented on the field-of-view extended sections. Body composition measurements include SM index (166.2, normal group), SM attenuation (17.5 HU, lower group), SM SD (41.0 HU, normal group), SAT index (189.7, higher group), SAT attenuation (−88.4 HU, normal group), and SAT SD (28.0 HU, higher group). Indexes were calculated as summed area (in square centimeters) across three levels divided by participant height squared (in square meters).
Body composition measurements were derived from baseline LDCT images to estimate body composition profiles at randomization. All body composition assessments were performed by an author (K.X., with 3 years of experience). Details on the development, quality review procedure, and intra- and interoperator reliability analysis of this AI algorithm are provided in Appendix S1.
Statistical Analysis
Descriptive statistics are presented as medians and IQRs for continuous variables and as numbers of participants and percentages for categorical variables. The correlations of AI-derived body composition measurements with body mass index (calculated as participant weight in kilograms divided by patient height in meters squared), fat-free mass index, and fat mass index were assessed with use of Spearman correlation coefficients, and the correlation coefficient (ρ), 95% CI, and P value are reported.
Confounding factors were selected for each end point individually based on relevant variables identified or included in previous studies. For lung cancer incidence and lung cancer death, the confounding factors included PLCOM2012 lung cancer risk model factors (23), such as age and body mass index, as well as quantitative emphysema (19). The complete list of the PLCOM2012 factors is provided in Appendix S1. For CVD death, the confounding factors included age; self-reported history of diabetes, heart disease or myocardial infarction, hypertension, and stroke; and image-derived coronary artery calcification severity level (20). For all-cause mortality, the confounding factors included the combination of all factors used in each of the cause-specific models.
Incidence rates of end point events were estimated with use of cumulative incidence functions, where deaths caused by unrelated diseases were considered competing events except for all-cause mortality (24). For regression analyses in the presence of competing events, we adopted the cause-specific hazard modeling approach, where the competing events were treated as censored (24). Female and male participants were modeled separately in all analyses.
To visually assess the associations between body composition measurements and end point events, the cumulative incidence functions were estimated for participants stratified by 25th and 75th percentiles of AI-derived body composition measurements. Participants were stratified into lower (<25th percentile), normal (between the 25th and 75th percentiles), or higher (>75th percentile) groups. Estimated cumulative incidence function curves are reported with 95% CIs. The Gray test was used to calculate a P value for the assessment of separation between cumulative incidence functions. Unadjusted hazard ratios (HRs) of the higher and lower groups, with the normal group as reference, were estimated by cause-specific Cox proportional hazards models. HRs (with 95% CIs) and P values are reported.
The associations of the AI-derived body composition measurements in their continuous forms with end point events were assessed with cause-specific Cox proportional hazards models in both unadjusted and fully adjusted settings. HR per change in IQR, 95% CI, and P value are reported.
The best subset of body composition measurements was determined as the combination of measurements that minimized the Akaike information criterion score of an adjusted model among all possible combinations (25). The added value of the body composition measurements was assessed by comparing the goodness of fit of the sex- and cause-specific models with or without the body composition measurements. The added value was assessed for both individual measurements and best subsets of measurements. Harrell C-indexes with the 95% CIs and Akaike information criterion scores are reported. Goodness-of-fit improvements were assessed with use of the likelihood ratio test for nested models. The χ2 index and P value are reported.
A priori, we defined P < .05 as indicative of a statistically significant result. Statistical analyses were performed by an author (K.X.). Additional notes on the statistical analysis, including multicollinearity assessment, interaction effects with sex and smoking status, false-discovery rate adjustment (Benjamini-Hochberg procedure, m = 8), and software packages and routines, are provided in Appendix S1.
