Abstract
Bovine fasciolosis caused by Fasciola hepatica and Fasciola gigantica, is a neglected tropical snail-borne trematode disease of cattle that poses an adverse effect on animals' health culminating in economic damage. Cross-sectional investigation on coprological and postmortem assessment and economic significance of bovine fasciolosis in cattle slaughtered at Tarcha Municipal Abattoir, Southern Ethiopia through simple random sampling techniques was conducted from December 2020 to August 2021. Out of 384 cattle examined, 20.3% and 29.94% were found harboring Fasciola through coprological examination and postmortem examination respectively. The leading cause of fasciolosis in the study area was Fasciola gigantica (53.9%) as compared to F. hepatica (46.1%). The investigation revealed that the sex, origin, and body condition of the cattle has a statistically significant effect on the prevalence of bovine fasciolosis. The odds of male animals infested with fasciolosis were 2.25 times higher than female cattle and those of poor body-conditioned cattle were three times higher than cattle with good body condition. In postmortem examination, an average of 6 flukes were found in a single infested liver and the mean fluke count was (10 ± 1.97) in severely infested livers while in lightly infested livers (3± 1.79). Considering postmortem examination as the gold standard for diagnosis of fasciolosis, the sensitivity of the direct sedimentation technique was found to be 68% and the specificity 100% with substantial agreement (k = 0.74) between the two methods. The estimated yearly direct financial losses incurred owing to fasciolosis was around 2,227,536 2, Birr (47,945.24 USD). Thus, bovine fasciolosis is one of the economically important animal diseases in the study area, which necessitates integrated control measures to tackle its effect on animal health and subsequent economic impact.
Keywords: Cattle, Economic importance, Fasciolosis, Prevalence, Tarcha
1. Introduction
Fasciolosis is one of the most common diseases that has a significant adverse effect on the health and productivity of ruminants (Abdulhakim and Addis, 2012; Yusuf et al., 2016). Fasciola hepatica, which predominates above 1800 m above sea level (masl), and Fasciola gigantica, which predominates below 1200 m.a.s.l, are two species of liver fluke (Fasciola) that cause bovine fasciolosis, as documented by stated (Berhe et al., 2009). It also affects humans, resulting in damage to liver tissues and the bile duct leading to inflammation and subsequent hepatomegaly or liver cirrhosis convoyed by diarrhea and anemia (Nyirenda et al., 2019). In infected ruminants, it poses enlargement and other pathological damages in the liver culminating in its condemnation during slaughter with resultant high economic loss in the livestock production sector and farmers (Jaja et al., 2017).
The amphibious L. truncatula and the water-dwelling L. natalensis snail species are essential for the survival of F. gigantica and F. hepatica, and the snails' bio network controls where the parasite is found (Legesse et al., 2017; Walker et al., 2008). Adult flukes reside in the bile duct and shed their eggs into the bile before entering the gut and being shed with faeces. Eggs hatch, a ciliated, motile miracidium infects the snail and there develop into sporocysts and then to radial stages and cercaria, which are shed by the snail, develop into infectious meta-cercaria on grass blades, which are subsequently consumed by the ultimate host, excyst in the small intestine, then make its journey to the liver through the gut wall, crossing the peritoneum (Zewde et al., 2019).
According to reports, domestic animal fasciolosis causes the global agricultural sector to lose an estimated US$ 2000 million annually (Hillyer and Apt, 1997). As stated by (Bekele and Getachew, 2010) fasciolosis poses huge financial losses in different parts of the world including the United Kingdom and Ireland alone greater than £18 million a year, a Swiss study estimated as €52 million a year or €299 per diseased animal mainly due to sub-clinical infection, in Kenya around 0.26 million USD annual losses due to liver condemnations in slaughtered cattle. In addition, the authors stated a high liver condemnation rate up to 100%, from cattle slaughtered in some abattoirs of Iringa, Tanzania. In their study in Adwa Municipal Abattoir, North Ethiopia they reported, the average annual financial loss attributed to the condemnation of the liver due to fasciolosis was 57,960 Ethiopian Birr (4674.2 USD). Other authors (Yusuf et al., 2016) from Haramaya, Ethiopia and (Zewde et al., 2019) from Wolaita Sodo, Ethiopia reported an annual loss of 4414.523 USD and $43, 024.458 Fasciola implicated liver condemnation in slaughtered cattle respectively.
Since the Ethiopian economy is mainly agriculture-based (Yigezu Wendimu, 2021), and Fasciola is widespread in Ethiopia (Birhan et al., 2019), Fasciola also poses tremendous economic loss indirectly associated with labor force reduction of tracking cattle, reduction of milk, meat, feed conversion efficiency, weight gain and fertility, death of animals and costs for treatment (Alemu, 2019; Jonsson et al., 2022; May et al., 2019; Wayessa et al., 2022). The annual economic loss attributable to the fasciolosis effect on animal productivity conventionally was estimated to be over USD 3.2 billion (Opio et al., 2021). Beyond this, a recent WHO research revealed that 180 million individuals are at risk of contracting fasciolosis and that 2.4 million people have the condition. Fasciolosis is officially recognized as an emerging human disease (WHO, 1995). According to (Alemu, 2019) human Fasciola infection was estimated to be from 2.4 to 1.7 million with 180 million at-risk populations across the world.
Several studies have been done in different parts of Ethiopia to determine the frequency of and assess the economic costs associated with fasciolosis (Abebe et al., 2010; Aragaw et al., 2012; Berhe et al., 2009; Fetene and Addis, 2014; Gebrie et al., 2015; Tadele and Worku, 2007; Zewde et al., 2019). However, no published work has been found on the coprological and postmortem assessment and economic significance of bovine fasciolosis in cattle slaughtered at Dawuro zone, Tarcha municipality abattoir. Thus, this study aimed to assess bovine fasciolosis through postmortem and coprological examination and estimate its financial losses in cattle slaughtered at the Tarcha municipality abattoir of Southern Ethiopia.
