Abstract
Mango is a widely favored fruit that offers high nutritional value. Mango has been studied to examine its influence on postprandial glucose, but few studies have used fresh mango compared to dried mango to measure blood glucose and satiety after consumption. Therefore, the objective of the present study was to investigate the effects of fresh versus dried mango consumption on satiety and postprandial glucose. A crossover design was implemented where 34 healthy adults (29 females and 5 males; 25.0 ± 6.0 years; BMI 23.8 ± 4.3 kg/m2) consumed either 100 kcal of fresh mango, dried mango, or white bread on three separate occasions. Following consumption, satiety was assessed every 15 min for 90 min and blood glucose was assessed every 30 min for 90 min. Consumption of fresh mango results showed a significant increase in satiety (tendency of greater fullness (P = 0.073) and less desire to eat (P < 0.05)) in participants. Fresh mango exhibited a more efficient decrease in postprandial glucose levels (P < 0.05) compared to dried mango or white bread, and fresh mango promoted a greater stability in blood glucose. Dried mango consumption also significantly lowered postprandial glucose compared to white bread (P < 0.05). These results suggest that fresh mango consumption may be beneficial in improving satiety responses and postprandial glucose control when compared to its dried alternative or white bread. The results of the study may help guide individuals who are overweight or obese and/or have type 2 diabetes by altering their food choices that ultimately could improve their health.
ClinicalTrials.gov Identifier: NCT03956602.
Keywords: Mango, Dried mango, Satiety, Postprandial glucose, Type 2 diabetes, Human
1. Introduction
Obesity and type 2 diabetes prevalence continue to rise in the United States [[1], [2], [3]]. According to the National Health and Nutrition Examination Survey (NHANES) from 2000 to 2017 the US obesity prevalence increased from 30.5% to 41.9% [1]. Further, the Center for Disease Control and Prevention (CDC) reports that 73.6% of Americans are overweight or obese [2]. Additionally, the CDC reported that 11.3% of all United Stated adults have diabetes, and Type 2 diabetes accounting for 95% of all cases of diabetes [3]. A primary risk factor of type 2 diabetes is overconsumption of calories which can lead to becoming overweight or obese [[4], [5], [6], [7]]. From 2013 to 2016 the CDC estimated 89% of individuals with type 2 diabetes were overweight or obese as defined by a body mass index (BMI) of 25 kg/m2 or higher [3]. A meta-analysis found that a Western diet, also known as the Standard American Diet (SAD), increases the risk of obesity [8] and developing type 2 diabetes by 41–44% [9]. The SAD largely consists of processed foods, red meat, high-fat dairy products, high-sugar foods, pre-packaged foods and is low in fruit and vegetable consumption.
Satiety is characterized by the absence of hunger. High satiety foods help lower the risk of overconsumption while possibly providing less calories and, reducing the risk of becoming overweight, obese, and developing type 2 diabetes. It is expected that high satiety is achieved when food remains in the stomach for a long period. It is well accepted that vegetables and fruits are considered highly satiating foods [[10], [11], [12]] and have been associated with a lower prevalence of obesity [13]. It has also been suggested that consumption of fruits and vegetables can positively impact blood glucose responses [14,15], and improve insulin sensitivity [[16], [17], [18]]. Fruits and vegetables have a high amount of fiber, which can slow carbohydrate digestion and help manage blood sugar levels. Fruit also contains varied amounts of sugars like fructose, and glucose [19]. Fructose that occurs naturally in fruits and vegetables produces a smaller postprandial rise in plasma glucose and serum insulin than other common carbohydrates [14]. Additionally, it has been shown that bioactive compounds present in fruits and vegetables improve glucose [15]. Specifically, it is suggested that fruits contain bioactive components that exhibit properties that aid in lowering blood glucose [15].
Mango is one of the most consumed fruit worldwide and is also commonly consumed in the United States [20]. It contains essential nutrients like fiber, vitamin A and C, magnesium, potassium, and many bioactive phytochemicals like mangiferin, polyphenols, carotenoids, and flavonoids. Recently, Pinneo et al. [21] found that fresh mango provides high satiety and improves postprandial glucose and insulin responses in healthy, overweight, and obese adults compared to a refined cookie. Various forms of mango puree demonstrate a slower rise and a faster decline in the postprandial glucose response when compared to pure glucose [22]. Mango by-product (constituting of mango peel and/or pulp) was shown to reduced serum glucose levels in diabetic rats [23,24].
The demand for less-perishable, nutritious and convenient snacks has led to the increased consumption of dried mango [25]. Dried mango contains similar nutrients and phytochemicals as fresh mangos but at different potencies due to the loss of moisture and drying processes. Evans et al. [15] found that regular consumption of freeze-dried mango by obese individuals provide a potential positive effect on fasting blood glucose [15]. Semkoff et al. [26] determined that consumption of freeze-dried mango in pre-diabetic individuals decreased blood glucose and increased insulin levels. There are no available studies assessing the effects of dried mango on satiety.
To the best of our knowledge there are no studies comparing fresh mango and dried mangos effects on satiety and blood glucose. Therefore, the objective of the present study was to investigate the effects of fresh versus dried mango consumption on satiety and postprandial glucose. We hypothesize that fresh mango consumption will increase satiety and improve postprandial glucose response compared to dried mango or the isocaloric control sample.