Results
Participant Characteristics
Of the 25 139 participants with available LDCT data, 4371 were excluded (Fig 2). The number of participants with missing data for each variable, summary of failure mode of the automated body composition assessment algorithm, and a comparison of demographic characteristics between excluded and included participants are provided in Tables S2, S3, and S4, respectively. For the 20 768 participants included in the statistical analysis (Fig 2), the median age at randomization was 61 years (IQR, 57–65 years); 12 317 (59.3%) were male, and 8451 (40.7%) were female. Most of these participants (19 789 [95.3%]) had their baseline screening scan completed within 60 days after the randomization. During the 6.6 years (IQR, 6.2–7.0 years) of lung cancer incidence follow-up, 865 participants were diagnosed with lung cancer. During the 12.3 years (IQR, 11.8–12.7 years) of mortality follow-up, 4180 participants died. Among the deceased, 913 died of lung cancer and 972 of CVD-related causes. The primary subtype of CVD-related death was death caused by ischemic heart diseases (517 participants) (Table S5). Participants who experienced adverse events during the follow-up period were older, more likely to have a current smoking status, and more likely to have severe emphysema and severe coronary artery calcifications (Table 1).
Figure 2:

Cohort selection flowchart. A total of 2963 participants were excluded from body composition analysis due to missing baseline CT data (n = 312), image data corruption (n = 81), images failing the section thickness or scan length requirement (n = 158), or images failing the requirement for soft-tissue reconstruction kernels (n = 2412). Then, 1408 participants were excluded due to images failing the quality review process (n = 233) or to missing clinical data or end point ascertainment (n = 1175). DICOM = Digital Imaging and Communications in Medicine, NIfTI = Neuroimaging Informatics Technology Initiative.
Table 1:
Cohort Baseline Characteristics
The summary statistics, including medians and IQRs, for sex-specific distributions of the AI-derived body composition measurements are given in Table S2. Differences in distributions between male and female participants were observed for all AI-derived body composition measurements (P < .001 for all measurements, Mann-Whitney U rank test). Pairwise joint distributions of SAT index with SM index, SAT attenuation, and SM attenuation are shown in Figure 3. Distinct differences in the joint distributions between SAT index and SM index were observed between male and female participants (Fig 3A). Strong negative correlations were observed between SAT index and SAT attenuation in both male (ρ = −0.68) and female participants (ρ = −0.61) (Fig 3B). Moderate negative correlations were observed between SAT index and SM attenuation in both male (ρ = −0.55) and female participants (ρ = −0.56) (Fig 3C).
Figure 3:
Pairwise associations of subcutaneous adipose tissue (SAT) index with SAT attenuation, skeletal muscle (SM) index, and SM attenuation of participants included in statistical analysis (20 768 participants: 12 317 male and 8451 female). Spearman correlation coefficients (ρ) were used to quantitatively assess the associations for male, female, and all participants separately. (A) Scatterplot shows the association between SAT index and SM index. Analysis indicated weak (0.20 ≤ |ρ| < 0.40) positive correlations between SAT index and SM index in male (ρ = 0.28) and female participants (ρ = 0.38) and a very weak (|ρ| < 0.20) negative correlation when combined (ρ = −0.10). Separation in the joint distributions of male and female participants was observed, with male participants showing a higher SM index and a lower SAT index, while female participants showed a lower SM index and a higher SAT index. (B) Scatterplot shows the association between SAT index and SAT attenuation. Strong (|ρ| ≥ 0.60) negative correlations were observed between SAT index and SAT attenuation in male (ρ = −0.68), female (ρ = −0.61), and all (ρ = −0.75) participants. Nonlinear correlation patterns can be observed in the joint distributions approximated by the scatterplot. (C) Scatterplot shows the association between SAT index and SM attenuation. Analysis indicated moderate (0.40 ≤ |ρ| < 0.60) negative correlations between SAT index and SM attenuation in male (ρ = −0.55) and female participants (ρ = −0.56) and a strong negative correlation when combined (ρ = −0.62).
Associations of AI-derived body composition measurements with anthropometric approximations for fat-free mass and fat mass indexes are detailed in Appendix S1 and Table 2.