2. Methods and materials
2.1. Study area
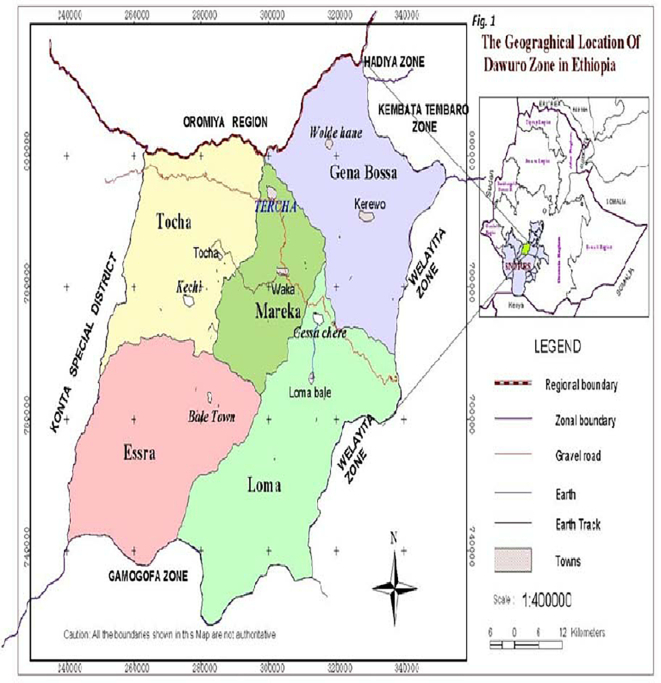
The study was carried out in the Dawro zone, southwest Ethiopia from November 2020 to August 2021. The area is situated in South Nation Nationalities and People's Region (SNNPR), which covers a total geographical area of approximately 4437 km2. The administrative hub of the zone, “Tarcha,” is located 500 km from Addis Abeba, the capital of Ethiopia and 322 km southwest of the regional headquarters Hawassa (Asfaw and Belachew, 2020). The zone is situated between 6.59 and 7.340 north latitudes and 36.68 and 37.520 east longitudes, with an elevation range of 501-3000 m. Kolla comprises 55.6% of the zone in terms of agroecology, followed by Weyna-Dega (41.4%) and Dega (3.3%). The Zones estimated livestock population was 113,554 sheep, 7081 horses, 1934 mules, 5064 donkeys, 28,557 traditional hives, and 157,996 chickens (CSA, 2017).
Map of the study area, Dawuro zone (Birhan et al., 2019).
2.2. Study animals
The study animal comprised indigenous beef cattle breeds, Borana breed (predominant beef cattle in the study site) and crossbreed cattle of both sexes, different ages, and body conditions originating from different agroecological zones. The age of the cattle was estimated by observing the incisor teeth according to (Yeates and Schmidt, 1994) and their body condition was categorized based on descriptions documented by (Nicholson and Butterworth, 1986).
2.3. Study design and sampling technique
The assessment of the prevalence of bovine fasciolosis through postmortem and coprological examination and estimation of its financial losses in cattle slaughtered at the Tarcha municipality abattoir of Southern Ethiopia was investigated through a cross-sectional study followed by a simple random sampling technique.
2.4. Sample size determination
The size of the sample for the investigation was determined by using the formula given by (Thrusfield, 2018) with an expected prevalence of 50% since there was no study conducted in the area. Substitution of the values in the formula gives the required sample size of 384 with a 95% confidential interval and 5% absolute precision.
When: N = required sample size;
Pexp = expected prevalence;
d = absolute precision.
2.5. Study methodology
2.5.1. Coprological examination
Each animal brought into the slaughterhouse for a normal meat inspection was given an identification number before being sampled and all the important data like sex, body condition, age, breed, and origin of the animals were recorded on the datasheet. Then faecal samples for coprological examination were taken from the animals' rectums while they are in the lairage and transferred to the securely closed universal bottles labeled accordingly. Then the samples were transported to the Tarcha veterinary clinic laboratory through an icebox and examined through the direct sedimentation technique for eggs of Fasciola species (Ahmad et al., 2020; Nyirenda et al., 2019). Immature flukes were identified by observing branching patterns of the caeca, ovary and testes (Valero et al., 2001; Valero et al., 2005).
2.5.2. Postmortem examination
The antemortem assessment involved meticulous documentation of the animals' ages, breeds, sexes, physical conditions, and places of origin. A unique identification number was also assigned to each research animal. Each liver had a thorough inspection to check for adult and immature Fasciola parasites (liver fluke). After palpating and observing the liver, sharp slices were made into the major bile ducts and into the parenchyma. The liver was then dissected into pieces of about 1 cm thickness and squeezed between the fingers to reveal flukes lodged in the tiny bile ducts (Opio et al., 2021; Zewde et al., 2019). After the bile ducts were sliced open with scissors, examined for liver flukes. Furthermore, the gall bladder was taken out of the liver, drained, and washed into a glass trough, after which the contents were checked for adult and immature liver flukes (Mequaninit and Mengesha, 2021; Nyirenda et al., 2019).
2.5.3. Fasciola species identification
The shape and size of each Fasciola species were used to identify them and classify them into respective species, and the results were recorded as F. hepatica and F. gigantica. The clues used to identify them are that F. gigantica is about 7.5 cm long with a narrow shoulder, while F. hepatica is around 3.5 cm long and 1.0 cm wide and has a leaf-like structure (Elsheikha and Khan, 2011). Immature flukes were identified into the respective species by, testes, ovary, oral sucker, ventral sucker and tegumental spines, pointed and molar shapes have been identified addressing to distinguish F. hepatica and F. gigantica species respectively after Hematoxylin and Eosin (H&E) staining technique (Fadavi et al., 2020; Lalor et al., 2021; Pandya et al., 2015).