2. Materials and methods
2.1. Participants
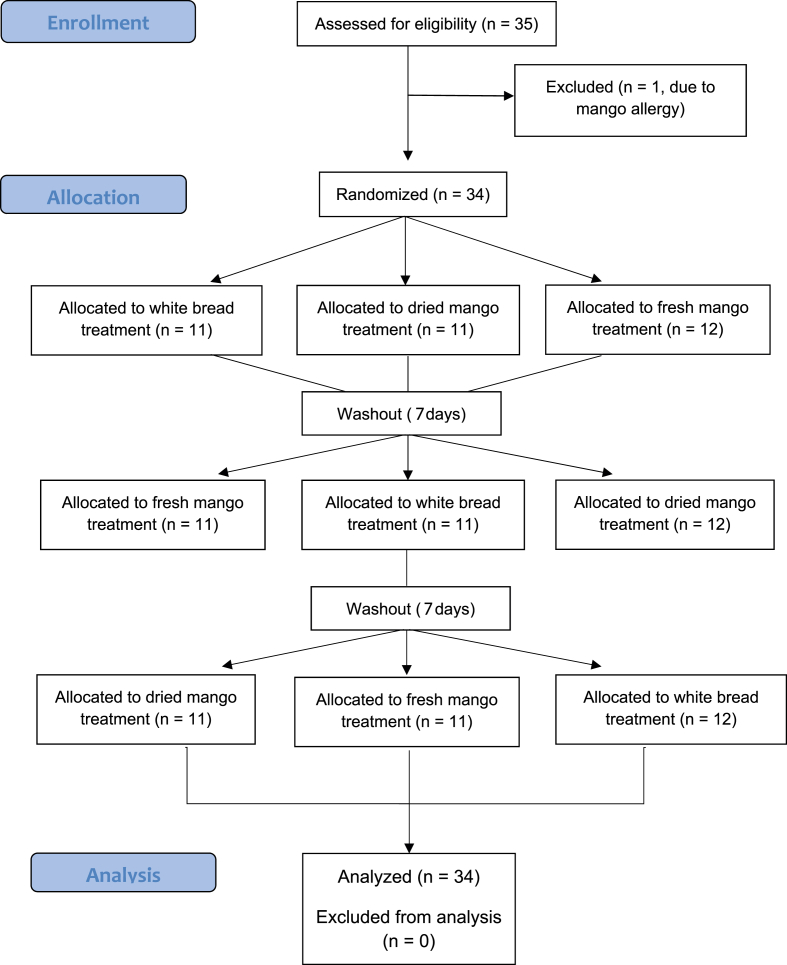
A total of 35 subjects were recruited to participate in this study and one person was excluded in the screening process due to mango allergy (Fig. 1). The study included 34 participants (29 females, and 5 males). Participants were between the ages of 18–50 years old. Participants were excluded from the study if they identified with one or more of the following criteria: a known chronic medical condition required dietary supplement use, smoking, pregnancy, and allergy to the intervention foods provided (e.g., mango or gluten). The study protocol was approved by the San Diego State University Institutional Review Board (IRB) and students provided informed consent prior to the study. The trial was registered at clinicaltrials.gov (NCT03956602).
Fig. 1.
CONSORT flow diagram of participant selection.
2.2. Study design
This crossover design included three interventions: white bread (control), dried mango, and fresh mango. Every subject participated in all three trials by consuming 100 kcal of white bread (Wonder brand, Flowers Foods Thomasville, GA) on day one of the experiment, dried mango (Kirkland, Costco, Issaquah, WA), and fresh mango (Alpine Fresh Inc., Miami, FL). Mango varieties include Tommy Atkins, Kent, or Keitt cultivars. Prepackaged ready-to-eat mangos were purchased and they contain no preservatives or additives. Each intervention was done a week apart.
At baseline the participants tested their blood pressure and pulse and measured their height, weight, waist circumference, hip circumference, and percent body fat. Blood pressure (BP) and pulse were measured after subjects had rested for at least 10 min. They were measured using a ReliOn automatic blood pressure monitor (Omron Healthcare Inc., Bannockburn, IL). BP or pulses that measured higher than normal range were measured again. Height and weight were recorded using a mechanical beam scale (Detecto, Webb City, MO). Body mass index (BMI) was calculated using the equation weight in kilograms divided by height in meters squared. Waist to hip ratio was calculated using waist measurement divided by hip measurement. Percent body fat was assessed using a handheld bioelectrical impedance analysis (Omron Healthcare, Inc.).
Prior to each intervention participants tested their fasting blood glucose using a blood-glucose finger prick meter at baseline and then every 30 min up to 90 min after snack consumption. Subjects were also instructed to complete a series of satiety questionnaires at baseline and every 15 min up to 90 min post consumption.
2.3. 24-hour recalls
Participants completed a 24-h recall prior to each intervention to consider diets from the previous day that could influence the intervention results. The information from the 24-h recall was entered into cronometer.com, a nutritional tracking application. This information was used to determine if any statistical corrections were necessary to account for dietary intake outside of the trials.
2.4. Satiety questionnaire
For each trial, subjects recorded their satiety measured using a 10 cm visual analogue scale (Flint et al., 2000). To measure satiety during the intervention's, participants completed a questionnaire at baseline, 15-, 30-, 45-, 60-, 75-, and 90-min. The questionnaire consisted of 5 questions related to satiety. Specifically, the questions were; “How hungry are you?”, “How full are you?”, “How strong is your desire to eat?”, “How much do you think you could eat right now?”, and “How thirsty are you?”, respectively.