Table 2:
Correlation of Body Composition Measurements with Anthropometric Indexes
Association of AI-derived Body Composition Measurements with End Points
Among male participants, separation in cumulative incidence functions stratified by SM attenuation was observed for all end points (Fig 4). In female participants, separation in cumulative incidence functions stratified by SM attenuation was observed for all end points except for lung cancer incidence (Fig 5). HRs of higher and lower groups with normal group as reference for each measurement and each end point in male and female participants are presented in Figure 6.
Figure 4:
Estimated cumulative incidence functions of each end point in male participants (n = 12 317) stratified by skeletal muscle (SM) attenuation values of 27.6 HU (25th percentile) and 35.1 HU (75th percentile) as lower (<27.6 HU), normal (between 27.6 HU and 35.1 HU), and higher (>35.1 HU) groups. Solid step lines represent estimated cumulative incidence functions, while semitransparent bands represent 95% CIs. P values were derived with use of the Gray test for separation between cumulative incidence functions. (A) Plot displays the estimated cumulative incidence of lung cancer for each group. Compared with the normal group, the lower group exhibited a higher incidence of lung cancer, while the higher group exhibited a lower incidence of lung cancer. (B) Plot displays the estimated cumulative incidence of lung cancer death for each group. Compared with the normal group, the lower group exhibited a higher incidence of lung cancer death, while the higher group exhibited a lower incidence of lung cancer death. (C) Plot displays the estimated cumulative incidence of cardiovascular disease (CVD) death for each group. Compared with the normal group, the lower group exhibited a higher incidence of CVD death, while the higher group exhibited a lower incidence of CVD death. (D) Plot displays the estimated cumulative incidence of all-cause death for each group. Compared with the normal group, the lower group exhibited a higher incidence of all-cause death, while the higher group exhibited a lower incidence of all-cause death.
Figure 5:
Estimated cumulative incidence functions of each end point in female participants (n = 8451) stratified by skeletal muscle (SM attenuation) values of 23.0 HU (25th percentile) and 30.6 HU (75th percentile) as lower (<23.0 HU), normal (between 23.0 HU and 30.6 HU), and higher (>30.6 HU) groups. Solid step lines represent estimated cumulative incidence functions, while semitransparent bands represent 95% CIs. P values were derived with use of the Gray test for separation between cumulative incidence functions. (A) Plot displays the estimated cumulative incidence of lung cancer for each group. Separation of cumulative incidence functions was not observed (P = .11). (B) Plot displays the estimated cumulative incidence of lung cancer death for each group. Compared with the lower and normal groups, the higher group exhibited a lower incidence of lung cancer death. (C) Plot displays the estimated cumulative incidence of cardiovascular disease (CVD) death for each group. Compared with the normal group, the lower group exhibited a higher incidence of CVD death, while the higher group exhibited a lower incidence of CVD death. (D) Plot displays the estimated cumulative incidence of all-cause death for each group. Compared with the normal group, the lower group exhibited a higher incidence of all-cause death, while the higher group exhibited a lower incidence of all-cause death.
Figure 6:
Estimated unadjusted hazard ratios (HRs) of the lower and higher groups, with the normal group as reference for each body composition measurement (rows) and each end point event (columns) in male and female participants. The groups were obtained by the stratification based on the sex-specific 25th and 75th percentiles for each measurement. (A) Plot displays the estimated unadjusted HRs in male participants (n = 12 317). (B) Plot displays the estimated unadjusted HRs in female participants (n = 8451). The dots represent the estimated HRs. The segments represent the 95% CIs of the estimated HRs. The HR of 1 (no difference in hazard) is displayed as a red line in each plot for reference. The numbers following each dot-segment combination show the numerical value of the HR, with the 95% CI in parentheses and the associated P value in square brackets. For instance, as indicated by the fourth row in the column “CVD Death (n = 680)” in A, the lower skeletal muscle (SM) attenuation group in male participants (<27.6 HU) was associated with higher risk for cardiovascular disease (CVD) death (HR, 2.27 [95% CI: 1.93, 2.67]; P < .001) when compared with the normal group, which is consistent with observations in Figure 4C based on cumulative incidence functions. SAT = subcutaneous adipose tissue.