2.6. Estimation of economic loss due to liver condemnation
For estimating the economic damage brought on by the condemnation of livers damaged by the liver fluke, all livers infected by Fasciola were deemed as being condemned. The annual loss from liver condemnation was calculated using the total number of animals slaughtered in the abattoir annually and the typical retail price of cattle liver at the abattoir. The average market price of the liver was established by conversing with butchers and abattoir workers, and the annual slaughter rate of the abattoir was calculated using past information from prior years available in the abattoir record. The annual financial loss incurred by the total condemnation of the liver was calculated according to the formula depicted by (Jaja et al., 2017; Ogurinade and Ogunrinade, 1980; Zewde et al., 2019) formerly.
2.7. Data analysis and management
All the data were entered into a Microsoft Excel 2016 spreadsheet, and then the data was analyzed using the STATA computer program version 14. Descriptive statistics were employed to explain the parasite's prevalence. The presence and the degree of correlation existing between risk factors and the prevalence of liver fluke in the study site were tested by using logistic regression and chi-square statistical tests. Those risk factors having a P-value <0.05 were regarded as statistically significant.
3. Results
3.1. Coprological assessment of bovine fasciolosis
Up on the coprological examination, 20.3% (78/384) of examined cattle were found positive for fasciolosis. The highest prevalence of fasciolosis was recorded in Mella 34.6% (27/84) followed by Bale 23.1% (18/95), Warra 17.9% (14/82), Dalba 15.4% (12/63) and least in Bato qelb 8.9% (7/60). While keeping cattle from Bale constant, those from Mella were found to have a 2.3 (OR = 2.30; CI = 1.13–4.71) times higher likelihood of getting infected with fasciolosis than those from Bato Qelb, Dalba, and Warra. The difference in the prevalence of fasciolosis among the origin of the cattle was statistically significant (P-value<0.05). The infection rate of fasciolosis was highest in medium body-conditioned cattle 39.7% (31/153) followed by those with good 35.9% (28/174) then by poor 24.4% (19/57) body-conditioned cattle. While cattle with good body condition were kept constant, those with poor body conditions were 3.24 (OR = 3.24; CI 1.57–6.68) times more susceptible to liver fluke infestation. The difference in the prevalence of fasciolosis among the cattle with different body conditions was statistically significant (P-value<0.05). Fasciolosis was highly prevalent in male (75.6%) than in female (24.4%) cattle. While female cattle were kept constant, the probability of male animals being infected with bovine fasciolosis was 1.99 (OR = 1.99; CI = 1.10–3.57) times higher. The difference in the infection rate of fasciolosis among both sexes of cattle was statistically significant (P-value<0.05). According to the age of cattle, adult cattle were found highly (61.5%) infected by fasciolosis than old (38.5%). The likelihood of old cattle contracting fasciolosis was 1.99 (OR = 1.06; CI = 0.57–1.97) times greater. Nevertheless, the difference in the prevalence of fasciolosis among the two age groups of the cattle was not statistically significant (P-value<0.05). Concerning the breed, a higher rate of fasciolosis was recorded in local breeds (91.1%) than in crossbreds (8.9%). The odds of local breeds contracting fasciolosis was 0.36 (OR = 1.06; CI = 0.57–1.97) while crossbreds were kept constant. However, the difference between the two breeds was not statistically significant (P-value>0.05) (Table 1).
Table 1.
Coprological assessment of bovine fasciolosis in association with its risk factors.
| Risk Factors | Category | No of animals examined | No positive animal | Prevalence (%) | χ2 value | P-value | OR [95% CI] |
|---|---|---|---|---|---|---|---|
| Sex | Male | 243 | 59 | 75.6 | 6.43 | 0.011 | 1.99 [1.10–3.57] |
| Female | 141 | 19 | 24.4 | 0.3202283–0.4168105 | |||
| Age | Old | 149 | 30 | 38.5 | 0.0048 | 0.945 | 1.06 [0.57–1.97] |
| Adult | 235 | 48 | 61.5 | Ref | |||
| Breed | Local | 365 | 71 | 91.1 | 3.37 | 0.066 | 0.36 [0.13–1.01] |
| Cross | 19 | 7 | 8.9 | Ref | |||
| BCS | Poor | 57 | 19 | 24.4 | 7.88 | 0.019 | 3.24 [1.57–6.68] |
| Medium | 153 | 31 | 39.7 | 1.33 [0.69–2.53] | |||
| Good | 174 | 28 | 35.9 | Ref | |||
| Origin | Bale | 95 | 18 | 23.1 | 10.73 | 0.030 | Ref |
| Bato qelb | 60 | 7 | 8.9 | 0.58 [0.22–1.52] | |||
| Dalba | 63 | 12 | 15.4 | 1.11[0.481–2.58] | |||
| Mella | 84 | 27 | 34.6 | 2.30 [1.13–4.71] | |||
| Warra | 82 | 14 | 17.9 | 0.89 [0.402–1.97] | |||
| Total | 384 | 78 | 20.3 |
3.2. Postmortem assessment of bovine fasciolosis
Up on postmortem examination, out of 384 cattle subjected to the study, 29.94% (115/384) were found infected by liver flukes, thus the overall prevalence of fasciolosis was 29.94%. Of these, 62 (53.91%) were infected with F. hepatica and 46.09% (53/384) with F. gigantica, with no mixed infection. According to the origin of cattle, in Bale and Warra, F. hepatica was the predominant species of Fasciola with a prevalence of 11.32% (6/53) and 28.3% (15/53), respectively. In Bale, Dalba and Mella, the higher infection was due to F. gigantica with a prevalence of 30.65% (19/62), 20.97% (13/62) and 24.19% (15/62) respectively. On the other hand, in Bata qelb, both species of Fasciola have almost the same prevalence F. hepatica 11.32% (6/53) F. gigantica 11.29% (7/62). From the study Kebeles, F. hepatica was highest 28.3% (15/53) in Warra and F. gigantica in Bale 30.65% (15/53) (Table 2).
Table 2.