2.5. Postprandial glucose response
The participants measured their glucose levels at baseline, and 30, 60, and 90 min after the trial food was consumed. Glucose was measured using a finger prick glucose meter (Nova Biomedical, Waltham, MA). The participants' glucose measurements were used to assess if there was a significant difference or impact on postprandial glucose following each trial.
2.6. Statistical analysis
Statistical analyses were conducted using the SPSS software (IBM, Armonk, New York). The data was analyzed using a repeated measure analysis of variance (ANOVA) to determine the effects of fresh mango, dried mango, and the control on satiety and glucose levels over time. Data are presented as means ± standard deviations (SDs) and results were considered significant at P ≤ 0.05.
3. Results
3.1. Anthropometrics and Demographics
The study included 29 females, and 5 males. The participants' average anthropometric information are as follows: age; 24.97 ± 5.97 years old and body mass index (BMI); 23.78 ± 4.28 kg/m2 (Table 1). Average systolic and diastolic blood pressure, waist circumference, hip circumference and waist to hip ratio (W/H ratio) are shown in Table 1. Subjects’ average BMI and blood pressure and waist to hip ratio were within normal range.
Table 1.
Demographics: age, height, weight, body mass index, blood pressure, waist, hip, waist to hip ratio, and body fat percentage.
| N = 34 (female 29; male 5) | |
|---|---|
| Age (yr) | 24.97 ± 5.97 |
| Height (cm) | 165.19 ± 7.56 |
| Weight (kg) | 64.91 ± 12.00 |
| BMI (kg/m2) | 23.78 ± 4.28 |
| SBP (mmHg) | 119.71 ± 17.21 |
| DBP (mmHg) | 78.26 ± 10.50 |
| Pulse (bpm) | 67.47 ± 10.23 |
| Waist circumference (cm) | 77.95 ± 8.28 |
| Hip circumference (cm) | 98.73 ± 9.58 |
| W/H ratio | 0.78 ± 0.07 |
| Body fat (%) | 22.72 ± 8.00 |
Data are presented as means ± SDs.
SD: Standard deviation; yr: year; cm: centimeters; kg: kilograms; BMI: body mass index; SBP: systolic blood pressure; mmHg: millimeters of mercury; DBP: diastolic blood pressure; bpm: beats per minute W/H: waist to hip.
3.2. Diet intakes
All three interventions had an equal caloric intake of 100 kcal, and each intervention was conducted a week apart. Participants completed a 24-h recall prior to each intervention to consider diets from the previous day that could influence the intervention results. The average kcal intake among the participants the day prior to the white bread consumption was 1752.94 ± 803.44 kcals, prior to the dried mango consumption was 1591.06 ± 558.08 kcals and prior to the fresh mango consumption was 1777.69 ± 589.67 kcals (Table 2). There were no statistically significant differences on intakes of kcal, carbohydrate, protein, fat, saturated fatty acids, monounsaturated fatty acids, polyunsaturated fatty acids, cholesterol, fiber, sugar, vitamins A, C, D, E, K, calcium, iron, and sodium among all three days from 24-h food recalls. There are no statistically significant differences in nutritional intake prior to each intervention among the participants that skew the intervention results, thus no adjustments were made. Further dietary intake macro and micronutrient intake amounts can be found in Table 2.
Table 2.
Food intake of subjects the day prior to the white bread, the dried mango, or the fresh mango consumption.
| White bread (N = 34) | Dried mango (N = 34) | Fresh mango (N = 34) | |
|---|---|---|---|
| Kcal | 1752.94 ± 803.44 | 1591.06 ± 558.08 | 1777.69 ± 589.67 |
| CHO (g) | 180.05 ± 84.35 | 167.07 ± 71.21 | 178.56 ± 82.03 |
| Protein (g) | 85.27 ± 51.65 | 78.53 ± 39.93 | 94.26 ± 56.66 |
| Fat (g) | 67.36 ± 36.82 | 60.40 ± 28.68 | 68.02 ± 25.69 |
| SFA (g) | 22.74 ± 14.06 | 17.57 ± 10.53 | 21.59 ± 9.00 |
| MUFA (g) | 14.95 ± 10.99 | 14.71 ± 12.12 | 17.15 ± 10.53 |
| PUFA (g) | 9.80 ± 6.71 | 10.04 ± 6.75 | 10.64 ± 6.64 |
| Cholesterol (mg) | 278.29 ± 219.86 | 238.10 ± 222.08 | 345.28 ± 259.29 |
| Fiber (g) | 25.35 ± 20.01 | 24.15 ± 16.42 | 22.70 ± 15.62 |
| Sugar (g) | 63.04 ± 46.55 | 52.99 ± 40.44 | 60.71 ± 44.98 |
| Vit A (IU) | 8672 ± 13351 | 10320 ± 18373 | 9590 ± 18109 |
| Vit C (mg) | 103.46 ± 117.40 | 105.78 ± 112.71 | 93.84 ± 92.44 |
| Vit D (IU) | 271.07 ± 446.05 | 157.11 ± 307.80 | 275.87 ± 424.74 |
| Vit E (mg) | 15.81 ± 46.50 | 6.83 ± 6.25 | 8.87 ± 9.32 |
| Vit K (μg) | 262.10 ± 559.70 | 261.32 ± 493.69 | 228.15 ± 581.21 |
| Ca (mg) | 810.62 ± 620.11 | 708.43 ± 387.62 | 702.82 ± 418.32 |
| Fe (mg) | 13.37 ± 9.97 | 11.30 ± 5.45 | 13.64 ± 9.89 |
| Na (mg) | 2561.97 ± 1733.67 | 2476.26 ± 1182.52 | 2873.88 ± 3110.50 |
Data are presented as means ± SDs.