Partially different results between male and female participants in the association between SM measurements and end points were also observed in univariable Cox proportional hazards regression analyses (Table S6). For instance, in male participants, higher SM attenuation was associated with decreased lung cancer incidence (HR, 0.81 per 7.6-HU increase [95% CI: 0.72, 0.90]; P < .001), and higher SM index (per 29.5-unit increase) was associated with decreased lung cancer death (HR, 0.74 [95% CI: 0.67, 0.83]; P < .001) and decreased CVD death (HR, 0.84 [95% CI: 0.76, 0.93]; P < .001). Similar associations were not observed in female participants.
In the fully adjusted models (Table 3), and when potential alpha level inflation due to multiple comparisons was taken into consideration, higher SM attenuation in male participants (per 7.6-HU increase) remained an independent predictor for decreased lung cancer death (HR, 0.78 [95% CI: 0.68, 0.88]; P < .001), CVD death (HR, 0.71 [95% CI: 0.64, 0.78]; P < .001), and all-cause mortality (HR, 0.69 [95% CI: 0.65, 0.73]; P < .001). Higher SM attenuation (per 7.6-HU increase) in female participants was also associated with decreased lung cancer death (HR, 0.76 [95% CI: 0.64, 0.91]; P = .002), CVD death (HR, 0.76 [95% CI: 0.64, 0.89]; P < .001), and all-cause mortality (HR, 0.71 [95% CI: 0.65, 0.77]; P < .001) (Table 3). Higher SM index remained an independent predictor only for decreased all-cause mortality in male participants (HR, 0.84 per 29.5-unit increase [95% CI: 0.80, 0.90]; P < .001). In addition, higher SM SD in male participants (per 3.2-HU increase) was associated with increased lung cancer death (HR, 1.25 [95% CI: 1.11, 1.41]; P < .001), CVD death (HR, 1.20 [95% CI: 1.09, 1.32]; P < .001), and all-cause mortality (HR, 1.29 [95% CI: 1.22, 1.37]; P < .001). In our adjusted analysis, higher SM SD in female participants (per 2.9-HU increase) was associated only with increased all-cause mortality (HR, 1.24 [95% CI: 1.15, 1.35]; P < .001). Higher SAT index in male participants (per 83.7-unit increase) was associated with increased all-cause death (HR, 1.13 [95% CI: 1.04, 1.23]; P = .004). Higher SAT attenuation in male participants (per 7.8-HU increase) was associated with increased CVD death (HR, 1.17 [95% CI: 1.07, 1.27]; P < .001) and all-cause mortality (HR, 1.18 [95% CI: 1.12, 1.23]; P < .001). Higher SAT attenuation in female participants (per 6.9-HU increase) was associated with increased all-cause death (HR, 1.17 [95% CI: 1.10, 1.24]; P < .001). Higher SAT SD in male participants (per 2.8-HU increase) was associated decreased all-cause mortality (HR, 0.92 [95% CI: 0.88, 0.97]; P = .002).
Table 3:
Sex- and Cause-specific Cox Proportional Hazards Regression Analysis of the Association of Body Composition Measurements with Lung Cancer Incidence, Lung Cancer Death, CVD Death, and All-Cause Death
Added Predictive Value of AI-derived Body Composition Measurements
The identified best subsets of AI-derived body composition measurements are annotated in Table 3. SM attenuation was the only measurement that was included in all sex- and cause-specific best subsets. Compared with the covariates-only models, improvements in goodness of fit after including the best subsets of body composition measurements were observed for lung cancer death (male participants: χ2 = 23.09, P < .001; female participants: χ2 = 15.04, P = .002), CVD death (male participants: χ2 = 69.94, P < .001; female participants: χ2 = 16.60, P < .001), and all-cause mortality (male participants: χ2 = 248.13, P < .001; female participants: χ2 = 94.54, P < .001), but not for lung cancer incidence (male participants: χ2 = 2.53, P = .11; female participants: χ2 = 1.73, P = .19) (Table 4). Evaluations for the added value of individual measurements are detailed in Table S7.