Frequency of Fasciola species regarding animal origin on postmortem examination.
| Fasciola species | Total examined |
No of positive |
Frequency |
CI |
|||
|---|---|---|---|---|---|---|---|
|
F. hepatica |
384 |
62 |
53.91% |
[0.1068717–0.1764551] |
|||
| F. gigantica | 384 | 53 | 46.09% | [0.1278103–0.201914] | |||
| Total | 384 | 115 | 29.94% | ||||
| Frequency of species of Fasciola in relation to the origin of the cattle | |||||||
| Fasciola Species | No. of Fasciola detected | Bale (%) | Dalba (%) | Mella (%) | Warra (%) | Bata qelb (%) | χ2(p) |
| F. hepatica | 53 | 15 (28.3) | 5 (9.4) | 12(22.64) | 15(28.3) | 6(11.32) | 9.48(0.3) |
| F. gigantica | 62 | 19(30.65) | 13(20.97) | 15(24.19) | 8 (12.9) | 7(11.29) | |
In terms of sex, male cattle had a greater infestation rate (35.8%) than female cattle (19.85%), and they are 2.25 (OR = 2.25; CI = 1.36–3.72) times more susceptible to fasciolosis. The difference in frequency of fasciolosis was statistically significant (P-value<0.5). Regarding the age of animals, there was almost a similar infection rate of fasciolosis in both olds 30.87% (46/149) and in adult animals (69/235). In association with the breed of the animals, it was higher in cross breeds 42.1% (8/19) than in local breeds 29.32% (107/365). The difference between the breeds was not statistically significant (P-value>0.05) Animals with poor body condition had the highest (47.37%) Fasciola infection rate, followed by those in medium physical condition (28.76%) and then those in good physical condition (25.29%). Holding animals with medium body condition constant, the likelihood of poor body condition cattle contracting fasciolosis infection was three (OR = 3; CI = 1.55–5.66) times higher than that in good body condition cattle. There was a statistically significant (P-value<0.5) difference in the infection rate of fasciolosis among animals with different body conditions (Table 3).
Table 3.
Frequency of bovine fasciolosis with possible risk factors.
| Risk Factors | Category | No of the animal examined | No positive animal | Prevalence (%) | χ2 value | P-value | OR [95% CI] |
|---|---|---|---|---|---|---|---|
| Sex | Male | 243 | 87 | 35.8 | 10.81 | 0.001 | 2.25 [1.36–3.72] |
| Female | 141 | 28 | 19.85 | Ref | |||
| Age | Adult | 235 | 69 | 29.36 | 0.099 | 0.75 | 0.77 [0.45–1.31] |
| Old | 149 | 46 | 30.87 | Ref | |||
| Breed | Local | 365 | 107 | 29.32 | 1.41 | 0.24 | 0.59 [0.22–1.59] |
| Cross | 19 | 8 | 42.1 | Ref | |||
| BCS | Poor | 57 | 27 | 47.37 | 10.15 | 0.006 | 3 [1.55–5.66] |
| Medium | 153 | 44 | 28.76 | 1.09 [0.63–1.89] | |||
| Good | 174 | 44 | 25.29 | Ref |
Regarding the cattle's origin, those from Bale 35.79% (34/95) had the highest overall prevalence of fasciolosis, followed by cattle from Mella 32.14% (27/84), Dalba 28.57% (18/63), Warra 28.05% (23/82), and Bato qelb 21.67% (13/60). The difference in the prevalence of fasciolosis among the study kebeles was not statistically significant (P-value>0.05) (Table 4).
Table 4.
Frequency of fasciolosis in relation to the origin of the cattle.
| Origin of animal | No. of animals examined | No of positive animal | Prevalence (%) | χ2 value | P-value | CI |
|---|---|---|---|---|---|---|
| Bale | 95 | 34 | 35.79 | 3.9 | 0.42 | [0.2066263–0.2932364] |
| Dalba | 63 | 18 | 28.57 | [0.1301543–0.2047254] | ||
| Mella | 84 | 27 | 32.14 | [0.1800524–0.263096] | ||
| Warra | 82 | 23 | 28.05 | [0.1752503–0.2575865] | ||
| Bato qelb | 60 | 13 | 21.67 | [0.1231323–0.1962813] |
3.2.1. Intensity and severity of liver infection
A fluke count on 115 affected livers from slaughtered cattle revealed an average fluke burden of 6 flukes per liver (range: 1–11). One to six flukes per liver were present in the majority (53%) and 7 to 11 flukes per liver were seen in 47% of the affected livers of the study animals (Table 5).
Table 5.
Fluke count in the infested livers.
| Fluke count interval | No. of livers | Mean | Relative proportion (%) |
|---|---|---|---|
| 1–6 | 61 | 6 | 53.0 |
| 7–11 | 54 | 47.0 | |
| Total | 115 | 100 |
Concerning the severity of the pathological lesions observed, 11.3% (13/115), 52.2% (60/115) and 36.5% (42/115) livers were, mildly, moderately, and seriously infested, respectively. The mean number of adult flukes counted in severely afflicted livers was 10 (SD,1.97), for those moderately infested was 7(SD, 1.83) and for livers with light infestation was 3 (SD,1.79) (Table 6).
Table 6.
Categorization of livers according to the severity of lesions and respective mean fluke burdens.
| Severity of liver lesions | No of livers | Relative proportion (%) | Mean fluke burden | SD | P |
|---|---|---|---|---|---|
| Lightly affected | 13 | 11.3 | 3 | 1.79 | 0.0001 |
| Moderately affected | 60 | 52.2 | 7 | 1.83 | |
| Severely affected | 42 | 36.5 | 10 | 1.97 |
3.2.2. The sensitivity and specificity of the faecal examination technique
From 384 cattle subjected to both coprological and postmortem liver examination, there were Fasciola eggs only 78 in the samples, whereas 115 of them had found harboring flukes in their livers. As equated to the sedimentation technique, the specificity and sensitivity of the postmortem examination were 100% and 68%, respectively with strong agreement amid the two tests (k = 0.74) (Table 7).