NS: Not significant; SD: standard deviation: Kcal: kilocalorie; CHO: carbohydrate; g: grams; SFA: saturated fatty acid; MUFA: monounsaturated fatty acids; PUFA: polyunsaturated fatty acids; mg: milligrams; IU: international unit; Vit: vitamin; μg: micrograms; Ca: calcium; Fe: iron; Na: sodium.
3.3. Satiety measurements
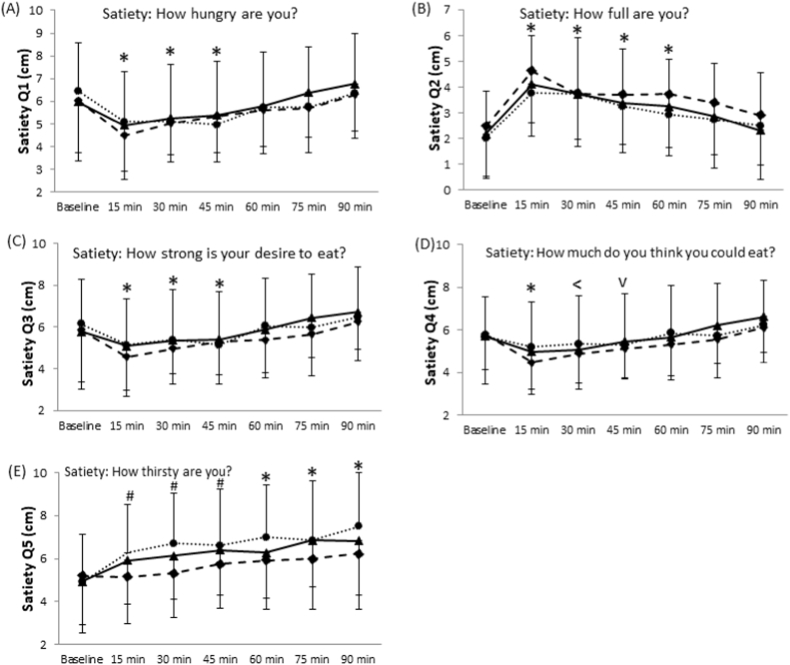
Question 1 (How hungry are you?) of the satiety questionnaire revealed a time effect (P < 0.001, Fig. 2A). All trials showed that hunger decreased at 15 min post consumption. The reduced hunger sensation reported by the participants was present until 45 min and began to increase at 60 min. There was no significant difference between baseline and hunger at 60, 75 and 90 min. Differences for main effect of trial were non-significant.
Fig. 2.
Effects of dried or fresh mango consumption on postprandial glucose.
Trials main effect P = 0.002, Time main effect P < 0.001, Interaction P = 0.054
*: different from the baseline at P < 0.05 in all three trials
#: different from the baseline at P < 0.05 in control (white bread) and dried mango trials
^:different between control (white bread) and mangos
+: different among trials
• Control (white bread); ▴ dried mango; ♦ fresh mango.
Results from question 2 (How full are you?) also showed a time effect (P < 0.001). The feeling of fullness increased from baseline to 15 min, and gradually decreased there after (Fig. 2B). While the trial effect was statistically insignificant, it should be noted that there was a trend of a trial effect (P = 0.073). Specifically, fresh mango consumption had a tendency of causing a greater fullness response.
Results for questions 3 (How strong is your desire to eat?) revealed a time effect among all trials (P < 0.001). There was a decreased desire at 15 min which is present until 45 min, and then this desire gradually increased over time (Fig. 2C). While insignificant, there was a lower desire to eat at 60 min after consumption of fresh mango compared to the control (P = 0.064). All other differences among time points were insignificant.
The results from question 4 (How much do you think you could eat right now?) demonstrated a time effect (P < 0.001). There was a significant reduction in the amount of food the subjects thought they could consume at 15 min compared to baseline in all three trials (Fig. 2D). The response lasted until 30 min in dried mango trial and until 45 min in fresh mango trial. There was a significant reduction in the amount of food the subjects thought they could consume 15 min after consuming fresh mango compared to both dried mango and the control (P < 0.05). Subjects felt that they could eat significantly less 75 and 90 min after the consumption of fresh mango compared to dried mango (P = 0.041 and P = 0.038, respectively).
Question 5 (How thirsty are you?) also displayed a time effect (P < 0.001) and trial effect (P = 0.001, Fig. 2E). For dried mango and white bread, participants reported greater thirst at 15 min which increased overtime. Fresh mango consumption showed an insignificant thirst response until 60 min compared to baseline. Thirst levels significantly lessened for fresh mango consumption over time compared to dried mango or the control. At both 60 and 90 min, thirst was significantly greater for bread than for dried mango (P < 0.01).