Table 4:
Added Value of the Body Composition Measurements in Predicting Lung Cancer Incidence, Lung Cancer Death, CVD Death, and All-Cause Death
Discussion
Noncontrast low-dose CT of the chest (LDCT) is the standard imaging modality in lung cancer screening and allows for opportunistic assessment of body composition. Previously, an artificial intelligence (AI) algorithm was developed for automated assessment of subcutaneous adipose tissue and skeletal muscle at lung cancer screening LDCT. The purpose of this study was to evaluate the added value of the AI-derived body composition measurements in risk prediction of lung cancer incidence, lung cancer death, cardiovascular disease (CVD) death, and all-cause mortality in a secondary analysis of the National Lung Screening Trial. Using the likelihood ratio test to compare the nested models with and without AI-derived body composition measurements, we showed that the AI-derived measurements improved risk prediction of lung cancer death, CVD death, and all-cause mortality but not of lung cancer incidence.
Field-of-view limitation is a known pitfall for body composition assessment using routine chest CT scans (5,12). Prior studies involving lung cancer screening LDCT primarily relied on regional tissue assessment approaches (14,15). In another NLST-based study (15), paraspinous muscles were automatically characterized using a single section at the level of the 12th thoracic vertebra in a subgroup of older participants (11 361 participants; baseline age, 60–69 years). The area and attenuation of the paraspinous muscles were associated with all-cause mortality in male but not female participants. In our study, we addressed the field-of-view limitation by automatically imputing biologically consistent body sections (16). Our analysis found the association of SM area and attenuation with all-cause mortality in male participants, as observed in the study by Lenchik et al (15), but also found similar associations among female participants. This difference can possibly be explained by substantial variations in measurement technique, cohort, and confounding factors. One limitation of our study was that we did not compare the derived measurements with those obtained by established regional tissue assessment methods. Some studies suggest that regional tissue assessments may inadequately represent overall assessments (5), yet direct comparison between regional tissue assessment and section-wise tissue assessment in terms of added predictive value in the same study cohort have not been reported, to our knowledge.
We observed that measurements associated with muscle adiposity, especially the attenuation of SM, play an important role in predicting mortality end points. This is consistent with previous reports on opportunistic body composition assessment using routine abdominal CT scans (9,26). Indeed, fat deposition within or around nonadipose tissues or organs has been associated with increased inflammation, metabolic disorders (eg, insulin resistance, type 2 diabetes, CVD), and physical impairment (27,28). Studies have also suggested that, compared with traditional sarcopenia characterized by reduced muscle bulk, SM fat infiltration (myosteatosis) may be a more consistent signature of deteriorating health conditions with aging and more predictive of health outcomes (11,28). Our findings support these earlier observations, as decreased SM attenuation was associated with increased incidence of lung cancer, CVD, and all-cause death in both male and female participants in the fully adjusted models, while associations between SM index and mortality were not observed in the fully adjusted models except for all-cause death in male participants. Interestingly, we also identified the predictive value of the SD of SM, which has rarely been considered in previous studies (5,11). This finding may support the use of this measurement in characterizing the increased heterogeneity of SM associated with myosteatosis. Another limitation of this study is that measurements for intermuscular adipose tissue, which is important for characterizing myosteatosis (7,27), were not explicitly obtained and studied.
Opportunistic assessment of imaging data beyond the initial study indication has great potential to extend the value of established population-based CT screening, especially when combined with fully automated AI solutions (11). Our analyses provide the evidence that fully automated body composition assessment in lung cancer screening can potentially improve the overall health of lung cancer screening population by identifying high-risk individuals for targeted interventions, such as medical optimization, physical conditioning, or lifestyle modifications. Another limitation of this present study is the lack of analysis of other incidental imaging data in lung cancer screening LDCT that are known to impact survival. These include bone mineral density and liver steatosis (11), as well as lung abnormalities other than emphysema (eg, interstitial lung abnormalities) (29,30). Finally, this study was an exploratory analysis. Subsequent confirmatory studies should be conducted to confirm the observed associations and added predictive values before adoption in routine clinical practice.