Table 7.
Descriptive statistics of coprological examination with postmortem examination.
| Faecal examination | Presence of Fasciola spp. in liver |
Total | |
|---|---|---|---|
| Fluke (+) | Fluke (−) | ||
| Eggs present (+) | 78 | 0 | 78 |
| Eggs absent (−) | 37 | 269 | 306 |
| Total | 115 | 269 | 384 |
3.3. Assessment of financial loss associated with liver condemnation
Direct financial loss incurred as the result of the condemnation of the liver owing to the damage by bovine fasciolosis was weighed from the total count of the number of defective and condemned livers owing to fasciolosis. The annual financial forfeiture due to the condemnation of the liver was calculated from the total annual slaughter (cattle) capacity of the abattoir, the average price of the liver in the abattoir and retail market and the prevalence of fasciolosis in the liver of cattle slaughtered at the abattoir. The annual financial loss associated with liver condemnation due to fasciolosis was estimated by using the following formula as depicted by (Jaja et al., 2017; Ogurinade and Ogunrinade, 1980; Zewde et al., 2019).
Where, ALLC: Annual loss from liver condemnation,
MCS: Mean number of cattle slaughtered per year,
MCL: Mean cost of one liver at the abattoir.
P: Prevalence of Fasciola in cattle liver at the abattoir.
According to information acquired throughout the study period through interviews and antemortem examination, the average number of animals slaughtered annually, and the average retail price of the bovine liver in the Tarcha municipal abattoir was 14,880 and 500 ETB (10.76 USD) respectively. The findings from this investigation demonstrate that bovine fasciolosis was present in 29.94% of cattle. In light of this, it was determined that the annual economic loss attributed to bovine fasciolosis-associated liver condemnation would be 2,227,536 ETB (47,945.24 USD).
4. Discussion
In the present investigation, postmortem examination revealed a significant fasciolosis prevalence when compared to coprological testing, which was analogous to the results of (Radostits et al., 2007; Zewde et al., 2019) who reported from Wolaita zone, southern Ethiopia. For quite a while, the coprological result wasn't outstanding as the result reported by (Edilawit et al., 2012; Sánchez-Andrade et al., 2002). The intermittent nature of the eggs' evacuation through the faeces was the reason for the coproscopy procedure's low sensitivity in detecting the condition (Briskey, 1998) and moreover, a prolonged pre-patent period of 8 to 15 weeks after the infection is required for the eggs to be shed in the faeces (Hillyer and Apt, 1997; Radostits et al., 2007; Sánchez-Andrade et al., 2002).
The sensitivity of the sedimentation diagnostic technique was judged to be 68% based on the results of the liver examination, and there was high agreement (kappa = 0.74) between the two tests. Comparable results were reported previously from Vietnam (66.7%) and Switzerland (69%), respectively by (Abunna et al., 2010; Mamo et al., 1857). The latter asserted that conventional coproscopy, with a sensitivity of roughly 92%, can be highly useful if there is repeated sampling.
The prevalence of bovine fasciolosis obtained through postmortem examination of the cattle (29.94%) was consistent with the studies of (Abunna et al., 2010; Legesse et al., 2017; Mamo et al., 1857), who reported a prevalence of 27.2% in Hawassa, 30% in Dangla, 26% in Bahir Dar, and 28% in Kombolcha Industrial Abattoir, respectively. However, it was lower than the reports of (Mamo et al., 1857) in Kembata Tembaro zone, (Tadele and Worku, 2007) in Jimma municipality, (Edilawit et al., 2012) in Sinnana, (Tolosa and Tigre, 2007) in Kuyu district North shoa, and (Petros et al., 2013) in Sheno who reported a prevalence of 40.62%, 46.6%, 47%, 54.2%, and 74.0%, respectively. Nonetheless, it exceeded the reports of (Abunna et al., 2010) and (Zewde et al., 2019) who documented (14.0%) and (20.24%) respectively, in Sodo municipal abattoir, Southern Ethiopia. These differences in prevalence from different areas may be attributed to variations in ecology, climate, and management systems as they have an effect on the vector as well as the parasite epidemiology and infection possibility (Aregay et al., 2013).
According to (Abunna et al., 2010), F. hepatica and F. gigantica infections happen in Ethiopia in regions above 1800 m above sea level and below 1200 m above sea level, respectively. Postmortem examination of the diseased livers of cattle brought from different places revealed that F. gigantica (53.9%) predominated over F. hepatica (46.1%). The current finding was incongruous with other investigations carried out in different parts of Ethiopia (Aregay et al., 2013; Berhe et al., 2009; Edilawit et al., 2012; Mamo et al., 1857; Petros et al., 2013; Tolosa and Tigre, 2007). However present investigation corroborates the finding of (Abunna et al., 2010) who asserted that F. gigantica was the most prevalent species of liver fluke affecting cattle. This discrepancy can be spurred out by the surrounding ecology, which favors the expansion of both parasitic species' intermediate hosts, snails (Urquhart et al., 1996).
The results of this study demonstrated that, in both postmortem and coprological evaluation, the animal's body condition and sex have a significant influence (p-value<0.05) on the occurrence of bovine fasciolosis. There was also a statistically significant link between the animal's origin and the presence of bovine fasciolosis on coproscopy like the finding of (Zewde et al., 2019), who reported significant differences in fasciolosis prevalence between both sexes of cattle. However, (Assefa et al., 2015; Metages et al., 2018; Petros et al., 2013) documented that sex has no bearing on the rate of infection. In this study, a management strategy, in which males are exposed to the outside for longer periods while keeping females indoors during the start of lactation, may be to blame for the greater infection rate in males.