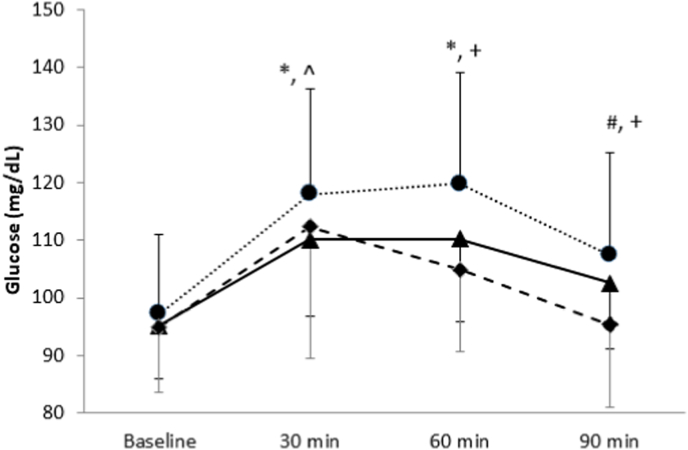
3.4. Glucose measurement
The results for glucose levels demonstrated a time effect (P < 0.001) and trial effect (P = 0.001; Fig. 3). Glucose levels increased from baseline until 30 min in all trials. Glucose levels remained elevated until 60 min and began to decline at 90 min in dried mango and the control. For fresh mango, postprandial glucose levels peaked at 30 min and then significantly declined at 60 and 90 min (P < 0.05). There was a significantly lower levels of blood glucose following dried mango and fresh mango consumption at 30 min compared to the control (P < 0.05). At 60- and 90-min time points, glucose was significantly lower in the fresh mango trial compared to white bread and dried mango trials (P < 0.05). Dried mango consumption significantly decreased postprandial glucose compared to white bread but was higher than fresh mango at 60- and 90-min post consumption (P < 0.05).
Fig. 3.
Effects of dried or fresh mango consumption on postprandial satiety responses. (A) How hungry are you?, Time main effect P < 0.001; (B) How full are you? Trial effect P = 0.073, Time main effect P < 0.001; (C) How strong is your desire to eat?, Time main effect P < 0.001; (D) How much do you think you could eat?, Time main effect P < 0.001; (E) How thirsty are you?, Trial effect P = 0.001, Time main effect P < 0.001.*, Different from the baseline in all three trials, P < 0.05. #: Different from the baseline in white bread and dried mango trials P < 0.05. <: Different from the baseline in dried and fresh mango trials, P < 0.05. v: Different from the baseline in fresh mango trial, P < 0.05. • Control (white bread); ▴ dried mango; ♦ fresh mango.
4. Discussion
There is an urgent need to focus on and promote foods with high satiation to address the obesity epidemic and lower the rate of type 2 diabetes in America. Mango is a nutritious fruit with many vitamins, minerals and phytochemicals including mangiferin, gallic acids, gallotannins, quercetin, isoquercetin, ellagic acid, and β-glucogallin. Consumption of dried mango is increasing due to its convenience, and nutritional benefits [25]. The present study was conducted to determine the effects of fresh versus dried mango consumption on the intensity and longevity of satiety, as well as postprandial glucose in healthy adults. To our knowledge, this is the first study comparing fresh versus dried mango consumption on satiety levels and postprandial glucose.
The results from our study revealed that overall, fresh mango had a greater satiety effect than dried mango and the white bread control. Specifically, fresh mango provides a greater sense of fullness and a lower desire to eat compared to the control and dried mango. It is well accepted that foods with higher moisture content promote satiety [[27], [28], [29], [30]]. Water content enhances satiation by slowing down gastric emptying which extends a sense of fullness. Therefore, the loss of water content in dried mango compared to fresh mango provides an explanation as to why fresh mango has a higher satiety effect than dried mango. Fresh mango not only contains more moisture but more fiber than the isocaloric dried mango (2.64 g vs 0.74 g). It is well accepted that fiber rich foods promote satiety [31,32]. Fiber promotes satiation due to its bulking and viscosity properties causing delayed gastric emptying which has a significant impact of satiation [[32], [33], [34]]. The high fiber and water content of fresh mango also qualify it as a high-volume fruit [35,36]. High volume foods likely maximize contact with gut receptors [35] including leptin, which signal to the brain a sense of fullness [37]. All these factors help explain the higher satiety effect of fresh mango versus dried mango in our study. Furthermore, various hormones are secreted from the gastrointestinal tract following fiber consumption, which promotes satiety [31]. For example, cholecystokinin (CCK) is secreted by the small intestine during the digestive process and is one hormone responsible for promoting satiation once food enters the small intestine [38]. Fiber has been shown to extend the release of CCK by prolonging the digestive process and gastric emptying [39], thus inducing satiation for a longer period [33]. Future studies are still needed to understand the satiety hormone responses between fresh mango vs dried mango.