In conclusion, with a previously developed artificial intelligence algorithm, we obtained the CT-based area and attenuation attributes of subcutaneous adipose tissue and skeletal muscle in a lung cancer screening cohort. These body composition measurements added predictive value for lung cancer death, cardiovascular disease death, and all-cause mortality, but not for lung cancer incidence. These results suggest that fully automated CT-based body composition assessment can potentially extend the value of lung cancer screening noncontrast low-dose CT of the chest beyond early lung cancer detection. Nevertheless, subsequent studies are needed to confirm the findings in this exploratory analysis.
Acknowledgments
Acknowledgments
The authors thank Valerie Welty, PhD, and Stephen A. Deppen, PhD, for important feedback on statistical analysis in this work. The authors thank the National Cancer Institute (NCI) for access to NCI’s data collected by the National Lung Screening Trial. The statements contained herein are solely those of the authors and do not represent or imply concurrence or endorsement by NCI.
This research is supported by the following awards: National Science Foundation CAREER 1452485; National Cancer Institute (NCI) grants R01 EB017230 and R01 CA253923; NCI grant U01 CA196405; grant UL1 RR024975-01 of the National Center for Research Resources and grant 2 UL1 TR000445-06 of the National Center for Advancing Translational Sciences; Martineau Innovation Fund grant through the Vanderbilt-Ingram Cancer Center Thoracic Working Group; NCI Early Detection Research Network grant 2U01CA152662; National Institutes of Health/National Heart, Lung, and Blood Institute training grant under award number 5T32HL110837; and the IBM PhD Fellowship.
Data sharing: Data analyzed during the study were provided by a third party. Requests for data should be directed to the provider indicated in the Acknowledgments.
Disclosures of conflicts of interest: K.X. No relevant relationships. M.S.K. No relevant relationships. T.Z.L. No relevant relationships. R.G. No relevant relationships. J.G.T. No relevant relationships. Y.H. No relevant relationships. T.A.L. No relevant relationships. J.J.C. Research grants to institution from GE Healthcare, Edison, IBM Watson Health (Merative), Varian (Siemens Healthineers), TheraTec, and the National Institutes of Health. F.M. No relevant relationships. B.A.L. No relevant relationships. K.L.S. Consulting fees from Aidence and Reveal Dx; leadership or fiduciary role in the National Lung Cancer Roundtable, Eastern Cooperative Oncology Group–American College of Radiology Imaging Network Cancer Research Group, and the American College of Radiology.
Abbreviations:
- AI
- artificial intelligence
- CVD
- cardiovascular disease
- HR
- hazard ratio
- LDCT
- noncontrast low-dose CT of the chest
- NLST
- National Lung Screening Trial
- SAT
- subcutaneous adipose tissue
- SM
- skeletal muscle
References
- 1. Heshka S , Ruggiero A , Bray GA , et al. Altered body composition in type 2 diabetes mellitus . Int J Obes 2008. ; 32 ( 5 ): 780 – 787 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dulloo AG , Jacquet J , Solinas G , Montani JP , Schutz Y . Body composition phenotypes in pathways to obesity and the metabolic syndrome . Int J Obes 2010. ; 34 ( Suppl 2 ): S4 – S17 . [DOI] [PubMed] [Google Scholar]
- 3. Eid AA , Ionescu AA , Nixon LS , et al. Inflammatory response and body composition in chronic obstructive pulmonary disease . Am J Respir Crit Care Med 2001. ; 164 ( 8 Pt 1 ): 1414 - 1418 . [DOI] [PubMed] [Google Scholar]
- 4. Goodpaster BH , Park SW , Harris TB , et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study . J Gerontol A Biol Sci Med Sci 2006. ; 61 ( 10 ): 1059 – 1064 . [DOI] [PubMed] [Google Scholar]
- 5. Troschel AS , Troschel FM , Best TD , et al. Computed tomography-based body composition analysis and its role in lung cancer care . J Thorac Imaging 2020. ; 35 ( 2 ): 91 – 100 . [DOI] [PubMed] [Google Scholar]