According to body conditions, animals in poor body conditions had the highest prevalence, followed by those in medium and good conditions, the difference was statistically significant (P < 0.05) with the prevalence of bovine fasciolosis which was in line with the findings of (Meharenet, 2018; Yusuf et al., 2016). Higher prevalence in cattle with poor body condition may be attributed to malnutrition or the presence of other chronic diseases that require a minimal parasite load to overcome host immunity. Additionally, a Fasciola infection is linked to poor physical body condition since the infection lowers blood and tissue fluid and even affects the parenchyma of the liver (in immature Fasciola), eventually resulting in protein. Furthermore, liver cirrhosis brought on by chronic fasciolosis may decrease bile flow to the duodenum, bile output, and fatty acid and lipid-soluble vitamin digestion and absorption, all of which contribute to weight loss.
The results of this investigation also showed that the prevalence of bovine fasciolosis is not significantly influenced by the breed or age of the animals. This demonstrated that all animals have an identical probability of contracting an infection. However, (Aregay et al., 2013; Meharenet, 2018) documented that, the age and breed of the animal have a substantial impact on the prevalence rate of bovine fasciolosis.
A mean fluke burden of 6 flukes per liver was found during a fluke count on infected livers, conversely (Soulsby, 1982) 50, (Zewde et al., 2019) 66 to 78 flukes per liver indicating a high pathogenicity than in the current study. Fluke loads of 40 to 140 have been shown to be typical in cattle in the USA (Dargie, 1987). Cattle in Iran, Nigeria, and Zambia had mean fluke loads of 68–100 (Schillhorn van Veen et al., 1980; Vercruysse and Claerebout, 2001). Significant production losses have also been reported to occur in infections with 30 flukes and/or herd prevalence of 25%, according to (Dargie, 1987; Vercruysse and Claerebout, 2001).
In contrast to (Dwinger et al., 1982), who observed significantly fewer flukes in highly damaged livers of beef cattle, the mean worm load with liver pathology in this investigation exhibited a statistically significant variance. These authors contend that severe fibrosis prevents the passage of immature flukes and that acquired resistance and bile duct calcification contribute to the ejection of flukes by generating an unfavorable milieu as reported by (Yilma and Mesfin, 2000).
In the current investigation, the economic loss incurred by bovine fasciolosis was estimated from the condemnation of the liver due to fasciolosis-inflicted abnormality. In the abattoir, all defective livers due to fasciolosis were considered unfit for human consumption and condemned. The annual financial losses attributed to the liver condemnation were estimated from the total number of animals (cattle) slaughtered in the study abattoir from the retrospective data of the abattoir and the average price of the single liver in the abattoir and retail market. For the estimation, the prevalence rate of fasciolosis in the current investigation was used. The direct economic loss as a result of total liver condemnation due to fasciolosis was estimated to be on average 2,227,536 2, birr (47,945.24 USD) per year, which was higher than the previous reports of (Abebe et al., 2010); (Tadele and Worku, 2007); (Bekele and Getachew, 2010); (Yusuf et al., 2016) and (Abunna et al., 2010) who reported 8312.5 USD, 6300 USD, 4674.2 USD, 4414 USD, and 4000 USD per annum, respectively.
5. Conclusion and recommendations
According to the current study, there was a significant prevalence of bovine fasciolosis, which resulted in a huge amount of liver being damaged and condemned, causing financial losses for local livestock farmers and adverse impacts on the animal production sector. During the investigation, both species of Fasciola Fasciola hepatica and Fasciola gigantica were documented in the study site. The sensitivity of the sedimentation method single examination and specificity was very good and there was strong agreement between both tests, which asserts coprological examination applicable method for the investigation of prevalence of the bovine fasciolosis. This higher prevalence rate necessitates that, it is essential to conduct control and prevention measures such as managing grazing, lowering the population of the intermediate host, diagnosing and treating sick animals with anthelmintics in order to tackle the infection and subsequent economic impression. Furthermore, it is better to molecular study to confirm the prevalent species of Fasciola in the study area.
Declaration of Competing Interest
All authors declared that there is no conflict of interest in publication of this research work.
References
- Abdulhakim Y., Addis M. An abattoir study on the prevalence of fasciolosis in cattle, sheep and goats in Debre Zeit town, Ethiopia. Glob. Vet. 2012;8:308–314. [Google Scholar]
- Abebe R., Abunna F., Berhane M., Mekuria S., Megersa B., Regassa A. Fasciolosis: prevalence, financial losses due to liver condemnation and evaluation of a simple sedimentation diagnostic technique in cattle slaughtered at Hawassa municipal abattoir, southern Ethiopia. Ethiopian Vet. J. 2010;14:39–52. [Google Scholar]