Fresh mango lowered postprandial glucose levels in comparison to the isocaloric dried mango and control. Fresh fruit has been linked to lowering blood glucose and decrease the risk of type 2 diabetes [40]. There are many available studies assessing the effects of mango, mango products and byproducts on fasting blood glucose and postprandial glucose. For example, it has been demonstrated that fresh mango improves postprandial glucose in healthy, overweight, and obese adults compared to a cookie control [21,41]. Wall‐Medrano et al. [42] found that the dietary fiber in mango byproducts (peel, seed, seed husk) can lower postprandial glucose by delaying the rate of glucose absorption. Moreover, mango peel extracts showed glucose-lowering effect for the prevention of type 2 diabetes [43]. Lastly, Evens et al. [15] found that 12-week consumption of freeze-dried mango lowered fasting blood glucose in obsess individuals. Overall, the results found in the present study align well with the findings of previous literature.
The mechanisms for mango and fruit lowering glucose are likely attributed to its dietary fiber. Fiber is not broken down into glucose by the small intestine like other digestible carbohydrates. Instead, it passes the gastrointestinal tract undigested into the colon where it is fermented by gut bacteria. Therefore, it does not lead to prolonged spiked glucose levels compared to foods that contain digestible carbohydrates and that do not contain high amounts of fiber. Another potential glucose lowering mechanism of mango could be due it its abundant bioactive compounds like mangiferin. Mangiferin has been shown to have antidiabetic properties by decreasing glucose levels and insulin resistance by enhancing insulin sensitivity [44,45]. Additionally, mangiferin is an inhibitor of glucosidase which are enzymes responsible for the breakdown of carbohydrates [44] Therefore, mangiferin could prevent a large amount of carbohydrates from being converted to glucose and absorbed in the intestine. Mango has been shown to up-regulate GLUT4 [23], which is an insulin-responsive glucose transporter responsible for maintaining postprandial glucose.
The current study is not without limitations. The short time frame for postprandial response and one-dose interventions are the primary limitations. Analyzing blood glucose and satiety for a more multiple time points would provide more clarity to this study. It would be also valuable if there are dose-dependent responses on satiety and glucose levels. In the future, a longer duration study where satiety hormones are evaluated like leptin and ghrelin and participants’ next meal choice is monitored would be beneficial to better understand the effects of the interventions on satiety.
The results of our study are important for controlling blood glucose and maintaining satiety to prevent over-consumption of calories. This information has the potential to impact individuals with diabetes and/or obesity. Food options that can quickly sustain glucose levels and increase satiety may limit risks present in individuals with diabetes. Similarly, it would be prudent for individuals who are overweight or obese to be mindful of kcal intake to mitigate further weight gain. Weight gain often goes hand in hand with metabolic syndrome which includes excess abdominal fat, high blood sugar, abnormal cholesterol and triglyceride levels and high blood pressure. This raises the risk for diabetes, heart attack and stroke. Foods that provide longer satiation are key to avoiding excess calories. The results of our study may help guide people with these conditions to better eating habits to improve their overall health. The information reported is also relevant for healthy people to prevent the development of these conditions and maintain their overall health.
Declaration of competing interests
The authors declare there are no competing interests.
Funding
This study was supported by The National Mango Board, United States [#603024]. The funding source did not have any involvement in the conduct of the research including study design, data collection, analysis and interpretation, and preparation of the article.
CRediT authorship contribution statement
Candice Stamper: Data curation, Visualization, and, Writing – original draft. Sama Safadi: Data curation, Writing – review & editing. Andrew Gehr: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, and, Writing – review & editing. Pia Asuncion: Data curation. Mee Young Hong: Conceptualization, Data curation, Funding acquisition, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, and, Writing – review & editing.
Acknowledgements
The authors would like to acknowledge the contributions of San Diego State University Nutrition 302L students who assisted in conducting and evaluating this research.
References
- 1.Stierman B, Afful J, Carroll MD, Chen TC, Davy O, Fink S, Fryar CD, Gu Q, Hales CM, Hughes JP, Ostchega Y. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. 10.15620/cdc:106273. Accessed on March 30, 2023. [DOI]