- 6. Ding J , Hsu F-C , Harris TB , et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA) . Am J Clin Nutr 2009. ; 90 ( 3 ): 499 – 504 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pishgar F , Shabani M , Quinaglia A C Silva T , et al. Quantitative analysis of adipose depots by using chest CT and associations with all-cause mortality in chronic obstructive pulmonary disease: longitudinal analysis from MESArthritis ancillary study . Radiology 2021. ; 299 ( 3 ): 703 – 711 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schorr M , Dichtel LE , Gerweck AV , et al. Sex differences in body composition and association with cardiometabolic risk . Biol Sex Differ 2018. ; 9 ( 1 ): 28 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee MH , Zea R , Garrett JW , Graffy PM , Summers RM , Pickhardt PJ . Abdominal CT body composition thresholds using automated AI tools for predicting 10-year adverse outcomes . Radiology 2023. ; 306 ( 2 ): e220574 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. US Preventive Services Task Force ; Krist AH , Davidson KW , et al. Screening for lung cancer: US preventive services task force recommendation statement . JAMA 2021. ; 325 ( 10 ): 962 – 970 . [DOI] [PubMed] [Google Scholar]
- 11. Pickhardt PJ . Value-added opportunistic CT screening: state of the art . Radiology 2022. ; 303 ( 2 ): 241 – 254 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu K , Gao R , Tang Y , et al. Extending the value of routine lung screening CT with quantitative body composition assessment . Proc SPIE Int Soc Opt Eng 2022. ; 12032 : 120321L . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. National Lung Screening Trial Research Team ; Aberle DR , Adams AM , et al. Reduced lung-cancer mortality with low-dose computed tomographic screening . N Engl J Med 2011. ; 365 ( 5 ): 395 – 409 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Digumarthy SR , De Man R , Canellas R , Otrakji A , Wang G , Kalra MK . Multifactorial analysis of mortality in screening detected lung cancer . J Oncol 2018. ; 2018 : 1296246 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lenchik L , Barnard R , Boutin RD , et al. Automated muscle measurement on chest CT predicts all-cause mortality in older adults from the National Lung Screening Trial . J Gerontol A Biol Sci Med Sci 2021. ; 76 ( 2 ): 277 – 285 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu K , Li T , Khan MS , et al. Body composition assessment with limited field-of-view computed tomography: a semantic image extension perspective . Med Image Anal 2023. ; 88 : 102852 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cagnon CH , Cody DD , McNitt-Gray MF , Seibert JA , Judy PF , Aberle DR . Description and implementation of a quality control program in an imaging-based clinical trial . Acad Radiol 2006. ; 13 ( 11 ): 1431 – 1441 . [DOI] [PubMed] [Google Scholar]
- 18. NLST – The Cancer Data Access System . National Cancer Institute; . https://cdas.cancer.gov/nlst/. Accessed February 23, 2023. [Google Scholar]
- 19. Labaki WW , Xia M , Murray S , et al. Quantitative emphysema on low-dose CT imaging of the chest and risk of lung cancer and airflow obstruction: an analysis of the National Lung Screening Trial . Chest 2021. ; 159 ( 5 ): 1812 – 1820 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zeleznik R , Foldyna B , Eslami P , et al. Deep convolutional neural networks to predict cardiovascular risk from computed tomography . Nat Commun 2021. ; 12 ( 1 ): 715 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuch B , Gneiting B , Döring A , et al. Indexation of left ventricular mass in adults with a novel approximation for fat-free mass . J Hypertens 2001. ; 19 ( 1 ): 135 – 142 . [DOI] [PubMed] [Google Scholar]