- Abunna F., Asfaw L., Megersa B., Regassa A. Bovine fasciolosis: coprological, abattoir survey and its economic impact due to liver condemnation at Soddo municipal abattoir, southern Ethiopia. Trop. Anim. Health Prod. 2010;42:289–292. doi: 10.1007/s11250-009-9419-3. [DOI] [PubMed] [Google Scholar]
- Ahmad I., Yakubu Y., Chafe U.M., Bolajoko B.M., Muhammad U. Prevalence of fasciolosis (liver flukes) infection in cattle in Zamfara, Nigeria: A slaughterhouse surveillance data utilizing postmortem examination. Vet. Parasitol. 2020;22 doi: 10.1016/j.vprsr.2020.100483. [DOI] [PubMed] [Google Scholar]
- Alemu B. Bovine Fasciolosis in Ethiopia-A review. J. Vet. Anim. Res. 2019;2:202. [Google Scholar]
- Aragaw K., Negus Y., Denbarga Y., Sheferaw D. Fasciolosis in slaughtered cattle in Addis Ababa abattoir, Ethiopia. Glob. Vet. 2012;8:115–118. [Google Scholar]
- Aregay F., Bekele J., Ferede Y., Hailemelekot M. Study on the prevalence of bovine fasciolosis in and around Bahir Dar, Ethiopia. Ethiopian Vet. J. 2013;17:1–11. [Google Scholar]
- Asfaw A., Belachew T. Effect of iodine deficiency on academic performance of school children in Dawro zone, Southwest Ethiopia: A prospective cohort study. Nutr. Diet. Suppl. 2020;12:157. [Google Scholar]
- Assefa A., Assefa Z., Beyene D., Desissa F. Prevalence of bovine fasciolosis in and around inchini town, west showa zone, Adaa Bega Woreda, Centeral Ethiopia. J. Vet. Med. Anim. Health. 2015;7:241–248. [Google Scholar]
- Bekele M., Getachew Y. Bovine Fasciolosis. Ethiopian J. Appl. Sci. Technol. 2010;1:39–47. [Google Scholar]
- Berhe G., Berhane K., Tadesse G. Prevalence and economic significance of fasciolosis in cattle in Mekelle area of Ethiopia. Trop. Anim. Health Prod. 2009;41:1503–1504. doi: 10.1007/s11250-009-9339-2. [DOI] [PubMed] [Google Scholar]
- Birhan M., Demewez G., Tewodros F., Tadegenge M. 2019. Prevalence and Economic Significance of Bovine Fasciolosis in Cattle Slaughtered at Debre. [Google Scholar]
- Briskey D. Diagnosis of liver fluke infections in cattle. Vet. Bull. 1998;68:1–4. [Google Scholar]
- CSA Livestock characteristics, agricultural sample survey. Addis Ababa, Ethiopia. Stat. Bull. 2017;2:9–13. [Google Scholar]
- Dargie J. The impact on production and mechanisms of pathogenesis of trematode infections in cattle and sheep. Int. J. Parasitol. 1987;17:453–463. doi: 10.1016/0020-7519(87)90121-4. [DOI] [PubMed] [Google Scholar]
- Dwinger R., Le Riche P., Kühne G. Fascioliasis in beef cattle in north-West Argentina. Trop. Anim. Health Prod. 1982;14:167–171. doi: 10.1007/BF02242150. [DOI] [PubMed] [Google Scholar]
- Edilawit W., Mekonnen A., Mulugeta T. An abattoir survey on the prevalence and monetary loss of fasciolosis among cattle in Wolaita Sodo town Ethiopia. Adv. Biol. Res. 2012;6:95–100. [Google Scholar]
- Elsheikha H.M., Khan H. Caister Academic Press; 2011. Essentials of Veterinary Parasitology. [Google Scholar]
- Fadavi A., Ashrafi K., Hassanpour H., Rokni M.B., Hosseini S.M., Bozorgomid A., Hosseinpour L., Najafi F., Mowlavi G. Identification of Fasciola species using Tegumental spines in tissue sections. Iran. J. Public Health. 2020;49:711. [PMC free article] [PubMed] [Google Scholar]
- Fetene A., Addis M. An abattoir survey on the prevalence and monetary loss of fasciolosis among cattle slaughtered at Dangila municipal abattoir, Ethiopia. J. Vet. Med. Anim. Health. 2014;6:309–316. [Google Scholar]
- Gebrie Y., Gebreyohannes M., Tesfaye A. Prevelance of bovine fasciolosis in and around Bahir Dar, north West Ethiopia. J. Parasitol. Vect. Biol. 2015;7:74–79. [Google Scholar]
- Hillyer G., Apt W. Food-borne trematode infections in the Americas. Parasitol. Today. 1997;3:87–88. [Google Scholar]
- Jaja I.F., Mushonga B., Green E., Muchenje V. Financial loss estimation of bovine fasciolosis in slaughtered cattle in South Africa. Parasite Epidemiol. Control. 2017;2:27–34. doi: 10.1016/j.parepi.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson N., MacLeod M., Hayward A., McNeilly T., Ferguson K., Skuce P. Liver fluke in beef cattle–impact on production efficiency and associated greenhouse gas emissions estimated using causal inference methods. Prevent. Vet. Med. 2022;200 doi: 10.1016/j.prevetmed.2022.105579. [DOI] [PubMed] [Google Scholar]
- Lalor R., Cwiklinski K., Calvani N.E.D., Dorey A., Hamon S., Corrales J.L., Dalton J.P., De Marco Verissimo C. Pathogenicity and virulence of the liver flukes Fasciola hepatica and Fasciola gigantica that cause the zoonosis Fasciolosis. Virulence. 2021;12:2839–2867. doi: 10.1080/21505594.2021.1996520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legesse S., Tsegaye S., Lamesgen S., Wolelaw Y., Garikipati D., Wondimagegn W. Coprological prevalence and associated risk factors of bovine fasciolosis in and around Zenzelma, Bahir Dar, Ethiopia. Eur. J. Exp. Biol. 2017;7 0–0. [Google Scholar]
- Mamo Y., Deneke Y., Ibrahim N. Prevalence of bovine Fasciolosis in and around Bedelle District, Ethiopia. Young. 1857;121(19):01. [Google Scholar]
- May K., Brügemann K., König S., Strube C. Patent infections with Fasciola hepatica and paramphistomes (Calicophoron daubneyi) in dairy cows and association of fasciolosis with individual milk production and fertility parameters. Vet. Parasitol. 2019;267:32–41. doi: 10.1016/j.vetpar.2019.01.012. [DOI] [PubMed] [Google Scholar]