- 2.Fryar C.D., Carroll M.D., Afful J. NCHS Health E-Stats; 2020. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2–19 years: United States, 1963–1965 through 2017–2018.https://www.cdc.gov/nchs/data/hestat/obesity-child-17-18/obesity-child.htm [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Centers for Disease Control and Prevention, US Department of Health and Human Services; Atlanta, GA: 2022. National diabetes statistics report; pp. 12–15.https://www.cdc.gov/diabetes/data/statistics-report/index.html 2022. [Google Scholar]
- 4.Apovian C.M., Okemah J., O'Neil P.M. Body weight considerations in the management of type 2 diabetes. Adv Ther. 2019;36:44–58. doi: 10.1007/s12325-018-0824-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maggio C.A., Pi-Sunyer F.X. Obesity and type 2 diabetes. Endocrinol Metabol Clin. 2003;32(4):805–822. doi: 10.1016/s0889-8529(03)00071-9. [DOI] [PubMed] [Google Scholar]
- 6.Mokdad A.H., Ford E.S., Bowman B.A., Dietz W.H., Vinicor F., Bales V.S., Marks J.S. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 7.Papandreou D., Magriplis E., Abboud M., Taha Z., Karavolia E., Karavolias C., Zampelas A. Consumption of raw orange, 100% fresh orange juice, and nectar-sweetened orange juice—effects on blood glucose and insulin levels on healthy subjects. Nutrients. 2019;11(9):2171. doi: 10.3390/nu11092171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rakhra V., Galappaththy S.L., Bulchandani S., Cabandugama P.K. Obesity and the western diet: how we got here. Mo Med. 2020;117(6):536–538. [PMC free article] [PubMed] [Google Scholar]
- 9.Xu G., Liu B., Sun Y., Du Y., Snetselaar L.G., Hu F.B., Bao W. Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: population based study. BMJ. 2018;362:K1497. doi: 10.1136/bmj.k1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fardet A. Minimally processed foods are more satiating and less hyperglycemic than ultra-processed foods: a preliminary study with 98 ready-to-eat foods. Food Funct. 2016;7:2338–2346. doi: 10.1039/C6FO00107F. [DOI] [PubMed] [Google Scholar]
- 11.Flood-Obbagy J.E., Rolls B.J. The effect of fruit in different forms on energy intake and satiety at a meal. Appetite. 2009;52(2):416–422. doi: 10.1016/j.appet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holt S.H., Brand Miller J.C., Petocz P., Farmakalidis E. A satiety index of common foods. Eur J Clin Nutr. 1995;49(9):675–690. [PubMed] [Google Scholar]
- 13.Slyper A. Oral processing, satiation and obesity: overview and hypotheses. Diabetes Metab Syndr Obes. 2021;14:3399–3415. doi: 10.2147/DMSO.S314379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bantle J.P. Dietary fructose and metabolic syndrome and diabetes. J Nutr. 2009 1;139(6):1263S. doi: 10.3945/jn.108.098020. 8S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans S.F., Meister M., Mahmood M., Eldoumi H., Peterson S., Perkins-Veazie P., Clarke S.L., Payton M., Smith B.J., Lucas E.A. Mango supplementation improves blood glucose in obese individuals. Nutr Metab Insights. 2014;7:77–84. doi: 10.4137/NMI.S17028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paquette M., Medina Larqué A.S., Weisnagel S.J., Desjardins Y., Marois J., Pilon G., Dudonne S., Marette A., Jacques H. Strawberry and cranberry polyphenols improve insulin sensitivity in insulin-resistant, non-diabetic adults: a parallel, double-blind, controlled and randomised clinical trial. Br J Nutr. 2017;117(4):519–531. doi: 10.1017/S0007114517000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solverson P.M., Rumpler W.V., Leger J.L., Redan B.W., Ferruzzi M.G., Baer D.J., Castonguay T.W., Novotny J.A. Blackberry feeding increases fat oxidation and improves insulin sensitivity in overweight and obese males. Nutrients. 2018;10(8):1048. doi: 10.3390/nu10081048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stull A.J., Cash K.C., Johnson W.D., Champagne C.M., Cefalu W.T. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J Nutr. 2010;140(10):1764–1768. doi: 10.3945/jn.110.125336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dreher M.L. Whole fruits and fruit fiber emerging health effects. Nutrients. 2018;10(12):1833. doi: 10.3390/nu10121833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Food and Agriculture Organization (FAO) 2019. Major tropical fruits: market review 2018.http://www.fao.org/3/ca5692en/ca5692en.pdf Retrieved from. [Google Scholar]
- 21.Pinneo S., O'Mealy C., Rosas M., Jr., Tsang M., Liu C., Kern M., Hooshmand S., Hong M.Y. Fresh mango consumption promotes greater satiety and improves postprandial glucose and insulin responses in healthy overweight and obese adults. J Med Food. 2022;25(4):381–388. doi: 10.1089/jmf.2021.0063. [DOI] [PubMed] [Google Scholar]
- 22.Elizondo-Montemayor L., Hernández-Brenes C., Ramos-Parra P.A., Moreno-Sánchez D., Nieblas B., Rosas-Pérez A.M., Lamadrid-Zertuche A.C. High hydrostatic pressure processing reduces the glycemic index of fresh mango puree in healthy subjects. Food Funct. 2015;6:1352–1360. doi: 10.1039/C4FO01005A. [DOI] [PubMed] [Google Scholar]
- 23.Perpétuo G.F., Salgado J.M. Effect of mango (Mangifera indica, L.) ingestion on blood glucose levels of normal and diabetic rats. Plant Foods Hum Nutr. 2003;58(3):1–2. doi: 10.1023/B:QUAL.0000040336.38013.83. [DOI] [Google Scholar]
- 24.Rodríguez-González S., Gutiérrez-Ruíz I.M., Pérez-Ramírez I.F., Mora O., Ramos-Gomez M., Reynoso-Camacho R. Mechanisms related to the anti-diabetic properties of mango (Mangifera indica L.) juice by-product. J Funct Foods. 2017;37:190–199. doi: 10.1016/j.jff.2017.07.058. [DOI] [Google Scholar]