- 22. Matelsky J , Kiar G , Johnson E , Rivera C , Toma M , Gray-Roncal W . Container-based clinical solutions for portable and reproducible image analysis . J Digit Imaging 2018. ; 31 ( 3 ): 315 – 320 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tammemägi MC , Katki HA , Hocking WG , et al. Selection criteria for lung-cancer screening . N Engl J Med 2013. ; 368 ( 8 ): 728 – 736 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Austin PC , Lee DS , Fine JP . Introduction to the analysis of survival data in the presence of competing risks . Circulation 2016. ; 133 ( 6 ): 601 – 609 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Z . Variable selection with stepwise and best subset approaches . Ann Transl Med 2016. ; 4 ( 7 ): 136 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pickhardt PJ , Graffy PM , Zea R , et al. Automated CT biomarkers for opportunistic prediction of future cardiovascular events and mortality in an asymptomatic screening population: a retrospective cohort study . Lancet Digit Health 2020. ; 2 ( 4 ): e192 – e200 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Terry JG , Hartley KG , Steffen LM , et al. Association of smoking with abdominal adipose deposition and muscle composition in Coronary Artery Risk Development in Young Adults (CARDIA) participants at mid-life: a population-based cohort study . PLoS Med 2020. ; 17 ( 7 ): e1003223 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Correa-de-Araujo R , Addison O , Miljkovic I , et al. Myosteatosis in the context of skeletal muscle function deficit: an interdisciplinary workshop at the National Institute on Aging . Front Physiol 2020. ; 11 : 963 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hata A , Schiebler ML , Lynch DA , Hatabu H . Interstitial lung abnormalities: state of the art . Radiology 2021. ; 301 ( 1 ): 19 – 34 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Putman RK , Hatabu H , Araki T , et al. Association between interstitial lung abnormalities and all-cause mortality . JAMA 2016. ; 315 ( 7 ): 672 – 681 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hofmanninger J , Prayer F , Pan J , Röhrich S , Prosch H , Langs G . Automatic lung segmentation in routine imaging is primarily a data diversity problem, not a methodology problem . Eur Radiol Exp 2020. ; 4 ( 1 ): 50 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Agatston AS , Janowitz WR , Hildner FJ , Zusmer NR , Viamonte M Jr , Detrano R . Quantification of coronary artery calcium using ultrafast computed tomography . J Am Coll Cardiol 1990. ; 15 ( 4 ): 827 – 832 . [DOI] [PubMed] [Google Scholar]
- 33. National Lung Screening Trial Research Team . Lung cancer incidence and mortality with extended follow-up in the National Lung Screening Trial . J Thorac Oncol 2019. ; 14 ( 10 ): 1732 – 1742 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rubin M . Do p values lose their meaning in exploratory analyses? It depends how you define the familywise error rate . Rev Gen Psychol 2017. ; 21 ( 3 ): 269 – 275 . [Google Scholar]
- 35. Althouse AD . Adjust for multiple comparisons? It’s not that simple . Ann Thorac Surg 2016. ; 101 ( 5 ): 1644 – 1645 . [DOI] [PubMed] [Google Scholar]









![Estimated unadjusted hazard ratios (HRs) of the lower and higher groups, with the normal group as reference for each body composition measurement (rows) and each end point event (columns) in male and female participants. The groups were obtained by the stratification based on the sex-specific 25th and 75th percentiles for each measurement. (A) Plot displays the estimated unadjusted HRs in male participants (n = 12 317). (B) Plot displays the estimated unadjusted HRs in female participants (n = 8451). The dots represent the estimated HRs. The segments represent the 95% CIs of the estimated HRs. The HR of 1 (no difference in hazard) is displayed as a red line in each plot for reference. The numbers following each dot-segment combination show the numerical value of the HR, with the 95% CI in parentheses and the associated P value in square brackets. For instance, as indicated by the fourth row in the column “CVD Death (n = 680)” in A, the lower skeletal muscle (SM) attenuation group in male participants (<27.6 HU) was associated with higher risk for cardiovascular disease (CVD) death (HR, 2.27 [95% CI: 1.93, 2.67]; P < .001) when compared with the normal group, which is consistent with observations in Figure 4C based on cumulative incidence functions. SAT = subcutaneous adipose tissue.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/c0aa/10374937/0113835b7f41/radiol.222937.fig6.jpg)