- Meharenet B. Prevalence of bovine fasciolosis and economic significance in and around Chora Wereda, Western Ethiopia. Acta Parasitol. Glob. 2018;9:107–111. [Google Scholar]
- Mequaninit G., Mengesha A. Prevalence of bovine Fasciolosis and associated economic loss in cattle slaughtered at Kombolcha industrial Abbatior. J. Vet. Med. Anim. Sci. 2021;4:1078. [Google Scholar]
- Metages Y., Mathewos M., Ademasu A., Mikias E. 2018. Abatoir Survey on Prevalence and Economic Significance of Fasciolosis in Small Ruminants Slaughtered in Addis Ababa Municipal Abatoir, Ethiopia. [Google Scholar]
- Nicholson M., Butterworth M.H. 1986. A Guide to Condition Scoring of Zebu Cattle. ILRI (aka ILCA and ILRAD) [Google Scholar]
- Nyirenda S.S., Sakala M., Moonde L., Kayesa E., Fandamu P., Banda F., Sinkala Y. Prevalence of bovine fascioliasis and economic impact associated with liver condemnation in abattoirs in Mongu district of Zambia. BMC Vet. Res. 2019;15:1–8. doi: 10.1186/s12917-019-1777-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogurinade A., Ogunrinade B.I. Economic importance of bovine fascioliasis in Nigeria. Trop. Anim. Health Prod. 1980;12:155–160. doi: 10.1007/BF02242647. [DOI] [PubMed] [Google Scholar]
- Opio L.G., Abdelfattah E.M., Terry J., Odongo S., Okello E. Prevalence of fascioliasis and associated economic losses in cattle slaughtered at lira municipality abattoir in northern Uganda. Animals. 2021;11:681. doi: 10.3390/ani11030681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya S., Hasnani J., Patel P. 2015. Research–Granthaalayah. [Google Scholar]
- Petros A., Kebede A., Wolde A. Prevalence and economic significance of bovine fasciolosis in Nekemte municipal abattoir. J. Vet. Med. Anim. Health. 2013;5:202–205. [Google Scholar]
- Radostits O., Gay K., Hinchcliff C., Constable P. A text book of the disease of cattle, horse, sheep, goats and pigs. Vet. Med. 2007;1516 [Google Scholar]
- Sánchez-Andrade R., Paz-Silva A., Suárez J., Panadero R., Pedreira J., López C., Díez-Baños P., Morrondo P. Influence of age and breed on natural bovine fasciolosis in an endemic area (Galicia, NW Spain) Vet. Res. Commun. 2002;26:361–370. doi: 10.1023/a:1016290727793. [DOI] [PubMed] [Google Scholar]
- Schillhorn van Veen T., Folaranmi D., Usman S., Ishaya T. Incidence of liver fluke infections (Fasciola gigantica andDicrocoelium hospes) in ruminants in northern Nigeria. Trop. Anim. Health Prod. 1980;12:97–104. doi: 10.1007/BF02242616. [DOI] [PubMed] [Google Scholar]
- Soulsby E. Arthropods and Protozoa of Domesticated Animals. 1982. Helminths; p. 291. [Google Scholar]
- Tadele T., Worku T. The prevalence and economic significance of bovine fasciolosis at Jimma abattoir. Ethiopia IJVM. 2007;3:1937–1943. [Google Scholar]
- Thrusfield M. John Wiley & Sons; 2018. Veterinary Epidemiology. [Google Scholar]
- Tolosa T., Tigre W. The prevalence and economic significance of bovine fasciolosis at Jimma abattoir, Ethiopia. Int. J. Vet. Med. 2007;3:1–7. [Google Scholar]
- Urquhart G., Armour J., Duncan J., Dunn A., Jennings F. Vol. 213. Black well science Ltd; Oxford, UK: 1996. Veterinary Parasitology; pp. 242–251. [Google Scholar]
- Valero M., Panova M., Mas-Coma S. Developmental differences in the uterus of Fasciola hepatica between livestock liver fluke populations from Bolivian highlands and European lowlands. Parasitol. Res. 2001;87:337–342. doi: 10.1007/pl00008588. [DOI] [PubMed] [Google Scholar]
- Valero M., Panova M., Mas-Coma S. Phenotypic analysis of adults and eggs of Fasciola hepatica by computer image analysis system. J. Helminthol. 2005;79:217–225. doi: 10.1079/joh2005301. [DOI] [PubMed] [Google Scholar]
- Vercruysse J., Claerebout E. Treatment vs non-treatment of helminth infections in cattle: defining the threshold. Vet. Parasitol. 2001;98:195–214. doi: 10.1016/s0304-4017(01)00431-9. [DOI] [PubMed] [Google Scholar]
- Walker S., Makundi A., Namuba F., Kassuku A., Keyyu J., Hoey E., Prödohl P., Stothard J., Trudgett A. The distribution of Fasciola hepatica and Fasciola gigantica within southern Tanzania–constraints associated with the intermediate host. Parasitology. 2008;135:495–503. doi: 10.1017/S0031182007004076. [DOI] [PubMed] [Google Scholar]
- Wayessa T.E., Gesite A., Garedew S.H. Prevalence, risk factors and financial losses due to bovine Fasciolosis in toke Kutaye District, west Shewa zone, Oromia, Ethiopia. Int. Clin. Med. Case Rep. J. 2022;1:1–13. [Google Scholar]
- WHO . World Health Organization; 1995. Control of Foodborne Trematode Infections: Report of a WHO Study Group. [PubMed] [Google Scholar]
- Yeates N.T.M., Schmidt P.J. 1994. Beef Cattle Production. Sydney, Australia. [Google Scholar]
- Yigezu Wendimu G. The challenges and prospects of Ethiopian agriculture. Cogent Food Agric. 2021;7:1923619. [Google Scholar]
- Yilma J., Mesfin A. Dry season bovine fasciolosis in northwestern part of Ethiopia. Rev. Med. Vet. 2000;151:493–500. [Google Scholar]
- Yusuf M., Nuraddis I., Tafese W., Deneke Y. Food Science and Quality Management; 2016. Prevalence of Bovine Fasciolosis in Municipal Abattoir of Haramaya, Ethiopia; p. 48. [Google Scholar]
- Zewde A., Bayu Y., Wondimu A. Prevalence of bovine fasciolosis and its economic loss due to liver condemnation at Wolaita Sodo Municipal Abattoir, Ethiopia. Vet. Med. Int. 2019;2019 doi: 10.1155/2019/9572373. [DOI] [PMC free article] [PubMed] [Google Scholar]