- 25.Sulistyawati I., Dekker M., Verkerk R., Steenbekkers B. Consumer preference for dried mango attributes: a conjoint study among Dutch, Chinese, and Indonesian consumers. J Food Sci. 2020;85(10):3527–3535. doi: 10.1111/1750-3841.15439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Semkoff J., Evans S., Janthachotikun S., Eldoumi H., Mahmood M., Meister M., Payton M., Peterson S., Perkins‐Veazie P., Clarke S., Smith B. The effect of mango supplementation on clinical parameters of pre‐diabetic individuals. Faseb J. 2015;29:602–612. doi: 10.1096/fasebj.29.1_supplement.602.12. [DOI] [Google Scholar]
- 27.Almiron‐Roig E., Chen Y., Drewnowski A. Liquid calories and the failure of satiety: how good is the evidence? Obes Rev. 2003;4(4):201–212. doi: 10.1046/j.1467-789X.2003.00112.x. [DOI] [PubMed] [Google Scholar]
- 28.Drewnowski A., Specter S.E. Poverty and obesity: the role of energy density and energy costs. Am J Clin Nutr. 2004;79(1):6–16. doi: 10.1093/ajcn/79.1.6. [DOI] [PubMed] [Google Scholar]
- 29.Rolls B.J., Castellanos V.H., Halford J.C., Kilara A., Panyam D., Pelkman C.L., Smith G.P., Thorwart M.L. Volume of food consumed affects satiety in men. Am J Clin Nutr. 1998;67(6):1170–1177. doi: 10.1093/ajcn/67.6.1170. [DOI] [PubMed] [Google Scholar]
- 30.Rolls B.J., Barnett R.A. Harper Collins; New York, NY: 2000. Volumetrics. A systematic lifetime approach to eating. [Google Scholar]
- 31.Akhlaghi M. The role of dietary fibers in regulating appetite, an overview of mechanisms and weight consequences. Crit Rev Food Sci Nutr. 2022:1–12. doi: 10.1080/10408398.2022.2130160. [DOI] [PubMed] [Google Scholar]
- 32.Slavin J., Green H. Dietary fibre and satiety. Nutr Bull. 2007;32:32–42. doi: 10.1111/j.1467-3010.2007.00603.x. [DOI] [Google Scholar]
- 33.Lyon M.R., Kacinik V. Is there a place for dietary fiber supplements in weight management? Curr Obes Rep. 2012;1:59–67. doi: 10.1007/s13679-012-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu K., Ke M.Y., Li W.H., Zhang S.Q., Fang X.C. The impact of soluble dietary fibre on gastric emptying, postprandial blood glucose and insulin in patients with type 2 diabetes. Asia Pac J Clin Nutr. 2014;23(2):210–218. doi: 10.6133/apjcn.2014.23.2.01. [DOI] [PubMed] [Google Scholar]
- 35.Norton G.N., Anderson A.S., Hetherington M.M. Volume and variety: relative effects on food intake. Physiol Behav. 2006;87(4):714–722. doi: 10.1016/j.physbeh.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 36.Rolls B.J., Roe L.S. Effect of the volume of liquid food infused intragastrically on satiety in women. Physiol Behav. 2002;76(4–5):623–631. doi: 10.1016/S0031-9384(02)00801-6. [DOI] [PubMed] [Google Scholar]
- 37.Yarandi S.S., Hebbar G., Sauer C.G., Cole C.R., Ziegler T.R. Diverse roles of leptin in the gastrointestinal tract: modulation of motility, absorption, growth, and inflammation. Nutrition. 2011;27(3):269–275. doi: 10.1016/j.nut.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Desai A.J., Dong M., Harikumar K.G., Miller L.J. Cholecystokinin-induced satiety, a key gut servomechanism that is affected by the membrane microenvironment of this receptor. Int J Obes. 2016;6(Suppl 1):S22–S27. doi: 10.1038/ijosup.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bourdon I., Olson B., Backus R., Richter B.D., Davis P.A., Schneeman B.O. Beans, as a source of dietary fiber, increase cholecystokinin and apolipoprotein b48 response to test meals in men. J Nutr. 2001;131(5):1485–1490. doi: 10.1093/jn/131.5.1485. [DOI] [PubMed] [Google Scholar]
- 40.Park H.A. Fruit intake to prevent and control hypertension and diabetes. Korean J Fam Med. 2021;42(1):9–16. doi: 10.4082/kjfm.20.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosas M., Jr., Pinneo S., O'Mealy C., Tsang M., Liu C., Kern M., Hooshmand S., Hong M.Y. Effects of fresh mango consumption on cardiometabolic risk factors in overweight and obese adults. Nutr Metabol Cardiovasc Dis. 2022;32(2):494–503. doi: 10.1016/j.numecd.2021.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Wall-Medrano A., Olivas-Aguirre F.J., Ayala-Zavala J.F., Domínguez-Avila J.A., Gonzalez-Aguilar G.A., Herrera-Cazares L.A., Gaytan-Martinez M. Health benefits of mango by-products. In book Food Wastes and By-products. 2020:159–191. doi: 10.1002/9781119534167.ch6. [DOI] [Google Scholar]
- 43.Preciado-Saldaña A.M., Domínguez-Avila J.A., Ayala-Zavala J.F., Astiazaran-Garcia H.F., Montiel-Herrera M., Villegas-Ochoa M.A., González-Aguilar G.A., Wall-Medrano A. Mango “Ataulfo” peel extract improves metabolic dysregulation in prediabetic wistar rats. Life. 2022;12(4):532. doi: 10.3390/life12040532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fomenko E.V., Chi Y. Mangiferin modulation of metabolism and metabolic syndrome. Biofactors. 2016;42(5):492–503. doi: 10.1002/biof.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miura T., Ichiki H., Hashimoto I., Iwamoto N., Kao M., Kubo M., Ishihara E., Komatsu Y., Okada M., Ishida T., Tanigawa K. Antidiabetic activity of a xanthone compound, mangiferin. Int J Phytomed. 2001;8(2):85–87. doi: 10.1078/0944-7113-00009. [DOI] [PubMed] [Google Scholar]